Summary
Background
KL-A167 is a fully humanized monoclonal antibody targeting programmed cell death-ligand 1. This phase 2 study aimed to evaluate the efficacy and safety of KL-A167 in Chinese patients with previously treated recurrent or metastatic (R/M) nasopharyngeal carcinoma (NPC).
Methods
This was a multicentre, single-arm, phase 2 study of KL-A167 in R/M NPC (KL167-2-05-CTP) (NCT03848286), conducted at 42 hospitals across the People's Republic of China. Eligible patients had histologically confirmed nonkeratinising R/M NPC, and had failed at least two lines of chemotherapy. Patients received KL-A167 900mg intravenously once every 2 weeks until confirmed disease progression, intolerable toxicity, or withdrawal of informed consent. The primary endpoint was objective response rate (ORR) assessed by the independent review committee (IRC) according to RECIST v1.1.
Findings
Between Feb 26th, 2019 and Jan 13th, 2021, 153 patients were treated. Totally, 132 patients entered full analysis set (FAS) and were evaluated for the efficacy. As of data cutoff date on Jul 13th, 2021, the median follow-up time was 21.7 months (95%CI 19.8–22.5). For FAS population, the IRC-assessed ORR was 26.5% (95%CI 19.2–34.9%), and disease control rate (DCR) was 56.8% (95%CI 47.9–65.4%). Median progression-free survival (PFS) was 2.8 months (95%CI 1.5–4.1) . Median duration of response was 12.4 months (95%CI 6.8–16.5), and median overall survival (OS) was 16.2 months (95%CI 13.4–21.3). When using the cutoff of 1000 copies/ml, 5000 copies/ml and 10,000 copies/ml for plasma EBV DNA titer, baseline low plasma EBV DNA was consistently related with better DCR, PFS and OS. Dynamic change of plasma EBV DNA was significantly associated with ORR and PFS. Among 153 patients, treatment related-adverse events (TRAEs) occurred in 73.2% of patients, and grade ≥3 TRAEs were in 15.0% of patients. No TRAE leading to death was reported.
Conclusion
In this study, KL-A167 showed promising efficacy and an acceptable safety profile in patients with previously treated R/M NPC. Baseline plasma EBV DNA copy number might be a potentially useful prognostic biomarker for KL-A167 treatment, and post-treatment EBV DNA decrease might be correlated with better response to KL-A167.
Funding
Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd., China National Major Project for New Drug Innovation (2017ZX09304015).
Keywords: KL-A167, Nasopharyngeal carcinoma, Efficacy, Safety, PD-L1, Biomarker
Research in context.
Evidence before this study
We searched PubMed between Jan 1st, 2010 and Jan 31st, 2022, for studies relevant to efficacy and safety of programmed cell death-1(PD-1) and programmed cell death-ligand 1 (PD-L1) inhibitors using the search terms “PD-1” OR “PD-L1” OR “pembrolizumab” OR “nivolumab” OR “camrelizumab” OR “toripalimab” OR “tislelizumab” OR “sintilimab” OR “atezolizumab” OR “durvalumab” OR “avelumab” AND “recurrent or metastatic” AND “previously treated” OR “pretreated” AND “nasopharyngeal carcinoma (NPC)”, including related terminology. No language restrictions were used in the search. One phase 1 trial and four phase 2 trials were identified to evaluate the clinical benefits of PD-1 inhibitors (pembrolizumab, nivolumab, camrelizumab and toripalimab) in previously treated recurrent or metastatic (R/M) NPC. All these studies demonstrated promising efficacy and a manageable safety profile of a PD-1 inhibitor monotherapy in R/M NPC. A phase 2, randomized study evaluated the efficacy and safety of spartalizumab, an anti-PD-1 inhibitor, versus chemotherapy per investigator's choice in platinum-refractory R/M NPC, and the primary endpoint of median PFS was not met. In addition, a phase 1a trial evaluated the PD-L1 inhibitor atezolizumab in a small number of patients with previously treated, advanced head and neck cancer including four patients with R/M NPC, and reported a preliminary antitumour activity of atezolizumab. However, there was no published study that specially evaluated the efficacy and safety of a PD-L1 inhibitor in R/M NPC patients before this study.
Added value of this study
To the best of our knowledge, this study is the first published and largest report evaluating the efficacy and safety of a PD-L1 inhibitor in previously treated R/M NPC patients. The PD-L1 monoclonal antibody KL-A167 showed promising efficacy in Chinese patients with previously treated R/M NPC. Additionally, KL-A167 had a favorable safety profile, with no new or unexpected safety signals reported. The results of this study also confirmed the potential prognostic value of baseline plasma EBV DNA for KL-A167 treatment, and demonstrated post-treatment EBV DNA decrease might be correlated with better response to KL-A167.
Implications of all the available evidence
Outcomes for R/M NPC patients are poor, and there are limited therapeutic options. This study contributes to providing evidence on the benefits of anti-PD-L1 therapy in R/M NPC. KL-A167 may potentially become a new therapeutic option for patients with R/M NPC whose disease has progressed after platinum-based chemotherapy. The findings of this study suggest plasma EBV DNA might be a potentially useful biomarker for predicting clinical efficacy of KL-A167 treatment.
Alt-text: Unlabelled box
Introduction
Nasopharyngeal carcinoma (NPC) is characterized by a distinct pattern of geographical distribution, which is particularly endemic to Southern China, Southeast Asia, and North Africa.1,2 The age-standardized rate was 3 cases per 100,000 in China compared to 0.4 case per 100,000 in populations that are mainly white.1 In endemic regions, nonkeratinising differentiated and undifferentiated carcinomas are the predominant pathological subtypes, accounting for more than 95% of cases, and intimately associated with Epstein-Barr virus (EBV) infection.1,3,4 By contrast, keratinising subtype is more frequent in non-endemic areas.1,3
In the past few decades, advances in imaging and radiotherapy techniques, and broader application of systemic therapy have led to remarkable improvements in treatment outcomes.5 Despite these improvements, retrospective studies in the era of intensity-modulated radiotherapy revealed that local failure occurred in 5% to 15% of patients, and distant metastases were observed in 15% to 30% of patients.6 The clinical outcomes of patients with recurrent or metastatic (R/M) NPC are heterogeneous and generally remain disappointing. Chemotherapy with platinum-based doublets remains the standard of care in the first-line setting for patients with disseminated disease, yielding a median overall survival (OS) of 11 to 28 months.7, 8, 9 However, there is no accepted standard treatment option for patients with R/M NPC who have failed the first-line treatment.
EBV-associated NPC is characterized by high programmed cell death-ligand 1 (PD-L1) expression (expressed in up to 90% of NPC) and a tumor stroma that is densely infiltrated by immune cells.10, 11, 12, 13 These characteristics of NPC render it potentially susceptible to programmed cell death (PD-1)/PD-L1 blockade. Several phase 1 trials or phase 2 trials evaluated anti-PD-1 monoclonal antibodies in pretreated R/M NPC, and reported objective response rates (ORR) ranging from 20.5% to 34%, indicating promising clinical efficacy of PD-1 inhibitors.14, 15, 16, 17, 18 PD-L1 inhibitors, which directly blocks the PD-L1 and PD-1 interaction but leaves the binding of PD-L2 and PD-1 intact, have a mechanism of action distinct from PD-1 inhibitors.11,19,20 In contrast to anti-PD-1 therapy, data on the potential benefits of PD-L1 inhibitors for NPC are very limited. An unpublished phase 1 trial (NCT02825940) including 20 Chinese patients with pre-treated NPC reported an ORR of 10% and disease control rate (DCR) of 65% for atezolizumab treatment.21 Therefore, the therapeutic value of PD-L1 inhibitors for NPC remains to be fully established.
