Abstract
Background
Immune-related adverse events (irAEs) are frequently reported during immune checkpoint inhibitor (ICI) therapy and are associated with long-term outcomes. It is unknown if the irAE occurrence is a valid surrogate of ICIs’ efficacy.
Methods
We identified articles reporting the results of randomized trials of experimental ICI therapy in solid tumors with a systematic search. The control arms could be placebo, cytotoxic/targeted therapy, or ICI therapy. We extracted the hazard ratios for overall survival (OS) with the number of OS events per arm and the number and percentages of overall and specific irAEs of grade 1-2 and grade 3-4 per arm. We estimated the treatment effect on the potential surrogate outcome with the odds ratio of the irAE rate between the experimental and the control arm. The statistical analysis consisted of weighted linear regression on a logarithmic scale between treatment effects on irAE rate and treatment effects on OS.
Results
Sixty-two randomized trials were included for a total of 79 contrasts and 42 247 patients. The analyses found no significant association between the treatment effects for overall grade 1-2 or grade 3-4 irAE rates or specific (skin, gastrointestinal, endocrine) irAE rates. In the non-small-cell lung cancer (NSCLC) trial subset, we observed a negative association between treatment effects on overall grade 1-2 irAEs and treatment effects on OS in studies with patients selected for programmed death-ligand 1 expression (R2 = 0.55; 95% confidence interval 0.20-0.95; R = −0.69). In the melanoma trial subset, a negative association was shown between treatment effects on gastrointestinal grade 3-4 irAEs and treatment effects on OS in trials without an ICI-based control arm (R2 = 0.77; 95% confidence interval 0.24-0.99; R = −0.89).
Conclusions
We found low-strength correlations between the ICI therapy effects on overall or specific irAE rates and the treatment effects on OS in several cancer types.
Key words: immune checkpoint inhibitors, immune-related adverse events, surrogate endpoints, trial-based meta-analysis
Highlights
-
•
Immune-related adverse events (irAEs) are prognostic in patients treated with immune checkpoint inhibitors.
-
•
We explored the irAEs’ surrogacy for overall survival with a trial-based meta-analysis.
-
•
We found correlations of varying magnitude (R ranged from −0.63 to 0.25). All estimated correlations were of low strength.
-
•
IrAEs do not seem to capture the whole effect of the immune checkpoint inhibitor therapy on overall survival.
Introduction
Immune checkpoint inhibitors (ICIs), which include anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4), anti-programmed cell death protein 1 (PD-1), and anti-programmed death-ligand 1 (PD-L1) monoclonal antibodies, represent significant progress in oncology, particularly in the treatment of solid tumors. These agents block the ability of malignancies to escape immune surveillance, thus reactivating the T-cell immune response against cancer. The activation of the immune system can also induce an immune-mediated inflammation of different organs and tissues, however, especially the skin, endocrine system, gastrointestinal (GI) tract, and lungs.1, 2, 3, 4
The immune-related adverse events (irAEs), secondary to ICI therapy, significantly impact treatment delivery. Most irAEs tend to be mild and self-limiting, but potentially life-threatening events can occur in a few severe cases (with toxicities of grade 3 or 4).3 Hence, either the European Society of Medical Oncology (ESMO) or the American Society of Clinical Oncology (ASCO) has issued guidelines for managing the various irAEs.5,6
Several studies evaluating different ICIs have shown an association between the development of irAEs and long-term survival, particularly in patients with melanoma,7 non-small-cell lung cancer (NSCLC),8,9 and urothelial cancers.10 A recent exploratory pooled analysis of 2503 patients from three randomized trials in non-squamous NSCLC (IMpower130, IMpower132, and IMpower150)11 showed that patients with irAEs (excluding rash, perceived as the least specific irAE) had longer overall survival (OS) compared with those without irAEs in the atezolizumab-containing arm [hazard ratio (HR) = 0.75; 95% confidence interval (CI) 0.65-0.87] but not in the control arm (HR = 0.90; 95% CI 0.71-1.12). NSCLC patients with irAEs of higher grade (grade 3 or 4) had inferior outcomes compared with those with lower grade (grade 1 or 2) irAEs.11
The previously mentioned individual-level relationship does not imply that the irAE occurrence might be a valid surrogate of immunotherapy efficacy. A surrogate endpoint to be validated has to correlate with the OS, and the effect of the intervention on the surrogate should predict the effect on the long-term clinical outcome.12,13 A meta-analysis of randomized, controlled trials represents an essential tool for validating surrogate endpoints.12,14,15
Currently, no candidate surrogate for long-term survival has been validated in ICI trials. Traditional intermediate endpoints, such as progression-free survival and objective response rate, failed to demonstrate either individual-level or trial-level association with OS in studies evaluating ICI therapy.16, 17, 18, 19
This study aimed to assess whether the treatment effects on irAEs can predict the treatment effects on the OS at the trial level, supporting the hypothesis of irAE occurrence as a surrogate of ICI treatment efficacy. The validation of this surrogacy would establish that the irAEs lie in the causal pathway of the ICI therapy effect on OS and thus capture most of the intervention’s impact on the clinical outcome. The secondary aims were to explore the heterogeneity of the trial-level associations according to specific trial characteristics: the study population (selected for tumor biomarkers, such as PD-L1 expression), the treatment type administered in the experimental arm [anti-CTLA-4 versus anti-programmed cell death protein 1 (anti-PD-1)/PD-L1 versus a combination of anti-CTLA4 and anti-PD-1/PD-L1 antibodies], the type of control arm (an ICI therapy versus a cytotoxic/targeted therapy/placebo control arm), and the treatment line (first line versus second or later line). For this purpose, we carried out a systematic literature search and a trial-based meta-analysis of randomized, controlled trials of experimental ICI therapy in patients with advanced solid tumors.
Methods
We planned a meta-analysis with aggregate data (AD) derived from randomized trials assessing the efficacy of ICI therapy and retrieved by a systematic search, according to the Reporting of Surrogate Endpoint Evaluation using Meta-analyses (ReSEEM) guidelines and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.20,21 The primary objective was the trial-level validation of the overall irAE rate as a potential surrogate endpoint of the effect on OS of the ICI therapy in advanced-stage solid tumors (i.e. evaluating whether the size of the treatment effect on irAEs allows one to predict the size of the effect on the final outcome). The primary analysis was planned in specific tumor types for which a significant number of randomized trials are available [i.e. NSCLC, melanoma, renal cell carcinoma, small-cell lung cancer (SCLC)].
Systematic search
We identified original articles reporting the results of randomized trials of ICI therapy in solid tumors from PubMed, EMBASE, and Cochrane Library database search from inception to 31 March 2021. Keywords included the following terms: ‘immunotherapy’, ‘immune checkpoint inhibitor therapy’, ‘anti-PD-1’, ‘anti-PD-L1’, ‘anti-CTLA-4’, ‘atezolizumab’, ‘avelumab’, ‘durvalumab’, ‘ipilimumab’, ‘nivolumab’, ‘pembrolizumab’, ‘tremelimumab’, ‘randomized OR randomised’, ‘phase 2 OR phase 3 trial’. Proceedings of the ASCO Annual Meeting, American Association for Cancer Research (AACR) Annual Meeting, and the ESMO Meeting were hand-searched for the years 2016 through 2020. We examined references of clinical trials, review articles, and editorials for other relevant articles. Commentaries, reviews, preclinical studies, observational studies, and articles not written in English were not eligible for the present study. We reported the detailed electronic search strategy and the PRISMA flow diagram in the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2023.100787.
Eligibility criteria
All citations were independently screened by three authors (DP, WFB, and VA). Eligible studies should have an ICI-based regimen as experimental arm (either ICI monotherapy or a combination of two different ICIs or an ICI plus a cytotoxic or targeted therapy). The control arm could be a cytotoxic/targeted therapy, placebo, or ICI-based therapy. We included randomized phase II or III trials that reported OS data and irAE rates according to the Common Terminology Criteria for Adverse Events (CTCAE). We excluded trials evaluating different forms of immunotherapy (i.e. antitumor vaccines). We included the most recent report of eligible randomized trials in the case of duplicate publications.
Data extraction
We recorded the following information from each selected trial: authors’ names, journal and year of publication, study design, and duration of follow-up. We initially defined the experimental and control arms for each included trial. We extracted the following data from each treatment arm: the number of patients randomly assigned to treatment and analyzed per arm, the HRs for OS with the number of OS events per arm, and the overall number and percentages of the irAEs per arm of any grade and grade 1-2 or 3-4. Furthermore, we extracted irAE rates for each specific organ (i.e. GI tract, skin, and endocrine system). In trials not reporting overall irAE rates, we summed up GI (diarrhea, colitis), skin (rash, pruritus, dermatitis, severe skin reaction), endocrine (hypothyroidism, hyperthyroidism, hypophysitis, adrenal insufficiency), and pulmonary (pneumonitis) toxicities per each arm.
