Mutations in the fms-like tyrosine kinase 3 (FLT3)-internal tandem duplication (ITD) and tyrosine kinase domain (TKD) are common in acute myeloid leukemia (AML), accounting for 30% and 7% of AML, respectively. These patients had an unfavorable prognosis even after allogeneic stem cell transplantation (SCT).1 However, in a recent report of the long-term follow-up of the ADMIRAL trial,2 gilteritinib maintenance therapy was associated with better survival rates than salvage chemotherapy in patients with relapsed or refractory (R/R) FLT3-mutated AML. Notably, 40% of patients (16/40) who received allogeneic SCT followed by gilteritinib maintenance survived for 2 years without relapse, whereas only 8% of patients who received SCT without gilteritinib maintenance survived, suggesting that gilteritinib maintenance prevents posttransplant relapse even in R/R settings. However, the survival rates of patients receiving posttransplant FLT3-inhibitors maintenance therapy in the R/R setting (ADMIRAL and QuANTUM-R3 [quizartinib maintenance]) were not as high as those of 2 studies conducted mainly in the first complete remission (CR) patients (2-year and 18-months relapse-free survival rates of 85% and 89% in RADIUS4 [midostaurin maintenance] and SORMAIN5 [sorafenib maintenance], respectively). Thus, to further improve the outcomes in patients with R/R, optimization of FLT3-inhibitor administration based on the biological characteristics of R/R FLT3-mutated AML may be required in clinical practice. In this study, we evaluated the effects of early initiation of post-SCT gilteritinib maintenance in patients with R/R FLT3-mutated AML.
We retrospectively analyzed 25 cases with R/R FLT3-mutated AML who received allogeneic SCT at our center between 1 January 2011 and 30 April 2022 (Table 1). Twenty-two patients had ITD mutations, and 5 had TKD mutations (2 had both). The planned duration of gilteritinib maintenance therapy was 2 years after SCT. The details of the methods are described in supplemental Methods. All patients attained CR after SCT, and none of the patients relapsed or died within 30 days after SCT. Fourteen patients received gilteritinib maintenance therapy after SCT, whereas 11 did not. Between the 2 groups, the characteristics, AML status, and SCT procedures were well balanced, except for the pre-SCT administration of FLT3-inhibitors. All 14 patients in the gilteritinib maintenance group and 5 of 11 patients in the non-maintenance group were administered FLT3-inhibitors before SCT as salvage therapy for R/R disease. Patients in the gilteritinib maintenance group were generally heavily treated (50% and 21.4% were in second CR/third CR/non-CR and second SCT/third SCT, respectively). Eleven (78.6%) patients were non-CR or measurable residual disease (MRD)-positive before SCT and remained MRD-positive post-SCT. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This study was approved by the institutional review board (2209-005).
Table 1.
Patient characteristics
| All patients |
With gilteritinib |
Without gilteritinib |
||
|---|---|---|---|---|
| Factors | n = 25 | n = 14 | n = 11 | P value |
| Age, y (median range]) | 52 (25-72) | 50 (32-72) | 53 (25-70) | .94 |
| Transplantation years before 2018, n (%) | 6 (24%) | 2 (14.3) | 4 (36.4) | .35 |
