Abstract
Background
Hearing loss and deafness are well-known sequelae from bacterial meningitis (ABM) and may result in social dysfunction and learning difficulties. Yet, the timely development of hearing loss and restitution is poorly studied, especially among adults. Hearing loss was revisited using otoacoustic emissions (OAEs) to determine the occurrence, magnitude, and development of hearing loss among adults with ABM.
Methods
Distortion product OAEs were measured in patients with ABM the day of admission and days 2, 3, 5–7, and 10–14 and at follow-up 30–60 days after discharge. Frequencies were categorized as low (1, 1.5, 2 kHz), mid (3, 4, 5 kHz), mid-high (6, 7, 8 kHz), and high (9, 10 kHz). Audiometry was performed on discharge and 60 days after. Results were compared with 158 healthy controls.
Results
OAE was obtained in 32 patients. ABM was due to S. pneumoniae in 12 patients (38%). All patients were treated with dexamethasone. OAE emission threshold levels (ETLs) were significantly decreased upon admission and at follow-up in all frequencies compared with healthy controls. A substantial and significant decrease in ETLs was found in S. pneumoniae meningitis. Sensorineural hearing loss (SNHL) >20 dB was present in 13 of 23 (57%) at discharge and in 11 of 18 patients (61%) 60 days after discharge. Hearing recovery decreased from day 3.
Conclusions
Hearing loss in ABM still affects >60% of patients despite treatment with dexamethasone. In S. pneumoniae meningitis, SNHL is profound and permanent. A window of opportunity for systemic or local treatments aiming to preserve cochlear function is proposed.
Keywords: bacterial meningitis, OAE, cochlea, hearing loss, otoacoustic emissions
Hearing loss in bacterial meningitis was measured using otoacoustic emissions and audiometry. Hearing loss was frequent irrespective of pathogen and profound in pneumococcal meningitis. Cochlea was the site of lesion. A timely window for preservation of cochlear function is proposed.
Acute bacterial meningitis (ABM) is one of the most common causes of acquired sensorineural hearing loss (SNHL), reported to occur in up to 54% of survivors, with the highest incidence among adults with S. pneumoniae meningitis [1–3]. SNHL is closely associated with long-term complications, including poor academic performance, depression, and impaired psychosocial adjustment [4].
Even though hearing loss and deafness are the most common sequelae from meningitis, clinical studies addressing new treatment strategies addressing this complication are sparse, and anti-inflammatory treatment with dexamethasone is essentially the only treatment currently available [1, 5–9]. Studies that were primarily carried out in children with H. influenzae and N. meningitidis documented the presence of hearing loss early during disease [1, 10, 11]. Still, the pattern is not well described, with highly variable SNHL either declining, fluctuating, or resolving with time intervals ranging from 1 month to 12 years [11–16]. Previously, the cause of SNHL was proposed to be secondary to brain damage or direct injury to nerve fibers [17, 18]. Subsequent experimental studies demonstrated the site of lesions showing suppurative labyrinthitis with cochlear damage and spread of infection from the subarachnoid space or directly from otitis media [19–23].
Cochlear inflammation has been shown to cause fibrosis and cochlear ossification developing 3–4 weeks after the onset of meningitis, increasing the risk of not being able to implant cochlear electrodes [24, 25]. This underlines the importance of timely identification of hearing loss and the need for further studies of preventive treatment strategies.
The present study targeted cochlea-derived hearing loss by measuring otoacoustic emissions (OAEs), which are low-intensity sounds generated by outer hair cells in the cochlea in response to sound stimuli [26, 27].
The aim of the present study was to clarify the temporal development and extent of SNHL in adults with ABM by repeated OAE measurements.
METHODS
In this prospective observational cohort study, SNHL was evaluated by audiometry and repeated measures of distortion product otoacoustic emissions (DPOAEs) in adults with ABM. Patients were recruited consecutively at the Department of Infectious Diseases, Nordsjællands Hospital, from November 2016 to July 2019 and from November 2016 to April 2017; patients were also recruited at the Department of Infectious Diseases, Aalborg University Hospital.
Control Cohort
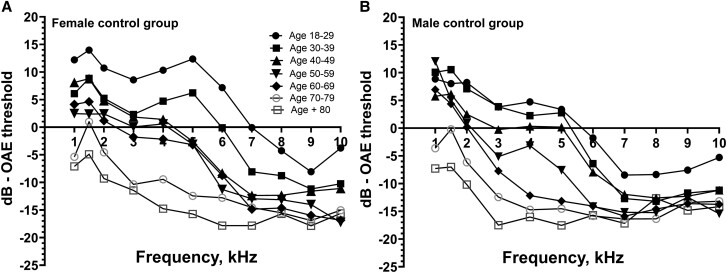
A control group of healthy subjects aged 18–80 years (n = 158) with even gender distribution divided into 8 groups in decennials were recruited for the assessment of age- and sex-related DPOAE loss (Figure 1). Subjects aged 18–65 years were recruited among blood donors at Nordsjællands Hospital. Subjects aged >65 years were recruited in the Department of Orthopedics at Nordsjællands Hospital among candidates for elective surgery (Figure 1).
Figure 1.
Emission threshold levels among healthy control group participants. Graph (A) shows ETLs among females, and graph (B) shows ETLs among males. The age groups are divided into decennials from 18 to 80+ years. Abbreviation: ETL, emission threshold level.
Exclusion criteria were familial deafness, head trauma requiring admission, significant history of noise exposure, ear surgery, previous administration of known ototoxic agents (eg, gentamycin), and prior central nervous system disease including meningitis. All subjects underwent otoscopy and tympanometry to rule out external and middle ear pathology.
