Abstract
Cell transplantation therapy using human induced pluripotent stem cell-derived neural stem/progenitor cells (hiPSC-NS/PCs) is a new therapeutic strategy for spinal cord injury (SCI). Preclinical studies have demonstrated the efficacy of hiPSC-NS/PCs transplantation in the subacute phase of SCI. However, locomotor recovery secondary to hiPSC-NS/PCs transplantation is limited in the chronic phase, suggesting that additional treatment, including rehabilitative training, is required to ensure recovery. The therapeutic potential of hiPSC-NS/PCs that qualify for clinical application is yet to be fully delineated. Therefore, in this study, we investigated the therapeutic effect of the combined therapy of clinical-grade hiPSC-NS/PCs transplantation and rehabilitative training that could produce synergistic effects in a rodent model of chronic SCI. Our findings indicated that rehabilitative training promoted the survival rate and neuronal differentiation of transplanted hiPSC-NS/PCs. The combination therapy was able to enhance the expressions of the BDNF and NT-3 proteins in the spinal cord tissue. Moreover, rehabilitation promoted neuronal activity and increased 5-HT-positive fibers at the lumbar enlargement. Consequently, the combination therapy significantly improved motor functions. The findings of this study suggest that the combined therapy of hiPSC-NS/PCs transplantation and rehabilitative training has the potential to promote functional recovery even when initiated during chronic SCI.
Keywords: chronic spinal cord injury, human induced pluripotent stem cell-derived neural stem/progenitor cells, cell transplantation, rehabilitative training, combination treatment
Graphical Abstract
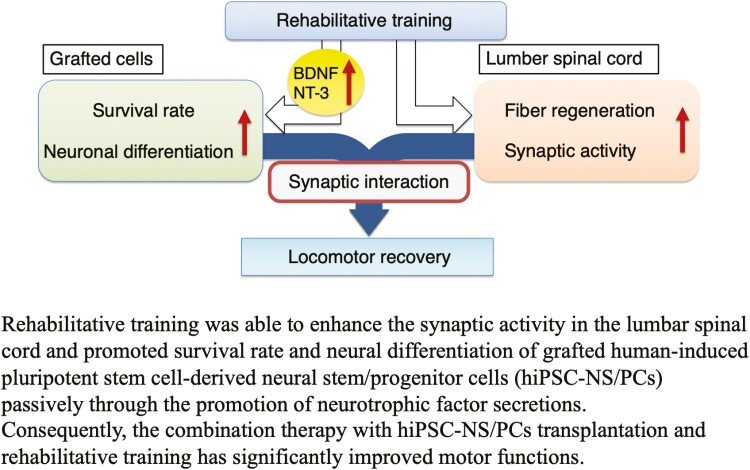
Graphical Abstract.
Significance Statement.
This study demonstrates the efficacy of the combination therapy of human induced pluripotent stem cell-derived neural stem/progenitor cells (hiPSC-NS/PCs) transplantation and rehabilitative training in chronic spinal cord injury (SCI). Rehabilitative training was able to enhance the survival and neuronal differentiation of transplanted hiPSC-NS/PCs, significantly improving motor function. These findings indicate that a therapeutic strategy that combines hiPSC-NS/PCs transplantation and rehabilitation could form the basis of regenerative therapy in cases of chronic SCI.
Introduction
Spinal cord injury (SCI) can cause severe damage to the central nervous system and can lead to motor paralysis or paresthesia. Fundamental and efficient treatment strategies for functional disabilities, such as paralysis, have yet to be established. Over the past two decades, cell transplantation therapy has emerged as a new treatment modality1-3. Recently, transplantation therapy using human induced pluripotent stem cell-derived neural stem/progenitor cells (hiPSC-NS/PCs) has gained significant attention due to the feasibility of genetic manipulation and mass production without ethical issues4. Transplantation of hiPSC-NS/PCs in the subacute phase of SCI has been reported to produce functional recovery via regeneration of some of the lost nerve connections, promoting remyelination, and by secreting trophic factors, as reported in conventional neural stem cells4-8. However, limited functional recovery was observed when hiPSC-NS/PCs were transplanted at the chronic phase, which is important because most SCI patients have chronic injuries9,10. It is essential, therefore, to explore therapeutic strategies that combine cell processing optimization with other therapies11.
Concerning optimizing cell processing, hiPSC-NS/PCs pretreated with a gamma-secretase inhibitor (GSI) that activates p38 MAPK to promote axonal regrowth showed remarkable functional recovery compared to controls in a cell line that has been used in subacute SCI12. Based on this finding, the first clinical trial of hiPSC-NS/PCs transplantation in subacute SCI patients involved a GSI pretreatment process13. While GSI-pretreated hiPSC-NS/PCs exerted specific effects, including axonal regrowth and remyelination at the chronically injured spinal cord14, the improvement in motor function was limited in the animal model.
Rehabilitative training is a therapy that can be performed without ethical constraints in human clinical practice. However, only a few preclinical studies have reported its usage in combination with cell transplantation therapy15, despite being suggested in numerous clinical studies. Furthermore, the combined therapy of rehabilitation with NS/PCs transplantation in the chronic phase has been examined in only one study16. Although Tashiro et al showed that combined treatment was significantly more effective for functional recovery than non-treatment, they could not demonstrate the superiority of the combination therapy over the single therapies. The mechanism by which combined treatment improves motor function after SCI, regardless of the phase, is yet to be fully elucidated17. Furthermore, there is currently no research that investigates the combined effect of rehabilitative training with any iPS cell-derived cell lines, which have attracted wide attention for their application in clinical practice.
The validation and optimization of the rehabilitative training carried out in preclinical investigations are crucial in terms of quality, quantity, feasibility, and reproducibility15. Recently, Shibata et al presented a treadmill training protocol based on the overload principle for chronic SCI mice and demonstrated improved gait performance with rehabilitative training alone18. They demonstrated that optimized rehabilitative training could improve motor function even when the intervention was initiated in the chronic phase. Although training alone did not induce sufficient functional recovery, further improvement would be expected when training is applied in combination with regenerative treatment.
No previous reports have investigated the combined use of validated rehabilitative training and GSI-pretreated or iPSC-derived cells transplantation. Furthermore, the mechanisms of the combination treatment initiated from the chronic phase are yet to be elucidated. Against this background, this study was conducted to clarify the combined effect of optimized cell transplantation and rehabilitative training. To this end, we transplanted GSI-pretreated hiPSC-NS/PCs, which are used in clinical practice13, into chronic phase SCI mice and compared the differences in therapeutic effect and histology with and without rehabilitative training intervention.
Materials and Methods
For further details see the Supplementary Material.
SCI Animal Model
Female NOD-SCID mice (8 weeks old, 16-20 g; Charles River Laboratories, Kanagawa, Japan) were used in this study. All experimental procedures were approved by the Experimental Animal Care Committee of KEIO University, School of Medicine (assurance no. 13020) and by the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD). Detailed methods are provided in the Supplementary Material.
Culture of hiPSC-NS/PCs
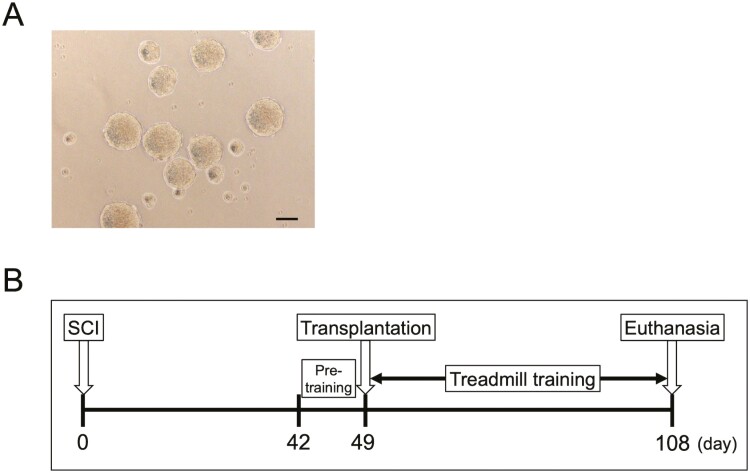
The YZWJs513 hiPSC line was used throughout this study. NS/PCs were prepared at a good manufacturing practice-grade cell processing facility at Osaka National Hospital, Japan. The clinical-grade human leukocyte antigen super-donor-derived, integration-free hiPSC line, YZWJs513, established by the iPSC Stock Project and organized by the Kyoto University Center for iPS Cell Research and Application, was used. The cells were cultured for 4 days in a floating culture, and the neurospheres were used for transplantation (Fig. 1A). Cells were treated with small-molecule GSI and N-[N-(3,5-difluorophenacetyl)-l-ananyl]-S-phenylglycine t-butyl ester (DAPT) (10 μM, Sigma-Aldrich, Inc., D5942) for 1 day before transplantation, as described in a previous study13. Detailed methods are provided in the Supplementary Material.
