Abstract
The fields of developmental psychopathology, developmental neuroscience, and behavioral genetics are increasingly moving toward a data sharing model to improve reproducibility, robustness, and generalizability of findings. This approach is particularly critical for understanding attention-deficit/hyperactivity disorder (ADHD), which has unique public health importance given its early onset, high prevalence, individual variability, and causal association with co-occurring and later developing problems. A further priority concerns multi-disciplinary/multi-method datasets that can span different units of analysis. Here, we describe a public dataset using a case-control design for ADHD that includes: multi-method, multi-measure, multi-informant, multi-trait data, and multi-clinician evaluation and phenotyping. It spans > 12 years of annual follow-up with a lag longitudinal design allowing age-based analyses spanning age 7–19 + years with a full age range from 7 to 21. Measures span genetic and epigenetic (DNA methylation) array data; EEG, functional and structural MRI neuroimaging;; and psychophysiological, psychosocial, clinical and functional outcomes data. The resource also benefits from an autism spectrum disorder add-on cohort and a cross sectional case-control ADHD cohort from a different geographical region for replication and generalizability. Datasets allowing for integration from genes to nervous system to behavior represent the “next generation” of researchable cohorts for ADHD and developmental psychopathology.
Keywords: Attention-deficit/hyperactivity disorder, Public dataset, Case-control longitudinal, Design, Genetic and epigenetic array, Neuroimaging, Pyschophysiological
1. Introduction
The fields of developmental psychopathology, developmental neuroscience, and behavioral genetics are increasingly moving toward a data sharing model. This direction is essential for establishing reproducibility, robustness, and generalizability of findings. Multi-disciplinary and multi-method data resources are particularly needed to uncover mechanisms related to mental disorders. Neuroimaging studies (e.g., brain-wide association studies) that relate complex behavior with imaging findings are accelerating the urgency to share imaging, behavioral, and clinical data in the context of psychopathologies. Similar to existing genetic datasets (e.g., Psychiatric Genomics Consortium (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2019), several large imaging data sets that are suitable for different kinds of studies are publicly available (e.g., the ABCD study (Casey et al., 2018, Garavan et al., 2018, Jernigan et al., 2018, Volkow et al., 2018), ENIGMA (Hoogman et al., 2019).
These public data sets have the advantage of large numbers (> 1000 participants), with several studies including epidemiological features. However, when such data sets represent consortia or multiple sites, they may face the challenge of examiner and data collection reliability varying across different settings, subtle but real effects that decrease effect sizes and sensitivity, and differences introduced by varying equipment and system timing for cognitive tasks. In addition, few data sets are multilevel (including clinical phenotypes, neurobiology, genetics, and epigenetics) and at the same time longitudinal. Even within multilevel data sets, clinical phenotyping may be limited, and the number of actual clinical cases in epidemiological samples is often low because they are mirroring population rates, with the purpose to track population effects rather than to isolate a clinical population. For example, the ABCD study (Casey et al., 2018, Garavan et al., 2018, Jernigan et al., 2018, Volkow et al., 2018) of nearly 12,000 children is estimated to have < 500 cases of ADHD (Cordova et al., 2022). Case-control data sets recruited from the community are enriched for clinical cases compared to the population rates, providing valuable and necessary complementary information for the national research effort on psychopathology and development.
An ADHD-rich cohort and data resource that is publicly-available, high-quality, and representing multi-unit longitudinal evaluation of a large sample should be of considerable value in making new discoveries about the contributors to ADHD course of illness, mechamisms and predictors. This paper explains and describes a new resource for this purpose and provides background and documentation to make the resource as useful as possible. ADHD is of unique public health importance, given its early onset during preschool and early school years (Kessler et al., 2014), the high prevalence in children worldwide (3–4%; Erskine et al., 2013), its consideration as preceding and likely causaly contributing to subsequent serious psychopathology (Groenman et al., 2017, Kessler et al., 2014, Kessler et al., 2006, Lee et al., 2011, Riglin et al 2020; Treur et al., 2019), disappointing long-term outcomes despite sophisticated treatments (Erskine et al., 2013, Hinshaw and Eugene Arnold, 2015, Swanson et al., 2017), and high morbidity including reduced life span. Notably, ADHD confers a 50–300% increased risk for substance use disorders, depression, psychosis, and anxiety disorders (Groenman et al., 2017, Kessler et al., 2006, Lee et al., 2011) and magnifies the chances of poor outcomes in school, work, and health (Forte et al., 2020). Indeed, ADHD is associated with a 50% higher chance of early death, multiplied to a 1000% increased risk if comorbid disorders develop (Dalsgaard et al., 2015). Although treatment can reduce these risks, it does not do so adequately. ADHD centers a critical early risk phenotype for understanding a wide range of psychopathology that emerges in the course of development.
Due to ADHD complexity, heterogeneity, and controversial developmental variations, multi-unit longitudinal data are necessary to establish a developmental understanding of psychopathology and to identify opportunities for resilience and recovery that are critical to a contemporary multi-level/multi-unit explanatory understanding (Nigg, 2023, in press). Large multi-unit samples are needed in part due to substantial within-group heterogeneity a problem common with many clinical diagnostic categories (Karalunas and Nigg, 2020, Nigg et al., 2020a). Furthermore, longitudinal datasets are particularly important because of shifting understanding of the developmental course of ADHD and its variability (Sudre et al., 2021, Thapar and Riglin, 2020). For example, robust longitudinal datasets help address current discussion about ADHD onset in later adolescence or adulthood (Asherson and Agnew-Blais, 2019). The lag longitudinal design has the advantage of substantially extending the age range that can be studied within a given period of time, facilitaging clinically relevant prediction models (Karalunas et al, 2022). Thus, while the current study spanned 2009–2021 (about 12 years), the age period included ranges from 7 to 21 (16 years).
Prior naturalistic outcome knowledge of ADHD has relied heavily on a set of foundational longitudinal studies of clinical and descriptive outcomes. However, those groundbreaking datasets were limited by sometimes large duration between assessments and frequent absence of baseline neurobiological data. Recognizing that mental illness will not be solved by a single strategy, finding, or biomarker, multi-unit analysis has become necessary over time to harness the power of cumulative processes in development (Nigg, in press). Put another way, integrating multiple units of analysis in densely observed longitudinal data sets should enable important new insights on the pathophysiology of developmental psychopathology that promote breakthrough prevention and treatment approaches. Yet, the field is in the early stages of establishing standard methods for combined analysis (e.g. high dimensional MRI and genetic data sets) and requires additional public data sets.
Here, we describe the Oregon-ADHD-1000, a newly available public data set on the NIMH Data Archive (NDA). It employs a case-control design for ADHD that is ideally suited to the type of multi-unit, process- oriented analyses needed due to the following features: up to 12 years of annual follow-up data; deep multi-method, multi-measure, multi-informant, multi-trait, multi-clinician clinical evaluation and phenotyping; over-representation (over 50% of longitudinal sample) of cases of ADHD; lag longitudinal design allowing age-based analyses spanning age 7–19 + years; extensive measures of neural and cognitive development (i.e., executive functioning and computationally derived cognitive phenotypes, longitudinal functional and structural MRI); GWAS and MWAS (methylation array) data; psychophysiological data (heart rate variability, electrodermal activity); psychosocial measures; and clinical and functional outcome data. In conjunction with larger population data sets, this data set provides a complementary resource for deeper study of functional implications of epidemiological findings, a test data set for developing hypotheses to be tested in other data sets, and a resource for case-control comparisons of the sort useful to clinical planning. This dataset represents a cohort that is unique for ADHD research in regard to the frequency and duration of follow-up that includes detailed clinical evaluation, repeat MRI, and genetic data. We also describe two companion cohorts that further enrich the resource: an available ASD-add-on cohort and a cross sectional replication case-control ADHD data set (the Michigan ADHD-1000) in a different region of the country for the clinical and behavioral measures.
Overall, this clinical case-control sample has advantages that are valuable for many purposes, by establishing confidence in the clinical status at baseline and the tracking of that status beyond baseline, by representing another perspective on what clinical decision making may entail, and by representing the “next generation” of ADHD outcome studies in relation to multi-unit evaluation, description, and mechanistic discovery. Our hope is that this paper will bring awareness and enhance usability of this unique data resource and its public accessibility for the broader research community, stimulating it useage in creative studies and in making new discoveries about ADHD.
2. Oregon-ADHD-1000: Methods
This resource encompasses participants from the community population (ADHD cohort) (the Oregon ADHD-1000), accompanied by a clinically-recruited population (ASD cohort, described later) and a replication ADHD cohort (Michigan-ADHD-1000, also described below). Participants were identifed and assigned to diagnostic groups in a case-finding, best estimate procedure. In the Oregon-ADHD-1000, ADHD and non-ADHD cases were evaluated in a lag longitudinal model covering developmental span from 7 to 19 + years of age. Data were collected over at least 8 and up to 12 years of follow-up per child with enriched young-adult outcome measures. Multiple data types were collected during the baseline visit and annual follow-up visits over. Loss to participation was 2–4% per year, although the onset of the COVID pandemic reduced the availalable in-person visits for cognitive and MRI studies at later ages and led to higher reliance on remote data collection in the final waves of data. A subset of the ADHD and non-ADHD samples were recruited to complete measures to match those of the ASD sample (subsequent section, below).
