Abstract
BRAF activation occurs as part of the mitogen-activated protein kinase (MAPK) cellular signaling pathway which leads to increased cellular proliferation and survival. Mutations in BRAF can result in unbridled activation of downstream kinases with subsequent uncontrolled cellular growth that formulate the basis for oncogenesis in multiple tumor types. Targeting BRAF by selective inhibitors has been one of the early successes in precision oncology. Agents have been explored either as monotherapy or in combination with MEK inhibition in BRAF V600-mutant pan-cancers and with EGFR inhibition in colorectal cancer. Spectrum of BRAF inhibition has evolved from being melanoma-specific to being a pan-cancer target. In this article, we review BRAF and MEK inhibitor drug development journey from tissue-specific melanoma, non-small-cell lung cancer, and anaplastic thyroid cancer to tissue-agnostic approvals.
Key words: BRAF, MEK, cancer, precision oncology, targeted therapy
Highlights
-
•
BRAF alterations lead to unbridled activation of the MAPK pathway which can result in cancer development and progression.
-
•
BRAF and MEK inhibitors led to paradigm change in management of BRAF-mutated melanoma, lung, and anaplastic thyroid cancers.
-
•
BRAF plus MEK inhibitor combination has shown clinical activity in >20 different BRAF V600-positive tumor types.
-
•
Dabrafenib and trametinib has received FDA accelerated approval for metastatic solid tumors with BRAF V600E mutations.
-
•
The recent tissue-agnostic approval is transformative as it prioritizes mutational status over tissue of origin.
Introduction
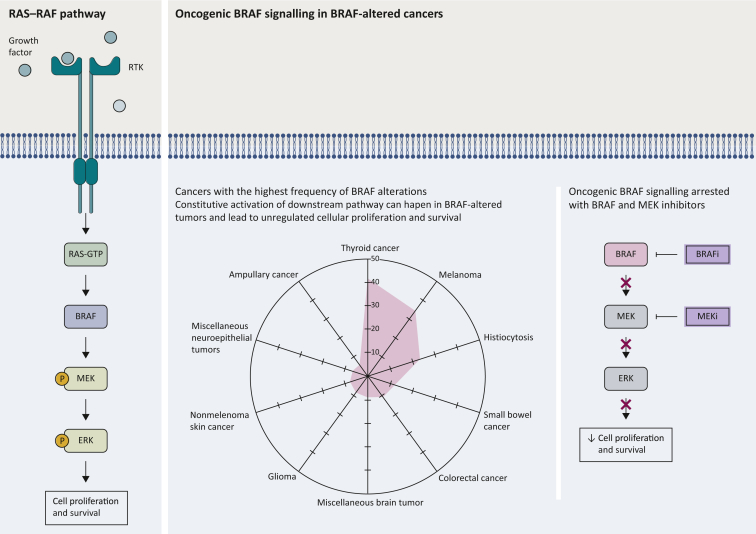
The RAF family proteins are serine/threonine protein kinases that constitute a cornerstone of the mitogen-activated protein kinase (MAPK) pathway which is frequently dysfunctional in many human cancers. Three RAF isoforms (A-RAF, B-RAF, and C-RAF) have been described as important regulators of cell growth, differentiation, and survival. RAF isoforms are activated through binding of small G proteins to the N-terminal of RAF proteins. Activation of RAF leads to downstream phosphorylation of MEK1 and MEK2 and their downstream ERK1 and ERK2 (Figure 1).1, 2, 3
Figure 1.
Targeting BRAF pathway: figure shows the BRAF signaling pathway and cancers with the highest frequency of BRAF alterations according to AACR GENIE. Included are only cancers where BRAF was profiled in at least 100 samples.
BRAFi, BRAF inhibitor; MEKi, MEK inhibitor.
BRAF alterations
BRAF mutations are the second most common alterations that occur in the MAPK pathway. Their role in cancer development was described in 2002 by Davies et al.;4 and since then, multiple efforts have led to substantial advances in characterizing their impact and improving their targetability.
In attempt to explore tissue distribution of BRAF alterations, we reviewed sequencing data of 153 554 samples from different tumor types in the AACR GENIE database v12.1.5 BRAF alterations were found in 9173 samples, accounting for 6% of all profiled samples. Prevalence varied significantly across different tumor types and histologies with the highest frequency of distribution observed in thyroid cancer (41%) (Figure 1).
BRAF mutations have been functionally categorized into three different classes that have variable levels of RAS dependency. Class I mutations, which include V600 E/K/D, are RAS independent and hence maintain high level of activity regardless of RAS signaling even in their monomeric status. Class II mutations include non-V600 mutations e.g. G469A, K601E, and L597Q as well as fusions and deletions. While also being RAS-independent, class II have intermediate monomeric kinase activity and usually require dimerization to function. Class III mutations, including D594 and G466, are RAS dependent and need to form dimers for activation. This class is strongly dependent on upstream activation and often occurs with upstream mutations including RAS, unlike class I and II which usually show mutual exclusivity due to the downstream inhibition of RAS via ERK.6, 7, 8, 9 In our AACR GENIE analysis, class I alterations were the most prevalent being present in 4869 of BRAF-altered samples (53%).
Detection of BRAF mutations
There is a wide variety of technologies that can be used for detection of BRAF alterations. For example, immunohistochemistry, PCR-based and sequencing-based technologies have all been validated for identification of BRAF mutations.10,11 In clinical trials of BRAF inhibitors, the cobas® 4800 BRAF V600 Mutation Test (Roche, Basel, Switzerland), Therascreen BRAF V600E RGQ polymerase chain reaction (PCR) Kit (QIAGEN, Hilden, Germany), Oncomine Dx Target Test (Thermo Fisher, Waltham, MA), BRAF PCR Assay (Response Genetics, Los Angeles, CA), and THxID™ BRAF assay (bioMérieux Clinical Diagnostics, Marcy l'Etoile, France) were used for confirmation of BRAF mutation presence in patients receiving targeted treatment options. More recently, interest has grown in detecting BRAF alterations in plasma or other body fluids via liquid biopsy technologies. This offers a minimally invasive tool for genetic assessment that can expand beyond baseline testing of targetability to evaluation of clonal evolution and emerging resistance mechanisms over time.12, 13, 14, 15, 16
Current actionable alterations
Different BRAF alterations have been evaluated for targetability in different tumor types17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 (Table 1). Current Food and Drug Administration (FDA) approvals include monotherapy with vemurafenib, dabrafenib, or trametinib. Moreover, the FDA approves combinations of (dabrafenib + trametinib), (encorafenib + cetuximab), (encorafenib + binimetinib), and (vemurafenib + cobimetinib) for treatment of patients with BRAF mutations (Figure 2). In this review, we will focus on BRAF V600-targeted therapeutic options that are currently FDA approved (level 1) either as monotherapy or in combinations for treatment of BRAF-altered cancers.
Table 1.
