Abstract
Purines and their derivatives, extensively distributed in the body, act as a class of extracellular signaling molecules via a rich array of receptors, also known as purinoceptors (P1, P2X, and P2Y). They mediate multiple intracellular signal transduction pathways and participate in various physiological and pathological cell behaviors. Since the function in myocardial ischemia–reperfusion injury (MIRI), this review summarized the involvement of purinergic signal transduction in diversified pathological processes, including energy metabolism disorder, oxidative stress injury, calcium overload, inflammatory immune response, platelet aggregation, coronary vascular dysfunction, and cell necrosis and apoptosis. Moreover, increasing evidence suggests that purinergic signaling also mediates the prevention and treatment of MIRI, such as ischemic conditioning, pharmacological intervention, and some other therapies. In conclusion, this review exhibited that purinergic signaling mediates the complex processes of MIRI which shows its promising application and prospecting in the future.
Keywords: Myocardial ischemia–reperfusion injury, Purinergic signaling, Pathological processes, Cardioprotective interventions
Introduction
As the most frequently occurring cardiovascular disease, myocardial ischemia poses a serious threat to human health owing to its high morbidity and mortality [1]. Therefore, it has been a pressing issue to the prevention and treatment of myocardial ischemia effectively. Currently, the main treatment for acute myocardial infarction (AMI) includes drug therapy, surgical coronary artery bypass grafting, and percutaneous coronary intervention (PCI). Bypass surgery and PCI are used to eliminate vascular blockage and restore normal vascular perfusion, which can greatly reduce the mortality of AMI. However, cardiac tissue damage is aggravated after reperfusion, which is called myocardial ischemia–reperfusion injury (MIRI) [2, 3]. This phenomenon also can be observed in other cardiac surgeries, such as valve replacement, heart transplantation, and surgery for congenital heart disease. Undoubtedly, further cardiac dysfunctions will be induced by this type of injury, such as myocardial stunning, reperfusion arrhythmia, myocyte death, and endothelial and microvascular dysfunction, including the no-reflow phenomenon and inflammatory response [4]. Moreover, it was reported that lethal reperfusion injury accounts for up to 50% of the final myocardial infarct size [5]. Consequently, MIRI is a grave, unsolved problem that hinders AMI patients from obtaining the best curative treatment.
Accumulating evidence indicates that purinergic signaling has great therapeutic potential against MIRI. Purinergic signaling involves purines and their derivatives, most notably adenosine and ATP, which were first considered as a class of extracellular signaling molecules by Geoffrey Burnstock in 1972 [6]. They are quite different from classic adrenergic and cholinergic neurotransmitters. By 1978, Burnstock proposed two separate families of receptors for purines, named P1 and P2 receptors [7]. P1 receptors are mainly activated by adenosine (ADO), while P2 receptors are activated by adenosine 5’-triphosphate (ATP) and adenosine 5’-diphosphate (ADP), uridine triphosphate (UTP), and uridine diphosphate (UDP). This theory has not been widely accepted until most purinergic receptors were cloned and characterized in the early 1990s [8]. To date, there have been four G protein-coupled subtypes of P1 receptors (A1, A2A, A2B, and A3), which are related to intracellular levels of cyclic adenosine monophosphate (cAMP). The four receptors are different from each other. The A1 and A3 signaling pathways are linked to inhibitory G proteins to downregulate cAMP, while the A2A and A2B signaling pathways are linked to stimulatory G proteins to upregulate cAMP. A1 and A2A have the highest affinity to ADO, and A3 and A2B have the lowest affinity to ADO [9]. Their activation depends on ADO concentration. The P2 receptors are a little more complicated and contain seven ion channel subtypes of P2X receptors (P2X1-7) and eight G protein-coupled subtypes of P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y). They are diffusely expressed in almost every system in the human body, which mediates multiple intracellular signal transduction pathways and triggers various cell behaviors since the abnormal purinergic signaling will result in a wide range of diseases, like neurological, rheumatic, cardiovascular, and cancer diseases [10]. In MIRI, as soon as the cardiac tissue is subjected to ischemic injury, intracellular ATP is released from the affected cells and gradually breaks down to ADP, adenosine 5’-monophosphate (AMP), and ADO. These endogenous ligands bind to and act on purinergic receptors in the cardiovascular system and associated circulating cells, thereby participating in the complicated pathological processes of MIRI. In this review, we will examine the role of purinergic signaling in MIRI and its application in clinical.
Expression of purinergic receptors in the cardiovascular system and associated immune cells
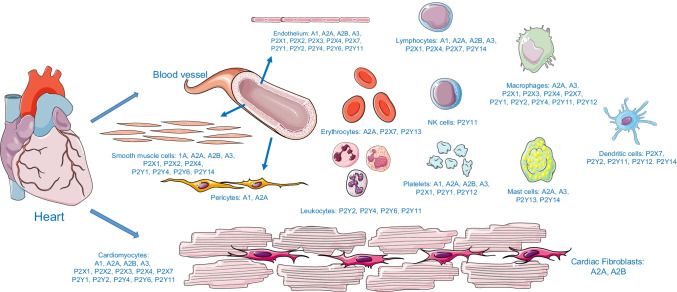
Since the first report on the effects of adenine compounds on disturbed cardiac rhythm was published in 1929 [11], thousands of articles on purinergic signaling in the cardiovascular system have emerged. Scientists have found that numerous cells in the heart and blood vessels can express one or more subtypes of purine receptors that affect heart function modulation, vascular tone, angiogenesis, and inflammation. Existing evidence has shown that the four P1 subtypes are differentially expressed in cardiomyocytes, cardiac fibroblasts, coronary vascular, and inflammatory cells, which mediate a range of generally beneficial actions [12]. Specifically, cardiomyocytes, endothelial cells, and vascular smooth muscle cells express all four P1 subtypes. Cardiac fibroblasts express the A2A and A2B subtypes; however, pericytes express the A1 and A2A subtypes. As for inflammatory cells, polymorphonuclear leukocytes express all four P1 subtypes, but mast cells and macrophages only express the A2A and A3 subtypes. They usually play a vasodilatory and cardioprotective role inside the body. Some studies suggest that almost all subtypes of P2X receptors are expressed in cardiomyocytes [13]. Furthermore, P2X1, P2X2, and P2X4 are expressed in vascular smooth muscle cells and endothelial cells, which contribute significantly to vascular contraction and relaxation responses, respectively [14]. The distribution of P2Y receptors is similar to that of P2X receptors. Some reviews reveal that many P2Y subtypes are also expressed in cardiomyocytes, including P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 [13, 15]. It is interesting to note that P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 are expressed in the vascular endothelium [14, 16], while P2Y2, P2Y4, P2Y6, and P2Y14 are expressed in the vascular smooth muscle [13, 17, 18]. All of the aforementioned P2Y subtypes participate in vascular contraction and relaxation. Furthermore, P2 receptors are expressed in erythrocytes, platelets, and immune cells, which play a major role in multiple physiological and pathological changes associated with cardiovascular diseases [13, 19]. The expression of purinergic receptors in the cardiovascular system cells and associated immune cells is summarized in Fig. 1.
Fig. 1.
Expression of purinergic receptors in the cardiovascular system and associated immune cells
Purinergic signaling in pathological processes of MIRI
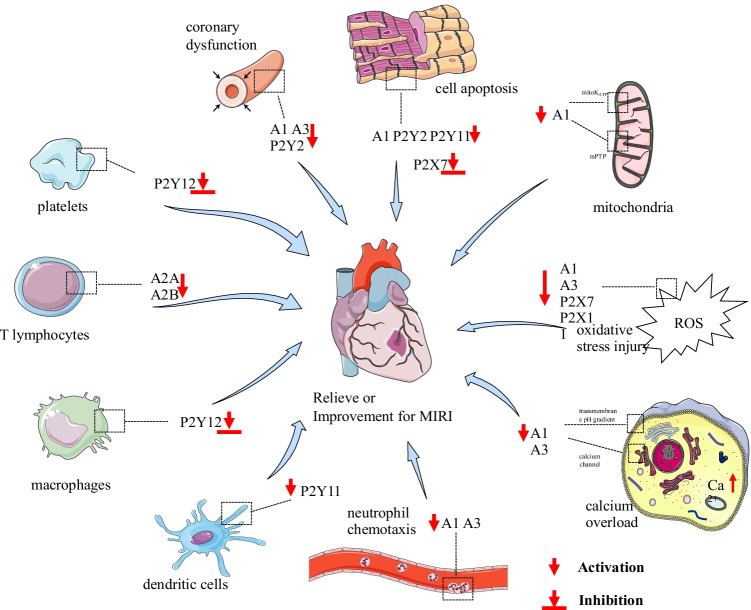
The microcirculation disturbance and surrounding tissue injury caused by myocardial ischemia–reperfusion cover the ischemic period, acute and subacute periods during reperfusion, and the chronic injury period after reperfusion. The disorder of energy metabolism initiates the pathological process of MIRI and results in the reduction of ATP synthesis furtherly in the ischemic period. In addition, the accelerating ATP deficiency in the intravascular and perivascular tissues, which, due to the continuous consumption of ATP by blood vessels and surrounding tissues, induces serious cytoskeleton depolymerization and cell necrosis. After the blocked blood vessels are recanalized, the injury enters the early stage of reperfusion. During this period, the supply of oxygen and nutrients is restored, and peroxide is produced in excess which acts in two aspects. On one side, the DNA and membrane structure of cardiac cells suffer great damage owing to lipid peroxidation. On the other side, peroxide triggers the release of inflammatory factors and increases the expression of adhesion molecules, which induce acute pathological changes, such as exudation, bleeding, thrombosis, and cell apoptosis. Within 24 h to 7 days after reperfusion, the damaged vascular endothelial cells and perivascular tissues release multiple chemokines and transforming growth factors. These substances induce collagen deposition by acting on fibroblasts and trigger the remodeling of perivascular tissue, which leads to the induction of a subacute pathological process dominated by organ fibrosis. After 7 days of reperfusion, some lymphocytes swim out of the blood vessels and contribute to the perivascular chronic inflammatory process. Based on the aforementioned results, it can be suggested that the complex pathological processes of MIRI can be roughly divided into the following categories: energy metabolism disorder, oxidative stress injury, calcium overload, inflammatory immune response, platelet aggregation, coronary vascular dysfunction, and cell necrosis and apoptosis. In this review, we will focus on the role of purinergic signaling in the aforementioned MIRI pathological processes, as it may reveal great therapeutic potential and help develop new therapeutic agents for MIRI (Fig. 2).
