Abstract
5-Azidomethyl-2’-deoxyuridine (5-AmdU, 1) has been successfully employed for metabolic labelling of DNA and fluorescent imaging of live cells. 5-AmdU also demonstrated significant radiosensitization in breast cancer cells via site-specific nitrogen-centered radical (π-aminyl (U-5-CH2-NH•, 2) and σ-iminyl (U-5-CH=N•, 3) formation. This work shows that these nitrogen-centered radicals are not formed via reduction of azido group in 6-azidomethyluridine (6-AmU, 4). Radical assignments were performed using electron spin resonance (ESR) in supercooled solutions, pulse radiolysis in aqueous solutions, and theoretical (DFT) calculations. Radiation-produced electron addition to 4 leads to the facile N3− loss forming stable neutral C-centered allylic radical (U-6-CH2•, 5) through dissociative electron attachment (DEA) via the transient negative ion, TNI, (U-6-CH2-N3•−), in agreement with DFT calculations. In contrast, TNI (U-5-CH2-N3•−) of 1, via facile N2 loss (DEA) and protonation from the surrounding water, forms radical 2. Subsequently, 2 undergoes rapid H-atom abstraction from 1 and produces the metastable intermediate α-azidoalkyl radical (U-5-CH•-N3). U-5-CH•-N3 converts facilely to radical 3. N3− loss from U-6-CH2-N3•− is thermodynamically controlled; whereas, N2 loss from U-5-CH2-N3•− is dictated by protonation from surrounding waters and resonance conjugation of the azidomethyl side chain at C5 with the pyrimidine ring.
Keywords: EPR, azidonucleosides, prehydrated electron, dissociative electron attachment, aminyl radical, iminyl radical, radical addition to double bond, H-atom abstraction, C-centered radicals
Graphical Abstract
Introduction
Azido-modified nucleosides have been of interest for over six decades and the finding that 3’-azido-3’-deoxythymidine (AZT or 3′-AZT) is a therapeutic agent1,2 for acquired immunodeficiency syndrome (AIDS) has sparked interest in their chemistry. Synthesis of azido-nucleosides, their reactions, and biological activities have been the subject of several comprehensive reviews3,4. Azidonucleosides have been mainly explored as substrates for the (a) synthesis of amino nucleosides (b) click chemistry, (c) bioconjugation and ligation, and (d) enzyme inhibitions including ribonucleotide reductases (RNR),5,6 among others. Recently, our group is employing azidonucleosides to augment the radiation-induced damage (i.e., radiosensitization) in cancer cells to improve the efficacy of tumor radiotherapy.7 Apart from our group, a few groups reported substantial radiosensitization provided with 3′-AZT.8–11 3′-AZT substantially enhanced γ-irradiation killing of EBV (Epstein-Barr virus)-transformed lymphoblastoid cells in vitro.12
Interaction of ionizing radiation with matter leads to ejection of high energy electrons along with ionizations and excitation events. These high-energy electrons cause further ionizations. These ionization events result in a cascade of medium and lower-energy electrons that lead to cause still further ionizations as well as numerous excited states.13–18 The DNA-radicals (cationic, anionic and neutral) that are produced through these ionization events and the excited states proximate to these radicals are responsible for most of the subsequent damage to the DNA.13–16 In addition, low-energy electrons (LEE) with energies below the ionization threshold can also directly damage DNA through a dissociative electron attachment process (DEA),13–26 causing sugar-phosphate bond cleavage leading to strand break formation.16,20,21,26
As the pyrimidines have a stronger electron affinity than purines,24–32 C5-modified pyrimidine derivatives, for example, C5-halopyrimidine derivatives like 5-bromo-2’-deoxyuridine (5-BrdU) are well-investigated as electron-affinic radiosensitizers in cancer radiotherapy.33–40 Cells incorporate 5-BrdU in their DNA almost as readily as thymidine and depending on the extent of 5-BrdU incorporation in cells; as a result, a 3–4-fold increase in radiation-induced cell killing has been reported.36 However, owing to the toxicity of 5-BrdU, it did not show an increase in patient survival during phase III clinical trials and the trials were called off.35,37–40 Comparison of the reaction of thymidine (Thd or 5-MedU) and radiation-produced electrons (in the gas phase or at low temperatures in solid state by LEE or presolvated electron (epre−) or at room temperature by solvated electron (esol−), and its previous epre−, and by LEE in solution (i.e., quasi-free electrons (eqf−)) with the corresponding reactions of radiation-produced electrons with 5-BrdU has elucidated the chemical mechanism of radiosensitization provided by 5-BrdU.35