Level of evidence for BRAF alterations’ targetability according to OncoKB17
| Level | Gene | Alterations | Cancer types | Drugs |
|---|---|---|---|---|
| 1 | BRAF | V600 | Erdheim–Chester disease | Vemurafenib |
| 1 | BRAF | V600 | Melanoma | Vemurafenib + atezolizumab + cobimetinib |
| 1 | BRAF | V600E | All solid tumors (excluding colorectal cancer) | Dabrafenib + trametinib |
| 1 | BRAF | V600E | Anaplastic thyroid cancer | Dabrafenib + trametinib |
| 1 | BRAF | V600E | Biliary tract cancer, NOS | Dabrafenib + trametinib |
| 1 | BRAF | V600E | Colorectal cancer | Encorafenib + cetuximab |
| 1 | BRAF | V600E | Melanoma | Dabrafenib |
| 1 | BRAF | V600E | Melanoma | Vemurafenib |
| 1 | BRAF | V600E | Non-small-cell lung cancer | Dabrafenib + trametinib |
| 1 | BRAF | V600E, V600K | Melanoma | Dabrafenib + trametinib |
| 1 | BRAF | V600E, V600K | Melanoma | Encorafenib + binimetinib |
| 1 | BRAF | V600E, V600K | Melanoma | Trametinib |
| 1 | BRAF | V600E, V600K | Melanoma | Vemurafenib + cobimetinib |
| 2 | BRAF | Fusions | Pilocytic astrocytoma | Selumetinib |
| 2 | BRAF | Oncogenic Mutations (excluding V600) | Erdheim–Chester disease | Cobimetinib, trametinib |
| 2 | BRAF | Oncogenic Mutations (excluding V600) | Langerhans cell histiocytosis | Cobimetinib, trametinib |
| 2 | BRAF | Oncogenic Mutations (excluding V600) | Rosai–Dorfman disease | Cobimetinib, trametinib |
| 2 | BRAF | V600 | Langerhans cell histiocytosis | Vemurafenib, dabrafenib |
| 2 | BRAF | V600 (excluding V600E and V600K) | Melanoma | Dabrafenib + trametinib |
| 2 | BRAF | V600 (excluding V600E and V600K) | Melanoma | Encorafenib + binimetinib |
| 2 | BRAF | V600 (excluding V600E and V600K) | Melanoma | Vemurafenib + cobimetinib |
| 2 | BRAF | V600E | Colorectal cancer | Encorafenib + panitumumab |
| 2 | BRAF | V600E | Diffuse glioma | Vemurafenib + cobimetinib |
| 2 | BRAF | V600E | Encapsulated glioma | Vemurafenib + cobimetinib |
| 2 | BRAF | V600E | Hairy cell leukemia | Vemurafenib |
| 2 | BRAF | V600E | Pilocytic astrocytoma | Selumetinib |
| 2 | BRAF | V600E | Pleomorphic xanthoastrocytoma, Pilocytic astrocytoma, Ganglioglioma | Vemurafenib + cobimetinib |
| 3 | BRAF | Fusions | Melanoma | Trametinib, cobimetinib |
| 3 | BRAF | Fusions | Ovarian cancer | Trametinib, cobimetinib |
| 3 | BRAF | K601 | Melanoma | Trametinib |
| 3 | BRAF | L597 | Melanoma | Trametinib |
| 3 | BRAF | Oncogenic Mutations (excluding V600) | Histiocytosis | Cobimetinib, trametinib |
| 3 | BRAF | V600 | Histiocytosis | Vemurafenib, dabrafenib |
| 4 | BRAF | G464, G469A, G469R, G469V | All solid tumors | PLX8394 |
| 4 | BRAF | K601 | All solid tumors | PLX8394 |
| 4 | BRAF | L597 | All solid tumors | PLX8394 |
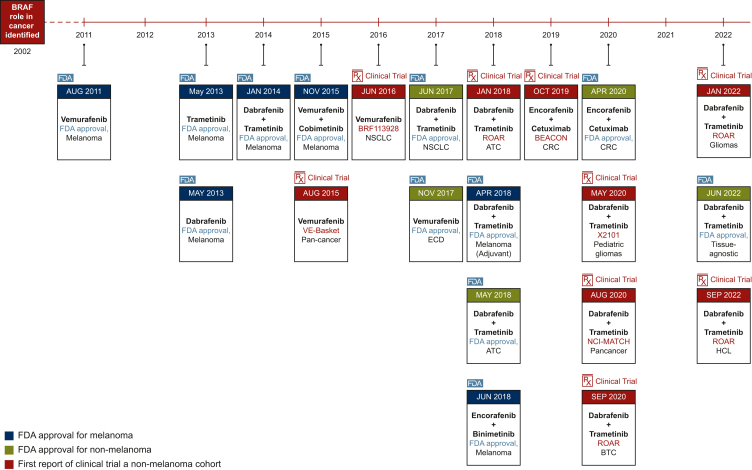
Figure 2.
Timeline of BRAF inhibitor drug development from melanoma to tissue-agnostic approval.
ATC, anaplastic thyroid carcinoma; BTC, biliary tract cancer; CRC, colorectal cancer; ECD, Erdheim–Chester disease; FDA, Food and Drug Administration; HCL, hairy cell leukemia; NSCLC, non-small-cell lung cancer.
BRAF-targeted monotherapy
Vemurafenib
The first FDA approval for a kinase inhibitor targeting BRAF was for vemurafenib in 2011. The drug is currently approved as monotherapy for treatment of patients with unresectable or metastatic melanoma who harbor BRAF V600E mutation and also for treatment of patients with Erdheim–Chester disease (ECD) who have a BRAF V600 mutation.57
Vemurafenib is a multikinase inhibitor that is available in oral form and possesses strong antitumor activity in cases with mutated BRAF. Some mutations, including V600 mutations, lead to continuous activation of the BRAF downstream pathway that becomes independent on upstream growth factor stimulation. In that setting, the inhibitory effect of vemurafenib can lead to cessation of cellular proliferation and tumor cell apoptosis. Preclinical data suggested paradoxical activation of the MAPK pathway by vemurafenib in BRAF wild-type tumors leading to increased cell proliferation which has been the basis of design of next generation inhibitors as well as combination with MEK and EGFR inhibitors.58,59
Multiple clinical trials have shown data favoring the use of vemurafenib as a single agent in different tumor types and led to current approvals (Table 2). Four trials are considered the basis for FDA approval of vemurafenib in treatment of BRAF V600E melanoma and non-melanoma cancers.57 The first randomized, controlled trial (BRIM-3; NCT01006980) explored the use of vemurafenib in treatment-naive patients with BRAF V600-mutated unresectable or metastatic melanoma. Patients in the vemurafenib arm showed improved overall response rate (ORR), progression-free survival (PFS), and overall survival (OS) compared with patients in the dacarbazine control arm.18, 19, 20 Further testing was done in previously treated patients in the phase II NCT00949702 trial where vemurafenib induced clinical response in more than half of included patients.21 Another phase II trial (NCT01378975) investigated the use of vemurafenib in patients with pretreated and treatment-naive brain metastasis from BRAF V600 melanoma and reported intracranial responses in 18% of patients.22
Table 2.
