Abstract
Objective
To evaluate the efficacy and safety of pentosan polysulfate sodium (PPS, Elmiron®) for dyslipidaemia and knee osteoarthritis (OA) related symptoms.
Method
This was a single-arm, open-label, prospective, non-randomised pilot study. People with painful knee OA and a history of primary hypercholesterolemia were included. PPS was taken orally in a dosage of 10 mg/kg once every 4 days for 5 weeks for two cycles. There was 5 weeks of no medication between the cycles. The main outcomes included the change in lipidemia levels, the change in knee OA-related symptoms assessed by pain numerical rating scale (NRS) and Knee Osteoarthritis Outcome Score (KOOS), and knee MRI semi-quantitative score. The changes were analysed using paired t-tests.
Results
38 participants were included, with a mean age of 62.2 years. We found a statistically significant decrease in total cholesterol (from 6.23 ± 0.74 to 5.95 ± 0.77 mmol/L; P = 0.01) and low-density lipoprotein (from 4.03 ± 0.61 to 3.82 ± 0.61 mmol/L; P = 0.009) from baseline to week 16. Knee pain NRS was significantly reduced at weeks 6, 16 and 26 from 6.39 ± 1.33 to 4.18 ± 1.99, 3.63 ± 2.28 and 4.38 ± 2.55, respectively (P < 0.001). However, there was no significant difference in terms of the primary outcome of triglyceride levels before and after treatment. The most common AEs were positive faecal occult blood tests, followed by headache and diarrhoea.
Conclusion
The findings suggest that PPS has promising effects on improving dyslipidaemia and symptomatic pain relief in people with knee OA.
Keywords: Knee osteoarthritis, Dyslipidaemia, Pentosan polysulfate sodium
1. Introduction
Osteoarthritis (OA) is a highly prevalent disease of the whole joint, involving structural changes in the articular cartilage, subchondral bone, ligaments, capsule, synovial membrane, and periarticular muscles [1]. Knee OA accounts for a substantial burden of osteoarthritis worldwide [2]. Studies showed knee OA with concomitant metabolic syndrome (MetS) have more significant radiological damage and functional disability [3]. Evidence from epidemiological and clinical studies has linked MetS to OA, including dyslipidaemia And lipidomics appears to be associated with OA severity [4]. A recent study showed that a substantial proportion of people with knee OA have dyslipidaemia [5]. Dyslipidaemia has been involved in metabolic-triggered inflammation in people with OA [6].
Pentosan polysulfate sodium (PPS) is a heparin analogue and glycosaminoglycans derivative and the active ingredient of Elmiron®, a drug approved for relieving bladder pain associated with interstitial cystitis [7,8]. PPS has exhibited potential treatment benefits for OA in previous studies [[9], [10], [11]]. One of the studies showed that PPS has anti-inflammatory activity by inducing chondrogenesis [9]. PPS at 1.3 mg/kg body weight, when injected into horses, significantly increased plasma total lipase activity [12]. Administration of PPS to hyperoxaluric rats significantly reduced tissue cholesterol and triglyceride levels [13]. These studies show that PPS is associated with antilipemia and prevents calcium oxalate crystallization in vitro and in vivo. Moreover, they concluded that PPS is a novel-inhibitor of hepcidin-facilitated osteoclast formation/function, which might be related to the treatment benefit for OA. In the context of the available evidence, oral PPS seems to be a promising treatment option for knee OA and dyslipidemia, with the potential to improve plasma lipid levels, clinical assessments, and cartilage metabolism [14]. However, the data on people with knee OA is scarce.
The primary objective of this pilot study was to evaluate the efficacy of PPS on the improvement of dyslipidaemia in people with knee OA. The secondary objective was to evaluate the efficacy and safety of PPS on pain, stiffness, and functional assessments, as well as structural changes.
2. Method
2.1. Study design
This was a single-arm, open-label, prospective, non-randomised pilot study with five study visits at screening, baseline, week 6, week 16 and week 26 conducted in a tertiary hospital (Royal North Shore Hospital, RNSH) in Sydney, Australia, from Feb 2019 to April 2020. Ethics approval was received from the Northern Sydney Local Health District (NSLHD) Human Research Ethics Committee (HREC/18/HAWKE/73). A signed consent form was obtained from all participants. The study was conducted according to Good Clinical Practice and the Declaration of Helsinki.
