Abstract
A series of N10-substituted acridone-2-carboxamide derivatives were synthesized and evaluated for their potent anti-cancer agents targeting AKT kinase. In vitro cytotoxicity activity of the target compounds was tested against breast cancer cell lines (MCF-7 and MDA-MB-231). Among the tested compounds, four compounds (7f, 8d, 8e, and 8f) exhibited promising anti-cancer activity against both cancer cell lines. Notably, compound 8f demonstrated the highest activity against MCF-7 and MDA-MB-231 at IC50 values of 4.72 and 5.53 μM, respectively. In vitro AKT kinase activity revealed that compounds 7f and 8f were the most potent AKT inhibitors with IC50 values of 5.38 and 6.90 μM, respectively. In addition, the quantitative ELISA method of testing confirmed that compound 8f effectively inhibited cell proliferation by suppressing the activation of p-AKT Ser473. Furthermore, molecular docking studies revealed that compound 8f can bind well to the active site of the AKT enzyme. The in silico ADME studies suggested that all synthesized molecules showed good oral bioavailability with a low-toxicity profile and can be used for further optimization as AKT kinase inhibitors in the treatment of breast cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-023-03524-z.
Keywords: Acridone-2-carboxamide, AKT, Molecular docking, Anti-cancer, SAR, Breast cancer
Introduction
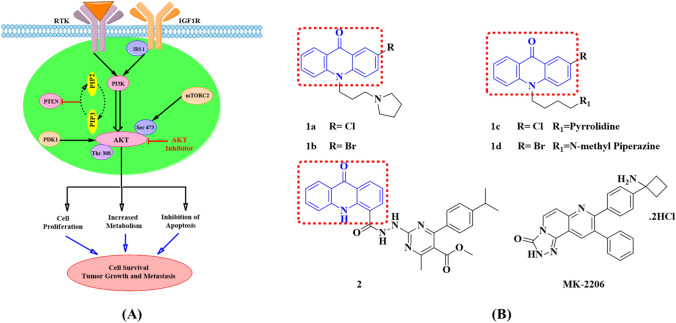
Cancer remains the leading cause of mortality in the world today. Worldwide, an estimated 19.3 million new cancer cases and almost 10.0 million cancer deaths were recorded in 2020. Female breast cancer has beaten lung cancer as the most commonly diagnosed cancer, with an estimated 2.3 million new cases (11.7%), followed by lung (11.4%), colorectal (10.0%), prostate (7.3%), and stomach (5.6%) cancers (Sung et al. 2021). At present, chemotherapy is the best approach used for the treatment of cancer. But the toxicity of available drugs and the development of resistance over existing drugs increases the need for the development of newer anti-cancer agents with low toxicity and high efficacy. Hence, many scientists are struggling to develop newer chemotherapeutic approach molecules (Belmont et al. 2007). In this direction, acridone is one of the heterocyclic structure having unique chemical properties which exists in both protonated and unprotonated forms, and their ability to intercalate with DNA, also act as a substrate to P-glycoprotein which is an essential target in reversing drug resistance in cancer (Belmont and Dorange 2008; Gensicka-Kowalewska et al. 2017). These derivatives have exhibited a wide array of pharmacological activities including anti-bacterial (Salimon et al. 2010), anti-malarial (Kelly et al. 2009; Wang et al. 2014), anti-viral (Tabarrini et al. 2006; Sepúlveda et al. 2012), anti-fungal (Ahua et al. 2004), anti-inflammatory (Chen et al. 2002), algicidal (Meepagala et al. 2005), anti-cancer (Zhang et al. 2017; Mahajan et al. 2015; Sathish et al. 2009), anti-allergic and as fluorescent probes (Chukaew et al. 2008; Qiu et al. 2009). Because of their unique properties, acridone moiety has drawn the attention of medicinal chemists. Many researchers have reported different substituted acridone derivatives which have shown potent anti-cancer activity against breast cancer (Kumar et al. 2014; Murahari et al. 2017; Rajendra Prasad et al. 2016; Babu et al. 2014). The AKT serine/threonine kinase, which is also known as protein kinase B (PKB), is a key regulator for PI3K/AKT/mTOR signaling pathway, which also plays a significant role in cell growth, metabolism, protein translation, cell survival, and autophagy (Mundi et al. 2016). Activation of this pathway contributes to the pathogenesis of many cancer types including breast cancer (Hill and Hemmings 2002; Sale et al. 2006; Morgensztern and McLeod 2005). The signaling through the phosphatidylinositol-3-kinase (PI3K)/AKT/mTOR pathway is a key mechanism of survival and therapeutic resistance across all breast cancer receptor subtypes (Fig. 1A) (Jansen et al. 2016). Inhibition of AKT activity prevents the growth and metabolic process in cancer cells and induces apoptosis which ultimately leads to cancer cell death. Hence, the inhibition of AKT might help in preventing the growth of cancer cells and would be a better therapeutic approach in the development of potent derivatives against cancer (Song et al. 2019; Martorana et al. 2021). The MK-2206 dihydrochloride is a selective allosteric pan-AKT inhibitor. It is an orally active allosteric inhibitor of AKT with an IC50 of 5 nM, 12 nM, and 65 nM against AKT1, AKT2, and AKT3, respectively. It is presently under phase II clinical studies for its anti-cancer activity (Fig. 1B) (Xing et al. 2019; Jo Chien et al. 2020).
Fig. 1.
(A) AKT as a potential target for cancer treatment. Its mechanism involves the activation of PI3K directly by receptor tyrosine kinase (RTK) or in conjugation with adaptor proteins such as IRS-1/2. The PI3K phosphorylates phosphotidyl inositol 4,5-biphosphate (PIP2) which is converted to phosphotidyl inositol 3,4,5-triphosphate (PIP3). The AKT binds to PIP3 at the plasma membrane and induces conformational changes which result in phosphorylation of AKT on two conserved residues where phosphorylation of Thr308 and Ser473 were promoted by a protein-dependent kinase (PDK-1) and the mammalian target of rapamycin complex 2 (mTORC-2), respectively. The phosphatase and tensin homolog (PTEN) leads to the de-phosphorylation of PIP3 and AKT. Phosphorylation at these residues leads to the activation of AKT which mediates various cellular functions such as cell proliferation, metabolism, protein synthesis, and cell survival. Hence, AKT is one of the potential and validated therapeutic target in cancer treatment. (B) Structures of acridone derivatives and MK-2206 as AKT inhibitors
Keeping in view the studies as AKT inhibitors the scientist designed newer moieties of acridone molecules as possible AKT inhibitors. Houghton et al.; showed that acridone-based derivatives as AKT inhibitors and patented the same. These acridone compounds (1a–1d) clearly showed their inhibitory AKT phosphorylation and kinase activity below 5 µM. This study suggested that the presence of an alkyl spacer at the N10 position is one of the essential structural requirement for a potent AKT inhibitor (Houghton et al. 2006). Murahari et al. also synthesized and evaluated hybrid systems of acridones with substituted pyrimidines for cytotoxicity against A-549, HeLa, MCF-7, and MDA-MB-231 cancer cell lines, respectively. Among the series, compound 2 mentioned in Fig. 1B exhibited AKT inhibition with an IC50 of 12 nM. This study led us and gave more insights into that hybrid molecules were selective and had the potential to inhibit AKT1. In addition to that, the results further suggested that these acridone derivatives could be used as leads for developing newer molecules in the field of anti-cancer research (Murahari et al. 2017).
Based on the literature and previous studies, an attempt to synthesize N10-substituted acridone-2-carboxamide derivatives with AKT kinase inhibition has been carried out. In the present study, we mainly focus on acridone derivatives and their anti-cancer activity against breast cancer with inhibition of AKT enzyme in breast cancer cells that are known to express the AKT kinases. We have synthesized newer N10-substituted acridones, characterized and screened for in vitro anticancer activity against human breast cancer cell lines MCF-7 and MDA-MB-231 for AKT inhibitory activity. Quantitative enzyme-linked immunosorbent assay (ELISA) analysis of phosphorylated AKT Ser473 (p-AKT) and total-AKT was carried out to determine the underlying molecular mechanism of the most active derivatives. We have performed molecular docking studies to identify the orientation, binding pattern, and amino acids at the active site of AKT. Furthermore, this study highlights acridone structural modification and has identified the potential acridone derivatives with promising AKT inhibitory activity and its structure–activity relationship (SAR) studies.
