Abstract
Extracellular vesicles (EVs), secreted membranous nano-sized particles, are critical intercellular messengers participating in nervous system homeostasis, while recent evidence implicates EVs in Alzheimer’s disease (AD) pathogenesis. Specifically, small EVs have been shown to spread toxic proteins, induce neuronal loss, and contribute to neuroinflammation and AD progression. On the other hand, EVs can reduce amyloid-beta deposition and transfer neuroprotective substances between cells, mitigating disease mechanisms. In addition to their roles in AD pathogenesis, EVs also exhibit great potential for the diagnosis and treatment of other brain disorders, representing an advantageous tool for Precision Medicine. Herein, we summarize the contribution of small EVs to AD-related mechanisms and disease progression, as well as their potential as diagnostic and therapeutic agents for AD.
Keywords: Alzheimer’s disease, Precision medicine, Extracellular vesicles, Exosomes, Progression, Diagnosis, Treatment
1. Introduction
After two decades of failed therapeutic trials and one of the lowest success rates for drug development in the entire field of medicine, the Alzheimer’s disease (AD) scientific community has been increasingly focusing on the early stages of AD and the long pre-symptomatic phase of the disorder to prevent or attenuate brain and synaptic damage before it becomes irreversible. During the long pre-symptomatic phase of AD, which may last up to 20 years (Dubois et al., 2016), different pathogenic processes and factors such as genetics, inflammation, lifetime stress, insulin resistance, and co-morbidities may influence disease progression and related brain damage (Golde, 2022). The emerging view of Precision Medicine suggests that AD should not be approached as a unitary biologic entity; instead, efforts should focus on a better understanding of variables that affect AD initiation and progression, which will improve our comprehension of the disease etiopathogenesis and pathophysiology (Berkowitz et al., 2018). This may lead to the identification of AD sub-types and the development of tailored novel treatments, as well as preventive interventions. In this context, extracellular vesicles (EVs), a class of nanosized secreted membranous particles that includes exosomes and microvesicles, are increasingly shown to play important roles in AD pathomechanisms and contribute to the propagation of AD pathology across the brain. Moreover, their ability to cross the blood-brain barrier (Chen et al., 2016; Saint-Pol et al., 2020), coupled with their cargo-protecting capacity and molecular signature similar to that of their originating cell, make exosomes and other small EVs unique biomarker tools in the new era of Precision Medicine that is considered to stimulate the development of 1) prognostic tests to implement therapies at the pre-symptomatic phase; 2) diagnostic tools with increased discrimination between neurological disorders; 3) cell-specific drug delivery systems for a personalized approach to complex disorders such as AD.
2. The biogenesis and secretion of EVs
Under healthy conditions, the nervous system and, more specifically, the brain is characterized by coordinated responses and homeostatic balance generated by proper communication between various cell types. Cells coordinate their actions by communicating with one another to maintain brain homeostasis. Conversely, if any of the processes underlying brain homeostasis is dysregulated, pathology can ensue.
In the past, intercellular communication in the nervous system was thought to be mediated mainly through synapses, specialized structures where cellular appendices of one cell lie in close proximity to their functional counterparts on another cell. Now, cell-to-cell communication is known to also occur through a variety of additional mechanisms: paracrine transmission of hormones and neurotransmitters (Barker and Smith, 1980); gap junctions that connect the cytoplasm of neighboring cells; tunneling nanotubes that join distanced cells; and most recently, extracellular vesicles (EVs), which are nanosized cargo-loaded vesicles released from cells. The first description of such vesicles occurred in the 1960s when Bonucci observed that chondrocytes secret small vesicles of 100 nm in size (Bonucci, 1967), and Wolf showed that platelets release EVs with clotting activity (Wolf, 1967), which confirmed previous suggestions of their existence (Chargaff and West, 1946; Hougie, 1955; O’Brien, 1955). Later, in the 1980s, studies on reticulocyte transferrin receptor turnover established that exosome biogenesis is essential for membrane-quality control (Pan and Johnstone, 1983; Harding and Stahl, 1983). Finally, at the turning of the millennium, Amigorena’s group first proposed a role for exosomes in the communication between cells of the immune system, thus making their debut as mediators of cell-cell communication (Zitvogel et al., 1998).
According to their biogenesis, extracellular vesicles were originally classified into three categories: microvesicles, apoptotic bodies, and exosomes (see Fig. 1). Briefly, exosomes derive from the endolysosomal pathway and the fusion of multivesicular bodies (MVBs) with the plasma membrane, while microvesicles and apoptotic bodies arise from plasma membrane budding and blebbing, respectively. However, as current isolation and characterization methods are not able to separate and distinguish between EVs based on their biogenesis, the International Society of Extracellular Vesicles released the “Minimal information for studies of extracellular vesicles 2018” (MISEV2018) guidelines, suggesting a different categorization of EVs based on physical characteristics (size or density), biochemical composition or cell of origin (Théry et al., 2018). Recently, a novel non-membranous nanovesicle, termed exomere, was reported. The isolation of these smaller than 50 nm nanovesicles was possible by applying asymmetric-flow field-flow fractionation (AF4), and their proteomic characterization revealed proteins associated with hypoxia, coagulation and metabolism, microtubule-associated proteins, and proteins related to mTOR signaling and glycolysis (Zhang et al., 2018).
Fig. 1.
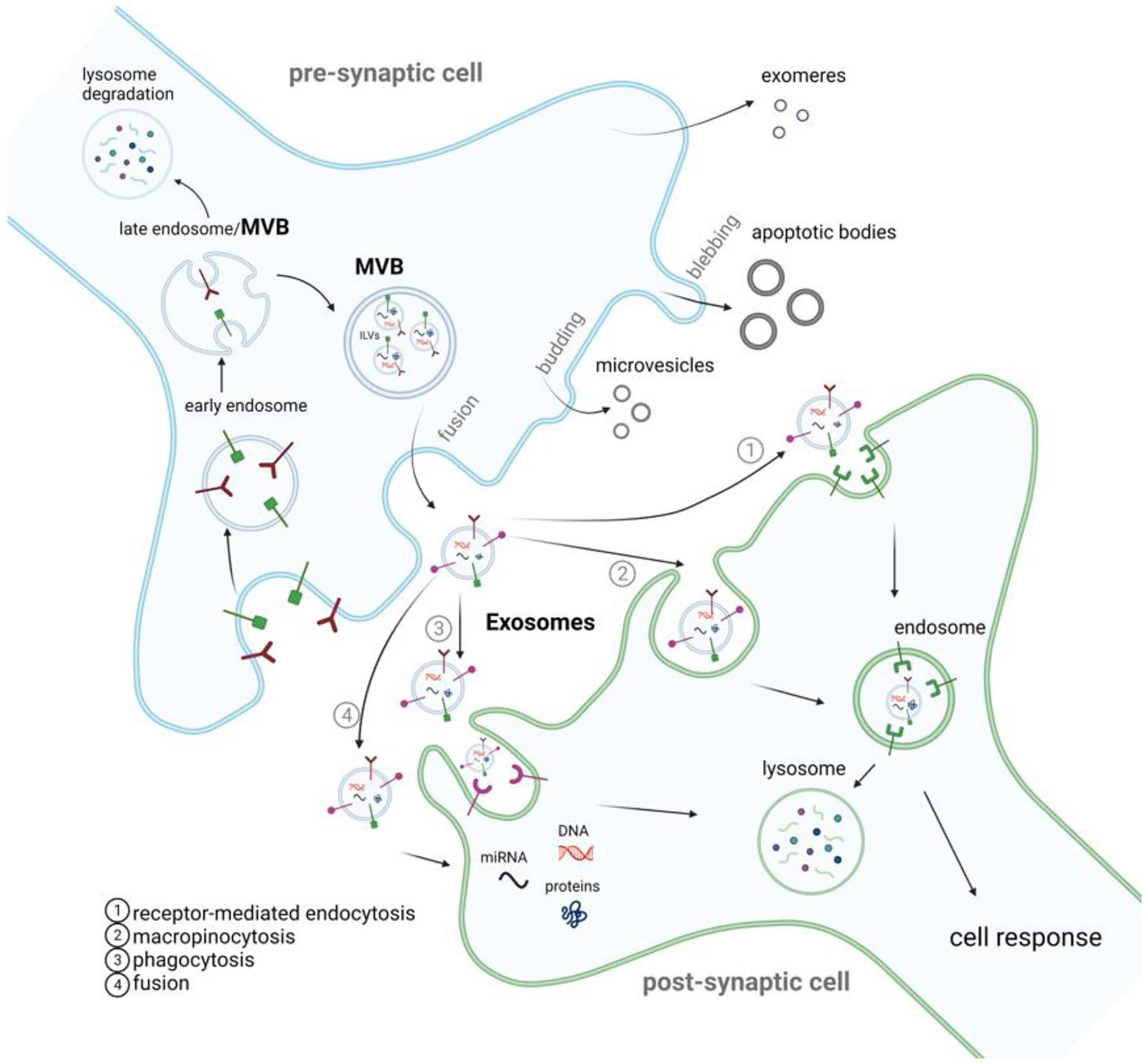
Extracellular vesicle life cycle. Extracellular vesicles (EVs) are membranous nano-sized particles released from the cell and are classically classified into exosomes, microvesicles, and apoptotic bodies. On the donor cell, exosomes derive from the endolysosomal pathway. Following endocytosis, early endosomes are generated and mature into late endosomes that will suffer further internal invagination to form intraluminal vesicles (ILVs). The MVBs fuse with the plasma membrane to release ILVs into the extracellular space as exosomes. Microvesicles are released through membrane budding, while apoptotic bodies are released by dying cells through the blebbing of the plasma membrane. Exomeres are a novel entity whose biogenesis is still being uncovered. Upon EVs release (the majority of studies have focused on small EVs – a mixture of exosomes and microvesicles), they are internalized by the recipient cell through several pathways such as receptor-mediated endocytosis (Dubois et al., 2016), macropinocytosis (Golde, 2022), phagocytosis (Berkowitz et al., 2018) and membrane fusion (Chen et al., 2016). Following their uptake by the recipient cell, EV cargo may undergo clearance or induce a cellular response.
EV biogenesis processes have often been categorized as ESCRT-dependent or ESCRT-independent, based on the contribution of the endosomal sorting complex required for transport (ESCRT) proteins. However, studies have shown that these systems may work synergistically to regulate exosome biogenesis (Babst, 2011; Gurunathan et al., 2021). For instance, different EV subpopulations might derive from different cellular pathways, while the cell type and its homeostatic state could also determine which cellular pathway is used for EV production. By far, the ESCRT-dependent mechanisms are the most extensively studied (Colombo et al., 2013; Katzmann et al., 2001), with ESCRT-independent mechanisms recently gaining attention after recent reports showed that lipids such as ceramide, as well as tetraspanins and heat shock proteins, can contribute to EV biogenesis (Stuffers et al., 2009; Matsuo, 2004). The fusion of MVBs with the plasma membrane to release exosomes is a complex process involving cytoskeletal proteins, molecular motors and switches, and fusion machinery (Hessvik and Llorente, 2018; Raposo and Stoorvogel, 2013).
3. Sorting of cargo into EVs
EV cargo is composed of bioactive molecules of great biological significance (e.g., proteins, lipids, nucleic acids) that have the capacity to influence the recipient cell. In addition, their composition greatly depends on the type and conditions of the parental cell and thus, reflects the proteomic, genomic, and homeostatic identity of that cell. The processes that govern protein or miRNA recruitment into exosomes are still poorly understood. It has been suggested that their cargo is passively accrued; however, recent studies have demonstrated multiple sorting systems that actively load molecules into exosomes (Fu et al., 2020). Protein loading into EVs uses different cellular mechanisms, including ESCRT components, ceramide, tetraspanins, and heat-shock proteins. ESCRT machinery recognizes ubiquitinylated proteins and is considered a crucial transporter of proteins to exosomes (Fu et al., 2020). Interestingly, recent evidence suggests a more prominent role of ESCRT-independent mechanism for cargo sorting. For instance, studies have suggested a mechanism of lateral cargo segregation dependent on lipid raft microdomains and lipids such as ceramide (Zhang et al., 2019). Similarly, tetraspanin-enriched microdomains containing CD81 were also shown to play a role in receptor sorting into small EVs (Perez-Hernandez et al., 2013). Furthermore, miRNAs loading into EVs may follow multiple sorting mechanisms (Janas et al., 2015). Similar to protein loading, miRNA sorting may be dependent on ESCRT-machinery (Gibbings et al., 2009). For instance, it has been described that miRNAs sequence motifs target them to exosomes. Indeed, heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1) recognizes these motifs and controls exosomal loading (Villarroya-Beltri et al., 2013), thus mutations on these motifs or changes in the sumoylation levels of hnRNPA2B1 protein affect miRNA sorting to exosomes. In addition, the 3′-end miRNA sequence was also suggested to contribute to this sorting (Koppers-Lalic et al., 2014). Another identified pathway is related to ceramide and neural sphingomyelinase 2, as expression of this enzyme relates to miRNA exosomal content (Kosaka et al., 2013). In addition, RNA-binding proteins, Ago2, miRNA induced silencing complex (miRISC) were shown to interact with miRNA and contribute to its differential sorting into exosomes (van Niel et al., 2018).
Similarly, the sorting of DNA and nuclear proteins into exosomes is a matter of intensive research and remains largely unclarified. It was recently described a role for micronuclei (markers of genomic instability) for nuclear content loading into EVs by interacting with MVBs precursors and tetraspanins (Yokoi et al., 2019). Thus, although the mechanisms involved in cargo sorting into EVs require further exploration, it is evident that EVs’ cargo packaging occurs both randomly and selectively. Our understanding of these mechanisms may enable the curated target of EV loading processe for future patient-tailored therapeutic approaches.
4. EV uptake
Following their secretion, EVs facilitate intercellular communication locally and systemically. This message-relaying system occurs through different mechanisms (see Fig. 1). First of all, there is tropism between donor and recipient cells, where a conserved cellular signature acts as a recognition motif for the uptake by the same cell type (Sancho-Albero et al., 2019). Moreover, EVs can carry membrane-specific motifs that enable their recognition in distant locations. Upon reaching the cell of interest, EVs may interact with cell surface receptors, activating intracellular signaling cascades. Alternatively, they might be uptaken by the recipient cell through macropinocytosis, endocytosis, membrane fusion, or receptor-ligand mediated interactions (McKelvey et al., 2015). As will be discussed below, EVs are suggested to be involved in the propagation of disease pathology between synaptically-connected cells and brain areas in different brain pathologies. Thus, understanding the mechanisms of secretion and uptake of EVs may contribute to the understanding of the initiation of neurodegenerative and neurological disorders towards the identification of novel therapeutic targets that may slow down or halt disease progression. On the other hand, a better understanding of exosome uptake mechanisms may aid in the development of more reliable and targeted exosome delivery for therapeutic purpose as their ability to cross the blood-brain barrier, together with their reduced immunogenicity and cargo-transport properties, make EVs promising “tools” for efficient and biocompatible drug delivery (Whitford and Guterstam, 2019; Schiffelers et al., 2012).
5. EVs in Alzheimer’s disease brain pathology
AD constitutes a worldwide public health crisis with a significant societal and financial burden. Indeed, it is estimated that approx. 50 million people worldwide suffer from AD and other types of dementia; this number will double by 2030 and surge to more than 115 million by 2050 (Prince et al., 2013). Moreover, it is estimated that the worldwide costs of dementia were US$818 billion in 2015, with 86% being spent in high-income countries (Wimo et al., 2017). Nowadays, AD is widely accepted as a multifactorial neurodegenerative disorder characterized by progressive cognitive impairment, including memory loss and behavioral changes. Among several non-modifiable risk factors for AD, age is the most significant, while certain genetic factors cause an autosomal-dominant form of early-onset AD (e.g., APP and presenilin mutations) and other non-deterministic genetic factors increase the risk for late-onset/sporadic disease (e.g., APOE) (Querfurth and LaFerla, 2010; Reiman et al., 2005). Education level is the most prominent among the modifiable risk factors, with years of education contributing to cognitive reserve and resilience to AD (Stern, 2012), probably by modulating synaptic density. Moreover, lifetime chronic stress and elevated glucocorticoids appear to increase the risk for AD (Rasmuson et al., 2001; O’Brien et al., 1996), with GC levels correlating with the rate of cognitive decline (Lucassen et al., 2014; Lupien et al., 1998) and influencing AD pathogenic mechanisms (Sotiropoulos et al., 2019; Justice, 2018; Mohammadi et al., 2021).
