Abstract
The replacement of fishmeal (FM) by insect meal (IM) in aquafeed formulation has been thoroughly studied lately, but little is known about their impact on nutrient metabolism of fish. This study evaluated the impact not only of partial but also total FM replacement by IM on intermediary metabolism of European sea bass (Dicentrarchus labrax). A fishmeal-based diet was used as a control (CTRL) and two other diets were formulated to include 20% and 40% of defatted Tenebrio molitor larvae meal (dTM), replacing 50% (TM50) and 100% (TM100) of fishmeal (FM), respectively. After a 16-week feeding trial, a multidisciplinary approach including assessment of histological, biochemical, molecular, and enzymatic parameters was adopted to investigate hepatic and plasmatic responses to the different dietary formulations. The results obtained demonstrated that dTM can be successfully used to replace 50% of FM in diets for European sea bass, without adversely affecting liver health or intermediary metabolism of nutrients. As for TM100, although no signs of steatosis were observed in the liver, the activity of glycolytic and lipogenic genes and enzymes increased when compared to CTRL diet (P < 0.05), resulting in higher levels of plasmatic non-esterified fatty acids and triacylglycerides (P < 0.05), which in the long-term may compromise fish health, thus precluding such a high degree of substitution for use in practical diets for European sea bass.
Keywords: animal nutrition, hepatic genes and enzymes, insect meal, intermediary metabolism, metabolites
Insect meal has been increasingly considered as protein source in diets for different animal models, including fish, but available studies are mainly focused on growth and nutrient utilization. As far as we are aware of, this is the first study thoroughly evaluating the impact of not only partial but also total fishmeal replacement by defatted Tenebrio molitor larvae meal on intermediary metabolism of market-sized European sea bass, using a multidisciplinary approach including histological, biochemical, molecular, and enzymatic tools.
Introduction
Over the last decades, the outstanding efforts made to find alternative protein sources to replace fishmeal (FM) in aquafeeds have attracted particular interest in fish physiological responses to the new dietary formulations (Aragão et al., 2022). In this regard, the evaluation of the organs involved in regulation of feed intake, digestion, absorption, and metabolism is of paramount importance to understand the feasibility of using new protein sources in aquafeeds formulations. Fish intestine plays a key role in the peripherical regulation of feed intake, digestion and absorption of nutrients and immunity response (Ray and Ringø, 2014; Blanco et al., 2021). After nutrient absorption the liver plays a primary role in the metabolism of dietary nutrients and its morphology and composition is highly dependent on the diet. Therefore, this organ must be the main target when it is intended to deepen knowledge about metabolic responses of fish to new dietary ingredients (Randazzo et al., 2021). The evaluation of hepatosomatic index (HSI) can give an indication of possible metabolic disorders in fish, such as hepatic steatosis (i.e., increased liver size due to lipids accumulation), which is the most common consequence of alterations in the metabolism of different nutrients in fish and may result in growth and health impairments of the animals (Asaoka et al., 2013; Jia et al., 2020). However, such hypothesis must be validated through different methodologies. Histological techniques are the most conventional ones used to identify morphological modifications and lipid deposition in liver, with Oil Red O being not only the most intuitive, but also the most accurate staining for that purpose (Levene et al., 2010; Riva et al., 2018). Additionally, new dietary formulations can also trigger metabolic alterations in the liver by the activation of a set of metabolic-related genes and enzymes with key roles in different pathways (e.g., glycolysis, gluconeogenesis, lipogenesis, lipolysis, β-oxidation, and amino acid anabolism and catabolism), providing information on metabolic responses of fish to new aquafeeds formulations, even in the absence of clear histo-morphological evidence (Viegas et al., 2014; Zhang et al., 2020).
Since the inclusion of insect meal (IM) from seven insect species in aquafeeds was approved by the European Union (EC Regulation No. 2017/893; European Commission, 2017), the interest in this protein source as alternative to FM has emerged (Alfiko et al., 2022; Tran et al., 2022). Generally, insects have high protein content (up to 75%), with a well-balanced essential amino acids profile; they are rich in minerals (e.g., iron and zinc) and vitamins (e.g., vitamin B12) and have biologically active compounds, such as chitin, antimicrobial peptides, and short-medium fatty acids (e.g., lauric acid) with beneficial effects on intestinal health of different fish species (Randazzo et al., 2021; Weththasinghe et al., 2021). On the other hand, and despite their high fat content, insects have limited amounts or even absence of n-3 long-chain polyunsaturated fatty acids (LC-PUFA), such as eicosapentaenoic and docosahexaenoic acids (EPA and DHA, respectively) (Nogales-Mérida et al., 2018). Since these nutrients are essential for carnivorous marine fish species (NRC, 2011), this may cause constraints when IM is used to replace high levels of FM in diets for such species. Hermetia illucens (HI) and Tenebrio molitor (TM) are the main insect species produced in Europe and have been the most studied as FM alternatives, in diets for both freshwater, and marine fish species with great importance for Mediterranean aquaculture like European sea bass (Dicentrarchus labrax) (Gasco et al., 2016; Henry et al., 2018; Nogales-Mérida et al., 2018; Pippinato et al., 2020). Previous results of the in vivo nutrients’ digestibility of HI and TM, both full-fat and defatted (-d), in European sea bass juveniles demonstrated that dTM is the most promising alternative to FM in diets for this fish species for having both the highest digestible protein (641 mg g−1 DM) and digestible essential amino acids (296 mg g−1 DM) content (Basto et al., 2020). The inclusion of dTM did not alter feed intake and its regulatory mechanisms in European sea bass (Basto et al., 2021a, 2022). However, when dTM was included at high levels (as total FM replacement) in diets for sea bass, induced alterations in hepatic and plasmatic metabolites, suggesting alterations in intermediary metabolism of fish that may compromise growth performance and/or health status of fish at long-term (Basto et al., 2021a, 2021b, 2022).
In this connection, the present study aimed to explore for the first time the impact of partial and total substitution of FM by dTM in a comprehensive approach, focusing on the underlying mechanisms involved in nutrients’ metabolism of European sea bass, through histological, biochemical, molecular, and enzymatic techniques.
Materials and Methods
Ethics statement
Experimental trial and sampling procedures were directed by accredited researchers (following FELASA category C recommendations), conducted according to the guidelines on the protection of animals used for scientific purposes (Directive 2010/63/EU from the European Union), and approved by the Ethical Committee of CIIMAR, overseen by the National Competence Authority (Direção Geral de Alimentação e Veterinária - DGAV).
Experimental diets
According to the nutritional requirements of European sea bass (NRC, 2011), three isoproteic, isolipidic and isoenergetic diets (47% protein, 20% lipids and 24 kJ g -1 on a dry matter basis, respectively) were formulated and extruded by SPAROS Lda. (Portugal). A control diet (CTRL) was formulated with 40% FM and 14% FO and two other experimental diets were formulated to replace 50% and 100% of FM by dTM, on protein basis (TM50 and TM100, respectively). Experimental diets with dTM inclusion (TM50 and TM100) were supplemented with monocalcium phosphate. All experimental diets were supplemented with dl-Methionine. l-Lysine, l-Threonine and l-Tryptophan were also added to TM100 diet. Feed ingredients and proximate composition of experimental diets are presented in Table 1. Experimental diets are the same in this and our previous study (Basto et al., 2022).
Table 1.
