This case-control study aims to quantify 1-year outcomes among individuals meeting a post–COVID-19 condition definition compared with a control group of individuals without COVID-19.
Key Points
Question
Do postacute sequelae of SARS-CoV-2 increase risks of 1-year adverse outcomes?
Findings
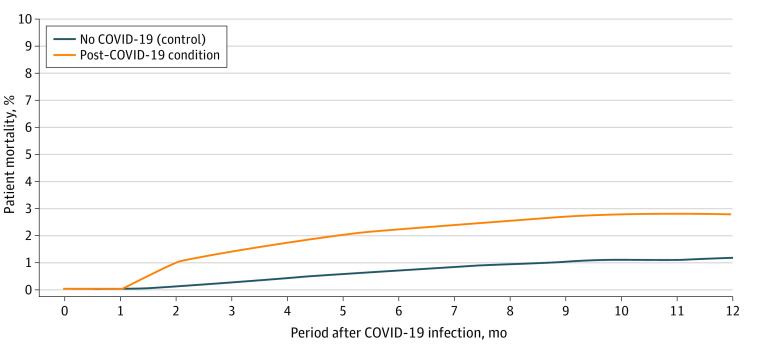
In this case-control study of 13 435 US adults with post–COVID-19 condition (PCC) and 26 870 matched adults without COVID-19, the adults with PCC experienced increased risks for a number of cardiovascular outcomes, such as ischemic stroke. During the 12-month follow-up period, 2.8% of the individuals with PCC vs 1.2% of the individuals without COVID-19 died, implying an excess death rate of 16.4 per 1000 individuals.
Meaning
Individuals with PCC may be at increased risk for adverse outcomes in the year following initial infection.
Abstract
Importance
Many individuals experience ongoing symptoms following the onset of COVID-19, characterized as postacute sequelae of SARS-CoV-2 or post–COVID-19 condition (PCC). Less is known about the long-term outcomes for these individuals.
Objective
To quantify 1-year outcomes among individuals meeting a PCC definition compared with a control group of individuals without COVID-19.
Design, Setting, and Participants
This case-control study with a propensity score–matched control group included members of commercial health plans and used national insurance claims data enhanced with laboratory results and mortality data from the Social Security Administration’s Death Master File and Datavant Flatiron data. The study sample consisted of adults meeting a claims-based definition for PCC with a 2:1 matched control cohort of individuals with no evidence of COVID-19 during the time period of April 1, 2020, to July 31, 2021.
Exposures
Individuals experiencing postacute sequelae of SARS-CoV-2 using a Centers for Disease Control and Prevention–based definition.
Main Outcomes and Measures
Adverse outcomes, including cardiovascular and respiratory outcomes and mortality, for individuals with PCC and controls assessed over a 12-month period.
Results
The study population included 13 435 individuals with PCC and 26 870 individuals with no evidence of COVID-19 (mean [SD] age, 51 [15.1] years; 58.4% female). During follow-up, the PCC cohort experienced increased health care utilization for a wide range of adverse outcomes: cardiac arrhythmias (relative risk [RR], 2.35; 95% CI, 2.26-2.45), pulmonary embolism (RR, 3.64; 95% CI, 3.23-3.92), ischemic stroke (RR, 2.17; 95% CI, 1.98-2.52), coronary artery disease (RR, 1.78; 95% CI, 1.70-1.88), heart failure (RR, 1.97; 95% CI, 1.84-2.10), chronic obstructive pulmonary disease (RR, 1.94; 95% CI, 1.88-2.00), and asthma (RR, 1.95; 95% CI, 1.86-2.03). The PCC cohort also experienced increased mortality, as 2.8% of individuals with PCC vs 1.2% of controls died, implying an excess death rate of 16.4 per 1000 individuals.
Conclusions and Relevance
This case-control study leveraged a large commercial insurance database and found increased rates of adverse outcomes over a 1-year period for a PCC cohort surviving the acute phase of illness. The results indicate a need for continued monitoring for at-risk individuals, particularly in the area of cardiovascular and pulmonary management.
Introduction
Among the many puzzling aspects of COVID-19 has been the variable recovery time and range of complications experienced by many individuals. Median recovery time for mild cases is approximately 2 weeks and for severe cases is 6 weeks.1 Numerous studies have identified a subset of patients for whom recovery time exceeds 6 weeks and who experience an increased risk of adverse outcomes.2,3,4 The condition among the subset of patients experiencing post–COVID-19 symptoms has been described in the lay press as “long COVID” and in the medical literature as experiencing postacute sequelae of SARS-CoV-2, or post–COVID-19 condition (PCC).5,6,7,8
The Centers for Disease Control and Prevention defines PCC as having new, returning, or ongoing health issues occurring more than 4 weeks after onset of initial infection.9 Estimates of PCC incidence vary widely, with published reports estimating that between 10% and 25% of symptomatic patients experience symptoms persisting beyond the acute phase of illness.10 A diagnosis of PCC is based on symptoms including fatigue, cough, pain (joint, throat, chest), loss of taste or smell, shortness of breath, thromboembolic conditions, neurocognitive difficulties, and depression.9
There are limitations to the initial studies assessing PCC rates and outcomes. Estimates were often based on hospitalized patients who had a higher severity of illness.11,12,13,14,15 Many reports were based on patient surveys that did not include comparison groups of similar individuals. Follow-up on individuals with milder cases has been difficult, given that individuals often self-manage. In addition, early reports were often research letters or field reports not subject to peer review.16,17 Finally, individuals at risk for PCC tend to have higher baseline risks due to preexisting conditions, resulting in selection bias for the exposure cohort.18,19,20
Subsequent to initial reports, additional work on PCC has been published, providing a more rigorous assessment of patient experiences. For example, a large panel survey study of symptomatic individuals with a matched control group showed that nearly half of respondents experienced lingering symptoms, most commonly fatigue, headache, and muscle weakness, even after 12 months.21 Other studies of PCC focused on neurological manifestations, depression, and anxiety.22,23
This case-control study provides a 12-month assessment of adverse outcomes for a cohort of individuals with PCC compared with a propensity-matched comparison group with similar baseline risks. The study provides a comprehensive view of individuals with and without initial hospitalizations. By leveraging a large health insurance claims database, we ascertained health status before the initial COVID-19 diagnosis, including assessment of baseline characteristics such as hypertension, obesity, depression, and chronic obstructive pulmonary disease (COPD). By using medical claims and socioeconomic variables, this study is unique in providing a comprehensive follow-up for a nationally representative sample. The findings will be useful in informing care coordination efforts for individuals with PCC, especially with respect to careful monitoring for cardiovascular and pulmonary risks after the period of acute infection.