Figure 1.
(A) Micrographs showing representative hiPSC-NS/PCs aggregates. Scale bar = 200 μm. (B) Schematic representation of the schedule of the whole experiment. All mice were induced with contusive SCI at Th10 and subjected to pre-training from 42 to 48 DPI. hiPSC-NS/PCs were transplanted into the injured spinal cord at 49 DPI, and treadmill training was conducted from 52 to 108 DPI in the TP + TMT group.
Experimental Design
Mice were randomly assigned to either the combination therapy with cell transplantation and treadmill training group (TP + TMT group, n = 19) or the cell transplantation single therapy group (TP group, n = 17). Mice with a Basso Mouse Scale (BMS) score of 4 or above at 42 days post-injury (DPI) were excluded from this study. All mice were subjected to pre-training for conditioning from 42 to 46 DPI (constant treadmill speed; 4 m/minute, 20 minutes/day). At 49 DPI, mice were transplanted with a total of 5.0 × 105/2 μL cells into 2 points on the rostral and caudal sides of the injured epicenter using a Hamilton syringe (87931, Hamilton, Reno, NV, USA) that used a 28G metal needle and a stereotaxic microinjector (KDS310, Muromachi-Kikai Co., Ltd., Tokyo, Japan). Mice in the TP + TMT group were subjected to treadmill training as rehabilitation therapy from 52 to 108 DPI (Fig. 1B). The treadmill training was performed individually for 20 min per day for 5 days per week. Training intensity was progressively increased according to the exercise tolerance of each individual mouse following the original training protocol described previously18. Details of the treadmill training method are provided in the Supplementary Material.
This study was conducted in 4 series in stages, with a total of 10 mice per series, including animals excluded at 42 DPI. Most of the animals that completed the experimental process were assigned for histological analyses (by sagittal section or axial section) or capillary electrophoresis analysis after euthanasia. However, a smaller but sufficient number of animals were used for electrophysiological analysis according to previous studies19,20, because electrical stimuli can affect histology or protein expression features. Details on the number of animals used for each experiment are provided in Supplementary Table S1.
Tissue Processing and Histological Analyses
Histological analyses were performed via H&E staining, picrosirius staining (ScyTek Laboratories, Inc.), and immunohistochemistry. Details of tissue processing and histological analyses are provided in the Supplementary Material.
Immunoelectron Microscopy Analysis
Immunoelectron microscopy analysis was performed on spinal cord sections. The detailed procedure is provided in the Supplementary Material.
Capillary Electrophoresis Analysis
The protein levels of BDNF, NT-3, GDNF, and IGF-1 in the injured epicenter and lumbar enlargement of the spinal cord were assessed at 108 DPI via capillary electrophoresis (004-600-N001; Simple Wes, Protein Simple, San Jose, CA, USA). The detailed methods are provided in the Supplementary Material.
Behavioral Analyses and Evaluation of Muscle Atrophy
The hind limb locomotor function was evaluated weekly for up to 15 weeks post-injury using BMS scores on the open field21. Body weight was also measured weekly up to 15 weeks post-injury. At 108 DPI, the rotarod test, quadrupedal gait analysis, and kinematics analysis were performed. The detailed methods are provided in the Supplementary Material.
Electrophysiology
The motor-evoked potential (MEP) was recorded using a Neuropack S1 MEB9402 signal processor (Nihon Kohden, Tokyo, Japan) at 108 DPI. The detailed methods are described in the Supplementary Material.
Quantification and Statistical Analyses
Statistical analysis of kinematics data was performed using R software (version 4.0.2, Foundation for Statistical Computing, Vienna, Austria), and the overall data similarity between the samples was visualized using Rtsne (version 0.15). For t-distributed stochastic neighbor embedding (t-SNE) analysis, 43 parameters (Supplementary Table S2) were computed as previously reported22. The other statistical analyses were performed using SPSS software (version 26.0.0.0; Japan IBM, Tokyo, Japan). All data were presented as the mean ± SEM. Statistical significance was defined at *P < .05, **P < .01, and ***P < .005. The Mann-Whitney U test was used for comparison between the 2 groups. BMS scores and body weight changes were analyzed with repeated-measures 2-way ANOVA, and the scores at each time point were compared using 2-tailed Student’s t test.
Results
Rehabilitative Training Enhanced the Survival of Grafted hiPSC-NS/PCs
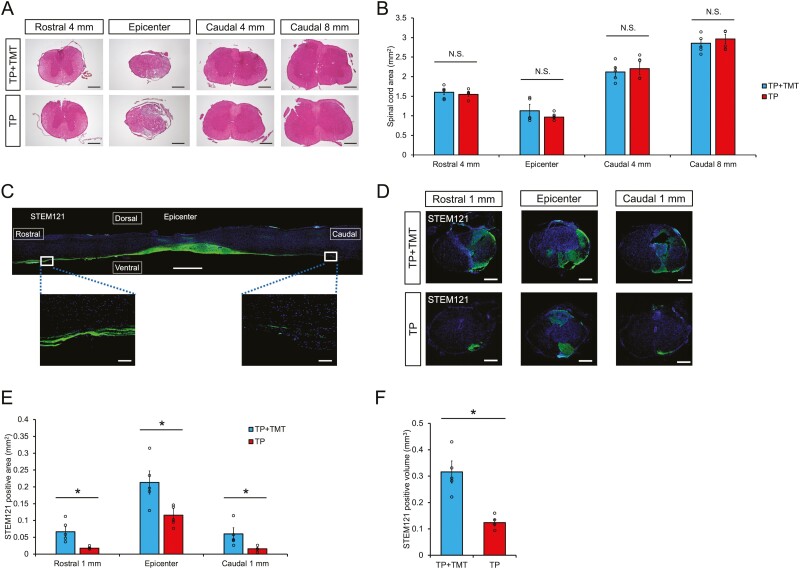
The cross-sectional areas of the chronically injured spinal cords were compared at 108 DPI using H&E staining (Fig. 2A). Quantitative analysis revealed no significant differences between the TP + TMT and TP groups at all measured sites (Fig. 2B). These results indicated that the combined therapy of hiPSC-NS/PCs transplantation and rehabilitative training had no impact on the retention of spinal cord tissue in the chronic phase.
Figure 2.
(A) Representative H&E-stained images of axial sections at the lesion epicenter and at the sites located 4 mm rostral and 4 or 8 mm caudal in each group. Scale bars = 300 μm. (B) Quantitative analysis of the spinal cord area measured in H&E-stained axial sections at each level. No significant differences were noted between the 2 groups at all measured sites. n = 5 each. (C) Representative image of a sagittal section stained for STEM121 in the TP + TMT group. STEM121 + grafted cells were elongated from the rostral and caudal sites. Scale bars, 3000 and 100 μm (enlarged images). (D) Representative images of axial sections stained for STEM121 at the lesion epicenter and 1 mm rostral and caudal sites in each group. Scale bars = 300 μm. (E) Quantitative analysis of STEM121 + area in axial sections at each level. STEM121 + areas were significantly increased in the TP + TMT group compared to the TP group at all measured sites. n = 5 each. (F) Quantitative analysis of STEM121 + volume. STEM121 + volume was observed to be significantly larger in the TP + TMT group than in the TP group. n = 5 each. *P < .05 according to Mann-Whitney U test. Data are presented as the mean ± SEM.