Patient Cohort
Patients with ABM were enrolled prospectively on admission and follow-up as outpatients. Otoscopy and tympanometry were performed to rule out external and middle ear pathology.
Inclusion Criteria
Patients were ≥18 years of age, had a clinical presentation strongly suggesting bacterial meningitis (headache, fever, stiffness of the neck, petechiae, confusion or impaired level of consciousness), and had ≥1 of the following:
Positive cerebrospinal fluid (CSF) culture.
Positive blood culture and ≥1 of the following CSF findings: >10 leukocytes (×106 cells/L); glucose index <0.3; CSF glucose <1.9 mmol/L or CSF lactate >3.5 mmol/L; CSF protein >2.2 g/L.
Presence of bacteria in gram stain of CSF or nonculture identification of bacteria in CSF by either gene amplification or antigen test.
>100 leukocytes (×106 cells/L) in CSF with neutrophil predominance (>50%) in combination with low CSF glucose or CSF/blood glucose ratio (<1.9 mmol/L and 0.3, respectively) or CSF lactate >3.5 mmol/L.
Exclusion Criteria
Exclusion criteria were nosocomial meningitis, concomitant endocarditis, brain abscess, and viral meningitis or encephalitis. Patients were screened by a structured interview to exclude hearing loss due to other causes as described above for healthy controls.
Patient Data
Patients were divided into 5 groups based on CSF bacteriological results: Streptococcus Pneumoniae, Haemophilus influenzae, Neisseria meningitidis, other pathogen (Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, non- and β-hemolytic streptococci), and unidentified pathogen. Clinical, biochemical, and microbiological data were recorded on admission.
Audiological Assessment
OAE (DPOAEs) was measured in both ears (excluding ears with middle ear pathology, eg, otitis media) on the day of admission (day 1), days 2 and 3, and between days 5 and 7 and 10 and 14. Patients were followed up in the outpatient clinic at least 30 days after discharge. OAEs were recorded using the Interacoustics Titan DPOAE 440 module. Eleven frequencies were measured in each ear: 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 9, and 10 kHz. The frequency ratio (f2/f1) was fixed at 1.22. OAE was performed with patients lying down with a 30° tilted head position. Frequencies were categorized as low (1, 1.5, 2 kHz), mid (3, 4, 5 kHz), mid-high (6, 7, 8 kHz), and high (9, 10 kHz). The emission threshold level (ETL) in each frequency category was calculated as the mean of the included frequencies.
A signal-to-noise ratio (SNR) of +3 dB was applied to the noise floor in low to mid-high frequencies and +6 dB in high frequencies. The distribution of final noise floor, and thus border of OAE detection, within each frequency category was low −10 dB, mid −15 dB, mid-high −13 dB, high −16 dB [28].
OAE Repeatability
Seven control group participants (median age [interquartile range {IQR}], 34 [30–52] years) were tested on 3 occasions (days 1, 14, and 90). From days 1 to 14, the mean variation ranged from 1.1 (+/−1.3 dB) to 1.4 dB (+/−0.9 dB). From days 1 to 90, mean variation ranged from 0.8 dB (+/−0.6 dB) to 0.7 dB (+/−1.1 dB). A change in ETL was determined to be ≥2.5 dB to be significant and a change <2.5 dB was registered as 0 (zero) [29].
Pure-Tone Audiometry
Patients were tested upon discharge and 60 days after discharge. Patients underwent pure-tone audiometry at the frequencies 0.125–8 kHz. Both air conduction and bone conduction were measured (Madsen Astera 2 Clinical Audiometer). The pure-tone average (PTA) was calculated as the average of the thresholds at 0.5, 1, 2, and 4 kHz. In case of a conductive hearing loss, bone conduction was applied. Hearing was classified in each ear as no hearing loss (≤20 dB HL), mild (21–40 dB HL), moderate (41–55 dB HL), moderately severe (56–70 dB HL), severe (71–90 dB HL), or profound (> 90 dB HL) [24]. The PTA of each patient was compared with PTA from an age- and sex-matched normative data set provided by ISO-7029 [30].
Statistical Evaluation and Data Handling
Statistical analyses were done in R studio, version 1.4.1717. The Fisher exact test and Mann-Whitney U test were used to compare groups, and a stratified Wilcoxon-Mann-Whitney combined analysis was used to perform comparisons of emissions with respect to age and sex. Spearman's rank test was applied for the correlation analysis between PTA and ETL levels. A P value <.05 was considered significant.
Loss of (otoacoustic) emissions upon admission (in dB) was calculated as the difference between the patient's ETL (mean of both ears) and the mean ETL in the age- and sex-matched control group.
Changes in ETL during admission were calculated as the difference from the patient's own ETL on admission. A recovery or decline of ≥2.5 dB was considered significant, and changes <2.5 dB were registered as 0 (zero). A recovery or decline in ETL was only registered at the first interval where this was observed (days 1–3, days 5–7, or days 10–14).
Patient Consent
The study was approved by the Danish Data Protection Agency (2012–58-0004, I-Suite 03637). Informed, written consent was obtained from subjects or relatives before enrollment. The study did not interfere with patient treatment, and OAE testing is harmless. The Committees on Biomedical Research Ethics for the Capital Region of Denmark (vek@regionh.dk) therefore did not require any registration (j.no. h-1-2012-086). The study was registered on clinicaltrials.gov (identifier: NCT03715569).
