Abstract
RNA editing in higher plant mitochondria modifies mRNA sequences by means of C-to-U conversions at highly specific sites. To determine the cis elements involved in recognition of an editing site in plant mitochondria, deletion and site-directed mutation constructs containing the cognate cox II mitochondrial gene were introduced into purified mitochondria by electroporation. The RNA editing status was analyzed for precursor and spliced transcripts from the test construct. We found that only a restricted number of nucleotides in the vicinity of the target C residue were necessary for recognition by the editing machinery and that the nearest neighbor 3′ residues were crucial for the editing process. We provide evidence that two functionally distinguishable sequences can be defined: the 16-nucleotide 5′ region, which can be replaced with the same region from another editing site, and a 6-nucleotide 3′ region specific to the editing site. The latter region may play a role in positioning the actual editing residue.
RNA editing refers to a process whereby the genetic message is changed at single nucleotides in a very specific manner. This process involves a variety of genetic systems and occurs by different mechanisms (reference 6 and references therein). In trypanosome kinetoplasts, RNA editing proceeds by insertion and deletion of uridine nucleotides in mRNAs (2); the insertion of C residues has been described for Physarum polycephalum mitochondria, and the insertion of some G residues occurs in paramyxovirus (25, 36). Another type of RNA editing is base conversion, occurring in mammalian nuclei (31) and plant organelles. C-to-U conversions have been described for higher plant mitochondria (9, 13, 16) and to a lesser extent for chloroplasts (18, 21).
RNA editing is a posttranscriptional event in plant organelles. It is essential in plant mitochondrion gene expression processes such as the maturation step of organellar transcripts (26, 27) or the synthesis of functional proteins, since the nucleotide conversions usually alter the coding properties of the mRNA (1). The editing systems in higher plant organelles, mitochondria, and chloroplasts share many similar features, but promiscuous chloroplast sequences are not edited in mitochondria (39); conversely, a mitochondrial sequence carrying an editing site does not sustain editing when transcribed into chloroplasts (35). These results indicate that editing recognition signals are specific to each organelle. The sequences flanking target C residues lack any apparent conserved consensus elements at the primary or secondary structure level. An essential problem is to define the signals that determine the specific recognition of every editing site.
A number of in vivo studies of transgenic chloroplasts have demonstrated that mRNA sequences flanking the editing site are involved in RNA editing (4, 5). RNA editing has been determined to proceed by deamination of the C residue in wheat (3) and pea (38). However, the molecular determinants for editing-site recognition have not yet been identified. Analysis of the naturally occurring fusion of coding sequences generating chimeric genes in the plant mitochondrial genome offers the opportunity to study a particular editing site in two different contexts (14, 22, 32). Using this approach, Williams et al. (37) have suggested that 5′ flanking sequences may be crucial for editing-site recognition.
Here we present data on specific editing-site recognition elements using a novel mitochondrial electroporation technique (11). Site-directed mutated mitochondrial editing target sequences were introduced into isolated mitochondria, and the matured products were analyzed. Our results show that the mitochondrial determinants for editing-site recognition are located close to the target C residue and that changes in the nearest-neighbor residues can dramatically affect the editing process. This is the first experimental approach showing the cis-acting elements required for mitochondrion RNA editing-site recognition.
MATERIALS AND METHODS
Plasmids.
All plasmids used for mitochondrion electroporation are derivatives of plasmid pCox II (see Fig. 1A), as previously described (11). The pCox II plasmid is based on the pBluscribe vector and contains 882 bp of the Triticum timopheevi cox II promoter region, 2,009 bp of the coding and intron sequences, and 533 bp of the terminator 3′ region from the T. timopheevi apocytochrome b (cob) gene (34). A 23-bp insert was introduced into the promoter region at position −60 in plasmid pCox II. This insertion provides a specific sequence to isolate the transgene transcripts by reverse transcription (RT)-PCR using primers 1 and 2. All of the mutants derived from pCox II were constructed using the QuickChange Site-Directed Mutagenesis kit (Stratagene). The cox II sequence from the T. timopheevi mitochondria used in this study is registered in the GenBank database under accession number AF336134.
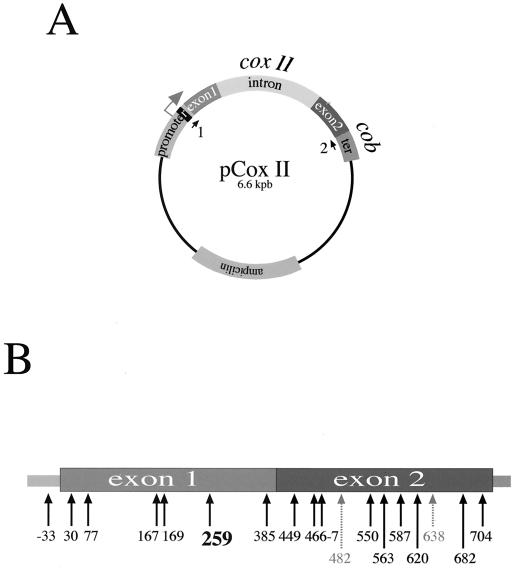
FIG. 1.