To evaluate the effect of combined therapy on scar volume, we compared the volumes of picrosirius red-stained collagen (fibrous scar tissue) in the 2 groups at 108 DPI (Supplementary Fig. S1A). Quantitative analysis revealed that there were no significant differences between the TP + TMT and TP groups in this immunodeficient strain (Supplementary Fig. S1B). This result indicated that the combination therapy had no effect on the fibrous scar tissue volume once the scar tissue had formed.
STEM121 immunostaining was used on the sagittal spinal section of the TP + TMT group to assess the survival of grafted cells (Fig. 2C). The results showed that grafted hiPSC-NS/PCs migrated rostrally and caudally from the epicenter and persisted in the host tissue for more than 8 weeks. Quantitative analysis in the axial section showed that the STEM121-positive area was significantly increased in the TP + TMT group compared to the TP group at the lesion epicenter and 1 mm rostral and 1 mm caudal areas (Fig. 2D, 2E; epicenter: 0.227 mm2 ± 0.047 mm2 vs. 0.116 mm2 ± 0.019 mm2, P < .05; rostral: 0.066 mm2 ± 0.015 mm2 vs. 0.017 mm2 ± 0.002 mm2, P < .05; caudal: 0.060 mm2 ± 0.018 mm2 vs. 0.016 mm2 ± 0.004 mm2, P < .05). The grafted cell volume was also significantly larger in the TP + TMT group compared to that in the TP group (Fig. 2F; 0.316 mm3 ± 0.042 mm3 vs. 0.124 mm3 ± 0.013 mm3, P < .05). Human nuclear antigen (HNA) immunostaining was used to assess the survival rate of transplanted cells (Supplementary Fig. S2A). Quantitative analysis of the survival rate of transplanted cells showed that the survival rate was significantly higher in the TP + TMT group compared to the TP group (Supplementary Fig. S2B; 9.69% ± 0.34% vs. 4.12% ± 0.31%, P < .01). These findings indicated that rehabilitative training enhanced the survival of grafted hiPSC-NS/PCs.
Rehabilitative Training Promoted the Neuronal Differentiation of Grafted hiPSC-NS/PCs into Mature Neurons
First, we analyzed the differentiation phenotype of hiPSC-NS/PCs in vitro. The results showed that most of the hiPSC-NS/PCs were differentiated into neurons, whereas some were differentiated into astrocytes; results that were consistent with those of previous reports (details of the data are provided in Supplementary Fig. S3)12,13.
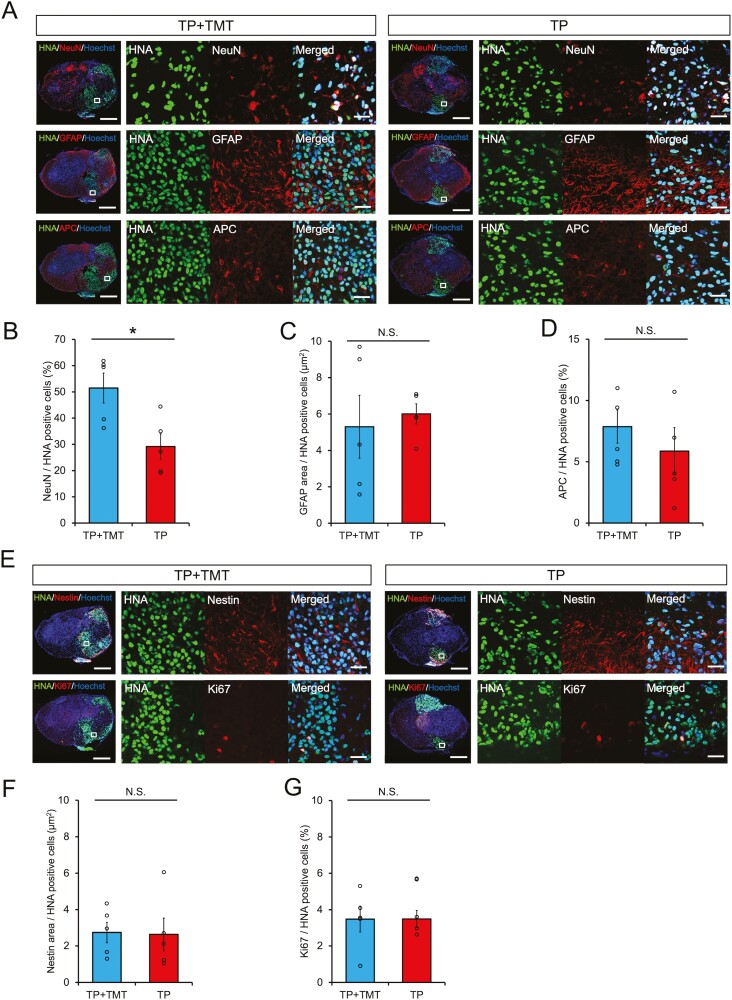
Second, we evaluated their differentiation phenotype in vivo at 108 DPI via immunostaining. Regardless of the experimental group, HNA-positive grafted cells appeared to be differentiated into three neural lineages: ELAVL3/4 (pan-neuronal marker)- or NeuN (mature neuron marker)-positive neurons, GFAP-positive astrocytes, and APC-positive oligodendrocytes (Fig. 3A and Supplementary Fig. S4A). Quantitative analysis revealed that the HNA/NeuN double-positive count was significantly increased in the TP + TMT group compared with the TP group (Fig. 3B; 51.48% ± 5.72% vs. 29.17% ± 4.85%, P < .05). The HNA/ELAVL3/4, HNA/APC double-positive count, and GFAP positive area results showed no significant differences between the 2 groups (Fig. 3C–3D and Supplementary Fig. S4B; ELAVL3/4: 84.60% ± 3.46% vs. 84.51% ± 3.58%, P = .602; GFAP: 5.30 μm2 ± 1.73 μm2 vs. 6.01 μm2 ± 0.55 μm2, P = .754; APC: 7.26% ± 1.26% vs. 5.31% ± 1.63%, P = .251). Immature cells, identified via the neural stem cell marker, Nestin, or the cell proliferation marker, Ki67, remained at low expression rates in both groups, and no significant differences were observed (Fig. 3E–3G; Nestin: 2.74 μm2 ± 0.57 μm2 vs. 2.63 μm2 ± 0.91 μm2, P = .465; Ki67: 3.48% ± 0.72% vs. 3.49% ± 0.47%, P = .917). These results indicated that rehabilitative training promoted neuronal differentiation of the grafted hiPSC-NS/PCs into mature neurons.
Figure 3.
(A) Representative images of neural cells differentiated from graft cells. HNA + grafted cells merged with NeuN (neurons), GFAP (astrocytes), and APC (oligodendrocytes). Scale bars = 300 and 20 μm (enlarged images). The histograms show the proportions of NeuN + cells among HNA + grafted cells (B), GFAP + area per HNA + grafted cells (C), and the proportions of APC + cells among HNA + grafted cells (D). Percentages of NeuN+/HNA + cells were significantly increased in the TP + TMT group compared with the TP group. n = 5 each. (E) Representative images of immature graft cells. HNA + grafted cells merged with Nestin (immature cell) and Ki67 (immature cell). Scale bars, 300 and 20 μm (enlarged images). The histograms show Nestin + area per HNA + grafted cells (F) and the proportions of Ki67 + cells among HNA + grafted cells (G). n = 5 each. *P < .05 according to Mann-Whitney U test. Data are presented as the mean ± SEM.
We also analyzed the characteristics of transplanted cell-derived neurons via immunostaining. The results showed that some of the transplanted neurons expressed glutamatergic or GABAergic markers, while others differentiated into inhibitory interneurons (Supplementary Fig. S5).