RESULTS
Patient Characteristics
In total, 32 patients with community-acquired ABM were included. The median age (IQR) was 67 (54–72) years, and 11 (34%) were female (Table 1). Etiology was confirmed in 28 (88%) patients and is presented in Table 1. Three patients were excluded because of bilateral otitis media (n = 1), preexisting hearing loss (n = 1), and deafness from previous S. pneumoniae meningitis (n = 1). Four patients were transferred to our hospital during disease, and DPOAE was only available at follow-up. Four patients did not attend scheduled follow-up. Mortality was 13% (n = 4/32). Data from 28 patients were therefore available on admission and for 24 patients at follow-up.
Table 1.
Patient Demographics
| Variables | Included Patients (n = 32) |
|---|---|
| Age, y | 67 [55–72] |
| Male sex | 21 (66) |
| Mortality | 4 (13) |
| Clinical findings | … |
| Duration of symptoms before admission, d | 2 [1–3] |
| Glasgow Coma Scale scorea | 11 [10–14] |
| Admission temperature, °C | 38.5 [37.5–39.2] |
| Focus of infection (otitis media) | 9 (30) |
| Focus of infection (pneumonia) | 3 (10) |
| Seizures before or on admission | 4 (13) |
| Brain imaging pathology | … |
| No. of patients with brain imaging pathology | 4 (13) |
| Brain infarction or hemorrhage | 2 (6.7) |
| Subdural empyema | 1 (3.3) |
| Brain edema | 1 (3.3) |
| Microbiology | … |
| Patients with positive CSF culture | 28 (88) |
| Streptococcus pneumoniae | 12 (38) |
| Neisseria meningitidis | 4 (13) |
| Haemophilus influenzae | 4 (13) |
| Staphylococcus aureus | 2 (6) |
| Listeria monocytogenes | 1 (3) |
| Other streptococci | 4 (13) |
| Escherichia coli | 1 (3) |
| Laboratory results | … |
| CRP, mg/L | 174 [89–278] |
| B-leukocytes, 109/L | 14.3 [11.6–22.2] |
| B-thrombocytes, ×109/L | 198 [158–261] |
| CSF leukocytes, ×106/L | 2520 [7762–7425] |
| CSF glucose, mmol/L | 2.8 [0.2–3.7] |
| CSF protein, g/L | 2.8 [1.5–5.3] |
| CSF lactate, mmol/L | 6.9 [3.9–15] |
Data are presented as No. (%) or median [IQR].
Abbreviations: CRP, C-reactive protein; CSF, cerebrospinal fluid.
Characteristics of 32 patients with bacterial meningitis.
Treatment
Initial antimicrobial treatment consisted of a combination of penicillin and a third-generation cephalosporin in 30 (94%) patients and carbapenem monotherapy in 2 (6%) patients. Adjunctive dexamethasone was administered to all patients, although it was tapered in 1 (Figure 1, Table 2).
Table 2.
Maximum Detectable Hearing Loss Based on Control Group Mean to Lowest Detectable OAE
| … | Frequency Group | Low 1, 1.5, 2 kHz | Mid 3, 4, 5 kHz | Mid-high 6, 7, 8 kHz | High 9, 10 kHz |
|---|---|---|---|---|---|
| … | Lowest OAE Level | −10 dB | −15 dB | −13 dB | −16 dB |
| Age group | Sex | … | … | … | … |
| 18–29 y | F | 22.3 db | 25.4 dB | 14 dB | 11.1 dB |
| M | 18.4 dB | 19.0 dB | 6.8 dB | 9.6 dB | |
| 30–39 y | F | 16.7 dB | 19.4 dB | 7.4 dB | 5.3 dB |
| M | 19.2 dB | 18.0 dB | 2.3 dB | 4.7 dB | |
| 40–49 y | F | 17.2 dB | 15.2 dB | 2.0 dB | 4.7 dB |
| M | 14.8 dB | 15.1 dB | 2.4 dB | 4.3 dB | |
| 50–59 y | F | 12.5 dB | 14.8 dB | 0.5 dB | 0.4 dB |
| M | 16.0 dB | 9.7 dB | 0 dB | 2.1 dB | |
| 60–69 y | F | 13.3 dB | 13.6 dB | 0.2 dB | 0 dB |
| M | 13.8 dB | 4.0 dB | 0 dB | 2.4 dB | |
| 70–79 y | F | 7.0 dB | 4.3 dB | 0 dB | 0 dB |
| M | 6.8 dB | 1.1 dB | 0 dB | 2.7 dB | |
| >80 y | F | 2.9 dB | 1.1 dB | 0 dB | 0 dB |
| M | 2.2 dB | 0 dB | 0 dB | 0.5 dB |
Only values in bold were included in the data-analysis of hearing loss in meningitis patients. The range from the control group mean emission threshold levels to the noise floor with an added SNR of 3 dB for the low to mid-high frequencies and 6 dB in the high frequency spectrum is shown for both sexes in decennials from 18 y to 80+.
Abbreviations: OAE, otoacoustic emission; SNR, signal-to-noise ratio.
In total, 158 healthy subjects were included in the control group. Measurements were conducted on both ears. No ETL difference between the right and left ear was found (Mann-Whitney test, P = .4) (Figure 2, Table 3).