Summary of trials leading to current FDA approvals for BRAF inhibitors. Independent review data are used whenever reported in the most updated analysis
| Vemurafenib | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PFS, months (median) |
OS, months (median) |
ORR (%) |
||||||||
| Study | Phase | Population | Design | Drug | Control | Drug | Control | Drug | Control | Cohort |
| BRIM-3 study (NCT01006980)18, 19, 20 | Phase III | Patients with treatment-naive BRAF V600-mutated unresectable or metastatic melanoma | Patients were randomized to receive vemurafenib 960 mg orally twice daily (n = 337) or dacarbazine 1000 mg/m2 i.v. every 3 weeks (n = 338) | 6.9 For V600E (5.9 for V600K) | 1.6 For V600E (1.7 for V600K) | 13.6 For V600E (14.5 for V600K) | 9.7 For V600E (7.6 for V600K) | 57 | 9 | |
| NCT0094970221a | Phase II | Patients with previously treated BRAF V600-mutated metastatic melanoma | Patients received vemurafenib 960 mg orally twice daily (n = 132) | 6.8 | 15.9 | 53 | ||||
| NCT0137897522b | Phase II | Patients with BRAF V600-mutated melanoma who have metastatic disease in the brain | Patients received vemurafenib 960 mg orally twice daily (n = 146) and were categorized into two cohorts. Cohort 1 included patients with no prior local therapy for brain metastasis (n = 90) whereas cohort 2 included patients who progressed after prior local therapy (n = 56) | 3.7 | 8.9 | 33 For EC RR (18 for IC RR) | Cohort 1 | |||
| 4 | 9.6 | 23 For EC RR (18 for IC RR) | Cohort 2 | |||||||
| VE-BASKET (NCT01524978)23, 24, 25 | Phase II | Patients with BRAF V600-mutated nonmelanoma solid tumors | Patients received vemurafenib 960 mg orally twice daily (n = 172). A separate analysis included 22 patients with ECD and was the basis for FDA approved indication24 | 5.8 | 17.6 | 33 | Overall cohort (Responses seen in 13 cancers) |
|||
| 7.3 | NR | 42 | NSCLC | |||||||
| NR | NR | 62 | ECD/LCH | |||||||
| 12 | CGC | |||||||||
| 29 | ATC | |||||||||
| 4.5 (3.7 for combo) | 9.3 (7.1 for combo) | 0 (4 with combo) | CRC | |||||||
| Dabrafenib | ||||||||||
| BREAK-3 Study (NCT01227889)26,27c | Phase III | Patients with BRAF V600E-mutated unresectable or metastatic melanoma | Patients were randomized to receive dabrafenib 150 mg orally twice daily (n = 187) or dacarbazine 1000 mg/m2 i.v. every 3 weeks (n = 63) | 6.9 | 2.7 | 18.2 | 15.6 | 50 | 6 | |
| BREAK-MB Study (NCT01266967)28d | Phase II | Patients with BRAF V600E- or V600K-mutated melanoma who have metastatic disease in the brain | Patients received dabrafenib 150 mg orally twice daily (n = 172) and were categorized into two cohorts. Cohort A included patients with no prior local therapy for brain metastasis (n = 89) whereas cohort B included patients who progressed after prior local therapy (n = 83) | 16.1 For V600E (8.1 for V600K) | 33.1 For V600E (16.3 for V600K) | 28 For V600E (0 for V600K) | Cohort A | |||
| 16.6 For V600E (15.9 for V600K) | 31.4 For V600E (21.9 for V600K) | 23 For V600E (11 for V600K) | Cohort B | |||||||
| Trametinib | ||||||||||
| METRIC study (NCT01245062)29,30 | Phase III | Patients with BRAF V600E- or V600K-mutated unresectable or metastatic melanoma | Patients were randomized to receive trametinib 2 mg orally once daily (n = 214) or chemotherapy [dacarbazine 1000 mg/m2 i.v. every 3 weeks or paclitaxel 175 mg/m2 i.v. every 3 weeks] (n = 108) | 4.9 | 1.5 | 15.6 | 11.3 | 29 | 9 | |
| Dabrafenib + trametinib | ||||||||||
| COMBI-d Study (NCT01584648)31,32 | Phase III | Patients with BRAF V600E- or V600K-mutated unresectable or metastatic melanoma | Patients were randomized to receive either a combination of dabrafenib (150 mg orally twice daily) and trametinib (2 mg orally once daily) (n = 211) or dabrafenib and placebo (n = 212) | 9.3 | 8.8 | NR | NR | 68 | 55 | |
| COMBI-v Study (NCT01597908)33 | Phase III | Patients with BRAF V600E- or V600K-mutated unresectable or metastatic melanoma | Patients were randomized to receive either combination of dabrafenib (150 mg orally twice daily) and trametinib (2 mg orally once daily) (n = 352) or vemurafenib 960 mg orally twice daily (n = 352) | 11.4 | 7.3 | NR | 17.2 | 64 | 51 | |
| COMBI-MB Study (NCT02039947)34 | Phase II | Patients with BRAF V600E- or V600K-mutated melanoma with brain metastasis | Patients (n = 125) received dabrafenib 150 mg orally twice daily and trametinib 2 mg orally once daily in four cohorts. Cohort A (n = 76) included asymptomatic patients with BRAF V600E mutation who had no prior local brain therapy. Cohort B (n = 16) included asymptomatic patients with BRAF V600E mutation who had prior local therapy. Cohort C (n = 16) included asymptomatic patients with BRAF V600D/K/R mutations who had no prior local brain therapy. Cohort D (n = 17) included patients with symptomatic disease regardless of local therapy or mutation subtype | 5.6 | 10.8 | 58 | Cohort A | |||
| 7.2 | 24.3 | 56 | Cohort B | |||||||
| 4.2 | 10.1 | 44 | Cohort C | |||||||
| 5.5 | 11.5 | 65 | Cohort D | |||||||
| COMBI-AD (NCT01682083)35 | Phase II | Patients with completely resected BRAF V600E- or V600K-mutated stage III melanoma | Patients were randomized to receive either combination of dabrafenib (150 mg orally twice daily) and trametinib (2 mg orally once daily) (n = 438) or placebo (n = 432) for 12 months | NR (RFS) | 16.6 (RFS) | NR | NR | 37 (Recurrence) | 57 (Recurrence) | |
| Study BRF113928 (NCT01336634)36, 37, 38, 39 | Phase II | Patients with BRAF V600E-mutated metastatic NSCLC | Patients (n = 171) received dabrafenib 150 mg orally twice daily in cohort A (n = 78) or dabrafenib 150 mg orally twice daily and trametinib 2 mg orally once daily in cohort B (n = 57) and cohort C (n = 36). Cohorts A and B included patients with at least one prior therapy. Cohort C included patients with treatment-naive disease | 5.5 | 12.6 | 33 | Cohort A | |||
| 10.2 | 18.2 | 68 | Cohort B | |||||||
| 10.8 | 17.3 | 64 | Cohort C | |||||||
| ROAR Trial; BRF117019 (NCT02034110)40, 41, 42, 43,56e | Phase II | Patients with BRAF V600E-mutated rare cancers, including anaplastic thyroid cancer (ATC) (n = 36), high-grade glioma (HGG) (n = 45), low-grade glioma (LGG) (n = 13), and biliary tract cancer (BTC) (n = 43) | Patients received dabrafenib 150 mg orally twice daily and trametinib 2 mg orally once daily | 5.5 | 14.5 | 53 | ATC | |||
| 9 | 14 | 47 | BTC | |||||||
| 14 | NR | 69 | LGG | |||||||
| 4.5 | 17.6 | 31 | HGG | |||||||
| NR | NR | 89 | HCL | |||||||
| NCI-MATCH Study (arm H); EAY131-H (NCT02465060)44 | Phase II | Patients with BRAF V600E-mutated cancers other than thyroid, melanoma, and CRC | Patients received dabrafenib 150 mg orally twice daily and trametinib 2 mg orally once daily (n = 35) | 11.4 | 28.6 | 38 | Overall cohort (Responses seen in 7 cancers) |
|||
| CTMT212X2101; Study X2101 (NCT02124772)45,55f | Phase I/II | Pediatric patients with BRAF V600-mutated solid tumors | Patients received dabrafenib 5.25 mg/kg/day and trametinib 0.032 mg/kg/day (n = 48) | 36.9 | 25 | LGG Cohort | ||||
| Encorafenib + binimetinib | ||||||||||
| COLUMBUS (NCT01909453)46, 47, 48g | Phase III | Patients with BRAF V600E- or V600K-mutated unresectable or metastatic melanoma | Patients were randomized to receive encorafenib 450 mg orally once daily in combination with binimetinib 45 mg twice daily (n = 192), encorafenib 300 mg orally once daily (n = 194), or vemurafenib 960 mg twice daily (n = 191) | 14.9 For combo |
7.3 For vemurafenib (9.6 for encorafenib) | 33.6 For combo |
16.9 For vemurafenib (23.5 for encorafenib) | 64 For combo |
41 For vemurafenib (52 for encorafenib) | |
| Encorafenib + cetuximab | ||||||||||
| BEACON CRC (NCT02928224)49, 50, 51 | Phase III | Patients with previously untreated BRAF V600E-mutated metastatic colorectal cancer | Patients were randomized to receive either a doublet combination of encorafenib (300 mg orally once daily) and cetuximab (400 mg/m2 initial dose then 250 mg/m2 once a week) (n = 220), triplet combination of encorafenib, cetuximab, and binimetinib (45 mg twice daily) (n = 224), or control of investigator’s choice [cetuximab + irinotecan or cetuximab + FOLFIRI] (n = 221) | 4.3 For doublet |
1.5 For control (4.5 for triplet) | 9.3 For doublet |
5.9 For control (9.3 for triplet) | 20 For doublet |
2 For control (27 for triplet) | |
| Vemurafenib + cobimetinib | ||||||||||
| coBRIM Trial (NCT01689519)52, 53, 54 | Phase III | Patients with previously untreated BRAF V600-mutated unresectable or metastatic melanoma | Patients (n = 495) received vemurafenib 960 mg orally twice daily and were randomized to receive cobimetinib 60 mg orally once daily D1-21 of an every 28-day cycle (n = 247) or matching placebo (n = 248) | 12.6 | 7.2 | 22.5 | 17.4 | 70 | 50 | |
ATC, anaplastic thyroid carcinoma; BTC, biliary tract cancer; CGC, cholangiocarcinoma; CRC, colorectal cancer; EC, extracranial; ECD, Erdheim–Chester disease; FDA, Food and Drug Administration; HCL, hairy cell leukemia; HGG, high-grade glioma; IC, intracranial; LCH, Langerhans cell histiocytosis; LGG, low-grade glioma; NR, not reached; NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Investigator-assessed ORR was 57% with a concordance of 83% between investigators’ assessment and assessment by independent review committee (IRC).