2.2. Study population
The diagnosis of knee OA was established by a study physician with a speciality in OA, based on the clinical and radiological criteria of the American College of Rheumatology Criteria for OA [15]. Participants were eligible if they met all inclusion criteria as below: i) people aged 45 years of age and over with body mass index less than 40 kg/m2 at screening visit; ii) history of primary hypercholesterolemia and total fasting cholesterol above 5.0 mmol/L at screening; iii) knee OA related symptoms for at least 6 months before screening visit; iv) index knee pain on most days over the last month (If both knees are affected by OA, then the most symptomatic knee will be considered as the index knee. If both knees are equally affected, the index knee will be determined by the Investigator); v) Knee Pain Severity Scale above 4 using an 11-point (0-10) numerical severity scale where 0 is no pain at all and 10 is worst possible pain in the last 48 h at baseline visit; vi) Kellgren-Lawrence Grade (KLG) 2 or 3 in the index knee based on knee radiograph performed at screening or within six months of the screening visit.
Participants who met any of the following criteria were excluded: i) history of fibromyalgia and inflammatory arthritis; ii) known hypersensitivity to PPS or related compounds (e.g., heparin); iii) unstable concurrent clinically significant medical conditions or abnormal laboratory findings; iv) contraindications for MRI (e.g., pacemaker, metal sutures, presence of shrapnel) or claustrophobia; v) history (within last 12 months) of bleeding (a gastric or duodenal ulcer or suspicion of gastrointestinal tract bleeding) or menorrhagia; vi) haemophilia; vii) recent surgery and/or anticipated invasive procedure (or surgery) within 6 months; viii) bilateral knee replacement; ix) concurrent heparin or oral anticoagulant therapy; x) concurrent therapy with lipid-modifying drugs for hypercholesterolemia; xi) pregnant or intend to get pregnant and breastfeeding; xii) using prohibited medications as below.
2.3. Prohibited medications
Prohibited pain medications were: i) oral and topical non-steroidal anti-inflammatory drugs (NSAIDs); ii) aspirin over 325 mg per day; iii) centrally-acting pain medications (e.g., pregabalin, gabapentin, duloxetine); iv) opioids (e.g., tramadol); v) muscle relaxants (e.g., tetrazepam, diazepam). Prohibited concomitant medications were: i) lipid-modifying drugs such as statins (e.g., atorvastatin, pravastatin, and simvastatin) or ezetimibe (Ezetrol); ii) anticoagulants including heparin, warfarin, apixaban (Eliquis), dabigatran (Pradaxa) and rivaroxaban (Xarelto); iii) biological disease-modifying anti-rheumatic drugs for arthritis; iv) steroid drugs for systematic use.
2.4. Intervention
Elmiron® was supplied in white opaque hard gelatine capsules containing 100 mg PPS, microcrystalline cellulose, and magnesium stearate, formulated for oral consumption provided by Sylvan Scientific Pty Ltd (Bondi Junction, NSW, Australia). Participants were asked to take the recommended dosage of PPS (i.e., 10 mg/kg once every 4 days for 5 weeks) based on their weight, followed by 5 weeks without the medication for two cycles (i.e., cycle 1 from weeks 1–5, and cycle 2 from weeks 11–15).
The study medication was dispensed through the clinical trial pharmacy at RNSH at baseline and week 6 visits. Participants were instructed to commence on the first 5-week PPS treatment at baseline and to repeat the course of PPS from week 11 following a phone call from the study coordinator. Paper diaries or e-report of compliance were collected at each time point to ensure compliance.
2.5. Outcomes
The primary outcome was the change in serum triglyceride from baseline to 16 weeks. The secondary outcomes were: i) the change of plasma lipids levels, including triglyceride (TG), high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol from baseline to 6, 16 and 26 weeks; ii) the change of knee OA symptoms assessed by pain NRS [16], and Knee Osteoarthritis Outcome Score [17] (KOOS, with 0 representing extreme knee problems and 100 representing no knee problems) at 6-weeks, 16 weeks, and 26 weeks; iii) structural changes assessed using semi-quantitative MRI Osteoarthritis Knee Score (MOAKS) at 26 weeks which was calculated for different subregions of bone marrow lesions and synovitis to assess in knee OA [18].
2.6. Rescue medication and other therapies
Participants were allowed to take paracetamol (e.g., Panadol or Panadol Osteo) with a maximum daily dose of 3 g for worsening pain, whether that pain was associated with OA or not (e.g., headache, fever). Participants were instructed to stop paracetamol 48 h before knee pain assessment scheduled at baseline, wk6, wk16 and wk26 visits.
Participants were advised to maintain a stable lifestyle regarding physical activity, and physical therapy throughout the study.
2.7. Clinical and laboratory assessments
Demographics, medical history, vital signs, and concomitant medications were collected at screening. The study investigator performed a general physical examination and bilateral knee examination to assess eligibility. A fixed flexion bilateral knee x-ray using SynaFlexer™ (Castlereagh Imaging Centre, Sydney) was obtained at the screening visit (or the prior 6 months) and was used for KLG assessment. Eligible participants were referred for index knee MRI at the same radiology clinic before commencing on PPS at baseline and within 14 days before the end of study visit (i.e., week 26 visit).