Methods
Study Design and Data
We analyzed administrative claims and laboratory results from the HealthCore Integrated Research Environment, which contains medical, pharmacy, and laboratory data from 14 commercial health plans with members who reside in all 50 US states and the District of Columbia. This study was conducted in compliance with relevant provisions of the Health Insurance Portability and Accountability Act. Only deidentified data were used, and the study was determined to be exempt from review by the WCG Institutional Review Board. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines were followed.24
PCC Cohort
The PCC cohort was drawn from an initial pool of 249 013 individuals 18 years and older who were diagnosed with COVID-19 between April 1, 2020, and July 31, 2020.22,23 Patients were included if they had a medical claim with an International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnosis code for COVID-19 (U071, B342, B9721, B9729, J1281) or a laboratory-confirmed positive COVID-19 test. The first observed diagnosis or positive test date was set as the index date. Individuals were required to have 6 months of continuous enrollment prior to the index date and to have survived at least 30 days following their diagnosis date. An index month was set by adding 30 days to the COVID-19 diagnosis date. Continuous enrollment was not required beyond the initial 30 days, as mortality was included as an outcome measure. Characteristics of the full COVID-19 cohort vs PCC cohort are summarized in eTable 1 in Supplement 1.
A claims-based definition for PCC was constructed based on symptoms drawn from Centers for Disease Control and Prevention publications and other peer-reviewed publications.3,4,5,6,9 Individuals in the PCC cohort received 3 or more diagnoses for COVID-19 or COVID-19 symptoms across more than 1 visit during weeks 5 to 12 after their index date, inclusive of telehealth visits (see eTable 2 in Supplement 1 for ICD-10 codes used in the definition). In 2021, several ICD-10 codes were issued to capture PCC (M35.81, U09.9)25; however, they were not available during the time period used for defining the cohort. From the initial pool of individuals confirmed positive for COVID-19, 14 086 (6.8%) were identified as having PCC and meeting cohort inclusion criteria (Figure 1).
Figure 1. Sample Construction.
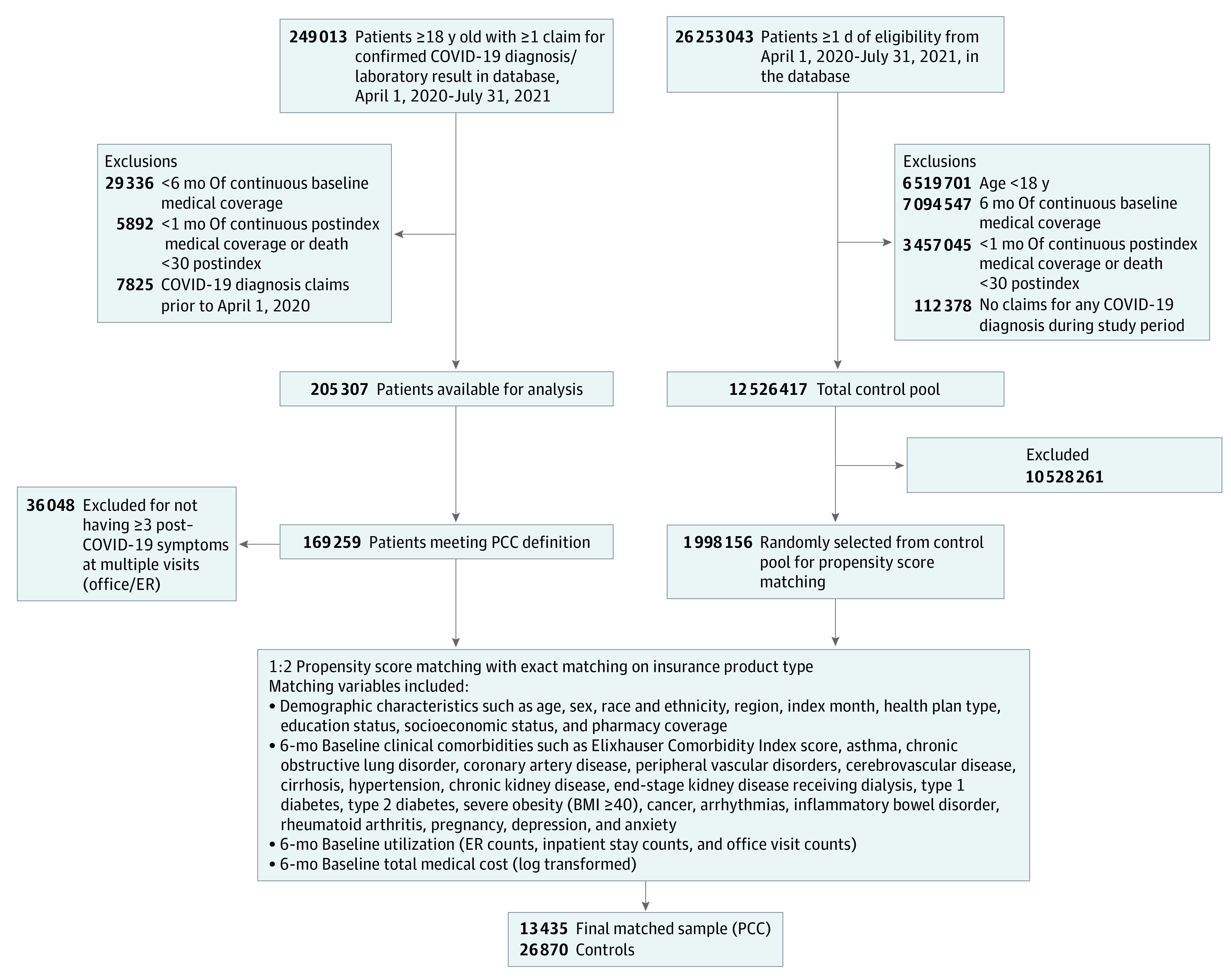
The sample construction diagram shows inclusion and exclusion criteria for assembly of the sample of individuals with post–COVID-19 condition (PCC) and matched controls without COVID-19. BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); ER, emergency room.