We note here that during and after incorporation of C5-halopyridines in cellular DNA, thymidylate synthetase causes efficient hepatic dehalogenation. This process hinders the accumulation of therapeutically sufficient amount of halopyrimidines and limits the use of halopyrimidines as effective radiosensitizers for tumor radiotherapy.35 Therefore, synthesis of C5-halopyridine-like molecules with similar or even better DEA efficiency, but without the drawbacks of dehalogenation by thymidylatse synthetase is very crucial. In other words, new base and nucleoside derivatives are synthesized that have similar or better DEA yields in the 0–3 eV region than the halopyrimidines35, e.g., azidonucleosides. Thus, development of non-toxic and efficient pyrimidine C5-modified analogs as radiosensitizers is of great interest.29,30,33–35,40
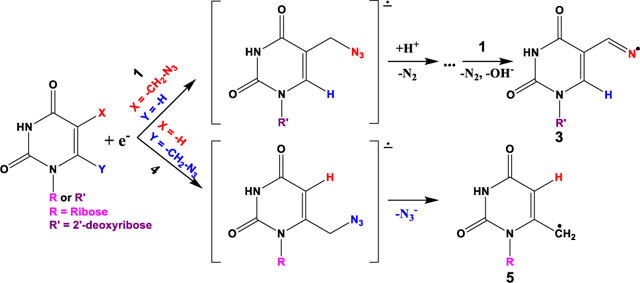
Neutral pi(π)-type aminyl radicals (RNH•) are biologically important and their generation via DEA to azidonucleosides has been investigated by our group through the use of synthesis, electron spin/paramagnetic resonance (ESR/EPR) spectroscopy and theoretical calculations.7,24,41–43 These studies have led to the following general findings: (a) Base-modified azidopyrimidine nucleosides are found to augment radiation damage to cancerous cells via radiation-produced site-specific electron-mediated π-aminyl (RNH• (U-5-CH2-NH•), 2)/σ-iminyl (R=N• (U-5-CH=N•), 3) radicals (Figure 1),7 and, (b) the site of azido substitution in the sugar moiety of azidosugars43 and in the azidopyrimidine nucleoside42 influences the reactivity (intramolecular vs. intermolecular H-atom abstraction) of the aminyl radical. For example, the addition of radiation-produced electrons to 5-azidomethyl-2’-deoxyuridine (5-AmdU, 1 Figure 1) 1 leads to the formation of RNH•, 2. Radical 2, via rapid H-atom abstraction from 1, leads to the formation of the intermediate α-azidoalkyl radical (U-5-CH•-N3). U-5-CH•-N3undergoes facile N2 loss and conversion to σ-iminyl radical, (R=N•), 3 (Scheme 1). 5-AmdU demonstrates effective radiosensitization in EMT6 breast cancer cells.7
Figure 1.
Structures of the compounds and of the radicals studied in this work. Structures of 5-AmdU (1)-mediated π-type aminyl (2) / σ-type iminyl radical (3); structure of 6-AmU (4).
Scheme 1.
Formation of neutral π-aminyl radical, RNH• (2, U-5-CH2-NH•) from 1, via one-electron reduction by radiation-produced electrons. RNH• from 1, undergoes bimolecular conversion to the σ-minyl radical, R=N• (3, U-5-CH=N•). Reproduced [or Adpated, Reprinted, etc.] with permission from Ref. [7], Copyright [2018] [Org. Lett.].
To expand these studies, 6-azidomethyluridine (6-AmU, 4, Figure 1) was investigated. Unlike C5-azidomethyl pyrimidine nucleosides,7 a C6-substituted pyrimidine cannot be incorporated in DNA by a polymerase33. We note that 4 is a ribonucleoside and 1 is 2′-deoxyribonucleoside as well as the radicals under investigation are pyrimidine base radicals only and not sugar radicals; the azidomethyl group is bonded to the C6 of the pyrimidine ring in 4 and the azidomethyl group is bonded to the C5 of the pyrimidine ring in 1. Aims of this work are:
to test whether the expected site-specific formation of the same nitrogen-centered (aminyl and iminyl) radicals generated either from the ribonucleoside 6-AmU (4) based on our previous studies7,43 of 1.
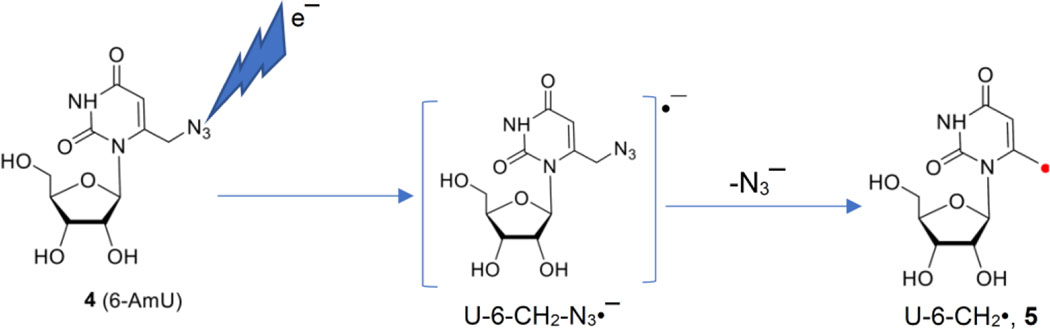
to test whether the initially formed unstable azide anion radical intermediate or the transient negative ion (TNI) (U-6-CH2-N3•−) leads to the formation of the allylic radical U-6-CH2•, 5, via unexpected facile loss of azide as an anion (N3−) via DEA at 77 K.
to test whether the same N3− loss via DEA happens at room temperature as well. That is, whether temperature could influence the DEA pathway (allylic radical formation due to thermodynamic control at low temperature vs. aminyl radical formation via N2 loss by kinetic control at room temperature).
Experimental:
Synthesis and spectroscopic characterization of 1 including the ESR, theoretical studies of assignments of radicals 2 and 3 along with the EMT6 breast cancer cellular studies showing evidences of radiosensitization by 1 are reported in our previous work.7 6-AmU (4) was synthesized from uridine employing Tanaka’s 6-hydroxymethylation methodolody44 following standard mesylation and azidation protocols as described in SI section.
For ESR studies, methods of preparation of homogeneous glassy samples of 4, γ-irradiation and storage of these glassy samples including the stepwise annealing of these glassy samples are described in the SI. The ESR equipment and the experimental set-up for recording the ESR spectra at 77 K including field calibration are described in the SI.
Pulse radiolysis experiments were performed using the ELYSE platform (Paris-Saclay University) 16,20,26,32,35 and the experimental details is provided in the supporting information.
Methods of theoretical calculations are described in the “Theoretical Calculations” part of the “Results and Discussion” section.
