Abstract
There are obvious differences between egg yolks of different varieties. Additionally, boiled eggs, which are widely liked and consumed globally, are nutrient rich. However, they absorb water in the esophagus during swallowing, and this result in an uncomfortable sensation. Here, we determined the moisture content and distribution as well as the protein contents and properties of 4 varieties of thermogelled egg yolks. Among the varieties, Green Shelled thermogelled egg yolk showed the highest protein content and solubility. Additionally, the ionic, hydrogen, and disulfide bonds corresponding to Rhode Island Red thermogelled egg yolk samples were the weakest, while the hydrophobic interaction force corresponding to the Hetian Dahei (HD) egg yolk samples was the weakest. Further, the distribution of the moisture contents of the 4 varieties was significantly different (P < 0.05). HD egg yolk showed the highest moisture content, and its bound and immobile moisture contents were significantly higher than those of the other 3 varieties. Egg yolk moisture content also affected free amino acid content, which was the highest for HD egg yolk. Therefore, owing to its high moisture content, HD egg yolk was conducive for chewing and swallowing and given its high free amino acid content, it also had a more suitable taste and flavor. The results of this study provide a theoretical basis for the application of egg yolks in food processing.
Key words: egg yolk, thermogel, moisture, protein, intermolecular force
INTRODUCTION
As one of the world's most staple foods, eggs, and egg products play an important role in the global economy (Gao et al., 2021). Notably, egg yolk, which the most nutrient-dense part of an egg contains approximately 50% water, 30% lipid, 16% protein, and small amounts of carbohydrates and minerals (Wang et al., 2020). It also comprises a complex system that endows it with excellent functional properties, especially in relation to the formation and stabilization of gels and emulsions (Guilmineau et al., 2005). Boiled eggs are an indispensable source of nutrition and are frequently eaten as part of breakfast. Compared with scrambled and fried eggs, boiled eggs can retain the nutrients of fresh eggs to the greatest extent (Harlina et al., 2018).
Additionally, egg yolk is used in different applications as their technical characteristics are versatile. Under the induction of external conditions, the yolk forms a three-dimensional network structure with unique textural properties (Santipanichwong and Suphantharika, 2009). These network structures provide storage space for moisture, fat, flavors, and other food components (Zhao et al., 2021), and also help retain moisture and flavors in the yolk gel to provide the desired texture and oral sensation (Li et al., 2020). It has also been reported that egg yolk protein properties influence the formation of yolk gel. Specifically, during the gelation process, the secondary structure of the egg yolk protein is destroyed, and protein-protein, protein-lipid, and protein-water interactions alter the spatial structure of yolk proteins (Zhao et al., 2016). Simultaneously, yolk gel is also affected by various molecular forces, including ionic bonds, hydrogen bonds, hydrophobic interactions, and disulfide bonds, which maintain the gel structure (Chen et al., 2015). Li et al. (2018) demonstrated that hydrophobic interactions and disulfide bonds play a major role in yolk gel formation, while hydrogen and ionic bonds play a complementary role in this regard. The relative contributions of these different chemical bonds to the gel network vary with different protein properties and environmental conditions (Mourtzinos and Kiosseoglou, 2005). While most studies have focused on the textural properties of yolk gels, to the best of our knowledge, there have been no studies on different egg yolks varieties focusing on changes in egg yolk moisture content and distribution, intermolecular forces, and amino acid properties owing to thermal induction.
Moisture content and distribution affect the properties and swallowing sensation of thermogelled egg yolk (TEY) (Li et al., 2018a). Due to the loss of water during heating, TEY absorbs water in the mouth and esophagus during chewing and swallowing, making its swallowing difficult and inducing a choking sensation. Therefore, the moisture content and distribution of TEY have an important impact on the sensation associated with egg yolk swallowing. Different egg yolks varieties have different moisture protein (free amino acids; FAA), fat, and nutrient contents (Sun et al., 2017), which directly affect their quality as well as their associated oral sensations (Goto et al., 2021). Yolk substances can also exhibit differences in moisture content owing to changing in their spatial structures. Studies have demonstrated that genotype also has an extremely important influence on yolk gel texture (Franco et al., 2020). Therefore, choosing the right yolk for food processing based on hen breed is of great significance. Low-field nuclear magnetic resonance (NMR) has been widely used in the study of moisture distribution on several food items, including eggs, meat, and dairy products (Marcone et al., 2013), and its usefulness in this regard has been validated in several studies. Additionally, low-field NMR can be used to measure the moisture content and mobility of different parts of egg yolk (Zhang et al., 2015). However, reports on the moisture content of TEY are limited.
Therefore, to assess TEY moisture content, 4 laying hen breeds were selected for this study. The breeds included the Rhode Island Red (RIR) breed, which is a globally famous breed of high-yielding laying hens (Hays, 1955), and the Dwarf Black (DB), Hetian Dahei (HD), and Green Shelled (GS) breeds, which are native to China and occupy the largest share of the Chinese market. The sensory perception of the yolks was investigated via texture analysis, the content and distribution of moisture in the yolk samples were determined by measuring their water holding capacities and by performing low-field NMR. Additionally, the properties of the yolk proteins and the effects of factors that affect water content and water distribution were explored via the measurement of protein contents, intermolecular forces, sulfhydryl (SH) contents, and FAA contents. The results obtained will help to guide the selection of laying hens to ensure high TEY moisture and will also provide a theoretical basis for using different egg yolk varieties in food processing and other related applications.
MATERIALS AND METHODS
Research on live animals met the guidelines approved by the institutional animal care and use committee (IACUC).
