Abstract
Giardia intestinalis is a pollutant of food and water, resistant to conventional disinfection treatments and its elimination requires effective methods action. Herein, mid-high-frequency ultrasound (375 kHz), which produces HO• and H2O2, was used as an alternative method of treatment to inactivate Giardia intestinalis cysts in water. The effect of ultrasound power (4.0, 11.2, 24.4 W) on the sonogeneration of radicals was tested, showing that 24.4 W was the condition most favorable to treat the parasite. The viability of the protozoan cysts was evaluated using the immunofluorescence technique and vital stains, showing this protocol was useful to quantify the parasite. The sonochemical method (at 375 kHz and 24.4 W) was applied at different treatment times (10, 20, and 40 min). A significant decrease in the protozoan concentration (reduction of 52.4% of viable cysts) was observed after 20 min of treatment. However, the extension of treatment time up to 40 min did not increase the inactivation. Disinfecting action was associated with attacks on the Giardia intestinalis cyst by sonogenerated HO• and H2O2 (which may induce structural damage, even the cell lysis). For future work is recommended to test combinations with UVC or Fenton process to enhance the inactivating action of this method.
-
•
Mid-high-frequency ultrasound produces HO• and H2O2 profitable to inactivate Giardia intestinalis.
-
•
Immunofluorescence technique and vital stains allowed us to quantify the parasite viability.
-
•
Giardia intestinalis cysts concentration decreased by 52.4% after only 20 min of sonication.
Keywords: Cysts, Parasite inactivation, Protozoan, Ultrasound, Water disinfection
Method name: Inactivation of Giardia intestinalis cysts in water using a sonochemical method based on mid-high-frequency waves
Graphical abstract
Specifications table
| Subject area: | Environmental Science |
| More specific subject area: | Water disinfection and alternative processes |
| Name of your method: | Inactivation of Giardia intestinalis cysts in water using a sonochemical method based on mid-high-frequency waves |
| Name and reference of original method: | Comparative Analysis of Ozone and Ultrasound Effect on the Elimination of Giardia spp. Cysts from Wastewater[1] |
| Resource availability: | All resources are detailed in this article |
Introduction
Giardia intestinalis (also named Giardia lamblia or Giardia duodenalis) is a flagellated protozoan, which lives in the small intestine of different vertebrates, including humans, and domestic and wild animals [2]. This is the second protozoan in frequency associated with outbreaks of waterborne diarrhea worldwide. Giardia intestinalis is the etiological agent of giardiasis, a disease with a worldwide distribution, which causes ∼300 million infections per year. A prevalence of 20–30% and 3–7% is reported in both developing and developed countries, respectively [3,4]. Giardia intestinalis is acquired mainly through the oro-fecal route by the consumption of food or water contaminated with cysts of this microorganism. Although direct person-to-person transmission (called the anthroponotic route) may also occur [2]. In fact, Giardia intestinalis is considered the second protozoan most frequently associated with waterborne diarrhea outbreaks worldwide after Cryptosporidium spp. [5,6].
Giardia intestinalis has a monoxenous life cycle with two parasitic stages: (1) trophozoite, the motile and feeding stage that is associated with damage in the intestinal epithelium; and (2) the cyst, which is the resistant and infective form. The trophozoite measures 12 to 20 µm long and 5 to 10 µm wide [3], it has two nuclei in the anterior part, 4 pairs of flagella that facilitate mobility, and a suction disk that allows the adhesion to the apical surface of the enterocytes [7]. The cyst has an ovoid shape, with a size of 8 to 12 µm long and 7 to 10 µm wide [3], containing four nuclei, a wall composed of two internal membranes, plus a filamentous outer membrane rich in N-acetyl galactosamine and structural proteins. These outer layers allow the parasite to resist adverse conditions in the environment [8]. Indeed, such morphological characteristics do Giardia intestinalis very recalcitrant to the action of classical methods such as filtration or chlorination [9]. Hence, efficient alternative disinfection methods to face Giardia intestinalis cysts are a need.