KL-A167, manufactured by Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd (Chengdu, China), is a fully humanized IgG1κ monoclonal antibody targeting PD-L1 that selectively blocks the interaction of PD-L1 and PD-1. On the basis of the phase 1 study of KL-A167 in lymphoma and solid tumors (data unpublished), the dose of 900 mg every 2 weeks was selected as recommended. Herein, we reported the results of this multicentre, single-arm, phase 2 pivotal study evaluating the efficacy and safety of KL-A167 in Chinese patients with previously treated R/M NPC. Additionally, associations between efficacy and baseline PD-L1 expression, as well as soluble PD-L1 (sPD-L1), baseline and dynamic plasma EBV DNA were explored.
Methods
Study design and participants
This was an open-label, multicentre, single-arm, phase 2 study of KL-A167 in patients with R/M NPC (KL167-2-05-CTP), conducted at 42 hospitals across the People's Republic of China. Eligible patients had histologically confirmed nonkeratinising NPC that was recurrent or metastatic. All patients were required to have stage IVb (defined by the eighth edition of the American Joint Committee on Cancer / International Union Against Cancer staging system for NPC) R/M NPC which had failed at least two lines of chemotherapy, and the first-line chemotherapy must include platinum-based regimens. Other key eligibility criteria included age ≥ 18 years, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1, at least one measurable lesion assessed by Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1), an estimated life expectancy ≥ 12 weeks, and adequate organ functions.
Patients who had previously received anti-PD-1, anti-PD-L1, anti-PD-L2, and/or anti-cytotoxic T-lymphocyte antigen antibody, chimeric antigen receptor T-cell immunotherapy, or any other agents targeting T-cell co-stimulation or checkpoint pathways were excluded. Other exclusion criteria included antitumor antibody therapy within 12 weeks and any other antitumor therapies within 4 weeks before the first dose of KL-A167, central nervous system metastases, any active autoimmune disease or history of autoimmune disease, active infection, and conditions requiring systemic treatment with either corticosteroids (>10 mg daily prednisone equivalent) or other immunosuppressive medications within 14 days of the first dose of KL-A167. The complete eligibility and exclusion criteria are provided in the protocol displayed in the Supplementary Material.
This study was conducted in accordance with the declaration of Helsinki and International Council for Harmonisation guidelines for Good Clinical Practice. The study protocol was approved by the independent ethics committee at each participating site. All patients provided written informed consent to participate before enrollment.
Procedures
Patients received KL-A167 treatment at the dose of 900 mg intravenously once every 2 weeks until confirmed disease progression, intolerable toxicity, or withdrawal of informed consent (whichever occurred first). Progressive disease (PD) could be confirmed by repeat assessments 4 weeks or more after response criteria were first met, at the discretion of the investigator. For patients who discontinued treatment due to other reasons except for documented disease progression, tumor assessments continued until documented disease progression, start of a new antitumor treatment, loss to follow-up or death. Dose reductions of KL-A167 were not allowed. Treatment with KL-A167 was discontinued if adverse events (AEs) failed to resolve to grade 0-1 within 12 weeks after the last dose.
All patients underwent tumor assessments at baseline, including computed tomography or magnetic resonance imaging of the nasopharyngeal, head, neck, chest, abdomen and pelvis. Response assessments were performed every 6 weeks for the first 24 months, and then could be performed every 12 weeks thereafter until disease progression, irrespective of treatment discontinuation. AEs were assessed at screening, continuously during treatment and for 30 days after completion of the withdrawal visit, and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. Immune-related adverse events (irAEs) were also reported and graded according to the National Comprehensive Cancer Network guideline for Management of Immunotherapy-Related Toxicities, Version 1.2020.22 Patients were followed up for survival throughout the treatment, and then every 30 days after completion of the safety follow-up visit.
Baseline biopsy specimens were obtained for exploratory biomarker analysis. Blood samples were collected for exploratory biomarker assessment at baseline, the time of each efficacy assessment, and the withdrawal visit.
Outcomes
The primary endpoint was ORR assessed by independent review committee (IRC) according to RECIST v1.1, defined as the proportion of patients who achieved confirmed complete response (CR) and partial response (PR). Secondary endpoints included investigator-assessed ORR per RECIST v1.1 and Immune-related Response Evaluation Criteria In Solid Tumors version 1.1 (irRECIST v1.1). Other secondary endpoints included DCR (defined as the proportion of patients who achieved CR, PR and stable disease [SD]), progression-free survival (PFS) (defined as the time from first KL-A167 dose to disease progression or death from any cause), duration of response (DOR) (defined as the time from the first documented CR and PR to the first documented disease progression), time to response (TTR) (defined as the time from first KL-A167 dose to the first documented CR and PR), OS (defined as the time from first KL-A167 dose to death from any cause), and safety.
Exploratory endpoints included associations between efficacy and PD-L1 expression, as well as sPD-L1 and plasma EBV DNA. PD-L1 expression was detected by immunohistochemistry (IHC) staining with SAB-028 antibody. PD-L1 positive expression was defined as membranous straining on ≥ 1% of tumor cells. sPD-L1 concentration was determined by enzyme-linked immunosorbent assay (ELISA). Plasma EBV DNA was measured by fluorescent quantitative polymerase chain reaction (PCR) at a central laboratory.
Statistical analysis
In the study protocol version 1.0 and version 1.1, the sample size of this study was estimated on the assumption that ORR to KL-A167 should be 26%, and the lower limit of 95% confidence interval (CI) of ORR was no less than 15% based on the Clopper-Pearson method. Considering a dropout rate of 20%, a total of 112 patients would be enrolled. However, the study protocol (Version 2.0 and Version 3.0) was amended to adjust the sample size based on the following assumptions, which obtained the consent from the Center for Drug Evaluation (CDE), National Medical Product Administration (NMPA) of China. A planned sample size of 139 provided approximately 90% power, with a two-sided α level of 0.05, to detect that the lower limit of 95% CI of ORR was no less than 15%, assuming an expected ORR of 26% for KL-A167 in R/M NPC. Considering a possible dropout rate of 10%, a sample size of 153 was required.
The intention-to treat (ITT) sample was defined as all patients enrolled in this study. Full analysis set (FAS) was defined as patients who received at least one dose of KL-A167 in the original protocol, but in the updated statistical analysis plan, it was amended to be defined as patients who received at least one dose of KL-A167, and must have failed at least two lines of prior therapy including the first-line platinum-containing therapy. Efficacy analyses were performed in FAS and the ITT sample. Safety was evaluated in safety set (SS) defined as all patients who received at least one dose of KL-A167 and had evaluable safety data. ORR and DCR with their two-sided exact 95% CIs were calculated with the Clopper-Pearson method. Median values and corresponding 95% CIs of PFS, OS, DOR and TTR were estimated using the Kaplan-Meier method. In exploratory biomarker analyses, categorical variables between groups were compared with Chi square test, and continuous variables were assessed using t-tests. All statistical analyses were done with SAS, version 9.4. This study is registered with ClinicalTrials.gov (NCT03848286).
Role of the funding resource
This study was designed by the sponsor and the principal investigator (Yuankai Shi). The sponsor provided funding and organizational support and had a role in data collection, data analysis, data interpretation and medical writing. All the authors had access to the raw data, reviewed and approved the final version of this article for submission. The corresponding author (Yuankai Shi) had the final responsibility for decisions related to the submission of these results for publication.