Materials and methods
All the chemicals and solvents utilized for the present study were purchased from the producers such as Sigma-Aldrich, SD-Fine, LOBA, and Hi-Media Laboratories Pvt. Ltd, Mumbai. The progress of reactions was monitored by thin layer chromatography (TLC) using Merck silica gel 60 F254 aluminum sheets. The spot identification was done using UV light. All the compounds were purified by passing through column chromatography packed with silica gel of 230–400 mesh size. The melting points of the compounds were determined on a Veego-programmable melting point apparatus (microprocessor-based). Purity analysis was carried out on the HPLC system of Shimadzu LC-20AD Prominence Liquid Chromatography. The separation process was carried out Phenomenex C18 bonded reverse-phase column. The mobile phase used was methanol and water which was degassed prior and the flow rate was maintained at 1.0 mL/min. Purity of all final compounds was found to be ≥ 95%. Pure compounds characterized by 1H NMR and 13C NMR were recorded in Bruker DRX 400 MHz Fourier transform NMR spectrometer in DMSO-d6 as a solvent and tetramethyl silane as an internal standard. Chemical shifts relative to deuterated solvent were expressed in parts per million (ppm). The IR spectra of the molecules were recorded on FTIR-ATR Perkin Elmer two Spectrum. The mass spectra at ESI–MS mode (positive and negative) were recorded with Agilent Technologies 6410 Triple Quad LC/MS. Chemical information like the molecular weight of title compounds was determined by obtaining ESI–MS spectroscopy using methanol as solvent.
Synthesis of target compounds
2-{(4-Carboxyphenyl) amino} benzoic acid (3)
2-Chlorobenzoic acid (5 g, 0.031 mmol), p-amino benzoic acid (4.81 g, 0.035 mmol), and copper powder (0.15 g, 0.0015 mmol) were mixed well in 30 mL of isoamyl alcohol, and anhydrous K2CO3 (6.72 g, 0.047 mmol) was added to it. Then the contents were mixed and kept on reflux for 6 h. After complete distillation of isoamyl alcohol, decolorizing carbon was added to the brown residual solution. The mixture was boiled for 15 min and filtered by suction. The filtrate was added, with stirring, to a mixture of 250 ml of conc. HCl and water (1:2). The precipitated acid was filtered with suction and a light brown color solid was obtained. This solid was recrystallized with ethanol to get the pure light cream-colored product. After drying to constant weight in the air, the yield was 48.21% (Rajendra Prasad et al. 2016). MS (ESI): m/z: 257.24 [M + H]+ 258.0, 279.9.
9(10H)-Acridone-2-carboxylic acid (4)
To compound 3 (3.5 g, 0.013 mmol) in a 250-mL round bottom flask (RBF), 15 mL of PPA was added. The contents of the flask were mixed well and kept for heating in a water bath at 100 °C. The reaction was monitored by TLC and after consumption of starting material, the reaction mixture was poured into cold distilled water, and the yellow color precipitate was obtained which was filtered, washed with distilled water, air-dried, and used for the next step as such (Rajendra Prasad et al. 2016). (Weighs 3.0 g, 93.75%). MS (ESI): m/z: 239.22 [M + H]+ 240.0.
10-Propyl/ butyl-9-oxo-9,10-dihydroacridine-2-carboxylic acid (5a/6a)
To a solution of 9(10H)-acridone-4-carboxylic acid (1 g, 0.0041 mmol) in DMF (20 mL) at 0 °C, anhydrous K2CO3 (1.13 g, 0.0082 mmol) was added and allowed to stir for 20 min. Then, n-bromobutane/n-bromopropane (0.86/0.77 g, 0.0061 mmol) was added and stirred at RT for 8–10 h. The reaction was monitored by TLC, and after completion of the reaction, the solution was poured into ice-chilled water. The precipitate was obtained which was filtered and washed with cold water, air-dried, and used for further process (Rajendra Prasad et al. 2016). 5a: (Weighs 0.82 g, 68.37%). MS (ESI): m/z: 282.15 [M + H]+ 283.20. 6a: (Weighs 0.9 g, 73.17%). MS (ESI): m/z: 295.33 [M + H]+ 296.20.
General procedure for compounds 7a–7g and 8a–8g
To a suspended solution of compound 5a/6a (1 mmol) in tetrahydrofuran (THF) (5 mL) at 0 °C thionyl chloride (1.5 mmol) was added dropwise and allowed to stir for 20 mins. Then the reaction mixture was refluxed for 3–4 h. The reaction was monitored by TLC and after complete consumption of the reactant, the reaction mixture was allowed to cool to RT, and then substituted anilines (1.1 mmol) with triethylamine (3 mmol) were added. The reaction mixture was allowed to stir at RT for 10–12 h. Then, the reaction mixture evaporated under reduced pressure to remove excess thionyl chloride and then extracted with ethyl acetate (30 mL × 3) and passed through sodium sulfate. Extracted fractions were collected and evaporated to dryness. The obtained crude product was purified either by recrystallization in ethanol or by column chromatography as suited to get the desired products (7a–7g) and (8a–8g). Spectral characterization of test compounds (1H NMR, 13C NMR, IR, and Mass) is mentioned in the supplementary information.
Biological evaluation
In vitro cell proliferation assay
Breast cancer cell lines MCF-7 and MDA-MB-231 were purchased from National Centre for Cell Sciences (NCCS), Pune, India. In vitro cytotoxic activity of all the synthesized N10-substituted acridone derivatives was evaluated using SRB assay. Doxorubicin was used as a standard. Briefly, cells were grown in DMEM media supplemented with 10% fetal bovine serum (FBS) and penicillin–streptomycin (50 U/mL, 50 mg/mL) at 37 °C, CO2 (5%) and air (95%). Logarithmically, growing cells were seeded in each well (5000–10,000 cells/well) using a 96-well plate and treated with compounds at different concentrations of the test compounds ranging from 0.1 to 100 μM. The plates were then incubated at 37 °C for 72 h in a 5% CO2 atmosphere, and a microscopic examination was performed after 24 h. The cells were fixed using ice-cold trichloroacetic acid (TCA) for 1 h at 4 °C. The cells were washed using distilled water to remove excess TCA and allowed to dry in the air. After 2 h, 50 μL of SRB solution was added to each well and allowed to stain at room temperature for 30 min. The plate was washed with 1% v/v acetic acid to remove the unbound dye and was allowed to dry in the air. About 100 μL of 10 mM unbuffered Tris Base (pH 10.5) was added to each well, and the plates were gently shaken for 5 min on a shaker platform to extract the bound SRB. The absorbance was measured using a microplate reader at a wavelength of 510 nm. The results were expressed as IC50 values in the μM range. IC50 is defined as the compound concentration required to reduce the viability of the cells by 50% with respect to the control (Vichai and Kirtikara 2006).
AKT kinase activity assay
All the reagents and working standards were prepared according to the ADI-EKS-400A assay kit (Enzo Life Sciences, Farmingdale, NY, USA). Stock solutions of N10-substituted acridone-2-carboxamides were prepared in dimethyl sulfoxide (DMSO) at a single concentration of 10 μM and stored at − 20 °C. The cells were treated with acridone derivatives, compound no.2, and 0.5% DMSO. Compound no. 2 and 0.5% DMSO were used as positive and negative control, respectively. The cell lysate was prepared by centrifugation of the cell pellets with RIPA lysis buffer at 8000 rpm and 4 °C for 20 min. The supernatants were collected, assayed for protein concentration estimation by Folin–Lowry method, and stored at − 80 °C for use. The AKT substrate microtiter plate was soaked with kinase assay dilution buffer at room temperature for 10 min. A volume of 30 μl of each of the diluted sample solutions and control was added to each empty well in duplicate. The reaction was initiated by adding 10 μl of ATP. The plate was covered with an adhesive plastic and was incubated for 90 min at room temperature. The contents were removed from the wells, and 40 μl of phosphor-specific substrate antibody was added to each well with an incubation period of 60 min. The contents were removed from the wells followed by washing them four times with 100 μl PBS buffer. In the next step, 40 μl of diluted secondary antibody was added and 30-min incubation was carried out. Washing was repeated with a volume of 100 μl PBS buffer. Then, 60 μl of 3,3ʹ,5,5-tetramethylbenzidine was added into each well (control, standard solutions, and derivatives samples). This was used to develop color in the wells with control, standard solutions, and derivatives samples. After sufficient color development, 20 μl of stop solution was added to the wells. Optical density was recorded with a plate reader at 450 nm. Data were analyzed, and results are presented as percentage AKT enzyme and compared to compound no. 2 as a reference AKT inhibitor. Then, potential molecules were further subjected to determine IC50 values using different concentrations (Chuang et al. 2015; Cui et al. 2016).
Quantitative ELISA analysis of p-AKT Ser473 and total-AKT
Further, in vitro quantitative estimation of phosphorylated (p-AKT Ser473) or total AKT protein was analyzed by using human p-AKT Ser473 and total AKT ELISA kit (Raybiotech, Cat No. PEL-AKT-S473-T-1). Briefly, MCF-7 cells were treated with compounds at different concentrations (0, 2.5, 5, and 10 µM) for 24 h. Cell lysates were prepared and used for the assay. One hundred microliters of lysates was added into each well and incubated for 2.5 h at RT. Then, these lysates were discarded and washed with buffer 4 times. One hundred microliters of primary antibody (p-AKT Ser473 and total AKT), as well as secondary antibody solutions, was added to measure phosphorylated or total protein in the cell lysates. Then, 100 μl of TMB substrate reagent was added followed by a stop solution. Optical density was measured at 450 nm. The activation status of AKT signaling proteins was evaluated according to the formula that the optical density (OD) value of p-AKT Ser473/the OD value of total AKT (Chen et al. 2021).