Currently, alternative methods for water treatment such as sonochemistry are being increasingly studied. Sonochemical systems belong to the advanced oxidation processes, which are based on the generation and use of strong disinfecting agents (e.g., radical species). Sonochemical treatments comprise the action of hydroxyl radicals generated by acoustic cavitation [10]. In this process, mid-high-frequency (200–1000 kHz) ultrasound waves [)))] lead to the cleavage of water vapor and oxygen molecules Eqs. (1)–((4)) [10], producing hydroxyl radicals that can inactivate microorganisms [11,12].
| H2O +))) → •H + •OH | (1) |
| O2 +))) → 2 •O | (2) |
| H2O + •O → 2 •OH | (3) |
| O2 + •H → •O + •OH | (4) |
Some previous works have reported the application of ultrasound-based processes to inactivate bacteria in water [13]. Also, protozoans like Cryptosporidium spp., even Giardia intestinalis, have been submitted to the effect of ultrasound waves [1,14,15]. However, those previous studies utilize low-frequency ultrasound (< 100 kHz), which has mechanical shaking forces as predominant effects more than the capability to produce radicals. Therefore, considering the concerns associated with Giardia intestinalis in water (especially related to health waterborne issues) and the capability of mid-high-frequency ultrasound to generate strong disinfecting agents (e.g., HO•), in this work the use of mid-high-frequency ultrasound (375 kHz) as an alternative method of treatment to inactivate Giardia intestinalis cysts is presented. Initially, the effect of ultrasound power on the sonogeneration of radicals was tested. Then, the viability of the protozoan cysts was evaluated using an immunofluorescence technique combined with a vital stain. Afterward, the sonochemical method was applied at different treatment times. Finally, the possible attacks of sonogenerated oxidizing species on Giardia intestinalis cells were discussed.
Equipment, materials, and analyses
The sonochemical treatment was performed at a Meinhardt Ultrasound reactor, containing 300 mL of the sample to be treated and operated at 375 kHz of frequency (a scheme of the reaction system is presented in Fig. 1). The temperature of the reactor was controlled at 20 ± 2 °C, using a Huber minichiller. The actual acoustic power inside the ultrasound reactor was determined calorimetrically [16]. All experiments were performed at least in triplicate.
Fig. 1.
Scheme of mid-high frequency ultrasound reaction system.
The accumulation of sonogenerated hydrogen peroxide was quantified through the iodometric/spectrophotometric method using potassium iodide (Merck) and ammonium heptamolybdate (Merk) as detailed in reference [17].
Giardia intestinalis cysts were collected from stool samples classified as positive for this protozoan from a veterinary diagnostic laboratory in Medellín (Approved by the Ethics Committee of Sede de Investigación Universitaria-SIU, Universidad de Antioquia. Act N° 130 of November 26, 2019). Samples were transported to the laboratory in an airtight screw cap container and were stored at 4 °C until the microscopic confirmation of the parasite cysts. Selected samples were concentrated with the MINI PARASEPⓇ SF – US kit, according to the fabricant instructions. After the concentration process, the number of cysts per mL was determined with a hemocytometer (Neubauer Improved Bright Line, Marienfeld). Cysts suspension was stored in refrigeration until its use in the sonochemical treatments. Milli-Q-water was inoculated with a proper volume of the cysts suspension to generate 300 mL of the sample (containing 104 cysts mL−1) to be treated by the sonochemical method.
The viability of Giardia intestinalis cysts was evaluated using a Propidium iodide (PI, obtained from Sigma) and 4′,6-diamidino-2-phenylindole (DAPI, purchased from Sigma) double staining. A commercial direct immunofluorescence kit Meridian (MerifluorⓇ) was used to evaluate changes in the cyst morphology. Briefly, 50 µl of each sample/control and 500 µl of Hanks' balanced salt solution (Sigma) were added to an Eppendorf tube, following incubation at 37 °C for 1 hour. Tubes were centrifuged at 14,000 rpm for 1 min and the resultant pellet was resuspended with 900 µL PBS (Sigma). Two additional washes with PBS were performed and the final pellet was resuspended in 100 µL PBS and vortexed. Then, 10 µL of PI and 10 µl of DAPI (5X) were added to the suspension obtained from each sample and control, and an incubation step at 37 °C for 2 h was done in the dark, followed by three washes with PBS. Next, samples were processed by the immunofluorescence assay according to the manufacturer´s instructions with some modifications. In brief: samples (50 µL) were placed on the slide spot and allowed to air dry. Methanol (Merck) was used to fix the sample. MAb-FITC (Merifluor-Meridian Bioscience, 50 µl) was applied and left to incubate for 25 min at 37 °C in a humid chamber. Slides were rinsed with 20X Wash Buffer (Merifluor-Meridian Bioscience) three times. Buffer excess was removed by tapping the long edge of the slide on a clean paper towel. After the slides were airdried, an additional DAPI staining step was performed. Slides were rinsed with 20X Wash Buffer three times. One drop of counterstain reagent was placed in each well. Slides were rinsed with 20X Wash Buffer three times. Then one drop of mounting medium was added to each well and a coverslip was applied. Slides were stored at 4 °C protected from light. Positive and negative controls were used in all the experiments.
