Abstract
Background
Takotsubo cardiomyopathy (TC) affects predominantly women. Prior studies have suggested that men might have worse short-term outcomes, but limited data are available regarding long-term outcomes. We hypothesized that men, compared to women, with TC have worse short- and long-term outcomes.
Methods
A retrospective study of patients diagnosed with TC between 2005 and 2018 in the Veteran Affairs system was performed. Primary outcomes were in-hospital death, 30-day risk of stroke, death, and long-term mortality.
Results
A total of 641 patients were included (444 men [69%]; 197 women [31%]). Men had a higher median age (65 vs 60 years; P < 0.001), and women were more likely to present with chest pain (68.7% vs 44.1%; P < 0.001). Physical triggers were more common in men (68.7% vs 44.1%, P < 0.001). Men had a higher in-hospital mortality rate (8.1% vs 1%; P < 0.001). On multivariable regression analysis, female sex was an independent predictor for improved in-hospital mortality, compared to men (odds ratio 0.25, 95% confidence interval 0.06-1.10; P = 0.04). On 30-day follow-up, no difference occurred in a combined outcome of stroke and death (3.9% vs 1.5%; P = 0.12). On long-term follow-up (3.7 ± 3.1 years), female sex was identified as an independent predictor of lower mortality (hazard ratio 0.71, 95% CI 0.51-0.97; P = 0.032). Women were more likely to have TC recurrence (3.6% vs 1.1%; P = 0.04)
Conclusions
In our study with a predominantly male population, men had less-favourable short- and long-term outcomes after TC, compared to those of women.
Graphical abstract
Résumé
Contexte
La cardiomyopathie de Takotsubo (CT) touche majoritairement les femmes. Or, des études antérieures semblent indiquer que les hommes pourraient connaître de pires résultats à court terme, mais peu de données portent sur les résultats à long terme. Nous avons formulé l’hypothèse selon laquelle les hommes atteints de CT obtiennent de moins bons résultats à court et à long terme que les femmes qui en sont atteintes.
Méthodologie
Nous avons réalisé une étude rétrospective auprès des patients qui étaient inscrits au système de soins de santé du département des Anciens Combattants des États-Unis et qui avaient reçu un diagnostic de CT entre 2005 et 2018. Les critères d’évaluations principaux étaient le taux de décès à l’hôpital, le risque d’AVC sur 30 jours, le taux de décès et le taux de mortalité à long terme.
Résultats
Au total, 641 patients ont été inclus dans l’étude (444 hommes [69 %]; 197 femmes [31 %]). L’âge médian était plus élevé chez les hommes (65 c. 60 ans; p < 0,001), et les femmes étaient plus susceptibles de présenter des douleurs à la poitrine (68,7 % c. 44,1 %; p < 0,001). Les déclencheurs physiques étaient plus fréquents chez les hommes (68,7 % c. 44,1 %; p < 0,001). Le taux de mortalité des hommes à l’hôpital était plus élevé (8,1 % c. 1 %; p < 0,001). L'analyse par régression multivariée a permis de constater que le sexe féminin était un indicateur prévisionnel indépendant d’un taux de mortalité plus faible à l’hôpital (rapport des cotes : 0,25; intervalle de confiance [IC] à 95 % : 0,06 à 1,10; p = 0,04). Lors du suivi au jour 30, aucune différence n’a été notée dans les résultats combinés d’AVC et de décès (3,9 % c. 1,5 %; p = 0,12). Lors du suivi à long terme (3,7 ± 3,1 ans), le sexe féminin a été ciblé comme un indicateur prévisionnel d’un plus faible taux de mortalité (rapport de risques instantanés : 0,71; IC à 95 % : 0,51 à 0,97; p = 0,032). Enfin, les femmes étaient plus susceptibles de connaître une récurrence de la maladie (3,6 % c. 1,1 %; p = 0,04).
Conclusions
Dans notre étude portant sur une population à prédominance masculine, les hommes atteints de CT ont obtenu des résultats à court et à long terme moins favorables que les femmes atteintes de ce syndrome.
Takotsubo (stress-induced) cardiomyopathy (TC) is characterized by transient left ventricular dysfunction, with distinct regional wall-motion abnormalities, that is not due to obstructive coronary artery disease (CAD).1 Although TC has been recognized for over 3 decades,2 our understanding of this unique disease is still evolving. The pathophysiology of TC is not well understood, but activation of the stress regions in the brain,3 excess catecholamines,4 endothelial dysfunction, and estrogen deficiency may play a role in triggering this condition.5,6 Previous studies have reported that TC predominantly affects women and is commonly preceded by intense emotional or physiological stress.7,8 The results of prior studies comparing outcome in men and women with TC suggest that in-hospital outcomes are worse for men. However, most prior studies were limited by having a small number of men, or using solely International Classification of Diseases (ICD) codes to determine inclusion, without chart review.7,9, 10, 11, 12 In addition, long-term outcomes have not been well studied. Also, uncertainty remains as to whether sex is an independent predictor for in-hospital mortality after adjustment for a significant difference in comorbidities between men and women.7,9,13, 14, 15
The US Veteran Affairs (VA) system serves mostly male patients.16 This primarily male population provides sufficient numbers of men to allow comparison of clinical characteristics and outcomes in a disease that predominantly affects women. We hypothesized that men with TC have worse short- and long-term outcomes, compared to those of women.
