Abstract
Upregulation of P2X3 receptor (P2X3R) has been strongly implicated in nociceptive signaling including bone cancer pain (BCP). The present study, using rat bone cancer model, aimed to explore the role of P2X3R in regulating rat pain behavior under the intervention of electroacupuncture (EA). The BCP model was successfully established by injection with MRMT-1 breast cancer cell into the medullary cavity of left tibia for 3 × 104 cells/3 μL PBS in rats as revealed by obvious bone destruction, decreased paw withdrawal thresholds (PWTs), and reduced paw withdrawal latencies (PWLs). Western blot analyses showed that P2X3R expression was significantly upregulated in ipsilateral lumbar 4–6 (L4-6) dorsal root ganglia (DRG), but the difference not seen in spinal cord dorsal horn (SCDH). With the in-depth study of P2X3R activation, we observed that intrathecal injection of P2X3R agonist α,β-meATP aggravated MRMT-1 induced BCP, while injection of P2X3R inhibitor A-317491 alleviated pain. Subsequently, we demonstrated that BCP induced mechanical allodynia and thermal hyperalgesia were attenuated after EA treatment. Under EA treatment, total P2X3R protein expression in ipsilateral DRGs was decreased, and it is worth mentioning that decreased expression of P2X3R membrane protein, which indicated that both the expression and membrane trafficking of P2X3R were inhibited by EA. The immunofluorescence assay showed that EA stimulation exerted functions by reducing the expression of P2X3R-positive cells in ipsilateral DRGs of BCP rats. Ca2+ imaging analysis revealed that the EA stimulation decreased the percentage of α,β-meATP responsive neurons in DRGs and inhibited calcium influx. Notably, the inhibitory effect of EA on mechanical allodynia and nociceptive flinches was abolished by intrathecal injection of α,β-meATP. These findings demonstrated EA stimulation ameliorated mechanical allodynia and thermal hyperalgesia in rat model of MRMT-1-induced BCP. EA exerts analgesic effect on BCP by reducing the overexpression and functional activity of P2X3R in ipsilateral DRGs of BCP rats. Our work first demonstrates the critical and overall role of P2X3R in EA’s analgesia against peripheral sensitization of MRMT-1-induced BCP and further supports EA as a potential therapeutic option for cancer pain in clinic.
Keywords: Electroacupuncture, Bone cancer-induced pain, P2X3 receptor, DRG
Introduction
In recent years, the incidence of malignant tumors has increased worldwide year by year, which are one of the major causes of life-threatening diseases in humans. The International Agency for Research on Cancer has reported that approximately 19.3 million new cancer cases and 10.0 million deaths due to cancer occurred in 2020 worldwide [1]. With the improvements of medical technology, survival periods of cancer patients have been extended, while cancer-induced bone pain has become a vital factor affecting patients’ quality of life. Breast cancer, lung cancer, bladder cancer, melanoma, renal cell carcinoma, thyroid cancers, and many other cancers can metastasize to bone and form osteolytic bone lesions, leading to significant bone cancer pain (BCP) [2]. Of these, the incidence of bone metastases from advanced breast cancer is up to 80% [3].
BCP is regarded as a multiple-mechanism of chronic pain with multiple features of both neuropathic and inflammatory pain, including spontaneous pain and breakthrough pain [4, 5]. Studies on the mechanisms of BCP have suggested its association with various factors, including inflammatory mediators (prostaglandins, endothelin, bradykinin, nerve growth factor), an acidic environment and carcinoma-evoked osteoclast activity [6, 7], etc. Evidence suggested bone metastasis of cancer cells could destroy the bone, and release large amounts of adenosine triphosphate (ATP) with other inflammatory cells to persistently activate the nociceptors, resulting in peripheral sensitization [8]. P2X3 receptors (P2X3R), ATP-gated membrane proteins, are selectively localized in small-medium dorsal root ganglia neurons and superficial laminae of spinal cord [9]. Accumulating studies have suggested that P2X3 receptors in primary sensory neurons are involved in pain transmission [10]. Notably, P2X3 receptors perform its function by transporting to the cell membrane [11, 12]. Animal studies have demonstrated that, within the peripheral nervous system, blockade of P2X3 receptors represents a novel therapeutic target for BCP [13].
Currently, the preferred medications used to treat cancer pain are opiates. However, long-term opioid analgesics treatment can lead to several adverse effects, such as addiction, nausea, constipation, drowsiness, and life-endangering respiratory depression [14]. Hence, better therapies for BCP alleviation with few side effects are urgently required. Several clinical studies have demonstrated acupuncture significantly reduced pain intensity in patients with cancer, and contributed to reducing opioid dosage [15, 16]. Electroacupuncture (EA) is one of acupuncture therapies in which electrical stimulation is applied to acupuncture points, which could not only relieve different types of pain, including cancer pain but also adverse drug reactions. EA, regarded as a complementary and alternative treatment, has been recommended by the National Comprehensive Cancer Network (NCCN) cancer treatment guidelines. It has been reported that EA exerted analgesic function via inducing the release of endogenous substances, such as beta-endorphin, dynorphin, enkephalin, substance P, and serotonin [17, 18]. In recent years, the purinergic signal pathway has been considered as a key signal pathway in EA’s analgesia. It was found that EA exerted analgesic effect by reducing P2X3R expression of rats with irritable bowel syndrome (IBS)-induced visceral pain, Freund’s complete adjuvant (CFA)-induced inflammatory pain, and chronic neuropathic pain, etc. [19–21]. Our previous study also demonstrated that EA stimulation produces analgesic effects in rat models of neuropathic pain by reducing P2X3R expression in DRG neurons [22]. It has been reported that enhanced translocation of P2X3R to the plasma membrane leads to up-regulating receptor function [11, 12]. However, very few studies focused on the plasma membrane P2X3 receptor. We have previously investigated that EA could significantly alleviate BCP-induced mechanical allodynia in rat [23], but the role and significance of P2X3R in EA’s analgesic effect on BCP is still unknown. The aim of this study is to more comprehensively investigate the key role of peripheral P2X3 receptor in EA analgesia on bone cancer-induced pain.
Materials and methods
Animals
Sprague–Dawley (SD) rats (female, weighing 180–220 g, specific-pathogen free grade) were purchased from the Zhejiang Chinese Medical University Laboratory Animal Research Center. SD rats were kept in a controlled environment, where standard rodent food and water were supplied ad libitum. Five rats were housed per cage at a constant temperature. The animal protocol was approved by the Ethics Committee of Zhejiang Chinese Medical University (IACUC Approval No. 20181022–01) and experiments followed the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Experimental design
The research was split into two parts. Radiographs and nociceptive behaviors were detected to confirm the successful establishment of the BCP model. In part one, rats were randomized into sham BCP (s-BCP) and BCP groups. Ipsilateral DRGs and SCDHs at L4-L6 level were collected used for western blot assay at days 11, 13, 15, 17, 20, and 24 after modeling. To further illustrate the involvement of P2X3R in the process of BCP, the animals were randomly divided into BCP + Veh, BCP + A31, s-BCP and BCP groups, each consisting of five rats. Rats in the BCP + A31 group were daily injected with the P2X3R inhibitor A317491 into ipsilateral hindpaw at day15-17. P2X3R activation induced nociceptive behaviors were measured by detecting α,β-meATP-induced flinches. The purpose of the second part of the study focused on the effect of EA on relieving MRMT-1 induced BCP, and this effect was related to the down-regulation of P2X3R expression and activity. Rats were randomly divided into the s-BCP, BCP + s-EA, and BCP + t-EA groups. The rats were sacrificed on the 17th day at the completion of nociceptive behaviors measurement, then paraformaldehyde prefixed or fresh L4-L6 DRGs in ipsilateral side were removed quickly for immunohistochemistry, western blot and Ca2+ imaging, respectively. To determine whether activation of P2X3R by intrathecal injection with α,β-meATP could attenuate EA analgesia in BCP, the rats were divided into BCP + EA + Veh and BCP + EA + α,β-meATP groups. Then paw withdrawal thresholds (PWTs) were examined dynamically at D10, D11, D12, D13, D15, and D17 after EA stimulation. At D18, intraplantar injection of α,β-meATP induced flinches were also measured to analyze the nociceptive behavior related to P2X3R activation.