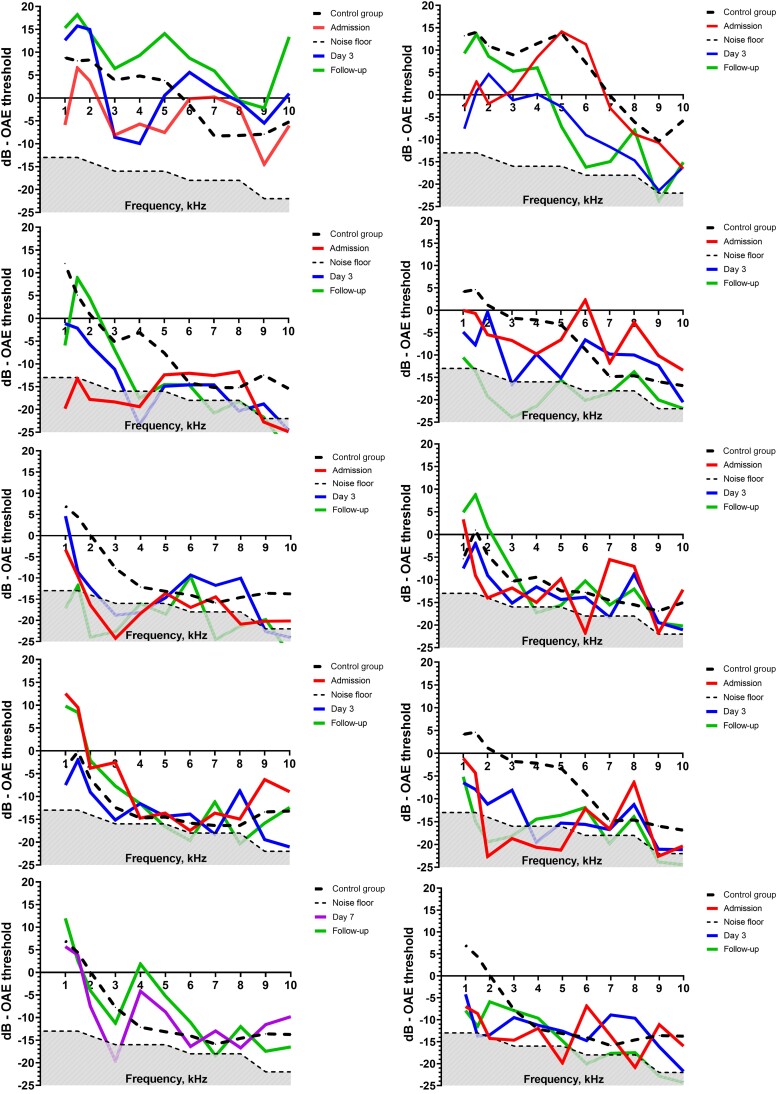
Figure 2.
Changes in ETLs from admission to follow-up showing the dB level of each frequency measured (1–10 kHz). The red line shows admission ETL; the blue line shows ETLs on day 3 (72 h after admission); the green line shows ETLs at final follow-up. The black dotted line shows the mean ETL level of age- and sex-matched controls. The gray scattered area at the bottom of each graph is the noise floor. The ETL level of detection was calculated from the noise floor with the addition of SNR +3 dB (low, mid, and mid-high frequencies) and +6 dB (high frequencies). A and B, A male (age 20) and female (age 18) with N. meningitidis meningitis. C and D, A male (age 50) and female (age 61) with S. pneumoniae meningitis. E and F, A male (age 65) and female (age 75) with H. influenzae meningitis. G and H, A male (age 71) and female (age 64) with other pathogens (S. aureus and E. coli, respectively). I and J, A female (age 67) and male (age 64) with meningitis with unidentified pathogens. Abbreviations: ETL, emission threshold level; SNR, signal-to-noise ratio.
Table 3.
Loss and Recovery of Emissions From Admission to Follow-up
| … | … | … | No. With Emission Loss | Age | Admission Emission Loss—ETLs in Db Compared With Mean Control Group Level, Median (Min/Max) | Emission Loss Follow-up | Final Follow-up Emission Loss—ETLs in Db Compared With Mean Control Group Level, Median (Min/Max) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathogen | Sex | No. | No./No. | Median and Range | Low Frequency 1, 1.5, 2 kHz | Mid Frequency 3, 4, 5 kHz | Mid-High Frequency 6, 7, 8 kHz | High Frequency 9, 10 kHz | No./No. | Low Frequency 1, 1.5, 2 kHz | Mid Frequency 3, 4, 5 kHz | Mid-High frequency 6, 7, 8 kHz | High Frequency 9, 10 kHz |
| All | F | 10 | 9/10 (90) | 67 (17 to 81) | 7.0 (2.4 to 13.3) | 7.6 (1.7 to 13.6) | 0.0a (0.0 to 1.2) | 0.0a (0.0 to 8.8) | 8/9 (72) | 7.0 (0.0 to 13.3) | 13.0 (0.0 to 14.0) | 14.0b (−) | 11.1b (−) |
| M | 18 | 13/18 (72) | 68 (19 to 82) | 10.3 (2.2 to 16.0) | 6.9 (1.1 to 12.4) | 0.0c (0.0 to 2.3) | 2.4d (0.0 to 4.7) | 7/11 (72) | 3.5 (0.0 to 13.8) | 1.8 (0.0 to 4.0) | 0.0a (−) | 2.4 (0.0 to 2.7) | |
| Streptococcus pneumoniae | F | 5 | 5/5 (100) | 67 (61 to 73) | 7.0 (5.1 to 13.3) | 4.3 (4.3 to 13.6) | BD | BD | 4/4 (100) | 7.9 (7.1 to 13.3) | 13.3 (6.9 to 13.6) | BD | BD |
| M | 4 | 3/4 (75) | 70 (50 to 82) | 13.8 (2.2 to 16.0) | 6.9 (4.0 to 9.7) | BD | 2.3 (2.1 to 2.4) | 3/3 (100) | 9.2 (3.5 to 13.8) | 1.8 (1.1 to 4.0) | BD | 2.1 (1.7 to 2.4) | |
| Haemophilus influenzae | F | 1 | 1/1 (100) | 75 (−) | 3.3 (−) | 1.2 (−) | BD | BD | 1/1 (100) | 0.0 (−) | 2.7 (−) | BD | BD |
| M | 3 | 3/3 (100) | 65 (64 to 66) | 13.6 (3.8 to 13.8) | 4.0 (4.0 to 4.0) | BD | 1.2 (0.0 to 2.4) | 3/3 (100) | 6.7 (2.3 to 13.3) | 0.0 (0.0 to 4.0) | BD | 2.4 (0.0 to 2.4) | |
| Neisseria meningitidis | F | 1 | 1/1 (100) | 18 (−) | 12.9 (−) | 7.6 (−) | 0.0 (−) | 8.8 (−) | 1/1 (100) | 1.8 (−) | 14.0 (−) | 14.0 (−) | 11.1 (−) |
| M | 2 | 2/2 (100) | 20 (19 to 20) | 3.5 (0.0 to 6.9) | 5.5 (0.0 to 11.1) | 0.0 (0.0 to 0.0) | 2.8 (0.0 to 4.6) | 0/2 (0) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) | |
| Other pathogen | F | 2 | 1/2 (50) | 73 (64 to 81) | 12.7 (−) | 13.6 (−) | BD | BD | 1/2 (50) | 13.3 (−) | 13.6 (−) | BD | BD |
| M | 6 | 3/6 (50) | 73 (50 to 79 | 6.5 (0.0 to 6.8) | 1.4 (1.1 to 6.3) | BD | 2.3 (2.1 to 2.4) | 1/3 (33) | 9.3 (−) | 1.8 (−) | 0.0 (−) | 2.4 (−) | |