(A) Scheme of the plasmid used in this study. pCox II contains a 2-kbp fragment from the T. timopheevi cox II ORF formed by two exons interrupted by a 1.2-kbp intron inserted in pBluescribe vector. The transgene is controlled by an 882-bp cox II promoter region from T. timopheevi and 533 bp of a cob 3′ terminator region from T. timopheevi mitochondria (34). The gray arrow indicates the transcription initiation region. The black box in the promoter region of pCox II represents the 23-bp insert used for specific PCR amplification. Arrows 1 and 2 show the positions of primers used to specifically amplify the transgene. (B) Scheme of the wheat cox II transcript. The spliced form of the cox II transcript is shown. Solid arrows indicate the positions of editing sites determined after electroporation of pCox II. Dotted arrows indicate nonedited residues. Numbers indicate the C residue changed to U by editing. Site C259 was chosen for mutation analyses.
PCR primers.
PCR primers were as follows: 1, GCGGTGCAGTCATACAGATCTGC; 2, TATCCAGATTTGGTACCAAAC.
Mutagenesis primers.
The mutagenesis primers were as follows (only sense primers are indicated): 23-bp insert, AACGCCGGACGTCAAGCGGTGCAGTCATACAGATCTGCGATCAGTCTCCTTTC; S1a, TCGAAATTATACGGACCATAT; S1b, TCGAAATTATGCGGACCATAT; S1c, TCGAAATTATCCGGACCATAT; S2a, GAAATTATTCAGACCATATTT; S2b, TACTATCGAAATTATTCTGACCATATTTCCAAGTG; S2c, GAAATTATTCCGACCATATTT; M1, AACTAATCCAATCCCGTTCATGGAACTACT; M2, AATCCCACAAAGGATACTACTATCGAAATT; M3, AAGGATTGTTCATGGGAAATTATTCGGACC; M4, TCATGGAACTACTATCGGACCATATTTCCA; M5, CTATCGAAATTATTCTCCAAGTGTCATTCT; M6, CCATATTTCCAAGTGGTTCATTGCTATACC; M7, CAAAGGATTGTTCATGGAACTCGAAATTATTCGGACCATA; M8, GATTGTTCATGGAACTACTAATTATTCGGACCATATTTCC; M9, TTCATGGAACTACTATCGAATCGGACCATATTTCCAAGTG; M10, ACTACTATCGAAATTATTCGTATTTCCAAGTGTCATTCTT; M11, TATCGAAATTATTCGGACCACCAAGTGTCATTCTTTTGTT; M12, AAATTATTCGGACCATATTTTGTCATTCTTTTGTTCATTG; M13, CGAAATTATTCCGGACCATAT; M14, GGAACTACTATCGAAATTATTAACCATGGCAATTAGGATCGGACCATATTTCCAAGTGTC; M15, GAACTACTATCGAAATTATTCTCAAGACGCAGCAACACCGGACCATATTTCCAAGTGTCA.
Mitochondrial purification.
Wheat embryos were obtained from Triticum aestivum var. Fortal seeds as previously described (19). Embryos were sterilized with 0.6% sodium hypochlorite and rapidly washed with sterile distilled water before use (33). Seven grams of embryos was set out on filter paper saturated with sterile water in a petri dish and incubated for 18 h at 22°C. The embryos were homogenized with a Polytron (Kinematica GmbH, Kriens-Luzern, Switzerland) in 150 ml of a solution containing 0.4 M mannitol, 25 mM morpholinepropanesulfonic acid (pH 7.8), 1 mM EGTA, 8 mM cysteine, and 1 mg of fatty-acid-free bovine serum albumin/ml. The extract was filtered through a 30-μm nylon membrane, and mitochondria were isolated as described previously (11, 24). Purified mitochondria were transferred to an Eppendorf tube, collected by centrifugation at 15,000 × g for 10 min, and washed twice with 0.33 M sucrose. All experiments were performed with freshly purified mitochondria.
Electroporation.