Microscopic examination was carried out with an epifluorescent microscope (NikonⓇ) equipped with DIC optics. Examinations were carried out at magnifications of 400x. Cysts morphology was confirmed by direct immunofluorescence with FITC and vital DAPI staining. Oval cysts with a size between 8 and 18 µm in length and 5 to 15 µm in width were observed. The viability parameter was confirmed by vital PI staining. Three filters were used for each fluorochrome: FITC (495 to 519 nm), which stained the cyst with green color; DAPI (365 to 420 nm) with a blue stain; and PI (520 to 560 nm), with a red stain, that reflects the damage of the cyst wall. The percentage of PI-negative cysts (viable cysts) was determined in each experiment, counting more than 100 cysts identified with immunofluorescence. The viability percentage was calculated using Eq. (5).
| (5) |
A univariate analysis was performed to describe the effect of the ultrasound treatment (375 kHz) at different exposure times on cyst viability by estimating average measures (mean ± standard deviation). A nonparametric analysis was performed to assess Spearman's correlation. Normality was verified by the Shapiro-Wilk test. Statistically significant differences were explored with the one-factor parametric ANOVA test and Tuckey's post-hoc test. All statistical analyzes were performed using the statistical package IBM SPSSⓇ.
Determination of the sonochemical activity of the ultrasound reactor
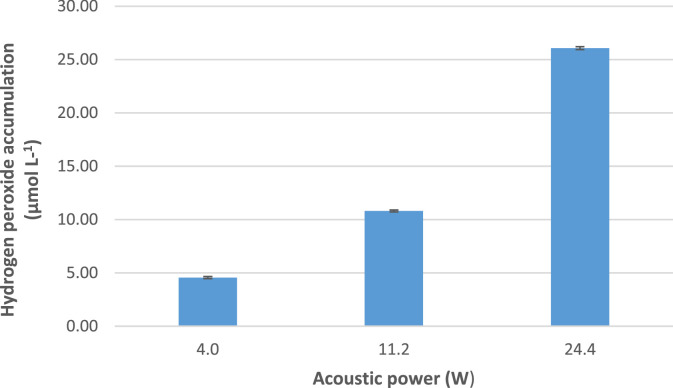
The sonochemical capability of the reactor to produce HO• as a function of the ultrasound power was assessed initially. We should mention that sonogenerated hydroxyl radicals can evolve into hydrogen peroxide (Eq. (6)). Thus, the measurement of H2O2 accumulation is used as an indirect indicator of sonochemical activity [17]. Fig. 2 shows that the H2O2 accumulation increased from 4.6 to 26.1 µmol L−1 (after 20 min of sonication) when the power augmented from 4.0 to 24.4 W, confirming the ability of the sonochemical reactor to produce HO•. It should be mentioned that when the acoustic power increases, the number of collapsing bubbles also increases, which results in the enhancement of the radicals formation, and subsequently the H2O2 accumulation is high [17].
| 2 HO• → H2O2 | (6) |
Fig. 2.
Power effect on the accumulation of sonogenerated H2O2. Experimental conditions: frequency: 375 kHz, sonication time: 20 min, volume: 300 mL.
Quantification of the viability of microorganisms
Before starting the sonochemical treatment of Giardia intestinalis, the concentration (in cysts mL−1) of this microorganism was quantified through double staining with PI and DAPI, and an immunofluorescence protocol. Fig. 3 shows the characteristics of the parasite cysts stained with PI, Giardia-antibodies labeled with fluorescein; and DAPI, confirming that through this protocol, it was possible to establish the viability of the Giardia intestinalis cysts.
Fig. 3.
Standardization of Giardia intestinalis quantification. A. Giardia intestinalis cysts stained with PI. B. Giardia intestinalis cysts stained with fluorescein-labeled antibodies. C. Giardia intestinalis, cysts stained with DAPI. Magnification of 1000x.
