Abstract
Background and Objective
Oxidative stress is one of the pathophysiological factors of pPROM and Vit. E being antioxidant may have preventive role. Study was conducted to estimate maternal serum vitamin E levels and cord blood oxidative stress markers in pPROM cases.
Methods
This was a case–control study including 40 pPROM cases and 40 controls. Maternal serum vitamin E levels were measured at recruitment. Cord blood was collected at delivery for estimation of telomere length and mtDNA copy number as oxidative stress markers. Levels were compared using student’s t test or Mann Whitney test. For correlation Pearson coefficient was used.
Results
Maternal serum vitamin E levels were normal in pPROM cases. Cord blood telomere length was more in pPROM than controls (428.99 ± 290.65 vs 322.35 ± 180.33) (p value 0.05). Cord blood mtDNA copy number was more in pPROM than controls (516.46 ± 443.55 vs 384.77 ± 328.27) (p value 0.13) though it was not significant. mtDNA copy number had negative correlation with Vit. E levels but it was statistically not significant (p value 0.49). There was no association of vitamin E levels with telomere length (p value 0.95).
Interpretation and Conclusion
pPROM was not associated with vitamin E deficiency. There was insignificant oxidative stress in cord blood as measured by mtDNA copy number but cord blood telomere length measurement did not detect any oxidative stress in pPPROM cases.
Keywords: mtDNA copy number, pPROM, Telomere length, Vitamin E
Introduction
Preterm (< 37 weeks of gestation) premature rupture of membranes (pPROM) is antecedent to 30–40% of all preterm births and can lead to significant perinatal morbidity and mortality [1]. The pathophysiology of pPROM is not clear. In pPROM, oxidative stress buildup can occur in response to insufficiency of antioxidants. Vitamin E being an antioxidant, prevents lipid peroxidation and protects the chorion and amnion from damage due to reactive oxygen species [2]. Inadequate dietary intake of vitamin E has been proposed as a cause of membrane damage in pPROM [3]. There are some studies on supplementation of vitamin E without assessing the vitamin E levels in pPROM [4, 5].
Cochrane review (2015) [6] revealed that routine supplementation of vitamin E in combination with other supplements during pregnancy did not improve materno-neonatal outcome. There was reduction in the number of placental abruption cases; however, it was unclear whether this finding was due to vitamin E or other agents used in the supplements. The review did not find any evidence for effect of vitamin E supplementation on pPROM.
Increased oxidative stress in pPROM and term births as compared to Preterm births has been reported in the literature [7] hence oxidative stress is proposed as a cause of premature senescence and aging of the membranes in pPROM. Telomeres are DNA–protein complexes located at the ends of chromosome and provide chromosomal stability throughout the cell cycle [8]. Telomeres are shortened with each cell division and once a critical shortening is attained, cell division stops and leads to cell senescence. Thus telomere length serves as a valid marker of biologic age [9]. Limited evidence shows that during fetal life there is decrease in the telomere length with advancing gestational age [10].
Oxidative stress accelerates the process of telomere length shortening; therefore, telomere length is considered as an oxidative stress (OS) biomarker [11]. Few studies have found that telomere length in pPROM cases is approximately equivalent to term births, thus suggesting premature aging of membranes in pPROM [12].
Mitochondia have multiple copies of small circular mitochondrial DNA (mtDNA ~ 16.5 kbp) [13]. mtDNA is sensitive to oxidative stress and is more prone to damage than nuclear DNA, as mtDNA lacks histone proteins and introns and has lower DNA repair activity [13]. It has been suggested that mitochondria compensate the mtDNA damage by increasing mtDNA copy number [14]; hence, mtDNA copy number has been suggested as an OS marker [15].
As there is no study in literature correlating maternal serum vitamin E levels with parameters of oxidative stress in pPROM, we planned this study to measure the maternal serum vitamin E levels and cord blood oxidative stress by telomere length and mtDNA copy number and to find association between them in pPROM cases.
Material and Methods
This was a case–control study conducted at Department of obstetrics and Gynecology in collaboration with Department of Biochemistry at University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi from November 2017 to April 2019.