Transplanted Cell-derived Neurons Connected to Host Neurons Via Synaptic Formation
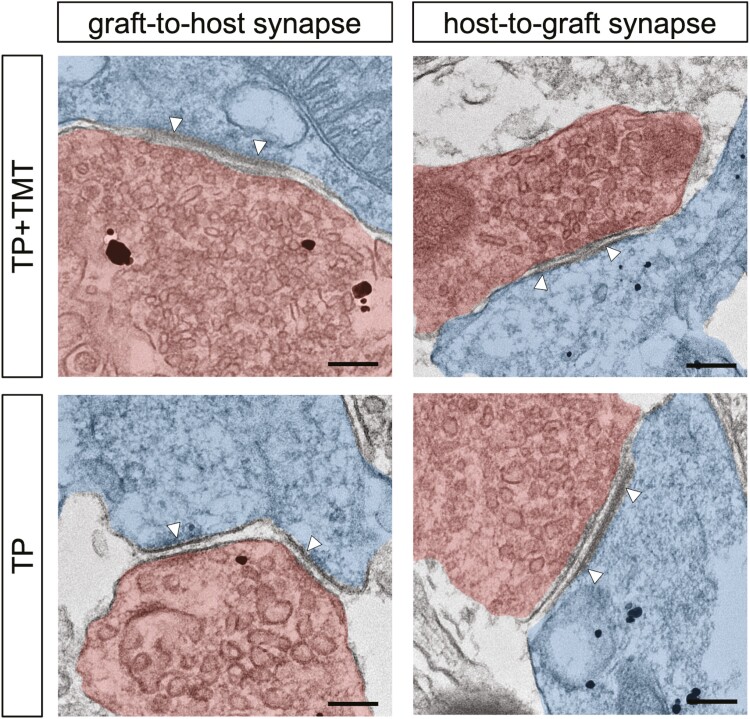
Immunoelectron microscopy labeling with an anti-human cell cytoplasm antibody (STEM121) revealed pre- and post-synaptic structures and synapses between STEM121 + transplanted cell-derived neurons and host mouse neurons in both groups (Fig. 4). These findings demonstrated the formation of reciprocal synaptic connections between the transplanted cell-derived neurons and the host.
Figure 4.
Immunoelectron microscopy image showing graft-to-host and host-to-graft synapse formation (white arrowheads) in the hiPSC-NS/PCs transplanted mouse spinal cords at 108 DPI. Grafted cells were detected by black dots observed upon anti-human cytoplasm antibody (STEM121) staining. STEM121 antibody labeling was observed in both presynaptic neurons (red) and postsynaptic neurons (blue) in both groups. Scale bars = 200 nm.
Combined Therapy of hiPSC-NS/PCs Transplantation and Rehabilitative Training Contributed to Synaptic Activity in the Lumbar Spinal Cord
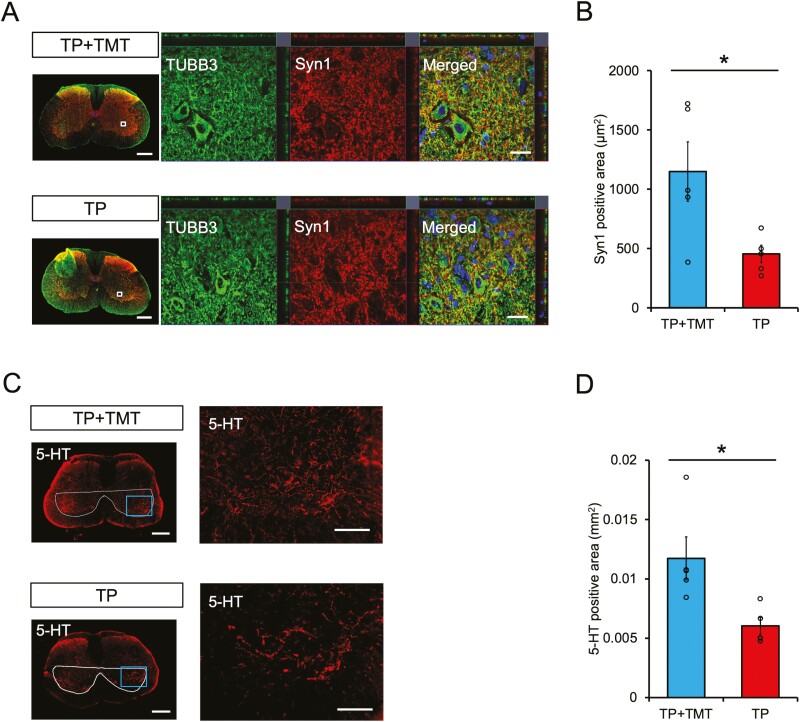
The neural activity in the central pattern generator at the lumbar enlargement, as assessed by the area of Syn1-positive boutons (Fig. 5A), was found to be significantly higher in the TP + TMT group compared to the TP group (Fig. 5B; 1 148.66 μm2 ± 250.37 μm2 vs. 454.26 μm2 ± 73.31 μm2, P < .05).
Figure 5.
(A) Representative axial section images in lumbar enlargement stained for βⅢ-tubulin and Syn1. Enlarged images of the area are shown in the white box. Scale bars = 300 and 20 μm (enlarged images). (B) Quantitative analysis of Syn1 + area in enlarged images of axial sections. Syn1 + areas were significantly larger in the TP+ TMT group than in the TP group. n = 5 each. (C) Representative images of the axial section in lumbar enlargement stained for 5-HT. The ventral horn is surrounded by a white line. Enlarged images of the area are shown in the blue box. Scale bars = 300 and 100 μm (enlarged images). (D) Quantitative analysis of 5-HT + area in the ventral horn of axial sections. 5-HT + areas were significantly larger in the TP + TMT group than in the TP group. n = 5 each. *P < .05 according to Mann-Whitney U test. Data are presented as the mean ± SEM.
Raphespinal tract innervation was evaluated by observation of 5-HT-positive fibers within the ventral horn in regions of lumbar enlargement (Fig. 5C). Quantitative analysis revealed that the 5-HT-positive area was significantly increased in the TP + TMT group compared with the TP group (Fig. 5D; 0.012 ± 0.002 mm2 vs. 0.006 ± 0.001 mm2, P < .05).
Combined Therapy of hiPSC-NS/PCs Transplantation and Rehabilitative Training Enhanced the Expression of Neurotrophic Factors in Injured Spinal Cord
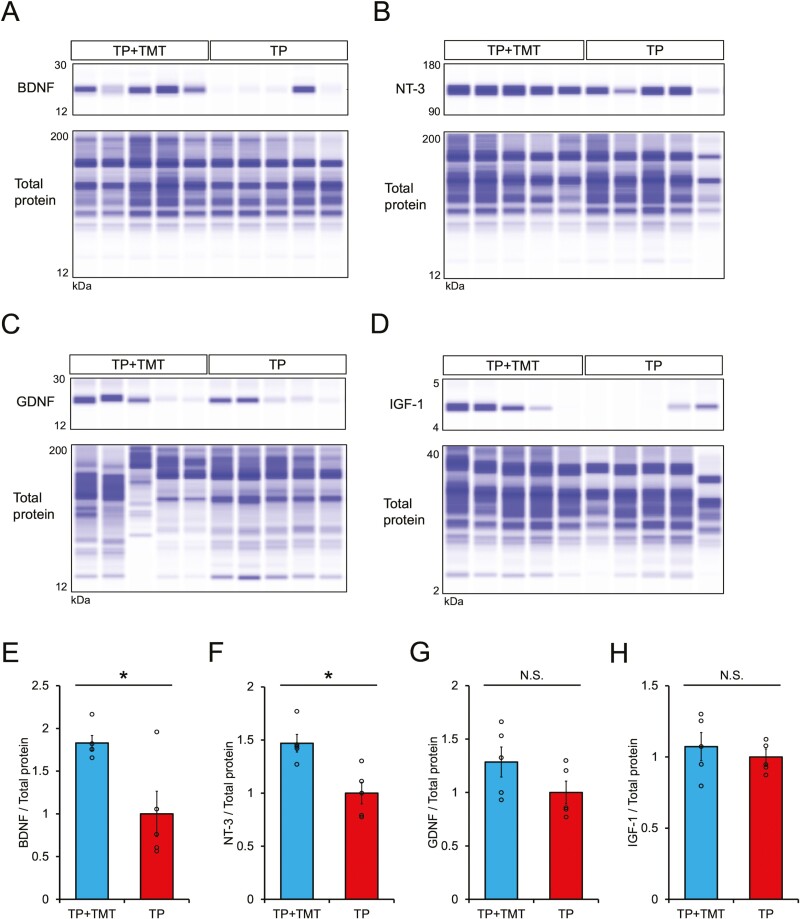
The expression of neurotrophic factors was assessed both in the lesion and in the lumbar enlargement (Fig. 6A–6D and Supplementary Fig. S6A–S6D). Quantitative analyses via capillary electrophoresis showed that the expression levels of the BDNF and NT-3 proteins at the lesion site were significantly higher in the TP + TMT group compared to the TP group (Fig. 6E–6H; BDNF: 1.83 ± 0.09 vs. 1.00 ± 0.27, P < .05; NT-3: 1.47 ± 0.08 vs. 1.00 ± 0.10, P < .05; GDNF: 1.29 ± 0.14 vs. 1.00 ± 0.11, P = .117; IGF-1: 1.07 ± 0.10 vs. 1.00 ± 0.06, P = .347). Similarly, quantitative analyses at the lumbar spinal cord revealed that the expressions of BDNF, NT-3, and IGF-1 were significantly increased in the TP + TMT group compared to the TP group (Supplementary Fig. S6E–S6H; BDNF: 1.82 ± 0.26 vs. 1.00 ± 0.17, P < .05; NT-3: 1.52 ± 0.04 vs. 1.00 ± 0.07, P < .05; GDNF: 1.60 ± 0.20 vs. 1.00 ± 0.15, P = .117; IGF-1: 2.40 ± 0.54 vs. 1.00 ± 0.26, P < .05). These findings suggest that the combined therapy was able to enhance the expression of neurotrophic factors in the spinal cord.
