Abstract
Depression is a common neuropsychiatric disorder with high incidence and disability. Electroacupuncture (EA) is effective in the treatment of depression. However, the underlying mechanisms are not fully understood. Social isolation stress during post-weaning period can impair purinergic signaling in the brain of rodents and has emerged as a major risk factor for depression. The purpose of this study was to investigate the involvement of P2Y1 receptor (P2Y1R) in the antidepressant-like effects of EA. In this study, C57BL/6 mice were randomly assigned to group-housed (GH) or social isolated (SI) groups at post-natal day 21. After 6 weeks of social isolation, EA was performed on acupoints “Bai-hui” (GV20) and “Yin-tang” (GV29), or non-acupoints for 4 weeks. The SI mice received either intracerebroventricular injection of a selective P2Y1R agonist, MRS2365 (1 nmol); or a selective P2Y1R antagonist, MRS2179 (2 μmol), before and after EA. We found that SI mice exhibited depression-like behaviors accompanied with anxiety-like behaviors. The expressions of P2Y1R were well co-localized with GFAP-positive astrocytes and increased in the prefrontal cortex and hippocampus of SI mice. After treated with MRS2179, the depression-like behaviors of SI mice were attenuated, but not with MRS2365. Meanwhile, we found that EA could attenuate social isolation caused depression- and anxiety-like behaviors, and inhibited the up-regulation of P2Y1R in the prefrontal cortex and hippocampus of SI mice. Notably, the positive effects of EA on depression-like behaviors of SI mice could be reversed by MRS2365, while MRS2365 had no effect on the anxiolytic-like effects of EA. Therefore, we provide new evidence that EA could ameliorate depression- and anxiety-like behaviors in social isolation stress mice, and P2Y1R was involved in the antidepressant-like effects of EA.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11302-021-09827-1.
Keywords: Electroacupuncture (EA), Depression, P2Y1 receptor (P2Y1R), Prefrontal cortex, Hippocampus
Introduction
Depression is one of the most common psychiatric disorders, with high incidence and disability [1]. The primary symptoms of depression include a variety of emotional, neurovegetative, and cognitive symptoms. In recent years, prevalence of depression in adolescence rises substantially, with 1-year prevalence rate exceeding 4% [2]. Adolescent depression severely limits psychosocial function of patients and diminishes quality of life; very few individuals show complete symptomatic and functional recovery in adults, with most reporting residual symptoms or impairment [3, 4]. Although several antidepressants can be recommended for depression, the significant side effects of these drugs limit their efficacy and application [5, 6]. Therefore, therapeutic approaches that show effectiveness with less side effects will hold considerable promise for the treatment of adolescent depression.
Accumulating evidence from systematic review and meta-analysis indicate that acupuncture is an effective therapeutic approach for improving symptoms of depression [7], and it is well-tolerated with little risk of serious adverse effects. It is established that the antidepressant-like effects of acupuncture may be mediated by its regulation on the dysregulated glutamate system [8], the synaptic plasticity [9], the ERK-CREB pathways [10], and the expression of neurotransmitter including serotonin receptors [11], brain-derived neurotrophic factor [12], and neuropeptide Y [13]. Clinically, several acupuncture interventions have been used for depression: manual acupuncture, electroacupuncture (EA), auricular acupuncture, and laser acupuncture. Among these therapies, EA is commonly used by modern acupuncturists and is increasingly accepted worldwide. EA is also preferred in animal experiments and has been confirmed to alleviate depression-like behaviors in mice [11, 14, 15]. Acupoints “Bai-hui” (GV20) and “Yin-tang” (GV29) are considered to be the optimized treatment acupoints for depression [16–18], as well as other mental disorders [19], or neurodegenerative diseases [20, 21]. However, the efficacy and mechanisms of EA treatment for depression in adolescence remain elusive.
Recently, purinergic signaling system has attracted increasing interests in the pathology of psychiatry. There is evidence that adenosine 5S-triphosphate (ATP) was involved in the modulation of depression-like behaviors in mice [22]. The actions of ATP are mediated by ionotropic P2X and metabotropic P2Y receptor subfamilies [23]. P2Y1 receptor (P2Y1R) is a member of metabotropic P2Y purinoceptors that are coupled to G proteins. Extracellular ATP promotes goal-directed (motivated) behaviors via dopamine release which may be induced by P2Y1R stimulation [24]. As such, P2Y1R might be related to the pathology of depression-like behaviors. Previous studies have indicated that purinergic receptors are identified to critically mediate the physiological mechanisms underlying EA analgesia [25]. However, whether P2Y1R is involved in the antidepressant-like effects of EA remains to be determined.
There is increasing awareness that early social isolation stress during post-weaning period can impair purinergic signaling in the brain of rodents and has emerged as a major risk factor for depression [26]. In this study, the depression model in mice was imitated by 6 weeks of social isolation stress in early adulthood (post-natal day (PND) 21–63), and we sought to investigate the effects of EA applied at GV20 and GV29 in social isolation stress mice and explore the potential role of purinergic P2Y1R in the antidepressant action of EA.
Materials and methods
Experimental animals
Twenty-one-day-old male C57BL/6 mice, weighted 6 ± 2 g, were obtained from the Animal Experimental Center of Tongji Hospital of Huazhong University of Science and Technology (License Number: SCXK (Jing) 20,160,006). All the animals were housed in a controlled environment of 22 ± 0.5 °C, and lighting conditions with a 12-h light/dark cycle (lights on at 7.00 am). Food and water were available ad libitum. All experimental procedures were approved by the Animal Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. All efforts were made to minimize the animals’ suffering and to reduce the number of animals used for experiments.
Social isolation model
As described in previous literature [27], the majority of social isolation models use the same rearing conditions in that the pups are singly housed after weaning and compared with group-housed littermates (4–6/cage). As such, in this study, the mice were weaned and grouped on the 21st day after birth (PND21). Then, all animals were randomly assigned to a social isolated (SI) group or a group-housed (GH) group. The SI mice were housed individually (one mice per cage) in small polypropylene plastic cages (240 × 170 × 120 mm) from PND21 to PND63, while the GH mice were group housed (five mice per cage) in standard polypropylene plastic cages (250 × 250 × 150 mm). In order to diminish handling and social interaction of SI mice to a minimum, all the animals were kept in the same room so they have visual auditory and olfactory contact with other animals, but the singly housed animals are deprived of social contact and play for at least 6 weeks.
Experiment design
The experimental design and timeline are portrayed in their entirety in Fig. 1.
Fig. 1.
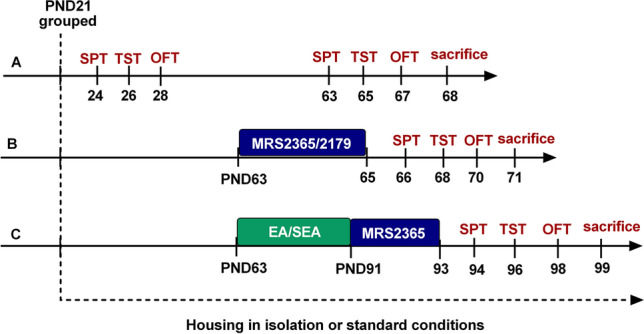
Experimental design—timeline and interventions. Timeline for experimental design showing age (weeks) of animals in each stage. EA electroacupuncture, OFT open field test, SEA sham electroacupuncture, SPT sucrose preference test, TST tail suspension test
In part A, we observed behavioral changes before and after 6 weeks of social isolation in the two groups: SI group and GH group. Behavioral tests including sucrose preference test (SPT), tail suspension test (TST), and open field test (OFT) were used to evaluate depression- and anxiety-like behaviors in both SI and GH animals.
In part B, the possible effects of P2Y1R agonists/antagonists on the SI model mice were assessed using the abovementioned tasks. In this regard, the SI mice were treated with MRS2179 or MRS2365 using lateral ventricle injection, once daily, continuous injection for 3 days (on PND 63–65).