Ingredients, chemical composition, and fatty acid profile of dTM and experimental diets.
| dTM | CTRL | TM50 | TM100 | |
|---|---|---|---|---|
| Ingredients (%) | ||||
| Fishmeal 1 | 40.0 | 20.0 | - | |
| Defatted Tenebrio molitor larvae meal | - | 20.5 | 40.4 | |
| Soy protein concentrate 2 | 10.5 | 10.5 | 10.5 | |
| Soybean meal 3 | 13.0 | 13.0 | 13.0 | |
| Rapeseed meal 4 | 5.0 | 5.0 | 5.0 | |
| Wheat meal 5 | 16.2 | 15.2 | 14.3 | |
| Fish oil 6 | 14.0 | 13.3 | 12.5 | |
| Vitamin and mineral premix 7 | 1.0 | 1.0 | 1.0 | |
| Vitamin C | 0.1 | 0.1 | 0.1 | |
| Vitamin E | 0.1 | 0.1 | 0.1 | |
| Monocalcium phosphate | - | 1.0 | 2.0 | |
| L-Lysine | - | - | 0.2 | |
| L-Threonine | - | - | 0.2 | |
| L-Tryptophan | - | - | 0.1 | |
| DL-Methionine | 0.1 | 0.2 | 0.3 | |
| Chemical composition (% DM) | ||||
| Dry matter | 97.8 | 93.1 | 92.6 | 92.5 |
| Protein | 71.0 | 46.9 | 47.3 | 47.2 |
| Lipids | 12.1 | 19.7 | 19.8 | 19.0 |
| Gross energy (kJ g-1 DM) | 24.3 | 23.2 | 23.5 | 24.0 |
| Ash | 4.8 | 10.2 | 8.1 | 6.3 |
| Phosphorus | 0.8 | 1.2 | 1.2 | 1.0 |
The abbreviations for the experimental diets stand for: CTRL—control diet; TM50 and TM100—diets with 50 and 100% fishmeal replacement by insect meal; 1 Peruvian fishmeal super prime: 71% crude protein (CP), 11% crude fat (CF), Exalmar, Peru; 2 Soy protein concentrate: 65% CP, 0.7% CF, ADM Animal Nutrition, The Netherlands; 3 Soybean meal 48: dehulled solvent extracted soybean meal: 48% CP, 2% CF, Cargill, Spain; 4 Rapseed meal: 36% CP, 3% CF, PREMIX Lda., Portugal; 5 Wheat meal: 10% CP, 1% CF, Casa Lanchinha, Portugal; 6 Sardine oil, Sopropêche, France; 7 Vitamin and mineral premix: WISIUM, ADM Portugal S.A., Portugal.
Experimental trial and fish sampling
The trial was conducted in fish holding facilities of CIIMAR (Porto, Portugal). After a three-weeks quarantine period, all fish were individually weighed and homogeneous groups of 15 fish (69 ± 8 g; CV = 8%) were established and distributed into 12 circular fiberglass tanks (160 L), in a seawater recirculation aquaculture system (RAS). The system was supplied with continuous water flow (6 L min−1) and maintained at 22 ± 1 °C, salinity of 35‰, oxygen level > 90% ± 1 saturation, and 12L:12D photoperiod. Levels of total ammonium, nitrite and nitrate (NH4+, NO2− and NO3−, respectively) as well as pH were daily supervised to guarantee levels within the recommended ranged for marine fish species (NH4+ ≤ 0.05 mg L−1; NO2- ≤ 0.5 mg L−1; NO3- ≤ 50 mg L−1; 7.5 ≤ pH ≤ 8.5). Experimental diets were tested in quadruplicate tanks and fish were fed by automatic feeders three times a day, seven days a week, as previously described by Basto et al. (2021b). At the end of 16 weeks of feeding trial, and after a 48-h fasting period, three fish/tank (i.e., n = 12 fish/treatment) were anesthetized (2-phenoxyethanol, 300 µL L−1) before being individually weighed and measured. Blood was sampled from the caudal vein using heparinized syringes, centrifuged (5,000 g for 5 min at 4 °C) and the resulting plasma supernatant was stored at −80 °C for further quantification of metabolite levels. Then, fish were euthanized by spinal cord section, viscera and liver were collected and weighed, and two portions of liver (≈ 150 mg each) were sampled and immediately frozen in dry ice and stored at −80 °C until mRNA relative abundance, metabolite levels and enzymatic activity analysis. Another portion of liver (≈ 1 cm3) was also collected and immediately frozen in isopentane, cooled by dry ice, and stored at −80 °C for further histological evaluation.
mRNA relative abundance analysis by RT-qPCR
Total RNA was extracted from liver using TRIzol reagent (Life Technologies, USA) and treated with RQ1DNAse (Promega, USA) following the manufacturer’s instructions. RNA purity (A260/A280 ratio) was evaluated by spectrophotometry, using a NanoDrop 2000c (Thermo, Vantaa, Finland), and only samples with A260/A280 ratio > 1.8 were used. Subsequently, superscript II reverse transcriptase and random hexamers (Promega, USA) were used to synthesize cDNA from 2 μg of total RNA. The relative expression of genes was assessed by real-time quantitative PCR (RT-qPCR), using the CFX96 Connect Real-Time PCR Detection System (Bio-Rad Laboratories Inc., USA). Reactions were carried out with 50–500 nM of each primer (forward and reverse), MAXIMA SYBR Green qPCR Master Mix (Life Technologies, USA) and 1 μL cDNA, in a total PCR reaction volume of 15 μL. Thermal cycling conditions were 95 °C for 15 min, followed by 40 cycles at 95 °C for 15 s, 56-64 °C for 30 s (annealing temperatures ate present in Table 2), and 72 °C for 30 s. At the end of each run, a post-amplification dissociation curve (55 °C temperature gradient at 0.5 °C/s from 55 to 95 °C) was obtained to ensure reaction specificity. Efficiency ranged between 90 and 105% (Table 2), and each unknown sample was run in duplicate, as well as negative controls without reverse transcriptase or without cDNA. Data were analyzed using the arithmetic mean of β-actin (actb), elongation factor 1α 1 (eef1α1), and ribosomal 18s RNA (18s) as housekeeping genes, according to Pfaffl (2001). The expression of housekeeping and target genes was measured using previously described sequences of primers for the same fish species (Viegas et al., 2013, 2014, 2015; Azeredo et al., 2015; Rimoldi et al., 2015; Cadiz et al., 2018; Basto et al., 2021a; Betancor et al., 2021), which are shown in Table 2. For acly, got1, fbp1 and hk1 new primers were designed using Primer3 software (http://bioinfo.ut.ee/primer3-0.4.0/) and sequenced as previously described by Basto et al. (2022), to ensure that sequences satisfactorily matched the reference GenBank sequences.
Table 2.