Establishment of BCP model in rats
The BCP rat model was established as we previously described [24]. We employed a modified modelling protocol, which was established by implanting MRMT-1 mammary gland carcinoma cells into the left tibia according to Medhurst et al. [25]. First, the rats were anesthetized using a mixture of oxygen and isoflurane (2–5% isoflurane in 100% oxygen) and placed in supine position. Then, MRMT-1 mammary gland carcinoma cells (3 × 104 cells in 3 µL) were slowly injected into the medullary cavity of the left tibia to induce bone cancer in rats. To prevent carcinoma cell leakage, microsyringe (Hamilton Co, Bonaduz, Switzerland) remained in position for 2 min after infusion, and the hole was sealed immediately with bone wax. Finally, after suturing, all rats received operation were intramuscularly injected of penicillin (20,000 units per rat per day for 3 days) to prevent infection. The rats of sham BCP group were injected with the equal volume of boiled carcinoma cells and followed the same procedure as described above.
EA treatment
In order to minimize stress in rats, a special cotton retainer is required, which is designed by our laboratory (Patent No. ZL 201,420,473,579.9, State Intellectual Property Office of the P.R.C). The rats in EA group and sham EA group were gently immobilized by the retainer with the hind legs exposed. Disposable stainless steel needles (0.25 mm diameter, 13 mm length, Suzhou Medical Appliance Factory, China) were inserted subcutaneously to the bilateral ST36 (5 mm lateral to the anterior tubercle of the tibia bone) and BL60 (at the level of the ankle joint, between the tip of the lateral malleolus and tendo calcaneus) acupoints with a depth of 5 mm. During needle retention, the needles were connected with HANS Acupuncture point Nerve Stimulator (LH-202H, Huawei Co., Ltd., Beijing, China). The EA stimulation persisted for 30 min with a dilatational wave (pulse width: 0.6 ms at 2 Hz, 0.2 ms at 100 Hz), 2/100 Hz alternative frequencies, and intensities ranging from 0.5, 1, and 1.5 mA (10 min each, total 30 min). The EA parameters were set according to our previous research [23, 26]. The rats of sham EA group received only superficial needle insertion into subcutaneous tissue of same acupoints and without EA stimulation.
Drug administration in vivo
Five days after the BCP surgery, a PE-10 polyethylene catheter was implanted into the subarachnoid space between the L4 and L5 vertebrae under anesthesia following a method by Storkson et al. [27]. The catheter was fixed onto the skin at the incision site. Rats were treated with penicillin (20,000 units in 200 μL; i.m.) to avoid infection. A317491 (300 nM in 10 μL; Sigma-Aldrich, Saint Louis, MO, USA) was injected into the left plantar of BCP rats for three consecutive days, which started on day 15 after BCP surgery. Eight days after the catheter implantation, a P2X3 receptor specific agonist α,β-meATP (30 nM, 20 μL; Sigma-Aldrich, Saint Louis, MO, USA) or equal volume of phosphate-buffered saline (PBS) was injected followed by additional 10 μL sterile PBS via the implanted catheter. Intrathecal administration was carried out once per day in five consecutive days started from 13th day to 17th day after BCP surgery. Mechanical pain behaviors and α,β-meATP-induced flinching behavior were examined dynamically at base and successive days after surgery.
Pain behavior measurement
Mechanical allodynia
Mechanical allodynia was determined by measuring the PWTs using von Frey filament (Stoelting Co., Thermo, Gilroy, CA, USA) stimulation. PWTs were detected before and after tibial inoculation of MRMT-1 carcinoma cells. Rats were acclimated for at least 30 min before each detection, and a series of von Frey filaments were applied to the plantar surface of the hindpaw for 5 s. Mechanical allodynia was quantified using the up-down method by measuring PWTs in response to von Frey filament stimulation. A maximum strength value was set at 50 g to prevent tissue damage.
Thermal hyperalgesia
Paw withdrawal latencies (PWLs) were determined using a plantar tester (Ugo Basile 37,370, Italy) and utilized to assess thermal hyperalgesia. The time required for the rat to remove its hind paw from the heat stimulus was recorded as the PWLs. In a quiet environment, rats were placed on the plexiglass plate of the plantar tester and habituated for 30 min before the test. A radiant light beam generated by a light bulb was directed into the central of rat’s hindpaw. The automatic cutoff time was set at 20 s to avoid excessive heating to cause injury. PWLs were calculated for each rat as a mean of five measurements with an interval of 5 min between two tests.
α,β-meATP-induced flinching behavior
As we described in earlier paper [22], rats were acclimated in individual standard cages for 1 h before EA. Intraplantar delivery of α,β-meATP (1 mM, 50 μL) into the palmar surface of left hindpaw was conducted immediately after the completion of EA or s-EA treatment. Subsequently, the rat received a flinching behavioral test. Flinching frequency was calculated in each 1-min time bin after α,β-meATP injection in order to determine the nociceptive behavior related to peripheral P2X3R activation. All behavioral tests were performed by an experimenter blinded to groupings.
X-ray detection
Rats were anesthetized by a mixture of oxygen and isoflurane at day 24 after tibial inoculation of MRMT-1 carcinoma cells and photographed with a PLX7000B HF mobile C-arm X-ray equipment (Perlove Medical Equipment Co., Ltd., Nanjing) for 2.5 s at 35 kV, 0.6 mA.
Extraction of total protein and membrane protein and western blot analysis
Rats were sacrificed at endpoint of experimental design under deep anesthesia with pentobarbital sodium (40 mg/kg; i.p.), and tissues were quickly dissected out then placed into oxygenated dissection solution. Membrane proteins in ipsilateral L4-6 DRGs were isolated by surface biotinylation, using Sulfo-NHS-LC-Biotin (1 mg/mL; Pierce Biotechnology, USA), which is a membrane-impermeable reagent. DRGs were biotinylated with 500 μL Sulfo-NHS-LC-Biotin for 30 min on ice, washed with RIPA five times. Following washing out unbound biotin, the DRGs were homogenized with RIPA buffer (RIPA:PMSF = 99:1) and centrifuged at 13,500 g for 12 min at 4 °C. The supernatants (i.e., total proteins) were collected for following protein assay (BCA) and western blot analysis. Total proteins (200 μg; supernatants) were incubated with 20 μL of 10 mg/mL streptavidin magnetic beads (Thermo Fisher, Waltham, MA, USA) on ice for 3 h with a shaker. After the supernatant was discarded, the beads were washed 3 times with an 800 μL lysis buffer and were mixed thoroughly with 10 μL RIPA lysis buffer and 10 μL 2 × sample buffer. Subsequently, the mixture was boiled at 98 °C for 3 min, and the supernatants (i.e., membrane proteins) were taken for the following assay. Total proteins from L4-6 SCDHs were extracted by the same method. Protein concentration was determined by a BCA protein assay kit. All samples were denatured by boiling at 100 °C with a loading buffer, loaded on a 8% SDS–polyacrylamide electrophoresis gel and transferred a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA). After being blocked with 5% bovine serum albumin (BSA) in TBST, the membrane was incubated with primary antibodies against P2X3R overnight at 4 °C. Sixteen hours later, the immunoblots were labeled using HRP-conjugated goat anti-rabbit IgG (1:10,000; Abcam, Cambridge, USA) for 2 h at room temperature. Immunoreactive proteins were detected by ECL reagents (Beyotime, Shanghai, China), and bands were analyzed by densitometry in Image Quant TL 7.0 analysis software (GE, USA).
Immunohistochemistry study
After anesthetized with pentobarbital sodium (40 mg/kg; i.p.), rats were transcardially perfused with 0.9% normal saline (4 ℃) and then 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for prefixation. The L4-6 DRGs were dissected followed by post-fixed in 4% PFA for 6 h and immersed in 30% sucrose solution overnight at 4 °C. The DRGs were embedded in O.C.T. Compound (Bayer Corp, Elkhart, IN) and cut at thickness of 14 µm using a frozen slicer (Thermo NX50, MA, USA). After being mounted on glass slides, sections were incubated overnight with Anti-P2X3R antibody (1:400; Alomone Labs, Jerusalem, Israel). The next day, sections were washed with PBS and incubated with Alexa Fluor-647 conjugated donkey anti-rabbit IgG (1:800; Abcam, Cambridge, USA) at 37℃ for 1 h. Sections were observed under a laser scanning confocal microscope (Nikon A1R, Nikon, Japan). The immunoreactivity was quantified with Image-Pro Plus 6.0 (Thermo, USA). The percentage of positive immunoreactivity was determined as the ratio of P2X3R positive DRG neurons in the total number of DRG neurons. The assessments were performed in five non-consecutive sections for each rat by calculating the mean values. At least three rats were analyzed in each group.