In part C, we observed the effects of P2Y1R agonists on the antidepressant-like effects of EA. After 6 weeks of social isolation, the SI mice were subjected to 4 weeks of EA/sham EA (SEA) treatment on PND 63–91. Then, the mice in EA + MRS2365 groups were treated with MRS2365 once daily for 3 days (on PND 91–93).
EA treatment
For EA treatment, EA stimulation was performed at acupoints “Bai-hui” (GV20) and “Yin-tang” (GV29), 20 min per session, one session every other day for 4 weeks. During each session, the mice were anesthetized by isoflurane at a concentration of 2–5% which could minimize stress caused by EA and restraints. The acupuncture needles (0.25 mm in diameter and 13 mm long; Beijing Zhongyan Taihe Medicine Company, Ltd.) were inserted transversely (puncturing with an angle at 15 angle) into GV20 and GV29 to a depth of 2–3 mm. GV20 is located at the midpoint between the auricular apices, while GV29 is located midway between the medial ends of the two eyebrows [17]. Then, Han’s acupoint nerve stimulator (Beijing Huawei Industrial Development Company, Han’s LH202H type) was used to give a dilatational wave at a frequency of 2/15 Hz and an intensity of 1 mA. For sham EA treatment, acupuncture needles were inserted 0.5 mm lateral to GV20 and GV29 without electrical stimulation.
Behavioral tests
Sucrose preference test
The mice were trained to adapt to drink 1% sucrose solution for 48 h, followed by deprivation of food and water for 12 h. Then, each mouse was housed in an individual cage. They had free access to two pre-weighed bottles for 4 h, one contained 50 ml 1% sucrose solution and the other contained 50 ml pure water. At the end of the test, the percentage of sucrose preference of each mouse was calculated using the following formula: sucrose preference (%) = sucrose solution consumption (g) / (water consumption + sucrose solution consumption) × 100%.
Tail suspension test
The mice were individually suspended by the tip of the tails with a piece of adhesive tape, placed on a horizontal bar 50 cm above the floor of an isolated area. Then, their spontaneous activity was recorded for 6 min. After struggling for a period of time, mice showed intermittent immobility. The total immobility time was measured manually in a blinded manner.
Open field test
Each mouse was randomly placed in the middle of a 50 × 50-cm black-walled open field test box, while concurrently activating the video-tracking system to record their spontaneous activity for 5 min. The total distance travelled in the open filed area and time spent in the center zone of the open field were recorded. The data was processed and analyzed by SuperMaze software (Xinsoft Super Maze Animal Behavior Analysis System, Shanghai, China).
Intracerebroventricular injection
Injection drugs were diluted in sterile isotonic saline (NaCl 0.9%; vehicle). The selective P2Y1 antagonist 20-deoxy-N6-methyladenosine 30,50-bisphosphate tetrasodium salt (MRS2179, 0900/10) was obtained from RD (Rndsystems, USA). The selective P2Y1 agonist [[(1R,2R,3S,4R,5S)-4-[6-Amino-2-(methylthio)-9H-purin-9-yl]-2,3-dihydroxybicyclo[3.1.0]hex-1-yl]methyl] diphosphoric acid mono ester trisodium salt (MRS2365, 2157/1) was also from RD (Rndsystems, USA).
Drugs (MRS2179 or MRS2365) were injected into the right lateral ventricle once daily for 3 consecutive days. Briefly, the mice were positioned in a stereotaxic frame; they were anesthetized with 2% isoflurane, and maintained with 1% isoflurane during the surgery. A scalp incision along the sagittal midline was performed to access the skull and the bone was perforated with a drill in the following coordinates: 1.0 mm anterior and 1.0 mm lateral from the bregma. Then, MRS2179 (2 μmol) or MRS2365 (1 nmol) was injected into the right lateral ventricle in a 1 μl volume, using a 5-μl microsyringe (Hamilton, USA) at a speed of 1 μl/min. Drug doses were calculated according to IC50 or EC50 value provided by the supplier (MRS2179 IC50 = 1.15 μM; MRS2365 EC50 = 0.4 nM) or according to published data [28, 29]. After injection, the microsyringe was kept in place for 10 min to allow proper diffusion of the drugs. Body temperature was maintained at 37 °C throughout the surgery using a heat lamp. The mice died of anesthesia were excluded.
Western blotting
The expression of P2Y1R in the prefrontal cortex and hippocampus was tested by western blotting. Mice were anesthetized with chloral hydrate 420 mg/kg bodyweight intraperitoneally, and brain tissue samples were collected. Total protein was extracted using RIPA lysis buffer containing protease inhibitor cocktail and phenylmethylsulfonyl fluoride (PMSF), after grinding by the tissue lyser (Servicebio, China). The samples were centrifugated at 12,000 rpm, 4℃ for 15 min. Then, the supernatant was collected and the protein concentration was measured by BCA protein quantification kit (Beyotime Biotechnology, China). After mixing with loading buffer and heating at 100℃ for 10 min, the protein sample was loaded on 10% SDS-PAGE gel for electrophoresis. After the proteins were transferred to nitrocellulose (NC) filter membrane, it was blocked in 5% nonfat milk for 1 h at room temperature. Primary antibody was incubated at 4℃ overnight; after washed 3 times, membranes were incubated with secondary antibodies for 1 h at room temperature. The antibodies and antibody dilutions used were listed as follows: mouse monoclonal anti-P2Y1R antibody (1:500; Santa Cruz/sc-377324, USA), horseradish peroxidase (HRP)–conjugated goat anti-mouse IgG (1:5000; Servicebio/GB23301, China), and β-actin (1:1000; Servicebio/GB11001, China). Finally, a Bio-Rad Chemi Doc XRS + imaging system was used to obtain band imaging. ImageJ software was used to quantify the integrated density of each band. The optical densities (OD) of signals were analyzed using ImageJ software. The integrate OD of the signals was semi-quantified and expressed as the ratio of OD from the tested proteins to OD from the control of β-actin.
Immunofluorescence labeling
Mice were anesthetized with chloral hydrate 420 mg/kg bodyweight intraperitoneally, and perfused quickly with pre-cooled 0.9% sterile saline solution and 4% paraformaldehyde. The brains were removed, stored in 4% paraformaldehyde at 4 °C overnight, and then were transferred into 30% sucrose at 4℃ for 3 days. The prefrontal cortex was sectioned with a cryostat at 10 μm thickness and stored at − 80 °C for further experiments and analysis. After washed in PBS for 3 times, the sections were immersed in 0.3% TritonX-100 for 20 min. Then, the slices were blocked in 5% bovine serum albumin (BSA) for 1 h at room temperature. Primary antibodies were applied overnight at 4℃. After washed 3 times, brain sections were incubated with secondary antibodies for 1 h at room temperature. DAPI was used to label the cell nucleus. Finally, brain slices were sealed with antifade mountant for imaging using a fluorescence microscope (Olympus, Japan). The antibodies used were listed as follows: mouse monoclonal anti-P2Y1R antibody (1:100; Santa Cruz/sc-377324, USA), rabbit anti-Iba1 (1:200; Wako/019–19,741, Japan), rabbit anti-GFAP (1:200; Abcam/ab7260, USA), rabbit anti-NeuN (1:200; Cell Signaling Technology/24307, USA), Alexa Fluor 488 conjugated Donkey anti-rabbit IgG (Jackson ImmunoResearch/711–545-152, USA), Alexa Fluor 594 conjugated Donkey anti-mouse IgG (Jackson ImmunoResearch/711–585-150, USA).
Statistical analysis
The statistical analysis was performed using SPSS 20.0 software (IBM, Chicago, IL, USA) and GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA). Variables were displayed as the mean ± SEM. We used t-test to compare two groups and one-way analysis of variance (ANOVA) to analyze three or more groups. For multiple comparisons, we used Dunnett’s multiple comparisons test for comparison of multiple treatment groups with a single control group, and used Tukey–Kramer’s multiple-comparison tests for comparison of each treatment group with every other groups. A probability level of P < 0.05 was considered to be statistically significant.