Propensity-Matched Control Group
The pre–COVID-19 health care utilization for the PCC cohort was used to create a matched cohort of individuals without COVID-19 (non–COVID-19 [NC]), allowing calculation of actual vs expected rates for postindex outcomes. An initial pool of potential control group members consisted of individuals without evidence of COVID-19 in claims or laboratory data between November 2019 and June 2021, a time period that predates widespread availability of home tests. Each potential control group member was assigned an index month in proportion to the distribution of dates for the PCC cohort, ensuring factors such as seasonality would be consistent between cohorts. The distribution by month is summarized in eTable 3 in Supplement 1. Once eligibility criteria were applied consistent with the PCC cohort, a pool of 1 998 156 individuals with no evidence of COVID-19 were available for matching (Figure 1).
A 1:2 propensity score match was performed using multivariate logistic regression. Matching variables included baseline characteristics of age, sex, region, neighborhood-based social determinants of health (race and ethnicity, education, socioeconomic status), Elixhauser Comorbidity Index score,26 comorbid conditions, index month, and 6-month baseline health care utilization and costs. Socioeconomic status quartiles were created using a composite score generated from 7 variables from the 2018 American Community Survey available at the census block group level, matching to member address.27 We used log-transformed cost values in propensity score matching to normalize cost data. The cohorts were selected using a greedy algorithm to ensure baseline comparability after matching. Propensity score balance checks were performed, and these results are included in the eFigure in Supplement 1. Due to the large comparison group available, 95% of the PCC cohort matched to the NC cohort, resulting in a final sample of 13 485 individuals with PCC and 26 870 individuals without COVID-19. Table 1 summarizes characteristics used for matching.
Table 1. Baseline Characteristics and All-Cause Health Care Utilization After 1:2 Propensity Score Matching Among Individuals With Post–COVID-19 Condition and Those Without COVID-19.
| Characteristic | No. (%) | Standardized difference | |
|---|---|---|---|
| With post–COVID-19 condition | Without COVID-19 | ||
| Overall cohort | |||
| No. of patients | 13 435 | 26 870 | NA |
| Age, mean (SD), y | 50.1 (15.1) | 50.2 (15.4) | −0.0065 |
| Age categories, y | |||
| 18-29 | 1452 (10.8) | 2900 (10.8) | 0.0005 |
| 30-44 | 3131 (23.3) | 6211 (23.1) | 0.0045 |
| 45-64 | 6939 (51.7) | 13 877 (51.6) | 0.0001 |
| 65-74 | 1212 (9.0) | 2498 (9.3) | −0.0095 |
| ≥75 | 701 (5.2) | 1384 (5.2) | 0.0030 |
| Sex | |||
| Female | 7874 (58.6) | 15 672 (58.3) | 0.0057 |
| Male | 5561 (41.4) | 11 198 (41.7) | −0.0061 |
| Residence regiona | |||
| Northeast | 4290 (31.9) | 8790 (32.7) | −0.0167 |
| Midwest | 1890 (14.1) | 3595 (13.4) | 0.0200 |
| South | 4098 (30.5) | 8126 (30.2) | 0.0057 |
| West | 3156 (23.5) | 6359 (23.7) | −0.0041 |
| Missing | 1 (0.0) | 0 | 0.0122 |
| Zip code–level measures of social determinants of health | |||
| Race [proportions from census block group data], median (IQR) | |||
| Asian | 0.02 (0.00-0.08) | 0.02 (0.00-0.07) | −0.0041 |
| Black | 0.04 (0.01-0.17) | 0.04 (0.01-0.17) | −0.0138 |
| White | 0.77 (0.55-0.90) | 0.77 (0.53-0.91) | 0.0246 |
| Otherb | 0.01 (0.00-0.07) | 0.01 (0.00-0.07) | −0.0184 |
| Hispanic ethnicity [proportions from census block group data], median (IQR) | 0.11 (0.03-0.29) | 0.10 (0.02-0.31) | −0.0265 |
| Education status high school or above, mean (SD) | 0.87 (0.12) | 0.86 (0.13) | 0.0799 |
| Socioeconomic status | |||
| 1 (Most disadvantaged) | 2737 (20.4) | 5531 (20.6) | −0.0053 |
| 2 | 2734 (20.4) | 5436 (20.2) | 0.0030 |
| 3 | 3264 (24.3) | 6531 (24.3) | −0.0003 |
| 4 (Least disadvantaged) | 4531 (33.7) | 9032 (33.6) | 0.0024 |
| 5 (Missing) | 169 (1.3) | 340 (1.3) | −0.0007 |
| Elixhauser Comorbidity Index score, median (IQR) | 2.0 (0-3) | 2.0 (0-3) | 0.0116 |
| Elixhauser Comorbidity Index categories | |||
| 0 | 3548 (26.4) | 6821 (25.4) | 0.0234 |
| 1 | 2903 (21.6) | 5769 (21.5) | 0.0033 |
| 2 | 2169 (16.1) | 4492 (16.7) | −0.0155 |
| 3 | 1534 (11.4) | 3267 (12.2) | −0.0230 |
| ≥4 | 3281 (24.4) | 6521 (24.3) | 0.0036 |
| Baseline comorbidities | |||
| Asthma | 1777 (13.2) | 3529 (13.1) | 0.0028 |
| Chronic obstructive lung disorder | 2561 (19.1) | 5138 (19.1) | −0.0015 |
| Any cardiovascular disease | 1710 (12.7) | 3356 (12.5) | 0.0072 |
| Coronary artery disease | 1173 (8.7) | 2317 (8.6) | 0.0038 |
| Peripheral vascular disorders | 697 (5.2) | 1406 (5.2) | −0.0020 |
| Cerebrovascular disease | 269 (2.0) | 517 (1.9) | 0.0056 |