AD histopathological hallmarks include extracellular senile plaques of amyloid β (Aβ) and intracellular neurofibrillary tangles of insoluble hyperphosphorylated Tau aggregates. In addition, synaptic loss is a major feature of this disease and the most significant correlate of cognitive decline. Similarly, Tau pathology degree and spatial distribution also correlate with disease severity and AD symptoms (Bejanin et al., 2017; Ossenkoppele et al., 2016). A recent human study has provided insight into the association between human Tau pathology and synaptic loss and altered synaptic function (Coomans et al., 2021), as it had previously been found in murine AD models (Lasagna-Reeves et al., 2011; Zhou et al., 2017). AD pathology is suggested to initiate at the entorhinal cortex, propagating to the hippocampus and eventually to the rest of the neocortex in a prion-like spreading pattern through mechanisms not yet fully understood. However, several studies have demonstrated the seeding potential of both free and exosomal Aβ and Tau, which play a role in the propagation of AD brain pathology (McAllister et al., 2020; Thompson et al., 2016). Different mechanisms are suggested to be involved in AD brain pathology, namely the disruption of cellular proteostasis (Labbadia and Morimoto, 2015), including protein folding and clearance mechanisms (Iliff et al., 2014; Tarasoff-Conway et al., 2015), astrogliosis, and inflammation. Below, we will focus on the relationship of EVs with these disrupted cellular processes (see Fig. 3), as well as their involvement in the propagation of Tau and Aβ (see Fig. 2).
Fig. 3.
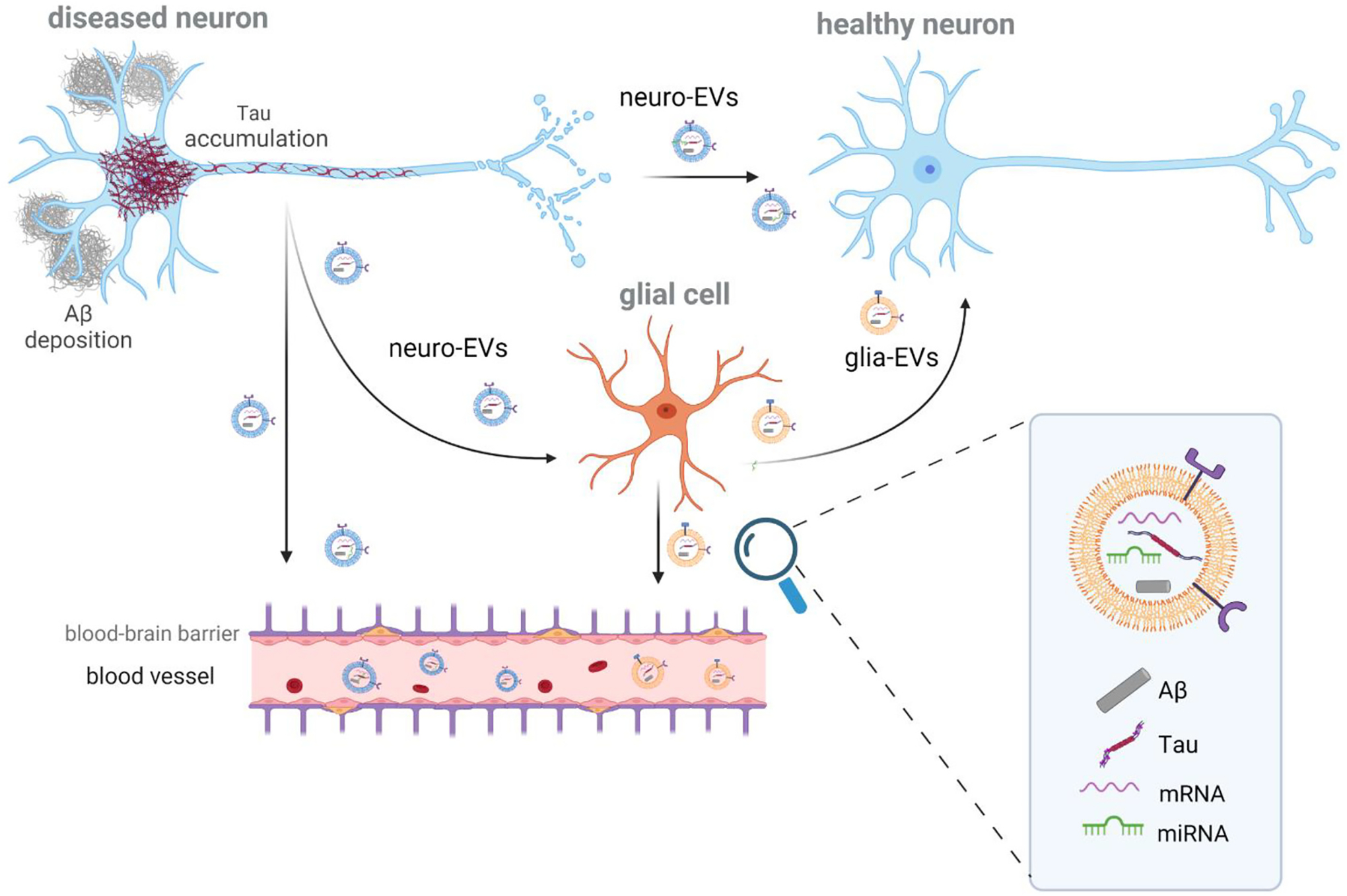
Role of extracellular vesicles in the spreading of Аβ and Tau pathology. Due to their endocytic nature, extracellular vesicles (EVs) reflect the genomic and proteomic status of the parental cell. For this reason, neuron-derived EVs (neuro-EVs) and glia-derived EVs (glia-EVs) may contain pathological forms of Tau and Aβ, which are capable of triggering AD pathology in the recipient cells. Besides proteins, EVs carry miRNAs and mRNAs that will also induce a cellular response into the recipient cell. The spreading of EVs can be released from the diseased neuron (donor cell) and be uptaken by the healthy neuron (recipient cell). Alternatively, glia cells can also uptake neuro-EVs and, in their turn, can secrete exosomes (glia-EVs) which will be uptaken by neurons. In addition, as EVs can cross the blood-brain barrier, both neuro- and glia-EVs are found in the peripheral blood, where they can be differentially isolated using immunoprecipitation for specific cell-specific transmembrane markers, e.g., L1CAM for neuro-EVs.
Fig. 2.
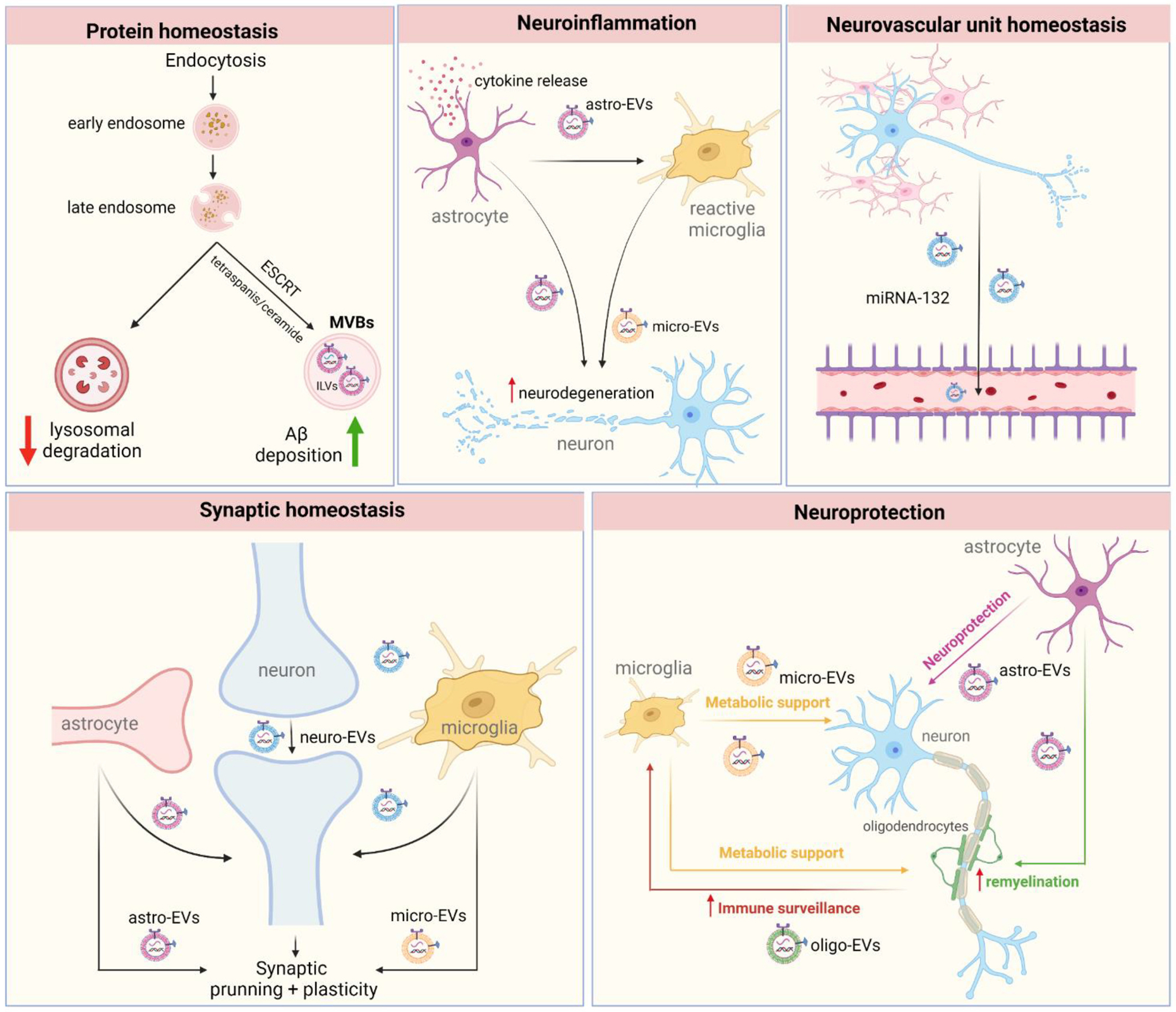
EVs interplay with different cellular mechanisms found to be disrupted in AD. 1) Protein degradation - The dysregulation of protein homeostasis due to impaired lysosome degradation may increase MVBs number in cells which may lead to an increase of EVs secretion, which in turn contributes to the enhanced Aβ deposition; 2) Neuroinflammation - Inflammatory activation associated with cytokines release leads to EVs secretion from astrocytes (astro-EVs) which, in this turn, will activate microglia contributing further to the pro-inflammatory state and ultimately to neurodegeneration. 3) Neurovascular unit homeostasis - blood-brain barrier dysfunction and increased permeability is found in AD, probably due to damage of the neurovascular unit. While the role of EVs in neurovascular dyshomeostasis in AD is under intense investigation, recent evidence suggests that neuron-derived EVs (neuro-EVs) carrying specific miRNAs (e.g., miR-132) are able to regulate endothelial integrity. 4) Synaptic plasticity and homeostasis – EVs are important cell-to-cell messengers, and emerging evidence suggests that EVs secreted by neurons, astrocytes, and microglia have been shown to regulate different levels of synaptic homeostasis, including synaptic pruning and maintenance, neurotransmitter release and reuptake, proteins of postsynaptic density; all important components of learning and memory that are damaged in AD. 5) Neuroprotection - Glial cells contribute to neuronal homeostasis and may tip the balance towards neuronal malfunction under pathological conditions. For instance, astrocyte-derived EVs (astro-EVs) transfer synapsin and neuroglobin to neurons, contributing to neurite growth. Moreover, microglia-derived EVs (micro-EVs) and astro-EVs are also shown to stimulate oligodendrocyte precursor cells, promoting (re)myelination. In their turn, oligodendrocytes-derived EVs (Oligo-EVs) and micro-EVs may support neuronal metabolic activity and axonal transport, providing different ways through which EVs are involved in neuroprotection and neurodegeneration.
5.1. Disruption of protein homeostasis and EVs
The main routes for cellular protein degradation are the ubiquitinproteasome system (UPS), the endolysosomal pathway, and autophagy (Cuervo et al., 2010). Proteasomes are protein complexes wherein misfolded proteins are degraded following their targeting by ubiquitination (Tanaka, 2009). The endolysosomal pathway engulfs cytosolic and membrane content targeting it to multivesicular bodies (MVBs), which may fuse with lysosomes for content degradation. Lysosomes, the terminal compartments of the endolysosomal and autophagy pathways, have an acidic lumen for the degradative activity of hydrolases. Finally, autophagy, activated by cellular stress (e.g., starvation, accumulation of misfolded proteins), is responsible for the degradation of macromolecules and organelles.
Neuronal endosomal traffic jams were first described in AD when abnormal early endosomal enlargement was detected at near-diagnostic precision (Cataldo et al., 2000). Since then, abnormalities of the endolysosomal pathway have been commonly reported, even before protein aggregation is observed, suggesting a key etiopathogenic role of degradative compartments in the downstream molecular alterations leading to neurodegeneration (Nixon et al., 2008). It’s interesting to note that chronic stress, a risk factor for AD, was shown to impair these degradative pathways contributing to the accumulation of hyperphosphorylated Tau and facilitating its aggregation (Vaz-Silva et al., 2018; Silva et al., 2019). Interestingly, exosomes derive from the endolysosomal pathway and represent an alternative pathway for the disposal of unwanted molecules besides lysosomal degradation. Indeed, EV secretion is enhanced when neuronal lysosomes are impaired (Guix et al., 2017; Miranda et al., 2018), which, in turn, may contribute to the release of toxic Aβ species and the formation of Aβ plaques (Eitan et al., 2016). Supporting this concept, inhibition of neutral sphingomyelinase 2, an enzyme required for exosome biogenesis, has been shown to decrease amyloid deposition (Dinkins et al., 2016). Recently, brains of patients with the ApoE ε4 allele were shown to have reduced levels of TSG101 and Rab35, molecules involved in the ESCRT pathway (part of the endolysosomal mechanism of degradation) and also implicated in exosome biogenesis and secretion. These findings suggest that the ApoE ε4 genotype may predispose to decreased exosome release, in turn inducing endolysosomal damage and leading to neuronal vulnerability and a higher risk of AD (Peng et al., 2019). On the other hand, the EV-delivery of toxic Tau species also contributes to the malfunction of degradative organelles, further contributing to Tau accumulation (Pedrioli et al., 2021).
5.2. EVs in Aβ and Tau propagation
The abovementioned studies show a clear role for the endolysosomal and other degradative pathways in AD pathology, supporting a potential role for exosomes and other EVs in the spreading of toxic forms of Aβ and Tau. Accumulating evidence suggests that Tau spreading in the brain occurs via prion-like mechanisms between synaptically-connected brain regions (Clavaguera et al., 2009; Clavaguera et al., 2020), e.g., from the entorhinal cortex to the hippocampus and then to the neocortex (Wang et al., 2020). The specific mechanisms underlying the propagation of pathological Tau remain unclear; however, Tau secretion from neurons has been described to occur through different routes, i.e., free secretion, or through nanotubes or inside EVs (Zhang et al., 2021). Indeed, there is ample evidence of the presence of Tau in EVs and its ability to induce Tau aggregation in recipient cells. For instance, plasma neuronal-derived EVs from AD patients have been shown to seed Tau aggregation and induce AD-like neuropathology in wild-type mouse brains (Winston et al., 2016). Recently, brain-derived EVs of AD patients were also shown to induce Tau lesions in vivo, supporting the hypothesis that brain derived-EVs participate in the prion-like propagation of Tau pathology (Leroux et al., 2022). Moreover, EVs isolated from the brains of Tau transgenic mice or cultured primary neurons expressing mutant Tau can stimulate the aggregation of Tau in recipient cells and increase Tau phosphorylation and soluble Tau oligomer formation (Baker et al., 2016; Polanco et al., 2016; Wang et al., 2017a). Recent proteomic evidence further consolidated our knowledge of the involvement of different cell types in AD pathology (Bai et al., 2021), which is mimicked by brain-derived EVs multi-omic analysis (Muraoka et al., 2021; You et al., 2022; Cohn et al., 2021). Indeed, not only neuronal EVs but also microglial or astrocytic EVs have been shown to drive Tau pathology propagation. For instance, overexpression of BIN1, the second most statistically-significant locus associated with late-onset AD, promoted Tau release via microglial EVs and exacerbation of Tau pathology in P301S-Tau transgenic (Tg) mice (Crotti et al., 2019). Recently, it was also shown that plaque-associated microglia hyper-secrete EVs, contributing to Tau propagation in a humanized AD mouse model (Clayton et al., 2021). Moreover, inhibition of P2X purinoceptor 7 (P2RX7), an ATP-evoked Na+/Ca2+channel predominantly expressed in microglia, suppresses disease phenotypes in the P301S-Tau Tg mice, probably by decreasing the release of microglial EVs (Ruan et al., 2020). Moreover, Asai and colleagues showed that microglia phagocyte and release Tau via EVs, while inhibition of either microglia function or exosome biogenesis was shown to halt Tau pathology propagation in P301S-Tau Tg mice (Asai et al., 2015).
