Abstract
Schizophyllum commune Fr. is a wild macro fungus species, which is often used as a food source by the indigenous Kaili tribe along the Palu-Koro fault, Central Sulawesi, Indonesia. This fungus has a wide variety in terms of the weathered wood substrate as a place to grow and is found in almost all types of ecosystems. Although its diversity has been investigated, there is no identification of the weathered wood type as a substrate for growth. Some communities in Indonesia have not also known its potential and benefits. Therefore, this research aims to determine the wood type that grows S. commune fungus, ethnomycology, mineral composition, proximate, and phytochemical compounds. It was carried out using the descriptive explanatory approach and the fungi location as well as wood substrate sampling, was determined through the purposive sampling technique in forest areas, agroforestry, and community gardens along the Palu-Koro fault, Central Sulawesi. The samples of unknown wood types were through the collection of tree parts, namely twigs, leaves, flowers, and fruits, which were brought to Herbarium Celebense, Tadulako University for identification. Analysis of mineral content, proximate, and fungal phytochemical compounds was carried out based on the method according to the existing protocol. The results showed that 92 types of rotted wood found where the fungus S. commune grew, belonged to 36 families. The nutritional content is also good, although it varies based on the type of wood growing media. Therefore, it can be used and processed into various health-beneficial food products. This showed that domestication of the fungus needs to be carried out to support its commercialization as food and medicine in the future.
Keywords: Schizophyllum commune, Substrate, Nutrition, Palu-Koro Fault, Ethnomycology, Nutritional content
1. Introduction
Macro fungus is currently gaining much attention due to its use as food ingredients (Dasanayaka and Wijeyaratne, 2017, Adejoye et al., 2007, Girma and Tasisa, 2018), drugs (Chandrawanshi et al., 2017, Kamalebo et al., 2018, Espejel-Sánchez et al., 2021), application in bioremediation (Das et al., 2021, Malik et al., 2021, Woldemariam, 2019), as well as in increasing crop production (Elsakhawy and El-Rahem, 2020, Owaid et al., 2017). There is also a high public interest and demand for macro fungus as a food source because of their important nutritional and therapeutic value for humans (Singh, 2017, Waktola and Temesgen, 2018). This fungus has a unique texture, aroma, and good taste that is different from food sourced from plants (Alemu, 2014). Some investigations also reported that several species of macro fungus are a source of bioactive compounds and antioxidants (Sánchez, 2017, Sande et al., 2019, Muszyńska et al., 2017, Zeb and Lee, 2021).
Schizophyllum was first described by Fries in 1815 as the species Schizophyllum commune (Fries, 1815), which is also known as the split-gill fungus because it resembles a split gill. Furthermore, it was most easily identified and recognized in eastern states hundreds of years ago as a medicine (Hobs, 2005). The name Schizophyllum comes from the Latin syllables Schizo, which means split, and phylum, meaning lamella (Carreño-Ruiz et al., 2019). The naming of the species “commune” was according to its meaning, general or widespread, and the occurrence of large intra- and inter-population genetic variations through the spread of spores over great distances and genetic drift (James et al., 1999, Chang and Miles, 2004). Furthermore, it is one of the non-timber forest products that has not been used optimally. This fungus is cosmopolitan, lives both saprophytic and pathogenic, and is easily found on rotting wood or trees in forests, agroforestry lands, plantations, and home gardens. It is very popular worldwide due to the ability to grow in sub-tropical and tropical countries (Yim et al., 2013) such as Mexico and Peninsular Malaysia (Takemoto et al., 2010), North East India, Sumatra Island, Indonesia, and Southern Thailand (Preecha and Thongliumnak, 2015). Most people in these countries use the fungus as food or medicine because it contains fiber, carbohydrates, protein, vitamins, lipids, and mineral elements that are good for the body (Adejoye et al., 2007, Krupodorova and Barshteyn, 2015), anti-diabetic (Chandrawanshi et al., 2019), schizophyllan of anti-cancer compounds, tumors, and immune triggers (Hao et al., 2010, Liu et al., 2015, Ooi and Liu, 2000, Tabata et al., 1981, Yamamoto et al., 1981), schizocommunin of bioactive compounds that can inhibit the growth of cervical cancer cells (Filip et al., 2019), cure infectious diseases caused by bacteria, fungi, and viruses (Mirfat et al., 2014, Kakumu et al., 1991, Komatsu et al., 1973). It is used for schizolysin production, where a hemolysin from this fungus can inhibit HIV disease (Han et al., 2010), the anti-pigmentation agent that is used as a natural ingredient in cosmetology products (Abdul Razak et al., 2018), as well as an ethanol producer (Horisawa et al., 2015).
In Indonesia, Schizophyllum commune Fr. has different local names, for example, in Kaili, Central Sulawesi, it is often called Tanggidi or Tanggojo (Yusran et al., 2021, Yusran et al., 2022a), Kulat Kritip in Dayak Ngaju, Central Kalimantan and Kulat Pokok Getah in West Kalimantan (Nion et al., 2012), Supa in Sundanese, Baduy tribe, Banten (Khastini et al., 2018), jamur gigit in Javanese, Tirau on the island of Sumatra (Kusrinah and Kasiamdari, 2015), kulat inditjeng in Sulawesi and Ngawate (Halmahera), Keho Kaladede in Ternate (Bisema 1968), Keho dlole in Tidore (Anwar et al., 2020) and jamur gerigit in West Papua (Nurlita et al., 2021). Currently, different investigations on S. commune have been conducted 30 times in 17 provinces in Indonesia (Nurlita et al., 2021), but the weathered wood type has not been identified as a substrate for growth. In several Indonesian regions, there are still communities that do not know its potential and benefits. These include Sulawesi, an island with a high endemism level, especially in Central Sulawesi Province. There is no report on the type of wood growing media, ethnomycology, mineral composition, proximate, and bioactive compounds. Sulawesi Island is one of the hotspots of the Wallacea Zone, where there are about 1,500 endemic vascular plant types and 30% of their original vegetation cover remains (Myers et al., 2000). Due to the huge potential of forests and trees as substrates for the growth of S. commune fungi in this province, it is necessary to have a clear record of the wood type diversity that becomes the substrate for the fungus to grow, including the ethnomycological analysis and the nutritional content influenced by the type of wood growing media. Therefore, this research was conducted to determine the type of substrate wood that grows S. commune fungus, the ethnomicology of its use by indigenous peoples in the area along the Palu-Koro fault, Central Sulawesi, mineral composition, proximate, and their phytochemical compounds based on the different type of weathered wood.
2. Materials and methods
2.1. Location
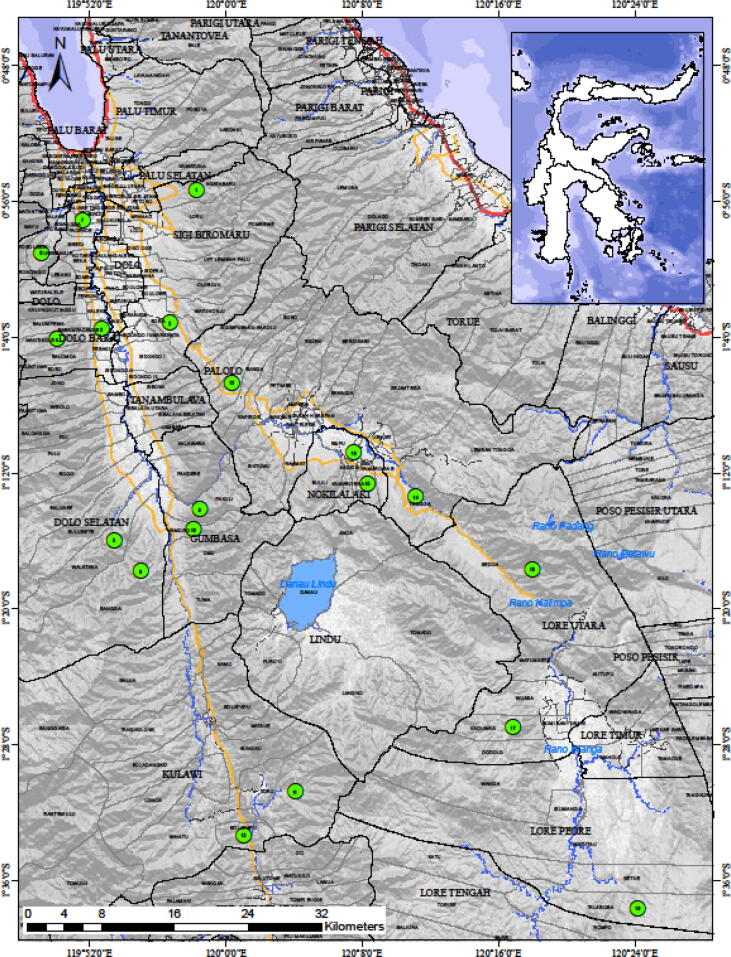
Observation and identification of the type of weathered wood growing media and sample collection of Schizophyllum commune were carried out in natural forests, agroforestry lands, or community gardens along the Palu-Koro fault, Central Sulawesi. This main active fault in Central Sulawesi stretches from the Palu Valley to North Luwu Regency for 220 Km (Patria and Putra, 2020, Socquet et al., 2006), with a higher population density than other areas in the province. The Kaili are the indigenous people and the most dominant. Observation and collection of fungus fruiting body samples were carried out starting from the Palu valley towards the south around Lore Lindu National Park, namely Ngata Baru and Bora villages (Sigi Biromaru Sub-district), as well as other villages which include Rarampadende and Mantikole (West Dolo Sub-district), Bangga and Walatana (South Dolo Sub-district), Binangga (Marawola Sub-district), Uwemanje (Kinovaro Sub-district), Pakuli and Simoro (Tanambulava Sub-district), Toro and Bolapapu (Kulawi Sub-district), Bobo and Tongoa (Palolo Sub-district), Kamarora A and Kadidia (Nokilalaki Sub-district), Sedoa and Kaduwaa (North Lore Sub-district) and Talabosa, Lore Peore Subdistrict (Napu Valley) as well as Doda and Lempe, Lore Tengah Subdistrict (Besoa Valley). The map of the research location is presented in Fig. 1.
Fig. 1.
Research location along the Palu-Koro fault, Central Sulawesi, Indonesia (Green dots).
The mineral and proximate content were analyzed at the Laboratory of Soil Science, Faculty of Agriculture and Laboratory of Animal Nutrition, as well as the Faculty of Animal Husbandry and Fisheries. Moreover, the analysis of phytochemical compounds was carried out at the Pharmacology Laboratory, Faculty of Mathematics and Natural Sciences, Tadulako University, Palu, Central Sulawesi, Indonesia.
2.2. Observation of weathered wood type, where Schizophyllum commune grows, collection of fungus sample fruiting bodies and their ethnomycology.
This exploratory research was carried out using the descriptive explanatory approach. The determination of the location for sampling the Schizopyllum commune fungus was carried out intentionally (purposive sampling) in forest areas, agroforestry, and community gardens along the Palu-Koro fault, starting from the valleys of Palu, Kulawi, Palolo, Napu, and Besoa, Central Sulawesi. The samples of weathered wood type were identified by interviewing the owner of the agroforestry/garden. Subsequently, for the wood type in the forest and unknown species, it is carried out by collecting the parts of the tree that are still alive such as leaves, flowers, fruit, and seeds in the field, and was brought to the Herbarium Celebense (CEB), Tadulako University for identification. Interviews were conducted with the community in each village, where the fungus was found and collected, to gain knowledge about the type of weathered wood that grows S. commune fungus. Its use as food and medicine, as well as the selling price in traditional markets across the villages, was also determined. Timber/tree type naming was based on Plants of the World Online (POWO) (2019) and Royal Botanical Garden (https://powo.science.kew.org/).