Nucleotide sequences of the PCR primers used to evaluate mRNA abundance by RT-PCR (qPCR).
| Gene | Forward | Reverse | Annealing temperature (ºC) | GeneBank acession number |
|---|---|---|---|---|
| acly | AGAGACGGCAGTAGCCAAAA | ATTGGCCACCAGTTCATCAT | 56.0 | * |
| actb | TCCTGCGGAATCCACGAGA | AACGTCGCACTTCATGATGCT | 60.9 | AY148350.1 |
| cpt1a | TGCCAAGAGGTCATCCAGAGTTCT | AGTCCACATCATCCGCCAGAGA | 64.2 | KF857302 |
| eef1a1 | CGTTGGCTTCAACATCAAGA | GAAGTTGTCTGCTCCCTTGG | 55.5 | AJ866727 |
| fasn | CGTCAAGCTCTCCATCCCTG | GGTGGTGTCTAGGCAGTGTC | 59.6 | MF566098 |
| fbp1 | GCTTTGACCCACTGGATGGT | GGCGGGATCAAGCATAAAGC | 58.4 | * |
| g6pc1 | TGAGACCCGGTTTTATGGAG | CATGCAGACCACCAGCTCTA | 57.0 | AM987970 |
| g6pd | GAGATGGTGCAGAACCTCATGG | CCACAGAAGACATCCAGGATGAG | 62.0 | JX073705 |
| gck | ATCGTCAGGGAACTCACACC | GAGTTCAGGCTTGCTTCACC | 58.3 | AM986860 |
| glud1 | CCATCAGCCAGGGAGGAATC | TGCTCATGTAAGACGCCTCG | 58.7 | KF857576 |
| got1 | CTGGGTGGTACAGGTGCTTT | AGCGTTGTGATTTTCCCAAG | 55.8 | * |
| gpt | TGAAGGAGGGGGTCAAGAAA | AGGGTAAGAACACAGAGCCA | 57.4 | JX073702 |
| gys2 | GACAAGGAGGCAGGTGAGAG | GAAGACGTGAGCACAGTGGA | 59.2 | * |
| hadh | CGGAAGTGGTCAGATGGGTG | TCGTCGCTTGTATCCACCAG | 59.0 | KF857303 |
| hk1 | CACCAATGCGTGCTACATGG | GTCGACGTCCCTGTCAAACT | 58.1 | * |
| pck1 | GCGCCATCAACACTAAAGGT | TTGTGCACTCTGTCCTCCAG | 57.2 | DV217087 |
| pklr | CTGTTTCCTGTGGAGGCAGT | CAGCACAGCATTTGAAGGAG | 55.8 | AM981422 |
| pygl | TTCCCAGACAAAGTCGCTGT | GTCTTGTGAGGTCCCATGC | 58.2 | * |
| 18s | CCGCTTTGGTGACTCTAGATAACC | CAGAAAGTACCATCGAAAGTTGATAGG | 59.0 | AY831388.1 |
acly, ATP citrate lyase; actcb, β-actin; cpt1a, carnitine palmitoyltransferase 1a; eef1a1, elongation factor 1α; fasn, fatty acid synthase; fbp1, fructose-1,6-biphosphatase; g6pc1, glucose-6-phosphatase; g6pd, glucose-6-phophate-dehydrogenase; gck, glucokinase; glud1, glutamate dehydrogenase; got1, aspartate aminotransferase 1; gpt, alanine transaminase; gys2, glycogen synthase liver form; hadh, hydroxyacyl-Coenzyme A dehydrogenase; hk1, hexokinase; pck1, phosphoenolpyruvate carboxylase cytosolic form; pklr, pyruvate kinase; pygl, glycogen phosphorylase liver form; 18s, ribosomal RNA 18s. The asterisk (*) means that accession number is pending validation by the National Center for Biotechnology.
Assessment of metabolite levels
For metabolite levels assessment, liver samples were homogenized and deproteinized by ultrasonic disruption in 7.5 vols of ice-cooled 0.6 M perchloric acid and neutralized with 1 M potassium bicarbonate. The homogenate was centrifuged at 10,000 g for 4.5 min at 4 °C, and the supernatant was used to assess tissue metabolite levels. Similarly, plasma samples were also deproteinized (0.6 M perchloric acid), neutralized (1 M potassium bicarbonate), and supernatant was collected after being centrifuged at 13,500 g for 4.5 min at 4 °C. Glucose, lactate, triglycerides (TAG), total cholesterol, and non-esterified fatty acids (NEFA) levels were determined enzymatically using commercial kits (1001190, 1001330, 1001313 and 1001090, Spinreact, Spain, and 434–91795 NEFA-HR (2) R1, and 436–91995 NEFA-HR (2) R2, Wako Chemicals, Germany, respectively), adapting manufacturer’s instructions to a microplate format. Total α-amino acid levels were assessed through the colorimetric ninhydrin method (Moore, 1968), with alanine as standard. Liver glycogen level was assessed using the Keppler and Decker (1974) method.
Assessment of enzyme activities
For enzymatic activity assessment, liver samples (1:9 w/v) were homogenized by ultrasonic disruption in ice-cooled buffer containing 50 mM Tris (pH 7.6), 5 mM EDTA, 2 mM 1,4-dithiothreitol, and a protease inhibitor cocktail (Merck KGaA, Germany). The homogenate was centrifuged at 9,000 g for 11 min at 4 °C, and the supernatant was used for enzyme assays. Enzyme activities were run in 96-well microplates and determined using a microplate reader Infinite 200 Pro (Tecan, Männedorf, Switzerland). Reaction rates of enzymes were determined by the increase or decrease in absorbance of NAD(P)H at 340 nm or, in the case of carnitine palmitoyl transferase 1a (Cpt1L; EC 2.3.1.21) activity, of 5,5-dithiobis-(2-nitrobenzoic acid)-CoA complex at 412 nm. To ensure that enzyme activities were assessed at maximum rates, optimal substrate concentrations were determined through preliminary tests, adapting to European sea bass the previously developed methods for rainbow trout (Oncorhynchus mykiss) (Polakof et al., 2007a, 2007b, 2008a, 2008b, 2008c; Librán-Pérez et al., 2012, 2013a, 2013b). The reactions were started by the addition of supernatant (10–25 μL), omitting the substrate in control wells (final volume 180–295 μL) and allowing the reactions to proceed at 37 °C for preestablished time periods (10–45 min). The results of enzymatic activity are expressed per protein level, which was assayed in triplicate in homogenates, according to the bicinchoninic acid method (Smith et al., 1985), with bovine serum albumin as standard (Merck KGaA, Germany). Hexokinase (Hk; EC 2.7.1.1) and glucokinase (Gck; EC 2.7.1.2) activities were assessed in a Tris–HCl buffer (80 mM, pH 7.0) containing 10.2 mM KCl, 37.5 mM MgCl2, 11.5 mM KH2PO4, 20 mM NaHCO3, 4 mM EDTA, 2.6 mM DTT, 2 mM NADP+, 7 mM ATP, 0.13 U mL−1 glucose 6-phosphate dehydrogenase, 0.13 U mL−1 6-phosphogluconate dehydrogenase, and 1.2 M (Hk) or 12 mM (Gck) d-glucose (omitted for controls). Pyruvate kinase (Pk; EC 2.7.1.40) activity was assessed in an imidazole buffer (50 mM, pH 7.4) containing 100 mM KCl, 10 mM MgCl2, 0.5 mM ADP, 0.15 mM NADH, 21 U mL−1 lactate dehydrogenase, and 2 mM phospho(enol)pyruvate (omitted for