Results
Six weeks of social isolation stress induced depression- and anxiety-like behaviors
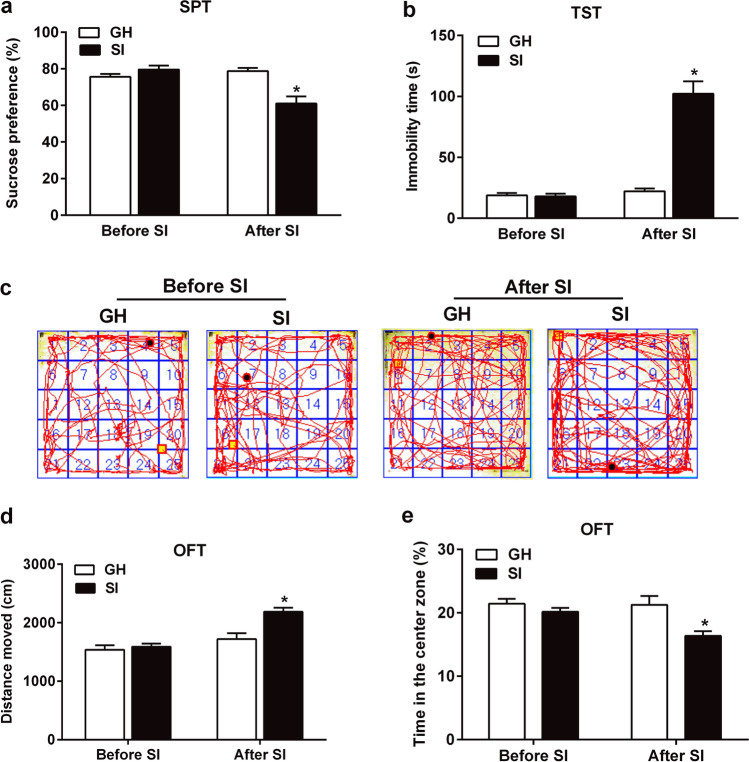
In order to investigate the changes in depression- and anxiety-like behaviors of SI mice, SPT, TST, and OFT were all tested in the SI and GH mice before and after social isolation. At baseline, no significant changes were observed in the sucrose consumption rate, the immobility time of TST, and the total moved distances and time spent in the center zone of the open field between the two groups.
After 6 weeks of social isolation, results obtained from the t-test analysis revealed that SI group showed a significantly reduced sucrose consumption rate in the SPT (t = 4.077, df = 14.0, P = 0.001, n = 8; Fig. 2a) and exhibited significantly increased immobility time in the TST (t = 7.713, df = 14.0, P < 0.001, n = 8; Fig. 2b), as compared to the mice in GH group. These changes exhibited depression-like behaviors. In the OFT, the SI mice showed longer moved distances (t = 3.819, df = 14.0, P < 0.001, n = 8; Fig. 2d), and spent lower time in the center zone of the open field than the GH group mice (t = 3.091, df = 14.0, P < 0.001, n = 8; Fig. 2e), which indicates that the SI mice also exhibit anxiety-like behaviors.
Fig. 2.
Six weeks of social isolation stress induced depressive- and anxious-like behavioral deficits. a Changes of sucrose preference percentage in the SPT before and after SI. b Changes of immobility time in the TST before and after SI. c Moving trajectory diagram in the OFT. d Changes of total distance of motor activity in the OFT before and after SI. e Changes of spent time in the center zone in the OFT before and after SI. The data were expressed as mean ± SEM (n = 8). *P < 0.05, SI group versus GH group. SI social isolated, GH group housed, SPT sucrose preference test, TST tail suspension test, OFT open field test
Expressions of P2Y1R were up-regulated in the prefrontal cortex and hippocampus of SI mice
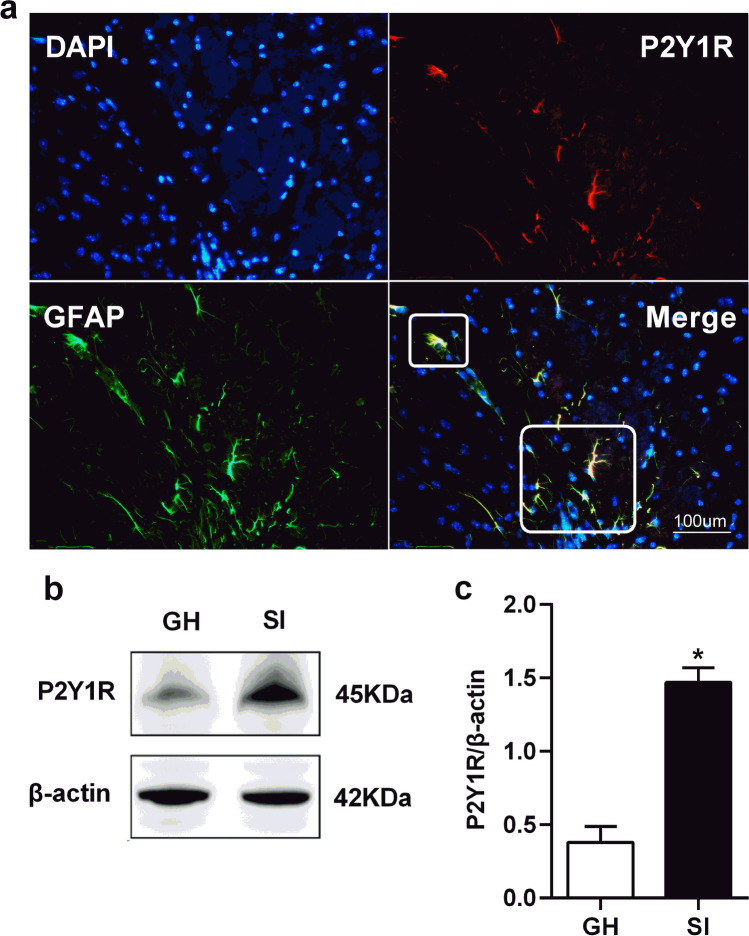
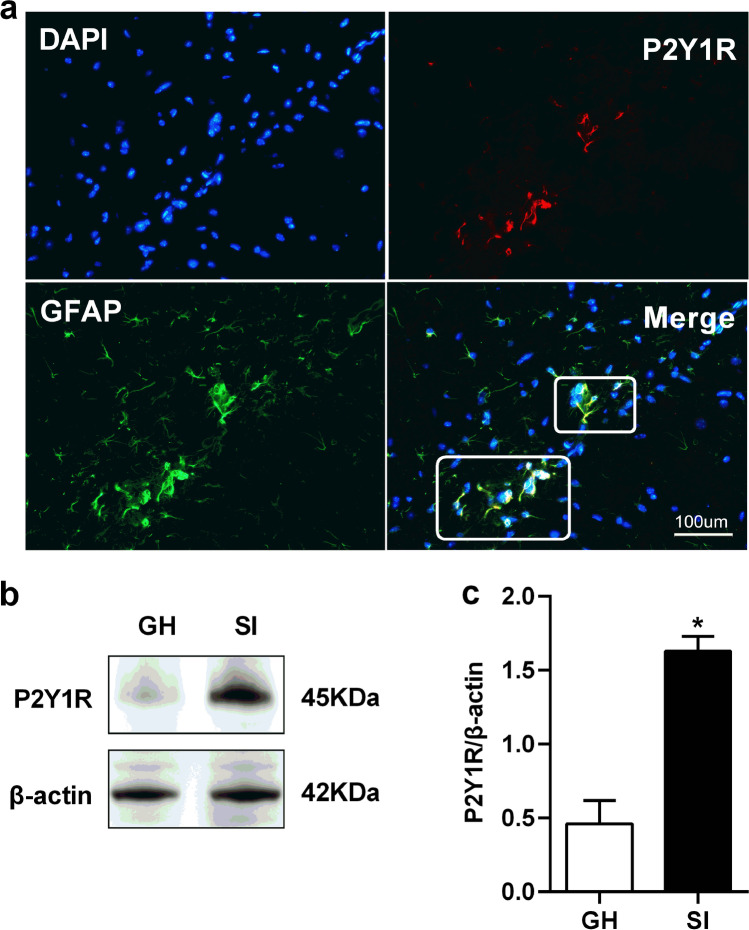
Western blotting and immunofluorescence labeling were used to detect the changes in the expression of P2Y1R of SI mice. Immunofluorescence labeling results showed that P2Y1R immunoreactivity was well co-localized with GFAP-positive astrocytes in the prefrontal cortex (Fig. 3a) and hippocampus (Fig. 4a) of SI mice, but not co-localized with NeuN and Iba1-positive microglia cell (Supplementary Fig. 1). Western blot analysis results revealed that the levels of P2Y1R protein in the SI group were significantly increased in the prefrontal cortex (t = 7.380, df = 8.0, P < 0.001, n = 5; Fig. 3c) and hippocampus (t = 6.269, df = 8.0, P < 0.001, n = 5; Fig. 4c) in comparison with the GH group.