Endpoints
We considered OS as the long-term clinical outcome of interest. The HR of death between the experimental and the control arm was used as the treatment effect estimate on the clinical outcome. We estimated the treatment effect on the potential surrogate outcome with the odds ratio (OR) of irAEs between the experimental and the control arm. The irAEs were collected as reported by the investigators according to CTCAE in each included study.
In addition, we carried out a sensitivity analysis using the attributable risk instead of the OR to estimate the treatment effects on the candidate surrogate endpoint, the irAE rate. Finally, a validation analysis was carried out, excluding trials that needed missing data imputation.
Statistical analysis
The statistical analysis consisted of weighted linear regression on a logarithmic scale between treatment effects on the OS and treatment effects on the irAE rates. Treatment effects on the clinical outcome (expressed as HRs) and on the surrogate outcome (expressed as ORs) were extracted from each trial or estimated when not reported using the survival curves (for HR) and the proportion of irAEs (for OR). Each study was weighted according to the total number of events (deaths) in patients included in each trial. When events were not explicitly reported, or the irAE event number was beyond the total number of patients, they were estimated using available information, as detailed in the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2023.100787. For trials with multiple arms, a contrast with the control arm was studied for each experimental arm (that is, the treatment effect was estimated for each experimental arm versus the control arm). As multiple contrasts from the same trial create ‘clustered data,’ each contrast coming from a trial with more than two arms was given a lower weight, obtained by equally dividing the number of events in the control arm among the experimental arms.
The coefficient of determination (R2) was used to assess the goodness of fit for each model and quantify the surrogacy level. The R2 threshold to identify a valid surrogate was set at 0.65 or higher according to Ciani et al.22 We estimated 95% CIs of the R2 values by bootstrap techniques.
We scored the strength of trial-level correlation according to the criteria proposed by the Institute of Quality and Efficiency in Health Care,23 and adapted by Prasad et al.24: low-strength correlation (R ≤ 0.70), medium-strength correlation (R > 0.7 to R < 0.85), and high-strength correlation (R ≥ 0.85).
Finally, an interaction analysis was used to test whether the surrogacy (that is, the slope of the weighted regression line) was different according to the following factors: type of experimental agent, selection of study population for PD-L1 expression or not, type of comparison, and treatment line. A further subgroup analysis was carried out only for significant results, dividing studies according to the factors mentioned above. This latter analysis was not pre-planned. P values <0.05 were selected as significant, and data were acquired and analyzed in R v4.1.1 software environment.25
Results
Trials included in the analysis
We identified 3150 citations with a systematic literature search. From a total of 98 randomized trials identified after level 1 screening, we included 62 studies (79 contrasts and 42 247 patients) (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2023.100787 and Table 1). We selected 6 randomized trials (8 contrasts) assessing ICI therapy in patients with upper GI cancer (esophageal, gastric, or gastroesophageal junction cancer),26, 27, 28, 29, 30, 31 6 trials (8 contrasts) in patients with renal cell carcinoma,32, 33, 34, 35, 36, 37 12 trials (17 contrasts) in patients with melanoma,38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 16 trials (18 contrasts) in NSCLC,50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 5 trials (7 contrasts) in SCLC,65, 66, 67, 68, 69 and 6 trials (9 contrasts) in urothelial cancers.70, 71, 72, 73, 74, 75 We included 11 trials (12 contrasts) in patients with other primary tumor sites [breast cancer (2 trials);76,77 glioblastoma (1 trial);78 mesothelioma (2 trials);79,80 prostate cancer (2 trials);81,82 hepatocellular carcinoma (1 trial);83 head and neck cancer (3 studies and 4 contrasts)].84, 85, 86
Table 1.
Trials included in the analysis
| Contrast | Tumor type | Experimental arm | Control arm | No. of patients | Median follow-up time (months) | OR (95% CI) | HR (95% CI) |
|---|---|---|---|---|---|---|---|
| Kojima et al.26 | Upper GI | Pembrolizumab | Paclitaxel, docetaxel, or irinotecan | 628 | 7.0 | 3.77 (2.27-6.26) | 0.89 (0.75-1.05) |
| Chau et al.27 | Upper GI | Nivolumab and chemotherapy | Chemotherapy | 645 | n.a. | 4.97 (3.47-7.11) | 0.74 (0.58-0.96) |
| Chau et al.27 | Upper GI | Nivolumab and ipilimumab | Chemotherapy | 649 | n.a. | 17.4 (11.7-25.87) | 0.78 (0.62-0.98) |
| Chen et al.28 | Upper GI | Nivolumab | Placebo | 493 | 27.3 | 8.69 (1.15-65.89) | 0.62 (0.51-0.76) |
| Shitara et al.29 | Upper GI | Pembrolizumab | Paclitaxel | 395 | 8.5 | 2.73 (1.6-4.66) | 0.82 (0.66-1.03) |
| Shitara et al.30 | Upper GI | Pembrolizumab | Cisplatin and fluorouracil | 506 | 29.4 | 3.2 (1.83-5.58) | 0.91 (0.74-1.1) |
| Shitara et al.30 | Upper GI | Pembrolizumab, cisplatin, and fluorouracil | Cisplatin and fluorouracil | 507 | 29.4 | 3.74 (2.16-6.49) | 0.85 (0.7-1.03) |
| Janjigian et al.31 | Upper GI | Nivolumab, oxaliplatin, and capecitabine | Oxaliplatin and capecitabine | 1581 | 12.1 | 5.5 (4.39-6.9) | 0.8 (0.68-0.94) |
| Ferris et al.84 | Head and neck | Nivolumab | MTX, docetaxel, or cetuximab | 361 | >24.2 | 1.52 (0.93-2.48) | 0.68 (0.54-0.86) |
| Cohen et al.85 | Head and neck | Pembrolizumab | MTX, docetaxel, or cetuximab | 495 | 7.3 | 2.53 (1.56-4.12) | 0.8 (0.65-0.98) |
| Siu et al.86 | Head and neck | Durvalumab and tremelimumab | Durvalumab | 194 | 6.5 | 2.92 (1.06-7.99) | 0.99 (0.69-1.43) |
| Siu et al.86 | Head and neck | Durvalumab and tremelimumab | Tremelimumab | 192 | 7.6 | 32.29 (1.94-538.89) | 0.72 (0.51-1.03) |
| Motzer et al.32 | RCC | Nivolumab 2 mg/kg | Nivolumab 0.3 mg/kg | 114 | 24.0 | 1.45 (0.69-3.07) | 0.8 (0.6-1.1) |
| Motzer et al.32 | RCC | Nivolumab 10 mg/kg | Nivolumab 0.3 mg/kg | 114 | 24.0 | 2.88 (1.34-6.2) | 0.9 (0.6-1.2) |