Calcium imaging
After rats were deeply anesthetized, the ipsilateral L4-6 DRGs were surgically removed and placed in a culture dish containing 2 mg neutral protease (5 mg/mL; Gibco, USA) and 1 mg collagenase Type 1A (2 mg/mL; Gibco, USA) in DMEM/F12 medium (1 mL; Gibco, USA). The culture dish was transferred to the incubator for 1 h (37 °C, 5% CO2). After the DRG cells were washed twice with DMEM/F12, centrifuged, and filtered with 40 μm cell strainer (Falcon, Massachusetts, USA), they were plated on culture dishes coated with laminin (Invitrogen, NY, USA) and poly-D-lysine (Sigma, Saint Louis, MO, USA) until use. Calcium imaging was performed within 4 h after DRGs preparation. Cells were loaded with 10 μM Fura-2-AM (Invitrogen, Carlsbad, CA, USA) in a loading buffer containing 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM HEPES, and incubated for 50 min at 37 ℃. At 24 s after perfusion, α,β-meATP (30 μmol/L, sigma, St Louis, MO, USA) was injected for 40 s at a flow rate of 1–2 mL/min. To identify viable neurons, 50 mM KCl was perfused after treatment. Ratiometric Ca2+ imaging was performed as described in our previous publication with a Nikon ECLIPSE Ti-S (Japan) microscope [28]. Images were captured with 0.5 ms exposures at 340 nm excitation wavelengths and 0.3 ms exposures at 380 nm excitation wavelengths. Ratiometric images were generated by using GraphPadV7.0 software. Only cells were considered responsive if the peak amplitude was above 20% of baseline level.
Statistical analysis
Data were presented as mean ± SEM. The independent t-test was used for comparisons between two groups. Comparisons among multiple groups were analyzed by one-way analysis of variance (ANOVA), followed by Tukey multiple correction tests for post hoc analysis. Differences were considered statistically significant at P values < 0.05. Prism (GraphPad software, La Jolla, CA) was used for plotting graphs and statistical analysis.
Results
Intra-tibia injection of MRMT-1 carcinoma cells induced mechanical allodynia and thermal hyperalgesia
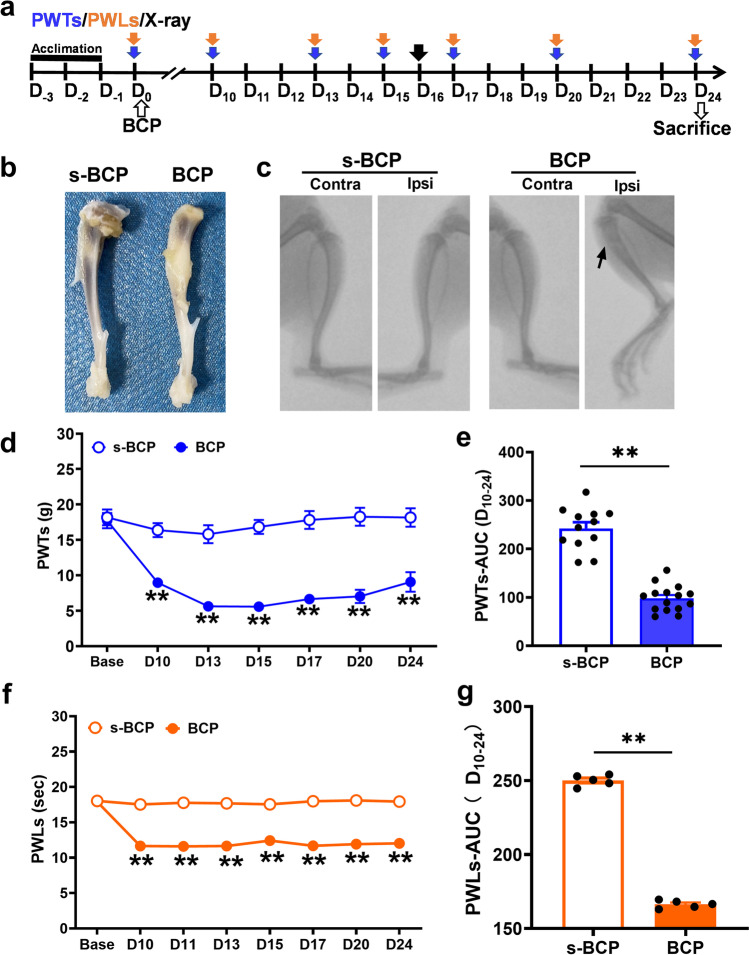
The rat model of bone cancer-induced pain was established by unilaterally implantation of MRMT-1 mammary gland carcinoma cells into the intramedullary cavity of the tibia according to modified protocol reported before [25]. Establishment of BCP modeling was confirmed by radiological and behavioral examination. As shown by anatomic and X-ray images, obvious bone destruction was observed about one third of the ipsilateral tibia of BCP rats at 16 days post-inoculation (Fig. 1b and c). However, no obvious alternation of the tibia structure was found in bilateral tibia of sterilized PBS-treated rats (s-BCP group) and in contralateral tibia of BCP rats. After intra-tibial innoculation of MRMT-1 mammary gland carcinoma cells, the rats produced a significant cancer-evoked pain behavior. Obvious sign of mechanical allodynia, manifested by a significant decrease in PWTs, developed at postoperative day 10 and lasted till day 24 (the end of observation time frame) in BCP rats, as compared with s-BCP rats (Fig. 1d and e). In addition, BCP rats also elicited an obvious reduction in PWLs, a sign of thermal hyperalgesia. The thermal hyperalgesia occurred at day 10 and persisted until day 24 post-inoculation as well (Fig. 1f and g). These results indicated the successful establishment of BCP modeling in rats.
Fig. 1.
Radiographs and nociceptive behaviors of BCP rats. a The diagramatic chart was shown in detail. Blue, orange and black arrow indicated the timepoints of PWTs, PWLs and X-ray. b Gross anatomy of the ipsilateral tibial bone from s-BCP and BCP rat. The comparison of the ipsilateral tibial bone from s-BCP and BCP rat on 24th day. c X-ray radiographs of the bilateral tibial bone on 16th day after carcinoma cell inoculation. d, e PWTs assessed by von Frey filament in BCP rats was lower than in s-BCP rats at D10, 13, 15, 17, 20, and 24 post-innoculation. Area under the curve (AUC, D10-24) of s-BCP and BCP rats further analyzed to illustrate the difference of the mechanical allodynia (n = 12 in s-BCP group, n = 15 in BCP group). f, g PWLs assessed by UGO’s infra-red test decreased in BCP rats in comparison with s-BCP rats. AUC of PWLs (D10-24) further illustrated the different thermal hyperalgesia of s-BCP and BCP (n = 5 per group). Data are represented as mean ± SEM, **P < 0.01, compared with the s-BCP group at the same timepoint
P2X3 receptor in the dorsal root ganglia not spinal cord dorsal horn was involved in MRMT-1-induced bone cancer pain
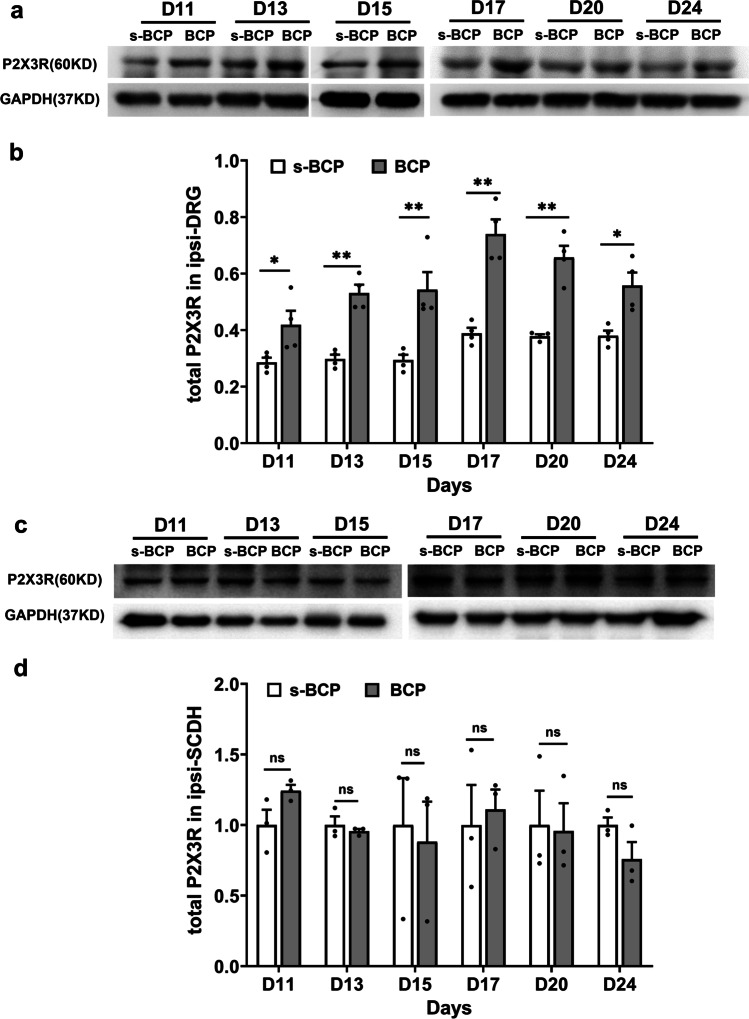
To investigate whether purinergic P2X3 receptor (P2X3R) participated in the development of MRMT-1 induced BCP, we used western blot to measure the P2X3R protein expression in ipsilateral DRGs and SCDHs. The protein expression of P2X3R in ipsilateral L4-6 DRGs increased at postoperative day 11 (D11), reached the peak at D17 and remained the higher level till D24 (P < 0.05 or P < 0.01, Fig. 2a and b). However, no significant difference in P2X3R protein expression was found in ipsilateral L4-6 SCDHs at days 11, 13, 15, 17, 20, and 24 after modeling (Fig. 2c and d). The results showed that peripheral not spinal P2X3R contributes to BCP.