Figure 6.
Digitized protein immunoblots of injured spinal cord tissue for BDNF (A), NT-3 (B), GDNF (C), and IGF-1 (D). n = 5 each. Quantitative analysis of BDNF (E), NT-3 (F), GDNF (G), and IGF-1 (H) relative protein expression levels normalized to the total protein level imaged in (A–D). Expressions of BDNF and NT-3 proteins in the injured spinal cord were enhanced in the TP + TMT group compared to the TP group. n = 5 each. *P < .05 according to Mann-Whitney U test. Data are presented as the mean ± SEM.
Combined Therapy of hiPSC-NS/PCs Transplantation and Rehabilitative Training Promoted Functional Recovery
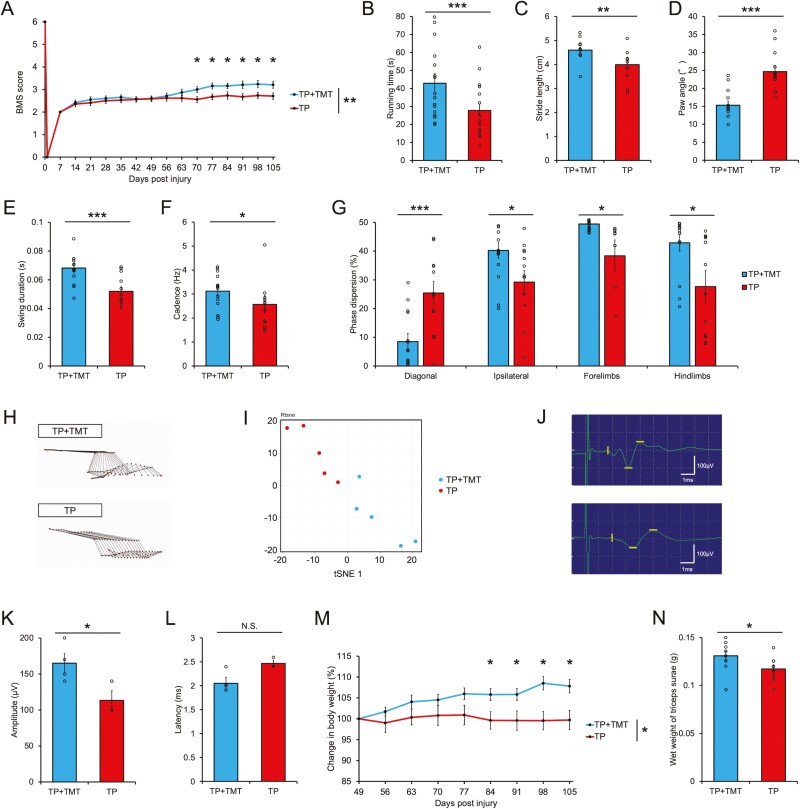
The BMS scores indicated significantly better recovery in the TP + TMT group than in the TP group (Fig. 7A; repeated-measures two-way ANOVA. P < .01; post hoc analyses with two-tailed Student’s t test, at 70 DPI: 3.00 ± 0.13 vs. 2.56 ± 0.13, P < .05; 77 DPI: 3.16 ± 0.13 vs. 2.67 ± 0.12, P < .05; 84 DPI: 3.16 ± 0.12 vs. 2.74 ± 0.16, P < .05; 91 DPI: 3.21 ± 0.16 vs. 2.68 ± 0.14, P < .05; 98 DPI: 3.24 ± 0.16 vs. 2.74 ± 0.14, P < .05; 105 DPI: 3.21 ± 0.15 vs. 2.71 ± 0.14, P < .05) (individual data trends are represented in Supplementary Fig. S7A).
Figure 7.
(A) Serial BMS score assessment of the hind limb motor functions among mice in the TP + TMT and TP groups. The BMS scores were noted to significantly improve in the TP + TMT group compared with the TP group. n = 19 for the TP + TMT group, n = 17 for the TP group. (B) Comparison of rotarod test results between the TP + TMT and TP groups. Running times were significantly longer in the TP + TMT group than in the TP group. n = 19 for the TP + TMT group, n = 17 for the TP group. The hind limb motion during quadrupedal gait was analyzed using the DigiGait system. The histograms show the following parameters: stride length (C), paw angle (D), swing duration (E), and cadence (F). Gait performances were significantly improved in the TP + TMT group compared with the TP group. n = 14 for the TP + TMT group, n = 12 for the TP group. (G) Interlimb coordination was analyzed with phase dispersions of diagonal, ipsilateral, forelimb, and hind limb pairs. Coordination was significantly improved for the TP + TMT group compared to the TP group for all limb pairs. n = 14 for the TP + TMT group, n = 12 for the TP group. H. Representative stick diagrams of hind limb movements in the TP + TMT and TP groups. (I) t-SNE analysis of the kinematic data in the TP + TMT and TP groups. The spatial arrangement of the points in the plot reflects the overall data similarity between the samples. n = 5 each. (J) Representative images of motor-evoked potential (MEP) waves, stimulated at Th5 spinal cord and recorded from the distal quadriceps muscle tendon in the TP + TMT and TP groups. (K) Quantitative analysis of the MEP amplitude in the TP + TMT and TP groups. The amplitude was significantly larger in the TP + TMT group than in the TP group. n = 4 for the TP + TMT group, n = 3 for the TP group. (L) Quantitative analysis of MEP latency in the TP + TMT and TP groups. The latency was shorter in the TP + TMT group than in the TP group, but the difference was not statistically significant. n = 4 for the TP + TMT group, n = 3 for the TP group. (M) Serial percentage change in body weight was calculated by dividing the current body weight by the body weight at 49 DPI in the TP + TMT and TP groups. The percentage change in body weight was significantly increased in the TP + TMT group compared with the TP group. n = 10 for the TP + TMT group, n = 9 for the TP group. (N) Histogram showing the wet weight of the triceps surae of mice in the TP + TMT and TP groups. The wet weight of the triceps surae was significantly greater in the TP + TMT group than in the TP group. n = 10 for the TP + TMT group, n = 9 for the TP group. *P < .05, **P < .01, and ***P < .005 according to repeated-measures 2-way ANOVA, followed by 2-tailed Student’s t tests (A and M) and Mann-Whitney U test (B–L and N). The data are presented as the mean ± SEM.