Fig. 3.
Expressions of P2Y1R were up-regulated in the prefrontal cortex. a The immunofluorescent staining of P2Y1R in the prefrontal cortex of SI mice. Scale bar = 100 um. b Representative western blots image of P2Y1R protein levels in the prefrontal cortex of GH and SI mice. c Quantifications of P2Y1R protein levels in the prefrontal cortex of GH and SI mice. All interest protein expressions were normalized to β-actin. Values are expressed as mean ± SEM (n = 5). *P < 0.05, SI group versus GH group. SI social isolated, GH group housed
Fig. 4.
Expressions of P2Y1R were up-regulated in the hippocampus. a The immunofluorescent staining of P2Y1R in the hippocampus of SI mice. Scale bar = 100 um. b Representative western blots image of P2Y1R protein levels in the hippocampus of GH and SI mice. c Quantifications of P2Y1R protein levels in the hippocampus of GH and SI mice. All interest protein expressions were normalized to β-actin. Values are expressed as mean ± SEM (n = 5). *P < 0.05, SI group versus GH group. SI social isolated, GH group housed
Effects of P2Y1R agonists and antagonists on social isolation caused depression- and anxiety-like behaviors
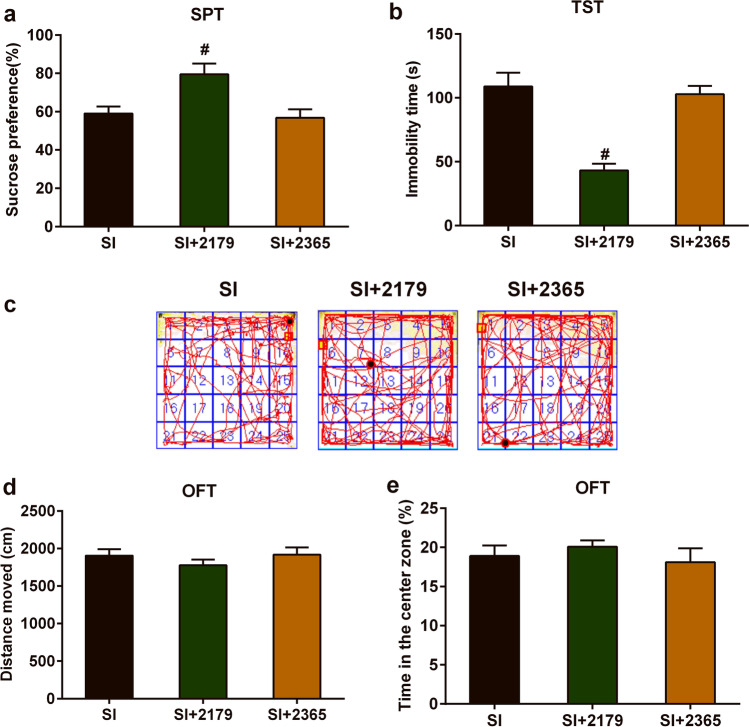
We tried to investigate the role of P2Y1R in the development of depressive- and anxiety-like behavior deficits caused by social isolation stress. One-way ANOVA showed that administration of MRS2179 into the lateral ventricle could impact the sucrose consumption rate in the SPT of SI mice (F(2,15) = 7.302, P < 0.001, n = 6; Fig. 5a) and the immobility time in the TST of SI mice (F(2,15) = 21.21, P < 0.001, n = 6; Fig. 5b). Dunnett’s multiple comparisons test showed that compared with the SI group, treatment with MRS2179 increased sucrose consumption rate in the SPT, with a group difference of 20.50 (95% confidence interval (CI) 4.53 to 36.47; P < 0.001); and decreased immobility time in the TST, with a group difference of − 65.8 (95% CI − 93.0 to − 38.5; P < 0.001). However, treatment with MRS2365 had no significant effects on SPT and TST of SI mice.
Fig. 5.
Effects of P2Y1R agonists and antagonists on social isolation–caused depressive- and anxiety-like behavioral deficits. a Changes in the SPT of SI mice after treated with MRS2179 or MRS2365. b Changes in the TST of SI mice after treated with MRS2179 or MRS2365. c Moving trajectory diagram in the OFT. d Changes of total distance of motor activity in the OFT of SI mice after treated with MRS2179 or MRS2365. e Changes of spent time in the center zone in the OFT of SI mice after treated with MRS2179 or MRS2365. The data were expressed as mean ± SEM (n = 5). #P < 0.05, SI + 2179 group versus SI group. SI social isolated, SPT sucrose preference test, TST tail suspension test, OFT open field test
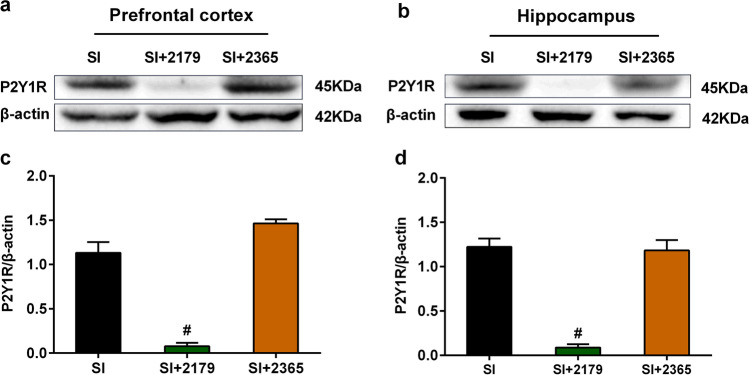
In the OFT, one-way ANOVA showed that administration of either MRS2179 or MRS2365 into the lateral ventricle could not affect the total moved distances and time spent in the center zone of SI mice (Fig. 5d, e). From Fig. 6, we found that administration of MRS2179 into the lateral ventricle of SI mice significantly decreased the expression of P2Y1R in the prefrontal cortex (Fig. 6a, c) and hippocampus (Fig. 6b, d), which indicated that administration of MRS2179 into the lateral ventricle is effective to antagonist expression of P2Y1 in the prefrontal cortex and hippocampus.
Fig. 6.