2.3. Fungus fruiting body collection and processing
The fruiting bodies of the fungi are cleaned of all adhering dirt with a soft brush without washing first. All fungus samples were cut into small pieces with a sharp knife and packaged separately in plastic bags according to the wood-growing media. The fungi were sun-dried when collected from areas, where electricity is not available. However, most of the fungus samples were dried by electric vacuum. Subsequently, the dried fruit bodies were dried again in the oven at 40 °C for 48 h and ground until smooth using a blender. The samples were stored in airtight plastic containing silica gel during the analysis period for further research purposes. Identification of S. commune was based on morphological and microscopic observations using the relevant literature, namely Kuo (2019) via https://www.mushroomexpert.com/schizophyllum_commune.html.
2.4. Analysis of proximate, minerals, and phytochemical compounds on fungi
A total of 15 species of the macro fungus Schizophyllum commune samples growing on various types of weathered wood as substrates, as well as macro fungi Lentinus sajor-caju and Auricularia auricula-judae as comparisons were collected from the forests, agroforestry lands, and community gardens in villages along the Palu-Koro fault, Central Sulawesi. The samples of the S. commune macro fungus are:
A = S. commune growing on the wood of Ficus benyamina L.
B = S. commune growing on the wood of Mangifera minor Blume.
C = S. commune growing on the wood of Leucaena leucocephala (Lamk) de Wit.
D = S. commune growing on the wood of Aleurites moluccana L. Wild.
E = S. commune growing on the wood of Tectona grandis L.f.
F = S. commune growing on the wood of Pinus merkusii Jung et de Vriese.
G = S. commune growing on the wood of Lannea coromandelica (Houtt.) Merr.
H = S. commune growing on the wood of Bambusa sp.
I = S. commune growing on the wood of Cassia siamea Lamk.
J = S. commune growing on the wood of Corypha sp.
K = S. commune growing on the wood of Tamarindus indica L.
L = S. commune growing on the wood of Anacardium occidentale L.
M = S. commune growing on the wood of Terminalia catappa L.
N = S. commune growing on the wood of Gmelina arborea Roxb.
0 = S. commune growing on the wood of Manihot utilissima L.
P = Lentinus sajor-caju growing on the wood of Cassea siamea Lamk.
Q = Auricularia auricula-judae growing on the wood of Theobroma cacao L.
2.4.1. Mineral analysis
The mineral content in the sample was analyzed using Atomic Absorption Spectroscopy (AOAC, 2010). The minerals identified are Nitrogen (N), Phosphorus (P), Potassium (K), Calcium (Ca), Magnesium (Mg), Sulfur (S), Sodium (Na), Iron (Fe), Zinc (Zn), and copper (Cu). A total of 2 g of the sample was put into the digestion flask, followed by the addition of 20 mL of 65% HNO3 and 10 mL of 70% HClO4 solution, shaken gently, and let stand for 24 h in the acid cupboard as well as digestion. Subsequently, the digested solution was filtered using Whatman paper no. 42 in a measuring flask and deionized water was added to the boundary line of the flask. The mineral content was determined using a standard curve according to the mineral to be analyzed.
2.4.2. Proximate analysis
Proximate analysis was carried out according to the AOAC (2010) method. The water content was tested using the thermogravimetric principle, where 1 g of the sample was put into a weighing bottle and heated in an oven at 105 °C for 1 h, put in a desiccator, and weighed. This process was repeated until the constant weight data were obtained for the last two measurements. Water content was calculated by the equation (weight of the initial sample-weight of the final sample)/weight of the initial sample * 100%.
Fungus protein content was tested by the Kjeldahl method. A total of 1 g of sample was put in a Kjeldahl flask and 25 mL of sulfuric acid, as well as Kjeldahl tablets, were added. The samples were digested at 400 °C for 2 h, cooled, and attached to the Kjeldahl apparatus circuit. The last step is titration to determine the Nitrogen content of the sample and multiplied by a correction factor to obtain the protein content.
Fat content was tested by the Soxhlet method, where a total of 1 g of sample was put into a Soxhlet flask and 25 mL of hexane solution was added. Soxhlet flasks are installed in the Soxhlet appliance suite. The hexane is expected to extract the fat in the sample and collect it at the bottom of the flask. After 4 h, the hexane solution was evaporated, and the flask was placed in the oven and weighed until it obtained a constant weight. The fat content was obtained from the calculation of the final weight-weight of the empty flask/sample weight * 100%.
Ash content was measured by the thermogravimetric method using a muffle furnace. Approximately 1 g of the sample was put in a crucible of known weight, placed into a muffle furnace for ashing, cooled, and weighed to a constant weight. Fiber analysis of fungus samples was carried out using the thermogravimetric method. A total of 1 g of sample was hydrolyzed with 1.25% H2SO4 and 1.25% NaOH. The residue obtained was washed successively with 20 mL of 10% K2SO4, 20 mL of hot distilled water, and 20 mL of 96% alcohol. The filter paper with the remaining residue was roasted at a temperature of 105 °C and weighed until a constant weight was obtained.
2.4.3. Phytochemical analysis of fungus samples
2.4.3.1. Fungus sample extraction
The ethanol extract of each fruiting body of the S. commune fungus was carried out by maceration extraction, namely by adding 5 l of pro-analytical 96% ethanol (pa) into a glass container containing 500 g of fungus powder. The powder was soaked for 3x24 hours and stirred every 1 × 24 hours (Kartini et al., 2018). Furthermore, the maceration results are accommodated in a container using a filter and funnel which are concentrated (Raaman, 2006).
2.4.3.2. Phytochemical analysis procedure of fungus samples
The procedure for analyzing the phytochemical compounds of fungus samples uses the following method:
-
a.
Flavonoid Test.
A total of 0.5 g of fungus samples that have been dissolved in ethanol were added to 2 mg of Mg powder. This was followed by the addition of 3 drops of concentrated hydrochloric acid (HCl). When a pink, brown, or reddish color is formed, it indicates the presence of flavonoids (Pooja and Vidyasagar, 2016).
-
b.
Phenolic Test
A total of 0.5 g of fungus samples were dissolved in 5 mL of water. Moreover, a few drops of 5% FeCl3 solution was added, and when there is a dark green or blue color change, it indicates the presence of phenolic compounds (Sahira and Cathrine, 2015, Pandey and Tripathi, 2014).
-
c.
Saponin Test
Saponins can be detected by the foam test in hot water. The stable foam was discovered for 5 min and did not disappear on the addition of 1 drop of 2 N HCl, indicating the presence of saponins (Nurhasnawati et al., 2019).
-
d.
Steroid/Triterpenoid Test
A total of 2 g of fungus samples were added with 25 mL of ethanol, heated, and filtered. The filtrate was evaporated and ether was added, while the ether layer was pipetted and tested on a spot plate. When 3 drops of Liebermann-Burchard reagent are added and a red/purple color was formed, this shows the presence of positive terpenoids and when green, it indicates steroids (Pooja and Vidyasagar, 2016).
-
e.
Alkaloid Test
A total of 0.5 g of fungus samples were dissolved in ethanol, dripped with HCl, and filtered. The filtrate was tested by adding two drops of Mayer and Dragendorff reagent in different test tubes. A positive reaction is indicated by the presence of a precipitate in the Mayer reagent and a red precipitate in the Dragendorff reagent (Pandey and Tripathi, 2014).
-
f.
Tanin Test
The fungus sample was added with 10 mL of distilled water, filtered, and the filtrate was added. Subsequently, a FeCl3 reagent was added, and the appearance of a dark blue or black color indicated the presence of tannin. A total of 1% gelatin solution containing sodium chloride was added to the sample, which gave a white precipitate, which indicates the presence of tannin (Pandey and Tripathi, 2014, Zohra et al., 2012).
2.5. Data Analysis
All experiments to determine the mineral composition and proximate were carried out in 3 replications. Data analysis used the statistical package fos social sciences 20 (SPSS 20) using one-way analysis of variant (Anova) and the smallest significant difference test was carried out at a probability of 5% to examine whether the treatments were significantly different. The results obtained are represented as the mean ± standard deviation.
3. Results
3.1. Diversity of substrate wood type that grows Schizophyllum commune and its ethnomycology
Based on observation data in the field, it was discovered that there were 92 types of weathered wood divided into 36 families, which became the substrate for the growth of Schizophyllum commune Fr. along the Palu-Koro fault area as shown in Table 1 below:
Table 1.
Type of wood as a substrate that grows S. commune.
