Abstract
Objectives
(1) To evaluate the use of hysteroscopy in the assessment of uterine pathologies, not evident on ultrasonography or hystero-salpingography in women with previous one or more IVF failures and (2) to determine whether correction of such pathologies during hysteroscopy improves clinical pregnancy rates in these women.
Methods
This is a prospective randomized study. The study population included were women with primary and secondary infertility registered at our center, fit into the inclusion and exclusion criteria of this study. The total 180 patients were included.
Results
Hysteroscopies were performed in 90 patients with at least one IVF cycle failure and another 90 patients taken as control with similar demographic parameters. The average duration of infertility between both the groups was not significant. Hysteroscopy was able to detect intrauterine pathologies in around 40% of the cases, which were treated in the same treatment phase. Early ultrasound findings with gestational sac and cardiac activity were found to be significant between two groups.
Conclusion
We noticed clinical improvement in IVF success rate after hysteroscopy. Hysteroscopy may be offered to the patients with previous one or more IVF failures, as clinically some of the previously undiagnosed pathologies could be detected and treated to achieve the positive outcomes.
Keywords: Hysteroscopy, Previous failure, In vitro fertilization, Rural
Introduction
The worldwide in vitro fertilization (IVF) success rate is around 30–40% despite new advancements in treatment methodology since the first IVF baby was born in 1978 [1]. The success of IVF mainly depends on good-quality embryo (seed) and endometrium (soil), whereas hysteroscopy may play a crucial role in an endometrial assessment [2]. The assumed causes of repeated failures can be categorized as (a) factors related to decreased endometrial receptivity, (b) factors related to defective embryonic development, and (c) others (such as endometriosis and presence of hydrosalpinges) [3]. The assessment of the endometrium for intrauterine abnormalities is a core part of evaluation as it may influence the complex process of embryo implantation. Nowadays, a routine 2D/3D ultrasonography (USG) is considered as a preferred tool for assessment of endometrial thickness and its vascularity. But there is a high prevalence (40–50%) of unsuspected intrauterine lesions such as uterine synechiae, uterine septum, endometritis, and uterine polyps in an asymptomatic patient, which were diagnosed by hysteroscopy, in numerous IVF studies noted in studies [3–7]. The presence of such intrauterine lesions may have a significant impact on embryo implantation as well as the pregnancy outcome in patients even after the treatment of their infertility [3]. As compared to transvaginal ultrasonography (TVS), hysteroscopy allows us to directly visualize the unsuspected lesions and to undergo its treatment at the same sitting [7, 8].
There are controversies regarding the use of routine hysteroscopy in all patients prior to an IVF cycle owing to its invasive way of the procedure. According to the international guidelines of the American Society for Reproductive Medicine (ASRM), hysteroscopy should be reserved for those having abnormalities on TVS and hystero-salpingography (HSG) considering its cost-effectivity [9]. This study aimed at assessing the role of hysteroscopy in whom there was no obvious uterine pathology detected on routine USG or HSG with previous IVF failure to detect unsuspected intrauterine lesions responsible for IVF failure in addition to assessing the effect of treating such lesions on the pregnancy outcome.
Aims and Objectives
The objectives of this study were (1) to evaluate the use of hysteroscopy in the assessment of uterine pathologies, not evident on ultrasonography (USG) or hystero-salpingography (HSG) in women with previous one or more IVF failures and (2) to determine whether correction of such pathologies during hysteroscopy improves clinical pregnancy rates in these women.