| Cirrhosis | 102 (0.8) | 217 (0.8) | −0.0055 |
| Hypertension | 5278 (39.3) | 10 593 (39.4) | −0.0028 |
| Chronic kidney disease | 1879 (14.0) | 3842 (14.3) | −0.0090 |
| End-stage kidney disease receiving dialysis | 159 (1.2) | 322 (1.2) | −0.0014 |
| Diabetes, type 1 | 189 (1.4) | 388 (1.4) | −0.0031 |
| Diabetes, type 2 | 2772 (20.6) | 5653 (21.0) | −0.0100 |
| Severe obesity (BMI ≥40) | 1385 (10.3) | 2781 (10.4) | −0.0013 |
| Cancer | 964 (7.2) | 1909 (7.1) | 0.0027 |
| Arrhythmias | 1664 (12.4) | 3315 (12.3) | 0.0015 |
| Inflammatory bowel disorder | 129 (1.0) | 262 (1.0) | −0.0015 |
| Rheumatoid arthritis | 364 (2.7) | 705 (2.6) | 0.0053 |
| Pregnancy | 190 (1.4) | 352 (1.3) | 0.0090 |
| Depression | 3170 (23.6) | 6431 (23.9) | −0.0080 |
| Anxiety | 3753 (27.9) | 7703 (28.7) | −0.0163 |
| Baseline all-cause 6-mo utilization, mean (SD) | |||
| Inpatient hospitalizations count | 0.18 (0.63) | 0.17 (0.59) | 0.0093 |
| Emergency department visits count | 0.35 (1.00) | 0.32 (1.00) | 0.0309 |
| Office visits count | 6 (6.1) | 6 (6.7) | 0.0539 |
| Baseline all-cause 6-mo overall medical cost PMPM, median (IQR), $ | 408.9 (124.4-1248.3) | 364.0 (99.4-1436.2) | −0.0049 |
| Hospitalized cohort | |||
| No. of patients | 3697 | 7394 | NA |
| Age, mean (SD), y | 57.4 (13.6) | 57.0 (13.7) | 0.0271 |
| Age categories, y | |||
| 18-29 | 111 (3.0) | 163 (2.2) | 0.0501 |
| 30-44 | 486 (13.2) | 1015 (13.7) | −0.0171 |
| 45-64 | 2123 (57.4) | 4255 (57.6) | −0.0025 |
| 65-74 | 611 (16.5) | 1240 (16.8) | −0.0065 |
| ≥75 | 366 (9.9) | 721 (9.8) | 0.0050 |
| Sex | |||
| Female | 1654 (44.7) | 3289 (44.5) | 0.0052 |
| Male | 2043 (55.3) | 4105 (55.5) | −0.0040 |
| Residence regiona | |||
| Northeast | 944 (25.5) | 1960 (26.5) | −0.0222 |
| Midwest | 693 (18.7) | 1353 (18.3) | 0.0115 |
| South | 1328 (35.9) | 2692 (36.4) | −0.0101 |
| West | 732 (19.8) | 1389 (18.8) | 0.0257 |
| Zip code–level measures of social determinants of health | |||
| Race [proportions from census block group data], median (IQR) | |||
| Asian | 0.01 (0.00-0.06) | 0.01 (0.00-0.06) | −0.0041 |
| Black | 0.06 (0.01-0.23) | 0.06 (0.01-0.24) | −0.0138 |
| White | 0.75 (0.49-0.90) | 0.76 (0.49-0.91) | 0.0246 |
| Otherb | 0.01 (0.00-0.07) | 0.01 (0.00-0.07) | 0.0035 |
| Hispanic ethnicity [proportions from census block group data], median (IQR) | 0.10 (0.02-0.29) | 0.09 (0.02-0.30) | −0.0265 |
| Education status high school or above, mean (SD) | 0.29 (0.12) | 0.28 (0.12) | 0.0833 |
| Socioeconomic status | |||
| 1 (Most disadvantaged) | 987 (26.7) | 1935 (26.2) | 0.0120 |
| 2 | 879 (23.8) | 1761 (23.8) | −0.0010 |
| 3 | 898 (24.3) | 1841 (24.9) | −0.0141 |
| 4 (Least disadvantaged) | 892 (24.1) | 1777 (24.0) | 0.0022 |
| 5 (Missing) | 41 (1.1) | 80 (1.1) | 0.0026 |
| Elixhauser Comorbidity Index score, median (IQR) | 2.0 (1-5) | 2.0 (1-4) | 0.0324 |
| Elixhauser Comorbidity Index categories | |||
| 0 | 814 (22.0) | 1575 (21.3) | 0.0174 |
| 1 | 596 (16.1) | 1233 (16.7) | −0.0150 |
| 2 | 534 (14.4) | 1082 (14.6) | −0.0054 |
| 3 | 462 (12.5) | 912 (12.3) | 0.0049 |
| ≥4 | 1291 (34.9) | 2592 (35.1) | −0.0028 |
| Baseline comorbidities | |||
| Asthma | 575 (15.6) | 1156 (15.6) | −0.0022 |
| Chronic obstructive lung disorder | 805 (21.8) | 1643 (22.2) | −0.0108 |
| Any cardiovascular disease | 713 (19.3) | 1356 (18.3) | 0.0242 |
| Coronary artery disease | 498 (13.5) | 988 (13.4) | 0.0032 |
| Peripheral vascular disorders | 309 (8.4) | 591 (8.0) | 0.0133 |
| Cerebrovascular disease | 102 (2.8) | 198 (2.7) | 0.0050 |
| Cirrhosis | 49 (1.3) | 92 (1.2) | 0.0072 |
| Hypertension | 1997 (54.0) | 4000 (54.1) | −0.0016 |
| Chronic kidney disease | 889 (24.1) | 1777 (24.0) | 0.0003 |
| End-stage kidney disease receiving dialysis | 111 (3.0) | 194 (2.6) | 0.0229 |
| Diabetes, type 1 | 73 (2.0) | 140 (1.9) | 0.0059 |
| Diabetes, type 2 | 1219 (33.0) | 2422 (32.8) | 0.0046 |
| Severe obesity (BMI ≥40) | 530 (14.3) | 1090 (14.7) | −0.0115 |
| Cancer | 347 (9.4) | 712 (9.6) | −0.0083 |
| Arrhythmias | 636 (17.2) | 1254 (17.0) | 0.0065 |
| Inflammatory bowel disorder | 36 (1.0) | 69 (0.9) | 0.0042 |
| Rheumatoid arthritis | 107 (2.9) | 218 (3.0) | −0.0032 |
| Pregnancy | 41 (1.1) | 78 (1.1) | 0.0052 |
| Depression | 589 (15.9) | 1192 (16.1) | −0.0052 |
| Anxiety | 626 (16.9) | 1267 (17.1) | −0.0054 |
| Baseline all-cause 6-mo utilization, mean (SD) | |||
| Inpatient hospitalizations count | 0.30 (0.87) | 0.27 (1.06) | 0.0093 |
| Emergency department visits count | 0.42 (1.10) | 0.36 (1.10) | 0.0309 |
| Office visits count | 5 (5.3) | 5 (5.6) | 0.0539 |
| Baseline all-cause 6-mo overall medical cost PMPM, median (IQR), $ | 373 (87.6-1543.4) | 328 (86.3-1563.5) | −0.0049 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable; PMPM, per member per month.