| No. | Local Name | Indonesian Name | Scientific Name | Family | Location |
|---|---|---|---|---|---|
| 1. | Taipa | Mangga | Mangifera foetida Lour. | Anacardiaceae | a,b,c,d,e,f |
| 2. | Taipa Dodoro | Mangga Dodor | Mangifera minor Blume | Anacardiaceae | a,b,c,d,e,f |
| 3. | Jambu Sera | Jambu Mete | Anacardium occidentale L. | Anacardiaceae | a,b,c,d,e,f |
| 4. | Kau Jawa | Kayu Jawa | Lannea coromandelica (Houtt.) Merr. | Anacardiaceae | a,b,c,d,e,f |
| 5. | Marantaipa | Rawa-rawa Pipit | Buchania arborescens (Blume) Blume | Anacardiaceae | a,b,c,d,e,f |
| 6. | Siuri | – | Koordersiodendron pinnatum (Blanco) Merr. | Anacardiaceae | c,f,g |
| 7. | Rao | Senkung | Dracontomelon dao (Blanco) Merr. & Rolfe | Anacardiaceae | g,h,i |
| 8. | Kadondo | Kedondong | Spondias dulcis Soland. Ex Forst.f. | Anacardiaceae | a,b,c,d,e,f |
| 9. | – | Kedondong Hutan | Spondias pinnata (L. F) Kurz | Anacardiaceae | a,b,c,d,e,f |
| 10. | Sarekaya | Sirsak | Annona muricata L. | Annonaceae | a,b,c,d,e,f |
| 11. | Sarekaya Ntovau | Srikaya | Annona squamosa L. | Annonaceae | a,b,c,d,e,f |
| 12. | Andolia (Kaili) | Kenanga | Cananga odorata (Lamk) Hook.f. & Thomson | Annonaceae | b,c,f |
| 13. | Lengaru | Pulai | Alstonia scholaris (L.) R. Br | Apocynaceae | a,b,c,d,e,f |
| 14. | – | – | Wrigtia pubescens R. Br. | Apocynaceae | f,h,g |
| 15. | Tirontasi (Kaili) | – | Gastonia serratifolia (Miq.) Philipson | Araliaceae | i,j,k,l |
| 16. | Lui | Silar | Corypha sp. | Arecaceae | a,b,c,d,e,f |
| 17. | Kaluku | Kelapa | Cocos nucifera L. | Arecaceae | a,b,c,d,e,f |
| 18. | Wanga | – | Pigafetta elata Becc. | Arecaceae | i,j |
| 19. | Kalosu | Pinang | Pinanga cease Blume | Arececeae | a,b,c,d,e,f,g,h,i |
| 20. | – | Kelapa Sawit | Elaeis guineensis Jacq. | Arecaceae | c,f,h |
| 21. | Nggonau | Aren | Arenga pinnata (Wurmb) Merr. | Arecaceae | a,b,c,d,e,f,g,h,I,j,k,l |
| 22. | – | Durian | Durio zibethinus Merr. | Bombacaceae | c,g,h,i |
| 23. | Guu (Tau Taa Wana) | Cemara Laut | Casuarina equisetifolia L. | Casuarinaceae | a,b |
| 24. | Donggala | Nyamplung | Calophyllum inophyllum L. | Clusiaceae | b,d |
| 25. | Talise | Ketapang | Terminalia catappa L. | Combretaceae | a,b,c,d,f |
| 26 | – | Simpur | Dillenia indica L. | Dilleniaceae | c,f |
| 27. | Marawola | – | Diospyros macrophylla Bl. | Ebenaceae | b,c,e,f |
| 28. | Sapiri | Kemiri | Aleurites moluccana L Wild. | Euphorbiaceae | e,f,g,h,I,j |
| 29. | Kasubi | Singkong | Manihot utilissima L. | Euphorbiaceae | a,b,c,d,e,f,g,h,I,j,k,l |
| 30. | Karet | Karet | Hevea brasiliensis Muell.Arg | Euphorbiaceae | c,f |
| 31. | Miapoa | Macaranga | Macaranga hispida (Blume) Müll.Arg | Euphorbiacea | b,c,e,f,g,h,I,j |
| 32. | Balintuma | Kerinjing | Bischofia javanica Blume | Euphorbiaceae | b,c,e,f |
| 33. | – | Kareumbi | Homalanthus populneus (Geiseler) Pax | Euphorbiaceae | h,I,j,k,l |
| 34. | Beru-beru | Flamboyan | Delonix regia (Bojer ex Hook) Raf. | Fabaceae | a,b,c,d,e,f |
| 35. | Johar | Johar | Cassia siamea Lamk. | Fabaceae | a,b,c,d,e,f |
| 36. | – | Nam-nam | Cynometra ramiflora L. | Fabaceae | a,b |
| 37. | Pete | Petai | Parkia speciosa Hassk. | Fabaceae | a,b,c,e,f |
| 38. | Poi | Asam Jawa | Tamarindus indica L. | Fabaceae | a,b,c,d,e,f |
| 39. | Sengon | Jeungjing | Paraserianthes falcataria (L.) Nielsen | Fabaceae | a,b,c,e,f |
| 40. | Tamalanja/ Kaupase |
Lamtoro | Leucaena leucocephala (Lamk) de Wit. | Fabaceae | a,b,c,d,e,f,h |
| 41. | Kau Colo | Trembesi | Samanea samman (Jacq.) Merr. | Fabaceae | a,b,c,d,e,f |
| 42. | Gamal | Gamal | Gliricidia sepium (Jacq.) Kunth ex Walp. | Fabaceae | a,b,c,d,e,f,g,h,i |
| 43. | Doda (Kulawi) | Dadap | Erythrina variegata L. | Fabaceae | a,b,c,d,e,f,g,h |
| 44. | Akasia | Mangium | Acacia mangium Willd. | Fabaceae | a,b,c,d |
| 45. | – | Akasia | Acacia auriculiformis A. Cunn. Ex Benth | Fabaceae | a,b,c,d,e,f |
| 46. | – | Angsana | Pterocarpus indicus Willd. | Fabaceae | a,e,d |
| 47. | Palili | – | Lithocarpus celebicus (Miq.) Rehder. | Fagaceae | g,h,i |
| 48. | Haleka (Besoa) | – | Castanopsis acuminatissima (Blume) Rheder | Fagaceae | j,k,l |
| 49, | Tavanjuka (Kaili) | Melinjo | Gnetum gnemon L. | Gnetaceae | a,b,c,d,e |
| 50. | – | – | Galbulimima belgraveana (F. Muell.) Sprach. | Himantandraceae | a,c,f,g,h |
| 51. | Jati Puti | Gmelina | Gmelina arborea Roxb | Lamiaceae | A,d,c,d,e,f |
| 52. | Avokad | Alpukat | Persea Americana Mill. | Lauraceae | a,b,c,e,f,g,h,I,j |
| 53. | Kau momi | Medang | Chinnamomum javanicum Blume | Lauraceae | g,h |
| 54. | Uru | Cempaka | Magnolia vrieseana (Miq). Baill ex Pierre | Magnoliaceae | g,h,I,j |
| 55. | Malapoga | – | Toona ciliata M. Roem | Meliaceae | f,h |
| 56. | Mindi | Mindi | Melia azedarach L. | Meliaceae | a,b,c,d,e,f |
| 57. | Cokolati | Kakao | Theobroma cacao L. | Malvaceae | a,b,c,e,f,g,h,I,j,k,l |
| 58. | Kalibau | Waru | Hibiscus tiliaceus L. | Malvaceae | a,b,c,d,e |
| 59. | Nunu | Beringin | Ficus benyamina L. | Moraceae | a,b,c,e,f,g,h,I,j |
| 60. | – | – | Ficus minahasae | Moraceae | h,I,j |
| 61. | Ganaga | Nangka | Artocarpus heterophyllus Lmk | Moraceae | a,b,c,d,e,f |
| 62. | Kuu | Sukun | Artocarpus altilis (Parkinson) Fosberg | Moraceae | a,b,c,d,e,f |
| 63. | Kamonji | – | Artocarpus elasticus Reinw. Ex Blume | Moraceae | a,b,c,d,e,f |
| 64. | Sule | Serut | Streblus asper Lour. | Moraceae | a,b,c,d,e,f |
| 65, | – | Leda | Eucalyptus sp. | Myrtaceae | h,I,j,k |
| 66. | Maku | Jambu Bol | Syzygium malaccensis (L.) Merr. & Perry | Myrtaceae | a,b,c,d,e,f |
| 67. | Alicope | Jembolan | Syzygium cumini (L.) Skeels | Myrtaceae | a,b,c,d,e,f |
| 68. | Jambu jembo | Jambu air | Syzygium samarangense (Blume) Merr. & L. M. Perry | Myrtaceae | a,b,c,d,e,f |
| 69. | Pala | Pala | Myristica fragrans Houtt. | Myristicaceae | e,f,g,h,i |
| 70. | Pinus | Tusam | Pinus merkusii Jung et de Vriese | Pinaceae | e,h |
| 71. | Avo | Bambu duri | Bambusa blumeana Schult.f | Poaceae | a,b,c,d,e,f |
| 72. | Avo | Bambu | Bambusa maculate Widjaja | Poaceae | a,b,c,d,e,f,g,h,I,j,k,l |
| 73. | Tovu | Tebu | Saccharum officinarum L. | Poaceae | a,b,c,d,e,f,g,h,I |
| 74. | Perande | – | Macadamia hildebrandii Van Steen. | Proteacea | g,j |
| 75. | Balintuma (Kaili) | – | Bischofia javanica Blume | Phyllanthaceae | e,f,g |
| 76. | Aropi (Kaili) | Buni | Antidesma bunius (L.) Spreng | Phyllanthaceae | c,f |
| 77. | – | Mengkudu | Morinda citrifolia L. | Rubiaceae | a,b,c,d,e,f |
| 78. | Jabon | Jabon | Anthocephalus chinensis (Lam.) A. Rich ex Walp. | Rubiaceae | c,f,h |
| 79. | Jabon | Jabon | Anthocephalus macrophyllus (Roxb) Havil | Rubiaceae | c,f,h |
| 80. | – | Kopi Robusta | Coffea robusta | Rubiaceae | b,c,e,f |
| 81. | Bila | Maja | Aegle marmelos (L.) Correa | Rutaceae | a,b,c,d,e,f |
| 82. | Lotu | Matoa | Pometia pinnata J.R. Forster & J.G. Forster | Sapindaceae | a,b,c,d,e,f |
| 83. | Rambutan | Rambutan | Nephelium lappaceum L. | Sapindaceae | a,b,c,d,e,f |
| 84. | Lengkeng | Klengkeng | Dimocarpus longan Lour. | Sapindaceae | a,b,c,d,e,f |
| 85. | Torode (Besoa, Napu) | Bayur | Pterospermum javanicum Jungh. | Sterculiaceae | g, h, I, j, k, l |
| 86. | – | Bayur Sulawesi | Pterospermum celebicum Miq. | Sterculiaceae | g, h, I, j, k, l |
| 87. | Lekatu (Kaili) | Binuang Laki | Duabanga moluccana Blume | Sonnerataceae | f, g, h, I, j, k, l |
| 88. | Tanjung | Tanjung | Mimusops elengi L. | Sapotaceae | a,b,d,f |
| 89. | Nantu | Nyatoh | Palaquium sp. | Sapotaceae | c,f,h, g,h |
| 90. | Nantu | Nyatoh | Palaquium obovatum (Griff.) Engl. | Sapotaceae | c,f,h |
| 91. | – | – | Gironniera subaequalis Planch. | Ulmaceae | i,j |
| 92. | Jati | Jati | Tectona grandis L.f | Verbenaceae | a,b,c,d,e,f,h |
Description: a = Ngata Baru and Bora villages (Sigi Biromaru Sub-district); b = Rarampadende and Mantikole (West Dolo Sub-district); c = Bangga and Walatana (South Dolo Sub-district); d = Binangga (Marawola Sub-district); e = Uwemanje (Kinovaro Sub-district); f = Pakuli and Simoro (Tanambulava Sub-district); g = Toro and Bolapapu (Kulawi Sub-district); h = Bobo and Tongoa (Palolo Sub-district); i = Kamarora A and Kadidia (Nokilalaki Sub-district). j = Sedoa and Kaduwaa (North Lore Sub-district), k = Talabosa, Lore Peore Subdistrict (Napu Valley); l = Doda and Lempe, Lore Tengah Subdistrict (Besoa Valley).
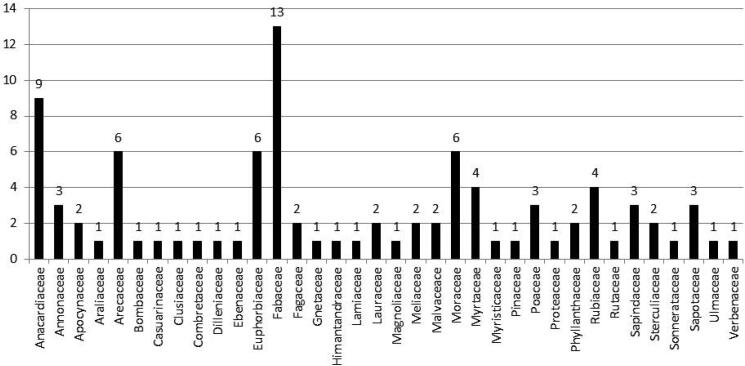
Out of the 36 wood families mentioned above, the Fabaceae family has the highest number of wood types, namely 13 species which are the substrate for the growth of S. commune fungi, followed by the Anacardiaceae with 9 species, 6 species of Arecaceae, Euphorbiaceae and Moraceae, 4 species of Myrtaceae and Rubiaceae, 3 species of Annonaceae, Poaceae, Sapindaceae and Sapotaceae, Apocynaceae, Fagaceae, Lauraceae, Meliaceae, Malvaceae, Phyllanthaceae and Sterculiaceae each as many as 2 species, and the rest are in the family Araliaceae, Bombaceae, Casuarinaceae, Clusiaceae, Combretaceae, Dilleniaceae, Ebenaceae, Gnetaceae, Himantandraceae, Lamiaceae, Magnoliaceae, Myristicaceae, Pinaceae, Proteaceae, Rutaceae, Sonnerataceae, Ulmaceae, and Verbenaceae with 1 species each as shown in Fig. 2. Furthermore, some examples of macro-morphological fruiting bodies of S. commune fungi growing on various types of weathered wood are presented in Fig. 3.
Fig. 2.
The number of weathered wood types as substrates for S. commune fungi in each different family.