Results and Discussion
ESR Studies:
The ESR investigations of 5-azidomethyl-2’-deoxyuridine (5-AmdU, 1 Figure 1) in supercooled (glassy) homogeneous solutions were presented in our previous studies (see Introduction and Scheme 1).7,43
To characterize the radical formation via DEA at 77 K from the ribonucleoside (6-AmU, 4), ESR studies were carried out by employing a γ-irradiated (irradiation at 77 K, absorbed dose = 500 Gy) homogeneous supercooled (glassy, 7.5 M LiBr/D2O) solution of 6-AmU (4). Results of this ESR study are presented in Figure 2.
Figure 2.
ESR spectrum (black) in (A) after radiation-produced one-electron addition to 6-AmU 4 (2.0 mg/mL) at 77 K (γ-irradiation, 500 Gy) in 7.5 M LiBr/D2O in dark. Spectrum (black) in (B) was obtained via warming the sample for 15 min at ca. 150 K in the dark. All spectra were recorded at 77 K. The red spectrum in (A) is the simulated spectrum (for simulation parameters, see text). The three reference markers in this figure and in subsequent figures are Fremy’s salt resonances with central marker is at g = 2.0056 and each of three markers is separated from one another by 13.09 G.
In Figure 2A, the 77 K ESR spectrum (black) obtained after addition of radiation-produced prehydrated electrons at 77 K to 6-AmU 4 in homogeneous oxygen free aqueous glassy solution at 77 K in the dark (7.5 M LiBr/D2O) is shown.
We have compared the black spectrum in Figure 2A with our reported spectra7,41–43 of the π-aminyl radical observed from azidonucleosides and azidosugars at 77 K in the dark. This comparison establishes that spectrum 2A does not show the line components due to axially symmetric anisotropic N-atom at the wings. Rather, the g-values (gxx, gyy, gzz = (2.0030, 2.0030, 2.0023)) demonstrate that this spectrum is due to a C-centered radical. Taking these g-values into account, applying a mixed (Lorentzian/Gaussian = 1) lineshape, an isotropic linewidth of 5.5 G, and using three α-H hyperfine coupling constant values, HFCCs, (Axx = −17.52, Axy = −7.02, Ayy = −12.06, Azz = −14.63; Axx = −7.78, Axy =−2.62, Ayy =−24.33, Azz = −16.54; Axx = −4.61, Ayy = −14.02, Azz = −10.51), a simulated spectrum (red) is obtained. Superimposition of the experimentally recorded (black) with the simulated spectrum (red) in Figure 2A shows a nice match. Based on these results, we have assigned these spectra to the allylic U-6-CH2•, 5. This radical is a C-centered radical and shows 3 α-H HFCCs – two at C6 and one at C5.
The spectrum (black) in Figure 2B is obtained after warming the sample at ca. 150 K in the dark for 15 min. This spectrum is only slightly changed from the experimental and the simulated spectra shown in Figure 2A. The g-value at the center of spectrum 2B matches with that of both experimental (black) and the simulated (red) spectrum in Figure 2A. Therefore, we have assigned the spectrum 2B to the allylic U-6-CH2•, 5 as well.
Thus, the ESR studies establish that contrary to our results with 1 where radiation-mediated epre−, formed in homogeneous aqueous glassy solution (7.5 M LiCl, 77 K) in the absence of oxygen, leads to the radical 2 that converts to 3 (Scheme 1), radiation-produced epre− addition to 4 leads to the unexpected loss of azide as an anion via DEA, from the initially formed TNI (U-6-CH2-N3•−), to generate the allylic radical 5 (Figure 3).
Figure 3.
The allylic radical U-6-CH2•, 5 formation via unexpected loss of azide as an anion (N3−) from the azide anion radical intermediate (U-6-CH2-N3•−) or TNI of 5 via dissociative electron attachment.
Pulse radiolysis:
Time-resolved radiolysis of aqueous solutions of 1 and 4 were conducted at room temperature at different concentrations (0.2, 0.5, and 1 mM) in the spectral range 280–700 nm at different timescales. The solutions were saturated using Ar and contained 0.2 M t-butanol to scavenge the hydrogen atom (H•) and hydroxyl radical (•OH) produced due to water radiolysis.16,26,29,30,32,45 Therefore, under these conditions, only the hydrated electron, , reacts with the solute (1 or 4).
Pulse Radiolysis of 1:
The increase in the decay of absorption at 600 nm correlates with the concentration of 1 (panel B, Figure 4). The rate constant of the reaction between and 1 is found to be (1 ± 0.2) × 1010 M−1 s−1; hence, this reaction is diffusion-controlled. The kinetics are wavelength dependent in the UV region (Figure 4), i.e., there could be multiple species involved with different absorption and kinetic properties (vide infra). To illustrate, we reported decays at three selected wavelengths: 300, 315 and 330 nm (panel A, Figure 4).
Figure 4.
Pulse radiolysis of Ar-saturated solutions of 1 in the presence of 0.2 M t-butanol. (A) Kinetics of species formed were observed at 300, 315, and 330 nm for the same solution ([1] = 1 mM). (B) The kinetics of electron reaction were observed at 600 nm for three different concentrations (0.2, 0.5, and 1 mM) of 1. (C) Absolute absorption spectra of the radicals obtained via eaq− -mediated reduction of 1. (D) The deconvoluted kinetics of eaq− and the radicals of 1 ([1] = 1 mM) (see inset) and the absorption spectra obtained by Bayesian analysis of full spectro-kinetics data matrix (Figure S1).