Molecular docking
Using AutoDock Vina in PyRx 0.8, the developed derivatives and AZD5363 (reference AKT inhibitor) were docked to the crystal structure of the PKB alpha in complex with AZD5363 (Dallakyan and Olson 2015). The structures of the acridone derivatives and AZD5363 were drawn using ChemDraw Ultra 12.0 (Mol File format). Using the open-Babel tool, the ligands were imported into the PyRx software. By using the Universal Force Field (UFF), each of the ligands was optimized in terms of reducing the amount of energy (Rappé et al. 1992). The ligands were then converted to pdbqt format and prepared for docking purpose. The crystal structure of the target protein was obtained from RCSB Protein Data Bank (PDB) with PDB ID: 4GV1 (https://www.rcsb.org/structure/4gv1, accessed on December 30, 2022). The reference inhibitor present in the crystal structure was 4-amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide. The protein structure was refined using Discovery Studio Visualizer (version 19.1.0.18287) and was purified and prepared for docking using the same program (San Diego: Accelrys Software Inc. 2012). The output file of the protein was saved in pdb file format and imported to PyRx to perform molecular docking studies. To aid molecular docking, a three-dimensional grid box (size_x = − 20.9277535202 Å; size_y = 4.86654471081 Å; size_z = 10.3527901031 Å) with an exhaustiveness value of eight was developed (Dallakyan and Olson 2015).
The strategy reported in previous papers was used to carry out the complete molecular docking method as well as to locate cavities and active amino acid residues (Almerico et al. 2008; Siddiqui et al. 2021; Khan et al. 2021; Unnisa et al. 2021; Jadhav et al. 2023). The exposed cavity of the enzyme is shown with the reference inhibitor in Fig. 2.
Fig. 2.
The reference AKT inhibitor in the allosteric location of the enzyme
The AutoDock Vina output results represented the docking scores as ΔG values. They were further converted to the predicted inhibition constants Ki (pred) (Shityakov and Förster 2014). The Ki pred values for analyzed docking poses were calculated from the ΔG parameters as follows:
where ∆G is the docking energy, Rcal (gas constant) is 1.98719 cal (mol K)−1, and TK (room temperature in kelvin) is 298 K (Iman et al. 2015).
ADMET studies
Swiss-ADME, a free web tool, was used to predict of adsorption, distribution, metabolism, and elimination (ADME) parameters of potential molecules (Daina et al. 2017). In addition to that, toxicity data was predicted by using the freely available web server ProTox II. Oral rat LD50 (median lethal dose) was predicted for the potential molecules treated in mg/kg body weight, followed by toxicity class (Drwal et al. 2014).
In the ProTox II server, toxicity data are given according to the globally harmonized system (GHS). There are 6 classes based on the toxic doses that are often represented in LD50. The toxicity classes are as follows; class I: fatal if swallowed (LD50 ≤ 5 mg/kg), class II: fatal if swallowed (5 < LD50 ≤ 50 mg/kg), class III: toxic if swallowed (50 < LD50 ≤ 300 mg/kg), class IV: harmful if swallowed (300 < LD50 ≤ 2000 mg/kg), class V: may be harmful if swallowed (2000 < LD50 ≤ 5000 mg/kg) and class VI: non-toxic (LD50 > 5000 mg/kg). Parameters, such as ‘average similarity’ and ‘prediction accuracy,’ were predicted depending on the chemical structure of acridone derivatives to compounds with known values in the database. Furthermore, parameters like organ toxicity, carcinogenicity, mutagenicity, and immunotoxicity were also predicted.
Result and discussions
Chemistry
As mentioned in Scheme 1, the synthesis of acridone starts with the Ullmann condensation of 2-chloro benzoic acid and p-amino benzoic acid in the presence of copper as a catalyst. Cyclization with polyphosphoric acid (PPA) yielded acridone-2-carboxylic acid. Further alkylation by treating with n-bromopropane/n-bromobutane gave N10-substituted acridone in the presence of solvent DMF. The final compounds were synthesized by coupling acridone-2-carboxylic acid with different substituted aromatic amines in THF in the presence of TEA which yielded two series, one with N-propyl substituted acridone-2-carboxamides (7a–7g) and the other with N-butyl substituted acridone-2-carboxamides (8a–8g) mentioned in Table 1. These compounds were synthesized by the outlined scheme. The molecular structures of the newly synthesized compounds were characterized by 1H NMR, 13C NMR, IR, and mass analytical techniques (Supplementary Data).
Scheme 1.
Synthesis of N10-substituted acridone-2-carboxamide derivatives. Reagents and conditions: (i) Cu, K2CO3, isoamyl alcohol, reflux, (ii) PPA, 100 °C, (iii) 1-bromopropane/1-bromobutane, K2CO3, DMF, 0 °C-RT, (iv) SOCl2, TEA, substituted aromatic amines, THF, 0 °C-Reflux
Table 1.
Synthesized N10-substituted acridone-2-carboxamide derivatives
| Comp code | R | R1 | Comp Code | R | R1 |
|---|---|---|---|---|---|
| 7a | Propyl | -Phenyl | 8a | Butyl | -Phenyl |
| 7b | Propyl | -4-Methylphenyl | 8b | Butyl | -4-Methylphenyl |
| 7c | Propyl | -4-Methoxyphenyl | 8c | Butyl | -4-Methoxyphenyl |
| 7d | Propyl | -4-Fluorophenyl | 8d | Butyl | -4-Fluorophenyl |
| 7e | Propyl | -4-Chlorophenyl | 8e | Butyl | -4-Chlorophenyl |
| 7f | Propyl | -4-Nitrophenyl | 8f | Butyl | -4-Nitrophenyl |
| 7 g | Propyl | -4-(Phenyldiazenyl)phenyl | 8 g | Butyl | -4-(Phenyldiazenyl)phenyl |
Biological evaluation
In vitro cytotoxicity assay
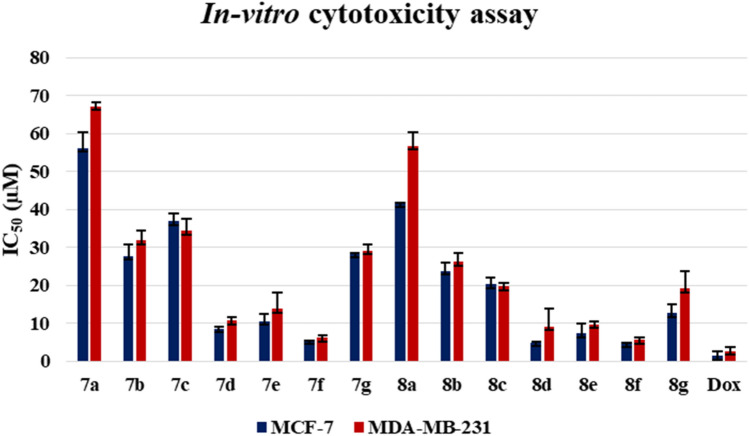
The anti-proliferative activity of the synthesized N10-substituted acridone derivatives having a carboxamide group at the 2nd position was determined using SRB assay, and doxorubicin was used as the positive control. The synthesized compounds (7a–7g, 8a–8g) were screened for in vitro cytotoxicity against human breast cancer cell lines such as MCF-7, MDA-MB-231, and normal human embryonic kidney cell line (HEK-293) was used as control. Treatment was carried out at nine different concentrations of the test compounds ranging from 0.1 to 100 μM (i.e., 0.1, 0.5, 1, 2.5, 5, 10, 25, 50, and 100 µM). The results of the in vitro cytotoxicity are expressed in terms of the compound concentration required to inhibit the growth of the cells by 50% with respect to the control (IC50 μM). The data from Fig. 3 and Table 2 show that some of the acridone molecules displayed good anti-proliferative activity with low IC50 values.
Fig. 3.
In vitro cytotoxicity assay result of N10-substituted acridone derivative
Table 2.