Fig. 2.
The expressions of P2X3R total protein in ipsilateral L4-6 DRGs and ipsilateral SCDHs. a Representative gel images of P2X3R total protein in ipsilateral DRGs of s-BCP and BCP rats at D11, 13, 15, 17, 20 and 24 after carcinoma cells inoculation. b Relative protein levels of total P2X3R in the two groups were quantified by ImageJ (n = 4 in each). The samples were taken from ipsilateral L4-6 DRGs. c Representative gel images of P2X3R total protein in ipsilateral L4-6 SCDHs of s-BCP and BCP rats at D11, 13, 15, 17, 20, and 24 after carcinoma cell inoculation. d Relative protein levels of total P2X3R in ipsilateral L4-6 SCDHs were quantified by ImageJ (n = 3 per group). P2X3R protein expression increased in ipsilateral L4-6 DRGs but remained unchanged in SCDHs. Data are represented as mean ± SEM, *P< 0.05, **P < 0.01, compared with the s-BCP group at the same timepoint
Inhibition of peripheral P2X3R alleviated MRMT-1-induced bone cancer pain while P2X3R activation aggravated pain
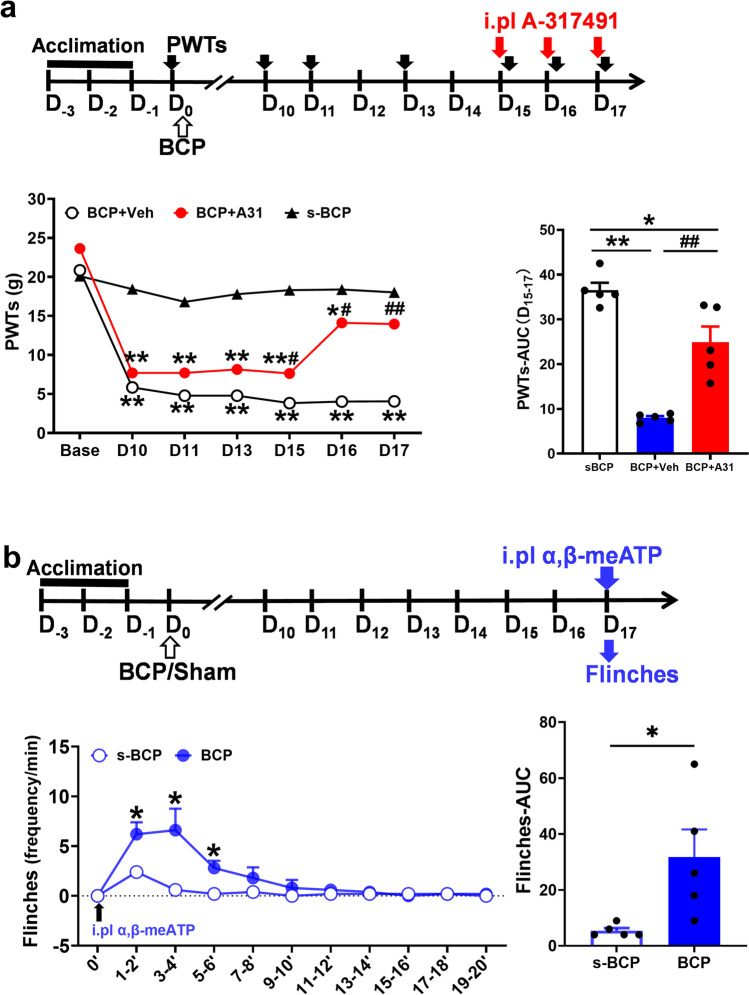
To further clarify the involvement of P2X3R activation aggravated BCP in rats, we observed the changes of PWTs and flinching behavior after intraplantar injection of P2X3R inhibitor A-317491 or its agonist α,β-meATP, respectively. Blocking P2X3R activation effectively reversed BCP. PWTs of BCP + A-317491 group were significantly higher than that of BCP + Vehicle group at D15-17 (P < 0.05 or P < 0.01). The flinching behavior induced by intraplantar injection of P2X3R agonist α,β-meATP at D17. Frequencies of paw flinching in BCP rats were higher than those of s-BCP rats at 1–6 min after α,β-meATP injection (P < 0.05) (Fig. 3b), which showed promoting P2X3R activation aggravated MRMT-1-induced BCP. The results further support that peripheral P2X3R activation promotes BCP, inhibition of peripheral P2X3R relieves MRMT-1-induced pain.
Fig. 3.
The effects of P2X3R inhibitor or agonist on rat pain behavior. a Experimental timeline showed the timepoints of PWTs (black solid arrow), model construction (black hollow arrow) and P2X3R inhibitor A317491 intervention (red solid arrow). PWTs assessed by von Frey filament in BCP + A31 rats was significantly higher than in BCP + Veh rats at D15, 16 and 17 post-innoculation. Area under the curve (AUC, D15-17) of BCP + Veh and BCP + A31 rats further analyzed to illustrate the difference of the mechanical allodynia (n = 5 per group). Data are represented as mean ± SEM, *P < 0.05, **P < 0.01, compared with the sBCP group at the same timepoint, #P < 0.05, ##P < 0.01, compared with the BCP + Veh group at the same timepoint. b Experimental timeline showed the timepoints of model construction (black hollow arrow), P2X3R agonist α,β-meATP intervention (blue solid arrow) and flinching test (blue hollow arrow). The number of flinches of BCP rats was significantly higher than in s-BCP rats between 1 and 6 min after plantar injection of α,β-meATP. Area under the curve (AUC, 0–20 min) of BCP and s-BCP rats further analyzed to illustrate the difference of the spontaneous pain resulted from peripheral P2X3R activation (n = 5 per group). Data are represented as mean ± SEM, *P < 0.05, **P < 0.01, compared with the s-BCP group at the same timepoint
EA stimulation attenuated mechanical allodynia and thermal hyperalgesia of BCP rats
To demonstrate the efficacy of EA in reducing BCP, the changes of PWTs and PWLs in ipsilateral hindpaw after EA stimulation were compared. The immediate analgesic effect of first EA stimulation was investigated at D11 post-inoculation, and accumulated analgesic effect was further investigated after the completion of several EA stimulations. PWTs in the BCP + t-EA group were higher than those in the BCP + s-EA group, were lower than in s-BCP control group at D10, 11, 13, 15, 17 (P < 0.01, Fig. 4b). AUC analysis during D11-17 further demonstrated an overall anti-allodynic effect of EA stimulation on BCP rats (Fig. 4c). In addition, true EA stimulation produced persistent relief of thermal hyperalgesia on BCP rats which was no difference compared with the s-BCP control group, whereas sham EA stimulation was without analgesic effect (P < 0.01, Fig. 4d). AUC analysis during D11-17 further demonstrated an overall effect of EA stimulation on thermal hyperalgesia of BCP rats (Fig. 4e).
Fig. 4.