The data presented in Figure 4 established that along with the decay of , an absorption band around 300 nm is formed with a weaker band around 530 nm (panels C and D, Figure 4). Combining our published ESR results on 17,43 and the DFT-calculated UV-VIS spectral results (vide infra), we assigned this absorption to 2. This is further supported by the kinetics in the first 200 ns when decays and 2 is formed and reach a maximum (see inset, panel D, Figure 4). The kinetics measured in the UV region showed that there is an isosbestic point at 315 nm (panels C and D, Figure 4) between the absorption of and the species formed successively after the decay of (panel D (inset), Figure 4). Therefore, we can deduce that the extinction coefficient of the absorption band of RNH• at 315 nm as ca. 1180 M−1 cm−1.
At 300 nm within 200 ns, we observed only an increase in absorbance (panel A, Figure 4). After the completion of the decay of , the global data analysis showed the presence of two transient species: 2 (maximum of absorbance 310 nm with a shoulder around 410 nm) and an absorption band around 540 nm was observed (panels C and D, Figures 4 and S1). The band at 540 nm is observed to be ca. 2 times less intense than that at 310 nm. The rate of formation of the final species depends on the concentration of 1 confirming the bimolecular reaction of radical 2 with 1 forming the α-azidoalkyl radical (U-C5-CH•-N3) supporting our ESR investigations on 17,43 and Scheme 1. From these data, by exploiting the isosbestic point and combining our published ESR results on 17,43 and the DFT-calculated UV-VIS spectral results (vide infra and Figures S6–S9), we assigned this absorption to the combination of the α-azidoalkyl radical (U-C5-CH•-N3) and σ-type iminyl radical, U-C5-CH=N•, 3 (Scheme 1).
Pulse radiolysis of Ar-saturated solutions of 1 ([1] = 1 mM) in the presence 0.2 M t-butanol at pH = 12 yielded results (Figures S2 and S3) similar to those presented in Figure 4 above. These results demonstrate that formation of radical 2, α-azidoalkyl radical (U-C5-CH•-N3) and σ-type iminyl radical, U-C5-CH=N•, 3 from 1 are not pH dependent. These results agree with our earlier ESR studies showing the RNH• formation from AZT at pHs ca. 5 and 12.41 Furthermore, these results demonstrate that the protonation from the water molecules surrounding the TNI (U-5-CH2-N3•−) plays an important role in the N2 loss that is involved DEA pathway. This is supported by the theoretical calculations (vide infra).
Pulse Radiolysis of 4:
Employing solutions of 4 with different concentrations (0.2, 0.5, and 1 mM), we investigated the kinetics between 280 and 700 nm at room temperature (Figure 5).
Figure 5.
Pulse radiolysis of Ar-saturated solution of 4 in the presence of 0.2 M t-butanol. Kinetics of species formed were observed at 300 nm and 340 nm for the same solution ([4] = 1 mM, panel A). The kinetics were observed at 600 nm for three different concentrations (0.2, 0.5, and 1 mM) of 4 (panel C). The deconvoluted kinetics of hydrated electron () and the radicals of 4 ([4] = 1 mM) (panel B) and the absorption spectra obtained by Bayesian analysis of full spectro-kinetics data matrix are shown (panel D, see also Figure S4). The absolute absorption spectrum of the radical species (5) was obtained via reduction of 4 using isosbestic point.
Similar to pulse radiolysis of 1, the decay of is accelerated in the presence of 4 (Figure 5, panel B and Figure S4). From the decay kinetics at 600 nm (panel C), the rate constant of the reaction between and 4 is found to be (5.5 ± 0.2) × 109 M−1 s−1 and this reaction is almost diffusion controlled similar to the corresponding reaction observed in 1. Although, this reaction is slower by a factor of ca. 2 than the corresponding reaction of with 1, reacts quantitatively with either 1 or 4 due to the diffusion controlled nature of this reaction.
The decay of is correlated with the formation of a species with an absorption spectrum showing a band at 295 nm, a shoulder around 340 nm and another absorption band located at 550 nm (panel D). In addition, the data analyses of all spectro-kinetics data showed the presence of only two species – and the species that resulted due to the reduction of 4 by (Figure S4 and panel B, Figure 5). The transient absorption data display a weak isosbestic point, thereby showing that the value of the extinction coefficient of and the extinction coefficient of the anion radical (U-6-CH2-N3•−)is almost the same (panel A, Figure 5, kinetics at 300 nm). Thus, the extinction coefficient of the absorption spectra of radical (panel D, Figure 5) formed via reduction of 4 by is deduced. This radical is stable at least for 1 μs.
A comparison of Figures 4 and 5 shows that there are two major differences in the reduction of 1 and 4. The reduction of 1 forms first a very short transient species with a half-lifetime of less than 100 ns. This transient species resulted in an absorption spectrum with bands at 310, a shoulder at 340 nm and a weak band at 540 nm (panels C and D, Figure 4). This absorption spectrum is assigned to the combination of the α-azidoalkyl radical (U-C5-CH•-N3) and σ-type iminyl radical, U-C5-CH=N•, 3.
However, the reduction of 4 by forms directly the radical representing an absorption spectrum with a band at 295 nm, a shoulder at 340 nm, and another band at 550 nm (panel D, Figure 5). This band at 550 nm is two times more intense than that observed by the reduction of 1 (Figure S5). Based on the ESR results (Figure 2) and DFT calculations (see below), this radical species from 4 is assigned to the allylic radical, 5 (Figure 3).