| Unidentified pathogen | F | 1 | 1/1 (100) | 67 (−) | 2.5 (−) | 9.1 (−) | BD | BD | 1/1 (100) | 1.0 (−) | 2.5 (−) | BD | BD |
| M | 3 | 1/3 (33) | 69 (37 to 69) | 15.8 (−) | 12.4 (−) | 2.3 (−) | 0.0 (−) | 0 | NA | NA | NA | NA | |
Data are shown as No. (%) or median with full range (min/max) in each frequency category. The emission loss in dB was calculated as the difference from the control group mean ETL to the patients’ ETLs. In cases with absent emissions, the difference was calculated from control group mean to the noise floor, where the SNR was +3 dB (low, mid, and mid-high frequencies) and +6 dB (high frequencies).
Abbreviations: BD, below level of detection; ETL, emission threshold level; NA, data not available; SNR, signal-to-noise ratio.
ETL was below age-related level of detection (BD) in 6 patients.
ETL was below age-related level of detection (BD) in 7 patients.
ETL was below age-related level of detection (BD) in 10 patients.
ETL was below age-related level of detection (BD) in 3 patients.
Admission
On admission, 22 (79%) of 28 patients had decreased ETL in ≥1 frequency group compared with healthy controls. ETL was significantly decreased in all frequency groups compared with age-matched and sex-matched healthy controls (male patients: Wilcoxon-Mann-Whitney test, P < .0001 in low and high frequencies; P = .003 in the mid frequency area; and P = .0001 in the mid-high frequency area; female patients: Wilcoxon-Mann-Whitney test, P < .0001 in low, mid, mid-high, and high frequencies). ETLs were below detection in all frequencies in 9 (32%) patients (5 S. pneumoniae, 2 H. influenzae, 2 other pathogens [E. coli]).
Nine (90%) of 10 female patients presented with decreased ETL on admission compared with 13 (72%) of 18 male patients (Fisher exact test, P > .05). Emission loss in dB was not different between female and male patients in any frequency category (Mann-Whitney test, P > .05).
Emission loss in dB was not different between pathogen groups in any frequency category and also not when comparing S. pneumoniae with all other pathogens combined (Kruskal-Wallis test, P > .05).
Follow-up
At final follow-up after discharge, 17 (71%) of 24 patients had decreased ETLs in ≥1 frequency group. ETLs remained significantly decreased in all frequency groups compared with controls (male patients: Wilcoxon-Mann-Whitney test, P < .0001 in low frequencies; P = .0003 in the mid frequency area; P = .001 in the mid-high frequency area; and P = .0002 in the high frequency area; female patients: Wilcoxon-Mann-Whitney test, P < .0001 in low, mid, mid-high, and high frequencies). ETL was below the border of detection in all frequencies in 5 (21%) of 24 patients (3 S. pneumoniae, 1 H. influenzae, 1 other pathogen [E. coli]). Eight (80%) of 10 female patients had decreased ETLs in ≥1 frequency area at the final follow-up compared with 9 (64%) of 14 male patients (Fisher exact test, P > .05).
Emission loss in dB was not different between pathogens in any frequency area (Kruskal-Wallis test, P > .05). Comparing S. pneumoniae meningitis with the other pathogen groups combined, yielded significantly higher emission loss in S. pneumoniae meningitis in the low (median [IQR], 8.5 [3.5–13.8] dB; vs median [IQR], 1.8 [0–13.3] dB; Mann-Whitney test, P = .021) and mid frequency areas (median [IQR], 4.3 [1.1–13.6] dB; vs median [IQR], 0 [0–3.7] dB; P = .046).