Investigator-assessed extracranial ORR was 32% and 23%, whereas intracranial ORR was 29% and 23% for cohorts 1 and 2, respectively.
Investigator-assessed ORR was 53% and 19% for dabrafenib and control, respectively.
In the original paper published in Lancet Oncology, investigator and IRC assessments were discordant in 72 (42%) patients. Per the investigators, the discordance between the investigator and review committee response assessment, particularly for intracranial disease, led to a blinded adjudication, in which investigator assessments were upheld in two-thirds of cases. Therefore, the reported investigator-assessed overall ORR for BREAK-MB was 38% and 31% in patients with V600E mutation in cohorts A and B, respectively (0% and 28% in patients with V600K); which is quite different from the IRC-assessed ORR which is reported in the Table 2. It is of note that the intracranial ORR was 39% and 31% for patients with V600E; and 7% and 22% for patients with V600K in cohorts A and B, respectively.
Investigator-assessed ORR was the primary endpoint in the ROAR study and was reported as 56%, 51%, 69%, 33%, and 89% in ATC, BTC, LGG, HGG, and HCL, respectively. Table contains IRC-reported ORR for ATC, BTC, LGG, and HGG but not HCL since it was not reported in the original study.
Investigator-assessed ORR was 53% in the combination group.
By local review, ORR was observed in 76%, 58%, and 49% in combination, encorafenib monotherapy, and vemurafenib groups, respectively.
Since BRAF V600 mutations are seen in multiple non-melanoma cancers, activity of monotherapy vemurafenib was explored in one of the first histology-independent trials ever designed—the VE-BASKET study.25 The trial showed activity of vemurafenib in non-small-cell lung cancer (NSCLC; 42%), ECD (43%), and multiple other rare tumors (including pleomorphic xanthoastrocytoma, anaplastic thyroid cancer (ATC), cholangiocarcinoma, salivary duct cancer, ovarian cancer, and clear-cell sarcoma). Such initial results were unprecedented and established BRAF as a potential pan-cancer target. In colorectal cancer (CRC), however, there was limited activity of vemurafenib monotherapy in patients harboring BRAF V600 mutation. A preclinical study suggested that unresponsiveness of CRC to BRAF inhibition was mediated through feedback activation of EGFR and might benefit from combination therapy of BRAF and EGFR inhibitors.60 So, the protocol was amended to add an arm of vemurafenib and cetuximab which successfully demonstrated response in CRC patients. Follow-up data of all the 172 included patients with 26 nonmelanoma cancer types showed responses in 13 different cancers.23 Striking responses were observed in patients with ECD/Langerhans cell histiocytosis who harbor BRAF V600 mutation which formed the basis for FDA approval of vemurafenib in ECD.23,24
Dabrafenib
Dabrafenib is an ATP-competitive inhibitor of RAF kinases that has potent activity against BRAF V600 mutations.61 It received its first FDA approval as a single agent in 2013 and is currently approved as monotherapy for treatment of patients with BRAF V600E-mutated unresectable or metastatic melanoma.62
Dabrafenib was investigated in multiple clinical trials for treatment of melanoma with BRAF V600E alteration. Two clinical trials represent the basis for FDA current approvals (Table 2). In the phase III randomized, controlled trial (BREAK-3; NCT01227889), dabrafenib-treated patients showed a significantly improved PFS and ORR compared with dacarbazine-treated patients.26,27 Another phase II trial (BREAK-MB study; NCT01266967) evaluated the intracranial efficacy of dabrafenib monotherapy in patients with BRAF V600E metastatic melanoma to the brain and reported intracranial responses and an acceptable safety profile in both untreated and previously treated cohorts.28
Most of the subsequent advances, however, in use of dabrafenib were related to its use in combination with the MEK inhibitor, trametinib, which is discussed later in this review. Combination therapy offered better clinical outcomes compared with monotherapy using either drug; and is, therefore, considered the current standard of care.
Trametinib
Trametinib is currently approved as monotherapy for treatment of patients with BRAF V600E or BRAF V600K metastatic or unresectable melanoma who have not received prior treatment with a BRAF inhibitor.63 It acts by reversible inhibition of MEK1 and MEK2 which are upstream regulators of ERK. Although trametinib does not exert a direct inhibitory effect on BRAF, its action would stop the constitutive activation of the BRAF pathway that occurs with different BRAF alterations including BRAF V600 mutations.64
The METRIC study (NCT01245062) is a randomized, controlled phase III trial that was the basis for the drug FDA approval. In this study, patients were randomized to receive either trametinib monotherapy or chemotherapy (dacarbazine or paclitaxel). Patients who received trametinib had a significantly longer PFS compared with patients who received chemotherapy (Table 2).29,30 Further success has been achieved by combining trametinib with the BRAF inhibitor, dabrafenib, which is discussed later in this review.