Null hypothesis for the present study was that vitamin E has no role in prevention of pPROM, hence the level was expected to be same in both pPROM cases and controls. We used the 2SD of vitamin E levels in normal pregnant women (17.74 ± 2.16 µmol/l) [16] for calculating the sample size. Total sample size calculated was 80 with equal number of pregnant women, i.e., 40 in each group in order to detect an effect size 0.3 with type 1 error fixed at 5% and power of study being 80%. For sample size calculation based on oxidative stress markers the telomere length from the study by Menon et al. was taken into account (in pPROM 9962.3 ± 3124.1 and term births 9011.1 ± 2497) [12]. Considering null hypothesis statement of no difference between telomere length in pPROM and term births, in order to detect a difference in telomere length equivalent to effect size 0.33 the required sample size was 8 in each group. Regarding mtDNA copy number there is no similar study in published literature. Therefore, a convenient sample size of 40 in each group was taken.
Study Population
Eighty pregnant women were recruited between 26 and 34 gestational weeks. Forty of these patients had preterm premature rupture of membranes (pPROM group) and 40 had healthy pregnancies delivering at term gestation (control group). pPROM with identifiable causes—cervical insufficiency, uterine anomaly; history of interventions like amniocentesis, chorionic villus sampling, and medical disorders like diabetes mellitus, hypertension and heart disease were excluded from the study.
Sample Collection
1 ml of maternal blood was collected in serum tube from pPROM cases (at the time of diagnosis) and gestational age-matched controls (at the time of recruitment) and stored in aliquots at −20 °C for estimation of vitamin E levels.
3 ml of cord blood at the time of delivery from both the groups was collected in an EDTA tube, and stored in aliquots at −20 °C before estimation of telomere length and mtDNA copy number.
Estimation of Vitamin E levels
Maternal serum vitamin E levels were estimated by using commercially available ELISA kit (E-EL-0018) based on competitive-ELISA method. Serum vitamin E levels were measured in µg/ml.
Estimation of Telomere Length and mtDNA Copy Number
Cord blood was thawed at room temperature before DNA extraction. DNA was extracted from cord blood using a kit (QIAamp DNA Blood Mini Kit, Qiagen) as per the protocol given by the manufacturer. Telomere length was measured as per the method described by Cawthon et al. [17]. Relative telomere length was determined by quantitative real time PCR. Telomere (T) PCR and single copy gene (S), i.e., β-globin gene PCR was performed as a multiplex PCR set up using SYBR green PCR mix (FNZ416L DyNAmo Color Flash SYBR Green QPCR Kit). The primer sequence used was: telomere (forward),
5′- ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT-3′ telomere (reverse),
5′-TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA-3′ and
β-globin (forward)
5′-CGGCGGCGGGCGGCGCGGGCTGGGCGGCTTCATCCACGTT
CACCTTG- 3′
β globin (reverse)
5′-GCCCGGCCCGCCGCGCCCGTCCCGCCGGAGGAGAAgTCTGCCGTT- 3′.
The Ct values thus obtained were used to determine the T/S ratio which is a measure of relative telomere length.
The relative mitochondrial DNA copy number was estimated by real time PCR (dye based chemistry) using DNA obtained from cord blood, and mitochondrial specific primers and nuclear specific primers. PCR was performed using SYBR green PCR mix (FNZ416L DyNAmo Color Flash SYBR Green QPCR Kit). Sequence of primer used was mtDNA (forward) 5′-CAC CCA AGA ACA GGG TTT GT-3′;mtDNA (reverse) 5′- TGG CCA TGG GTA TGT TGT TA-3′ and nucDNA (forward) 5′- TGC TGT CTC CAT GTT TGA TGT ATC T-3′; nucDNA (reverse) 5;- TCT CTG CTC CCC ACC TCT AAG T-3′. The ratio of CT values gives relative mitochondrial DNA content which in turn reflects the mitochondrial DNA copy number. To determine the mitochondrial DNA content, relative to nuclear DNA the following equations were used: ΔCT = (nucDNA CT−mtDNA CT) and Relative mitochondrial content = 2 × 2ΔCT.
Data Analysis
SPSS 22.0 software was used for statistical analysis. Baseline characteristics, maternal serum vitamin E levels and cord blood oxidative stress markers, i.e., telomere length and mtDNA copy number being continuous variables continuous variables, were presented as mean ± SD or median and were compared between the groups by using student’s t test or Mann Whitney test. For categorical variables, data were presented as frequency % and for comparison Chi-square test or Fischer exact test was used wherever appropriate. To correlate levels of vitamin E with telomere length and mtDNA copy number Pearson coefficient was used. p value ≤ 0.05 was considered significant.