Expression of P2Y1R in the prefrontal cortex and hippocampus after SI mice were treated with MRS2179 or MRS2365. a, b Representative western blots image of P2Y1R protein levels in the prefrontal cortex and hippocampus after SI mice were treated with MRS2179 or MRS2365. c, d Quantifications of P2Y1R protein levels in the prefrontal cortex and hippocampus after SI mice were treated with MRS2179 or MRS2365. All interest protein expressions were normalized to β-actin. Values are expressed as mean ± SEM (n = 4). #P < 0.05, SI + 2179 group versus SI group. SI social isolated
Effects of EA on social isolation caused depression- and anxiety-like behaviors and the expression of P2Y1R in the brain
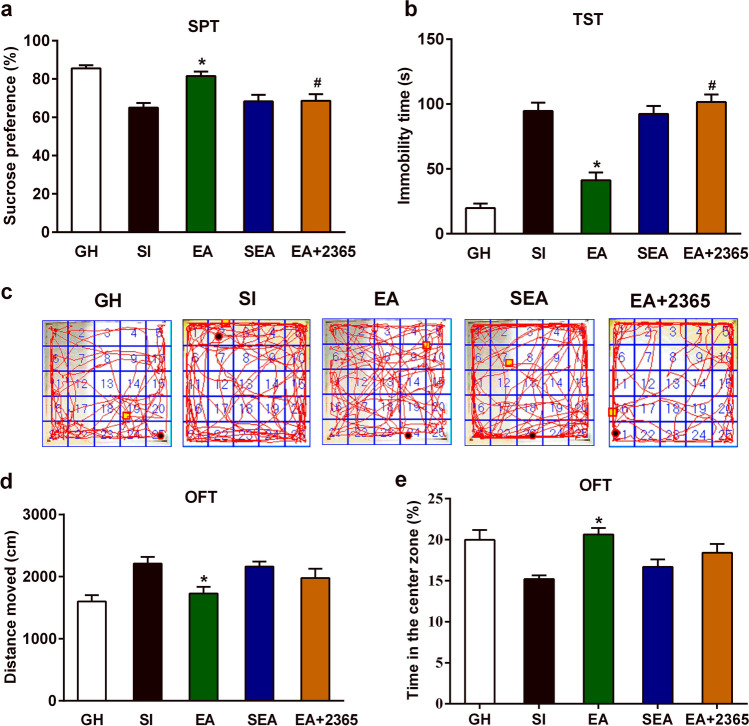
Subsequently, we observed the effects of EA on depression- and anxiety-like behaviors of SI mice. One-way ANOVA showed that EA could impact sucrose consumption rate in the SPT (F(4,25) = 11.31, P < 0.001, n = 6; Fig. 7a), immobility time in the TST (F(4,25) = 42.26, P < 0.001, n = 6; Fig. 7b), total moved distances in the OFT (F(4,25) = 5.694, P < 0.001, n = 6; Fig. 7d), and time spent in the center zone of OFT (F(4,25) = 6.100, P = 0.001, n = 6; Fig. 7e). Tukey–Kramer’s multiple-comparison tests demonstrated that compared with the SI group, EA significantly increased sucrose consumption rate in the SPT (95% CI 5.23 to 27.67; P < 0.001) and the time spent in the center zone of OFT (95% CI 1.636 to 9.231; P < 0.001). Meanwhile, it decreased immobility time in the TST (95% CI − 76.91 to − 29.78; P < 0.001) and moved distances in the OFT (95% CI − 944.20 to − 18.82; P < 0.001). These results suggested that 4 weeks of EA treatment could alleviate depression- and anxiety-like behaviors caused by social isolation.
Fig. 7.
EA attenuated social isolation–caused depressive- and anxiety-like behavioral deficits and P2Y1R agonists reversed the antidepressant effects of EA. a Changes of sucrose preference percentage in the SPT of five groups on PND94: GH, SI, EA, SEA, and EA + 2365 groups. b Changes of immobility time in the TST of five groups on PND94: GH, SI, EA, SEA, and EA + 2365 groups. c Moving trajectory diagram in the OFT. d Changes of distance of motor activity in the OFT of five groups on PND94: GH, SI, EA, SEA, and EA + 2365 groups. e Changes of spent time in the center zone in the OFT of five groups on PND94: GH, SI, EA, SEA, and EA + 2365 groups. The data were expressed as mean ± SEM (n = 6). *P < 0.05, EA group versus SI group. #P < 0.05, EA + 2365 group versus EA group. EA electroacupuncture, GH group housed, OFT open field test, SI social isolated, SPT sucrose preference test, SEA sham electroacupuncture, TST tail suspension test
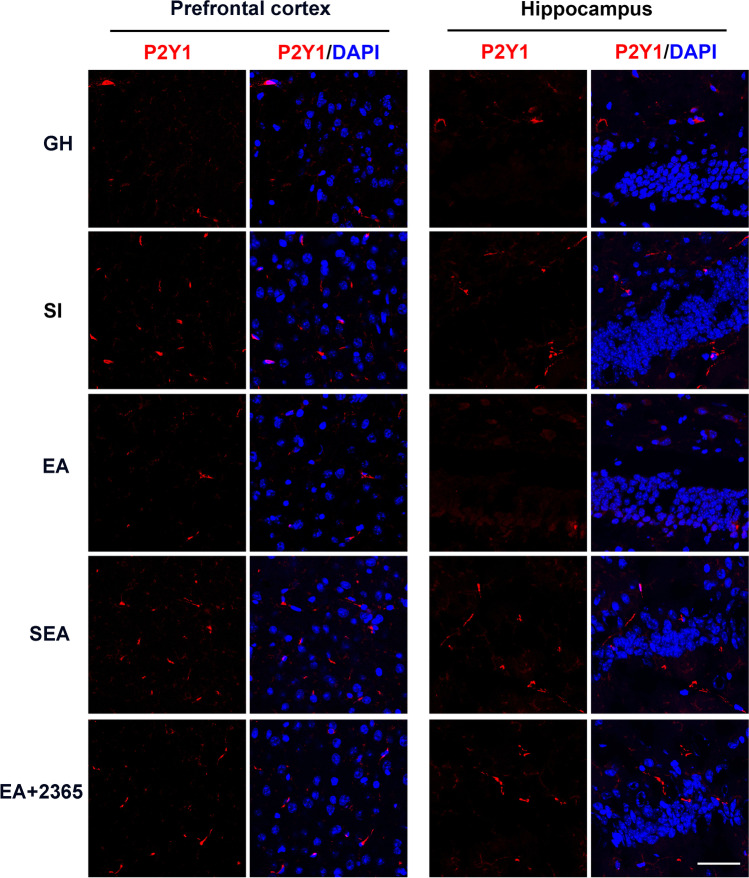
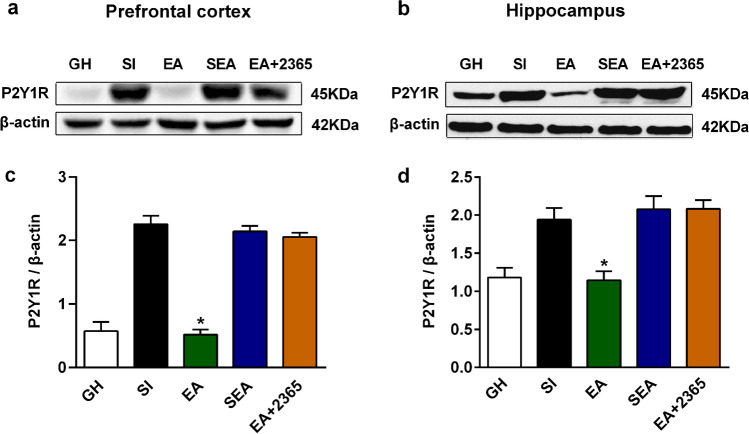
Immunofluorescence labeling results showed that the expression of P2Y1R in the prefrontal cortex and hippocampus of the EA group was less than that of the SI group (Fig. 8). Furthermore, Tukey–Kramer’s multiple-comparison tests confirmed that EA could significantly decrease the protein level of P2Y1R in the prefrontal cortex (P < 0.001, n = 5; Fig. 9c) and hippocampus (P < 0.001, n = 5; Fig. 9d), as compared with the SI group. However, sham EA could not affect the expression of P2Y1R in the prefrontal cortex and hippocampus.
Fig. 8.