Materials and Methods
This is a prospective randomized study, which was conducted at Wardha Test Tube Baby Center, a Unit of Obstetrics and Gynaecology Department, at Jawaharlal Nehru Medical College (JNMC) and Acharya Vinoba Bhave Rural Hospital (AVBRH), Sawangi, Wardha district of India, for the duration of one year from September 2020 to August 2021. The patients in this study were included from the rural population registered at our center having primary or secondary infertility who were fulfilling the fixed inclusion and exclusion criteria of our study. The total sample size was 180 patients out of whom 90 patients underwent hysteroscopy and another 90 patients were taken as a control in whom hysteroscopy was not performed. The inclusion criteria were as follows: (1) women under 45 years of age, (2) women who have undergone one or more embryo implantation failures in previous IVF cycles, and (3) women having normal uterine findings on transvaginal USG or HSG. The exclusion criteria were as follows: (1) women aged above 45 years, (2) women undergoing the first cycle of IVF, and (3) women with an already diagnosed intrauterine pathology. In this study, women with primary and secondary infertility with one or more implantation failures were subjected for accurate history and clinical examination and investigated for routine blood and radiological parameters. The written informed consent was obtained prior to the procedure explaining its details, and the selected cases were subjected for hysteroscopy. The hysteroscopic findings such as endometrial adhesions, endometrial hyperplasia, submucous polyp or fibroids, and T-shaped uterus were diagnosed and treated accordingly with its corrective procedures (Fig. 1). These patients then underwent repeated IVF treatment in the next cycle again. The pregnancy outcome of all these patients was recorded by measuring their beta HCG level on day 14 of embryo transfer. The early scan (two weeks after the positive beta HCG test) showing live pregnancy with cardiac activity was recorded. The data were compared for a positive outcome of IVF treatment post-hysteroscopy with that of control subjects.
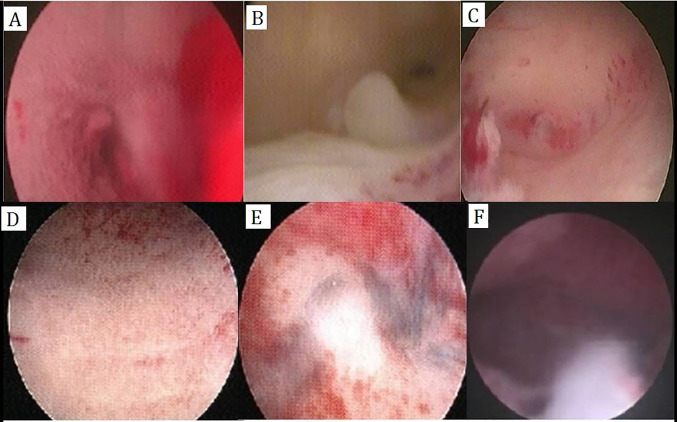
Fig. 1.
The hysteroscopic images show: (A) submucous myoma, (B) endometrial polyp, (C) uterine septum, (D) endometritis, (E) intrauterine adhesions, and (F) T-shaped uterine cavity
Results
The hysteroscopy was performed in 90 patients with a history of at least one IVF cycle failure, and another 90 patients were taken as control having almost similar demographic parameters (Table 1). The average duration of infertility between both these groups was founded to be nonsignificant. Although, the hysteroscopy was able to detect intrauterine pathologies in 36 cases (40%), which were treated for its abnormal pathologies in the same sitting, and another 54 cases (60%) were found to have normal hysteroscopic findings. The most common hysteroscopic finding that we noticed in this study was intrauterine adhesions, while endometritis and endometrial polyp were the second most common (Table 2). Following the treatment of this intrauterine pathologies, patient underwent another next embryo transfer, the results of which were noted and compared (Table 3). As compared to 18 out of 54 patients with normal hysteroscopic findings, 24 out of 36 patients with corrected abnormal hysteroscopic findings had a beta HCG positive rate. Thirty-six out of 90 cases of non-hysteroscopy group also showed positive results (Table 4). The gestational sac with cardiac activity was seen in 12 (13.3%) in the normal hysteroscopy finding group, 15 (16.6%) in abnormal hysteroscopy group, i.e., total 27 cases (30%) with hysteroscopy group, but 21 cases (23.3%) without hysteroscopy group (Table 5).
Table 1.
Demographic distribution of patients in HSC and non-HSC groups
| Factors | HSC group (n = 90) | Non-HSC group (n = 90) | P value |
|---|---|---|---|
| Mean age (in years) | 32.90 (± 1.97) | 33.57 (± 3.21) | 0.657, not significant |
| Mean duration of infertility (in years) | 7.60 (± 3.87) | 8.13 (± 3.98) | 0.22, not significant |
| Previous only one IVF failure | 18 (20%) | 30 (33.3%) | 0.000003, significant |
| Previous more than one IVF failures | 72 (80%) | 60 (66.6%) | 0.122, not significant |
Table 2.