Observation and Results
Forty women with preterm premature rupture of membranes (26–34 gestational weeks) constituted the pPROM group and 40 gestational age-matched healthy women delivering at term constituted the control group. Baseline characteristics were similar between both the groups with respect to maternal age, BMI and gestational age at the time of recruitment. Most of the women in both the groups belonged to middle socioeconomic class. Parity was different as controls were not parity matched (Table 1).
Table 1.
Comparison of baseline characteristics in both the groups
| Baseline characteristic | pPROM N = 40 (mean ± SD) | Control N = 40 (mean ± SD) | p value |
|---|---|---|---|
| Age (years) | 26 ± 4.48 | 25.1 ± 3.43 | 0.345 |
| Parity | 0.85 ± 0.86 | 0.38 ± 0.58 | 0.009* |
| BMI (kg/m2) | 24.8 ± 2.2 | 24.0 ± 1.8 | 0.066 |
| Socioeconomic status | |||
| Upper middle | 10 (25%) | 17 (42.5%) | 0.316 |
| Lower middle | 20 (50%) | 18 (45%) | |
| POG (weeks) | 30.8 ± 2.04 | 30.4 ± 1.80 | 0.411 |
| Neonatal gestation (weeks) | 31.52 ± 2.09 | 39.24 ± 1.06 | < 0.001 |
| Mode of delivery | |||
| NVD | 35 (87.5%) | 38 (95%) | 0.153 |
| Instrumental | 0 | 1 (2.5%) | |
| LSCS | 5 (12.5%) | 1 (2.5%) | |
Maternal serum vitamin E levels were within normal range in pPROM cases and statistically not different from controls (21.36 ± 11.01 µg/ml v/s 19.54 ± 12.90 µg/ml) (P value 0.5). (Table 2).
Table 2.
Maternal serum vitamin E levels and cord blood telomere length and mtDNA copy number in both the groups
| pPROM (mean ± SD) | Control (mean ± SD) | p value | |
|---|---|---|---|
| Serum vitamin E levels (µg/ml) | 21.36 ± 11.01 | 19.54 ± 12.90 | 0.50 |
| Cord blood Telomere length (T/S ratio) | 428.99 ± 290.65 | 322.35 ± 180.33 | 0.05 |
| ≤ 32 weeks pPROM | 417.6 ± 250.3 | 322.35 ± 180.33 | 0.07 |
| > 32 weeks pPROM | 447.98 ± 356.82 | 322.35 ± 180.33 | 0.12 |
| Cord blood Mitochondrial DNA copy number | 516.46 ± 443.55 | 384.77 ± 328.27 | 0.13 |
Mean gestational age at the time of delivery in pPROM cases was 31.52 ± 2.09 (Table 1). Mode of delivery was similar in both the groups (Table 1).
Telomere length in cord blood leukocytes in pPROM cases (428.99 ± 290.65) was significantly more than controls (322.35 ± 180.33) (p value 0.05). Even in very preterm PROM (≤ 32 weeks) telomere length was more than controls (Table 2). Thus gestational age effect was seen on telomere length which was not modified by premature senescence due to oxidative stress.
Mitochondrial DNA (mtDNA) copy number in cord blood leukocytes in pPROM cases (516.46 ± 443.55) was more than controls (384.77 ± 328.27), although statistically not significant (p value 0.13) (Table 2), thus suggesting oxidative stress.