2.1. Participant recruitment and diagnosis
2.2. Recruitment
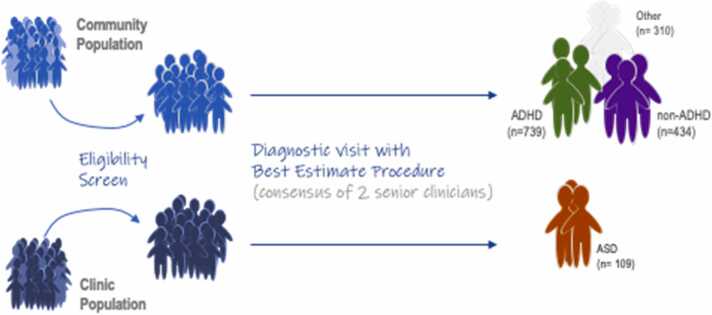
The present sample was intended to complement the benefits and minimize the shortcomings of large epidemiological samples and clinic-referred samples for purposes of evaluating psychopathology (rather than normative development) over time. Although sampling from clinic referrals has the advantage of capturing severe and comorbid cases, the approach has the disadvantage of potentially creating artifactual associations (e.g., Berkson’s bias) and was not selected for this dataset. Therefore, the present sample was obtained using a modified multi-stage, best- estimate, case-finding procedure in the community (Kraemer et al., 2005) (Fig. 1). Human subject protection and ethics approval were obtained from the local University Institutional Review Board.
Fig. 1.
Overview of Participant Recruitment Fig. 1 illustrates the participant flow into this study using a case-finding, best-estimate procedure. On the left top moving to the right, the primary Oregon-ADHD-1000 cohort was recruited through widespread public outreach and mass mailings and then screened to find cases of ADHD and non-ADHD typically developing youth who met study inclusion criteria. “Other” represents cases in which only one reporter rated notable symptoms, or the case was subthreshold/NOS (e.g. 4 symptoms with impairment), or the child was taking a disqualifying medication (e.g.anti-depressant) at baseline. The same procedure was used for the generalizability sample named the Michigan-ADHD-1000 (not depicted to maintain readability; described below). On the bottom left to right, the ASD cohort (also described later in the text) was recruited from hospital records and then diagnostic status was confirmed by a similar best-estimate multi-clinician review procedure as used in the ADHD-1000. Please refer to the main text for full details on recruitment and diagnosis.
To implement this procedure, families were recruited from the community, with ADHD deliberately oversampled to implement a case-controlled design and ensure adequate clinical range variation to detect mechanistic signal (Benca et al., 2017) and to enable us to examine ADHD heterogeneity. To preserve the representativeness of the sample, sex or other demographics were not oversampled. Thus, we expected groups to differ on sex ratio (at least at the earlier ages, IQ, and social disadvantage, all of which have been previously associated with ADHD (Faraone et al., 2005; Miller et al, 2018). Families were recruited via mass mailings to all homes with children in the target age range in a 50-mile radius from the campus. The mailings requested volunteers with or without possible ADHD. Volunteers (n = 2144) were screened by phone to evaluate eligibility as determined by current medications, other clinical conditions, and interest in participation. Complete details are provided in the Supplementary Information. The primary cohort described was collected in western Oregon and is the Oregon-ADHD-1000.
2.3. Diagnostic visit
Following the phone screening for eligibility, a parent/legal guardian provided written informed consent and children provided written assent . An in person, on site clinical evaluation was conducted using standardized, well-normed rating scales from parent and teacher, parent semi-structured clinical interview, child intellectual testing, and clinical observation. Best estimate research diagnoses and final eligibility were established by a team of two experienced clinicians (a child psychiatrist and a child psychologist) who independently arrived at the diagnosis using all available information, including: parent and teacher rating scales, semi-structured clinical interview by a masters-degree trained clinician with inter-interviewer reliability established (K-SADS or SCID, below), a short form of the WISC-IV to estimate full scale IQ, achievement testing screeners, and clinician and psychometrician written observations. Aside from two clinically relevant paper and pencil cognitive tests (Stroop and Trailmaking), the battery of chronometric computerized cognitive tests as well as personality/temperament ratings were were not utilized in diagnostic decision-making, to maintain independence of dependent and independent variables in the analyses of neurocognitive development.
In addition to the multi-method, multi-informant clinical evaluation, the data set includes temperament ratings on children (and personality ratings at older ages), measures of family environment, multi-informant data on behavioral and functional outcomes, treatment history, a comprehensive neurocognitive battery, peripheral psychophysiological measurements, as well as multi-modal neuroimaging, genotyping, and epigenetic data (Kozlowski et al., 2022). A complete list of measures is available in the online supplement.
2.4. Exclusion criteria
At baseline, children were excluded for non-stimulant psychotropic medications, history of seizures or head injury, parent-teacher rating discrepancy making diagnosis uncertain, and diagnosis of intellectual disability (IQ < 75) or other major medical conditions, including: psychosis, mania, current major depressive episode, Tourette’s syndrome, and autism. Youth were excluded at baseline if they currently met criteria for a full major depressive episode, due to the difficulty of accurately evaluating ADHD symptoms in those youth. In follow up visits, depression was not exclusionary. As expected, depression developed in many of the youth, enabling study of depressed mood and related outcomes over time. Complete details including specific medications at baseline, are provided in the Supplementary Information. Medication and treatment status were recorded annually.
2.5. Quality control processes
Extensive procedures were implemented to ensure high data quality in all aspects of the data from collection to processing. For the clinical and diagnostic data, K-SADS interviewers underwent initial expert training and then a master trainer maintained validity, fidelity, and reliability with each subsequent generation of interviewers over the course of the study. Interviewers were required to achieve a threshold kappa > =0.70 with the master trainer. Then, a percentage of their interviews were recorded and reviewed by a master trainer to ensure continued fidelity and to prevent procedural drift. Research assistants administering paper and pencil materials (IQ screen, academic screen, certain cognitive tests) underwent extensive training and had to pass a check-out procedure with a licensed clinical psychologist before seeing participants to collect data. Their sessions were videotaped and fidelity checks conducted on a regular basis as well. The diagnostic team clinicians (psychiatrist and clinical psychologist) reviewed cases independently and achieved kappa > 0.87 for ADHD diagnoses at all years of the study and > 0.70 for nearly all common disorders. These same procedures were in place both for the Oregon-ADHD-1000 and the Michigan-ADHD cohorts. Computerized data (cognitive experiments and tasks, physiological recordings) were carefully screened for data quality. Computerized chronometric and accuracy data were filtered for out of range values, and for evidence of task non-participation, as detailed in the measure descriptions in the online supplement. Paper and pencil questionnaire data and paper and pencil cognitive measures were double entered by two research assistants and discrepancies in their entries were resolved by a data manager reviewing the raw data.
Genetic data and neuroimaging data were also carefully processed using best practices as detailed below.
3. Data types
Baseline evaluations were collected in 1483 children in the ADHD longitudinal cohort (ADHD n = 739; non-ADHD n = 434; Subthreshold/NOS n = 310). The participant demographics for the Oregon and Michigan ADHD cohorts are presented in Table 1.
Table 1.
Demographic information for the Oregon-ADHD-1000 and the Michigan-ADHD-1000 data sets at baseline.
| Oregon ADHD-1000 |
Michigan ADHD-1000 |
|||
|---|---|---|---|---|
| % Female |
39% |
44% |
||
| Mean Agea (SD) |
9.4 years (1.6) |
13.6 years (5.4) |
||
| Race / Ethnicity | Not Hispanic/Latino | Hispanic / Latino | Not Hispanic/Latino | Hispanic / Latino |
| American Indian / Alaska Native | 3 (0.2%) | 2 (0.1%) | 14 (1.1%) | 0 |
| Asian | 30 (2.1%) | 1 (0.07%) | 8 (0.6%) | 0 |
| Native Hawaiian / Pacific Islander | 5 (0.3%) | 2 (0.1%) | 2 (0.2%) | 0 |
| Black / African American | 38 (2.6%) | 3 (0.2%) | 123 (9.2%) | 0 |
| White | 1148 (79.2%) | 54 (3.7%) | 984 (73.9%) | 0 |
| More than One Race | 123 (8.5%) | 34 (2.3%) | 122 (9.2%) | 0 |
| Unknown / Not Reported | 5 (0.3%) | 2 (0.1%) | 17 (1.3%) | 61 (4.6%) |
| Mean Family Incomeb (SD) | 4.5 (2.0) | 3.8 (1.9) | ||
Age at baseline
Family income measures are based on the following scale: 1 =less than $25,000, 2 = $25,000-$35,000, 3 = $35,000-$50,000, 4 = $50,000-$75,000, 5 = $75,000-$100,000, 6 = $100,000-$130,000, 7 = $130,000-$150,000, 8 =more than $150,000.
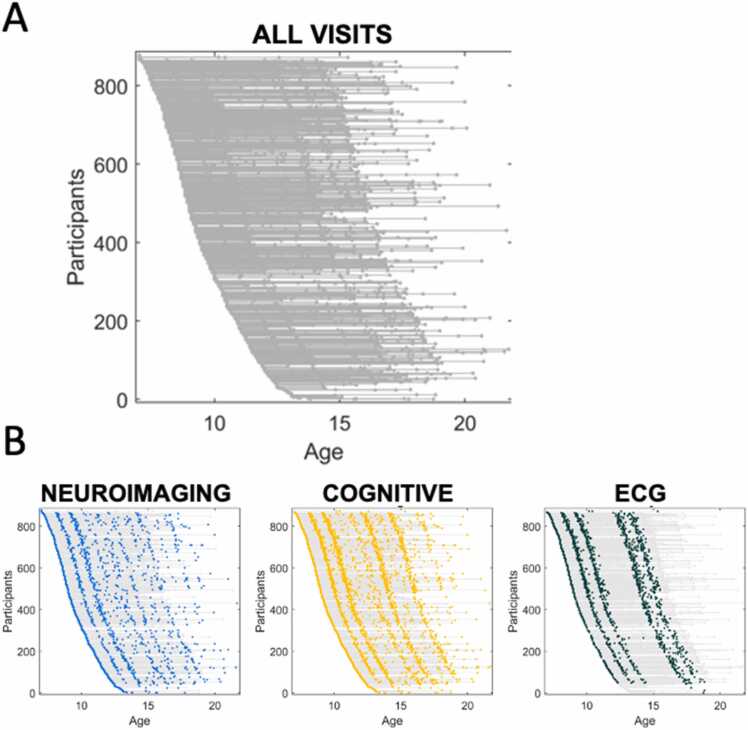
A summary of data types collected for each cohort is presented in Table 2. After the baseline assessment, 849 children participated in a second testing visit, and 646 children continued for annual follow-up for up to 12 years (Fig. 2).