Fig. 3.
The morphology of the Schizophyllum commune macro fungus growing on several species of weathered wood (A = Pinus merkussi, B = Mangifera foetida, C = Gmelina arborea, D = Macaranga hispida, E = Lannea coromandelica, F = Melia azedarach, G = Samanea saman, H = Artocarpus elasticus, I = Anacardium occidentale, J = Spondias dulcis, K = Arenga pinnata, L = Theobroma cacao, M = Corypha sp, N = Bambusa maculate, 0 = Cocos nucifera, P = Tectona grandis.
The S. commune fungus has been used by the Kaili tribe along the Palu-Koro fault area and its surroundings for generations. The Kaili community process this fungus in a simple way, for example, stir-fry with chicken eggs, chilies, tomatoes, and salt, making a soup mixed with vegetables such as cabbage, potatoes, chickpeas, or cooked with coconut milk, chili, and seasonings, which is locally called Utadada Tanggidi. Some people from the Lore tribe in the Napu Valley, Poso Regency, also process by washing with clean water, mixing it with chili and salt, finely ground, and eating the fungus with warm rice. When the harvest is abundant, people usually dry the fruit bodies of this fungus, store them for a certain period in jars and cook the fruit bodies again by soaking them in clean water for a few minutes to bloom and soften the texture. Generally, this fungus is traded in traditional markets that spread in the Palu valley and its surroundings. The fungus is sold at an uncertain price depending on the season, which is cheap during the rainy season because it is easy to find and grows abundantly on rotting wood. Meanwhile, the price is high during the dry season, because people have to look for the fungus deep in the forest, where it grows a lot due to the microclimate conditions that are very supportive of its growth. In the rainy season, the price per bowl (±250 g) varies from 2,500–5000 IDR (1 US Dollar = 15,000 IDR) and will increase to 10,000–20,000 IDR per bowl during the dry season. This fungus is sold in fresh condition and sometimes in a processed form that is ready to be consumed, especially processed mixed with coconut milk (Utadada Tanggidi), which is a favorite dish among the Kaili tribe along the Palu-Koro fault as shown in Fig. 4. For the Kaili tribal community, it is the most preferred and is one of the favorite side dishes because, in addition to its delicious taste, the fungus is easy to obtain since the price is cheap.
Fig. 4.
The Schizophyllum commune Fr. fungus in traditional markets along the Palu Koro fault area, Central Sulawesi, the fruit bodies of the fungi are sold in fresh condition (a,b) and Processed Fungus ‘Uta Dada’ (c) (Photo taken by Yusran Yusran).
3.2. Mineral content of the Schizophyllum commune fungus growing on various types of weathered wood
The results of the analysis of the mineral content of Schizophyllum commune, Lentinus sajor-caju and Auriculari auricular-judae growing on various types of weathered wood are presented in Table 2.
Table 2.
Mineral composition some samples of Schizophyllum commune, Lentinus sajor-caju and Auriculari auricular-judae growing on various types of weathered wood.
| Treatment | Parameters |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | N (%) | P (%) | K (%) | Ca (%) | Mg (%) | S (%) | Na (%) | Fe (%) | Zn (%) | Cu (ppm) | |
| A | 6.23 ± 0.03e | 0.30 ± 0.050c | 0.106 ± 0.020f | 1.23 ± 0.03c | 0.237 ± 0.001b | 0.14 ± 0.010b | 0.14 ± 0.010a | 0.03 ± 0.020d | 0.06 ± 0.010b | 0.007 ± 0.0005b | 0.20 ± 0.05ab |
| B | 6.51 ± 0.02g | 0.25 ± 0.005b | 0.125 ± 0.005h | 1.33 ± 0.01d | 0.401 ± 0.005cd | 0.27 ± 0.001b | 0.13 ± 0.002e | 0.14 ± 0.002j | 0.05 ± 0.002b | 0.005 ± 0.0001ab | 0.20 ± 0.02ab |
| C | 6.52 ± 0.04g | 0.28 ± 0.003e | 0.099 ± 0.001e | 1.13 ± 0.02b | 0.215 ± 0.003a | 0.05 ± 0.002bc | 0.15 ± 0.005f | 0.17 ± 0.010c | 0.03 ± 0.005a | 0.012 ± 0.001d | 0.20 ± 0.05ab |
| D | 6.17 ± 0.02d | 0.34 ± 0.005d | 0.128 ± 0.001h | 1.55 ± 0.01f | 0.493 ± 0.001d | 0.400 ± 0.05c | 0.17 ± 0.005c | 0.09 ± 0.005 k | 0.14 ± 0.002d | 0.017 ± 0.001f | 0.20 ± 0.01ab |
| E | 6.52 ± 0.05g | 0.20 ± 0.010a | 0.100 ± 0.010e | 1.28 ± 0.01d | 0.298 ± 0.002a | 0.07 ± 0.002b | 0.12 ± 0.005d | 0.11 ± 0.002f | 0.03 ± 0.005a | 0.012 ± 0.001d | 0.18 ± 0.005a |
| F | 6.49 ± 0.01f | 0.23 ± 0.005ab | 0.097 ± 0.001e | 1.00 ± 0.05a | 0.109 ± 0.001b | 0.11 ± 0.005b | 0.11 ± 0.002f | 0.17 ± 0.005a | 0.03 ± 0.005a | 0.013 ± 0.0005de | 0.21 ± 0.005b |
| G | 6.50 ± 0.05 fg | 0.31 ± 0.005cd | 0.118 ± 0.001g | 1.48 ± 0.01e | 0.274 ± 0.002e | 0.45 ± 0.005b | 0.14 ± 0.005g | 0.27 ± 0.005e | 0.09 ± 0.005c | 0.009 ± 0.001c | 0.20 ± 0.05ab |
| H | 6.51 ± 0.01g | 0.25 ± 0.005b | 0.110 ± 0.005f | 1.47 ± 0.01e | 0.330 ± 0.002e | 0.26 ± 0.010bc | 0.15 ± 0.005a | 0.04 ± 0.001h | 0.14 ± 0.002d | 0.009 ± 0.0005c | 0.20 ± 0.01ab |
| I | 6.49 ± 0.02f | 0.23 ± 0.003ab | 0.074 ± 0.001d | 1.01 ± 0.01a | 0.314 ± 0.001c | 0.25 ± 0.005b | 0.10 ± 0.010a | 0.04 ± 0.001g | 0.05 ± 0.001b | 0.009 ± 0.0005c | 0.19 ± 0.002a |
| J | 6.50 ± 0.05 fg | 0.31 ± 0.010cd | 0.129 ± 0.001h | 1.84 ± 0.01e | 0.329 ± 0.001e | 0.47 ± 0.005c | 0.19 ± 0.010c | 0.08 ± 0.005h | 0.23 ± 0.005e | 0.015 ± 0.001e | 0.22 ± 0.01b |
| K | 6.43 ± 0.02f | 0.24 ± 0.030b | 0.119 ± 0.020g | 1.29 ± 0.01d | 0.270 ± 0.010b | 0.12 ± 0.020bc | 0.15 ± 0.020b | 0.07 ± 0.002e | 0.17 ± 0.025de | 0.013 ± 0.001de | 0.19 ± 0.01a |
| L | 5.88 ± 0.02a | 0.24 ± 0.010b | 0.057 ± 0.004c | 1.14 ± 0.06b | 0.248 ± 0.016c | 0.23 ± 0.010a | 0.12 ± 0.003c | 0.08 ± 0.008d | 0.10 ± 0.015cd | 0.014 ± 0.001e | 0.17 ± 0.02a |
| M | 6.24 ± 0.02e | 0.21 ± 0.020a | 0.025 ± 0.010a | 1.09 ± 0.07b | 0.201 ± 0.010b | 0.12 ± 0.010b | 0.12 ± 0.002a | 0.03 ± 0.010b | 0.08 ± 0.010c | 0.013 ± 0.001de | 0.18 ± 0.03a |
| N | 6.64 ± 0.05h | 0.24 ± 0.026b | 0.037 ± 0.006b | 1.17 ± 0.05c | 0.357 ± 0.012d | 0.37 ± 0.020b | 0.12 ± 0.020a | 0.03 ± 0.007i | 0.14 ± 0.040d | 0.015 ± 0.001e | 0.17 ± 0.02a |
| O | 6.09 ± 0.05c | 0.25 ± 0.015b | 0.115 ± 0.002g | 1.12 ± 0.04b | 0.241 ± 0.010b | 0.39 ± 0.078b | 0.11 ± 0.005a | 0.04 ± 0.010d | 0.07 ± 0.010bc | 0.010 ± 0.008e | 0.26 ± 0.02bc |
| P | 6.50 ± 0.05h | 0.20 ± 0.040a | 0.109 ± 0.005f | 1.47 ± 0.11e | 0.207 ± 0.003b | 0.11 ± 0.035b | 0.14 ± 0.030b | 0.06 ± 0.030b | 0.07 ± 0.010bc | 0.004 ± 0.002a | 2.02 ± 0.04c |
| Q | 5.98 ± 0.01b | 0.20 ± 0.010a | 0.062 ± 0.001c | 1.51 ± 0.001e | 0.326 ± 0.001d | 0.38 ± 0.005b | 0.12 ± 0.004a | 0.04 ± 0.002h | 0.02 ± 0.003a | 0.005 ± 0.0003ab | 0.20 ± 0.01ab |
Description: The mean value is the average of three replications, ± is the Standard Deviation. The numbers followed by the same letter in the same column mean that they are not significantly different in the Least Significant Difference test at the 5% level.
3.3. Proximate content of Schizophyllum commune samples growing on various types of weathered wood
The analysis results of the proximate content of each sample of Schizophyllum commune, Lentinus sajor-caju and Auriculari auricular-judae are presented in Table 3.
Table 3.
Proximate content of the Schizophyllum commune, Lentinus sajor-caju and Auriculari auricular-judae growing on various types of weathered wood.
| Treatment | Parameter |
||||
|---|---|---|---|---|---|
| Water content (%) | Fat (%) | Proteins (%) | Fiber (%) | Ash (%) | |
| A | 10.14 ± 0.03c | 1.00 ± 0.02c | 13.29 ± 0.02c | 4.07 ± 0.007h | 15.99 ± 0.02c |
| B | 11.02 ± 0.02e | 1.20 ± 0.02e | 14.43 ± 0.03h | 1.22 ± 0.03a | 5.79 ± 0.01a |
| C | 11.39 ± 0.21f | 1.01 ± 0.01c | 13.57 ± 0.02d | 2.15 ± 0.03d | 8.05 ± 0.01a |
| D | 10.52 ± 0.08d | 1.36 ± 0.02g | 16.07 ± 0.05j | 4.48 ± 0.09i | 13.88 ± 0.05c |
| E | 8.64 ± 0.13b | 1.05 ± 0.02c | 13.71 ± 0.03e | 1.45 ± 0.08b | 16.53 ± 0.06d |
| F | 11.84 ± 0.06g | 0.90 ± 0.01b | 13.98 ± 0.01f | 3.98 ± 0.02h | 17.98 ± 0.01a |
| G | 10.01 ± 0.04c | 1.29 ± 0.04f | 17.00 ± 0.02l | 1.45 ± 0.08b | 8.69 ± 0.04b |
| H | 11.16 ± 0.05ef | 0.82 ± 0.02a | 14.81 ± 0.02i | 2.00 ± 0.01c | 9.22 ± 0.002b |
| I | 10.92 ± 0.03e | 1.34 ± 0.04f | 17.60 ± 0.05m | 2.83 ± 0.04e | 10.07 ± 0.02b |
| J | 8.31 ± 0.02a | 1.11 ± 0.03d | 19.21 ± 0.02o | 10.98 ± 0.01k | 18.54 ± 0.05e |
| K | 10.15 ± 0.03c | 1.30 ± 0.07f | 18.58 ± 0.03n | 4.90 ± 0.05j | 15.02 ± 0.01d |
| L | 12.38 ± 0.09h | 0.91 ± 0.02b | 13.95 ± 0.07f | 3.17 ± 0.01f | 16.04 ± 0.11d |
| M | 10.75 ± 0.52de | 0.90 ± 0.04b | 16.21 ± 0.18k | 2.86 ± 0.12e | 19.25 ± 0.57e |
| N | 13.24 ± 0.11i | 1.25 ± 0.06e | 14.18 ± 0.08g | 3.57 ± 0.09g | 11.75 ± 0.02c |
| O | 11.30 ± 0.04f | 1.57 ± 0.09h | 13.98 ± 0.08d | 2.23 ± 0.07d | 14.15 ± 0.24d |
| P | 13.12 ± 0.03i | 1.67 ± 0.02i | 12.58 ± 0.02b | 19.9 ± 0.05l | 10.92 ± 0.03b |
| Q | 13.34 ± 0.02i | 1.02 ± 0.01c | 12.22 ± 0.05a | 31.1 ± 0.01m | 9.75 ± 0.02b |
Description: The mean value is the average of three replications, ± is the Standard Deviation. The numbers followed by the same letter in the same column mean that they are not significantly different on the 5% level of the Least Significant Difference test.