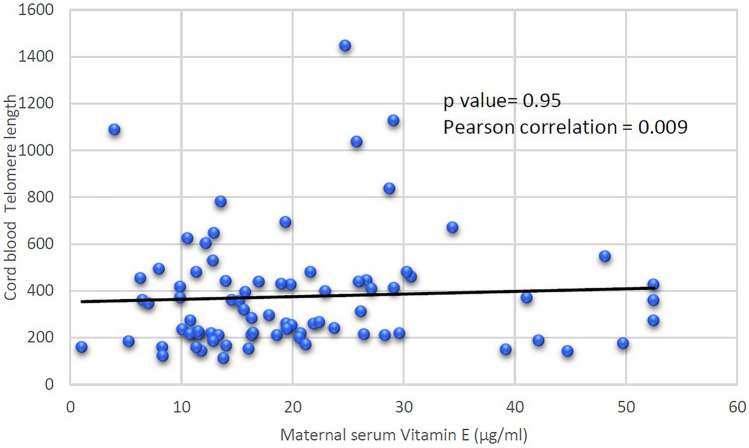
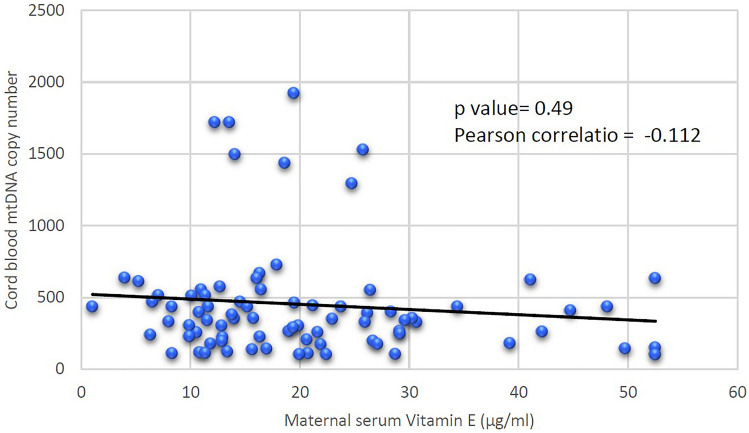
Maternal serum vitamin E levels had no association with cord blood telomere length (p value 0.95 Pearson coefficient 0.009) (Fig. 1). There was negative association of maternal serum vitamin E levels with cord blood mtDNA copy number (p value 0.49 Pearson coefficient −0.112), although statistically not significant (Fig. 2).
Fig. 1.
Association of maternal serum vitamin E levels with cord blood telomere length in pPROM
Fig. 2.
Association of maternal serum vitamin E levels with cord blood mtDNA copy number in pPROM
Discussion
pPROM is a disease of fetal membranes where premature activation of senescence predisposes them to rupture [17]. Oxidative stress causes damage to telomere length [18], mtDNA and other cellular structures in materno-fetal unit leading to premature senescence of membranes in pPROM [19]. Vitamin C and E being natural antioxidants scavenge reactive oxygen species and may prevents premature rupture of membranes. Normal serum vitamin E levels in adult population range from 5.5 to 17 µg/ml. It is deficient when level is < 3 µg/ml. There being paucity in literature regarding vitamin E levels in pPROM cases, we studied vitamin E levels in this population and its association with oxidative stress markers.
Our study revealed that mean maternal serum vitamin E levels were normal in both the groups (21.36 ± 11.01 µg/ml vs 19.54 ± 12.9 µg/ml) (p value 0.5). We could find only one study about vitamin E levels in pPROM in published literature wherein Mathews et al. have measured component of vitamin E (tocopherol-α and γ) in maternal serum and found that levels were not deficient in both the groups [20] similar to present study.
Telomere length is a novel marker to assess the premature aging which may be induced by oxidative stress. Telomeres are more vulnerable to oxidative damage due to guanine rich sequences [21]. After ∼60 cell divisions, as predicted by Hayflick [22] human telomeres become short, leading to senescence of membranes [8]. Oxidative stress accelerates the rate of telomere shortening and advances the cellular aging, hence loss of telomere function induces cell cycle arrest and senescence of membranes in pPROM [11].
Smaller is the telomere length more is oxidative stress. Relative telomere length was calculated by T/S ratio in our study and we found that cord blood relative telomere length was (428.99 ± 290.65) significantly more in pPROM cases than term births (322.35 ± 180.33 p value = 0.05).
Recently there have been few studies measuring telomere length in pPROM.
Menon et al. [12] reported that the cord blood leukocyte telomere length in pPROM > 32 weeks of gestation was marginally longer than term births. In same study, they also reported that cord blood leukocyte telomere length in pPROM ≤ 32 weeks of gestation was equivalent to term births indicating premature senescence of membranes in very preterm (< 32 weeks) PROM.
Ferrari et al. [23] studied the placental tissue telomere length in term births, preterm births (pPROM and PTBs with intact membranes) and unexplained stillbirths and found that telomere length was significantly shorter in stillbirths as compared to term and preterm births with intact membranes. They also reported that telomere length in stillbirths was similar to pPROM (< 37 weeks).
Both these studies reported shortening of telomere length in pPROM suggestive of oxidative stress, which was different from our study.
Lack of shortening of telomere length in our study indicates that either there was no oxidative stress induced premature senescence of membranes or increased oxidative stress (that might be present in mother with pPROM resulting in the damage to the membranes) was not reflected in newborns, probably because of compensatory mechanism of DNA damage repair in cord blood [24].