The gait performance and appearance were assessed using the rotarod test and quadrupedal gait analysis using the DigiGait system at 108 DPI. The rotarod test revealed that the mice in the TP + TMT group continued running for significantly longer than those in the TP group (Fig. 7B; 42.93 s ± 5.32 s vs. 27.78 s ± 3.51 s, P < .005). Quadrupedal gait analysis showed that there was a significantly better improvement in the TP + TMT group than in the TP group in terms of stride length, paw angle, swing duration, and cadence of the hind limb (Fig. 7C–7F; stride length: 4.60 cm ± 0.12 cm vs. 3.99 cm ± 0.18 cm, P < .05; paw angle: 15.30° ± 1.10° vs. 24.57° ± 1.71°, P < .005; swing duration: 0.068 s ± 0.003 s vs. 0.052 s ± 0.003 s, P < .005; cadence: 3.12 Hz ± 0.20 Hz vs. 2.57 Hz ± 0.37 Hz, P < .05) and in stride length and swing duration of the forelimb (Supplementary Fig. S7B–S7E; stride length: 4.12 cm ± 0.15 cm vs. 3.37 cm ± 0.19 cm, P < .01; swing duration: 0.080 s ± 0.003 s vs. 0.053 s ± 0.004 s, P < .005). The other parameters did not significantly differ between the TP + TMT and TP groups (data not shown).
The interlimb coordination, as represented by the phase dispersion (percentage of anchor step cycle) between limbs indicated significant improvement in the TP + TMT group compared to the TP group in all limb pairs (Fig. 7G; diagonal limb pairs: 8.48% ± 2.92% vs. 25.42% ± 4.01%, P < .05; ipsilateral limb pairs: 40.24% ± 2.72% vs. 29.22% ± 4.03%, P < .05; forelimb pairs: 49.46% ± 0.71% vs. 38.36% ± 5.54%, P < .05; hind limb pairs: 42.91% ± 2.93% vs. 27.67% ± 5.68%, P < .05).
Stick diagrams of kinematics analysis during quadrupedal treadmill walking depicted a more coordinated and consistent gait in the TP + TMT group compared to the TP group (Fig. 7H). t-SNE analysis revealed a qualitative difference in the gait motion between the 2 groups (Fig. 7I).
The electrophysiological analysis using the MEP indicated that the spinal conductivity was more recovered in the TP + TMT group compared to the TP group (Fig. 7J); the amplitude was significantly larger (Fig. 7K; 165.00 μV ± 13.23 μV vs. 113.33 μV ± 13.33 μV, P < .05), and the latency was shorter; however, the difference was not statistically significant (Fig. 7L; 2.05 ms ± 0.13 ms vs. 2.47 ms ± 0.07 ms, P = .064).
The recovery of reduced muscle volume as represented by body weight and wet weight of the triceps surae muscle was noted to be greater in the TP + TMT group than in the TP group. The percentage change in body weight following the initiation of intervention was significantly increased in the TP + TMT group compared to the TP group (Fig. 7M, repeated-measures two-way ANOVA. P < .05; post hoc analyses with two-tailed Student’s t test, on day 84: 105.80% ± 1.41% vs. 99.61% ± 2.10%, P < .05; day 91: 105.82% ± 1.40% vs. 99.57% ± 2.31%, P < .05; day 98: 108.52% ± 1.61% vs. 99.56% ± 2.20%, P < .05; day 105: 107.82% ± 1.60% vs. 99.70% ± 2.32%, P < .05) (individual data trends of the percentage change in body weight are represented in Supplementary Fig. S7F and serial raw data for body weight are represented in Supplementary Fig. S7G), and the wet weight of the triceps surae was significantly greater (Fig. 7N; 0.131 g ± 0.005 g vs. 0.117 g ± 0.004 g, P < .05). These findings suggested that the combined therapy recovered muscle mass and spinal conduction resulting in improved hind limb motor function.
Discussion
In this study, we examined the effectiveness of a combination therapy that included clinical-grade GSI-pretreated hiPSC-NS/PCs transplantation and a validated rehabilitation protocol that addressed a central clinical principle (the overload principle) in chronic SCI. The survival and neuronal differentiation of transplanted hiPSC-NS/PCs and the histological efficacy induced by the combination therapy resulted in significant improvement in terms of motor function. Thus, the combination of GSI-pretreated hiPSC-NS/PCs transplantation and regenerative rehabilitation could be effective for even chronic SCI patients as part of a treatment strategy.
In human clinical practice, it has been documented that multi-modal or robot-assisted gait training alone can significantly improve gait and trunk function, even in the chronic phase of SCI23-25. We previously presented a treadmill training protocol that adjusts the intensity of training based on the overload principle for chronic SCI mice and demonstrated limited improvement in gait performance18. However, such improvement was not remarkable in terms of practical activity; therefore, a completely new treatment strategy was required, such as regenerative medicine using iPS cell technology18,26,27. No significant improvement was observed in preclinical research where the combined effect of neural stem cells and rehabilitative training in chronic SCI was investigated, although a significant recovery between the combination treatment and the control was reported compared with the transplantation-only group16. Thus, the current study is the first to report that regenerative rehabilitation far surpasses the effect of transplantation monotherapy. Our findings indicate the specific effects of GSI-pretreated NS/PCs transplantation and the verified rehabilitative training.
The outcomes of combination therapy in regenerative treatments have recently been reported by Tashiro et al, both in the injured spinal cord where cells were transplanted and also distant from the lesion17. Improved survival was one benefit of rehabilitation training on transplanted cells28,29. Regardless of the timing of the injury, the survival rate of grafted cells rapidly declines within 2 weeks of transplantation30, and preventing this decline is crucial to improve motor function28. This study reports for the first time that combination rehabilitation prevents transplanted cell loss in the chronic phase as in the subacute phase.
The differentiation of transplanted cells into neurons and oligodendrocytes is another factor that is essential for functional recovery31. Previous research focusing on the subacute stage of treatment demonstrated that transplanted neural stem cells differentiated into more mature neurons and oligodendrocytes with rehabilitative training28,29. In contrast, the current study’s results were limited to an increase in the differentiation of hiPSC-NS/PCs into mature neurons with rehabilitation, which is consistent with the findings of a prior study that focused on the chronic phase of treatment16. These findings imply that the timing of the training sessions may alter how the differentiation of grafted neural stem cells is affected by rehabilitation. On the other hand, we also observed that transplanted cell-derived neurons differentiated into glutamatergic and GABAergic neurons and inhibitory interneurons in both groups. Further studies are needed to determine whether rehabilitation alters the character of transplanted cell-derived neurons. In any case, the synaptic connection of transplanted cells differentiated into mature neurons to the host neuronal network, as shown in the immunoelectron microscopy analysis, indicated successful cell transplantation32. Furthermore, the improved survival of transplanted cells and their differentiation into mature neurons might contribute significantly to functional improvement. However, differences were observed between in vivo and in vitro results in terms of the percentage of positive NeuN, an indicator of mature neuron. We attribute this result to the fact that the time point evaluated after transplantation in vivo was 8 weeks, whereas the incubation period for differentiation in vitro was 5 weeks, and the environment to which the cells were exposed was different.
Our results revealed that rehabilitation expanded 5-HT-positive fibers in the lumbar enlargement and promoted neuronal activity as measured via Syn1 expression. Syn1 is frequently used as a pre-synaptic marker for activity-dependent synaptic plasticity and its functioning33. Syn1 was downregulated below the lesion, but treadmill training restored its expression in the transplanted hiPSC-NS/PCs animals, in line with a study on non-transplanted animals34. The raphespinal tract, identified with 5-HT staining, is one of the supraspinal tracts that originates from the brain stem and is crucial for forelimb and hind limb coordination, contributing to the gait quality in quadrupedal rodents35. We observed an improvement in the coordination value together with the restoration of the 5-HT-positive area in the animals that received the combined treatment. The present findings demonstrated that the combined treatment increased 5-HT fibers in the lumbar spinal cord, especially in the case of chronic SCI. It has been documented previously that hiPSC-NS/PCs transplantation or rehabilitation training alone can boost 5-HT fibers20,36. However, the mechanisms behind the development of 5-HT-positive fibers are yet to be determined. The tracing technique may help to clarify this.