Effect of EA on mechanical allodynia and thermal hyperalgesia of BCP rats. a Experimental timeline showed the timepoints of PWTs (blue arrow), PWLs (orange arrow), and EA intervention (black lightning). b, d EA stimulation increased PWTs and PWLs in BCP rats at D10, 11, 13, 15, and 17 post-innoculation. c, e Area under the curve (AUC, D11-17) of PWTs and PWLs further analyzed according to b and d, respectively. Data are represented as mean ± SEM (n = 5 per group). **P < 0.01, compared with the s-BCP group at the same timepoint. ##P < 0.01, compared with the BCP + s-EA group at the same timepoint
EA stimulation decreased the overexpression of P2X3R expression in DRGs of BCP rats
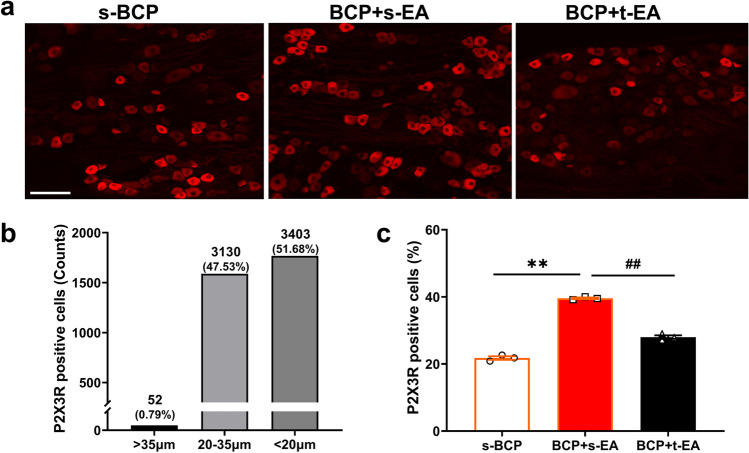
Since it was known that EA stimulation relieved BCP, further to explored the role and significance of peripheral P2X3R contribute to EA’s analgesia on MRMT-1-induced BCP. Our immunofluorescence results revealed that more than 99% of P2X3R positive cells were mainly distributed in the medium and small DRG neurons (Fig. 5a and b). Moreover, the percentage of P2X3R positive DRG neurons significantly increased in BCP + s-EA rats compared with s-BCP control rats (P < 0.01, Fig. 5a and c). Repeated EA stimulation significantly decreased the percentage of P2X3R positive DRG neurons induced by BCP (P < 0.01, Fig. 5a and c). We further measured the protein expression of P2X3R in ipsilateral DRGs by western blot, the ratio of membrane and total protein (mem/total) was then calculated to indirectly represent the membrane transport of P2X3R. As shown in Fig. 6a and b, both total and membrane protein expression of P2X3R were increased in ipsilateral L4-6 DRGs of BCP + s-EA rats (P < 0.01 or P < 0.05). Compared with the BCP + s-EA group, repeated EA stimulation significantly reduced both total and membrane/total ratio of P2X3R protein expression in ipsilateral L4-6 DRGs, it indicated that EA stimulation reduced overexpression and membrane transport of P2X3R in DRGs.
Fig. 5.
EA stimulation reduced the upregulation of P2X3R expression in DRG neurons of BCP rats. a Representative fluorescent images of P2X3R positive cells in ipsilateral L4-6 DRGs (bar = 100 μm) at D17 post-innoculation. b The number of positive cells with different diameters and sizes were counted from all of slices, indicating that most of P2X3R positive neurons distributed in the medium and small DRG neurons. c The rate of P2X3R positive cells was subsequently calculated in each group. Data are represented as mean ± SEM (n = 3 per group), **P < 0.01, compared with the s-BCP group. ##P < 0.01, compared with the BCP + s-EA group
Fig. 6.
EA stimulation decreased protein expression of P2X3R in DRGs of BCP rats. a Representative gel images of P2X3R total protein and membrane protein on 17th day after carcinoma cell inoculation. b Relative protein levels of total and plasma membrane P2X3R in the three groups were quantified by ImageJ. Data are represented as mean ± SEM (n = 5 per group), *P < 0.05, **P < 0.01, compared with the s-BCP group; #P < 0.05, ##P < 0.01, compared with the BCP + s-EA group
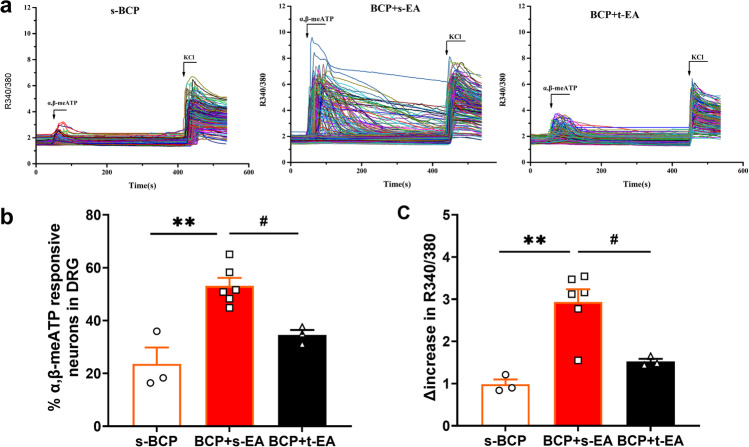
EA stimulation eliminated the enhancement of P2X3R activity in DRG neurons of BCP rats
Since the overexpression of P2X3R in DRGs was downregulated by EA stimulation, we further detected the effect of EA on functional activities of P2X3R in acutely dissociated L4-6 DRG neurons from rats by using Ca2+ imaging. α,β-meATP, the selective P2X3R agonist, was used to probe P2X3R activities. Perfusion of α,β-meATP elicited more percentage of α,β-meATP responsive neurons from BCP + s-EA rats in comparison with s-BCP rats, whereas repeated EA stimulation significantly reduced this increase resulted from BCP modeling (P < 0.01 or P < 0.05, Fig. 7a and b). The peak amplitude of the Ca2+ traces were further analyzed among s-BCP, BCP + s-EA, and BCP + t-EA group. Compared with the s-BCP rats, it produced more increase in the peak amplitude of the Ca2+ traces in DRG neurons from BCP + s-EA rats (P < 0.01, Fig. 7c). Moreover, repeated EA stimulation significantly reduced α,β-meATP-induced increase in peak amplitude of Ca2+ traces (P < 0.05, Fig. 7c). Thus, Ca2+ imaging results indicated that repeated EA stimulation attenuated the upregulated functional activity of P2X3R in DRG neurons induced by BCP modeling.
Fig. 7.
EA stimulation attenuated the increase in amplitude of α,β-meATP-induced Ca2+ response in DRG neurons from BCP rats. a Overlaid Ca2+ imaging traces of DRG neurons isolated from the s-BCP, BCP + s-EA, and BCP + t-EA groups of rats: DRG neurons were perfused with α,β-meATP (30 μM), followed with KCL (50 mM). The arrows and bars indicate the time point and duration of each drug application, respectively. b Bar graphs showing the percentage of α,β-meATP responsive neurons in DRG neurons from the s-BCP, BCP + s-EA, and BCP + t-EA groups. c Bar graphs showing the Δ increase in peak 340/380 ratio before and after α,β-meATP application. Data are represented as mean ± SEM. *P < 0.05, **P < 0.01, compared with the s-BCP group; #P < 0.05, ##P < 0.01, compared with the BCP + s-EA group. n = 3–6 test/group, and each group contains more than 300 neurons derived from 3 rats
Peripheral injection of α,β-meATP reversed EA’s analgesia on BCP rats
To determine whether the activation of peripheral P2X3R could recede the analgesic effect of EA stimulation, the intervention of α,β-meATP on EA’s analgesia in BCP rats was investigated. As shown in Fig. 8a, BCP rats were intrathecally implanted with PE-10 polyethylene catheter at D5 and followed by 5-day recovery, then treated by EA stimulation once per day from D11 to D18. α,β-meATP, a specific P2X3R agonist, was injected intrathecally once per day before EA stimulation at D13-17 and then PWTs were measured after EA stimulation. At D18, α,β-meATP-induced flinching behavior was then observed. After 1, 3, and 5 times of intrathecal injection of α,β-meATP to L4-5 DRG level, the analgesic effect of EA on MRMT-1-induced BCP was significantly weakened compared with vehicle-treated rats (P < 0.05 or P < 0.01, Fig. 8b). Area under the curve (AUC, D13-17) analysis further demonstrated activation of peripheral P2X3R attenuated anti-allodynic effect of EA stimulation on BCP rats (P < 0.01, Fig. 8c). The frequency of α,β-meATP-induced flinches in the EA + me ATP group was higher than that in the EA + Veh group, especially at 1–2’ and 3–4’ minutes bins after intraplantar injection (P < 0.05 or P < 0.01, Fig. 8d). Area under the curve (AUC, 0–20 min) analysis further indicated an overall effect of P2X3R agonist significantly reversed EA’s analgesic effect on α,β-meATP-induced spontaneous pain (P < 0.01, Fig. 8e).
