Abstract
Bleomycin is a unique antibiotic agent with cytotoxic activity and is used successfully in various malignant diseases, such as Hodgkin lymphoma and germ cell tumors. Drug-induced lung injury (DILI) is one of the major limitations of bleomycin administration in particular clinical settings. The incidence varies among patients and depends on a variety of risk factors, such as cumulative drug dose, underlying malignant disease, and concurrent radiation. The clinical presentations are non-specific for bleomycin-induced lung injury (BILI), depending on the onset and severity of symptoms. There is no established guideline for the best treatment of DILI and the treatment is based on the time and severity of pulmonary symptoms. It is important to consider BILI in any patient with pulmonary clinical manifestations who has been treated with bleomycin.
Here, we report a 19-year-old woman who is a known case of Hodgkin lymphoma. She was treated with a bleomycin-containing chemotherapy regimen. On the 5th month of therapy, she was admitted to hospital with severe acute pulmonary symptoms and decreased oxygen saturation. She was treated successfully with high-dose corticosteroid without any significant sequelae.
Keywords: Acute Lung Injury, Pulmonary, Bleomycin
INTRODUCTION
Bleomycin is a unique antibiotic agent with an antitumor activity and is used successfully for the treatment of various malignancies, including germ cell tumors and Hodgkin lymphoma (1,2). Drug-induced lung injury is the major limitation of bleomycin therapy. The incidence varies among patients undergoing chemotherapy and depends on various risk factors, including cumulative drug dose, underlying malignant disease, age, renal function, and concomitant use of other agents (3,4).
The rate of BILI in patients with Hodgkin lymphoma is about 10%, and fatal pulmonary toxicity is 4–5% (5). The clinical manifestations of BILI are non-specific and commonly mild dyspnea due to diffuse alveolar damage with non-specific interstitial pneumonitis (NSIP). There is little evidence to guide the best treatment of DILI and cessation of bleomycin is the mainstay of therapy (2,5, 6). Here, we report a reversible severe bleomycin-induced pneumonitis in a patient with Hodgkin lymphoma.
CASE SUMMARIES
A 19-year-old woman came with complaints of progressive involuntary weight loss and fatigue from three months ago. She did not mention any past medical and drug history. In physical examination, she was a young oriented woman with stable vital signs. Multiple bilateral cervical lymphadenopathies were detected, but there was no organomegaly.
An excisional biopsy was performed that was consistent with classic Hodgkin lymphoma, a nodule-sclerosis variant. Her staging chest and abdominal-pelvic computed tomography (CT) scan were normal and the final diagnosis was stage 2b of classic Hodgkin lymphoma. She was a candidate for treatment with six cycles (12 injection sessions) of ABVD chemotherapy regimen, including doxorubicin 37.5 milligrams (mg), bleomycin 15 mg, vinblastine nine mg, dacarbazine 560 mg. These chemotherapy drugs should be injected every two weeks for a total of 12 injection sessions.
Our patient completed eight sessions (Four cycles) of chemotherapy with a good response without any significant complication except one episode of neutropenic fever; so, granulocyte colony-stimulating factor (G-CSF) was administered after every chemotherapy session. At the end of 9th chemotherapy session, after the drug injection was completed, she complained of mild dyspnea and palpitation. She was not febrile and her vital signs were stable, so oral propranolol was prescribed with suspicion of anxiety. Her dyspnea worsened after two hours, and oxygen saturation (O2 sat) decreased to 60%. On physical examination, she had right-sided fine crackles. Her chest X-ray (CXR) revealed air space opacity at right lung with involvement of right middle lobe (RML) and right lower lobe (RLL) (Figure 1-A). Complementary lung CT scan showed patches of ground-glass opacities (GGO) at both lungs, more confluent on the right side (Figure 2).
Figure 1.