| WBC count at SCT, /μL (median range]) | 110 (0-6420) | 245 (0-2960) | 80 (0-6420) | .48 |
| Hb at SCT, g/dL (median [range]) | 8.6 (6.8-13.2) | 8.6 (6.8-13.2) | 8.7 (7.1-12.2) | .93 |
| Plt at SCT, 104/μL (median [range]) | 4.2 (1.5-33.2) | 5.0 (1.5-11.5) | 3.9 (2.2-33.2) | .96 |
| LDH at SCT, U/L (median [range]) | 244 (98-512) | 285 (124-512) | 190 (98-322) | .007 |
| sCre at SCT, mg/dL (median [range]) | 0.53 (0.32-0.85) | 0.56 (0.32-0.85) | 0.52 (0.4-0.77) | .41 |
| T-Bil at SCT, mg/L (median [range]) | 0.69 (0.29-2.3) | 0.76 (0.4-1.17) | 0.58 (0.29-2.3) | .17 |
| Conventional karyotype, n (%) | ||||
| Normal karyotype | 17 (68.0) | 10 (71.4) | 7 (63.6) | 1 |
| FLT3-ITD/TKD, n (%) | ||||
| ITD | 20 (80.0) | 11 (78.6) | 9 (81.8) | 1 |
| TKD | 3 (12.0) | 2 (14.3) | 1 (9.1) | |
| Both | 2 (8.0) | 1 (7.1) | 1 (9.1) | |
| Previous administration of FLT3-inhibitor before SCT, n (%) | ||||
| YES | 19 (76.0) | 14 (100) | 5 (45.5) | .003 |
| AML status at SCT, n (%) | ||||
| CR, WT1 neg | 6 (24.0) | 3 (21.4) | 3 (27.3) | .44 |
| CR, WT1 pos | 12 (48.0) | 9 (64.3) | 4 (36.4) | |
| non-CR | 6 (24.0) | 2 (14.3) | 4 (36.4) | |
| Number of SCT, n (%) | ||||
| First SCT | 19 (76.0) | 11 (78.6) | 8 (72.7) | .79 |
| Second SCT | 5 (20.0) | 2 (14.3) | 3 (27.3) | |
| Third SCT | 1 (4.0) | 1 (7.1) | 0 (0.0) | |
| Conditioning intensity, n (%) | ||||
| MAC | 17 (68.0) | 9 (64.3) | 8 (72.7) | 1 |
| HCT-CI (%)∗ | ||||
| 0 | 4 (20.0) | 2 (25.0) | 2 (16.7) | .88 |
| 1 | 11 (55.0) | 5 (62.5) | 6 (50.0) | |
| 2 | 2 (10.0) | 1 (12.5) | 1 (8.3) | |
| 3 ≤ | 3 (15.0) | 0 ( 0.0) | 3 (27.3) | |
| Donor selection, n (%) | ||||
| 8/8 matched related | 5 (20.0) | 3 (21.4) | 2 (18.2) | .85 |
| 8/8 matched unrelated | 3 (12.0) | 1 (7.1) | 2 (18.2) | |
| 7/8 mismatched unrelated | 1 (4.0) | 1 (7.1) | 0 (0.0) | |
| ≤6/8 haplo-identical | 7 (28.0) | 3 (21.4) | 4 (36.4) | |
| Cord blood | 9 (36.0) | 6 (42.9) | 3 (27.3) | |
| Post-SCT status at day 28-45, n (%) | ||||
| CR, MRD neg | 8 (32.0) | 3 (21.4) | 5 (45.5) | .14 |
| CR, MRD pos | 16 (64.0) | 11 (78.6) | 5 (45.5) | |
| CR, not MRD evaluated | 1 (4.0) | 0 (0.0) | 1 (9.1) |
| Factors at gilteritinib administration post-SCT | ||||
|---|---|---|---|---|
| WBC count/μL (median [range]) | 3960 (1300-6830) | |||
| Hb, g/dL (median [range]) | 9.1 (7.5-11.3) | |||
| Plt, 104/μL (median [range]) | 5.8 (1.4-21.0) | |||
| Antifungal drug at initiation of gilteritinib | ||||
| FLCZ | 7 (50.0) | |||
| VRCZ | 2 (14.3) | |||
| PSCZ | 2 (14.3) | |||
| L-Amb | 1 (7.1) | |||
| MCFG | 2 (14.3) | |||
| Days from SCT to gilteritinib administration (median [range]) | 36 (21-110) | |||
| Gilteritinib initial dose, mg/day, n (%) | ||||
| 40 | 8 (57.1) | |||
| 80 | 3 (21.4) | |||
| 120 | 3 (21.4) | |||
| Gilteritinib maximum dose, mg/d (median [range]) | 120 (40-120) | |||
AML, acute myeloid leukemia; FLCZ, fluconazole; FLT3-ITD/TKD, fms-like tyrosine kinase 3-internal tandem duplication/tyrosine kinase domain; Hb, hemoglobin; HCT-CI, hematopoietic cell transplant-comorbidity index; MAC, myeloablative conditioning regimen; MCFG, micafungin; L-Amb, liposomal amphotericin B; LDH, lactate dehydrogenase; Plt, platelet; PSCZ, posaconazole; sCre, serum creatinine; SCT, stem-cell transplantation; T-Bil, total bilirubin; VRCZ, voriconazole; WBC, white blood cell.