controls). Phosphoenolpyruvate carboxykinase cytosolic form (Pepck1; EC 4.1.1.32) activity was assessed in a Tris–HCl buffer (50 mM, pH 7.5) containing 1 mM MnCl2, 20 mM NaHCO3, 1.5 mM phospho(enol)pyruvate, 0.3 mM NADH, 1.7 U mL−1 malate dehydrogenase, and 0.5 mM 2’-deoxyguanosine-5-diphosphate (omitted for controls). Fructose 1,6-bisphosphatase (Fbpase; EC 3.1.3.11) activity was assessed in an imidazole buffer (85 mM, pH 7.7) containing 5 mM MgCl2, 0.5 mM NADP+, 2.5 U mL−1 6-phosphoglucose isomerase, 0.8 U mL−1 glucose 6-phosphate dehydrogenase, and 7 mM fructose 1,6-bisphosphate (omitted for controls). Glucose 6-phosphatase (G6pase; EC 3.1.3.9) activity was assessed in an imidazole buffer (100 mM, pH 6.5) containing 25 mM d-glucose 6-phosphate (omitted for controls). Glycogen synthase liver form (Gsase; EC 2.4.1.11) activity was assessed in an imidazole buffer (50 mM, pH 7.5) containing 150 mM KCl, 15 mM MgCl2, 2 mg mL−1 glycogen, 1.5 mM phospho(enol)pyruvate, 0.3 mM NADH, 4.6 mM D-glucose 6-phosphate, 1.4 U mL−1 lactate dehydrogenase, 1.4 U mL−1 pyruvate kinase, and 100 mM uridine diphosphoglucose (omitted for controls). Glycogen phosphorylase liver form (Gpase; EC 2.4.1.1) activity was assessed in a phosphate buffer (50 mM, pH 7.0) containing 27 mM MgSO4, 24.2 mM EDTA, 2.5 mM AMP, 0.5 mM NADP+, 1.7 U mL−1 phosphoglucomutase, 6.8 U mL−1 glucose 6-phosphate dehydrogenase, 10 μM α-D-glucose 1,6-bisphosphate, and 40 mg mL−1 glycogen (omitted for controls). Glucose-6-phosphate-dehydrogenase (G6pdh; EC 1.1.1.49) activity was assessed in an imidazole buffer (78 mM, pH 7.7) containing 5 mM MgCl2, 0.5 mM NADP+, and 20 mM D-glucose 6-phosphate (omitted for controls). ATP citrate lyase (Acly; EC 4.1.3.8) activity was assessed in a Tris–HCl buffer (50 mM, pH 7.8) containing 100 mM KCl, 10 mM MgCl2, 20 mM sodium citrate, 10 mM β-mercaptoethanol, 5 mM ATP, 0.3 mM NADH, 7 U mL-1 malate dehydrogenase, and 8 mM Coenzyme A (omitted for controls). Fatty acid synthase (Fas; EC 2.3.1.85) activity was assessed in a phosphate buffer (0.1 mM K2HPO4 and 0.1 mM KH2PO4, pH 6.5) containing 0.1 mM NADPH, 25 μM acetyl-CoA, and 40 μM malonyl-CoA (omitted for controls). Carnitine palmitoyl transferase 1 (Cpt1L; EC 2.3.1.21) activity was assessed in a Tris–HCl buffer (75 mM, pH 8.0) containing 1.5 mM EDTA, 0.25 mM DTNB, 35 μM palmitoyl-CoA, and 5.5 mM l-carnitine (omitted for controls). Aspartate aminotransferase 1 (Ast1; EC 2.6.1.1) activity was assessed in an imidazole buffer (50 mM, pH 7.8) containing 10 mM α-ketoglutarate, 0.05 mM pyridoxal 5’-phosphate, 0.3 mM NADH and 1.5 mM l-aspartate (omitted for controls). Alanine transaminase (Aat1; EC 2.6.1.2) activity was assessed in an imidazole buffer (50 mM, pH 7.8) containing 10 mM α-ketoglutarate, 0.025 mM pyridoxal 5’-phosphate, 0.2 mM NADH, 21 U mL-1 lactate dehydrogenase and 14 mM l-alanine (omitted for controls). Glutamate dehydrogenase (Gdh; EC 1.4.1.2) activity was ssessed in an imidazole buffer (50 mM, pH 7.8) containing 250 mM ammonium acetate, 0.10 mM NADH, 1 mM ADP and 0.35 mM α-ketoglutarate (omitted for controls).
Histological analysis
Frozen sections of liver (12 μm) were obtained with a cryostat and stained with Oil Red O, using a commercial kit (010303, DIAPATH S.p.A., Italy) according to manufacturer’s instructions. To evaluate the degree of accumulation of lipid droplets each section was totally examined at a 200× magnification under a light microscope (Olympus BX51, 194 GmbH, Germany) coupled with a camera (Olympus DP50, GmbH, Germany), using an imaging software (Olympus cellSens Dimension Desktop). A semi-quantitative approach was used, according to the following five scores and criteria: Score 0—absence of lipid droplets; Score 1 (low) – on average, < 1/3 of the hepatocyte cytoplasm shows lipid droplets; Score 2 (moderate) – on average, 1/3 < × < 2/3 of the hepatocyte cytoplasm shows lipid droplets; Score 3 (high) – on average, > 2/3 of the hepatocyte cytoplasm shows lipid droplets; Score 4 (extreme) – 3/3 of the hepatocyte cytoplasm shows lipid droplets.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 26.0 (IBM corporation, USA). All variables were tested for normality and homogeneity of variances using Kolmogorov-Smirnov and Levene’s tests, respectively, followed by one-way ANOVA. When one-way ANOVA showed significance (P < 0.05), groups were compared using Tukey’s HSD multiple comparison test.
Results
Growth performance, somatic indexes and histological evaluation of liver
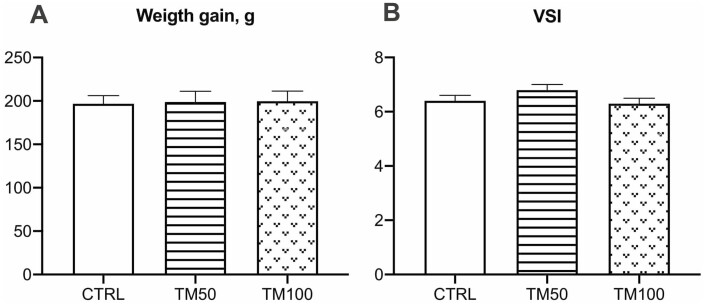
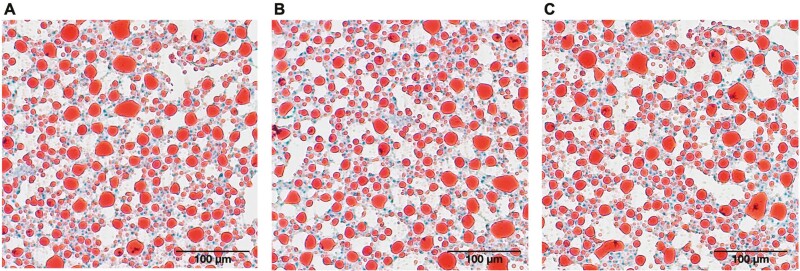
After 16 weeks of feeding trial, the dietary inclusion of dTM did not affect fish growth nor HSI (Figure 1A-B). In general, European sea bass hepatocytes showed moderate accumulation of lipid droplets (Score 2), and no significant differences were observed among dietary treatments (Figure 2).
Figure 1.
Weight gain (A) and hepatosomatic index (B) of European sea bass fed for 16 weeks with control diet containing 40% fishmeal (CRTL) or with experimental diets formulated with 20% and 40% of dTM resulting in 50% (TM50) or 100% (TM100) fishmeal replacement. Bars represent means ± standard deviation of the mean (n = 4 for weight gain (mean of body weight of 15 fish per tank) and n = 24 fish per treatment for hepatosomatic index).
Figure 2.
Representative pictures of fat deposit stained with Oil Red in histological liver sections of European sea bass fed for 16 weeks with control diet containing 40% fishmeal (CRTL, A) or with experimental diets formulated with 20% and 40% of dTM resulting in 50% (TM50, B) or 100% (TM100, C) fishmeal replacement.