Results
Patient baseline characteristics
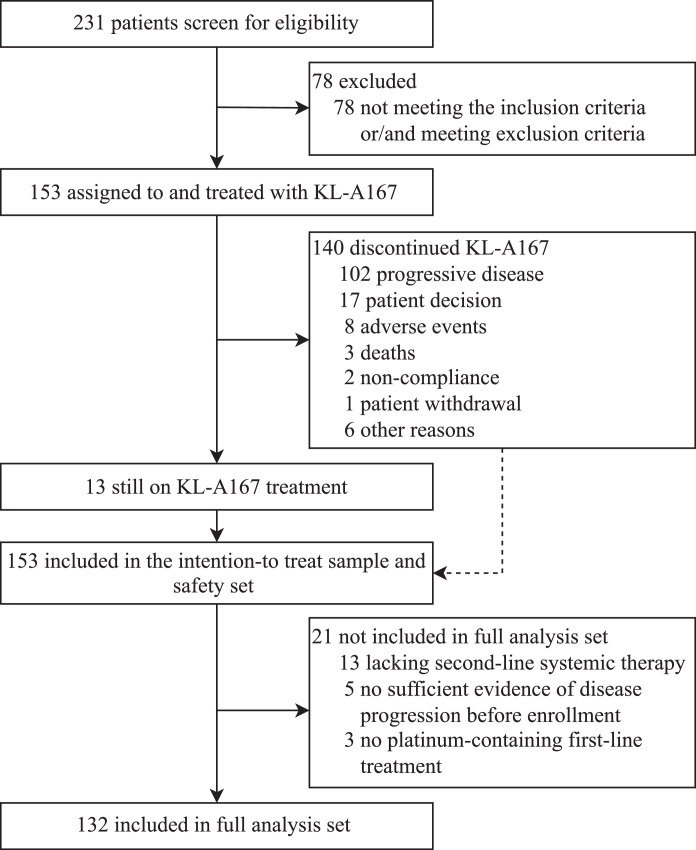
Between Feb 26th, 2019 and Jan 13th, 2021, 231 patients were screened for eligibility across 42 hospitals in the People's Republic of China; 153 patients received at least one dose of KL-A167 (Supplementary Table S1). All 153 patients were included in the ITT sample and SS, and 132 patients entered FAS (Figure 1). The data cutoff date was Jul 13th, 2021. Median follow-up time was 21.7 months (95%CI 19.8–22.5) for FAS, and 21.7 months (95%CI 20.0–22.5) for the ITT sample.
Figure 1.
Trial profiles.
Baseline characteristics of patients in FAS are shown in Table 1. The median age was 49 years (range 26–68), and 109 (82.6%) of 132 patients were male. All patients had received at least 2 prior lines of chemotherapy, and 42 (31.8%) of 132 patients had received at least three lines of chemotherapy. All 132 patients had nonkeratinising NPC, of which 84 (63.6%) patients were undifferentiated nonkeratinising, and 21 (15.9%) were differentiated nonkeratinising. At study entry, 58 (43.9%) of 132 patients had liver metastases. For the ITT sample, 98 (64.1%) patients were undifferentiated nonkeratinising, and 71 (46.4%) had liver metastases. Data on previous chemotherapy drugs used for advanced disease were shown in Supplementary Table S2. Detailed baseline characteristics of the ITT sample are also presented in Table 1.
Table 1.
Patient characteristics in FAS and the ITT sample.
| Characteristic | FAS (n=132) | ITT (n=153) |
|---|---|---|
| N (%) | N (%) | |
| Age, years | ||
| Median (range) | 49.0 (26.0–68.0) | 49.0 (20.0–68.0) |
| <65 | 126 (95.5) | 147 (96.1) |
| ≥65 | 6 (4.5) | 6 (3.9) |
| Gender | ||
| Male | 109 (82.6) | 125 (81.7) |
| Female | 23 (17.4) | 28 (18.3) |
| ECOG PS | ||
| 0 | 52 (39.4) | 59 (38.6) |
| 1 | 80 (60.6) | 94 (61.4) |
| Smoking history | ||
| Non-smoker | 87 (65.9) | 98 (64.1) |
| Current or former smoker | 45 (34.1) | 55 (35.9) |
| Prior radiotherapy | ||
| Yes | 126 (95.5) | 146 (95.4) |
| No | 6 (4.5) | 7 (4.6) |
| Prior radical chemoradiotherapy | ||
| Yes | 100 (75.8) | 117 (76.5) |
| No | 32 (24.2) | 36 (23.5) |
| Number of prior lines of chemotherapy | ||
| 1 | NA | 15 (9.8) |
| 2 | 90 (68.2) | 92 (60.1) |
| 3 or more | 42 (31.8) | 43 (28.1) |
| Unknown | NA | 3 (2.0) |
| Histology (WHO) | ||
| Undifferentiated nonkeratinising | 84 (63.6) | 98 (64.1) |
| Differentiated nonkeratinising | 21 (15.9) | 24 (15.7) |
| Nonkeratinising (differentiation unknown) | 27 (20.5) | 31 (20.3) |
| Liver metastases | ||
| Yes | 58 (43.9) | 71 (46.4) |
| No | 74 (56.1) | 82 (53.6) |
| Time since initial diagnosis, months | ||
| Median (range) | 33.1 (0.9–212.4) | 33.1 (0.2–212.4) |
| <36 | 72 (54.5) | 85 (55.6) |
| ≥36 | 60 (45.5) | 68 (44.4) |
| Time since last systemic therapy, months | ||
| Median (range) | 1.7 (0.03–16) | 1.7 (0.03–16) |
| ≤3 | 103 (78.0) | 119 (77.8) |
| 3-6 | 18 (13.6) | 21 (13.7) |
| >6 | 11 (8.3) | 13 (8.5) |
| The number of involved sites | ||
| <3 | 74 (56.1) | 84 (54.9) |
| ≥3 | 58 (43.9) | 69 (45.1) |
| The number of lesions | ||
| <5 | 24 (18.2) | 25 (16.3) |
| ≥5 | 108 (81.8) | 128 (83.7) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group; PS, performance status; NA, not applicable; WHO, World Health Organization.
Efficacy
As of data cutoff date on Jul 13th, 2021, of 153 patients, 89 (58.2%) deaths were observed, 13 (8.5%) patients remained on KL-A167 treatment, and 140 (91.5%) patients discontinued treatment. The major reasons for treatment discontinuation were PD (n=102), patient decision (n=17) and AEs (n=8). Patients received a median of 9 cycles (range 1–52) of KL-A167, with a median duration of exposure to KL-A167 of 4.1 months (range 0.5–24.3). Of 153 patients, 58 (37.9%) patients received KL-A167 treatment for ≥ 6 months and 27 (17.6%) patients were treated for ≥ 12 months.