Methods
Data source and study population
Using data from the Compensation and Pension Record Interchange (CAPRI) system, we identified patients who had the diagnosis of TC between January 2005 and January 2018 across all hospitals in the VA healthcare system in the US. The CAPRI system of electronic health records contains national data and allows electronic search, as well as review of notes, procedures, imaging reports, and lab results across various VA hospitals. We initially narrowed our search by identifying patients with the following ICD codes: Takotsubo syndrome—ICD 10 code I51. 81; ICD 9 code 429.83. We used a combination of electronic data searching (using ICD codes) and manual verification to identify demographic data and baseline clinical variables at the time of diagnosis. We then removed any duplicate charts. We manually reviewed each medical record. Patients who did not meet the revised Mayo Clinic criteria and the International Takotsubo Diagnostic Criteria were excluded.1,17 All patients were required to have a coronary angiography performed, unless the procedure was deemed unnecessary by the treating cardiologist, with the reviewers’ agreement (agreement given if characteristic echocardiography changes were present, recent coronary angiography was performed, the risks of coronary angiography were deemed to outweigh the benefits, and no ST abnormalities suggestive of injury and/or no typical pattern of troponin elevation were present). Patient data were collected individually by at least 2 investigators. If any disagreement about the diagnosis occurred between the reviewers, then consensus was reached with the rest of the investigational team. Patients with no echocardiogram or ventriculography consistent with TC were excluded.
The following information was collected: demographic data, comorbidities, medications, trigger factor, presenting symptoms, electrocardiography results, echocardiogram results (at presentation and serial echocardiography), left heart catheterization, type of TC, in-hospital events, 30-day events, and long-term outcomes (up to July 2020, to provide us with about 2.5 years of follow-up for the patients included most recently in our study). In patients with missing data from the echocardiogram report, diagnosis was based on ventriculography data obtained from heart catheterization. The Central Arkansas VA Health System’s institutional review board approved the study.
We categorized trigger factors as physical or emotional. Physical trigger factors included acute medical/surgical illness, accidents, physical altercations, trauma, and others.
Types of TC
Based on previously described types, TC was classified as apical, mid-ventricular, basal, or focal.7 The type of TC was determined based on the description of wall-motion abnormality in echocardiography or angiography reports.
Outcomes
We collected data for in-hospital complications, including cardiogenic shock, arrhythmias, cardiac arrest, respiratory failure, and death. We also collected data on the use of vasopressor/inotropic drugs, left ventricular assist devices, and invasive and noninvasive ventilation. Primary outcomes of interest were as follows: (i) in-hospital mortality; (ii) 30-day risk of stroke, and death; and (iii) long-term mortality.
Statistical analysis
Patients were classified into 2 groups—men and women. Categorical variables were reported as counts and percentages; differences were assessed using the χ2 test. Continuous variables were presented as median with interquartile range (IQR); and differences were compared using the Mann-Whitney test. Kaplan-Meier analysis was used to compare all-cause mortality for the unadjusted data.
We performed univariate analysis for gender, comorbidities, clinical characteristics, trigger factor, labs at the time of diagnosis (hemoglobin level, white blood cell count, glomerular filtration rate, Troponin level, brain natriuretic peptide level), ejection fraction (EF), use of admission medications (beta blockers, angiotensin-converting enzyme inhibitors/ angiotensin II receptor blockers [ACE-Is/ARBs], antiplatelets/anticoagulants, statin), medical treatment, the presence of cardiogenic shock, and respiratory failure requiring ventilatory support to evaluate in-hospital mortality. We subsequently performed multivariate logistic regression analysis on variables with a P value ≤ 0.05 to identify independent variables that predicted in-hospital mortality. Similarly, we performed univariable analysis, then multivariate analysis for 30 days, for combined death and stroke. We used the discharge medications instead of the admission medications.
For long-term follow-up, we performed univariable analysis for the above variables, except for using discharge medications instead of admission medications. We then performed Cox regression analysis to adjust for baseline variables and calculate the adjusted hazard ratios for mortality on long-term follow-up. A 2-sided P value ≤ 0.05 was considered significant. Analysis was performed using MedCalc Statistical Software version 18.1.1 (MedCalc Software, Ostend, Belgium).
Patient and public involvement
Neither patients nor the public were involved in the design, conduct, reporting, and dissemination plans of our research.