Liver and plasma metabolite levels
The hepatic levels of glycogen, glucose, lactate, total α-amino acid, NEFA, triglyceride, and cholesterol did not change with dietary inclusion of dTM (Table 3). Plasmatic levels of NEFA and TAG significantly increased in fish fed TM50 or TM100 diets when compared to those fed CTRL diet, whereas plasmatic levels of glucose, lactate, total α-amino acid, and cholesterol remained similar among dietary treatments (Table 3).
Table 3.
Liver and plasma metabolite levels of European sea bass fed for 16 weeks with control diet containing 40% fishmeal (CRTL) or with experimental diets formulated with 20% and 40% of dTM resulting in 50% (TM50) or 100% (TM100) fishmeal replacement.
| CTRL | TM50 | TM100 | P-value | |
|---|---|---|---|---|
| Liver (mmol g -1 ) | ||||
| Glycogen | 34.8 ± 2.7 | 36.4 ± 2.6 | 34.7 ± 3.7 | 0.126 |
| Glucose | 31.3 ± 0.2 | 31.2 ± 0.7 | 32.8 ± 1.0 | 0.083 |
| Lactate | 1.3 ± 0.2 | 1.4 ± 0.2 | 1.2 ± 0.2 | 0.690 |
| Total α-amino acids | 23.8 ± 2.4 | 21.5 ± 1.8 | 23.8 ± 2.8 | 0.750 |
| NEFA | 0.3 ± 0.04 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.972 |
| TAG | 3.2 ± 0.2 | 3.1 ± 0.3 | 3.5 ± 0.3 | 0.775 |
| Cholesterol | 0.3 ± 0.03 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.964 |
| Plasma (mmol L -1 ) | ||||
| Glucose | 6.3 ± 0.4 | 6.4 ± 0.7 | 6.9 ± 0.5 | 0.706 |
| Lactate | 9.3 ± 0.8 | 7.9 ± 0.8 | 8.8 ± 1.1 | 0.592 |
| Total α-amino acids | 5.0 ± 0.3 | 5.1 ± 0.3 | 6.1 ± 0.6 | 0.203 |
| NEFA | 0.1 ± 0.01 a | 0.2 ± 0.02 b | 0.2 ± 0.02 b | 0.016 |
| TAG | 3.7 ± 0.1 a | 5.6 ± 0.6 b | 5.6 ± 0.5 b | 0.019 |
| Cholesterol | 7.9 ± 0.4 | 8.5 ± 0.8 | 8.6 ± 0.7 | 0.749 |
The abbreviations stand for: NEFA, non-esterified fatty acids; TAG, triglyceride. Values are presented as mean ± standard deviation of the mean (n = 12 fish per treatment). Significant differences (P < 0.05) among groups are denoted with different letters.
Metabolic-related genes and enzymes in liver
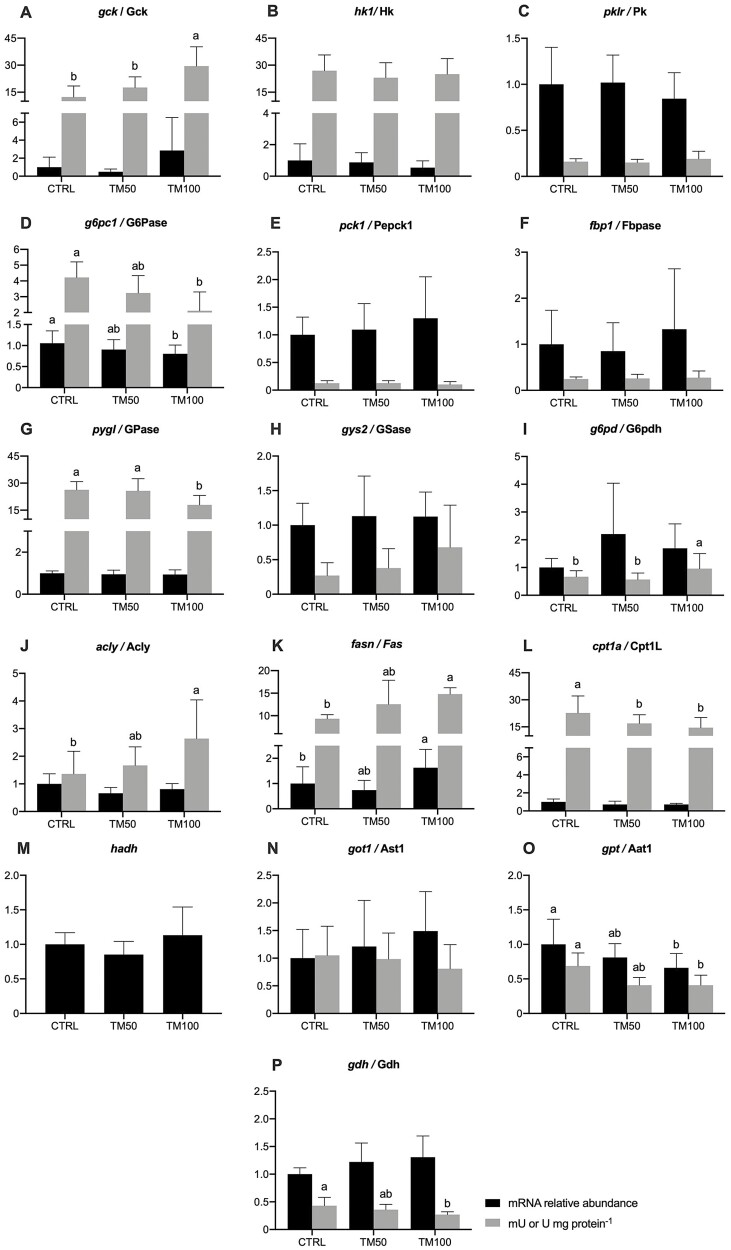
Although the mRNA abundance of glycolysis-related genes (gck, hk1 and pklr) did not significantly vary with dietary inclusion of dTM (Figure 3A-C), the activity of Gck significantly increased in fish fed TM100 when compared to those fed CTRL and TM50 (Figure 3A). Similarly to results of mRNA abundance, the activity of Hk and Pk remained similar between fish fed CTRL and dTM diets (Figure 3B-C). Amongst the gluconeogenesis-related genes and their encoded enzymes, only g6pc1/G6pase significantly decreased in fish fed TM100 when compared to those fed CTRL diet (Figure 3D), whereas pck1/Pepck1 and fbp1/Fbpase remained similar among all dietary treatments (Figure 3E-F). The mRNA abundance of glycogenolysis-related gene pygl did not change with dTM dietary inclusion, but the activity of Gpase was significantly lower in fish fed TM100 than in fish fed CTRL or TM50 diets (Figure 3G). The glycogenesis-related gene gys2 and its encoded enzyme Gsase remained similar among dietary treatments (Figure 3H). The mRNA abundance of pentose phosphate pathway-related gene g6pd also did not change with dietary inclusion of dTM, but the activity of its encoded enzyme G6pdh significantly increased in fish fed TM100 when compared to those fed CTRL and TM50 (Figure 3I). Likewise, total FM replacement by dTM also resulted in increased activity of the lipogenic enzyme Acly compared with controls, whereas mRNA relative abundance of acly did not vary among dietary treatments (Figure 3J). The mRNA abundance of fasn and the activity of its encoded enzyme Fas significantly increased in fish fed and TM100 when compared to those fed CTRL (Figure 3K). The β-oxidation-related gene cpt1a showed no significant differences whereas its encoded enzyme Cpt1L displayed lower activities in fish fed TM50 or TM100 when compared with fish fed with CRTL diet (Figure 3L). The mRNA abundance of hadh did not vary significantly with dietary inclusion of dTM (Figure 2M). Concerning amino acid metabolism-related genes and enzymes, the mRNA abundance of got1 and its encoded enzyme Ast1 remained similar among dietary treatments (Figure 3N). On the other hand, both mRNA abundance of gpt and the activity of its encoded enzyme Aat1 significantly decreased in fish fed TM100 diets when compared to those fed CTRL diet (Figure 3O). Finally, the mRNA abundance of glud1 was not altered by feeding experimental diets, but the activity of its encoded enzyme Gdh was significantly lower in fish fed TM100 than in those fed CTRL diet (Figure 3P).