These results show that the water content of Schizophyllum commune that grows wild on various types of weathered wood is lower than Lentinus sajor-caju and Auricularia auricula-judae. It depends on the conditions in which it grows, the water needed to live, and the ability to absorb water from the growing substrate. Although the mean water content of S. commune is lower, samples E (S. commune grown on Tectona grandis L.f) and J (S. commune grown on Corypha sp. wood) had the lowest water content. The highest water content was in sample N, which was not significantly different from the control.
3.4. Content of phytochemical compounds in Schizophyllum commune samples growing on various types of weathered wood
The qualitative analysis results of the phytochemical compounds on each sample of Schizophyllum commune based on the wood type are presented in Table 4.
Table 4.
The content of phytochemical compounds in samples of S. commune, Lentinus sajor-caju and Auriculari auricular-judae growing on various types of weathered wood.
| Treatment | Flavonoid |
Alkaloid |
Tanin |
Phenolic | Saponins | Steroids/Terpenoids | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| AlCl3 | mg powder | Ammonia | Mayer | Dragen dorff | FeCl3 | Gelatin | ||||
| A | + | + | + | + | + | + | + | + | – | – |
| B | + | + | + | + | + | + | + | + | – | – |
| C | + | + | + | + | + | + | + | + | – | – |
| D | + | + | + | + | + | + | + | + | – | – |
| E | + | + | + | + | + | + | + | + | – | – |
| F | + | + | + | + | + | + | + | + | – | – |
| G | + | + | + | + | + | + | + | + | – | – |
| H | + | + | – | + | + | + | + | + | – | – |
| I | + | + | + | + | + | + | + | + | – | – |
| J | + | + | + | + | + | + | + | + | – | – |
| K | + | + | + | + | + | + | + | + | – | – |
| L | – | – | + | + | + | + | + | + | – | – |
| M | + | + | + | + | + | + | + | + | – | – |
| N | + | + | + | – | + | + | + | + | – | – |
| O | + | + | – | + | + | + | + | + | – | – |
| P | + | + | + | + | + | + | + | + | – | – |
| Q | + | + | + | + | – | + | + | + | – | – |
Information: + = Yes, – = None/not detected.
The results showed that the content of phytochemical compounds in S. commune samples was relatively not different from one another as shown in Table 4. All samples analyzed were positive for flavonoid, alkaloid, tannin, and phenolic compounds, but did not contain saponin or steroid/terpenoid compounds. In particular, flavonoid compounds were not found in samples of fungi growing on Anacardium occidentale L wood, which were detected using AlCl3 reagent and mg powder, or in those on Bambusa sp. and Manihot utilissima L.
4. Discussion
These study results are greater than the previous survey conducted by Yusran et al., (2021), which discovered only 16 types of weathered wood that became the substrate for this fungus to grow in Lore Lindu National Park, Indonesia. Furthermore, Herliyana et al., (2011) reported that this is a rotting fungus of several types of wood such as Sengon (Paraserianthes falcataria), Karet (Hevea brasiliensis), Tusam (Pinus merkusii), and Mangium (Acacia mangium). In this study, the large number of wood species found to be substrates for S. commune fungus was due to observations and explorations carried out in a wider area, both in forest areas, agroforestry, community plantations and agricultural land ranging from lowlands to highlands (0- > 1000 m above sea level) along the Palu-Koro fault, Central Sulawesi, Indonesia. Compared to previous studies which were only in the Lore Lindu National Park area, the size of the area in this study made it possible to find a greater number of wood species which became substrates for the S. commune fungus, because the types of wood produced by trees that grow spread out and are influenced by altitude above sea level. There are certain types of wood that are the substrate for this fungus and only grow at high altitudes, for example Pigafetta elata Becc. and conversely, there are wood-producing trees that only grow in the lowlands. These results indicate that this fungus can grow from the lowlands to the highlands of more than 1000 m asl. The macro fungus has the most growing places and is found in almost all ecosystems. Raper and Miles, 1958, Cooke, 1961, stated that it is widely found and can grow on rotting dead wood of at least 150 different genera of flowering plants. According to de Jong (2006), the fungus can also colonize softwood and grass silage.
The yield and productivity of macro fungi depend on the species, substrate, and environmental conditions (Teoh et al., 2017). Meanwhile, S. commune will grow well on carbon nutrient sources of sucrose, maltose, and others (Niederpruem and Hunt, 1964). These results indicate that the type of substrate wood, where the fungus grows significantly affects the size and color of the growing fruiting bodies. This showed in the fungus growing on the wood of Lannea coromandelica (Houtt.) Merr. The weathered ones produce fruit bodies that are larger than those grown on other weathered woods and have a grayish color, while the fungus on Corypha sp. has a whiter color. Macroscopically, this fungus has the characteristics of small and short fruiting bodies, hairy lamellae, with a white, or grayish color. Dasanayaka and Wijeyaratne (2017) reported that the edible fungus of S. commune can grow on a substrate of 7 species of wood, namely Alstonia macrophylla, Artocarpus heterophyllus, Harpullia arborea, Mangifera indica, Dillenia indica, Nephelium lappaceum, and Terminalia catappa. Cultivation on Artocarpus heterophyllus wood substrate gave the highest production compared to the other 6 wood substrates.
According to Whitten et al., 1987, Keßler et al., 2002, almost 15% of the 5000 vascular plant species recorded are endemic to the island of Sulawesi, including more than 2100 woody plant species. The forest area along the Palu-Koro fault is also very rich in plant diversity, therefore, it has the potential as a substrate for food and medicinal macro fungi to grow. One of the important conservation areas along the Palu-Koro fault is Lore Lindu National Park. A total of 166 tree species were also found in sub-montane and lower montane primary forests, Lore Lindu National Park, Central Sulawesi (Culmsee and Pitopang, 2009). The richness of plant species in this area is a huge potential for the growth and production of S. commune fungi as well as other species of food and medicinal macro fungi, with their future development efforts.
Generally, the macro fungus can grow on various types of substrates, but the level of use of the substrate and the growth of the fungus depend on the species of the fungus itself (Kumla et al., 2020). Almost all agricultural waste contains lignocellulosic as the main component needed by fungi and based on the type of plant (Suwannarach et al., 2022). This research is the first to report specifically in large quantities about the type of substrate wood, where the S. commune fungus grows in Indonesia.
The bioaccumulation of elements in the fungal body and the biological importance of the accumulation process is strongly influenced by many factors, which are still poorly understood. The fundamental factors that have been known include natural geochemical factors from the source rock, environmental pollution by heavy metals, the environment of the fungus, and the bioaccumulation of trace elements in the fruiting bodies (Kozarski et al., 2015). This research shows that the mineral content of the S. commune fungus varies based on the type of weathered wood as the substrate growing media. Furthermore, mineral K has the highest content compared to other minerals, followed by Ca, Mg, and Cu. This is due to the high level of potassium in weathered wood, which is the substrate for the fungus. Alananbeh et al., (2014) stated that the highest mineral in Pleurotus ostreatus is K, followed by Mg, Ca, and Zn. The presence of K in the highest concentrations was reported in wild fungi that live on weathered wood substrates (Sanmee et al., 2003).
The concentration of Magnesium and Calcium in fungi is also influenced by environmental factors, as reported in Sanmee et al., 2003, Uzun et al., 2011. Calcium is needed by fungi for the growth of mycelia, neutralizing acidic substrate conditions, and increasing the calcium content for consumption (Suzuki, 2020). In the human body calcium functions as a form of bone tissue, teeth, and various other organs, which helps muscle tissue contraction, regulates heart rate, and nerve function. Meanwhile, magnesium plays a role in biological oxidation processes and respiration as well as affects blood pressure and blood sugar levels (Rózsa et al., 2021).
Fungi are known as good accumulators for zinc with fairly high content. The zinc levels in this research are equivalent to other reports by Işıloğlu et al., 2001, Altaf et al., 2020. This element is required by fungi for their metabolism and used for various physiological functions such as cell growth and regeneration in humans. Zinc deficiency is a big problem and needs attention alongside iron and vitamin A deficiency (Gupta et al., 2020), therefore, fungi can be used to reduce the level of deficiency in humans.
There is a need to observe the high Cu content in Lentinus sajor-caju, which grows on decayed Cassia siamea wood collected from community gardens. It is suspected to be heavy metal contamination from pesticides used by the community in controlling nuisance organisms. Although the concentration of 2 ppm is not harmful to body health (Uzun et al., 2011), in consuming food macro fungi, the public needs to consider the origin and place of collection. This is to determine whether it is from agricultural and plantation areas, where pesticides are very often applied to avoid contaminating the fruit bodies of the collected fungi.
Falandysz and Borovička (2013) reported that wild-grown edible macro-fungi can accumulate minerals essential for humans at greater levels than cultivated fungi as influenced by the growing habitat (Krupodorova and Sevindik, 2020). Furthermore, Bhattacharyya et al., (2014) stated that trace elements such as Zn, Cu, Fe, and Mn, although required in very small amounts, will have an essential role as a co-factor of enzymes to carry out biological functions in almost all metabolic processes of the human body.
Based on a previous report, the water content can be influenced by several factors, namely harvesting time, maturity level, and environmental climatic conditions (Beluhan & Ranogajec, 2011). The difference in water content in the mushroom samples in this study was more due to climate differences, especially temperature, humidity and rainfall at the locations where these mushrooms were found, which were influenced by the altitude above sea level. The altitude above sea level greatly affects temperature and humidity, which of course greatly affects the growth of fungi. In addition, the research location in the highlands has higher rainfall than in the lowlands (Palu valley) which is known as a dry area. The next factor that affects the moisture content of the mushrooms is the age of the mushrooms when they are harvested. The fruit bodies are harvested without knowing their age because they grow naturally, which of course will have different levels of maturity, which will affect their water content. The water content needs to be considered because at a high value, it is susceptible to damage such as browning, off-flavor, and the growth of microorganisms after harvesting, thereby reducing the shelf life (Niu et al., 2020).