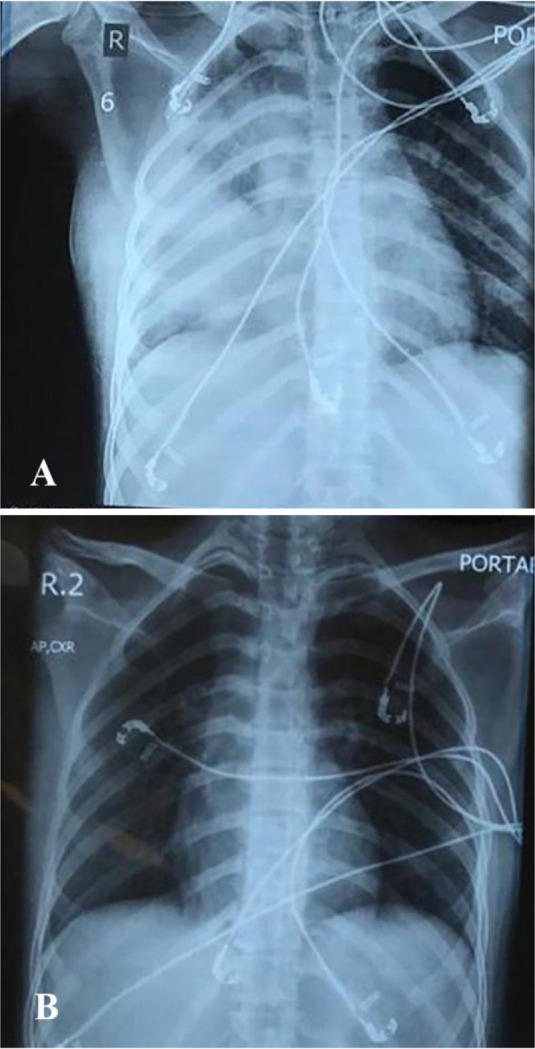
A- CXR with air space opacity at right lung and involvement of right middle lobe (RML) and right lower lobe (RLL). B- near normal CXR 48 hours after therapy.
Figure 2.
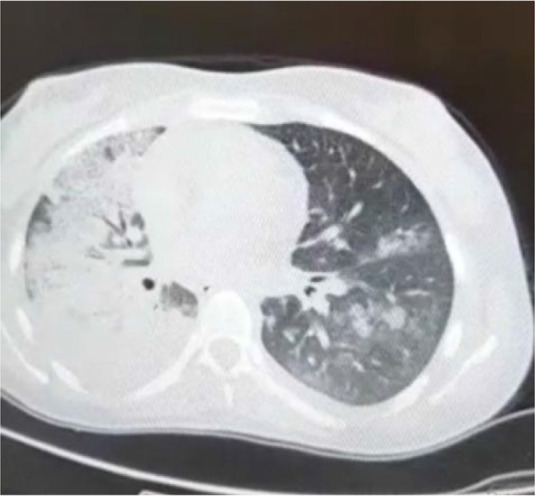
Lung CT scan revealed patches of ground glass opacities at both lungs more confluent on right side
According to the sudden onset of dyspnea and history of recent bleomycin injection, DILI was the first suspected diagnosis. We could not definitely exclude infection, but the clinical course did not support that our patient had no fever and was not neutropenic. Her PCR (polymerase chain reaction) for Coronavirus was negative. Finally, we administered corticosteroids (dexamethasone, eight mg every eight hours) with the diagnosis of DILI. Empiric antibiotic therapy with Levofloxacin was also initiated. After 48 hours, her performance status and O2 sat were improved significantly and the CXR was nearly normal (Figure 1-B). The remaining chemotherapy sessions were continued without bleomycin and fortunately, the patient did not have any other complications.
DISCUSSION
Cytotoxic chemotherapy agents have frequent adverse effects on multiple organs, and the lungs are a common target because they have a large surface area and are one of the major sites for metabolism of drugs (2). Bleomycin is a cytotoxic agent that is used in multiple malignant diseases, including Hodgkin lymphoma and can induce lung toxicity in up to 10 percent of patients. The incidence depends on multiple factors, including bleomycin cumulative doses of more than 400 units, age over 40, renal insufficiency, thoracic irradiation, and concomitant use of G-CSF may influence the risk of BILI (2,3). The present patient did not have any mentioned risk factors except for G-CSF use for neutropenia. She was a young woman without any underlying comorbidity and her cumulative bleomycin dose was 135 units.