BRAF-targeted combination therapies
Rationale behind combination therapies
Drug combinations targeting different steps in a signaling pathway have been long used in cancer therapy for potentiating the antineoplastic effect.65 In the BRAF pathway, different agents have been tested for use as monotherapies with a mechanism of action including inhibition of either BRAF or MEK kinases. Despite promising results in monotherapy trials as discussed above, acquired resistance to BRAF inhibitors can develop and limit their duration of response.66,67 Moreover, single-agent inhibitors have been associated with the development of secondary skin cancers which was linked to activation of the MAPK pathway that can happen with monotherapy.18,68
Most of the current approved combination options include a BRAF and a MEK inhibitor. The dual inhibition of upstream BRAF and downstream MEK kinases can lead to interruption of the BRAF signaling pathway that associates with increased cellular proliferation in BRAF-mutated cancers. Compared with single-agent BRAF and MEK inhibitors, a combination of BRAF and MEK inhibition showed a lower incidence of skin hyperproliferation and a delayed emergence of resistance; which led to improved PFS. Another currently approved combination uses an EGFR antibody along with a BRAF inhibitor, with the aim of overcoming EGFR-mediated adaptive feedback signaling that leads to reactivation of the MAPK pathway following BRAF-targeted monotherapy.69
Current drugs included as part of FDA-approved combination therapies include the BRAF inhibitors vemurafenib, dabrafenib, and encorafenib, the MEK inhibitors trametinib, binimetinib, and cobimetinib, and the EGFR inhibitor cetuximab. Since BRAF resistance mechanisms are complex,70 other studies have explored combinations including mammalian target of rapamycin (mTOR) inhibitors, MET inhibitors, and chemotherapy, but showed limited successes.71, 72, 73, 74
Dabrafenib + trametinib
Via a synergistic effect, dabrafenib and trametinib work by exerting a targeted inhibition of two different kinases in the BRAF pathway: BRAF and MEK. Preclinical data suggested that the combination led to greater activity against BRAF V600-mutated tumors compared with either drug given as monotherapy.75,76
Based on data from clinical trials (Table 2), the dabrafenib and trametinib combination is currently approved by FDA for treatment of patients with unresectable or metastatic melanoma and BRAF V600E or V600K mutation, metastatic NSCLC and BRAF V600E mutation, locally advanced or metastatic ATC with BRAF V600E mutation and no locoregional treatment options, and locally advanced melanoma with involved lymph nodes and V600E or V600K mutation following complete resection as part of adjuvant therapy.62
The COMBI-d (NCT01584648) and COMBI-v (NCT01597908) were phase III trials that evaluated the use of dabrafenib and trametinib combination compared with dabrafenib or vemurafenib as controls in patients with metastatic or unresectable melanoma. Both trials demonstrated a significant improvement in PFS and OS as well as a lower rate of incidence for hyperproliferative skin adverse events.31, 32, 33 A pooled long-term analysis of both trials suggested PFS and OS rates of 19% and 34% at 5 years as well as a complete response in 19% of included patients.77 The COMBI-MB phase II trial (NCT02039947) provided substantial evidence on intracranial activity and extracranial clinical benefit from the dabrafenib/trametinib combination in patients with melanoma and brain metastasis as well as a tolerable safety profile.34 The first approval for use in the adjuvant setting came based on data from COMBI-AD trial (NCT01682083) that assessed the use of dabrafenib and trametinib combination versus placebo in patients with stage III melanoma who had lymph node disease and BRAF V600E or V600K mutations. Data suggested a lower risk of recurrence in patients receiving the combination therapy compared with placebo controls.35
Tissue-agnostic activity of dabrafenib + trametinib
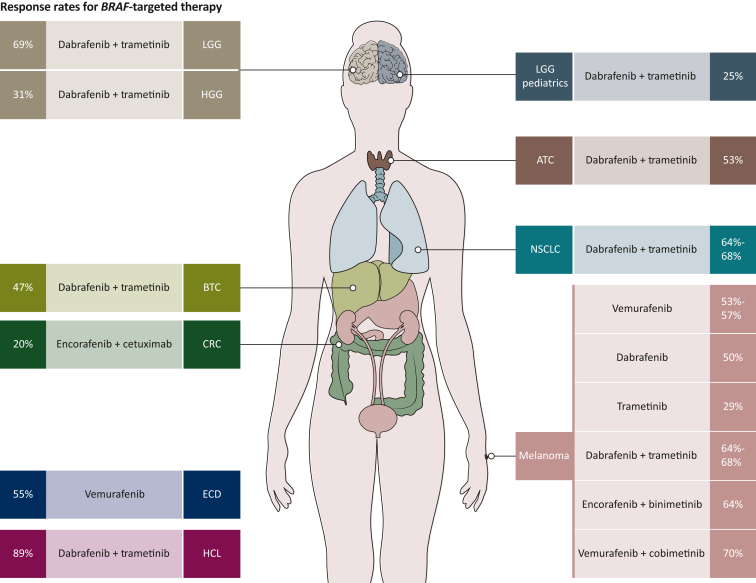
Expanding the reach of BRAF targetability beyond melanoma started with the study BRF113928 (NCT01336634) which was a phase II trial that established the efficacy of dabrafenib and trametinib combination in previously treated and untreated patients with BRAF V600E-mutant NSCLC leading to subsequent approval.36, 37, 38, 39 BRAF inhibitor tissue-agnostic drug development, however, has been quite challenging stemming from the unresponsiveness of CRC patients to monotherapy BRAF inhibition, which raised a question if BRAF V600 can be a tissue-agnostic target. Beyond melanoma and lung, BRAF V600 is also seen in a variety of tumor types; and although monotherapy with vemurafenib showed responses, resistance quickly emerges and leads to refractory disease progression.78 With BRAF and MEK combination emerging as standard of care in melanoma tumors, the ROAR (Rare Oncology Agnostic Research) trial (BRF117019; NCT02034110) was designed as a basket trial to investigate the use of combined dabrafenib and trametinib in none different tumor types harboring BRAF V600 alterations.40, 41, 42, 43,56 Given the dramatic responses in ATC, one of the most lethal forms of thyroid cancers, and in an area of huge unmet need, the combination received FDA approval as the first targeted therapy for BRAF V600-mutant anaplastic thyroid carcinoma.40,41 A notable response rate (51% ORR per investigator assessment) was also seen in BRAF V600-mutant biliary tract cancers leading to inclusion of the combination as a potential treatment option in National Comprehensive Cancer Network (NCCN) guidelines.43 Subsequently, promising data in brain tumors from low-grade glioma (ORR = 69%) and high-grade glioma (ORR = 33%) validated the expanded spectrum of dabrafenib/trametinib antitumor activity. In addition to solid tumors, the combination was active in hairy cell leukemia with an investigator-assessed ORR of 89%.56 Contemporaneously, arm H of the NCI-MATCH trial (EAY131-H; NCT02465060) tested the combination of dabrafenib and trametinib in a pan-cancer cohort and showed promising results in seven distinct tumor types with ORR of 38%.44 Another study (Study X2101; NCT02124772) evaluated the use of the combination in pediatric patients and has so far reported a response rate of 25% in patients with low-grade gliomas45,55,79 (Figure 3).
Figure 3.
Response rates in different tumor types for BRAF inhibitors as monotherapy and in combination with MEK inhibitors in multiple tumors and EGFR inhibitors in colorectal cancer. Independent review data are used whenever reported in the most updated analysis.
ATC, anaplastic thyroid carcinoma; BTC, biliary tract cancer; CRC, colorectal cancer; ECD, Erdheim–Chester disease; HCL, hairy cell leukemia; HGG, high-grade glioma; LGG, low-grade glioma; NSCLC, non-small-cell lung cancer.