Materials
Eggs lay within 24 h by HD, DB, RIR, and GS breeds were collected from Hebei Rongde Breeding Company. The eggs were randomly selected during sampling, and for this experiment, all the hens were housed in individual cages under the same conditions, and were fed the same diet throughout the experimental period. The bovine serum albumin (BSA > 98% pure) and bicinchonininc Acid (BCA) were obtained from Sigma-Aldrich, Co., Ltd. (St. Louis, MO). The 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) and β-mercaptoethanol were purchased from Aldrich (Sigma-Aldrich, Co., Ltd.). All other analytical grade chemicals were purchased from Solarbio Science & Technology Co., Ltd. (Beijing, China).
Sample Processing
The TEY pretreatment procedure was as follows: the eggs were added 1 min after water had boiled, and were thereafter, boiled for 5 min (100°C). Three minutes after turning off the heat, the eggs were taken out, and after breaking their shells, the TEY were manually separated from the egg whites and prepared for the experiments. In brief, after the eggs were broken, the whites were removed using a yolk separator. Thereafter, the removed yolks were then rubbed on filter papers to remove the remaining egg whites and lace. Finally, the yolk membrane was punctured, and the yolk liquid collected. The egg yolk water content of the 4 varieties is 47%, with no significant difference.
Texture Profile Analysis
A TA-XT2i texture profile analyzer (Texture Technologies, Scarsdale, NY) with a P50 probe was used to evaluate the texture characteristics the TEY samples. The speed of the analyzer was 2.0 mm/s before the test, 1.0 mm/s during the test, and 2.0 mm/s after the test, and the distance was 10.0 mm. The texture profile analysis (TPA) parameters were then obtained using the Texture Exponent software package of the analyzer.
Protein Content and Solubility Measurements
Using a previously reported method (Abugoch et al., 2008) with some modifications, 1-g yolk samples were diluted 15-fold with deionized water, and stirred magnetically for 10 min. Thereafter, the pH of the samples were adjusted to 7.0. In the next step, each sample was divided into 2 parts, and one part was centrifuged at 12,000 × g for 10 min at 25°C after which 200 µL of the supernatant and the uncentrifuged samples were analyzed using the BCA protein quantitative analysis kit to determine protein contents, with BSA as a standard. Protein solubility was expressed as the ratio of the protein content of the supernatant obtained after centrifugation to that of the uncentrifuged sample. The content in the supernatant is the total protein content.
Intermolecular Force Measurements
Five solutions were prepared as follows: 0.05 M sodium chloride (A); 0.6 M sodium chloride (B); a mixed solution of 0.6 M sodium chloride and 1.5 M urea (C); a mixed solution of 0.6 M sodium chloride and 8 M urea (D); and a mixed solution of 0.6 M sodium chloride, 8 M urea and 0.05 M β-mercaptoethanol (E). Thereafter, 0.2 g TEY samples of each variety were mixed with 1 mL of each of these 5 solutions. The mixtures were then homogenized in a homogenizer at 5,000 rpm for 1 min and allowed to stand for 1 h at 4°C. Thereafter, the samples were centrifuged at 10,000 rpm for 15 min, and protein concentrations in the supernatants were determined using the BCA protein quantitative assay kit. Each S4 fraction was dialyzed against S1 to avoid interference with β-mercaptoethanol. The differences in protein contents of solutions A and B, B and C, C and D, and D and E represented the ionic bonds, hydrogen bonds, hydrophobic interactions, and disulfide bonds in the samples, respectively.
Determination of Sulfhydryl Group Content
The free SH and surface SH contents of the yolk samples were determined using the Ellman method following the procedure reported by Hansen (Hansen and Winther, 2009). In brief, approximately 1.0-g yolk samples were dissolved in a 100 mL Tris-Gly buffer solution (0.86 M Tris, 0.09 M glycine, 4 mM EDTA; pH 8.0) to measure the free SH content, while an 8-M urea-Tris-Gly buffer solution was used to measure the surface SH content. Next, 5-mL sample solutions were extracted using a pipette, and 0.1 mL of DTNB (40 mg 5,5′-dithiobis(2-nitrobenzoic acid)) dissolved in 10 mL 0.1 M Tris-Glycine buffer (pH 8.0) was added followed by mixing. After 20 min of reaction at room temperature (25°C), the samples were centrifuged at 5,000 × g for 15 min, and the absorbance of the supernatant was measured at 412 nm using the buffer as a blank. The SH content was then calculated as SH = 73.53 × A412 × D/C, where A412 represents the absorbance of the sample, D represents the dilution number, and C represents the sample concentration in mg/mL. The unit of SH content is μmol/g. This procedure was performed in triplicates for each egg yolk variety.
Water Holding Capacity Measurement
A 5.0-g gel samples from each variety were accurately weighed and their masses were recorded as M1. Thereafter, samples were wrapped with 3 layers of filter paper and placed in 50-mL centrifuge tubes followed by centrifugation at 8,000 × g for 20 min at 4°C. Next, the centrifuged samples were weighed and their masses were recorded as M2. Finally, the water-holding capacity (WHC) of the samples was determined according to the expression: .
Low-Field NMR Spin-Spin Relaxation Measurements
The NMR T2 relaxation time was measured via low-field NMR (MesoMR23-060H-I, Suzhou Newmark Analytical Instruments Co., Suzhou, China) with a slight modification based on the method used by Sheng (Sheng et al., 2018). Specifically, a 1 × 1 × 1.2-cm gel column sample was collected using a gel sampler and loaded into an NMR tube. Before the test, the instrument system parameters were adjusted and then the sample was placed in the 60-mm diameter instrument probe coil to start the test. The decay of transverse magnetization was tested using a Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence, with the sequence parameters as follows: duration of 90° and 180° pulses, 21 and 42 μs, respectively; temperature, 32°C; echo time, 0.2 ms; and number of echoes, 2,500. The collected relaxation signals were analyzed using the InvFit inversion software that comes with the instrument, and the T2 distribution map of the sample was obtained.