EA inhibited the up-regulated expression of P2Y1R in the prefrontal cortex and hippocampus from immunofluorescent staining. The immunofluorescent staining of P2Y1R and DAPI in the prefrontal cortex and hippocampus in the following five groups: GH, SI, EA, SEA, EA + 2365 groups. Scale bar = 20 μm. EA electroacupuncture, GH group housed, SI social isolated, SEA sham electroacupuncture
Fig. 9.
EA inhibited the up-regulated expression of P2Y1R in the prefrontal cortex and hippocampus from western blotting. a, b Representative western blots image of P2Y1R protein levels in the prefrontal cortex and hippocampus in the following five groups: GH, SI, EA, SEA, EA + 2365 groups. c, d Quantifications of P2Y1R protein levels in the prefrontal cortex and hippocampus in the following five groups: GH, SI, EA, SEA, EA + 2365 groups. All interest protein expressions were normalized to β-actin. Values are expressed as mean ± SEM (n = 4). #P < 0.05, EA group versus SI group. EA electroacupuncture, GH group housed, SI social isolated, SEA sham electroacupuncture
P2Y1R agonists reversed the effects of EA on social isolation–caused depression-like behaviors
Tukey–Kramer’s multiple-comparison tests showed that compared with the EA group, the EA + 2365 group had a decreased sucrose consumption rate in the SPT, with a group difference of − 12.85 (95% CI − 24.07 to − 1.63; n = 6, P < 0.001; Fig. 7a); and an increased immobility time in the TST, with a group difference of 60.23 (95% CI 36.66 to 83.79; n = 6, P < 0.001; Fig. 7b). However, there are no significant differences between EA and EA + 2365 groups in the OFT (Fig. 7d, e). These results suggested that treatment with MRS2365 could reversed the effects of EA on social isolation–caused depression-like behaviors, but could not affect the effects of EA on social isolation–caused anxiety-like behaviors.
Discussion
Although EA has been widely used for neuropsychiatric disorders in the clinic, the mechanisms underlying the efficacy of EA on depression are not fully understood. Recently, a large body of evidence shows the important role of purinergic system in the neurobiology of psychiatry disorders, including depression [23]. In this regard, we explored the potential role of P2Y1R, a member of purinergic receptors, in the antidepressant effects of EA on social isolation–induced depression mice. We found that after 6 weeks of early social isolation, the SI mice exhibited obvious depression- and anxiety-like behaviors and showed increased expression of P2Y1R in the prefrontal cortex and hippocampus. P2Y1R antagonist could suppress social isolation–caused depression-like behaviors, while P2Y1R agonist could not. Furthermore, we found that insertion and electrical stimulation of acupuncture needles at GV20 and GV29 could suppress social isolation–caused depression- and anxiety-like behaviors, as well as inhibited the up-regulation of P2Y1R in the prefrontal cortex and hippocampus. However, the antidepressant-like effects of EA could be reversed by P2Y1R agonist. Taken together, our findings indicated that P2Y1R might be involved in the antidepressant actions of EA.
Social isolation stress in the development of depression and the role of EA in the treatment of depression
Increasing evidence indicates that social isolation has been an important risk in the development of several neuropsychiatric disorders including depression [30, 31]. In clinic, due to a small social network, social isolation people usually have a feeling of loneliness and are more likely to be depressed. Increasing social interaction is beneficial in reducing the prevalence of depression [32]. In animal experiments, previous studies showed that social isolation stress during post-weaning period could induce depressive- and anxious-like behavior in some behavioral tasks [33–35]. Social isolation could induce activation of hypothalamic–pituitary–adrenal axis, and reduction in copper and zinc levels and catalase activity, as well as lower brain-derived neurotrophic factor (BDNF) protein level in the hippocampus of mice [36, 37]; those changes are thought to be closely related to the development of depression.
In the present study, three well-known behavioral tests (SPS, TST, OFT) were applied to evaluate the anhedonia, despair symptoms, locomotor activity, and associated anxiety-like behaviors of SI mice, respectively. Consistent with the abovementioned studies, we found that mice subjected to 6 weeks of social isolation stress during PND 21–63 exhibited a depression-related phenotype characterized by anhedonia, behavioral despair, and increased movement, anxiety, and alterations of purinergic P2Y1R.
Treatment with 4 weeks of EA ameliorated depression-like behaviors of SI mice as evaluated by behavioral tests including SPS and TST. The antidepressant effects of EA on SI mice suggested that EA might be an effective therapeutic approach for depression in adolescence. The effects of acupuncture therapy for various diseases depend on application of acupuncture stimulation at specific acupoint or different combinations of acupoints. Consistent with previous studies on depression [16, 38], we observed the antidepressant-like effects of EA on SI mice when EA is applied at acupoints GV20 and GV29. In 1984, Luo et al. for the first time reported that application of EA at GV20 and GV29 was beneficial for patients with depression [39]. After that, acupoint compatibility of GV20 and GV29 was widely used for various mental diseases [39]. Based on a recent Chinese literature, GV20 and GV29 were the most frequently used acupoints for depression; the usage frequency was 81.25% and 40.10%, respectively [40]. According to traditional acupuncture theory, the two acupoints are located in the head. Acupoint GV20 belongs to Du meridian, where the qi and spirit are infused. Traditional acupuncture theory believes that GV20 is the intersection of multiple meridians. It has many effects, such as clearing heat for resuscitation, strengthening the brain and tranquilizing the spirit, and lifting the Yang qi, as well as calming the liver and extinguishing the wind. Acupoint GV29 is an extraordinary point, where the qi of Du meridian is infused, and has the effect of tranquilizing and allaying excitement.
Treatment with EA also ameliorated anxiety-like behaviors of SI mice as evaluated by OFT. The anxiolytic-like effects of EA on SI mice suggested that EA had the dual-directional regulation of mood disorders. Indeed, acupoints have dual-directional regulating effects. It has been confirmed by animal experiments that EA stimulation applied at GV20 and GV29 also alleviated adolescent cocaine exposure–induced anxiety-like behaviors [41].
P2Y1R is involved in the antidepressant-like effects of EA
The purinergic system may play a role in the pathophysiology and the treatment of mood disorders, including depression [42]. Purinergic signals may influence neurotransmitter systems and hormonal pathways of the hypothalamic–pituitary–adrenal axis, providing a potential therapeutic target for depression. It is established that purinergic receptors could be differentiated into two families, P1 and P2 receptors, activated by adenosine and ATP, respectively [43]. The impact of purinergic signaling on depression is mainly related to the changes in adenosine and ATP-mediated signaling at P1 and P2 receptors, respectively [44].
P2Y1R is a member of P2 receptors, which belongs to the P2Y G protein–coupled receptor family (including P2Y1, 2, 4, 6, 11, 12 subtypes) [45]. The widespread and abundant distribution of metabotropic P2Y receptors in the mammalian brain suggests important functions of these receptors in the central nervous system. In part A of this study, we observed the expression of P2Y1R was significantly increased in the prefrontal cortex and hippocampus. Previous studies [46] have examined the intracellular signaling pathways underlying the regulation of synaptic function by stress and antidepressant treatments. Repeated stress decreases the expression of BDNF in limbic and cortical brain regions, notably the hippocampus and prefrontal cortex; these studies indicated the importance of the hippocampus and prefrontal cortex in depression.
P2Y1R is highly expressed on astrocytes, and involved in regulating the activities of glutamate and norepinephrine [47, 48]. These neurotransmitters have been shown to be involved in the pathogenesis of depression. In previous studies, it has been demonstrated that P2Y1R is involved in the regulation of depression [42], anxiety [49], Alzheimer’s disease [50], arterial thrombosis [51], and brain ischemia [52]. But to date, its role in the antidepressant actions of acupuncture is unclear. Our findings indicated that P2Y1R was implicated in the antidepressant actions of EA. Treatment with EA reversed the up-regulation of P2Y1R in the prefrontal cortex and hippocampus of SI mice. MRS2179 is a competitive antagonist at P2Y1R, while MRS 2365 is a highly potent, selective P2Y1R agonist. We found that before EA, treatment with MRS2179 attenuated the depression-like behaviors of SI mice, but could not affect the anxiety-like behaviors of SI mice. We also found that after EA, treatment with MRS2365 significantly reversed the antidepressant-like effects of EA on SI mice, but could not affect the anxiolytic-like effects of EA on SI mice. These results might shed new light on the mechanism of EA in the treatment of depression.