Northeast: Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, Vermont, New Jersey, New York, and Pennsylvania; Midwest: Indiana, Illinois, Michigan, Ohio, Wisconsin, Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, and South Dakota; South: Delaware, District of Columbia, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, West Virginia, Alabama, Kentucky, Mississippi, Tennessee, Arkansas, Louisiana, Oklahoma, and Texas; West: Arizona, Colorado, Idaho, New Mexico, Montana, Utah, Nevada, Wyoming, Alaska, California, Hawaii, and Oregon.
Other race included American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, and other reported race.
Outcomes and Covariates
The outcomes were selected based on internal health plan analysis during the first year of the pandemic, when it became apparent that there was a high rate of cardiovascular-related utilization observed among health plan members diagnosed with COVID-19. This coincided with case reports but predated other published work in the area. Outcomes of interest included claims-based utilization tied to cardiovascular disorders (heart failure, cardiac arrhythmias, peripheral artery disease, pulmonary embolism/deep vein thrombosis, coronary artery disease, and stroke), as well as chronic respiratory disorders (chronic obstructive lung disorders [eg, COPD] and asthma) and mortality. All clinical outcomes were ascertained using ICD-10 codes, with additional utilization information such as hospitalizations identified via claims. The Social Security Administration’s Death Master File and Datavant Flatiron data files were used to capture mortality that may have occurred after any health plan disenrollment. In setting the study measurement period, 12-month outcomes were assessed according to each individual index date (falling between April 1, 2020, and July 31, 2020), with study follow-up ending July 31, 2021.
Statistical Analysis
All variables were summarized using descriptive statistics, with mean, SD, and median for continuous data, and frequencies and proportions for categorical data. All outcome measures were assessed using t tests for continuous data and Pearson χ2 tests for categorical data. Relative risks (RRs) with 95% CIs were calculated for binary outcomes. Statistical significance was set at a 2-sided P = .05. The mortality analysis was carried out using Kaplan-Meier survival estimates to get mortality percentages for each month. Variables were created using the Instant Health Data platform (Panalgo). Statistical analyses were performed using SAS Enterprise Guide, version 8.3 (SAS Institute).
Subset Analysis
Many initial PCC studies reported outcomes for individuals who had an initial hospitalization. To compare results with prior studies, we performed a subset analysis for 3697 individuals with hospitalizations in the first month after COVID-19 diagnosis compared with the matched NC cohort.
Results
Sample Description
The study sample included 13 435 adults with PCC and 26 870 matched adults without COVID-19. The mean (SD) age for the PCC cohort was 50.1 (15.1) years, with 58.7% female. The PCC cohort included individuals from across the US, with representation from the Northeast (31.9%), South (30.5%), Midwest (14.1%), and West (23.5%). In the sample, 33.7% of the individuals were in the least disadvantaged socioeconomic status quartile, and 20.4% were in the most disadvantaged socioeconomic status quartile.
The PCC cohort had a relatively high level of chronic conditions before developing COVID-19, with the following conditions commonly observed: hypertension (39.2%), depression (23.7%), diabetes (20.5%), COPD (19.1%), asthma (13.3%), and severe obesity, defined as body mass index (calculated as weight in kilograms divided by height in meters squared) of 40 or higher (10.3%). The mean (SD) Elixhauser Comorbidity Index score for the cohort was 2.4 (2.7), with 52.0% of the cohort having 2 or more preindex comorbidities. The PCC cohort experienced average health care costs of $2093 per month in the preindex period.
The propensity-matched NC cohort had similar rates and costs for all metrics during the preindex period. The standardized mean difference for all characteristics of the propensity-matched cohorts was below 0.10 (Table 1).
The most common symptoms observed during follow-up for the PCC cohort included shortness of breath (41%), anxiety (31%), muscle aches/weakness (30%), depression (25%), and fatigue (21%). See eTable 4 in Supplement 1 for a full distribution of post–COVID-19 symptoms for the PCC cohort.
Outcomes
There was a consistent elevation in adverse outcomes in the PCC cohort relative to the NC cohort (Table 2). During the follow-up period, the PCC cohort, compared with the NC cohort, experienced increased health care utilization for cardiac arrhythmias (postperiod rate, 29.4% vs 12.5%), with an increase in RR of 2.35 (95% CI, 2.26-2.45); pulmonary embolism (postperiod rate, 8.0% vs 2.2%), with an increase in RR of 3.64 (95% CI, 3.23-3.92); ischemic stroke (postperiod rate, 3.9% vs 1.8%), with an increase in RR of 2.17 (95% CI, 1.98-2.52); coronary artery disease (postperiod rate, 17.1% vs 9.6%), with an increase in RR of 1.78 (95% CI, 1.70-1.88); heart failure (postperiod rate, 11.8% vs 6.0%), with an increase in RR of 1.97 (95% CI, 1.85-2.10); COPD (postperiod rate, 32.0% vs 16.5%), with an increase in RR of 1.94 (95% CI, 1.88-2.00); and asthma (postperiod rate, 24.2% vs 12.4%), with an increase in RR of 1.95 (95% CI, 1.86-2.03). With regard to mortality, 2.8% of the PCC cohort vs 1.2% of the NC cohort died during the follow-up period. This difference implies an excess death rate of 16.4 per 1000 individuals (Figure 2).