Electrotransfer experiments were carried out with a Bio-Rad Gene Pulser at 4°C in 0.1-cm-electrode-gap cuvettes (Bio-Rad). The settings were 25 μF, 400 Ω, and 13 kV/cm. One microgram of plasmid, purified with a Qiagen plasmid Midi kit, was added to 1 mg of mitochondria in 50 μl of 0.33 M sucrose. After electroporation, the mitochondrial suspension was withdrawn and the cuvette was washed with an additional 50 μl of 0.33 M sucrose, which was added to the mitochondrial suspension. Mitochondria were collected by centrifugation at 15,000 × g for 10 min and then resuspended in 250 μl of an expression buffer containing 330 mM mannitol, 90 mM KCl, 10 mM MgCl2, 12 mM Tricine (pH 7.2), 5 mM KH2PO4, 1.2 mM EGTA, 1 mM GTP, 2 mM dithiothreitol, 2 mM ADP, 10 mM sodium succinate, and 0.15 mM (each) CTP and UTP. Mitochondria were incubated at 25°C for 18 h with constant stirring at 150 rpm. After incubation, the mitochondrial pellet was recovered by centrifugation at 15,000 × g for 15 min at 4°C. Mitochondrial RNA was purified with 200 μl of TRIzol reagent (Gibco-BRL) according to the protocol suggested by the supplier. The RNA was resuspended in 20 μl of diethylpyrocarbonate-treated water.
RT-PCR.
One microgram of RNA was treated with 2 U of amplification-grade DNase I (Gibco-BRL). cDNA synthesis was performed with 200 U of Superscript II RT (Gibco-BRL) using 100 ng of random hexamers as proposed by the supplier. PCRs were performed with primers 1 and 2 using Advantage 2 polymerase mix (Clontech) as follows: 95°C for 1 min; 5 cycles at 95°C for 30 s and 68°C for 1 min; 30 cycles at 95°C for 30 s, 58°C for 30 s, and 68°C for 30 s; and finally, 68°C for 1 min.
DNA sequencing.
Sequence analyses were performed directly on the RT-PCR product or, after cloning, on the pGEM-T (Promega) vectors using either the Thermo Sequenase radiolabeled terminator cycle sequencing kit (Amersham) or the BigDye terminator cycle sequencing kit (Applied Biosystems).
RESULTS
The structure of the plasmid used in this study is shown in Fig. 1A. The transgene was obtained from an alloplasmic male-sterile line of wheat (T. aestivum) containing the T. timopheevi cytoplasm (34). The exon sequences found in T. timopheevi cox II are identical to those of the endogenous T. aestivum gene (10), and few differences are found in noncoding sequences (11). To detect specifically the exogenous cox II gene and the respective transcript, a 23-nucleotide (nt) sequence was inserted at position −60 (see Materials and Methods). The T. timopheevi cox II open reading frame (ORF) possesses the same 17 editing sites found in the endogenous gene transcript scattered in both exons and another editing site located −33 nt from the start codon. We previously verified that 15 out of the 17 potential editing sites were faithfully edited in the transcript from the chimeric cox II transgene after electroporation in isolated wheat mitochondria (11). The editing sites in the chimeric cox II transcripts are indicated in Fig. 1B.
To define the cis recognition elements, we focused our study on editing site C259, located in the first exon of cox II. To ascertain that we were following a functional process, we analyzed the editing events occurring in the spliced transcripts. Along with the target site C259, sites C167, C169, and C385 were systematically analyzed in each experiment.
Editing recognition site is located in the vicinity of the C residue.
Deletion mutants were obtained by sequentially suppressing a block of 10 residues upstream and downstream of the C259 editing site. Mutants with deletions at positions −37 to −28 (Fig. 2, lane M1) or −30 to −20 (lane M2) upstream of the C259 target site were edited. By contrast, deletions at −20 (Fig. 2, lane M3) or −10 (lane M4) abolished editing. Similarly, the 3′ mutations at positions +18 to +27 (Fig. 2, lane M6) of the editing site were edited, whereas deletion of residues +1 to +10 (lane M5) inhibited the editing of site C259. Thus, the editing recognition sequences seemed to operate close to the target C. Based on this observation, analysis was performed on shorter deletion mutants inside this region. Deleting residues −16 to −12 (Fig. 2, lane M7) reduced the efficiency of the editing of C259. Deletions −11 to −7 (Fig. 2, lane M8) and −6 to −2 (lane M9) inhibited editing. At the 3′ side of C259, deletion of residues +7 to +11 (Fig. 2, lane M11) or +12 to +16 (lane M12) reduced the efficiency of editing, whereas deletion of residues +2 to +6 (lane M10) completely abolished editing. Therefore, the recognition signal is situated between positions −16 nt upstream and +6 nt downstream of the target C residue.
FIG. 2.