Fasting blood and urine samples were collected at screening, baseline, weeks 6, 16 and 26 for the assessment of full blood count, liver function, lipids (HDL, LDL, total cholesterol, and RG), PAGE analysis of LDL, lipoprotein(a), GGT, plasminogen activator inhibitor-1 (PAI-1), Alpha -2AP, coagulation studies (i.e., PT, APTT and INR, D-dimer), bone serum markers (i.e., ALP, BSAP, BGP (Osteocalcin), collagen serum markers (CTXII, NTX1, P1NP, IL-6, APC), COMP, ADAMTS-5, faecal occult blood test, serum creatinine, Glucose and CRP, urine analysis by dipstick, urine DPD NTX 1. These samples were collected by Pathology North (Northern Sydney Collection Centre) and analysed at the Sylvan Scientific laboratories.
2.8. Safety evaluation
At each visit, the study physician (trained rheumatologist) assessed adverse events and laboratory findings.
2.9. Statistical analysis
Cheras and Ghosh [19] detected a 25% drop in serum TG in dogs given oral PPS. Due to the uncertainty in estimating baseline serum TG levels in this population, percentage changes from the baseline levels have been used instead of using exact values. A sample size of 30 patients will provide >90% power to detect a 25% change in serum TG from baseline based on a standard deviation of 30% (of the baseline value) and a significance level of 0.05.
The primary analysis was according to the intention-to-treat principle. Baseline characteristics are presented as descriptive data. Continuous variables were summarised as mean and standard deviation (SD) or median (range) where appropriate. Categorical variables were presented as frequency (percentage). Missing data were replaced by mean value.
The change in the outcome measures over the study period was analysed using a paired t-test and presented as mean with standard deviation. General estimating equation (GEE) models including time, clinical outcome and adjusting for baseline lipid level were used to investigate the relationship between the change in lipid levels and change in clinical outcomes across assessments.
Where normality assumptions were not met, appropriate transformations of the data or other strategies (use of categories and/or non-parametric tests) were employed.
3. Results
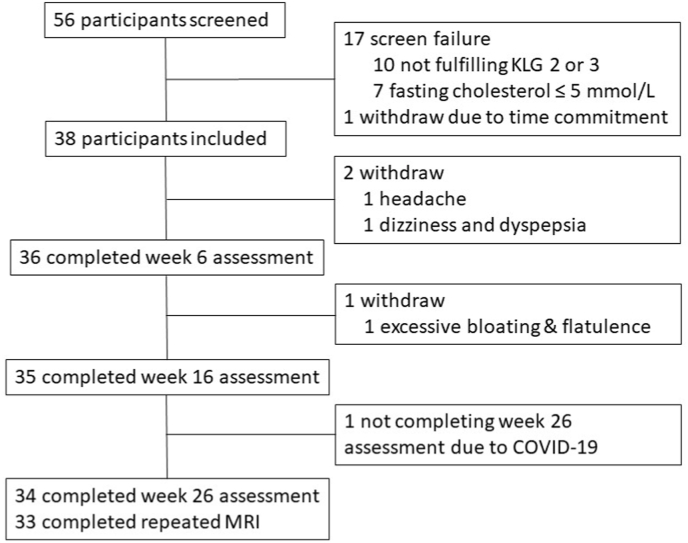
A total of 56 participants were screened and 38 eligible participants were enrolled in this study. Participants’ demographic characteristics at baseline are presented in Table 1. The mean (SD) age of participants was 62.2 (6.6) years, 27 (71.1%) were women. Most participants (94.7%) were Caucasian. A flow chart for the study participants is shown in Fig. 1.
Table 1.
Baseline characteristics.