FAA Content Measurement
To measure FAA contents, 1-g TEY samples were added to 5% sulfosalicylic acid to precipitate the proteins (1:4), which thereafter, were refrigerated at 4°C for 60 min, and then centrifuged using a high-speed refrigerated centrifuge at 18,000 rpm for 30 min. The thus obtained supernatant was collected, and after filtration using a 0.22 to 0.45 μm filtration membrane, was analyzed using an amino acid analyzer (L-8900 System, Hitachi Inc., Tokyo, Japan).
Data Analysis
The experimental design was completely randomized. Each sample was tested 3 times under the same conditions, and the average value was taken. Further, the data were presented as the mean ± standard deviation (SD). Statistical analysis was performed using SPSS software version 19.0 (IBM Corporation, Armonk, NY). Analysis of variance (ANOVA) was performed to determine differences among the means corresponding to the 4 groups, whiles pairs of means were compared by performing Duncan's multiple range tests. Statistical significance was set at P < 0.05.
RESULTS AND DISCUSSION
Texture Profile Analysis
Variety affects the sensory perception and texture of egg yolk (Antonelo et al., 2020). As an important indicator for evaluating the performance of food gels, TPA has attracted increasing attention from researchers and in this study, the results of the TPA of 4 varieties of TEY samples is illustrated in Table 1, from which it is evident that the 4 varieties showed significant differences in texture characteristics (P < 0.05). The hardness of the GS-derived sample was significantly higher than those of the samples derived from the other 3 varieties (P < 0.05). Further, the HD-derived sample showed the lowest springiness and cohesiveness. Notably, its springiness was significantly lower than those of the RIR- and GS-derived samples (P < 0.05), indicating that TEY variety significantly affects texture.
Table 1.
Texture characteristics of thermogelled egg yolk samples.
| HD | DB | RIR | GS | |
|---|---|---|---|---|
| Hardness | 275.93 ± 17.88a | 262.49 ± 18.12a | 261.62 ± 18.11a | 399.13 ± 35.89b |
| Springiness | 0.45 ± 0.05b | 0.55 ± 0.04ab | 0.61 ± 0.04a | 0.58 ± 0.04a |
| Cohesiveness | 0.25 ± 0.05a | 0.27 ± 0.04a | 0.37 ± 0.05a | 0.36 ± 0.07a |
Abbreviations: HD, Hetian Dahei; DB, Dwarf Black; RIR, Rhode Island Red; GS, Green Shelled.
Significant differences between mean values (P < 0.05).
Differences in Protein Content and Solubility
Protein solubility and protein content, which are important protein indicators, are related to protein function (Li et al., 2018a). Figure 1 illustrates the protein content and solubility of the 4 egg yolk varieties. From this figure, it is evident that the protein content of the egg yolk samples from the different varieties decreased significantly after heat treatment (Figure 1). Protein content directly affects protein solubility. Further, the protein content of the GS-derived sample was significantly higher than those of the samples derived from the 3 other breeds. The protein content of RIR TEY was significantly lower than those of the samples derived from the other 3 varieties, indicating that GS TEY had the high protein content, hence the highest nutritional value. These observations are consistent with those reported in previous studies, which have shown that different varieties contain different types and amounts of proteins (Zhou et al., 2021). Compared with liquid egg yolk samples, TEY samples show significantly reduced protein solubilities due to protein aggregation during heating, which makes them less soluble (Guerrero et al., 2004). Similarly, our results indicated that TEY protein solubility decreased with decreasing protein content. Specifically, the protein solubility of the GS-derived sample was the highest, while that corresponding to the RIR-derived sample was the lowest, indicating that variety also affects protein solubility.
Figure 1.
Changes in protein solubility and content before and after heating. (A) TEY protein content, (B) liquid egg yolk protein content, (C) TEY protein solubility, and (D) liquid egg yolk protein solubility. Abbreviation: TEY, thermogelled egg yolk.
Differences in Intermolecular Forces Between TEY Varieties
During heating, yolk gel formation is inevitably induced and maintained by certain intermolecular interactions, including covalent (disulfide) and non-covalent interactions (Wang et al., 2020). To elucidate TEY aggregation behavior, the interaction forces that exist between the 4 kinds of protein molecules were measured. As certain chemicals are added to protein gels, the constituent proteins that contribute to gel formation dissolve (Jiang and Xiong, 2013). Based on the different destructive effects of these different reducing agents on various interaction forces between protein molecules, 0.6 M NaCl can destroy ionic bonds, 1.5 M urea can destroy hydrogen bonds, 8 M urea can destroy hydrogen bonds and hydrophobic forces, and 0.05 M β- Mercaptoethanol can break disulfide bonds (Yang et al., 2019). Thus, in this study, we obtained the contents of ionic bonds, hydrogen bonds, hydrophobic interactions, and disulfide bonds in the TEY samples using these different solutions.
As illustrated in Figure 2, during the heating process, the contents of the intermolecular forces formed by proteins varied in the order: disulfide bonds > hydrophobic interactions > hydrogen bonds > ionic bonds. Disulfide bonds and hydrophobic interactions had the highest content among all the intermolecular interactions observed in the TEY samples, suggesting that disulfide bonds and hydrophobic interactions may be the main intermolecular forces in TEY. This result is consistent with those reported in previous studies (Li et al., 2021). After the egg yolk is heated, TEY formation is predominantly caused by covalent bonds. Among the 4 varieties, liquid egg yolk samples derived from RIR showed the lowest protein content (Figure 1), which possibly resulted in them showing the lowest ionic bond, hydrogen bond, and disulfide bond contents. It is also worth noting that the content of hydrophobic interactions were the lowest in HD-derived TEY, possibly because of its lower hydrophobic functional groups content (Mizuno and Lucey, 2007). The lower repulsive force of hydrophobic interactions can promote the formation of disulfide bonds (Felix et al., 2017); thus, the HD-derived TEY had higher disulfide bond content. These observations indicated that the TEY varieties showed differences in intermolecular forces, implying that variety influences the formation of interaction forces in yolk gel samples to varying degrees. The molecular force is formed between proteins. When the protein content is less, the molecular force of egg yolk is less. At the same time, less molecular force makes the yolk structure loose and its hardness low (Zhao et al., 2017).