Distribution of various hysteroscopic findings in HSC group (n = 90)
| Findings in HSC group | Frequency (n = 90) | Percentage | |
|---|---|---|---|
| Normal HSC findings | 54 | 60 | |
| Abnormal HSC findings | 36 | 40 | |
| Abnormal findings | |||
| Endometrial polyp | 6 | 16.7 | |
| Endocervical polyp | 3 | 8.3 | |
| Endometritis | 6 | 16.7 | |
| Intrauterine adhesions | 12 | 33.3 | |
| Submucous myoma | 3 | 8.3 | |
| T-shaped uterus | 3 | 8.3 | |
| Uterine fundal septum | 3 | 8.3 | |
Table 3.
IVF cycle characteristics in HSC and non-HSC groups
| HSC group (n = 90) | Non-HSC group (n = 90) | P value | |
|---|---|---|---|
| Mean number of oocytes retrieved | 9.27 (± 1.89) | 8.57 (1.67) | 0.166, not significant |
| Mean number of oocytes fertilized | 7.07 (± 1.55) | 6.23 (1.45) | 0.166, not significant |
| Mean number of embryos transferred | 2.9 (± 0.8) | 2.6(2.6) | 0.12, not significant |
Table 4.
Patients with abnormal hysteroscopic findings and positive beta HCG (n = 24)
| Abnormal hysteroscopic findings (number) | Positive beta HCG (n = 24) (%) |
|---|---|
| Endometrial polyp (6) | 3 (12.5) |
| Endocervical polyp (3) | 3 (12.5%) |
| Endometritis (6) | 3 (12.5%) |
| Intrauterine adhesions (12) | 6 (25%) |
| Submucous myoma (3) | 3 (12.5%) |
| T-shaped uterus (3) | 3 (12.5%) |
| Uterine fundal septum (3) | 3 (12.5%) |
| Total | 24 |
Table 5.
Distribution of pregnancy outcome
| Parameters | HSC group (n = 90) n(%) |
Non-HSC Group (n = 90) n (%) |
P value | ||
|---|---|---|---|---|---|
| Normal HSC (n = 54) n (%) |
Abnormal HSC (n = 36) n (%) |
Total (n = 90) n (30%) |
|||
| Beta HCG (positive) | 18 (33.3) | 24 (66.66) | 42 (46.6) | 36 (40.0) |
[for normal v/s abnormal HSC group = 0.073, not significant] [for HSC v/s non-HSC group = 0.602, not significant] |
| Transvaginal ultrasonography (Sac Seen) | 15 (27.8) | 18 (50.0) | 33 (36.6) | 30 (33.3) |
[for normal v/s abnormal HSC group = 0.215, not significant] [for HSC v/s non-HSC group = 0.015, significant] |
| Cardiac activity on USG scan | 12 (13.3) | 15 (16.6) | 27 (30) | 21 (23.3) |
[for normal v/s abnormal HSC group = 0.157, not significant] [for HSC v/s non-HSC group = 0.023, significant] |
Discussion
The abnormalities of the uterine cavity may be responsible for adversely affecting the post-IVF pregnancy rates [10]. In such cases, hysteroscopy is the important investigation for the evaluation of the uterine cavity and detecting an undiagnosed pathology inside it. Most of the pathology can be accurately diagnosed with this technique, and subsequently, further management of such pathology can be immediately done along with the biopsy for histopathological examination [11]. According to the literature, some of the authors support the idea of performing hysteroscopy after recurrent embryo transfer failure, whereas some authors do not. Schiano et al. reported the uterine abnormality in approximately 50% of the cases such as cervical abnormalities, endometrial polyps, and submucous leiomyomas, after hysteroscopic evaluation in those patients who had undergone two unsuccessful assisted reproduction techniques (ART) attempts [12]. Elestohy et al. reported such abnormalities in around 44% of his cases [3]. Bakas et al. also diagnosed intrauterine abnormalities in around 43% of the cases [7]. Similarly, Demirol et al. noticed hysteroscopic intrauterine abnormalities in 26% cases [13]. As all these intrauterine masses such as polyps, fibroids, and septum are known to affect the embryo implantation in several ways, such as abnormal vascularization, chronic inflammation, and uterine contractions, it ultimately results in IVF cycle failure.
The age of the patient is also an important factor affecting the chances of getting an abnormal uterine pathology which seems to increase with an advancing age as evident in the literature [6]. Ajayi et al.’s study showed that uterine pathologies were lower in patients with age below 30 years, but its incidence subsequently increased in number with the advancing age above 30 years. Abnormal findings in HSC groups were (a) submucous myoma, (b) endometrial polyp, (c) uterine septum, (d) endometritis, (e) intrauterine adhesions, and (f) T-shaped uterine cavity. This pathology was treated in a respective manner. The management of submucous myoma was done by hysteroscopic myomectomy. Endometrial polyps were treated by polypectomy. The cases of uterine septum were managed with hysteroscopic septal resection. Intrauterine adhesions, the most common pathology in our study, were treated by adhesiolysis, whereas T-shaped uterine cavity was managed using lateral metroplasty.