Similarly, EVs derived from AD patients’ brain that contained Aβ oligomers were found to transfer such toxic Aβ species to recipient neurons in culture-inducing toxicity (Sardar Sinha et al., 2018). Concerning the role of astrocyte-derived EVs, recent evidence showed that, after engulfing Aβ fibrils resistant to lysosomal degradation (Söllvander et al., 2016), astrocytes caused neurotoxicity by releasing EVs that contained an N-terminus-truncated form of Aβ42, which is even more resistant to degradation, more prone to aggregation and more toxic (De Kimpe et al., 2013). Moreover, microglial EVs can also contain toxic forms of Aβ1–42 and Aβ1–40, supporting a detrimental role for microglia in AD, which can mediate the spreading of neurotoxic Aβ species throughout the brain (Joshi et al., 2014). Moreover, it is important to note that Aβ spreading is also dependent on neuronal EVs that appear to accelerate Aβ amyloidogenesis (Yuyama et al., 2012). On the other side, neuronal EVs were also shown to exhibit a protective role by inhibiting Aβ oligomerization and enhancing microglia-mediated Aβ clearance in vitro (Yuyama et al., 2012). By trapping Aβ through membrane glycosphingolipids and then transporting it into microglia, neuronal EVs were also shown to decrease Aβ pathology (Yuyama et al., 2014).
Although the vast majority of evidence monitoring the transmission of protein aggregates in neurodegenerative disorders focuses on the brain, studies have also described this transmission from the periphery to the central nervous system. For example, intraperitoneal administration of brain extracts containing Aβ or Tau triggers their accumulation in the brain, while parabiosis between Aβ transgenic and wild-type mice results in Aβ accumulation in the brain of wild-type animals suggesting that Аβ or Tau circulating in the blood can enter the central nervous system (Peng et al., 2020). However, the role of EVs in this periphery-to-central nervous system propagation remains unclear.
5.3. Exosomes and neuronal stem cells in brain homeostasis
Emerging evidence suggests a role for EVs and exosomes originated from neuronal stem cells (NSCs) as possible players of brain homeostasis. NSCs are multipotent cells characterized by self-renewal, paracrine transmission, and inducible differentiation into three cell lineages of the central nervous system: neurons, astrocytes, and oligodendrocytes (Ling et al., 2020).
NSCs-derived EVs appear as key “players” in the EVs-based cross-talk among the different adult neurogenic niches such as the hypothalamus, hippocampal subgranular zone, and sub-ventricular zone (SVZ) of the lateral ventricles (Sardar Sinha et al., 2018). A recent study supports the essential role of NSC-derived EVs in regulating the transition of NSCs from the quiescence to proliferation by regulating protein translation related to the cell cycle. In addition, proteomic analysis of the cargo of NSCs-derived EVs revealed that they are enriched in growth-factor proteins, while treatment of neuronal progenitor cells (NPCs) with NSC-derived EVs promotes NPC proliferation (Ma et al., 2019).
Despite their participation in paracrine communication, EVs from NSCs are also proposed as promising “actors” under pathological conditions with therapeutic potential (Lee et al., 2018a). An increasing number of studies show that NSC-derived EVs carrying a “pallet” of proteins and miRNAs are involved in biological processes necessary for maintaining brain homeostases, such as inflammation, oxidation, and apoptosis (Upadhya et al., 2020a). For instance, SVZ NSC-derived EVs have immunomodulatory and inflammatory properties acting as microglia morphogens, e.g., activating the STAT1 pathway in target cells (Ruan et al., 2021). Moreover, NSC-derived EVs were also shown to have a neuroprotective effect in vitro against Parkinson’s disease (Lee et al., 2022).
5.4. EVs role in synaptic plasticity and neuronal function
Complex behavioral responses, such as learning and memory, require the dynamic refinement of synaptic connections by eliminating specific synapses in a process known as synaptic pruning (Goda and Davis, 2003). Glial cells have been shown to play an important role in this process (Eroglu and Barres, 2010) in a complement-dependent manner and are triggered by neuronal activity (Hong et al., 2016; Schafer et al., 2012). Indeed, neuronal EVs secreted by depolarized PC12 cells increased the expression of C3 (complement system protein of immune response) in microglial cells, resulting in enhanced clearance of neurites (Bahrini et al., 2015). Moreover, neuronal exosomes carrying PRR7 (a transmembrane protein) were shown to contribute to the elimination of excitatory synapses in neighboring neurons by i) blocking the secretion of Wnt via exosomes (secreted Wnt plays a critical role in synapse formation and maintenance) and ii) promoting proteasomal degradation of post-synaptic density (PSD) proteins (Lee et al., 2018b). Also, astrocytic EVs regulate neuronal protein expression and dendritic complexity by delivering several miRNAs (miR-26a-5p, miR-29c, miR-130a) to neurons (Lafourcade et al., 2016; Zou et al., 2015; Zhang et al., 2016). In addition, in response to ATP and IL-10 (anti-inflammatory cytokine), astrocytic EVs are thought to exhibit a role in neuronal plasticity as they are enriched in proteins involved in neurite outgrowth, axonal guidance, synaptogenesis, and long-term synaptic potentiation regulating gap junction, and CREB signaling (Datta Chaudhuri et al., 2020; Patel and Weaver, 2021; Peng et al., 2022). Besides being involved in the regulation of synapse pruning and maintenance, EVs also influence synaptic transmission. Different studies have shown a role for EVs in regulating postsynaptic retrograde signaling (Korkut et al., 2013) and presynaptic modulation. Furthermore, microglial EVs have been shown to increase neurotransmitter release, enhance excitatory neuro-transmission by transferring endocannabinoids to neurons, and promote sphingolipid metabolism (Antonucci et al., 2012; Gabrielli et al., 2015). EVs also play a role in the “tripartite synapse”, in which neuronal EVs transfer miR124-3p to astrocytes, thereby inactivating other miRNAs and increasing the expression of astrocytic GLT1 transporter (Morel et al., 2013; Men et al., 2019), which, in turn, favorites for glutamate uptake from the synaptic cleft. Noteworthy, EV release itself is stimulated by synaptic activity and glutamate in neurons and oligodendrocytes (Lachenal et al., 2011) and by ATP or inflammatory stimulus in other glial cells.
In addition to regulating synaptic transmission, EVs also regulate cellular mechanisms involved in neuroprotection and myelination. For example, it is shown that astrocytic EVs facilitate neuroprotection (Upadhya et al., 2020b; Guitart et al., 2016; Pascua-Maestro et al., 2019) by transferring neuroglobin (Venturini et al., 2019) and synapsin-1 (Wang et al., 2011) to neurons that contribute to neurite outgrowth and neuronal survival. Microglial and astrocytic EVs were also found to promote (re)myelination by stimulating oligodendrocyte precursor cell migration and differentiation (Lombardi et al., 2019; Willis et al., 2020). In addition, oligodendroglial EVs protect neurons from stress conditions, supporting higher metabolic activity and axonal transport (Frühbeis et al., 2013; Frühbeis et al., 2020; Mukherjee et al., 2020; Krämer-Albers et al., 2007; Fröhlich et al., 2014). Moreover, oligodendroglial EVs communicate with other glial cells for clearance and immune surveillance of the microenvironment. Indeed, microglia cells are the scavengers of the nervous system, phagocyting toxic substances, and EVs. While the uptake of neuronal EVs loaded with misfolded proteins may activate microglial cells, glioma EVs and oligodendroglial EVs are shown to maintain microglia in an immunologically silent state (Paolicelli et al., 2019; Fitzner et al., 2011; Abels et al., 2019; Maas et al., 2020). Microglial EVs have also been shown to contribute to the metabolic support of neurons, providing supplementary energy substrates during synaptic activity (Potolicchio et al., 2005). In addition, under several conditions, EVs from microglia were shown to contribute to neuronal survival by transferring miR-124, which induces neurite outgrowth (Song et al., 2019; Huang et al., 2018; Li et al., 2019).
As mentioned previously, one of the hallmarks of AD is synaptic loss and impaired synaptic homeostasis. The mechanisms that lead to this dysfunction have yet to be understood. Aβ oligomers and plaques are known to disrupt synaptic function (Laurén et al., 2009; Walsh et al., 2002; Shankar et al., 2008), and some studies indicate that EVs neutralize these effects (Yuyama et al., 2012; Bulloj et al., 2010; An et al., 2013), while others suggest that they have a role in mediating Aβ neurotoxicity (Elsherbini et al., 2020a; Elsherbini et al., 2020b). Indeed, Aβ in astrocytic EVs is internalized by neurons and was shown to induce mitochondrial clustering and dysfunction, increasing DRP1 levels and activating caspases, ultimately leading to neuronal death (Elsherbini et al., 2020a; Elsherbini et al., 2020b).
5.5. EVs and brain inflammation
Genome-wide association studies of AD patients have reported that more than 2/3 of single nucleotide polymorphisms occur in genes that encode proteins expressed in microglia and are related to inflammation, endosomal pathways, and cholesterol metabolism (ElAli and Rivest, 2016). Indeed, AD has been associated with chronic innate inflammation in the CNS, where activated microglia and reactive astrocytes increase the production of cytokines and chemokines and lead to the infiltration of immune cells, culminating in secondary neurodegeneration (Calsolaro and Edison, 2016; Czirr and Wyss-Coray, 2012). Although the initial activation of microglia is beneficial for clearing toxic Aβ from the AD brain, over time, the chronic stimulation of microglia by Aβ may also be deleterious, leading to prolonged inflammation, excessive Aβ deposition, and acceleration of the neurodegenerative process (Sala Frigerio et al., 2019; Hickman et al., 2008). Astrocytic EV release is promoted by pro-inflammatory cytokines (Wang et al., 2017b), which recruits peripheral leukocytes (Dickens et al., 2017), indicating a role for astrocytic EVs in the modulation of neuroinflammation. Astrocytic EVs containing miR-138 can activate microglia via TLR7 signaling (Liao et al., 2020), while those enriched in miR-873a-5p or miR-9 have been shown to attenuate microglia-mediated neuroinflammation (Long et al., 2020; Yang et al., 2018a). In addition, exosomes from reactive astrocytes are enriched in miRNAs that target neurogenesis and synaptogenesis (Datta Chaudhuri et al., 2020; Chaudhuri et al., 2018), increasing neuronal uptake and reducing neurite outgrowth, branching, and neuronal firing (You et al., 2020), as well as having axonotrophic effects following spinal cord injury (Adolf et al., 2019). These findings suggest a beneficial role for astrocytic EVs in neuronal function under inflammatory conditions. Microglial EVs and their cargo reflect the phenotype of the parent cells and may promote inflammation under some circumstances (depending on cytokine cargo, e.g., IL1-β, TNF-α) (Yang et al., 2018b). Previous studies have demonstrated that abnormal elevation of glutaminase 1 in microglia promotes their switch to a pro-inflammatory phenotype and exosome release in an AD mouse model (Gao et al., 2019). In addition, active microglia deliver EV-associated microRNA 146-a-5p to neurons promoting synaptic weakening (Prada et al., 2018) and neuronal death (Beneventano et al., 2017; Tang et al., 2016). On the other hand, neuron-derived EVs with high levels of miR-21-5p trigger neuroinflammation by activating microglia (Yin et al., 2020). Recently, EVs from mesechrymal stem cells (MSC-EVs) were also shown to inhibit glial activation through miRNAs, reversing brain inflammation (Markoutsa et al., 2022; Losurdo et al., 2020). In summary, neuroinflammation is an important aspect of AD pathogenesis, and whether exosomes promote or inhibit inflammatory responses depends on their cargoes and the cellular conditions under which they are released. Clearly, the mechanisms underlying beneficial and detrimental effects of EVs on inflammation in the context of Alzheimer’s disease need further investigation.
5.6. Neurovascular homeostasis and EVs
The neurovascular unit (NVU) is an anatomical structure composed of neurons, glial cells, brain endothelial cells, pericytes, and smooth muscle cells. Its function is to promote an effective cerebral blood flow, maintaining neuronal metabolic activity and a functional blood-brain barrier (BBB) (Soto-Rojas et al., 2021). Dysfunction of the NVU is an early event in AD and a reliable predictor of cognitive deficits (Thal et al., 2003; Nation et al., 2019; van de Haar et al., 2016). Aβ deposits have been associated with deterioration of the NVU, decreased cerebral lymphatic and blood flow, leading to reduced Aβ clearance (Bakker et al., 2016; Kimbrough et al., 2015). Similarly, Tau perivascular deposits have also been reported with Tau-mediating vascular defects preceding neuronal loss (Canepa and Fossati, 2021). Little is known about the role of EVs in NVU homeostasis, although there are reports that EVs contribute to neurovascular repair processes (Zhang et al., 2015; Dong et al., 2022). In addition, EV-associated miR-132 from neurons has been shown to regulate endothelial cadherin and maintain vascular integrity (Xu et al., 2017). Recently, astrocytic-EVs derived from AD brains were shown to impair neuroglial and vascular components (González-Molina et al., 2021). A deeper understanding of the crosstalk between NVU elements and the role of EVs in NVU homeostasis could promote novel interventions to halt or slow disease progression.
6. Technical difficulties related to the isolation and study of brain EVs
The literature shows that neuronal and glial EVs impact brain homeostasis and function. The seemingly contradictory roles of EVs in these processes derive from the fact that it is challenging to study exosomes secreted by neurons or glial cells in vivo. Therefore, most of the studies to date have taken in vitro approaches to explore the role of EVs in AD pathomechanisms, which may not fully recapitulate the endogenous mechanisms in the brain. In addition, the conditions in which EVs are collected and isolated greatly influence their content and biological function (Moloudizargari et al., 2021). Therefore, the collection and characterization of physiologically relevant exosomes are of the utmost importance. However, it is still challenging to identify, isolate and quantify exosomes efficiently, accurately, and selectively. For instance, the first protocol for brain exosome isolation proposed in 2012 (Perez-Gonzalez et al., 2012), and its following adaptations (Vella et al., 2017), rely on tissue digestion. Although it has provided invaluable insight into the role of EVs and, particularly, exosomes in brain pathology, this method of exosome isolation is based on brain tissue digestion which may contaminate the exosome fraction by cellular disruption/damage; here, intracellular components (e.g., immature vesicles intraluminal vesicles (ILVs) that could otherwise be targeted for degradation instead of exocytosis – see Fig. 1) and artificially produced vesicles may be isolated together with EVs/exosome fraction (Vella et al., 2017). As it is not possible to discriminate between ILVs that would be secreted (exosomes) from those that would be degraded, tissue dissociation approaches may lead to less pure or contaminated EVs yield, leading to the conflicting, incongruent, or inaccurate characterization of exosomes profile. Our team has recently developed a novel protocol for the purification of small EVs that are spontaneously released by brain tissue. This novel protocol allows for the isolation of small EVs from different brain areas in both mouse and human, avoiding the mechanical disruption and subsequent enzymatic digestion required for the current method of EV isolation from brain tissue, which may contaminate the EV fraction with immature vesicles and influence EV cargo.
Moreover, the seemingly opposing roles for EVs described above may be due to the fact that these effects are mediated by a different sub-population of EVs. Thus, great efforts are being undertaken to provide novel tools for the study of such subpopulations of vesicles. Novel techniques such as asymmetric-flow field-flow fractionation allow for differential isolation of subpopulations of small vesicles (Zhang et al., 2018), and a new commercial device, ExoView, allows for the study of specific subpopulations based on their membrane proteins – e.g., tetraspanin-specific, neuronal or microglial specific (Silva et al., 2021). In addition, novel “exosome reporter” mice have enabled the isolation and characterization of cell type-specific EVs in vivo (McCann et al., 2020), the temporal and spatial labeling of exosomes (Luo et al., 2020), and the study of neuron-glia communication (Men et al., 2019). Moreover, in vivo tracking of EVs has enabled the dissection of their trafficking and communication routes – indeed, several labeling methods have been developed for this purpose (Gangadaran et al., 2018; Carpintero-Fernández et al., 2017). Another particularly interesting technique is immunoprecipitation, which takes advantage of the membranous content of EVs to capture specific populations; for instance, it is common to use L1CAM or NCAM to immunoprecipitate neuron-derived EVs, while astrocytic EVs precipitate with GLAST. This is essential to study the role of specific EV populations in a given biological process, including brain pathology, and their particular contribution to AD biomarker discovery (Delgado-Peraza et al., 2021).
7. EVs as a tool for clinical studies and medical practice
Due to their small size, EVs are able to cross the blood-brain barrier and the CSF-brain barrier, although unequivocal demonstration of this crossing under healthy conditions is largely lacking (Chen et al., 2016; Haqqani et al., 2013; Matsumoto et al., 2017). Nevertheless, EVs with brain cell cargos have been found in several biofluids (e.g., CSF, plasma, urine, saliva). This feature provides a unique opportunity for biomarker research, as we can easily access brain-derived EVs, which may mirror brain pathology in the periphery, e.g., peripheral blood (Delgado-Peraza et al., 2021). In addition, their low immunogenicity also makes them perfect candidates for customized drug delivery systems.