We also measured mtDNA copy number as oxidative stress marker, which is a novel and sensitive marker of oxidative stress. In present study cord blood leukocyte mtDNA copy number in pPROM cases (516.46 ± 443.55) was more than term births (384.77 ± 328.27) (p value = 0.13). Although not significant, this does indicates that there was mitochondrial compensation to oxidative stress induced mtDNA damage in pPROM cases.
There is only one study evaluating mtDNA copy number in cord blood which was to see the effect of lead exposure on oxidative stress. They found increased mtDNA copy number in cases with higher lead exposure and also found that pPROM cases were associated with increased mtDNA copy number [25].
In our study oxidative stress was reflected by increased mtDNA copy number (though not significant) and not by telomere length shortening. This may imply that mtDNA copy number is a better marker of oxidative stress (OS) in cord blood in pPROM.
Considering oxidative stress as a cause of pPROM, maternal serum vitamin E levels were correlated with these two oxidative stress markers. We found no association of maternal serum vitamin E (natural antioxidant) levels with cord blood leukocyte telomere length (pearson coefficient = 0.009 p value = 0.95). On the other hand decreasing maternal serum vitamin E levels were associated with oxidative stress as detected by increasing mtDNA copy number in pPROM cases, though statistically not significant (pearson coefficient −0.11 p value = 0.49). This indicates a probable protective role of vitamin E as an antioxidant against oxidative stress induced mtDNA damage in pPROM cases. In our knowledge, this was the first study on association of maternal serum vitamin E levels with cord blood oxidative stress markers, i.e., telomere length and mtDNA copy number in pPROM cases. In present study, sample size was adequate as calculated by telomere length and vitamin E levels. But for mtDNA copy number this was the first such study. On the basis of differences in the mtDNA copy number between both the groups, 138 sample size in each group is needed to detect significant oxidative stress. Therefore further studies are needed to find the association of vitamin E levels with oxidative stress markers in pPROM cases.
Conclusions
Maternal serum vitamin E levels were not deficient in both the cases and controls. There was evidence of oxidative stress induced mtDNA damage as depicted by increased mtDNA copy number in pPROM cases and it had a negative correlation with Vit. E levels thus suggesting a protective role of vitamin E for pPROM. But as measured by cord blood telomere length no oxidative stress was detected in pPROM cases. Therefore further studies are needed to understand the role of oxidative stress and potential benefit of Vit. E supplementation in pPROM cases.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Dr RK, Dr A S and Dr MM. The first draft of the manuscript was written by Dr RK, Dr RM and Dr AS. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding was received for the study.
Declarations
Conflict of interest
The authors declare that they have no potential conflict of interest.
Ethical Approval
The study was approved by the Institutional Ethical Committee—Human Research of University College of Medical Sciences and GTB Hospital, Delhi. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed Consent
Written voluntary informed consent to participate in the study was obtained from all the participants.
Footnotes
Rupa Kumari (MD), Senior Resident, Department of Obstetrics and Gynecology, University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, India; Amita Suneja (MD), Director Professor and Head, Department of Obstetrics and Gynecology, University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, India; Mohit Mehndiratta, Associate Professor, Department of Biochemistry, University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, India; Kiran Guleria (MD), Director Professor, Department of Obstetrics and Gynecology, University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, India; Rashmi Malik, Associate Professor, Department of Obstetrics and Gynecology, University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, India.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rupa Kumari, Email: rupalhmc@gmail.com.
Amita Suneja, Email: amita_suneja@yahoo.co.in.
Mohit Mehndiratta, Email: drmohitofficial@gmail.com.
Kiran Guleria, Email: kiranguleria@yahoo.co.in.