In vitro cytotoxicity of synthesized compounds
| Sr. no. | Compound code | IC50 (μM)a | ||
|---|---|---|---|---|
| MCF-7 | MDA-MB-231 | HEK-293 | ||
| 1 | 3a | > 100 | > 100 | ND |
| 2 | 4a | > 100 | > 100 | ND |
| 3 | 7a | 50.14 ± 4.12 | 53.19 ± 1.12 | 58.20 ± 1.96 |
| 4 | 7b | 27.76 ± 3.15 | 31.82 ± 2.61 | 41.82 ± 3.60 |
| 5 | 7c | 36.82 ± 2.16 | 34.32 ± 3.16 | 38.32 ± 2.43 |
| 6 | 7d | 8.56 ± 0.57 | 10.58 ± 1.01 | 32.59 ± 1.03 |
| 7 | 7e | 10.54 ± 1.77 | 13.68 ± 4.26 | 31.68 ± 3.86 |
| 8 | 7f | 5.41 ± 0.07 | 6.14 ± 0.72 | 36.18 ± 0.96 |
| 9 | 7g | 28.26 ± 0.27 | 29.14 ± 1.68 | 39.14 ± 1.36 |
| 10 | 8a | 41.63 ± 0.19 | 46.68 ± 3.65 | 50.02 ± 2.65 |
| 11 | 8b | 23.72 ± 2.12 | 26.20 ± 2.18 | 29.82 ± 2.39 |
| 12 | 8c | 20.16 ± 1.77 | 19.68 ± 0.93 | 34.83 ± 1.35 |
| 13 | 8d | 4.90 ± 0.11 | 9.16 ± 4.64 | 26.21 ± 3.24 |
| 14 | 8e | 7.24 ± 2.56 | 9.60 ± 0.93 | 34.32 ± 2.18 |
| 15 | 8f | 4.72 ± 0.15 | 5.53 ± 0.65 | 36.53 ± 0.16 |
| 16 | 8g | 12.63 ± 2.27 | 19.14 ± 4.64 | 29.14 ± 2.34 |
| 17 | bDox | 1.28 ± 1.19 | 2.6 ± 1.08 | ND |
IC50 = required concentration of compound for inhibition of cell proliferation by 50%
ND =Not determined
aThe values are the mean ± SD of at least three independent experiments
bDOX = doxorubicin, positive control compound
Among the synthesized series N-butyl derivative of acridone showed significant cytotoxicity. Results obtained from the biological study revealed that compounds 8d, 8e, and 8f were found to be active against both the breast cancer cell lines (MCF-7 and MDA-MB-231) with cytotoxicity of less than 10 µM. The compounds 8b, 8c, and 8g showed moderate to good cytotoxicity in the range of 12–30 µM against tested cell lines. N-propyl-substituted series compounds 7d and 7f exhibited better cytotoxicity against MCF-7 with IC50 less than 10 µM, whereas compound 7f displayed good cytotoxicity against MDA-MB-231 with an IC50 of 6.14 µM. The compounds 7b, 7e, and 7g showed cytotoxicity in the range of 20–30 µM against the MCF-7 breast cancer cell line. The structure–activity relationship suggested that the substitution of the methoxy group and azo-benzene on the phenyl ring lowered the cytotoxicity against both cell lines.
In addition, the cellular toxicity of the synthesized compound was again evaluated against normal HEK-293 cells, and results revealed that none of the compounds showed a significant effect on the growth of normal cells.
In vitro AKT kinase assay
Further, all synthesized molecules were evaluated against AKT kinase activity using compound 2 (Fig. 1B) as a reference standard. In vitro AKT kinase inhibition assay was performed at 10 μM concentration by using a commercially available ADI-EKS-400A assay kit. Amongst the tested series, seven compounds (7e, 7f, 7g, 8d, 8e, 8f, and 8g) showed more than 50% inhibition while compound 8f was found to be the most potent AKT inhibitor with 68.98% inhibition. Compounds showing more than 50% inhibition were re-evaluated for IC50 determination with seven concentrations (i.e., 0.01, 0.1, 0.5, 1, 5, 10, and 25 µM) in triplicate. The IC50 values were expressed in μM using a mean of triplicate and are mentioned in Table 3. Results obtained from evaluation revealed that compounds 8f and 7f displayed the most potent AKT inhibitory activity with IC50 values of 5.38 and 6.90 μM, respectively. These data revealed that the electron-withdrawing group at the para-position of the benzene ring improved the AKT enzyme inhibitory activity more significantly. These findings suggested that the inhibition of AKT might mainly contribute to the good activities of compounds against MCF-7 and MDA-MB-231 cell lines.
Table 3.
The AKT inhibitory activity of synthesized compounds
| Comp. code | IC50 (μM)a |
|---|---|
| 7e | 15.32 ± 0.66 |
| 7f | 6.90 ± 0.23 |
| 7g | 12.94 ± 0.28 |
| 8d | 11.96 ± 0.02 |
| 8e | 10.35 ± 0.12 |
| 8f | 5.38 ± 0.13 |
| 8g | 8.39 ± 0.05 |
| 2b | 0.10 ± 0.62 |
aThe values are the mean ± SD of at least three independent experiments
bCompound 2 = reference standard
We have compared our results with previous studies where they showed that acridone-based derivatives comprised of hydrophobic aromatic ring with hydrogen bond acceptor carbonyl, and alkyl spacer connecting the two nitrogen moieties could be an effective AKT inhibitor. In the present study, we observed comparable results with previous findings and indicated that our designed N-butyl substituted derivatives also showed potent AKT kinase inhibitory activity with the same acridone scaffold (Houghton et al. 2006). Our study revealed the importance of butyl spacer at the N10 position which is responsible for an increase in the AKT-specific anti-cancer activity and gave us more structural insights to increase their potency by modification.
Quantitative estimation of p-AKT Ser473 and total-AKT
In addition, we performed a quantitative ELISA analysis of total AKT and p-AKT Ser 473 to explore the effect of compounds 7f and 8f on the signal transduction mechanism. As shown in Fig. 4, MCF-7 cells were treated with increasing concentrations (2.5, 5, and 10 µM) of compounds 7f and 8f for evaluation of levels of p-AKT Ser 473 and total AKT. This assay results demonstrated that compounds showed a dose-dependent decrease in phosphorylation of AKT as compared to total AKT. It has been also found that compound 8f effectively inhibited the activation of p-AKT Ser473. Results finding conclude that both compounds inhibit cell proliferation by suppressing the activation of AKT signaling.
Fig. 4.
The effects of compounds 7f and 8f on phosphorylation of AKT in MCF-7 cells. The cells were treated with compounds at different concentrations (0, 2.5, 5, and 10 µM) for 24 h. Quantitative ELISA assay results of compounds 7f and 8f on A levels of p-AKT Ser473, B levels of total AKT, C the activation of p-AKT Ser473. A significant difference in p-AKT Ser473 was indicated between the control group and the 8f treated group. n.s, no significant difference, *P < 0.05, **P < 0.01, Error bars, mean ± SD of three independent experiments. Compounds inhibit cancer cell growth by suppressing the activation of AKT signaling
Determination of the significance of the correlation coefficient
Results obtained from in vitro cytotoxicity assay and AKT kinase assay demonstrated that compounds 7f and 8f displayed good cytotoxicity along with potent inhibitory activity toward AKT. A correlation between the IC50 value of both the cell lines (MCF-7 and MDA-MB-231) and % AKT inhibition has been studied and is depicted in Fig. 5. The results indicated that there was a good correlation between the IC50 value of both cell lines and % AKT inhibition among all the compounds with regression coefficient R2 = 0.7602 and R2 = 0.7718, respectively. Similarly, on comparison of lipophilicity of active compounds (7f, 8e, 8f, and 8g) with AKT inhibitory activity, it was observed that a significant increase in AKT inhibition as the decrease in the lipophilic character of molecules.
Fig. 5.
Correlation between IC50 of MCF-7, MDA-MB-231 cell line, and % AKT inhibition of all the compounds
Structure–activity relationship
Based on in vitro cytotoxicity and AKT inhibitory activity of all the synthesized N10-substituted acridone-2-carboxamides against breast cancer cell lines, the following assumptions about the SAR are clearly described in Fig. 6.
-
(i)
The presence of the carboxamide group at the 2nd position and alkyl substitution at the N10 position of the acridone ring is very important for anti-cancer activity.
-
(ii)
N-butyl-substituted acridone-2-carboxamides were found more active than N-propyl-substituted acridone-2-carboxamides.
-
(iii)
An increase in the length of the alkyl chain from propyl to butyl at the N10 position of acridone showed significant enhancement in the anti-cancer activity.
-
(iv)
Compound with the substituted nitro group at the 4th position phenyl ring showed more potent anti-cancer activity in both MCF-7 and MDA-MB-231 cell lines.
-
(v)
Compound with substitution of nitro, fluoro, and chloro group on phenyl at 4th position showed more potent anti-cancer along with AKT inhibitory activity.
-
(vi)
The replacement of the 4-chlorophenyl with 4-fluorophenyl showed a remarkable increase in the cytotoxicity of both MCF-7 and MDA-MB-231 cell lines.
-
(vii)
The carboxamide linker attached between the phenyl ring and acridone ring showed a rise in activity against both the cell lines.
-
(viii)
The derivative of 4-methoxyphenyl and 4-methyl phenyl have less activity compared to 4-chlorophenyl, 4-fluorophenyl, and 4-nitrophenyl derivatives.
-
(ix)
Among the different para substituents, the order of activity was found to be NO2 > F > Cl > CH3 > OCH3.