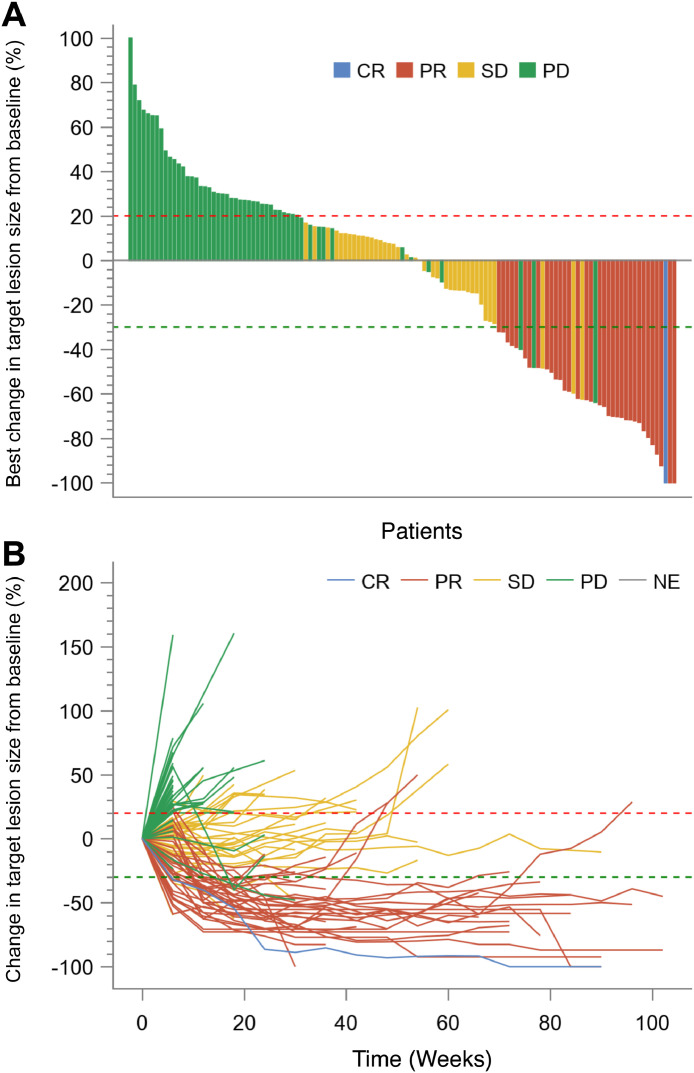
The IRC-assessed and investigator-assessed efficacy data for FAS and the ITT sample are presented in Table 2, with similar efficacy between FAS and the ITT sample. Of 132 patients in FAS, 35 (26.5% [95%CI 19.2–34.9%]) patients achieved an objective response assessed by IRC according to RECIST v1.1, with one (0.8%) patient achieving CR and 34 (25.8%) patients having PR (Table 2). IRC-assessed DCR was 56.8% (75/132, 95%CI 47.9–65.4%). Decrease in target lesion size was observed in 58 (37.9%) patients (Figure 2A). For patients in FAS, investigator-assessed ORR per RECIST v1.1 was 24.2% (32/132, 95%CI 17.2–32.5%), and investigator-assessed DCR was 54.5% (72/132, 95%CI 45.7–63.2%) (Table 2). The changes in target lesion size assessed by investigator for FAS are shown in Supplementary Figure S1A.
Table 2.
Response assessed in FAS and the ITT sample according to RECIST v1.1.
| Response | FAS (n=132) |
ITT (n=153) |
|||
|---|---|---|---|---|---|
| IRC-assessed | Investigator-assessed | IRC-assessed | Investigator-assessed | ||
| CR, n (%) | 1 (0.8) | 1 (0.8) | 1 (0.7) | 1 (0.7) | |
| PR, n (%) | 34 (25.8) | 31 (23.5) | 35 (22.9) | 32 (20.9) | |
| SD, n (%) | 40 (30.3) | 40 (30.3) | 46 (30.1) | 46 (30.1) | |
| PD, n (%) | 51 (38.6) | 54 (40.9) | 64 (41.8) | 67 (43.8) | |
| NE, n (%) | 6 (4.5) | 6 (4.5) | 7 (4.6) | 7 (4.6) | |
| ORR, n (% [95% CI]) | 35 (26.5 [19.2–34.9]) | 32 (24.2 [17.2–32.5]) | 36 (23.5 [17.1–31.1]) | 33 (21.6 [15.3–28.9]) | |
| DCR, n (% [95% CI]) | 75 (56.8 [47.9–65.4]) | 72 (54.5 [45.7–63.2]) | 82 (53.6 [45.4–61.7]) | 79 (51.6 [43.4–59.8]) | |
| Median TTR, months (95%CI) | 2.7 (1.4–2.8) | 2.7 (1.4–2.8) | 2.7 (1.4–2.8) | 2.7 (1.4–2.8) | |
| DOR | |||||
| Events, n (%) | 20 (57.1)* | 25 (78.1)* | 21 (58.3)* | 26 (78.8)* | |
| Median, months (95%CI) | 12.4 (6.8–16.5) | 9.6 (6.8–13.8) | 12.4 (6.8–16.5) | 9.6 (6.8–13.8) | |
| 12 months, % (95%CI) | 52.1 (33.5–67.8) | 42.4 (24.5–59.2) | 50.5 (32.3–66.1) | 40.9 (23.5–57.5) | |
| PFS | |||||
| Events, n (%) | 106 (80.3) | 115 (87.1) | 126 (82.4) | 136 (88.9) | |
| Median, months (95%CI) | 2.8 (1.5–4.1) | 2.8 (1.4–4.2) | 2.7 (1.4–4.0) | 2.7 (1.4–4.1) | |
| 12 months, % (95%CI) | 19.2 (12.5–27.0) | 17.2 (11.0–24.6) | 16.6 (10.8–23.5) | 14.7 (9.4–21.2) | |
*The percent was calculated based on the number of patients achieving CR or PR as denominator.
Abbreviation: FAS, full analysis set; ITT, intention-to-treat; IRC, Independent Review Committee; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response, SD, stable disease; PD, progressive disease; NE, not evaluable; ORR, objective response rate; DCR, disease control rate; TTR, time to response; DOR, duration of response; PFS, progression-free survival; CI, confidence interval.
Figure 2.
Tumor response per IRC according to RECIST v1.1. (A) Best change in sum of target lesion diameter from baseline for patients in FAS (n=132). (B) Spider plot of change in tumor size over time from baseline for patients in FAS (n=132). The blue line represents CR; red line represents PR; gold line represents SD; green line represents PD. The red dashed horizontal line represents 20% tumor increase, and the green dashed horizontal line represents 30% tumor reduction. Note: In Figure 2A, among 132 patients in the FAS, six patients terminated the study before performing efficacy assessment, and one patient did not have measurable target lesion at baseline assessed by IRC. Additionally, among the remaining 125 patients, the best change in sum of longest target lesion diameter from baseline was 0% for one patient. Therefore, a total of 124 bars for 124 patients are displayed in the waterfall plot. IRC, independent review committee; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1; FAS, full analysis set; CR, complete response; PR, partial response, SD, stable disease; PD, progressive disease; NE, not evaluable.
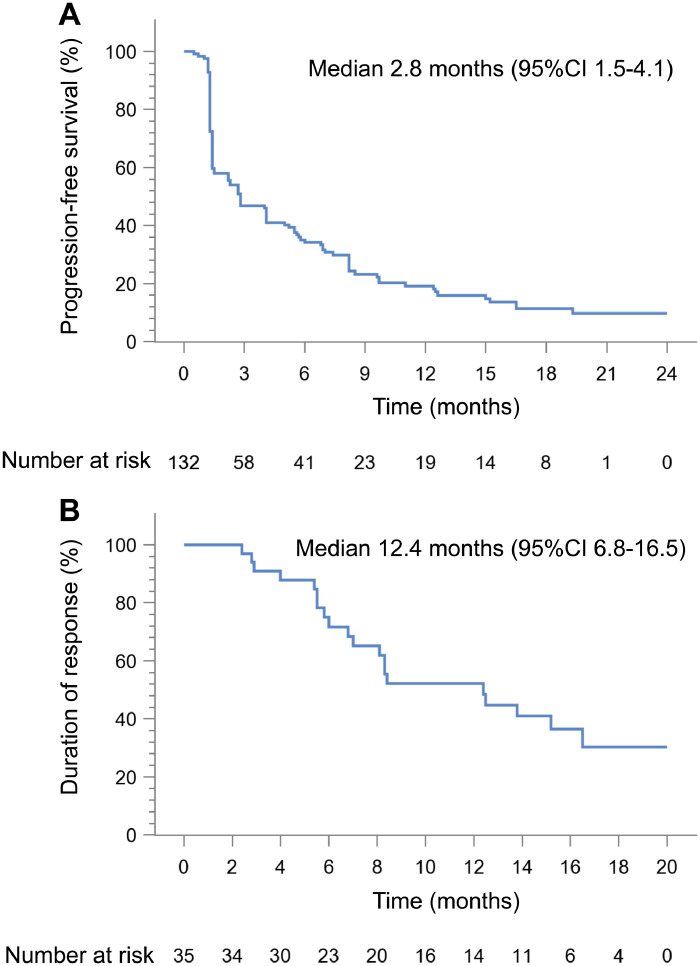
Of FAS, median TTR assessed by IRC was 2.7 months (95%CI 1.4–2.8). The IRC-assessed median PFS was 2.8 months (95%CI 1.5–4.1), with 12-month PFS rate of 19.2% (12.5–27.0%) (Figure 3A). Response for the 35 patients with CR or PR was durable, with median DOR of 12.4 months (95%CI 6.8–16.5) (Figure 2B and Figure 3B). Assessed by investigator, median TTR was 2.7 months (95%CI 1.4–2.8), median PFS was 2.8 months (95%CI 1.4–4.2), and median DOR was 9.6 months (95%CI 6.8–13.8), (Table 2, Supplementary Figure S1B and Figure S2). Median OS was 16.2 months (95%CI 13.4–21.3), with 12-month OS rate of 60.5% (95%CI 51.3–68.6%) and 24-month OS rate of 39.1% (95%CI 29.7–48.4%) (Supplementary Figure S3).