Table 2. Twelve-Month Postindex Health Care Utilization for Various Outcomes of Interest Among the Matched Individuals With Post–COVID-19 Condition (PCC) and Controls Without COVID-19.
| Outcome | No. (%) | Relative risk (95% CI) | McNemars P value | |
|---|---|---|---|---|
| With PCC | Without COVID-19 | |||
| Study cohort | ||||
| No. of patients | 13 435 | 26 870 | NA | NA |
| COPD | 4302 (32.0) | 4431 (16.5) | 1.94 (1.88-2.00) | <.001 |
| Asthma (moderate/severe) | 3245 (24.2) | 3341 (12.4) | 1.95 (1.86-2.03) | <.001 |
| Pulmonary embolism/DVT | 1073 (8.0) | 603 (2.2) | 3.64 (3.23-3.92) | <.001 |
| Cardiac arrhythmia | 3956 (29.5) | 3360 (12.5) | 2.35 (2.26-2.45) | <.001 |
| Coronary artery disease | 2301 (17.1) | 2574 (9.6) | 1.78 (1.70-1.88) | <.001 |
| Peripheral vascular disease | 1336 (9.9) | 1683 (6.3) | 1.57 (1.48-1.70) | <.001 |
| Ischemic stroke | 530 (3.9) | 475 (1.8) | 2.17 (1.98-2.52) | <.001 |
| Heart failure | 1587 (11.8) | 1614 (6.0) | 1.97 (1.84-2.10) | <.001 |
| Hospitalized cohort | ||||
| No. of patients | 3697 | 7394 | NA | NA |
| COPD | 1593 (43.1) | 1423 (19.3) | 2.24 (2.11-2.38) | <.001 |
| Asthma (moderate/severe) | 1167 (31.6) | 1086 (14.7) | 2.15 (2.00-2.31) | <.001 |
| Pulmonary embolism/DVT | 712 (19.3) | 230 (3.1) | 6.23 (5.36-7.15) | <.001 |
| Cardiac arrhythmia | 1912 (51.7) | 1285 (17.4) | 2.97 (2.81-3.16) | <.001 |
| Coronary artery disease | 1069 (28.9) | 1071 (14.5) | 1.99 (1.85-2.15) | <.001 |
| Peripheral vascular disease | 638 (17.3) | 657 (8.9) | 1.94 (1.75-2.15) | <.001 |
| Ischemic stroke | 308 (8.3) | 200 (2.7) | 3.07 (2.59-3.66) | <.001 |
| Heart failure | 948 (25.6) | 749 (10.1) | 2.53 (2.32-2.76) | <.001 |
Abbreviations: COPD, chronic obstructive pulmonary disease; DVT, deep vein thrombosis; NA, not applicable.
Figure 2. Twelve-Month Mortality Among Individuals With Post–COVID-19 Condition vs Those Without COVID-19.
The 12-month mortality for individuals with post–COVID-19 condition was substantially higher than in matched controls without COVID-19.
Subset With Hospitalizations
Within the PCC cohort, 27.5% of individuals (n = 3697) experienced hospitalizations in the first month. The mean (SD) age for the hospitalized subset of the PCC cohort was 57.4 (13.6) years, 6 years older than the overall PCC cohort, and 55.2% female. This hospitalized subset of patients had higher levels of chronic conditions before developing COVID-19 relative to the overall PCC cohort, including hypertension (54.1%), type 2 diabetes (30.7%), COPD (22.2%), asthma (15.6%), and severe obesity (14.7%). By contrast, this hospitalized subset had lower levels of depression (16.1%). The mean (SD) Elixhauser Comorbidity Index score for the cohort was 3.2 (3.2), with 61.9% of the cohort having 2 or more comorbidities in the preindex period (Table 1). In the preindex period, the hospitalized PCC subset experienced mean health care costs of $2792 per month; 15.5% of the cohort experienced an inpatient stay during the preindex period. The matched NC cohort had similar rates and costs for all metrics during the preindex period compared with the hospitalized PCC cohort (Table 1).
Outcomes Among Hospitalized Subset
The hospitalized subset experienced higher rates of adverse outcomes relative to the NC cohort (Table 2). During the follow-up period, the hospitalized PCC cohort, compared with the NC cohort, experienced increased health care utilization for cardiac arrhythmias (postperiod rate, 51.7% vs 17.4%), with an increase in RR of 2.97 (95% CI, 2.81-3.16); pulmonary embolism (postperiod rate, 19.3% vs 3.1%), with an increase in RR of 6.23 (95% CI, 5.36-7.15); ischemic stroke (postperiod rate, 8.3% vs 2.7%), with an increase in RR of 3.07 (95% CI, 2.59-3.66); coronary artery disease (postperiod rate, 28.9% vs 14.5%), with an increase in RR of 1.99 (95% CI, 1.85-2.15); heart failure (postperiod rate, 25.6% vs 10.1%), with an increase in RR of 2.53 (95% CI, 2.32-2.76); COPD (postperiod rate, 43.1% vs 19.2%), with an increase in RR of 2.24 (95% CI, 2.11-2.38); and asthma (postperiod rate, 31.6% vs 14.7%), with an increase in RR of 2.15 (95% CI, 2.00-2.31).
Discussion
There has been considerable attention devoted to issues facing individuals with PCC. This study provides new insights into risks for adverse outcomes in individuals with PCC after accounting for higher levels of pre–COVID-19 disease burden. Based on published literature, the most common symptoms experienced by individuals with PCC include fatigue, headache, and attention disorder.6 While these symptoms are concerning, results from this study also indicated a statistically significant increased risk for a range of cardiovascular conditions as well as mortality. While these risks were heightened for individuals who experienced a more severe acute episode of COVID-19 (ie, requiring hospitalization), it is essential to note that most individuals (72.5%) in the cohort did not experience hospitalization during the acute phase. Many of these conditions will have lasting effects on quality of life.