-
(x)
Although derivatives with a substituent such as azo-benzene at the 4th position on the phenyl ring have shown moderate anti-cancer activity, they showed potent AKT inhibitory activity
Fig. 6.
Structure–activity relationship of the newly synthesized acridone derivatives as AKT inhibitors and anti-cancer agent
These results indicate that the position of substituents, electron-negativity, and steric effect in the benzene ring might change the cytotoxic profile of acridone compounds.
Molecular docking analysis
For the evaluation of binding energy and interaction poses of acridone derivatives, a molecular docking study was done. In this study, we docked all the selected molecules with AKT enzyme to shortlist and analyze the molecular interaction modes based on interaction energy. From the docking results, we found that all the tested compounds showed binding energies in the range of − 8.4 to − 9.4 kcal/mol and reflected hydrogen bonding interaction and hydrophobic interactions (pi–anion, pi–pi t-shaped, pi–sigma, pi–sulfur, alkyl, and pi–alkyl). The docking interactions of all the ligands with active amino acid residues of the AKT enzyme are given in Table 4. The superimposed docking poses of the molecules in the active binding pocket are illustrated in Fig. 7. The docking scores and binding poses of all derivatives were compared with the co-crystallized inhibitor (AZD5363). The lower binding energy (more -ve) score indicates stronger and more favorable binding between the protein and ligand. The seven compounds (7a, 7g, 8a, 8b, 8d, 8e, and 8g) showed lower interaction energies compared to AZD5363 (Singh et al. 2022). The 2D-and 3D-docking interactions of the most potent compounds are depicted in Fig. 8A, whereas the 2D and 3D-docking poses of the remaining compounds are given in the Supplementary file in Table S1.
Table 4.
Docking Study of compounds with PDB ID: 4GV1
| Active amino acid residues | Bond length (Å) | Interaction category | Interaction type | Ligand energy | Docking score |
|---|---|---|---|---|---|
| (Kcal/mol) | |||||
| 7a | |||||
| Lys179 | 4.22716 | Electrostatic | pi–Cation | 308.32 | − 9.1 |
| Val164 | 3.79428 | Hydrophobic | pi–Sigma | ||
| Val164 | 3.73416 | ||||
| Val164 | 3.40038 | ||||
| Met281 | 3.68926 | Other | pi–Sulfur | ||
| Val164 | 4.71944 | Hydrophobic | pi–Alkyl | ||
| Ala177 | 4.07878 | ||||
| Ala230 | 5.39781 | ||||
| Phe438 | 5.33088 | ||||
| 7b | |||||
| Asp292 | 4.42558 | Electrostatic | pi–Anion | 306.84 | − 8.8 |
| Asp292 | 3.44331 | ||||
| Val164 | 3.83443 | Hydrophobic | pi–Sigma | ||
| Met281 | 3.81847 | Other | pi–Sulfur | ||
| Leu156 | 4.67076 | Hydrophobic | Alkyl | ||
| Ala177 | 4.02738 | ||||
| Ala230 | 4.03833 | ||||
| Met281 | 4.89469 | ||||
| Lys179 | 5.29003 | ||||
| Leu181 | 4.43764 | ||||
| Leu156 | 5.15554 | pi–Alkyl | |||
| Ala177 | 4.41821 | ||||
| Phe161 | 5.41406 | ||||
| Tyr229 | 4.55216 | ||||
| Phe438 | 4.92419 | ||||
| 7c | |||||
| Gly157 | 3.33778 | Hydrogen bond | Carbon hydrogen bond | 321.17 | − 8.4 |
| Glu234 | 3.37134 | Electrostatic | pi–Anion | ||
| Glu234 | 3.18157 | ||||
| Phe236 | 4.27334 | Hydrophobic | pi–Alkyl | ||
| 7d | |||||
| Glu191 | 3.18543 | Halogen | Halogen (fluorine) | 307.25 | − 8.6 |
| Val164 | 3.93772 | Hydrophobic | pi–Sigma | ||
| Val164 | 3.95879 | ||||
| Val164 | 3.71584 | ||||
| Val164 | 3.78111 | ||||
| Met281 | 3.82098 | Other | pi–Sulfur | ||
| Ala177 | 4.15334 | Hydrophobic | pi–Alkyl | ||
| Phe438 | 5.17675 | ||||
| 7e | |||||
| Val164 | 3.93589 | Hydrophobic | pi–Sigma | 306.31 | − 8.6 |
| Val164 | 3.96262 | ||||
| Val164 | 3.72883 | ||||
| Val164 | 3.79365 | ||||
| Met281 | 3.81556 | Other | pi–Sulfur | ||
| Ala177 | 4.14909 | Hydrophobic | pi–Alkyl | ||
| Phe161 | 4.5388 | ||||
| Phe438 | 5.19921 | ||||
| 7f | |||||
| Val164 | 3.95393 | Hydrophobic | pi–Sigma | 320.02 | − 8.7 |
| Val164 | 3.80327 | ||||
| Val164 | 3.90008 | ||||
| Met281 | 3.80029 | Other | pi–Sulfur | ||
| Ala177 | 4.14092 | Hydrophobic | pi–Alkyl | ||
| Phe438 | 5.0053 | ||||
| 7g | |||||
| Glu191 | 3.29651 | Hydrogen bond; electrostatic | Salt bridge; attractive charge | 369.11 | − 9.2 |
| Glu191 | 4.68868 | Electrostatic | Attractive charge | ||
| Asp274 | 5.18243 | ||||
| Val164 | 3.98827 | Hydrophobic | pi–Sigma | ||
| Val164 | 3.91342 | ||||
| Val164 | 3.89115 | ||||
| Val164 | 3.7836 | ||||
| Met281 | 3.83158 | Other | pi–Sulfur | ||
| His194 | 4.95226 | Hydrophobic | pi–pi T-shaped | ||
| Ala177 | 4.11743 | pi–Alkyl | |||
| Leu295 | 5.19839 | ||||
| Phe438 | 5.18846 | ||||
| 8a | |||||
| Lys179 | 3.19462 | Hydrogen bond; electrostatic | pi–Cation; pi–donor hydrogen bond | 310.6 | − 9.2 |
| Val164 | 3.82571 | Hydrophobic | pi–Sigma | ||
| Val164 | 3.7804 | ||||
| Val164 | 3.46463 | ||||
| Met281 | 3.65649 | Other | pi–Sulfur | ||
| Leu156 | 5.49735 | Hydrophobic | pi–Alkyl | ||
| Val164 | 4.73074 | ||||
| Ala177 | 4.11239 | ||||
| Ala230 | 5.38106 | ||||
| Phe438 | 5.02257 | ||||
| Phe442 | 5.2436 | ||||
| 8b | |||||
| Asp292 | 4.06557 | Electrostatic | pi–Anion | 306.83 | − 9.4 |
| Asp292 | 3.42469 | ||||
| Val164 | 3.7686 | Hydrophobic | pi–Sigma | ||
| Met281 | 3.86312 | Other | pi–Sulfur | ||
| Leu156 | 4.45999 | Hydrophobic | Alkyl | ||
| Ala177 | 3.95696 | ||||
| Ala230 | 4.3323 | ||||
| Met281 | 4.91842 | ||||
| Leu181 | 4.11 | ||||
| Ile186 | 5.30867 | ||||
| Leu156 | 5.34604 | pi–Alkyl | |||
| Ala177 | 4.57421 | ||||
| Phe161 | 4.95008 | ||||
| Tyr229 | 4.77413 | ||||
| Phe438 | 4.86978 | ||||
| 8c | |||||
| Val164 | 3.91902 | Hydrophobic | pi–Sigma | 321.88 | − 8.5 |
| Val164 | 3.99995 | ||||
| Val164 | 3.7388 | ||||
| Val164 | 3.84935 | ||||
| Met281 | 3.78377 | Other | pi–Sulfur | ||
| Ala177 | 4.23042 | Hydrophobic | pi–Alkyl | ||
| Phe438 | 4.92818 | ||||
| Phe442 | 5.31265 | ||||
| 8d | |||||
| Ala230 | 2.00269 | Hydrogen bond; halogen | Conventional hydrogen bond; halogen (fluorine) | 335.39 | − 9.0 |
| Ala230 | 3.5106 | Halogen | Halogen (fluorine) | ||
| Asp292 | 3.74855 | Electrostatic | pi–Anion | ||
| Met281 | 3.71765 | Other | pi–Sulfur | ||
| Leu181 | 4.35037 | Hydrophobic | Alkyl | ||
| Val164 | 5.32128 | pi–Alkyl | |||
| Leu156 | 5.19865 | ||||
| Val164 | 4.61367 | ||||
| Ala177 | 4.176 | ||||
| Phe161 | 4.9188 | ||||
| 8e | |||||
| Asp292 | 4.13228 | Electrostatic | pi–Anion | 306.34 | − 9.1 |
| Asp292 | 3.43642 | ||||
| Val164 | 3.76648 | Hydrophobic | pi–Sigma | ||
| Met281 | 3.87118 | Other | pi–Sulfur | ||
| Leu156 | 4.44955 | Hydrophobic | Alkyl | ||
| Ala177 | 4.03439 | ||||
| Ala230 | 4.26056 | ||||
| Met281 | 4.94049 | ||||
| Leu181 | 4.09395 | ||||
| Ile186 | 5.2944 | ||||
| Leu156 | 5.3767 | pi–Alkyl | |||
| Ala177 | 4.65857 | ||||
| Phe161 | 4.95098 | ||||
| Tyr229 | 4.61982 | ||||
| Phe438 | 4.77444 | ||||
| 8f | |||||
| Glu191 | 2.22352 | Hydrogen Bond | Conventional Hydrogen Bond | 322.75 | − 8.9 |