n = 20
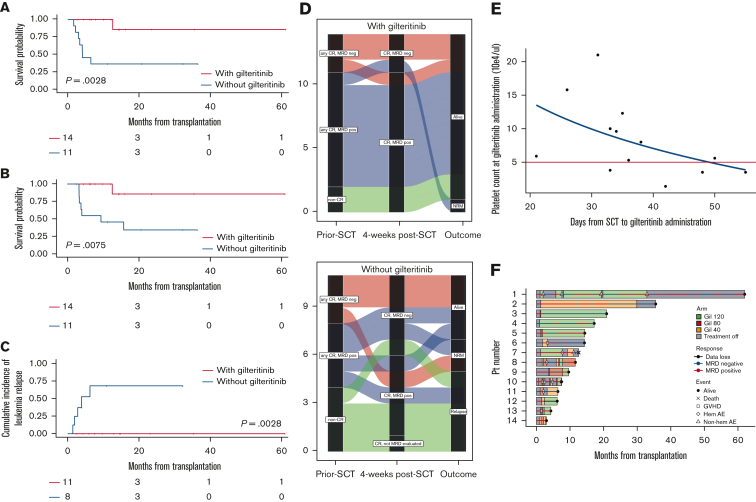
The median follow-up period was 11.2 months, whereas the median and 1-year RFS were not reached (NR) and 70.3% (95% confidence interval [CI], 47.3-84.7), respectively. The median and 1-year overall survival (OS) were NR and 73.9% (95% CI, 50.6-87.4), respectively. Patients with gilteritinib maintenance showed significantly longer RFS and OS compared with those without gilteritinib maintenance (1-year RFS, 100% vs 36.4% [95% CI, 100-100 vs 11.2-62.7; P = .0028], Figure 1A; and 1-year OS, 100% vs 45.5% [95% CI, 100-100 vs 16.7-70.7; P = .0075], Figure 1B). To avoid time-lead bias, we reanalyzed the survival curve using a time-dependent covariate hazard model. Patients in the gilteritinib maintenance group showed longer RFS and OS compared with those without gilteritinib (RFS hazard ratio [HR], 0.089; P = .004 and OS HR, 0.20; P = .069, supplemental Figure 1). In the gilteritinib maintenance group, none of the patients relapsed until the last follow-up, and 1 patient died of hemolytic anemia of unknown etiology during AML remission. In the group without gilteritinib maintenance, 5 patients relapsed and 7 died. The median RFS and OS of this group were 6.5 months (95% CI, 2.2-NR) and 9.5 months (95% CI, 3.3 months-NR), respectively. In particular, among patients with MRD-positive or non-CR before SCT (n = 19), patients with gilteritinib maintenance showed a lower 1-year cumulative incidence of AML relapse than those without gilteritinib maintenance (0% vs 68.8%, 95% CI, 0-0 vs 35.9-95.2, P = .0028, Figure 1C). Meanwhile, none of the patients with an MRD-negative presentation before SCT relapsed. In addition, when only patients who received their first SCT were analyzed (n = 19), patients who maintained gilteritinib (n = 11) had a 1-year longer RFS and OS than those who did not use gilteritinib (n = 8) (1-year RFS, 100% vs 50%; P = .059, and 1-year OS, 100% vs 50%; P = .087, supplemental Figure 2).
Figure 1.