Fungi are foods with high protein content, hence, S. commune has a higher protein content compared to the control. Sample J, which is S. commune growing on Corypha sp. wood had the highest protein content of 19.21%. These results are in line with Ao & Deb (2019) who investigated wild macro fungi in India with high protein content. Ouzouni et al. (2009) showed that fungi have a high protein content compared to the type of ingredients commonly consumed as vegetables, such as green leafy and others. Moreover, other results also show that S. commune contains 16–27% protein (Aletor, 1995, Longvah and Deosthale, 1998, Yusran et al., 2022b). High protein content has the potential to be developed into various food products and consumed to increase people's protein intake.
The fat content of the fungus samples showed a range between 0.82% and 1.57%, which is influenced by metabolic factors and food reserves. The low-fat content shows that S. commune can be developed as a low-fat healthy food. This is in line with other research, which explains that fungi have low-fat content (Jacinto-Azevedo et al., 2021, Fogarasi et al., 2018). Moreover, fungi are widely processed for various food products and are believed to provide many health benefits (González et al., 2020). Low levels of fat are reported to loss of weight and the risk of increasing blood pressure (Sun & Niu, 2020). The fiber content of S. commune with different growth substrates was in the range of 1–10%, which is lower compared to the control. According to Ao & Deb (2019), fungi are good for human health because they are rich in fiber. This present research proves that fungi with various substrates of weathered wood as growth media have good nutritional levels, therefore, they can be used and processed into several food-beneficial products.
Similarly, Herawati et al., (2021) discovered that the macro fungus S. commune that grows wild is positive for flavonoid compounds, steroids, tannins, and coumarins. Okwulehie et al., 2007 also detected the presence of alkaloids, flavonoids, phenols, saponins, and tannins in the S. commune growing on rotten mango (Mangifera indica) wood. This is different from Acanto and Helen Cuaderes, (2021), who stated that S. commune growing in the forest of Minapasuk, Calatrava, Negros Occidental, Philippines contains saponin compounds. Furthermore, Wirth et al., (2021) showed that the fungus contained terpenoid compounds in form of sesquiterpenes.
According to Basso et al., (2020), the content of phytochemical compounds of macro fungus differs based on the type of weathered wood as a substrate for growth media. Furthermore, the detection of phytochemical compounds in the sample is also influenced by several factors, including the solvents and reagents (Shaikh and Patil, 2020). Teoh and Don (2013) analysis using methanol as an extraction solvent discovered the presence of flavonoid compounds, saponins, and phenols in this fungus, but did not contain alkaloids. Moreover, S. commune can produce antioxidant compounds through the shikimate pathway from the central metabolism that generates chorismate precursors. This can eventually form secondary anti-oxidant metabolites such as isoflavones, flavones, flavonols, anthocyanins, tannins, coumarin derivatives, and other phenolic compounds (Boonthatui et al., 2021). The composition of the phenolics content in macrofungi is also influenced by genetic and environmental factors (Yildiz et al., 2017), strains or species of macrofungi, the composition of growing media, harvest time, management techniques, handling conditions, and preparation of substrate for their cultivation, as well as soil/substrate composition or host associated with the wild fungi species (Heleno et al., 2010).
Based on the previous research, it was reported that S. commune with its phytochemical compounds has pharmacological activity. Stan et al., (2021) stated that the aqueous extract has anti-dengue activity, while the ethanolic extract has strong antioxidant activity related to phenolic compounds (Basso et al., 2020, Mongkontanawat and Thumrongchote, 2021). The potential immunostimulator and antitumor activity of glucan-associated S. commune have also been reported (Vannucci et al., 2013). Fungal antioxidants can vary based on the fruiting body, mycelium, and growth medium (Dulay et al., 2016).
5. Conclusion
This research showed that Schizophyllum commune Fr. grows on 92 types of woods that had rotted. This fungus has variations in the color, size, and production of the fruiting bodies, which depend on the weathered wood substrate of the growing medium. The mineral content, proximate, and phytochemical compounds of the macro fungus S. commune also varied depending on the type of wood growing media. The results are very useful for local people who are malnourished and have low incomes to consume wild food fungi such as Tanggidi (S. commune) because of its high nutritional content and antioxidants. The fungus has affordable selling prices and can be found growing naturally on various types of weathered wood in the area along the Palu-Koro fault, Central Sulawesi, Indonesia. It was also discovered that macro fungi with various decayed wood substrates as growth media have good nutritional levels, therefore, they can be used and processed into various food products that are beneficial for health. Based on these results, it is recommended to carry out chemical compounds analysis on each type of wood to realize the prospect of its use as a substrate in the cultivation of S. commune fungi. Therefore, there is a need to domesticate this fungus to support its commercialization as food and medicine in the future.
Funding information
This research was funded by the Directorate General of Research and Development Strengthening, Ministry of Research, Technology and Higher Education, Indonesia, through the Higher Education Applied Research Grant (PTUPT), No. SP DIPA-042.06.1.401516/2019, 5 December 2018.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to all participants, namely Aprianto Manggu, Wili Yanto Andisi, Rizal Munandar, Gilno Vanderlik Waro, Angelius Akmon, Robi Gembu, and Abdul Gafur who assisted during the experiment, especially in data and sample collection in the field as well as mineral and proximate analysis of fungus samples in the laboratory. The authors are also grateful to Haris Priyana for the assistance in completing the research site map.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Yusran Yusran, Email: yusran@untad.ac.id.
Erniwati Erniwati, Email: erniwatiii@untad.ac.id.
Akhmad Khumaidi, Email: ahmadkhumaidi@untad.ac.id.
Ramadanil Pitopang, Email: Ramadhanil@untad.ac.id.
Ignasius Radix Astadi Praptono Jati, Email: radix@ukwms.ac.id.
References
- Abdul Razak D.L., Jamaluddin A., Abd. Rashid N.Y., Mohd Fadzil N.A., Sani N.A., Abdul Manan M. Comparative Evaluation of Schizophyllum commune Extracts as Potential Cosmeceutical Bio-1ngridient. Int. J. Agric. Sci. 2018;5(1):2348–3997. [Google Scholar]
- Acanto R.B., Helen Cuaderes V.S. Antimicrobial Activity And Phytochemical Screening of Split Gill Mushroom (Schizophyllum commune) Ethanolic Extract. Int. J. Sci. Technol. Res. 2021;10:4–9. [Google Scholar]
- Adejoye O.D., Adebayo-Tayo B.C., Ogunjobi A.A., Afolabi O.O. Physicochemical Studies on Schizophyllum commune (Fries) a Nigerian Edible Fungus. World Appl. Sci. J. 2007;2:73–76. [Google Scholar]
- Alananbeh K.M., Bouqellah N.A., Al Kaff N.S. Cultivation of oyster mushroom Pleurotus ostreatus on date-palm leaves mixed with other agro-wastes in Saudi Arabia. Saudi J. Biol. Sci. 2014 doi: 10.1016/j.sjbs.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemu F. Cultivation of Lentinus edodes on teff straw (agricultural residue) at Dilla University, Ethiopia. Appl. Microbiol. 2014;1:49–59. [Google Scholar]
- Aletor V.A. Compositional studies on edible tropical species of mushrooms. Food Chem. 1995;54:265–268. [Google Scholar]
- Altaf U., Lalotra P., Sharma Y.P. Nutritional and mineral composition of four wild edible mushrooms from Jammu and Kashmir, India. Indian Phytopathol. 2020;73:313–320. doi: 10.1007/s42360-020-00230-1. [DOI] [Google Scholar]
- Anwar R., Nasichah A.Z., Roini C. Pengetahuan masyarakat Kecamatan Tidore Utara tentang pemanfaatan makroskopis sebagai potensi lokal daerah. Saintifik@: Jurnal Pendidikan MIPA. 2020;6:86–92. [Google Scholar]
- Ao T., Deb C.R. Nutritional and antioxidant potential of some wild edible mushrooms of Nagaland. India. J. Food Sci. Technol. 2019;56(2):1084–1089. doi: 10.1007/s13197-018-03557-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC, 2010. Official methods of analysis of the association of the analytical chemists. Maryland, USA.
- Basso V., Schiavenin C., Mendonça S., de Siqueira F.G., Salvador M., Camassola M. Chemical features and antioxidant profile by Schizophyllum commune produced on different agroindustrial wastes and byproducts of biodiesel production. Food Chem. 2020;329 doi: 10.1016/j.foodchem.2020.127089. [DOI] [PubMed] [Google Scholar]
- Beluhan S., Ranogajec A. Chemical composition and non-volatile components of Croatian wild edible mushrooms. Food Chem. 2011;124(3):1076–1082. [Google Scholar]
- Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisema J.M. PT Kinta; Jakarta: 1968. Djamur: jang dapat dimakan, jang beracun dan pengusahaan djamur merang. [Google Scholar]
- Boonthatui Y., Chongsuwat R., Kittisakulnam S. Production of antioxidant bioactive compounds during mycelium growth of Schizophyllum commune on different cereal media. CMUJ. Nat. Sci. 2021;20(2):e2021032. [Google Scholar]
- Carreño-Ruiz S.D., Lázaro A.A.A., García S.C., Hernández R.G., Chen J., Navarro G.K.G., Fajardo L.V.G., Pérez N.D.C.J., Cruz M.T.D.L., Blanco J.C., Cappello R.E. New record of Schizophyllum (Schizophyllaceae) from Mexico and the confirmation of its edibility in the humid tropics. Phytotaxa. 2019;413(2):137–148. doi: 10.11646/phytotaxa.413.2.3. [DOI] [Google Scholar]
- Chandrawanshi N.G., Tandia D.K., Jadhav S.K. Nutraceutical properties evaluation of Schizophyllum commune. Indian J. Sci. Res. 2017;13(2):57–62. [Google Scholar]
- Chandrawanshi N.K., Tandia D.K., Jadhav S.K. Determination of Anti-Diabetic Property of organic and nonorganic solvent extracts of Schizophyllum commune. New Bio. World. 2019;1(1):5–8. [Google Scholar]
- Chang, S., Miles, G.P., 2004. Mushrooms: Cultivation, Nutritional Value, Medicinal Effects and Environmental Impact, p. 436. Boca Raton, FL, CRC Press. 10.1201/9780203492086. [DOI]
- Cooke W.B. The genus Schizophyllum. Mycologia. 1961;53:575–599. doi: 10.2307/3756459. [DOI] [Google Scholar]
- Culmsee H., Pitopang R. Tree diversity in sub-montane and lower montane primary rain forests in Central Sulawesi. Blume. 2009;54:119–123. doi: 10.3767/000651909X475473. [DOI] [Google Scholar]
- Das S., Bisoyi S.K., Anmoldeep P.D., Srivastava S. Role of Cultivated Mushrooms in Bioremediation: A Review. Biol. For. 2021;13(1):160–168. [Google Scholar]
- Dasanayaka P.N., Wijeyaratne S.C. Cultivation of Schizophyllum commune mushroom on different wood substrates. J. Trop. Fores. Env. 2017;7(1):65–73. [Google Scholar]
- de Jong, J.F, 2006. Aerial Hyphae of Schizophyllum commune: Their Function and Formation. PhD thesis, Univ. Utrecht.