| Motzer et al.32 | RCC | Nivolumab 10 mg/kg | Nivolumab 2 mg/kg | 108 | 24.0 | 1.98 (0.92-4.3) | n.a. |
| Motzer et al.33 | RCC | Nivolumab and ipilimumab | Sunitinib | 1096 | 32.4 | 0.88 (0.65-1.21) | 0.71 (0.59-0.86) |
| Rini et al.34 | RCC | Atezolizumab and bevacizumab | Sunitinib | 915 | 24.0 | 1.44 (1.11-1.87) | 0.93 (0.76-1.14) |
| Powles et al.35 | RCC | Pembrolizumab and axitinib | Sunitinib | 861 | 30.6 | 1.85 (1.41-2.44) | 0.53 (0.38-0.74) |
| Motzer et al.36 | RCC | Lenvatinib and pembrolizumab | Sunitinib | 712 | 26.6 | 3.47 (2.53-4.75) | 0.66 (0.49-0.88) |
| Choueiri et al.37 | RCC | Nivolumab and cabozantinib | Sunitinib | 651 | 18.1 | 9.11 (6.02-13.79) | 0.6 (0.44-0.82) |
| Hodi et al.38 | Melanoma | Ipilimumab and gp100 | gp100 | 539 | 19.1 | 2.98 (1.96-4.53) | 0.68 (0.55-0.85) |
| Hodi et al.38 | Melanoma | Ipilimumab | gp100 | 273 | 22.5 | 3.36 (2.02-5.58) | 0.66 (0.51-0.87) |
| Maio et al.39 | Melanoma | Ipilimumab and dacarbazine | Dacarbazine | 502 | 9.9 | 5.64 (3.8-8.35) | 0.69 (0.57-0.84) |
| Wolchok et al.40 | Melanoma | Ipilimumab 10 mg/kg | Ipilimumab 0.3 mg/kg | 145 | 9.5 | 6.64 (3.2-13.8) | 0.77 (0.52-1.13) |
| Wolchok et al.40 | Melanoma | Ipilimumab 10 mg/kg | Ipilimumab 3 mg/kg | 144 | 9.7 | 1.29 (0.64-2.62) | 0.88 (0.59-1.29) |
| Wolchok et al.40 | Melanoma | Ipilimumab 3 mg/kg | Ipilimumab 0.3 mg/kg | 145 | 8.5 | 5.13 (2.51-10.5) | 0.88 (0.6-1.28) |
| Long et al.41 | Melanoma | Pembrolizumab | Ipilimumab | 834 | 45.9 | 1.58 (1.09-2.27) | 0.73 (0.61-0.89) |
| Hamid et al.42 | Melanoma | Pembrolizumab 2 mg/kg | Investigator-choice chemotherapy | 359 | 28.0 | 12.27 (3.68-40.92) | 0.86 (0.67-1.1) |
| Hamid et al.42 | Melanoma | Pembrolizumab 10 mg/kg | Investigator-choice chemotherapy | 360 | 28.0 | 15.09 (4.56-49.93) | 0.74 (0.57-0.96) |
| Hodi et al.43 | Melanoma | Nivolumab and ipilimumab | Ipilimumab | 142 | 24.5 | 1.84 (0.7-4.8) | 0.74 (0.43-1.26) |
| Wolchok et al.44 | Melanoma | Nivolumab | Ipilimumab | 631 | >36.0 | 0.58 (0.42-0.82) | 0.63 (0.48-0.81) |
| Wolchok et al.44 | Melanoma | Nivolumab and ipilimumab | Ipilimumab | 629 | >36.0 | 2.59 (1.7-3.95) | 0.55 (0.42-0.72) |
| Ribas et al.45 | Melanoma | Tremelimumab | Temozolomide or dacarbazine | 655 | n.a. | n.a. | 0.88 (NA-NA) |
| Weber et al.46 | Melanoma | Nivolumab → ipilimumab | Ipilimumab → nivolumab | 138 | 17.2 | n.a. | 0.48 (0.29-0.8) |
| Ascierto et al.47 | Melanoma | Ipilimumab 10 mg/kg | Ipilimumab 3 mg/kg | 727 | 12.8 | 2.4 (1.76-3.28) | 0.84 (0.7-0.99) |
| Larkin et al.48 | Melanoma | Nivolumab | Investigator’s choice chemotherapy | 405 | 24.0 | 5.92 (3.58-9.79) | 0.95 (0.73-1.24) |
| Ascierto et al.49 | Melanoma | Nivolumab | Ipilimumab | 906 | 51.0 | n.a. | 0.87 (0.66-1.14) |
| Horn et al.50 | NSCLC | Nivolumab | Docetaxel | 272 | n.a. | 0.87 (0.51-1.5) | 0.62 (0.47-0.8) |
| Brahmer et al.53 | NSCLC | Pembrolizumab | Platinum-doublet chemotherapy | 305 | 19.1 | 9.05 (4.12-19.87) | 0.63 (0.46-0.88) |
| Carbone et al.51 | NSCLC | Nivolumab | Platinum-based chemotherapy | 541 | 13.5 | 2.6 (1.78-3.79) | 1.07 (0.86-1.33) |
| Borghaei et al.52 | NSCLC | Pembrolizumab, carboplatin, and pemetrexed | Carboplatin and pemetrexed | 123 | 23.9 | 2.22 (0.82-6.03) | 0.56 (0.32-0.95) |
| Horn et al.50 | NSCLC | Nivolumab | Docetaxel | 582 | n.a. | 1.28 (0.91-1.79) | 0.75 (0.63-0.91) |
| Herbst et al.54 | NSCLC | Pembrolizumab 2 mg/kg | Docetaxel | 688 | 13.1 | 5.76 (3.04-10.9) | 0.71 (0.58-0.88) |
| Herbst et al.54 | NSCLC | Pembrolizumab 10 mg/kg | Docetaxel | 689 | 13.1 | 6.58 (3.49-12.38) | 0.61 (0.49-0.75) |
| Gandhi et al.55 | NSCLC | Pembrolizumab, pemetrexed, and platinum-based drug | Pemetrexed and platinum-based drug | 616 | 10.5 | 2.09 (1.28-3.4) | 0.49 (0.38-0.64) |
| Socinski et al.56 | NSCLC | Atezolizumab, bevacizumab, carboplatin, and paclitaxel | Bevacizumab, carboplatin, and paclitaxel | 692 | 15.4 | 4.19 (3.06-5.75) | 0.78 (0.64-0.96) |
| Jotte et al.57 | NSCLC | Atezolizumab, carboplatin, and nab-paclitaxel | Carboplatin and nab-paclitaxel | 683 | 25.8 | 4.42 (3-6.49) | 0.88 (0.73-1.05) |
| Paz-Ares et al.58 | NSCLC | Pembrolizumab, carboplatin, and paclitaxel or nab-paclitaxel | Carboplatin and paclitaxel or nab-paclitaxel | 559 | 7.8 | 4.44 (2.69-7.31) | 0.64 (0.49-0.85) |
| Herbst et al.59 | NSCLC | Atezolizumab | Platinum-based chemotherapy | 572 | 17.5 | 3.35 (2.24-5) | 0.83 (0.65-1.07) |
| Rizvi et al.60 | NSCLC | Durvalumab | Platinum-based chemotherapy | 325 | 30.2 | 4.44 (2.32-8.49) | 0.76 (0.56-1.02) |
| Rizvi et al.60 | NSCLC | Durvalumab and tremelimumab | Platinum-based chemotherapy | 325 | 30.2 | 11.18 (6.03-20.76) | 0.85 (0.61-1.17) |
| West et al.61 | NSCLC | Atezolizumab, carboplatin, and nab-paclitaxel | Carboplatin and nab-paclitaxel | 679 | 18.8 | 3.21 (2.13-4.83) | 0.79 (0.64-0.98) |
| Paz-Ares et al.62 | NSCLC | Nivolumab, ipilimumab, and platinum-based chemotherapy | Platinum-based chemotherapy | 719 | 13.2 | 18.85 (11.46-31) | 0.66 (0.55-0.8) |
| Mok et al.63 | NSCLC | Pembrolizumab | Platinum-based chemotherapy | 1274 | 16.7 | 5 (3.52-7.12) | 0.81 (0.71-0.93) |
| Govindan et al.64 | NSCLC | Ipilimumab, carboplatin, and paclitaxel | Carboplatin and paclitaxel | 749 | 12.1 | 2.01 (1.49-2.7) | 0.91 (0.77-1.07) |
| Liu et al.65 | SCLC | Atezolizumab, carboplatin, and etoposide | Carboplatin and etoposide | 403 | 22.9 | 2.18 (1.42-3.35) | 0.76 (0.6-0.95) |
| Reck et al.66 | SCLC | Ipilimumab (concurrent), carboplatin, and paclitaxel | Carboplatin and paclitaxel | 88 | >11.1 | n.a. | 0.95 (0.59-1.54) |
| Reck et al.66 | SCLC | Ipilimumab (phased), carboplatin, and paclitaxel | Carboplatin and paclitaxel | 87 | >11.1 | 0.37 (0.15-0.89) | 0.75 (0.46-1.23) |
| Rudin et al.67 | SCLC | Pembrolizumab, etoposide, and platinum | Etoposide and platinum | 453 | 21.6 | 2.85 (1.68-4.83) | 0.8 (0.64-0.98) |
| Goldman et al.68 | SCLC | Durvalumab, tremelimumab, etoposide, and platinum | Etoposide and platinum | 537 | 25.1 | 20.89 (9.47-46.1) | 0.82 (0.68-1) |
| Goldman et al.68 | SCLC | Durvalumab, etoposide, and platinum | Etoposide and platinum | 537 | 25.1 | 9.25 (4.12-20.77) | 0.75 (0.62-0.91) |
| Reck et al.69 | SCLC | Ipilimumab, etoposide, and platinum | Etoposide and platinum | 954 | 10.3 | 3.51 (2.68-4.6) | 0.94 (0.81-1.09) |
| Fradet et al.70 | Urothelial | Pembrolizumab | Taxane or vinflunine | 542 | 27.7 | 3.4 (1.91-6.06) | 0.7 (0.57-0.85) |