In summary, our investigation indicates that P2Y1R is involved in the modulation of depression. Repeated EA treatment at GV20 and GV29 could ameliorate depression-like behaviors, which might be mediated by regulating purinergic P2Y1R in the prefrontal cortex and hippocampus.
Supplementary Information
Below is the link to the electronic supplementary material.
Dr. Lingling Yu
received the B.S., M.S., and Ph.D. degrees in acupuncture from Hubei University of Traditional Chinese Medicine, Wuhan, China in 2005, 2009, 2012, respectively. Currently, she is working in the Institute of Integrated Traditional Chinese and Western Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. Her research interests include mechanisms of acupoints sensitization, acupoints specificity, acupuncture analgesia, and effects of acupuncture on depression and anxiety.MO
Author contribution
LY and MX designed the experiments. LY conducted the EA treatment and performed the experiments. JH and HZ performed the data analysis. LY wrote the manuscript. YW added the experimental data and GC mainly takes charge of checking format and grammar errors in the revised manuscript. All authors helped revise the manuscript and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (82174500, 82004491), China Postdoctoral Science Foundation (2017M612472), and Young Excellent Medical Scholarship of Hubei Province (2020 LJRC011).
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Code availability
Not applicable.
Declarations
Ethical approval
The experimental procedure was approved by the Animal Ethics Committee of Tongji Hospital, Huazhong University of Science and Technology.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
Lingling Yu declares that she has no conflict of interest.
Wang Yao declares that he has no conflict of interest.
Hong Zhang declares that he has no conflict of interest.
Man Li declares that she has no conflict of interest.
Chen Guang declares that he has no conflict of interest.
Jiahuan Hao declares that he has no conflict of interest.
Minjie Xie declares that she has no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jiahuan Hao, Email: 438573667@qq.com.
Minjie Xie, Email: xie_minjie@126.com.
References
- 1.Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2018;392(10159):1789–858. 10.1016/s0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed]
- 2.Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. Lancet (London, England) 2012;379(9820):1056–1067. doi: 10.1016/s0140-6736(11)60871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fava GA, Ruini C, Belaise C. The concept of recovery in major depression. Psychol Med. 2007;37(3):307–317. doi: 10.1017/s0033291706008981. [DOI] [PubMed] [Google Scholar]
- 4.Conradi HJ, Ormel J, de Jonge P. Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychol Med. 2011;41(6):1165–1174. doi: 10.1017/s0033291710001911. [DOI] [PubMed] [Google Scholar]
- 5.Dale E, Bang-Andersen B, Sánchez C. Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochem Pharmacol. 2015;95(2):81–97. doi: 10.1016/j.bcp.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Sullivan NR, McAllister CE, Van de Kar LD, Muma NA. Estradiol accelerates the effects of fluoxetine on serotonin 1A receptor signaling. Psychoneuroendocrinology. 2013;38(7):1145–1157. doi: 10.1016/j.psyneuen.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan YY, Lo WY, Yang SN, Chen YH, Lin JG. The benefit of combined acupuncture and antidepressant medication for depression: a systematic review and meta-analysis. J Affect Disord. 2015;176:106–117. doi: 10.1016/j.jad.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 8.Tu CH, MacDonald I, Chen YH. The effects of acupuncture on glutamatergic neurotransmission in depression, anxiety, schizophrenia, and Alzheimer’s disease: a review of the literature. Front Psych. 2019;10:14. doi: 10.3389/fpsyt.2019.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh JY, Kim YK, Kim SN, Lee B, Jang JH, Kwon S, et al. Acupuncture modulates stress response by the mTOR signaling pathway in a rat post-traumatic stress disorder model. Sci Rep. 2018;8(1):11864. doi: 10.1038/s41598-018-30337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Liang J, Wang JR, Hu L, Tu Y, Guo JY. Acupuncture activates ERK-CREB pathway in rats exposed to chronic unpredictable mild stress. evidence-based complementary and alternative medicine : eCAM. 2013;2013:469765. 10.1155/2013/469765. [DOI] [PMC free article] [PubMed]
- 11.Lee MJ, Ryu JS, Won SK, Namgung U, Jung J, Lee SM, et al. Effects of acupuncture on chronic stress-induced depression-like behavior and its central neural mechanism. Front Psychol. 2019;10:1353. doi: 10.3389/fpsyg.2019.01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yun SJ, Park HJ, Yeom MJ, Hahm DH, Lee HJ, Lee EH. Effect of electroacupuncture on the stress-induced changes in brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett. 2002;318(2):85–88. doi: 10.1016/s0304-3940(01)02492-2. [DOI] [PubMed] [Google Scholar]
- 13.Eshkevari L, Egan R, Phillips D, Tilan J, Carney E, Azzam N, et al. Acupuncture at ST36 prevents chronic stress-induced increases in neuropeptide Y in rat. Exp Biol Med (Maywood) 2012;237(1):18–23. doi: 10.1258/ebm.2011.011224. [DOI] [PubMed] [Google Scholar]
- 14.Kwon S, Lee B, Yeom M, Sur BJ, Kim M, Kim ST, et al. Modulatory effects of acupuncture on murine depression-like behavior following chronic systemic inflammation. Brain Res. 2012;1472:149–160. doi: 10.1016/j.brainres.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Guo X, Pan F, Wang B, Li W, Xia C, Ju Z. Effect of electroacupuncture on mice model of permenopausal depressive disorder. Saudi journal of biological sciences. 2019;26(8):2030–2036. doi: 10.1016/j.sjbs.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Y, He J, Guo L, Yao L, Zheng X, Yang Z, et al. Transcriptome analysis on maternal separation rats with depression-related manifestations ameliorated by electroacupuncture. Front Neurosci. 2019;13:314. doi: 10.3389/fnins.2019.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H, Zhang X, Wang Y, Zhang H, Li J, Yang X, et al. Mechanisms underlying the antidepressant response of acupuncture via PKA/CREB signaling pathway. Neural Plast. 2017;2017:4135164. doi: 10.1155/2017/4135164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y, Tu Y, Guo Y, Jiang HL, Li YH, Wang Y, et al. [Acupuncture improved depressive behavior by regulating expression of hippocampal apoptosis-related factors in psychological stress-induced depression rats]. Zhen ci yan jiu = Acupuncture research. 2019;44(6):412–8. 10.13702/j.1000-0607.190098. [DOI] [PubMed]
- 19.Lin L, Yu L, Xiang H, Hu X, Yuan X, Zhu H, et al. Effects of acupuncture on behavioral stereotypies and brain dopamine system in mice as a model of Tourette syndrome. Front Behav Neurosci. 2019;13:239. doi: 10.3389/fnbeh.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu A, Tang Y, Zeng Q, Wang X, Tian H, Zhou Y, et al. Electroacupuncture enhances cognition by promoting brain glucose metabolism and inhibiting inflammation in the APP/PS1 mouse model of Alzheimer’s disease: a pilot study. J Alzheimers Dis. 2020;77(1):387–400. doi: 10.3233/jad-200242. [DOI] [PubMed] [Google Scholar]
- 21.Ding N, Jiang J, Xu A, Tang Y, Li Z. Manual acupuncture regulates behavior and cerebral blood flow in the SAMP8 mouse model of Alzheimer’s disease. Front Neurosci. 2019;13:37. doi: 10.3389/fnins.2019.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao X, Li LP, Wang Q, Wu Q, Hu HH, Zhang M, et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med. 2013;19(6):773–777. doi: 10.1038/nm.3162. [DOI] [PubMed] [Google Scholar]