Theoretical Calculations:
To support the assignments of the radicals using ESR investigations of homogeneous glassy solutions and pulse radiolysis in dilute aqueous solutions at ambient temperature, theoretical calculations were performed. Density Functional Theory (DFT) using the B3LYP functional and the 6–31G++** basis set were employed for structure optimization of radicals in their ground state. Time dependent density functional theory (TD-DFT) at the same level of theory was applied to calculate the vertical excited states and UV-vis spectra of the C-centered allylic radicals, viz, U-C5-CH2• (from 1) and 5, (from 4), as well the radicals with heteroatoms formed from the ribonucleoside of 1, viz, the aminyl radical (2), the α-azidoalkyl radical (U-C5-CH•-N3) and the iminyl radical (3). All the calculations were performed in the aqueous phase employing the integral equation formalism of the polarized continuum model (IEF-PCM) by Tomasi et al.46 Gaussian 16 suite of programs47 is used for the calculations, Jmol48 a free molecular viewer is used for the structure drawings and spectra were plotted using the Gabedit freeware49.
(a) HFCCs calculation of the allylic radical (5):
The B3LYP-PCM/6–31G++** calculated isotropic HFCCs (Aiso) of three hydrogens (the two hydrogens of CH2 group attached at C6 and the H5 attached at C5) of 5 from 4 are: H5 (−11.4 G), H6′ (−16.2 G) and H6′′ (−15.3 G), respectively. The calculated anisotropic HFCCs (Aaniso) of these hydrogens are: H5 [−5.0, −1.0, 6.0] G, H6′ [−8.8, −0.3, 9.1] G and H6′′ [−7.8, −0.7, 8.5] G, respectively. The total HFCCs (Aiso + Aaniso) are H5 [−16.4, −12.4, −5.4] G, H6′ [−25, −16.5, −7.1] G and H6′′ [−23.1, −16, −6.8] G. The corresponding experimental HFCCs are (Axx = −4.61, Ayy = −14.02, Azz = −10.51; Axx = −17.52, Ayy = −12.06, Azz = −14.63; Axx = −7.78, Ayy =−24.33, Azz = −16.54); both sets of HFCCs compare fairly.
(b) Calculated UV-vis spectra:
The B3LYP-PCM/6–31G++** calculated vertical excited state spectrum of 5 is presented in Figure 6 shows absorbance peaks at ca. 272 nm, ca. 348, ca. 390 and ca. 503 nm, respectively. The peak at ca. 272 nm has the maximum absorbance. The spectrum obtained employing pulse radiolysis of 4 (Figure 5) has maxima absorbance at 295, 340, and 540 nm, respectively. The calculated spectrum is blue-shifted by ca. 20 nm and the calculated spectrum after 20 nm shift has peaks at 292 nm, 368 nm, 410 nm and 523 nm, respectively, which is in good agreement with experiment. A small discrepancy between theory and experiment is expected50 as the present calculations do not consider vibronic and thermal effects.
Figure 6.
The B3LYP-PCM/6–31G++** calculated vertical excited state spectrum of the allylic radical, 5 from 4.
The spectra of the aminyl radical (2), α-azidoalkyl radical (U-C5-CH•-N3) and σ-type iminyl radical, R-CH=N• (3) are shown in the SI (Figures S6–S8).
We have also calculated the theoretical excited state spectrum of the allylic U-5-CH2• from 5-AmU anion radical via azide anion loss (Figure S9). This spectrum does not match with those shown in Figures 5 and 6.
In addition, theoretical calculations show that the TNI of 4 undergoes a barrier free N3− loss. On the other hand, in the B3LYP-PCM/6–31G++** calculated and optimized structure of 1 using the same method, we find that in the azidomethyl moiety at C5, the N1-N2 bond starts at 1.23 A. In the TNI (U-5-CH2-N3•−) of 1, i.e., upon adding one electron to 1, this bond distance becomes 1.33 A and following this, there is a 12 kcal/mol barrier to cause cleavage of the N1-N2 bond leading to N2 loss. From our earlier ESR work on AZT at different pHs (ca. 5 and 12)41 and pulse radiolysis of 1 at different pHs (7 and 11, this work), it is possible that the protonation of the TNI (U-5-CH2-N3•−) of 1 is likely to complete the N1-N2 bond cleavage process required for the N2 loss. To check this possibility, we calculated (at B3LYP/6–31G**) the barrier for N2 loss after protonation of the N1 nitrogen of U-5-CH2-N3•− by the water (solvent) molecules and found a nominal barrier of 1.6 kcal/mol (Figure S10). This small barrier confirms that protonation of U-5-CH2-N3•− by the surrounding water molecules will drive the N2 loss reaction to completion.
Conclusions:
ESR studies in the homogeneous glassy solution, pulse radiolysis in aqueous solution at ambient temperature, and DFT (for HFCCs) along with TDDFT (for UV-Vis spectra) calculations were performed to characterize the radical formed via DEA from 4. These studies led to unequivocal identification of highly stable allylic radical 5 (Figure 3). Formation of 5 at 77 K and at the ambient temperature establishes that N3− elimination from the TNI of 4 is thermodynamically and not kinetically controlled. Resonance stabilization of 5 owing to the presence of the azidomethyl moiety at position C6 of the base and solvation of N3− by surrounding water molecules contribute to the facile N3− loss from the TNI of 4.
The compound 4 enabled us to compare the radiation chemical results (ESR, pulse radiolysis, and theory) with the already published results7,43 (ESR and theory) for 1. The ESR experiments were carried out using glassy solution whereas pulse radiolysis experiments were conducted in aqueous solutions at ambient temperatures. ESR and pulse radiolysis results show that the mechanisms are the same in the glassy and in the aqueous samples and this comparison established that (a) the radicals formed from these two compounds (1 (radicals 2 and 3, Scheme 1) and 4 (radical 5, Figure 3) via the same reaction (DEA) are different, and (b) the reaction mechanism of DEA along with the subsequent reactions of the radicals formed are also different (N2 loss and protonation from the surrounding water (Scheme 1) vs. N3− loss (Figure 3)).