Results
Baseline characteristics
A total of 641 patients with the diagnosis of TC were included in our analysis, of whom 444 (69%) were men and 197 (31%) were women. Table 1 compares baseline characteristics between the 2 groups. Men were significantly older than women (median [IQR] age 65 years [60-72.5] vs 60 years [55-66]; P < 0.001), with a higher prevalence of CAD (18.7% vs 11.2%; P = 0.02), chronic kidney disease (CKD) (16.9% vs 7.1%, P = 0.001), chronic obstructive pulmonary disease (COPD) (48% vs 38.1%, P = 0.02), and history of malignancy (19.8% vs 8.1%; P < 0.001). In addition, men had a higher prevalence of reported alcohol use (39.9% vs 19.8%; P < 0.001). Women had higher median body mass index (26 vs 24.8 kg/m2; P = 0.01) and were more likely to have bipolar disease (12.7% vs 6.1%; P = 0.005) and fibromyalgia (20.8% vs 10.6%; P < 0.001). No significant difference between the 2 groups was present in baseline medications at presentation.
Table 1.
Differences in baseline characteristics between men and women
| Demographic variable | Men (n = 444) | Women (n = 197) | P |
|---|---|---|---|
| Age, median (IQR), y | 65 (60–72.5) | 60 (55–66) | < 0.001 |
| Diabetes | 142 (32) | 55 (27.9) | 0.3 |
| Hypertension | 266 (59.9) | 108 (54.8) | 0.23 |
| Hyperlipidemia | 289 (65.1) | 140 (71.1) | 0.14 |
| CKD | 75 (16.9) | 14 (7.1) | 0.001 |
| COPD | 213 (48) | 75 (38.1) | 0.02 |
| CAD | 83 (18.7) | 22 (11.2) | 0.02 |
| PVD | 57 (12.8) | 7 (3.6) | < 0.001 |
| CHF | 47 (10.6) | 16 (8.1) | 0.33 |
| Atrial fibrillation | 41 (9.2) | 12 (6.1) | 0.18 |
| History of malignancy | 88 (19.8) | 16 (8.1) | < 0.001 |
| CVA | 96 (21.6) | 30 (15.2) | 0.06 |
| Bipolar disease | 27 (6.1) | 25 (12.7) | 0.005 |
| Smoking history | 270 (60.8) | 101 (51.3) | 0.02 |
| White race | 340 (81.3) | 151 (83.9) | 0.42 |
| Black race | 69 (15.5) | 22 (11.2) | 0.14 |
| Medications on admission | |||
| Beta-blocker | 148 (33.8) | 59 (30.3) | 0.38 |
| ACE-Is/ARB | 174 (39.7) | 76 (39) | 0.86 |
| Antiplatelets | 199 (45.4) | 74 (37.9) | 0.08 |
| Anticoagulation | 40 (9.1) | 16 (8.2) | 0.71 |
| Statin | 191 (43.6) | 83 (42.6) | 0.81 |
| Calcium channel blocker | 74 (16.9) | 28 (14.4) | 0.42 |
| Trigger factor | |||
| Emotional trigger | 33 (8.1) | 40 (22.6) | < 0.001 |
| Physical trigger | 281 (68.7) | 78 (44.1) | < 0.001 |
| No trigger factor identified | 95 (23.2) | 59 (33.3) | 0.01 |
| Location | |||
| Initial admission for TC | 280 (65.1) | 161 (83) | < 0.001 |
| Already admitted to inpatient general ward | 87 (20.2) | 20 (10.3) | < 0.001 |
| Already admitted to ICU | 57 (13.3) | 7 (3.6) | < 0.001 |
| Operating room | 6 (1.4) | 6 (3.1) | 0.15 |
Values are n (%), unless otherwise indicated.
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; ICU, intensive care unit; IQR, interquartile range; PVD, peripheral vascular disease; TC, Takotsubo cardiomyopathy.
Trigger factor and presenting symptoms
Physical stress was a more common trigger factor in men than in women (68.7% vs 44.1%; P < 0.001), whereas typical emotional stress was more likely in women than in men (22.6% vs 8.1%; P < 0.001). In our cohort, 26.2% had no evident trigger and it was more common in women than in men (33.3% vs 23.2%; P = 0.01).
Chest pain was the most common presenting symptom in both groups, but it was more common in women (60.3% vs 40.5%; P < 0.001). Compared to women, men were more likely to present with shortness of breath (18.8% vs 10.9%; P = 0.02), weakness (6.7% vs 2.2%; P = 0.02), and respiratory failure or pulmonary edema (3.9% vs 0.5%; P = 0.02). Women were more likely to have TC as a primary diagnosis at presentation to the hospital (83% vs 65.1%; P < 0.001).
Electrocardiogram, echocardiogram, and coronary angiogram on admission
As shown in Table 2, no significant differences between the groups were present in ST-T changes, by electrocardiogram. Men were more likely to present with atrial fibrillation or atrial flutter (8.9% vs 1.5%; P = 0.004).
Table 2.