- 23.Krügel U. Purinergic receptors in psychiatric disorders. Neuropharmacology. 2016;104:212–225. doi: 10.1016/j.neuropharm.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Krügel U, Spies O, Regenthal R, Illes P, Kittner H. P2 receptors are involved in the mediation of motivation-related behavior. Purinergic Signalling. 2004;1(1):21–29. doi: 10.1007/s11302-004-4745-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang Y, Yin HY, Liu J, Rubini P, Illes P. P2X receptors and acupuncture analgesia. Brain Res Bull. 2019;151:144–152. doi: 10.1016/j.brainresbull.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Andrejew R, Paim M, Moritz CEJ, Carreño F, Rates SMK, Elisabetsky E, et al. Post-weaning social isolation impairs purinergic signaling in rat brain. Neurochem Int. 2021;148:105111. doi: 10.1016/j.neuint.2021.105111. [DOI] [PubMed] [Google Scholar]
- 27.Marsden CA, King MV, Fone KC. Influence of social isolation in the rat on serotonergic function and memory–relevance to models of schizophrenia and the role of 5-HT6 receptors. Neuropharmacology. 2011;61(3):400–407. doi: 10.1016/j.neuropharm.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Suyama S, Sunabori T, Kanki H, Sawamoto K, Gachet C, Koizumi S, et al. Purinergic signaling promotes proliferation of adult mouse subventricular zone cells. J Neurosci. 2012;32(27):9238–9247. doi: 10.1523/jneurosci.4001-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alves M, De Diego GL, Conte G, Jimenez-Mateos EM, D’Orsi B, Sanz-Rodriguez A, et al. Context-specific switch from anti- to pro-epileptogenic function of the P2Y(1) receptor in experimental epilepsy. J Neurosci. 2019;39(27):5377–5392. doi: 10.1523/jneurosci.0089-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Courtin E, Knapp M. Social isolation, loneliness and health in old age: a scoping review. Health Soc Care Community. 2017;25(3):799–812. doi: 10.1111/hsc.12311. [DOI] [PubMed] [Google Scholar]
- 31.Domènech-Abella J, Mundó J, Haro JM, Rubio-Valera M. Anxiety, depression, loneliness and social network in the elderly: longitudinal associations from The Irish Longitudinal Study on Ageing (TILDA) J Affect Disord. 2019;246:82–88. doi: 10.1016/j.jad.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 32.Famitafreshi H, Karimian M. Modulation of catalase, copper and zinc in the hippocampus and the prefrontal cortex in social isolation-induced depression in male rats. Acta Neurobiol Exp. 2019;79(2):184–192. doi: 10.21307/ane-2019-016. [DOI] [PubMed] [Google Scholar]
- 33.Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW. The neuroendocrinology of social isolation. Annu Rev Psychol. 2015;66:733–767. doi: 10.1146/annurev-psych-010814-015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haj-Mirzaian A, Nikbakhsh R, Ramezanzadeh K, Rezaee M, Amini-Khoei H, Haj-Mirzaian A, et al. Involvement of opioid system in behavioral despair induced by social isolation stress in mice. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2019;109:938–44. 10.1016/j.biopha.2018.10.144. [DOI] [PubMed]
- 35.Brenes JC, Fornaguera J, Sequeira-Cordero A. Environmental enrichment and physical exercise attenuate the depressive-like effects induced by social isolation stress in rats. Front Pharmacol. 2020;11:804. doi: 10.3389/fphar.2020.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JW, Kirkpatrick B. Social isolation in animal models of relevance to neuropsychiatric disorders. Biol Psychiat. 1996;40(9):918–922. doi: 10.1016/0006-3223(95)00546-3. [DOI] [PubMed] [Google Scholar]
- 37.Zaletel I, Filipović D, Puškaš N. Hippocampal BDNF in physiological conditions and social isolation. Rev Neurosci. 2017;28(6):675–692. doi: 10.1515/revneuro-2016-0072. [DOI] [PubMed] [Google Scholar]
- 38.Zhao J, Tian H, Song H, Wang X, Luo T, Ai L, et al. Effect of electroacupuncture on reuptake of serotonin via miRNA-16 expression in a rat model of depression. Evidence-based complementary and alternative medicine : eCAM. 2019;2019:7124318. doi: 10.1155/2019/7124318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Zhongfa, Wang Junfeng, Du Guiping, Zhao Xueying, Luo hechun. Application of electroacupuncture at Baihui and Yintang points in Department of Mental Diseases. Chinese Acupuncture and Moxibustion(Zhongguo zhenjiu). 2001;21(10):633–636.
- 40.Yue Z, Yaping O, Shuming Z, Hongfeng W. Acupoints compatibility of acupuncture and moxibustion treating depression based on data mining. J Changchun Univ Chin Med. 2018;34(1):176–178. [Google Scholar]
- 41.Nie J, Wei X, Xu X, Li N, Li Y, Zhao Y, et al. Electro-acupuncture alleviates adolescent cocaine exposure-enhanced anxiety-like behaviors in adult mice by attenuating the activities of PV interneurons in PrL. FASEB J. 2020;34(9):11913–11924. doi: 10.1096/fj.202000346RR. [DOI] [PubMed] [Google Scholar]
- 42.Zou Y, Yang R, Li L, Xu X, Liang S. Purinergic signaling: a potential therapeutic target for depression and chronic pain. Purinergic Signalling. 2021 doi: 10.1007/s11302-021-09801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burnstock G. Purine and purinergic receptors. Brain and neuroscience advances. 2018;2:2398212818817494. doi: 10.1177/2398212818817494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartoli F, Burnstock G, Crocamo C, Carrà G (2020) Purinergic signaling and related biomarkers in depression. Brain Sci 10(3). 10.3390/brainsci10030160 [DOI] [PMC free article] [PubMed]
- 45.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22(4):182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 46.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(3):238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franke H, Krügel U, Grosche J, Heine C, Härtig W, Allgaier C, et al. P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2004;127(2):431–441. doi: 10.1016/j.neuroscience.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Domercq M, Brambilla L, Pilati E, Marchaland J, Volterra A, Bezzi P. P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-alpha and prostaglandins. J Biol Chem. 2006;281(41):30684–30696. doi: 10.1074/jbc.M606429200. [DOI] [PubMed] [Google Scholar]
- 49.Kittner H, Franke H, Fischer W, Schultheis N, Krügel U, Illes P. Stimulation of P2Y1 receptors causes anxiolytic-like effects in the rat elevated plus-maze: implications for the involvement of P2Y1 receptor-mediated nitric oxide production. Neuropsychopharmacology. 2003;28(3):435–444. doi: 10.1038/sj.npp.1300043. [DOI] [PubMed] [Google Scholar]
- 50.Delekate A, Füchtemeier M, Schumacher T, Ulbrich C, Foddis M, Petzold GC. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer’s disease mouse model. Nat Commun. 2014;5:5422. doi: 10.1038/ncomms6422. [DOI] [PubMed] [Google Scholar]
- 51.Wong PC, Watson C, Crain EJ. The P2Y1 receptor antagonist MRS2500 prevents carotid artery thrombosis in cynomolgus monkeys. J Thromb Thrombolysis. 2016;41(3):514–521. doi: 10.1007/s11239-015-1302-7. [DOI] [PubMed] [Google Scholar]
- 52.Kuboyama K, Harada H, Tozaki-Saitoh H, Tsuda M, Ushijima K, Inoue K. Astrocytic P2Y(1) receptor is involved in the regulation of cytokine/chemokine transcription and cerebral damage in a rat model of cerebral ischemia. J Cereb Blood Flow Metab. 2011;31(9):1930–1941. doi: 10.1038/jcbfm.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Not applicable.