EV cargo with biomarker potential for Alzheimer’s disease diagnosis spans multiple biomolecules, i.e., proteins, miRNA, mRNA, and crDNA, reflecting the biological processes associated with AD brain pathology. Due to their cargo-protecting abilities, EV-related proteins have been studied for their diagnostic potential (Arioz et al., 2021) as well as their capacity to distinguish pathologies or offer a prognosis for dementia (Jia et al., 2021). Therefore, small EVs, such as exosomes, represent a form of liquid biopsy that seems to be a powerful tool in the new era of Precision Medicine (Hampel et al., 2019) and of particular importance for the early detection of AD. Moreover, as described above, EVs seems to participate in disease progression and may also be promising candidates for novel therapies as will be discussed below.
8. The biomarker potential of EV miRNAs
miRNAs are small non-coding RNAs that modulate RNA expression. Recently, the dysregulation of miRNA has been associated with AD pathological status (Olsson et al., 2016), and while they may be found freely in body fluids, they are significantly more stable in small EVs, such as exosomes (Cheng et al., 2014; Kim et al., 2017). Thus, a growing number of studies have looked at the exosomal miRNA potential for diagnosis (Xing et al., 2021; He et al., 2021; Dong et al., 2021; Dong et al., 2020). Interestingly, the delivery of some miRNA via exosomes has also been studied with promising therapeutic results and without short-term side effects (He et al., 2021).
So far, exosomal miRNA studies in AD have been cross-sectional, focusing on plasma, serum, and CSF exosomes allowing the distinction of AD from other neurodegenerative disorders (e.g., Parkinson’s disease [PD], Dementia with Lewis Bodies [DLB]), and healthy conditions. Indeed, Nie et al. (Nie et al., 2020) identified eight miRNA that were differently expressed in AD patients, with let-7e-5p and miR-548 being differently expressed in comparison with PD patients and thus, being potential differencing markers. Similarly, Gámez-Valero et al. (Gámez-Valero et al., 2019) have identified two miRNAs that differentiate AD from DLB patients (miR-451a and miR-21-5p), while four miRNAs are downregulated in AD patients compared to healthy controls. Interestingly, miR-23a-3p is downregulated in this study, while others (Nie et al., 2020; Serpente et al., 2020) have shown its upregulation in AD patients. These incongruencies may be due to the different isolation methods used and miRNA sequencing techniques (SEC and NGS (Gámez-Valero et al., 2019) vs. Exosome isolation Kit and small RNA seq (Nie et al., 2020)). Furthermore, a study by Lugli et al. (Lugli et al., 2015) proposes a panel of 7 miRNAs for the diagnosis of AD with an accuracy near 90%. Serpente et al. and Cha et al. (Serpente et al., 2020; Cha et al., 2019) have isolated neuronal-derived EVs from the plasma, identifying that miR-23a-3p, miR-223-3p, miR-190a-5p were upregulated in AD subjects, while miR-132 was able to distinguish AD patients from controls but not MCI-AD from healthy subjects. This may suggest that different miRNAs would be altered in different stages of dementia. Briefly, serum-derived exosomal miRNA have also demonstrated diagnostic potential with Cheng et al., proposing a model with miRNAs and other serum factors with 87% sensitivity and 77% specificity (Cheng et al., 2015). Similarly, Barbagallo et al. created a model to discriminate among different neurodegenerative processes using a panel of exosomal miRNAs (Barbagallo et al., 2020). Furthermore, Li et al. generated a model, including miRNAs and pSer396-Tau, that was able to distinguish AD from vascular dementia and MCI (Li et al., 2020). Another study by Yang et al. demonstrated the ability of miR-200b to distinguish between MCI and AD, while it provided novel evidence of the importance of miR-135a, miR-200a, and miR-42a in AD progression. Moreover, plasma EVs miR-233 in AD patients are shown to be correlated with cognitive scores of the patients (Mini-Mental State Examination and Clinical Dementia Rate scores) (Wei et al., 2018). Lastly, the most common biofluid used in AD diagnosis is CSF, from which exosomes have also been isolated and studied. Based on CSF exosome analysis, Riancho et al. identified exosomal miR-9-5p and miR-598 as potential AD diagnostic markers (Riancho et al., 2017), while another study by McKeever et al. provided evidence of miRNAs biomarkers in early- and late-onset AD patients (McKeever et al., 2018). A summary of the studies that identified exosomal miRNAs in AD patients is provided in Table 1.
Table 1.
Exosomal miRNAs as biomarkers for AD.
| Study | Source | Down-regulated miRNAs | Up-regulated miRNAs | Known target genes (relevant to AD pathogenesis) |
|---|---|---|---|---|
| Riancho et al., 2017 | CSF | miR-9-5p | ||
| CSF | miR-16-2 | |||
| McKeever et al., 2018 | CSF | miR-16-5p | ADAM10; APH1A; APP; MME; CDK5R1 | |
| Gui et al., 2015 | CSF | miR-29c | BACE1; GAPDH; LPL; CASP7; GSK3β | |
| McKeever et al., 2018 | CSF | miR-125-5p | APP | |
| Gui et al., 2015 | CSF | miR-132-5p | ||
| McKeever et al., 2018 | CSF | miR-125b-5p | ||
| Gui et al., 2015 | CSF | miR-136-3p | ||
| Liu et al., 2014 | CSF | miR-193b | ||
| Gui et al., 2015 | CSF | miR-331-5p | ||
| McKeever et al., 2018 | CSF | miR-451a | ||
| Gui et al., 2015 | CSF | miR-485-5p | ||
| Riancho et al., 2017 | CSF | miR-598 | ||
| McKeever et al., 2018 | CSF | miR-605-5p | ||
| Nie et al., 2020 | Plasma | let-7e-5p | ||
| Gámez-Valero et al., 2019 | Plasma | let-7i-5p | APH1A; IDE; LRP1; CASP3 | |
| Nie et al., 2020 Serpente et al., 2020 Gámez-Valero et al., 2019 | Plasma | miR-23a-3p | miR-23a-3p | ADAM10; PSEN1; NCSTN; APH1A; CASP3; CASP7 |
| Lugli et al., 2015 | Plasma | miR-23b-3p | ADAM10; APH1A; GAPDH; NCSTN; PSEN1; ASP3; CASP7 | |
| Lugli et al., 2015 | Plasma | miR-24-3p | APH1A; NCSTN; CAPN1; GSK3β | |
| Lugli et al., 2015 | Plasma | miR-29b-3p | BACE1; GAPDH; LPL; CASP7; GSK3β | |
| Serpente et al., 2020 | Plasma | miR-100-3p | ||
| Nie et al., 2020 | Plasma | miR-125a-5p | ||
| Lugli et al., 2015 | Plasma | miR-125b-5p | APP | |
| Gámez-Valero et al., 2019 | Plasma | miR-126-3p | CAPN1 | |
| Cha et al., 2019 | Plasma | miR-132 | APH1A; CAPN2; CASP7; GSK3β | |
| Lugli et al., 2015 | Plasma | miR-138-5p | ||
| Lugli et al., 2015 | Plasma | miR-139-5p | ||
| Lugli et al., 2015 | Plasma | miR-141-3p | APH1B; GAPDH; PSEN1 | |
| Lugli et al., 2015 | Plasma | miR-150-5p | ||
| Gámez-Valero et al., 2019 | Plasma | miR-151a-3p | GAPDH; APH1A | |
| Lugli et al., 2015 | Plasma | miR-152-3p | ||
| Lugli et al., 2015 | Plasma | miR-185-5p | BACE2; GAPDH; LPL; MME; GSK3β | |
| Serpente et al., 2020 | Plasma | miR-190a-5p | ||
| Nie et al., 2020 | Plasma | miR-204-5p | ||
| Cha et al., 2019 | Plasma | miR-212 | CAPN2; GSK3β | |
| Serpente et al., 2020 | Plasma | miR-223-3p | ||
| Lugli et al., 2015 | Plasma | miR-338-3p | ||
| Lugli et al., 2015 | Plasma | miR-342-3p | APH1B; MME; LPL; GSK3β | |
| Lugli et al., 2015 | Plasma | miR-342-5p | GAPDH; LRP1 | |
| Nie et al., 2020 | Plasma | miR-369-5p | ||
| Nie et al., 2020 | Plasma | miR-375 | ||
| Nie et al., 2020 | Plasma | miR-423-5p | ||
| Lugli et al., 2015 | Plasma | miR-659-5p | ||
| Nie et al., 2020 | Plasma | miR-1468-5p | ||
| Lugli et al., 2015 | Plasma | miR-3065-5p | ||
| Lugli et al., 2015 | Plasma | miR-3613-3p | ||
| Lugli et al., 2015 | Plasma | miR-3916 | ||
| Lugli et al., 2015 | Plasma | miR-4772-3p | ||
| Lugli et al., 2015 | Plasma | miR-5001-3p | ||
| Li et al., 2020 Cheng et al., 2015 | Serum | miR-15a-5p | ADAM10; APH1A; APP; BACE1; MME; CDK5R1 | |
| Li et al., 2020 Cheng et al., 2015 | Serum | miR-15b-3p | ||
| Li et al., 2020 Cheng et al., 2015 | Serum | miR-18b-5p | APH1B; GAPDH | |
| Cheng et al., 2020 Li et al., 2020 Cheng et al., 2015 | Serum | miR-20a-5p | APP | |
| Barbagallo et al., 2020 | Serum | miR-29a | APH1A; GAPDH; BACE1; BACE2; LPL; GSK3β; CASP7 | |
| Li et al., 2020 Cheng et al., 2015 | Serum | miR-30e-5p | APP; NAE1; CASP3 | |
| Cheng et al., 2020 | Serum | miR-32-5p | ||
| Barbagallo et al., 2020 | Serum | miR-34b | ||
| Li et al., 2020 Cheng et al., 2015 | Serum | miR-93-5p | APP; GAPDH; LRP1; GSK3β | |
| Li et al., 2020 Cheng et al., 2015 | Serum | miR-101-3p | ADAM10; APP; PSEN1; CAPN2; CASP3; GSK3β | |
| Li et al., 2020 Cheng et al., 2015 | Serum | miR-106a-5p | APP; ADAM17; CAPN2 | |
| Li et al., 2020 Cheng et al., 2015 | Serum | miR-106b-5p | APP; CASP7 | |
| Yang et al., 2018c | Serum | miR-135a | ||
| Li et al., 2020 Cheng et al., 2015 | Serum | miR-143-3p | ADAM10; LRP1 | |
| Yang et al., 2018c | Serum | miR-193b | ||
| Cheng et al., 2020 | Serum | miR-219a | ||
| Wei et al., 2018 | Serum | miR-223 | ||
| Li et al., 2020 Cheng et al., 2015 | Serum | miR-335-5p | APBB1; LRP1; CASP7 | |
| Li et al., 2020 Cheng et al., 2015 | Serum | miR-342-3p | APH1B; MME; LPL; GSK3β | |
| Li et al., 2020 (Cheng et al., 2015) | Serum | miR-361-5p | ADAM10; APH1B; LRP1 | |
| Cheng et al., 2020 | Serum | miR-374a-5p | ||
| Yang et al., 2018c | Serum | miR-384 | ||
| Li et al., 2020 Cheng et al., 2015 | Serum | miR-424-5p | APBB1; APH1A; APP; LRP1; CDK5R1; GSK3β | |
| Li et al., 2020 Cheng et al., 2015 | Serum | miR-582-5p | IDE | |
| Cheng et al., 2020 | Serum | miR-585-5p | ||
| Cheng et al., 2020 | Serum | miR-941 | ||
| Li et al., 2020 Cheng et al., 2015 | Serum | miR-1306-5p | ||
| Cheng et al., 2020 Li et al., 2020 Cheng et al., 2015 | Serum | miR-3065-5p | ||
| Cheng et al., 2020 | Serum | miR-3157-5p |
9. EVs proteins as potential biomarkers for AD
Similar to exosomal miRNA content, the protein load of brain-derived exosomes constitutes a promising pool of molecules with great biomarker potential for AD (see Table 2 and Fig. 4). These EVs proteins include Aβ and Tau, synaptic proteins, lysosomal function, inflammation, transcription, and other processes (Kim et al., 2021). Besides their diagnostic value in the clinical phase, it is of extreme importance to uncover biomarkers for the early detection of AD. Thus, several groups have undertaken longitudinal studies that unraveled some potential markers for early detection of AD, namely lysosomal proteins (Goetzl et al., 2015), synaptic proteins (Jia et al., 2021), and others, including Aβ42, phosphorylated Tau, total Tau, that have prognostic value with a lead time of 10 years (Fiandaca et al., 2015; Kapogiannis et al., 2015; Eren et al., 2020; Kapogiannis et al., 2019). In line with the idea for better AD biomarkers for disease progression and treatment-response, synaptic proteins in exosomes are shown to decline as the disease progresses (Goetzl et al., 2018a; Chanteloup et al., 2019). Moreover, complement protein levels in astrocyte-derived exosomes may predict conversion from MCI to AD (Winston et al., 2019). Interestingly, Tau protein in neuron-derived EVs accompanies the changes in the scores of cognitive function and depression in AD patients (Mini-Mental State Examination and Geriatric Depression Scale, respectively (Nam et al., 2020). Changes in the levels of the above exosomal proteins upon treatment are promising game-changers in clinical trials for new drugs for Alzheimer’s disease.
Table 2.
Exosomal protein as biomarkers in AD.
| Study | Exosomal proteins | Change | Specimen | Patients |
|---|---|---|---|---|
| Gu et al., 2020) (Jia et al., 2019 Abner et al., 2016 Goetzl et al., 2016a Winston et al., 2016 Fiandaca et al., 2015 | Aβ42 | Increased | Plasma | AD |
| Gu et al., 2020) (Jia et al., 2019 Kapogiannis et al., 2019 Goetzl et al., 2016a Abner et al., 2016 Winston et al., 2016 Fiandaca et al., 2015 | p-T181-Tau | Increased | Plasma | AD |
| Jia et al., 2019 Fiandaca et al., 2015 | t-Tau | Increased | Plasma | AD |
| Goetzl et al., 2016a Winston et al., 2016 Fiandaca et al., 2015 | p-S396-Tau | Increased | Plasma | AD |
| Abner et al., 2016 Goetzl et al., 2015 | cathepsin D | Increased | Plasma | AD & MCI |
| Goetzl et al., 2016a | BACE-1 | Increased | Plasma | AD & MCI |
| Goetzl et al., 2016a | γ-secretase | Increased | Plasma | AD, MCI & early dementia |
| Goetzl et al., 2016a | sAPPβ | Increased | Plasma | AD, MCI & early dementia |
| Goetzl et al., 2016a | sAPPα | Increased | Plasma | AD, MCI & early dementia |
| Goetzl et al., 2015 | Heat-Shock Protein 70 (HSP70) | Decreased | Plasma | AD |
| Jia et al., 2021 Abner et al., 2016 Winston et al., 2016 | Neurogranin (NRGN) | Decreased | Plasma | AD |
| Jia et al., 2021 Agliardi et al., 2019 | Synaptosome Associated Protein 25 (SNAP25) | Decreased | Blood | AD, amnestic MCI |
| Jia et al., 2021 Goetzl et al., 2016b | Growth Associated Protein 43 (GAP43) | Decreased | Blood | AD, amnestic MCI |
| Jia et al., 2021 Goetzl et al., 2016b | Synaptotagmin-1 | Decreased | Blood | AD, amnestic MCI |
| Goetzl et al., 2016b | Synaptophysin | Decresead | Plasma | AD |
| Goetzl et al., 2016b | Synaptopodin | Decreased | Plasma | AD |
| Goetzl et al., 2016b | pS9-synapsin | Decreased | Plasma | AD |
| Goetzl et al., 2016b | Synapsin | Decreased | Plasma | AD |
| Kapogiannis et al., 2015 | Total insulin receptor substrate 1 (t-IRS-1) | Decreased | Plasma | Preclinical AD |
| Kapogiannis et al., 2019 Kapogiannis et al., 2015 | Phosphorylated-serine 312-type 1 insulin receptor substrate (p-S312-IRS-1) | Increased | Plasma | Preclinincal AD |
| Kapogiannis et al., 2019 Kapogiannis et al., 2015 | ratio of P-serine 312-IRS-1 to P-pan-tyrosine-IRS-1 (p-S312-IRS-1/p-Y-IRS-1) | Increased | Plasma | Preclinincal AD |
| Goetzl et al., 2015 | LAMP-1 | Increased | Plasma | AD |
| Goetzl et al., 2018b | Complement proteins | Increased | Plasma | AD |
| Abner et al., 2016 Winston et al., 2016 | REST | Increased Decreased |
Plasma | AD |
| Goetzl et al., 2016b | MOG | Decreased | Plasma | AD |
| Goetzl et al., 2016a | GDNF | Decreased | Plasma | AD, MCI & early dementia |
| Goetzl et al., 2016a | GFAP | Decreased | Plasma | AD, MCI & early dementia |
| Goetzl et al., 2016a | GluSyn | Decreased | Plasma | AD, MCI & early dementia |
| Goetzl et al., 2016a | Neuron-specific enolase | Decreased | Plasma | AD, MCI & early dementia |
| Goetzl et al., 2016a | Septin-8 | Decreased | Plasma | AD, MCI & early dementia |
| Gu et al., 2020 | Metalloproteinase 9 (MMP-9) | Increased | Plasma | AD |
Fig. 4.