Table 2.
Summary of data types available for each cohort.
| DATATYPE | COHORT |
||
|---|---|---|---|
| OREGON ADHD-1000 | OREGON-ASD | MICHIGAN ADHD-1000 | |
| Personality and temperament | • | • | • |
| Cognition | • | • | • |
| Peripheral Psychophysiology | • | • | |
| Neuroimaging | • | • | |
| Electroencephalography | • | ||
| Genetic | • | • | |
| DNA methylation | • | • | |
| Longitudinal Annual Follow-up | • | ||
Fig. 2.
Repeated Assessment of Multiple Datatypes Each participant is represented by an individual line with each visit represented as a dot along the line (A). The longitudinal collection of each datatype is represented in B. Details on individual visits per participant are provided in the Supplementary Information. Blue=neuroimaging, yellow=cognitive tasks (e.g. executive functioning), green=peripheral psychophysiology as represented by electrocardiography(ECG).
Supplementary Information provides the number of individual participants at each time point frequency as well as retention data. Overall, participant loss averaged 2.5% per year, resulting in 64% retained at 12 years. Note that 29 additional 7-year-old participants had varying levels of participation due to the pandemic, as described in the Supplementary Information.
3.1. Clinical evaluation
As described above, DSM-IV and DSM-5 diagnoses were made independently by each clinician to assign to ADHD (n = 739), non-ADHD(n = 434) and Other (n = 310; principally cases in which only one reporter rates notable symptoms, or the case was subthreshold/NOS (e.g. 4 symptoms with impairment), or the child was taking a disqualifying medication such as an anti-depressant) groups at baseline using a best estimate procedure. To count symptoms, the clinicians used the following rule: If both parent and teacher ratings exceeded T-score > = 60 on at least one ADHD scale and both rated at least 3 symptoms as “often” or “very often” on the ADHD rating scale (or for parents the K-SADS), the “or” algorithm could be employed (Lahey et al., 1994). When either of the informant’s rating fell below this mark, and clinicians judged that this was not explained by successful medication treatment during the school day, then the case was excluded from follow-up if this occurred at baseline or assigned a subthreshold or NOS/other diagnostic code if occurring at follow-up. The team required that all other DSM-IV and DSM-5 criteria be met, as described completely in the Supplementary Information.
The full diagnostic evaluation was repeated annually, with questionnaires completed at 6, 12, 18, and 24 months and then annually. When face-to-face evaluation could not be conducted, then a modified evaluation was conducted remotely using rating scales and questionnaires and a semi-structured clinical interview conducted by telephone. At the follow-up visits, parents completed a K-SADS interview. At an average age of 12 years for one cohort and at 16 years for another cohort, children also completed a self-report K-SADS with a clinician. After age 18 years, the youth completed a SCID-I interview and SCID-II questionnaire. At the later ages, additional adolescent-appropriate follow-up and outcome measures were added. In addition to repeating the baseline measures, outcome and development measures included assessment of treatment received (pharmacological and psychosocial) annually; pubertal status from early on (enabling tracking of its emergence); alcohol and substance use; and social, academic, and health functioning, enabling mapping of trajectories of development in these spheres. See Supplementary Information for a full list of measures administered in each follow-up year.
3.2. Additional Sub-phenotyping
The data set is designed to enable integration with dimensional analyses of sub-phenotypes following the logic of the NIMH Research Domain Criteria (RDoC) initiative (Clark et al., 2017, Cuthbert, 2020). These dimensional domains include arousal (signal detection, activation), executive function (working memory, response inhibition, set shifting), information gain, other neurocognitive measures, temperament and personality (pertaining to positive and negative affective valence), as well as broad band self- and other ratings of psychopathology using normative scales. Measures of parent psychopathology, and child developmental history, are also included. Several of the relevant constructs have been assembled into validated latent variables in relation to conceptualized constructs, via confirmatory factor analyses, including validated latent variables for parent- and teacher-rated ADHD symptoms, arousal/alertness, response inhibition, response output speed, and working memory (see Latent Variable section below and supplement for particulars).
3.3. Personality and temperament
The study conceptualization included consideration of the link from ADHD to temperament and personality in childhood and during development building on a substantial literature relating psychopathology with personality or temperament (see Nigg et al., 2020c). These were measured by parent-ratings and, at older ages, self-ratings . Thus, for children under the age of 12, only parent report was obtained, both to reduce burden on the child participants and due to the likely higher validity of parent ratings in that age period. At ages 12 and up, child self-report of temperament was also obtained in both data sets.
Although direct lab task observation of temperament can be assessed by trained observers (Tackett et al., 2019), parent ratings are advantageous given they are in position to observe children across multiple contexts (Rothbart, 2012), have been utilized in studies related to the development of temperament in children across the lifespan and viewed as a valid indicator of temperament traits (Clark and Watson, 2021, Shiner et al., 2021).
Temperament measurement was derived from Rothbart’s tripartite temperament model (Rothbart, 2012, Rothbart et al., 2000), which maps quite well onto major developmental theories of personality. The broader construct of personality, complementing the temperament measures, was measured with the versatile California Q-Sort (Caspi and Block, 1992) which enables retrieval of multiple domains of personality from different perspectives (Eisenberg et al., 2003, 1996; Martel et al., 2022). Publications from this and the companion data set have thus been able to utilize different theoretical lenses. Temperament was evaluated with the Rothbart rating scale, either the parent-report Temperament in Middle Childhood Questionnaire (TMCQ) or the parent- and self-report Early Adolescent Temperament Questionnaire (EATQ) depending on age (see Supplementary Information for details, factor structure, and measures by age). Because the factor structure of these measures has been uncertain, the online supplement provides a confirmatory and exploratory factor model for both measures in this data set and recommended modified scale usage (Kozlowski et al., 2022). The model validated here distinguishes ADHD, surgency, and two components of negative affectivity defined as (a) sadness, worry, fear and (b) irritability/anger (Nigg et al., 2020b).
3.4. Cognition
After selection for enrollment at baseline, children completed a second baseline visit in which the cognitive measures were obtained after a medication washout of > = 7 half-lives. We administered the following tasks (a) Stop-Go task (Nigg, 1999, Schachar et al., 1995), (b) Identical Pairs Continuous Performance Task (Cornblatt and Malhotra, 2001), (c) Spatial span forward and backward (De Luca et al., 2003), (d) Digit span forward and backward from the WISC-IV, (e) N-back, including 0-back, 1-back, and 2-back conditions, (f) Delis, Kaplan, and Kramer (DKEF; Delis et al., 2001) version of the Stroop task (word, color, and color-word), (g) DKEF Trailmaking test (number, letter, and shifting), and (h) a motor time reproduction task at fast (500 ms) interval from which we derived clock precision (clock variation; Wing, 2002; Wing and Kristofferson, 1973a, Wing and Kristofferson, 1973b). See the online Supplementary Information for details of task procedures, data cleaning, quality control, and validity checks.
The cognitive measures were selected at study launch in 2007/2008 with the intent to cover the major cognitive and attentional theories of ADHD at that time. These included (a) executive functioning with a special focus on working memory and response inhibition, (b) arousal/activation (or in more contemporary terminology, attentional alertness (Petersen and Posner, 2012), with a focus on signal detection and more recently computational measures of information gain (Karalunas and Huang-Pollock, 2013), (c) temporal information processing (time reproduction task), (d) output speed, and (e) reward delay discounting. As detailed in the supplement, standard computerized measures were used drawing from both clinical neuropsychology and cognitive science using chronometric methods. Careful data cleaning was undertaken and computational models were utilized for task decomposition whenever possible. In particular, the race model for response inhibition (Verbruggen et al., 2019), signal detection theory to evaluate detection sensitivity (d’) seen as an index of arousal or alertness (Wickens, 2001), and (B’) as an index of activation on the continuous performance task (Sergeant et al., 1999); the drift diffusion model to evaluate information accumulation and properly model speed-accuracy trade-offs on sequential reaction time data under a formal psychometric theory (Ratcliff et al., 2016); a hyperbolic model of delay discounting (Mazur, 1987, Mitchell et al., 2015) and the Wing-Kristofferson model for temporal information processing (Wing and Kristofferson, 1973a). Measures of intellectual function are also obtained using a validated and reliable short form of the WISC-IV congruent with the years of data collection. Academic functioning is estimated with short forms of the WIAT-III (version matched to years of data collection).