Fig. 2.
Purinergic signaling in pathological processes of MIRI
Energy metabolism disorder
As previously described, the energy metabolism disorder is the initial contributing factor to MIRI [20]. The normal function of cardiomyocytes is inextricably linked to the energy provided by ATP. During the ischemic period, the energy metabolism of the ischemic myocardium will change from aerobic oxidation to anaerobic glycolysis when the coronary blood flow decreases to a certain extent, which reduces ATP production and energy supply. In this situation, the cardiomyocytes are in a state of energy starvation, thus inducing serious cardiac dysfunction. Simultaneously, anaerobic glycolysis results in a great increase in intracellular lactic acid levels, which leads to a decrease in pH and accelerates the acidosis of cardiomyocytes. No doubt, those trigger a series of pathological changes. It is worth noting that the energy metabolism disorder also occurs throughout the reperfusion period owing to the mitochondrial damage caused by ischemia. Therefore, the timely treatment of the myocardial energy metabolism disorder is an important method to alleviate MIRI. As early as the 1980s, scientists found that the administration of exogenous ADO could increase ATP levels in the post-ischemic myocardium [21], a phenomenon that could not be replicated in in vivo models [22]. Moreover, ADO could also stimulate myocardial glycolysis, which maintains cell viability by increasing the cellular uptake of glucose [23, 24]. This protective effect may be mediated by the P1 receptor. For example, some studies have found that the overexpression of the A1 receptor can reduce ATP loss and improve the bioenergetic state during severe ischemic insult and reperfusion, which may contribute to improved functional tolerance [25]. This protective effect of the A1 receptor may be mediated by the activation of the intrinsic mitochondrial KATP channel, which is a core link of energy metabolism in cardiomyocytes [26–28]. A recent study suggested that the remote cardioprotective effect of the transfer of coronary effluent from an ischemic preconditioned heart is mediated by P1 receptor activation, which preserves mitochondrial integrity and function in cardiomyocytes [29]. Therefore, purinergic signaling may be a potential target for alleviating energy metabolism disorders in MIRI.
Oxidative stress injury
Reactive oxygen species (ROS) incur important pathological changes in two aspects in MIRI. On the one hand, ROS can reduce the activities of Na/K ATPase and Ca2+ ATPase and cause lipid peroxidation, which damages the cell membrane directly and destroys the integrity of the cardiomyocytes causing myocardial necrosis and apoptosis. On the other hand, ROS initiate the expression of inflammatory mediators, resulting in neutrophil infiltration and capillary injury. This excessive inflammatory reaction is another significant pathological change that aggravates the myocardial injury. Furthermore, ROS can create a dissipation of the mitochondrial membrane potential and induce long-term opening of the mitochondrial permeability transition pore (mPTP), which inevitably leads to cell energy metabolism disorder. Thus, based on this point, it is believed that the inhibition of excessive oxidative stress is a vital strategy for MIRI prevention and treatment. A large amount of studies show that overexpressing the A1 receptor can preserve mitochondrial function and salvage cardiomyocytes from cell death by inhibiting the opening of mPTP and modulating KATP channels in MIRI [30, 31]. Similarly, the activation of A3 receptor activation can regulate KATP channels to attenuate post-ischemic dysfunction and provide cardioprotection [32, 33], which may involve the activation of the NF-κB, transcription of iNOS, and synthesis of NO in the heart [34, 35]. In addition to the P1 receptor, P2 subtypes also contribute to oxidative stress injury in MIRI. For example, one study showed that extracellular ATP addition at the reoxygenation stage confers a cardioprotective effect against hypoxia/reoxygenation injury, which is mediated by the P2Y11 receptor in human cardiomyocytes via reducing mitochondrial ROS production and activating the PKCε signaling pathway [36]. In addition, the pannexin-1/P2X7 compound can be activated in MIRI, which promotes the release of endogenous cardioprotectants, such as adenosine and sphingosine 1-phosphate. These substances can trigger a protective effect through the PI3k/Akt survival pathway to delay mPTP opening and reduce myocardial apoptosis [37]. Thus, purinergic signaling plays a major role in oxidative stress injury in MIRI.
Calcium overload
Changes in intracellular calcium homeostasis play an important role in MIRI. Under physiological conditions, the concentrations of intracellular and extracellular calcium ions are relatively stable. Once myocardial cells are ischemic and hypoxic, metabolism shifts from cellular respiration to anaerobic glycolysis, causing a transmembrane pH gradient change. Abundant sodium ions flow into the cells. When the reperfusion stage occurs, the energy supply is restored, and the pH gradient returns to normal. This recovery promotes the transmembrane exchange of sodium and calcium ions. Finally, the influx of extracellular calcium into cells results in calcium overload This is a potential mechanism for oxygen-free radical and neutrophil infiltration, which is also closely related to cardiac purinergic signaling. Some studies have revealed that cardiac A1 receptor overexpression is associated with a decreased rate of active calcium transport into the sarcoplasmic reticulum [38]. This reduction in active calcium uptake can contribute to increased myocardial resistance to ischemia. Interestingly, the A3 receptor has a similar modulation effect on calcium channels in the sarcoplasmic reticulum, thereby exerting a myocardial protection effect against MIRI [39]. Purinergic signaling is a conventional therapeutic target for intracellular calcium homeostasis in MIRI.
Inflammatory immune response
The activation of inflammatory immune response is another crucial pathological change during MIRI; in the early stage of reperfusion, injured cardiomyocytes activate the innate immune response, which induces various inflammatory factors and chemokines releasing, and creating a pro-inflammatory environment finally. Some circulating inflammatory cells, such as neutrophils, macrophages, and lymphocytes, are recruited at the site of the injured myocardium both directly and indirectly. This infiltration aggravates and spreads the inflammatory response. Approximately 4 days later, the injured cardiomyocytes exhibit anti-inflammatory and restorative properties. During this pathological change, multiple anti-inflammatory and immunosuppressive factors are released which promote vascular regeneration and myocardial tissue repair. In this long and complicated period, purinergic signaling plays both a positive and negative regulatory role via selective receptor activation. However, there are controversies among studies about the role of purinergic signaling. For example, some studies have suggested that the activation of the A1 receptor can stimulate neutrophil chemotaxis [40], which promotes a pro-inflammatory immune response in MIRI. However, other studies have shown that the overexpression of the A1 receptor can lower the levels of tissue myeloperoxidase activity, an index of neutrophil accumulation, thus resulting in smaller infarct size in MIRI [41]. Moreover, the result of one study has verified that A3 receptor activation can attenuate MIRI by decreasing neutrophil-endothelial cell interactions [42]; however, the results of other studies suggest that the activation of the A3 receptor leads to pro-inflammatory activity and contributes to MIRI [43, 44]. A2 receptor subtypes are considered more involved in inflammatory immune responses in ischemia–reperfusion injury. CD73 on T cell-derived adenosine acts on A2A and A2B receptors in an autocrine and paracrine manner [45]. The activation of A2A and A2B receptors can inhibit the release of pro-inflammatory cytokines, including TNF-α, INF-γ, IL-1α, IL-1β, IL-2, and IL-6. In contrast, some anti-inflammatory cytokines, such as IL-10, are secreted via the stimulation of the A2A receptor [46, 47]. Furthermore, P2 receptors are also important players in this pathological change. For instance, some studies have reported that pro-inflammatory factors, such as IL-1β, IL-18, and ROS, can be secreted excessively via the P2X7 receptor [48–50]. The potential mechanism may be the K+ efflux and Ca2+ influx induced by the openness of the P2X7 receptor, which triggers NLRP3 inflammasome assembly and then converts pro-caspase-1 into active caspase-1. This inflammatory response of the P2X7 receptor contributes to myocardial injury and myocardial fibrosis, which results in decreased cardiac function [51]. Paradoxically, some other studies revealed that P2X7/pannexin-1 pore mediated the cardiac protective effect of conditioning intervention; thus, the inhibition of pannexin-1 or P2X7 could abrogate the protective effect of ischemia–reperfusion conditioning and result in increased infarct sizes [52–54]. Furthermore, the P2Y11 receptor in human dendritic cells also plays a pivotal role in mediating the inflammatory response following ischemia–reperfusion injury, which could be beneficial in AMI [55]. The stimulation of this subtype could also modulate the secretome of cardiac fibroblasts, regulate inflammatory immune reactions, and reduce hypoxia/reoxygenation injury [56]. Another important purinergic receptor is P2Y12. One study verified that P2Y12 inhibition in macrophages can attenuate inflammation and cardiac remodeling induced by MIRI [57]. Based on the aforementioned, the knowledge between purinergic signaling and multiple inflammatory immune cells needs to be furtherly explored to understand their roles and mechanisms.