| Val164 | 3.91845 | Hydrophobic | pi–Sigma | ||
| Val164 | 3.75942 | ||||
| Val164 | 3.87206 | ||||
| Met281 | 3.78408 | Other | pi–Sulfur | ||
| Ala177 | 4.21607 | Hydrophobic | pi–Alkyl | ||
| Phe438 | 4.99086 | ||||
| Phe442 | 5.29146 | ||||
| 8g | |||||
| Glu191 | 2.92519 | Hydrogen bond; electrostatic | Salt bridge; attractive charge | 370.05 | 9.3 |
| Glu191 | 4.66536 | Electrostatic | Attractive charge | ||
| Asp274 | 5.30331 | ||||
| Val164 | 3.99864 | Hydrophobic | pi–Sigma | ||
| Val164 | 3.8869 | ||||
| Val164 | 3.88578 | ||||
| Val164 | 3.75251 | ||||
| Met281 | 3.81559 | Other | pi–Sulfur | ||
| His194 | 4.92973 | Hydrophobic | pi–pi T-shaped | ||
| Ala177 | 4.19138 | pi–Alkyl | |||
| Leu295 | 5.25291 | ||||
| Phe438 | 4.9485 | ||||
| Phe442 | 5.3082 | ||||
| Reference inhibitor | |||||
| Gly162 | 2.37153 | Hydrogen Bond | Conventional Hydrogen Bond | 466.3 | − 8.8 |
| Lys179 | 2.74348 | Hydrogen bond; electrostatic | pi–Cation; pi–donor hydrogen bond | ||
| Glu191 | 3.78125 | Electrostatic | pi–Anion | ||
| Asp292 | 3.99079 | ||||
| Phe161 | 5.84153 | Hydrophobic | pi–pi T-0shaped | ||
| Leu156 | 5.47474 | pi–Alkyl | |||
| Val164 | 4.38863 | ||||
| Met281 | 5.49417 | ||||
Fig. 7.
The superimposed binding poses of molecules in the active binding pocket of the AKT enzyme
Fig. 8.
(A) 2D and 3D docking poses of acridone molecules with their binding interactions (a) compound 8b, (b) compound 8g, (c) compound 8f, (d) AZD5363 (Reference Inhibitor) with AKT (PDB: 4GV1). The dotted lines in different colors reflected various types of interactions such as hydrogen bonding (green), charge or polar interactions (orange) pi–alkyl/alkyl (light pink), pi–sulfur (yellow) van der Waals (light green), and pi–pi and pi–sigma (violet) interaction. (B) Relationships between binding energy and Ki (inhibition constant) of acridone compounds
The binding interactions and binding mode of native ligand AZD5363 were used to validate the binding modes of developed molecules. Here, we have considered the docking mode of the native ligand as standard and all the docking results were validated and compared with it. Moreover, we have compared our results with already published literature in the field. A total of 9 conformers were generated from each molecule, where the best binding affinity was displayed at 0.0 root mean square deviation (RMSD) values. The RMSD value for AZD5363 (reference inhibitor) was found to be 0.0 Å, and all the docked complexes showed the same RMSD value.
Out of docked molecules, many displayed more potent interactions than reference inhibitor and are discussed in the section given below: AZD5363 showed − 8.8 kcal/mol binding affinity and formed one conventional hydrogen bond with Gly162 and one pi–cation hydrogen bond with Lys179. It has developed some hydrophobic interactions (pi–anion, pi–pi t-shaped, and pi–alkyl) with Glu191, Asp292, Phe161, Leu156, val164, and Met281. We found that compounds 7g and 8g exhibited − 9.2 and − 9.3 kcal/mol binding affinity with the target enzyme, respectively. These molecules formed one electrostatic hydrogen bond (salt-bridge) with Glu191 and developed many hydrophobic interactions (pi–sigma, pi–sulfur, pi–pi T-shaped, and pi–alkyl) with Val164, Met281, His194, Ala177, Leu295, and Phe438, respectively.
7a exhibited − 9.1 kcal/mol binding affinity and showed many hydrophobic interactions. It was formed with one pi–cation and three pi–sigma bonds with Lys179 and Val164, respectively. It also showed some pi–alkyl interactions with Val164, Ala177, Ala230, Phe438, and one pi–sulfur bond with Met281. The compound 8a displayed − 9.2 kcal/mol binding affinity and formed one potent hydrogen bond (pi–cation) with Lys179 and many hydrophobic (pi–sigma, pi–sulfur, and pi–alkyl) interactions with Val164, Met281, Leu156, Ala177, Ala230, Phe438, and Phe442. Compound 8b exhibited the lowest binding free energy of − 9.4 kcal/mol and developed many hydrophobic interactions (pi–anion, pi–sigma, pi–sulfur, alkyl, and pi–alkyl) with Asp292, Val164, Met281, Leu156, Ala177, Ala230, Leu181, Ile186, Phe161, Tyr229, and Phe438 (Fig. 8A). Compound 8d exhibited − 9 kcal/mol binding affinity and formed one conventional hydrogen bond through fluorine with Ala230 and numerous hydrophobic (pi–anion, pi–sulfur, alkyl, and pi–alkyl) with Asp292, Met281, Leu181, Val164, Leu156, Ala177, and Phe161. Compound 8e developed many hydrophobic interactions (pi–anion, pi–sigma, pi–sulfur, alkyl, and pi–alkyl) with Asp292, Val164, Met281, Leu156, Ala177, Ala230, Leu181, Ile186, Phe161, Tyr229, Phe438 and exhibited − 9.1 kcal/mol binding affinity. The key residues frequently involved in hydrogen bonding and hydrophobic interactions were Glu191, Lys179, Asp292, Val164, Met281, His194, Ala177, Leu295, Phe438, Phe442 Asp274, Ala230 (Table 4).
The interpretation of molecular docking data showed that the complex with acridone molecules had lower binding energy and stronger interactions than the complex with a co-crystallized inhibitor. On comparison of docking interactions based on their distance, we found that bond distance/length was smaller in the case of most of the compounds as compared to the reference inhibitor. And also ligand energy in the interaction table indicates the stability of the molecules. Hence we can say that these compounds were found stable as compared to the reference inhibitor.
The Ki (inhibitor constant) have been tried to compare with the native ligand. Based on the predicted binding energy, compounds 7f, 7g, 8b, 8e, 8f, and 8g with − 8.7, − 9.2, − 9.4, − 9.1, − 8.9, and − 9.3 kcal/mol binding energy, respectively, are more potent than AZD5363 as a reference inhibitor, with − 8.88 kcal/mol binding energy. According to the Ki value, compounds 7f, 7g, 8b, 8e, 8f, and 8g with 1.13, 0.48, 0.34, 0.57, 0.81, and 0.41 µM inhibition constant can inhibit the enzyme more efficiently when compared to AZD5363 with 0.95 µM inhibition constant. Compounds 7g, 8b, 8e, 8f, and 8g have inhibition constants and binding energy less than those of AZD5363. The lower the Ki value for a particular drug at a particular receptor, the stronger its binding affinity for that receptor. The relationship between binding energy and Ki is shown in Fig. 8B. This relationship was linear with R2 = 0.97, which means each compound with more binding energy has a higher inhibition constant.
On comparison of results from molecular docking and with AKT kinase activity, it was found that replacement of N-propyl with N-butyl substitution in most of the compounds displayed lower binding energy and good potency in AKT enzyme assay results also.
As N-butyl-substituted derivatives showed better docking scores and AKT kinase inhibitory activity, it can be proposed that these derivatives could be effective heterocycle for discovering future potent anti-cancer agents targeting AKT kinase enzyme.