| Characteristics | Category | |
|---|---|---|
| Age (years, mean [SD]) | 62.2 (6.6) | |
| Gender n (%) | Female | 27 (71.1) |
| Ethnicity n (%) | Caucasian | 36 (94.7) |
| Alcohol consumption n (%) | Yes | 30 (78.9) |
| Most symptomatic knee n (%) | Right Knee | 23 (60.5) |
| Left Knee | 15 (39.5) | |
| Years since official diagnosis (median [Min, Max]) | 4.3 (0.2, 29.1) | |
| History of knee trauma in index knee n (%) | No | 24 (63.2) |
| Yes | 14 (36.8) | |
| History of surgical intervention in index knee n (%) | No | 28 (73.7) |
| Yes | 10 (26.3) | |
| Use of any NSAIDS in the previous four weeks n (%) | No | 21 (55.3) |
| Yes | 17 (44.7) | |
| Physically active lifestyle n (%) | Yes | 36 (94.7) |
| KOOS ADL score (mean [SD]) | 58.7 (17.0) | |
| KOOS Pain score (mean [SD]) | 51.3 (12.5) | |
| KOOS QoL score (mean [SD]) | 32.9 (15.7) | |
| KOOS sport and recreation score (mean [SD]) | 28.6 (19.1) | |
| NRS pain score of index knee (mean [SD]) | 6.4 (1.3) | |
| KLG right knee n (%) | Grade 2 | 13 (34.2) |
| Grade 3 | 19 (50.0) | |
| KLG left knee n (%) | Grade 2 | 9 (23.7) |
| Grade 3 | 20 (52.6) | |
| Study knee selected n (%) | Right Knee | 22 (57.9) |
| Left Knee | 16 (42.1) | |
| Clinical findings in study knee: | ||
| Knee Pain n (%) | Yes | 36 (94.7) |
| Stiffness <30 min n (%) | Yes | 22 (57.9) |
| Crepitus n (%) | Yes | 36 (94.7) |
| Bony tenderness n (%) | Yes | 36 (94.7) |
| Bony enlargement n (%) | Yes | 34 (89.5) |
| No palpable warmth n (%) | Yes | 34 (89.5) |
| Swelling n (%) | Yes | 28 (73.7) |
Abbreviations: SD, standard deviation; NSAIDS, non-steroidal anti-inflammatory drugs; KOOS, Knee injury and Osteoarthritis Outcome Score; ADL, activities of daily living; QoL, quality of life; NRS, Numerical Rating Scale; KLG, Kellgren and Lawrence Grade.
Fig. 1.
Flowchart of the study profile.
3.1. Primary and secondary endpoints
In terms of the primary outcome, there was no observed reduction in serum triglyceride levels from baseline 1.10 (0.42) to week 16 1.17 (0.45) (p > 0.05) (Table 2).
Table 2.
Analysis of lipids and other blood variables.
| Variables | Baseline |
Week 6 |
Week 16 |
Week 26 |
|||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | P-value | Mean (SD) | P-value | Mean (SD) | P-value | |
| Total Cholesterol (mmol/L) | 6.23 (0.74) | 6.14 (0.67) | 0.06 | 5.95 (0.77) | 0.01 | 5.92 (0.78) | 0.005 |
| HDL Cholesterol (mmol/L) | 1.70 (0.43) | 1.68 (0.36) | 0.18 | 1.60 (0.34) | 0.05 | 1.70 (0.55) | >0.95 |
| LDL Cholesterol (mmol/L) | 4.03 (0.61) | 3.96 (0.62) | 0.122 | 3.82 (0.61) | 0.009 | 3.87 (0.73) | 0.08 |
| Triglycerides (mmol/L) | 1.10 (0.42) | 1.09 (0.49) | 0.80 | 1.17 (0.45) | 0.13 | 1.12 (0.44) | 0.40 |
| CRP (mg/L) | 3.55 (7.53) | 3.17 (5.13) | 0.91 | 2.77 (5.46) | 0.30 | 3.33 (8.15) | 0.71 |
| APTT (sec) | 28.84 (2.54) | 28.54 (2.51) | 0.34 | 28.17 (2.41) | 0.12 | 28.49 (2.46) | 0.21 |
| Prothrombin Time (sec) | 13.13 (0.91) | 13.19 (0.75) | 0.69 | 13.20 (0.93) | 0.72 | 13.31 (0.74) | 0.21 |
| White Blood Count (109/L) | 5.81 (1.68) | 5.53 (1.30) | 0.17 | 5.72 (1.68) | 0.95 | 5.64 (1.53) | 0.46 |
| D-dimer (mg/L) | 0.44 (0.26) | 0.39 (0.16) | 0.53 | 0.37 (0.16) | 0.19 | 0.38 (0.14) | 0.22 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; CRP, C-reactive protein; APTT, activated partial thromboplastin time.
Total cholesterol significantly decreased at weeks 16 (P = 0.01) and 26 (P = 0.005). LDL cholesterol significantly decreased at week 16 (P = 0.009) after adjustment for multiple comparisons. No statistically significant difference was found in other lipid or other blood variables.
There were statistically significant decreases in the knee pain NRS score from week 6 until week 26 (Table 3). There were also significant improvements in all KOOS subscales (P < 0.001; Table 4).
Table 3.
Knee pain improvement assessed by Numeric Rating Scale.
| Time | n | Mean (SD) | Change from baseline (median, range) | P-value |
|---|---|---|---|---|
| Baseline | 38 | 6.39 (1.33) | – | – |
| Week 6 | 34 | 4.18 (1.99) | −2.15 (−2.84, −1.45) | <0.001 |
| Week 16 | 35 | 3.63 (2.28) | −2.74 (−3.46, −2.03) | <0.001 |
| Week 26 | 34 | 4.38 (2.55) | −1.94 (−2.80, −1.09) | <0.001 |
Table 4.