Differences in clinical presentation between men and women
| Presentation/symptoms | Men (n = 444) | Women (n = 197) | P |
|---|---|---|---|
| Chest pain | 168 (40.5) | 111 (60.3) | < 0.001 |
| Shortness of breath | 78 (18.8) | 20 (10.9) | 0.02 |
| Cough | 48 (11.6) | 8 (4.3) | 0.005 |
| Gastrointestinal | 8 (1.9) | 6 (3.3) | 0.31 |
| Weakness | 28 (6.7) | 4 (2.2) | 0.022 |
| Respiratory failure/pulmonary edema | 16 (3.9) | 1 (0.5) | 0.02 |
| Arrhythmia | 14 (3.4) | 5 (2.7) | 0.67 |
| Shock | 15 (3.6) | 4 (2.2) | 0.35 |
| Cardiac arrest | 24 (5.8) | 10 (5.4) | 0.92 |
| ECG on presentation | |||
| ST-segment elevation | 131 (39.5) | 46 (36.5) | 0.56 |
| ST-segment depression | 25 (7.5) | 12 (9.5) | 0.48 |
| T wave changes | 99 (31.9) | 44 (37.9) | 0.24 |
| Lab tests | |||
| WBC, cells/μL | 9.9 (7.2–13.9) | 9.2 (7–12.3) | 0.41 |
| Hemoglobin, g/dL | 12.4 (10.4–14.6) | 13.3 (11.3–14.4) | 0.22 |
| Troponin I, ng/mL | 1.7 (0.6–4.4) | 1.6 (0.7–3.8) | 0.81 |
| BNP, pg/mL | 622.5 (187–1654) | 615 (370–1246) | 0.98 |
| GFR, ml/min | 60 (58–92.5) | 64 (55–81) | 0.62 |
| Echo on presentation | |||
| EF ≤ 35 | 231 (63.5) | 83 (54.6) | 0.06 |
| EF, % [n] | 32 (30–35) [364] | 35 (32–40) [152] | 0.03 |
| Coronary angiography done | 318 (71.6) | 161 (81.7) | 0.007 |
| LVEDP, mm Hg [n] | 20 (15–26) [76] | 21.5 (16–26) [36] | 0.82 |
| Type of TC | |||
| Apical | 301 (95) | 137 (98.6) | 0.07 |
| Mid-ventricular | 5 (1.6) | 0 | 0.33 |
| Basal | 11 (3.5) | 2 (1.4) | 0.23 |
Values are n (%) or median (interquartile range), unless otherwise indicated.
BNP, brain natriuretic peptide; ECG, electrocardiogram; EF, ejection fraction; GFR, glomerular filtration rate; LVEDP, left ventricular end diastolic pressure; TC, Takotsubo cardiomyopathy; WBC, white blood cell.
On the admission echocardiogram, men had a lower median (IQR) EF (32% [25%-40%] vs 35% [25%-45%]; P = 0.03). The most common type of TC identified was apical in both groups (94.7% in men vs 98.4% in women; P = 0.08).
Coronary angiogram was more likely to be performed in women than in men (81.7% vs 71.6%; P = 0.007). All patients who presented after a cardiac arrest underwent coronary angiography.
In-hospital course/outcomes
As shown in Table 3, men were more likely to require invasive or noninvasive ventilation (30.2% vs 14.6%; P = 0.001) and vasopressors/inotropes (17.2% vs 8.4%; P = 0.01). Men also had higher median (IQR) length of stay (7 days [3-14] vs 4 days [2-8]; P < 0.001) and were more likely to sustain cardiac arrest (12.4% vs 4.9%; P = 0.01).
Table 3.
In-hospital events in men and women with Takotsubo cardiomyopathy
| In-hospital events | Men (n = 444) | Women (n = 197) | P |
|---|---|---|---|
| Cardiogenic shock | 55 (15) | 16 (11.2) | 0.26 |
| Invasive/noninvasive positive pressure ventilation | 112 (30.2) | 21 (14.6) | 0.001 |
| Mechanical circulatory support (IABP/Impella) | 11 (3) | 4 (2.8) | 0.95 |
| Pharmacologic inotrope/vasopressor support | 62 (17.2) | 12 (8.4) | 0.01 |
| LOS, d | 7 (3–14) | 4 (2–8) | < 0.001 |
| In-hospital death | 36 (8.1) | 2 (1) | < 0.001 |
| Cardiac arrest | 46 (12.4) | 7 (4.9) | 0.01 |
| Combined cardiac arrest/respiratory failure/cardiogenic shock | 141 (36.4) | 31 (20.1) | < 0.001 |
Values are n (%), or median (interquartile range), unless otherwise indicated. Impella pump is from company (Abiomed, Danvers, MA).
IABP, intra-aortic balloon pump; LOS, length of stay.
Men were more likely to die during initial admission (8.1% vs 1%; P = 0.001).
In the total study population, patients who were already being admitted to the hospital for another medical/surgical reason had a higher mortality rate, compared to that of patients who were diagnosed with TC on presentation to the hospital (12.6% vs 3.2%; P < 0.001).
Age, male sex, presence of physical stress as a trigger factor, and the use of Intubation/noninvasive ventilation were independently associated with in-hospital mortality (Table 4). COPD, atrial fibrillation, and CKD showed trends toward increased mortality but this trend did not reach the level of statistical significance.
Table 4.