Inactivation of Giardia intestinalis cysts by the sonochemical action
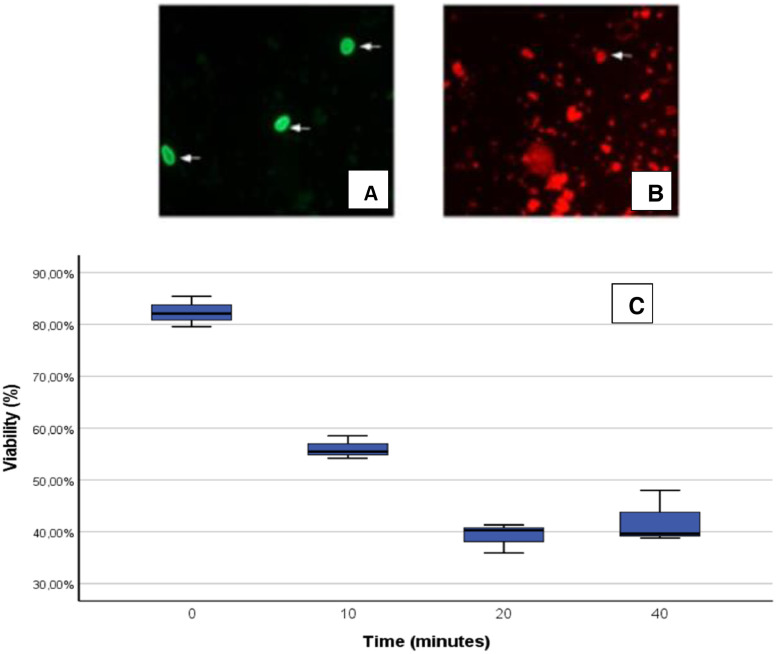
The Giardia intestinalis cysts were treated by the high-frequency ultrasound method. The viability percentage of the cysts was determined at 0, 10, 20, and 40 min of treatment. Fig. 4A and B show the microscopic results at 10 min of application of the sonochemical disinfection method, evidencing the capability of the analytical procedure to quantify the viability of Giardia cysts. In turn, Fig. 4C presents the viability evolution. At time 0 min the cysts viability percentage was 82.4 ± 2%. Meanwhile, at 10, 20, and 40 min of ultrasound treatment, the viability percentages were 56 ± 1%, 39.2 ± 2%, and 42.1 ± 5%, respectively. An inverse correlation (p = -0.864, p < 0.05) was observed between the treatment time and cyst viability, indicating that increasing the treatment time effectively reduced the cyst viability. Statistically significant differences were found between 0, 10, and 20 min of treatment (p < 0.05). However, when comparing the percentages of viability between times 20 and 40 min, no statistically significant differences were established (p = 0.725), which suggested that after 20 min there was no significant effect of the ultrasound treatment on the viability of the cysts. Indeed, the highest reduction in the cyst viability percentage was found at 20 min of treatment, which was statistically significant compared to the non-treated sample (i.e., 0 min) (p = 0.000).
Fig. 4.
Evolution of Giardia intestinalis cysts under treatment by the high-frequency ultrasound method. A. Treated Giardia intestinalis cysts (white arrows) by ultrasound for 10 min and marked with fluorescein antibodies. B. One of the positive cysts (white arrow) for PI staining (non-viable), magnification of 400x. C. Box and whisker plot of the evolution of Giardia intestinalis cysts during the ultrasound treatment at 375 kHz. The cysts viability percentages are shown as the median from triple assays.
The ultrasound method at a mid-high frequency (375 kHz) reduced the concentration of viable cysts of Giardia intestinalis from 8240 ± 200 to 3920 ± 200 cysts mL−1 after only 20 min of treatment (i.e., a reduction of 52.4%), indicating that this new method is also effective and can be used as an alternative to the low frequency (<100 kHz) systems [1,14]. Besides, our results showed that the time to achieve a significant decrease in the viability of the cysts corresponded to 20 min, due to the absence of statistical differences in the viability percentages between this time and 40 min of treatment. This finding is important for the operation of the ultrasound method since a short treatment time allows less electric energy consumption, and prolongation of the useful life of the reactor, which turns out profitable for such a method [18].