- Dulay R.M.R., Vicente J.J.A., Dela C.A.G., Gagarin J.M., Fernando W., Kalaw S.P., Reyes R.G. Antioxidant activity and total phenolic content of Volvariella volvacea and Schizophyllum commune mycelia cultured in indigenous liquid media. Mycosphere. 2016;7(2):131–138. doi: 10.5943/mycosphere/7/2/4. [DOI] [Google Scholar]
- Elsakhawy T.A., El-Rahem W.T.A. Evaluation of Spent Mushroom Substrate Extract as a Biofertilizer for Growth Improvement of Rice (Oryza sativa L) Egypt. J. Soil. Sci. 2020;60(1):31–42. [Google Scholar]
- Espejel-Sánchez K.I., Espinosa-Solares T., Reyes-Trejo B., HernándezRodríguez G., Cunill-Flores J.M., Guerra-Ramírez D. Nutritional value and thermal degradation of bioactive compounds in wild edible mushrooms. Rev. Chapingo Ser. Cienc. For. Ambiente. 2021;27(3):337–354. doi: 10.5154/r.rchscfa.2020.12.078. [DOI] [Google Scholar]
- Falandysz J., Borovička B. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2013;97:477–501. doi: 10.1007/s00253-012-4552-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip R., Shaw T.A., Nishida A., Pezacki J.P. Fungal Natural Alkaloid Schizocommunin Activates The Aryl Hydrocarbon Receptor Pathway. Med. Chem. Commun. 2019;10:985–990. doi: 10.1039/C9MD00138G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarasi M., Socaci S.A., Dulf F.V., Diaconeasa Z.M., Fărcaș A.C., Tofană M., Semeniuc C.A. Bioactive compounds and volatile profiles of five Transylvanian wild edible mushrooms. Molecules. 2018;23(12):3272. doi: 10.3390/molecules23123272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E.M. Gerhardi Bonnieri; Copenhagen, Denmark: 1815. Observationes mycologicae. [Google Scholar]
- Girma W., Tasisa T. Application of Mushroom as Food and Medicine. Adv. Biotech Micro. 2018;11(4) doi: 10.19080/AIBM.2018.11.555817. [DOI] [Google Scholar]
- González A., Cruz M., Losoya C., Nobre C., Loredo A., Rodríguez R., Contreras J., Ruth Belmares R. Edible mushrooms as a novel protein source for functional foods. Food Func. 2020;11(9):7400–7414. doi: 10.1039/D0FO01746A. [DOI] [PubMed] [Google Scholar]
- Gupta S., Brazier A.K.M., Lowe N.M. Zinc deficiency in low-and middle-income countries: prevalence and approaches for mitigation. J. Hum. Nutr. Diet. 2020;33(5):624–643. doi: 10.1111/jhn.12791. [DOI] [PubMed] [Google Scholar]
- Han C., Zhang G., Wang H., Ng T.B. Schizolysin, a hemolysin from the split gill mushroom Schizophyllum commune. FEMS Microbiol Lett. 2010;309(2):115–121. doi: 10.1111/j.1574-6968.2010.02022.x. [DOI] [PubMed] [Google Scholar]
- Hao L., Xing X., Li Z., Zhang J., Sun J., Jia S., Qiao C., Wu T. Optimization of effect factors for mycelia growth and exopolysaccharide production by Schizophyllum commune. Appl. Biochem. Biotechnol. 2010;160(2):621–631. doi: 10.1007/s12010-008-8507-6. [DOI] [PubMed] [Google Scholar]
- Heleno S.A., Barros L., Sousa M.J., Martins A., Ferreira I.C.F.R. Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Chem. 2010;119(4):1443–1450. doi: 10.1016/j.foodchem.2009.09.025. [DOI] [Google Scholar]
- Herawati E., Ramadhan R., Ariyani F., Marjenah, Kusuma I.W., Suwinarti W., Mardji D., Amirta R., Arung E.T. Phytochemical screening and antioxidant activity of wild mushrooms growing in tropical regions. Biodivers. 2021;22(11):4716–4721. doi: 10.13057/biodiv/d221102. [DOI] [Google Scholar]
- Herliyana, E.N, Maryam, L.F, Hadi, Y.S, 2011. Schizophyllum commune Fr. Sebagai Jamur Uji Ketahanan Kayu Standar Nasional Indonesia Pada Empat Jenis Kayu Rakyat: Sengon (P. falcataria), Karet (H. brasiliensis), Tusam (P. merkusii), Mangium (A. mangium). J. Silvikultur Tropika. 2(3), 176–180. doi: 10.29244/j-siltrop.2.3.%2525p. [DOI]
- Hobs C.R. The Chemistry, Nutritional Value, Immunopharmacology, and Safety of the Traditional Food of Medicinal Split-Gill Fungus Schizophyllum commune Fr.:Fr (Schizophyllaceae). A Literature Review. Int. J. Med. Mushrooms. 2005;7(1–2):127–140. doi: 10.1615/IntJMedMushr.v7.i12.130. [DOI] [Google Scholar]
- Horisawa S., Ando H., Ariga O., Sakuma Y. Direct ethanol production from cellulosic materials by consolidated biological processing using the wood rot fungus Schizophyllum commune. Bioresour. Technol. 2015;197:37–41. doi: 10.1016/j.biortech.2015.08.031. [DOI] [PubMed] [Google Scholar]
- Işıloğlu M., Yılmaz F., Merdivan M. Concentrations of trace elements in wild edible mushrooms. Food Chem. 2001;73(2):169–175. doi: 10.1016/S0308-8146(00)00257-0. [DOI] [Google Scholar]
- Jacinto-Azevedo B., Valderrama N., Henríquez K., Aranda M., Aqueveque P. Nutritional value and biological properties of Chilean wild and commercial edible mushrooms. Food Chem. 2021;356 doi: 10.1016/j.foodchem.2021.129651. [DOI] [PubMed] [Google Scholar]
- James T.Y., Porter D., Hamrick J.L., Vilgalys R. Evidence for limited intercontinental gene flow in the cosmopolitan mushroom. Schizophyllum commune. Evolution. 1999;53(6):1665–1677. doi: 10.1111/j.1558-5646.1999.tb04552.x. [DOI] [PubMed] [Google Scholar]
- Kakumu S., Ishikawa T., Wakita T., Yoshioka K., Ito Y., Shinagawa T. Effect of sizofiran, a polysaccharide, on interferon gamma, antibody production and lymphocyte proliferation specific for hepatitis B virus antigen in patients with chronic hepatitis B. Int. J. Immunopharmacol. 1991;13(7):969–975. doi: 10.1016/0192-0561(91)90050-h. [DOI] [PubMed] [Google Scholar]
- Kamalebo H.M., Malale H.N.S.W., Ndabaga C.M., Deegreef J., Kesel A.D. Uses and importance of wild fungi: traditional knowledge from the Tshopo province in the Democratic Republic of the Congo. J. Ethnobiol. Ethnomed. 2018;14(13):1–12. doi: 10.1186/s13002-017-0203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartini K., Khumaidi A., Khaerati K., Ihwan I. Ethanolic extract of eboni leaf decrease blood glucose level in alloxan induced male rats. J Vet. 2018;19(3):329–334. [Google Scholar]
- Keßler P.J.A., Bos M., Sierra Daza S.E.C., Kop A., Willemse L.P.M., Pitopang R., Gradstein S.R. Checklist of woody plants of Sulawesi, Indonesia. Blumea. Supplement. 2002;14:1–160. [Google Scholar]
- Khastini R.O., Wahyuni I., Saraswati I. Ethnomycology of bracket fungi in Baduy tribe Indonesia. Biosaintifika. 2018;10(2):423–431. doi: 10.15294/biosaintifika.v10i2.14082. [DOI] [Google Scholar]
- Komatsu N., Nagumo N., Okubo S., Koike K. Protective effect of schizophyllan, a polysaccharide of Schizophyllum commune, on bacterial infections of mice. Jpn. J. Antibiot. 1973;26:277–283. [PubMed] [Google Scholar]
- Kozarski M., Klaus A., Jakovljevic D., Todorovic N., Vunduk J., Petrović P., Niksic M., Vrvic M.M., van Griensven L. Antioxidants of Edible Mushrooms. Molecules. 2015;20:19489–19525. doi: 10.3390/molecules201019489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupodorova T.A., Barshteyn V.Y. Amaranth Flour as a New Alternative Substrate for Schizophyllum commune Fr.: Fr. and Cordyceps sinensis (Berk.) Sacc. Growth J. Siberian Fed. Univ. Biol. 2015;8(1):32–44. doi: 10.17516/1997-1389-2015-8-1-32-44. [DOI] [Google Scholar]
- Krupodorova T., Sevindik M. Antioxidant potential and some mineral contents of wild edible mushroom Ramaria stricta. AgroLife Sci. J. 2020;9(1):186–191. [Google Scholar]
- Kumla J., Suwannarach N., Sujarit K., Penkhrue W., Kakumyan P., Jatuwong K., Vadthanarat S., Lumyong S. Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules. 2020;25(12):2811. doi: 10.3390/molecules25122811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, M. 2019. Schizophyllum commune. Retrieved from mushroomexpert.com. https://www.mushroomexpert.com/schizophyllum_commune.html (accessed in April 2019).
- Kusrinah, Kasiamdari R.S. Morphological Characteristics and Kinship Relationship of Mushroom Schizophyllum commune Fr. J. Nat. Sci. Res. 2015;1(2):65–71. doi: 10.21580/jnsmr.2015.1.2.1620. [DOI] [Google Scholar]
- Liu X., Frydenvang K., Liu H., Zhai L., Chen M., Olsen C.E., Christensen S.B. Iminolactones from Schizophyllum commune. J. Nat. Prod. 2015;78:1165–1168. doi: 10.1021/np500836y. [DOI] [PubMed] [Google Scholar]
- Longvah T., Deosthale Y.G. Compositional and nutritional studies on edible wild mushrooms from norteasth India. Food Chem. 1998;63(3):331–334. doi: 10.1016/S0308-8146(98)00026-0. [DOI] [Google Scholar]
- Malik N.A., Kumar J., Wani M.S., Tantray Y.R., Ahmad T. In: Dar G.H., Bhat R.A., Mehmood M.A., Hakeem K.R., editors. Vol 2. Springer; Cham: 2021. Role of Mushrooms in the Bioremediation of Soil. (Microbiota and Biofertilizers). [DOI] [Google Scholar]
- Mirfat A.H.S., Noorlidah A., Vikineswary S. Antimicrobial Activities of Split Gill Mushroom Schizophyllum commune Fr. m. J. Res. Commun. 2014;2(7):113–124. [Google Scholar]
- Mongkontanawat N., Thumrongchote D. Effect of strains and extraction methods on β-glucan production, antioxidant properties, and FTIR Spectra from mushroom fruiting bodies of Schizophyllum commune Fr. Thailand. Food Resc. 2021;5(4):410–415. doi: 10.26656/fr.2017.5(4).259. [DOI] [Google Scholar]
- Muszyńska B., Kała K., Rojowski J., Grzywacz A., Opoka W. Composition and Biological Properties of Agaricus bisporus Fruiting Bodies – a Review. Pol. J. Food Nutr. Sci. 2017;67(3):173–181. doi: 10.1515/pjfns-2016-0032. [DOI] [Google Scholar]
- Myers N., Miitermeier R.A., Mittermeier C.G., Da Fosenca G.A., Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Niederpruem D.J., Hunt S. Polyols in Schizophyllum commune. Am. J. Bot. 1964;54(2):241–245. doi: 10.2307/2440804. [DOI] [Google Scholar]
- Nion Y.S., Djaya A.A., Kadie E.M., Lune S., Wijaya C.H. Siklus Hidup Jamur Konsumsi Lokal Kulat Kritip (Schizophyllum commune) Pada Daerah Bergambut dan Daerah Bertanah Mineral serta Potensi Nutrisinya (Cycles life of local edible mushroom Kulat Kritip (Schizophyllum commune) on peat soil and mineral soil also its nutrition potency) J. Biologi Indones. 2012;8(20):399–406. doi: 10.14203/jbi.v8i2.3060. [DOI] [Google Scholar]
- Niu Y., Yun J., Bi Y., Wang T., Zhang Y., Liu H., Zhao F. Predicting the shelf life of postharvest Flammulina velutipes at various temperatures based on mushroom quality and specific spoilage organisms. Postharvest Biol. Technol. 2020;167 doi: 10.1016/j.postharvbio.2020.111235. [DOI] [Google Scholar]
- Nurhasnawati H., Sundu R., Supriningrum R., Kuspradini H., Arung E.T. Antioxidant activity, total phenolic and flavonoid content of several indigenous species of ferns in East Kalimantan, Indonesia. Biodivers. 2019;20(2):576–580. doi: 10.13057/biodiv/d200238. [DOI] [Google Scholar]
- Nurlita A.I., Putra I.P., Ikhsan M. Catatan Pemanfaatan Schizophyllum commune di Kampung Udapi Hilir. Papua Barat. Integrated Lab. J. 2021;9(1):18–28. doi: 10.14421/ilj.2021.090104. [DOI] [Google Scholar]
- Okwulehie I.C., Nwosu C.P., Okoroafor C.J. Pharmaceutical and Nutritional Prospects of Two Wild Macro-Fungi Found in Nigeria. Biotechnol. 2007;6(4):567–572. doi: 10.3923/biotech.2007.567.572. [DOI] [Google Scholar]
- Ooi V.E.C., Liu F. Immunomodulation and Anti-Cancer Activity of Polysaccharide- Protein Complexes. Curr. Med. Chem. 2000;7(7):715–729. doi: 10.2174/0929867003374705. [DOI] [PubMed] [Google Scholar]
- Ouzouni P.K., Petridis D., Koller W.D., Riganakos K.A. Nutritional value and metal content of wild edible mushrooms collected from West Macedonia and Epirus, Greece. Food Chem. 2009;115(4):1575–1580. doi: 10.1016/j.foodchem.2009.02.014. [DOI] [Google Scholar]
- Owaid M.N., Abed I.A., Al-Saeedi S.S.S. Applicable properties of the bio-fertilizer spent mushroom substrate in organic systems as a byproduct from the cultivation of Pleurotus spp. Inform. Proc. in Agric. 2017;4(1):78–82. doi: 10.1016/j.inpa.2017.01.001. [DOI] [Google Scholar]
- Pandey A., Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J. Pharmacogn. Phytochem. 2014;2(5):115–119. [Google Scholar]
- Patria A., Putra P.S. Development of the Palu-Koro Fault in NW Palu Valley, Indonesia. Geosci. Lett. 2020;7 doi: 10.1186/s40562-020-0150-2. [DOI] [Google Scholar]
- Plants of the World Online (POWO), 2019, October 30. Royal Botanical Garden (https://powo.science.kew.org/).