Multivariate regression for in-hospital mortality
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Age, y | 1.04 | 1.00–1.09 | 0.01 |
| Female sex | 0.25 | 0.06–1.10 | 0.04 |
| Diabetes mellitus | 0.80 | 0.37–1.76 | 0.69 |
| CKD | 0.33 | 0.10–1.10 | 0.07 |
| COPD | 0.48 | 0.23–1.04 | 0.06 |
| CAD | 0.90 | 0.35–2.37 | 0.91 |
| PVD | 1.26 | 0.42–3.76 | 0.80 |
| Atrial fibrillation | 2.35 | 0.80–6.94 | 0.07 |
| History of malignancy | 1.61 | 0.73–3.59 | 0.24 |
| Physical stress | 4.69 | 1.51–14.60 | 0.004 |
| Intubation/noninvasive ventilation | 3.79 | 1.72–8.36 | < 0.001 |
| Cardiogenic shock | 1.98 | 0.86–4.54 | 0.19 |
| Ejection fraction < 35% | 1.68 | 0.75–3.79 | 0.17 |
CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; OR, odds ratio; PVD, peripheral vascular disease.
30-day outcomes
At 30-day follow-up (Table 5), no significant difference was present in readmission rate, or in a combined outcome of stroke and death, between the 2 groups (3.9% vs 1.5%; P = 0.12). Men had a higher rate of arrhythmias (2.7% vs 0; P = 0.02).
Table 5.
Events at 30 days after discharge of men and women with Takotsubo cardiomyopathy
| 30-d events | Men (n = 408) | Women (n = 193) | P |
|---|---|---|---|
| Stroke | 3 (0.7) | 1 (0.5) | 0.75 |
| Arrhythmia | 11 (2.7) | 0 | 0.02 |
| Death | 13 (3.2) | 2 (1) | 0.12 |
| Combined stroke/ death | 16 (3.9) | 3 (1.5) | 0.12 |
| Readmission | 87 (21.3) | 32 (16.6) | 0.17 |
Values are n (%), unless otherwise indicated.
Patients who had TC while in the hospital for medical/surgical illness had higher mortality rates at 30 days after hospital discharge than did patients who were diagnosed with TC on presentation to the hospital (5.7% vs 1.2%; P = 0.002). Older age at diagnosis of TC, CKD, COPD, peripheral vascular disease (PVD), and need for intubation/noninvasive ventilation were all independent predictors for the 30-day combined outcomes of stroke and death (Table 6).
Table 6.
Multivariate regression for mortality and stroke at 30 days
| Covariate | OR | 95% CI | P |
|---|---|---|---|
| Age at diagnosis | 1.07 | 1.03–1.11 | < 0.001 |
| Female sex | 0.52 | 0.14–1.91 | 0.32 |
| Diabetes mellitus | 0.67 | 0.28–1.62 | 0.38 |
| CKD | 2.93 | 1.23–7.00 | 0.02 |
| COPD | 0.40 | 0.18–0.92 | 0.03 |
| CAD | 1.15 | 0.45–2.94 | 0.78 |
| PVD | 3.08 | 1.17–8.09 | 0.02 |
| Atrial fibrillation | 1.71 | 0.52–5.62 | 0.38 |
| History of malignancy | 1.61 | 0.67–3.87 | 0.29 |
| Ejection fraction < 35% | 1.08 | 0.47–2.52 | 0.85 |
| Physical stress as a trigger factor | 1.88 | 0.72–4.88 | 0.20 |
| Intubation/noninvasive ventilation | 4.22 | 1.71–10.43 | 0.002 |
| Cardiogenic shock | 1.14 | 0.35–3.70 | 0.83 |
CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; OR, odds ratio; PVD, peripheral vascular disease.
Long-term outcomes
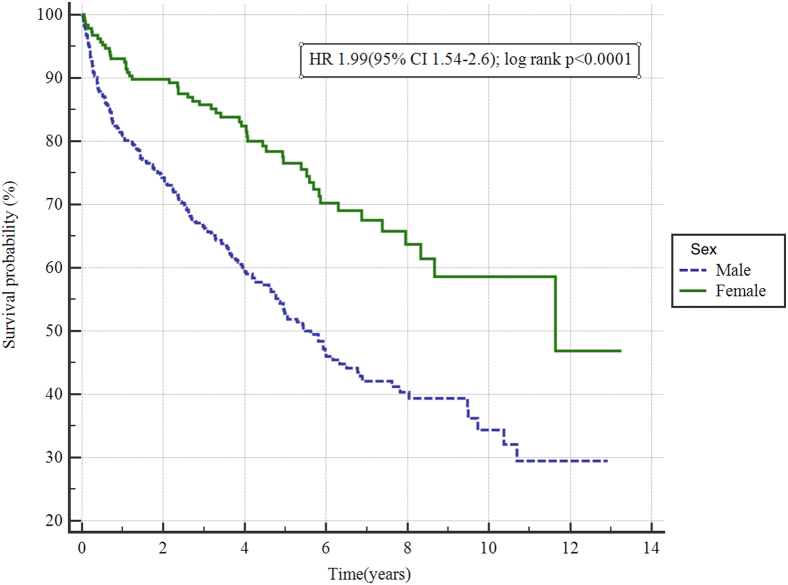
After a median ± standard deviation follow-up time of 3.7 ± 3.1 years, mortality was higher among men than women (hazard ratio 1.99 [95% confidence interval 1.54-2.6]; P < 0.001; Fig. 1).
