Abstract
Tissue injury is a common clinical problem, which may cause great burden on patients' life. It is important to develop functional scaffolds to promote tissue repair and regeneration. Due to their unique composition and structure, microneedles have attracted extensive attention in various tissues regeneration, including skin wound, corneal injury, myocardial infarction, endometrial injury, and spinal cord injury et al. Microneedles with micro-needle structure can effectively penetrate the barriers of necrotic tissue or biofilm, therefore improving the bioavailability of drugs. The use of microneedles to deliver bioactive molecules, mesenchymal stem cells, and growth factors in situ allows for targeted tissue and better spatial distribution. At the same time, microneedles can also provide mechanical support or directional traction for tissue, thus accelerating tissue repair. This review summarized the research progress of microneedles for in situ tissue regeneration over the past decade. At the same time, the shortcomings of existing researches, future research direction and clinical application prospect were also discussed.
Keywords: Microneedles, Tissues regeneration, Drug delivery
Graphical abstract

1. Introduction
Tissue repair or regeneration is one of the most complicated biological process, and refers to the restoration of damaged tissue to a normal state with full functions [[1], [2], [3]]. This process usually consists of three sequential stages of inflammation, tissue formation and maturation, and involves kinds of cells including immune cells, epithelial cells, endothelial cells [[4], [5], [6]]. Although small injuries can be healed through the body's adjustment, special regenerative therapy is usually required for non-self-healable pathological defects or large-scale damages [[7], [8], [9]]. Every year, millions of people die from damage or failure of organs or tissues worldwide, which prompts researchers to invest a lot of energy to explore advanced regenerative therapy [[10], [11], [12]]. Recently, with the in-depth exploration of tissue repair mechanisms and the development of biomaterials, the concept of in situ tissue regeneration (ISTR) has been introduced.
ISTR refers to the use of engineering biomaterials carrying biological active molecules (drugs, growth factors, genes, polypeptides, and cells, etc.) to direct endogenous progenitor or stem cells to repair damaged tissues [13,14]. Compared with ex vivo systems, ISTR is relatively convenient and eliminates the need for harvested cells, making it more suitable for clinical translation [15,16]. For example, NeuraGen and Neurotube for nerve repair, INFUSE bone grafts for dental or orthopedic applications [13]. Due to the complexity of the internal environment and tissue structure, biomaterials applied for ISTR should have comprehensive characteristics. For example, skin repair materials are required to possess tissue regeneration bioactivity, antibacterial effect and anti-inflammatory ability [[17], [18], [19]]. Angiogenesis is preferred for regeneration of vascularized tissues or organs such as heart and muscle [20], but should be inhibited during regeneration of avascular tissues such as cartilage and cornea [13,21]. To date, scaffolds including nanoparticles, fibers, hydrogels, microneedles (MNs), sponges and 3D printed scaffolds prepared from polymers, ceramics, metals and composites materials have been extensively investigated for ISTR [13,16]. Among them, MNs for minimally invasive delivery of therapeutic agents have become an emerging technology to promote tissue repair and healing, providing a novel development opportunity and challenge for ISTR.
The MN is a novel drug delivery device consisting of a substrate (area of mm2 to cm2) and micro-needles with a height of tens of microns to several nanometers [[22], [23], [24], [25], [26]]. Compared with traditional drug delivery methods such as injection and oral administration, MNs can painlessly penetrate tissue barriers [27], thereby improving the bioavailability of the drug and patients' adaptability, and avoiding the liver's over-mediated metabolism [13,28]. And MNs can also extract tissue interstitial fluid to obtain disease-related biomarkers [[29], [30], [31]]. As a result, MNs have been widely used to treat and detect various diseases, including skin cancer, infections, diabetes, obesity, and ocular diseases [[32], [33], [34], [35], [36], [37], [38]]. In the field of regenerative medicine, there are two main application pathways of MNs. One is transdermal delivery of active substances with MNs to promote tissue regeneration. For example, hyaluronic acid (HA) MNs to enhance transdermal uptake of exosomes for Achilles tendon repair [39], polyvinyl alcohol (PVA) MNs loaded with carbonized wormwood and inflammatory factors for skeletal muscles repair [40], and PVA MNs loaded with platelet derived growth factor for tendon healing [41]. Another is to use MNs as the scaffold for ISTR to enhance the healing ability of damaged tissues [13,42,43], which is also the focus of the present review.
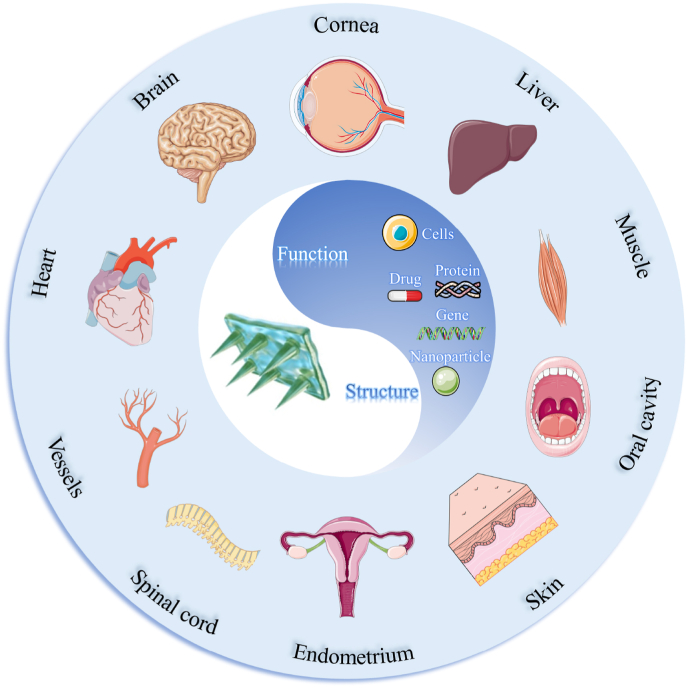
MNs can overcome necrotic tissue barriers or microbial membranes to deliver active substances minimally invasively to deep local tissues [44,45]. In addition, through the rational design of needle tips structure and basing, controlled release of active substances can be achieved conveniently and effectively. And MNs with special needle structures such as barbs can effectively close wounds and achieve rapid hemostasis or wound healing. Moreover, the basing of the MNs can act as sealant to prevent infection and tissue adhesions. In recent years, with the increasing understanding of the mechanisms of corneal healing, skin repair, nerve and heart regeneration and the rapid development of micro-/nano-technology, MNs-based ISTR has become a promising therapeutic strategy for tissue regeneration. MNs offer unique advantages in terms of improved patient convenience and drug bioavailability. And there are numerous reviews have discussed the preparation and classification of MNs and their applications in biomedical fields such as transdermal drug delivery, antibacterial, wound healing, and biosensing [42,44,46,47]. However, there are still few reviews that summarize and describe the principles and mechanisms of tissue repair using MNs. Herein, we systematically summarized and analyzed the major design concepts of MNs for ISTR and the applications of functional MNs in promoting the repair of damaged tissues such as skin wounds, corneal injuries, myocardial infarction (MI), and endometrial injuries (as shown in Fig. 1). And the shortcomings and clinical translation prospects of existing MNs-based tissue repair materials were also discussed.
Fig. 1.
Schematic illustration of functional MNs for ISTR.
2. Design principles of functional MNs for ISTR
2.1. Types of MNs
The first patent for MN was submitted by Gerstel and Place in 1976, but it was not until 1998 that the first paper on MN to enhance transdermal absorption of drugs was published [26,47]. Since then, the research on MNs has shown a spurt in development [48]. And the main methods for preparing MNs are: micromolding, mapping lithography, thermal drawing, electro-drawing, magnetorheological drawing lithography, additive manufacturing (3D printing) [42,[49], [50], [51], [52]]. Based on the mechanism of drug delivery, MNs can be classified as solid, hollow, coated, dissolving, and hydrogel-forming (as shown in Fig. 2) [53,54].
Fig. 2.
Representative five types of MNs (Reproduced with permission of Ref. [54]).
Solid MNs are mainly prepared from materials such as silicon, stainless steel or titanium and are commonly used as pretreatment strategies to form microchannels on the skin surface to improve drug diffusion efficiency [25,55,56]. However, poor biocompatibility, residual of sharp solid waste and uncontrollable drug dose limit their application in tissue regeneration [57,58]. The surface of coated MNs possesses drug coating formed by dip-coating or spraying methods, and the drugs are released after insertion [59]. The main disadvantages of coated MNs are the drug coating is easily wiped off during insertion and the drug loading capacity is insufficient [47,60]. Hollow MNs are usually used in combination with syringes, and the drugs are continuously delivered to deep tissues through a hole in the needle tip [54,59]. The main problems with hollow MNs are the risk of blockage or even blowout of the needle and the complexity of the preparation process [55,56]. Hollow and solid MNs can be viewed as miniature syringes that do not involve new drug dosage forms, so regulatory approval is more straightforward and quick. As a result, the U.S. National Food and Drug Administration (FDA) has approved two hollow MNs products for vaccination [49]. Currently, in the field of ISTR, hollow MNs and coated MNs are mainly used for corneal repair.
Dissolving, and hydrogel-forming MNs are the two types of MNs most frequently used in ISTR, due to their simple preparation process, flexible structural design or controlled drug release rate. Their common preparation technique is the solution-cast micromolding method, which uses a cavity prepared from polydimethylsiloxane (PDMS) as a master mold [42]. The materials used to prepare dissolving MNs are mainly dissolvable polymers (including HA, PVA, carboxymethyl cellulose (CMC), gelatin, sodium alginate (SA) and polyvinylpyrrolidone (PVP)) or biodegradable polymers (polycaprolactone (PCL), polylactic acid (PLA) and poly(lactic-co-glycolic acid) (PLGA)) [49,61]. After the dissolving MNs are inserted into the tissue, the drugs are released with the dissolution or degradation of the needle tip [55]. The selection of materials is critical for the properties of dissolving MNs such as mechanical strength, dissolution rate and tissue penetration. For example, compared with MNs prepared by SA, CMC and gelatin, HA MNs possess a faster dissolution rate, better mechanical strength and less shrinkage [62]. And MNs prepared from synthetic polymers such as PLGA and PLA typically have higher mechanical properties and slower drug release rates. The materials commonly used for the preparation of dissolving MNs and their advantages and disadvantages can be found in the published reviews [36,63].
Hydrogel MNs are made by cross-linking hydrophilic polymers such as poly(ethylene glycol) diacrylat (PEGDA) and gelatin methacrylamide (GelMA) [64,65]. The advantages and disadvantages of these materials can be found in the previously published reviews [66]. Hydrogels have high mechanical strength in the dry state and can penetrate tissues. After being inserted into the tissue, the MNs swell and then the drugs diffuse into the tissue [47,55,56,59]. Compared to dissolving MNs, hydrogel MNs have a slower and more controlled drug release rate [67]. In general, the mechanical strength of hydrogel MNs is directly proportional to the concentration and cross-linking time of the material, and the release rate of the drug tends to be inversely proportional [68].
MNs can painlessly and minimally invasively penetrate a variety of tissues, including skin and cornea, without causing pain or inflammatory response [69,70]. Compared to conventional injections or clinical implants, MNs allow controlled delivery of the active substance to the target site with minimal tissue damage, making them more suitable for self-administration by patients. Their multi-stage structure consisting of a needle tip and base greatly facilitates the design and regulation of the release rate of the active substance. When applied to ISTR, MNs are initially used only as a drug delivery tool. With the development of micro-/nano-technology and better understanding of tissue repair mechanisms, different functional MNs are designed. Currently, the main roles of MNs in the tissue repair process are: 1. enhancing the delivery efficiency of active substances to improve bioavailability, 2. regulating the release rate of multiple active substances to achieve on-demand release, and 3. providing mechanical support or physical stimuli to regulate cellular behavior for rapid wound healing.
2.2. Enhancing local delivery efficiency
Due to the lack of penetration ability of conventional scaffolds such as hydrogels and sponges, necrotic tissue barriers or biofilms at damaged tissues may limit the permeability of active substances and lead to low bioavailability and poor therapeutic efficacy. Here, MNs with micro-needles structure provide an effective solution without using syringes which may cause inflammation and pain. For instance, by means of simple finger pressure, MNs can penetrate the skin tissue and deliver the active substance to the wound bed [71]. And MNs can effectively encapsulate cells or bioactive substances and maintain their good activity. As shown in Fig. 3A and B, Prof. Zhao's group fabricated methacrylated HA (HAMA) hydrogel MNs encapsulated with bioactive platelet-derived growth factor D (PDGF-D) and human adipose stem cells (ADSCs) [72]. The HAMA MNs could maintain the activity of ADSCs over 90% for 24 h, enhance the biological function of ADSCs to secret growth factor and promote diabetic wound healing by accelerating vascularization, tissue regeneration and collagen deposition. In addition, mature biofilms form a protective microenvironment to hinder the penetration of antimicrobial substances, while MNs can effectively penetrate biofilms [29,[73], [74], [75], [76]]. However, for MNs, effective drug delivery to thin tissues with little background support (e.g., the cornea) has been a challenge. As shown in Fig. 3C, Ryu et al. designed a pen system with a spring-loaded applicator to provide impact force to insert a single MN into the corneal tissue [77]. This MN system allowed for a more localized and minimally invasive delivery of sunitinib to the target site compared to subcutaneous needles. In a suture-induced corneal angiogenesis model, the MN system effectively inhibited corneal neovascularization in vivo.
Fig. 3.
A) Schematic illustrations of the MNs loaded with ADSCs and PDGF-D. B) Application of MNs for diabetic wound treatment (Reproduced with permission of Ref. [72]). C) Schematic diagram of transfer-molded MN for localized and minimally invasive ocular drug delivery (Reproduced with permission of Ref. [77]).
By utilizing the microenvironment of damaged tissue sites (e.g., enzymes, pH) or in vitro stimulus such as near-infrared (NIR), intelligent MNs can be designed to control the release rate of active substances. Xia et al. encapsulated gelatin nanoparticles modified with the gelatinase-responsive antimicrobial photothermal peptide AMP-Cypate in dissolving MNs [78]. When applied to the infected site, the MNs broke through the epidermis and stratum corneum and released the nanoparticles after dissolving. And the nanoparticles could release AMP-Cypate with photothermal antimicrobial properties in response to gelatinase secreted by S. aureus. Similarly, Wang et al. prepared NIR photothermally responsive NO-releasing MNs by embedding graphene oxide particles loaded with nitric oxide and metal organic frameworks into the porous structure of PEGDA hydrogel MNs [79]. Zhao et al. embedded black phosphorus quantum dots and oxygen-loaded hemoglobin into GelMA hydrogel MNs [80]. Upon NIR irradiation, the MNs could responsively release oxygen to treat diabetic wounds.
It must be noted that due to the variability of mechanical strength and thickness of different tissues, the physical properties required for MNs to effectively deliver the active substance to the target tissue are different. For the design and preparation of MNs for tissue regeneration, the dimensions of the needles of MNs (including shape, height, area of the needle tip, and mechanical strength, etc.) are the first things worth considering. Due to the good tissue penetration of the conical structure [81], the needle shapes of MNs currently used for tissue regeneration are mainly conical or pyramidal (quadrangular conical). The mechanical strength of MNs is mainly related to the shape of MNs and the area or materials of the needle tip [82]. Studies have shown that the strength of MNs exceeding 0.05 N/needle can penetrate tissues such as skin, cornea, endometrium and heart [[83], [84], [85]], while most of the current MNs have a strength exceeding 0.1 N/needle and are sufficient for use.
Overall, recent researches have demonstrated the spatial precision of MNs for drug delivery while overcoming the complex and dynamic barriers of various tissues including eye and skin [86]. Clinical studies of regenerative therapies based on active substances such as stem cells and growth factors have been stalled by their susceptibility to inactivation and low transplantation efficiency, and MNs may offer a promising solution for them. MNs with unique micro-needle structures can integrate bioactive materials and host tissues well, thus improving the contact area and depth of the biomaterial with the tissue, and enabling better regulation of cellular behavior to promote damaged tissue repair. However, parameters such as material, height, number and mechanical strength of tips should be fully considered when designing and preparing MNs for different damaged tissue repair.
2.3. Regulating the release of multiple active substances
Due to the complexity of the tissue environment, additional demands are placed on the functions of MNs used for tissue repair. For example, MNs for bacterial infected wound repair require antimicrobial properties, pro-angiogenesis and tissue regenerative properties, and MNs for MI repair require pro-angiogenic activity and inhibition of myocardial fibrosis.