Figure 3.
Kaplan-Meier estimates of PFS and DOR per IRC according to RECIST v1.1. (A) PFS for patients in FAS (n=132). (B) DOR for patients who responded in FAS (n=35). PFS, progression-free survival; DOR, duration of response; IRC, independent review committee; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1; FAS, full analysis set
Post hoc, subgroup analyses of efficacy according to patient baseline characteristics were conducted in FAS. Objective responses were observed across all subgroups including subsets with poor prognosis. The IRC-assessed ORR for patients with liver metastases was 17.2% (8.6–29.4%), and ORR for patients with time interval since last systemic therapy ≤ 3 months was 25.2% (17.2–34.8%) (Supplementary Table S3).
Supplementary Table S4 summarizes the efficacy assessed by investigator according to irRECIST v1.1. For FAS, the ORR was 24.2% (95%CI 17.2–32.5%), with one (0.8%) patient achieving immune-related CR and 31 (23.5%) having immune-related PR. Disease control was achieved in 75 (56.8% [95%CI 47.9–65.4]) patients. Median TTR was 2.7 months (95%CI 1.4–2.8), and median PFS was 2.8 months (95%CI 1.4–4.2). Median DOR was 9.6 months (95%CI 6.8–13.8), with the 12-month DOR rate of 42.4% (95%CI 24.5–59.2%). The efficacy assessed by investigator according to irRECIST v1.1 for the ITT sample were similar to those for FAS (Supplementary Table S4).
Safety
Among 153 patients in SS, 147 (96.1%) experienced at least one treatment-emergent adverse events (TEAEs), with 65 (42.5%) patients having grade ≥ 3 TEAEs (Supplementary Table S5). Treatment related-adverse event (TRAEs) of any grade occurred in 112 (73.2%) patients, and grade ≥ 3 TRAEs occurred in 23 (15.0%) patients. The most common (≥ 5%) TRAEs of any grade included hypothyroidism (n = 36, 23.5%), anemia (n = 20, 13.1%), white blood cell count decrease (n = 16, 10.5%), aspartate aminotransferase (AST) increase (n = 14, 9.2%), alanine aminotransferase (ALT) increase (n = 13, 8.5%), fever (n = 11, 7.2%), hypoalbuminemia (n = 10, 6.5%), thrombocytopenia (n = 10, 6.5%), weight decreased (n = 9, 5.9%), fatigue (n = 9, 5.9%), blood creatine phosphokinase increased (n = 8, 5.2%), γ-glutamyltransferase (γ-GGT) elevation (n = 8, 5.2%) and proteinuria (n = 8, 5.2%). The most common grade ≥ 3 TRAEs were anemia (n = 7, 4.6%), γ-GGT elevation (n = 4, 2.6%), white blood cell count decrease (n = 2, 1.3%), AST increase (n = 2, 1.3%), and thrombocytopenia (n = 2, 1.3%). Detailed TRAEs are listed in Table 3.
Table 3.
TRAEs in SS (n=153).
| TRAEs | Any grade | Grade 1 | Grade 2 | Grade ≥ 3 |
|---|---|---|---|---|
| Hypothyroidism | 36 (23.5) | 14 (9.2) | 22 (14.4) | 0 (0) |
| Anemia | 20 (13.1) | 8 (5.2) | 5 (3.3) | 7 (4.6) |
| White blood cell count decrease | 16 (10.5) | 7 (4.6) | 7 (4.6) | 2(1.3) |
| Aspartate aminotransferase increase | 14 (9.2) | 10 (6.5) | 2 (1.3) | 2 (1.3) |
| Alanine aminotransferase increase | 13 (8.5) | 11 (7.2) | 2 (1.3) | 0 (0) |
| Pyrexia | 11 (7.2) | 9 (5.9) | 2 (1.3) | 0 (0) |
| Hypoalbuminemia | 10 (6.5) | 9 (5.9) | 1 (0.7) | 0 (0) |
| Thrombocytopenia | 10 (6.5) | 4 (2.6) | 4 (2.6) | 2(1.3) |
| Weight decrease | 9 (5.9) | 5 (3.3) | 4 (2.6) | 0 (0) |
| Fatigue | 9 (5.9) | 7 (4.6) | 1 (0.7) | 1 (0.7) |
| Blood creatine phosphokinase increase | 8 (5.2) | 6 (3.9) | 2 (1.3) | 0 (0) |
| γ-glutamyltransferase elevation | 8 (5.2) | 2 (1.3) | 2 (1.3) | 4(2.6) |
| Proteinuria | 8 (5.2) | 6 (3.9) | 2 (1.3) | 0 (0) |
| α-hydroxybutyrate dehydrogenase elevation | 5 (3.3) | 2 (1.3) | 2 (1.3) | 1 (0.7) |
| Lymphocyte count decrease | 5 (3.3) | 0 (0) | 4 (2.6) | 1 (0.7) |
| Chest discomfort | 3 (2.0) | 2 (1.3) | 0 (0) | 1 (0.7) |
| Troponin I elevation | 4 (2.6) | 1 (0.7) | 2 (1.3) | 1 (0.7) |
| Creatine phosphokinase-MB elevation | 4 (2.6) | 3 (2.0) | 0 (0) | 1 (0.7) |
| Alkaline phosphatase increase | 4 (2.6) | 0 (0) | 3 (2.0) | 1 (0.7) |
| Bone marrow failure | 3 (2.0) | 2 (1.3) | 0 (0) | 1 (0.7) |
| Diarrhea | 3 (2.0) | 2 (1.3) | 0 (0) | 1 (0.7) |
| Conjugated bilirubin elevation | 3 (2.0) | 0 (0) | 2 (1.3) | 1 (0.7) |
| Hyperuricemia | 2 (1.3) | 1 (0.7) | 0 (0) | 1 (0.7) |
| Pneumonia | 2 (1.3) | 1 (0.7) | 0 (0) | 1 (0.7) |
| Dyspnea | 2 (1.3) | 1 (0.7) | 0 (0) | 1 (0.7) |
| Diabetes | 1 (0.7) | 0 (0) | 0 (0) | 1 (0.7) |
| Upper gastrointestinal perforation | 1 (0.7) | 0 (0) | 0 (0) | 1 (0.7) |
| Immune mediated myocarditis | 1 (0.7) | 0 (0) | 0 (0) | 1 (0.7) |
| Bacterial infection of lower respiratory tract | 1 (0.7) | 0 (0) | 0 (0) | 1 (0.7) |
| Fungal infection of lower respiratory tract | 1 (0.7) | 0 (0) | 0 (0) | 1 (0.7) |
| Fasciitis | 1 (0.7) | 0 (0) | 0 (0) | 1 (0.7) |
| Radiation-induced brain injury | 1 (0.7) | 0 (0) | 0 (0) | 1 (0.7) |
Abbreviation: TRAEs, treatment-related adverse events; SS, safety set.
Note: Data are n (%). TRAEs of any grade were reported in at least 5% of patients, or all the grade ≥3 TRAEs were reported. No grade 5 TRAE was observed.