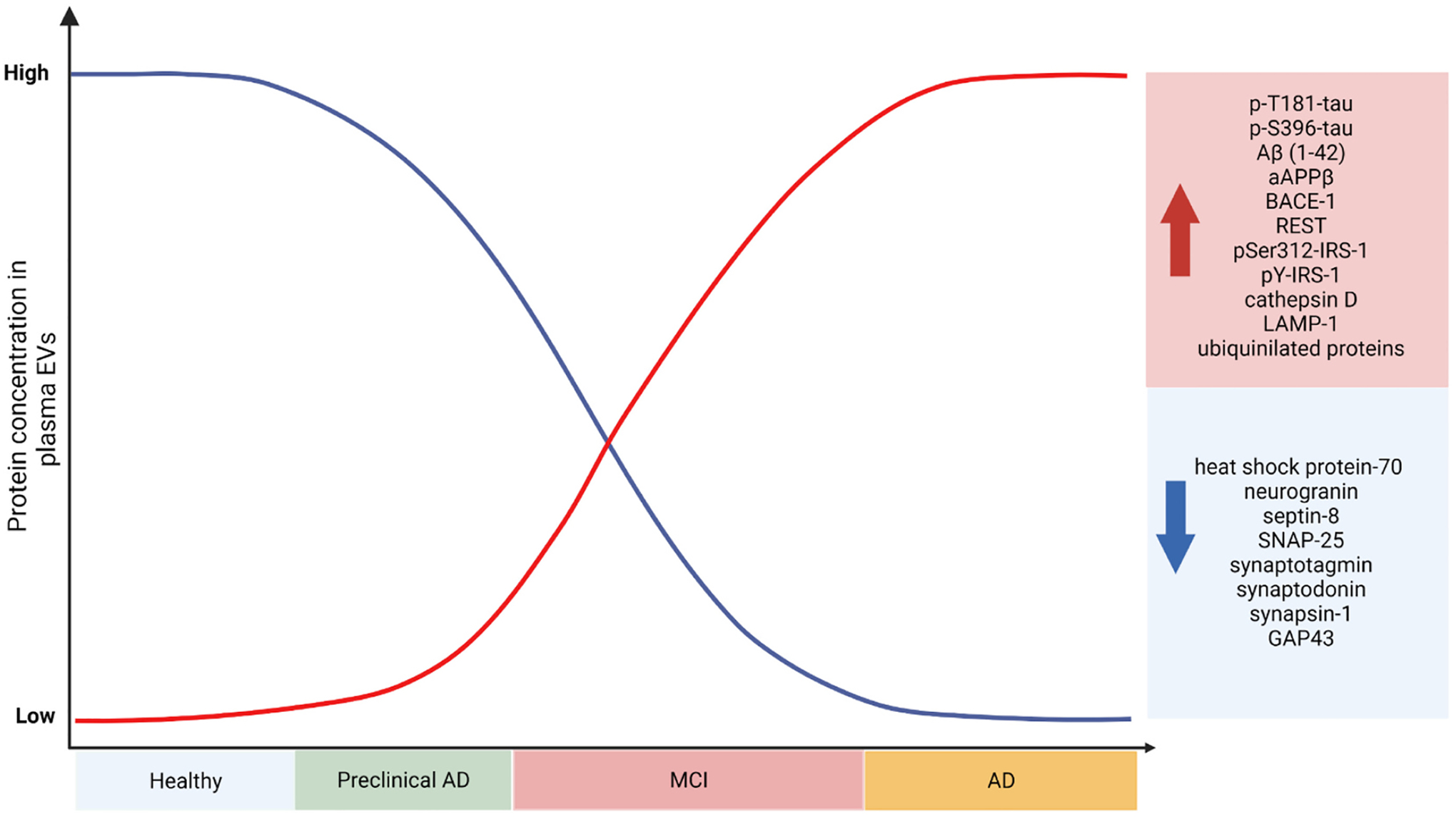
EVs proteins in AD diagnostics. Retrospective and cross-sectional studies of human cohorts have revealed the potential of EV contents as diagnostic and prognostic tools. While the AD biomarker potential of miRNA in EVs has recently started to be investigated, EV protein content has been assessed under multiple clinical conditions and tasks, including their diagnostic and prognostic potential. Based on previous clinical studies (see also Table 2), one group of EVs proteins was detected to be increased as dementia progresses (e.g., total Tau, specific Tau phospho-epitopes, Aβ, ubiquitinylated proteins) while another group of EVs proteins, mostly synapse-related proteins, are found to be decreased in AD. Further longitudinal studies of large and polycentric cohorts of healthy and AD patients are necessary in order to clarify the protein and miRNA cargo of EVs along the progress of AD and identify specific target or groups of targets of protein and/or miRNA EVs cargo with high biomarker value for AD.
10. EVs as AD drug targets and therapy delivery systems
Since EVs seem to play a role in disease propagation and progression, they may also be good candidates for novel therapeutic interventions. Indeed, genetic ablation or chemical inhibition of neutral sphingomyelinase 2 (nSMase2), an enzyme involved in EVs biogenesis, was found to reduce Aβ and Tau deposits, slowing down disease progression (Dinkins et al., 2016; Asai et al., 2015). Moreover, repetitive treatment with nSMase2 inhibitor GW4869 provided protection against behavioral and neuropathological deficits following immune system activation in AD preclinical studies (Sobue et al., 2018). In addition, knocking down ESCRT proteins involved in MVB biogenesis, TSG101, and VPS4A were found to reduce both the spread of Aβ oligomers and the downstream cellular toxicity (Sardar Sinha et al., 2018). Another study showed that inhibition of P2RX7 in microglial cells hindered exosome release and offered protection against Tau pathology (Ruan et al., 2020). Recently, dual nSMase2/Acetylcholinesterase Inhibitors were developed with promising results for the treatment of AD (Bilousova et al., 2020). For targeting disease-promoting EVs, potential points for intervention could include: 1) exosome biogenesis, 2) their release from parent cells, and 3) exosome uptake by recipient cells. In the cancer field, which has generated critical discoveries in the EVs field, several attempts have already been made or are ongoing in order to target the above steps involved in exosome biogenesis and spreading (Moloudizargari et al., 2021).
Moreover, EVs have also garnered interest as drug delivery agents for personalized medicine since their discovery. For instance, intracerebrally administered EVs can act as potent Aβ scavengers by trapping Aβ and promoting its degradation by microglia, representing a novel therapeutic intervention for AD (Yuyama et al., 2014). Moreover, their ability to carry bioactive molecules, to circulate in body fluids, and cross physiological barriers (e.g., BBB), as well as their low immunogenicity, make them appropriate tools to deliver chemical or biological compounds. So far, EVs have been engineered to carry chemotherapeutic agents, siRNAs, miRNAs, Cas9 protein, and other compounds. These processes involve medium conditioning with drugs of interest or techniques like electroporation, saponin permeabilization, hypotonic dialysis, and passive incubation (Rufino-Ramos et al., 2017; Sutaria et al., 2017). For instance, delivery of EVs derived from curcumin-treated cells can ameliorate AD neuropathology and memory impairment in an AD mouse model by inhibiting Tau hyperphosphorylation and neuronal death (Wang et al., 2019). Given the critical role of neuroinflammation in AD, the EV-based delivery of compounds that modulate brain inflammation but could not necessarily cross the BBB (such as curcumin) was a major breakthrough in the research field of AD (Zhuang et al., 2011). In addition, current efforts focus on the delivery of RNA species inside small EVs (Iranifar et al., 2019). For instance, a recent study showed that microglia-derived exosomes that function as a vehicle to deliver Tetraspanin 2 siRNA across the BBB are able to modulate the neuroinflammatory response (Reynolds and Mahajan, 2020). Moreover, the use of EVs as drug delivery tool is also focusing on their delivery to specific cell types. For instance, the exosomal delivery of BACE-1 siRNA to neurons, microglia, and oligodendrocytes leads to a 55% reduction of Aβ levels in the mouse brain (Alvarez-Erviti et al., 2011).
Another aspect of the therapeutical potential of EVs relies on the use of EVs from mesenchymal and adipocyte stem cells which have also exhibited beneficial effects for the treatment of AD (Hao et al., 2014; Perets et al., 2019; Liew et al., 2017). Mesenchymal stem cells (MSC) possess immunomodulatory and tissue regenerative properties and, therefore, have been suggested as therapeutic tools (Pittenger et al., 2019). MSC-derived exosomes have been shown to decrease Aβ levels but also inhibit the Aβ-driven downregulation of synaptic plasticity-related genes (Chen et al., 2021) or microglial activation (Kaniowska et al., 2022). Other studies have also shown that MSC-derived EVs have beneficial effects in in vitro AD models by protecting neurons from oxidative stress and synaptic damage induced by Aβ oligomers (Bodart-Santos et al., 2019; de Godoy et al., 2018). EVs from adipose stem cells (ADSCs-derived EVs) have also been shown to alleviate neuronal damage and promote neurogenesis in an AD mouse model (Ma et al., 2020). Moreover, ADSC-derived EVs were shown to carry enzymatically active Neprilysin (NEP), an Aβ-degrading enzyme that was proven to decrease levels of Aβ in the brain of an AD model, reducing cell apoptosis (Lee et al., 2018a; Katsuda et al., 2013). Lastly, EVs derived from stem cells of other origins, e.g., human umbilical cord MSCs (hUMSCs) and bone marrow mesenchymal stem cells (BM-MSC), were recently shown to exhibit beneficial effects against Aβ (Yang et al., 2020; Jeong et al., 2021).
Despite the above important advances in the field, EV-based therapies still have a long way to go, as many therapeutic approaches fail in clinical trials. For instance, while autologous injection of EVs leads to very low immunogenicity, EVs produced by cell lines or genetically distinct individuals may induce immune responses. As mentioned in the section “Technical difficulties related to the isolation and study of brain EVs,” our characterization methods have profiled a heterogeneous population of small EVs of similar size, including microvesicles and exosomes, and thus it remains to be identified specific (surface or cargo) content that can help us discriminate these two EV subpopulations which could, in turn, improve our engineering strategies for the production and therapeutic delivery of EVs. Another significant hurdle is the scalability of EVs production for treatment in large cohorts of AD patients, as we lack an efficient isolation method to obtain large quantities of pure EVs (e.g., from cell cultures or biofluid free of molecular and cellular contaminants). Moreover, there is an ongoing debate, in the clinical context, related to the optimal dosage (whether the EV number or content in EVs) and the route of EVs administration, as well as the timeframe for EVs treatment which is critical to define the most efficient therapeutic scheme (Lv et al., 2017). In addition, the long-term biological safety of EVs still needs to be assessed in order to investigate their potential adverse effects and efficacy of the administration in AD patients.
11. Conclusion
EVs represent an important vehicle for intercellular messaging in the brain. while emerging evidence suggest that EVs regulate multiple physiological processes that are impaired in the AD brain. Cell-type-specific EVs have differential effects on the progression of the disease, with some ameliorating and others worsening it. the development of novel EV-related tools and animal models will advance our EV studies in vivo, improving our understanding of how EVs contribute to both physiological and pathological mechanisms of the brain. Thus, a better understanding of EVs biogenesis and spreading, as well as cargo sorting, will help our research efforts to regulate and engineer EVs against AD neurodegeneration, as well as facilitate the discovery of specific and accurate biomarkers for early detection of AD and dementia-precipitating processes, representing a valuable tool in the new era of Precision Medicine.
Acknowledgments
This work has been funded by Portuguese national funds, through the Foundation for Science and Technology (FCT) - project UIDB/50026/2020 and UIDP/50026/2020; and by the projects NORTE-01-0145-FEDER-000013 and NORTE-01-0145-FEDER-000023, the Project Estratégico co-funded by FCT (PEst-C/SAU/LA0026/2013) and the European Regional Development Fund COMPETE (FCOMP-01-0124-FEDER-037298; POCI-01-0145-FEDER-007038) supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). This work was also supported by Jerôme Lejeune-Sisley-D’Ornano Foundation Postdoc Research Grant. Patricia Gomes was supported by FCT grant PD/BD/135271/2017 via MD-PhD program (Portugal) and Anastasia Vamvaka Iakovou by ELIDEK PhD fellowship grant 5900/2020 (Greece).