| Powles et al.71 | Urothelial | Atezolizumab | Taxane or vinflunine | 234 | 17.3 | 2.16 (1.21-3.84) | 0.87 (0.63-1.21) |
| Powles et al.72 | Urothelial | Durvalumab | Gemcitabine and platinum | 690 | 41.2 | 11.43 (4.87-26.82) | 0.99 (0.83-1.17) |
| Powles et al.72 | Urothelial | Durvalumab and tremelimumab | Gemcitabine and platinum | 686 | 41.2 | 30.13 (13.04-69.6) | 0.85 (0.72-1.02) |
| Galsky et al.73 | Urothelial | Atezolizumab and platinum-based chemotherapy | Platinum-based chemotherapy | 851 | 11.8 | 1.9 (1.44-2.51) | 0.83 (0.69-1) |
| Galsky et al.73 | Urothelial | Atezolizumab | Platinum-based chemotherapy | 762 | 11.8 | 1.12 (0.83-1.52) | 1.02 (0.83-1.24) |
| Powles et al.74 | Urothelial | Maintenance avelumab | Best supportive care | 700 | >19.0 | 28.26 (11.34-70.42) | 0.69 (0.56-0.86) |
| Powles et al.75 | Urothelial | Pembrolizumab, gemcitabine and platinum | Gemcitabine and platinum | 703 | 31.7 | 11.82 (5.61-24.91) | 0.86 (0.72-1.02) |
| Powles et al.75 | Urothelial | Pembrolizumab | Gemcitabine and platinum | 659 | 31.7 | 11.01 (5.18-23.39) | 0.92 (0.77-1.11) |
| Schmid et al.76 | Breast | Atezolizumab and nab-paclitaxel | Nab-paclitaxel | 902 | 18.0 | 1.9 (1.46-2.48) | 0.86 (0.72-1.02) |
| Winer et al.77 | Breast | Pembrolizumab | Single-drug chemotherapy | 622 | 31.4 | 6.37 (2.95-13.73) | 0.97 (0.82-1.15) |
| Reardon et al.78 | Glioblastoma | Nivolumab | Bevacizumab | 369 | 9.5 | 3.13 (1.89-5.18) | 1.04 (0.83-1.3) |
| Finn et al.83 | HCC | Pembrolizumab | Placebo | 413 | 12.2 | 2.5 (1.26-4.97) | 0.78 (0.61-1) |
| Scherpereel et al.79 | Mesothelioma | Nivolumab and ipilimumab | Nivolumab | 125 | 10.4 | 0.46 (0.31-0.69) | 0.92 (0.76-1.12) |
| Maio et al.80 | Mesothelioma | Tremelimumab | Placebo | 571 | n.a. | n.a. | n.a. |
| Kwon et al.81 | Prostate | Ipilimumab | Placebo | 799 | 9.6 | 6.23 (4.55-8.54) | 0.85 (0.72-1) |
| Beer et al.82 | Prostate | Ipilimumab | Placebo | 602 | n.a. | 8.55 (5.81-12.59) | 1.11 (0.88-1.39) |
CI, confidence interval; GI, gastrointestinal; HCC, hepatocellular carcinoma; HR, hazard ratio for overall survival (experimental versus control arm); MTX, methotrexate; n.a./NA, not available; NSCLC, non-small-cell lung cancer; OR, odds ratio for the overall any grade immune-related adverse event (experimental versus control arm); RCC, renal cell carcinoma; SCLC, small-cell lung cancer.
The median follow-up of the included studies ranged from 6.5 to 51 months, with a median of 19.0 months [interquartile range (IQR), 11.8-27.5 months]. In 20 out of 79 contrasts (25.3%), the study population was selected for expressing PD-L1 in at least 1% of tumor or immune cells. We provided treatments’ details in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.100787.
Relationship between treatment effects on irAEs and treatment effects on the clinical outcome (OS)
We estimated the relationship between treatment effects on irAEs versus treatment effects on OS and estimated regression equations based on data from all included studies (Table 1).
NSCLC
The analysis, regressing the log(HR) for OS on the log(OR) for irAEs, using the complete set of data, demonstrated no association between the two effects for either the overall (any grade, grade 1-2, grade 3-4) irAE rates or the specific organ (skin, GI, endocrine) irAE rates (Figure 1A-C; Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel A to I). Sensitivity and validation analysis gave similar results. The regression coefficients obtained from the main, sensitivity, and validation analyses were similar according to toxicity grade (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel A).
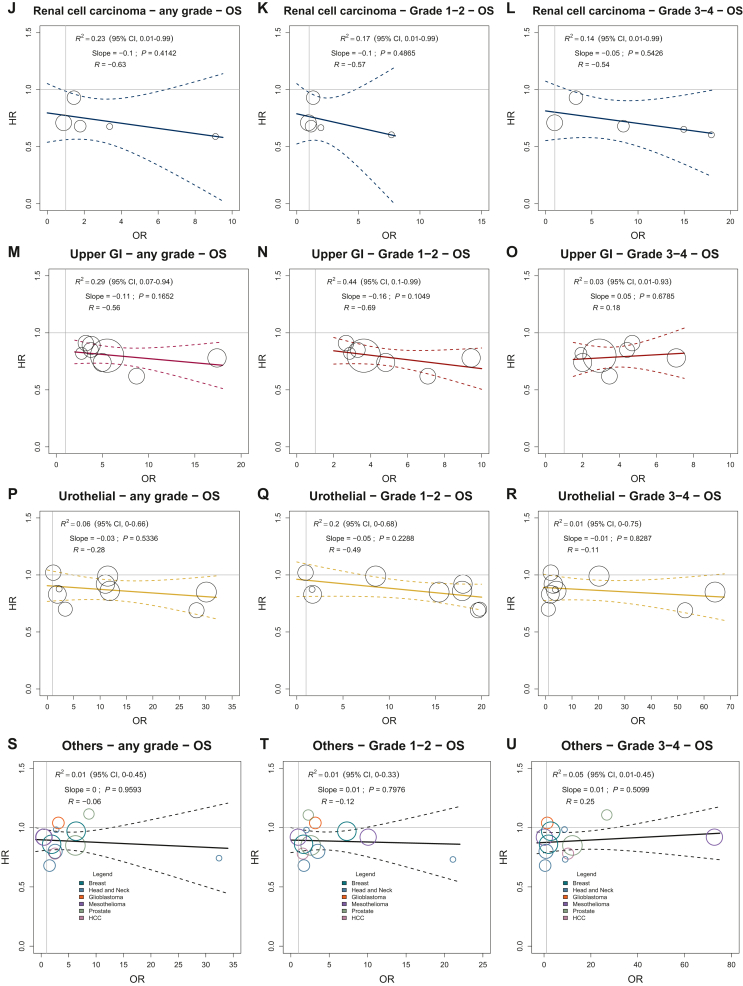
Figure 1.
Treatment effects on immune-related adverse events versus treatment effects on overall survival in specific tumor sites according to toxicity grade. Treatment effects are expressed as ORs for irAEs and HRs for OS. Every circle represents a comparison of the experimental group versus the control group, with the size of the circles representing the weight of the comparison, proportional to the number of events (deaths). The straight lines represent the weighted regressions, which show the effects on OS predicted by the observed effects on irAEs.
CI, confidence interval; GI, gastrointestinal; HCC, hepatocellular carcinoma; HR, hazard ratio; irAEs, immune-related adverse events; NSCLC, non-small-cell lung cancer; OR, odds ratio; OS, overall survival; SCLC, small-cell lung cancer.
The trials included in the analyses were classified as reported in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.100787. Regarding interaction analysis, the surrogacy (that is, the slope of the weighted regression line) was significantly different according to the study population selected for PD-L1 expression or not, treatment line, and type of experimental regimen factors (P value for interaction: 0.0496, 0.0027, and 0.0004, respectively). Concerning the selection of study population for the PD-L1 factor, a negative association between treatment effects on the overall grade 1-2 irAE rate and treatment effects on OS was observed in studies with the population selected for PD-L1 expression (that means: as irAE OR of experimental versus control arm increases, HR for OS of experimental versus control arm is predicted to decrease). The R2 value of the weighted regression line was 0.55 (95% CI 0.20 - 0.95; P value = 0.03), indicating that 55% of the variability among treatment effects on the OS is explained by the effects observed on the overall irAE rate of grade 1-2. Moreover, a negative low-strength correlation was observed in this trial subset (R = −0.69) (Figure 2B). In contrast, a positive association (that means: as irAE OR of experimental versus control arm increases, HR for OS of experimental versus control arm is predicted to increase) was observed between treatment effects on the endocrine grade 3-4 irAEs and treatment effects on OS in trials evaluating anti-PD1/PD-L1 antibody as experimental arm (R2 = 0.72; 95% CI 0.10-0.98; P value = 0.02). A medium-strength correlation was estimated (R = 0.76) (Figure 2E). Only two trials that studied the combination of anti-PD1/PD-L1 and anti-CTLA4 antibodies as experimental arm were available, however, for this subgroup analysis (Figure 2F). Moreover, due to the large estimated R2 CI values, all the subgroup analyses must be considered preliminary and warrant further validation.
Figure 2.