Prediction of ADMET parameters
Furthermore, the drug-likeness properties of potential molecules were computed by using Swiss-ADME software. The data obtained from the Swiss-ADME software are mentioned in Table 5. The tested compounds (7e, 7f, 7g, 8d, 8e, 8f, and 8g) had 1 hydrogen bond donor (HBD) and 2–4 hydrogen bond acceptors (HBA), molecular weight ranging from 356.4 to 474.5 and log P value less than 5 except (7g and 8g) which is slightly higher than the limit. All of them had a TPSA within the limits suggested for good bioavailability (20–130 Å2) and displayed positive values for the bioavailability score which was 0.55. In reference to Lipinski’s rule of 5, all compounds had log P ≤ 5 except (7g and 8g), molecular weight ≤ 500, number of hydrogen bond donors ≤ 5, and number of hydrogen bond acceptors ≤ 10. By looking at the results from the predicted pharmacokinetics and drug-likeness, compounds 7f and 8f with nitro substitution can be considered as promising lead and good candidates as “drug-like” properties with promising bioavailability.
Table 5.
ADME parameters of N10-substituted acridone compounds
| Descriptors | Compound code | ||||||
|---|---|---|---|---|---|---|---|
| 7e | 7f | 7g | 8d | 8e | 8f | 8g | |
| MW (g/mol) | 390.86 | 401.41 | 460.53 | 388.43 | 404.89 | 415.44 | 474.55 |
| No. of HBA | 2 | 4 | 4 | 3 | 2 | 4 | 4 |
| No. of HBD | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| TPSA | 51.1 | 96.92 | 75.82 | 51.1 | 51.1 | 96.92 | 75.82 |
| Consensus Log P | 4.62 | 3.39 | 5.67 | 4.69 | 4.92 | 3.7 | 5.99 |
| ESOL Log S | − 5.91 | − 5.37 | − 6.95 | − 5.7 | − 6.14 | − 5.6 | − 7.19 |
| GI absorption | High | High | Low | High | High | High | Low |
| ESOL class | MS | MS | PS | MS | MS | MS | PS |
| BBB permeation | Yes | No | No | Yes | Yes | No | No |
| Bioavailability score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| Lipinski violations | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
MW molecular weight; TPSA topological polar surface area; log P logarithm of the partition coefficient; ESOL Log S ESOL model logarithm of molar solubility in water; ESOL class solubility class in log S scale; MS moderately soluble; PS poorly soluble; GI gastrointestinal; BBB blood–brain barrier
For the prediction of toxicity of these potential molecules (7e, 7f, 7g, 8d, 8e, 8f, and 8g), ProTox II software was used. The toxic dose was predicted in the form of oral rat LD50 (lethal dose) (mg/kg). The data obtained are given in Table 6 relating to ProTox II software. Amongst all the investigated compounds, predicted LD50 was observed at ≥ 500 mg/kg for most of the compounds. According to the developer’s limits, these acridone derivatives come under the category of class 4 toxicity and none of the compounds have toxicity fragments. These compounds were found inactive for organ toxicity specifically hepatotoxicity. And also most of the compounds were found inactive for carcinogenicity, immunotoxicity, and cytotoxicity. Hence, this study suggests that N-substituted acridone derivatives can be taken as lead molecules and further modifications can be done for the design of better compounds with improved cytotoxicity.
Table 6.
Oral toxicity prediction results of N-substituted acridone molecules
| Compound code | Predicted LD50 (mg/kg) | Predicted toxicity class | Average similarity (%) | Prediction accuracy (%) | Toxic fragments | Organ toxicity hepatotoxicity |
|---|---|---|---|---|---|---|
| 7e | 800 | 4 | 60.29 | 68.07 | Nil | Inactive |
| 7f | 500 | 4 | 59.16 | 67.38 | Nil | Inactive |
| 7g | 800 | 4 | 53.62 | 67.38 | Nil | Inactive |
| 8d | 500 | 4 | 56.23 | 67.38 | Nil | Inactive |
| 8e | 800 | 4 | 59.66 | 67.38 | Nil | Inactive |
| 8f | 500 | 4 | 59.1 | 67.38 | Nil | Inactive |
| 8g | 800 | 4 | 53.55 | 67.38 | Nil | Inactive |
Conclusion
In this study, a series of N10-substituted acridone-2-carboxamide derivatives were synthesized and evaluated against MCF-7 and MDA-MB-231 cells. In vitro cytotoxicity evaluation showed that some of these compounds exhibited low micromolar IC50 values. Among them, compound 8f showed potent cytotoxicity against both breast cancer cell lines. Compound 8d displayed potent cytotoxicity against MCF-7 cells. In vitro AKT kinase assay exhibited that compounds (7e, 7f, 7g, 8d, 8e, 8f, and 8g) displayed promising activity against AKT kinase. This study revealed that compound 8f can be considered a kinase inhibitor because of its potent AKT inhibitory activity. Quantitative estimation of p-AKT Ser473 and total-AKT results further confirmed that 8f inhibited MCF-7 cell proliferation by blocking AKT downstream PI3K/AKT signaling pathway. The SAR indicated that the electron-withdrawing group at the 4th position of the benzene played an important role in the anti-proliferative activities of the compounds. The results of the molecular docking study showed that the binding modes of compounds (7f and 8f) were like that of reference inhibitor which supported their mode of action. Furthermore, in silico ADMET studies of synthesized compounds predicted that they are promising molecules for oral administration having better pharmacokinetics and drug-likeness properties, which recommended that they can be considered as good candidates for being lead compounds. The result clearly shows the potential of acridone carboxamide derivatives discussed in this work as a lead scaffold that can be exploited further to design and develop safer and more potent anticancer agents targeting AKT kinase.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to the Department of Health Research (DHR), Government of India, New Delhi, Grant/Award Number: no. V.25011/547‐HRD/2016‐HR for providing funding for research.
Author contributions
TTY contributed to the study conduction, synthesis, data collection, analysis and interpretation of results, and draft/manuscript preparation; PDP was involved in the synthesis and data collection; GMS performed the molecular docking and data collection; MSK assisted in the methodology suggestions for biological studies, suggestions, and supervision, writing—reviewing and editing, and critical review of the manuscript; MC reviewed, edited, and critically revised the manuscript; MYC contributed to the conceptual design, reviewing and editing, and approval of the final version. All authors reviewed the results and approved the final version of the manuscript.
Data availability
The data used to support the findings of this study are included within the manuscript.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Research involving human participants and/or animals:
The authors confirm that there is no involvement of human participants and/or animals in conducting research.
Informed consent
Not applicable.
References
- Ahua KM, Ioset JR, Ransijn A, et al. Antileishmanial and antifungal acridone derivatives from the roots of Thamnosma rhodesica. Phytochemistry. 2004;65:963–968. doi: 10.1016/j.phytochem.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Almerico AM, Tutone M, Lauria A. Docking and multivariate methods to explore HIV-1 drug-resistance: a comparative analysis. J Comput Aided Mol Des. 2008 doi: 10.1007/s10822-008-9186-7. [DOI] [PubMed] [Google Scholar]
- Babu YR, Bhagavanraju M, Reddy GD, et al. Design and synthesis of quinazolinone tagged acridones as cytotoxic agents and their effects on EGFR tyrosine kinase. Arch Pharm (weinheim) 2014;347:624–634. doi: 10.1002/ardp.201400065. [DOI] [PubMed] [Google Scholar]
- Belmont P, Bosson J, Godet T, Tiano M. Acridine and acridone derivatives, anticancer properties and synthetic methods: where are we now? Anticancer Agents Med Chem. 2007;7:139–169. doi: 10.2174/187152007780058669. [DOI] [PubMed] [Google Scholar]
- Belmont P, Dorange I. Acridine/acridone: a simple scaffold with a wide range of application in oncology. Expert Opin Ther Pat. 2008;18:1211–1224. doi: 10.1517/13543776.18.11.1211. [DOI] [Google Scholar]
- Chen J, Wang Y, Zhao D, et al. Chrysin serves as a novel inhibitor of DGKα/FAK interaction to suppress the malignancy of esophageal squamous cell carcinoma (ESCC) Acta Pharm Sin B. 2021;11:143–155. doi: 10.1016/j.apsb.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Lu CM, Chen IL, et al. Synthesis and antiinflammatory evaluation of 9-anilinoacridine and 9-phenoxyacridine derivatives. J Med Chem. 2002;45:4689–4694. doi: 10.1021/jm020102v. [DOI] [PubMed] [Google Scholar]
- Chuang CH, Cheng TC, Leu YL, et al. Discovery of akt kinase inhibitors through structure-based virtual screening and their evaluation as potential anticancer agents. Int J Mol Sci. 2015;16:3202–3212. doi: 10.3390/ijms16023202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukaew A, Ponglimanont C, Karalai C, et al. Potential anti-allergic acridone alkaloids from the roots of Atalantia monophylla. Phytochemistry. 2008;69:2616–2620. doi: 10.1016/j.phytochem.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Cui Z, Li X, Li L, et al. Design, synthesis and evaluation of acridine derivatives as multi-target Src and MEK kinase inhibitors for anti-tumor treatment. Bioorg Med Chem. 2016;24:261–269. doi: 10.1016/j.bmc.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. doi: 10.1038/SREP42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Methods Mol Biol. 2015;1263:243–250. doi: 10.1007/978-1-4939-2269-7_19. [DOI] [PubMed] [Google Scholar]
- Drwal MN, Banerjee P, Dunkel M, et al. ProTox: a web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res. 2014;42:53–58. doi: 10.1093/nar/gku401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensicka-Kowalewska M, Cholewiński G, Dzierzbicka K. Recent developments in the synthesis and biological activity of acridine/acridone analogues. RSC Adv. 2017;7:15776–15804. doi: 10.1039/c7ra01026e. [DOI] [Google Scholar]
- Hill MM, Hemmings BA. Inhibition of protein kinase B/Akt. implications for cancer therapy. Pharmacol Ther. 2002;93:243–251. doi: 10.1016/S0163-7258(02)00193-6. [DOI] [PubMed] [Google Scholar]
- Houghton PJ, Thimmaiah KN, Easton JB (2006) Substituted phenoxazines and acridones as inhibitors of akt. World patents WO2006094207A2. 2006 Sept 8.