Ten patients (6.5%) had serious TRAEs. TRAEs leading to treatment interruption occurred in 27 (17.6%) patients. A total of 4 (2.6%) patients experienced TRAEs leading to treatment discontinuation including diabetes, bacterial infection of lower respiratory tract, fungal infection of lower respiratory tract, AST increase, radiation-induced brain injury, and immune mediated myocarditis. No TRAE leading to death was reported (Supplementary Table S5).
The irAEs of any grade were reported in 31 (20.3%) patients, and the incidence of grade ≥ 3 irAEs was 3.9%. No grade 4 or 5 irAE was observed. The most frequently reported irAEs of any grade were hypothyroidism (n = 20, 13.1%), hyperthyroidism (n = 3, 2.0%), thyroxine decrease (n = 2, 1.3%), thyroid stimulating hormone increase (n = 2, 1.3%) and platelet count decrease (n = 2, 1.3%). The grade ≥ 3 irAEs included platelet count decrease (n = 2, 1.3%), diabetes (n = 1, 0.7%), immune mediated myocarditis (n = 1, 0.7%), lymphocyte count decrease (n = 1, 0.7%), and γ-GGT elevation (n = 1, 0.7%). Detailed irAEs are summarized in Supplementary Table S6.
Biomarker analysis
Among 132 patients in FAS, 110 (83.3%) were PD-L1 positive, 17 (12.9%) were PD-L1 negative and the status of PD-L1 expression for 5 (3.8%) patients was unknown. There was no statistically significant difference between PD-L1 positive patients and PD-L1 negative patients regarding ORR, DCR, PFS and OS (Supplementary Table S7). In total, 131 of 132 (99.2%) patients had baseline sPD-L1 data. There was no significant association between baseline sPD-L1 concentration and tumor response (P = 0.504) and disease control (P = 0.108) (Supplementary Table S8).
Data on baseline plasma EBV DNA copy number was available in 131 of 132 (99.2%) patients. Patients with baseline plasma EBV DNA < 1000 copies/ml (n = 51) tended to have a better ORR than those with EBV DNA ≥ 1000 copies/ml (n = 80) (35.3% vs 21.3%, P = 0.077). Patients with baseline plasma EBV DNA < 1000 copies/ml showed a better DCR (72.5% vs 47.5%, P = 0.005), median PFS (6.9 months vs 1.5 months, P = 0.012) and median OS (25.0 months vs 13.5 months, P<0.001) than patients with EBV DNA ≥ 1000 copies/ml. When using the cutoff of 5000 copies/ml and 10,000 copies/ml for plasma EBV DNA titer, better DCR and OS were observed in patients with EBV DNA < 5000 copies/ml; better DCR, PFS and OS were shown in those with EBV DNA < 10,000 copies/ml (Supplementary Table S9).
The results of dynamic plasma EBV DNA were available for 123 of 132 (93.2%) patients. The associations of the maximum change of plasma EBV DNA from baseline to post-treatment with efficacy were analyzed (Supplementary Table S10). Patients with no plasma EBV DNA increase after treatment (n = 32) had a significantly higher ORR (50.0% vs 20.9%, P = 0.002), and a longer median PFS (5.5 months vs 2.8 months, P = 0.021) than those with plasma EBV DNA increase (n = 91). No significant association between the maximum change of plasma EBV DNA from baseline to post-treatment and DCR was observed. Furthermore, patients with >50% EBV DNA decrease after treatment (n = 13) had a significantly better ORR (53.8% vs 36.8% vs 19.4%, P = 0.016) than patients with ≤50% EBV DNA decrease but ≤100% increase after treatment (n = 38), and those with >100% EBV DNA increase after treatment (n = 72) (Supplementary Table S10).
Discussion
Outcomes for patients with R/M NPC are poor, and there are limited therapeutic options, despite the approvals of PD-1 inhibitors for the treatment of this patient population.14, 15, 16, 17,23 To the best of our knowledge, this study is the first published and largest report evaluating the efficacy and safety of a PD-L1 inhibitor in previously treated R/M NPC. In this study, KL-A167 monotherapy showed promising efficacy and a manageable safety profile in patients with previously treated R/M NPC.
Chemotherapy with single agent, including gemcitabine, capecitabine and docetaxel, severs as a suitable second-line treatment option for R/M NPC patients who were previously treated with platinum-based chemotherapy, with reported ORRs of 23.5–48% and median OS of 7.6–12.8 months.24, 25, 26 Several clinical trials have evaluated anti-PD-1 therapies in R/M NPC, wherein anti-PD-1 antibodies demonstrated promising clinical efficacy and safety profile.14, 15, 16, 17, 18,23 In a phase 1 trial enrolling 91 Chinese pretreated patients with R/M NPC, camrelizumab monotherapy exhibited an ORR of 34% and DCR of 59%.16 In the phase 2 study of camrelizumab (CAPTAIN study) including 156 Chinese patients with previously treated R/M NPC, the IRC-assessed ORR was 28.2% and DCR was 54.5%.18 The POLARIS-02 study evaluated toripalimab in 190 previously treated R/M NPC, and reported an ORR of 20.5% and DCR of 40.0%.17 For 92 patients who failed at least two lines of systemic chemotherapy, toripalimab resulted in an ORR of 23.9% and DCR of 41.3%.17 Our study evaluated the efficacy and safety of a PD-L1 inhibitor for the first time. In our study, all 132 patients in FAS had failed at least two lines of chemotherapy, and had nonkeratinising NPC. Partly distinct from our study, the POLARIS-02 study enrolled 51.6% (98/190) of patients who had only one prior line of systemic chemotherapy, and a small proportion of patients (4.2%) with keratinising NPC.17 It remains unclear whether there was a significant association between histological subtypes and clinical efficacy of anti-PD-1 or anti-PD-L1 therapies. Despite these differences, the efficacy with KL-A167 in our study were comparable with that reported with PD-1 inhibitors,14, 15, 16, 17, 18 with an IRC-assessed ORR of 26.5% and DCR of 56.8% for patients in FAS. There was a high degree of agreement between efficacy assessed by IRC and that assessed by investigator, both in FAS and the ITT sample. The similar response rates assessed by investigator per irRECIST further supported the promising efficacy of KL-A167 in previously treated R/M NPC. Additionally, comparable to PD-1 inhibitors,14, 15, 16, 17,23 the median DOR per IRC of KL-A167 was 12.4 months, indicating that patients could achieve durable benefit from KL-A167.
In addition to inhibiting the binding of PD-L1 to PD-1, PD-L1 inhibitors provide the advantage of blocking the PD-L1 and B7.1 (also called CD80) binding, therefore further enhancing anticancer immunity.27,28 In contrast to targeting PD-1, targeting PD-L1 may not inhibit T-cell responses through the PD-1/PD-L2 axis. However, PD-L2 expression, only in a tiny minority of human tumors, is far less prevalent than PD-L1, and anti-PD-L2 blockade had a much weaker antitumor effect compared with anti-PD-L1 blockade.19 Moreover, direct targeting of PD-L1 leaves the PD-L2 and PD-1 interaction intact, which could reduce the likelihood of developing autoimmune adverse events.19,20 Unlike PD-1 studies, data on the potential benefits of PD-L1 inhibitors are limited in NPC. Previously, a phase 1a trial, which evaluated atezolizumab in 32 previously treated, advanced head and neck cancer patients including four patients with R/M NPC, reported an ORR of 22% and median DOR of 7.4 months.29 Another unpublished phase 1 trial (NCT02825940) evaluated the clinical benefit of atezolizumab in patients with locally advanced or metastatic solid tumors who had exhausted all available standard treatment options. In that study, only 20 Chinese NPC patients were enrolled, and the ORR was 10% and DCR was 65%.21 Our study, with a much larger series of R/M NPC patients, suggested the promising efficacy of KL-A167, which contributes to providing evidence on the benefits of anti-PD-L1 therapy in R/M NPC.