Simply, multifunctional MNs can be obtained by incorporating multiple active substances into polymer solutions using a micromolding method. By this method, Professor Wang's group prepared HA MNs co-loaded with recombinant humanized type III collagen (rhCol III) and naproxen (Nap) encapsulated PLGA nanoparticles [87]. After being inserted into the wound bed, the prepared MNs would rapidly dissolve within few minutes, thereby releasing rhCol III and PLGA nanoparticles. And PLGA nanoparticles could slowly release Nap (release of more than 70% of the drug over 14 d) in the dermis to effectively inhibit the inflammatory response in the wound site. The side effects caused by the explosive release of dissolved MNs can be better avoided by using the slow-release properties of nanoparticles. In another research, with the dissolution of MNs, ovofloxacin directly doped in PVP MNs was almost completely released within 15 min, whereas protein drugs encapsulated in PLGA microspheres were consistently released for a long time (more than 86% of the drug over 15 d) [88]. The advantage of this design was that the rapid release of antibiotics effectively inhibited bacterial infection, while the sustained release of bFGF promoted tissue regeneration throughout the repair process.
The second route is to apply the concept of coated MNs and prepare multilayer composite MNs to regulate the release rate of drugs. For example, as illustrated in Fig. 4A, ferrum-mesenchymal stem cell-derived artificial nanovesicles (Fe-MSC-NVs) with pro-angiogenic activity were encapsulated in the internal HA core of the MN tip, while polydopamine (PDA) with antioxidant function was encapsulated in the HAMA hydrogel shell [89]. With the rapid dissolution of HA, more than 95% of Fe-MSC-NVs were released within 5 h; whereas for PDA in HAMA hydrogels, only about 80% was released after 70 h. PDMS molds for the preparation of such bilayer MNs required plasma treatment to enable polymers with hydrophilic and adhesive properties to adhere to the mold surface, ultimately forming a stable and robust shell [90]. The above two methods achieve the release of multiple active substances through the MNs tips, however, the loading amount raises concerns due to the small size of the tip.
Fig. 4.
A) Schematic illustrations of Fe-MSC-NVs/PDA MN for diabetic wound healing (Reproduced with permission of Ref. [89]). B) Schematic presentation of MN-PBNs-VEGF for diabetic wound healing (Reproduced with permission of Ref. [93]).
Another approach is to embed other active substances in the base of MNs at the same time, which can effectively provide programmed treatment to damaged tissues [91]. In addition, this type of MN allows better control of the spatial distribution of the drugs in the target tissue, an advantage that other types of materials such as hydrogels or sponges do not have. For example, antimicrobial materials in the base of MNs can effectively remove bacteria from the wound surface and prevent infection, while pro-repair materials at the needle tips can be delivered to the dermis for enhanced bioactivity and retention [92]. As shown in Fig. 4B, the prussian blue nanoenzyme (PBN) and vascular endothelial growth factor (VEGF) were encapsulated in the tips, while polymyxin was encapsulated in the base of silk fibroin methacryloyl (SilMA) MNs. The base of MNs could release polymyxin to inhibit bacterial infection. Due to the interconnected porous structure of hydrogel MNs, PBN and VEGF could be slowly released into the wound tissue after the MNs swelling [93]. The use of the base, which plays a mechanically supportive role, as a drug carrier allows the preparation of multifunctionally integrated MNs and can, to a certain extent, solve the problem of low loading of dissolving and hydrogel MNs.
Interestingly, when the needles and base of the MNs are made of materials with different dissolution or degradation rates, the drug release rate can be flexibly controlled. For example, a bilayer MN consisted of tetracycline hydrochloride (TCH) loaded HA needles, and deferoxamine (DFO) loaded chitosan and silk fibroin base. The TCH was completely released from HA needles in 25 min to promote early antimicrobial activity, while the release of DFO in the base was relatively slower [94]. It is worth mentioning that the interface between the needles and the base may have an impact on the release rate and delivery efficiency of the drug. For example, the addition of air bubbles or materials that dissolve quickly at the union of the two layers can enable rapid separation or push the needles into deeper tissue [91].
For dissolving MNs, encapsulating drugs in materials such as nanoparticles, micelles, and hydrogels that can release drugs continuously and slowly can better circumvent the negative effects of their explosive drug release and control the release rate of multiple drug combinations. Bilayer MNs prepared from a combination of dissolvable and hydrogel materials are an important research direction for the future. This is because they allow for increased drug loading and ease of use, while also intelligently controlling the rate of drug release according to the requirements of tissue repair process. Compared to direct doping method, bilayer MNs offer more flexible active substance combinations and easier control of release rates. However, the preparation process of bilayer MNs is more tedious and the reproducibility of the product may be less demanding. Considering the sequential nature of the tissue regeneration process, the on-demand release of active substances is particularly important. However, the preparation of MNs with drug release rates that can perfectly match the tissue healing process is still a considerable challenge due to the limitations of MNs preparation technology.
2.4. Providing mechanical support or physical adjustment
In addition to acting as a carrier of active substances, MNs, a minimally invasive medical device, can also modulate cellular behavior and provide a favorable environment for tissue repair. Luo et al. found silk fibroin (SF) MNs induced impairment of mechanical communication between fibroblasts and ECM, as well as reduced mechanical stress and contraction generated by fibroblasts [95]. In addition, SF MNs attenuated integrin-FAK signaling, thereby downregulating the expression of TGF-β1, α-SMA, collagen I, and fibronectin. However, although this report investigated the therapeutic effects of three different sizes of MNs (arrays of 5 × 5, 10 × 10, and 15 × 15 with heights of 500, 1000, and 1500 μm), it lacked consideration of the physicochemical properties such as the shape and mechanical strength of MNs. In conclusion, as a minimally invasive option, the SF MNs had great potential to provide cost-effective and convenient therapy for scar-free wound healing. For the treatment of MI, MNs can also act as myocardial patches to reduce wall stress and strain in the infarcted area and maintain left ventricular function and morphology [96].
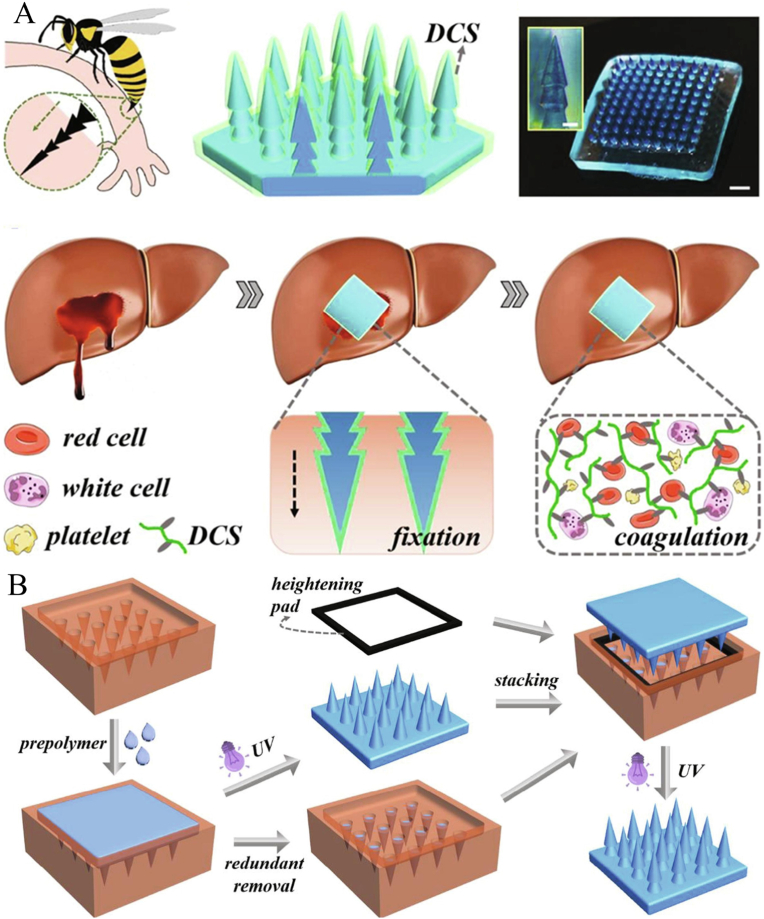
Mimicking nature's layered architecture and sophisticated strategies, bionic MNs with special structures are designed and manufactured to adhere tightly to tissue surfaces or generate traction to close wounds [42]. For example, inspired by the expandable proboscis of endoparasites, Cha et al. developed a bilayer adhesive hydrogel MN consisting of an expandable mussel adhesion protein shell and a non-expandable SF core [97]. The prepared MNs could replace sutures for rapid closure of gastrointestinal/skin wounds and release pro-regenerative or anti-inflammatory agents to the target tissue. Inspired by the layered microstructure of foot or insect bites, Prof. Zhao's group prepared a dodecyl-modified chitosan (DCS) coated pagoda-like multilayer hydrogel MNs by repeatedly stacking MN and molds (as shown in Fig. 5) [98]. The prepared MNs exhibited excellent hemostatic properties in a rabbit model of acute tissue injury (e.g. liver hemorrhage, spleen hemorrhage and kidney hemorrhage). Similarly, other bionic MNs inspired by lamprey-teeth [99], claws of eagles [100], and shark tooth [101], had been designed and manufactured to produce traction to close wounds and promote wound healing. However, the preparation of bionic MNs often requires complex processes or places higher demands on the precision of the equipment, which may be a major obstacle to their clinical translation and industrial production.
Fig. 5.
A) Design and hemostatic function of the bioinspired pagoda-like MNs. B) Schematic illustration of the fabrication process (Reproduced with permission of Ref. [98]).
In general, relevant studies have shown that MNs can provide physical stimulation, traction, and support to repair damaged tissues. However, the specific therapeutic mechanism remains to be further demonstrated. To date, most studies on MNs have focused on MNs as minimally invasive devices for drug delivery, and few have investigated the effects of the structure or materials of MNs on tissue repair. Surprisingly, with the development of micro and nano processing technology, some bionic MNs with specific structures have been designed and prepared for ISTR. These MNs have shown promising therapeutic effects and potential in areas such as wound closure, myocardial repair and hemostasis of the liver. However, the complexity of their preparation process also poses a challenge for their further research and development.
3. MNs for ISTR
3.1. MNs for skin repair
Skin wound care is a major social and economic burden, with millions of people suffering from poor wound healing each year worldwide. Approximately 1–2% of the population in developed countries is reported to suffer from chronic wounds [37]. The normal skin wound healing process includes hemostasis, inflammation, proliferation and tissue remodeling, involving various types of cells and bioactive factors [44]. Diseases such as diabetes, nasty skin tumors or infections can delay wound healing and eventually result in the development of chronic wounds [102]. In chronic wounds, the presence of scabs and microbial membranes, exudate drainage, and a hostile chemical microenvironment rich in various enzymes can undermine the therapeutic efficacy of topical drug delivery [103]. Recent studies have shown that MNs can painlessly puncture the epidermis to form microscopic pores which facilitate the rapid diffusion of biologically active substances (drugs, cells, growth factors, genes, etc.) to the wound bed [43,46,[104], [105], [106]]. Table 1 summarizes representative recent studies of MNs for skin tissue regeneration. As we well known, the total thickness of the dermis and stratum corneum of the skin is about 200 μm, and the length of MNs for skin tissue regeneration is typically 500–1000 μm [26], taking into account the compressive deformation of MNs and the elasticity of the skin.
Table 1.
MNs for skin tissue regeneration.
| Materials | Structure | Bioactive substances | Mechanisms | Ref. |
|---|---|---|---|---|
| Base: gelatin Needles: HAMA |
Arrays: - Height: 800 μm Shape: conical Strength: Failure force about 2 N |
Platelet derived growth factor D, human adipose-derived stem cells | Ensuring sufficient nutrient supply to sustain viability of cells >90% for 24 h. | [72] |
| Needles: GelMA Base: polyvinyl acetate |
Arrays:- Height:- Shape: conical Strength: >0.5 N/needle |
Oxygen | The base would soon disappear by dissolving within 10 min; Using the photothermal effect of the black phosphorus and the reversible oxygen-binding property of hemoglobin, the needle tip released oxygen under NIR irradiation. |
[80] |
| Needle: outer PLGA shell and internal GelMA Base: doublesided tape |
Arrays:- Height: 700 μm Shape: conical Strength: Failure force of 1–1.5 N |
Mesenchymal stem cells | >90% of MNs fall off over 1 min; Cell viability remained above 90% up to 24 h in MNs; Delivery of cell-secreted VEGF. |
[86] |
| HA | Arrays: 10 × 10 Height: 600 μm Shape: pyramidal Strength: 3.98–5.72 N |
Recombinant humanized collagen type III, naproxen | Drugs were rapidly released to the wound site within a few minutes. | [87] |
| PVP | Arrays: 10 × 10 Height: 530 μm Shape: conical Strength: 3.98–5.72 N |
bFGF, ofloxacin | Ofloxacin was quickly released to inhibit infection due to the fast dissolution of MN; bFGF loaded in PLGA microspheres was slowly released to further promote wound healing. |
[88] |
| HA, HAMA | Arrays: 20 × 20 Height: 860 μm Shape: pyramidal Strength: |
PDA nanoparticles, Fe-MSC-NVs | Fe-MSC-NVs loaded inner HA core of the MN tips accelerated angiogenesis; PDA nanoparticles in the outer HAMA shell could be sustainably released at the lesion to suppress the ROS |
[89] |
| Base: PVP Needles: chitosan |
Arrays: 20 × 20 Height:- Shape: conical Strength: >0.25 N/needle |
Magnesium, Panax notoginseng saponins |
The back dissolved within 9 min to release Mg2+; The hydrogel needles slowly released the drug into the dermis. |
[91] |
| Poly(γ-glutamic acid) | Arrays: 10 × 10 Height: 500 μm Shape: pyramidal Strength:- |
Magnesium MOF, graphene oxide-silver nanocomposites |
The base inhibited bacterial infection; The needles slowly released Mg2+ and gallic acid in the deep layer of the dermis. |
[92] |
| Silk fibroin methacryloyl | Arrays: 385 needles Height: 700 μm Shape: conical Strength:- |
Prussian blue nanozymes, VEGF, polymyxin | Polymyxin in the base inhibited bacterial infections; Nanozymes and VEGF loaded needles facilitated diabetic wound healing. |
[93] |
| PVA | Arrays: 10 × 10 Height: 750 μm Shape: pyramidal Strength:- |
α-amylase, levofloxacin-loaded polydopamine nanoparticles | The needle tip penetrated the biofilm; Under NIR irradiation, amylase was released to expose bacteria in the biofilm and combine the photothermal effect and antibiotics to kill the bacteria. | [107] |
| Chitosan | Arrays: 20 × 20 Height: 600 μm Shape: conical Strength:- |
VEGF | VEGF loaded temperature responsive hydrogel in needles released drugs induced by the inflammation response at the site of wounds. | [113] |
| Poly(γ-glutamic acid) | Arrays: 10 × 10 Height: 500 μm Shape: pyramidal Strength: >1 N/needles |
MXenes, asiaticoside | MXenes improved the mechanical strength and reduced the drug release rate of MNs; Penetrating cuticle for subcutaneous drug delivery. |
[115] |
| GelMA, 4-(2-acrylamidoethylcarbamoyl)-3-fluorophenylboronic acid | Arrays: 11 × 11 Height: 600 μm Shape: conical Strength:- |
Insulin | Glucose-responsive insulin release to control glucose in diabetic patients. | [117] |
| Needles: GelMA Base: silk fibroin methacryloyl |
Arrays: 20 × 20 Height: 600 μm Shape: conical Strength:- |
AgNPs, mesenchymal stem cell- derived exosomes, | Adhering steadily to the skin around the wound edges to form an anti-infective surface; Delivering exosomes to the dermis. |
[119] |
| Base: alginate, gelatin, HA Needles: HA |
Arrays: 20 × 20 Height: 540 μm Shape: pyramidal Strength: >0.1 N/needle |
Curcumin, Indocyanine Green | The needles would rapidly dissolve and release drugs to remove tumor under NIR irradiation. Hydrogel base covered the wound and promoted the cells proliferation. |
[121] |
| Silk fibroin, silicon | Arrays:- Height:- Shape: conical Strength: 0.5 N/Needle |
VEGF | Porous structures enhanced the drug loading capability; Liquid diffused along the microfluidic channel for detecting biomarkers in wound secretion; Microelectronic circuits were integrated to sense the motion state of wound areas. |
[123] |
| HA | Arrays:- Height: 600 μm Shape: conical Strength: |
Platinum–ruthenium nanoalloys and porous graphitic carbon nitride C3N5 nanosheets | Improving transdermal drug delivery efficiency | [124] |
Bacterial infected wounds have become a major global healthcare problem, with 60–90% of hard-to-heal wounds associated with biofilms [107]. Fortunately, antimicrobial MNs have been shown to provide effective treatment for infected wounds. The most common method of preparing antimicrobial MNs is to encapsulate antimicrobial agents in the tip or backing of MNs. For example, Yan et al. incorporated the photosensitizer Zn2GeO4:Cu2+ (ZGC) into dissolving MNs [108]. The prepared MNs penetrated biofilms in a minimally invasive manner and delivered pre-UV-treated ZGC to infected wounds. The ZGC could produce multiple reactive oxygen species (ROS) (1O2, hydroxyl radicals and superoxide radicals) continuously over 48 h, effectively eliminate methicillin-resistant Staphylococcus aureus (MRSA) biofilms, reduce in vivo inflammation and promote wound healing. Similarly, antimicrobial agents such as water-soluble antibiotics (ofloxacin [88], asiatic acid [109]) or antimicrobial nanomaterials (Metal organic framework (MOF) [110], silver nanoparticles (AgNPs) [111], and nanozyme [112]) have been successfully encapsulated into MNs and demonstrated excellent antimicrobial properties and the ability to promote infected skin wounds regeneration. Another way to prepare antimicrobial MNs is to use antimicrobial polymers such as chitosan as the main material [113,114]. Zhao et al. fabricated an antibacterial and angiogenic chitosan hydrogel MNs by encapsulating the VEGF-loaded temperature-sensitive hydrogels into the porous network of needles [113]. The prepared MNs could release VEGF induced by the rise of the body surface temperature attributing to the inflammation response.