3.5. Peripheral Electrocardiology (ECG)
Peripherally measured cardiac data were obtained using a 7-lead electrocardiography (ECG) and impedance cardiography (ICG) configuration. These were used to derive respiratory sinus arrythmia (RSA) and cardiac pre-ejection period (PEP), measures thought respectively to relate to tap parasympathetic and sympathetic regulation of heart rate, and thus, interpretable in regard to self-regulation (Meye and Digirolamo, 2007). The measures were obtained during a resting baseline and throughout several of the cognitive tasks (spatial span, delay discounting, stop-go task) and an emotion task. Briefly, in the emotion induction and suppression procedure, children viewed a sequence of video clips appropriate to the age of children at that year of data collection (details in the supplement). The procedures included conditions in which children were instructed to visibly show the emotion elicited by the video clips or to intentionally suppress expression of the emotion and successfully challenged regulation(Musser et al., 2011). Procedural details including inter-person reliability and validation are provided in the Supplementary Information.
3.6. MRI Neuroimaging
Participants were scanned at OHSU's Advanced Imaging Research Center (AIRC) on a 3.0 T Siemens Tim Trio Magnetom scanner using a 12-channel head coil and completed one T1 weighted structural image as well as 3 5-minute resting state scans (see Supplementary Information for detailed information on fMRI data acquisition and approach to sequence change during the study period), providing 15 min of functional data per participant per follow-up assessment. All of the data were processed using a modified version of the Human Connectome Project (HCP) image processing pipeline (Glasser et al., 2013, Mills et al., 2018). After processing, we used a manual curation process to further assess the data quality. See Supplementary Information for detailed information on quality control.
3.7. Electroencephalographic Neural Measurement
Two-hundred seventy-two children (nADHD at baseline = 169) also completed a single time point of EEG data collection at either Year 5, 6, or 8 of the larger longitudinal study (ages 11–17 years old). EEG was recorded with either 32 (n = 177) or 64 (n = 95) Ag-AgCl active electrodes based on the international 10–20 system using an Easycap (Easycap GmbH; configurations available at https://www.easycap.de/author/easycap/). The EEG signal was amplified with Brain Products’ ActiCHamp system and digitally recorded at 500 Hz using PyCorder v1.0.9. Impedance levels for each electrode was at or below 50 kΩ during data collection. EEG was referenced online to the central midline electrode site (Cz).
During the EEG visits, children completed a series of tasks intended to measure aspects of emotional and cognitive control, as well as their interaction. This included: 1) an 8-minute resting baseline task, which was divided into four, 2-minute blocks alternating eyes-closed and eyes-open conditions (Alperin et al., 2019, Karalunas et al., 2022, Ostlund et al., 2021); 2) 3 separate conditions (positive [happy], negative [fear], neutral) of an emotional go/no-go task (in counterbalanced order) intended to measure effects of emotion on inhibitory control and reaction time/reaction time variability; and 3) a novel whole-report version of the well-known change detection paradigm to measure working memory capacity and performance variability independent of motor response (Adam et al., 2015).
3.8. Developmental, environmental, and clinical treatment measures
Extensive developmental and family data were also obtained which have been productive in mapping ADHD risk, as well as ADHD environment and genetic interplay as illustrated with the following. Parents completed a detailed developmental history form that retrospectively tracked prenatal risk factors (Martel et al., 2022, Wiggs et al., 2016) and early developmental milestones, including history of breast-feeding (Stadler et al., 2016). Family context was evaluated serially with common measures including the Family Environment Scale (Elmore et al., 2016, Moos and Moos, 1987), Child Perception of Inter-Parental Conflict Scale (Nigg et al., 2009), and a parenting styles questionnaire (Martel et al., 2011). In addition, for an observational measure a parent five-minute speech sample was obtained, transcribed, and coded providing a measure of the expressed emotion (Musser et al., 2016).
Treatment history was evaluated by parent- and self-report every six months for the first 3 years and then annually, providing detailed information on medications, psychotherapy, and other treatment including doses, duration, and type. Treatment is thus available as a useful time-varying covariate or moderator.
3.9. Genetic
A subset of the children (N = 770, including 105 sibling pairs) provided saliva samples and were genotyped. Saliva samples were collected during study visits beginning at baseline (mid-session, after at least one hour without food) using Oragene (R) cups (DNA Genotek Inc., Kanata, ON, Canada) and DNA was isolated using manufacturer protocols. DNA were extracted by the Integrated Genomics Laboratory at OHSU under the direction of Chris Harrington, Ph.D. The online supplement provides details on extraction.
Genotype data allows for genome-wide association studies, polygenic risk score analyses Hermosillo et al. (2020); Mooney et al. (2020); Nigg et al. (2020a); Nigg et al. (2018)) and other investigations into the causal structure of ADHD and related traits (e.g. GxE analyses and Mendelian randomization). Genotyping and DNA methylation (MWAS) chips were conducted as below and repeat MWAS data are pending. PsychChip v1.1 array (N = 603132 SNPs), developed by Illumina, Inc. in collaboration with the Psychiatric Genetics Consortium. For most children, multiple samples have been obtained. For those samples genotyped to date, all individuals had genotype call rates > 98%. The following criteria were used to exclude SNPs: call rate < 97%, Hardy-Weinberg equilibrium deviation (p < 1e-6), ambiguous strand information, or differential call rates between genotyping batches or ethnic groups. SNPs located in regions of suggestive copy number anomalies as calculated by B-allele frequency were also removed. Additional SNPs determined by Illumina to perform poorly across populations were excluded. The final data set included 552,352 SNPs.
Non-genotypes SNPs were imputed with IMPUTE2 (Howie et al., 2009)using 1000 genomes (1KG phase 3) as the reference panel (https://mathgen.stats.ox.ac.uk/impute/1000GP_Phase3.html). Autosomal chromosomes (N = 537,976 SNPs) were preprocessed and phased using SHAPEIT (Delaneau et al., 2013). Variant positions and alleles were checked against the reference panel and SNPs that were missing, or mismatches were removed (115543 SNPs). Imputation was done on 3 Mb chunks with 1 Mb buffers on either side. Genotype probabilities were converted to best-guess genotypes with genotype set to missing if the probability < 0.8. The final data after imputation included 16,284,035 SNPs. See Supplementary Information for detailed information on validation analyses.
3.10. DNA methylation
DNA methylation was assessed at a single time point (mean age=9.85 years) for 752 children (including 101 sibling pairs), 743 of which also have genotype data (Mooney et al., 2020). Multiple repeat samples are available for most of this group, although currently methylation has been assayed at two timepoints approximately two years apart for only a subset (N = 134), demonstrating massive epigenetic change during that time frame (data forthcoming; data available in the NDA). Genomic DNA was isolated from saliva, bisulfite converted, and DNA methylation was measured on the MethylationEPIC BeadChip (Illumina, Inc.) using a standard protocol. Genome Studio v2011.1 (Illumina, Inc.) was used to investigate sample hybridization quality and to extract probe signal intensities. The data QC procedures included: manual inspection of beta distributions, curation of control probes using the Illumina BeadArray Controls Reporter, manual inspection of total CpG intensity distributions, sex prediction, outlier sample detection, and comparison of SNP probes on the MethylationEPIC with the genotype data (described above). Data were normalized using smooth quantile normalization. See Supplementary Information for detailed information on validation of the DNA Methylation data.
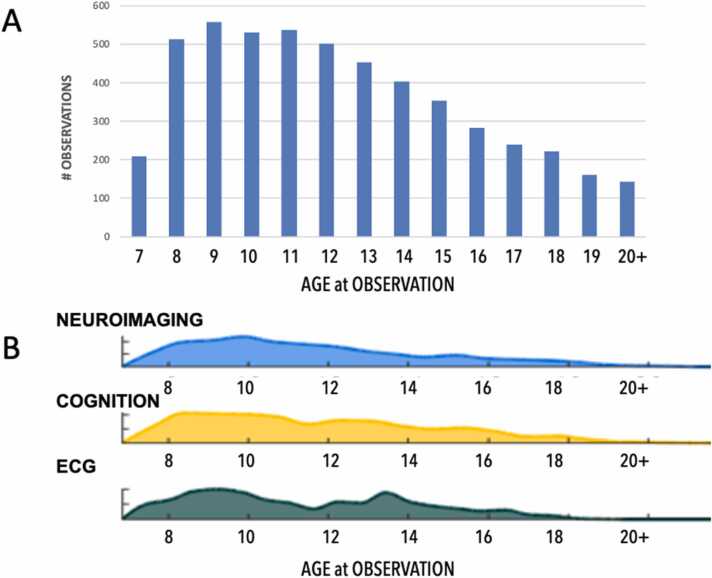
Fig. 3 represents the longitudinal collection of data across multiple data types.
Fig. 3.
Data types available at each age This resource provides numerous data types across a wide range of ages, enabling multi-level age-based analyses. (A) Total observations at each year of age and (B) the distribution of those observations across Neuroimaging, Cognition and Electrocardiology (ECG) data types. All available data types are provided in the Supplementary Information.
4. Oregon-ADHD-1000 data reduction resources
4.1. Latent variables
One benefit of the extensive clinical characterization of our sample is the ability to create latent variables measuring primary constructs of interest. Those variables are already available for some constructs and were developed using a combination of exploratory and confirmatory factor analytic procedures. For latent variable scores that are available in the public dataset, we direct readers to the following citations for details of modeling: 1) ADHD symptoms [e.g.,(Nigg et al., 2020b); 2) temperament/emotional constructs, such as irritability, surgency, and sadness/anxiety (e.g.,(Nigg et al., 2020b); and 3) executive function/cognition, including arousal, inhibitory control, processing speed, and working memory (Nigg et al., 2018). Latent variables will need to be extended into additional study years but should provide a reasonable starting point for many analyses.