Figure 3.
mRNA abundance and activity of key genes and enzymes of intermediary metabolism in liver of European sea bass for 16 weeks with control diet containing 40% fishmeal (CRTL) or with experimental diets formulated with 20% and 40% of dTM resulting in 50% (TM50) or 100% (TM100) fishmeal replacement. Bars represent means ± standard deviation of the mean (n = 12 fish per treatment). Significant differences (P < 0.05) among groups are denoted with different letters.
Discussion
In previous studies, we demonstrated that partial (up to 80%) and total dietary FM replacement by dTM neither affected homeostatic regulation of feed intake nor growth performance of European sea bass, but hepatic and plasmatic metabolites suggested alterations in intermediary metabolism when FM was totally replaced by dTM (Basto et al., 2021a, 2021b, 2022). Therefore, we aimed to thoroughly characterize those metabolic changes. The present study showed that a diet devoid of FM and with a high IM inclusion (TM100) induced the glycolytic, glycogenolytic, pentose phosphate and lipogenic pathways, whereas inhibited the gluconeogenesis and FAs β-oxidation.
In the present study, partial (50%) and total FM replacement by dTM also did not affect weight gain of European sea bass after 16 weeks of feeding trial. However, fish fed TM50 and TM100 diets displayed higher levels of NEFA and TAG in plasma than those of fish fed the control diet. Since liver is the main organ involved in intermediary metabolism in fish, we assessed changes in the mRNA abundance and activities of main enzymes involved in glucose, lipid, and amino acid metabolism to ascertain the origin of the changes in plasma.
It is well known that plasmatic glucose levels are maintained in homeostasis with those present in liver due to the existence of specific diffusion through glucose transporters (Polakof et al., 2012). In the liver, excess of glucose may be stored as glycogen through the action of Gsase enzyme (glycogenesis), or catabolized through the glycolytic pathway, followed by tricarboxylic acid (TCA) cycle and respiratory chain for energy production, or through the pentose phosphate pathway resulting in the production of NADPH and ribose 5-phosphate, needed for de novo lipid biosynthesis (lipogenesis) or nucleotide synthesis, respectively (Polakof et al., 2012). On the other hand, under hypoglycaemic conditions, glucose requirements can be satisfied by glycogen depletion to glucose through the action of Gpase (glycogenolysis), or gluconeogenesis from non-carbohydrate sources through the action of Pepck and Fbpase (Polakof et al., 2012). In the present study, we observed that total FM replacement by dTM led to an increase in the use of glucose in liver, as supported by increased Gck activity. The lack of changes in Hk relates to the more important role of Gck in liver of fish (Polakof et al., 2012).
The increased availability of glucose 6-phosphate resulting from the increased activity of Gck can be used through three different pathways (Enes et al., 2009; Polakof et al., 2012). First, this glucose is not apparently used through glycolysis since no changes occurred in the mRNA abundance and activity of the glycolytic enzyme Pk. The lack of changes observed in parameters indicative of glycolytic capacity occurred in parallel with the lack of changes observed in parameters related to gluconeogenesis, such as the mRNA abundance and activity of the enzymes Fbpase and Pepck1. As a second possibility, the increased availability of glucose 6-phosphate could have been used to synthesize glycogen through glycogenesis. However, no main changes occurred in glycogen levels as well as in mRNA abundance and activity of the glycogenic enzyme Gsase (though a decrease occurred in the activity of the glycogenolytic enzyme Gpase). The third pathway through which increased availability of glucose 6 phosphate could have been used is glucose release to the plasma. This capacity is however inhibited in liver, based on changes displayed by the responsible enzyme G6pase in terms not only of changes in mRNA abundance but also of enzyme activity. Finally, the increased glucose 6-phosphate levels resulting from Gck activity could have been used through pentose phosphate pathway involved in the production of reducing power in the form of NADPH. This pathway seems to be activated since increased activity of the main regulatory enzyme of the pathway, i.e., G6pdh increased in fish fed TM100. As a whole, changes occurring in glucose metabolism in liver indicate that feeding TM100 result in increased use of glucose, which is not being use to provide energy to hepatic cells (no changes in glycolytic/gluconeogenic capacities), to store glycogen (no changes in glycogen levels as well as in glycogenic/glycogenolytic capacities) or to be released into plasma (no changes in levels of plasma glucose and the activity of the liver enzyme G6pase). The increased use of glucose is therefore apparently directed to the pentose phosphate pathway thus providing NADPH, which is usually associated with increased capacity for lipid synthesis.
The increased capacity of liver to synthesize lipid, based on changes in glucose metabolism described above, must be supported by changes in the mRNA abundance and/or activity of enzymes involved in lipid metabolism. Acly represents the starting point of lipogenesis, which is initiated by conversion of citrate into acetyl-CoA and oxaloacetate through the action this enzyme. Afterwards, occurs the carboxylation of acetyl-CoA to malonyl-CoA by acetyl-CoA carboxylase, and the final step of lipogenesis is characterized by the action of Fas under malonyl-CoA (Zhang et al., 2020). In its turn, malonyl-CoA inhibits Cpt1L, the first rate-limiting enzyme of β-oxidation. In these processes, significant amounts of NADPH are required for reducing power, and it is supplied by the action of dehydrogenases of the pentose phosphate pathway, such as G6pdh (Weil et al., 2013), as commented above. Accordingly, the mRNA abundance and the activity of the lipogenic enzyme Fas, as well as the activity of the lipogenic enzyme Acly clearly increased in fish fed with TM100 compared with controls. A rise in mRNA abundance of lipogenic enzymes also occurred in liver of rice field eel (Monopterus albus) in which FM was partially replaced by HI (Hu et al., 2020) whereas in gilthead sea bream (Sparus auratus) a 30% replacement of FM by TM did not affect liver lipogenic capacity (Mastoraki et al., 2022). This increased lipogenic capacity matches clearly with the increased activity of G6pdh mentioned above. Furthermore, we also assessed in liver the activity and/or mRNA abundance of enzymes involved in lipolysis in liver. While no changes occurred for mRNA abundance of hadh (transcript of the Hadh lipolytic enzyme), a clear decrease occurred in the activity of Cpt1, one of the enzymes involved in fatty acid oxidation in liver. This is indicating that not only lipogenic pathway is activated but also that lipolytic pathway is inhibited. Guerreiro et al. (2020) observed that 52% FM substitution by partially defatted HI in meagre (Argyrosomus regius) decreased mRNA abundance of hadh, which is involved in FAs β-oxidation. Considering that no changes occurred in liver lipid levels, as demonstrated by both biochemical and histological measurements, the present results suggest that a fast mobilization of synthesized lipids occur in liver, and these lipids are exported into plasma to be used in other tissues. This would help to explain why NEFA and TAG levels increase in plasma (but not in liver) of fish fed TM100 diet. These results are in line with those obtained in our previous studies in the same species and may explain the increased whole-body fat content observed when FM was totally replaced by dTM (Basto et al., 2021b, 2022). In other studies, increased levels of lipid occurred in liver such as in zebrafish (Danio rerio) fed with a diet in which FM was replaced by HI (Zarantoniello et al., 2020) pointing to differences in the speed of FA mobilization between species and IM used. Besides, when dTM was used as single protein source in diets for European sea bass for 10 weeks, it was observed a down-regulation trend of peroxisome proliferator-activated receptor-α (pparα) (Basto et al., 2021b), which is a nuclear hormone receptor that regulates β-oxidation of fatty acids (Li et al., 2020), highlighting that total FM replacement by dTM inhibits β-oxidation pathway, in a way comparable to that herein observed.