The global prevalence of adult diabetes is expected to increase to 7.7% by 2030, and diabetic complications pose a serious threat to patients' health, leading to reduced quality of life, increased healthcare costs and mortality [64,115]. In particular, approximately 15–25% of diabetes will develop a diabetic foot ulcer (DFU) during their lifetime [92,116]. DFU is often accompanied by persistent inflammation, bacterial infection, poor angiogenesis and tissue regeneration, making it often difficult to cure DFU with traditional treatment [117,118]. As illustrated in Fig. 6, Zhao et al. prepared an adherent MNs encapsulated with mesenchymal stem cell-derived exosomes (MSC-exos) and AgNPs for improving diabetic wound healing [119]. The tips of the MNs consist of GelMA hydrogel that could continuously release anti-inflammatory and pro-angiogenic MSC-exos to the wound bed, therefore accelerating healing by improving cell function, remodeling blood vessels and restoring the immune system. In addition, the AgNPs loaded backing composed of SF allowed MNs to adhere well to the skin and anchor to the wound site.
Fig. 6.
Illustration of the MSC-exos loaded MN patch for promoting wound healing (Reproduced with permission of Ref. [119]).
MNs can effectively penetrate the skin, increase drug distribution to deeper tumor sites, and minimize leakage of therapeutic drugs to adjacent tissues, thus improving the therapeutic effect of cutaneous epidermal tumors [120]. On the other hand, MNs with tissue repair function can simultaneously promote skin wound healing after tumor eradication [121]. Yu et al. prepared HA MNs functionalized with silica-coated melanin nanoparticles for simultaneous tumor photothermal therapy (PTT) and promotion of skin tissue regeneration [122]. The prepared MNs could remove residual subcutaneous tumor cells and inhibit bacterial infection in the wound bed by PTT. In addition, they could also modulate inflammatory responses, upregulate angiogenic gene expression and promote skin tissue regeneration by scavenging ROS and releasing proto-silicate ions.
In conclusion, MNs can provide effective treatment for difficult-to-heal wounds such as bacterially infected wounds, diabetic wounds, and wounds caused by tumor resection. Considering the complexity of the wound physiological environment, bilayer MNs with flexible drug combinations and controlled drug release rates are one of the hot spots for future research. However, it is important to consider that the thickness of the skin layer varies from patient to patient, making the penetration depth of MNs potentially very different, leading to a wide range of abnormalities in the spatial distribution of active ingredients. Furthermore, most MNs for tissue regeneration are prepared by using fixed-shape molds. Despite the simplicity and reproducibility of the preparation process, also makes it difficult to provide effective treatment of irregular wounds with MNs. Although the shape and size of the mold can be personalized using image recognition [125], it also increases the difficulty of the process. And for acute wounds or burns what the dermis is exposed, minimally invasive MNs may damage capillaries and lead to increased inflammation. Finally, porous MNs such as hydrogel MNs can aspirate biomarker-rich skin interstitial fluid and can monitor tissue healing during treatment [123,126], but their sensitivity and timeliness still need to be improved.
3.2. MNs for corneal regeneration
The cornea, a multi-layered transparent tissue without blood vessels, is the most densely distributed nerve tissue in the body, with approximately 7000 injurious receptors per square millimeter [[127], [128], [129]]. Corneal damage is the second leading cause of blindness in developing countries, and approximately 10 million patients worldwide have been diagnosed with bilateral corneal blindness [130]. Despite genes, stem cells, gels and drops have been developed, human donor corneal transplant is the most effective treatment for corneal blindness and restoring patients' vision (with a cure rate of more than 80%) over a century. In 116 countries, approximately 185,000 corneal transplants are performed each year, yet 13 million people are still waiting for corneal transplants [[130], [131], [132]]. Therefore, there is an urgent need to find novel strategy to promote corneal regeneration. The micron-sized MNs can overcome the disadvantages (frequent, invasive and low bioavailability) of traditional injections, and have been widely used for the treatment of ocular diseases such as corneal injury, glaucoma, age-related macular degeneration and uveitis [30,[133], [134], [135], [136]]. MNs have been used for the treatment of ocular diseases over 10 years, and there are still no reports of serious side effects of MN injections on eye tissue.
Table 2 summarizes representative recent studies of MNs for corneal regeneration. Considering the cornea has a certain curvature (39–45 D), MNs for corneal repair usually have a small number of needles. It is worth noting that there is some controversy regarding the length of MNs for corneal tissue. Some studies suggested that the length of MNs should be matched within the maximum injection depth (43–63 μm) corresponding to the human corneal epithelium (approximately 50 μm thick) without causing irreversible corneal stromal scarring [137,138]. However, in a separate study, MNs with a length of 600 μm and an insertion depth of approximately 150 μm did not cause significant inflammation or angiogenesis in animal tests [90]. Therefore, more detailed studies are still needed to explore the requirements of different tissues for the length and insertion depth of MNs.
Table 2.
MNs for corneal regeneration.
| Materials | Structure | Bioactive substances | Mechanisms | Ref. |
|---|---|---|---|---|
| Frozen glycerin | Arrays: 3 × 3 Height: 400–440 μm Shape: pyramidal Strength: 00.3–0.4 N/neelde |
Bdellovibrio bacteriovorus | Bacterial activity exceeded 80% after 14 d of storage. | [85] |
| Needle: outer MeHA shell and internal HA Base: HA |
Arrays: 3 × 3 Height: 500 μm Shape: pyramidal Strength: 0.4 N/neelde |
Anti-angiogenic monoclonal antibody (DC101), diclofenac | Rapid dissolution of the outer layer of the needle tip, releasing >80% of the drug within 5 min; Slow release of the inner hydrogel layer (over 3 d). |
[90] |
| Needle: silicon Base: PVA |
Arrays: Height: 60 μm Shape: Circular Strength: |
Bevacizumab | Painless and long-term sustained delivery of ocular drugs over the course of months. | [137] |
| PLGA, SU8 resin | Arrays: 1 needle Height: 150 μm Shape: Tapered Strength: 00.3–0.4 N/neelde |
Antibiotics | The drug-tip is separated from base and released drugs after the hydrolysis of PLGA occurred in cornea. | [143] |
| Poly(D,l-lactide), HA | Arrays: 20 × 20 Height: 390 μm Shape: pyramidal Strength: |
Fluconazole | Penetrating the corneal epithelial layer reversibly; Increasing the residence time of drug in the conjunctival sac | [144] |
Keratitis caused by bacterial or fungal infections is one of the leading causes of corneal clouding and is the fourth leading cause of blindness worldwide [[139], [140], [141], [142]]. Antifungal drugs or antibiotic-loaded MNs have been shown to possess higher bioavailability and better therapeutic efficacy compared to eye drops [143,144]. To address bacterial drug resistance, Xu et al. prepared a novel frozen MNs by filling PDMS molds with glycerol/PBS/predatory Bdellovibrio bacteriovorus (B. bacteriovorus) bacterial suspensions and freezing them at −80 °C (as shown in Fig. 7) [85]. The frozen MNs could release B. bacteriovorus to remove gram-negative bacteria. This novel frozen MNs could provide an efficient delivery method for therapeutic organisms such as stem cells, which can adequately maintain their activity. However, such MNs need to be stored in a cryogenic environment before use, raising the cost of storage and transportation.
Fig. 7.
Illustration of cryoMNs for ocular delivery of predatory bacteria in treating eye infection (Reproduced with permission of Ref. [85]).
Alkali burns or inflammation can cause abnormal angiogenesis in corneal tissue, leading to vision loss. Chen et al. prepared a bilayer MN with biphasic drug release kinetics to treat corneal alkali burns [90]. The MN consisted with a methacrylated HA (MeHA) hydrogel shell containing anti-angiogenic monoclonal antibody (DC101) and HA core containing anti-inflammatory compound (diclofenac). The prepared MN could rapidly release diclofenac and continuously release DC101 to inhibit angiogenesis.
Although MNs have been shown to be a safe and effective delivery device for corneal repair, there is still some controversy regarding the needle length of MNs. In addition, the curvature of the cornea and the nature of the background-free support place certain demands on the way MNs are applied and the flexibility of the backing material. The available literature discusses less about the insertion efficiency of MNs for corneal regeneration, especially when the array number of MNs is large. Therefore, MNs for corneal tissue repair need to be designed in a more scientific manner. Although some studies designed a micro-needle pen with a spring to provide puncture force [77], this pen could only deliver a single MN. In addition, the angle of the pen was more difficult to control for different locations of the cornea.
3.3. MNs for endometrial repair
The endometrium is the epithelial layer on the surface of the uterine cavity and plays an important role in embryo implantation and pregnancy maintenance. Common events such as cervical dilatation/scraping and inflammation predispose to impaired endometrial regeneration, scar formation, fibrosis, and intrauterine adhesions (IUA) [[145], [146], [147]]. IUA is a gynecological condition in which scar tissue forms in the uterus, causing a range of symptoms, including recurrent miscarriages, amenorrhea, menorrhagia, cyclic pelvic pain, and even infertility [69,148,149]. The incidence of IUA has been reported to range from 6% to 30% in women who have undergone all types of abortions. In addition, the incidence of IUA can be as high as 25% in women who have undergone dilation and curettage after delivery [150,151]. Table 3 summarizes recent studies of MNs for endometria tissue regeneration. Typically, the length of MNs used for endometrial tissue repair falls within range of 500–1000 μm.
Table 3.
MNs for endometrial regeneration.
| Materials | Structure | Bioactive substances | Mechanisms | Ref. |
|---|---|---|---|---|
| GelMA | Arrays: Height: Shape: conical Strength: 0.3 N/needle |
CeO2 nanozyme, stem cell | antioxidant CeO2 loaded base removed excessive ROS; Hydrogel needles improved stem cell activity, targeting efficiency and dwell time. |
[152] |
| Arrowhead needles: GelMA; Base: bottom layer was N-acryloyl glycinamide (NAGA), top layer was PEGMA |
Arrays: Height: 760 μm Shape: Arrowhead Strength: 0.2 N/needle |
bFGF | The arrowhead needles allowed MNs steadily adhering to the tissues and released growth factors; Backing layer prevented tissue adhesions |
[153] |
| GelMA, | Arrays: Height: 600 μm Shape: conical Strength: 0.2 N/needle |
Lactoferrin, human endometrium-derived adventitial cells | Cells could rapidly form more biologically active three-dimensional cell spheres within the micropores surrounding the needles. Lactoferrin inhibited bacterial infections |
[154] |
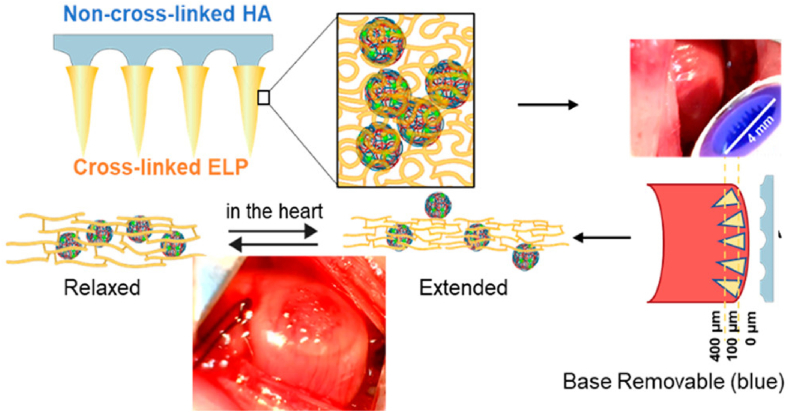
In order to scavenge ROS and improve the regenerative capacity of endometrium, Zhao et al. embedded antioxidant cerium oxide (CeO2) nanoenzymes and stem cells in the backing layer and tip of GelMA MNs, respectively [152]. The experimental results showed that the prepared MNs could promote the morphological reconstruction of damaged uterus, improve the pregnancy rate and restore the reproductive function. In addition, their group designed a new type of composite MNs with the stylized tip and anisotropic surface adhesion (as shown in Fig. 8) [153]. The tips of the MNs could form an interlock with the tissue, allowing the MNs to adhere stably to the tissue. And the tip was fabricated with cell-adhesive hydrogel containing growth factors, while its backing was an anti-adhesive polyethylene glycol methacrylate (PEGMA) hydrogel. Therefore, the prepared MNs could promote cell adhesion to repair damaged tissues while inhibiting tissue contact on the other surface to prevent undesirable adhesion.
Fig. 8.
A) Preparation of arrowhead composite MNs. B) Schematic illustrations of the properties of the arrowhead composite MNs and their applications in endometrium repair and IUAs prevention (Reproduced with permission of Ref. [153]).
In another study, Ding et al. prepared a layered GelMA hydrogel MNs containing lactoferrin (LF) with anti-inflammatory activity and antibacterial properties and human endometrium-derived epithelial cells (En-ADVs) [154]. The study designed a series of micropores on MNs in which EnADVs could be cultured as three-dimensional (3D) cell spheres. Compared to conventional monolayers or 3D dissociated cells, 3D cell spheroids exhibited enhanced maintenance of pluripotency, cell proliferation, cell migration, and promotion of angiogenesis.
Given their unique structure and good biocompatibility, MNs can effectively deliver active substances to achieve endometrial regeneration while acting as a physical barrier to prevent tissue adhesion. However, there are relatively few studies on MNs for the treatment of IUA, and large-scale animal experiments and clinical trials are still needed to verify their effectiveness. Future research in the field of endometrial damage repair by MNs may favor flexible combinations between multiple active substances (such as drugs, cells, growth factors and exosomes) and MNs, providing a wealth of options for the treatment of IUA. And it is important to consider the delivery system of MNs and the way they are inserted into the tissue when they are applied clinically to promote endometrial repair.
3.4. MNs for cardiac repair
Ischemic cardiovascular diseases such as MI are the leading cause of death worldwide. MI is a process of adverse cardiac remodeling triggered by apoptosis and necrosis of myocardial cells, activation of inflammatory response, degradation of extracellular matrix (ECM) and tissue fibrosis following acute or chronic blockage of coronary arteries [69,155,156]. There is still no effective treatment to promote cardiac regeneration [157]. Common clinical treatments mainly include drug therapy, heart transplantation and left ventricular assist device implantation [158]. However, due to the limited regenerative capacity of the heart, existing treatments can only temporarily maintain cardiac function and do not repair infarcted myocardial tissue [102]. There are also disadvantages such as heart size mismatch, vascular lesions, and shortage of supply [158]. Therefore, the development of scaffolds for cardiac tissue regeneration is expected to address these deficiencies and has important clinical implications. Recently, MNs have emerged as a promising tool with great versatility for delivering a variety of active substances to treat MI. Table 4 summarizes recent studies of MNs for cardiac tissue repair. And the length of MNs used for cardiac tissue repair is also 500–1000 μm, which is similar to skin and endometrium.