Deletion mutants. The sequence in the neighborhood of the C259 editing site is shown. Deletion mutants M1 to M6 are missing 10 nt in the regions indicated. Mutants M7 to M12 are missing 5 nt. The sequence analyses of RT-PCR transcript products after electroporation of the respective constructs are shown in panels. +, occurrence of editing as found in wild-type construct; −, absence of nonencoded U residue; +/−, reduced editing compared to the wild type. Small gray arrowheads mark the position of the C259 editing target. Large open arrowheads show the positions of the deleted residues.
Deletion mutations at C259 do not affect editing at other sites.
The editing statuses of sites C167, C169, and C385 were analyzed in transcripts generated from each mutant plasmid. In all cases, sites C167, C169, and C385 were correctly edited (not shown), suggesting that long-range signals do not operate in recognition of the editing site. It should be mentioned that the sequential deletion of 10 residues altered the reading frame of cox II mRNA, but only the modification of site C259 was impaired, indicating that the editing process is not connected with mRNA translation.
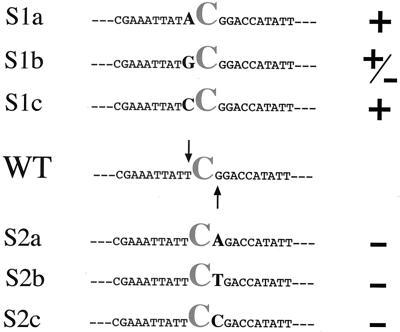
Changing the 5′ neighboring residue slightly modifies C editing.
To test whether the identity of the neighboring residue upstream of C259 affected editing, we constructed a series of pCox II derivatives in which the wild-type 5′ T258 residue was changed to A, G, or C in mutants Sla, Slb, and Slc, respectively. Analysis of mutant transcripts revealed that editing of C259 was unaffected when the 5′ neighboring residue was an A, G, or C. By contrast, the editing efficiency was reduced when the 5′ nucleotide was a G (Fig. 3). These results indicate that the 5′ neighboring residue may affect editing but does not play a critical role in it.
FIG. 3.
Point mutations. Single mutations at the 5′ (S1a to S1c) and 3′ (S2a to S2c) nearest neighboring residues are indicated by arrows. WT, wild-type C259 adjacent sequence. +, occurrence of editing as found in wild-type construct; −, absence of nonencoded U residue; +/−, reduced editing compared to the wild type.
Modifications of 3′ neighbor residues dramatically affect editing.
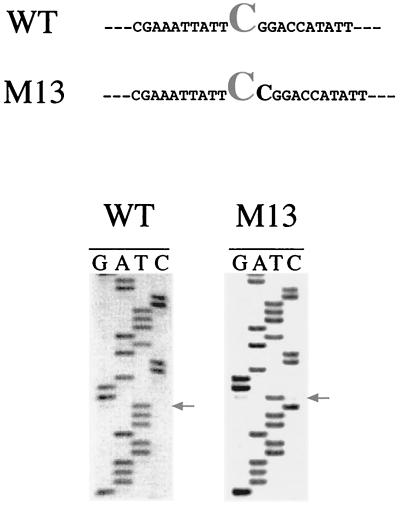
When a C, T, or A replaced the nearest neighbor 3′ G residue, editing of C259 was completely abolished (Fig. 3). Since residues located 3′ from the C editing target had a dramatic effect on the editing process, we decided to increase by 1 nt the distance from the downstream cis elements. We inserted an additional C residue at this motif immediately downstream of the target cytidine. Unexpectedly, the target site was not edited, but the inserted C260 residue was efficiently converted to U (Fig. 4). This suggests that the 3′ recognition element might be involved in determining the position of the target C.
FIG. 4.
C259 3′ insertion mutant. The wild-type C259 sequence (WT) and the mutant bearing a 3′ C insertion (M13) are shown. The sequence analyses of the respective transcripts (RT-PCR products) are shown in the lower panels. Arrows show the U residue generated by editing.
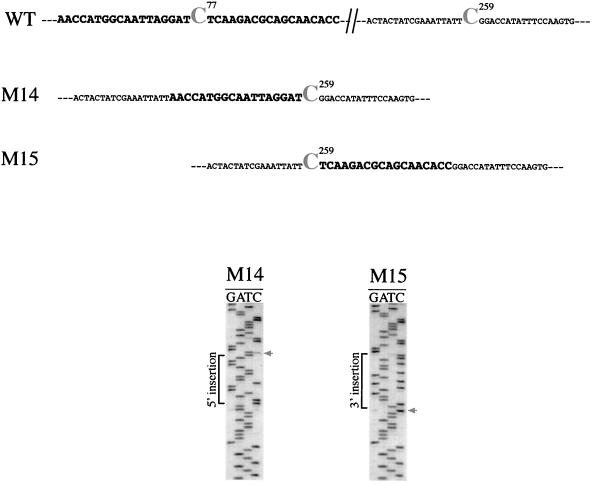
Replacing 5′ and 3′ cis elements of the C259 editing site.