4.2. Latent trajectory class assignments
A further goal that motivated development of this cohort was to enumerate and characterize latent trajectory classes defined by differential developmental patterns over time. These models are useful for obtaining an initial picture of developmental trajectories (Nagin, 1999). Analyses from Waves 1–3 have revealed three meaningful trajectory classes for ADHD symptoms, including persisting and remitting classes (Karalunas et al., 2022). Similar trajectory classes have been modeled for comorbid symptoms, such as anxiety, depression, and oppositional defiant disorder. They should prove a useful starting point for investigations of the relations between symptom course and the behavioral, cognitive, and imaging data. Thus, we also provide the derived class assignments for these trajectories in the dataset. All modeling details can be found in the referenced publications.
4.3. Temperament sub-phenotype profiles
Recently, work from our team has focused on creation of sub-profiles of ADHD that address irritability and emotional dysregulation via ratings of temperament (Karalunas et al., 2014, Karalunas et al., 2019). Profiles show at least moderate stability over the first several years of the study, are robust to modeling approach used (e.g., community detection, latent profile analysis), and have been informative for clinical prediction. Details of modeling, stability, and prediction are available in publications. We include these temperament sub-phenotype assignments in the public dataset as a starting point for related analyses.
5. Oregon ASD add-on sample methods and data types
The ASD sample provides unique relevance given the status of ADHD and ASD as the two major, early onset, behaviorally-based neurodevelopmental disorder of childhood (APA, 2013, DSM5) and given (A) the change in DSM-5 allowing for comorbid identification (APA, 2013), (b) evidence that ADHD and ASD co-segregate in families (Miller et al., 2019, Musser et al., 2014), (c) ongoing interest in identifying neurobiological, cognitive, and behavioral overlaps and distinctions in these neurobehavioral disorders and the diagnostic complexity that comorbid cases present following the logic of RDoC, in relation to distinct or overlapping dimensions of development (Taurines et al., 2012, Rommelse et al., 2017, Uddin et al., 2017, Antshel and Russo, 2019, Kangarani-Farahani et al., 2022).
6. Recruitment
ASD participants (n = 109) ages 7–16 years, were recruited through search of hospital records and outreach to eligible participants and through mailings and from OHSU's site for the Autism Treatment network housed in the OHSU Institute on Development and Disability, as depicted earlier in Fig. 1. Those potential participants who volunteered were phone screened for basic eligibility. Those that were eligible were screened via an on-campus diagnostic evaluation.
7. Diagnostic visit
Following the phone assessment, a parent/legal guardian provided written informed consent and children provided written assent. Participants received a consensus diagnosis from a team of two licensed psychologists and a psychiatrist using research reliable clinical interviews, including: the same K-SADs interview described earlier and the Autism Diagnostic Interview (ADI-R; Rutter, 2003), as well as the Autism Diagnostic Observation Schedule (ADOS-2; ADOS-2: Autism Diagnostic Observation Schedule: Manual 2012), and parent and teacher answered questionnaires, including: the Social Responsiveness Scale (SRS-2; Constantino and Gruber, 2012), Children’s Communication Checklist (CCC-2; Bishop, 2021), and a developmental history. They also completed the behavioral checklists noted for the ADHD cohort including the Strengths and Difficulties Questionnaire (Goodman, 1997) and the Conners ADHD Rating scale. Similar quality control processes were followed as described for the ADHD cohorts. All ASD participants also underwent the same evaluation for possible ADHD as the participants in the primary ADHD-1000 study, including parent K-SADS interview, parent and teacher symptom readings, and diagnostic team best estimate evaluation.
8. Exclusion criteria
ASD participants were excluded if they were taking long-acting psychoactive medication, had (by parent report) genetic abnormalities, neurological impairments, major medical issues or physical disabilities, or by K-SADS interview had significantly impairing Axis I comorbidities (i.e., bipolar disorder, schizophrenia), closed head injury, seizure disorders, or if they had an estimated IQ < 75 by our IQ screen.
9. Cognitive and neuroimaging procedures
Those participants meeting criteria for the ASD cohort completed the same battery of cognitive measures as described earlier for the ADHD and non-ADHD groups. They also completed the same neuroimaging protocol as described below.
10. Michigan-ADHD-1000 replication sample methods and data types
An additional benefit of this resource is the availability of a prior cohort of youth recruited in Michigan between 2000 and 2009, prior to the Oregon longitudinal cohort, but using identical recruitment and enrollment procedures. However, because the population was different—small town and rural population in central Michigan versus largely urban Oregon population, the samples differ across several factors (socioeconomic, cultural, ethnic variation, as well as clinical severity, which was somewhat more severe in the Michigan sample). Thus, the Michigan sample provides powerful data for generalizability analysis on cross sectional findings. This cross-sectional sample includes n = 755 children ages 6–12 years, n = 380 adolescents ages 13–17 years, as well as a sample of n = 196 adults age 18–30 years with total N available for generalizability = 1331(including n = 126 sibling pairs) with clinical, personality, neuropsychology, environmental (pre/perinatal, proximal family), genotype (N = 848, including 126 sibling pairs), and DNA methylation (N = 443, including 47 sibling pairs) data. The inclusion of this replication dataset provides opportunities to conduct confirmation analyses across two large-scale, case-control samples of youth recruited using identical procedures. Notably, differences in clinical severity, socioeconomic, and cultural variation across these samples can provide robust tests of generalizability of mechanisms across multiple individual and family-level variables. See Supplementary Information for detailed information on confirmation across two samples (Michigan and OHSU) with identical procedures.
The data types available in each of these cohorts are summarized in Table 1.
11. Discussion
ADHD centers a critical early risk phenotype that is both costly in its own right and sets the stage, too often, for poor long-term outcome. The mechanisms informing those outcomes, as well as the heterogeneity that characterizes both the ADHD syndrome and its development paths, require ongoing study that considers multiple units of analysis and with sufficiently large samples of youth affected by ADHD to evaluate sample heterogeneity. For such purpose, this data set provides longitudinal assessments of multiple datatypes across a broad range of development that will enable multi-level analysis within and between participants (Fig. 3).
ADHD research from a developmental perspective has relied heavily either on longitudinal case-control studies using descriptive data or on population data sets with relatively few well-characterized clinical cases. The present resource therefore fills an important gap for developmental psychopathology research by providing a longitudinal case-control cohort with a substantial number of deeply phenotyped and clinically well-characterized cases of ADHD at baseline (indeed, more cases of ADHD than in many widely used population cohorts). It departs from the first generation of ADHD cohort studies by incorporating community (not clinic) recruitment, repeat chronometric neurocognitive studies, repeat multi-method neuroimaging, and genetic and epigenetic data.
The development and availability of more sophisticated treatments based on single strategies or single biomarkers have not impacted long-term outcomes (Erskine et al., 2013, Hinshaw and Eugene Arnold, 2015, Swanson et al., 2017). Given the complexity and variable presentation of ADHD characteristics, multi-level analysis in densely observed longitudinal cohorts will provide new insight on the pathophysiology of developmental psychopathology that impact breakthrough prevention and treatment approaches. This dataset intends to contribute to this effort by establishing state of the art data collection across numerous datatypes in a case-controlled longitudinal cohort. The ability to combine genetic, imaging, and various kinds of phenotype data is increasingly useful for efforts to understand the within-person mechanisms involved in ADHD and this resource is well suited to that aim.
In addition to the value of these datatypes as described above, this dataset highlights three additional features that are critical to the field: depth of phenotyping, emphasis on data quality, and the hurdles of open science.
Particular care was given to detailed phenotyping: clinical (multi-method, multi-informant and uniquely, expert clinician review), temperament/personality, as well as neuropsychological and neurobiological measures readily comparable to other data sets in the literature. Neuroimaging data and genetic data are available to complete the phenotype and mechanistic study. Thus, it is possible to consider a range of extended phenotypes, refined phenotypes, and endophenotypes in relation to ongoing issues such as cognitive function, emotional functioning and regulation, as well as neurobiology and physiology.
Extensive steps were taken to ease enrollment for groups historically under-represented or under-identified in ADHD research, including in person visits to local community and church centers. In both the Oregon and Michigan data sets, the demographics of the sample match the study catchment quite closely. However, like with other local samples, the catchment areas may not be representative of other communities in the U.S, specifically related to gender and racial disparities (Hinshaw et al., 2022, Coker et al., 2016).
Second, data quality was a major consideration in assembling the resource. For the clinical data, extensive training, ongoing measurement of inter-rater and inter-clinician reliability, and regular validity and fidelity checks on data collection procedures were undertaken. The best estimate team reviewed cases independently and maintained acceptable agreement and kappa agreement rates. For cognitive data, careful data cleaning and curating was undertaken to restrict the inclusion of data noise whether due to child non-compliance, task misunderstanding, or other common data collection source of error. For physiology and EEG data, data cleaning procedures similarly included standardized (manualized) formal training procedures and assessment of inter-rater reliability for subjective decision points that go well beyond what is standard in these fields. For neuroimaging data, reliability training and manual quality control were completed by all raters to confirm criteria required for imaging data to be included in analysis. For genetic data, likewise, extensive data processing and quality control checks were undertaken, including standard quality filters for both samples and SNPs (e.g., call rate, departure from Hardy-Weinberg equilibrium), assessment of batch effects, detection of chromosomal anomalies, confirmation of sample sex, and identification of cryptic relatedness among samples.