Figure 1.
Long-term mortality in men and women with Takotsubo cardiomyopathy: Kaplan-Meier analysis for mortality on long-term follow-up. On median ± standard deviation follow-up of 3.7 ± 3.1 years, men were found to have significantly higher mortality rates (hazard ratio [HR] 1.99; 95% confidence interval [CI] 1.54-2.6; P < 0.001).
Age at diagnosis of TC, male sex, diabetes mellitus, CKD, COPD, PVD, history of malignancy, physical stress as a trigger factor, and receiving intubation/noninvasive ventilation were all independently associated with increased mortality on long-term follow-up (Table 7).
Table 7.
Cox regression analysis of factors affecting long-term all-cause mortality of patients with Takotsubo cardiomyopathy
| Covariate | HR | 95% CI | P |
|---|---|---|---|
| Age at diagnosis | 1.03 | 1.02–1.04 | < 0.001 |
| Female sex | 0.71 | 0.51–0.97 | 0.03 |
| Diabetes mellitus | 1.38 | 1.05–1.83 | 0.02 |
| CKD | 1.47 | 1.06–2.04 | 0.02 |
| COPD | 1.44 | 1.11–1.86 | 0.006 |
| CAD | 1.30 | 0.94–1.80 | 0.12 |
| PVD | 1.48 | 1.03–2.13 | 0.03 |
| Atrial fibrillation | 1.12 | 0.72–1.75 | 0.60 |
| History of malignancy | 2.05 | 1.52–2.75 | < 0.001 |
| Ejection fraction < 35% | 1.11 | 0.85–1.44 | 0.44 |
| Physical stress as a trigger factor | 1.75 | 1.33–2.31 | < 0.001 |
| Intubation/noninvasive ventilation | 1.66 | 1.14–2.11 | 0.006 |
| Cardiac arrest | 1.16 | 0.35–3.81 | 0.81 |
| ACE-Is/ARB on discharge | 0.90 | 0.70–1.16 | 0.42 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; PVD, peripheral vascular disease.
Patients who were discharged on ACE-Is/ARBs had lower mortality rates (hazard ratio 0.84 [95% confidence interval 0.57-0.98]; P = 0.03). However, on Cox regression analysis, discharging patients on ACE-Is/ARBs did not predict improved outcome during long-term follow-up. No significant difference in mortality was present between patients who were discharged on beta-blockers compared to those not on beta-blockers. The overall incidence of recurrent TC was 1.8%. Men were less likely to have recurrence than women (1.1% vs 3.6%; P = 0.04).
Discussion
The major findings in this large retrospective study of patients in a VA healthcare system are the following: (i) men had higher in-hospital mortality than women; (ii) no significant difference occurred between men and women in the combined outcome of stroke and death or in readmission rate at 30 days after discharge; and (iii) men had higher all-cause mortality but lower recurrence rate in long-term follow-up than women.
To our knowledge, this study includes the largest cohort of men with TC (n = 444).7,9,10,18, 19, 20, 21
Clinical characteristics/trigger factors
In our cohort, the men were older and had higher rates of medical comorbidities on presentation, including CAD, consistent with the findings of previous studies.7,10 Also significant differences were present in trigger factors between men and women. Previous studies have shown conflicting results regarding the predominant trigger factor in patients diagnosed with TC.7,9,10,19 In our study, physical stress was more commonly identified as the trigger factor in both men and women, but it was more common in men. The reason is unclear, but in previous studies, a physical trigger was associated with higher levels of norepinephrine in patients with Takotsubo, which might play a role in the pathophysiology of the disease.22 We also observed a high proportion of patients in both groups with no identifiable trigger factor, consistent with previous reports.7 Similar results were obtained when we compared men above the age of 51 years to women in a similar age group (above the median age of menopause). More than 60% of the men in our study presented with symptoms other than chest pain; this finding is contrary to results of previous studies that showed chest pain was the presenting symptom in men in 57%-100% of patients.7,9,19 This discrepancy is likely due to the fact that more men developed TC in the midst of other medical or surgical illness, making symptom elicitation challenging. However, a true sex difference cannot be ruled out. This finding was consistent with TC triggered by an exacerbation of an underlying disorder or by a medical/surgical procedure.18 Patients with an emotional or no trigger factor were more likely to present with chest pain than were patients with a physical trigger, and this finding was similar for men and women in our study.