On the other hand, the reduction in the viability of Giardia intestinalis cysts could be associated with the attacks of sonogenerated reactive oxygen species (ROS) such as HO• and H2O2 (as proposed in Fig. 5). The first action of such ROS may cause damage to the wall structure of the protozoan. It is well known that ROS such as hydroxyl radical and hydrogen peroxide can oxidize membranes and cellular components [12], allowing a decrease in the Giardia intestinalis viability after the ultrasound treatment through a process called sonoporation. During the sonochemical action, pores can be formed in the cell wall and membranes, facilitating both extracellular and intracellular oxidation [18], thus, the loss of cell integrity and cyst death is plausible. However, at long treatment times, there is a large amount of non-viable cysts (even higher than the viable ones) that can also be attacked by ROS (which are non-selective and cannot differentiate viable Giardia intestinalis from the non-viable microorganisms), producing a competence that decreases the availability of ROS to act on the remaining viable cysts. Therefore, a plateau in the inactivation can be reached (as observed in Fig. 4 at 40 min of treatment). This fact could limit the feasibility in practice of this method. However, a strategy to achieve the complete reduction of the viability of cysts is the combination with other methods, which could be performed in future works. For instance, the addition of UVC light and/or ferrous ions to the sonochemical system (the sono-photo-Fenton method) [10,12], is a plausible option. The addition of UVC light could promote direct inactivating action on the parasite [19] plus the extra production of HO• by homolysis of sonogenerated hydrogen peroxide Eq. (7)). Besides, the presence of iron (II) and UV light in the ultrasound reaction system could promote in situ the photo-Fenton process (Eqs. (8) and (9), which also lead to the production of disinfecting radicals [10,12].
| H2O2 + UVC → 2HO• | (7) |
| Fe2++ H2O2 → Fe3+ + HO• + HO− | (8) |
| Fe3++ H2O + UVlight → Fe2++ HO• + H+ | (9) |
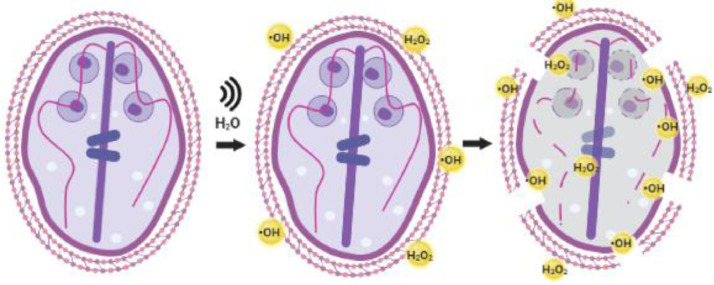
Fig. 5.
Schematic proposal of the mechanism of action of the sonochemical method on Giardia intestinalis cysts. The sonication of the H2O produces HO• and H2O2, which come into contact with the cysts, affecting the structure of their wall, leading to the loss of cell integrity, and the subsequent inactivation.
Complementary tests, to evaluate the infectivity of the cysts after treatment by the mid-high-frequency ultrasound method, are also recommended. Moreover, the leading role of hydroxyl radical or hydrogen peroxide in the inactivation of the Giardia intestinalis cysts, using specific scavengers of ROS or electron paramagnetic resonance spectroscopy (EPR) analyses, should be stated in further research works.
Finally, a comparison of our results with literature about the inactivation of cysts using ultrasound waves was done (Table 1). It can be noted that the previous works used ultrasound of low frequencies (22 or 42 kHz). From Table 1 can be highlighted that the method at the mid-high frequency (375 kHz) also achieved partial inactivation of the parasite, as obtained at low frequencies (e.g., 42 kHz). However, we should mention that at high frequencies (as in our research), the ultrasound method has mainly a chemical effect (i.e., action oxidant species), while at low frequencies a mechanical effect predominates [18]. Interestingly, the combination of low-frequency ultrasound (42 kHz) with ozone strongly accelerates the inactivation of the cysts. A similar enhancement of the inactivation of the microorganism could be expected, if the mid-high frequency ultrasound (375 kHz) is combined with ozone, UV light, and/or ferrous ions [10,12].
Table 1.