Improvement in knee injury and osteoarthritis outcome score.
| Subscale | Baseline |
Week 6 |
Week 16 |
Week 26 |
|||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | P-value | Mean (SD) | P-value | Mean (SD) | P-value | |
| Activities of daily living | 58.67 (17.00) | 67.82 (15.21) | 0.003 | 73.11 (17.24) | <0.001 | 69.33 (17.68) | 0.001 |
| Pain | 51.32 (12.45) | 60.54 (14.68) | <0.001 | 66.43 (17.77) | <0.001 | 62.17 (18.78) | <0.001 |
| Quality of Life | 32.89 (15.70) | 45.77 (20.51) | <0.001 | 52.32 (19.65) | <0.001 | 45.77 (20.51) | <0.001 |
| Sport and recreation | 28.55 (19.06) | 41.76 (23.09) | <0.001 | 47.57 (22.83) | <0.001 | 40.29 (21.64) | 0.001 |
Semi-quantitative MRI scoring through the MOAKS instrument was calculated for different subregions for scoring the bone marrow lesions (with/without cysts) and Hoffa-synovitis and effusion synovitis joint score to assess structural changes in knee OA. Thirty-three participants (86.8%) completed baseline and week 26 MRI scans. The maximum scores over the 15 articular subregions were calculated at baseline and 6 months with no significant changes observed over time (Table 5).
Table 5.
MOAKS-BML Size and Synovitis score.
| Baseline (N = 37) |
6 Month (N = 33) |
|||
|---|---|---|---|---|
| Outcome | Grading | n (%) | n (%) | P-valueb |
| Maximuma BML size (including volume of any associated cyst), % | 0: None | 2 (5.4) | 1 (3.0) | 0.652 |
| 1: <33% | 7 (18.9) | 8 (24.2) | ||
| 2: 33–66% | 11 (29.7) | 13 (39.4) | ||
| 3: >66% | 17 (45.9) | 11 (33.3) | ||
| Maximuma BML size (excluding cyst), % | 0: None | 2 (5.4) | 1 (3.0) | 0.647 |
| 1: <33% | 0 (0.0) | 1 (3.0) | ||
| 2: 33–66% | 3 (8.1) | 1 (3.0) | ||
| 3: >66% | 32 (86.5) | 30 (90.9) | ||
| Whole joint synovitis/effusion score | 0 Normal | 11 (30.6) | 10 (31.3) | 0.855 |
| 1 Mild | 9 (25.0) | 7 (21.9) | ||
| 2 Moderate | 11 (30.6) | 8 (25.0) | ||
| 3 Severe | 5 (13.9) | 7 (21.9) | ||
| Maximuma number of BMLs | 0 | 2 (5.4) | 1 (3.0) | 0.113 |
| 1 | 5 (13.5) | 6 (18.2) | ||
| 2 | 10 (27.0) | 9 (27.3) | ||
| 3 | 18 (48.6) | 9 (27.3) | ||
| 4 | 0 (0.0) | 5 (15.2) | ||
| 5 | 2 (5.4) | 3 (9.1) | ||
Maximum over all knee subregions.
Chi-squared test.
3.2. Exploratory biomarkers
Bone and cartilage biomarkers such as CTXII, NTX1, P1NP and UDPD were evaluated at each study visit. There was a significant decrease in urine UDPD, serum P1NP and serum CTXII from baseline to week 26 (Table 6).
Table 6.
Analysis of bone and cartilage biomarkers.
| Baseline (N = 38) |
Week 6 (N = 37) |
Week 16 (N = 36) |
Week 26 (N = 34) |
||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | P-value | Mean (SD) | P-value | Mean (SD) | P-value | |
| UDPD (U) | 7.36 (2.21) | 7.29 (2.20) | 0.572 | 6.77 (2.12) | 0.427 | 6.41 (2.43) | 0.042 |
| NTX1 (U) | 90.2 (53.1) | 102 (62.3) | 0.309 | 75.5 (47.9) | 0.164 | 79.2 (45.8) | 0.305 |
| CTXII (S) | 0.488 (0.411) | 0.474 (0.439) | 0.610 | 0.521 (0.446) | 0.147 | 0.420 (0.349) | 0.057 |
| NTX1 (S) | 18.6 (5.18) | 18.6 (5.93) | 0.929 | 18.1 (5.41) | 0.473 | 19.2 (6.98) | 0.579 |
| P1NP (S) | 73.9 (14.4) | 71.1 (15.3) | 0.097 | 69.5 (15.2) | 0.014 | 69.4 (15.3) | 0.033 |
Abbreviations: UDPD; Urine deoxypyrinidoline; NTX1; N terminal telo peptide of type I collagen; CTXII; C terminal telo peptide of type II collagen; P1NP; Procollagen type I N propeptide; (S); Serum; (U); Urine.