To examine whether the cis elements are specific for each editing site, chimeric plasmids between sites C77 and C259 were constructed. Plasmids M14 and M15, contained 18 nt upstream and downstream from site C77, respectively, were inserted at the corresponding positions of C259. As shown in Fig. 5, the chimeric C77-C259 site from the M14 transcripts was faithfully edited. By contrast, the chimeric C259-C77 site from the M15 transcripts was not edited. These results indicate that 5′ but not 3′ sequences may be interchangeable between different editing sites. It should be noted that the negative effect of the downstream sequence might reflect the fact that the 3′ residue is a T, the nucleotide that strongly inhibited editing in 3′ single-mutant experiments (see above). Therefore, an appropriate combination of upstream and downstream sequences may be required for efficient editing.
FIG. 5.
Exchange of 5′ and 3′ regions between two different editing sites. Eighteen nucleotides from the 5′ or the 3′ region of the C77 site were inserted immediately upstream (M14) or downstream (M15) of C259. Insertions are indicated in bold. The sequence analyses of the respective transcripts (RT-PCR products) are shown in the respective panels. The arrows show the editing targets.
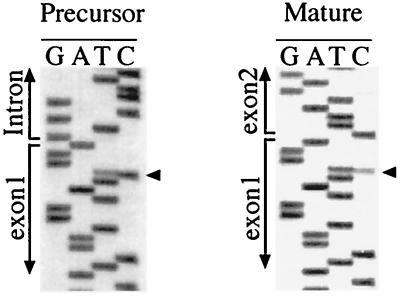
C385 is edited in precursor and spliced transcripts.
The editing site C385, located three nucleotides before the end of exon 1, constitutes an interesting model for analysis. It can be found in two natural 3′ environments. In precursors, the downstream sequence is GGAGTT, and in the spliced transcript, it is GGACTG; these sequences differ by two residues (underlined). Residue C385 was edited in both precursor and mature transcripts (Fig. 6).
FIG. 6.
Editing status of C385 in precursor and spliced wild-type cox II transcripts. The editing status of site C385 was analyzed by sequencing the RT-PCR products generated after electroporation of pCox II. Arrowheads indicate the edited residue. The exon 1-intron and the exon 1-exon 2 junctions are shown.
DISCUSSION
Most, if not all, information regarding cis recognition has been obtained to date from in vivo analysis of steady-state transcripts in different plant models or in the same plant under different conditions, but nothing is known about the recognition process of editing target sequences. To address this issue, we used a model employing the expression of exogenous DNA constructs introduced by electroporation into isolated mitochondria (11). This concept allows the use of a mutational approach to gain information on the sequence domains involved in RNA editing-site recognition. Moreover, the choice of the intron-containing cox II gene makes it possible to focus the analysis on either the precursor or the mature transcripts.
When comparing the nucleotide preference in the vicinity of different editing sites to the same position of unedited C residues in the total RNA population of Arabidopsis mitochondria, no evident consensus sequence is observed (12). Most of the residues located at the −1 position in different editing sites of wheat cox II transcripts were T, and a few were A, thus suggesting a strong bias in the 5′ neighbor nucleotide. By contrast, at the +1 position, no residue preference was evident. From this observation one might conclude that a specific nucleotide is selected at the 5′ position for editing. However, our experimental results obtained with point mutations 5′ and 3′ of C259 invalidate such a conclusion. The bias observed probably does not reflect constraints generated by the RNA editing process but rather the requirements necessary for other events, such as the correct translation of an active protein.
Deletion and point mutation analyses of RNA editing have been reported for stable transformed transgenic chloroplasts (5, 7, 8, 15). For plant mitochondria, such an approach is not possible. Some authors have defined the cis determinant motif for editing-site recognition by comparing the editing status of the transcripts of mitochondrial chimeric genes with that of their normal counterparts (14, 22, 32, 37). Kubo and Kadowaki (20), analyzing the normal and chimeric atp6 gene sequences in rice mitochondria, reported that the 5′ sequence adjacent to the editing site contains cis information required for RNA editing, whereas the 3′ flanking sequence contributes little to editing-site recognition. Using an analogous approach, Mulligan et al. (30) recently reported a similar conclusion when analyzing the editing status in transcripts of the ribosomal protein S12 (rps 12) and in transcripts of a second copy created by recombination very near the editing sites.