Open data access is a pillar of the broader Open Science movement and a key, but not always appreciated, corollary is the need for researchers to have access to detailed understanding of the motivating drives behind study design and the detailed research procedures. With the resource here, we intend to facilitate not only the open availability of the data, but access to the meaningful details of study design and data collection procedures that may substantively affect how data should be used and interpreted. Relatedly, at least one of the goals of the Open Science movement is to speed scientific discovery via promoting tests of replication and generalizability of findings. Here, we contribute directly to these critical components of scientific discovery via the availability of a generalizability cohort (the Michigan ADHD-1000) with nearly identical methods of behavioral data collection, which adds a unique dimension compared to other public datasets. On the neuroimaging side, the ability to match methods to the ABCD study is also a valuable feature. Open science practices are advanced by the availability of this clinically rich dataset along with a well-matched replication sample. In this way, researchers can test rich, multiple levels of analysis conceptualizations and pathways to disorders, as well as replicate in a large, demographically distinct sample.
12. Conclusion
In conclusion, we describe a case-control data source for ADHD with multi-method, multi-measure, multi-informant, multi-trait, multi-clinician evaluation and phenotyping over 12 years of annual follow-up spanning age 7–19 + years. In addition, this data set benefits from an autism spectrum disorder add-on cohort and a cross sectional replication case-control ADHD data set from a different geographical region. By establishing confidence in clinical status at and beyond baseline, this public data source may enable the next generation of multi-unit evaluation in ADHD. This data source is available on the NIMH Data Archive (NDA) under # 1938, THE OREGON ADHD-1000: A LONGITUDINAL DATA RESOURCE ENRICHED FOR CLINICAL CASES AND MULTIPLE LEVELS OF ANALYSIS DOI: 10.15154/1528485. The study page provides instructions on locating the ASD and Michigan-ADHD-1000 data. Additional availability is planned on Open Neuro (www.openneuro.org).
Funding sources
Critical philanthropic support was provided by the Abracadbra Foundation (Stephen and Patricia Sharp). Multiple NIMH grants supported the development of these cohorts: 1R01-MH59105 (Nigg); R01-MH63146 (Nigg); R01-MH070004 (Friderici); 1R01MH86654 (Nigg-Fair); R01MH099064 (Nigg); 1R37MH59105 (Nigg); 2R56MH086654 (Nigg); R01MH115357 (Fair-Nigg); 2R37MH59105 (Nigg); R01 MH096773 (Fair); K23 MH108656 (Karalunas). An integrated SQL data base at OHSU has housed all the data and is supported by the Oregon Clinical and Translational Research Institute funded by a grant from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR002369. The material in this paper does not necessarily reflect the opinion of the National Institutes of Health or the National Institute of Mental Health.
CRediT authorship contribution statement
Authors had the following contributions: study concept and design – JN, DF, SK, MM, BW; development of methodology and writing - all; review and revision of the paper - all; acquisition of the data – all; statistical analysis – not applicable; read and approved the final paper – all.
Research data
The data set described in this manuscript will be publicly available on the NIMH Data Archive (NDA) under # 1938, THE OREGON ADHD-1000: A LONGITUDINAL DATA RESOURCE ENRICHED FOR CLINICAL CASES AND MULTIPLE LEVELS OF ANALYSIS DOI: 10.15154/1528485. The study page provides instructions on locating the ASD and Michigan-ADHD-1000 data.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. Damien A. Fair reports a relationship with NOUS Imaging Inc that includes: board membership and equity or stocks.
Acknowledgements
The authors would like to thank the participants and their families; Ajit Jetmalani, M.D., Eric Fombonne, M.D., Jennifer Ablow, Ph.D., multiple study collaborators and consultants who provided invaluable insight over the course of these studies, and numerous volunteers and research staff who made this study possible and dedicated countless hours to ensuring maximum data integrity and dependability. Genetic data were processed at the OHSU Integrated Genomics Lab under the guidance of Chris Harrington, Ph.D., with critical technical support from Kristina Vartanian.
Declarations
JTN, SLK, JT, MAN, MMM, PR, MAM, BW, BJN, CS, LN, and EDM have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. DAF has a financial interest with NOUS Imaging Inc. and may financially benefit if the company is successful in marketing FIRMM software products and may receive royalty income based on FIRMM technology developed at Oregon Health and Sciences University and Washington University and licensed to NOUS Imaging Inc.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2023.101222.
Contributor Information
Joel T. Nigg, Email: niggj@ohsu.edu.
Damien A. Fair, Email: faird@umn.edu.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
Public data set on the NIMH Data Archive (NDA).
References
- Adam K.C.S., Mance I., Fukuda K., Vogel E.K. The contribution of attentional lapses to individual differences in visual working memory capacity. J. Cogn. Neurosci. 2015;27:1601–1616. doi: 10.1162/jocn_a_00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alperin B.R., Smith C.J., Gustafsson H.C., Figuracion M.T., Karalunas S.L. The relationship between alpha asymmetry and ADHD depends on negative affect level and parenting practices. J. Psychiatr. Res. 2019;116:138–146. doi: 10.1016/j.jpsychires.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antshel K.M., Russo N. Autism spectrum disorders and ADHD: overlapping phenomenology, diagnostic issues, and treatment considerations. Curr. Psychiatry Rep. 2019;21:34. doi: 10.1007/s11920-019-1020-5. [DOI] [PubMed] [Google Scholar]
- Asherson P., Agnew-Blais J. Annual research review: does late-onset attention-deficit/hyperactivity disorder exist? J. Child Psychol. Psychiatry. 2019;60:333–352. doi: 10.1111/jcpp.13020. [DOI] [PubMed] [Google Scholar]
- Benca C.E., Derringer J.L., Corley R.P., Young S.E., Keller M.C., Hewitt J.K., Friedman N.P. Predicting cognitive executive functioning with polygenic risk scores for psychiatric disorders. Behav. Genet. 2017;47:11–24. doi: 10.1007/s10519-016-9814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. Children’s Communication Checklist (CCC-2) Encycl. Autism Spectr. Disord. 2021 doi: 10.1007/978-3-319-91280-6_1929. [DOI] [Google Scholar]
- Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M., et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018 doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi Block, Block Klopp. A" common-language" version of the California Child Q-Set for personality assessment. Psychol. Assess. 1992;4:512–523. [Google Scholar]
- Clark L.A., Watson D. In: Handbook of Personality: Theory and Research. John O.P., Robins R.W., editors. The Guilford Press; 2021. Temperament: theory and research; pp. 145–175. [Google Scholar]
- Clark L.A., Cuthbert B., Lewis-Fernández R., Narrow W.E., Reed G.M. Three approaches to understanding and classifying mental disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC) Psychol. Sci. Public Interest. 2017;18:72–145. doi: 10.1177/1529100617727266. [DOI] [PubMed] [Google Scholar]
- Coker T.R., Elliott M.N., Toomey S.L., Schwebel D.C., Cuccaro P., Tortolero Emery S., Davies S.L., Visser S.N., Schuster M.A. Racial and ethnic disparities in ADHD diagnosis and treatment. Pediatrics. 2016;138 doi: 10.1542/peds.2016-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino, J.N., Gruber, C.P., 2012. Social Responsiveness Scale: SRS-2.
- Cordova M.M., Antovich D.M., Ryabinin P., Neighbor C., Mooney M.A., Dieckmann N.F., Miranda-Dominguez O., Nagel B.J., Fair D.A., Nigg J.T. Attention-deficit/hyperactivity disorder: restricted phenotypes prevalence, comorbidity, and polygenic risk sensitivity in ABCD baseline cohort. J. Am. Acad. Child Adolesc. Psychiatry. 2022 doi: 10.1016/j.jaac.2022.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt B.A., Malhotra A.K. Impaired attention as an endophenotype for molecular genetic studies of schizophrenia. Am. J. Med. Genet. 2001;105:11–15. [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Electronic address: plee0@mgh.harvard.edu, Cross-Disorder Group of the Psychiatric Genomics Consortium, 2019. Genomic Relationships, Novel Loci, and Pleiotropic Mechanisms across Eight Psychiatric Disorders. Cell 179, 1469–1482.e11. [DOI] [PMC free article] [PubMed]
- Cuthbert B.N. The role of RDoC in future classification of mental disorders. Dialog-. Clin. Neurosci. 2020;22:81–85. doi: 10.31887/DCNS.2020.22.1/bcuthbert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard S., Østergaard S.D., Leckman J.F., Mortensen P.B., Pedersen M.G. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385:2190–2196. doi: 10.1016/S0140-6736(14)61684-6. [DOI] [PubMed] [Google Scholar]
- De Luca C.R., Wood S.J., Anderson V., Buchanan J.-A., Proffitt T.M., Mahony K., Pantelis C. Normative data from the CANTAB. I: development of executive function over the lifespan. J. Clin. Exp. Neuropsychol. 2003;25:242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Delaneau O., Zagury J.-F., Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nat. Methods. 2013;10:5–6. doi: 10.1038/nmeth.2307. [DOI] [PubMed] [Google Scholar]
- Delis, D.C., Kaplan, E., Kramer, J.H., 2001. Delis-Kaplan Executive Function System. https://doi.org/10.1037/t15082–000.