Clinical impacts of early initiation of low-dose gilteritinib maintenance on posttransplant outcomes in patients with R/R FLT3mut AML. (A) RFS from transplantation (1-year RFS, 100% [95% CI, 100-100] in the gilteritinib group vs 36.4% [95% CI, 11.2-62.7] in the non-gilteritinib group; P = .0028). The median RFS in the gilteritinib and non-gilteritinib group was NR and 6.5 months (95% CI, 2.2-NR), respectively. (B) OS after transplantation (1-year OS, 100% [95% CI, 100-100] in the gilteritinib group vs 45.5% [95% CI, 16.7-70.7] in the non-gilteritinib group; P = .0075). The median OS in the gilteritinib and non-gilteritinib group was NR and 9.5 months (95% CI, 3.3-NR), respectively. (C) Cumulative relapse rate in patients with MRD-positive or non-CR before SCT (n = 19) (1-year relapse rate, 0% [95% CI, 0-0] in the gilteritinib group vs 68.8% [95% CI, 35.9-95.2] in the non-gilteritinib group, P = .0028). (D) Alluvial diagrams according to pre-SCT status, post-SCT status at week 4 (day 28-45), and outcome with gilteritinib maintenance therapy (upper panel) or not (lower panel). Each line indicates the clinical course of the patient. No patient with residual MRD or non-CR pre-SCT in the gilteritinib group (blue and green lines) relapsed, whereas 5 patients in the non-gilteritinib group (blue and green lines) relapsed. Moreover, none of the patients with MRD-positive post-SCT in the gilteritinib group relapsed, whereas 3 patients with MRD-positive post-SCT in the non-gilteritinib group relapsed. (E) Scatterplot of platelet counts during gilteritinib maintenance therapy post-SCT and days of resumption after SCT. A non-linear regression was observed. Five patients restarted gilteritinib treatment when their platelet count was less than 50 000/μL (area under the red line). One patient who resumed gilteritinib 110 days after SCT is not shown in this figure. (F) Swimmer plot of patients who received gilteritinib maintenance therapy. We observed 14 discontinuations in 7 patients (50.0%), mainly due to thrombocytopenia (n = 3), neutropenia (n = 2), anemia (n = 1), and acute GVHD (n = 2). Patient 7 died of hematolytic anemia without leukemia relapse. The cause of hemolytic anemia was considered potential graft-versus-host effect rather than gilteritinib adverse events. No other factors of hemolytic anemia, such as collagen disease, parvovirus B19, other viral reactivation or infections, secondary malignancies, Coombs test, drug, and disseminated intravascular coagulation, were detected.
Alluvial diagrams illustrate the pre- and post-SCT status and outcomes according to whether gilteritinib was maintained (Figure 1D, upper panel) or not (Figure 1D, lower panel). Eleven patients in CR with MRD-positive post-SCT in the gilteritinib group survived without relapse. Notably, 2 patients with non-CR before SCT were alive without relapse (green flow). In contrast, in the group without gilteritinib maintenance, all 4 patients with non-CR before SCT relapsed.
To clarify the characteristics of the administration method in our practice, we examined the correlation between the timing of the resumption of gilteritinib administration and the platelet count (Figure 1E). We started gilteritinib as soon as the platelet count exceeded 50,000/μL. In addition, even in patients whose platelets persisted below 50,000/μL 40 days posttransplant, low-dose gilteritinib was started to prevent early relapse. The median time from SCT to the initiation of post-HSCT gilteritinib maintenance therapy was 36 days (range, 21-110 days). Most patients started on reduced doses of gilteritinib (median, 40 mg/day; range, 40-120 mg/day), which were gradually increased with intermittent dose interruptions as needed. The duration of gilteritinib administration and its adverse events in each patient are shown in the Swimmer plot in Figure 1F. We observed 14 discontinuations in 7 patients (50.0%), mainly due to thrombocytopenia (n = 3), neutropenia (n = 2), anemia (n = 1), and acute graft-versus-host disease (GVHD) (n = 2) (supplemental Table 1). None of the patients relapsed during temporal gilteritinib discontinuation. Late engraftment failure was not observed during the gilteritinib maintenance therapy.
In this study, we showed the results suggesting that early initiation of gilteritinib maintenance, even at a low starting dose, could improve the prognosis of patients with R/R FLT3-mutated AML even after adjusting for a time-dependent covariate hazard model to avoid time-lead bias. To determine the factors that led to our method of improving clinical outcomes, we compared our practice of gilteritinib maintenance with previous clinical studies.