Figure 2.
Thermogelled egg yolk intermolecular interaction forces. (A) Ionic bond, (B) hydrogen bond, (C) hydrophobic interaction force, and (D) disulfide bond.
Differences in Surface Sulfhydryl and Free Sulfhydryl Contents
SH significantly affects the functional properties of food proteins and plays an important role in the formation of specific structures in protein gels (Xu et al., 2018). It has also been reported that it is the most active functional group of proteins, as it forms disulfide bonds via oxidation (Omana et al., 2011). Additionally, disulfide bond formation contributes to the stability of egg yolk proteins and improves the textural properties of the proteins after heat treatment. Figure 3 illustrates the changes in the free SH and surface SH contents of the different varieties of liquid egg yolk and TEY. Notably, the TEY samples showed higher free SH contents than the liquid samples (Sun et al., 2021). The sulfhydryl group in egg yolk has strong polarity. These sulfhydryl groups absorb polar water molecules around protein molecules through hydrogen bonds and intermolecular interactions. Low content of sulfhydryl group leads to the reduction of hydrogen bonds in egg yolk (Hwang et al., 2007). Disulfide bond is the main force for globular protein to form gel, and the change of sulfhydryl group in yolk is mainly due to yolk protein (Chen et al., 2016). The HD- and GS-derived TEY samples showed decreased surface SH contents, while the DB- and RIR-derived samples showed increased surface SH contents. Additionally, the free SH content corresponding to the HD-derived liquid egg yolk sample was significantly higher than those of the liquid egg yolk samples obtained from the other 3 varieties. However, the corresponding HD-derived TEY sample showed a lower free SH content. This observation may be due to the oxidation of the SH group and the occurrence of an SH-disulfide exchange reaction (Visschers and de Jongh, 2005), which resulted in the formation of a new disulfide bond (Figure 2). Further, considering all the TEY samples, the lowest free SH content corresponded to the GS-derived sample, which rather showed the highest disulfide bond content (Figure 2). Thus, in the GS-derived sample, possibly, several SH groups were oxidized to form disulfide bonds, resulting in it showing the lowest free SH group content (Nguyen and Burley, 1984). The RIR-derived sample free showed a decreased SH group content, minimal disulfide bond formation, and part of the free SH groups was converted into surface SH groups via ionic bonds, resulting in the observed increase in the number of surface SH groups (Beveridge et al., 1974).
Figure 3.
Free sulfhydryl group and surface sulfhydryl group contents of four liquid and thermogelled egg yolk varieties. (A) Free sulfhydryl group and (B) surface sulfhydryl group.
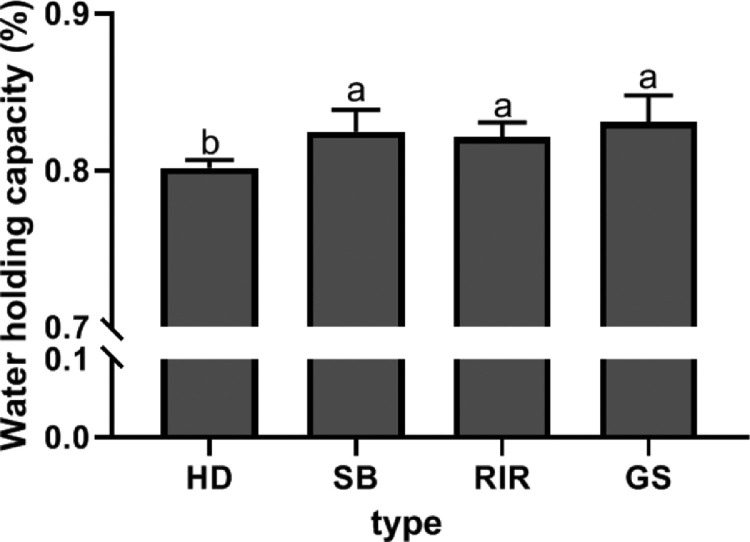
Differences in Water-Holding Capacity
WHC can effectively reflect the interaction between protein and water in the gel network structure (Khemakhem et al., 2019). As shown in Figure 4, the WHC of the HD-derived sample was significantly lower than those of the samples obtained from the other three breeds. This observation could be attributed the fact that the HD-derived sample showed the weakest hydrophobic interactions (Figure 2), and released moisture more easily after centrifugation. Reportedly, variety also affects the ionization and net charge of protein molecules, which in turn affect protein molecule interactions as well as the water binding ability of the protein (Deng et al., 2020). The decrease in WHC indicated that the TEY samples contain more water, which reduces their springiness and cohesiveness (Table 1). The reduction of sulfhydryl group content reduces the ability of protein to combine with water, which is beneficial to the release of water from egg yolk. As TEY passes through the mouth and esophagus, it absorbs the water in the mouth and esophagus, causing difficulties in swallowing as well as a poor oral sensation. The HD-derived TEY contained a lot more water than the other samples. Thus, it was associated with a lower level of water absorption in the mouth esophagus and was sensed as delicate and smooth.
Figure 4.
Water-holding capacities of four thermogelled egg yolk varieties.