Our results confirm, in part, these observations; the mutants with deletions up to 10 nt upstream of the target site were defective in editing. However, these results clearly indicate that important information for RNA editing-site recognition in plant mitochondria resides immediately downstream of the target C in a range of 6 nt. It is noteworthy that the region required for RNA editing described here is very close to that described for a chloroplastic psbL site in transplastomic tobacco, which encompasses 16 nt upstream and 5 nt downstream of the target C (8).
The role of nucleotides flanking the editing site was assessed by mutating the C259 nearest-neighbor residues. A change in the +1 G dramatically abolished editing. By contrast, no changes were observed by changing the −1 T residue to an A or C. However, editing was less efficient when the −1 residue was G (mutant S1b). This situation is different from that described for chloroplast psbL (8) sites, where a change in the 5′ neighboring A into a C residue completely abolishes editing and where the 3′ neighboring residue is not essential but affects efficiency. By contrast, Bock et al. (4) found that changing the 5′ neighboring T residue into a G reduces the efficiency of site V in transplastomic ndhB transcripts. The latter finding is similar to that from the result we obtained with mutant S1b.
The importance of the 3′ region is strongly supported by the results obtained with a mutant containing an extra C residue downstream of site C259, suggesting that the cis determinant located 3′ from the editing site might play a role in determining the choice of target C. This situation is different from that described for chloroplast ndhB, in which the identity of the editing site (site V) is defined by its distance from an essential upstream sequence element (15).
An important point is raised by the results obtained with mutants in which the 5′ or the 3′ region of site C77 replaces the corresponding region of site C259. The C77 5′ sequence can efficiently replace the C259 5′ region in spite of the complete divergence in sequence. By contrast, the C77 3′ region is unable to replace the C259 downstream region. This strongly suggests that a specific recognition site is located 3′ from the C259 editing site. However, we cannot exclude the possibility that the recognition elements of site C77 reside essentially in the 5′ region without a significant contribution of 3′ sequences, as described for the chloroplast ndhB editing site (4, 17). Further analysis of the C77 editing site is required to clarify these findings. It should be noted that this region appears able to tolerate small variations, since site C385 was edited in two slightly different downstream hexanucleotides. Taken together, these results indicate that the different editing sites found in organellar transcripts may represent some variations on the fine architecture of the editing and that these variations should be considered when establishing a general model of editing-site recognition.
Our results provide the first experimental evidence that recognition of the C editing target residue is defined by neighbor sequences in plant mitochondrial transcripts. An upstream 16-nt sequence is necessary for editing, and 6 residues located immediately downstream constitute an essential element for positioning the target C. An important result of this study is the discovery of the modular nature of the editing recognition elements. Indeed, upstream elements may be replaced by sequences required for a different editing site, suggesting that plant mitochondria possess an editing mechanism in which catalytic deaminase activity acts on all editing residues and specific factors are responsible for recognition of each particular site. This situation is reminiscent of that described for apolipoprotein B, where the deaminase (apobec-1) requires an additional protein cofactor for activity. These cofactors have the ability to bind both the catalytic protein and the RNA substrate (23, 28, 29). The results presented here and those described for chloroplast RNA editing-site recognition elements (4, 8, 15, 17) strongly support the common origin of plant organellar editing machinery and suggest that the latter might share some general elements necessary for editing.
ACKNOWLEDGMENTS
We thank Simon Litvak and Dominique Bégu for helpful discussions and critical reading of the manuscript and Evelyne Sargos and Beata Matusiak for technical assistance.
This research was supported by the French Ministère de l'Enseignement Supérieur et de la Recherche, the Université Victor Segalen Bordeaux 2, the French Ministére de l'Agriculture et de la Pêche, the Pôle Génie Biologique et Medical Aquitaine, and ECOS (France)-CONICYT (Chile) cooperation program grant C98B01.