- Iman M, Saadabadi A, Davood A. Molecular docking analysis and molecular dynamics simulation study of ameltolide analogous as a sodium channel blocker. Turk J Chem. 2015;39:306–316. doi: 10.3906/kim-1402-37. [DOI] [Google Scholar]
- Jadhav PB, Jadhav SB, Zehravi M, et al. Virtual screening, synthesis, and biological evaluation of some carbohydrazide derivatives as potential DPP-IV inhibitors. Molecules. 2023 doi: 10.3390/molecules28010149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen VM, Mayer IA, Arteaga CL. Is there a future for AKT inhibitors in the treatment of cancer? Clin Cancer Res. 2016;22:2599. doi: 10.1158/1078-0432.CCR-16-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Chien A, Tripathy D, Albain KS, et al. MK-2206 and standard neoadjuvant chemotherapy improves response in patients with human epidermal growth factor receptor 2-positive and/or hormone receptor-negative breast cancers in the I-SPY 2 trial. J Clin Oncol. 2020;38:1059–1069. doi: 10.1200/JCO.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JX, Smilkstein MJ, Brun R, et al. Discovery of dual function acridones as a new antimalarial chemotype. Nature. 2009;459:270–273. doi: 10.1038/nature07937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SL, Siddiqui FA, Shaikh MS, et al. Discovery of potential inhibitors of the receptor-binding domain (RBD) of pandemic disease-causing SARS-CoV-2 spike glycoprotein from triphala through molecular docking. Curr Chin Chem. 2021 doi: 10.2174/2666001601666210322121802. [DOI] [Google Scholar]
- Kumar R, Kaur M, Bahia MS, Silakari O. Synthesis, cytotoxic study and docking based multidrug resistance modulator potential analysis of 2-(9-oxoacridin-10(9H)-yl)-N-phenyl acetamides. Eur J Med Chem. 2014;80:83–91. doi: 10.1016/j.ejmech.2014.04.030. [DOI] [PubMed] [Google Scholar]
- Mahajan A, Rane R, Amritkar A, et al. Synthesis of novel amides based on acridone scaffold with interesting antineoplastic activity. Anticancer Agents Med Chem. 2015;15:555–564. doi: 10.2174/1871520614666141130130130. [DOI] [PubMed] [Google Scholar]
- Martorana F, Motta G, Pavone G, et al. AKT inhibitors: new weapons in the fight against breast cancer? Front Pharmacol. 2021;12:1–13. doi: 10.3389/fphar.2021.662232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meepagala KM, Schrader KK, Wedge DE, Duke SO. Algicidal and antifungal compounds from the roots of Ruta graveolens and synthesis of their analogs. Phytochemistry. 2005;66:2689–2695. doi: 10.1016/J.PHYTOCHEM.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Morgensztern D, McLeod HL. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 2005;16:797–803. doi: 10.1097/01.cad.0000173476.67239.3b. [DOI] [PubMed] [Google Scholar]
- Mundi PS, Sachdev J, McCourt C, Kalinsky K. AKT in cancer: new molecular insights and advances in drug development. Br J Clin Pharmacol. 2016;110:943–956. doi: 10.1111/bcp.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murahari M, Prakash KV, Peters GJ, Mayur YC. Acridone-pyrimidine hybrids- design, synthesis, cytotoxicity studies in resistant and sensitive cancer cells and molecular docking studies. Eur J Med Chem. 2017;139:961–981. doi: 10.1016/j.ejmech.2017.08.023. [DOI] [PubMed] [Google Scholar]
- Qiu B, Guo L, Chen Z, et al. Synthesis of N-4-butylamine acridone and its use as fluorescent probe for ctDNA. Biosens Bioelectron. 2009;24:1281–1285. doi: 10.1016/j.bios.2008.07.055. [DOI] [PubMed] [Google Scholar]
- Rajendra Prasad VVS, Deepak Reddy G, Kathmann I, et al. Nitric oxide releasing acridone carboxamide derivatives as reverters of doxorubicin resistance in MCF7/Dx cancer cells. Bioorg Chem. 2016;64:51–58. doi: 10.1016/j.bioorg.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Rappé AK, Casewit CJ, Colwell KS, et al. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J Am Chem Soc. 1992;114:10024–10035. doi: 10.1021/ja00051a040. [DOI] [Google Scholar]
- Sale E, Hodgkinson C, Jones N, Sale G. Role of protein kinase B in breast cancer. Breast Cancer Res. 2006;8:P23. doi: 10.1186/bcr1578. [DOI] [Google Scholar]
- Salimon J, Salih N, Yousif E, et al. Synthesis and pharmacological evaluation of 9(10H)-acridone bearing 1,3,4-oxadiazole derivatives as antimicrobial agents. Arab J Chem. 2010;3:205–210. doi: 10.1016/j.arabjc.2010.06.001. [DOI] [Google Scholar]
- San Diego: Accelrys Software Inc. (2012) Discovery Studio Modeling Environment, Release 3.5. Accelrys Softw. Inc.
- Sathish NK, Prasad VVSR, Raghavendra NM, et al. Synthesis of novel 1,3-diacetoxy-acridones as cytotoxic agents and their DNA-binding studies. Sci Pharm. 2009;77:19–32. doi: 10.3797/scipharm.0811-03. [DOI] [Google Scholar]
- Sepúlveda CS, García CC, Fascio ML, et al. Inhibition of Junin virus RNA synthesis by an antiviral acridone derivative. Antiviral Res. 2012;93:16–22. doi: 10.1016/j.antiviral.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Shityakov S, Förster C. In silico structure-based screening of versatile P-glycoprotein inhibitors using polynomial empirical scoring functions. Adv Appl Bioinform Chem. 2014;7:1–9. doi: 10.2147/AABC.S56046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui FA, Khan SL, Marathe RP, Nema NV. Design, synthesis, and in silico studies of novel N-(2-aminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide derivatives targeting receptor-binding domain (RBD) of SARS-CoV-2 spike glycoprotein and their evaluation as antimicrobial and antimalarial agents. Lett Drug Des Discov. 2021;18:915–931. doi: 10.2174/1570180818666210427095203. [DOI] [Google Scholar]
- Singh R, Kumar S, Bhardwaj VK, Purohit R. Screening and reckoning of potential therapeutic agents against DprE1 protein of Mycobacterium tuberculosis. J Mol Liq. 2022;358:119101. doi: 10.1016/j.molliq.2022.119101. [DOI] [Google Scholar]
- Song M, Bode AM, Dong Z, Lee MH. AKt as a therapeutic target for cancer. Cancer Res. 2019;79:1019–1031. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Tabarrini O, Manfroni G, Fravolini A, et al. Synthesis and anti-BVDV activity of acridones as new potential antiviral agents. J Med Chem. 2006;49:2621–2627. doi: 10.1021/jm051250z. [DOI] [PubMed] [Google Scholar]
- Unnisa A, Khan SL, Sheikh FAH, et al. In-silico inhibitory potential of triphala constituents against cytochrome P450 2E1 for the prevention of thioacetamide-induced hepatotoxicity. J Pharm Res Int. 2021 doi: 10.9734/jpri/2021/v33i43a32499. [DOI] [Google Scholar]
- Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- Wang C, Wan J, Mei Z, Yang X. Acridone alkaloids with cytotoxic and antimalarial activities from Zanthoxylum simullans Hance. Pharmacogn Mag. 2014;10:73–76. doi: 10.4103/0973-1296.126669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Lin NU, Maurer MA, et al. (2019) Phase II trial of AKT inhibitor MK-2206 in patients with advanced breast cancer who have tumors with PIK3CA or AKT mutations, and/or PTEN loss/PTEN mutation. Breast Cancer Res. 2019;211(21):1–12. doi: 10.1186/S13058-019-1154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wang N, Zhang C, et al. Novel multi-substituted benzyl acridone derivatives as survivin inhibitors for hepatocellular carcinoma treatment. Eur J Med Chem. 2017;129:337–348. doi: 10.1016/j.ejmech.2017.02.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are included within the manuscript.