Platelet aggregation
Platelet aggregation has recently emerged as a popular pathological symptom of MIRI. Activated platelets may aggregate and form microthrombi in small cardiac vessels and capillaries, leading to cardiac tissue damage. Activated platelets also contribute to reperfusion injury by enhancing platelet-leucocyte aggregation, the release of potent vasoconstrictors, and the secretion of pro-inflammatory molecules [58]. So far, three subtypes of P2 receptors in platelets have been recognized, including two receptors of ADP (P2Y1 and P2Y12) and one of ATP (P2X1), which are all involved in platelet aggregation. Therefore, the pharmacological inhibition of platelets is considered a standard treatment for AMI patients, especially by P2Y12 receptor inhibitors. Among these inhibitors, clopidogrel is the most commonly used drug in clinics, which effectively reduces coronary occlusion without thrombus formation and is recommended by many clinical guidelines [59]. Scientists have found that a few newly discovered P2Y12 inhibitors, such as prasugrel [60], cangrelor [61, 62], and ticagrelor [63], exhibit great ability to ameliorate myocardial damage beyond their well-studied anti-thrombotic effects. However, it does not mean that clopidogrel can be replaced completely. Recent trials have shown that in patients aged 70 years or older who suffered high bleeding risk, clopidogrel is a favorable alternative to ticagrelor and prasugrel as it leads to fewer bleeding events without an increase in the combined endpoint of all-cause death, myocardial infarction, stroke, and bleeding [64–66]. However, the potential mechanism of action of clopidogrel, which seems to extend beyond platelet inhibition, has not yet been fully explored. Some studies have reported that the cardioprotective effect of cangrelor is dependent on platelets, sphingosine phosphorylation, and certain other blood components [67, 68]. Prasugrel can reduce ischemia-induced ventricular arrhythmias via PI3k/Akt signaling pathways [69]. Ticagrelor can block the adenosine re-uptake transporter ENT1, thereby raising tissue adenosine levels to reduce cardiac injury [70, 71]. Therefore, it is worthwhile to pay more attention to purinergic signaling in platelet function during MIRI.
Coronary vascular dysfunction
Previous studies have verified that ischemia–reperfusion can generate substantial coronary vascular events, such as vasospasm, thrombosis or re-stenosis, and endothelial injury. These dysfunctions contribute significantly to myocardial depression and impaired cardiac reflow, which are the key determinants of infarct size in MIRI. Given the specific vascular protective effect of ADO, scientists have attempted to explore the relevance of purinergic signaling in coronary vascular dysfunction. In the case of the P1 receptor, some studies have shown that vascular injury is intrinsically limited by the endogenous activation of the A1 receptor, while the exogenous A3 receptor activation further limits post-ischemic dysfunction [72]. Thus, the pretreatment with an agonist of the A1 receptor can alter the spatial distribution of myocardial blood flow, which might reflect a downregulation of metabolic state, thereby contributing to cardioprotective effects [73]. Even more, the coronary microvascular tone can also be activated by receptors of A2A and A2B. Adenosine-mediated. Previous research revealed that ischemia–reperfusion can attenuate coronary vasodilatation induced by the A2A agonist in the dog [74]. What is more, A2B-mediated relaxation in isolated coronary small arteries can also be blunted in swine with myocardial infarction [75]. The downstream targets of P1 receptors are H2O2, KATP, KV, and KCa2+ channels [76]. Besides, the increased extracellular ATP level after the ischemic injury can also protect heart endothelial cells against acute reperfusion injury [77]. Another study has suggested that the coronary endothelium-dependent relaxation may be partly mediated by P2 receptors after ischemia–reperfusion [78]. For example, the P2Y2 receptor on the coronary artery can be reduced to minimize coronary contraction following ischemia–reperfusion injury [79]. Therefore, purinergic receptors may represent new avenues for the treatment of MIRI resulting from coronary vascular dysfunction.
Cell necrosis and apoptosis
Cell necrosis and apoptosis are the most direct pathological manifestations of MIRI. Both terms describe different types of cardiac cell death. Cell necrosis refers to passive death caused by physical or chemical damage injuries, such as hypoxia and malnutrition. Cell apoptosis is also known as programmed cell death, refers to cell death controlled by apoptosis genes activated by certain conditions, and is a useful strategy for better adaptation to the living environment. These pathological processes can be modulated by purinergic signaling. Numerous clinical trials have verified that ADO infusion can result in a significant reduction in infarct size [80, 81]. This cardioprotective effect is mainly mediated by P1 receptors. For example, some studies have reported that the stimulation of the A1 receptor can reduce necrosis and infarct size in MIRI [41, 82] and inhibit cardiac cell apoptosis by regulating the expression of Bcl-2/Bax and caspase 3 and their activity [83–85]. In addition, P2 receptors are also involved in cell death in MIRI. For example, P2X7 plays a role in myocardial impairment by increasing apoptosis, while the administration of a specific P2X7 receptor antagonist can reduce the HSP70 protein levels in cardiac cells [86]. In contrast, the P2Y2 receptor may exert a protective effect against MIRI. A treatment with a specific P2Y2 receptor agonist reduces cell death and increases the expression of the anti-apoptotic protein Bcl-2 [86]. These two regulatory agents can decrease the pro-apoptotic protein caspase-8 levels [86]. A recent study revealed that the stimulation of the P2Y11 receptor can also reduce the apoptotic markers after cardiac transplantation, such as the Bax/Bcl-2 ratio, which results in significantly prolonged cardiac allograft survival [87]. All these results suggest that purinergic signaling is closely related to cell necrosis and apoptosis, which are the last, but not least, pathological links to MIRI.
Purinergic signaling in cardioprotective interventions for MIRI
Purine signaling has become a potential target for the prevention and treatment of MIRI, owing to its involvement in the multiple pathological manifestations of MIRI. Thus far, we reviewed the various interventions reported and found that many of them employ purinergic signaling as action mechanisms (Table 1).
Table 1.
Purinergic signaling in cardioprotective interventions in MIRI
| Ischemic conditioning | Pharmacological interventions | Other therapies | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clopidogrel, prasugrel, cangrelor, ticagrelor | Neutrophil-derived netrin-1 | Alogliptin | Vitamin B6 pyridoxal 5-phosphate | miR-150 | Metformin | Dipyridamole | Intrathecal morphine preconditioning | Mycelia of Cordyceps sinensis | Resveratrol | Alcohol | Aerobic exercise | Electro-acupuncture | ||
| A1 | √ | √ | √ | √ | √ | √ | √ | √ | ||||||
| A2A | √ | √ | ||||||||||||
| A2B | √ | √ | √ | |||||||||||
| A3 | ? | |||||||||||||
| P2X7 | √ | √ | √ | √ | ||||||||||
| P2Y6 | √ | |||||||||||||
| P2Y11 | √ | |||||||||||||
| P2Y12 | √ | |||||||||||||
Ischemic conditioning
Ischemic conditioning is a crucial intervention in MIRI, including ischemic preconditioning, ischemic postconditioning, and remote ischemic conditioning (pre and post). Each preconditioning has its own merits. Ischemic preconditioning was first discovered in 1986 [88, 89]. Some physicians have discovered that preconditioning the myocardium using short episodes of sublethal ischemia could delay the onset of necrosis during a subsequent lethal ischemic insult. Since then, scientists have been trying hard to explore the underlying mechanism of this endogenous myocardial protection effect. To date, they have reached the following consensus: the protective effect produced by ischemic preconditioning includes early preconditioning and the second window of protection. Early preconditioning refers to infarction delayed by 1–2 h after the first ischemic stimulation, while the second window of protection refers to a protective effect that lasts 12–72 h [90]. ADO is considered a classic trigger and mediator [91] that is involved in the preconditioning of rabbit [92], dog [93], pig [94], and human myocardia [95]. This cardioprotective effect of ADO in preconditioning is related to all P1 receptor subtypes. Among them, A1 and A3 receptors can not only modulate the activation of the mitochondrial KATP channel and the opening of mPTP in cardiomyocytes [96, 97], but also activate phospholipase C or protein kinase C (PKC) directly [98–100]. However, some researchers still claim that the A3 receptor is not necessary for ischemic preconditioning, as it may incur injury in MIRI; thus this requires more in-depth research in the future [101]. The myocardial protective mechanisms of A2A and A2B receptors are completely different. Some researchers have suggested that A2A may inhibit endothelial-neutrophil interactions in MIRI [102], while A2B activation reduces MIRI by promoting anti-inflammatory macrophage differentiation via the PI3K/Akt pathway [103, 104]. Subcellular ERK isoform signaling is also involved in P1 receptor preconditioning to reduce myocardial infarct size, especially via the A1 and A2A receptors [105]. In 1993, researchers confirmed that ischemic preconditioning and its protective effect can occur between different parts of the same organ or between different organs, which was proposed as remote ischemic conditioning [106]. In 2003, it was shown that brief cycles of coronary occlusion during the early minutes of reperfusion can reduce the infarct size, which becomes equivalent to that seen after ischemic preconditioning [107]. These two interventions have emerged as novel therapeutic strategies for MIRI, but the underlying mechanisms remain unclear. Some studies have shown that this cardioprotective effect is associated with the activation of the PI3K/Akt pathway and the prevention of mPTP formation via the A2B receptor during reperfusion [108, 109]. Furthermore, the pre- and postconditioning of P2X7 receptor agonists can protect the heart against ischemia–reperfusion injury by opening pannexin-1/P2X7 channels [53].
Pharmacological intervention
The development of pharmacology has enhanced the exploitation of many adjuvant drugs for purinergic receptors. The emergence of these drugs is not only convenient for laboratory research, but also is the potential to play a role in clinical application against MIRI, especially in terms of selective agonists and antagonists acting on various receptor subtypes. The most well known are P2Y12 inhibitors, such as clopidogrel [110], prasugrel [69], cangrelor [68], and ticagrelor [63], which have been discussed in detail in a previous section of this review. It is worth noting that purines coexist with other classical transmitters, thereby purinergic receptors have multiple cross-talk with other signaling pathways. In other words, some non-purinergic molecules can also activate purinergic receptors and exert the same cardioprotective effect against MIRI. For instance, neutrophil-derived netrin-1 attenuates MIRI through myeloid adenosine A2B signaling [111]. The same cardioprotective effect has been observed in the dipeptidyl peptidase 4 inhibitor, alogliptin, which suppresses MIRI via the A1-PKC-CREB signaling pathways [112]. P2Y11 and P2Y6 receptors are candidate receptors of cardiac pharmacological preconditioning induced by vitamin B6 and its metabolite, pyridoxal 5-phosphate [113], which may be associated with the reduction of sarcoplasmic reticulum Ca2+ transport activities [114].
Additionally, one recent study affirmed that some circulating micro-RNAs, such as miR-150, protect the heart from ischemic injury by regulating cell death through the direct repression of pro-inflammatory P2X7 in cardiomyocytes [115].