Adverse events of KL-A167 were mainly grade 1 or 2 in our study, and could be manageable with standard clinical practice. Although direct comparison between studies is not possible, the safety profile of KL-A167 was generally consistent with that observed in other PD-1 or PD-L1 inhibitors.14, 15, 16, 17, 18,21,23,29 No new safety signals for KL-A167 were noted. Common TRAEs for KL-A167 included hypothyroidism, anemia, and white blood cell count decrease, which were generally grade 1 or 2. The discontinuation rate of KL-A167 as a result of TRAEs was low (2.6%), and no TRAE leading to death occurred. Of note, rash, which was frequently observed with pembrolizumab (25.9%),14 camrelizumab (16.0%),16 toripalimab (6.3%) 17 and atezolizumab (16.0%),29 occurred at a much lower frequency (2.6%) for KL-A167. Additionally, the incidence of specific irAEs was low. Taken together, KL-A167 monotherapy has a manageable safety profile in patients with previously treated R/M NPC.
To date, robust predictive biomarkers for treatment responses of anti-PD-1 or PD-L1 therapy have not been well established in NPC. Previous evidence has supports the correlation between PD-L1 expression in tumor cells or tumor-infiltrating immune cells and clinical outcomes to immune checkpoint inhibitor therapy in various cancers, however, the predictive role of PD-L1 expression in NPC needs to be defined. Consistent with previous studies of PD-1 inhibitors in NPC,15, 16, 17, 18 there was no significant association between PD-L1 expression and clinical efficacy of KL-A167 therapy in our study. Besides, no obvious association between baseline sPD-L1 concentration and tumor response was observed in our study. The differences in PD-L1 IHC assays and cutoffs may limit the use of PD-L1 as a robust biomarker in NPC.
The role of plasma EBV DNA as a clinically useful biomarker in the detection, monitoring, prognostication, guiding adjuvant or induction chemotherapy, and surveillance for NPC has been well defined.5,30,31 However, the value of plasma EBV DNA in predicting response to anti-PD-1 or PD-L1 therapy in NPC has been less investigated. In POLARIS-02 study, patients with baseline EBV titer ≤ 10,000 IU/mL showed numerically higher ORR than those with EBV DNA titer >10,000 IU/mL (54.5% vs 39.5%, P = 0.088). Besides, there was a significant correlation between an early decrease in plasma EBV DNA copy number and favorable response.17 In CAPTAIN study, patients with negative EBV DNA level at baseline had higher ORR (41.0% vs 23.9%) and longer median PFS (6.0 vs 2.7 months) than those with positive EBV DNA level, and patients with ≥ 50% EBV DNA level decrease showed better ORR and PFS than those with < 50% decrease.18 Our study evaluated potential correlations of EBV DNA status with clinical outcomes of a PD-L1 inhibitor in R/M NPC for the first time. In our study, baseline plasma EBV DNA < 1000 copies/ml was associated with better DCR, PFS and OS, although its correlation with ORR did not reach statistical significance (P = 0.077). Notably, when the cutoff values of 5000 copies/ml and 10,000 copies/ml of plasma EBV DNA were used, similar results were observed. Importantly, our results also demonstrated the dynamic change of plasma EBV DNA were significantly associated with ORR and OS of KL-A167, and post-treatment EBV DNA decrease might be correlated with better response to KL-A167. Taken together, baseline and dynamic change of plasma EBV DNA may be a potentially useful biomarker for predicting efficacy of KL-A167 treatment. More investigations are needed to further confirm the predictive value of plasma EBV DNA on response to KL-A167 treatment in NPC.
Our study has several limitations. First, this study was single-arm without a control group for comparison. Another limitation was that all patients enrolled in our study had a histology of World Health Organization (WHO) type 2 (nonkeratinising NPC). Therefore, whether our results could be applied to the histology of the WHO type 1 (keratinising squamous NPC) or type 3 (basaloid squamous NPC) remains unclear. Despite these limitations, the sample size of our study is large, increasing the ability to evaluate the clinical benefit of KL-A167. Of note, the recent KEYNOTE-122 study (NCT02611960) compared pembrolizumab monotherapy versus investigator's choice chemotherapy in 233 patients with platinum-pretreated R/M NPC.32 The primary endpoint, OS, was not significantly different between the two arms, with the median OS of 17.2 months (95%CI 11.7–22.9) for pembrolizumab and 15.3 months (95%CI 10.9–18.1) for chemotherapy (HR = 0.90, 95% CI, 0.67–1.19; P = 0.2262).32 Another phase 2 study compared the efficacy and safety of spartalizumab versus chemotherapy per investigator's choice in R/M NPC that progressed on/after platinum-based chemotherapy.33 The primary endpoint of median PFS was not met (1.9 months in the spartalizumab arm versus 6.6 months in the chemotherapy arm, P = 0.915), whereas median OS and median DOR were significantly longer with spartalizumab.33 Despite these negative results, the results of our study demonstrate that KL-A167 therapy still had promising efficacy in R/M NPC that failed at least two lines of chemotherapy and thus, has a place in the treatment paradigm of R/M NPC.
In conclusion, in this study, KL-A167 showed promising efficacy and an acceptable safety profile in patients with previously treated R/M NPC. Based on the results of this study, KL-A167 may potentially serve as a new therapeutic option for patients with R/M NPC whose disease has progressed after platinum-based chemotherapy. Baseline plasma EBV DNA copy number might be a potentially useful prognostic biomarker for KL-A167 treatment, and post-treatment EBV DNA decrease might be correlated with better response to KL-A167. More investigations are warranted to validate the predictive value of plasma EBV DNA on response to KL-A167 treatment in NPC. A phase 3 trial comparing KL-A167 combined with cisplatin and gemcitabine versus placebo combined with cisplatin and gemcitabine as first-line treatment for R/M NPC is ongoing (NCT05294172).
Contributors
Yuankai Shi was the leading principal investigator, contributed to study conception and design, study supervision, data acquisition and interpretation, manuscript writing, editing and revision. Xintian Qin, Xingchen Peng, Aiping Zeng, Jingao Li, Chuanben Chen, Sufang Qiu, Suming Pan, Yulong Zheng, Jing Cai, Xiaopin Chen, Shenhong Qu, Lizhu Lin, Jianli Huang, Hui Wu, Ying Lu, Wei Wang, Changlu Hu, Xia He, Zhonghua Yu, Xiaojian Liu, Bo Xie, Anwen Liu, Guangyuan Hu, Shanghua Jing, Qingyuan Zhang, Renhua Guo, Qi Li, Jinsheng Hong, Feng Jin, Juan Meng, Jianhua Shi, Peiguo Wang, Jiuwei Cui, Kunyu Yang, Xuebang Zhang, Xiaojiang Li, Liangfang Shen, Yuxiang He, Limin Zhai, Xiuhua Sun enrolled patients and collected the data. Junyou Ge, Yan Qing and Dekang Zong were involved in data analysis and data interpretation. All authors had full access to the data in the study, critically reviewed and approved the submission.
Data sharing statement
Data generated and analyzed in this study could be available upon reasonable request to the corresponding author Yuankai Shi.
Declaration of interests
Junyou Ge, Yan Qing and Dekang Zong are employees of Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd (Chengdu, China). All other authors declare no potential conflicts of interest.
Acknowledgements
This study was funded by Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (Chengdu, China), and also supported in part by China National Major Project for New Drug Innovation (2017ZX09304015). The authors would like to thank all the participating patients, their families, and the study teams for supporting this study. The authors also would like to thank Dr. Haizhu Chen (National Cancer Center and National Clinical Research Center for Cancer and Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College) for providing medical writing assistance with this article, funded by Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (Chengdu, China).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100617.