REFERENCES
- 1.Bégu D, Graves P V, Domec C, Arselin G, Litvak S, Araya A. RNA editing of wheat mitochondrial ATP synthase subunit 9: direct protein and cDNA sequencing. Plant Cell. 1990;2:1283–1290. doi: 10.1105/tpc.2.12.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benne R, Van den Burg J, Brakenhoff J P, Sloof P, Van Boom J H, Tromp M C. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell. 1986;46:819–826. doi: 10.1016/0092-8674(86)90063-2. [DOI] [PubMed] [Google Scholar]
- 3.Blanc V, Litvak S, Araya A. RNA editing in wheat mitochondria proceeds by a deamination mechanism. FEBS Lett. 1995;373:56–60. doi: 10.1016/0014-5793(95)00991-h. [DOI] [PubMed] [Google Scholar]
- 4.Bock R, Hermann M, Kössel H. In vivo dissection of cis-acting determinants for plastid RNA editing. EMBO J. 1996;15:5052–5059. [PMC free article] [PubMed] [Google Scholar]
- 5.Bock R, Hermann M, Fuchs M. Identification of critical nucleotide positions for plastid RNA editing-site recognition. RNA. 1997;3:1194–1200. [PMC free article] [PubMed] [Google Scholar]
- 6.Brennicke A, Marchfelder A, Binder S. RNA editing. FEMS Microbiol Rev. 1999;23:297–316. doi: 10.1111/j.1574-6976.1999.tb00401.x. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhuri S, Carrer H, Maliga P. Site-specific factor involved in the editing of the psbL mRNA in tobacco plastids. EMBO J. 1995;14:2951–2957. doi: 10.1002/j.1460-2075.1995.tb07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhuri S, Maliga P. Sequences directing C to U editing of the plastid psbL mRNA are located within a 22 nucleotide segment spanning the editing site. EMBO J. 1996;15:5958–5964. [PMC free article] [PubMed] [Google Scholar]
- 9.Covello P S, Gray M W. RNA editing in plant mitochondria. Nature. 1989;341:662–666. doi: 10.1038/341662a0. [DOI] [PubMed] [Google Scholar]
- 10.Covello P S, Gray M W. Differences in editing at homologous sites in messenger RNAs from angiosperm mitochondria. Nucleic Acids Res. 1990;18:5189–5196. doi: 10.1093/nar/18.17.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farré J C, Araya A. Gene expression in isolated plant mitochondria: high fidelity of transcription, splicing and editing of a transgene product in electroporated organelles. Nucleic Acids Res. 2001;29:2484–2491. doi: 10.1093/nar/29.12.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giege P, Brennicke A. RNA editing in Arabidopsis mitochondria effects 441 C to U changes in ORFs. Proc Natl Acad Sci USA. 1999;96:15324–15329. doi: 10.1073/pnas.96.26.15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gualberto J M, Lamattina L, Bonnard G, Weil J H, Grienenberger J M. RNA editing in wheat mitochondria results in the conservation of protein sequences. Nature. 1989;341:660–662. doi: 10.1038/341660a0. [DOI] [PubMed] [Google Scholar]
- 14.Gualberto J M, Bonnard G, Lamattina L, Grienenberger J M. Expression of the wheat mitochondrial nad3-rps12 transcription unit: correlation between editing and mRNA maturation. EMBO J. 1991;3:1109–1120. doi: 10.1105/tpc.3.10.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermann M, Bock R. Transfer of plastid RNA-editing activity to novel sites suggests a critical role for spacing in editing-site recognition. Proc Natl Acad Sci USA. 1999;96:4856–4861. doi: 10.1073/pnas.96.9.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiesel R, Wissinger B, Schuster W, Brennicke A. RNA editing in plant mitochondria. Science. 1989;246:1632–1634. doi: 10.1126/science.2480644. [DOI] [PubMed] [Google Scholar]
- 17.Hirose T, Sugiura M. Involvement of a site-specific trans-acting factor and a common RNA-binding protein in the editing of chloroplast mRNAs: development of a chloroplast in vitro RNA editing system. EMBO J. 2001;20:1144–1152. doi: 10.1093/emboj/20.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoch B, Maier R M, Appel K, Igloi G L, Kössel H. Editing of a chloroplast mRNA by creation of an initiation codon. Nature. 1991;353:178–180. doi: 10.1038/353178a0. [DOI] [PubMed] [Google Scholar]
- 19.Johnston F B, Stern H. Mass isolation of viable wheat embryos. Nature. 1957;179:160–161. doi: 10.1038/179160b0. [DOI] [PubMed] [Google Scholar]
- 20.Kubo N, Kadowaki K. Involvement of 5′ flanking sequence for specifying RNA editing sites in plant mitochondria. FEBS Lett. 1997;413:40–44. doi: 10.1016/s0014-5793(97)00873-9. [DOI] [PubMed] [Google Scholar]