Therefore, purinergic signaling may be a potential mechanism of some pharmacological interventions. Metformin, a beneficial medicine for diabetes, can preserve myocardial function after ischemia and reduce infarct size. However, this effect could be completely abolished by a P1 receptor antagonist, which verifies the critical dependence of metformin on the stimulation of the P1 receptor [116]. Cilostazol, an anticoagulant drug, can reduce the myocardial infarct size by increasing ADO and NOx levels, attenuating superoxide production, and opening the mitochondrial KATP channels [117]. A clinically usable nucleoside transport inhibitor, dipyridamole, exerts a sustained cardioprotective effect via A1 receptor signaling during ischemia [118]. Moreover, opioid receptors interact closely with P1 receptors. For example, intrathecal morphine preconditioning can be used to reduce the infarct size in MIRI, but the cardioprotective effect can be reversed by the intravenous and intrathecal administration of a P1 receptor antagonist [119]. In induced postconditioning, an ultra-short-acting opioid receptor agonist, remifentanil has cross-talk with the P1 receptor [120]; both of these conditioning types depend on mitochondrial KATP and ROS in MIRI [121].
In light of these converging pathways between purinergic signaling and other pathways, the coadministration of multiple drugs will undoubtedly enhance the cardioprotective effect against MIRI. One study revealed that there is an additive effect on local myocardial adenosine levels in ischemia–reperfusion injury when ticagrelor and rosuvastatin are coadministered, which may be mediated by adenosine-induced effects, including the downregulation of pro-inflammatory mediators and upregulation of anti-inflammatory ones [122]. Another study reported that the caspase-1 inhibitor VX-765 combined with the P2Y12 receptor antagonist cangrelor, both administered at the reperfusion stage in MIRI, can preserve cardiac function and reduce infarct size after reperfusion [123]. The pre-ischemic coadministration of the sodium–hydrogen exchanger inhibitor cariporide and the adenosine agonist AMP579 can act additively to reduce the myocardial infarct size [124]. In addition, triple combining interventions by cangrelor, cariporide, and cooling can increase greatly myocardial salvage with an infarct size of only 3%, which is much better than the effect of two drugs used alone [125].
Other therapies
Beside the traditional ischemic conditioning and pharmacological intervention against MIRI, some novel therapies have been developed in recent years, which often exhibit significant cardioprotective effects; however, their underlying mechanisms have not been fully explored and explained. Fortunately, purinergic signaling may provide a novel reference for their interpretation. For instance, the mycelia of cultured Cordyceps sinensis, which is a Chinese herb frequently used, have a suppressive effect on ischemic contracture. Additionally, they provide cardioprotection through enhancing P1 receptor activation in MIRI [126]. Furthermore, resveratrol (a polyphenol produced in grapes and present in wine) can protect the heart from MIRI in the long term, by stimulating the production of ADO and activating A1 and A3 receptors [127].
It is worth noting that the cardioprotective effect of purinergic signaling also can be influenced by other active ingredients. One study showed that caffeinated coffee can abrogate the infarct size limiting effect of atorvastatin by blocking the P1 receptors and preventing the phosphorylation of Akt. However, caffeinated coffee does not affect the infarct size of rats not treated with atorvastatin [128]. A previous research suggests that alcohol consumption can mimic the cardioprotective effect of preconditioning by the A1 receptor [129]; this effect warrants reconsideration and further research.
Purinergic signaling is also involved in other complementary therapies. As early as the 1990s, researchers found that ADO can affect coronary vasodilation during exercise [130]. Recent studies have shown that aerobic exercise can reverse cardiac remodeling by reducing inflammation, fibrosis, and apoptosis, thereby partly inhibiting P2X7 receptor expression in cardiomyocytes [131]. Acupuncture preconditioning has been verified as a potential therapy for MIRI, owing to its popularity in Asian countries [132, 133]. This potential mechanism of treatment requires further exploration. Purinergic signaling has been considered the initiation pathway in acupuncture therapy since 2009 [134–136]. Many researchers believe that purinergic signaling may be the regulatory target of acupuncture preconditioning for MIRI [137, 138], and electro-acupuncture may achieve a cardioprotective effect by modulating the expression of A2A and A2B receptors in myocardial tissue [139].
Reflection and prospect of purinergic signaling against MIRI
Based on this review, it can be suggested that purinergic signaling plays various roles in the pathophysiology of MIRI. ATP is released from ischemic cardiomyocytes in the form of autocrine or paracrine messengers, then activates P1 and P2 receptors, and mediates a series of pathological reactions. By the mutual promotion and inhibition in the corresponding relationships in different stages, these reactions are not isolated. They promote and inhibit each other and then play a corresponding role in different stages. During the ischemic phase, continuous local ischemia and hypoxia weaken the level of cellular oxidative phosphorylation and lead to energy exhaustion, due to which cellular energy demands remain unmet. At the same time, intracellular anaerobic glycolysis increases significantly to maintain ATP levels, thereby resulting in lactic acid accumulation and cell solute acidification. The activity of sodium-hydrogen exchange, sodium-calcium exchange, and calcium channels in the sarcoplasmic reticulum decreases, which leads to intracellular calcium overload. Subsequently, the cardiac cytoskeleton is depolymerized, and apoptosis and necrosis pathways are activated. All these activities result in metabolic collapse and myocardial cell death. Although the supply of oxygen and nutrients is restored during the reperfusion stage, a second wave of damage is induced. On the one hand, large numbers of peroxides are produced to damage the DNA and membrane structure through lipid peroxidation. They also initiate a variety of intracellular signal transduction pathways and induce the release of pro-inflammatory factors. On the other hand, persistent calcium overload activates the opening of the mPTP, again affecting energy production. This results in either apoptotic or necrotic cell death being induced. After 4 days of reperfusion, the damaged vascular endothelial cells release multiple chemokines, which cooperate with inflammatory factors and immune cells. These substances act on fibroblasts to initiate tissue remodeling and promote cardiac function recovery. During this complicated process, scientists first concerned the function of P1 receptors, which may be due to the modulative effect of ADO has been observed as early as the 1920s. Compared to ATP and ADP, the molecular structure of ADO is more stable. The mechanism of the A1 subtype is clearly demonstrated, which reveals that it is involved in many critical pathological links in MIRI, especially calcium overload, oxidative stress injury, and the opening of mPTP. Intracellular signaling pathways include PKC, PI3 kinase, and MAPKs. However, other subtypes of P1 receptors are involved in the inflammatory and immune pathways in MIRI. In particular, the A2b receptor, which can only be activated under hypoxic conditions, shows a strong cardioprotective effect in MIRI. P2 receptors, such as P2X4, P2X7, P2Y2, P2Y11, and P2Y12 also play important roles in MIRI. They express in different cells and affect almost all the pathological links in MIRI. To some extent, regulating the activity of these P2 receptors to some extent can have a beneficial effect on ischemic cardiomyocytes. These compounds seem to constitute a class of promising therapeutic targets for MIRI. For example, targeting the P2Y12 receptor with clopidogrel, prasugrel, or ticagrelor is the most successful strategy to date.
Nevertheless, many aspects of purinergic signaling in regulating MIRI are still not fully understood due to a number of discrepant observations, which are as follows: (1) The essential function of purinergic signaling requires further accurate verification, in terms of the pathological link it acts on, the signaling pathway it is involved in, the species it works on, etc. These aspects should be conducted inspections in the future. (2) The MIRI is a complex process that lasts for a long time, including aggravating injury, self-healing, and recovery period, respectively. Thus, one receptor may play different roles at different stages in MIRI. Temporal changes of purinergic signaling require additional attention. (3) Purinergic signaling cross-talks with other signaling pathways. For example, several studies have reported the cardioprotective properties of P2Y12 receptor inhibitors, irrespective of their anti-thrombotic activity. The interaction effect among signaling pathways is also very important and can help predict the therapeutic potential and possible side effects. (4) Some non-drug interventions, such as ischemic conditioning, exercise, and acupuncture, are with powerful cardioprotective effects mediated by purinergic signaling. This may depend on the integrative effect of multiple receptors expressed both in the cardiovascular and nervous systems; this needs to be researched further and verified clinically.
Author contribution
Yi Zhuang, Mei-ling Yu, and Sheng-feng Lu discussed the concepts, contributed to literature and analysis, and wrote the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81774210), “Six Major Talent Summit” of Jiangsu Province (YY-033), “Qing Lan Project” of Jiangsu Province (2020), and Acupuncture & Chronobiology Key Laboratory of Sichuan Province (No. 2021005).
Data availability
Not applicable.
Declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yi Zhuang, Email: 270743@njucm.edu.cn.
Mei-ling Yu, Email: nberry@njucm.edu.cn.
Sheng-feng Lu, Email: lushengfeng@njucm.edu.cn.