Fig. 8.
Peripheral injection of α,β-meATP reversed EA’s analgesia on BCP rats. a Experimental timeline showed the timepoints of PWTs (blue solid arrow), model construction (black hollow arrow), EA intervention (black lightning), P2X3R agonist α,β-meATP intervention (red hollow arrow) and flinching test (red solid arrow). b PWTs assessed by von Frey filament in EA + me ATP rats was lower than in EA + Veh rats at D13, 15, and 17 post-innoculation. c Area under the curve (AUC, D13-17) analysis of b (n = 8 per group). d The frequency of flinches in EA + meATP rats was higher than in EA + Veh rats between 1 and 4 min after plantar injection of α,β-me ATP. e Area under the curve (AUC, 0–20 min) analysis of d (n = 7 per group). Data are represented as mean ± SEM, **P < 0.01, compared with the EA + Veh group at the same timepoint
Discussion
Cancer pain, regarded as an intractable pain, is widely recognized as a clinically significant complication of cancer. Unrelieved pain, especially breakthrough cancer pain, severely reduced daily functionality and life quality of cancer patients. There is no ending to seeking more effective methods with less side-effects for improvements in pain management. The establishment of an optimized animal model to mimic the clinical disease is a good beginning to wipe it out. In this study, the classic BCP model was established for exploring the mechanisms of cancer pain induced by bone metastasis, in which MRMT-1 gland carcinoma cells were inoculated into the medullary cavity [25]. This led to significant bone destruction, reduced PWTs to von Frey filament stimulation and decreased PWLs to heat stimulation (Fig. 1), suggesting the successful establishment of BCP.
The involvement of purinergic signaling in the initiation and transmission of cancer pain was demonstrated as early as 1996, as abrasive movement of tumors resulted in the release of ATP that may activate P2X3 receptors on afferent nerve endings [29]. As is well known, P2X3R not only distributed in peripheral nervous systems, such as primary afferent central terminals and DRG, but also in the presynaptic part of the spinal cord and the midbrain periaqueductal gray (PAG) [30–32], indicating that P2X3 receptors are involved in nociceptive conduction and modulation in both the peripheral and central nervous systems. Since DRG neurons are primary afferent neurons responsible for transmitting sensory information to the spinal cord and brain due to their unique pseudo-unipolar structure [33], studies have exclusively focused on the expression changes of P2X3R in DRGs after BCP model establishment during the past two decades. As reported, the number of P2X3R positive cells, as well as the mRNA level and protein level of P2X3R expression were significantly increased in DRGs of rats after tibial injection of Walker 256 carcinoma cells [34, 35]. The upregulation of P2X3R has also been observed on CGRP-IR (calcitonin gene-related peptide immunoreactivity) fibers in mice following NCTC clone 2472 fibrosarcoma cell implantation [36]. In this study, we found that total P2X3R protein expression in L4-6 DRGs was upregulated after BCP modeling, which was consistent with a previous study [35], yet, its protein expression in L4-6 SCDHs of BCP rats remained unchanged at D11, 13, 15, 17, 20, and 24. Furthermore, by means of calcium imaging, we observed that intracellular calcium amplitude followed by P2X3R activation significantly increased in DRG neurons of BCP + s-EA rats compared with the s-BCP rats, suggesting that P2X3R functional activity is enhanced in DRGs of BCP rats. We further found that blocking peripheral P2X3R significantly relieved MRMT-1-induced pain, whereas α, β-meATP-induced flinching behavior obviously enhanced in BCP rats. These results suggest that intra-tibia injection of MRMT-1 carcinoma cells resulted in upregulated expression of P2X3R and enhanced function of P2X3R in ipsilateral DRGs rather than SCDHs. Up to now, the association between upregulated expression of P2X3R in SCDHs and BCP is solely supported by indirect evidence that spinal administration of selective P2X3R antagonist AF-353 dose-dependently reduced bone cancer-induced dorsal horn neuronal hyperexcitability in vivo [37]. We believe that the observed discrepancies might in part be explained by the altered expressions and activities of P2X3R during the different stages of BCP, which was confirmed in a study that the analgesic effect of P2X3R antagonist differed between the early and late stage of femoral cancer-induced bone pain [38]. Mechanisms underlying the discrepancy of P2X3R expression in spinal level was worthy of further exploration in future studies.
Various experimental studies have proved the efficacy of EA in BCP [23, 39, 40]. Studies have shown that EA at ST36 and BL60 exerted a significant anti-nociceptive effect on inflammatory, neuropathic pain conditions as well as BCP [20, 41, 42]. Our previous studies have demonstrated significant analgesic effects of EA on BCP induced by Walker 256 [23, 24], without being affected by different current frequencies and treating frequencies [26]. However, it has been demonstrated that low-frequency EA treatments induced the release of β-endorphin (β-EP) and endomorphin (EM), thereby activating μ-opioid receptor in the brain, whereas high-frequency EA treatments stimulate the release of dynorphin, thus activating μ-opioid receptor in the spinal cord [43–45]. Therefore, in order to obtain a global effect that include the release of four opioid receptors, we chose EA with 2/100 Hz frequency so as to maximize the analgesic effect [46]. Our behavioral studies showed that EA treatment alleviated mechanical allodynia and thermal hyperalgesia induced by BCP, as previously reported [23, 24, 47].
A recent review summarizing the present knowledge about the role of P2X3Rs in acupuncture analgesia has shown that peripheral and central P2X3Rs are involved in EA analgesia [48]. Although P2X3R located in small or mediate size DRG neurons played an important role in peripheral sensitization of the various pain conditions including cancer pain [49], visceral pain [20], chronic neuropathic pain [19], and so on, the role of peripheral P2X3R in EA’s analgesia on BCP remains unknown. Our study complemented peripheral mechanism of P2X3R on BCP, demonstrating that EA exerts an analgesic effect in BCP by ameliorating the overexpression and functional activity of P2X3R in ipsilateral DRGs of BCP rats. First, western blot analyses showed EA suppressed up-regulation of total protein expression and DRG plasma membrane protein expression of P2X3R in ipsilateral L4-6 DRGs of BCP rats. It is well established that P2X3 receptors activated by transporting to the cell membrane and then exerted its function [11, 12]. Bao’s findings have shown calmodulin-dependent protein kinase II (CaMKII) played an important role in driving ligand-induced membrane trafficking of P2X3 receptor [50]. Wnt5b/Ryk has been reported to up-regulate membrane expression of P2X3 receptors by activating CaMKII in DRGs, leading to hyperalgesia in BCP mice, which disclosed that membrane translocation of P2X3 receptors was critical to the pathogenesis of BCP [51]. Obviously, targeting membrane translocation of P2X3 receptors will be an effective approach to suppress hyperalgesia in BCP models. This was in agreement with our findings about the analgesic effect of EA on BCP in a rat model. The similar effect of EA on peripheral P2X3R by suppressing DRG plasma membrane P2X3 receptor upregulation has been found in rats with diabetic neuropathic pain [52]. Second, immunofluorescence was conducted to determine changes in P2X3R positive cells of BCP rats treated by EA, which proved that EA reduced the expression of P2X3R positive cells in ipsilateral DRGs of BCP rats. Third, Ca2+ imaging proved that EA can inhibit calcium inflow induced by P2X3R agonist α,β-meATP, suggesting that EA exerts analgesic action through suppression of P2X3R activation in DRGs of BCP rats. Finally, we further validated it by pharmacological experiment in vivo. Intrathecal injection of α,β-meATP, which is binding to P2X3R in DRGs, reversed EA's analgesia on mechanical allodynia. Thus, our study provides the first evidence showing that EA can reduce overexpression and functional activity of P2X3R in DRGs from BCP rats.
To summarize, these data identify the increased expression of P2X3R in DRG contributes to the development of BCP, EA exerts an analgesic effect in BCP by ameliorating the overexpression and functional activity of P2X3R in ipsilateral DRGs of BCP rats. Peripheral P2X3 receptor might be an important therapeutic target for EA’s analgesia against peripheral sensitization of BCP. Therefore, these findings provide more evidence for the study of EA’s analgesia on cancer pain and its potential mechanisms.
Conclusions
Our study demonstrates that EA stimulation ameliorates mechanical allodynia and thermal hyperalgesia in rat model of MRMT-1-induced BCP. EA exerts analgesic effect on BCP by reducing the overexpression and functional activity of P2X3R in ipsilateral DRGs of BCP rats. Our work first demonstrates the critical and overall role of P2X3R in EA’ s analgesia against peripheral sensitization of MRMT-1-induced BCP and further supports EA as a potential therapeutic option for cancer pain in clinic.