Table 4.
MNs for cardiac repair.
| Materials | Structure | Bioactive substances | Mechanisms | Ref. |
|---|---|---|---|---|
| PVA | Arrays: 20 × 20 Height: 600 μm Shape: conical Strength: 2 N/needle |
Cardiac stromal cells | Serving as the channels to allow for communication between the patch and the host myocardium, and transport nutrients and cytokines. | [159] |
| Needle: Elastin-like peptide Base: HA |
Arrays:- Height: 600 μm Shape: conical Strength: |
Mesenchymal stromal cell-secreted factors | The base was detached once MNs contacted the pleural fluid; Hydrogel tips in the myocardium allowed for continuous release of cytokines. |
[160] |
| PVA | Arrays: 44.75 ± 1.28 needles Height: 850 μm Shape: conical Strength: Young's modulus of 12.28 ± 0.80 MPa |
Adeno-associated virus | Achieving homogeneous distribution of adeno-associated virus delivery. | [161] |
| GelMA | Arrays:- Height:- Shape: pyramidal Strength:- |
Carbon nanotube, cardiomyocytes | Adhering to the heart and released the encapsulated drugs; Ensuring the simultaneous contraction of cardiomyocytes distributed on the patch, made these cells to keep synergies with the heart in vivo. |
[162] |
| PVA | Arrays: 11 × 11 Height: 600 μm Shape: conical Strength: puncture force of 4.7 N |
VEGF, graphene oxide | Folded MNs could be implanted minimally invasively and could pierce the myocardium and wrap the heart when fully expanded under NIR irradiation; Sustained release of VEGF. |
[163] |
| GelMA | Arrays: 385 needles Height: 570 μm Shape: conical Strength: 0.4 N/needle |
Galunisertib | Sustainably released galunisertib for more than 2 weeks and provided mechanical support. | [164] |
MNs can act as a channel between the patch and myocardial tissue. Prof. Cheng's group prepared PVA MNs carrying cardiac stromal cells [159]. The MNs could aspirate interstitial fluid from the heart to provide nutrients to the cells, while releasing paracrine factors to repair the heart. The results of animal experiments demonstrated that the prepared MNs could promote MI healing by promoting angiogenesis, reducing scar size and enhancing cardiac function. Subsequently, to avoid the cardiac burden caused by the sutures of the MNs, they designed a detachable MNs (as shown in Fig. 9) [160]. The tip of this MNs consisted of PLGA nanoparticles loaded with mesenchymal stromal cell secretory factor (MSCF) and elastin-like peptide hydrogel, and the basing of the MNs was dissolvable HA. Once in contact with tissue fluid, the backing would fall off quickly, so no sutures were required. Instead, the tips could be firmly inserted into the infarcted myocardium and release MSCF to reduce myocardial apoptosis, restore myocardial volume, and decrease myocardial fibrosis. Similarly, other active substances including adeno-associated virus (AAV) and VEGF, IL-10 were also encapsulated into MNs for MI treatment [[161], [162], [163]].
Fig. 9.
MSCF loaded detachable MNs for cardiac repair (Reproduced with permission of Ref. [160]).
On the other hand, MNs can also act as myocardial patches, providing mechanical support for heart to reduce myocardial stress and inhibit unfavorable ventricular remodeling [164]. However, MNs are currently provided through invasive open-heart surgery, which increases the risk of infection and cardiac burden on patients. Inspired by the venomous stinger of honeybees, Prof. Zhu's group prepared bionic MNs with a barbed structure and embedded the MNs in an elastomeric film [96]. The prepared MNs could firmly fix to the beating heart, reduce the wall stress in the infarcted area and maintain the function and morphology of the left ventricle. In addition, the MNs could be minimally invasively implanted into the beating porcine heart using thoracoscopy in less than 10 min without sutures and adhesives. However, the process was studied in the normal heart.
In conclusion, MNs can promote MI repair by delivering active substances to infarcted myocardial tissue or providing mechanical support. MNs allow better integration of biomaterials and infarcted myocardial tissue than conventional patches. Tissue-adherent MNs can be delivered in a minimally invasive manner, thus avoiding suturing and open-heart surgery. However, most of the animal models are acute MI caused by ligation of the left anterior descending branch. In the future, more models are still needed to further validate the therapeutic effect of MNs. On the other hand, whether MNs can damage the normal tissue surrounding the infarcted myocardial tissue, destroy capillaries or cause inflammation still needs to be rigorously verified.
3.5. Others
In addition to skin, cornea, endometrium, and myocardium, MNs have been also widely explored as controlled local delivery platform of active substances for the repair of damaged tissues such as spinal cord injury (SCI), stroke, and periodontal diseases. And relevant studies are summarized in Table 5.
Table 5.
MNs for other tissues regeneration.
| Tissue | Materials | Structure | Bioactive substances | Mechanisms | Ref. |
|---|---|---|---|---|---|
| Spinal cord | Silicon coated with gold and polypyrrole | Arrays: 10 × 10 Height: 500 μm Shape: conical Strength:- |
Dexamethasone phosphate | Transdural and electronically controlled delivery of drugs to the intrathecal space. | [170] |
| GelMA | Arrays: 45 needles Height: 600 μm Shape: conical Strength: <1.5 N/needle |
Mesenchymal stem cell | Maintaining the activity of cells for more than 7 d; Achieving sustained delivery of exosome. |
[171] | |
| GelMA | Arrays: 20 × 20 Height: 300 μm Shape: conical Strength: Young's modulus of 500 kPa |
Mesenchymal stem cell-derived exosome | Improving the exosome retention rate, achieved sustained release over 6 d. | [172] | |
| Periodontal tissue | GelMA and gelatin | Arrays: 11 × 11 Height: 600 μm Shape: conical Strength: about 4 N |
Tetracycline, IL-4 and TGF-β | Quickly dissolvable gelatin base for burst release of antibiotic; Antibiotic loaded nanoparticles and cytokine loaded silica microparticles in needles for sustained release. |
[176] |
| Oral mucosal | HA | Arrays: 10 × 10 Height: 350 μm Shape: conical Strength: penetrate the oral mucosa |
dexamethasone acetate, vitamin C and tetracaine hydrochloride | Fully released the encapsulated drug within 10 s. | [177] |
| HA | Arrays: 15 × 15 Height: 700 μm Shape: conical Strength: |
Betamethasone | Fully released the encapsulated drug within 3 min. | [178] | |
| Brain | GelMA | Arrays: 10 × 10 Height: 600 μm Shape: conical Strength: >0.07 N/needle |
Adeno-associated virus | For sustained and controlled delivery of adeno-associated virus (AAV) expressing human VEGF (AAV-VEGF) that achieves homogenous distribution and high transfection efficiency in ischemic brains. | [182] |
| Skeletal muscle | PLGA needles coated tungsten | Arrays: 5 × 5 Height: 900 μm Shape: pyramidal Strength: modulus of 77 MPa |
Aspirin and ibuprofen | The base was combined with wireless power transmission system; Transmission of periodic electrical stimulation to regulate cell behavior and tissue regeneration, release anti-inflammatory drug. |
45 |
| Cartilage | – | – | cellular microspheroids | Microspheroids on MNs were cultured to permit fusion into a tissue construct. | [187] |
| Liver | GelMA | Arrays: 11 × 11 Height: 577 μm Shape: conical Strength: 0.054 N/needle |
silicate nanoplatelets | The needle-shaped structure increases the contact area with blood. Adhering to the wound through an interlocking mechanism. |
[190] |
| HA | Arrays: Height: 750 μm Shape: pyramidal Strength: ∼0.2 N/needle |
Tranexamic acid | Peripheral MN for minimally and site-selectively invasive hemostatic drug delivery. The released drugs were transported from adjacent tissue to the target site through blood flow in capillaries. |
[191] | |
| Blood vessel | 208CTH-F | Arrays: Height: 200 μm Shape: octagonal based cones Strength: |
paclitaxel | MNs covered on the surface of drug-eluting balloons. Balloon expansion allowed MNs to penetrate the inner layer of the vessel wall and released the drug on the surface. |
[193] |
| PLGA | Arrays: 3 × 3 Height: 650 μm Shape: conical Strength: |
Rhodamine B, paclitaxel | The combination with the cuff-shaped device allowed MNs to be applied to the outer surface of the vessel; Drug released by MNs were uniformly distributed in the vascular tunica media over 2 weeks. |
[194] | |
| PLGA | Arrays: 4 × 4 Height: 480, 640 and 780 μm Shape: pyramidal Strength: Young's moduli of 29.7 MPa |
rhodamine B, sirolimus | The MNs are mounted on a flexible PLGA mesh that released drug to the vessel wall and minimized the mechanical stresses applied to the vessel. | [195] | |
| Needle: PCL, PLGA, lauric acid Base: Curable resin PCLMA |
Arrays: Height: 500 μm Shape: conical Strength: >0.2 N/neelde |
paclitaxel | MNs were mounted on the surface of the balloon; After irradiation by a circular NIR laser inside the catheter, the drug-carrying tip was dislodged and embedded in the vascular system and gradually released the drug for more than six months. |
[196] |
3.5.1. Spinal cord
SCI is one of the most serious neurodegenerative diseases in modern society, causing sensory impairment and/or paraplegia in patients [165,166]. SCI can be caused by a variety of reasons, including traffic accidents, industrial accidents, and sports injuries [[167], [168], [169]]. Improving neurological recovery in SCI patients has remained a challenge over the past few decades. MNs has been shown to deliver the active substance more effectively without damaging nearby spinal tissue than traditional local repeat injections [170,171]. As illustrated in Fig. 10, Xin et al. cultured mesenchymal stem cells in a hydrogel and obtained exosomes (3D-Exo) containing a large number of proteins and miRNAs involved in local microenvironment regulation [172]. And they implanted 3D-Exo loaded GelMA hydrogel MNs into a rat SCI model. The Young's modulus of MN@3D-Exo determined by atomic force microscopy (AFM) was 500 kPa, which matched with normal spinal cord (200−600 kPa). The in vivo results showed that local implantation of MNs significantly improved the retention rate of 3D-Exo, achieved slow release of active ingredients, and promoted the recovery of neural function. It is important to note that the mechanical properties of MNs should match those of the spinal cord in order to avoid causing tissue damage. In addition, the optimal time window for implantation of MNs remains to be further investigated.
Fig. 10.
3D-Exo loaded MNs for SCI Repair (Reproduced with permission of Ref. [172]).
3.5.2. Oral tissues
Periodontitis is a chronic inflammatory disease that leads to progressive destruction of periodontal tissue (including alveolar bone, periodontal ligament and root cementum) and is the leading cause of tooth loss in adults [173]. For various reasons (such as oral hygiene, defect size, infection, and many others), injured periodontal tissue may not be able to repair itself through wound healing and tissue regeneration [174,175]. Therefore, advanced regenerative therapy need to be developed to restore the original structure and function of periodontal tissue. To clear microbial infections and modulate immune responses, Li et al. developed a biodegradable MN that could be implanted into gingival tissue in a painless and suture free manner [176]. The prepared MN consisted of a fast-dissolving adhesive backing for immediate release of tetracycline and biodegradable GelMA hydrogel tips containing tetracycline loaded PLGA nanoparticles and cytokine loaded silica particles. The tips could continuously release antibiotics to completely inhibit bacterial growth, and release IL-4 and TGF-b to induce polarization of anti-inflammatory macrophages and formation of regulatory T cells. In addition, MNs have also been shown to painlessly penetrate the tongue abdomen mucosa and release drugs to treat oral ulcers, another common oral inflammatory disease [177]. Wang et al. prepared dissolving HA MNs containing betamethasone phosphate sodium (BSP) and betamethasone dipropionate (BDP) [178]. HA MNs could release BSP and BDP to the base of the ulcer (to a depth of more than 200 μm) in less than 3 min. The experimental results demonstrated that MNs could improve the comfort and efficacy of oral ulcer treatment compared to commercially available topical injections and creams.
3.5.3. Brain
Stroke is an ischemic or hemorrhagic disease mainly occurring in the microvascular system of the brain, resulting in cerebrovascular damage and irreversible damage to neurological function. And stroke is one of the major causes of death and severe disability worldwide [179,180]. Intravenous thrombolysis to restore blood flow to the ischemic site is the main treatment for ischemic stroke. However, thrombolysis can also lead to a sharp increase in oxygen concentration at the injured site after ischemia-reperfusion, which may aggravate brain injury [181]. In order to promote angiogenesis and functional reconstruction in infarction brain tissue, Wang's group innovatively proposed a GelMA MN for continuous and controlled local delivery of human VEGF expression adeno-associated virus (AAV) [182]. Ischemic stroke models were established in adult rats, and an AAV-VEGF-loaded MNs cortex was inserted into the ischemic core and penumbra of these rats one day after the ischemic attack. The in vivo results showed that MNs did not cause significant inflammatory response, and achieved successful transfection and uniform distribution in the cerebral cortex within three weeks after surgery. And the prepared MNs increased VEGF expression and enhanced functional angiogenesis and neurogenesis.
3.5.4. Muscle
Electrical stimulation is a physical and convenient treatment that has been widely used to promote cell proliferation and tissue regeneration [183,184]. In a pioneering study, Yu et al. prepared conductive MN electrodes by hot pressing PLGA MNs loaded with aspirin and ibuprofen onto Mg foils and then depositing W or Mg onto MN [45]. When combined with radio frequency (RF) based wireless power transmission systems, the MNs could be externally controlled and programmed to generate target waveforms for target tissues. Drugs were continuously released from the MNs during the dissolution of the device to prevent inflammation. In a rat model of muscle injury, the device not only shortened the duration of muscle regeneration, but also reduced the inflammatory response.
3.5.5. Cartilage
Cartilage defects due to osteoarthritis are a serious tissue lesion for which effective treatment is still lacking [185]. As a minimally invasive drug delivery device, MNs are often used for transdermal delivery of anti-inflammatory drugs or immunosuppressants to treat arthritis and promote cartilage repair [186]. D'Lima et al. assembled stem cell microspheroids of 500 μm diameter individually on MNs in a predetermined arrangement to form a construct of desired size and shape [187]. Microspheroids on MNs were cultured for 4–5 days to permit fusion into a tissue construct. This tissue construct showed good pro-chondral repair ability in the repair of ex vivo osteoarthritic human cartilage and in vivo rabbit osteochondral defects.
3.5.6. Liver
Limited by the poor effectiveness of traditional clinical hemostatic techniques such as suturing, uncontrolled or excessive visceral bleeding caused by acute trauma is a common and serious medical problem, often resulting in patient death [188,189]. In section 2.4, we have discussed the ability of a biomimetic pagoda-like MNs to immediately repair acute tissue injuries such as hepatic hemorrhage, splenic hemorrhage, and renal hemorrhage by physical interlocking [98]. In addition, recent studies have found that the needle-like structure of MNs could increase the contact area of the material with blood, thus further accelerating liver repair and hemostasis [190]. A silicate nanosheet-loaded GelMA MNs exhibited exciting hemostatic effects and could reduce clotting time by 89%, exceeding most commercially available solid-based hemostatic agents. And in a rat liver hemorrhage model, the MNs reduced bleeding by approximately 92% compared to the untreated injury group. Furthermore, to avoid secondary damage to the liver injury site, a peripheral MNs was designed by Lim et al. [191] The drug released by this MNs was transported from adjacent tissues to the target site via blood flow in the capillaries.
3.5.7. Blood vessel
The microscopic thickness of the outer and inner vessel membranes results in low bioavailability of traditional intravascular drug delivery methods such as balloons and stents [51]. The high rate of restenosis and neointima formation has prompted investigators to diligently pursue new peritascular or intravascular delivery methods, and MNs offer a potential alternative [35,192]. Unlike traditional MNs used for other tissue repair, MNs used for vascular repair usually require special structural design, combined with flexible substrates or balloons, to ensure that MNs can penetrate the vascular wall without penetrating [193,194]. Due to the highly curved or complex vascular structures, which have repetitive pulsatile movements, base of the MNs for vascular repair needs to be more flexible so that they can better fit the vascular wall and avoid vascular damage. For example, a type of PLGA MN mounted on the surface of a PLGA fabric mesh could wrap around the outer vascular wall to enhance drug delivery efficiency and minimize the mechanical stresses applied to the vessel [195]. Most of the earliest MNs used for vascular repair were coated microneedles with limited drug loading and treatment period. Wang et al. equipped the tip detachable MN on the balloon surface to achieve longer-lasting drug release for more than half a year [196]. The drug loaded tips of the MN consisted of the biodegradable material PLGA/PCL and lauric acid with low melting point. The annular laser fiber in the inner axis of the catheter emitted NIR to heat and melt the lauric acid. After the molten lauric acid was converted to a solid state, the bond between the MN tip and the base was weakened. And the drug loaded tip could be separated from the base by pulling the balloon catheter by hand.