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/s0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernink FJ, Timmers L, Beek AM, Diamant M, Roos ST, Van Rossum AC, Appelman Y. Progression in attenuating myocardial reperfusion injury: an overview. Int J Cardiol. 2014;170(3):261–269. doi: 10.1016/j.ijcard.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Yellon DM. Targeting myocardial reperfusion injury–the search continues. N Engl J Med. 2015;373(11):1073–1075. doi: 10.1056/NEJMe1509718. [DOI] [PubMed] [Google Scholar]
- 4.Moens AL, Claeys MJ, Timmermans JP, Vrints CJ. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol. 2005;100(2):179–190. doi: 10.1016/j.ijcard.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 6.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24(3):509–581. [PubMed] [Google Scholar]
- 7.Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Straub RW, Bolis L, editors. Cell membrane receptors for drugs and hormones: a multidisciplinary approach. New York: Raven Press; 1978. pp. 107–118. [Google Scholar]
- 8.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50(3):413–492. [PubMed] [Google Scholar]
- 9.Laubach VE, French BA, Okusa MD. Targeting of adenosine receptors in ischemia-reperfusion injury. Expert Opin Ther Targets. 2011;15(1):103–118. doi: 10.1517/14728222.2011.541441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Z, Xie N, Illes P, Virgilio FD, Ulrich H, Semyanov A, Verkhratsky A, Sperlagh B, Yu SG, Huang CH, Tang Y. From purines to purinergic signalling: molecular functions and human diseases. Signal Transduct Target Ther. 2021;6(1):162. doi: 10.1038/s41392-021-00553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drury AN, Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68(3):213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Headrick JP, Ashton KJ, Rose’meyer RB, Peart JN. Cardiovascular adenosine receptors: expression, actions and interactions. Pharmacol Ther. 2013;140(1):92–111. doi: 10.1016/j.pharmthera.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Burnstock G. Purinergic signaling in the cardiovascular system. Circ Res. 2017;120(1):207–228. doi: 10.1161/CIRCRESAHA.116.309726. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Z, Matsumoto T, Jankowski V, Pernow J, Mustafa SJ, Duncker DJ, Merkus D. Uridine adenosine tetraphosphate and purinergic signaling in cardiovascular system: An update. Pharmacol Res. 2019;141:32–45. doi: 10.1016/j.phrs.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wernly B, Zhou Z. More purinergic receptors deserve attention as therapeutic targets for the treatment of cardiovascular disease. Am J Physiol Heart Circ Physiol. 2020;319(4):H723–H729. doi: 10.1152/ajpheart.00417.2020. [DOI] [PubMed] [Google Scholar]
- 16.Ralevic V. Purinergic signalling in the cardiovascular system-a tribute to Geoffrey Burnstock. Purinergic Signal. 2021;17(1):63–69. doi: 10.1007/s11302-020-09734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ralevic V, Dunn WR. Purinergic transmission in blood vessels. Auton Neurosci. 2015;191:48–66. doi: 10.1016/j.autneu.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Abbas ZSB, Latif ML, Dovlatova N, Fox SC, Heptinstall S, Dunn WR, Ralevic V. UDP-sugars activate P2Y14 receptors to mediate vasoconstriction of the porcine coronary artery. Vascul Pharmacol. 2018;103–105:36–46. doi: 10.1016/j.vph.2017.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev. 2014;66(1):102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- 20.Sadek HA, Nulton-Persson AC, Szweda PA, Szweda L. Cardiac ischemia/reperfusion, aging, and redox-dependent alterations in mitochondrial function. Arch Biochem Biophys. 2003;420(2):201–208. doi: 10.1016/j.abb.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Ely SW, Mentzer RM, Jr, Lasley RD, Berne RM. Functional and metabolic evidence of enhanced myocardial tolerance to ischemia and reperfusion with adenosine. J Thorac Cardiovasc Surg. 1985;90(4):549–556. doi: 10.1016/S0022-5223(19)38568-X. [DOI] [PubMed] [Google Scholar]
- 22.Ely SW, Berne RM. Protective effects of adenosine in myocardial ischemia. Circulation. 1992;85(3):893–904. doi: 10.1161/01.cir.85.3.893. [DOI] [PubMed] [Google Scholar]
- 23.Wyatt DA, Edmunds MC, Berne RM, Lasley RD, Mentzer RM., Jr Adenosine stimulates glycolytic flux in isolated perfused rat hearts by A1-adenosine receptors. Am J Physiol. 1989;257(6 Pt 2):H1952–1957. doi: 10.1152/ajpheart.1989.257.6.H1952. [DOI] [PubMed] [Google Scholar]
- 24.Mainwaring R, Lasley R, Rubio R, Wyatt DA, Mentzer RM., Jr Adenosine stimulates glucose uptake in the isolated rat heart. Surgery. 1988;103(4):445–449. [PubMed] [Google Scholar]
- 25.Headrick JP, Gauthier NS, Berr SS, Morrison RR, Matherne GP. Transgenic A1 adenosine receptor overexpression markedly improves myocardial energy state during ischemia-reperfusion. J Mol Cell Cardiol. 1998;30(5):1059–1064. doi: 10.1006/jmcc.1998.0672. [DOI] [PubMed] [Google Scholar]
- 26.Pomerantz BJ, Robinson TN, Morrell TD, Heimbach JK, Banerjee A, Harken AH. Selective mitochondrial adenosine triphosphate-sensitive potassium channel activation is sufficient to precondition human myocardium. J Thorac Cardiovasc Surg. 2000;120(2):387–392. doi: 10.1067/mtc.2000.107521. [DOI] [PubMed] [Google Scholar]
- 27.Cerniway RJ, Morrison RR, Byford AM, Lankford AR, Headrick JP, Van Wylen DG, Matherne GP. A1 adenosine receptor overexpression decreases stunning from anoxia-reoxygenation: role of the mitochondrial K(ATP) channel. Basic Res Cardiol. 2002;97(3):232–238. doi: 10.1007/s003950200016. [DOI] [PubMed] [Google Scholar]
- 28.Miura T, Liu Y, Kita H, Ogawa T, Shimamoto K. Roles of mitochondrial ATP-sensitive K channels and PKC in anti-infarct tolerance afforded by adenosine A1 receptor activation. J Am Coll Cardiol. 2000;35(1):238–245. doi: 10.1016/s0735-1097(99)00493-3. [DOI] [PubMed] [Google Scholar]
- 29.Leung CH, Wang LX, Nielsen JM, Tropak MB, Fu YY, Kato H, Callahan J, Redington AN, Caldarone CA. Remote cardioprotection by transfer of coronary effluent from ischemic preconditioned rabbit heart preserves mitochondrial integrity and function via adenosine receptor activation. Cardiovasc Drugs Ther. 2014;28(1):7–17. doi: 10.1007/s10557-013-6489-2. [DOI] [PubMed] [Google Scholar]
- 30.Cleveland JC, Jr, Meldrum DR, Rowland RT, Banerjee A, Harken A. Adenosine preconditioning of human myocardium is dependent upon the ATP-sensitive K+ channel. J Mol Cell Cardiol. 1997;29(1):175–182. doi: 10.1006/jmcc.1996.0262. [DOI] [PubMed] [Google Scholar]
- 31.Headrick JP, Gauthier NS, Morrison R, Matherne GP. Cardioprotection by K(ATP) channels in wild-type hearts and hearts overexpressing A(1)-adenosine receptors. Am J Physiol Heart Circ Physiol. 2000;279(4):H1690–1697. doi: 10.1152/ajpheart.2000.279.4.H1690. [DOI] [PubMed] [Google Scholar]
- 32.Thourani VH, Nakamura M, Ronson RS, Jordan JE, Zhao ZQ, Levy JH, Szlam F, Guyton RA, Vinten-Johansen J. Adenosine A(3)-receptor stimulation attenuates postischemic dysfunction through K(ATP) channels. Am J Physiol. 1999;277(1):H228–235. doi: 10.1152/ajpheart.1999.277.1.H228. [DOI] [PubMed] [Google Scholar]
- 33.Tracey WR, Magee W, Masamune H, Oleynek JJ, Hill RJ. Selective activation of adenosine A3 receptors with N6-(3-chlorobenzyl)-5’-N-methylcarboxamidoadenosine (CB-MECA) provides cardioprotection via KATP channel activation. Cardiovasc Res. 1998;40(1):138–145. doi: 10.1016/s0008-6363(98)00112-6. [DOI] [PubMed] [Google Scholar]
- 34.Zhao TC, Kukreja RC. Late preconditioning elicited by activation of adenosine A(3) receptor in heart: role of NF- kappa B, iNOS and mitochondrial K(ATP) channel. J Mol Cell Cardiol. 2002;34(3):263–277. doi: 10.1006/jmcc.2001.1510. [DOI] [PubMed] [Google Scholar]
- 35.Zhao T, Xi L, Chelliah J, Levasseur JE, Kukreja RC. Inducible nitric oxide synthase mediates delayed myocardial protection induced by activation of adenosine A(1) receptors: evidence from gene-knockout mice. Circulation. 2000;102(8):902–907. doi: 10.1161/01.cir.102.8.902. [DOI] [PubMed] [Google Scholar]
- 36.Benoist L, Chadet S, Genet T, Lefort C, Heraud A, Danila MD, Muntean DM, Baron C, Angoulvant D, Babuty D, Bourguignon T, Ivanes F. Stimulation of P2Y11 receptor protects human cardiomyocytes against hypoxia/reoxygenation injury and involves PKCε signaling pathway. Sci Rep. 2019;9(1):11613. doi: 10.1038/s41598-019-48006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z, He L, Li L, Chen L. The P2X7 purinergic receptor: an emerging therapeutic target in cardiovascular diseases. Clin Chim Acta. 2018;479:196–207. doi: 10.1016/j.cca.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 38.Zucchi R, Cerniway RJ, Ronca-Testoni S, Morrison RR, Ronca G, Matherne GP. Effect of cardiac A(1) adenosine receptor overexpression on sarcoplasmic reticulum function. Cardiovasc Res. 2002;53(2):326–333. doi: 10.1016/s0008-6363(01)00471-0. [DOI] [PubMed] [Google Scholar]