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- Abels ER, Maas SLN, Nieland L, Wei Z, Cheah PS, Tai E, et al. , 2019. Sep. Glioblastoma-associated microglia reprogramming is mediated by functional transfer of extracellular miR-21. Cell Rep. 28 (12), 3105–3119.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abner EL, Jicha GA, Shaw LM, Trojanowski JQ, Goetzl EJ, 2016. May. Plasma neuronal exosomal levels of Alzheimer’s disease biomarkers in normal aging. Ann Clin Transl Neurol. 3 (5), 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolf A, Rohrbeck A, Münster-Wandowski A, Johansson M, Kuhn HG, Kopp MA, et al. , 2019. Apr. Release of astroglial vimentin by extracellular vesicles: modulation of binding and internalization of C3 transferase in astrocytes and neurons. Glia. 67 (4), 703–717. [DOI] [PubMed] [Google Scholar]
- Agliardi C, Guerini FR, Zanzottera M, Bianchi A, Nemni R, Clerici M, 2019. Aug. SNAP-25 in serum is carried by exosomes of neuronal origin and is a potential biomarker of Alzheimer’s disease. Mol. Neurobiol 56 (8), 5792–5798. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA, 2011. Apr. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol 29 (4), 341–345. [DOI] [PubMed] [Google Scholar]
- An K, Klyubin I, Kim Y, Jung J, Mably AJ, O’Dowd ST, et al. , 2013. Exosomes neutralize synaptic-plasticity-disrupting activity of Aβ assemblies in vivo. Mol. Brain 6 (1), 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci F, Turola E, Riganti L, Caleo M, Gabrielli M, Perrotta C, et al. , 2012. Mar 7. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism: microglial MVs increase sphingolipid metabolism in neurons. EMBO J 31 (5), 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arioz BI, Tufekci KU, Olcum M, Durur DY, Akarlar BA, Ozlu N, et al. , 2021. Jun. Proteome profiling of neuron-derived exosomes in Alzheimer’s disease reveals hemoglobin as a potential biomarker. Neurosci. Lett 755, 135914. [DOI] [PubMed] [Google Scholar]
- Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, et al. , 2015. Nov. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci 18 (11), 1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M, 2011. Aug. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr. Opin. Cell Biol 23 (4), 452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrini I, Song J. Hoon, Diez D, Hanayama R, 2015. Jul. Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Sci. Rep 5 (1), 7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai B, Vanderwall D, Li Y, Wang X, Poudel S, Wang H, et al. , 2021. Dec. Proteomic landscape of Alzheimer’s disease: novel insights into pathogenesis and biomarker discovery. Mol. Neurodegener 16 (1), 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S, Polanco JC, Götz J, 2016. Oct 4. Extracellular vesicles containing P301L mutant tau accelerate pathological tau phosphorylation and oligomer formation but do not seed mature neurofibrillary tangles in ALZ17 mice. Gozes I, editor. J. Alzheimers Dis 54 (3), 1207–1217. [DOI] [PubMed] [Google Scholar]
- Bakker ENTP, Bacskai BJ, Arbel-Ornath M, Aldea R, Bedussi B, Morris AWJ, et al. , 2016. Mar. Lymphatic clearance of the brain: perivascular, paravascular and significance for neurodegenerative diseases. Cell. Mol. Neurobiol 36 (2), 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbagallo C, Mostile G, Baglieri G, Giunta F, Luca A, Raciti L, et al. , 2020. May. Specific signatures of serum miRNAs as potential biomarkers to discriminate clinically similar neurodegenerative and vascular-related diseases. Cell. Mol. Neurobiol 40 (4), 531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JL, Smith TG, 1980. Three modes of intercellular neuronal communication. In: Progress in Brain Research [Internet]. Elsevier, pp. 169–192 [cited 2021 Aug 15]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0079612308600643. [DOI] [PubMed] [Google Scholar]
- Bejanin A, Schonhaut DR, La Joie R, Kramer JH, Baker SL, Sosa N, et al. , 2017. Dec 1. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain 140 (12), 3286–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneventano M, Spampinato SF, Merlo S, Chisari M, Platania P, Ragusa M, et al. , 2017. Nov 9. Shedding of microvesicles from microglia contributes to the effects induced by metabotropic glutamate receptor 5 activation on neuronal death. Front. Pharmacol 8, 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz CL, Mosconi L, Scheyer O, Rahman A, Hristov H, Isaacson RS, 2018. Jul 13. Precision medicine for Alzheimer’s disease prevention. Healthc. Basel Switz 6 (3), E82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilousova T, Simmons BJ, Knapp RR, Elias CJ, Campagna J, Melnik M, et al. , 2020. Jun 19. Dual neutral Sphingomyelinase-2/acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease. ACS Chem. Biol 15 (6), 1671–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodart-Santos V, de Carvalho LRP, de Godoy MA, Batista AF, Saraiva LM, Lima LG, et al. , 2019. Dec. Extracellular vesicles derived from human Wharton’s jelly mesenchymal stem cells protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. Stem Cell Res Ther 10 (1), 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonucci E, 1967. Sep. Fine structure of early cartilage calcification. J. Ultrastruct. Res 20 (1–2), 33–50. [DOI] [PubMed] [Google Scholar]
- Bulloj A, Leal MC, Xu H, Castaño EM, Morelli L, 2010. Jan 6. Insulin-degrading enzyme sorting in exosomes: a secretory pathway for a key brain amyloid-β degrading protease. Lovell MA, editor. J. Alzheimers Dis 19 (1), 79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calsolaro V, Edison P, 2016. Jun. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement 12 (6), 719–732. [DOI] [PubMed] [Google Scholar]
- Canepa E, Fossati S, 2021. Jan 7. Impact of tau on neurovascular pathology in Alzheimer’s disease. Front. Neurol 11, 573324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpintero-Fernández P, Fafián-Labora J, O’Loghlen A, 2017. Nov 28. Technical advances to study extracellular vesicles. Front. Mol. Biosci 4, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA, 2000. Jul. Endocytic pathway abnormalities precede amyloid β deposition in sporadic Alzheimer’s disease and down syndrome. Am. J. Pathol 157 (1), 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha DJ, Mengel D, Mustapic M, Liu W, Selkoe DJ, Kapogiannis D, et al. , 2019. Nov 26. miR-212 and miR-132 are downregulated in neurally derived plasma exosomes of Alzheimer’s patients. Front. Neurosci 13, 1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanteloup G, Cordonnier M, Moreno-Ramos T, Pytel V, Matías-Guiu J, Gobbo J, et al. , 2019. Oct 15. Exosomal HSP70 for monitoring of frontotemporal dementia and Alzheimer’s disease: clinical and FDG-PET correlation. J. Alzheimers Dis 71 (4), 1263–1269. [DOI] [PubMed] [Google Scholar]
- Chargaff E, West R, 1946. Nov. The biological significance of the thromboplastic protein of blood. J. Biol. Chem 166 (1), 189–197. [PubMed] [Google Scholar]
- Chaudhuri AD, Dastgheyb RM, Yoo SW, Trout A, Talbot CC Jr., Hao H, et al. , 2018. Mar. TNFα and IL-1β modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death Dis 9 (3), 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV, et al. , 2016. Dec. Elucidation of exosome migration across the blood–brain barrier model in vitro. Cell. Mol. Bioeng 9 (4), 509–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YA, Lu CH, Ke CC, Chiu SJ, Jeng FS, Chang CW, et al. , 2021. May 24. Mesenchymal stem cell-derived exosomes ameliorate Alzheimer’s disease pathology and improve cognitive deficits. Biomedicines 9 (6), 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Sharples RA, Scicluna BJ, Hill AF, 2014. Jan. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J. Extracell. Vesicles 3 (1), 23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Doecke JD, Sharples RA, Villemagne VL, Fowler CJ, Rembach A, et al. , 2015. Oct. Prognostic serum miRNA biomarkers associated with Alzheimer’s disease shows concordance with neuropsychological and neuroimaging assessment. Mol. Psychiatry 20 (10), 1188–1196. [DOI] [PubMed] [Google Scholar]
- Cheng L, Vella LJ, Barnham KJ, McLean C, Masters CL, Hill AF, 2020. Sep. Small RNA fingerprinting of Alzheimer’s disease frontal cortex extracellular vesicles and their comparison with peripheral extracellular vesicles. J. Extracell. Vesicles 9 (1), 1766822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, et al. , 2009. Jul. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol 11 (7), 909–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Duyckaerts C, Haïk S, 2020. Apr. Prion-like properties of tau assemblies. Curr. Opin. Neurobiol 61, 49–57. [DOI] [PubMed] [Google Scholar]
- Clayton K, Delpech JC, Herron S, Iwahara N, Ericsson M, Saito T, et al. , 2021. Dec. Plaque associated microglia hyper-secrete extracellular vesicles and accelerate tau propagation in a humanized APP mouse model. Mol. Neurodegener 16 (1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn W, Melnik M, Huang C, Teter B, Chandra S, Zhu C, et al. , 2021. Dec 2. Multiomics analysis of microglial extracellular vesicles from human Alzheimer’s disease brain tissue reveals disease-associated signatures. Front. Pharmacol 12, 766082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. , 2013. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci 126 (Pt 24), 5553–5565 (Jan 1;jcs.128868). [DOI] [PubMed] [Google Scholar]
- Coomans EM, Schoonhoven DN, Tuncel H, Verfaillie SCJ, Wolters EE, Boellaard R, et al. , 2021. Dec. In vivo tau pathology is associated with synaptic loss and altered synaptic function. Alzheimers Res. Ther 13 (1), 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotti A, Sait HR, McAvoy KM, Estrada K, Ergun A, Szak S, et al. , 2019. Dec. BIN1 favors the spreading of tau via extracellular vesicles. Sci. Rep 9 (1), 9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Wong ESP, Martinez-Vicente M, 2010. Protein degradation, aggregation, and misfolding: autophagy and neurodegeneration. Mov. Disord 25 (S1), S49–S54. [DOI] [PubMed] [Google Scholar]
- Czirr E, Wyss-Coray T, 2012. Apr 2. The immunology of neurodegeneration. J. Clin. Invest 122 (4), 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta Chaudhuri A, Dasgheyb RM, DeVine LR, Bi H, Cole RN, Haughey NJ, 2020. Jan. Stimulus-dependent modifications in astrocyte-derived extracellular vesicle cargo regulate neuronal excitability. Glia 68 (1), 128–144. [DOI] [PubMed] [Google Scholar]
- de Godoy MA, Saraiva LM, de Carvalho LRP, Vasconcelos-dos-Santos A, Beiral HJV, Ramos AB, et al. , 2018. Feb. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. J. Biol. Chem 293 (6), 1957–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kimpe L, van Haastert ES, Kaminari A, Zwart R, Rutjes H, Hoozemans JJM, et al. , 2013. Jun. Intracellular accumulation of aggregated pyroglutamate amyloid beta: convergence of aging and Aβ pathology at the lysosome. AGE 35 (3), 673–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Peraza F, Nogueras-Ortiz CJ, Volpert O, Liu D, Goetzl EJ, Mattson MP, et al. , 2021. Apr 23. Neuronal and astrocytic extracellular vesicle biomarkers in blood reflect brain pathology in mouse models of Alzheimer’s disease. Cells 10 (5), 993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens AM, Tovar-y-Romo LB, Yoo SW, Trout AL, Bae M, Kanmogne M, et al. , 2017. Apr 4. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci. Signal 10 (473) eaai7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkins MB, Enasko J, Hernandez C, Wang G, Kong J, Helwa I, et al. , 2016. Aug 17. Neutral Sphingomyelinase-2 deficiency ameliorates Alzheimer’s disease pathology and improves cognition in the 5XFAD mouse. J. Neurosci 36 (33), 8653–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Zheng D, Nao J, 2020. Sep 8. Circulating exosome microRNAs as diagnostic biomarkers of dementia. Front. Aging Neurosci 12, 580199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Gu H, Guo Q, Liang S, Xue J, Yao F, et al. , 2021. Jul. Profiling of serum exosome MiRNA reveals the potential of a MiRNA panel as diagnostic biomarker for Alzheimer’s disease. Mol. Neurobiol 58 (7), 3084–3094. [DOI] [PubMed] [Google Scholar]
- Dong X, Jiang D, Wang L, Zhao J, Yu L, Huang Y, et al. , 2022. Apr. VPS28 regulates brain vasculature by controlling neuronal VEGF trafficking through extracellular vesicle secretion. iScience 25 (4), 104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. , 2016. Mar. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement J. Alzheimers Assoc 12 (3), 292–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan E, Hutchison ER, Marosi K, Comotto J, Mustapic M, Nigam SM, et al. , 2016. Dec. Extracellular vesicle-associated Aβ mediates trans-neuronal bioenergetic and Ca2+−handling deficits in Alzheimer’s disease models. Npj Aging Mech Dis 2 (1), 16019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElAli A, Rivest S, 2016. Jul. Microglia in Alzheimer’s disease: a multifaceted relationship. Brain Behav. Immun 55, 138–150. [DOI] [PubMed] [Google Scholar]
- Elsherbini A, Kirov AS, Dinkins MB, Wang G, Qin H, Zhu Z, et al. , 2020. Dec. Association of Aβ with ceramide-enriched astrosomes mediates Aβ neurotoxicity. Acta Neuropathol. Commun 8 (1), 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsherbini A, Qin H, Zhu Z, Tripathi P, Crivelli SM, Bieberich E, 2020. Dec. In vivo evidence of exosome-mediated Aβ neurotoxicity. Acta Neuropathol. Commun 8 (1), 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren E, Hunt JFV, Shardell M, Chawla S, Tran J, Gu J, et al. , 2020. Jun 26. Extracellular vesicle biomarkers of Alzheimer’s disease associated with sub-clinical cognitive decline in late middle age. Alzheimers Dement 16 (9), 1293–1304 alz.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Barres BA, 2010. Nov. Regulation of synaptic connectivity by glia. Nature 468 (7321), 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, et al. , 2015. Jun. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement 11 (6), 600–607.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, et al. , 2011. Feb 1. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci 124 (3), 447–458. [DOI] [PubMed] [Google Scholar]
- Fröhlich D, Kuo WP, Frühbeis C, Sun JJ, Zehendner CM, Luhmann HJ, et al. , 2014. Sep 26. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos. Trans. R. Soc. B Biol. Sci 369 (1652), 20130510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, et al. , 2013. Jul 9. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte–neuron communication. Barres BA, editor. PLoS Biol 11 (7) e1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühbeis C, Kuo-Elsner WP, Müller C, Barth K, Peris L, Tenzer S, et al. , 2020. Dec 22. Oligodendrocytes support axonal transport and maintenance via exosome secretion. Emery B, editor. PLOS Biol 18 (12) e3000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Wang Y, Xia X, Zheng JC, 2020. Oct. Exosome engineering: current progress in cargo loading and targeted delivery. NanoImpact 20, 100261. [Google Scholar]
- Gabrielli M, Battista N, Riganti L, Prada I, Antonucci F, Cantone L, et al. , 2015. Dec. Active endocannabinoids are secreted on the surface of microglial microvesicles. SpringerPlus 4 (S1), L29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gámez-Valero A, Campdelacreu J, Vilas D, Ispierto L, Reñé R, Álvarez R, et al. , 2019. Dec. Exploratory study on microRNA profiles from plasma-derived extracellular vesicles in Alzheimer’s disease and dementia with Lewy bodies. Transl. Neurodegener 8 (1), 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadaran P, Hong CM, Ahn BC, 2018. Feb 28. An update on in vivo imaging of extracellular vesicles as drug delivery vehicles. Front. Pharmacol 9, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Zhao S, Xia X, Li C, Li C, Ji C, et al. , 2019. Jun 28. Glutaminase C regulates microglial activation and pro-inflammatory exosome release: relevance to the pathogenesis of Alzheimer’s disease. Front. Cell. Neurosci 13, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O, 2009. Sep. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat. Cell Biol 11 (9), 1143–1149. [DOI] [PubMed] [Google Scholar]
- Goda Y, Davis GW, 2003. Oct. Mechanisms of synapse assembly and disassembly. Neuron 40 (2), 243–264. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, et al. , 2015. Jul 7. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 85 (1), 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl EJ, Mustapic M, Kapogiannis D, Eitan E, Lobach IV, Goetzl L, et al. , 2016. Nov. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J 30 (11), 3853–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl EJ, Kapogiannis D, Schwartz JB, Lobach IV, Goetzl L, Abner EL, et al. , 2016. Dec. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J 30 (12), 4141–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl EJ, Abner EL, Jicha GA, Kapogiannis D, Schwartz JB, 2018. Feb. Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer’s disease. FASEB J 32 (2), 888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl EJ, Schwartz JB, Abner EL, Jicha GA, Kapogiannis D, 2018. Mar. High complement levels in astrocyte-derived exosomes of Alzheimer disease: ADE complement proteins in AD. Ann. Neurol 83 (3), 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde TE, 2022. Dec. Alzheimer’s disease – the journey of a healthy brain into organ failure. Mol. Neurodegener 17 (1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Molina LA, Villar-Vesga J, Henao-Restrepo J, Villegas A, Lopera F, Cardona-Gómez GP, et al. , 2021. Feb 19. Extracellular vesicles from 3xTg-AD mouse and Alzheimer’s disease patient astrocytes impair neuroglial and vascular components. Front. Aging Neurosci 13, 593927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu D, Liu F, Meng M, Zhang L, Gordon ML, Wang Y, et al. , 2020. Sep. Elevated matrix metalloproteinase-9 levels in neuronal extracellular vesicles in Alzheimer’s disease. Ann Clin Transl Neurol 7 (9), 1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui Y, Liu H, Zhang L, Lv W, Hu X, 2015. Nov 10. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget 6 (35), 37043–37053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart K, Loers G, Buck F, Bork U, Schachner M, Kleene R, 2016. Mar. Improvement of neuronal cell survival by astrocyte-derived exosomes under hypoxic and ischemic conditions depends on prion protein. Glia 64 (6), 896–910. [DOI] [PubMed] [Google Scholar]