- Eisenberg N., Valiente C., Fabes R.A., Smith C.L., Reiser M., Shepard S.A., Losoya S.H., Guthrie I.K., Murphy B.C., Cumberland A.J. The relations of effortful control and ego control to children’s resiliency and social functioning. Dev. Psychol. 2003;39:761–776. doi: 10.1037/0012-1649.39.4.761. [DOI] [PubMed] [Google Scholar]
- Elmore A.L., Nigg J.T., Friderici K.H., Jernigan K., Nikolas M.A. Does 5HTTLPR genotype moderate the association of family environment with child attention-deficit hyperactivity disorder symptomatology? J. Clin. Child Adolesc. Psychol. 2016;45:348–360. doi: 10.1080/15374416.2014.979935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine H.E., Ferrari A.J., Nelson P., Polanczyk G.V., Flaxman A.D., Vos T., Whiteford H.A., Scott J.G. Research review: epidemiological modelling of attention-deficit/hyperactivity disorder and conduct disorder for the Global Burden of Disease Study 2010. J. Child Psychol. Psychiatry. 2013 doi: 10.1111/jcpp.12144. [DOI] [PubMed] [Google Scholar]
- Faraone S.V., Perlis R.H., Doyle A.E., Smoller J.W., Goralnick J.J., Holmgren M.A., Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Forte A., Orri M., Galera C., Pompili M., Turecki G., Boivin M., Tremblay R.E., Côté S.M. Developmental trajectories of childhood symptoms of hyperactivity/inattention and suicidal behavior during adolescence. Eur. Child Adolesc. Psychiatry. 2020;29:145–151. doi: 10.1007/s00787-019-01338-0. [DOI] [PubMed] [Google Scholar]
- Garavan H., Bartsch H., Conway K., Decastro A., Goldstein R.Z., Heeringa S., Jernigan T., Potter A., Thompson W., Zahs D. Recruiting the ABCD sample: Design considerations and procedures. Dev. Cogn. Neurosci. 2018;32:16–22. doi: 10.1016/j.dcn.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L., Xu J., Jbabdi S., Webster M., Polimeni J.R., Van Essen D.C., Jenkinson M. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. The strengths and difficulties questionnaire: a research note. J. Child Psychol. Psychiatry. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Groenman A.P., Janssen T.W.P., Oosterlaan J. Childhood psychiatric disorders as risk factor for subsequent substance abuse: a meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56:556–569. doi: 10.1016/j.jaac.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Hermosillo R.J.M., Mooney M.A., Fezcko E., Earl E., Marr M., Sturgeon D., Perrone A., et al. Polygenic risk score–derived subcortical connectivity mediates attention-deficit/hyperactivity disorder diagnosis. Biol. Psychiatry. Cogn. Neurosci. Neuroimag. 2020;5:330–341. doi: 10.1016/j.bpsc.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw S.P., Eugene Arnold L., For the MTA Cooperative Group Attention‐deficit hyperactivity disorder, multimodal treatment, and longitudinal outcome: evidence, paradox, and challenge. WIREs Cogn. Sci. 2015 doi: 10.1002/wcs.1324. [DOI] [PubMed] [Google Scholar]
- Hinshaw S.P., Nguyen P.T., O'Grady S.M., Rosenthal E.A. Annual Research Review: Attention-deficit/hyperactivity disorder in girls and women: underrepresentation, longitudinal processes, and key directions. J. Child Psychol. Psychiatry. 2022;63:484–496. doi: 10.1111/jcpp.13480. [DOI] [PubMed] [Google Scholar]
- Hoogman M., Muetzel R., Guimaraes J.P., Shumskaya E., Mennes M., Zwiers M.P., et al. Brain imaging of the cortex in ADHD: a coordinated analysis of large-scale clinical and population-based samples. Am. J. Psychiatry. 2019;176:531–542. doi: 10.1176/appi.ajp.2019.18091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie B.N., Donnelly P., Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan T.L., Brown S.A., Dowling G.J. The adolescent brain cognitive development study. J. Res. Adolesc. 2018;28:154–156. doi: 10.1111/jora.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangarani-Farahani M., Izadi-Najafabadi S., Zwicker J.G. How does brain structure and function on MRI differ in children with autism spectrum disorder, developmental coordination disorder, and/or attention deficit hyperactivity disorder? Int. J. Dev. Neurosci. 2022;82:681–715. doi: 10.1002/jdn.10228. [DOI] [PubMed] [Google Scholar]
- Karalunas S.L., Huang-Pollock C.L. Integrating impairments in reaction time and executive function using a diffusion model framework. J. Abnorm. Child Psychol. 2013;41:837–850. doi: 10.1007/s10802-013-9715-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas S.L., Nigg J.T. Heterogeneity and subtyping in attention-deficit/hyperactivity disorder-considerations for emerging research using person-centered computational approaches. Biol. Psychiatry. 2020;88:103–110. doi: 10.1016/j.biopsych.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas S.L., Gustafsson H.C., Fair D., Musser E.D., Nigg J.T. Do we need an irritable subtype of ADHD? Replication and extension of a promising temperament profile approach to ADHD subtyping. Psychol. Assess. 2019;31:236–247. doi: 10.1037/pas0000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas S.L., Fair D., Musser E.D., Aykes K., Iyer S.P., Nigg J.T. Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: toward biologically based nosologic criteria. JAMA Psychiatry. 2014;71:1015–1024. doi: 10.1001/jamapsychiatry.2014.763. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Karalunas S.L., Ostlund B.D., Alperin B.R., Figuracion M., Gustafsson H.C., Deming E.M., Foti D., Antovich D., Dude J., Nigg J., Sullivan E. Electroencephalogram aperiodic power spectral slope can be reliably measured and predicts ADHD risk in early development. Dev. Psychobiol. 2022;64 doi: 10.1002/dev.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Adler L.A., Berglund P., Green J.G., McLaughlin K.A., Fayyad J., Russo L.J., Sampson N.A., Shahly V., Zaslavsky A.M. The effects of temporally secondary co-morbid mental disorders on the associations of DSM-IV ADHD with adverse outcomes in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A) Psychol. Med. 2014 doi: 10.1017/s0033291713002419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Adler L., Barkley R., Biederman J., Conners C.K., Demler O., Faraone S.V., Greenhill L.L., Howes M.J., Secnik K., Spencer T., Ustun T.B., Walters E.E., Zaslavsky A.M. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am. J. Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski M.B., Antovich D., Karalunas S.L., Nigg J.T. Temperament in middle childhood questionnaire: New data on factor structure and applicability in a child clinical sample. Psychol. Assess. 2022;34(12):1081–1092. doi: 10.1037/pas0001180. PMID: 36174168; PMCID: PMC9772251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer H.C., Karen Kraemer Lowe, Kupfer D.J. Oxford University Press; 2005. To Your Health: How to Understand What Research Tells Us about Risk. [Google Scholar]
- Lahey BB., Applegate B., McBurnett K., Biederman J., Greenhill L., Hynd GW., Barkley RA., Newcorn J., Jensen P., Richters J., et al. DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents. Am J Psychiatry. 1994;151(11):1673–1685. doi: 10.1176/ajp.151.11.1673. PMID: 7943460. [DOI] [PubMed] [Google Scholar]
- Lee S.S., Humphreys K.L., Flory K., Liu R., Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clin. Psychol. Rev. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel M.M., Nikolas M., Jernigan K., Friderici K., Waldman I., Nigg J.T. The dopamine receptor D4 gene (DRD4) moderates family environmental effects on ADHD. J. Abnorm. Child Psychol. 2011;39:1–10. doi: 10.1007/s10802-010-9439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel M.M., Elkins A.R., Eng A.G., Goh P.K., Bansal P.S., Smith-Thomas T.E., Thaxton M.H., Ryabinin P., Mooney M.A., Gustafsson H.C., Karalunas S.L., Nigg J.T. Longitudinal temperament pathways to ADHD between childhood and adolescence. Res Child Adolesc. Psychopathol. 2022 doi: 10.1007/s10802-022-00902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur J.E. In: The Effect of Delay and of Intervening Events on Reinforcement Value. Commons M.L., Mazur J.E., Nevin J.A., Rachlin H., editors. Pyschology Press; 1987. An adjusting procedure for studying delayed reinforcement; pp. 55–73. [Google Scholar]
- Meye F.J., Digirolamo G.J. In: Psychological Medicine. 3rd edn., Cacioppo J.T., Tassinary L.G., Berntson. G.G., editors. Vol. 37. Cambridge University Press; 2007. Handbook of Psychophysiology; pp. 1818–1819. [Google Scholar]
- Miller LL., Gustafsson HC., Tipsord J., Song M., Nousen E., Dieckmann N., Nigg JT. Is the Association of ADHD with Socio-Economic Disadvantage Explained by Child Comorbid Externalizing Problems or Parent ADHD? J Abnorm Child Psychol. 2018;46(5):951–963. doi: 10.1007/s10802-017-0356-8. PMID: 29128953; PMCID: PMC5948120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M., Musser E.D., Young G.S., Olson B., Steiner R.D., Nigg J.T. Sibling recurrence risk and cross-aggregation of attention-deficit/hyperactivity disorder and autism spectrum disorder. JAMA Pediatr. 2019;173:147–152. doi: 10.1001/jamapediatrics.2018.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills B.D., Grayson D.S., Shunmugavel A., Miranda-Dominguez O., Feczko E., Earl E., Neve K.A., Fair D.A. Correlated gene expression and anatomical communication support synchronized brain activity in the mouse functional connectome. J. Neurosci. 2018;38:5774–5787. doi: 10.1523/JNEUROSCI.2910-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S.H., Wilson V.B., Karalunas S.L. Comparing hyperbolic, delay-amount sensitivity and present-bias models of delay discounting. Behav. Process. 2015;114:52–62. doi: 10.1016/j.beproc.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney M.A., Ryabinin P., Wilmot B., Bhatt P., Mill J., Nigg J.T. Large epigenome-wide association study of childhood ADHD identifies peripheral DNA methylation associated with disease and polygenic risk burden. Transl. Psychiatry. 2020 doi: 10.1038/s41398-020-0710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos R.H., Moos B.S. In: Handbook of Measurements for Marriage and Family. Freman N., Sherman R., editors. Routledge; 1987. Family environment scale. [Google Scholar]