- 39.Zucchi R, Yu G, Ghelardoni S, Ronca F, Ronca-Testoni S. A3 adenosine receptor stimulation modulates sarcoplasmic reticulum Ca(2+) release in rat heart. Cardiovasc Res. 2001;50(1):56–64. doi: 10.1016/s0008-6363(00)00318-7. [DOI] [PubMed] [Google Scholar]
- 40.Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest. 1990;85(4):1150–1157. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Z, Cernniway RJ, Byford AM, Berr SS, French BA, Matherne GP. Cardiac overexpression of A1-adenosine receptor protects intact mice against myocardial infarction. Am J Physiol Heart Circ Physiol. 2002;282(3):H949–955. doi: 10.1152/ajpheart.00741.2001. [DOI] [PubMed] [Google Scholar]
- 42.Jordan JE, Thourani VH, Auchampach JA, Robinson JA, Wang NP, Vinten-Johansen J. A(3) adenosine receptor activation attenuates neutrophil function and neutrophil-mediated reperfusion injury. Am J Physiol. 1999;277(5):H1895–1905. doi: 10.1152/ajpheart.1999.277.5.H1895. [DOI] [PubMed] [Google Scholar]
- 43.Cerniway RJ, Yang Z, Jacobson MA, Linden J, Matherne GP. Targeted deletion of A(3) adenosine receptors improves tolerance to ischemia-reperfusion injury in mouse myocardium. Am J Physiol Heart Circ Physiol. 2001;281(4):H1751–1758. doi: 10.1152/ajpheart.2001.281.4.H1751. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314(5806):1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 45.Borg N, Alter C, Görldt N, Jacoby C, Ding Z, Steckel B, Quast C, Bönner F, Friebe D, Temme S, Flögel U, Schrader J. CD73 on T Cells orchestrates cardiac wound healing after myocardial infarction by purinergic metabolic reprogramming. Circulation. 2017;136(3):297–313. doi: 10.1161/CIRCULATIONAHA.116.023365. [DOI] [PubMed] [Google Scholar]
- 46.Boros D, Thompson J, Larson DF. Adenosine regulation of the immune response initiated by ischemia reperfusion injury. Perfusion. 2016;31(2):103–110. doi: 10.1177/0267659115586579. [DOI] [PubMed] [Google Scholar]
- 47.Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol Ther. 2007;114(2):208–221. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Wu Y, Zhang Y, Zhang J, Zhai T, Hu J, Luo H, Zhou H, Zhang Q, Zhou Z, Liu F. Cathelicidin aggravates myocardial ischemia/reperfusion injury via activating TLR4 signaling and P2X7R/NLRP3 inflammasome. J Mol Cell Cardiol. 2020;139:75–86. doi: 10.1016/j.yjmcc.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123(6):594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 50.Sandanger Ø, Ranheim T, Vinge LE, Bliksøen AK, Finsen AV, Dahl CP, Askevold ET, Florholmen G, Christensen G, Fitzgerald KA, Lien E, Valen G, Espevik T, Aukrust P, Yndestad A. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2013;99(1):164–174. doi: 10.1093/cvr/cvt091. [DOI] [PubMed] [Google Scholar]
- 51.Zhou J, Zhou Z, Liu X, Yin HY, Tang Y, Cao X. P2X7 receptor-mediated inflammation in cardiovascular disease. Front Pharmacol. 2021;12:654425. doi: 10.3389/fphar.2021.654425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vessey DA, Li L, Kelley M. Pannexin-I/P2X7 purinergic receptor channels mediate the release of cardioprotectants induced by ischemic pre- and postconditioning. J Cardiovasc Pharmacol Ther. 2010;15(2):190–195. doi: 10.1177/1074248409360356. [DOI] [PubMed] [Google Scholar]
- 53.Vessey DA, Li L, Kelley M. P2X7 receptor agonists pre- and postcondition the heart against ischemia-reperfusion injury by opening pannexin-1/P2X7 channels. Am J Physiol Heart Circ Physiol. 2011;301(3):H881–887. doi: 10.1152/ajpheart.00305.2011. [DOI] [PubMed] [Google Scholar]
- 54.Vessey DA, Li L, Kelley M. Ischemic preconditioning requires opening of pannexin-1/P2X(7) channels not only during preconditioning but again after index ischemia at full reperfusion. Mol Cell Biochem. 2011;351(1–2):77–84. doi: 10.1007/s11010-011-0713-9. [DOI] [PubMed] [Google Scholar]
- 55.Chadet S, Ivanes F, Benoist L, Salmon-Gandonnière C, Guibon R, Velge-Roussel F, Babuty D, Baron C, Roger S, Angoulvant D. Hypoxia/reoxygenation inhibits P2Y11 receptor expression and its immunosuppressive activity in human dendritic cells. J Immunol. 2015;195(2):651–660. doi: 10.4049/jimmunol.1500197. [DOI] [PubMed] [Google Scholar]
- 56.Lefort C, Benoist L, Chadet S, Piollet M, Heraud A, Babuty D, Baron C, Ivanes F, Angoulvant D. Stimulation of P2Y11 receptor modulates cardiac fibroblasts secretome toward immunomodulatory and protective roles after hypoxia/reoxygenation injury. J Mol Cell Cardiol. 2018;121:212–222. doi: 10.1016/j.yjmcc.2018.07.245. [DOI] [PubMed] [Google Scholar]
- 57.Wang L, Li N, Wang F, Cui L. P2Y12 inhibition in macrophages reduces ventricular arrhythmias in rats after myocardial ischemia-reperfusion. Adv Clin Exp Med. 2021;30(4):413–420. doi: 10.17219/acem/133139. [DOI] [PubMed] [Google Scholar]
- 58.Ziegler M, Wang X, Peter K. Platelets in cardiac ischaemia/reperfusion injury: a promising therapeutic target. Cardiovasc Res. 2019;115(7):1178–1188. doi: 10.1093/cvr/cvz070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patti G, Micieli G, Cimminiello C, Bolognese L. The role of clopidogrel in 2020: a reappraisal. Cardiovasc Ther. 2020;2020:8703627. doi: 10.1155/2020/8703627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yokota T, Higuma T, Endo T, Nishizaki F, Hanada K, Yokoyama H, Yamada M, Okumura K, Tomita H. Prasugrel versus clopidogrel for residual thrombus burden in patients with ST-segment elevation myocardial infarction: an optical coherence tomography study. Coron Artery Dis. 2018;29(8):663–669. doi: 10.1097/mca.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 61.Pandit A, Aryal MR, Pandit AA, Jalota L, Hakim FA, Mookadam F, Lee HR, Tleyjeh IM. Cangrelor versus clopidogrel in percutaneous coronary intervention: a systematic review and meta-analysis. EuroIntervention. 2014;9(11):1350–1358. doi: 10.4244/eijv9I11A226. [DOI] [PubMed] [Google Scholar]
- 62.Barrabés JA, Inserte J, Mirabet M, Quiroga A, Hernando V, Figueras J, Garcia-Dorado D. Antagonism of P2Y12 or GPIIb/IIIa receptors reduces platelet-mediated myocardial injury after ischaemia and reperfusion in isolated rat hearts. Thromb Haemost. 2010;104(1):128–135. doi: 10.1160/TH09-07-0440. [DOI] [PubMed] [Google Scholar]
- 63.Ye Y, Birnbaum GD, Perez-Polo JR, Nanhwan MK, Nylander S, Birnbaum Y. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol. 2015;35(8):1805–1814. doi: 10.1161/ARVBAHA.115.305655. [DOI] [PubMed] [Google Scholar]
- 64.Gimbel M, Qaderdan K, Willemsen L, Hermanides R, Bergmeijer T, Very E, Heestermans T, Gin MTJ, Waalewijn R, Hofma S, Hartog F, Jukema W, Birgelen C, Voskuil M, Kelder J, Deneer V, Berg JT. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet. 2020;395(10233):1374–1381. doi: 10.1016/S0140-6736(20)30325-1. [DOI] [PubMed] [Google Scholar]
- 65.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Investigators P, Freij A, Thórsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 66.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, Servi SD, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 67.Bell RM, Sivaraman V, Kunuthur SP, Cohen MV, Downey JM, Yellon DM. Cardioprotective properties of the platelet P2Y12 receptor inhibitor, Cangrelor: protective in diabetics and reliant upon the presence of blood. Cardiovasc Drugs Ther. 2015;29(5):415–418. doi: 10.1007/s10557-015-6609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohen MV, Yang XM, White J, Yellon DM, Bell RM, Downey JM. Cangrelor-mediated cardioprotection requires platelets and sphingosine phosphorylation. Cardiovasc Drugs Ther. 2016;30(2):229–232. doi: 10.1007/s10557-015-6633-2. [DOI] [PubMed] [Google Scholar]
- 69.Dost T. Cardioprotective properties of the platelet P2Y(12) receptor inhibitor prasugrel on cardiac ischemia/reperfusion injury. Pharmacol Rep. 2020;72(3):672–679. doi: 10.1007/s43440-019-00046-5. [DOI] [PubMed] [Google Scholar]
- 70.Aungraheeta R, Conibear A, Butler M, Kelly E, Nylander S, Mumford A, Mundell SJ. Inverse agonism at the P2Y12 receptor and ENT1 transporter blockade contribute to platelet inhibition by ticagrelor. Blood. 2016;128(23):2717–2728. doi: 10.1182/blood-2016-03-707844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Armstrong D, Summers C, Ewart L, Nylander S, Sidaway JE, Giezen J. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther. 2014;19(2):209–219. doi: 10.1177/1074248413511693. [DOI] [PubMed] [Google Scholar]
- 72.Zatta AJ, Matherne GP, Headrick JP. Adenosine receptor-mediated coronary vascular protection in post-ischemic mouse heart. Life Sci. 2006;78(21):2426–2437. doi: 10.1016/j.lfs.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 73.Huang CH, Kim SJ, Ghaleh B, Kudej RK, Shen YT, Bishop SP, Vatner SF. An adenosine agonist and preconditioning shift the distribution of myocardial blood flow in conscious pigs. Am J Physiol. 1999;276(2):H368–375. doi: 10.1152/ajpheart.1999.276.2.H368. [DOI] [PubMed] [Google Scholar]