Elevated levels of the neurotrophic factors, BDNF and NT-3, in the injured spinal tissue were factors that may enhance the benefits of the combined treatment stated above. Training before and after transplantation may have helped this process, as BDNF is a protein associated with plasticity that is known to be elevated due to short-term rehabilitation and is implicated in neuronal activity, survival, and remodeling37-40. Exercise-induced neuroplasticity is tightly correlated with the levels of BDNF in the neuromuscular system41,42. In addition, NT-3 is a growth factor known to be upregulated by many forms of exercise rehabilitation and promotes axonal sprouting, neuronal differentiation, and neuroprotection43,44. These mechanisms may shed light on the findings mentioned above. Interestingly, neurotrophic factors are upregulated via both NS/PCs transplantation and rehabilitation as represented by BDNF and NT-3. These neurotrophic factors may enhance the therapeutic effects of both NS/PCs transplantation and rehabilitation. One of the key mechanisms of the combined effect of transplantation and rehabilitation appears to be this complementing process via trophic support11. In addition, Kusano et al reported that neural stem cells expressing NT-3 significantly improved motor recovery in chronic phase SCI45. Although it was previously believed that the host tissue secretes neurotrophic factors14,18, our research suggests that combining exogenous neurotrophic factors along with hiPSC-NS/PCs and rehabilitation may improve function further.
The motor function was evaluated from various aspects, including general open-field locomotion using BMS, quadrupedal gait appearance using the footprint in the DigiGait system, and joint motion by kinematics captured with a high-resolution camera. In addition, the endurance was assessed via the rotarod test, the coordination was assessed with phase dispersion analysis, and disused muscle atrophy was assessed by the muscle weight. When the results from the electrophysiological study were also taken into account, the combined therapy promoted motor functional recovery, as evidenced by the fact that those parameters were better improved in the rehabilitation-combined animals than in the transplantation-alone group. GSI-pretreated hiPSC-NS/PCs in combination with rehabilitation showed significantly improved effects, resulting in the acquisition of practical locomotor function, although treatment with GSI-pretreated hiPSC-NS/PCs transplantation alone also showed a partial improvement of motor function at the chronic phase in a previous report14. The improvement in motor function may have been due, in part, to the strengthening of the local circuit synapses in the lumbar spinal cord and the recovery in lower leg muscle volume resulting from rehabilitation therapy alone. However, as shown by the synaptic interaction between the transplanted and host cells and the results of the electrophysiological analysis, improved survival and enhanced neuronal differentiation of the transplanted cells may have led to increased synaptic connection. Therefore, the combination of transplantation and rehabilitation may play an essential role in integrating lower and upper motor neurons. These findings suggest that hiPSC-NS/PCs transplantation combined with rehabilitative training is an efficient treatment in the chronic phase of SCI with low treatment sensitivity.
This study had several limitations. First, this study used a moderate contusive injury to the thoracic spinal cord as the type of SCI model. Future studies should investigate whether the same outcomes are attained for different injury sites and severities. Second, this study’s results do not explain the enhanced synaptic formation between the transplanted cells and host caused as a result of rehabilitative training. Although the results of the electrophysiological analysis implied that the synaptic formation between the transplanted cells and the host was facilitated by rehabilitative training, future studies are required to provide direct evidence of these findings. Third, no direct evidence existed that elevated neurotrophic factors promoted the survival rate, neuronal differentiation of transplanted cells, and subsequent improvement in motor function. Further studies are required to elucidate these mechanisms. However, neurotrophic factors were elevated in the injured spinal cord and the lumbar spinal cord, where there was evidence of increased neural activity, suggesting that neurotrophic factors may have had a positive impact on functional improvement. Finally, it is unclear which factors most significantly contributed to the improvement in motor function because the current study compared two groups with cell transplantation. The contribution to motor function from training alone may be significant, as indicated by the result of increased muscle weight. However, investigation into the effects of rehabilitative training on transplanted cells is essential to advance regenerative medicine.
Conclusion
The combination of hiPSC-NS/PCs transplantation and rehabilitative training was able to enhance the synaptic activity in the lumbar spinal cord and promoted the survival rate and neuronal differentiation of grafted cells passively through the promotion of neurotrophic factor secretions. Consequently, the combined therapy of GSI-pretreated hiPSC-NS/PCs transplantation and the verified rehabilitation protocol promoted motor functional recovery. Therefore, this therapeutic strategy could be expected to form the basis of regenerative treatment in cases of chronic SCI.
Supplementary Material
Acknowledgments
We appreciate the assistance and instruction provided by Drs. M. Ishikawa, O. Tsuji, S. Nori, Y. Tanimoto, Y. Hoshino, R. Shibata, Y. Kamata, K. Kajikawa, T. Nishijima, Y. Saijyo, Y. Suematsu, T. Yoshida, K. Ito, T. Tanaka, A. Toga, and R. Ogaki, all of whom are members of the Spinal Cord Research Team at the Department of Orthopaedic Surgery and Physiology, Keio University School of Medicine, Tokyo, Japan. We also appreciate K. Yasutake, M. Akizawa, T. Kobayashi, and T. Harada for their assistance with the experiments and animal care. This work was supported by the Japan Agency for Medical Research and Development (AMED; grant nos. JP20bk0104114, JP19bk0104017, and JP15bm0204001 to H.O. and M.N.).
Contributor Information
Takahiro Shibata, Department of Orthopaedic Surgery, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan; Department of Physiology, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan.
Syoichi Tashiro, Department of Rehabilitation Medicine, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan.
Shinsuke Shibata, Division of Microscopic Anatomy, Graduate School of Medical and Dental Sciences, Niigata University, Niigata City, Niigata, Japan; Electron Microscope Laboratory, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan.
Munehisa Shinozaki, Department of Physiology, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan.
Tomoko Shindo, Electron Microscope Laboratory, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan.
Shogo Hashimoto, Department of Orthopaedic Surgery, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan; Department of Physiology, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan.
Momotaro Kawai, Department of Orthopaedic Surgery, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan.
Takahiro Kitagawa, Department of Orthopaedic Surgery, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan.
Kentaro Ago, Department of Orthopaedic Surgery, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan.
Morio Matsumoto, Department of Orthopaedic Surgery, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan.
Masaya Nakamura, Department of Orthopaedic Surgery, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan.
Hideyuki Okano, Department of Physiology, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan.
Narihito Nagoshi, Department of Orthopaedic Surgery, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan.
Funding
Japan Agency for Medical Research and Development (AMED), Grant/Award Numbers: JP20bk0104114, JP19bk0104017, JP15bm0204001.
Conflict of Interest
H.O. declared employment with Keio University School of Medicine, advisory role with SanBio Co. Ltd., and K Pharma, Inc., and research funding from SanBio Co. Ltd., and K Pharma, Inc. All of the other authors declare no potential conflicts of interests.
Data Availability
The data that support the findings of this study are available on request from the corresponding authors.
Author Contributions
T.S.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; S.S., M.S., T.S., S.H., M.K., T.K., K.A.: data analysis and interpretation; M.M., M.N., H.O.: financial support, reviewing and editing the manuscript; N.N.: reviewing and editing the manuscript, final approval of manuscript; S.T.: conception and design, administrative support, reviewing and editing the manuscript, final approval of manuscript.