Rashmi Malik, Email: rashmi.malik2011@gmail.com.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plessinger MA, Woods JR, Jr, Miller RK. Pretreatment of human amnion-chorion with vitamins C and E prevents hypochlorous acid-induced damage. Am J Obstet Gynecol. 2000;183(4):979–985. doi: 10.1067/mob.2000.106676. [DOI] [PubMed] [Google Scholar]
- 3.Woods JR, Jr, Plessinger MA, Miller RK. Vitamins C and E: missing links in preventing preterm premature rupture of membranes? Am J Obstet Gynecol. 2001;185(1):5–10. doi: 10.1067/mob.2001.115868. [DOI] [PubMed] [Google Scholar]
- 4.Spinnato JA, Freire S, Pinto e Silva JL, Rudge MV, Martins-Costa S, Koch MA, et al. Antioxidant supplementation and premature rupture of the membranes: a planned secondary analysis. Am J Obstet Gynecol. 2008;199(4):433e1–8. doi: 10.1016/j.ajog.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gungorduk K, Asicioglu O, Gungorduk OC, Yildirim G, Besimoğlu B, Ark C. Does vitamin C and vitamin E supplementation prolong the latency period before delivery following the preterm premature rupture of membranes? a randomized controlled study. Am J Perinatol. 2014;31(3):195–202. doi: 10.1055/s-0033-1343774. [DOI] [PubMed] [Google Scholar]
- 6.Rumbold A, Ota E, Hori H, Miyazaki C. Crowther CA (2015) Vitamin E supplementation in pregnancy. Cochrane Database Syst Rev. 2015;9:CD004069. doi: 10.1002/14651858.CD004069.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kacerovsky M, Tothova L, Menon R, Vlkova B, Musilova I, Hornychova H, et al. Amniotic fluid markers of oxidative stress in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015;28(11):1250–1259. doi: 10.3109/14767058.2014.951628. [DOI] [PubMed] [Google Scholar]
- 8.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 9.Houben JM, Moonen HJ, van Schooten FJ, Hageman GJ. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med. 2008;44(3):235–246. doi: 10.1016/j.freeradbiomed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich U, Schwab M, Griese EU, Fritz P, Klotz U. Telomeres in Neonates: new insights in Fetal hematopoiesis. Pediatr Res. 2001;49:252–256. doi: 10.1203/00006450-200102000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/S0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 12.Menon R, Yu J, Basanta-Henry P, Brou L, Berga SL, Fortunato SJ, Taylor RN. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS ONE. 2012;7(2):e31136. doi: 10.1371/journal.pone.0031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carelli V, Maresca A, Caporali L, Trifunov S, Zanna C, Rugolo M. Mitochondria: Biogenesis and mitophagy balance in segregation and clonal expansion of mitochondrial DNA mutations. Int J Biochem Cell Biol. 2015;63:21–24. doi: 10.1016/j.biocel.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Meyer JN, Hartman JH, Mello DF. Mitochondrial toxicity. Toxicol Sci. 2018;162(1):15–23. doi: 10.1093/toxsci/kfy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong J, Cayir A, Trevisi L, Sanchez-Guerra M, Lin X, Peng C, et al. Traffic-Related air pollution, blood pressure, and adaptive response of Mitochondrial abundance. Circulation. 2016;133(4):378–387. doi: 10.1161/CIRCULATIONAHA.115.018802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suhail M, Patil S, Khan S, Siddiqui S. Antioxidant vitamins and lipoperoxidation in non-pregnant, pregnant, and gestational diabetic women: erythrocytes osmotic fragility profiles. J Clin Med Res. 2010;2(6):266–273. doi: 10.4021/jocmr454w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37(3):e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menon R, Richardson LS. Preterm prelabor rupture of the membranes: a disease of the fetal membranes. Semin Perinatol. 2017;41(7):409–419. doi: 10.1053/j.semperi.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longini M, Perrone S, Vezzosi P, Marzocchi B, Kenanidis A, Centini G, et al. Association between oxidative stress in pregnancy and preterm premature rupture of membranes. Clin Biochem. 2007;40(11):793–797. doi: 10.1016/j.clinbiochem.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Mathews F, Neil A. Antioxidants and preterm prelabour rupture of the membranes. BJOG. 2005;112(5):588–594. doi: 10.1111/j.1471-0528.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin C, Yang D. Human Telomeric G-Quadruplex structures and G-Quadruplex-interactive compounds. Methods Mol Biol. 2017;1587:171–196. doi: 10.1007/978-1-4939-6892-3_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayflick L. Mortality and immortality at the cellular level. A review Biochemistry (Mosc) 1997;62(11):1180–1190. [PubMed] [Google Scholar]
- 23.Ferrari F, Facchinetti F, Saade G, Menon R. Placental telomere shortening in stillbirth: A sign of premature senescence? J Matern Fetal Neonatal Med. 2016;29(8):1283–1288. doi: 10.3109/14767058.2015.1046045. [DOI] [PubMed] [Google Scholar]
- 24.Menon R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Front Immunol. 2014;5:567. doi: 10.3389/fimmu.2014.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Guerra M, Peng C, Trevisi L, Cardenas A, Wilson A, Osorio-Yáñez C, et al. Altered cord blood mitochondrial DNA content and pregnancy lead exposure in the progress cohort. Environ Int. 2019;125:437–444. doi: 10.1016/j.envint.2019.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]