Pulse radiolysis at various pHs, and TDDFT calculations (this work) along with our previous ESR and DFT studies7,43 establish that the TNI of 1 forms the aminyl radical (2, U-C5-CH2NH•) via N2 loss followed by rapid solvent protonation of the incipient nitrene anion radical. For the N2 loss, the resultant nitrene anion radical (U-C5-CH2N•−) and the subsequent aminyl radical (2) have hyperconjugation stabilization due to two methylene protons. This renonance stabilization and protonation from the surrounding solvent (water molecules) explain our previous observation of aminyl radical formation in the azidonucleosides where the azido group is in the sugar moiety and in aliphatic 3-azido-1-propanol via DEA through N2 loss. In addition, both ESR7,43 and pulse radiolysis support formation of α-azidoalkyl radical (U-C5-CH•-N3) from 1 and its conversion to σ-type iminyl radical, 3 (Scheme 1).
In conclusion, our work combining synthesis, ESR, pulse radiolysis, and theory establish that the position of azidomethyl group or the azido group in the compound and the surrounding water molecules dictate the elimination part (N2 or N3− loss) of DEA reactionfrom the TNI. N2 loss from the TNI is the dominant mechanism of the DEA observed in the azido-subtituted compounds that we have investigated, so far. TNI of 4 is the only case to date in which we have observed N3− loss
leading to the formation of the resonance conjugation stabilized allylic radical 5. Combining Scheme 1 and Figure 3, the findings of our work are summarized in the Scheme 2 below.
Scheme 2.
Summary of the results eastablishing that the position of azidomethyl group or the azido group in the compound and the surrounding water molecules dictate the elimination part (N2 or N3− loss) of DEA reactionfrom the TNI.
Supplementary Material
ACKNOWLEDGEMENTS
SW, AK, MDS, and AA thank the National Cancer Institute of the National Institutes of Health (Grant RO1CA045424) for support. AA thanks the National Science Foundation under Grant No. CHE-1920110. AA also thanks Université Paris-Saclay for the Visiting Professorship at the Institut de Chimie Physique.
Footnotes
Associated Content:
Supporting information: This contains the following: (a) relevant information for the synthesis of compound 4, (b) sample preparation and methodologies for ESR studies, (b) methodology and additional data of pulse radiolysis, (c) additional data of theoretical including TD-DFT calculations. The Supporting Information is available free of charge at http://pubs.acs.org/.
Notes
The authors declare no competing financial interest.
References
- 1.Horwitz JP; Chua J; Noel M. Nucleosides V. The Monomesylates of 1-(2’-Deoxy-β-D-lyxofuranosyl)thymine1,2. J. Org. Chem 1964, 29, 2076–2078. [Google Scholar]
- 2.Mitsuya H; Weinhold KJ; Furman PA; St Clair MH; Lehrman SN; Gallo RC; Bolognesi D; Barry DW; Broder S. 3’-Azido-3’-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 7096–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pathak T. Azidonucleosides: Synthesis, Reactions, and Biological Properties. Chem. Rev 2002, 102, 1623–1668. [DOI] [PubMed] [Google Scholar]
- 4.Müggenburg F; Müller S. Azide-modified Nucleosides as Versatile Tools for Bioorthogonal Labeling and Functionalization. The Chemical Record, e202100322. [DOI] [PubMed] [Google Scholar]
- 5.van der Donk WA; Stubbe J; Gerfen GJ; Bellew BF; Griffin RG EPR Investigations of the Inactivation of E. coli Ribonucleotide Reductase with 2’-Azido-2’-deoxyuridine 5’-Diphosphate: Evidence for the Involvement of the Thiyl Radical of C225-R1. J. Am. Chem. Soc 1995, 117, 8908–8916. [Google Scholar]
- 6.Fritscher J; Artin E; Wnuk S; Bar G; Robblee JH; Kacprzak S; Kaupp M; Griffin RG; Bennati M; Stubbe J. Structure of the Nitrogen-Centered Radical Formed during Inactivation of E. coli Ribonucleotide Reductase by 2’-Azido-2’-deoxyuridine-5’-diphosphate: Trapping of the 3’-Ketonucleotide. J. Am. Chem. Soc 2005, 127, 7729–7738. [DOI] [PubMed] [Google Scholar]
- 7.Wen Z; Peng J; Tuttle P; Ren Y; Garcia C; Debnath D; Rishi S; Hanson C; Ward S; Kumar A. et al. Electron-mediated Aminyl and Iminyl Radicals from C5-Azido-Modified Pyrimidine Nucleosides Augment Radiation Damage to Cancer Cells. Org. Lett, 2018, 20, 7400–7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagetia GC; Aruna R. Correlation of micronuclei-induction with the cell survival in HeLa cells treated with a base analogue, azidothymidine (AZT) before exposure to different doses of gamma-radiation. Toxicology Lett. 2003, 139, 33–43. [DOI] [PubMed] [Google Scholar]