Several studies have found increased risks of cardiovascular disease28,29,30 among people post–COVID-19, looking at cohorts with varying degrees of severity of illness. For example, an American Heart Association study of patients with COVID-19 treated in the emergency department or hospitalized found that 1.3% of patients (103 of 8163) developed acute ischemic stroke during their hospital stay.31
A European study of 2292 individuals presenting at the emergency department with mild to moderate COVID-19 found increased thrombosis risk in the subsequent 28 days: a rate of 2.3% in the presence of moderate COVID-19 and 0.6% for individuals with mild COVID-19. After adjusting for comorbid conditions, patients in the moderate cohort had an absolute increase in risk for thrombosis of 1.69%.32
While these studies inform risk in the immediate aftermath of acute illness (eg, within 30 days), fewer studies address long-term event outcomes. A 2022 study of outcomes among 153 760 patients from the US Department of Veterans Affairs national health care database who were followed up for 12 months after COVID-19 infection compared with a control group with no evidence of COVID-19 found substantial cardiovascular burden in the following year, including increased risk of stroke (hazard ratio [HR], 1.52), ischemic heart disease (HR, 1.75), and thromboembolic disorders (HR, 2.93).33 The Veterans Affairs population included all levels of illness severity as opposed to a focus on individuals with PCC. Consequently, while risk of increased cardiovascular events increased among all COVID-19 survivors, the present study highlights the greater risks for individuals experiencing PCC.
Prior studies have found increases in 1-year mortality among individuals who were initially hospitalized.34,35 A study using electronic health record data showed that 12-month adjusted all-cause mortality was statistically significantly higher for patients who presented with severe COVID-19 compared with individuals who had not had COVID-19 (HR, 2.50). Another study of US Veterans Health Administration data showed that beyond the first 30 days of illness, individuals with COVID-19 had increased risk of mortality (HR of 1.59, and an adjusted excess burden of death estimated at 8.39 cases per 1000 patients at 6 months) compared with a matched cohort of individuals who had not had COVID-19.36 In contrast, the present study revealed numbers substantially higher than earlier reports for several reasons: a focus on PCC, the ability to evaluate data comprehensively across all care settings, comprehensive mortality information, and a 12-month follow-up period.
This study focused on cardiovascular, pulmonary, and mortality outcomes as the events that could be well captured from administrative data sources. As the number of PCC cases grows, it is essential to learn more about this subset of patients with COVID-19 for several reasons. Gaining additional insight into the risks and trajectory of the disease is essential for clinicians caring for these individuals, especially a need for primary prevention for individuals at higher risk. At a health-systems level, it is also necessary to develop resources and guidance for individuals at risk for serious complications. For example, following the dissemination of early results within Elevance Health, Inc, a care management program was developed and deployed to individuals identified as being at risk for PCC.
From a health policy perspective, these results also indicate a meaningful effect on future health care utilization, and even potential implications for labor force participation. Gaining knowledge on the scope and trajectory of PCC is relevant for policy makers, given the recent guidance by the US Department of Health and Human Services that classifies “long COVID” as a disability if it substantially limits major life activities.37
Limitations
There are several limitations in undertaking a claims-based evaluation for a condition where individuals experience a wide range of symptoms, many of which are less likely to be captured in claims data (eg, fatigue, ageusia). Because claims data provide information on people who receive care, the PCC cohort evaluated in this study excluded people who experienced symptoms but were self-managing. Second, the definition of PCC used in this study is more stringent and represents a higher severity population compared with studies using survey data. This would imply a higher incidence of adverse outcomes compared with a broader definition. However, research using broad definitions of PCC may underestimate the consequences for high-risk populations. A third limitation lies in the generalizability of findings, given that the outcomes are for individuals first experiencing COVID-19 in 2020. The time period for identification of members in the study predates the availability of vaccines. Following the availability of vaccines, individuals may have had different health care utilization patterns, due to potential mitigating effects of vaccines on PCC. Given the widespread nature of COVID-19, there may be concerns with the extent to which the control group also had COVID-19 exposures. However, the study period predated the widespread availability of home tests, lending more confidence in the creation of the control group. In the event that individuals in the control group had COVID-19 exposure, this would bias the results toward a null finding. Changes to the virus over time may affect outcomes for individuals. It would be helpful to repeat this evaluation on future cohorts to determine whether patterns remain consistent. We could not capture outcomes for individuals in either the PCC or control cohort who disenrolled from a health plan for reasons other than death. To the extent that individuals disenrolled for health-related reasons (eg, leaving employment), severe outcomes may be underestimated. To account for potential bias, we carried out a sensitivity analysis to assess outcomes for individuals with continuous enrollment and observed consistent risk differences for that cohort as well (eTable 5 in Supplement 1). Finally, this study focused on a commercially insured population, and future efforts should focus on individuals with Medicaid or other coverage to account for influence of access to care.
Conclusions
In this case-control study of 13 435 US adults with PCC and 26 870 matched adults without COVID-19, individuals with PCC experienced elevated rates of adverse health events and mortality over the 12-month follow-up period, after accounting for risk factors present pre–COVID-19. To our knowledge, this analysis is the largest national study of commercially insured individuals with PCC including a full year of follow-up. Assessing ongoing needs of this population will be crucial, especially as it relates to the onset of new chronic conditions following the initial illness. These findings will improve understanding of care needed for individuals with PCC, as well as inform health care systems directing resources toward surveillance, follow-up, and case management to this population.