As a novel in situ delivery platform, MNs have demonstrated excellent ability to promote tissue regeneration in animal models of SCI, stroke, periodontitis and muscle injury. However, due to the lack of sufficient experimental data and clinical research support, the therapeutic effect of MNs needs to be further verified. At the same time, MNs are expected to be applied in more other tissues, bringing new opportunities for healing damaged tissues.
4. Future perspectives and conclusions
After more than ten years of exploration, a variety of multi-functional MNs represented by dissolving or hydrogel MNs have been developed for regeneration of various tissues, including skin, cornea, endometrium, myocardium, muscle, spinal cord, oral tissues and blood vessel. Among these, difficult-to-heal wound repair such as diabetic ulcers and bacterially infected wounds are currently the most extensively researched areas for MNs. In general, MNs offer several unique advantages over traditional scaffolds such as films, sponges and hydrogels.
-
1.
The micro-needle structure provides strong penetration to help biomaterials pierce the necrotic tissues, biofilms, vascular wall, corneal epithelial layer and other tissue barrier, thereby improving the drug bioavailability.
-
2.
Flexible combinations of materials (dissolvable or degradable, and hydrogels) and structures (needle tips and bases) allow control of drug release rates, and drug spatial distribution on the tissues surface or deep layer, and damaged area or adjacent normal tissue.
-
3.
Regulating cell behavior by generating physical stimuli or providing engagement/adhesion for rapid wound closure.
-
4.
With their flexible preparation methods (solvent casting, 3D printing and drawing lithography et al.), material properties (strength, flexibility and melting point et al.) and geometry, MNs can be delivered to the target tissue site by thumb press, vacuum pump, spring-loaded pen, balloon and catheter, etc., thus meeting the requirements of clinical applications.
Most of the current studies are using MNs as a device for drug delivery to improve delivery efficiency. But studies on the effects of the physical properties of MNs, including geometry, needle height, tip radius, needle density and material, on the tissue repair process are still lacking. As a drug delivery platform, the problem of low drug loading rate of MNs is still not well solved, therefore MNs require careful research on efficient drug delivery methods, intelligent drug release mechanism and pharmacokinetics. In addition, the in vivo tissue repair performance of MNs was mainly evaluated in small animals such as mice, rats and rabbits. Due to their anatomical, physiological and biomechanical structure, they differ from humans. Therefore, human volunteers will need to be recruited in the future to test the therapeutic effects of MNs.
Although MNs show promising potential in the field of tissue repair, unfortunately, most of the products currently on the market are for cosmetic and vaccination purposes. In addition, a search of the U.S. clinical trials database (clinicaltrials.gov) using the keywords ‘(microneedle) OR (microneedling) OR (micro needle) OR (microneedles)’ showed that more than 3/4 of the clinical trials are hollow or solid MNs in vaccination, dermatology, pain management, etc. And no clinical trials have been found on the use of MNs for ISTR. MNs used for ISTR have the ability to interact with and alter the microenvironment in vivo, resulting in the need for regulatory approval of all aspects of safety and performance (including host tissue acceptability, effects on gene expression and signaling, effects on local microenvironments, and safety or therapeutic efficacy of degradation products). In addition, MNs have been relatively poorly studied in tissues other than difficult-to-heal wounds. As a result, more verification and validation tests for MNs used in ISTR require more effort and resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the funding listed as follows: the Fundamental Research Funds for the Central Universities (No.YJ2021115), the 111 Project (The Program of Introducing Talents of Discipline to Universities (B16033)), and the Sichuan Science and Technology Major Project (2018SZDZX0011), Natural Science Foundation of Sichuan Province(2023NSFSC0998), and National Key Research and Development Programs, China (2022YFC2402800).
Contributor Information
Li Yang, Email: yanglisc@scu.edu.cn.
Shibo Tang, Email: tangshibo@vip.163.com.
Yunbing Wang, Email: yunbing.wang@scu.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Christman K.L. Biomaterials for tissue repair. Science. 2019;363(6425):340–341. doi: 10.1126/science.aar2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eming S.A., Wynn T.A., Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356(6342):1026–1030. doi: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- 3.Hu C., Yang L., Wang Y. Recent advances in smart-responsive hydrogels for tissue repairing. MedComm – Biomaterials and Applications. 2022;1(2):e23. doi: 10.1002/mba2.23. [DOI] [Google Scholar]
- 4.Forbes S.J., Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat. Med. 2014;20(8):857–869. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- 5.Scott E.W. Stem cell reviews and reports: adult stem cells and tissue regeneration section. Stem Cell Reviews and Reports. 2017;13(1):2. doi: 10.1007/s12015-017-9724-6. 2. [DOI] [PubMed] [Google Scholar]
- 6.Galliot B., Crescenzi M., Jacinto A., Tajbakhsh S. Trends in tissue repair and regeneration. Development. 2017;144(3):357–364. doi: 10.1242/dev.144279. [DOI] [PubMed] [Google Scholar]
- 7.Cao B., Li Y., Yang T., Bao Q., Yang M., Mao C. Bacteriophage-based biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2019;145:73–95. doi: 10.1016/j.addr.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao A.S., Mooney D.J. Regenerative medicine: current therapies and future directions. Proc. Natl. Acad. Sci. USA. 2015;112(47):14452–14459. doi: 10.1073/pnas.1508520112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L., Hao R., Qin J., Song J., Chen X., Rao F., Zhai J., Zhao Y., Zhang L., Xue J. Electrospun fibers control drug delivery for tissue regeneration and cancer therapy. Advanced Fiber Materials. 2022;4(6):1375–1413. doi: 10.1007/s42765-022-00198-9. [DOI] [Google Scholar]
- 10.Zhao Y., Song S., Ren X., Zhang J., Lin Q., Zhao Y. Supramolecular adhesive hydrogels for tissue engineering applications. Chem. Rev. 2022;122(6):5604–5640. doi: 10.1021/acs.chemrev.1c00815. [DOI] [PubMed] [Google Scholar]
- 11.Spicer C.D. Hydrogel scaffolds for tissue engineering: the importance of polymer choice. Polym. Chem. 2020;11(2):184–219. doi: 10.1039/C9PY01021A. [DOI] [Google Scholar]
- 12.Nam S., Mooney D. Polymeric tissue adhesives. Chem. Rev. 2021;121(18):11336–11384. doi: 10.1021/acs.chemrev.0c00798. [DOI] [PubMed] [Google Scholar]
- 13.Gaharwar A.K., Singh I., Khademhosseini A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020;5(9):686–705. doi: 10.1038/s41578-020-0209-x. [DOI] [Google Scholar]
- 14.Peng L., Abbasi N., Xiao Y., Xie Z. Black phosphorus: degradation mechanism, passivation method, and application for in situ tissue regeneration. Adv. Mater. Interfac. 2020;7(23) doi: 10.1002/admi.202001538. [DOI] [Google Scholar]
- 15.Abdulghani S., Mitchell G.R. Biomaterials for in situ tissue regeneration: a review. Biomolecules. 2019;9(11):750. doi: 10.3390/biom9110750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safina I., Embree M.C. Biomaterials for recruiting and activating endogenous stem cells in situ tissue regeneration. Acta Biomater. 2022;143:26–38. doi: 10.1016/j.actbio.2022.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li T., Chang J., Zhu Y., Wu C. 3D printing of bioinspired biomaterials for tissue regeneration. Advanced Healthcare Materials. 2020;9(23) doi: 10.1002/adhm.202000208. [DOI] [PubMed] [Google Scholar]
- 18.Wang X., Chang J., Wu C. Bioactive inorganic/organic nanocomposites for wound healing. Appl. Mater. Today. 2018;11:308–319. doi: 10.1016/j.apmt.2018.03.001. [DOI] [Google Scholar]
- 19.Xue J., Wang X., Wang E., Li T., Chang J., Wu C. Bioinspired multifunctional biomaterials with hierarchical microstructure for wound dressing. Acta Biomater. 2019;100:270–279. doi: 10.1016/j.actbio.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Hu C., Liu W., Long L., Wang Z., Zhang W., He S., Lu L., Fan H., Yang L., Wang Y. Regeneration of infarcted hearts by myocardial infarction-responsive injectable hydrogels with combined anti-apoptosis, anti-inflammatory and pro-angiogenesis properties. Biomaterials. 2022;290 doi: 10.1016/j.biomaterials.2022.121849. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z., Mao X., Ye X., Li S., Wu T., Wang Q., Zhang J., Chen L., Tang N., He H., Wang Z., McAlinden C., Wang Q., Chen S., Gao R., Huang J. A novel and biocompatible nanofiber of VEGF peptide for enhanced corneal neovascularization suppression. Chem. Eng. J. 2021;416 doi: 10.1016/j.cej.2021.129081. [DOI] [Google Scholar]
- 22.Cai B., Gong Y., Wang Z., Wang L., Chen W. Microneedle arrays integrated with living organisms for smart biomedical applications. Theranostics. 2021;11(20):10012–10029. doi: 10.7150/thno.66478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma G., Wu C. Microneedle, bio-microneedle and bio-inspired microneedle: a review. J. Contr. Release. 2017;251:11–23. doi: 10.1016/j.jconrel.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Chen M., Quan G., Sun Y., Yang D., Pan X., Wu C. Nanoparticles-encapsulated polymeric microneedles for transdermal drug delivery. J. Contr. Release. 2020;325:163–175. doi: 10.1016/j.jconrel.2020.06.039. [DOI] [PubMed] [Google Scholar]
- 25.Singh V., Kesharwani P. Recent advances in microneedles-based drug delivery device in the diagnosis and treatment of cancer. J. Contr. Release. 2021;338:394–409. doi: 10.1016/j.jconrel.2021.08.054. [DOI] [PubMed] [Google Scholar]
- 26.Long L.-y., Zhang J., Yang Z., Guo Y., Hu X., Wang Y. Transdermal delivery of peptide and protein drugs: strategies, advantages and disadvantages. J. Drug Deliv. Sci. Technol. 2020;60 doi: 10.1016/j.jddst.2020.102007. [DOI] [Google Scholar]
- 27.Mohammed Y., Holmes A., Kwok P.C.L., Kumeria T., Namjoshi S., Imran M., Matteucci L., Ali M., Tai W., Benson H.A.E., Roberts M.S. Advances and future perspectives in epithelial drug delivery. Adv. Drug Deliv. Rev. 2022;186 doi: 10.1016/j.addr.2022.114293. [DOI] [PubMed] [Google Scholar]
- 28.Qu F., Geng R., Liu Y., Zhu J. Advanced nanocarrier- and microneedle-based transdermal drug delivery strategies for skin diseases treatment. Theranostics. 2022;12(7):3372–3406. doi: 10.7150/thno.69999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ullah A., Jang M., Khan H., Choi H.J., An S., Kim D., Kim Y.-R., Kim U.-K., Kim G.M. Microneedle array with a pH-responsive polymer coating and its application in smart drug delivery for wound healing. Sensor. Actuator. B Chem. 2021;345 doi: 10.1016/j.snb.2021.130441. [DOI] [Google Scholar]
- 30.Chang H., Zheng M., Chew S.W.T., Xu C. Advances in the formulations of microneedles for manifold biomedical applications. Advanced Materials Technologies. 2020;5(4) doi: 10.1002/admt.201900552. [DOI] [Google Scholar]
- 31.Leng F., Zheng M., Xu C. 3D-printed microneedles with open groove channels for liquid extraction. Explorations. 2021;1(3) doi: 10.1002/EXP.20210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J., Liu X., Fu Y., Song Y. Recent advances of microneedles for biomedical applications: drug delivery and beyond. Acta Pharm. Sin. B. 2019;9(3):469–483. doi: 10.1016/j.apsb.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J., Zhang H., Hu T., Xu C., Jiang L., Shrike Zhang Y., Xie M. Recent advances of microneedles used towards stimuli-responsive drug delivery, disease theranostics, and bioinspired applications. Chem. Eng. J. 2021;426 doi: 10.1016/j.cej.2021.130561. [DOI] [Google Scholar]
- 34.Yang J., Yang J., Gong X., Zheng Y., Yi S., Cheng Y., Li Y., Liu B., Xie X., Yi C., Jiang L. Recent progress in microneedles-mediated diagnosis, therapy, and theranostic systems. Advanced Healthcare Materials. 2022;11(10) doi: 10.1002/adhm.202102547. [DOI] [PubMed] [Google Scholar]
- 35.Mbituyimana B., Ma G., Shi Z., Yang G. Polymer-based microneedle composites for enhanced non-transdermal drug delivery. Appl. Mater. Today. 2022;29 doi: 10.1016/j.apmt.2022.101659. [DOI] [Google Scholar]
- 36.Zhang X.P., He Y.T., Li W.X., Chen B.Z., Zhang C.Y., Cui Y., Guo X.D. An update on biomaterials as microneedle matrixes for biomedical applications. J. Mater. Chem. B. 2022;10(32):6059–6077. doi: 10.1039/D2TB00905F. [DOI] [PubMed] [Google Scholar]
- 37.Yao Z., Xue T., Xiong H., Cai C., Liu X., Wu F., Liu S., Fan C. Promotion of collagen deposition during skin healing through Smad3/mTOR pathway by parathyroid hormone-loaded microneedle. Mater. Sci. Eng. C. 2021;119 doi: 10.1016/j.msec.2020.111446. [DOI] [PubMed] [Google Scholar]
- 38.Sun L., Fan L., Bian F., Chen G., Wang Y., Zhao Y. MXene-integrated microneedle patches with innate molecule encapsulation for wound healing. Research. 2021 doi: 10.34133/2021/9838490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu A., Wang Q., Zhao Z., Wu R., Wang M., Li J., Sun K., Sun Z., Lv Z., Xu J., Jiang H., Wan M., Shi D., Mao C. Nitric oxide nanomotor driving exosomes-loaded microneedles for Achilles tendinopathy healing. ACS Nano. 2021;15(8):13339–13350. doi: 10.1021/acsnano.1c03177. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C., Jia S., Huang J., Peng H., Zhang J., Liu L., Zhang W., Xin H., Wang X. A carbonized wormwood modified photothermal microneedle patch for the repair of damaged skeletal muscles. J. Mater. Chem. B. 2021;9(38):8014–8020. doi: 10.1039/D1TB00610J. [DOI] [PubMed] [Google Scholar]
- 41.Liu X., Li Y., Wang S., Lu M., Zou J., Shi Z., Xu B., Wang W., Hu B., Jin T., Wu F., Liu S., Fan C. PDGF-loaded microneedles promote tendon healing through p38/cyclin D1 pathway mediated angiogenesis. Materials Today Bio. 2022;16 doi: 10.1016/j.mtbio.2022.100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makvandi P., Maleki A., Shabani M., Hutton A.R.J., Kirkby M., Jamaledin R., Fang T., He J., Lee J., Mazzolai B., Donnelly R.F., Tay F.R., Chen G., Mattoli V. Bioinspired microneedle patches: biomimetic designs, fabrication, and biomedical applications. Matter. 2022;5(2):390–429. doi: 10.1016/j.matt.2021.11.021. [DOI] [Google Scholar]