With >20 different tumor types showing antitumor activity with dabrafenib and trametinib combination in ROAR, NCI-MATCH, and Study X2101 studies, and considering the totality of evidence, the combination received US FDA accelerated approval for the treatment of adult and pediatric patients ≥6 years of age with unresectable or metastatic solid tumors with BRAF V600E mutation who have progressed following prior treatment and have no satisfactory alternative treatment options.62,79,80 The exception for tissue-agnostic indication was CRC because of known intrinsic resistance mechanism. This approval was distinct being the first tissue-agnostic approval for a treatment targeting a specific genetic mutation. Prior tissue-agnostic approvals were based on NTRK fusions, microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) cancers, and tumors with high tumor mutational burden (TMB).80
Encorafenib + binimetinib
Encorafenib is a kinase inhibitor that acts on both BRAF V600E and wild-type BRAF as well as other kinases to suppress cellular signaling and lead to tumor regression. It is currently approved in combination with binimetinib, a selective MEK inhibitor, for treatment of patients with unresectable or metastatic melanoma who harbor a BRAF V600E or BRAF V600K mutation.81
Compared with either drug alone, the combination led to higher antitumor activity and more delayed emergence of resistance in BRAF V600E-mutant melanoma xenografts. The COLUMBUS trial (NCT01909453) represents the current basis for FDA approval of the combination (Table 2). In this phase III trial, patients with metastatic or unresectable melanoma who harbored BRAF V600E or V600K mutations were randomized to receive the encorafenib/binimetinib combination, encorafenib monotherapy, or vemurafenib. Results suggested greater response rate as well as PFS and OS in the combination group when compared with vemurafenib control.46, 47, 48
Encorafenib + cetuximab
Encorafenib has also been approved in combination with the monoclonal anti-EGFR antibody, cetuximab, for treatment of adult patients with metastatic CRC who have a BRAF V600E mutation.81 In BRAF-mutated CRC, reactivation of the MAPK pathway via EGFR-mediated feedback has been suggested as a potential mechanism of resistance to BRAF inhibitors.69 Therefore, the use of combination was postulated to lead to blockade of the EGFR pathway and potentiate the effect of the BRAF-targeting encorafenib.
BEACON CRC (NCT02928224) was the phase III study that led to the FDA approval (Table 2). The study showed improved survival rates and response in patients treated with the combination of encorafenib plus cetuximab compared with control. The effect was comparable to that of a triplet regimen including encorafenib/cetuximab/binimetinib and was markedly different from that of control regimens including cetuximab and conventional chemotherapy.49, 50, 51
Vemurafenib + cobimetinib
The combination of vemurafenib and cobimetinib was approved in 2015 for treatment of patients with unresectable or metastatic melanoma who harbor a BRAF V600E or V600K mutation.57 Cobimetinib is a selective inhibitor of MEK kinases which when combined with the BRAF inhibitory effect of vemurafenib led to a halt of the activated MAPK pathway in BRAF-mutant cancers.
Vemurafenib and cobimetinib combination was tested in the setting of the coBRIM trial (NCT01689519) where patients were randomized in a phase III randomized, controlled trial to receive vemurafenib alone or in combination with cobimetinib. Patients in the vemurafenib/cobimetinib arm had greater ORR, PFS, and OS compared with patients in the control arm (Table 2).52, 53, 54
Landscape of ongoing clinical trials and future directions
The list of targeted therapeutic options that work by inhibiting the BRAF pathway is growing (Table 1). Even more drugs are currently explored in preclinical studies and early phase clinical trials for treatment of BRAF-altered cancers. As of 9 November 2022 and using clinicaltrials.gov82 as a data source, we were able to identify 369 studies registered as either phase I, phase II, or phase III trials. Of those, only 76 trials had results available or published. This can provide a glimpse on the future potential of BRAF targetability expansion in different tumor types.
Future directions would primarily be focused on two main trajectories. First, researchers are trying to improve the inherent drug characteristics including pharmacokinetics and pharmacodynamics that can lead to better efficacy. For example, researchers have been trying to explore the possibility of improving brain penetration abilities of BRAF inhibitors. Although the current BRAF inhibitors have some brain penetration, brain-penetrant BRAF inhibitors with superior blood–brain barrier penetration, e.g. PF-07284890 (NCT04543188) have been developed, and are being explored in early phase trials.83 Second, researchers have explored other approaches for targeting the BRAF pathway. For example, drugs with an expanded spectrum of activity against non-V600 mutations have been explored, e.g. PLX8394 (NCT02012231).84, 85, 86 Selective degraders of mutant BRAF for the treatment of BRAF-driven cancers, e.g. CFT1946, are also being developed in BRAF-altered cancers.87
Conclusion
BRAF is an important cornerstone in the development and treatment of cancer. Several agents are currently approved for treatment of patients with BRAF alterations and work by inhibiting either BRAF or MEK kinases. Combination therapies can overcome limitations of BRAF inhibitor monotherapy and improve PFS. The spectrum of BRAF inhibition has expanded in the past decade from melanoma to tissue-agnostic indications. In the years to come, better options may be available for currently druggable and undruggable BRAF alterations.
Acknowledgements
VS is an Andrew Sabin Family Foundation fellow at the University of Texas MD Anderson Cancer Center. VS acknowledges the support of the Jacquelyn A. Brady Fund.
Figures 1 and 2 are created using tools from Biorender.com.
Funding
This work was supported by a United States National Institutes of Health (NIH) grant [grant numbers R01CA242845, R01CA273168 to VS]; MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas [grant number RP1100584], the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy [grant number 1U01 CA180964], an NCATS (Center for Clinical and Translational Sciences) grant [grant number UL1 TR000371], and the MD Anderson Cancer Center Support Grant [grant number P30 CA016672].
Disclosure
VS reports research funding/grant support for clinical trials from AbbVie, Agensys, Inc., Alfasigma, Altum, Amgen, Bayer, Berg Health, Blueprint Medicines Corporation, Boston Biomedical, Inc., Boston Pharmaceuticals, Celgene Corporation, D3 Bio, Inc., Dragonfly Therapeutics, Inc., Exelixis, Fujifilm, GlaxoSmithKline, Idera Pharmaceuticals, Inc., Incyte Corporation, Inhibrx, Loxo Oncology, MedImmune, MultiVir, Inc., NanoCarrier, Co., National Comprehensive Cancer Network, NCI-CTEP, Northwest Biotherapeutics, Novartis, PharmaMar, Pfizer, Relay Therapeutics, Roche/Genentech, Takeda, Turning Point Therapeutics, UT MD Anderson Cancer Center, and Vegenics Pty Ltd.; travel support from ASCO, ESMO, Helsinn Healthcare, Incyte Corporation, Novartis, and PharmaMar; consultancy/advisory board participation for Helsinn Healthcare, Jazz Pharmaceuticals, Incyte Corporation, Loxo Oncology/Eli Lilly, MedImmune, Novartis, QED Therapeutics, Relay Therapeutics, Daiichi-Sankyo, and R-Pharm US; and other relationship with Medscape. MG has declared no conflicts of interest.