Shu-xin Tian
received a bachelor’s degree and a master’s degree from Zhejiang Chinese Medical University, Hangzhou, China. She is currently working toward the Ph.D. degree in acupuncture and moxibustion with the Third School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, China. Her research interests include neurobiology and acupuncture research.

Author contribution
Yi Liang designed the experiments. Yang-qian Cai performed the animal experiments. Ming-hui Wu, Si-jia Zeng, Wen Wang, and You Zhou analyzed the data. Shu-xin Tian and Ren-yi Shi wrote the initial manuscripts. Ting Xu participated in figure preparations. All authors contributed to revising the article and approved the final manuscript.
Funding
This research was funded by National Natural Science Foundation of China (82174510 and 81674061), by Zhejiang Provincial Natural Science Funds (LGF21H270006), and by the Key Foundation of the Zhejiang Health Committee (2021ZZ017).
Data availability
All data included in this study are available upon request by contact with the corresponding author.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study was approved by the ethics committee of Zhejiang Chinese Medical University, Hangzhou, China (Approval No. IACUC-20181022–01).
Informed consent
We have obtained written informed consent from all study participants.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shu-xin Tian, Ting Xu, and Ren-yi Shi contributed equally to this work.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Edwards CM, Johnson RW. From good to bad: the opposing effects of PTHrP on tumor growth, dormancy, and metastasis throughout cancer progression. Front Oncol. 2021;11:644303. doi: 10.3389/fonc.2021.644303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwakwa KA, Sterling JA. Integrin αvβ3 signaling in tumor-induced bone disease. Cancers. 2017;9(7):84. doi: 10.3390/cancers9070084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan HL, Liu BL, Lin W, Zhang YQ. Modulation of Nav1.8 by lysophosphatidic acid in the induction of bone cancer pain. Neurosci Bull. 2016;32(5):445–454. doi: 10.1007/s12264-016-0060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H, Yan H, Li X, Liu J, Cao S, Huang B, Huang D, Wu L. inhibition of connexin 43 and phosphorylated NR2B in spinal astrocytes attenuates bone cancer pain in mice. Front Cell Neurosci. 2018;12:129. doi: 10.3389/fncel.2018.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng FF, Xu Y, Dan QQ, Wei L, Deng YJ, Liu J, He M, Liu W, Xia QJ, Zhou FH, Wang TH, Wang XY. Intrathecal injection of lentivirus-mediated glial cell line-derived neurotrophic factor RNA interference relieves bone cancer-induced pain in rats. Cancer Sci. 2015;106(4):430–437. doi: 10.1111/cas.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang E, Lee S, Yi MH, Nan Y, Xu Y, Shin N, Ko Y, Lee YH, Lee W, Kim DW. Expression of granulocyte colony-stimulating factor 3 receptor in the spinal dorsal horn following spinal nerve ligation-induced neuropathic pain. Mol Med Rep. 2017;16(2):2009–2015. doi: 10.3892/mmr.2017.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai WL, Bao YN, Fan JF, Ma B, Li SS, Zhao WL, Yu BY, Liu JH. Blockade of spinal dopamine D1/D2 receptor suppresses activation of NMDA receptor through Gαq and Src kinase to attenuate chronic bone cancer pain. J Adv Res. 2021;28:139–148. doi: 10.1016/j.jare.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Köles L, Fürst S, Illes P. Purine ionotropic (P2X) receptors. Curr Pharm Des. 2007;13(23):2368–2384. doi: 10.2174/138161207781368747. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Xu H, Zou L, Xie J, Wu H, Wu B, Yi Z, Lv Q, Zhang X, Ying M, Liu S, Li G, Gao Y, Xu C, Zhang C, Xue Y, Liang S. LncRNA uc.48+ is involved in diabetic neuropathic pain mediated by the P2X3 receptor in the dorsal root ganglia. Purinergic Signal. 2016;12(1):139–148. doi: 10.1007/s11302-015-9488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma W, Quirion R. Targeting cell surface trafficking of pain-facilitating receptors to treat chronic pain conditions. Expert Opin Ther Targets. 2014;18(4):459–472. doi: 10.1517/14728222.2014.887683. [DOI] [PubMed] [Google Scholar]
- 12.Robinson LE, Murrell-Lagnado RD. The trafficking and targeting of P2X receptors. Front Cell Neurosci. 2013;7:233. doi: 10.3389/fncel.2013.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guedon JG, Longo G, Majuta LA, Thomspon ML, Fealk MN, Mantyh PW. Dissociation between the relief of skeletal pain behaviors and skin hypersensitivity in a model of bone cancer pain. Pain. 2016;157(6):1239–1247. doi: 10.1097/j.pain.0000000000000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crockett SD, Greer KB, Heidelbaugh JJ, Falck-Ytter Y, Hanson BJ, Sultan S. American Gastroenterological Association Institute guideline on the medical management of opioid-induced constipation. Gastroenterology. 2019;156(1):218–226. doi: 10.1053/j.gastro.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 15.He Y, Guo X, May BH, Zhang AL, Liu Y, Lu C, Mao JJ, Xue CC, Zhang H. Clinical evidence for association of acupuncture and acupressure with improved cancer pain: a systematic review and meta-analysis. JAMA Oncol. 2020;6(2):271–278. doi: 10.1001/jamaoncol.2019.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paley CA, Bennett MI, Johnson MI. Acupuncture for cancer-induced bone pain? Evid Based Complement Alternat Med. 2011;2011:671043. doi: 10.1093/ecam/neq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HJ, Lee JH, Lee EO, Lee HJ, Kim KH, Kim SH, Lee KS, Jung HJ, Kim SH. Substance P and beta-endorphin mediate electro-acupuncture induced analgesia in mouse cancer pain model. J Exper Clin Cancer Res. 2009;28(1):102. doi: 10.1186/1756-9966-28-102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Tian XY, Bian ZX, Hu XG, Zhang XJ, Liu L, Zhang H. Electro-acupuncture attenuates stress-induced defecation in rats with chronic visceral hypersensitivity via serotonergic pathway. Brain Res. 2006;1088(1):101–108. doi: 10.1016/j.brainres.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Wang WS, Tu WZ, Cheng RD, He R, Ruan LH, Zhang L, Gong YS, Fan XF, Hu J, Cheng B, Lai YP, Zou EM, Jiang SH. Electroacupuncture and A-317491 depress the transmission of pain on primary afferent mediated by the P2X3 receptor in rats with chronic neuropathic pain states. J Neurosci Res. 2014;92(12):1703–1713. doi: 10.1002/jnr.23451. [DOI] [PubMed] [Google Scholar]
- 20.Weng ZJ, Wu LY, Zhou CL, Dou CZ, Shi Y, Liu HR, Wu HG. Effect of electroacupuncture on P2X3 receptor regulation in the peripheral and central nervous systems of rats with visceral pain caused by irritable bowel syndrome. Purinergic Signal. 2015;11(3):321–329. doi: 10.1007/s11302-015-9447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiang X, Wang S, Shao F, Fang J, Xu Y, Wang W, Sun H, Liu X, Du J, Fang J. Electroacupuncture stimulation alleviates CFA-induced inflammatory pain via suppressing P2X3 expression. Int J Mol Sci. 2019;20(13):3248. doi: 10.3390/ijms20133248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang Y, Gu Y, Shi R, Li G, Chen Y, Huang LM. Electroacupuncture downregulates P2X3 receptor expression in dorsal root ganglia of the spinal nerve-ligated rat. Mol Pain. 2019;15:1744806919847810. doi: 10.1177/1744806919847810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang Y, Du JY, Fang JF, Fang RY, Zhou J, Shao XM, Jiang YL, Chen YT, Fang JQ. Alleviating mechanical allodynia and modulating cellular immunity contribute to electroacupuncture’s dual effect on bone cancer pain. Integr Cancer Ther. 2018;17(2):401–410. doi: 10.1177/1534735417728335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Zhou Y, Cai Y, Wang S, Shao F, Du J, Fang J, Liu J, Shao X, Liu B, Fang J, Liang Y. Phosphoproteomic profiling of rat’s dorsal root ganglia reveals mTOR as a potential target in bone cancer pain and electro-acupuncture’s analgesia. Front Pharmacol. 2021;12:593043. doi: 10.3389/fphar.2021.593043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medhurst SJ, Walker K, Bowes M, Kidd BL, Glatt M, Muller M, Hattenberger M, Vaxelaire J, O'Reilly T, Wotherspoon G, Winter J, Green J, Urban L. A rat model of bone cancer pain. Pain. 2002;96(1–2):129–140. doi: 10.1016/s0304-3959(01)00437-7. [DOI] [PubMed] [Google Scholar]