- Guix FX, Sannerud R, Berditchevski F, Arranz AM, Horŕe K, Snellinx A, et al. , 2017. Dec. Tetraspanin 6: a pivotal protein of the multiple vesicular body determining exosome release and lysosomal degradation of amyloid precursor protein fragments. Mol. Neurodegener 12 (1), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan S, Kang MH, Kim JH, 2021. Feb. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int. J. Nanomedicine 16, 1281–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Goetzl EJ, Kapogiannis D, Lista S, Vergallo A, 2019. Mar 29. Biomarker-drug and liquid biopsy co-development for disease staging and targeted therapy: cornerstones for Alzheimer’s precision medicine and pharmacology. Front. Pharmacol 10, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao P, Liang Z, Piao H, Ji X, Wang Y, Liu Y, et al. , 2014. Mar. Conditioned medium of human adipose-derived mesenchymal stem cells mediates protection in neurons following glutamate excitotoxicity by regulating energy metabolism and GAP-43 expression. Metab. Brain Dis 29 (1), 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haqqani AS, Delaney CE, Tremblay TL, Sodja C, Sandhu JK, Stanimirovic DB, 2013. Method for isolation and molecular characterization of extracellular microvesicles released from brain endothelial cells. Fluids Barriers CNS 10 (1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C, Stahl P, 1983. Jun. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem. Biophys. Res. Commun 113 (2), 650–658. [DOI] [PubMed] [Google Scholar]
- He M, Zhang H. nan, Tang Z. chu, Gao S. guang, 2021. May 16. Diagnostic and therapeutic potential of exosomal MicroRNAs for neurodegenerative diseases. Baroncelli L, editor. Neural Plast 2021, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessvik NP, Llorente A, 2018. Jan. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci 75 (2), 193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, El Khoury J, 2008. Aug 13. Microglial dysfunction and defective -amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci 28 (33), 8354–8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, et al. , 2016. May 6. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352 (6286), 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougie C, 1955. Apr. The activation of platelets by plasma. Br. J. Haematol 1 (2), 213–222. [DOI] [PubMed] [Google Scholar]
- Huang S, Ge X, Yu J, Han Z, Yin Z, Li Y, et al. , 2018. Jan. Increased miR-124–3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J 32 (1), 512–528. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. , 2014. Dec 3. Impairment of Glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci 34 (49), 16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranifar E, Seresht BM, Momeni F, Fadaei E, Mehr MH, Ebrahimi Z, et al. , 2019. Mar. Exosomes and microRNAs: new potential therapeutic candidates in Alzheimer disease therapy. J. Cell. Physiol 234 (3), 2296–2305. [DOI] [PubMed] [Google Scholar]
- Janas T, Janas MM, Sapoń K, Janas T, 2015. Jun 4. Mechanisms of RNA loading into exosomes. FEBS Lett 589 (13), 1391–1398. [DOI] [PubMed] [Google Scholar]
- Jeong H, Kim OJ, Oh SH, Lee S, Reum Lee HA, Lee KO, et al. , 2021. Dec 3. Extracellular vesicles released from Neprilysin gene-modified human umbilical cord-derived mesenchymal stem cell enhance therapeutic effects in an Alzheimer’s disease animal model. Mandraffino G, editor. Stem Cells Int 2021, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Qiu Q, Zhang H, Chu L, Du Y, Zhang J, et al. , 2019. Aug. Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement 15 (8), 1071–1080. [DOI] [PubMed] [Google Scholar]
- Jia L, Zhu M, Kong C, Pang Y, Zhang H, Qiu Q, et al. , 2021. Jan. Blood neuro-exosomal synaptic proteins predict Alzheimer’s disease at the asymptomatic stage. Alzheimers Dement 17 (1), 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P, Turola E, Ruiz A, Bergami A, Libera DD, Benussi L, et al. , 2014. Apr. Microglia convert aggregated amyloid-β into neurotoxic forms through the shedding of microvesicles. Cell Death Differ 21 (4), 582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice NJ, 2018. Feb. The relationship between stress and Alzheimer’s disease. Neurobiol. Stress 8, 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniowska D, Wenk K, Rademacher P, Weiss R, Fabian C, Schulz I, et al. , 2022. Mar. Extracellular vesicles of mesenchymal stromal cells can be taken up by microglial cells and partially prevent the stimulation induced by β-amyloid. Stem Cell Rev. Rep 18 (3), 1113–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, et al. , 2015. Feb. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J 29 (2), 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis D, Mustapic M, Shardell MD, Berkowitz ST, Diehl TC, Spangler RD, et al. , 2019. Nov 1. Association of extracellular vesicle biomarkers with Alzheimer disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol 76 (11), 1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, et al. , 2013. Dec 20. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep 3 (1), 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann DJ, Babst M, Emr SD, 2001. Jul. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106 (2), 145–155. [DOI] [PubMed] [Google Scholar]
- Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M, 2017. Jul. RNA in extracellular vesicles: RNA in extracellular vesicles. Wiley Interdiscip. Rev. RNA 8 (4), e1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Shin KY, Chang KA, 2021. Jul 3. Brain-derived Exosomal proteins as effective biomarkers for Alzheimer’s disease: a systematic review and Meta-analysis. Biomolecules 11 (7), 980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough IF, Robel S, Roberson ED, Sontheimer H, 2015. Dec. Vascular amyloidosis impairs the gliovascular unit in a mouse model of Alzheimer’s disease. Brain 138 (12), 3716–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppers-Lalic D, Hackenberg M, Bijnsdorp IV, van Eijndhoven MAJ, Sadek P, Sie D, et al. , 2014. Sep. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep 8 (6), 1649–1658. [DOI] [PubMed] [Google Scholar]
- Korkut C, Li Y, Koles K, Brewer C, Ashley J, Yoshihara M, et al. , 2013. Mar. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron 77 (6), 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T, 2013. Apr. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic MicroRNAs regulate cancer cell metastasis. J. Biol. Chem 288 (15), 10849–10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer-Albers EM, Bretz N, Tenzer S, Winterstein C, Möbius W, Berger H, et al. , 2007. Nov. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteomics Clin. Appl 1 (11), 1446–1461. [DOI] [PubMed] [Google Scholar]
- Labbadia J, Morimoto RI, 2015. Jun 2. The biology of Proteostasis in aging and disease. Annu. Rev. Biochem 84 (1), 435–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, et al. , 2011. Feb. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci 46 (2), 409–418. [DOI] [PubMed] [Google Scholar]
- Lafourcade C, Ramírez JP, Luarte A, Fernández A, Wyneken U, 2016. Jan. MiRNAs in Astrocyte-Derived Exosomes as Possible Mediators of Neuronal Plasticity. J. Exp. Neurosci 10, 1–9, 10s1:JEN.S39916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Clos AL, Jackson GR, Kayed R, 2011. Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol. Neurodegener 6 (1), 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM, 2009. Feb. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature 457 (7233), 1128–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Ban JJ, Yang S, Im W, Kim M, 2018. Jul. The exosome of adipose-derived stem cells reduces β-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer’s disease. Brain Res 1691, 87–93. [DOI] [PubMed] [Google Scholar]
- Lee SH, Shin SM, Zhong P, Kim HT, Kim DI, Kim JM, et al. , 2018. Dec. Reciprocal control of excitatory synapse numbers by Wnt and Wnt inhibitor PRR7 secreted on exosomes. Nat. Commun 9 (1), 3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Choi Y, Lee HJ, Hwang DW, Lee DS, 2022. Dec. Human neural stem cell-derived extracellular vesicles protect against Parkinson’s disease pathologies. J. Nanobiotechnology 20 (1), 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux E, Perbet R, Caillierez R, Richetin K, Lieger S, Espourteille J, et al. , 2022. Feb. Extracellular vesicles: major actors of heterogeneity in tau spreading among human tauopathies. Mol. Ther 30 (2), 782–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Huang S, Yin Z, Zhu J, Ge X, Han Z, et al. , 2019. Aug. Increases in miR-124–3p in microglial exosomes confer neuroprotective effects by targeting FIP200-mediated neuronal autophagy following traumatic brain injury. Neurochem. Res 44 (8), 1903–1923. [DOI] [PubMed] [Google Scholar]
- Li F, Xie XY, Sui XF, Wang P, Chen Z, Zhang JB, 2020. Apr. Profile of pathogenic proteins and MicroRNAs in plasma-derived extracellular vesicles in Alzheimer’s disease: a pilot study. Neuroscience 432, 240–246. [DOI] [PubMed] [Google Scholar]
- Liao K, Niu F, Hu G, Yang L, Dallon B, Villarreal D, et al. , 2020. Nov. Morphine-mediated release of miR-138 in astrocyte-derived extracellular vesicles promotes microglial activation. J. Extracell. Vesicles 10 (1), e12027 [Internet]. [cited 2021 Aug 22];10(1). Available from: https://onlinelibrary.wiley.com/doi/10.1002/jev2.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew LC, Katsuda T, Gailhouste L, Nakagama H, Ochiya T, 2017. Jan 1. Mesenchymal stem cell-derived extracellular vesicles: a glimmer of hope in treating Alzheimer’s disease. Int. Immunol 29 (1), 11–19. [DOI] [PubMed] [Google Scholar]
- Ling X, Zhang G, Xia Y, Zhu Q, Zhang J, Li Q, et al. , 2020. Jan. Exosomes from human urine-derived stem cells enhanced neurogenesis via miR-26a/HDAC6 axis after ischaemic stroke. J. Cell. Mol. Med 24 (1), 640–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CG, Song J, Zhang YQ, Wang PC, 2014. Nov. MicroRNA-193b is a regulator of amyloid precursor protein in the blood and cerebrospinal fluid derived exosomal microRNA-193b is a biomarker of Alzheimer’s disease. Mol. Med. Rep 10 (5), 2395–2400. [DOI] [PubMed] [Google Scholar]
- Lombardi M, Parolisi R, Scaroni F, Bonfanti E, Gualerzi A, Gabrielli M, et al. , 2019. Dec. Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure. Acta Neuropathol. (Berl) 138 (6), 987–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X, Yao X, Jiang Q, Yang Y, He X, Tian W, et al. , 2020. Dec. Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. J. Neuroinflammation 17 (1), 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losurdo M, Pedrazzoli M, D’Agostino C, Elia CA, Massenzio F, Lonati E, et al. , 2020. Sep 1. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. Stem Cells Transl. Med 9 (9), 1068–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen PJ, Pruessner J, Sousa N, Almeida OFX, Van Dam AM, Rajkowska G, et al. , 2014. Jan. Neuropathology of stress. Acta Neuropathol. (Berl) 127 (1), 109–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli G, Cohen AM, Bennett DA, Shah RC, Fields CJ, Hernandez AG, et al. , 2015. Oct 1. Plasma exosomal miRNAs in persons with and without Alzheimer Disease: altered expression and prospects for biomarkers. Zhang B, editor. PLoS One 10 (10) e0139233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Dai Y, Chen Z, Yue X, Andrade-Powell KC, Chang J, 2020. Dec. Spatial and temporal tracking of cardiac exosomes in mouse using a nano-luciferase-CD63 fusion protein. Commun Biol 3 (1), 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NPV, et al. , 1998. May. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat. Neurosci 1 (1), 69–73. [DOI] [PubMed] [Google Scholar]
- Lv LL, Feng Y, Li ZL, Tang TT, Liu BC, 2017. Therapeutic application of extracellular vesicles in kidney disease: promises and challenges. J. Cell Mol. Med 22 (2), 728–737 [Internet]. Oct 30 [cited 2022 Jun 17]; Available from: https://onlinelibrary.wiley.com/doi/10.1111/jcmm.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Wang K, Pan J, Fan Z, Tian C, Deng X, et al. , 2019. Apr. Induced neural progenitor cells abundantly secrete extracellular vesicles and promote the proliferation of neural progenitors via extracellular signal–regulated kinase pathways. Neurobiol. Dis 124, 322–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Huang M, Zheng M, Dai C, Song Q, Zhang Q, et al. , 2020. Nov. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer’s disease. J. Control. Release 327, 688–702. [DOI] [PubMed] [Google Scholar]
- Maas SLN, Abels ER, Van De Haar LL, Zhang X, Morsett L, Sil S, et al. , 2020. Dec. Glioblastoma hijacks microglial gene expression to support tumor growth. J. Neuroinflammation 17 (1), 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markoutsa E, Mayilsamy K, Gulick D, Mohapatra SS, Mohapatra S, 2022. Feb. Extracellular vesicles derived from inflammatory-educated stem cells reverse brain inflammation—implication of miRNAs. Mol. Ther 30 (2), 816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto J, Stewart T, Sheng L, Li N, Bullock K, Song N, et al. , 2017. Dec. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol. Commun 5 (1), 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo H, 2004. Jan 23. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 303 (5657), 531–534. [DOI] [PubMed] [Google Scholar]
- McAllister BB, Lacoursiere SG, Sutherland RJ, Mohajerani MH, 2020. May. Intracerebral seeding of amyloid-β and tau pathology in mice: factors underlying prion-like spreading and comparisons with α-synuclein. Neurosci. Biobehav. Rev 112, 1–27. [DOI] [PubMed] [Google Scholar]
- McCann JV, Bischoff SR, Zhang Y, Cowley DO, Sanchez-Gonzalez V, Daaboul GD, et al. , 2020. Reporter mice for isolating and auditing cell type-specific extracellular vesicles in vivo. Genesis 58 (7), e23369 [Internet]. Jul [cited 2021 Aug 22];58(7). Available from: https://onlinelibrary.wiley.com/doi/10.1002/dvg.23369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeever PM, Schneider R, Taghdiri F, Weichert A, Multani N, Brown RA, et al. , 2018. Dec. MicroRNA expression levels are altered in the cerebrospinal fluid of patients with young-onset Alzheimer’s disease. Mol. Neurobiol 55 (12), 8826–8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA, 2015. Nov 2. Exosomes: mechanisms of uptake. J. Circ. Biomark (4), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men Y, Yelick J, Jin S, Tian Y, Chiang MSR, Higashimori H, et al. , 2019. Dec. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat. Commun 10 (1), 4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda AM, Lasiecka ZM, Xu Y, Neufeld J, Shahriar S, Simoes S, et al. , 2018. Dec. Neuronal lysosomal dysfunction releases exosomes harboring APP C-terminal fragments and unique lipid signatures. Nat. Commun 9 (1), 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi S, Zandi M, Dousti Kataj P, Karimi Zandi L., 2021. Jul 5. Chronic stress and Alzheimer’s disease. Biotechnol. Appl. Biochem 69 (4), 1451–1458 bab.2216. [DOI] [PubMed] [Google Scholar]
- Moloudizargari M, Asghari MH, Goel A, 2021. Oct. The therapeutic triad of extracellular vesicles: as drug targets, as drugs, and as drug carriers. Biochem. Pharmacol 192, 114714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S, et al. , 2013. Mar. Neuronal Exosomal miRNA-dependent translational regulation of Astroglial glutamate transporter GLT1. J. Biol. Chem 288 (10), 7105–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee C, Kling T, Russo B, Miebach K, Kess E, Schifferer M, et al. , 2020. Aug. Oligodendrocytes provide antioxidant defense function for neurons by secreting ferritin heavy chain. Cell Metab 32 (2), 259–272.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka S, Jedrychowski MP, Iwahara N, Abdullah M, Onos KD, Keezer KJ, et al. , 2021. Mar 5. Enrichment of neurodegenerative microglia signature in brain-derived extracellular vesicles isolated from Alzheimer’s disease mouse models. J. Proteome Res 20 (3), 1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam E, Lee YB, Moon C, Chang KA, 2020. Jul 15. Serum tau proteins as potential biomarkers for the assessment of Alzheimer’s disease progression. Int. J. Mol. Sci 21 (14), 5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, et al. , 2019. Feb. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med 25 (2), 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie C, Sun Y, Zhen H, Guo M, Ye J, Liu Z, et al. , 2020. May 7. Differential expression of plasma exo-miRNA in neurodegenerative diseases by next-generation sequencing. Front. Neurosci 14, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, Yang DS, Lee JH, 2008. Jul. Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy 4 (5), 590–599. [DOI] [PubMed] [Google Scholar]
- O’Brien JR, 1955. Apr. The platelet-like activity of serum. Br. J. Haematol 1 (2), 223–228. [PubMed] [Google Scholar]
- O’Brien JT, Ames D, Schweitzer I, Mastwyk M, Colman P, 1996. Jan. Enhanced adrenal sensitivity to adrenocorticotrophic hormone (ACTH) is evidence of HPA axis hyperactivity in Alzheimer’s disease. Psychol. Med 26 (1), 7–14. [DOI] [PubMed] [Google Scholar]
- Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, et al. , 2016. Jun. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 15 (7), 673–684. [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, et al. , 2016. May. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 139 (5), 1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BT, Johnstone RM, 1983. Jul. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33 (3), 967–978. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bergamini G, Rajendran L, 2019. May. Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience 405, 148–157. [DOI] [PubMed] [Google Scholar]
- Pascua-Maestro R, González E, Lillo C, Ganfornina MD, Falcón-Pérez JM, Sanchez D, 2019. Jan 10. Extracellular vesicles secreted by astroglial cells transport apolipoprotein D to neurons and mediate neuronal survival upon oxidative stress. Front. Cell. Neurosci 12, 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MR, Weaver AM, 2021. Mar. Astrocyte-derived small extracellular vesicles promote synapse formation via fibulin-2-mediated TGF-β signaling. Cell Rep 34 (10), 108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrioli G, Barberis M, Magrin C, Morone D, Piovesana E, Senesi G, et al. , 2021. Sep 1. Tau seeds in extracellular vesicles induce tau accumulation in degradative organelles of cells. DNA Cell Biol 40 (9), 1185–1199. [DOI] [PubMed] [Google Scholar]