3.3. Paracetamol consumption
24 (64.9%) participants took paracetamol during the study. 76.7% was used for knee pain.
3.4. Safety
28 (73.6%) participants experienced adverse events (Supplementary Table 1). Only one participant (2%) developed a serious adverse event which was planned elective ankle surgery requiring hospitalization. Three participants discontinued (7.9%) and chose to withdraw due to the adverse events which were associated with possible and expected events. 40 out of 55 adverse events were mild. The most common AEs were positive faecal occult blood test result (n = 9: 32%) followed by headache (n = 4; 14%) and diarrhoea (n = 2; 5.2%).
4. Discussion
This pilot study showed promising treatment effects of PPS for improving dyslipidaemia and clinical symptoms related to knee OA such as knee pain, stiffness, and disability though no significant change was found in the primary outcome of triglyceride levels. PPS in a dose of 10 mg/kg was well tolerated without serious AE related to the drug, indicating that it is safe to consume. Participants showed excellent adherence and compliance to the study drug in both intervention dose cycles. No statistically significant changes were observed in the MRI scores for bone marrow lesions and synovitis-effusion scores over six months.
Although the exact mechanism of dyslipidemia in the pathogenesis of OA is still missing, the hypothesis is that lipid deposition in the joint may trigger OA development, on the basis of findings that substantial lipid deposits in osteoarthritic cartilage and chondrocytes at an early stage of OA before apparent histological changes [20]. Also, higher total and non-HDL cholesterol were associated with increased cartilage degeneration [21]. To our knowledge, this is the first study investigating the efficacy and safety of oral PPS in people with dyslipidaemia and knee OA. This study found that PPS significantly reduced blood levels of total and LDL cholesterol, which confirmed the previous study findings in animals [19]. In OA dogs, PPS significantly decreased serum levels of TG to an extent that is similar to the control group following subcutaneous administration of the drug (3 mg/kg) and the effect was maintained four weeks after the injection. This effect of downregulation of lipid levels is probably related to the raised lipoprotein lipase and hepatic lipase after treatment of PPS in people with OA [19]. This is the first study clinically demonstrating efficacy in modifying lipid levels in people with knee OA.
Significant knee pain improvement assessed by both NRS and KOOS pain subscale was achieved at week 6 and maintained until 26 weeks. This finding is consistent with a previous report using weekly intramuscular injections of PPS (3 mg/kg), in which they found pain improvement was achieved 8 weeks from initiating therapy [19]. Other studies also reported similar results in pain relief after four to six weekly subcutaneous injections of PPS in a dose of 2–3 mg/kg [10,14]. The limitation of injectable formulas reported in previous studies is injection-related injury or reaction. Well-tolerated oral delivery is much easier for people to consume. Due to a previous report of potentially low oral bioavailability [22], we chose a relatively large oral dose to enhance the bioavailability. Animal studies show that PPS may reduce joint inflammation by altering the local expression of key functional genes characterised for their involvement in growth factor signalling and lymphocyte activation as well as its positive effect on the anti-inflammatory IL-35 level [23,24].
The dosage of PPS in this trial is different from the above mentioned subcutaneous and intramuscular injections [10,25]. Preclinical pharmacokinetic studies revealed that PPS had a location-dependent half-life and will be cleared from these various tissues at different rates. PPS was a more effective inhibitor of tumour growth in rats when they received it weekly rather than being exposed to it continually. Furthermore, continuous chronic oral administration to patients with advanced cancer suggested accumulation of PPS [26]. Another study showed that intermittent dosing (weekly rather than daily) was more efficacious at reducing tumour volumes in MDA-MB 231 human breast cancer cell xenografts [27]. Taken together, PPS can achieve the same therapeutic effect at lower concentrations given less often, as the extended half-life in cells is contributing to a prolonged therapeutic effect.
Moreover, our results confirmed the potential effect of improving cartilage metabolism by decreasing the level of urine UDPD, serum P1NP and serum CTXII, which is consistent with previous claims of PPS reducing proteolytic and angiogenic activity within the joint space [14,28]. In vitro studies showed that PPS promotes proliferation and chondrogenesis of mesenchymal precursor cell as well as reduces cartilage matrix breakdown, offering new strategies for cartilage regeneration and repair in osteoarthritic joints [29,30]. The evidence makes PPS a potential prototypic disease-modifying agent for OA.