- 43.Ali Zahid A., Chakraborty A., Shamiya Y., Ravi S.P., Paul A. Leveraging the advancements in functional biomaterials and scaffold fabrication technologies for chronic wound healing applications. Mater. Horiz. 2022;9(7):1850–1865. doi: 10.1039/D2MH00115B. [DOI] [PubMed] [Google Scholar]
- 44.Jamaledin R., Yiu C.K.Y., Zare E.N., Niu L.-N., Vecchione R., Chen G., Gu Z., Tay F.R., Makvandi P. Advances in antimicrobial microneedle patches for combating infections. Adv. Mater. 2020;32(33) doi: 10.1002/adma.202002129. [DOI] [PubMed] [Google Scholar]
- 45.Huang Y., Li H., Hu T., Li J., Yiu C.K., Zhou J., Li J., Huang X., Yao K., Qiu X., Zhou Y., Li D., Zhang B., Shi R., Liu Y., Wong T.H., Wu M., Jia H., Gao Z., Zhang Z., He J., Zheng M., Song E., Wang L., Xu C., Yu X. Implantable electronic medicine enabled by bioresorbable microneedles for wireless electrotherapy and drug delivery. Nano Lett. 2022;22(14):5944–5953. doi: 10.1021/acs.nanolett.2c01997. [DOI] [PubMed] [Google Scholar]
- 46.Long L., Liu W., Hu C., Yang L., Wang Y. Construction of multifunctional wound dressings with their application in chronic wound treatment. Biomater. Sci. 2022;10(15):4058–4076. doi: 10.1039/D2BM00620K. [DOI] [PubMed] [Google Scholar]
- 47.Sabri A.H., Kim Y., Marlow M., Scurr D.J., Segal J., Banga A.K., Kagan L., Lee J.B. Intradermal and transdermal drug delivery using microneedles – fabrication, performance evaluation and application to lymphatic delivery. Adv. Drug Deliv. Rev. 2020;153:195–215. doi: 10.1016/j.addr.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Ingrole R.S.J., Azizoglu E., Dul M., Birchall J.C., Gill H.S., Prausnitz M.R. Trends of microneedle technology in the scientific literature, patents, clinical trials and internet activity. Biomaterials. 2021;267 doi: 10.1016/j.biomaterials.2020.120491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ali M., Namjoshi S., Benson H.A.E., Mohammed Y., Kumeria T. Dissolvable polymer microneedles for drug delivery and diagnostics. J. Contr. Release. 2022;347:561–589. doi: 10.1016/j.jconrel.2022.04.043. [DOI] [PubMed] [Google Scholar]
- 50.Liu C., Zhao Z., Lv H., Yu J., Zhang P. Microneedles-mediated drug delivery system for the diagnosis and treatment of melanoma. Colloids Surf. B Biointerfaces. 2022;219 doi: 10.1016/j.colsurfb.2022.112818. [DOI] [PubMed] [Google Scholar]
- 51.Lee K., Goudie M.J., Tebon P., Sun W., Luo Z., Lee J., Zhang S., Fetah K., Kim H.-J., Xue Y., Darabi M.A., Ahadian S., Sarikhani E., Ryu W., Gu Z., Weiss P.S., Dokmeci M.R., Ashammakhi N., Khademhosseini A. Non-transdermal microneedles for advanced drug delivery. Adv. Drug Deliv. Rev. 2020;165–166:41–59. doi: 10.1016/j.addr.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh P., Carrier A., Chen Y., Lin S., Wang J., Cui S., Zhang X. Polymeric microneedles for controlled transdermal drug delivery. J. Contr. Release. 2019;315:97–113. doi: 10.1016/j.jconrel.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 53.Amani H., Shahbazi M.-A., D'Amico C., Fontana F., Abbaszadeh S., Santos H.A. Microneedles for painless transdermal immunotherapeutic applications. J. Contr. Release. 2021;330:185–217. doi: 10.1016/j.jconrel.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 54.Liu T., Chen M., Fu J., Sun Y., Lu C., Quan G., Pan X., Wu C. Recent advances in microneedles-mediated transdermal delivery of protein and peptide drugs. Acta Pharm. Sin. B. 2021;11(8):2326–2343. doi: 10.1016/j.apsb.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang D., Chen M., Sun Y., Jin Y., Lu C., Pan X., Quan G., Wu C. Microneedle-mediated transdermal drug delivery for treating diverse skin diseases. Acta Biomater. 2021;121:119–133. doi: 10.1016/j.actbio.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 56.Sharma S., Hatware K., Bhadane P., Sindhikar S., Mishra D.K. Recent advances in microneedle composites for biomedical applications: advanced drug delivery technologies. Mater. Sci. Eng. C. 2019;103 doi: 10.1016/j.msec.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Gupta J., Gill H.S., Andrews S.N., Prausnitz M.R. Kinetics of skin resealing after insertion of microneedles in human subjects. J. Contr. Release. 2011;154(2):148–155. doi: 10.1016/j.jconrel.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donnelly R.F., Morrow D.I.J., McCrudden M.T.C., Alkilani A.Z., Vicente-Pérez E.M., O'Mahony C., González-Vázquez P., McCarron P.A., Woolfson A.D. Hydrogel-forming and dissolving microneedles for enhanced delivery of photosensitizers and precursors. Photochem. Photobiol. 2014;90(3):641–647. doi: 10.1111/php.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu H., Mah Jian Qiang J., Wang C.G., Chan C.Y., Zhu Q., Ye E., Li Z., Loh X.J. Flexible polymeric patch based nanotherapeutics against non-cancer therapy. Bioact. Mater. 2022;18:471–491. doi: 10.1016/j.bioactmat.2022.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gill H.S., Prausnitz M.R. Coated microneedles for transdermal delivery. J. Contr. Release. 2007;117(2):227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sartawi Z., Blackshields C., Faisal W. Dissolving microneedles: applications and growing therapeutic potential. J. Contr. Release. 2022;348:186–205. doi: 10.1016/j.jconrel.2022.05.045. [DOI] [PubMed] [Google Scholar]
- 62.Bauleth-Ramos T., El-Sayed N., Fontana F., Lobita M., Shahbazi M.-A., Santos H.A. Recent approaches for enhancing the performance of dissolving microneedles in drug delivery applications. Mater. Today. 2023 doi: 10.1016/j.mattod.2022.12.007. [DOI] [Google Scholar]
- 63.Huang Y., Yu H., Wang L., Shen D., Ni Z., Ren S., Lu Y., Chen X., Yang J., Hong Y. Research progress on cosmetic microneedle systems: preparation, property and application. Eur. Polym. J. 2022;163 doi: 10.1016/j.eurpolymj.2021.110942. [DOI] [Google Scholar]
- 64.Yuan M., Liu K., Jiang T., Li S., Chen J., Wu Z., Li W., Tan R., Wei W., Yang X., Dai H., Chen Z. GelMA/PEGDA microneedles patch loaded with HUVECs-derived exosomes and Tazarotene promote diabetic wound healing. J. Nanobiotechnol. 2022;20(1):147. doi: 10.1186/s12951-022-01354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demir B., Rosselle L., Voronova A., Pagneux Q., Quenon A., Gmyr V., Jary D., Hennuyer N., Staels B., Hubert T., Abderrahmani A., Plaisance V., Pawlowski V., Boukherroub R., Vignoud S., Szunerits S. Innovative transdermal delivery of insulin using gelatin methacrylate-based microneedle patches in mice and mini-pigs. Nanoscale Horizons. 2022;7(2):174–184. doi: 10.1039/D1NH00596K. [DOI] [PubMed] [Google Scholar]
- 66.Turner J.G., White L.R., Estrela P., Leese H.S. Hydrogel-forming microneedles: current advancements and future trends. Macromol. Biosci. 2021;21(2) doi: 10.1002/mabi.202000307. [DOI] [PubMed] [Google Scholar]
- 67.Xue Y., Chen C., Tan R., Zhang J., Fang Q., Jin R., Mi X., Sun D., Xue Y., Wang Y., Xiong R., Lu H., Tan W. Artificial intelligence-assisted bioinformatics, microneedle, and diabetic wound healing: a “new deal” of an old drug. ACS Appl. Mater. Interfaces. 2022;14(33):37396–37409. doi: 10.1021/acsami.2c08994. [DOI] [PubMed] [Google Scholar]
- 68.Le Z., Yu J., Quek Y.J., Bai B., Li X., Shou Y., Myint B., Xu C., Tay A. Design principles of microneedles for drug delivery and sampling applications. Mater. Today. 2022 doi: 10.1016/j.mattod.2022.10.025. [DOI] [Google Scholar]
- 69.Li F., Zhang J., Yi K., Wang H., Wei H., Chan H.F., Tao Y., Li M. Delivery of stem cell secretome for therapeutic applications. ACS Appl. Bio Mater. 2022;5(5):2009–2030. doi: 10.1021/acsabm.1c01312. [DOI] [PubMed] [Google Scholar]
- 70.Chen B.Z., Zhao Z.Q., Shahbazi M.-A., Guo X.D. Microneedle-based technology for cell therapy: current status and future directions. Nanoscale Horizons. 2022;7(7):715–728. doi: 10.1039/D2NH00188H. [DOI] [PubMed] [Google Scholar]
- 71.Tansathien K., Suriyaamporn P., Ngawhirunpat T., Opanasopit P., Rangsimawong W. A novel approach for skin regeneration by a potent bioactive placental-loaded microneedle patch: comparative study of deer, goat, and porcine placentas. Pharmaceutics. 2022;14(6):1221. doi: 10.3390/pharmaceutics14061221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu Y., Wu X., Zhang X., Zu Y., Tan Q., Zhao Y. Living microneedle patch with adipose-derived stem cells embedding for diabetic ulcer healing. Adv. Funct. Mater. 2023;33(1) doi: 10.1002/adfm.202209986. [DOI] [Google Scholar]
- 73.Arshad M.S., Zahra A.T., Zafar S., Zaman H., Akhtar A., Ayaz M.M., Kucuk I., Maniruzzaman M., Chang M.-W., Ahmad Z. Antibiofilm effects of macrolide loaded microneedle patches: prospects in healing infected wounds. Pharmaceut. Res. 2021;38(1):165–177. doi: 10.1007/s11095-021-02995-0. [DOI] [PubMed] [Google Scholar]
- 74.Ma C.J., He Y., Jin X., Zhang Y., Zhang X., Li Y., Xu M., Liu K., Yao Y., Lu F. Light-regulated nitric oxide release from hydrogel-forming microneedles integrated with graphene oxide for biofilm-infected-wound healing. Biomaterials Advances. 2022;134 doi: 10.1016/j.msec.2021.112555. [DOI] [PubMed] [Google Scholar]
- 75.Yang X., Jia M., Li Z., Ma Z., Lv J., Jia D., He D., Zeng R., Luo G., Yu Y. In-situ synthesis silver nanoparticles in chitosan/Bletilla striata polysaccharide composited microneedles for infected and susceptible wound healing. Int. J. Biol. Macromol. 2022;215:550–559. doi: 10.1016/j.ijbiomac.2022.06.131. [DOI] [PubMed] [Google Scholar]
- 76.Su Y., Mainardi V.L., Wang H., McCarthy A., Zhang Y.S., Chen S., John J.V., Wong S.L., Hollins R.R., Wang G., Xie J. Dissolvable microneedles coupled with nanofiber dressings eradicate biofilms via effectively delivering a database-designed antimicrobial peptide. ACS Nano. 2020;14(9):11775–11786. doi: 10.1021/acsnano.0c04527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song H.B., Lee K.J., Seo I.H., Lee J.Y., Lee S.-M., Kim J.H., Kim J.H., Ryu W. Impact insertion of transfer-molded microneedle for localized and minimally invasive ocular drug delivery. J. Contr. Release. 2015;209:272–279. doi: 10.1016/j.jconrel.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 78.Lei X., Li M., Wang C., Cui P., Qiu L., Zhou S., Jiang P., Li H., Zhao D., Ni X., Wang J., Xia J. Degradable microneedle patches loaded with antibacterial gelatin nanoparticles to treat staphylococcal infection-induced chronic wounds. Int. J. Biol. Macromol. 2022;217:55–65. doi: 10.1016/j.ijbiomac.2022.07.021. [DOI] [PubMed] [Google Scholar]
- 79.Yao S., Wang Y., Chi J., Yu Y., Zhao Y., Luo Y., Wang Y. Porous MOF microneedle array patch with photothermal responsive nitric oxide delivery for wound healing. Adv. Sci. 2022;9(3) doi: 10.1002/advs.202103449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang X., Chen G., Liu Y., Sun L., Sun L., Zhao Y. Black phosphorus-loaded separable microneedles as responsive oxygen delivery carriers for wound healing. ACS Nano. 2020;14(5):5901–5908. doi: 10.1021/acsnano.0c01059. [DOI] [PubMed] [Google Scholar]
- 81.Fonseca D.F.S., Vilela C., Pinto R.J.B., Bastos V., Oliveira H., Catarino J., Faísca P., Rosado C., Silvestre A.J.D., Freire C.S.R. Bacterial nanocellulose-hyaluronic acid microneedle patches for skin applications: in vitro and in vivo evaluation. Mater. Sci. Eng. C. 2021;118 doi: 10.1016/j.msec.2020.111350. [DOI] [PubMed] [Google Scholar]
- 82.Davis S.P., Landis B.J., Adams Z.H., Allen M.G., Prausnitz M.R. Insertion of microneedles into skin: measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004;37(8):1155–1163. doi: 10.1016/j.jbiomech.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 83.Lim S., Park T.Y., Jeon E.Y., Joo K.I., Cha H.J. Double-layered adhesive microneedle bandage based on biofunctionalized mussel protein for cardiac tissue regeneration. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121171. [DOI] [PubMed] [Google Scholar]
- 84.Park J.-H., Allen M.G., Prausnitz M.R. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J. Contr. Release. 2005;104(1):51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 85.Cui M., Zheng M., Wiraja C., Chew S.W.T., Mishra A., Mayandi V., Lakshminarayanan R., Xu C. Ocular delivery of predatory bacteria with cryomicroneedles against eye infection. Adv. Sci. 2021;8(21) doi: 10.1002/advs.202102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee K., Xue Y., Lee J., Kim H.-J., Liu Y., Tebon P., Sarikhani E., Sun W., Zhang S., Haghniaz R., Çelebi-Saltik B., Zhou X., Ostrovidov S., Ahadian S., Ashammakhi N., Dokmeci M.R., Khademhosseini A. A patch of detachable hybrid microneedle depot for localized delivery of mesenchymal stem cells in regeneration therapy. Adv. Funct. Mater. 2020;30(23) doi: 10.1002/adfm.202000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Long L.-y., Liu W., Li L., Hu C., He S., Lu L., Wang J., Yang L., Wang Y.-b. Dissolving microneedle-encapsulated drug-loaded nanoparticles and recombinant humanized collagen type III for the treatment of chronic wound via anti-inflammation and enhanced cell proliferation and angiogenesis. Nanoscale. 2022;14(4):1285–1295. doi: 10.1039/D1NR07708B. [DOI] [PubMed] [Google Scholar]
- 88.Chen Y., Yu W., Qian X., Li X., Wang Y., Ji J. Dissolving microneedles with a biphasic release of antibacterial agent and growth factor to promote wound healing. Biomater. Sci. 2022;10(9):2409–2416. doi: 10.1039/D2BM00281G. [DOI] [PubMed] [Google Scholar]
- 89.Ma W., Zhang X., Liu Y., Fan L., Gan J., Liu W., Zhao Y., Sun L. Polydopamine decorated microneedles with Fe-MSC-Derived nanovesicles encapsulation for wound healing. Adv. Sci. 2022;9(13) doi: 10.1002/advs.202103317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Than A., Liu C., Chang H., Duong P.K., Cheung C.M.G., Xu C., Wang X., Chen P. Self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drug delivery. Nat. Commun. 2018;9(1):4433. doi: 10.1038/s41467-018-06981-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ning T., Yang F., Chen D., Jia Z., Yuan R., Du Z., Liu S., Yu Y., Dai X., Niu X., Fan Y. Synergistically detachable microneedle dressing for programmed treatment of chronic wounds. Advanced Healthcare Materials. 2022;11(11) doi: 10.1002/adhm.202102180. [DOI] [PubMed] [Google Scholar]