- 9.Coucke PA; Cottin E; Decosterd LA Simultaneous alteration of de novo and salvage pathway to the deoxynucleoside triphosphate pool by (e)-2’-deoxy-(fluoromethylene)cytidine (fmdc) and zidovudine (azt) results in increased radiosensitivity in vitro. Acta Oncologica 2007, 46, 612–620. [DOI] [PubMed] [Google Scholar]
- 10.Liao Z-K; Zhou F-X; Luo Z-G; Zhang W-J; Jie X; Bao J; Han G; Zhang M-S; Xie C-H; Zhou Y-F Radio-activation of htert promoter in larynx squamous carcinoma cells: An ‘indirected-activator’ strategy in radio-gene-therapy. Oncol. Rep 2008, 19, 281–286. [PubMed] [Google Scholar]
- 11.Zhou F-X; \Liao Z-K; Dai J; Jie X; Xie C-H; Luo Z-G; Liu S-Q.; Zhou Y-F Radiosensitization effect of zidovudine on human malignant glioma cells. Biochem. Biophys. Res. Commun 2007, 354, 351–356. [DOI] [PubMed] [Google Scholar]
- 12.Westphal EM; Blackstock W; Feng W; Israel B; Kenney SC Activation of lytic Epstein-Barr virus (EBV) infection by radiation and sodium butyrate in vitro and in vivo: a potential method for treating EBV-positive malignancies. Cancer Res. 2000, 60, 5781–5788. [PubMed] [Google Scholar]
- 13.Becker D; Adhikary A; Sevilla MD In Recent Trends in Radiation Chemistry; Rao BSM, Wishart J. Eds.; World Scientific Publishing Co., Singapore, New Jersey, London, 2010, 509–542. [Google Scholar]
- 14.Becker D; Adhikary A; Sevilla MD In Particle Charged and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces; Hatano Y, Katsumura Y, Mozumder A. (Eds.)), CRC Press, Taylor & Francis Group, Boca Raton, London, New York, 2010, 503–541. [Google Scholar]
- 15.Adhikary A; Becker D; Sevilla MD Electron spin resonance of radicals in irradiated DNA. In Applications of EPR in radiation research, Eds. Lund A, Shiotani M, Springer-Verlag, Berlin, Heidelberg, 2014, 299–352. [Google Scholar]
- 16.Ma J; Denisov SA; Adhikary A; Mostafavi M. Ultrafast Processes Occurring in Radiolysis of Highly Concentrated Solutions of Nucleosides/Tides. Int. J. Mol. Sci 2019, 20, 4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker D; Kumar A; Adhikary A; Sevilla MD γ- and Ion-beam DNA Radiation Damage: Theory and Experiment. In DNA Damage DNA Repair and Disease Vol 2, Eds. Dizdaroglu M, Llyod RS, Royal Society of Chemistry (RSC), London, UK, 2020, 426–457. [Google Scholar]
- 18.Bernhard WA In ‘Radical and Radical Ion Reactivity in Nucleic Acid Chemistry’, Ed. Greenberg MM, John Wiley & Sons, Inc., New Jersey, 2009, 41–68. [Google Scholar]
- 19.Close DM In’ Radiation-induced Molecular Phenomena in Nucleic Acids: A Comprehensive Theoretical and Experimental Analysis’, Eds. Shukla MK, Leszczynski J, Springer-Verlag, Berlin, Heidelberg, New York, 2008, 493–529. [Google Scholar]
- 20.Ma J; Kumar A; Muroya Y; Yamashita S; Sakurai T; Denisov SA; Sevilla MD; Adhikary A; Seki S; Mostafavi M. Observation of Dissociative Quasi-Free Electron Attachment to Nucleoside via Excited Anion Radical in Solution. Nat. Commun 2019, 10 (1), 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boudaïffa B; Cloutier P; Hunting D; Huels MA; Sanche L. Resonant formation of DNA strand breaks by low-energy (3–20 eV) electrons. Science 2000, 287, 1658–1660. [DOI] [PubMed] [Google Scholar]
- 22.Fabrikant II; Eden S; Mason NJ; Fedor J. Recent Progress in Dissociative Electron Attachment: From Diatomics to Biomolecules, 1st ed.; Elsevier Inc., 2017, 66. 10.1016/bs.aamop.2017.02.002. [DOI] [Google Scholar]
- 23.Alizadeh E; Sanche L. Precursors of Solvated Electrons in Radiobiological Physics and Chemistry. Chem. Rev 2012, 112, 5578–5602. [DOI] [PubMed] [Google Scholar]
- 24.Kumar A; Sevilla MD Low-Energy Electron (LEE)-Induced DNA Damage: Theoretical Approaches to Modeling Experiment. In Handbook of Computational Chemistry, Ed. Leszczynski J, Springer, Dordrecht, The Netherlands, 2015, 1–63. [Google Scholar]
- 25.Kumar A; Becker D; Adhikary A; Sevilla MD Reaction of Electrons with DNA: Radiation Damage to Radiosensitization. Int. J. Mol. Sci 2019, 20, 3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma J; Denisov S; Adhikary A; Mostafavi M. Comment un électron induit un dommage oxydatif dans l’ADN. l’Actualité Chimique, 2020, 450, 13–18. [PMC free article] [PubMed] [Google Scholar]
- 27.Gu J; Leszczynski J; Schaefer III HF Interactions of electrons with bare and hydrated biomolecules: From nucleic acid bases to DNA segments. Chem. Rev 2012, 112, 5603–5640. [DOI] [PubMed] [Google Scholar]
- 28.Aflatooni K; Gallup GA; Burrow PD Electron attachment energies of the DNA bases. J. Phys. Chem. A 1998, 102, 6205–6207. [Google Scholar]
- 29.von Sonntag C. Free-radical-induced DNA damage and its repair: a chemical perspective; Springer: Berlin, 2006; ISBN 978–3-540–26120-9. [Google Scholar]
- 30.von Sonntag C. The Chemical Basis of Radiation Biology, Taylor and Francis, London, 1987. [Google Scholar]