Cochlear Dynamics—Recovery and Decline in OAE
Changes in ETLs, defined as an increase or decrease >2.5 dB in any frequency area, were found in 17 (63%) of 27 patients between admission and day 3; 6 (22%) patients from days 5 to 7; 1 (4%) patient from days 10 to 14. Two patients had emissions below the border of detection from admission to follow-up. Data from 1 patient were only available on admission and follow-up (Supplementary Tables 1 and 2).
Audiometry
Audiometry at discharge was available in 23 (82%) of 28 patients, and audiometry after discharge was available in 18 (64%) of 28 patients (median, 60 days) (Table 4). The 5 patients who did not show up for their second audiometry had either no hearing loss (n = 3) or mild hearing loss (n = 2) in the first audiometry. SNHL was present in 13 (57%) of 23 patients upon the first audiometry and 11 (61%) of 18 patients upon the second audiometry. Cases with severe or profound hearing loss were all due to S. pneumoniae meningitis. SNHL was bilateral in all cases except for 1 patient. In the 18 patients who underwent the second audiometry, hearing was unchanged in 14 patients, improved in 3 patients, and deteriorated in 1 patient. Two patients with S. pneumoniae meningitis were candidates for cochlear implants (but declined).
Table 4.
Results of Audiometry
| No. Patients | ||
|---|---|---|
| Severity of Hearing Loss | First Audiometry, No. (%) | Second Audiometry, No. (%) |
| No HL | 10 (43) | 7 (39) |
| Mild HL | 5 (22) | 5 (28) |
| Moderate HL | 5 (22) | 3 (17) |
| Severe HL | 2 (9) | 2 (11) |
| Profound HL | 1 (4) | 1 (5) |
Classification of sensorineural hearing loss in patients with bacterial meningitis with and without age and sex adjustment. Classification is based on PTA and ΔPTA in the worse ear. No hearing loss (≤20 dB HL), mild (21–40 dB HL), moderate (41–55 dB HL), moderately severe (56–70 dB HL), severe (71–90 dB HL), profound (>90 dB HL). Audiometries were performed 13 days (median) and 60 days (median) after hospitalization. First audiometry (n = 23), second audiometry (n = 18).
Abbreviations: HL, hearing loss; PTA, pure-tone average.
PTA from the first audiometry had significantly higher thresholds compared with the PTA from the age- and sex-matched control data set (mean, 29 dB vs 14 dB; Mann-Whitney test, P = .008).
OAE Correlation With Pure Tone Audiometry
The mean ETLs at the final follow-up in frequencies 1–4 kHz were significantly correlated to PTA results of the first audiometry (P = .003; Spearmann rank, −0.63).
DISCUSSION
The present study is the first to measure otoacoustic emissions through the course of disease in adult patients with bacterial meningitis. Our findings confirm that cochlear injury is the primary cause of hearing loss and deafness in meningitis. Compared with a healthy age- and sex-matched control group, we found that decreased cochlear emissions affected 79% of patients on admission and still 71% at final follow-up.
Repeated OAE measurements revealed that 60% of emission changes—recoveries and declines—occurred within 72 hours of admission, thus suggesting a narrow window for additional preventive measures. This finding is consistent with a previous study in children demonstrating early loss of click stimulus response [1, 10, 11].
Dexamethasone has both clinically and experimentally been shown to significantly reduce hearing loss [31–33]. Still, SNHL was marked among our patients, all of whom received dexamethasone.
Our control group data were in accordance with previous data showing better hearing among women and declining hearing with age [34]. Gender differences were not further analyzed given the higher baseline emission threshold levels observed among healthy female controls.
The limited recovery of cochlear function in patients with S. pneumoniae meningitis is likely related to obliteration of cochlear lumen that may occur in the weeks after hearing loss has developed [24, 25]. Also, ossification of cochlea following bacterial meningitis has been demonstrated experimentally [22]. Experimental studies have greatly improved our understanding of the pathophysiology of suppurative labyrinthitis in ABM and hearing loss, elucidating the spread of bacterial and inflammatory debris from the cerebrospinal fluid and vice versa [20–23]. A bilateral open cochlear aqueduct or hematogenic spread is supported by our data, in which hearing loss was observed in both ears with no significant difference between the right and left ears.
SNHL, defined by PTA, was present in 13 (57%) patients in the first audiometry. This is higher than previously reported but is supported by recent data from a retrospective study [35]. Another recent systematic review reported a much lower incidence of hearing loss of 14% and an incidence of 5% for profound hearing loss following bacterial meningitis [31]. The high frequency of SNHL may by the result of the high number of patients with S. pneumoniae meningitis, which is known to result in the highest incidence of meningitis-related hearing loss [3]. This is consistent with our results, where severe or profound hearing loss was observed predominantly among patients surviving S. pneumoniae meningitis (Figure 2). However, cases due to other pathogens were few, and not all our patients underwent audiometry; this may have biased our results. Although the worst hearing outcome was observed in S. pneumoniae meningitis, not all patients had SNHL defined by PTA. Therefore, DPOAE measurements in patients with normal hearing defined by PTA may provide an early indication of hearing loss in patients at risk of presbycusis.
Our study was limited by the ability of OAE to detect emission loss in the frequencies above 5 kHz due to age-related hearing loss naturally occurring in our study group. The limited sample size restricted further analysis of factors associated with emission loss. Calculating emission loss as the mean value from both ears and not only the “worse ear” may also contribute to lower levels of emission loss. The clinical impact of the measured changes in ETLs may be subclinical, and a definite border of significance was not clarified. With respect to this, our application of SNR +3 to +6 dB reduced the predefined spectrum of detectable emissions. However, an analysis of reproducibility of emissions close to the noise floor was beyond the scope of this study. Lastly, we cannot exclude preexisting hearing loss, and despite careful interview the number of patients excluded due to preexisting hearing loss was low. Essentially, only a premorbid hearing test could fully clarify this issue.