References
- 1.Wellbrock C., Karasarides M., Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 2.Pearson G., Robinson F., Beers Gibson T., et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 3.Subbiah V., Baik C., Kirkwood J.M. Clinical development of BRAF plus MEK inhibitor combinations. Trends Cancer. 2020;6:797–810. doi: 10.1016/j.trecan.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Davies H., Bignell G.R., Cox C., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 5.AACR Project GENIE Consortium AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017;7:818–831. doi: 10.1158/2159-8290.CD-17-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dankner M., Rose A.A.N., Rajkumar S., Siegel P.M., Watson I.R. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene. 2018;37:3183–3199. doi: 10.1038/s41388-018-0171-x. [DOI] [PubMed] [Google Scholar]
- 7.Yaeger R., Corcoran R.B. Targeting alterations in the RAF-MEK pathway. Cancer Discov. 2019;9:329–341. doi: 10.1158/2159-8290.CD-18-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dankner M., Wang Y., Fazelzad R., et al. Clinical activity of mitogen-activated protein kinase-targeted therapies in patients With non-V600 BRAF-mutant tumors. JCO Precis Oncol. 2022;6 doi: 10.1200/PO.22.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendifar A., Blais E.M., Wolpin B., et al. Retrospective case series analysis of RAF family alterations in pancreatic cancer: real-world outcomes from targeted and standard therapies. JCO Precis Oncol. 2021;5 doi: 10.1200/PO.20.00494. PO.20.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martins-de-Barros A.V., Anjos R.S.D., Silva C.C.G., et al. Diagnostic accuracy of immunohistochemistry compared with molecular tests for detection of BRAF V600E mutation in ameloblastomas: systematic review and meta-analysis. J Oral Pathol Med. 2022;51:223–230. doi: 10.1111/jop.13278. [DOI] [PubMed] [Google Scholar]
- 11.Vanni I., Tanda E.T., Spagnolo F., Andreotti V., Bruno W., Ghiorzo P. The current state of molecular testing in the BRAF-mutated melanoma landscape. Front Mol Biosci. 2020;7:113. doi: 10.3389/fmolb.2020.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouda M.A., Ong E., Huang H.J., et al. Ultrasensitive detection of BRAF V600E mutations in circulating tumor DNA of patients with metastatic thyroid cancer. Endocrine. 2022;76:491–494. doi: 10.1007/s12020-022-03004-z. [DOI] [PubMed] [Google Scholar]
- 13.Gouda M.A., Polivka J., Huang H.J., et al. Ultrasensitive detection of BRAF mutations in circulating tumor DNA of non-metastatic melanoma. ESMO Open. 2022;7 doi: 10.1016/j.esmoop.2021.100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs M.G., Malapelle U., Andre F., et al. Practical considerations for the use of circulating tumor DNA in the treatment of patients with cancer: a narrative review. JAMA Oncol. 2022;8(12):1830–1839. doi: 10.1001/jamaoncol.2022.4457. [DOI] [PubMed] [Google Scholar]
- 15.Iyer P.C., Cote G.J., Hai T., et al. Circulating BRAF V600E cell-free DNA as a biomarker in the management of anaplastic thyroid carcinoma. JCO Precis Oncol. 2018;2:1–11. doi: 10.1200/PO.18.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gouda M.A., Subbiah V. Precision oncology for biliary tract tumors: it’s written in blood. Ann Oncol. 2022;33(12):1209–1211. doi: 10.1016/j.annonc.2022.09.157. [DOI] [PubMed] [Google Scholar]
- 17.Chakravarty D., Gao J., Phillips S.M., et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017;2017 doi: 10.1200/PO.17.00011. PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman P.B., Hauschild A., Robert C., et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman P.B., Robert C., Larkin J., et al. Vemurafenib in patients with BRAFV600 mutation-positive metastatic melanoma: final overall survival results of the randomized BRIM-3 study. Ann Oncol. 2017;28:2581–2587. doi: 10.1093/annonc/mdx339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McArthur G.A., Chapman P.B., Robert C., et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323–332. doi: 10.1016/S1470-2045(14)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sosman J.A., Kim K.B., Schuchter L., et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McArthur G.A., Maio M., Arance A., et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicentre study. Ann Oncol. 2017;28:634–641. doi: 10.1093/annonc/mdw641. [DOI] [PubMed] [Google Scholar]
- 23.Subbiah V., Puzanov I., Blay J.Y., et al. Pan-cancer efficacy of vemurafenib in BRAF (V600)- mutant non-melanoma cancers. Cancer Discov. 2020;10:657–663. doi: 10.1158/2159-8290.CD-19-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diamond E.L., Subbiah V., Lockhart A.C., et al. Vemurafenib for BRAF V600-mutant Erdheim-Chester disease and Langerhans cell histiocytosis: analysis of data from the histology-independent, phase 2, open-label VE-BASKET study. JAMA Oncol. 2018;4:384–388. doi: 10.1001/jamaoncol.2017.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyman D.M., Puzanov I., Subbiah V., et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauschild A., Grob J.J., Demidov L.V., et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 27.Hauschild A., Grob J.J., Demidov L.V., et al. An update on BREAK-3, a phase III, randomized trial: dabrafenib (DAB) versus dacarbazine (DTIC) in patients with BRAF V600E-positive mutation metastatic melanoma (MM) J Clin Oncol. 2013;31:9013. [Google Scholar]
- 28.Long G.V., Trefzer U., Davies M.A., et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 29.Flaherty K.T., Robert C., Hersey P., et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 30.Robert C., Flaherty K., Nathan P., et al. Five-year outcomes from a phase 3 METRIC study in patients with BRAF V600 E/K-mutant advanced or metastatic melanoma. Eur J Cancer. 2019;109:61–69. doi: 10.1016/j.ejca.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 31.Long G.V., Flaherty K.T., Stroyakovskiy D., et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631–1639. doi: 10.1093/annonc/mdx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long G.V., Stroyakovskiy D., Gogas H., et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 33.Robert C., Karaszewska B., Schachter J., et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 34.Davies M.A., Saiag P., Robert C., et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18:863–873. doi: 10.1016/S1470-2045(17)30429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long G.V., Hauschild A., Santinami M., et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 36.Planchard D., Besse B., Groen H.J.M., et al. Phase 2 study of dabrafenib plus trametinib in patients with BRAF V600E-mutant metastatic NSCLC: updated 5-year survival rates and genomic analysis. J Thorac Oncol. 2022;17:103–115. doi: 10.1016/j.jtho.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Planchard D., Besse B., Groen H.J.M., et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17:984–993. doi: 10.1016/S1470-2045(16)30146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Planchard D., Kim T.M., Mazieres J., et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:642–650. doi: 10.1016/S1470-2045(16)00077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Planchard D., Smit E.F., Groen H.J.M., et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–1316. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 40.Subbiah V., Kreitman R.J., Wainberg Z.A., et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36:7–13. doi: 10.1200/JCO.2017.73.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subbiah V., Kreitman R.J., Wainberg Z.A., et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study. Ann Oncol. 2022;33:406–415. doi: 10.1016/j.annonc.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen P.Y., Stein A., van den Bent M., et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutant low-grade and high-grade glioma (ROAR): a multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 2022;23:53–64. doi: 10.1016/S1470-2045(21)00578-7. [DOI] [PubMed] [Google Scholar]
- 43.Subbiah V., Lassen U., Elez E., et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21:1234–1243. doi: 10.1016/S1470-2045(20)30321-1. [DOI] [PubMed] [Google Scholar]
- 44.Salama A.K.S., Li S., Macrae E.R., et al. Dabrafenib and trametinib in patients with tumors with BRAF(V600E) mutations: results of the NCI-MATCH trial subprotocol H. J Clin Oncol. 2020;38:3895–3904. doi: 10.1200/JCO.20.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geoerger B., Bouffet E., Whitlock J.A., et al. Dabrafenib plus trametinib combination therapy in pediatric patients with BRAF V600-mutant low-grade glioma: safety and efficacy results. J Clin Oncol. 2020;38 doi: 10.1200/JCO.22.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dummer R., Ascierto P.A., Gogas H.J., et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315–1327. doi: 10.1016/S1470-2045(18)30497-2. [DOI] [PubMed] [Google Scholar]
- 47.Dummer R., Ascierto P.A., Gogas H.J., et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603–615. doi: 10.1016/S1470-2045(18)30142-6. [DOI] [PubMed] [Google Scholar]
- 48.Dummer R., Flaherty K.T., Robert C., et al. COLUMBUS 5-year update: a randomized, open-label, phase III trial of encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF V600-mutant melanoma. J Clin Oncol. 2022;40:4178–4188. doi: 10.1200/JCO.21.02659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kopetz S., Grothey A., Yaeger R., et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 50.Tabernero J., Grothey A., Van Cutsem E., et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39:273–284. doi: 10.1200/JCO.20.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Cutsem E., Huijberts S., Grothey A., et al. Binimetinib, encorafenib, and cetuximab triplet therapy for patients with BRAF V600E-mutant metastatic colorectal cancer: safety lead-in results from the phase III BEACON colorectal cancer study. J Clin Oncol. 2019;37:1460–1469. doi: 10.1200/JCO.18.02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ascierto P.A., Dreno B., Larkin J., et al. 5-year outcomes with cobimetinib plus vemurafenib in BRAFV600 mutation-positive advanced melanoma: extended follow-up of the coBRIM study. Clin Cancer Res. 2021;27:5225–5235. doi: 10.1158/1078-0432.CCR-21-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ascierto P.A., McArthur G.A., Dreno B., et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248–1260. doi: 10.1016/S1470-2045(16)30122-X. [DOI] [PubMed] [Google Scholar]
- 54.Larkin J., Ascierto P.A., Dreno B., et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 55.Bouffet E., Geoerger B., Moertel C., et al. Efficacy and safety of trametinib monotherapy or in combination with dabrafenib in pediatric BRAF V600-mutant low-grade glioma. J Clin Oncol. 2023;41:664–674. doi: 10.1200/JCO.22.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kreitman R.J., Moreau P., Ravandi F., et al. Dabrafenib plus trametinib in patients with relapsed/refractory BRAF V600E mutation-positive hairy cell leukemia. Blood. 2022 doi: 10.1182/blood.2021013658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ZELBORAF® (vemurafenib) tablet for oral use: FDA Packaging Insert. In: FDA, ed.; 2020.