Comparison of inactivation of Giardia intestinalis cysts by diverse ultrasound-based methods.
| Reference | Matrix | Ultrasound frequency (kHz) |
Main results |
|---|---|---|---|
| [1] | Effluent of a wastewater treatment plant | 42 |
Reduction of 62.5% in the number of cysts with 60 min of treatment. The combination of ultrasound with ozonation leads to a reduction of 100% of parasites after 10 min of treatment |
| [14] | Solid waste landfill leachate and sewage sludge | 22 |
Ultrasound treatment performed for 20 min disintegrated solids and reduced the burden of cysts to undetectable levels. |
| This work | Distilled water inoculated with the protozoan | 375 kHz | Reduction of the concentration of viable cysts from 8240 ± 200 to 3920 ± 200 cysts mL−1 after 20 min of treatment (i.e., reduction of 52.4% of cysts at 20 min of sonication) |
Final remarks
The mid-high-frequency ultrasound system produces ROS (such as HO• and H2O2), making the sonochemical method an interesting alternative to low-frequency systems for the inactivation of Giardia intestinalis cysts. Indeed, a significant reduction in the viability of Giardia intestinalis cysts was achieved at short treatment times. It was proposed that sonogenerated ROS can attack the protozoan affecting the structure of their wall, leading to the loss of cell integrity but it is necessary to perform specific analysis to support the inactivation mechanism, evaluating parameters such as membrane peroxidation, wall proteins modifications, and DNA fragmentation. Future research could focus on the evaluation of ultrasound combined with ozone, or UV light and iron (sono-photo-Fenton methods), to enhance the effectiveness for eliminating Giardia intestinalis cysts in aqueous media completely, making the sonochemical method more viable in practice.
CRediT authorship contribution statement
Laura María Gómez-Quintero: Investigation, Methodology, Writing – original draft. Marlon Alexis Múnera-Marín: Investigation, Methodology, Writing – original draft. María Alejandra Urán-Serna: Investigation, Methodology, Writing – original draft. Efraím A. Serna-Galvis: Conceptualization, Methodology, Data curation, Formal analysis, Writing – review & editing. Ana Luz Galván-Diaz: Conceptualization, Supervision, Writing – review & editing, Resources, Funding acquisition. Ricardo A. Torres-Palma: Conceptualization, Resources, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Escuela de Microbiología Grant (Convocatoria para Apoyar Trabajos de Grado en Los Programas de Pregrado de la Escuela de Microbiología-2018), Universidad de Antioquia. We thank the School of Microbiology for providing us with the facilities and equipment for the development of this study, and the TestLab Veterinary Clinical Diagnostic Laboratory for providing us with the Giardia-positive samples. The authors from GIRAB acknowledge the support provided by Universidad de Antioquia UdeA through “Programa de Sostenibilidad” and by MINCIENCIAS COLOMBIA for the support provided through project No. 1115–852–69594 (PRO—CEC-AGUA). E. A. Serna-Galvis thanks his Ph.D. fellowship during July 2015-June 2019 (Convocation 647/2014).
Contributor Information
Ana Luz Galván-Diaz, Email: ana.galvan@udea.edu.co.
Ricardo A. Torres-Palma, Email: ricardo.torres@udea.edu.co.
Data availability
Data will be made available on request.