First, gilteritinib restarted earlier than in the ADMIRAL trial (median initiating day, 36 days in our study vs 55 days in the ADMIRAL trial). Considering that the actual median resumption was day 55 in patients who entered the ADMIRAL protocol, which prescribed the resumption of gilteritinib by day 60, it was assumed that the majority of patients in the trial resumed gilteritinib after day 50. In contrast, our cohort resumed gilteritinib by day 30 to 40 in most cases, nearly 3 weeks earlier than the ADMIRAL trial. Because relapse frequently increases 30 days after transplant, especially in patients with MRD-positive FLT3-mutated AML,5 early initiation of gilteritinib maintenance could be preferable for residual disease control. One concern with resuming gilteritinib treatment soon after transplantation is that it may induce thrombocytopenia. However, gilteritinib is less active than other FLT3-inhibitors in terms of suppressing the signals required for normal hematopoiesis, such as canonical FLT3 and KIT,6 allowing the restart of gilteritinib even with a low platelet count (<50 000/μL). Indeed, some patients in our cohort required temporary gilteritinib discontinuation because of thrombocytopenia, but none of the patients relapsed during discontinuation. Modification of gilteritinib dosing and timing in patients with thrombocytopenia could allow for early administration of gilteritinib, which may contribute to the suppression of early relapse. Second, most patients in our study started on reduced doses of gilteritinib (median 40 mg), which were gradually increased to 120 mg. In the ADMIRAL trial, most enrolled patients were prescribed a 120 mg dose for post-SCT maintenance, except for 18% (7/40) who had reduced doses because of adverse events. Because CYP-competing agents, including antifungal drugs, are commonly used in the early posttransplant period, dose adjustment using a step-up method may be effective for the safe continuation of maintenance therapy.
The biological rationale for the early initiation of post-SCT gilteritinib maintenance therapy may not only be the direct anti-tumor effect on FLT3-mutated AML cells but also indirect immune-based effects. Recent murine studies have reported that FLT3-inhibitors attenuated the expression of PD-1 and TIGIT in donor CD8+ T-cell and enhance graft-versus-leukemia (GVL) effects by increasing IL-15 production in vivo in the acute phase post-SCT.7,8 In the early posttransplant period, GVL enhancement is particularly necessary because of the survival of residual tumor cells and immune exhaustion in donor T-cells.9 Gilteritinib may act on the tumor microenvironment and donor T-cell immunity to reinforce GVL in this important phase of SCT, which might finally eliminate the residual MRD-positive disease. Moreover, considering that we reduced the gilteritinib dose in many cases, the actual dose sufficient to induce GVL should be reconsidered in a clinical setting.
In contrast, the early administration of gilteritinib did not exacerbate GVHD; only 2 patients (14.3%) temporarily discontinued gilteritinib due to acute GVHD of the skin, and 4 patients (28.6%) developed controllable moderate-to-severe cGVHD (data not shown), similar to that in previous clinical trials.2,4,5,10 This may suggest the GVL-specific immune effect by the early gilteritinib administration as the murine study showed.8
In this study, we proposed the early start of gilteritinib maintenance to prevent early relapse; however, the appropriate duration of gilteritinib maintenance to prevent late relapse remains unclear. A previous study exploring sorafenib maintenance posttransplant demonstrated that relapses after the discontinuation of 2-years of sorafenib maintenance were not observed in patients without MRD at transplant and increased in patients with MRD at transplant.5 To think of the data, especially in cases with MRD, it may be desirable to continue maintenance therapy even beyond 2 years, as FLT3 inhibitors can act on the residual tumor to produce IL-15 and maintain GVL.7,8 However, our data at this time cannot definitively support the hypothesis because this study focuses on maintenance therapy in the early period post-SCT and includes only 2 patients who survive longer than 2 years (the median treatment duration of 6.5 months). To explore the maintenance effect until the late period, further long-term follow-up study is warranted.
This study was limited by its retrospective and single-center nature, without a validation analysis in independent cohorts. First, the non-maintenance group included more patients who received SCT in the early time period when gilteritinib was not approved than the maintenance group, although no statistical significance was observed. Because transplant outcome has continued to improve over time, the difference in the transplantation period is considered a potential bias in this study. Second, approximately half of the patients in the nonmaintenance group received FLT3 inhibitors before SCT, as compared with all patients in the maintenance group. Pre-SCT administration of FLT3 inhibitors may not only contribute to reducing MRD but may also make transplantation safer by avoiding cytotoxic chemotherapy until just before SCT. In other words, the results suggest the advantages of using gilteritinib not only after SCT but also before SCT for patients with R/R, and further studies are expected in the future. Nine patients (40.9%) presented with an unknown FLT3-ITD allelic ratio, which was another limitation; the updated European LeukemiaNet guideline excluded the allelic ratio from the risk classification.11
In conclusion, our study showed preliminary but promising results for the early initiation of gilteritinib maintenance in patients with FLT3-mutated AML post-SCT in clinical practice. Despite poor patient backgrounds and MRD-positive peri-SCT statuses, early initiation of gilteritinib maintenance with reduced doses resulted in improved RFS and OS. Based on these results, we would suggest careful adjustments to the starting timing and dose modification of gilteritinib maintenance therapy for each patient to further improve the outcome of patients with R/R FLT3-mutated AML with residual MRD peri-SCT. Subsequent studies are warranted to validate our results and to elucidate the detailed mechanisms underlying our new insights.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
Acknowledgments: The authors thank the patients with hematologic malignancy, their families, and the medical staff of the Department of Hematology and Oncology of Okayama University Hospital. They also thank Hiroki Kobayashi (Department of Hematology and Oncology, Okayama University Hospital) and Kentaro Narita (Department of Hematology and Oncology, Kameda Medical Center) for their advice from a statistical perspective and Editage (https://www.editage.jp/) for English language editing. The authors did not receive financial support from any organization for the submitted work. This work was supported by a research fund from the Japan Society for the Promotion of Science KAKENHI (grant number 26461449).