- Musser E.D., Backs R.W., Schmitt C.F., et al. Emotion regulation via the autonomic nervous system in children with attention-deficit/hyperactivity disorder (ADHD) J. Abnorm. Psychol. 2011;39:841–852. doi: 10.1007/s10802-011-9499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser E.D., Karalunas S.L., Dieckmann N., Peris T.S., Nigg J.T. Attention-deficit/hyperactivity disorder developmental trajectories related to parental expressed emotion. J. Abnorm. Psychol. 2016;125:182–195. doi: 10.1037/abn0000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser E.D., Hawkey E., Kachan-Liu S.S., Lees P., Roullet J.-B., Goddard K., Steiner R.D., Nigg J.T. Shared familial transmission of autism spectrum and attention-deficit/hyperactivity disorders. J. Child Psychol. Psychiatry. 2014;55:819–827. doi: 10.1111/jcpp.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin D.S. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol. Methods. 1999;4:139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Nigg J.T. The ADHD response-inhibition deficit as measured by the stop task: replication with DSM–IV combined type, extension, and qualification. J. Abnorm. Child Psychol. 1999;27:393–402. doi: 10.1023/a:1021980002473. [DOI] [PubMed] [Google Scholar]
- Nigg J.T., Karalunas S.L., Feczko E., Fair D.A. Toward a revised nosology for attention-deficit/hyperactivity disorder heterogeneity. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2020;5:726–737. doi: 10.1016/j.bpsc.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg J.T., Sibley M.H., Thapar A., Karalunas S.L. Development of ADHD: etiology, heterogeneity, and early life course. Annu Rev. Dev. Psychol. 2020;2:559–583. doi: 10.1146/annurev-devpsych-060320-093413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg J.T., Nikolas M., Miller T., Burt S.A., Klump K.L., von Eye A. Factor structure of the Children’s Perception of Interparental Conflict Scale for studies of youths with externalizing behavior problems. Psychol. Assess. 2009;21:450–456. doi: 10.1037/a0016564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg J.T., Gustafsson H.C., Karalunas S.L., Ryabinin P., McWeeney S.K., Faraone S.V., Mooney M.A., Fair D.A., Wilmot B. Working memory and vigilance as multivariate endophenotypes related to common genetic risk for attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2018 doi: 10.1016/j.jaac.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg J.T., Karalunas S.L., Gustafsson H.C., Bhatt P., Ryabinin P., Mooney M.A., Faraone S.V., Fair D.A., Wilmot B. Evaluating chronic emotional dysregulation and irritability in relation to ADHD and depression genetic risk in children with ADHD. J. Child Psychol. Psychiatry. 2020 doi: 10.1111/jcpp.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg, J.T., (2023). Toward an epigenetic and common pathway theory of mental disorder. Journal of Psychopathology and Clinical Science. In Press. [DOI] [PMC free article] [PubMed]
- Ostlund B.D., Alperin B.R., Drew T., Karalunas S.L. Behavioral and cognitive correlates of the aperiodic (1/f-like) exponent of the EEG power spectrum in adolescents with and without ADHD. Dev. Cogn. Neurosci. 2021;48 doi: 10.1016/j.dcn.2021.100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S.E., Posner M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012 doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Antovich D, Miller N, Nigg JT. (2022). Prospective prediction of developing internalizing disorders in ADHD. J Child Psychol Psychiatry. Dec 4. doi: 10.1111/jcpp.13731. Online ahead of print.PMID: 36464786. [DOI] [PMC free article] [PubMed]
- Ratcliff R., Smith P.L., Brown S.D., McKoon G. Diffusion decision model: current issues and history. Trends Cogn. Sci. 2016;20:260–281. doi: 10.1016/j.tics.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riglin L., Leppert B., Dardani C., Thapar AK., Rice F., O’Donovan MC., Thapar A. ADHD and depression: investigating a causal explanation. Psychological Medicine. 2020:1–8. doi: 10.1017/S0033291720000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelse N., Buitelaar J.K., Hartman C.A. Structural brain imaging correlates of ASD and ADHD across the lifespan: a hypothesis-generating review on developmental ASD–ADHD subtypes. J. Neural Transm. 2017;124:259–271. doi: 10.1007/s00702-016-1651-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart M.K. Guilford Press; 2012. Becoming Who We Are: Temperament and Personality in Development. [Google Scholar]
- Rothbart M.K., Ahadi S.A., Evans D.E. Temperament and personality: origins and outcomes. J. Pers. Soc. Psychol. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Rutter, M., 2003. Autism Diagnostic Interviewed Revised ADI-R. Western Psychological Services (WPS).
- Schachar R., Tannock R., Marriott M., Logan G. Deficient inhibitory control in attention deficit hyperactivity disorder. J. Abnorm. Child Psychol. 1995;23:411–437. doi: 10.1007/BF01447206. [DOI] [PubMed] [Google Scholar]
- Sergeant J.A., Oosterlaan J., van der Meere J. In: Handbook of Disruptive Behavior Disorders. Quay H.C., Hogan A.E., editors. Springer; US, Boston, MA: 1999. Information processing and energetic factors in attention-deficit/hyperactivity disorder; pp. 75–104. [Google Scholar]
- Shiner R.L., Soto C.J., De Fruyt F. Personality assessment of children and adolescents. Annu. Rev. Dev. Psychol. 2021;3:113–137. [Google Scholar]
- Stadler D.D., Musser E.D., Holton K.F., Shannon J., Nigg J.T. Recalled initiation and duration of maternal breastfeeding among children with and without ADHD in a well characterized case-control sample. J. Abnorm. Child Psychol. 2016;44:347–355. doi: 10.1007/s10802-015-9987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre G., Sharp W., Kundzicz P., Bouyssi-Kobar M., Norman L., Choudhury S., Shaw P. Predicting the course of ADHD symptoms through the integration of childhood genomic, neural, and cognitive features. Mol. Psychiatry. 2021;26:4046–4054. doi: 10.1038/s41380-020-00941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J.M., Eugene Arnold L., Molina B.S.G., Sibley M.H., Hechtman L.T., Hinshaw S.P., Abikoff H.B., Stehli A., Owens E.B., Mitchell J.T., Nichols Q., Howard A., Greenhill L.L., Hoza B., Newcorn J.H., Jensen P.S., Vitiello B., Wigal T., Epstein J.N., Tamm L., Lakes K.D., Waxmonsky J., Lerner M., Etcovitch J., Murray D.W., Muenke M., Acosta M.T., Arcos-Burgos M., Pelham W.E., Kraemer H.C., the MTA Cooperative Group Young adult outcomes in the follow-up of the multimodal treatment study of attention-deficit/hyperactivity disorder: symptom persistence, source discrepancy, and height suppression. J. Child Psychol. Psychiatry. 2017 doi: 10.1111/jcpp.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackett J.L., Lang J.W.B., Markon K.E., Herzhoff K. A correlated traits, correlated methods model for thin-slice child personality assessment. Psychol. Assess. 2019;31:545–556. doi: 10.1037/pas0000635. [DOI] [PubMed] [Google Scholar]
- Taurines R., Schwenck C., Westerwald E., Sachse M., Siniatchkin M., Freitag C. ADHD and autism: differential diagnosis or overlapping traits? A selective review. Atten. Defic. Hyperact. Disord. 2012;4:115–139. doi: 10.1007/s12402-012-0086-2. [DOI] [PubMed] [Google Scholar]
- Thapar A., Riglin L. The importance of a developmental perspective in Psychiatry: what do recent genetic-epidemiological findings show? Mol. Psychiatry. 2020;25:1631–1639. doi: 10.1038/s41380-020-0648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treur JL., Demontis D., Smith GD., Sallis H., Richardson TG., Wiers RW., Munafò MR. Investigating causality between liability to ADHD and substance use, and liability to substance use and ADHD risk, using Mendelian randomization. Addiction Biology. 2019 doi: 10.1111/adb.12849. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Dajani D.R., Voorhies W., Bednarz H., Kana R.K. Progress and roadblocks in the search for brain-based biomarkers of autism and attention-deficit/hyperactivity disorder. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2017.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F., Aron A.R., Band G.P., Beste C., Bissett P.G., Brockett A.T., et al. A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. Elife. 2019:8. doi: 10.7554/eLife.46323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Koob G.F., Croyle R.T., Bianchi D.W., Gordon J.A., Koroshetz W.J., Pérez-Stable E.J., Riley W.T., Bloch M.H., Conway K., Deeds B.G., Dowling G.J., Grant S., Howlett K.D., Matochik J.A., Morgan G.D., Murray M.M., Noronha A., Spong C.Y., Wargo E.M., Warren K.R., Weiss S.R.B. The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev. Cogn. Neurosci. 2018;32:4–7. doi: 10.1016/j.dcn.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens T.D. Oxford University Press; 2001. Elementary Signal Detection Theory. [Google Scholar]
- Wiggs K., Elmore A.L., Nigg J.T., Nikolas M.A. Pre- and perinatal risk for attention-deficit hyperactivity disorder: Does neuropsychological weakness explain the link? J. Abnorm. Child Psychol. 2016;44:1473–1485. doi: 10.1007/s10802-016-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing A.M. Voluntary timing and brain function: an information processing approach. Brain Cogn. 2002 doi: 10.1006/brcg.2001.1301. [DOI] [PubMed] [Google Scholar]
- Wing A.M., Kristofferson A.B. Response delays and the timing of discrete motor responses. Percept. Psychophys. 1973 doi: 10.3758/bf03198607. [DOI] [Google Scholar]
- Wing A.M., Kristofferson A.B. The timing of interresponse intervals. Percept. Psychophys. 1973 doi: 10.3758/bf03205802. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Public data set on the NIMH Data Archive (NDA).