- Pooja S., Vidyasagar G. Phytochemical screening for secondary metabolites of Opuntia dillenii Haw. J. Med. Plants. Stud. 2016;4(5):39–43. [Google Scholar]
- Preecha C., Thongliumnak S. Bag opening technique for bag spawn culture of spit gill mushroom (Schizophyllum commune) Int. J. Agric. Technol. 2015;11(2):367–372. [Google Scholar]
- Raaman N. New India Publishing Agensi; India: 2006. Phytochemical Techniques; p. 306. [Google Scholar]
- Raper, J.R., Miles, P.G., 1958. The Genetics of Schizophyllum Commune. 43(3), 530–546. doi: 10.1093/genetics/43.3.530. [DOI] [PMC free article] [PubMed]
- Rózsa M., Măniuțiu D.N., Egyed E. Influence of magnesium (Mg) source on the Cordyceps militaris (L.) mushroom mycelium growth. Curr. Trends Nat. Sci. 2021;10:333–340. [Google Scholar]
- Sahira B.K., Cathrine L. General Techniques Involved in Phytochemical Analysis. Int. J. Adv. Res. Chem. Sci. 2015;2(4):25–32. [Google Scholar]
- Sánchez C. Reactive oxygen species and antioxidant properties from mushrooms. Synth. Syst. Biotechnol. 2017;2(1):13–22. doi: 10.1016/j.synbio.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sande D., de Oliveira G.P., e Moura M.A.F., Martins B.A., Lima M.T.N.S., Takahashi J.A. Edible mushrooms as a ubiquitous source of essential fatty acids. Food Res. Int. 2019;125 doi: 10.1016/j.foodres.2019.108524. [DOI] [PubMed] [Google Scholar]
- Sanmee R., Dell B., Lumyong P., Izumori K., Lumyong S. Nutritive value of popular wild edible mushrooms from northern Thailand. Food Chem. 2003;82(4):527–532. doi: 10.1016/S0308-8146(02)00595-2. [DOI] [Google Scholar]
- Shaikh J.R., Patil M. Qualitative tests for preliminary phytochemical screening: An overview. Int. J. Chem. Stud. 2020;8(2):603–608. doi: 10.22271/chemi.2020.v8.i2i.8834. [DOI] [Google Scholar]
- Singh R. A Review on Different Benefits of Mushroom. IOSR J. Pharm. Biol. Sci. 2017;12(1):107–111. doi: 10.9790/3008-120102107111. [DOI] [Google Scholar]
- Socquet A., Simons W., Vigny C., McCafrey R., Subarya C., Sarsito D., Ambrosius B., Spakman W. Microblock rotations and fault coupling in SE Asia triple junction (Sulawesi, Indonesia) from GPS and earthquake slip vector data. J. Geophys. Res. Solid Earth. 2006;111:B08409. doi: 10.1029/2005JB003963. [DOI] [Google Scholar]
- Stan D., Enciu A.M., Mateescu A.L., Ion A.C., Brezeanu A.C., Stan D., Tanase C. Natural compounds with antimicrobial and antiviral effect and nanocarriers used for their transportation. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.723233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Niu Z. A mushroom diet reduced the risk of pregnancy-induced hypertension and macrosomia: a randomized clinical trial. Food Nutr. Res. 2020;64:4451. doi: 10.29219/fnr.v64.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwannarach N., Kumla J., Zhao Y., Kakumyan P. Impact of Cultivation Substrate and Microbial Community on Improving Mushroom Productivity: A Review. Biology. 2022;11:569. doi: 10.3390/biology11040569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki D. Effects of Phosphoryl Oligosaccharides of Calcium (POs-Ca) on Mycelial Growth and Fruiting Body Development of the Edible Mushroom,Pleurotus ostreatus. J. Appl. Glycosci. 2020;67(3):67–72. doi: 10.5458/jag.jag.JAG-2020_0001. 10.5458%2Fjag.jag.JAG-2020_0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata K., Ito W., Kojima T., Kawabata S., Misaki A. Ultrasonic degradation of schizophyllan, an antitumor polysaccharide produced by Schizophyllum commune. Fries. Carbohydr Res. 1981;89(1):121–135. doi: 10.1016/s0008-6215(00)85234-9. [DOI] [PubMed] [Google Scholar]
- Takemoto, S., Nakamura, H., Erwin, Imamura, Y., Shimane, T., 2010. Schizophyllum commune as a Ubiquitous plant parasite. JARQ 44(4), 357–364. https://www.jircas.affrc.go.jp.
- Teoh Y.P., Don M.M. In vitro anti fungal activities and phytochemical analysis of filamentous white-rot fungi, Schizophyllum commune. Sains Malays. 2013;42(9):1267–1272. [Google Scholar]
- Teoh Y.P., Don M.M., Ujang S. Production of Biomass by Schizophyllum commune and Its Antifungal Acivity towards rubberwood-degrading fungi. Sains Malays. 2017;46(1):123–128. doi: 10.17576/jsm-2017-4601-16. [DOI] [Google Scholar]
- Uzun Y., Genccelep H., Kaya A., Akcay M.E. The Mineral Contents of Some Wild Edible Mushrooms. Ekoloji Dergisi. 2011;20(80):6–12. [Google Scholar]
- Vannucci L., Krizan J., Sima P., Stakheev D., Caja F., Rajsiglova L., Horak V., Saieh M. Immunostimulatory properties and antitumor activities of glucans. Int. J. Oncol. 2013;43(2):357–364. doi: 10.3892/ijo.2013.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waktola G., Temesgen T. Application of mushrooms as food and medicine. Adv. Biotech. Microbiol. 2018;11 doi: 10.19080/AIBM.2018.11.555817. [DOI] [Google Scholar]
- Whitten A.J., Muslim M., Henderson G.S. Gadjah Mada University Press; Yogyakarta, Indonesia: 1987. The Ecology of Sulawesi. [Google Scholar]
- Wirth S., Krause K., Kunert M., Broska S., Paetz C., Boland W., Kothe E. Function of sesquiterpenes from Schizophyllum commune in interspecific interactions. PLoS ONE. 2021;16(1):1–14. doi: 10.1371/journal.pone.0245623. e0245623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldemariam W.G. Mushrooms in the Bio-Remediation of Wastes from Soil. Adv. Life Sci. Tech. 2019;76:41–46. doi: 10.7176/ALST. [DOI] [Google Scholar]
- Yamamoto T., Yamashita T., Tsubura E. Inhibition of pulmonary metastasis of Lewis lung carcinoma by a glucan, schizophyllan. Inv. Metast. 1981;1:71–84. [PubMed] [Google Scholar]
- Yildiz S., Yilmaz A., Can Z., Kiliç C., Yildiz Ü.C. Total phenolic, flavonoid, tannin contents and antioxidant properties of Pleurotus ostreatus and Pleurotus citrinopileatus cultivated on various sawdust. GIDA. 2017;42:315–323. doi: 10.15237/gida.GD16099. [DOI] [Google Scholar]
- Yim H.S., Chye F.Y., Rao V., Low J.Y., Matanjun P., How S.E.C.W. Optimization of Extraction Time and Temperature on Antioxidant Activity of Schizophyllum commune Aqueous Extract Using Response Surface Methodology. J. Food Sci. Technol. 2013;50:275–283. doi: 10.1007/s13197-011-0349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusran Y., Erniwati E., Wahyuni D., Ramadhanil R., Khumaidi A. Diversity of macro fungus across three altitudinal ranges in Lore Lindu National Park, Central Sulawesi, Indonesia and their utilization by local residents. Biodiversity. 2021;22:199–210. doi: 10.13057/biodiv/d220126. [DOI] [Google Scholar]
- Yusran Y., Erniwati E., Khumaidi A., Pitopang R., Rahmawati R., Korja I.N., Labiro E., Rukmi R., Muthmainnah M., Maksum H., Setyawati R. Diversity of Macro Fungus Species in Several Types of Land Use Around Lore Lindu National Park, Central Sulawesi, Indonesia. Int. J. Des. Nat. Ecodyn. 2022;17:341–351. doi: 10.18280/ijdne.170303. [DOI] [Google Scholar]
- Yusran Y., Erniwati E., Khumaidi A., Setiarto R.H.B. Effect of Cooking on the Proximate Composition and Minerals Content of Wild Edible Macro Fungi from Lore Lindu National Park, Central Sulawesi, Indonesia. African. J. Food Agric. Nutr. Dev. 2022;22:20523–20541. doi: 10.18697/ajfand.110.21660. [DOI] [Google Scholar]
- Zeb M., Lee C.H. Medicinal Properties and Bioactive Compounds from Wild Mushrooms Native to North America. Molecules. 2021;26:251. doi: 10.3390/molecules26020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohra S.F., Meriem B., Samira S., Alsayadi Muneer M.S. Phytochemical Screening and identification of some compounds from Mallow. J. Nat. Prod. Plant Resour. 2012;2:512–516. [Google Scholar]