There are a few limitations in this study. This is a pilot study with a small sample size. Other confounding factors, particularly lifestyle changes, including increased activity and changes to diet on cholesterol levels may have also influenced the results. The frequency of the study visits (i.e., five visits in 26 weeks) may subconsciously alter their physical activity and other lifestyle factors. Whilst those experiencing a (perceived or real) benefit from treatment may, for example, feel more able to exercise, which may impact on the measured outcomes and contribute to the ‘treatment effect’. The very small sample size in this study, further limits the interpretation of any treatment effect in the context of such variation. As this study was only powered for the measure of change in triglyceride levels, the significant change in knee pain measured by NRS is treated as exploratory rather than explanatory (confirmatory). It is an open label study and both investigators and patients were aware of the study treatment, which might introduce bias. That is, the patient reported symptomatic relief may be influenced by the fact that they were aware that they were receiving an active treatment. Whilst significant reductions in pain NRS and KOOS subscales were observed in this study, these were of similar magnitude to changes seen over similar periods in the placebo arm of recent placebo-controlled knee OA pharmacological RCTs. Moreover, it is not a randomised clinical trial (RCT) and there is no control group, the placebo response could be playing an important role in this open-label study. Therefore, the findings of this study might be influenced by potential placebo effect, as the placebo effects can substantially alter estimates of the relative efficacies of active treatments [31]. As such, it is difficult to ascertain whether the significant difference is a real treatment effect. To further validate our findings, a larger and more robust RCT with an appropriate control group will be required. Moreover, despite the safety profile in this small pilot study, physicians who prescribe PPS should be aware of the potential association of retinal pigmentary changes and maculopathy and be vigilant about assessing eye health in pentosan users [32,33].
5. Conclusion
Oral treatment with PPS at a dose of 10 mg/kg showed promising treatment effects to improve dyslipidaemia and clinical symptoms related to knee OA. These findings suggest further studies in this area are necessary.
Registration
Australian New Zealand Clinical Trials Registry reference: ACTRN12619000047190.
Contributions
XL drafted the paper. SV, TF, WWO, and DJH conceived and conducted the study. SV performed data analysis and study report. All authors reviewed and approved the final version of the manuscript.
Funding
This study was funded by Sylvan Scientific Pty Ltd.
Role of the funder and collaborated pharmaceutical company
Sylvan Scientific provided the study medication only and was not involved in the study design, data analysis and interpretation and manuscript preparation and submission. Sylvan Scientific reviewed and provided feedback on the manuscript.
Declaration of competing interest
DJH is supported by an NHMRC Practitioner Fellowship and provides consulting advice for Merck Serono, TLC Bio, Tissuegene, Lilly and Pfizer.
Acknowledgement
We thank all study participants for being involved in this study.
Handling Editor: H Madry
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2023.100343.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 2.Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pragasam S.S.J., Venkatesan V. Metabolic syndrome predisposes to osteoarthritis: lessons from model system. Cartilage. 2021;13(1_suppl):1598s–1609s. doi: 10.1177/1947603520980161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loef M., et al. The association of the lipid profile with knee and hand osteoarthritis severity: the IMI-APPROACH cohort. Osteoarthritis Cartilage. 2022;30(8):1062–1069. doi: 10.1016/j.joca.2022.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Samaan S.F., Taha S.I. The impact of metabolic syndrome on quality of life among individuals with knee osteoarthritis living in Egypt. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2022;15 doi: 10.1177/11795441221097361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X., et al. Metabolic triggered inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23(1):22–30. doi: 10.1016/j.joca.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Wiedemann A. [Oral therapy for interstitial cystitis: pentosan polysulfate sodium] Aktuelle Urol. 2021;52(6):556–560. doi: 10.1055/a-1629-0199. [DOI] [PubMed] [Google Scholar]
- 8.Simon M., et al. Metabolism of [3H]pentosan polysulfate sodium (PPS) in healthy human volunteers. Xenobiotica. 2005;35(8):775–784. doi: 10.1080/00498250500230586. [DOI] [PubMed] [Google Scholar]
- 9.Solanki P., et al. Repurposing pentosan polysulfate sodium as hyaluronic acid linked polyion complex nanoparticles for the management of osteoarthritis: a potential approach. Med. Hypotheses. 2021;157 doi: 10.1016/j.mehy.2021.110713. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh P., et al. Effects of pentosan polysulfate in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled pilot study. Curr. Ther. Res. Clin. Exp. 2005;66(6):552–571. doi: 10.1016/j.curtheres.2005.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh P. The pathobiology of osteoarthritis and the rationale for the use of pentosan polysulfate for its treatment. Semin. Arthritis Rheum. 1999;28(4):211–267. doi: 10.1016/s0049-0172(99)80021-3. [DOI] [PubMed] [Google Scholar]