In-hospital outcomes
Men with TC required higher rates of use of invasive and noninvasive ventilation and catecholamines. Length of stay was longer for men, and they were more likely to sustain a cardiac arrest or die in the hospital. This finding is consistent with those of a previous large multicentre study by Templin et al.7 A very recently published study by Arcari et al. also found higher in-hospital mortality, increased frequency of cardiogenic shock, and longer length of stay in men, compared to women.21 In addition, in a previous study, patients with TC who developed cardiac arrest were more likely to be men.23 Although the reason is unclear, a higher number of medical comorbidities in men might be a contributing factor. In a previous small study of patients diagnosed with TC in the intensive care unit, half of the patients were men.24 A point of interest is that men were less likely than women to receive coronary angiography during their hospitalization; we think this difference might have been related to the aversion of some clinicians to perform invasive procedures in patients who are more acutely ill at diagnosis, such as the men in our cohort. In addition, male sex was a predictor of in-hospital mortality on multivariate analysis in our study. Prior studies have conflicting results regarding in-hospital mortality. Weidner et al. and Schneider et al. showed no difference in in-hospital mortality for men vs women; neither study evaluated independent predictors for in-hospital mortality.9,13 Murakami et al. found that male sex was an independent predictor of in-hospital composite cardiac events, whereas Budnik et al. found that sex was not a predictor of in-hospital complications, in a univariate analysis.14,15 Brinjikji et al. identified male sex as an independent predictor of in-hospital mortality, but patients included in the study, and outcomes, were based on chart coding only.11 Templin et al. reported male sex and a physical trigger as predictors for their composite endpoint (catecholamine use, cardiogenic shock, use of invasive/noninvasive ventilation, use of cardiopulmonary resuscitation, and death). But male sex was not an independent predictor on multivariate analysis. Of note, age above 70 years was found to be an independent predictor of better outcomes in the same study.7 Male sex was identified as an independent predictor of in-hospital mortality on multivariate analysis in the German Italian Spanish Takotsubo (GEIST) Registry. 21
Short-term outcomes
At 30-day follow-up, male sex was not an independent predictor of mortality in our study. Previous studies have conflicting data. Increased mortality rates in the matched cohort of men at 60 days, compared to women, was reported in the GEIST Registry.21 By contrast, male sex was not an independent predictor in the International Takotsubo Registry at 30 days.20
Long-term outcomes
In our study, men were less likely than women to have recurrence on long-term follow-up (1.1% vs 3.6%; P = 0.04). Previous studies have shown conflicting data regarding recurrence rate for men vs women. No difference in recurrence rate was present between men and women in the GEIST Registry and in the International Takotsubo Registry (0.8% vs 1.9%; P = 0.22),7,21 but in a meta-analysis, nearly all cases of recurrence occurred in women who received 72 months of follow-up.25 An explanation might be the repetitive nature of emotional stress that could trigger recurrent episodes in women.
A limited number of studies have been done on long-term outcomes in patients with TC. In our study, men had higher overall mortality than women. Other independent predictors of mortality in our study, aside from male sex, were age at diagnosis of TC, diabetes mellitus, CKD, COPD, PVD, history of malignancy, physical stress as a trigger factor, and receiving intubation/noninvasive ventilation during the hospital admission for the TC diagnosis. Weidner et al.13 assessed long-term mortality in patients with TC, and male sex and EF < 35% were identified as independent predictors for all-cause mortality, but the study had a small number of men (n = 16). In our study, EF < 35% at diagnosis was not an independent predictor of mortality. In the recently published study involving patients in the GEIST Registry, male sex, age, diabetes, pulmonary disease, malignancies, physical trigger, low EF, and cardiogenic shock were identified as independent predictors of long-term mortality.21 The long-term mortality rate was higher in our study, compared to that in the GEIST Registry. This difference could be due to the higher number of patients with significant lung disease and physical triggers in our study. The independent predictors of long-term mortality in the International Takotsubo Registry were male sex, age > 70 years, malignancies, EF < 45%, physical triggers, and no triggers.20
Limitations
Despite its large size and multicentre cohort, our study was based on retrospective record review and thus is prone to bias. ICD codes were used to identify cases, so some cases may not have been identified. However, this limitation affects both groups, and therefore is unlikely to affect the results. In contrast to studies that used ICD codes for adjudicating outcomes, patient-level data and outcomes were manually extracted in our study. This manual review allowed us to identify outcomes in cases in which patients were admitted outside the VA system, because we had access to scanned records and could collect follow-up data, due to the ability to track individual patients across the healthcare system, even when they moved to a different city. However, some events could have been missed. Multivariate analysis for inpatient mortality was underpowered due to the low number of events compared to the number of variables included.
In addition, due to the predominance of men in the population at the VA, the study cohort might not be representative of the general population. Patients were included only if they met the latest criteria for TC diagnosis, but cases of coronary spasm and myocardial infarction with nonobstructive coronary arteries (MINOCA) could have been included due to the limitations of the criteria.
Conclusions
Men diagnosed with TC are more likely than women to have a physical trigger, become more acutely ill, have a more complicated hospital course, and have higher mortality rates on long-term follow-up. However, women were more likely to have recurrent TC and were more likely to present with chest pain. Future prospective studies should focus on the mechanisms of these differences to elucidate how sex affects the TC clinical course, which may help physicians make better treatment decisions and more accurate prognoses.
Acknowledgments
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The Central Arkansas VA Health System’s institutional review board approved the study.
See page 127 for disclosure information.