One should note that the hysteroscopy is also associated with procedural complications such as uterine perforation, excessive bleeding, cervical trauma, fluid overload, and electrolyte imbalance; however, no such complications were noted in any of the patients registered in our study. Also, Pereira et al. reported that there was no significant difference in pregnancy rate and delay in an IVF cycle after hysteroscopy [14]. So, similar to the studies, in this study, we found that performing hysteroscopy before the first IVF cycle, a high number (around 40%) of intrauterine abnormalities were noticed in patients with previous IVF failure which were undiagnosed previously even after conventional TVS and HSG. All the post-hysteroscopy diagnosed abnormalities were effectively treated in our cases. The embryo transfer during IVF may fail to implant, which depends on the quality of embryo, receptivity of the endometrium, and integrity of the uterus. For the uterine integrity and other pathologies, we can assess by hysteroscopy and endometrial biopsy. In our study, we found a clinical increase in pregnancy rate in patients where endometrial abnormalities were evaluated and effectively treated.
The results of our study are compared with other similar eight studies found in the literature (Table 6). The overall analysis showed that the clinical pregnancy rate was positively higher in the HSC group compared with that in the non-HSC group (Table 6). Regarding miscarriage rate, only four studies provided miscarriage rate data and were included in the analysis (two were RCTs and two were NRS). The results revealed no significant difference in the miscarriage rate between the two groups (HSC and Non-HSC), which was in contrast to our results where the miscarriage rate was more in the control group. The overall analysis of the implantation rate revealed that it was significantly higher in the HSC group compared with the control (non-HSC) group (Table 6).
Table 6.
Primary outcomes of various studies mentioned in the literature
| Sr. no. | Study | Study design | Intervention | No. of patients | Clinical pregnancy rate[a] | Miscarriage rate | Implantation rate |
|---|---|---|---|---|---|---|---|
| 1 | Kanazawa et al. [15] | Retrospective cohort | Hysteroscopy | 45 | 65/45 (35.6%) | 7/16 (43.8%) | 18/70 (25.8%) |
| Control | 90 | 20/90 (22.2%) | 9/20 (45.0%) | 22/119 (18.5%) | |||
| 2 | Pabuçcu et al. [16] | Retrospective cohort | Hysteroscopy | 119 | N/A | 10/119 (8.4%) | 22.40% |
| Control | 224 | N/A | 18/244 (7.3%) | 18.70% | |||
| 3 | El-Toukhy et al. [17] | RCT | Hysteroscopy | 322 | 114/301 (38%) | 29/133 (22%) | 129/410 embryos (32%) |
| Control | 318 | 110/290 (38%) | 33/136 (24%) | 134/423 embryos (32%) | |||
| 4 | Gao et al. [18] | NRS | Hysteroscopy | 334 | 140/334 (41.9%) | N/A | 176/739 (23.8%) |
| Control | 338 | 109/338 (32.2%) | N/A | 135/726 (18.6%) | |||
| 5 | Hosseini et al. [19] | NRS | Hysteroscopy | 142 | 72/142 (50.7%) | N/A | N/A |
| Control | 221 | 64/211 (30.3%) | N/A | N/A | |||
| 6 | Makrakis et al. [20] | NRS | Hysteroscopy | 414 | 145/414 (35.0%) | N/A | N/A |
| Control | 414 | 104/414 (25.1%) | N/A | N/A | |||
| 7 | Ramaraju et al. [21] | RCT | Normal hysteroscopy | 160 | 71/160 (44.4%) | 23/71 (32.3%) | N/A |
| Abnormal hysteroscopy | 95 | 38/95 (39.6%) | 13/38 (35.1%) | N/A | |||
| Control | 265 | 69/265 (26.2%) | 25/69 (36.2%) | N/A | |||
| 8 | Demirol et al. [13] | RCT | Normal hysteroscopy | 154 | 50/154 (32.5%) | N/A | N/A |
| Abnormal hysteroscopy | 56 | 17/56 (30.4%) | N/A | N/A | |||
| Control | 211 | 45/211 (21.6%) | N/A | N/A | |||
| 9 | Our prospective study in rural population | Normal hysteroscopy | 54 | 12/54(22.2%) | 3/20(13.6%) | 12/112 embryos (10.7%) | |
| Abnormal hysteroscopy | 36 | 15/36(41.66%) | 2/13(15.38%) | 15/103 embryos (14.56%) | |||
| Control | 90 | 21/90(23.3%) | 9/30(30%) | 21/234 embryos (8.9%) | |||
N/A = Not available, NRS = nNn-randomized study, RCT = Randomized controlled trial. a = Data reported as number of patients (%)
Conclusion
In this study, hysteroscopy was able to detect around 40% of intrauterine pathologies, which were undiagnosed by other conventional methods such as USG or HSG. We noticed clinical improvement in IVF success rate post-hysteroscopy (30% v/s 23.3%), which was statistically significant. According to the results of our study, hysteroscopy should be offered to all those women with previous one or more IVF failures because some clinically undiagnosed pathologies can be detected and treated accordingly achieving positive outcomes in subsequent IVF cycles.