- 12.Wijekoon S., et al. Pentosan polysulfate regulates hepcidin 1-facilitated formation and function of osteoclast derived from canine bone marrow. PLoS One. 2022;17(3):e0265596. doi: 10.1371/journal.pone.0265596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subha K., Varalakshmi P. Effect of sodium pentosan polysulphate on tissue lipids in control and glycollate treated rats. Pharmacol. Res. 1993;27(4):289–297. doi: 10.1006/phrs.1993.1029. [DOI] [PubMed] [Google Scholar]
- 14.Kumagai K., et al. Sodium pentosan polysulfate resulted in cartilage improvement in knee osteoarthritis--an open clinical trial. BMC Clin. Pharmacol. 2010;10:7. doi: 10.1186/1472-6904-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altman R., et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 16.Hawker G.A., et al. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-mpq), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP) Arthritis Care Res. 2011;63(Suppl 11):S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 17.Roos E.M., Lohmander L.S. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual. Life Outcome. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter D.J., et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011;19(8):990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh P., Cheras P.A. Vascular mechanisms in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2001;15(5):693–709. doi: 10.1053/berh.2001.0188. [DOI] [PubMed] [Google Scholar]
- 20.Zhang K., et al. High-density lipoprotein cholesterol and apolipoprotein A1 in synovial fluid: potential predictors of disease severity of primary knee osteoarthritis. Cartilage. 2021;13(1_suppl):1465s–1473s. doi: 10.1177/19476035211007919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashmeik W., et al. Investigating the association of metabolic biomarkers with knee cartilage composition and structural abnormalities using MRI: a pilot study. Cartilage. 2021;13(1_suppl):630s–638s. doi: 10.1177/1947603520946376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong L., et al. Enhanced ileal absorption of a hydrophilic macromolecule, pentosan polysulfate sodium (PPS) J. Biomater. Sci. Polym. Ed. 2004;15(5):671–682. doi: 10.1163/156856204323046924. [DOI] [PubMed] [Google Scholar]
- 23.Rudd P.A., et al. Pentosan polysulfate sodium prevents functional decline in chikungunya infected mice by modulating growth factor signalling and lymphocyte activation. PLoS One. 2021;16(9):e0255125. doi: 10.1371/journal.pone.0255125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashour R.H., et al. Pentosan Polysulfate Sodium augments the therapeutic effect of 5-Aminosalicylic Acid in DSS colitis model; the role of IL-35 expression. Int. Immunopharm. 2022;106 doi: 10.1016/j.intimp.2022.108620. [DOI] [PubMed] [Google Scholar]
- 25.Read R.A., Cullis-Hill D., Jones M.P. Systemic use of pentosan polysulphate in the treatment of osteoarthritis. J. Small Anim. Pract. 1996;37(3):108–114. doi: 10.1111/j.1748-5827.1996.tb02355.x. [DOI] [PubMed] [Google Scholar]
- 26.Marshall J.L., et al. Phase I trial of orally administered pentosan polysulfate in patients with advanced cancer. Clin. Cancer Res. 1997;3(12 Pt 1):2347–2354. [PubMed] [Google Scholar]
- 27.Yunmbam M.K., Wellstein A. The bacterial polysaccharide tecogalan blocks growth of breast cancer cells in vivo. Oncol. Rep. 2001;8(1):161–164. [PubMed] [Google Scholar]
- 28.Ghosh P., Smith M. Osteoarthritis, genetic and molecular mechanisms. Biogerontology. 2002;3(1–2):85–88. doi: 10.1023/a:1015219716583. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh P., et al. Pentosan polysulfate promotes proliferation and chondrogenic differentiation of adult human bone marrow-derived mesenchymal precursor cells. Arthritis Res. Ther. 2010;12(1):R28. doi: 10.1186/ar2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troeberg L., et al. Calcium pentosan polysulfate is a multifaceted exosite inhibitor of aggrecanases. Faseb. J. 2008;22(10):3515–3524. doi: 10.1096/fj.08-112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith M.M., Hayes A.J., Melrose J. Pentosan polysulfate, a semisynthetic heparinoid disease-modifying osteoarthritic drug with roles in intervertebral disc repair biology emulating the stem cell instructive and tissue reparative properties of heparan sulfate. Stem Cell. Dev. 2022;31(15–16):406–430. doi: 10.1089/scd.2022.0007. [DOI] [PubMed] [Google Scholar]
- 32.Lardieri A., et al. Pentosan associated retinal pigmentary changes: FDA's perspective on an emerging postmarketing safety finding. Int Urogynecol J. 2021;32(11):2891–2897. doi: 10.1007/s00192-021-04970-0. [DOI] [PubMed] [Google Scholar]
- 33.Atanasoff T.L., Schleis M.N., Keller J.A. Maculopathy secondary to chronic use of pentosan polysulfate sodium in treatment of interstitial cystitis. Clin. Exp. Optom. 2022:1–3. doi: 10.1080/08164622.2022.2111200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.