CONCLUSIONS
In ABM, hearing loss is the result of cochlear dysfunction and injury. Emission loss is bilateral, affecting 70% of patients determined by OAE and 56% by audiometry. Hearing loss was primarily observed in S. pneumoniae meningitis, but all pathogens can lead to permanent hearing loss. Our data suggest that local or systemic treatment strategies aimed at preserving cochlear function should be initiated upon admission and no later than day 3.
Our findings underline the importance of audiological evaluation in patients with ABM and provide a framework for future clinical studies.
Supplementary Material
Acknowledgments
Clinical specialist nurse Lea Norman provided technical expertise in supporting OAE testing. Data manager Lars Pedersen, Nordsjællands Hospital, maintained the clinical SQL database.
Financial support. This study was funded by grants from the Nordsjællands Hospital Foundation, Oticon Foundation, Jascha Foundation, Tvergaards Foundation, Helene Rudes Foundation, and Kaptain Løjtnant Harald Jensen og Hustrus Foundation.
Contributor Information
Elisa Skovgaard Jensen, Department of Otorhinolaryngology, Nordsjællands Hospital, University of Copenhagen, Hillerød, Denmark.
Per Cayé-Thomasen, Department of Otorhinolaryngology Head & Neck Surgery and Audiology, University Hospital Copenhagen Rigshospitalet, Copenhagen, Denmark; Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Jacob Bodilsen, Department of Infectious Diseases, University Hospital Aalborg, Aalborg, Denmark.
Henrik Nielsen, Department of Infectious Diseases, University Hospital Aalborg, Aalborg, Denmark.
Lennart Friis-Hansen, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Department of Clinical Biochemistry, Bispebjerg Hospital, University of Copenhagen, Copenhagen, Denmark.
Thomas Christensen, Department of Neurology, University Hospital Copenhagen Rigshospitalet, Copenhagen, Denmark.
Malina Christiansen, Department of Otorhinolaryngology, Nordsjællands Hospital, University of Copenhagen, Hillerød, Denmark.
Malene Kirchmann, Department of Otorhinolaryngology, Nordsjællands Hospital, University of Copenhagen, Hillerød, Denmark.
Christian Thomas Brandt, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Department of Pulmonary and Infectious Diseases, Nordsjællands Hospital, University of Copenhagen, Hillerød, Denmark; Department of Infectious Diseases, Zealand University Hospital, University of Copenhagen, Roskilde, Denmark.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Dodge PR, Davis H, Feigin RD, et al. Prospective evaluation of hearing impairment as a sequela of acute bacterial meningitis. N Engl J Med 1984; 311:869–74. [DOI] [PubMed] [Google Scholar]
- 2. Heckenberg SGB, Brouwer MC, van der Ende A, Hensen EF, van de Beek D. Hearing loss in adults surviving pneumococcal meningitis is associated with otitis and pneumococcal serotype. Clin Microbiol Infect 2012; 18:849–55. [DOI] [PubMed] [Google Scholar]
- 3. Worsøe L, Cayé-Thomasen P, Brandt CT, Thomsen J, Østergaard C. Factors associated with the occurrence of hearing loss after pneumococcal meningitis. Clin Infect Dis 2010; 51:917–24. [DOI] [PubMed] [Google Scholar]
- 4. Smith RJH, Bale JF, White KR. Sensorineural hearing loss in children. Lancet 2005; 365:879–90. [DOI] [PubMed] [Google Scholar]
- 5. Peltola H, Roine I, Fernández J, et al. Hearing impairment in childhood bacterial meningitis is little relieved by dexamethasone or glycerol. Pediatrics 2010; 125:e1–8. [DOI] [PubMed] [Google Scholar]
- 6. Lempinen L, Laulajainen-Hongisto A, Aarnisalo AA, et al. Hearing impairment in Angolan children with acute bacterial meningitis with and without otitis media. Acta Paediatr 2022; 111:1585–93. [DOI] [PubMed] [Google Scholar]
- 7. Wald ER, Kaplan SL, Mason EO, et al. Dexamethasone therapy for children with bacterial meningitis. Meningitis Study Group. Pediatrics 1995; 95:21–8. [PubMed] [Google Scholar]
- 8. Odio CM, Faingezicht I, Paris M, et al. The beneficial effects of early dexamethasone administration in infants and children with bacterial meningitis. N Engl J Med 1991; 324:1525–31. [DOI] [PubMed] [Google Scholar]
- 9. Lebel MH, Freij BJ, Syrogiannopoulos GA, et al. Dexamethasone therapy for bacterial meningitis. Results of two double-blind, placebo-controlled trials. N Engl J Med 1988; 319:964–71. [DOI] [PubMed] [Google Scholar]
- 10. Rodenburg-Vlot MBA, Ruytjens L, Oostenbrink R, Goedegebure A, van der Schroeff MP. Systematic review: incidence and course of hearing loss caused by bacterial meningitis: in search of an optimal timed audiological follow-up. Otol Neurotol 2016; 37:1–8. [DOI] [PubMed] [Google Scholar]