References
- 1. Shinozaki M, Nagoshi N, Nakamura M, et al. Mechanisms of stem cell therapy in spinal cord injuries. Cells. 2021;10(10):2676. 10.3390/cells10102676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Okano H, Kaneko S, Okada S, et al. Regeneration-based therapies for spinal cord injuries. Neurochem Int. 2007;51(2-4):68-73. 10.1016/j.neuint.2007.04.013 [DOI] [PubMed] [Google Scholar]
- 3. Zhu Y, Uezono N, Yasui T, et al. Neural stem cell therapy aiming at better functional recovery after spinal cord injury. Dev Dyn. 2018;247(1):75-84. [DOI] [PubMed] [Google Scholar]
- 4. Nori S, Okada Y, Yasuda A, et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci USA. 2011;108(40):16825-16830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamata Y, Isoda M, Sanosaka T, et al. A robust culture system to generate neural progenitors with gliogenic competence from clinically relevant induced pluripotent stem cells for treatment of spinal cord injury. Stem Cells Transl Med. 2021;10(3):398-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagoshi N, Khazaei M, Ahlfors JE, et al. Human spinal oligodendrogenic neural progenitor cells promote functional recovery after spinal cord injury by axonal remyelination and tissue sparing. Stem Cells Transl Med. 2018;7(11):806-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawabata S, Takano M, Numasawa-Kuroiwa Y, et al. Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Rep. 2016;6(1):1-8. 10.1016/j.stemcr.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobayashi Y, Okada Y, Itakura G, et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One. 2012;7(12):e52787e52787. 10.1371/journal.pone.0052787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsuji O, Sugai K, Yamaguchi R, et al. Concise review: laying the groundwork for a first-in-human study of an induced pluripotent stem cell-based intervention for spinal cord injury. Stem Cells. 2019;37(1):6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzuki H, Sakai T.. Current concepts of stem cell therapy for chronic spinal cord injury. Int J Mol Sci. 2021;22(14):7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tashiro S, Nakamura M, Okano H.. The prospects of regenerative medicine combined with rehabilitative approaches for chronic spinal cord injury animal models. Neural Regen Res. 2017;12(1):43-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okubo T, Iwanami A, Kohyama J, et al. Pretreatment with a gamma-secretase inhibitor prevents tumor-like overgrowth in human iPSC-derived transplants for spinal cord injury. Stem Cell Rep. 2016;7(4):649-663. 10.1016/j.stemcr.2016.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sugai K, Sumida M, Shofuda T, et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen Ther. 2021;18:321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okubo T, Nagoshi N, Kohyama J, et al. Treatment with a gamma-secretase inhibitor promotes functional recovery in human iPSC-derived transplants for chronic spinal cord injury. Stem Cell Rep. 2018;11(6):1416-1432. 10.1016/j.stemcr.2018.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tashiro S, Tsuji O, Shinozaki M, et al. Current progress of rehabilitative strategies in stem cell therapy for spinal cord injury: a review. NPJ Regen Med. 2021;6(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tashiro S, Nishimura S, Iwai H, et al. Functional recovery from neural stem/progenitor cell transplantation combined with treadmill training in mice with chronic spinal cord injury. Sci Rep. 2016;6:30898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tashiro S, Nakamura M, Okano H.. Regenerative rehabilitation and stem cell therapy targeting chronic spinal cord injury: a review of preclinical studies. Cells. 2022;11(4):685. 10.3390/cells11040685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shibata T, Tashiro S, Shinozaki M, et al. Treadmill training based on the overload principle promotes locomotor recovery in a mouse model of chronic spinal cord injury. Exp Neurol. 2021;345:113834. 10.1016/j.expneurol.2021.113834 [DOI] [PubMed] [Google Scholar]
- 19. Kitagawa T, Nagoshi N, Kamata Y, et al. Modulation by DREADD reveals the therapeutic effect of human iPSC-derived neuronal activity on functional recovery after spinal cord injury. Stem Cell Rep. 2022;17(1):127-142. https://.2021.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ito S, Nagoshi N, Tsuji O, et al. LOTUS inhibits neuronal apoptosis and promotes tract regeneration in contusive spinal cord injury model mice. eNeuro. 2018;5(5):ENEURO.0303-ENEU18.2018. 10.1523/eneuro.0303-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Basso DM, Fisher LC, Anderson AJ, et al. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23(5):635-659. 10.1089/neu.2006.23.635 [DOI] [PubMed] [Google Scholar]
- 22. Kawai M, Imaizumi K, Ishikawa M, et al. Long-term selective stimulation of transplanted neural stem/progenitor cells for spinal cord injury improves locomotor function. Cell Rep. 2021;37(8):110019. [DOI] [PubMed] [Google Scholar]
- 23. Gant KL, Nagle KG, Cowan RE, et al. Body system effects of a multi-modal training program targeting chronic, motor complete thoracic spinal cord injury. J Neurotrauma. 2018;35(3):411-423. 10.1089/neu.2017.5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sawada T, Okawara H, Matsubayashi K, et al. Influence of body weight-supported treadmill training with voluntary-driven exoskeleton on the quality of life of persons with chronic spinal cord injury: a pilot study. Int J Rehabil Res. 2021;44(4):343-349. [DOI] [PubMed] [Google Scholar]
- 25. Okawara H, Tashiro S, Sawada T, et al. Neurorehabilitation using a voluntary driven exoskeletal robot improves trunk function in patients with chronic spinal cord injury: a single-arm study. Neural Regen Res. 2022;17(2):427-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamanaka S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. 2020;27(4):523-531. 10.1016/j.stem.2020.09.014 [DOI] [PubMed] [Google Scholar]
- 27. Okano H, Sipp D.. New trends in cellular therapy. Development. 2020;147(18):dev192567. [DOI] [PubMed] [Google Scholar]
- 28. Hwang DH, Shin HY, Kwon MJ, et al. Survival of neural stem cell grafts in the lesioned spinal cord is enhanced by a combination of treadmill locomotor training via insulin-like growth factor-1 signaling. J Neurosci. 2014;34(38):12788-12800. 10.1523/jneurosci.5359-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Younsi A, Zheng G, Scherer M, et al. Treadmill training improves survival and differentiation of transplanted neural precursor cells after cervical spinal cord injury. Stem Cell Res. 2020;45:101812. 10.1016/j.scr.2020.101812 [DOI] [PubMed] [Google Scholar]
- 30. Nishimura S, Yasuda A, Iwai H, et al. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain. 2013;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nori S, Nakamura M, Okano H.. Plasticity and regeneration in the injured spinal cord after cell transplantation therapy. Prog Brain Res. 2017;231:33-56. [DOI] [PubMed] [Google Scholar]
- 32. Shimogawa T, Sakaguchi H, Kikuchi T, et al. Therapeutic effects of combined cell transplantation and locomotor training in rats with brain injury. NPJ Regen Med. 2019;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Houle JD, Cote MP.. Axon regeneration and exercise-dependent plasticity after spinal cord injury. Ann N Y Acad Sci. 2013;1279:154-163. 10.1111/nyas.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR.. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145-167. 10.1146/annurev.neuro.27.070203.144308 [DOI] [PubMed] [Google Scholar]
- 35. Slawinska U, Miazga K, Jordan LM.. The role of serotonin in the control of locomotor movements and strategies for restoring locomotion after spinal cord injury. Acta Neurobiol Exp (Wars). 2014;74(2):172-187. [DOI] [PubMed] [Google Scholar]
- 36. Zhang W, Yang B, Weng H, et al. Wheel running improves motor function and spinal cord plasticity in mice with genetic absence of the corticospinal tract. Front Cell Neurosci. 2019;13:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vavrek R, Girgis J, Tetzlaff W, et al. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain. 2006;129(Pt 6):1534-1545. [DOI] [PubMed] [Google Scholar]
- 38. Cote MP, Azzam GA, Lemay MA, et al. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma. 2011;28(2):299-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ying Z, Roy RR, Edgerton VR, Gómez-Pinilla F.. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193(2):411-419. 10.1016/j.expneurol.2005.01.015 [DOI] [PubMed] [Google Scholar]
- 40. Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR.. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13(6):1078-1084. 10.1046/j.0953-816x.2001.01484.x [DOI] [PubMed] [Google Scholar]
- 41. Tashiro S, Shinozaki M, Mukaino M, et al. BDNF induced by treadmill training contributes to the suppression of spasticity and allodynia after spinal cord injury via upregulation of KCC2. Neurorehabil Neural Repair. 2015;29(7):677-689. [DOI] [PubMed] [Google Scholar]
- 42. Gomez-Pinilla F, Huie JR, Ying Z, et al. BDNF and learning: Evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience. 2007;148(4):893-906. 10.1016/j.neuroscience.2007.05.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ghosh A, Greenberg ME.. Distinct roles for bFGF and NT-3 in the regulation of cortical neurogenesis. Neuron. 1995;15(1):89-103. 10.1016/0896-6273(95)90067-5 [DOI] [PubMed] [Google Scholar]
- 44. Keefe KM, Sheikh IS, Smith GM.. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int J Mol Sci. 2017;18(3):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kusano K, Enomoto M, Hirai T, et al. Transplanted neural progenitor cells expressing mutant NT3 promote myelination and partial hindlimb recovery in the chronic phase after spinal cord injury. Biochem Biophys Res Commun. 2010;393(4):812-817. 10.1016/j.bbrc.2010.02.088 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding authors.