- 92.Yin M., Wu J., Deng M., Wang P., Ji G., Wang M., Zhou C., Blum N.T., Zhang W., Shi H., Jia N., Wang X., Huang P. Multifunctional magnesium organic framework-based microneedle patch for accelerating diabetic wound healing. ACS Nano. 2021;15(11):17842–17853. doi: 10.1021/acsnano.1c06036. [DOI] [PubMed] [Google Scholar]
- 93.Guan G., Zhang Q., Jiang Z., Liu J., Wan J., Jin P., Lv Q. Multifunctional silk fibroin methacryloyl microneedle for diabetic wound healing. Small. 2022;18(51) doi: 10.1002/smll.202203064. [DOI] [PubMed] [Google Scholar]
- 94.Gao S., Zhang W., Zhai X., Zhao X., Wang J., Weng J., Li J., Chen X. An antibacterial and proangiogenic double-layer drug-loaded microneedle patch for accelerating diabetic wound healing. Biomater. Sci. 2023;11(2):533–541. doi: 10.1039/D2BM01588A. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Q., Shi L., He H., Liu X., Huang Y., Xu D., Yao M., Zhang N., Guo Y., Lu Y., Li H., Zhou J., Tan J., Xing M., Luo G. Down-regulating scar formation by microneedles directly via a mechanical communication pathway. ACS Nano. 2022;16(7):10163–10178. doi: 10.1021/acsnano.1c11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu Y., Ren T., Zhang H., Jin Q., Shen L., Shan M., Zhao X., Chen Q., Dai H., Yao L., Xie J., Ye D., Lin T., Hong X., Deng K., Shen T., Pan J., Jia M., Ling J., Li P., Zhang Y., Wang H., Zhuang L., Gao C., Mao J., Zhu Y. A honeybee stinger-inspired self-interlocking microneedle patch and its application in myocardial infarction treatment. Acta Biomater. 2022;153:386–398. doi: 10.1016/j.actbio.2022.09.015. [DOI] [PubMed] [Google Scholar]
- 97.Jeon E.Y., Lee J., Kim B.J., Joo K.I., Kim K.H., Lim G., Cha H.J. Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle protein patch for regenerative internal/external surgical closure. Biomaterials. 2019;222 doi: 10.1016/j.biomaterials.2019.119439. [DOI] [PubMed] [Google Scholar]
- 98.Zhang X., Chen G., Cai L., Wang Y., Sun L., Zhao Y. Bioinspired pagoda-like microneedle patches with strong fixation and hemostasis capabilities. Chem. Eng. J. 2021;414 doi: 10.1016/j.cej.2021.128905. [DOI] [Google Scholar]
- 99.Deng Y., Yang C., Zhu Y., Liu W., Li H., Wang L., Chen W., Wang Z., Wang L. Lamprey-teeth-inspired oriented antibacterial sericin microneedles for infected wound healing improvement. Nano Lett. 2022;22(7):2702–2711. doi: 10.1021/acs.nanolett.1c04573. [DOI] [PubMed] [Google Scholar]
- 100.Zhang X., Chen G., Sun L., Ye F., Shen X., Zhao Y. Claw-inspired microneedle patches with liquid metal encapsulation for accelerating incisional wound healing. Chem. Eng. J. 2021;406 doi: 10.1016/j.cej.2020.126741. [DOI] [Google Scholar]
- 101.Guo M., Wang Y., Gao B., He B. Shark tooth-inspired microneedle dressing for intelligent wound management. ACS Nano. 2021;15(9):15316–15327. doi: 10.1021/acsnano.1c06279. [DOI] [PubMed] [Google Scholar]
- 102.Zhang X., Meng Y., Gong B., Wang T., Lu Y., Zhang L., Xue J. Electrospun nanofibers for manipulating soft tissue regeneration. J. Mater. Chem. B. 2022;10(37):7281–7308. doi: 10.1039/D2TB00609J. [DOI] [PubMed] [Google Scholar]
- 103.Barnum L., Samandari M., Schmidt T.A., Tamayol A. Microneedle arrays for the treatment of chronic wounds. Expet Opin. Drug Deliv. 2020;17(12):1767–1780. doi: 10.1080/17425247.2020.1819787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chun Y.Y., Tan W.W.R., Vos M.I.G., Chan W.K., Tey H.L., Tan N.S., Tan T.T.Y. Scar prevention through topical delivery of gelatin-tyramine-siSPARC nanoplex loaded in dissolvable hyaluronic acid microneedle patch across skin barrier. Biomater. Sci. 2022;10(14):3963–3971. doi: 10.1039/D2BM00572G. [DOI] [PubMed] [Google Scholar]
- 105.Su Y., McCarthy A., Wong S.L., Hollins R.R., Wang G., Xie J. Simultaneous delivery of multiple antimicrobial agents by biphasic scaffolds for effective treatment of wound biofilms. Advanced Healthcare Materials. 2021;10(12) doi: 10.1002/adhm.202100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park S.Y., Lee H.U., Lee Y.-C., Kim G.H., Park E.C., Han S.H., Lee J.G., Choi S., Heo N.S., Kim D.L., Huh Y.S., Lee J. Wound healing potential of antibacterial microneedles loaded with green tea extracts. Mater. Sci. Eng. C. 2014;42:757–762. doi: 10.1016/j.msec.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 107.Yu X., Zhao J., Fan D. A dissolving microneedle patch for Antibiotic/Enzymolysis/Photothermal triple therapy against bacteria and their biofilms. Chem. Eng. J. 2022;437 doi: 10.1016/j.cej.2022.135475. [DOI] [Google Scholar]
- 108.Gong J.-H., Chen L.-J., Zhao X., Yan X.-P. Persistent production of reactive oxygen species with Zn2GeO4:Cu nanorod-loaded microneedles for methicillin-resistant Staphylococcus aureus infectious wound healing. ACS Appl. Mater. Interfaces. 2022;14(15):17142–17152. doi: 10.1021/acsami.2c02503. [DOI] [PubMed] [Google Scholar]
- 109.Chi J., Sun L., Cai L., Fan L., Shao C., Shang L., Zhao Y. Chinese herb microneedle patch for wound healing. Bioact. Mater. 2021;6(10):3507–3514. doi: 10.1016/j.bioactmat.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yao S., Chi J., Wang Y., Zhao Y., Luo Y., Wang Y. Zn-MOF encapsulated antibacterial and degradable microneedles array for promoting wound healing. Advanced Healthcare Materials. 2021;10(12) doi: 10.1002/adhm.202100056. [DOI] [PubMed] [Google Scholar]
- 111.Zhao M., Zhou M., Gao P., Zheng X., Yu W., Wang Z., Li J., Zhang J. AgNPs/nGOx/Apra nanocomposites for synergistic antimicrobial therapy and scarless skin recovery. J. Mater. Chem. B. 2022;10(9):1393–1402. doi: 10.1039/D1TB01991K. [DOI] [PubMed] [Google Scholar]
- 112.Sun Y., Liu J., Wang H., Li S., Pan X., Xu B., Yang H., Wu Q., Li W., Su X., Huang Z., Guo X., Liu H. NIR laser-triggered microneedle-based liquid band-aid for wound care. Adv. Funct. Mater. 2021;31(29) doi: 10.1002/adfm.202100218. [DOI] [Google Scholar]
- 113.Chi J., Zhang X., Chen C., Shao C., Zhao Y., Wang Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020;5(2):253–259. doi: 10.1016/j.bioactmat.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu X., Wang C., Wang Y., Li L., Gao X., Zhu T., An P., Meng Z., Wang W., Wu T., Hao Y. Microneedle array patch made of kangfuxin/chitosan/fucoidan complex enables full-thickness wound healing. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.838920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang P., Wang Y., Yi Y., Gong Y., Ji H., Gan Y., Xie F., Fan J., Wang X. MXenes-integrated microneedle combined with asiaticoside to penetrate the cuticle for treatment of diabetic foot ulcer. J. Nanobiotechnol. 2022;20(1):259. doi: 10.1186/s12951-022-01468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Singh N., Armstrong D.G., Lipsky B.A. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 117.Guo Z., Liu H., Shi Z., Lin L., Li Y., Wang M., Pan G., Lei Y., Xue L. Responsive hydrogel-based microneedle dressing for diabetic wound healing. J. Mater. Chem. B. 2022;10(18):3501–3511. doi: 10.1039/D2TB00126H. [DOI] [PubMed] [Google Scholar]
- 118.Zeng Z., Jiang G., Sun Y., Aharodnikau U.E., Yunusov K.E., Gao X., Liu T., Solomevich S.O. Rational design of flexible microneedles coupled with CaO2@PDA-loaded nanofiber films for skin wound healing on diabetic rats. Biomater. Sci. 2022;10(18):5326–5339. doi: 10.1039/D2BM00861K. [DOI] [PubMed] [Google Scholar]
- 119.Gan J., Zhang X., Ma W., Zhao Y., Sun L. Antibacterial, adhesive, and MSC exosomes encapsulated microneedles with spatio-temporal variation functions for diabetic wound healing. Nano Today. 2022;47 doi: 10.1016/j.nantod.2022.101630. [DOI] [Google Scholar]
- 120.Li X., Zhao Z., Zhang M., Ling G., Zhang P. Research progress of microneedles in the treatment of melanoma. J. Contr. Release. 2022;348:631–647. doi: 10.1016/j.jconrel.2022.06.021. [DOI] [PubMed] [Google Scholar]
- 121.Shan Y., Tan B., Zhang M., Xie X., Liao J. Restorative biodegradable two-layered hybrid microneedles for melanoma photothermal/chemo co-therapy and wound healing. J. Nanobiotechnol. 2022;20(1):238. doi: 10.1186/s12951-022-01426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lei Q., He D., Ding L., Kong F., He P., Huang J., Guo J., Brinker C.J., Luo G., Zhu W., Yu Y. Microneedle patches integrated with biomineralized melanin nanoparticles for simultaneous skin tumor photothermal therapy and wound healing. Adv. Funct. Mater. 2022;32(22) doi: 10.1002/adfm.202113269. [DOI] [Google Scholar]
- 123.Gao B., Guo M., Lyu K., Chu T., He B. Intelligent silk fibroin based microneedle dressing (i-SMD) Adv. Funct. Mater. 2021;31(3) doi: 10.1002/adfm.202006839. [DOI] [Google Scholar]
- 124.Shi S., Jiang Y., Yu Y., Liang M., Bai Q., Wang L., Yang D., Sui N., Zhu Z. Piezo-augmented and photocatalytic nanozyme integrated microneedles for antibacterial and anti-inflammatory combination therapy. Adv. Funct. Mater. 2022;n/a(n/a) doi: 10.1002/adfm.202210850. [DOI] [Google Scholar]
- 125.Wang Y., Lu H., Guo M., Chu J., Gao B., He B. Personalized and programmable microneedle dressing for promoting wound healing. Advanced Healthcare Materials. 2022;11(2) doi: 10.1002/adhm.202101659. [DOI] [PubMed] [Google Scholar]
- 126.Shan J., Zhang X., Kong B., Zhu Y., Gu Z., Ren L., Zhao Y. Coordination polymer nanozymes-integrated colorimetric microneedle patches for intelligent wound infection management. Chem. Eng. J. 2022;444 doi: 10.1016/j.cej.2022.136640. [DOI] [Google Scholar]
- 127.Bikbova G., Oshitari T., Baba T., Yamamoto S. Neuronal changes in the diabetic cornea: perspectives for neuroprotection. BioMed Research International, 2016. 2016 doi: 10.1155/2016/5140823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sacchetti M., Lambiase A. Neurotrophic factors and corneal nerve regeneration, Neural Regen. Res. 2017;12(8) doi: 10.4103/1673-5374.213534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ljubimov A.V., Saghizadeh M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015;49:17–45. doi: 10.1016/j.preteyeres.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mohan R.R., Kempuraj D., D'Souza S., Ghosh A. Corneal stromal repair and regeneration. Prog. Retin. Eye Res. 2022;91 doi: 10.1016/j.preteyeres.2022.101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McTiernan C.D., Simpson F.C., Haagdorens M., Samarawickrama C., Hunter D., Buznyk O., Fagerholm P., Ljunggren M.K., Lewis P., Pintelon I., Olsen D., Edin E., Groleau M., Allan B.D., Griffith M. LiQD Cornea: Pro-regeneration collagen mimetics as patches and alternatives to corneal transplantation. Science Advances. 2020;6(25) doi: 10.1126/sciadv.aba2187. eaba2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kumar A., Yun H., Funderburgh M.L., Du Y. Regenerative therapy for the cornea. Prog. Retin. Eye Res. 2022;87 doi: 10.1016/j.preteyeres.2021.101011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gupta P., Yadav K.S. Applications of microneedles in delivering drugs for various ocular diseases. Life Sci. 2019;237 doi: 10.1016/j.lfs.2019.116907. [DOI] [PubMed] [Google Scholar]
- 134.Vora L.K., Moffatt K., Tekko I.A., Paredes A.J., Volpe-Zanutto F., Mishra D., Peng K., Raj Singh Thakur R., Donnelly R.F. Microneedle array systems for long-acting drug delivery. Eur. J. Pharm. Biopharm. 2021;159:44–76. doi: 10.1016/j.ejpb.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 135.Bariya S.H., Gohel M.C., Mehta T.A., Sharma O.P. Microneedles: an emerging transdermal drug delivery system. J. Pharm. Pharmacol. 2012;64(1):11–29. doi: 10.1111/j.2042-7158.2011.01369.x. [DOI] [PubMed] [Google Scholar]
- 136.Lee K., Park S., Jo D.H., Cho C.S., Jang H.Y., Yi J., Kang M., Kim J., Jung H.Y., Kim J.H., Ryu W., Khademhosseini A. Self-plugging microneedle (SPM) for intravitreal drug delivery. Advanced Healthcare Materials. 2022;11(12) doi: 10.1002/adhm.202102599. [DOI] [PubMed] [Google Scholar]
- 137.Park W., Nguyen V.P., Jeon Y., Kim B., Li Y., Yi J., Kim H., Leem J.W., Kim Y.L., Kim D.R., Paulus Y.M., Lee C.H. Biodegradable silicon nanoneedles for ocular drug delivery. Science Advances. 2022;8(13) doi: 10.1126/sciadv.abn1772. eabn1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Basu S., Hertsenberg A.J., Funderburgh M.L., Burrow M.K., Mann M.M., Du Y., Lathrop K.L., Syed-Picard F.N., Adams S.M., Birk D.E., Funderburgh J.L. Human limbal biopsy–derived stromal stem cells prevent corneal scarring. Sci. Transl. Med. 2014;6(266) doi: 10.1126/scitranslmed.3009644. 266ra172-266ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bourne R.R.A., Flaxman S.R., Braithwaite T., Cicinelli M.V., Das A., Jonas J.B., Keeffe J., Kempen J.H., Leasher J., Limburg H., Naidoo K., Pesudovs K., Resnikoff S., Silvester A., Stevens G.A., Tahhan N., Wong T.Y., Taylor H.R., Bourne R., Ackland P., Arditi A., Barkana Y., Bozkurt B., Braithwaite T., Bron A., Budenz D., Cai F., Casson R., Chakravarthy U., Choi J., Cicinelli M.V., Congdon N., Dana R., Dandona R., Dandona L., Das A., Dekaris I., Del Monte M., Deva J., Dreer L., Ellwein L., Frazier M., Frick K., Friedman D., Furtado J., Gao H., Gazzard G., George R., Gichuhi S., Gonzalez V., Hammond B., Hartnett M.E., He M., Hejtmancik J., Hirai F., Huang J., Ingram A., Javitt J., Jonas J., Joslin C., Keeffe J., Kempen J., Khairallah M., Khanna R., Kim J., Lambrou G., Lansingh V.C., Lanzetta P., Leasher J., Lim J., Limburg H., Mansouri K., Mathew A., Morse A., Munoz B., Musch D., Naidoo K., Nangia V., Palaiou M., Parodi M.B., Pena F.Y., Pesudovs K., Peto T., Quigley H., Raju M., Ramulu P., Resnikoff S., Robin A., Rossetti L., Saaddine J., Sandar M.Y.A., Serle J., Shen T., Shetty R., Sieving P., Silva J.C., Silvester A., Sitorus R.S., Stambolian D., Stevens G., Taylor H., Tejedor J., Tielsch J., Tsilimbaris M., van Meurs J., Varma R., Virgili G., Volmink J., Wang Y.X., Wang N.-L., West S., Wiedemann P., Wong T., Wormald R., Zheng Y. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Global Health. 2017;5(9):e888–e897. doi: 10.1016/S2214-109X(17)30293-0. [DOI] [PubMed] [Google Scholar]
- 140.Iyer S.A., Tuli S.S., Wagoner R.C. Fungal keratitis: emerging trends and treatment outcomes. Eye Contact Lens. 2006;32(6):267–271. doi: 10.1097/01.icl.0000249595.27520.2e. [DOI] [PubMed] [Google Scholar]