- 11. Richardson MP, Reid A, Tarlow MJ, Rudd PT. Hearing loss during bacterial meningitis. Arch Dis Child 1997; 76:134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roine I, Pelkonen T, Cruzeiro ML, et al. Fluctuation in hearing thresholds during recovery from childhood bacterial meningitis. Pediatr Infect Dis J 2014; 33:253–7. [DOI] [PubMed] [Google Scholar]
- 13. Brookhouser PE, Worthington DW, Kelly WJ. Fluctuating and/or progressive sensorineural hearing loss in children. Laryngoscope 1994; 104:958–64. [DOI] [PubMed] [Google Scholar]
- 14. Brookhouser PE, Auslander MC, Meskan ME. The pattern and stability of postmeningitic hearing loss in children. Laryngoscope 1988; 98:940–8. [DOI] [PubMed] [Google Scholar]
- 15. Külahli I, Oztürk M, Bilen C, Cüreoglu S, Merhametsiz A, Cağil N. Evaluation of hearing loss with auditory brainstem responses in the early and late period of bacterial meningitis in children. J Laryngol Otol 1997; 111:223–7. [DOI] [PubMed] [Google Scholar]
- 16. Grimwood K, Nolan TM, Bond L, Anderson VA, Catroppa C, Keir EH. Risk factors for adverse outcomes of bacterial meningitis. J Paediatr Child Health 1996; 32:457–62. [DOI] [PubMed] [Google Scholar]
- 17. Vienny H, Despland PA, Lütschg J, Deonna T, Dutoit-Marco ML, Gander C. Early diagnosis and evolution of deafness in childhood bacterial meningitis: a study using brainstem auditory evoked potentials. Pediatrics 1984; 73:579–86. [PubMed] [Google Scholar]
- 18. Jiang ZD, Liu XY, Wu YY, Zheng MS, Liu HC. Long-term impairments of brain and auditory functions of children recovered from purulent meningitis. Dev Med Child Neurol 1990; 32:473–80. [DOI] [PubMed] [Google Scholar]
- 19. Kay R. The site of the lesion causing hearing loss in bacterial meningitis: a study of experimental streptococcal meningitis in Guinea-pigs. Neuropathol Appl Neurobiol 1991; 17:485–93. [DOI] [PubMed] [Google Scholar]
- 20. Brandt CT, Cayé-Thomasen P, Lund SP, et al. Hearing loss and cochlear damage in experimental pneumococcal meningitis, with special reference to the role of neutrophil granulocytes. Neurobiol Dis 2006; 23:300–11. [DOI] [PubMed] [Google Scholar]
- 21. Cayé-Thomasen P, Worsøe L, Brandt CT, et al. Routes, dynamics, and correlates of cochlear inflammation in terminal and recovering experimental meningitis. Laryngoscope 2009; 119:1560–70. [DOI] [PubMed] [Google Scholar]
- 22. Møller MN, Brandt C, Østergaard C, Caye-Thomasen P. Bacterial invasion of the inner ear in association with pneumococcal meningitis. Otol Neurotol 2014; 35:e178–86. [DOI] [PubMed] [Google Scholar]
- 23. Møller MN, Brandt C, Østergaard C, Caye-Thomasen P. Endolymphatic sac involvement in bacterial meningitis. Eur Arch Otorhinolaryngol 2015; 272:843–51. [DOI] [PubMed] [Google Scholar]
- 24. Philippon D, Bergeron F, Ferron P, Bussières R. Cochlear implantation in postmeningitic deafness. Otol Neurotol 2010; 31:83–7. [DOI] [PubMed] [Google Scholar]
- 25. Caye-Thomasen P, Dam MS, Omland SH, Mantoni M. Cochlear ossification in patients with profound hearing loss following bacterial meningitis. Acta Otolaryngol (Stockh) 2012; 132:720–5. [DOI] [PubMed] [Google Scholar]
- 26. Engdahl B, Tambs K, Hoffman HJ. Otoacoustic emissions, pure-tone audiometry, and self-reported hearing. Int J Audiol 2013; 52:74–82. [DOI] [PubMed] [Google Scholar]
- 27. Christiansen M, Jensen ES, Brandt CT, Kirchmann M. Otoacoustic emissions in patients with bacterial meningitis. Int J Audiol 2020; 59:647–53. [DOI] [PubMed] [Google Scholar]
- 28. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988; 16:128–40. [DOI] [PubMed] [Google Scholar]
- 29. Konrad-Martin D, Poling GL, Dreisbach LE, et al. Serial monitoring of otoacoustic emissions in clinical trials. Otol Neurotol 2016; 37:e286–94. [DOI] [PubMed] [Google Scholar]
- 30. ISO 7029:2017 . Acoustics Statistical Distribution of Hearing Thresholds Related to Age and Gender. ASHA; 2017. [Google Scholar]
- 31. Brouwer MC, McIntyre P, Prasad K, van de Beek D. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev 2015; 9:CD004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Worsøe L, Brandt CT, Lund SP, Østergaard C, Thomsen J, Cayé-Thomasen P. Systemic steroid reduces long-term hearing loss in experimental pneumococcal meningitis. Laryngoscope 2010; 120:1872–9. [DOI] [PubMed] [Google Scholar]
- 33. Worsøe L, Brandt CT, Lund SP, Ostergaard C, Thomsen J, Cayé-Thomasen P. Intratympanic steroid prevents long-term spiral ganglion neuron loss in experimental meningitis. Otol Neurotol 2010; 31:394–403. [DOI] [PubMed] [Google Scholar]
- 34. Engdahl B. Otoacoustic emissions in the general adult population of nord-trøndelag, Norway: I. Distributions by age, gender, and ear side. Int J Audiol 2002; 41:64–77. [DOI] [PubMed] [Google Scholar]
- 35. Persson F, Bjar N, Hermansson A, Gisselsson-Solen M. Hearing loss after bacterial meningitis, a retrospective study. Acta Otolaryngol (Stockh) 2022; 142:298–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.