- 58.Bollag G., Hirth P., Tsai J., et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang H., Higgins B., Kolinsky K., et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 2010;70:5518–5527. doi: 10.1158/0008-5472.CAN-10-0646. [DOI] [PubMed] [Google Scholar]
- 60.Prahallad A., Sun C., Huang S., et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 61.Laquerre S., Amone M., Moss K., et al. A selective Raf kinase inhibitor induces cell death and tumor regression of human cancer cell lines encoding B-Raf(V600E) mutation. Mol Cancer Ther. 2009;8:B88. [Google Scholar]
- 62.TAFINLAR® (dabrafenib) capsules, for oral use: FDA Packaging Insert. U.S. Food and Drug Administration website. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/202806s022lbl.pdf. Accessed November 9, 2022.
- 63.MEKINIST® (trametinib) tablets, for oral use: FDA Packaging Insert. U.S. Food and Drug Administration website. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/204114s024lbl.pdf. Accessed November 9, 2022.
- 64.Gilmartin A.G., Bleam M.R., Groy A., et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res. 2011;17:989–1000. doi: 10.1158/1078-0432.CCR-10-2200. [DOI] [PubMed] [Google Scholar]
- 65.Gilad Y., Gellerman G., Lonard D.M., O’Malley B.W. Drug combination in cancer treatment-from cocktails to conjugated combinations. Cancers (Basel) 2021;13:669. doi: 10.3390/cancers13040669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi H., Hugo W., Kong X., et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4:80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Allen E.M., Wagle N., Sucker A., et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4:94–109. doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boussemart L., Routier E., Mateus C., et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691–1697. doi: 10.1093/annonc/mdt015. [DOI] [PubMed] [Google Scholar]
- 69.Corcoran R.B., Ebi H., Turke A.B., et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227–235. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sen S., Meric-Bernstam F., Hong D.S., Hess K.R., Subbiah V. Co-occurring genomic alterations and association with progression-free survival in BRAFV600-mutated nonmelanoma tumors. J Natl Cancer Inst. 2017;109:djx094. doi: 10.1093/jnci/djx094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Janku F., Sakamuri D., Kato S., et al. Dose-escalation study of vemurafenib with sorafenib or crizotinib in patients with BRAF-mutated advanced cancers. Cancer. 2021;127:391–402. doi: 10.1002/cncr.33242. [DOI] [PubMed] [Google Scholar]
- 72.Subbiah V., Sen S., Hess K.R., et al. Phase I study of the BRAF inhibitor vemurafenib in combination with the mammalian target of rapamycin inhibitor everolimus in patients with BRAF-mutated malignancies. JCO Precis Oncol. 2018;2 doi: 10.1200/PO.18.00189. PO.18.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhatty M., Kato S., Piha-Paul S.A., et al. Phase 1 study of the combination of vemurafenib, carboplatin, and paclitaxel in patients with BRAF-mutated melanoma and other advanced malignancies. Cancer. 2019;125:463–472. doi: 10.1002/cncr.31812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sen S., Tanaka R., Khatua S., et al. Dual inhibition of BRAF and mTOR in BRAF(V600E) -mutant pediatric, adolescent, and young adult brain tumors. Cold Spring Harb Mol Case Stud. 2020;6:a005041. doi: 10.1101/mcs.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paraiso K.H., Fedorenko I.V., Cantini L.P., et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010;102:1724–1730. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Su F., Viros A., Milagre C., et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robert C., Grob J.J., Stroyakovskiy D., et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381:626–636. doi: 10.1056/NEJMoa1904059. [DOI] [PubMed] [Google Scholar]
- 78.Adashek J.J., Menta A.K., Reddy N.K., et al. Tissue-agnostic activity of BRAF plus MEK inhibitor in BRAF V600-mutant tumors. Mol Cancer Ther. 2022;21:871–878. doi: 10.1158/1535-7163.MCT-21-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.FDA grants accelerated approval to dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation. Available at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid. Accessed November 9, 2022.
- 80.Mullard A. BRAF plus MEK inhibitor combo secures tumour-agnostic FDA approval. Nat Rev Drug Discov. 2022;21:548. doi: 10.1038/d41573-022-00117-y. [DOI] [PubMed] [Google Scholar]
- 81.BRAFTOVI® (encorafenib) capsules, for oral use: FDA Packaging Insert. U.S. Food and Drug Administration website. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210496lbl.pdf. Accessed November 9, 2022.
- 82.Available at www.clinicaltrials.gov. Accessed November 9, 2022.
- 83.Subbiah V., Gutierrez M., Anders C.K., et al. Trial in progress: phase 1a/b study of PF-07284890 (brain-penetrant BRAF inhibitor) with/without binimetinib in patients with BRAF V600-mutant solid tumors. J Clin Oncol. 2021;39 [Google Scholar]
- 84.Yao Z., Gao Y., Su W., et al. RAF inhibitor PLX8394 selectively disrupts BRAF dimers and RAS-independent BRAF-mutant-driven signaling. Nat Med. 2019;25:284–291. doi: 10.1038/s41591-018-0274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Janku F., Sherman E.J., Parikh A.R., et al. Interim results from a phase 1/2 precision medicine study of PLX8394-a next generation BRAF inhibitor. Eur J Cancer. 2020;138:S2–S3. [Google Scholar]
- 86.Gouda M.A., Sherman E.J., Gilcrease G.W., et al. Activity, safety and circulating tumour DNA (ctDNA) dynamics of paradox breaker BRAF inhibitor PLX8394 in patients with advanced cancer. Ann Oncol. 2020;31 S465. [Google Scholar]
- 87.Sowa M.E., Kreger B., Baddour J., et al. Preclinical evaluation of CFT1946 as a selective degrader of mutant BRAF for the treatment of BRAF driven cancers. Cancer Res. 2022;82:2158. [Google Scholar]