Acknowledgements
The authors express their gratitude to the patients for their cooperation in this study and keeping regular follow-up. They also thank the junior doctors and the departmental staff for data acquisition.
Author Contributions
MP and DS were responsible for study conception and design, data acquisition, statistical analysis, and manuscript drafting. SS contributed to interpretation. JT contributed to manuscript drafting. All authors read and approved the final manuscript.
Funding
The authors received no financial support for the research.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Ethical approval from institutional ethical committee, JNMC and AVBRH, Wardha, was taken.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Dr. Minakshi Pounikar, MS Obstetrics and Gynaecology (Assistant Professor), Department of Obstetrics and Gynaecology, Jawaharlal Nehru Medical College and AVBRH hospital, Sawangi, Wardha, India; Dr. Deepti Shrivastava, MS Obstetrics and Gynaecology (Professor), Department of Obstetrics and Gynaecology, Jawaharlal Nehru Medical College and AVBRH hospital, Sawangi, Wardha, India; Dr. Sapna Sharma, Diploma in Obstetrics and Gynaecology (Senior Resident), Department of Obstetrics and Gynaecology, Jawaharlal Nehru Medical College and AVBRH hospital, Sawangi, Wardha, India; Dr. Jitendra Tadghare, MCh Neurosurgery (Assistant Professor), Department of Neurosurgery, Jawaharlal Nehru Medical College and AVBRH hospital, Sawangi, Wardha, India.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360(3):236–243. doi: 10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- 2.Taylor E, Gomel V. The uterus and fertility. Fertil Steril. 2008;89(1):1–16. doi: 10.1016/j.fertnstert.2007.09.069. [DOI] [PubMed] [Google Scholar]
- 3.Elsetohy KA, Askalany AH, Hassan M, et al. Routine office hysteroscopy prior to ICSI vs ICSI alone in patients with normal transvaginal ultrasound: a randomized controlled trial. Arch Gynecol Obstet. 2015;291(1):193–199. doi: 10.1007/s00404-014-3397-z. [DOI] [PubMed] [Google Scholar]
- 4.Cenksoy P, Ficicioglu C, Yildirim G, et al. Hysteroscopic findings in women with recurrent IVF failures and the effect of correction of hysteroscopic findings on subsequent pregnancy rates. Arch Gynecol Obstet. 2013;287(2):357–360. doi: 10.1007/s00404-012-2627-5. [DOI] [PubMed] [Google Scholar]
- 5.Doldi N, Persico P, Di Sebastiano F, et al. Pathological findings in hysteroscopy before in-vitro fertilization-embryo transfer. Gynecol Endocrinol. 2005;21(4):235–237. doi: 10.1080/09513590500366696. [DOI] [PubMed] [Google Scholar]
- 6.Xiaoyan M, Ling W, Qiuju C, et al. Effect of hysteroscopy before starting in-vitro fertilization for women with recurrent implantation failure: a meta-analysis and systematic review. Medicine (Baltimore) 2019;98(7):e14075. doi: 10.1097/MD.0000000000014075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakas P, Hassiakos D, Grigoriadis C, et al. Role of hysteroscopy prior to assisted reproduction techniques. J Minim Invasive Gynecol. 2014;21(2):233–237. doi: 10.1016/j.jmig.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Bosteels J, Weyers S, Puttemans P, et al. The effectiveness of hysteroscopy in improving pregnancy rates in subfertile women without other gynaecological symptoms: a systematic review. Hum Reprod Update. 2010;16(1):1–11. doi: 10.1093/humupd/dmp033. [DOI] [PubMed] [Google Scholar]