Low-Field Nuclear Magnetic Resonance Analysis
The low-field NMR technology, which has advantages, including rapid detection, nondestructive to sample, and a low sample amount requirement, has been widely used in the field of food analysis (Liu et al., 2019), and reportedly, different relaxation times are indicative of different protein, water, and lipid contents (Liu et al., 2019). Therefore, this technology can be used to evaluate water retention in food and detect changes in moisture content in different food material types with great sensitivity (Marcone et al., 2013). Specifically, the low-field NMR T2 relaxation parameters corresponding to the four TEY varieties are shown in Table 2. The T2 spectral curves of the different egg yolk sample showed 3 proton peaks, namely, the T21, T22, and T23 peaks. This phenomenon is consistent with the results of a study conducted by Bao et al. (2020). T21, which had a short relaxation time (0.01–1 ms), represented water and lipid protons that were tightly bound to macromolecules; T22, with relaxation time of 4 to 140 ms, represented water and lipid protons that did not easily migrate; and T23, with a longer relaxation time (140–500 ms) represented more mobile water and lipid protons (Shao et al., 2016; Yang et al., 2016).
Table 2.
Low-field nuclear magnetic resonance results corresponding to thermogelled egg yolk samples.
| HD | DB | RIR | GS | |
|---|---|---|---|---|
| T21 | 0.33 ± 0.02a | 0.31 ± 0.01a | 0.31 ± 0.01a | 0.32 ± 0.03a |
| T22 | 19.06 ± 0.88a | 16.67 ± 1.35a | 16.67 ± 1.35a | 17.14 ± 1.56a |
| T23 | 254.49 ± 11.74a | 228.44 ± 10.82b | 228.44 ± 10.82b | 234.69 ± 10.82ab |
| PT21 | 420.19 ± 38.9a | 359.28 ± 3.22b | 365.14 ± 26.32b | 395.42 ± 20.34ab |
| PT22 | 6,599.39 ± 57.27a | 6,563.43 ± 92.42a | 6,456.84 ± 219.05a | 6,536.4 ± 85.22a |
| PT23 | 160.79 ± 6.7b | 183.73 ± 10.63a | 187.69 ± 12.5a | 185.9 ± 13.14a |
Abbreviations: HD, Hetian Dahei; DB, Dwarf Black; RIR, Rhode Island Red; GS, Green Shelled.
Significant differences between mean values (P < 0.05).
As illustrated in Table 2, the relaxation time of the TEY sample derived from the different breeds was different. Compared with the T21, T22, and T23 proton peaks corresponding to samples obtained from other 3 species, those corresponding to the HD-derived sample showed longer relaxation times, indicating that these protons (water and lipid) were less constrained and had more degrees of freedom (Xu et al., 2019). Additionally, the relaxation times of the T21 and T22 peaks corresponding to the HD-derived TEY may be due to the loose structure of the HD yolk particles and the larger cross-linking gap between the constituent protein molecules, giving water and lipids more space for movement, and resulting in less steric confinement and greater freedom for water and lipids (Xu et al., 2019). The longer relaxation time of the T23 peak corresponding to the HD-derived sample indicated larger voids between HD yolk particles, resulting in less restriction and a much greater degree of freedom for flowing water and lipids. Additionally, the T21, T22, and T23 peaks corresponding to the DB- and RIR-derived TEY samples showed similar relaxation times, indicating the existence of a few yolks with the same degrees of freedom for water and lipids. We also observed that the relaxation time trends of the T21, T22, and T23 peaks corresponding to the different varieties were the same. However, the relaxation times corresponding to the DB- and RIR-derived samples were the fastest, followed that of the GS-derived sample and then that of the HD-derived sample. This observation indicated that in the DB- and RIR-derived samples, GS-derived sample, and HD-derived sample, the water and lipid protons are closely bound by macromolecules, migrated less easily, and had more degrees of freedom (could flow more easily, affecting each other), respectively. The relaxation time of the T21 peak was delayed as the relaxation times of the T22 and T23 peaks (Au et al., 2016).
Peak areas (PT21, PT22, and PT23 for the relaxation times T21, T22, and T23, respectively) reflect the changes in water distribution in various states (Wang et al., 2004). The peak area corresponding to the HD-derived sample was the largest at T21 (Table 2), indicating that the protein molecules in HD showed the strongest hydration and the most bound water and lipids. Consistent with Figure 2, hydrophobic interactions were minimal in the HD-derived samples; thus, the ability of the associated protein molecules to bind water was enhanced, hence the increased PT21. Simultaneously, the increase in protein molecule-hydration capacity decreased the cohesiveness of the egg yolk (Table 1). After heat treatment, proteins aggregated via intermolecular forces to form gels with a network structure. The HD-sample showed a higher content of disulfide bonds and denser protein networks, and some non-flowable water was trapped, resulting in an increase in non-flowable water content as well as reduced free-flow water (Ma et al., 2021). Therefore, the PT22 corresponding to HD TEY was the largest, while its PT23 was the smallest. Thus, the relaxation spectra and relaxation parameters of the TEY varieties varied greatly, indicating that the 3 types of moisture contained in these different TEY varieties were quite different; this is consistent with the results of a previous study (Kruk et al., 2021).
Differences in Amino Acid Content
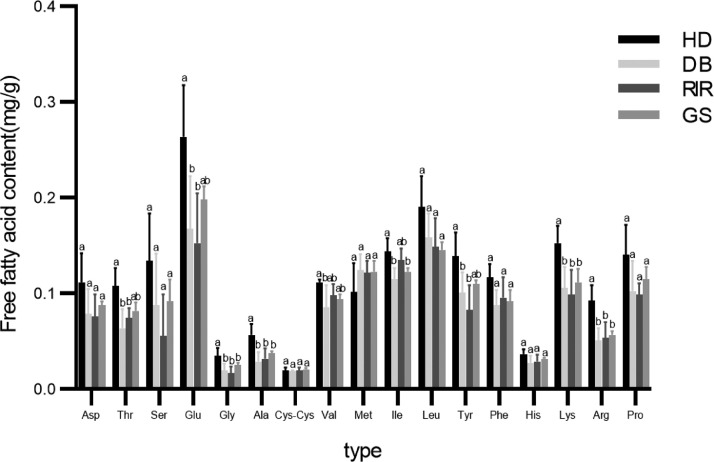
FAAs play a key role in the flavor development of foods subjected to heat treatment (Mottram, 2007) and greatly influence the flavor of yolks (Goto et al., 2021). Additionally, Gly and Ala are important parts of the umami of preserved eggs (Gao et al., 2021). It has also been observed that FAAs affect the senses, for example, beef affects the senses through Ala and Gly (Antonelo et al., 2020). Therefore, to explore the effect of amino acids on TEY, the FAA contents of the TEY samples were determined (Figure 5). In this regard, a total of 17 FAAs, of which nine were significantly different among the 4 TEY varieties, were identified. This indicated that the FAA contents of the TEY samples were controlled by genotype, which is consistent with the results of previous studies (Goto et al., 2022). The composition of protein is amino acid. The decrease of protein content may be the reason for the increase of free amino acid.