- 74.Cox BF, Greenland BD, Perrone MH, Merkel LA. Ischaemia/reperfusion selectively attenuates coronary vasodilatation to an adenosine A2- but not to an A1-agonist in the dog. Br J Pharmacol. 1994;111:1233–1239. doi: 10.1111/j.1476-5381.1994.tb14877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Z, de Wijs-Meijler D, Lankhuizen I, Jankowski J, Jankowski V, Jan Danser AH, Duncker DJ, Merkus D. Blunted coronary vasodilator response to uridine adenosine tetraphosphate in post-infarct remodeled myocardium is due to reduced P1 receptor activation. Pharmacol Res. 2013;77:22–29. doi: 10.1016/j.phrs.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Wernly B, Cao X, Mustafa SJ, Tang Y, Zhou Z. Adenosine and adenosine receptor-mediated action in coronary microcirculation. Basic Res Cardiol. 2021;116(1):22. doi: 10.1007/s00395-021-00859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gündüz D, Kasseckert SA, Härtel FV, Aslam M, Abdallah Y, Schäfer M, Piper HM, Noll T, Schäfer C. Accumulation of extracellular ATP protects against acute reperfusion injury in rat heart endothelial cells. Cardiovasc Res. 2006;71(4):764–773. doi: 10.1016/j.cardiores.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 78.García-Villalón ÁL, Granado M, Monge L, Fernández N, Carreño-Tarragona G, Amor S. Purinergic component in the coronary vasodilatation to acetylcholine after ischemia-reperfusion in perfused rat hearts. J Vasc Res. 2014;51(4):283–289. doi: 10.1159/000365928. [DOI] [PubMed] [Google Scholar]
- 79.Kristiansen SB, Skovsted GF, Berchtold LA, Radziwon-Balicka A, Dreisig K, Edvinsson L, Sheykhzade M, Haanes KA. Role of pannexin and adenosine triphosphate (ATP) following myocardial ischemia/reperfusion. Scand Cardiovasc J. 2018;52(6):340–343. doi: 10.1080/14017431.2018.1552793. [DOI] [PubMed] [Google Scholar]
- 80.Yetgin T, Uitterdijk A, Hekkert MTL, Merkus D, Krabbendam-Peters I, Beusekom HMM, Falotico R, Serruys P, Manintveld OC, Geuns RJM, Zijlstra F, Duncker DJ. Limitation of infarct size and no-reflow by intracoronary adenosine depends critically on dose and duration. JACC Cardiovasc Interv. 2015;8(15):1990–1999. doi: 10.1016/j.jcin.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 81.Bulluck H, Sirker A, Loke YK, Garcia-Dorado D, Hausenloy DJ. Clinical benefit of adenosine as an adjunct to reperfusion in ST-elevation myocardial infarction patients: an updated meta-analysis of randomized controlled trials. Int J Cardiol. 2016;202:228–237. doi: 10.1016/j.ijcard.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morrison RR, Jones R, Byford AM, Stell AR, Peart J, Headrick JP, Matherne GP. Transgenic overexpression of cardiac A(1) adenosine receptors mimics ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2000;279(3):H1071–1078. doi: 10.1152/ajpheart.2000.279.3.H1071. [DOI] [PubMed] [Google Scholar]
- 83.Crawford M, Ford S, Henry M, Matherne GP, Lankford A. Myocardial function following cold ischemic storage is improved by cardiac-specific overexpression of A1-adenosine receptors. Can J Physiol Pharmacol. 2005;83(6):493–498. doi: 10.1139/y05-038. [DOI] [PubMed] [Google Scholar]
- 84.Regan SE, Broad M, Byford AM, Lankford AR, Cerniway RJ, Mayo MW, Matherne GP. A1 adenosine receptor overexpression attenuates ischemia-reperfusion-induced apoptosis and caspase 3 activity. Am J Physiol Heart Circ Physiol. 2003;284(3):H859–866. doi: 10.1152/ajpheart.00251.2002. [DOI] [PubMed] [Google Scholar]
- 85.Zhao ZQ, Budde JM, Morris C, Wang NP, Velez DA, Muraki S, Guyton RA, Vinten-Johansen J. Adenosine attenuates reperfusion-induced apoptotic cell death by modulating expression of Bcl-2 and Bax proteins. J Mol Cell Cardiol. 2001;33(1):57–68. doi: 10.1006/jmcc.2000.1275. [DOI] [PubMed] [Google Scholar]
- 86.Granado M, Amor S, Montoya JJ, Monge L, Fernández N, García-Villalón AL. Altered expression of P2Y2 and P2X7 purinergic receptors in the isolated rat heart mediates ischemia-reperfusion injury. Vascul Pharmacol. 2015;73:96–103. doi: 10.1016/j.vph.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 87.Bourguignon T, Benoist L, Chadet S, Miquelestorena-Standley E, Fromont G, Ivanes F, Angoulvant D. Stimulation of murine P2Y11-like purinoreceptor protects against hypoxia/reoxygenation injury and decreases heart graft rejection lesions. J Thorac Cardiovasc Surg. 2019;158(3):780–790.e1. doi: 10.1016/j.jtcvs.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 88.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 89.Reimer KA, Murry CE, Yamasawa I, Hill ML, Jennings RB. Four brief periods of myocardial ischemia cause no cumulative ATP loss or necrosis. Am J Physiol. 1986;251(6 Pt 2):H1306–1315. doi: 10.1152/ajpheart.1986.251.6.H1306. [DOI] [PubMed] [Google Scholar]
- 90.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88(3):1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 91.Yellon DM, Baxter GF, Garcia-Dorado D, Heusch G, Sumeray MS. Ischaemic preconditioning: present position and future directions. Cardiovasc Res. 1998;37(1):21–33. doi: 10.1016/s0008-6363(97)00214-9. [DOI] [PubMed] [Google Scholar]
- 92.Mullane K, Bullough D. Harnessing an endogenous cardioprotective mechanism: cellular sources and sites of action of adenosine. J Mol Cell Cardiol. 1995;27(4):1041–1054. doi: 10.1016/0022-2828(95)90073-x. [DOI] [PubMed] [Google Scholar]
- 93.Auchampach JA, Gross GJ. Adenosine A1 receptors, KATP channels, and ischemic preconditioning in dogs. Am J Physiol. 1993;264(5 Pt 2):H1327–1336. doi: 10.1152/ajpheart.1993.264.5.H1327. [DOI] [PubMed] [Google Scholar]
- 94.Schulz R, Rose J, Post H, Heusch G. Involvement of endogenous adenosine in ischaemic preconditioning in swine. Pflugers Arch. 1995;430(2):273–282. doi: 10.1007/BF00374659. [DOI] [PubMed] [Google Scholar]
- 95.Walker DM, Walker JM, Pugsley WB, Pattison CW, Yellon DM. Preconditioning in isolated superfused human muscle. J Mol Cell Cardiol. 1995;27(6):1349–1357. doi: 10.1016/s0022-2828(05)82397-1. [DOI] [PubMed] [Google Scholar]
- 96.McCully JD, Toyoda Y, Uematsu M, Stewart RD, Levitsky S. Adenosine-enhanced ischemic preconditioning: adenosine receptor involvement during ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2001;280(2):H591–602. doi: 10.1152/ajpheart.2001.280.2.H591. [DOI] [PubMed] [Google Scholar]
- 97.Toyoda Y, Friehs I, Parker RA, Levitsky S, McCully JD. Differential role of sarcolemmal and mitochondrial K(ATP) channels in adenosine-enhanced ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2000;279(6):H2694–2703. doi: 10.1152/ajpheart.2000.279.6.H2694. [DOI] [PubMed] [Google Scholar]
- 98.Cohen MV, Downey JM. Adenosine: trigger and mediator of cardioprotection. Basic Res Cardiol. 2008;103(3):203–215. doi: 10.1007/s00395-007-0687-7. [DOI] [PubMed] [Google Scholar]
- 99.Mubagwa K, Flameng W. Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardiovasc Res. 2001;52(1):25–39. doi: 10.1016/s0008-6363(01)00358-3. [DOI] [PubMed] [Google Scholar]
- 100.Giannella E, Mochmann HC, Levi R. Ischemic preconditioning prevents the impairment of hypoxic coronary vasodilatation caused by ischemia/reperfusion: role of adenosine A1/A3 and bradykinin B2 receptor activation. Circ Res. 1997;81(3):415–422. doi: 10.1161/01.res.81.3.415. [DOI] [PubMed] [Google Scholar]
- 101.Guo Y, Bolli R, Bao W, Wu WJ, Black RG, Jr, Murphree SS, Salvatore CA, Jacobson MA, Auchampach JA. Targeted deletion of the A3 adenosine receptor confers resistance to myocardial ischemic injury and does not prevent early preconditioning. J Mol Cell Cardiol. 2001;33(4):825–830. doi: 10.1006/jmcc.2001.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vinten-Johansen J, Thourani VH, Ronson RS, Jordan JE, Zhao ZQ, Nakamura M, Velez D, Guyton RA. Broad-spectrum cardioprotection with adenosine. Ann Thorac Surg. 1999;68(5):1942–1948. doi: 10.1016/s0003-4975(99)01018-8. [DOI] [PubMed] [Google Scholar]
- 103.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12(3–4):181–188. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 104.Tian Y, Piras BA, Kron IL, French BA, Yang Z (2015) Adenosine 2B receptor activation reduces myocardial reperfusion injury by promoting anti-inflammatory macrophages differentiation via PI3K/Akt pathway. Oxid Med Cell Longev 2015: 58529710.1155/2015/585297 [DOI] [PMC free article] [PubMed]
- 105.Reid EA, Kristo G, Yoshimura Y, Ballard-Croft C, Keith BJ, Mentzer RM, Jr, Lasley RD. In vivo adenosine receptor preconditioning reduces myocardial infarct size via subcellular ERK signaling. Am J Physiol Heart Circ Physiol. 2005;288(5):H2253–2259. doi: 10.1152/ajpheart.01009.2004. [DOI] [PubMed] [Google Scholar]
- 106.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87(3):893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 107.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579–588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 108.Hausenloy DJ, Iliodromitis EK, Andreadou I, Papalois A, Gritsopoulos G, Anastasiou-Nana M, Kremastinos DT, Yellon DM. Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc Drugs Ther. 2012;26(2):87–93. doi: 10.1007/s10557-011-6364-y. [DOI] [PubMed] [Google Scholar]
- 109.Cohen MV, Downey JM. Ischemic postconditioning: from receptor to end-effector. Antioxid Redox Signal. 2011;14(5):821–831. doi: 10.1089/ars.2010.3318. [DOI] [PubMed] [Google Scholar]