Explorative interaction analyses according to prespecified factors in NSCLC trial subset. Treatment effects are expressed as ORs for irAEs and HRs for OS. Every circle represents a comparison of the experimental group versus the control group, with the size of the circles representing the weight of the comparison, proportional to the number of events (deaths). The straight lines represent the weighted regressions, which show the effects on OS predicted by the observed effects on irAEs.
CI, confidence interval; CTLA-4, cytotoxic T-lymphocyte antigen 4; HR, hazard ratio; ICI, immune checkpoint inhibitor; irAEs, immune-related adverse events; NaN, not a number; NSCLC, non-small-cell lung cancer; OR, odds ratio; OS, overall survival; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1.
SCLC
The analysis, regressing the log(HR) for OS on the log(OR) for irAEs using the complete set of data, did not demonstrate any association between the two effects for both the overall (any grade, grade 1-2, grade 3-4) irAE rates and the specific organ (skin, GI, endocrine) irAE rates (Figure 1D-F; Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel A to I).
Sensitivity and validation analysis gave similar results for irAEs of grade 1-2 and 3-4. The regression coefficients obtained from the main, sensitivity, and validation analyses were similar. As regards the irAEs of any grade, validation analysis results were in line with those obtained in the main analysis. In contrast, sensitivity analysis gave discordant results, but the large 95% CIs suggest a low precision in the regression coefficient estimation (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel B).
Concerning the interaction analysis, the surrogacy was significantly different according to the type of experimental agent. In all the comparisons, however, at least one trial subgroup (i.e. the trial subset with an anti-CTLA-4 antibody as experimental arm) included only two randomized trials. Therefore, this finding deserves further validation (Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel A to L).
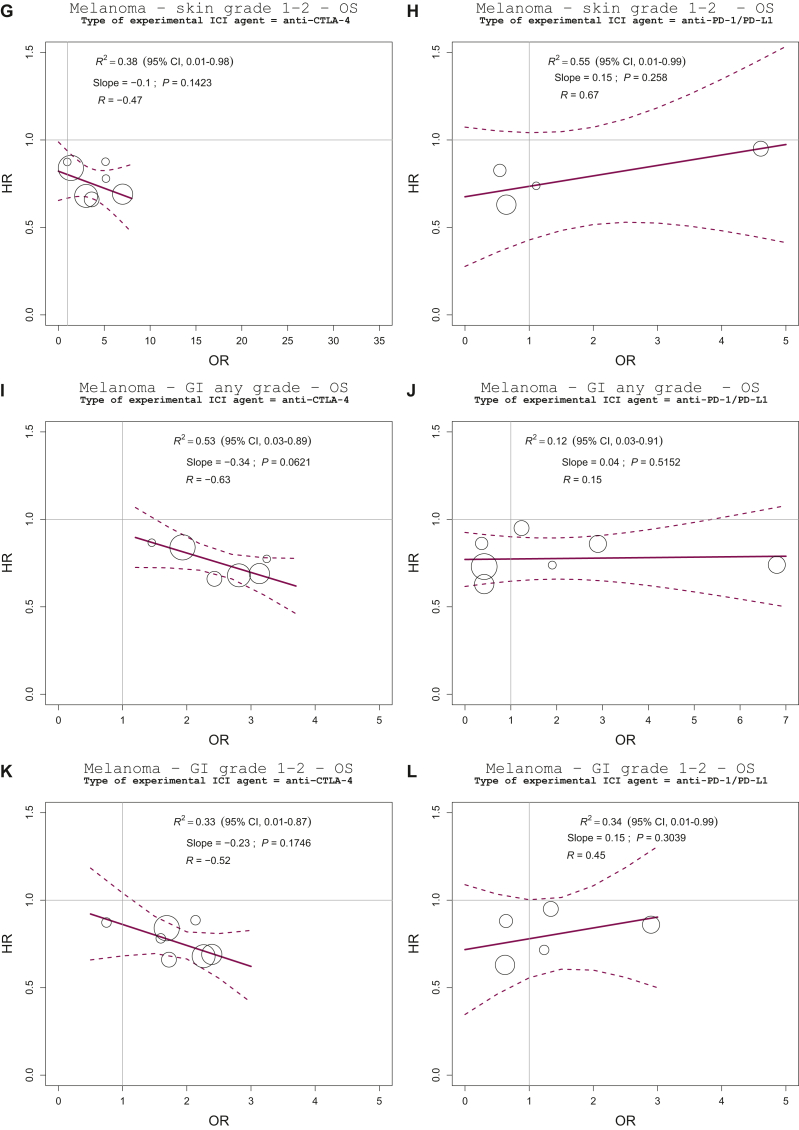
Melanoma
A total of 10 out of 17 contrasts (58.8%) compared two different ICI-based regimens, whereas 7 contrasts (41.2%) had a control arm that was not ICI-based (chemotherapy or gp100 peptide vaccine). Anti-CTLA-4, anti-PD-1/PD-L1, and a combination of anti-PD-1/PD-L1 and anti-CTLA-4 agents were employed as experimental arms in eight (47.0%), eight (47.0%), and one contrast (5.9%), respectively (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.100787).
The analysis, regressing the log(HR) for OS on the log(OR) for irAE using the complete set of data, demonstrated no association between the two effects for either the overall (any grade, grade 1-2, grade 3-4) irAE rates or the specific organ (skin, GI, endocrine) irAE rates (Figure 1G-I; Supplementary Figure S6, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel A to I). Sensitivity and validation analysis gave similar results for the irAEs of grade 3-4. Regarding the irAEs of any grade, validation analysis showed opposite results compared with those from the primary analysis. We estimated, however, a large 95% CI for the regression coefficient in the validation analysis (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel C).
Regarding interaction analysis, the association between treatment effects was significantly different according to the type of comparison and experimental ICI agent (P value for interaction: 0.0365, 0.0114, 0.0082, 0.0490, 0.0347, and 0.0082, for overall grade 3-4, skin any grade and grade 1-2, GI any grade, grade 1-2 and grade 3-4 irAE rate, respectively).
The regression lines, estimated separately for the type of control arm, indicated a weak association between treatment effects on the overall grade 3-4 irAE rate and treatment effects on OS in studies with a non-ICI-based control arm (R2 = 0.44, 95% CI 0.17-0.99; slope = −0.11; P value = 0.15) (Figure 3A). We observed a significant negative association between treatment effects on the GI grade 3-4 irAE rate and treatment effects on OS in studies with a non-ICI-based control arm. The R2 value of the weighted regression line was 0.77 (95% CI 0.24-0.99; P value = 0.02), indicating that 77% of the variability among treatment effects on OS was explained by the observed effects on the GI grade 3-4 irAE rate. In this case, we estimated a high-strength correlation that reached the cut-off level for surrogacy (R = −0.89) (Figure 3C). Due to the large estimated R2 CI values, however, these findings must be considered preliminary and warrant further validation.
Figure 3.
Explorative interaction analyses according to prespecified factors in melanoma trial subset. Treatment effects are expressed as ORs for irAEs and HRs for OS. Every circle represents a comparison of the experimental group versus the control group, with the size of the circles representing the weight of the comparison, proportional to the number of events (deaths). The straight lines represent the weighted regressions, which show the effects on OS predicted by the observed effects on irAEs.
CI, confidence interval; CTLA-4, cytotoxic T-lymphocyte antigen 4; HR, hazard ratio; ICI, immune checkpoint inhibitor; irAEs, immune-related adverse events; OR, odds ratio; OS, overall survival; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1.
In the trial subset with an anti-CTLA-4 single agent as experimental arm, we observed negative low-strength correlations between treatment effects on the skin any grade and grade 1-2 irAEs, the GI any grade and grade 1-2 irAE rate, and treatment effects on OS (R = −0.47, −0.47, −0.63, and −0.52, respectively) (Figure 3E, G, I, and K). These correlations did not reach the cut-off level for claiming surrogacy despite the significant interaction of the relationship among treatment effects according to the type of experimental ICI agent.
Renal cell carcinoma
The analysis, regressing the log(HR) for OS on the log(OR) for irAE using the complete set of data, did not demonstrate any association between the two effects for either the overall (any grade, grade 1-2, grade 3-4) irAE rates or the specific organ (skin, GI, endocrine) irAE rates (Figure 1J-L; Supplementary Figure S7, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel A to I). Sensitivity and validation analyses gave similar results (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel D). Due to missing values for trials with treatment line ≥2 and comparison between two ICI-based regimens, we did not carry out the interaction analysis.
Upper GI
The analysis, regressing the log(HR) for OS on the log(OR) for irAEs using the complete set of data, did not demonstrate any significant association between the two effects for either the overall (any grade, grade 1-2, grade 3-4) irAE rates or the specific organ (skin, GI, endocrine) irAE rates (Figure 1M-O; Supplementary Figure S8, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel A to I). We observed a weak association, not statistically significant, between treatment effects on the overall grade 1-2 irAE rate and treatment effects on OS. The R2 value of the weighted regression line was 0.44 (95% CI 0.10-0.99; slope = −0.16; P value = 0.10). The correlation between treatment effects was of low strength (R = −0.69) (Figure 1N). Due to the low number of available studies, the validation analysis was not carried out (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel E).