Figure 5.
Contents of the free amino acids. Abbreviations: DB, Dwarf Black; GS, Green Shelled; HD, Hetian Dahei; RIR, Rhode Island Red.
Except for Met and Cys-Cys, HD-derived TEY showed the highest FAA content (Figure 5), indicating that it contained the freest amino acids. This may be because the peptide chain in the HD TEY was broken resulting in the release of FAAs. It has been also reported that the presence of FAAs enhances the umami taste of HD-derived egg yolk (Mori et al., 2020), and the presence of Ala and Gly has also been shown to decrease the cohesiveness of HD-derived TEY (Goto et al., 2022). Amino acids can be classified as polar and non-polar amino acids (Wu, 2021). The polar amino acid contents of the DB- and RIR-derived TEY samples were lower than their non-polar amino acid contents, while the opposite trend was observed for the HD- and GS-derived samples (Figure 6). This observation could be attributed to the moisture contents of the samples. The HD-derived sample showed the highest polar and non-polar amino acid contents, but its polar amino acid content was higher than its non-polar amino acid content. This difference was much higher than that observed for the other 3 varieties. Based on these findings (Figures 4 and 5), HD TEY had the highest water content, and as polar amino acids are soluble in water, their content increased in this sample. Additionally, given that Cys-Cys is the product formed following the oxidation of 2 cysteines to disulfide bonds through their side-chain SH groups, a higher Cys-Cys content led to an increase in disulfide bonds in GS TEY (Figure 2).
Figure 6.
Polar and non-polar amino acid contents of four varieties of thermogelled egg yolk samples.
In conclusion, there were significant differences in protein properties and moisture distribution among the four TEY varieties investigated in this study, that is, the hen varieties influenced the protein content, solubility, and intermolecular interactions in the TEY samples, and GS TEY showed the highest protein content and nutritional value. Further, the SH group contents of the different varieties of liquid egg yolk samples were also different and resulted in different disulfide bonds contents. Our results also indicated that hydrophobic interaction forces affected the moisture content of the TEY samples. This interaction force was the weakest in HD TEY, which showed the highest moisture content, and was associated with the highest bound and non-flowable water contents. Further, the increase in the water content of HD TEY contributed to the increase of in its FAA content. These findings demonstrated that the high protein content of GS TEY contributed to the improvement of its nutritional value, while the high moisture content of HD TEY made it conducive for chewing and swallowing. Further, the HD TEY, given its high FAA content, had a more suitable taste and flavor. This study however, had some limitations. First, only 4 TEY varieties investigated. Additionally, the substances that affect TEY moisture content were not sufficiently clarified, and the hydrophobicity of the TEY samples needs to be further investigated as well. Notwithstanding, this study provides a theoretical basis for the application of egg yolk in food products.
ACKNOWLEDGMENTS
This work was supported by the China Agriculture Research System (grant number CARS-40). Sponsors had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
DISCLOSURES
The authors declare that they have no conflict of interest.
REFERENCES
- Abugoch L.E., Romero N., Tapia C.A., Silva J., Rivera M. Study of some physicochemical and functional properties of quinoa (Chenopodium Quinoa Willd) protein isolates. J. Agrc. Food Chem. 2008;56:4745–4750. doi: 10.1021/jf703689u. [DOI] [PubMed] [Google Scholar]
- Antonelo D.S., Cônsolo N.R.B., Gômez J.F.M., Beline M., Goulart R.S., Corte R.R.P.S., Colnago L.A., Schilling M.W., Gerrard D.E., Silva S.L. Metabolite profile and consumer sensory acceptability of meat from lean Nellore and Angus × Nellore crossbreed cattle fed soybean oil. Food Res. Int. 2020;132 doi: 10.1016/j.foodres.2020.109056. [DOI] [PubMed] [Google Scholar]
- Au C., Wang T., Acevedo N.C. Development of a low resolution 1H NMR spectroscopic technique for the study of matrix mobility in fresh and freeze-thawed hen egg yolk. Food Chem. 2016;204:159–166. doi: 10.1016/j.foodchem.2016.02.085. [DOI] [PubMed] [Google Scholar]
- Bao Z., Kang D., Li C., Zhang F., Lin S. Effect of salting on the water migration, physicochemical and textural characteristics, and microstructure of quail eggs. LWT. 2020;132 [Google Scholar]
- Beveridge T., Toma S.J., Nakai S. Determination of sh- and ss-groups in some food proteins using Ellman's reagent. J. Food Sci. 1974;39:49–51. [Google Scholar]
- Chen Z., Li J., Tu Y., Zhao Y., Luo X., Wang J., Wang M. Changes in gel characteristics of egg white under strong alkali treatment. Food Hydrocolloid. 2015;45:1–8. [Google Scholar]
- Chen Z., Shi X., Xu J., Du Y., Yao M., Guo S. Gel properties of SPI modified by enzymatic cross-linking during frozen storage. Food Hydrocolloid. 2016;56:445–452. [Google Scholar]