- 141.Srinivasan M. Fungal keratitis. Curr. Opin. Ophthalmol. 2004;15(4) doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 142.Jian H.-J., Wu R.-S., Lin T.-Y., Li Y.-J., Lin H.-J., Harroun S.G., Lai J.-Y., Huang C.-C. Super-cationic carbon quantum dots synthesized from spermidine as an eye drop formulation for topical treatment of bacterial keratitis. ACS Nano. 2017;11(7):6703–6716. doi: 10.1021/acsnano.7b01023. [DOI] [PubMed] [Google Scholar]
- 143.Park S., Lee K., Kang H., Lee Y., Lee J., Kim J.H., Song H.B., Ryu W. Single administration of a biodegradable, separable microneedle can substitute for repeated application of eyedrops in the treatment of infectious keratitis. Advanced Healthcare Materials. 2021;10(11) doi: 10.1002/adhm.202002287. [DOI] [PubMed] [Google Scholar]
- 144.Shi H., Zhou J., Wang Y., Zhu Y., Lin D., Lei L., Vakal S., Wang J., Li X. A rapid corneal healing microneedle for efficient ocular drug delivery. Small. 2022;18(4) doi: 10.1002/smll.202104657. [DOI] [PubMed] [Google Scholar]
- 145.Lv H., Wu B., Song J., Wu W., Cai W., Xu J. Hydrogel, a novel therapeutic and delivery strategy, in the treatment of intrauterine adhesions. J. Mater. Chem. B. 2021;9(33):6536–6552. doi: 10.1039/D1TB01005K. [DOI] [PubMed] [Google Scholar]
- 146.Cai Y., Wu F., Yu Y., Liu Y., Shao C., Gu H., Li M., Zhao Y. Porous scaffolds from droplet microfluidics for prevention of intrauterine adhesion. Acta Biomater. 2019;84:222–230. doi: 10.1016/j.actbio.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 147.Ji W., Hou B., Lin W., Wang L., Zheng W., Li W., Zheng J., Wen X., He P. 3D Bioprinting a human iPSC-derived MSC-loaded scaffold for repair of the uterine endometrium. Acta Biomater. 2020;116:268–284. doi: 10.1016/j.actbio.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 148.Xin L., Wei C., Tong X., Dai Y., Huang D., Chen J., Ma L., Zhang S. In situ delivery of apoptotic bodies derived from mesenchymal stem cells via a hyaluronic acid hydrogel: a therapy for intrauterine adhesions. Bioact. Mater. 2022;12:107–119. doi: 10.1016/j.bioactmat.2021.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang S., Chang Q., Li P., Tong X., Feng Y., Hao X., Zhang X., Yuan Z., Tan J. Concentrated small extracellular vesicles from menstrual blood-derived stromal cells improve intrauterine adhesion, a pre-clinical study in a rat model. Nanoscale. 2021;13(15):7334–7347. doi: 10.1039/D0NR08942G. [DOI] [PubMed] [Google Scholar]
- 150.Kou L., Jiang X., Xiao S., Zhao Y.-Z., Yao Q., Chen R. Therapeutic options and drug delivery strategies for the prevention of intrauterine adhesions. J. Contr. Release. 2020;318:25–37. doi: 10.1016/j.jconrel.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 151.Zhu R., Gan L., Wang S., Duan H. A cohort study comparing the severity and outcome of intrauterine adhesiolysis for Asherman syndrome after first- or second-trimester termination of pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019;238:49–53. doi: 10.1016/j.ejogrb.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 152.Zhu Y., Li S., Li Y., Tan H., Zhao Y., Sun L. Antioxidant nanozyme microneedles with stem cell loading for in situ endometrial repair. Chem. Eng. J. 2022;449 doi: 10.1016/j.cej.2022.137786. [DOI] [Google Scholar]
- 153.Zhang X., Chen G., Wang Y., Fan L., Zhao Y. Arrowhead composite microneedle patches with anisotropic surface adhesion for preventing intrauterine adhesions. Adv. Sci. 2022;9(12) doi: 10.1002/advs.202104883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li S., Li Y., Yu F., Li N., Liu C., Mao J., Sun H., Hu Y., Zhu Y., Zhou M., Ding L. Human endometrium-derived adventitial cell spheroid-loaded antimicrobial microneedles for uterine regeneration. Small. 2022;18(31) doi: 10.1002/smll.202201225. [DOI] [PubMed] [Google Scholar]
- 155.Zhuang Y., Cui W. Biomaterial-based delivery of nucleic acids for tissue regeneration. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113885. [DOI] [PubMed] [Google Scholar]
- 156.Fang J., Li J.J., Zhong X., Zhou Y., Lee R.J., Cheng K., Li S. Engineering stem cell therapeutics for cardiac repair. J. Mol. Cell. Cardiol. 2022;171:56–68. doi: 10.1016/j.yjmcc.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 157.Yang Q., Fang J., Lei Z., Sluijter J.P.G., Schiffelers R. Repairing the heart: state-of the art delivery strategies for biological therapeutics. Adv. Drug Deliv. Rev. 2020;160:1–18. doi: 10.1016/j.addr.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 158.Wang Y., Li G., Yang L., Luo R., Guo G. Development of innovative biomaterials and devices for the treatment of cardiovascular diseases. Adv. Mater. 2022;34(46) doi: 10.1002/adma.202201971. [DOI] [PubMed] [Google Scholar]
- 159.Tang J., Wang J., Huang K., Ye Y., Su T., Qiao L., Hensley M.T., Caranasos T.G., Zhang J., Gu Z., Cheng K. Cardiac cell–integrated microneedle patch for treating myocardial infarction. Science Advances. 2018;4(11) doi: 10.1126/sciadv.aat9365. eaat9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hu S., Zhu D., Li Z., Cheng K. Detachable microneedle patches deliver mesenchymal stromal cell factor-loaded nanoparticles for cardiac repair. ACS Nano. 2022;16(10):15935–15945. doi: 10.1021/acsnano.2c03060. [DOI] [PubMed] [Google Scholar]
- 161.Shi H., Xue T., Yang Y., Jiang C., Huang S., Yang Q., Lei D., You Z., Jin T., Wu F., Zhao Q., Ye X. Microneedle-mediated gene delivery for the treatment of ischemic myocardial disease. Sci. Adv. 2020;6(25) doi: 10.1126/sciadv.aaz3621. eaaz3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sun L., Zhu X., Zhang X., Chen G., Bian F., Wang J., Zhou Q., Wang D., Zhao Y. Induced cardiomyocytes-integrated conductive microneedle patch for treating myocardial infarction. Chem. Eng. J. 2021;414 doi: 10.1016/j.cej.2021.128723. [DOI] [Google Scholar]
- 163.Fan Z., Wei Y., Yin Z., Huang H., Liao X., Sun L., Liu B., Liu F. Near-infrared light-triggered unfolding microneedle patch for minimally invasive treatment of myocardial ischemia. ACS Appl. Mater. Interfaces. 2021;13(34):40278–40289. doi: 10.1021/acsami.1c09658. [DOI] [PubMed] [Google Scholar]
- 164.Chen H., Fan L., Peng N., Yin Y., Mu D., Wang J., Meng R., Xie J. Galunisertib-loaded gelatin methacryloyl hydrogel microneedle patch for cardiac repair after myocardial infarction. ACS Appl. Mater. Interfaces. 2022;14(36):40491–40500. doi: 10.1021/acsami.2c05352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang Q., Shi B., Ding J., Yan L., Thawani J.P., Fu C., Chen X. Polymer scaffolds facilitate spinal cord injury repair. Acta Biomater. 2019;88:57–77. doi: 10.1016/j.actbio.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 166.Kiyotake E.A., Martin M.D., Detamore M.S. Regenerative rehabilitation with conductive biomaterials for spinal cord injury. Acta Biomater. 2022;139:43–64. doi: 10.1016/j.actbio.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 167.Silva D., Sousa R.A., Salgado A.J. Hydrogels as delivery systems for spinal cord injury regeneration. Materials Today Bio. 2021;9 doi: 10.1016/j.mtbio.2021.100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wu Z., Zhao Z., Yu Y., Hu X., Xu W., Zeng Z., Sun Y.E., Cheng L. New strategies for the repair of spinal cord injury. Chin. Sci. Bull. 2014;59(31):4041–4049. doi: 10.1007/s11434-014-0484-2. [DOI] [Google Scholar]
- 169.Luo Y., Xue F., Liu K., Li B., Fu C., Ding J. Physical and biological engineering of polymer scaffolds to potentiate repair of spinal cord injury. Mater. Des. 2021;201 doi: 10.1016/j.matdes.2021.109484. [DOI] [Google Scholar]
- 170.Huang J., Yap N., Walter M., Green A., Smith C., Johnson J., Saigal R. 3D-Printed polypyrrole microneedle arrays for electronically controlled transdural drug release. ACS Biomater. Sci. Eng. 2022;8(4):1544–1553. doi: 10.1021/acsbiomaterials.1c01305. [DOI] [PubMed] [Google Scholar]
- 171.Fang A., Wang Y., Guan N., Lin L., Guo B., Cai W., Chen X., Ye J., Abdelrahman Z., Li X., Zuo Y., Zheng H., Wu Z., Jin S., Xu K., Gu X., Yu B., Wang X. Porous microneedle patch with sustained exosome delivery repairs severe spinal cord injury. bioRxiv. 2022 doi: 10.1101/2022.07.18.500400. 2022.2007.2018.500400. [DOI] [Google Scholar]
- 172.Han M., Yang H., Lu X., Li Y., Liu Z., Li F., Shang Z., Wang X., Li X., Li J., Liu H., Xin T. Three-dimensional-cultured MSC-derived exosome-hydrogel hybrid microneedle array patch for spinal cord repair. Nano Lett. 2022;22(15):6391–6401. doi: 10.1021/acs.nanolett.2c02259. [DOI] [PubMed] [Google Scholar]
- 173.Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 174.Chen F.-M., Zhang J., Zhang M., An Y., Chen F., Wu Z.-F. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 2010;31(31):7892–7927. doi: 10.1016/j.biomaterials.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 175.Polimeni G., Xiropaidis A.V., Wikesjö U.M.E. Biology and principles of periodontal wound healing/regeneration. Periodontol. 2000. 2006;41(1):30–47. doi: 10.1111/j.1600-0757.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- 176.Zhang X., Hasani-Sadrabadi M.M., Zarubova J., Dashtimighadam E., Haghniaz R., Khademhosseini A., Butte M.J., Moshaverinia A., Aghaloo T., Li S. Immunomodulatory microneedle patch for periodontal tissue regeneration. Matter. 2022;5(2):666–682. doi: 10.1016/j.matt.2021.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang Y., Sheng A.a., Jiang X., Yang S., Lin L., Yang M., Zhu F., Hu Y., Li J., Chang L. 2022. Multidrug dissolvable microneedle patch for the treatment of recurrent oral ulcer, Bio-Design and Manufacturing. [DOI] [Google Scholar]
- 178.Guo X., Zhu T., Yu X., Yi X., Li L., Qu X., Zhang Z., Hao Y., Wang W. Betamethasone-loaded dissolvable microneedle patch for oral ulcer treatment. Colloids Surf. B Biointerfaces. 2023;222 doi: 10.1016/j.colsurfb.2022.113100. [DOI] [PubMed] [Google Scholar]
- 179.Zeng C., Chen Z., Yang H., Fan Y., Fei L., Chen X., Zhang M. Advanced high resolution three-dimensional imaging to visualize the cerebral neurovascular network in stroke. Int. J. Biol. Sci. 2022;18(2):552–571. doi: 10.7150/ijbs.64373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Campbell B.C.V., Khatri P. Stroke, The Lancet. 2020;396(10244):129–142. doi: 10.1016/S0140-6736(20)31179-X. [DOI] [PubMed] [Google Scholar]
- 181.Kong J., Chu R., Wang Y. Neuroprotective treatments for ischemic stroke: opportunities for nanotechnology. Adv. Funct. Mater. 2022;32(52) doi: 10.1002/adfm.202209405. [DOI] [Google Scholar]
- 182.Liu Y., Long L., Zhang F., Hu X., Zhang J., Hu C., Wang Y., Xu J. Microneedle-mediated vascular endothelial growth factor delivery promotes angiogenesis and functional recovery after stroke. J. Contr. Release. 2021;338:610–622. doi: 10.1016/j.jconrel.2021.08.057. [DOI] [PubMed] [Google Scholar]
- 183.Chen C., Bai X., Ding Y., Lee I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019;23(1):25. doi: 10.1186/s40824-019-0176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hayes A.J., Melrose J. Electro-stimulation, a promising therapeutic treatment modality for tissue repair: emerging roles of sulfated glycosaminoglycans as electro-regulatory mediators of intrinsic repair processes. Advanced Therapeutics. 2020;3(11) doi: 10.1002/adtp.202000151. [DOI] [Google Scholar]
- 185.Cucchiarini M., Madry H. Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair. Nat. Rev. Rheumatol. 2019;15(1):18–29. doi: 10.1038/s41584-018-0125-2. [DOI] [PubMed] [Google Scholar]
- 186.Yu K., Yu X., Cao S., Wang Y., Zhai Y., Yang F., Yang X., Lu Y., Wu C., Xu Y. Layered dissolving microneedles as a need-based delivery system to simultaneously alleviate skin and joint lesions in psoriatic arthritis. Acta Pharm. Sin. B. 2021;11(2):505–519. doi: 10.1016/j.apsb.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Grogan S.P., Dorthé E.W., Glembotski N.E., Gaul F., D'Lima D.D. Cartilage tissue engineering combining microspheroid building blocks and microneedle arrays. Connect. Tissue Res. 2020;61(2):229–243. doi: 10.1080/03008207.2019.1617280. [DOI] [PubMed] [Google Scholar]
- 188.Long L.-y., Hu C., Liu W., Wu C., Lu L., Yang L., Wang Y.-b. Microfibrillated cellulose-enhanced carboxymethyl chitosan/oxidized starch sponge for chronic diabetic wound repair. Biomaterials Advances. 2022;135 doi: 10.1016/j.msec.2022.112669. [DOI] [PubMed] [Google Scholar]
- 189.Yuk H., Varela C.E., Nabzdyk C.S., Mao X., Padera R.F., Roche E.T., Zhao X. Dry double-sided tape for adhesion of wet tissues and devices. Nature. 2019;575(7781):169–174. doi: 10.1038/s41586-019-1710-5. [DOI] [PubMed] [Google Scholar]
- 190.Haghniaz R., Kim H.-J., Montazerian H., Baidya A., Tavafoghi M., Chen Y., Zhu Y., Karamikamkar S., Sheikhi A., Khademhosseini A. Tissue adhesive hemostatic microneedle arrays for rapid hemorrhage treatment. Bioact. Mater. 2023;23:314–327. doi: 10.1016/j.bioactmat.2022.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lee J., Park S., Le P.T., Lee G., Lee H.W., Yun G., Jeon J., Park J., Pham D.T., Park Y.S., Lim H., Kim C., Hwang T.S., Kim S.W., Lim G. Peripheral microneedle patch for first-aid hemostasis. Advanced Healthcare Materials. 2022;n/a(n/a) doi: 10.1002/adhm.202201697. [DOI] [PubMed] [Google Scholar]
- 192.Parhi R. Recent advances in microneedle designs and their applications in drug and cosmeceutical delivery. J. Drug Deliv. Sci. Technol. 2022;75 doi: 10.1016/j.jddst.2022.103639. [DOI] [Google Scholar]
- 193.Lee K., Lee J., Lee S.G., Park S., Yang D.S., Lee J.-J., Khademhosseini A., Kim J.S., Ryu W. Microneedle drug eluting balloon for enhanced drug delivery to vascular tissue. J. Contr. Release. 2020;321:174–183. doi: 10.1016/j.jconrel.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 194.Lee K.J., Park S.H., Lee J.Y., Joo H.C., Jang E.H., Youn Y.-N., Ryu W. Perivascular biodegradable microneedle cuff for reduction of neointima formation after vascular injury. J. Contr. Release. 2014;192:174–181. doi: 10.1016/j.jconrel.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 195.Lee J., Kim D.-H., Lee K.J., Seo I.H., Park S.H., Jang E.H., Park Y., Youn Y.-N., Ryu W. Transfer-molded wrappable microneedle meshes for perivascular drug delivery. J. Contr. Release. 2017;268:237–246. doi: 10.1016/j.jconrel.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 196.Huang L., Fang H., Zhang T., Hu B., Liu S., Lv F., Zeng Z., Liu H., Zhou W., Wang X. Drug-loaded balloon with built-in NIR controlled tip-separable microneedles for long-effective arteriosclerosis treatment. Bioact. Mater. 2023;23:526–538. doi: 10.1016/j.bioactmat.2022.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.