- 9.Taşkin EA, Berker B, Ozmen B, et al. Comparison of hysterosalpingography and hysteroscopy in the evaluation of the uterine cavity in patients undergoing assisted reproductive techniques. Fertil Steril. 2011;96(2):349–352. doi: 10.1016/j.fertnstert.2011.05.080. [DOI] [PubMed] [Google Scholar]
- 10.Eldar-Geva T, Meagher S, Healy DL, et al. Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil Steril. 1998;70:687–691. doi: 10.1016/S0015-0282(98)00265-9. [DOI] [PubMed] [Google Scholar]
- 11.Farhi J, Ashkenazi J, Feldberg D, et al. Effect of uterine leiomyomata on the results of in-vitro fertilization treatment. Hum Reprod. 1995;10:2576–2578. doi: 10.1093/oxfordjournals.humrep.a135748. [DOI] [PubMed] [Google Scholar]
- 12.Schiano A, Jourdain O, Papaxanthos A, et al. The value of hysteroscopy after repeated implantation failures with in vitro fertilization. Contracept Fertil Sex. 1999;27(2):129–132. [PubMed] [Google Scholar]
- 13.Demirol A, Gurgan T. Effect of treatment of intrauterine pathologies with office hysteroscopy in patients with recurrent IVF failure. Reprod Biomed Online. 2004;8:590–594. doi: 10.1016/S1472-6483(10)61108-X. [DOI] [PubMed] [Google Scholar]
- 14.Pereira N, Amrane S, Estes JL, et al. Does the time interval between hysteroscopic polypectomy and start of in vitro fertilization affect outcomes? Fertil Steril. 2016;105(2):539–544. doi: 10.1016/j.fertnstert.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Kanazawa E, Nakashima A, Yonemoto K, et al. Injury to the endometrium prior to the frozen-thawed embryo transfer cycle improves pregnancy rates in patients with repeated implantation failure. J Obstet Gynaecol Res. 2017;43(1):128–134. doi: 10.1111/jog.13182. [DOI] [PubMed] [Google Scholar]
- 16.Pabuçcu EG, Yalçın İ, Bodur T, et al. Impact of office hysteroscopy in repeated implantation failure: experience of a single center. J Turk Ger Gynecol Assoc. 2016;17(4):197–200. doi: 10.5152/jtgga.2016.16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Toukhy T, Campo R, Khalaf Y, et al. Hysteroscopy in recurrent invitro fertilisation failure (TROPHY): a multicentre, randomized controlled trial. Lancet. 2016;387:2614–2621. doi: 10.1016/S0140-6736(16)00258-0. [DOI] [PubMed] [Google Scholar]
- 18.Gao M, Sun Y, XieH,, et al. Hysteroscopy prior to repeat embryo transfer may improve pregnancy outcomes for asymptomatic women with repeated implantation failure. J Obstet Gynaecol Res. 2015;41(10):1569–76. doi: 10.1111/jog.12773. [DOI] [PubMed] [Google Scholar]
- 19.Hosseini MA, Ebrahimi N, Mahdavi A, et al. Hysteroscopy in patients with repeated implantation failure improves the outcome of assisted reproductive technology in fresh and frozen cycles. J Obstet Gynaecol Res. 2014;40(5):1324–1330. doi: 10.1111/jog.12315. [DOI] [PubMed] [Google Scholar]
- 20.Makrakis E, Hassiakos D, Stathis D, et al. Hysteroscopy in women with implantation failures after in vitro fertilization: findings and effect on subsequent pregnancy rates. J Minim Invasive Gynecol. 2009;16(2):181–187. doi: 10.1016/j.jmig.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Rama Raju GA, Shashi KG, Krishna KM, Prakash GJ, Madan K. Assessment of uterine cavity by hysteroscopy in assisted reproduction programme and its influence on pregnancy outcome. Arch Gynecol Obstet. 2006;274:160–164. doi: 10.1007/s00404-006-0174-7. [DOI] [PubMed] [Google Scholar]