- Deng C., Shao Y., Xu M., Yao Y., Wu N., Hu H., Zhao Y., Tu Y. Effects of metal ions on the physico-chemical, microstructural and digestion characteristics of alkali-induced egg white gel. Food Hydrocolloid. 2020;107 [Google Scholar]
- Felix M., Romero A., Rustad T., Guerrero A. Physicochemical, microstructure and bioactive characterization of gels made from crayfish protein. Food Hydrocolloid. 2017;63:429–436. [Google Scholar]
- Franco D., Rois D., Arias A., Justo J.R., Marti-Quijal F.J., Khubber S., Barba F.J., López-Pedrouso M., Lorenzo J.Manuel. Effect of breed and diet type on the freshness and quality of the eggs: a comparison between mos (Indigenous Galician Breed) and Isa brown hens. Foods (Basel, Switzerland) 2020;9:342. doi: 10.3390/foods9030342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Hu X., Li R., Zhao Y., Tu Y., Zhao Y. Screening of characteristic umami substances in preserved egg yolk based on the electronic tongue and UHPLC-MS/MS. LWT. 2021;152 [Google Scholar]
- Goto T., Ohya K., Takaya M. Genotype affects free amino acids of egg yolk and albumen in Japanese indigenous breeds and commercial Brown layer chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Shimamoto S., Takaya M., Sato S., Takahashi K., Nishimura K., Morii Y., Kunishige K., Ohtsuka A., Ijiri D. Impact on genetic differences among various chicken breeds on free amino acid contents of egg yolk and albumen. Sci. Rep.-Uk. 2021;11:2270. doi: 10.1038/s41598-021-81660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero A., Carmona J., Martinez I., Cordobes F., Partal P. Effect of pH and added electrolyte on the thermal-induced transitions of egg yolk. Rheol. Acta. 2004;43:539–549. [Google Scholar]
- Guilmineau F., Krause I., Kulozik U. Efficient analysis of egg yolk proteins and their thermal sensitivity using sodium dodecyl sulfate polyacrylamide gel electrophoresis under reducing and nonreducing conditions. J. Agr. Food Chem. 2005;53:9329–9336. doi: 10.1021/jf050475f. [DOI] [PubMed] [Google Scholar]
- Hansen R.E., Winther J.R. An introduction to methods for analyzing thiols and disulfides: reactions, reagents, and practical considerations. Anal. Biochem. 2009;394:147–158. doi: 10.1016/j.ab.2009.07.051. [DOI] [PubMed] [Google Scholar]
- Harlina P.W., Ma M., Shahzad R., Gouda M.M., Qiu N. Effect of clove extract on lipid oxidation, antioxidant activity, volatile compounds and fatty acid composition of salted duck eggs. J. Food Sci. Technol. 2018;55:4719–4734. doi: 10.1007/s13197-018-3367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays F.A. Breeding Rhode Island reds for viability*. Poult. Sci. 1955;34:904–908. [Google Scholar]
- Hwang J., Lai K., Hsu K. Changes in textural and rheological properties of gels from tilapia muscle proteins induced by high pressure and setting. Food Chem. 2007;104:746–753. [Google Scholar]
- Jiang J., Xiong Y.L. Extreme pH treatments enhance the structure-reinforcement role of soy protein isolate and its emulsions in pork myofibrillar protein gels in the presence of microbial transglutaminase. Meat Sci. 2013;93:469–476. doi: 10.1016/j.meatsci.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Khemakhem M., Attia H., Ayadi M.A. The effect of pH, sucrose, salt and hydrocolloid gums on the gelling properties and water holding capacity of egg white gel. Food Hydrocolloid. 2019;87:11–19. [Google Scholar]
- Kruk D., Florek-Wojciechowska M., Oztop M., Ilhan E., Wieczorek Z. Water dynamics in eggs by means of nuclear magnetic resonance relaxometry. J. Magn. Reson. 2021;327 doi: 10.1016/j.jmr.2021.106976. [DOI] [PubMed] [Google Scholar]
- Li J., Ma J., Wagar E.A., Liang D., Meng Q.H. A rapid ultra-performance LC-MS/MS assay for determination of serum unbound fraction of voriconazole in cancer patients. Clin. Chim. Acta. 2018;486:36–41. doi: 10.1016/j.cca.2018.07.022. [DOI] [PubMed] [Google Scholar]
- Li J., Wang J., Zhai J., Gu L., Su Y., Chang C., Yang Y. Improving gelling properties of diluted whole hen eggs with sodium chloride and sodium tripolyphosphate: study on intermolecular forces, water state and microstructure. Food Chem. 2021;358 doi: 10.1016/j.foodchem.2021.129823. [DOI] [PubMed] [Google Scholar]
- Li J., Xu L., Su Y., Chang C., Yang Y., Gu L. Flocculation behavior and gel properties of egg yolk/κ-carrageenan composite aqueous and emulsion systems: effect of NaCl. Food Res. Int. 2020;132 doi: 10.1016/j.foodres.2020.108990. [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu Y., Han K., Cai Y., Ma M., Tong Q., Sheng L. Effect of nano-TiO2 on the physical, mechanical and optical properties of pullulan film. Carbohyd. Polym. 2019;218:95–102. doi: 10.1016/j.carbpol.2019.04.073. [DOI] [PubMed] [Google Scholar]
- Ma Z., Ma Y., Wang R., Chi Y. Influence of antigelation agents on frozen egg yolk gelation. J. Food Eng. 2021;302 [Google Scholar]
- Marcone M.F., Wang S., Albabish W., Nie S., Somnarain D., Hill A. Diverse food-based applications of nuclear magnetic resonance (NMR) technology. Food Res. Int. 2013;51:729–747. [Google Scholar]