- 110.Tscharre M, Egger F, Machata M, Rohla M, Michael N, Neumayr M, Zweiker R, Hajos J, Adlbrecht C, Suppan M, Helmreich W, Eber B, Huber K, Weiss TW. Contemporary use of P2Y12-inhibitors in patients with acute coronary syndrome undergoing percutaneous coronary intervention in Austria: a prospective, multi-centre registry. PLoS One. 2017;12(6):e0179349. doi: 10.1371/journal.pone.0179349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li J, Conrad C, Mills TW, Berg NK, Kim B, Ruan W, Lee JW, Zhang X, Yuan X, Eltzschig HK. PMN-derived netrin-1 attenuates cardiac ischemia-reperfusion injury via myeloid ADORA2B signaling. J Exp Med. 2021;218(6):e20210008. doi: 10.1084/jem.20210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ihara M, Asanuma H, Yamazaki S, Kato H, Asano Y, Shinozaki Y, Mori H, Minamino T, Asakura M, Sugimachi M, Mochizuki N, Kitakaze M. An interaction between glucagon-like peptide-1 and adenosine contributes to cardioprotection of a dipeptidyl peptidase 4 inhibitor from myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2015;308(10):H1287–1297. doi: 10.1152/ajpheart.00835.2014. [DOI] [PubMed] [Google Scholar]
- 113.Millart H, Alouane L, Oszust F, Chevallier S, Robinet A. Involvement of P2Y receptors in pyridoxal-5’-phosphate-induced cardiac preconditioning. Fundam Clin Pharmacol. 2009;23(3):279–292. doi: 10.1111/j.1472-8206.2009.00677.x. [DOI] [PubMed] [Google Scholar]
- 114.Dhalla NS, Takeda S, Elimban V. Mechanisms of the beneficial effects of vitamin B6 and pyridoxal 5-phosphate on cardiac performance in ischemic heart disease. Clin Chem Lab Med. 2013;51(3):535–543. doi: 10.1515/cclm-2012-0553. [DOI] [PubMed] [Google Scholar]
- 115.Tang Y, Wang Y, Park KM, Hu Q, Teoh JP, Broskova Z, Ranganathan P, Jayakumar C, Li J, Su H, Tang Y, Ramesh G, Kim IM. MicroRNA-150 protects the mouse heart from ischaemic injury by regulating cell death. Cardiovasc Res. 2015;106(3):387–397. doi: 10.1093/cvr/cvv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paiva M, Riksen NP, Davidson SM, Hausenloy DJ, Monteiro P, Goncalves L, Providência L, Rongen GA, Smits P, Mocanu MM, Yellon DM. Metformin prevents myocardial reperfusion injury by activating the adenosine receptor. J Cardiovasc Pharmacol. 2009;53(5):373–378. doi: 10.1097/FJC.0b013e31819fd4e7. [DOI] [PubMed] [Google Scholar]
- 117.Bai Y, Muqier MH, Iwasa M, Sumi S, Yamada Y, Ushikoshi H, Aoyama T, Nishigaki K, Takemura G, Uno B, Minatoguchi S. Cilostazol protects the heart against ischaemia reperfusion injury in a rabbit model of myocardial infarction: focus on adenosine, nitric oxide and mitochondrial ATP-sensitive potassium channels. Clin Exp Pharmacol Physiol. 2011;38(10):658–665. doi: 10.1111/j.1440-1681.2011.05550.x. [DOI] [PubMed] [Google Scholar]
- 118.Figueredo VM, Diamond I, Zhou HZ, Camacho SA. Chronic dipyridamole therapy produces sustained protection against cardiac ischemia-reperfusion injury. Am J Physiol. 1999;277(5):H2091–2097. doi: 10.1152/ajpheart.1999.277.5.H2091. [DOI] [PubMed] [Google Scholar]
- 119.Yao L, Wong GTC, Xia ZY, Irwin MG. Interaction between spinal opioid and adenosine receptors in remote cardiac preconditioning: effect of intrathecal morphine. J Cardiothorac Vasc Anesth. 2011;25(3):444–448. doi: 10.1053/j.jvca.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 120.Ha JY, Lee YC, Park SJ, Jang YH, Kim JH. Remifentanil postconditioning has cross talk with adenosine receptors in the ischemic-reperfused rat heart. J Surg Res. 2015;195(1):37–43. doi: 10.1016/j.jss.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 121.Peart JN, Gross GJ. Adenosine and opioid receptor-mediated cardioprotection in the rat: evidence for cross-talk between receptors. Am J Physiol Heart Circ Physiol. 2003;285(1):H81–89. doi: 10.1152/ajpheart.00985.2002. [DOI] [PubMed] [Google Scholar]
- 122.Birnbaum Y, Birnbaum GD, Birnbaum I, Nylander S, Ye Y. Ticagrelor and rosuvastatin have additive cardioprotective effects via adenosine. Cardiovasc Drugs Ther. 2016;30(6):539–550. doi: 10.1007/s10557-016-6701-2. [DOI] [PubMed] [Google Scholar]
- 123.Audia JP, Yang XM, Crockett ES, Housley N, Haq EU, O’Donnell K, Cohen MV, Downey JM, Alvarez DF. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res Cardiol. 2018;113(5):32. doi: 10.1007/s00395-018-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kristo G, Yoshimura Y, Ferraris SP, Jahania SA, Mentzer RM, Jr, Lasley RD. The preischemic combination of the sodium-hydrogen exchanger inhibitor cariporide and the adenosine agonist AMP579 acts additively to reduce porcine myocardial infarct size. J Am Coll Surg. 2004;199(4):586–594. doi: 10.1016/j.jamcollsurg.2004.05.274. [DOI] [PubMed] [Google Scholar]
- 125.Yang XM, Cui L, Alhammouri A, Downey JM, Cohen MV. Triple therapy greatly increases myocardial salvage during ischemia/reperfusion in the in situ rat heart. Cardiovasc Drugs Ther. 2013;27(5):403–412. doi: 10.1007/s10557-013-6474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yan XF, Zhang ZM, Yao HY, Guan Y, Zhu JP, Zhang LH, Jia YL, Wang RW. Cardiovascular protection and antioxidant activity of the extracts from the mycelia of Cordyceps sinensis act partially via adenosine receptors. Phytother Res. 2013;27(11):1597–1604. doi: 10.1002/ptr.4899. [DOI] [PubMed] [Google Scholar]
- 127.Bradamante S, Barenghi L, Piccinini F, Bertelli AAE, Jonge RD, Beemster P, Jong JWD. Resveratrol provides late-phase cardioprotection by means of a nitric oxide- and adenosine-mediated mechanism. Eur J Pharmacol. 2003;465(1–2):115–123. doi: 10.1016/s0014-2999(03)01441-9. [DOI] [PubMed] [Google Scholar]
- 128.Ye Y, Abu Said GH, Lin Y, Manickavasagam S, Hughes MG, McAdoo DJ, Perez-Polo RJ, Birnbaum Y. Caffeinated coffee blunts the myocardial protective effects of statins against ischemia-reperfusion injury in the rat. Cardiovasc Drugs Ther. 2008;22(4):275–282. doi: 10.1007/s10557-008-6105-z. [DOI] [PubMed] [Google Scholar]
- 129.Miyamae M, Diamond I, Weiner MW, Camacho SA, Figueredo VM. Regular alcohol consumption mimics cardiac preconditioning by protecting against ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 1997;94(7):3235–3239. doi: 10.1073/pnas.94.7.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ishibashi Y, Duncker DJ, Zhang J, Bache RJ. ATP-sensitive K+ channels, adenosine, and nitric oxide-mediated mechanisms account for coronary vasodilation during exercise. Circ Res. 1998;82(3):346–359. doi: 10.1161/01.res.82.3.346. [DOI] [PubMed] [Google Scholar]
- 131.Chen X, Li H, Wang K, Liang X, Wang W, Hu X, Huang Z, Wang Y. Aerobic exercise ameliorates myocardial inflammation, fibrosis and apoptosis in high-fat-diet rats by inhibiting P2X7 purinergic receptors. Front Physiol. 2019;10:1286. doi: 10.3389/fphys.2019.01286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xiao Y, Chen W, Zhong Z, Ding L, Bai H, Chen H, Zhang H, Gu Y, Lu S. Electroacupuncture preconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting mitophagy mediated by the mTORC1-ULK1-FUNDC1 pathway. Biomed Pharmacother. 2020;127:110148. doi: 10.1016/j.biopha.2020.110148. [DOI] [PubMed] [Google Scholar]
- 133.Xiao N, Li Y, Shao ML, Cui HF, Zhang CY, Kong SP, Zhang X, Yu HJ, Tan QW (2020) Jiaji (EX-B2)-based electroacupuncture preconditioning attenuates early ischaemia reperfusion injury in the rat myocardium. Evid Based Complement Alternat Med 2020: 885403310.1155/2020/8854033 [DOI] [PMC free article] [PubMed]
- 134.Burnstock G. Acupuncture: a novel hypothesis for the involvement of purinergic signalling. Med Hypotheses. 2009;73(4):470–472. doi: 10.1016/j.mehy.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 135.Lv ZY, Yang YQ, Yin LM. Role of purinergic signaling in acupuncture therapeutics. Am J Chin Med. 2021;49(3):645–659. doi: 10.1142/s0192415x21500294. [DOI] [PubMed] [Google Scholar]
- 136.Tang Y, Illes P. Tribute to Prof. Geoffrey Burnstock: his contribution to acupuncture. Purinergic Signal. 2021;17(1):71–77. doi: 10.1007/s11302-020-09729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dai QF, Wang YY, Liu Q, Xin JJ, Lu FY, Cui JJ, Wu SY, Zhou C, Zhao YX, Gao JH, Yu XC. A potential role of adenosine A2b receptor in mediating acupuncture pretreatment induced cardioprotection via influencing intracellular calcium regulator. Zhen Ci Yan Jiu. 2018;43(9):576–580. doi: 10.13702/j.1000-0607.180089. [DOI] [PubMed] [Google Scholar]
- 138.Ren Y, Chen Z, Wang R, Yu Y, Li D, He Y. Electroacupuncture improves myocardial ischemia injury via activation of adenosine receptors. Purinergic Signal. 2020;16(3):337–345. doi: 10.1007/s11302-020-09704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lu S, Tang Y, Ding Y, Yu M, Fu S, Zhu B. Effects of electroacupuncture on the expression of adenosine receptors in the heart tissue of myocardial ischemia rats. Zhongguo Zhen Jiu. 2018;38(2):173–179. doi: 10.13703/j.0255-2930.2018.02.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.