Contributions: T.T. conceived and designed the study, collected data, performed the statistical analysis, wrote the manuscript, and provided patient care; K.-i.M. designed and supervised the research and edited the manuscript; H.U., A.M., C.M., K.K., T.K., H.F., N.A., D.E., H.N., K.F., and N.F. collected the data and provided patient care; Y.M. supervised this study and edited the manuscript; and all authors reviewed and approved the manuscript.
Footnotes
Data generated during and/or analyzed during the current study are available upon reasonable request from authors, Toshiki Terao (tarao.toshiki.0127@gmail.com) and Ken-ich Matsuoka (k-matsu@md.okayama-u.ac.jp).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Brunet S, Labopin M, Esteve J, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30(7):735–741. doi: 10.1200/JCO.2011.36.9868. [DOI] [PubMed] [Google Scholar]
- 2.Perl AE, Larson RA, Podoltsev NA, et al. Follow-up of patients with R/R FLT3-mutation-positive AML treated with gilteritinib in the phase 3 ADMIRAL trial. Blood. 2022;139(23):3366–3375. doi: 10.1182/blood.2021011583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganguly S, Cortes JE, Krämer A, et al. Clinical outcomes in patients with FLT3-ITD-mutated relapsed/refractory acute myelogenous leukemia undergoing hematopoietic stem cell transplantation after quizartinib or salvage chemotherapy in the QuANTUM-R trial. Transplant Cell Ther. 2021;27(2):153–162. doi: 10.1016/j.bbmt.2020.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Maziarz RT, Levis M, Patnaik MM, et al. Midostaurin after allogeneic stem cell transplant in patients with FLT3-internal tandem duplication-positive acute myeloid leukemia. Bone Marrow Transplant. 2021;56(5):1180–1189. doi: 10.1038/s41409-020-01153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burchert A, Bug G, Fritz LV, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN) J Clin Oncol. 2020;38(26):2993–3002. doi: 10.1200/JCO.19.03345. [DOI] [PubMed] [Google Scholar]
- 6.Levis M, Perl AE. Gilteritinib: potent targeting of FLT3 mutations in AML. Blood Adv. 2020;4(6):1178–1191. doi: 10.1182/bloodadvances.2019000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathew NR, Baumgartner F, Braun L, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med. 2018;24(3):282–291. doi: 10.1038/nm.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Hasegawa Y, Hashimoto D, et al. Gilteritinib enhances graft-versus-leukemia effects against FLT3-ITD mutant leukemia after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2022;57(5):775–780. doi: 10.1038/s41409-022-01619-4. [DOI] [PubMed] [Google Scholar]
- 9.Asakura S, Hashimoto D, Takashima S, et al. Alloantigen expression on non-hematopoietic cells reduces graft-versus-leukemia effects in mice. J Clin Invest. 2010;120(7):2370–2378. doi: 10.1172/JCI39165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xuan L, Wang Y, Huang F, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised phase 3 trial. Lancet Oncol. 2020;21(9):1201–1212. doi: 10.1016/S1470-2045(20)30455-1. [DOI] [PubMed] [Google Scholar]
- 11.Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 ELN recommendations from an international expert panel. Blood. 2022;140(12):1345–1377. doi: 10.1182/blood.2022016867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.