Appendix. Supplementary materials
References
- 1.Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet (London, England) 2019;394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet (London, England) 2016;387(10022):1012–1024. doi: 10.1016/S0140-6736(15)00055-0. [DOI] [PubMed] [Google Scholar]
- 4.Young LS, Dawson CW. Epstein-Barr virus and nasopharyngeal carcinoma. Chin J Cancer. 2014;33(12):581–590. doi: 10.5732/cjc.014.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong KCW, Hui EP, Lo KW, et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat Rev Clin Oncol. 2021;18(11):679–695. doi: 10.1038/s41571-021-00524-x. [DOI] [PubMed] [Google Scholar]
- 6.Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol: Off J Am Soc Clin Oncol. 2015;33(29):3356–3364. doi: 10.1200/JCO.2015.60.9347. [DOI] [PubMed] [Google Scholar]
- 7.Ngan RK, Yiu HH, Lau WH, et al. Combination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: report of a phase II study. Ann Oncol: Off J Eur Soc Med Oncol. 2002;13(8):1252–1258. doi: 10.1093/annonc/mdf200. [DOI] [PubMed] [Google Scholar]
- 8.Chua DT, Yiu HH, Seetalarom K, et al. Phase II trial of capecitabine plus cisplatin as first-line therapy in patients with metastatic nasopharyngeal cancer. Head Neck. 2012;34(9):1225–1230. doi: 10.1002/hed.21884. [DOI] [PubMed] [Google Scholar]
- 9.Hong S, Zhang Y, Yu G, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin as first-line therapy for recurrent or metastatic nasopharyngeal carcinoma: final overall survival analysis of GEM20110714 phase III study. J Clin Oncol: Off J Am Soc Clin Oncol. 2021;39(29):3273–3282. doi: 10.1200/JCO.21.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Q, Cai MY, Chen CL, et al. Tumor cells PD-L1 expression as a favorable prognosis factor in nasopharyngeal carcinoma patients with pre-existing intratumor-infiltrating lymphocytes. Oncoimmunology. 2017;6(5) doi: 10.1080/2162402X.2017.1312240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YQ, Chen YP, Zhang Y, et al. Prognostic significance of tumor-infiltrating lymphocytes in nondisseminated nasopharyngeal carcinoma: A large-scale cohort study. Int J Cancer. 2018;142(12):2558–2566. doi: 10.1002/ijc.31279. [DOI] [PubMed] [Google Scholar]
- 12.Ono T, Azuma K, Kawahara A, et al. Prognostic stratification of patients with nasopharyngeal carcinoma based on tumor immune microenvironment. Head Neck. 2018;40(9):2007–2019. doi: 10.1002/hed.25189. [DOI] [PubMed] [Google Scholar]
- 13.Hsu MC, Hsiao JR, Chang KC, et al. Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod Pathol: Off J US Can Acad Pathol. 2010;23(10):1393–1403. doi: 10.1038/modpathol.2010.130. [DOI] [PubMed] [Google Scholar]
- 14.Hsu C, Lee SH, Ejadi S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol: Off J Am Soc Clin Oncol. 2017;35(36):4050–4056. doi: 10.1200/JCO.2017.73.3675. [DOI] [PubMed] [Google Scholar]
- 15.Ma BBY, Lim WT, Goh BC, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the mayo clinic phase 2 consortium (NCI-9742) J Clin Oncol: Off J Am Soc Clin Oncol. 2018;36(14):1412–1418. doi: 10.1200/JCO.2017.77.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang W, Yang Y, Ma Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19(10):1338–1350. doi: 10.1016/S1470-2045(18)30495-9. [DOI] [PubMed] [Google Scholar]
- 17.Wang FH, Wei XL, Feng J, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase ii clinical trial (POLARIS-02) J Clin Oncol: Off J Am Soc Clin Oncol. 2021;39(7):704–712. doi: 10.1200/JCO.20.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Zhou T, Chen X, et al. Efficacy, safety, and biomarker analysis of camrelizumab in previously treated recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN study) J Immunother Cancer. 2021;9(12):e003790. doi: 10.1136/jitc-2021-003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res: Off J Am Assoc Cancer Res. 2012;18(24):6580–6587. doi: 10.1158/1078-0432.CCR-12-1362. [DOI] [PubMed] [Google Scholar]
- 20.Akbari O, Stock P, Singh AK, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010;3(1):81–91. doi: 10.1038/mi.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen L, Zhang L, Hu X, et al. Atezolizumab monotherapy in Chinese patients with locally advanced or metastatic solid tumours. Ann Oncol. 2018;29:ix49. [Google Scholar]
- 22.Thompson JA, Schneider BJ, Brahmer J, et al. NCCN guidelines insights: management of immunotherapy-related toxicities, version 1.2020. J Natl Compr Cancer Netw: JNCCN. 2020;18(3):230–241. doi: 10.6004/jnccn.2020.0012. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Huang X, Bai Y-X, et al. Preliminary Results with Tislelizumab, an Investigational Anti-PD-1 Antibody, in Chinese Patients with Nasopharyngeal Cancer (NPC) J Clin Oncol. 2019;37(no. 15_suppl):2556. [Google Scholar]
- 24.Foo KF, Tan EH, Leong SS, et al. Gemcitabine in metastatic nasopharyngeal carcinoma of the undifferentiated type. Ann Oncol: Off J Eur Soc Med Oncol. 2002;13(1):150–156. doi: 10.1093/annonc/mdf002. [DOI] [PubMed] [Google Scholar]
- 25.Chua DT, Sham JS, Au GK. A phase II study of capecitabine in patients with recurrent and metastatic nasopharyngeal carcinoma pretreated with platinum-based chemotherapy. Oral Oncol. 2003;39(4):361–366. doi: 10.1016/s1368-8375(02)00120-3. [DOI] [PubMed] [Google Scholar]
- 26.Ngeow J, Lim WT, Leong SS, et al. Docetaxel is effective in heavily pretreated patients with disseminated nasopharyngeal carcinoma. Ann Oncol: Off J Eur Soc Med Oncol. 2011;22(3):718–722. doi: 10.1093/annonc/mdq425. [DOI] [PubMed] [Google Scholar]
- 27.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JJ, Omiya R, Matsumura Y, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116(8):1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colevas AD, Bahleda R, Braiteh F, et al. Safety and clinical activity of atezolizumab in head and neck cancer: results from a phase I trial. Ann Oncol: Off J Eur Soc Med Oncol. 2018;29(11):2247–2253. doi: 10.1093/annonc/mdy411. [DOI] [PubMed] [Google Scholar]
- 30.Lee AWM, Lee VHF, Ng WT, et al. A systematic review and recommendations on the use of plasma EBV DNA for nasopharyngeal carcinoma. Eur J Cancer. 2021;153:109–122. doi: 10.1016/j.ejca.2021.05.022. [DOI] [PubMed] [Google Scholar]
- 31.Trevisiol C, Gion M, Vaona A, et al. The appropriate use of circulating EBV-DNA in nasopharyngeal carcinoma: comprehensive clinical practice guidelines evaluation. Oral Oncol. 2021;114 doi: 10.1016/j.oraloncology.2020.105128. [DOI] [PubMed] [Google Scholar]
- 32.Chan A, Lee V, Hong R, et al. 858O Results of KEYNOTE-122: a phase III study of pembrolizumab (pembro) monotherapy vs chemotherapy (chemo) for platinum-pretreated, recurrent or metastatic (R/M) nasopharyngeal carcinoma (NPC) Ann Oncol. 2021;32:S786. doi: 10.1016/j.annonc.2022.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Even C, Wang HM, Li SH, et al. Phase II, randomized study of spartalizumab (PDR001), an anti-PD-1 antibody, versus chemotherapy in patients with recurrent/metastatic nasopharyngeal cancer. Clin Cancer Res: Off J Am Assoc Cancer Res. 2021;27(23):6413–6423. doi: 10.1158/1078-0432.CCR-21-0822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.