According to Engelking (2015), Ast1, Aat1 and Gdh are considered the most relevant enzymes involved in amino acid metabolism. Aat1 and Ast1 are responsible for transamination of alanine and aspartate to pyruvate and glutamate, respectively. Gdh is a mitochondrial enzyme that reversibly converts glutamate to α-ketoglutarate and NH4+. Except for Ast1, changes observed in the other enzymes after feeding diets are comparable, with decreased activity (also mRNA abundance in the case of gpt) in fish fed TM100 diet compared with controls. This reduced activity clearly indicates a reduced capacity of the liver to oxidize amino acid both for fuelling purposes and/or to synthesize glucose through gluconeogenesis, which is one of the main pathways in which amino acids are involved in the metabolism of teleost liver (Polakof et al., 2012). Since amino acids are a primary source of carbon for gluconeogenesis, such decline of Aat1 activity contributes to explain the reduction of gluconeogenesis in fish fed TM100, as previously observed through the reduced activity of G6Pase. Comparable decreases in the oxidative capacity of amino acids occurred in gilthead sea bream (Sparus aurata) and tench (Tinca tinca) fed with a diet with a partial replacement of FM by TM (Fabrikov et al., 2020). However, in other studies no changes occurred in the capacity for oxidizing amino acids. Thus, Mastoraki et al. (2020) evaluated the effect of 30% FM replacement by different IM on amino acids metabolism of European sea bass and concluded that partially defatted HI and full-fat TM or Musca domestica did not affect the activity of Ast1, Aat1 and Gdh. In gilthead sea bream, 50% of FM replacement by full-fat TM or HI also did not change the activity of Ast1, Aat1, and Gdh (Fabrikov et al., 2021), and a similar lack of changes also occurred when 30% of FM was replaced by TM (Mastoraki et al., 2022). In mammals, serum activities of Ast1, Aat1 and their ratio are commonly evaluated as biomarkers for liver health (Gwaltney-Brant, 2016). Thus, our findings suggest that partial or complete replacement of FM by dTM might have a protective effect in the liver of European sea bass. Similar results were obtained by Belghit et al. (2019) when FM was totally replaced by partially defatted HI in diets for sea-water phase of Atlantic salmon (Salmo salar). Regarding Gdh, Skiba-Cassy et al. (2016) suggested that dietary excess of methionine in diets for rainbow trout triggered amino acid deamination and an increase in the activity of this enzyme. In this regard, the observed decrease of Gdh activity in the liver of fish fed TM100 may suggest the deficit of some amino acid. Besides, since the activity of Aat1 decreased in fish fed TM100, lower concentrations of glutamate were expected, and thus a decreased activity of Gdh. However, further studies are needed to better understand the impact of different dietary formulations on either Ast1, Aat1 or Gdh.
Conclusions
Despite no signs of steatosis were observed in the liver of fish fed TM100, this dietary treatment induced a clear increase in the liver capacity to use glucose and a decrease in the capacity of using amino acids. These changes result in redirecting liver metabolism to an enhanced lipogenic capacity reflected in high levels of plasmatic NEFA and TAG, which in the long-term may compromise fish health. Thus, despite 100% replacement of FM by dTM render comparable growth and weight gain, the important number of metabolic changes described above preclude to consider such a level of replacement for the practical formulation of diets for European sea bass. Contrarily to the current findings, Chemello et al. (2020) demonstrated that total FM replacement by partially defatted TM did not alter activity of Ast1, Aat1, Gdh, G6pdh, and Fas in the liver of rainbow trout. However, it is important to point out that in the study conducted by Chemello et al. (2020) a total FM replacement was achieved with the inclusion of 20% IM, whereas in the present study to totally substitute FM it was necessary to include 40% of dTM. Thus, the results obtained by Chemello et al. (2020) are not completely comparable with those herein obtained.
In contrast to fish fed with TM100, no significant changes occurred in any of the parameters assessed in liver of fish fed TM50 compared with fish fed the control diet supporting that glucose, lipid, and amino acid metabolism in liver are not altered by 50% replacement of FM by dTM. In rainbow trout, the activity of different enzymes involved in intermediary metabolism of nutrients were also not influenced up to 50% of FM replacement by full-fat TM or HI (Melenchón et al., 2020, 2022). Therefore, dTM can be successfully used to replace 50% of FM in aquafeeds for European sea bass without negatively affecting fish hepatic health and intermediary metabolism of nutrients. This is important for considering such level of replacement in the formulation of practical diets for European sea bass.
Supplementary Material
Acknowledgments
This study was supported by the Spanish Research Agency through the European Regional Development Fund (ERDF) ((PID2019-103969RB-C31/AEI/10.13039/501100011033) and Xunta de Galicia (Axudas para a consolidación e estruturación de unidades de investigación competitivas e outras accións de fomento nas universidades do SUG, ED431B 2022/01) to JLS; project ATLANTIDA (ref. NORTE-01-0145-FEDER000040), supported by the Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement and through the ERDF; and by ANIMAL4AQUA Project, funded by Portugal 2020, financed by ERFD through COMPETE—POCI-01-0247-FEDER—017610. Ana Basto was financially supported by FCT, Portugal (SFRH/BD/138593/2018). Financial support from FCT to CIIMAR within the scope of UIDB/04423/2020 and UIDP/04423/2020 is also acknowledged. Funding for open access charge: Universidade de Vigo/CISUG.
Glossary
Abbreviations
- FM
fishmeal
- dTM
defatted Tenebrio molitor larvae meal
Contributor Information
Ana Basto, CIIMAR/CIMAR-LA, Interdisciplinary Centre of Marine and Environmental Research -University of Porto, Matosinhos 4450-208, Portugal; ICBAS, School of Medicine and Biomedical Sciences – University of Porto, Porto 4050-313, Portugal; Centro de Investigación Mariña, Laboratorio de Fisioloxía Animal, Departamento de Bioloxía Funcional e Ciencias da Saúde, Facultade de Bioloxía, Universidade de Vigo, E-36310 Vigo, Spain.
Luisa M P Valente, CIIMAR/CIMAR-LA, Interdisciplinary Centre of Marine and Environmental Research -University of Porto, Matosinhos 4450-208, Portugal; ICBAS, School of Medicine and Biomedical Sciences – University of Porto, Porto 4050-313, Portugal.
Vera Sousa, CIIMAR/CIMAR-LA, Interdisciplinary Centre of Marine and Environmental Research -University of Porto, Matosinhos 4450-208, Portugal; ICBAS, School of Medicine and Biomedical Sciences – University of Porto, Porto 4050-313, Portugal.
Marta Conde-Sieira, Centro de Investigación Mariña, Laboratorio de Fisioloxía Animal, Departamento de Bioloxía Funcional e Ciencias da Saúde, Facultade de Bioloxía, Universidade de Vigo, E-36310 Vigo, Spain.
José L Soengas, Centro de Investigación Mariña, Laboratorio de Fisioloxía Animal, Departamento de Bioloxía Funcional e Ciencias da Saúde, Facultade de Bioloxía, Universidade de Vigo, E-36310 Vigo, Spain.