- Peng KY, Pérez-González R, Alldred MJ, Goulbourne CN, Morales-Corraliza J, Saito M, et al. , 2019. Jan 1. Apolipoprotein E4 genotype compromises brain exosome production. Brain 142 (1), 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Trojanowski JQ, Lee VMY, 2020. Apr. Protein transmission in neurodegenerative disease. Nat. Rev. Neurol 16 (4), 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D, Wang Y, Xiao Y, Peng M, Mai W, Hu B, et al. , 2022. Extracellular vesicles derived from astrocyte-treated with haFGF 14–154 attenuate Alzheimer phenotype in AD mice. Theranostics 12 (8), 3862–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perets N, Betzer O, Shapira R, Brenstein S, Angel A, Sadan T, et al. , 2019. Jun 12. Golden exosomes selectively target brain pathologies in neurodegenerative and neurodevelopmental disorders. Nano Lett 19 (6), 3422–3431. [DOI] [PubMed] [Google Scholar]
- Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E, 2012. Dec. The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J. Biol. Chem 287 (51), 43108–43115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Hernandez D, Gutiérrez-Vázquez C, Jorge I, López-Martín S, Ursa A, Sánchez-Madrid F, et al. , 2013. Apr. The intracellular Interactome of Tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J. Biol. Chem 288 (17), 11649–11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI, 2019. Dec. Mesenchymal stem cell perspective: cell biology to clinical progress. Npj Regen Med 4 (1), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanco JC, Scicluna BJ, Hill AF, Götz J, 2016. Jun. Extracellular vesicles isolated from the brains of rTg4510 mice seed tau protein aggregation in a threshold-dependent manner. J. Biol. Chem 291 (24), 12445–12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potolicchio I, Carven GJ, Xu X, Stipp C, Riese RJ, Stern LJ, et al. , 2005. Aug 15. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J. Immunol 175 (4), 2237–2243. [DOI] [PubMed] [Google Scholar]
- Prada I, Gabrielli M, Turola E, Iorio A, D’Arrigo G, Parolisi R, et al. , 2018. Apr. Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol. (Berl) 135 (4), 529–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP, 2013. Jan. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 9 (1), 63–75.e2. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM, 2010. Jan 28. Alzheimer’s disease. N. Engl. J. Med 362 (4), 329–344. [DOI] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W, 2013. Feb 18. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol 200 (4), 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmuson S, Andrew R, Näsman B, Seckl JR, Walker BR, Olsson T, 2001. Mar. Increased glucocorticoid production and altered cortisol metabolism in women with mild to moderate Alzheimer’s disease. Biol. Psychiatry 49 (6), 547–552. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. , 2005. Jun 7. From the cover: correlations between apolipoprotein E 4 gene dose and brain-imaging measurements of regional hypometabolism. Proc. Natl. Acad. Sci 102 (23), 8299–8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JL, Mahajan SD, 2020. Sep. Transmigration of tetraspanin 2 (Tspan2) siRNA via microglia derived exosomes across the blood brain barrier modifies the production of immune mediators by microglia cells. J. NeuroImmune Pharmacol 15 (3), 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riancho J, Vázquez-Higuera JL, Pozueta A, Lage C, Kazimierczak M, Bravo M, et al. , 2017. Mar 21. MicroRNA profile in patients with Alzheimer’s disease: analysis of miR-9–5p and miR-598 in raw and exosome enriched cerebrospinal fluid samples. J. Alzheimers Dis 57 (2), 483–491. [DOI] [PubMed] [Google Scholar]
- Ruan Z, Delpech JC, Venkatesan Kalavai S, Van Enoo AA, Hu J, Ikezu S, et al. , 2020. Dec. P2RX7 inhibitor suppresses exosome secretion and disease phenotype in P301S tau transgenic mice. Mol. Neurodegener 15 (1), 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Z, Pathak D, Venkatesan Kalavai S, Yoshii-Kitahara A, Muraoka S, Bhatt N, et al. , 2021. Feb 12. Alzheimer’s disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain 144 (1), 288–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufino-Ramos D, Albuquerque PR, Carmona V, Perfeito R, Nobre RJ, Pereira de Almeida L, 2017. Sep. Extracellular vesicles: novel promising delivery systems for therapy of brain diseases. J. Control. Release 262, 247–258. [DOI] [PubMed] [Google Scholar]
- Saint-Pol J, Gosselet F, Duban-Deweer S, Pottiez G, Karamanos Y, 2020. Apr 1. Targeting and crossing the blood-brain barrier with extracellular vesicles. Cells 9 (4), 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala Frigerio C, Wolfs L, Fattorelli N, Thrupp N, Voytyuk I, Schmidt I, et al. , 2019. Apr. The major risk factors for Alzheimer’s disease: age, sex, and genes modulate the microglia response to Aβ plaques. Cell Rep 27 (4), 1293–1306.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Albero M, Navascués N, Mendoza G, Sebastián V, Arruebo M, Martín-Duque P, et al. , 2019. Dec. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J. Nanobiotechnology 17 (1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardar Sinha M, Ansell-Schultz A, Civitelli L, Hildesjö C, Larsson M, Lannfelt L, et al. , 2018. Jul. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. (Berl) 136 (1), 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. , 2012. May. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74 (4), 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffelers R, Kooijmans. Vader, van Dommelen S, Solinge Van, 2012. Mar. Exosome mimetics: a novel class of drug delivery systems. Int. J. Nanomedicine 1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpente M, Fenoglio C, D’Anca M, Arcaro M, Sorrentino F, Visconte C, et al. , 2020. Jun 10. MiRNA profiling in plasma neural-derived small extracellular vesicles from patients with Alzheimer’s disease. Cells 9 (6), 1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. , 2008. Aug. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med 14 (8), 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JM, Rodrigues S, Sampaio-Marques B, Gomes P, Neves-Carvalho A, Dioli C, et al. , 2019. Aug. Dysregulation of autophagy and stress granule-related proteins in stress-driven tau pathology. Cell Death Differ 26 (8), 1411–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AM, Lázaro-Ibáñez E, Gunnarsson A, Dhande A, Daaboul G, Peacock B, et al. , 2021. Quantification of protein cargo loading into engineered extracellular vesicles at single-vesicle and single-molecule resolution. J. Extracell. Vesicles 10 (10), e12130 [Internet]. Aug [cited 2021 Aug 22];10(10). Available from: https://onlinelibrary.wiley.com/doi/10.1002/jev2.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue A, Ito N, Nagai T, Shan W, Hada K, Nakajima A, et al. , 2018. May. Astroglial major histocompatibility complex class I following immune activation leads to behavioral and neuropathological changes. Glia 66 (5), 1034–1052. [DOI] [PubMed] [Google Scholar]
- Söllvander S, Nikitidou E, Brolin R, Söderberg L, Sehlin D, Lannfelt L, et al. , 2016. Dec. Accumulation of amyloid-β by astrocytes result in enlarged endosomes and microvesicle-induced apoptosis of neurons. Mol. Neurodegener 11 (1), 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Li Z, He T, Qu M, Jiang L, Li W, et al. , 2019. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 9 (10), 2910–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulos I, Silva JM, Gomes P, Sousa N, Almeida OFX, Takashima A, Wolozin B, Buee L, 2019. Stress and the etiopathogenesis of Alzheimer’s disease and depression. In: Tau Biology [Internet]. Advances in Experimental Medicine and Biology, Vol. 1184. Springer; Singapore, Singapore, pp. 241–257 [cited 2021 Jun 13]. Available from: http://link.springer.com/10.1007/978-981-32-9358-8_20. [DOI] [PubMed] [Google Scholar]
- Soto-Rojas LO, Pacheco-Herrero M, Martínez-Gómez PA, Campa-Córdoba BB, Apátiga-Pérez R, Villegas-Rojas MM, et al. , 2021. Feb 18. The neurovascular unit dysfunction in Alzheimer’s disease. Int. J. Mol. Sci 22 (4), 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, 2012. Nov. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11 (11), 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuffers S, Sem Wegner C, Stenmark H, Brech A, 2009. Jul. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 10 (7), 925–937. [DOI] [PubMed] [Google Scholar]
- Sutaria DS, Badawi M, Phelps MA, Schmittgen TD, 2017. May. Achieving the promise of therapeutic extracellular vesicles: the devil is in details of therapeutic loading. Pharm. Res 34 (5), 1053–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, 2009. The proteasome: overview of structure and functions. Proc. Jpn. Acad. Ser. B 85 (1), 12–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L. li, Wu Y. bo, Fang C. qin, Qu P, Gao Z. liang, 2016. Jan. NDRG2 promoted secreted miR-375 in microvesicles shed from M1 microglia, which induced neuron damage. Biochem. Biophys. Res. Commun 469 (3), 392–398. [DOI] [PubMed] [Google Scholar]
- Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, et al. , 2015. Aug. Clearance systems in the brain—implications for Alzheimer disease. Nat. Rev. Neurol 11 (8), 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Ghebremedhin E, Orantes M, Wiestler OD, 2003. Dec. Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J. Neuropathol. Exp. Neurol 62 (12), 1287–1301. [DOI] [PubMed] [Google Scholar]
- Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. , 2018. Dec 1. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7 (1), 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AG, Gray E, Heman-Ackah SM, Mäger I, Talbot K, Andaloussi SE, et al. , 2016. Jun. Extracellular vesicles in neurodegenerative disease — pathogenesis to biomarkers. Nat. Rev. Neurol 12 (6), 346–357. [DOI] [PubMed] [Google Scholar]
- Upadhya R, Madhu LN, Attaluri S, Gitaí DLG, Pinson MR, Kodali M, et al. , 2020. Sep. Extracellular vesicles from human iPSC-derived neural stem cells: miRNA and protein signatures, and anti-inflammatory and neurogenic properties. J. Extracell. Vesicles 9 (1), 1809064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhya R, Zingg W, Shetty S, Shetty AK, 2020. Jul. Astrocyte-derived extracellular vesicles: Neuroreparative properties and role in the pathogenesis of neurodegenerative disorders. J. Control. Release 323, 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Haar HJ, Burgmans S, Jansen JFA, van Osch MJP, van Buchem MA, Muller M, et al. , 2016. Nov. Blood-brain barrier leakage in patients with early Alzheimer disease. Radiology 281 (2), 527–535. [DOI] [PubMed] [Google Scholar]
- van Niel G, D’Angelo G, Raposo G, 2018. Apr. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol 19 (4), 213–228. [DOI] [PubMed] [Google Scholar]
- Vaz-Silva J, Gomes P, Jin Q, Zhu M, Zhuravleva V, Quintremil S, et al. , 2018. Endolysosomal degradation of tau and its role in glucocorticoid-driven hippocampal malfunction. EMBO J 37 (20), e99084 [Internet]. Oct 15 [cited 2021 Jun 13];37 (20). Available from: https://onlinelibrary.wiley.com/doi/10.15252/embj.201899084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella LJ, Scicluna BJ, Cheng L, Bawden EG, Masters CL, Ang CS, et al. , 2017. Dec 1. A rigorous method to enrich for exosomes from brain tissue. J Extracell Vesicles 6 (1), 1348885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturini A, Passalacqua M, Pelassa S, Pastorino F, Tedesco M, Cortese K, et al. , 2019. Dec 2. Exosomes from astrocyte processes: signaling to neurons. Front. Pharmacol 10, 1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernandez D, Vázquez J, Martin-Cofreces N, et al. , 2013. Dec. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun 4 (1), 2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. , 2002. Apr. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416 (6880), 535–539. [DOI] [PubMed] [Google Scholar]
- Wang S, Cesca F, Loers G, Schweizer M, Buck F, Benfenati F, et al. , 2011. May 18. Synapsin I is an Oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J. Neurosci 31 (20), 7275–7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Balaji V, Kaniyappan S, Krüger L, Irsen S, Tepper K, et al. , 2017. Dec. The release and trans-synaptic transmission of Tau via exosomes. Mol. Neurodegener 12 (1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Ye L, Lu H, Chen H, Zhang Y, Huang Y, et al. , 2017. Dec. TNF-α promotes extracellular vesicle release in mouse astrocytes through glutaminase. J. Neuroinflammation 14 (1), 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sui H, Zheng Y, Jiang Y, Shi Y, Liang J, et al. , 2019. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the tau protein through the AKT/GSK-3β pathway. Nanoscale 11 (15), 7481–7496. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu EJ, Liu Q, Li SH, Li T, Zhou QZ, et al. , 2020. Sep 1. Tau acetylation in entorhinal cortex induces its chronic hippocampal propagation and cognitive deficits in mice. J. Alzheimers Dis 77 (1), 241–255. [DOI] [PubMed] [Google Scholar]
- Wei H, Xu Y, Xu W, Zhou Q, Chen Q, Yang M, et al. , 2018. May. Serum Exosomal miR-223 serves as a potential diagnostic and prognostic biomarker for dementia. Neuroscience 379, 167–176. [DOI] [PubMed] [Google Scholar]
- Whitford W, Guterstam P, 2019. May. Exosome manufacturing status. Future Med. Chem 11 (10), 1225–1236. [DOI] [PubMed] [Google Scholar]
- Willis CM, Nicaise AM, Bongarzone ER, Givogri M, Reiter CR, Heintz O, et al. , 2020. Dec. Astrocyte support for oligodendrocyte differentiation can be conveyed via extracellular vesicles but diminishes with age. Sci. Rep 10 (1), 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, Winblad B, et al. , 2017. Jan. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 13 (1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston CN, Goetzl EJ, Akers JC, Carter BS, Rockenstein EM, Galasko D, et al. , 2016. Jan. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement. Diagn. Assess. Dis. Monit 3 (1), 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston CN, Goetzl EJ, Schwartz JB, Elahi FM, Rissman RA, 2019. Dec. Complement protein levels in plasma astrocyte-derived exosomes are abnormal in conversion from mild cognitive impairment to Alzheimer’s disease dementia. Zetterberg H, editor. Alzheimers Dement. Diagn. Assess. Dis. Monit 11 (1), 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf P, 1967. May. The nature and significance of platelet products in human plasma. Br. J. Haematol 13 (3), 269–288. [DOI] [PubMed] [Google Scholar]
- Xing W, Gao W, Lv X, Xu X, Zhang Z, Yan J, et al. , 2021. Mar 1. The diagnostic value of exosome-derived biomarkers in Alzheimer’s disease and mild cognitive impairment: a meta-analysis. Front. Aging Neurosci 13, 637218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Zhang Y, Du XF, Li J, Zi HX, Bu JW, et al. , 2017. Jul. Neurons secrete miR132-containing exosomes to regulate brain vascular integrity. Cell Res 27 (7), 882–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Niu F, Yao H, Liao K, Chen X, Kook Y, et al. , 2018. Sep. Exosomal miR-9 released from HIV tat stimulated astrocytes mediates microglial migration. J. NeuroImmune Pharmacol 13 (3), 330–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Boza-Serrano A, Dunning CJR, Clausen BH, Lambertsen KL, Deierborg T, 2018. Dec. Inflammation leads to distinct populations of extracellular vesicles from microglia. J. Neuroinflammation 15 (1), 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Liu CG, Gao SC, Zhang Y, Wang PC, 2018. Feb. The serum exosome derived MicroRNA-135a, 193b, and 384 were potential Alzheimer’s disease biomarkers. Biomed. Environ. Sci 31 (2), 87–96. [DOI] [PubMed] [Google Scholar]
- Yang L, Zhai Y, Hao Y, Zhu Z, Cheng G, 2020. Jan. The regulatory functionality of exosomes derived from hUMSCs in 3D culture for Alzheimer’s disease therapy. Small 16 (3), 1906273. [DOI] [PubMed] [Google Scholar]
- Yin Z, Han Z, Hu T, Zhang S, Ge X, Huang S, et al. , 2020. Jan. Neuron-derived exosomes with high miR-21–5p expression promoted polarization of M1 microglia in culture. Brain Behav. Immun 83, 270–282. [DOI] [PubMed] [Google Scholar]
- Yokoi A, Villar-Prados A, Oliphint PA, Zhang J, Song X, De Hoff P, et al. , 2019. Nov. Mechanisms of nuclear content loading to exosomes. Sci. Adv 5 (11) eaax8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y, Borgmann K, Edara VV, Stacy S, Ghorpade A, Ikezu T, 2020. Sep. Activated human astrocyte-derived extracellular vesicles modulate neuronal uptake, differentiation and firing. J. Extracell. Vesicles 9 (1), 1706801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y, Muraoka S, Jedrychowski MP, Hu J, McQuade AK, Young-Pearse T, et al. , 2022. Human neural cell type-specific extracellular vesicle proteome defines disease-related molecules associated with activated astrocytes in Alzheimer’s disease brain. J. Extracell. Vesicles 11 (1), e12183 [Internet]. Jan [cited 2022 Jun 17];11(1). Available from: https://onlinelibrary.wiley.com/doi/10.1002/jev2.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama K, Sun H, Mitsutake S, Igarashi Y, 2012. Mar. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J. Biol. Chem 287 (14), 10977–10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama K, Sun H, Sakai S, Mitsutake S, Okada M, Tahara H, et al. , 2014. Aug. Decreased amyloid-β pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J. Biol. Chem 289 (35), 24488–24498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, et al. , 2015. Apr. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg 122 (4), 856–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen M, Qiu Z, Hu K, McGee W, Chen X, et al. , 2016. Jul. MiR-130a regulates neurite outgrowth and dendritic spine density by targeting MeCP2. Protein Cell 7 (7), 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, et al. , 2018. Mar. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol 20 (3), 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Liu H, Tang WH, 2019. Dec. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9 (1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cao Y, Ma L, Wei Y, Li H, 2021. Jul 28. Possible mechanisms of tau spread and toxicity in Alzheimer’s disease. Front Cell Dev Biol 9, 707268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, McInnes J, Wierda K, Holt M, Herrmann AG, Jackson RJ, et al. , 2017. Aug. Tau association with synaptic vesicles causes presynaptic dysfunction. Nat. Commun 8 (1), 15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. , 2011. Oct. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther 19 (10), 1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. , 1998. May. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat. Med 4 (5), 594–600. [DOI] [PubMed] [Google Scholar]
- Zou H, Ding Y, Shi W, Xu X, Gong A, Zhang Z, et al. , 2015. Apr. MicroRNA-29c/PTEN pathway is involved in mice brain development and modulates neurite outgrowth in PC12 cells. Cell. Mol. Neurobiol 35 (3), 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]