The interaction analysis showed that surrogacy was not different according to the treatment line factor. The other stratification factors were not considered in the interaction analysis.
Since we could not examine the impact of missing data imputation on the primary analysis, these findings must be regarded as preliminary and warrant further validation.
Urothelial cancer
The analysis, regressing the log(HR) for OS on the log(OR) for irAEs using the complete set of data, demonstrated no association between the two effects for both the overall (any grade, grade 1-2, grade 3-4) irAE rates and the specific organ (skin, GI, endocrine) irAE rates (Figure 1P-R; Supplementary Figure S9, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel A to I). Sensitivity and validation analyses gave similar results for the irAEs of any grade and grade 3-4 (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel F). Due to the low number of trials, the interaction analysis was not carried out.
Other tumors
We grouped ICI therapy randomized studies of cancer types with fewer than four trials (head and neck cancer, breast cancer, prostate cancer, hepatocellular carcinoma, glioblastoma, and mesothelioma). In this subset of 12 contrasts, the experimental arm was an anti-PD-1/PD-L1 antibody in six comparisons, an anti-CTLA-4 antibody in three studies, and a combination of anti-PD-1/PD-L1 and anti-CTLA-4 agents in 3 contrasts (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.100787).
The analysis, regressing the log(HR) for OS on the log(OR) for irAE using the complete set of data, did not demonstrate any association between the two effects for either overall (any grade, grade 1-2, grade 3-4) irAE rates or specific organ (skin, GI, endocrine) irAE rates (Figure 1S-U; Supplementary Figure S10, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel A to I). Sensitivity and validation analyses gave similar results. The regression coefficients obtained from the main, sensitivity, and validation analyses were similar according to the immune-related toxicity grade. Also, a large 95% CI of the regression coefficient was observed in sensitivity analysis for irAEs of any grade (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel G).
The surrogacy was significantly different according to the type of experimental agent and control arm (ICI-based or not) factors (P value for interaction: 0.0347 and 0.0499, respectively).
The regression lines, estimated separately for the different experimental regimens, indicated a positive association, not statistically significant, between treatment effects on the overall any grade irAE rate and treatment effects on OS in the trial subset assessing an anti-PD-1/PD-L1 antibody as experimental arm (R2 = 0.52, 95% CI 0.08-0.99; slope = 0.19; P value = 0.10; R = 0.73) (Supplementary Figure S11, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel B). In the trial subset with the combination of anti-PD-1/PD-L1 and anti-CTLA-4 antibodies as experimental arm, based on three randomized studies, we observed a weak association, not statistically significant, between treatment effects on the overall any grade irAE rate and treatment effects on OS (R2 = 0.60, 95% CI 0.09-0.90; slope = −0.05; P value = 0.44; R = −0.8) (Supplementary Figure S11, available at https://doi.org/10.1016/j.esmoop.2023.100787, panel C).
In the subset of three trials comparing two different ICI-based regimens, the regression line showed a weak association, not statistically significant, between treatment effects on the overall grade 1-2 irAEs and treatment effects on OS (R2 = 0.79, 95% CI 0.31-0.99; slope = −0.08; P value = 0.31; R = −0.91) (Supplementary Figure S11, available at https://doi.org/10.1016/j.esmoop.2023.100787; panel E).
Discussion
Several clinical studies and an individual patient data (IPD) meta-analysis have shown an association between the occurrence of the irAEs and the survival outcomes of patients undergoing ICI therapy. To assess whether a new intervention significantly affects survival based on the effect on a surrogate measure, however, a reliable and plausible prediction equation should be estimated by a meta-analysis of several randomized trials.87
The present meta-analysis collected randomized studies that tested ICI therapy across several advanced-stage cancers, such as NSCLC, SCLC, melanoma, renal cell carcinoma, urothelial, and upper GI cancers. The median follow-up of the included studies (19.0 months; IQR, 11.8-27.5 months) was numerically longer than previous meta-analyses assessing the surrogacy of other intermediate endpoints in ICI-based trials.17,19,88
The present analysis showed correlations between the ICI therapy effects on the overall irAE rate and treatment effects on OS of varying magnitude (R ranged from −0.63 to 0.25) in different cancer types. All estimated correlations, however, were of low strength, according to the predefined R values necessary for validating a candidate surrogate endpoint. The coefficients of determination of the calculated linear regression models (R2) were between 0.01 and 0.29, meaning that, in general, less than one-third of the variability of ICI therapy survival benefit could be explained by the variability in the treatment effects on the irAE rate.
We aimed to explore the surrogacy of the irAEs starting from the biological plausibility of the relationship between the irAEs and the OS outcome.89 The biological hypothesis underlying the potential surrogacy of irAEs in patients treated with immunotherapy is that the stronger the autoimmune response induced by ICI therapy is, the more robust is antitumor immunity. In that case, the irAE development could lie in the main causal pathway of the disease process and thus would entirely capture the ICI effect on the OS. In addition, it is possible that certain irAEs are more directly related to antitumor efficacy than others, so that, along this line, not all irAEs are required to occur to obtain a benefit from ICI therapy.90 Therefore, in the primary analysis, we explored the surrogacy of irAEs according to the severity (grade 1-2 or grade 3-4) and specific organ involvement (skin, GI, and endocrine system). We only observed a low-strength negative correlation (R = −0.69) among the treatment effects on the overall grade 1-2 irAE rate and treatment effects on OS in the upper GI trial subset, which was not statistically significant (P value = 0.10).
Considering that a candidate surrogate should be specific for the disease setting and the type of treatment administered, we also planned an interaction analysis by patient selection for PD-L1 expression or not, class of drugs used (anti-PD-1/PD-L1 versus anti-CTLA-4 versus a combination of anti-PD-1/PD-L1 and anti-CTLA-4 antibodies), type of control arm (ICI-based or not) and treatment line. The results of the different analyses suggest some hypothetical correlations. In the NSCLC trial subset, we did show a significant negative correlation between the overall irAEs of grade 1-2 and OS in trials with patients selected for PD-L1 expression (R = −0.69). In the melanoma trial subgroup with a non-ICI-based control arm, we reported a negative medium-strength correlation between treatment effects on the overall grade 3-4 irAEs and treatment effects on OS (R = −0.72) that was not statistically significant and otherwise a significant high-strength negative correlation between treatment effects on GI grade 3-4 irAEs and treatment effects on OS (R = −0.89).
Immunotherapy with ICIs has significantly improved the outcomes of patients with different cancers, although biomarkers available to predict ICI efficacy remain unsatisfactory.91 As a whole, our findings did not support the hypothesis of surrogacy of irAEs in cancer patients undergoing immunotherapy. Therefore, irAEs’ development possibly captures only part of the mechanism of action by which ICI therapy improves survival. Other important determinants of ICI efficacy could be related, for example, to tumor characteristics, such as PD-L1 expression, tumor mutational burden, tumor-infiltrating lymphocytes, or specific gene signatures.91 The weak surrogacy of grades 1 or 2 irAEs confined to NSCLC patients with PD-L1 positivity, observed in our study, goes in this direction.
Indeed, our results suggest also that ICI treatment effects on grade 3 or 4 irAE rates might be positively associated with treatment effects on OS in at least some patient subsets. This observation might be explained by the fact that cancer patients can experience severe morbidity and increased mortality related to immune toxicities, which may confound the survival difference between patients with and without irAEs. In addition, treatment interruptions or discontinuations for severe immune toxicities may reduce the effectiveness of ICI therapy.92 Clinicians should promptly manage the lower grade irAEs with supportive care as recommended by current guidelines,6 to prevent the development of more severe toxicities.
The present study has several limitations. Firstly, we relied on data summarized in the published literature. One limit of using AD in meta-analyses is that errors in estimating treatment effects on the surrogate and the long-term outcome cannot be fully accounted for, resulting in a systematic bias toward lower association values.93 Moreover, the irAE rate is a binary endpoint whose correlation with OS can be quantified with HRs, log-rank test, or survival ORs from a joint copula only in IPD and not in AD meta-analyses.20
Secondly, the premise of our trial-based surrogate validation study was that the ‘outcome surrogacy’ of the irAEs (that is, the demonstration of a strong correlation between the irAE occurrence and OS) had been adequately demonstrated in different cancer types.9,11,94,95 In melanoma patients receiving ipilimumab, however, OS was not affected by the occurrence of irAEs in a retrospective and mono-institutional study.96 Therefore, whether the irAE development correlates with improved outcomes is partly controversial.