References
- 1.Prasad A., Lerman A., Rihal C.S. Apical ballooning syndrome (Tako-tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Sato H., Uchida T., Dote K., Ishihara M. Tokyo Kagakuhyoronsha Publ; 1990. Tako-tsubo-like left ventricular dysfunction due to multivessel coronary spasm; pp. 56–64. [Google Scholar]
- 3.Suzuki H., Matsumoto Y., Kaneta T., et al. Evidence for brain activation in patients with Takotsubo cardiomyopathy. Circ J. 2014;78:256–258. doi: 10.1253/circj.cj-13-1276. [DOI] [PubMed] [Google Scholar]
- 4.Akashi Y.J., Nakazawa K., Sakakibara M., et al. 123I-MIBG myocardial scintigraphy in patients with “Takotsubo” cardiomyopathy. J Nucl Med. 2004;45:1121–1127. [PubMed] [Google Scholar]
- 5.Naegele M., Flammer A.J., Enseleit F., et al. Endothelial function and sympathetic nervous system activity in patients with Takotsubo syndrome. Int J Cardiol. 2016;224:226–230. doi: 10.1016/j.ijcard.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Pelliccia F., Kaski J.C., Crea F., Camici P.G. Pathophysiology of Takotsubo syndrome. Circulation. 2017;135:2426–2441. doi: 10.1161/CIRCULATIONAHA.116.027121. [DOI] [PubMed] [Google Scholar]
- 7.Templin C., Ghadri J.R., Diekmann J., et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 8.Bybee K.A., Kara T., Prasad A., et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141:858–865. doi: 10.7326/0003-4819-141-11-200412070-00010. [DOI] [PubMed] [Google Scholar]
- 9.Schneider B., Athanasiadis A., Stöllberger C., et al. Gender differences in the manifestation of Tako-tsubo cardiomyopathy. Int J Cardiol. 2013;166:584–588. doi: 10.1016/j.ijcard.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Agdamag A.C., Patel H., Chandra S., et al. Sex differences in Takotsubo syndrome: a narrative review. J Women’s Heal. 2020;29:1122–1130. doi: 10.1089/jwh.2019.7741. [DOI] [PubMed] [Google Scholar]
- 11.Brinjikji W., El-Sayed A.M., Salka S. In-hospital mortality among patients with Takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164:215–221. doi: 10.1016/j.ahj.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Khera R., Light-Mcgroary K.A., Zahr F., Horwitz P.A., Girotra S. Trends in hospitalization for Takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53–63. doi: 10.1016/j.ahj.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weidner K.J., El-Battrawy I., Behnes M., et al. Sex differences of in-hospital outcome and long-term mortality in patients with Takotsubo cardiomyopathy. Ther Clin Risk Manag. 2017;13:863–869. doi: 10.2147/TCRM.S131760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murakami T., Yoshikawa T., Maekawa Y., et al. Gender differences in patients with Takotsubo cardiomyopathy: multi-center registry from Tokyo CCU Network. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budnik M., Nowak R., Fijałkowski M., et al. Sex-dependent differences in clinical characteristics and in-hospital outcomes in patients with Takotsubo syndrome. Polish Arch Intern Med. 2019;130:25–30. doi: 10.20452/pamw.14970. [DOI] [PubMed] [Google Scholar]
- 16.National Center for Veterans Analysis and Statistics Profile of veterans: 2017. 2019. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2017.pdf Available at:
- 17.Ghadri J.-R., Wittstein I.S., Prasad A., et al. International expert consensus document on Takotsubo syndrome (part i): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39:2032–2046. doi: 10.1093/eurheartj/ehy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuchihashi K., Ueshima K., Uchida T., et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. J Am Coll Cardiol. 2001;38:11–18. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 19.Wittstein I.S., Thiemann D.R., Lima J.A.C., et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 20.Ghadri J.R., Kato K., Cammann V.L., et al. Long-term prognosis of patients with Takotsubo syndrome. J Am Coll Cardiol. 2018;72:874–882. doi: 10.1016/j.jacc.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Arcari L., Núñez-Gil I.J., Stiermaier T., et al. Gender differences in Takotsubo syndrome. J Am Coll Cardiol. 2022;79:2085–2093. doi: 10.1016/S0735-1097(22)03076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sobue Y., Watanabe E., Ichikawa T., et al. Physically triggered Takotsubo cardiomyopathy has a higher in-hospital mortality rate. Int J Cardiol. 2017;235:87–93. doi: 10.1016/j.ijcard.2017.02.090. [DOI] [PubMed] [Google Scholar]
- 23.Gili S., Cammann V.L., Schlossbauer S.A., et al. Cardiac arrest in Takotsubo syndrome: results from the InterTAK Registry. Eur Heart J. 2019;40:2142–2151. doi: 10.1093/eurheartj/ehz170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haghi D., Fluechter S., Suselbeck T., et al. Takotsubo cardiomyopathy (acute left ventricular apical ballooning syndrome) occurring in the intensive care unit. Intensive Care Med. 2006;32:1069–1074. doi: 10.1007/s00134-006-0111-z. [DOI] [PubMed] [Google Scholar]
- 25.Singh K., Carson K., Usmani Z., et al. Systematic review and meta-analysis of incidence and correlates of recurrence of Takotsubo cardiomyopathy. Int J Cardiol. 2014;174:696–701. doi: 10.1016/j.ijcard.2014.04.221. [DOI] [PubMed] [Google Scholar]