- 26.Du J, Fang J, Chen Y, Wu S, Liang Y, Fang J. Parametric optimization of electroacupuncture against bone-cancer pain in rats and its intervention on mRNA expression of opioid receptor and precursor. Zhongguo Zhen Jiu. 2015;35(2):161–168. [PubMed] [Google Scholar]
- 27.Størkson RV, Kjørsvik A, Tjølsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Meth. 1996;65(2):167–172. doi: 10.1016/0165-0270(95)00164-6. [DOI] [PubMed] [Google Scholar]
- 28.Yin C, Liu B, Li Y, Li X, Wang J, Chen R, Tai Y, Shou Q, Wang P, Shao X, Liang Y, Zhou H, Mi W, Fang J, Liu B. IL-33/ST2 induces neutrophil-dependent reactive oxygen species production and mediates gout pain. Theranostics. 2020;10(26):12189–12203. doi: 10.7150/thno.48028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burnstock G. A unifying purinergic hypothesis for the initiation of pain. Lancet (London, England) 1996;347(9015):1604–1605. doi: 10.1016/s0140-6736(96)91082-x. [DOI] [PubMed] [Google Scholar]
- 30.Xiao Z, Ou S, He WJ, Zhao YD, Liu XH, Ruan HZ. Role of midbrain periaqueductal gray P2X3 receptors in electroacupuncture-mediated endogenous pain modulatory systems. Brain Res. 2010;1330:31–44. doi: 10.1016/j.brainres.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q, Zhao Y, Guo Y, Cao DY, Tang XD, Tian YL, Yao FR, Wang HS. Activation and sensitization of C and Adelta afferent fibers mediated by P2X receptors in rat dorsal skin. Brain Res. 2006;1102(1):78–85. doi: 10.1016/j.brainres.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 32.Nakatsuka T, Gu JG. P2X purinoceptors and sensory transmission. Pflugers Arch. 2006;452(5):598–607. doi: 10.1007/s00424-006-0057-6. [DOI] [PubMed] [Google Scholar]
- 33.Huang LY, Gu Y, Chen Y. Communication between neuronal somata and satellite glial cells in sensory ganglia. Glia. 2013;61(10):1571–1581. doi: 10.1002/glia.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou YL, Jiang GQ, Wei J, Zhang HH, Chen W, Zhu H, Hu S, Jiang X, Xu GY. Enhanced binding capability of nuclear factor-κB with demethylated P2X3 receptor gene contributes to cancer pain in rats. Pain. 2015;156(10):1892–1905. doi: 10.1097/j.pain.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu JX, Yuan XM, Wang Q, Wei W, Xu MY. Rho/ROCK acts downstream of lysophosphatidic acid receptor 1 in modulating P2X3 receptor-mediated bone cancer pain in rats. Mol Pain. 2016 doi: 10.1177/1744806916644929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilchrist LS, Cain DM, Harding-Rose C, Kov AN, Wendelschafer-Crabb G, Kennedy WR, Simone DA. Re-organization of P2X3 receptor localization on epidermal nerve fibers in a murine model of cancer pain. Brain Res. 2005;1044(2):197–205. doi: 10.1016/j.brainres.2005.02.081. [DOI] [PubMed] [Google Scholar]
- 37.Kaan TK, Yip PK, Patel S, Davies M, Marchand F, Cockayne DA, Nunn PA, Dickenson AH, Ford AP, Zhong Y, Malcangio M, McMahon SB. Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain. 2010;133(9):2549–2564. doi: 10.1093/brain/awq194. [DOI] [PubMed] [Google Scholar]
- 38.Hansen RR, Nasser A, Falk S, Baldvinsson SB, Ohlsson PH, Bahl JM, Jarvis MF, Ding M, Heegaard AM. Chronic administration of the selective P2X3, P2X2/3 receptor antagonist, A-317491, transiently attenuates cancer-induced bone pain in mice. Eur J Pharmacol. 2012;688(1–3):27–34. doi: 10.1016/j.ejphar.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Zhang RX, Li A, Liu B, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Electroacupuncture attenuates bone cancer pain and inhibits spinal interleukin-1 beta expression in a rat model. Anesth Analg. 2007;105(5):1482–1488. doi: 10.1213/01.ane.0000284705.34629.c5. [DOI] [PubMed] [Google Scholar]
- 40.Xu M, Fei Y, He Q, Fu J, Zhu J, Tao J, Ni C, Xu C, Zhou Q, Yao M, Ni H. Electroacupuncture Attenuates Cancer-Induced Bone Pain via NF-κB/CXCL12 Signaling in Midbrain Periaqueductal Gray. ACS Chem Neurosci. 2021;12(18):3323–3334. doi: 10.1021/acschemneuro.1c00224. [DOI] [PubMed] [Google Scholar]
- 41.Cheng RD, Tu WZ, Wang WS, Zou EM, Cao F, Cheng B, Wang JZ, Jiang YX, Jiang SH. Effect of electroacupuncture on the pathomorphology of the sciatic nerve and the sensitization of P2X3 receptors in the dorsal root ganglion in rats with chronic constrictive injury. Chin J Integr Med. 2013;19(5):374–379. doi: 10.1007/s11655-013-1447-1. [DOI] [PubMed] [Google Scholar]
- 42.Zhou YF, Ying XM, He XF, Shou SY, Wei JJ, Tai ZX, Shao XM, Liang Y, Fang F, Fang JQ, Jiang YL. Suppressing PKC-dependent membrane P2X3 receptor upregulation in dorsal root ganglia mediated electroacupuncture analgesia in rat painful diabetic neuropathy. Purinergic Signal. 2018;14(4):359–369. doi: 10.1007/s11302-018-9617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin JG, Chen WL. Acupuncture analgesia: a review of its mechanisms of actions. Am J Chin Med. 2008;36(4):635–645. doi: 10.1142/s0192415x08006107. [DOI] [PubMed] [Google Scholar]
- 44.Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361(1–3):258–261. doi: 10.1016/j.neulet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 45.Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26(1):17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 46.Sun S, Chen WL, Wang PF, Zhao ZQ, Zhang YQ. Disruption of glial function enhances electroacupuncture analgesia in arthritic rats. Exp Neurol. 2006;198(2):294–302. doi: 10.1016/j.expneurol.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Zhang RX, Li A, Liu B, Wang L, Xin J, Ren K, Qiao JT, Berman BM, Lao L. Electroacupuncture attenuates bone-cancer-induced hyperalgesia and inhibits spinal preprodynorphin expression in a rat model. Eur J Pain. 2008;12(7):870–878. doi: 10.1016/j.ejpain.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang Y, Yin HY, Liu J, Rubini P, Illes P. P2X receptors and acupuncture analgesia. Brain Res Bull. 2019;151:144–152. doi: 10.1016/j.brainresbull.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 49.Wu JX, Xu MY, Miao XR, Lu ZJ, Yuan XM, Li XQ, Yu WF. Functional up-regulation of P2X3 receptors in dorsal root ganglion in a rat model of bone cancer pain. Eur J Pain. 2012;16(10):1378–1388. doi: 10.1002/j.1532-2149.2012.00149.x. [DOI] [PubMed] [Google Scholar]
- 50.Chen XQ, Zhu JX, Wang Y, Zhang X, Bao L. CaMKIIα and caveolin-1 cooperate to drive ATP-induced membrane delivery of the P2X3 receptor. J Mol Cell Biol. 2014;6(2):140–153. doi: 10.1093/jmcb/mju011. [DOI] [PubMed] [Google Scholar]
- 51.He JJ, Wang X, Liang C, Yao X, Zhang ZS, Yang RH, Fang D. Wnt5b/Ryk-mediated membrane trafficking of P2X3 receptors contributes to bone cancer pain. Exp Neurol. 2020;334:113482. doi: 10.1016/j.expneurol.2020.113482. [DOI] [PubMed] [Google Scholar]
- 52.Fei X, He X, Tai Z, Wang H, Qu S, Chen L, Hu Q, Fang J, Jiang Y. Electroacupuncture alleviates diabetic neuropathic pain in rats by suppressing P2X3 receptor expression in dorsal root ganglia. Purinergic Signal. 2020;16(4):491–502. doi: 10.1007/s11302-020-09728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this study are available upon request by contact with the corresponding author.