Another limitation concerns the assessment of irAEs, which cannot be subject to verification. The irAEs are usually reported by patients, noted, and graded by investigators according to the CTCAE system. We collected only clinician-reported AEs that were classified as irAEs in the published studies. Patients, however, generally report adverse symptoms earlier and more frequently than clinicians. Consequently, the most accurate information on irAEs would derive from the patient, using validated patient-reported outcome (PRO) CTCAEs.97 Nevertheless, there is a need to strengthen the validity of PRO measures used to monitor patients receiving ICI therapy.98 In addition, it may be difficult to distinguish the immune pathogenesis of an adverse event from other causes, especially if the ICI is combined with other therapies. This bias might have influenced the results of our analyses.
In the present meta-analysis, we used the HR of death between the experimental and control arm as the treatment effect estimate on the clinical outcome OS. Reporting HRs may not adequately represent the ICI treatment benefit due to delayed separation or crossing of survival curves observed in many randomized trials of ICI therapy.99 We cannot rule out that further analyses with more studies might better account for the potential loss of power due to the non-proportionality of survival curves in ICI trials. Moreover, we planned to explore the heterogeneity of the trial-level associations according to specific trial characteristics, but many of these subgroup analyses are difficult to interpret due to the limited number of available studies.
Finally, we have set the irAE rate as a potential efficacy endpoint of ICI therapy in the present meta-analysis, although it is typically a safety endpoint in the included randomized studies. As intermediate efficacy endpoints are generally validated to replace OS as the primary endpoint in trials assessing novel therapies, this cannot be the case for the irAE rate. Therefore, our analyses have only the speculative intent to provide further valuable insight into the ICI therapy’s mechanism of action and not suggest alternative primary endpoints for future clinical studies.13
In conclusion, the present study showed that the irAE rate should not be considered a valid surrogate for OS when evaluating ICI therapy efficacy in NSCLC, SCLC, melanoma, upper GI cancers, renal cell carcinoma, urothelial cancers, and other solid tumors. To our knowledge, this is the first trial-based meta-analysis addressing this intriguing topic. Further research using IPD from a significant number of randomized studies might contribute to examining in depth the association of the irAEs with long-term outcomes at both patient and trial level.
Funding
None declared.
Disclosure
VA has reported advisory role for Gilead. AB has reported paid speech or advisory role for Novartis AAA, Janssen, Amgen, HRA, Astellas, and Ipsen. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Koon H., Atkins M. Autoimmunity and immunotherapy for cancer. N Engl J Med. 2006;354(7):758–760. doi: 10.1056/NEJMe058307. [DOI] [PubMed] [Google Scholar]
- 2.Zimmer L., Goldinger S.M., Hofmann L., et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:210–225. doi: 10.1016/j.ejca.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann L., Forschner A., Loquai C., et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Boutros C., Tarhini A., Routier E., et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 5.Haanen J., Carbonnel F., Robert C., et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv264–iv266. doi: 10.1093/annonc/mdy162. [DOI] [PubMed] [Google Scholar]
- 6.Schneider B.J., Naidoo J., Santomasso B.D., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO Guideline Update. J Clin Oncol. 2021;39(36):4073–4126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 7.Gogas H., Ioannovich J., Dafni U., et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354(7):709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 8.Sun X., Roudi R., Dai T., et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis. BMC Cancer. 2019;19(1):558. doi: 10.1186/s12885-019-5701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berner F., Bomze D., Diem S., et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 2019;5(7):1043–1047. doi: 10.1001/jamaoncol.2019.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawai T., Sato Y., Makino K., et al. Immune-related adverse events predict the therapeutic efficacy of pembrolizumab in urothelial cancer patients. Eur J Cancer. 2019;116:114–115. doi: 10.1016/j.ejca.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Socinski M.A., Jotte R.M., Cappuzzo F., et al. Pooled analyses of immune-related adverse events (irAEs) and efficacy from the phase 3 trials IMpower130, IMpower132, and IMpower150. J Clin Oncol. 2021;39(suppl 15) doi: 10.1001/jamaoncol.2022.7711. 9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buyse M., Molenberghs G. Criteria for the validation of surrogate endpoints in randomized experiments. Biometrics. 1998;54(3):1014–1029. [PubMed] [Google Scholar]
- 13.Fleming T.R., DeMets D.L. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125(7):605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 14.Baker S.G. Five criteria for using a surrogate endpoint to predict treatment effect based on data from multiple previous trials. Stat Med. 2018;37(8):1406. doi: 10.1002/sim.7603. [DOI] [PubMed] [Google Scholar]
- 15.Katz R. Biomarkers and surrogate markers: an FDA perspective. NeuroRx. 2004;1(2):189–195. doi: 10.1602/neurorx.1.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J., Liang W., Liang H., Wang X., He J. Endpoint surrogacy in oncological randomized controlled trials with immunotherapies: a systematic review of trial-level and arm-level meta-analyses. Ann Transl Med. 2019;7(11):244. doi: 10.21037/atm.2019.04.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kok P.S., Cho D., Yoon W.H., et al. Validation of progression-free survival rate at 6 months and objective response for estimating overall survival in immune checkpoint inhibitor trials: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman H.L., Schwartz L.H., William W.N., Jr., et al. Evaluation of classical clinical endpoints as surrogates for overall survival in patients treated with immune checkpoint blockers: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2018;144(11):2245–2261. doi: 10.1007/s00432-018-2738-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mushti S.L., Mulkey F., Sridhara R. Evaluation of overall response rate and progression-free survival as potential surrogate endpoints for overall survival in immunotherapy trials. Clin Cancer Res. 2018;24(10):2268–2275. doi: 10.1158/1078-0432.CCR-17-1902. [DOI] [PubMed] [Google Scholar]
- 20.Xie W., Halabi S., Tierney J.F., et al. A systematic review and recommendation for reporting of surrogate endpoint evaluation using meta-analyses. JNCI Cancer Spectr. 2019;3(1):pkz002. doi: 10.1093/jncics/pkz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciani O., Davis S., Tappenden P., et al. Validation of surrogate endpoints in advanced solid tumors: systematic review of statistical methods, results, and implications for policy makers. Int J Technol Assess Health Care. 2014;30(3):312–324. doi: 10.1017/S0266462314000300. [DOI] [PubMed] [Google Scholar]
- 23.Institute for Quality and Efficiency in Health Care: Executive Summaries. Institute for Quality and Efficiency in Health Care (IQWiG) © IQWiG (Institute for Quality and Efficiency in Health Care); Cologne, Germany: 2005. Validity of surrogate endpoints in oncology: Executive summary of rapid report A10-05, Version 1.1. [PubMed] [Google Scholar]
- 24.Prasad V., Kim C., Burotto M., Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med. 2015;175(8):1389–1398. doi: 10.1001/jamainternmed.2015.2829. [DOI] [PubMed] [Google Scholar]
- 25.R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. https://www.r-project.org/ Available at.