eTable 1. Baseline characteristics of initial Covid population vs post covid conditions (PCC) cohort
eTable 2. List of symptoms and Covid diagnosis used to identify PCC cases with their ICD 10 codes and description
eTable 3. Index month distributions among the overall cohort
eFigure. Propensity score balance checks for common support
eTable 4. Distribution of PCC symptoms during the weeks 5 to 12 post index among the PCC cohort
eTable 5. Sensitivity analysis among members with 6 months continuous enrollment
Data Sharing Statement
References
- 1.Rubin R. As their numbers grow, COVID-19 “long haulers” stump experts. JAMA. 2020;324(14):1381-1383. doi: 10.1001/jama.2020.17709 [DOI] [PubMed] [Google Scholar]
- 2.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group . Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Huang L, Wang Y, et al. 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tenforde MW, Kim SS, Lindsell CJ, et al. ; IVY Network Investigators; CDC COVID-19 Response Team; IVY Network Investigators . Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993-998. doi: 10.15585/mmwr.mm6930e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. doi: 10.1038/s41598-021-95565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wostyn P. COVID-19 and chronic fatigue syndrome: is the worst yet to come? Med Hypotheses. 2021;146:110469. doi: 10.1016/j.mehy.2020.110469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munblit D, O’Hara ME, Akrami A, Perego E, Olliaro P, Needham DM. Long COVID: aiming for a consensus. Lancet Respir Med. 2022;10(7):632-634. doi: 10.1016/S2213-2600(22)00135-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long COVID or post-COVID conditions. Centers for Disease Control and Prevention . Updated December 16, 2022. Accessed January 26, 2023. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
- 10.Post-COVID conditions: information for healthcare providers. Centers for Disease Control and Prevention . Updated December 16, 2022. Accessed January 26, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html
- 11.Wanga V, Chevinsky JR, Dimitrov LV, et al. Long-term symptoms among adults tested for SARS-CoV-2—United States, January 2020-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(36):1235-1241. doi: 10.15585/mmwr.mm7036a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabrera Martimbianco AL, Pacheco RL, Bagattini ÂM, Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: a systematic review. Int J Clin Pract. 2021;75(10):e14357. doi: 10.1111/ijcp.14357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81(6):e4-e6. doi: 10.1016/j.jinf.2020.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. doi: 10.7326/M20-5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rando HM, Bennett TD, Byrd JB, et al. Challenges in defining Long COVID: striking differences across literature, electronic health records, and patient-reported information. medRxiv. Preprint posted online March 26, 2021. doi: 10.1101/2021.03.20.21253896 [DOI]
- 17.Deer RR, Rock MA, Vasilevsky N, et al. Characterizing long COVID: deep phenotype of a complex condition. EBioMedicine. 2021;74:103722. doi: 10.1016/j.ebiom.2021.103722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626-631. doi: 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su Y, Yuan D, Chen DG, et al. ; ISB-Swedish COVID-19 Biobanking Unit . Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881-895.e20. doi: 10.1016/j.cell.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffith GJ, Morris TT, Tudball MJ, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020;11(1):5749. doi: 10.1038/s41467-020-19478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hastie CE, Lowe DJ, McAuley A, et al. Author correction: outcomes among confirmed cases and a matched comparison group in the Long-COVID in Scotland study. Nat Commun. 2022;13(1):6540. doi: 10.1038/s41467-022-34344-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefanou MI, Palaiodimou L, Bakola E, et al. Neurological manifestations of long-COVID syndrome: a narrative review. Ther Adv Chronic Dis. 2022;13:20406223221076890. doi: 10.1177/20406223221076890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roessler M, Tesch F, Batram M, et al. Post-COVID-19-associated morbidity in children, adolescents, and adults: a matched cohort study including more than 157,000 individuals with COVID-19 in Germany. PLoS Med. 2022;19(11):e1004122. doi: 10.1371/journal.pmed.1004122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; Iniciativa STROBE . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Rev Esp Salud Publica. 2008;82(3):251-259. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 25.Evaluating and caring for patients with post-COVID conditions: interim guidance. Centers for Disease Control and Prevention . Updated June 14, 2021. Accessed January 26, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-background.html
- 26.Simard M, Sirois C, Candas B. Validation of the combined comorbidity index of Charlson and Elixhauser to predict 30-day mortality across ICD-9 and ICD-10. Med Care. 2018;56(5):441-447. doi: 10.1097/MLR.0000000000000905 [DOI] [PubMed] [Google Scholar]
- 27.Ulmer C, McFadden B, Nerenz DR, eds. Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement. National Academies Press; 2009. [PubMed] [Google Scholar]
- 28.Abbasi J. The COVID heart—one year after SARS-CoV-2 infection, patients have an array of increased cardiovascular risks. JAMA. 2022;327(12):1113-1114. doi: 10.1001/jama.2022.2411 [DOI] [PubMed] [Google Scholar]
- 29.Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. 2021;398(10300):599-607. doi: 10.1016/S0140-6736(21)00896-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farshidfar F, Koleini N, Ardehali H. Cardiovascular complications of COVID-19. JCI Insight. 2021;6(13):e148980. doi: 10.1172/jci.insight.148980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qureshi AI, Baskett WI, Huang W, et al. Acute ischemic stroke and COVID-19: an analysis of 27 676 patients. Stroke. 2021;52(3):905-912. doi: 10.1161/STROKEAHA.120.031786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douillet D, Riou J, Penaloza A, et al. Risk of symptomatic venous thromboembolism in mild and moderate COVID-19: a comparison of two prospective European cohorts. Thromb Res. 2021;208:4-10. doi: 10.1016/j.thromres.2021.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583-590. doi: 10.1038/s41591-022-01689-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mainous AG III, Rooks BJ, Wu V, Orlando FA. COVID-19 post-acute sequelae among adults: 12 month mortality risk. Front Med (Lausanne). 2021;8:778434. doi: 10.3389/fmed.2021.778434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Bari M, Tonarelli F, Balzi D, et al. COVID-19, vulnerability, and long-term mortality in hospitalized and nonhospitalized older persons. J Am Med Dir Assoc. 2022;23(3):414-420.e1. doi: 10.1016/j.jamda.2021.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259-264. doi: 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 37.Guidance on “long COVID” as a disability under the ADA, section 504, and section 1557. US Department of Health and Human Services . Accessed January 26, 2023. https://www.hhs.gov/civil-rights/for-providers/civil-rights-covid19/guidance-long-covid-disability/index.html#footnote9_7rjxrql
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline characteristics of initial Covid population vs post covid conditions (PCC) cohort
eTable 2. List of symptoms and Covid diagnosis used to identify PCC cases with their ICD 10 codes and description
eTable 3. Index month distributions among the overall cohort
eFigure. Propensity score balance checks for common support
eTable 4. Distribution of PCC symptoms during the weeks 5 to 12 post index among the PCC cohort
eTable 5. Sensitivity analysis among members with 6 months continuous enrollment
Data Sharing Statement