References
- 1.Marques Passos T., Moreira da Silva L.H., Marmo Moreira L., Amaro Zângaro R., da Silva Santos R., Barrinha Fernandes F., de Lima C.J., Barrinha Fernandes A. Comparative analysis of ozone and ultrasound effect on the elimination of Giardia spp. cysts from wastewater. Ozone Sci. Eng. 2014;36:138–143. doi: 10.1080/01919512.2013.864227. [DOI] [Google Scholar]
- 2.García-Montoya G.M., Botero-Garces J.H. Giardiasis in Colombia: a review of the current knowledge. Curr. Trop. Med. Rep. 2018;5:154–161. doi: 10.1007/s40475-018-0152-8. [DOI] [Google Scholar]
- 3.Leung A.K.C., Leung A.A.M., Wong A.H.C., Sergi C.M., Kam J.K.M. Giardiasis: an overview. Recent Pat. Inflamm. Allergy Drug Discov. 2019;13:134–143. doi: 10.2174/1872213X13666190618124901. [DOI] [PubMed] [Google Scholar]
- 4.Esch K.J., Petersen C.A. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin. Microbiol. Rev. 2013;26:58–85. doi: 10.1128/CMR.00067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karanis P., Kourenti C., Smith H. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J. Water Health. 2007;5:1–38. doi: 10.2166/wh.2006.002. [DOI] [PubMed] [Google Scholar]
- 6.Efstratiou A., Ongerth J.E., Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2011–2016. Water Res. 2017;114:14–22. doi: 10.1016/j.watres.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Einarsson E., Ma'ayeh S., Svärd S.G. An up-date on Giardia and giardiasis. Curr. Opin. Microbiol. 2016;34:47–52. doi: 10.1016/j.mib.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Aguilar-Díaz H., Carrero J.C., Argüello-García R., Laclette J.P., Morales-Montor J. Cyst and encystment in protozoan parasites: optimal targets for new life-cycle interrupting strategies? Trends Parasitol. 2011;27:450–458. doi: 10.1016/j.pt.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Bartelt L.A., Sartor R.B. Advances in understanding Giardia: determinants and mechanisms of chronic sequelae. F1000Prime Rep. 2015;7 doi: 10.12703/P7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montoya-Rodríguez D.M., Serna-Galvis E.A., Ferraro F., Torres-Palma R.A. Degradation of the emerging concern pollutant ampicillin in aqueous media by sonochemical advanced oxidation processes - parameters effect, removal of antimicrobial activity and pollutant treatment in hydrolyzed urine. J. Environ. Manag. 2020:261. doi: 10.1016/j.jenvman.2020.110224. [DOI] [PubMed] [Google Scholar]
- 11.Giannakis S., Ruales-Lonfat C., Rtimi S., Thabet S., Cotton P., Pulgarin C. Castles fall from inside: evidence for dominant internal photo-catalytic mechanisms during treatment of Saccharomyces cerevisiae by photo-fenton at near-neutral pH. Appl. Catal. B Environ. 2016;185:150–162. doi: 10.1016/j.apcatb.2015.12.016. [DOI] [Google Scholar]
- 12.Giannakis S. Analogies and differences among bacterial and viral disinfection by the photo-Fenton process at neutral pH: a mini review. Environ. Sci. Pollut. Res. 2018;25:27676–27692. doi: 10.1007/s11356-017-0926-x. [DOI] [PubMed] [Google Scholar]
- 13.Lin L., Wang X., Li C., Cui H. Inactivation mechanism of E. coli O157:H7 under ultrasonic sterilization. Ultrason. Sonochem. 2019;59 doi: 10.1016/j.ultsonch.2019.104751. [DOI] [PubMed] [Google Scholar]
- 14.Graczyk T.K., Kacprzak M., Neczaj E., Tamang L., Graczyk H., Lucy F.E., Girouard A.S. Occurrence of cryptosporidium and Giardia in sewage sludge and solid waste landfill leachate and quantitative comparative analysis of sanitization treatments on pathogen inactivation. Environ. Res. 2008;106:27–33. doi: 10.1016/j.envres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Abeledo-Lameiro M.J., Ares-Mazás E., Goméz-Couso H. Use of ultrasound irradiation to inactivate cryptosporidium parvum oocysts in effluents from municipal wastewater treatment plants. Ultrason. Sonochem. 2018;48:118–126. doi: 10.1016/j.ultsonch.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Kimura T., Sakamoto T., Leveque J.M., Sohmiya H., Fujita M., Ikeda S., Ando T. Standardization of ultrasonic power for sonochemical reaction. Ultrason. Sonochem. 1996;3:S157–S161. doi: 10.1016/S1350-4177(96)00021-1. [DOI] [Google Scholar]
- 17.Serna-Galvis E.A., Silva-Agredo J., Giraldo-Aguirre A.L., Torres-Palma R.A. Sonochemical degradation of the pharmaceutical fluoxetine: effect of parameters, organic and inorganic additives and combination with a biological system. Sci. Total Environ. 2015;524–525:354–360. doi: 10.1016/j.scitotenv.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 18.Matafonova G., Batoev V. Review on low- and high-frequency sonolytic, sonophotolytic and sonophotochemical processes for inactivating pathogenic microorganisms in aqueous media. Water Res. 2019;166 doi: 10.1016/j.watres.2019.115085. [DOI] [PubMed] [Google Scholar]
- 19.Einarsson E., Svärd S.G., Troell K. UV irradiation responses in Giardia intestinalis. Exp. Parasitol. 2015;154:25–32. doi: 10.1016/j.exppara.2015.03.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.