- 26.Kojima T., Shah M.A., Muro K., et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138–4148. doi: 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
- 27.Chau I., Doki Y., Ajani J.A., et al. Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): First results of the CheckMate 648 study. J Clin Oncol. 2021;39(suppl 18) LBA4001. [Google Scholar]
- 28.Chen L.T., Satoh T., Ryu M.H., et al. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update data. Gastric Cancer. 2020;23(3):510–519. doi: 10.1007/s10120-019-01034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shitara K., Ozguroglu M., Bang Y.J., et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–133. doi: 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 30.Shitara K., Van Cutsem E., Bang Y.J., et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571–1580. doi: 10.1001/jamaoncol.2020.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janjigian Y.Y., Shitara K., Moehler M., et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motzer R.J., Rini B.I., McDermott D.F., et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33(13):1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motzer R.J., Rini B.I., McDermott D.F., et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20(10):1370–1385. doi: 10.1016/S1470-2045(19)30413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rini B.I., Powles T., Atkins M.B., et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404–2415. doi: 10.1016/S0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 35.Powles T., Plimack E.R., Soulieres D., et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563–1573. doi: 10.1016/S1470-2045(20)30436-8. [DOI] [PubMed] [Google Scholar]
- 36.Motzer R., Alekseev B., Rha S.Y., et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 37.Choueiri T.K., Powles T., Burotto M., et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodi F.S., O’Day S.J., McDermott D.F., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maio M., Grob J.J., Aamdal S., et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol. 2015;33(10):1191–1196. doi: 10.1200/JCO.2014.56.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolchok J.D., Neyns B., Linette G., et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 41.Long G.V., Schachter J., Ribas A., et al. 4-year survival and outcomes after cessation of pembrolizumab (pembro) after 2-years in patients (pts) with ipilimumab (ipi)-naive advanced melanoma in KEYNOTE-006. J Clin Oncol. 2018;36(suppl 15) 9503. [Google Scholar]
- 42.Hamid O., Puzanov I., Dummer R., et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37–45. doi: 10.1016/j.ejca.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 43.Hodi F.S., Chesney J., Pavlick A.C., et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17(11):1558–1568. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribas A., Kefford R., Marshall M.A., et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber J.S., Gibney G., Sullivan R.J., et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol. 2016;17(7):943–955. doi: 10.1016/S1470-2045(16)30126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ascierto P.A., Del Vecchio M., Robert C., et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18(5):611–622. doi: 10.1016/S1470-2045(17)30231-0. [DOI] [PubMed] [Google Scholar]
- 48.Larkin J., Minor D., D’Angelo S., et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol. 2018;36(4):383–390. doi: 10.1200/JCO.2016.71.8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ascierto P.A., Del Vecchio M., Mandala M., et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(11):1465–1477. doi: 10.1016/S1470-2045(20)30494-0. [DOI] [PubMed] [Google Scholar]
- 50.Horn L., Spigel D.R., Vokes E.E., et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057) J Clin Oncol. 2017;35(35):3924–3933. doi: 10.1200/JCO.2017.74.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carbone D.P., Reck M., Paz-Ares L., et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borghaei H., Langer C.J., Gadgeel S., et al. 24-Month overall survival from KEYNOTE-021 cohort G: pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2019;14(1):124–129. doi: 10.1016/j.jtho.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Brahmer J.R., Rodriguez-Abreu D., Robinson A.G., et al. Progression after the next line of therapy (PFS2) and updated OS among patients (pts) with advanced NSCLC and PD-L1 tumor proportion score (TPS) ≥50% enrolled in KEYNOTE-024. J Clin Oncol. 2017;35(suppl 15) 9000. [Google Scholar]
- 54.Herbst R.S., Baas P., Kim D.W., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 55.Gandhi L., Rodriguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 56.Socinski M.A., Jotte R.M., Cappuzzo F., et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 57.Jotte R., Cappuzzo F., Vynnychenko I., et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;15(8):1351–1360. doi: 10.1016/j.jtho.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 58.Paz-Ares L., Luft A., Vicente D., et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 59.Herbst R.S., Giaccone G., de Marinis F., et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 60.Rizvi N.A., Cho B.C., Reinmuth N., et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020;6(5):661–674. doi: 10.1001/jamaoncol.2020.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.West H., McCleod M., Hussein M., et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 62.Paz-Ares L., Ciuleanu T.E., Cobo M., et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 63.Mok T.S.K., Wu Y.L., Kudaba I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 64.Govindan R., Szczesna A., Ahn M.J., et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35(30):3449–3457. doi: 10.1200/JCO.2016.71.7629. [DOI] [PubMed] [Google Scholar]
- 65.Liu S.V., Reck M., Mansfield A.S., et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133) J Clin Oncol. 2021;39(6):619–630. doi: 10.1200/JCO.20.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reck M., Bondarenko I., Luft A., et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24(1):75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 67.Rudin C.M., Awad M.M., Navarro A., et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–2379. doi: 10.1200/JCO.20.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldman J.W., Dvorkin M., Chen Y., et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65. doi: 10.1016/S1470-2045(20)30539-8. [DOI] [PubMed] [Google Scholar]
- 69.Reck M., Luft A., Szczesna A., et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740–3748. doi: 10.1200/JCO.2016.67.6601. [DOI] [PubMed] [Google Scholar]
- 70.Fradet Y., Bellmunt J., Vaughn D.J., et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol. 2019;30(6):970–976. doi: 10.1093/annonc/mdz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Powles T., Duran I., van der Heijden M.S., et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 72.Powles T., van der Heijden M.S., Castellano D., et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1574–1588. doi: 10.1016/S1470-2045(20)30541-6. [DOI] [PubMed] [Google Scholar]
- 73.Galsky M.D., Arija J.A.A., Bamias A., et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10236):1547–1557. doi: 10.1016/S0140-6736(20)30230-0. [DOI] [PubMed] [Google Scholar]
- 74.Powles T., Park S.H., Voog E., et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 75.Powles T., Csoszi T., Ozguroglu M., et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7):931–945. doi: 10.1016/S1470-2045(21)00152-2. [DOI] [PubMed] [Google Scholar]
- 76.Schmid P., Rugo H.S., Adams S., et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 77.Winer E.P., Lipatov O., Im S.A., et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(4):499–511. doi: 10.1016/S1470-2045(20)30754-3. [DOI] [PubMed] [Google Scholar]
- 78.Reardon D.A., Brandes A.A., Omuro A., et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the checkMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. doi: 10.1001/jamaoncol.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scherpereel A., Mazieres J., Greillier L., et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019;20(2):239–253. doi: 10.1016/S1470-2045(18)30765-4. [DOI] [PubMed] [Google Scholar]
- 80.Maio M., Scherpereel A., Calabro L., et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017;18(9):1261–1273. doi: 10.1016/S1470-2045(17)30446-1. [DOI] [PubMed] [Google Scholar]
- 81.Kwon E.D., Drake C.G., Scher H.I., et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beer T.M., Kwon E.D., Drake C.G., et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35(1):40–47. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 83.Finn R.S., Ryoo B.Y., Merle P., et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 84.Ferris R.L., Blumenschein G., Jr., Fayette J., et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51. doi: 10.1016/j.oraloncology.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cohen E.E.W., Soulieres D., Le Tourneau C., et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 86.Siu L.L., Even C., Mesia R., et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-Low/Negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol. 2019;5(2):195–203. doi: 10.1001/jamaoncol.2018.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ciani O., Buyse M., Garside R., et al. Meta-analyses of randomized controlled trials show suboptimal validity of surrogate outcomes for overall survival in advanced colorectal cancer. J Clin Epidemiol. 2015;68(7):833–842. doi: 10.1016/j.jclinepi.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 88.Nie R.C., Chen F.P., Yuan S.Q., et al. Evaluation of objective response, disease control and progression-free survival as surrogate end-points for overall survival in anti-programmed death-1 and anti-programmed death ligand 1 trials. Eur J Cancer. 2019;106:1–11. doi: 10.1016/j.ejca.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 89.Ciani O., Buyse M., Drummond M., Rasi G., Saad E.D., Taylor R.S. Use of surrogate end points in healthcare policy: a proposal for adoption of a validation framework. Nat Rev Drug Discov. 2016;15(7):516. doi: 10.1038/nrd.2016.81. [DOI] [PubMed] [Google Scholar]
- 90.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune Checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 91.Sharma P., Siddiqui B.A., Anandhan S., et al. The next decade of immune Checkpoint therapy. Cancer Discov. 2021;11(4):838–857. doi: 10.1158/2159-8290.CD-20-1680. [DOI] [PubMed] [Google Scholar]
- 92.Ksienski D., Wai E.S., Croteau N., et al. Efficacy of nivolumab and pembrolizumab in patients with advanced non-small-cell lung cancer needing treatment interruption because of adverse events: a retrospective multicenter analysis. Clin Lung Cancer. 2019;20(1):e97–e106. doi: 10.1016/j.cllc.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 93.Buyse M., Molenberghs G., Paoletti X., et al. Statistical evaluation of surrogate endpoints with examples from cancer clinical trials. Biom J. 2016;58(1):104–132. doi: 10.1002/bimj.201400049. [DOI] [PubMed] [Google Scholar]
- 94.Eggermont A.M.M., Kicinski M., Blank C.U., et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6(4):519–527. doi: 10.1001/jamaoncol.2019.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang K., Seo J., Tiu B.C., et al. Association of cutaneous immune-related adverse events with increased survival in patients treated with anti-programmed cell death 1 and anti-programmed cell death ligand 1 therapy. JAMA Dermatol. 2022;158(2):189–193. doi: 10.1001/jamadermatol.2021.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horvat T.Z., Adel N.G., Dang T.O., et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dueck A.C., Mendoza T.R., Mitchell S.A., et al. Validity and reliability of the US national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) JAMA Oncol. 2015;1(8):1051–1059. doi: 10.1001/jamaoncol.2015.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Da Silva Lopes A.M., Colomer-Lahiguera S., Mederos Alfonso N., et al. Patient-reported outcomes for monitoring symptomatic toxicities in cancer patients treated with immune-checkpoint inhibitors: a Delphi study. Eur J Cancer. 2021;157:225–237. doi: 10.1016/j.ejca.2021.08.026. [DOI] [PubMed] [Google Scholar]
- 99.Anagnostou V., Yarchoan M., Hansen A.R., et al. Immuno-oncology trial endpoints: capturing clinically meaningful activity. Clin Cancer Res. 2017;23(17):4959–4969. doi: 10.1158/1078-0432.CCR-16-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.