- Mizuno R., Lucey J.A. Properties of milk protein gels formed by phosphates. J. Dairy Sci. 2007;90:4524–4531. doi: 10.3168/jds.2007-0229. [DOI] [PubMed] [Google Scholar]
- Mori H., Takaya M., Nishimura K., Goto T. Breed and feed affect amino acid contents of egg yolk and eggshell color in chickens. Poult. Sci. 2020;99:172–178. doi: 10.3382/ps/pez557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram D.S. The Maillard Reaction: Source of Flavour in Thermally Processed Foods. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability. Springer Berlin; Heidelberg: 2007. pp. 269–283. [Google Scholar]
- Mourtzinos I., Kiosseoglou V. Protein interactions in comminuted meat gels containing emulsified corn oil. Food Chem. 2005;90:699–704. [Google Scholar]
- Nguyen T., Burley R.W. Studies on the apoproteins of the major lipoprotein of the yolk of hen's eggs. v. protein sulfhydryl groups: accessibility in the lipoprotein and the effect of temperature. Australian J. Biol. Sci. 1984;37:7–16. [PubMed] [Google Scholar]
- Omana D.A., Plastow G., Betti M. The use of β-glucan as a partial salt replacer in high pressure processed chicken breast meat. Food Chem. 2011;129:768–776. doi: 10.1016/j.foodchem.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Santipanichwong R., Suphantharika M. Influence of different β-glucans on the physical and rheological properties of egg yolk stabilized oil-in-water emulsions. Food Hydrocolloid. 2009;23:1279–1287. [Google Scholar]
- Shao J., Deng Y., Song L., Batur A., Jia N., Liu D. Investigation the effects of protein hydration states on the mobility water and fat in meat batters by LF-NMR technique. LWT - Food Sci. Technol. 2016;66:1–6. [Google Scholar]
- Sheng L., Li P., Wu H., Liu Y., Han K., Gouda M., Tong Q., Ma M., Jin Y. Tapioca starch-pullulan interaction during gelation and retrogradation. LWT. 2018;96:432–438. [Google Scholar]
- Sun C., Liu J., Li W., Xu G., Yang N. Divergent proteome patterns of egg albumen from domestic chicken, duck, goose, turkey, quail and pigeon. Proteomics. 2017;17 doi: 10.1002/pmic.201700145. [DOI] [PubMed] [Google Scholar]
- Sun Y., Wang Q., Jin H., Li Z., Sheng L. Impact of ozone-induced oxidation on the textural, moisture, micro-rheology and structural properties of egg yolk gels. Food Chem. 2021;361 doi: 10.1016/j.foodchem.2021.130075. [DOI] [PubMed] [Google Scholar]
- Visschers R.W., de Jongh H.H.J. Disulphide bond formation in food protein aggregation and gelation. Biotechnol. Adv. 2005;23:75–80. doi: 10.1016/j.biotechadv.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Wang R., Ma Y., Ma Z., Du Q., Zhao Y., Chi Y. Changes in gelation, aggregation and intermolecular forces in frozen-thawed egg yolks during freezing. Food Hydrocolloid. 2020;108 [Google Scholar]
- Wang X., Choi S., Kerr W.L. Water dynamics in white bread and starch gels as affected by water and gluten content. LWT - Food Sci. Technol. 2004;37:377–384. [Google Scholar]
- Xu L., Zhao Y., Xu M., Yao Y., Wu N., Du H., Tu Y. Changes in physico-chemical properties, microstructure, protein structures and intermolecular force of egg yolk, plasma and granule gels during salting. Food Chem. 2019;275:600–609. doi: 10.1016/j.foodchem.2018.09.078. [DOI] [PubMed] [Google Scholar]
- Wu G. Amino acids: chemistry and classification, Reference Module in Food Science, Elsevier; Amsterdam, Netherlands: 2021. [Google Scholar]
- Xu L., Zhao Y., Xu M., Yao Y., Nie X., Du H., Tu Y. Changes in aggregation behavior of raw and cooked salted egg yolks during pickling. Food Hydrocolloid. 2018;80:68–77. [Google Scholar]
- Yang Y., Wan M., Ma Y., Zhang W. An improved method for tool point dynamics analysis using a bi-distributed joint interface model. Int. J. Mech. Sci. 2016;105:239–252. [Google Scholar]
- Yang Y., Zhao Y., Xu M., Wu N., Yao Y., Du H., Liu H., Tu Y. Changes in physico-chemical properties, microstructure and intermolecular force of preserved egg yolk gels during pickling. Food Hydrocolloid. 2019;89:131–142. [Google Scholar]
- Zhang Z., Yang Y., Tang X., Chen Y., You Y. Chemical forces and water holding capacity study of heat-induced myofibrillar protein gel as affected by high pressure. Food Chem. 2015;188:111–118. doi: 10.1016/j.foodchem.2015.04.129. [DOI] [PubMed] [Google Scholar]
- Zhao J., Sun F., Li Y., Liu Q., Kong B. Modification of gel properties of soy protein isolate by freeze-thaw cycles are associated with changes of molecular force involved in the gelation. Process Biochem. 2017;52:200–208. [Google Scholar]
- Zhao Y., Feng F., Yang Y., Xiong C., Xu M., Tu Y. Gelation behavior of egg yolk under physical and chemical induction: a review. Food Chem. 2021;355 doi: 10.1016/j.foodchem.2021.129569. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Chen Z., Li J., Xu M., Shao Y., Tu Y. Formation mechanism of ovalbumin gel induced by alkali. Food Hydrocolloid. 2016;61:390–398. [Google Scholar]
- Zhou L., Yang F., Zhao M., Zhang M., Liu J., Marchioni E. Determination and comparison of phospholipid profiles in eggs from seven different species using UHPLC-ESI-Triple TOF-MS. Food Chem. 2021;339 doi: 10.1016/j.foodchem.2020.127856. [DOI] [PubMed] [Google Scholar]