The clinical presentation of BILI is not specific and the common earliest symptom is dyspnea and the common sign is fine crackles (2,3), and the present patient had these manifestations. Symptoms of BILI usually develop sub-acutely over one to six months of bleomycin therapy initiation. Our case was in the 5th month of chemotherapy. Other less common types of BILI include hypersensitivity pneumonitis with more rapid onset, acute chest pain syndrome during bleomycin infusion and fibrotic form with indolent onset of dyspnea after completion of therapy (1,3, 4). The rate of severe and fatal lung injury is about 4–5% (5). Our case had severe and life-threatening lung toxicity. She was near intubation with the O2 sat under 70%. Figure 2 showed the chest CT findings of this case with diffused GGO and air space consolidation. The most suspicious underlying histopathology was diffused alveolar damage. Other CT-Scan findings that can be associated with bleomycin toxicity include extensive reticular markings and tractional bronchiectasis in lung fibrosis, GGO and subpleural reticular markings in non-specific interstitial pneumonia, diffused GGO with centrilobular nodules in hypersensitive pneumonitis, and peribronchial air space consolidation and nodular densities in organizing pneumonia (3).
The diagnosis of BILI is based on history, clinical pattern, findings of pulmonary function tests, and the exclusion of infection, tumor lung involvement, cardiogenic pulmonary edema, and radiation-induced pulmonary toxicity. In the setting of uncertain diagnosis, other interventions may be helpful, like bronchoalveolar lavage (BAL) and lung biopsy (3,5). This patient was not febrile or neutropenic, her COVID-19 PCR test was negative and the clinical pulmonary manifestations progressed rapidly, so DILI was the first diagnosis. There is no established guideline for BILI management and permanent discontinuation of the drug is the mainstay of treatment. Glucocorticoid administration may be beneficial in patients with acute BILI with respiratory symptoms. The rate of corticosteroid response depends on the type of pulmonary damage, and the best response is seen in acute bleomycin-induced inflammatory pneumonitis. However, we administer corticosteroids in clinical practice based on the patient’s clinical presentation without knowing the exact pathology of pulmonary damage.
The acute clinical course of our patient was consistent with acute inflammatory reaction and she responded well to corticosteroids. As infection could not be excluded initially, empiric antibiotics were administered, but the rapid clinical and radiological recovery did not support the underlying infection (1,2). The exact corticosteroid dose is not established, but a reasonable option is to start with prednisolone 1 mg/kg per day (1). Because our patient had severe respiratory distress, we initiated therapy with intravenous dexamethasone and continued with prednisolone after two days and tapered it in two weeks. Other therapies that may be of benefit in the lung toxicity of bleomycin in patients who do not respond to corticosteroids, are tyrosine kinase inhibitor imatinib, and anti-TNF-α such as infliximab (5).
CONCLUSION
Bleomycin-induced lung injury (BILI) may present with acute clinical manifestations. Corticosteroids are a good therapeutic choice in this setting and can induce complete clinical and radiological recovery.
REFERENCES
- 1.Sleijfer S. Bleomycin-induced pneumonitis. Chest 2001;120(2):617–24. [DOI] [PubMed] [Google Scholar]
- 2.Balaji O, Amita D, Patil N, Thomas J. Bleomycin-induced reversible acute interstitial pneumonia. Asian Journal of Pharmaceutical and Clinical Research 2017;10(5):6–8. [Google Scholar]
- 3.Lauritsen J, Kier MG, Bandak M, Mortensen MS, Thomsen FB, Mortensen J, et al. Pulmonary Function in Patients With Germ Cell Cancer Treated With Bleomycin, Etoposide, and Cisplatin. J Clin Oncol 2016;34(13):1492–9. [DOI] [PubMed] [Google Scholar]
- 4.Martin WG, Ristow KM, Habermann TM, Colgan JP, Witzig TE, Ansell SM. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin's lymphoma. J Clin Oncol 2005;23(30):7614–20. [DOI] [PubMed] [Google Scholar]
- 5.Ge V, Banakh I, Tiruvoipati R, Haji K. Bleomycin-induced pulmonary toxicity and treatment with infliximab: A case report. Clin Case Rep 2018;6(10):2011–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Limper AH. Chemotherapy-induced lung disease. Clin Chest Med 2004;25(1):53–64. [DOI] [PubMed] [Google Scholar]