- 21.Kudla J, Igloi G L, Metzlaff M, Hagemann R, Kössel H. RNA editing in tobacco chloroplasts leads to the formation of a translatable psbL mRNA by a C to U substitution within the initiation codon. EMBO J. 1992;11:1099–1103. doi: 10.1002/j.1460-2075.1992.tb05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar R, Levings C S., III RNA editing of a chimeric maize mitochondrial gene transcript is sequence specific. Curr Genet. 1993;23:154–159. doi: 10.1007/BF00352015. [DOI] [PubMed] [Google Scholar]
- 23.Lau P P, Zhu H J, Nakamuta M, Chan L. Cloning of an apobec-1-binding protein that also interacts with apolipoprotein B mRNA and evidence for its involvement in RNA editing. J Biol Chem. 1997;272:1452–1455. doi: 10.1074/jbc.272.3.1452. [DOI] [PubMed] [Google Scholar]
- 24.Leaver C J, Hack E, Forde B G. Protein synthesis by isolated plant mitochondria. Methods Enzymol. 1983;97:476–484. doi: 10.1016/0076-6879(83)97156-2. [DOI] [PubMed] [Google Scholar]
- 25.Mahendran R, Spottswood M R, Miller D L. RNA editing by cytidine insertion in mitochondria of Physarum polycephalum. Nature. 1991;349:434–438. doi: 10.1038/349434a0. [DOI] [PubMed] [Google Scholar]
- 26.Marchfelder A, Brennicke A, Binder S. RNA editing is required for efficient excision of tRNA(Phe) from precursors in plant mitochondria. J Biol Chem. 1996;271:1898–1903. doi: 10.1074/jbc.271.4.1898. [DOI] [PubMed] [Google Scholar]
- 27.Maréchal-Drouard L, Cosset A, Remacle C, Ramamonjisoa D, Dietrich A. A single editing event is a prerequisite for efficient processing of potato mitochondrial phenylalanine tRNA. Mol Cell Biol. 1996;16:3504–3510. doi: 10.1128/mcb.16.7.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta A, Driscoll D M. A sequence-specific RNA-binding protein complements apobec-1 to edit apolipoprotein B mRNA. Mol Cell Biol. 1998;18:4426–4432. doi: 10.1128/mcb.18.8.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta A, Kinter M T, Sherman N E, Driscoll D M. Molecular cloning of apobec-1 complementation factor, a novel RNA-binding protein involved in the editing of apolipoprotein B mRNA. Mol Cell Biol. 2000;20:1846–1854. doi: 10.1128/mcb.20.5.1846-1854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulligan R M, Williams M A, Shanahan M T. RNA editing-site recognition in higher plant mitochondria. J Hered. 1999;90:338–344. doi: 10.1093/jhered/90.3.338. [DOI] [PubMed] [Google Scholar]
- 31.Niswender C M. Recent advances in mammalian RNA editing. Cell Mol Life Sci. 1998;54:946–964. doi: 10.1007/s000180050225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nivison H T, Sutton C A, Wilson R K, Hanson M R. Sequencing, processing, and localization of the petunia CMS-associated mitochondrial protein. Plant J. 1994;5:613–623. doi: 10.1111/j.1365-313x.1994.00613.x. [DOI] [PubMed] [Google Scholar]
- 33.Ricard B, Lejeune B, Araya A. Studies on wheat mitochondrial DNA organization. Comparison of mitochondrial DNA from normal and cytoplasmic male sterile varieties of wheat. Plant Sci. 1986;43:141–149. [Google Scholar]
- 34.Saalaoui E, Litvak S, Araya A. The apocytochrome b from an alloplasmic line of wheat (T. aestivum, cytoplasm-T. timopheevi) exists in two differently expressed forms. Plant Sci. 1990;66:237–246. [Google Scholar]
- 35.Sutton C A, Zoubenko O V, Hanson M R, Maliga P. A plant mitochondrial sequence transcribed in transgenic tobacco chloroplasts is not edited. Mol Cell Biol. 1995;15:1377–1381. doi: 10.1128/mcb.15.3.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vidal S, Curran J, Kolakofsky D. A stuttering model for paramyxovirus P mRNA editing. EMBO J. 1990;9:2017–2022. doi: 10.1002/j.1460-2075.1990.tb08330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams M A, Kutcher B M, Mulligan R M. Editing-site recognition in plant mitochondria: the importance of 5′-flanking sequences. Plant Mol Biol. 1998;36:229–237. doi: 10.1023/a:1005961718612. [DOI] [PubMed] [Google Scholar]
- 38.Yu W, Schuster W. Evidence for a site-specific cytidine deamination reaction involved in C to U RNA editing of plant mitochondria. J Biol Chem. 1995;270:18227–18233. doi: 10.1074/jbc.270.31.18227. [DOI] [PubMed] [Google Scholar]
- 39.Zeltz P, Kadowaki K, Kubo N, Maier R M, Hirai A, Kössel H. A promiscuous chloroplast DNA fragment is transcribed in plant mitochondria but the encoded RNA is not edited. Plant Mol Biol. 1996;31:647–656. doi: 10.1007/BF00042236. [DOI] [PubMed] [Google Scholar]