- 31.Li X; Cai Z; Sevilla MD DFT Calculations of the Electron Affinities of Nucleic Acid Bases: Dealing with Negative Electron Affinities. J. Phys. Chem. A 2002, 106, 1596–1603. [Google Scholar]
- 32.Ma J; Wang F; Denisov SA; Adhikary A; Mostafavi M. Reactivity of Prehydrated Electrons towards Nucleobases and Nucleotides in Aqueous Solution. Sci. Adv 2017, 3, e1701669, DOI: 10.1126/sciadv.1701669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozak W; Demkowicz S; Daśko M; Rachon J; Rak J. Modifications at the C(5) Position of Pyrimidine Nucleosides. Russ. Chem. Rev 2020, 89, 281–310. [Google Scholar]
- 34.Chomicz L; Petrovici A; Archbold IJ; Adhikary A; Kumar A; Sevilla MD; Rak J. An ESR and DFT study of Hydration of the 2′-Deoxyuridine-5-yl Radical: Possible Hydroxyl Radical Intermediate. Chem. Commun 2014, 50, 14605–14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma J; Bahry T; Denisov S; Adhikary A; Mostafavi M. Quasi-free Electron-mediated Radiation Sensitization by C5-Halopyrimidines. J. Phys. Chem. A 2021, 125, 7967–7975 and references therein [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb CF; Jones GDD; Ward JF; Moyer DJ; Aguilera JA; Ling LL Mechanisms of Radiosensitization in Bromodeoxyuridine-substituted Cells. Int. J. Radiat Biol 1993, 64, 695–705. [DOI] [PubMed] [Google Scholar]
- 37.Lehnert S. Radiosensitizers and radiochemotherapy in the treatment of cancer. (CRC Press, Taylor & Francis Group, Boca Raton: 2015, 93–118. [Google Scholar]
- 38.Dabaja BS; McLaughlin P; Ha CS; Pro B; Meyers CA; Seabrooke LF; Wilder RB; Kyritsis AP; Preti HA; Yung WKA et al. Primary central nervous system lymphoma: Phase I evaluation of infusional bromodeoxyuridine with whole brain accelerated fractionation radiation therapy after chemotherapy. Cancer 2003, 98, 1021–1028. [DOI] [PubMed] [Google Scholar]
- 39.Prados MD; Seiferheld W; Sandler HM; Buckner JC; Phillips T; Schultz C; Urtasun R; Davis R; Gutin P; Cascino TL et al. Phase III randomized study of radiotherapy plus procarbazine, lomustine, and vincristine with or without BUdR for treatment of anaplastic astrocytoma: final report of RTOG 9404. Int. J. Radiat. Oncol. Biol. Phys 2004, 58, 1147–1152. [DOI] [PubMed] [Google Scholar]
- 40.Schurmann R; Vogel S; Ebel K; Bald I. The physico-chemical basis of DNA radiosensitization - implications for cancer radiation therapy. Chem. Eur. J 2018, 24, 1–10. [DOI] [PubMed] [Google Scholar]
- 41.Adhikary A; Khanduri D; Pottiboyina V; Rice CT; Sevilla MD Formation of Aminyl Radicals on Electron Attachment to AZT: Abstraction from the Sugar Phosphate Backbone versus One-Electron Oxidation of Guanine. J. Phys. Chem. B 2010, 114, 9289–9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mudgal M; Dang T; Sobczak A; Lumpuy D; Dutta P; Ward S; Ward K; Alahmadi M; Kumar A; Sevilla MD et al. Site of Azido Substitution in the Sugar Moiety of Azidopyrimidine Nucleosides Influences the Reactivity of Aminyl Radicals Formed by Dissociative Electron Attachment. J. Phys. Chem. B 2020, 124, 11357–11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mudgal M; Rishi S; Lumpuy DA; Curran KA; Verley KL; Sobczak AJ; Dang TP; Sulimoff N; Kumar A; Sevilla MD et al. Prehydrated One-Electron Attachment to Azido-Modified Pentofuranoses: Aminyl Radical Formation, Rapid H-Atom Transfer, and Subsequent Ring Opening. J. Phys. Chem. B 2017, 121, 4968–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka H; Hayakawa H; Iijima S; Haraguchi K; Miyasaka T, Lithiation of 3’,5’-O-(tetraisopropyldisiloxane-1,3-diyl)-2’-deoxyuridine: synthesis of 6-substituted 2’-deoxyuridines. Tetrahedron 1985, 41, 861–866. [Google Scholar]
- 45.Wojnárovits L; Takács E. Rate constants of sulfate radical anion reactions with organic molecules: A review. Chemosphere 2019, 220, 1014–1032. [DOI] [PubMed] [Google Scholar]
- 46.Tomasi J; Mennucci B; Cammi R. Quantum Mechanical Continuum Solvation Models. Chem. Rev 2005, 105, 2999–3094. [DOI] [PubMed] [Google Scholar]
- 47.Frisch MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Scalmani G; Barone V; Petersson GA; Nakatsuji H. et al. Gaussian 16, Revision A.03; Gaussian.Com; Gaussian, Inc., Wallingford CT, 2016. [Google Scholar]
- 48.Jmol: an open-source browser-based HTML5 viewer and stand-alone Java viewer for chemical structures in 3D http://jmol.sourceforge.net/ (accessed 2021 -10 -02).
- 49.Allouche A-R Gabedit—A Graphical User Interface for Computational Chemistry Softwares. J. Comput. Chem 2011, 32, 174–182. [DOI] [PubMed] [Google Scholar]
- 50.Kumar A; Sevilla MD Excited States of One-Electron Oxidized Guanine-Cytosine Base Pair Radicals: A Time Dependent Density Functional Theory Study. J. Phys. Chem. A 2019, 123, 3098–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.