Conflict of Interest Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors contribution
Ana Basto: Methodology, Validation, Formal analysis, Investigation, Writing—Original Draft, Visualization. Luísa M. P. Valente: Conceptualization, Methodology, Resources, Writing—Review & Editing, Supervision, Project Administration, Funding Acquisition. Vera Sousa: Investigation, Formal Analysis, Validation. Marta Conde-Sieira: Conceptualization, Methodology, Formal analysis, Writing—Review & Editing, Supervision. José L. Soengas: Conceptualization, Methodology, Resources, Writing—Review & Editing, Supervision, Project Administration, Funding Acquisition.
Data Availability
None of the data were deposited in an official repository. The data that support the study findings are available from the authors upon request.
Literature Cited
- Alfiko, Y., Xie D., Astuti R. T., Wong J., and Wang L... 2022. Insects as a feed ingredient for fish culture: status and trends. Aquac. Fish. 7:166–178. doi: 10.1016/j.aaf.2021.10.004. [DOI] [Google Scholar]
- Aragão, C., Gonçalves A. T., Costas B., Azeredo R., Xavier M. J., and Engrola S... 2022. Alternative proteins for fish diets: implications beyond growth. Animals. 12:1211. doi: 10.3390/ani12091211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka, Y., Terai S., Sakaida I., and Nishina H... 2013. The expanding role of fish models in understanding non-alcoholic fatty liver disease. Dis. Model. Mech. 6:905–914. doi: 10.1242/dmm.011981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azeredo, R., Perez-Sanchez J., Sitja-Bobadilla A., Fouz B., Tort L., Aragao C., Oliva-Teles A., and Costas B... 2015. European sea bass (Dicentrarchus labrax) immune status and disease resistance are impaired by arginine dietary supplementation. PLoS One. 10:e0139967. doi: 10.1371/journal.pone.0139967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basto, A., Calduch J., Oliveira B., Petit L., Sá T., Maia M. R. G., Cabral-Fonseca S., Matos E., Pérez-Sánchez J., and Valente L. M. P... 2021b. The use of defatted Tenebrio molitor larvae meal as a main protein source is supported in European sea bass (Dicentrarchus labrax) by data on growth performance, lipid metabolism and flesh quality. Front. Physiol. 12. doi: 10.3389/fphys.2021.659567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basto, A., Matos E. L., and Valente M. P... 2020. Nutritional value of different insect larvae meals as protein sources for European sea bass (Dicentrarchus labrax) juveniles. Aquaculture. 521. doi: 10.1016/j.aquaculture.2020.735085. [DOI] [Google Scholar]
- Basto, A., Valente L. M. P., Conde-Sieira M., and Soengas J. L... 2021a. Central regulation of food intake is not affected by inclusion of defatted Tenebrio molitor larvae meal in diets for European sea bass (Dicentrarchus labrax). Aquaculture. 544. doi: 10.1016/j.aquaculture.2021.737088. [DOI] [Google Scholar]
- Basto, A., Valente L. M. P., Soengas J. L., and Conde-Sieira M... 2022. Partial and total fishmeal replacement by defatted Tenebrio molitor larvae meal do not alter short- and mid-term regulation of food intake in European sea bass (Dicentrarchus labrax). Aquaculture. 560. doi: 10.1016/j.aquaculture.2022.738604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belghit, I., Liland N. S., Gjesdal P., Biancarosa I., Menchetti E., Li Y., Waagbø R., Krogdahl A., and Lock E.-J... 2019. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture. 503:609–619. doi: 10.1016/j.aquaculture.2018.12.032. [DOI] [Google Scholar]
- Betancor, M. B., MacEwan A., Sprague M., Gong X., Montero D., Han L., Napier J. A., Norambuena F., Izquierdo M., and Tocher D. R... 2021. Oil from transgenic Camelina sativa as a source of EPA and DHA in feed for European sea bass (Dicentrarchus labrax L.). Aquaculture. 530:735759. doi: 10.1016/j.aquaculture.2020.735759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco, A. M., Calo J. J., and Soengas L... 2021. The gut-brain axis in vertebrates: implications for food intake regulation. J. Exp. Biol. 224. doi: 10.1242/jeb.231571. [DOI] [PubMed] [Google Scholar]
- Cadiz, L., Zambonino-Infante J. L., Quazuguel P., Madec L., Le Delliou H., and Mazurais D... 2018. Metabolic response to hypoxia in European sea bass (Dicentrarchus labrax) displays developmental plasticity. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 215:1–9. doi: 10.1016/j.cbpb.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Chemello, G., Renna M., Caimi C., Guerreiro I., Oliva-Teles A., Enes P., Biasato I., Schiavone A., Gai F., and Gasco L... 2020. Partially defatted Tenebrio molitor larva meal in diets for grow-out rainbow trout, Oncorhynchus mykiss (Walbaum): effects on growth performance, diet digestibility and metabolic responses. Animals. 10:229. doi: 10.3390/ani10020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enes, P., Panserat S., Kaushik S., and Oliva-Teles A... 2009. Nutritional regulation of hepatic glucose metabolism in fish. Fish Physiol. Biochem. 35:519–539. doi: 10.1007/s10695-008-9259-5. [DOI] [PubMed] [Google Scholar]
- Engelking, L. R. 2015. Chapter 8 - amino acid catabolism. In: Engelking, L. R., editor, Textbook of veterinary physiological chemistry. 3rd ed. Boston: Academic Press; p. 45–51. [Google Scholar]
- Fabrikov, D., Sánchez-Muros M. J., Barroso F. G., Tomás-Almenar C., Melenchón F., Hidalgo M. C., Morales A. E., Rodriguez-Rodriguez M., and Montes-Lopez J... 2020. Comparative study of growth performance and amino acid catabolism in Oncorhynchus mykiss, Tinca tinca and Sparus aurata and the catabolic changes in response to insect meal inclusion in the diet. Aquaculture 529:735731. doi: 10.1016/j.aquaculture.2020.735731. [DOI] [Google Scholar]
- Fabrikov, D., Vargas-Garcia M. D. C., Barroso F. G., Sanchez-Muros M. J., Cacua Ortiz S. M., Morales A. E., Cardenete G., Tomas-Almenar C., and Melenchon F... 2021. Effect on intermediary metabolism and digestive parameters of the high substitution of fishmeal with insect meal in Sparus aurata feed. Insects. 12. doi: 10.3390/insects12110965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasco, L., Henry M., Piccolo G., Marono S., Gai F., Renna M., Lussiana C., Antonopoulou E., Mola P., and Chatzifotis S... 2016. Tenebrio molitor meal in diets for European sea bass (Dicentrarchus labrax L.) juveniles: growth performance, whole body composition and in vivo apparent digestibility. Anim. Feed Sci. Technol. 220:34–45. doi: 10.1016/j.anifeedsci.2016.07.003. [DOI] [Google Scholar]
- Guerreiro, I., Castro C., Antunes B., Coutinho F., Rangel F., Couto A., Serra C. R., Peres H., Pousão-Ferreira P., Matos E.,. et al. 2020. Catching black soldier fly for meagre: growth, whole-body fatty acid profile and metabolic responses. Aquaculture. 516:734613. doi: 10.1016/j.aquaculture.2019.734613. [DOI] [Google Scholar]
- Gwaltney-Brant, S. M. 2016. Chapter 7 - nutraceuticals in hepatic diseases. In: Gupta, R. C., editor. Nutraceuticals. Boston: Academic Press; p. 87–99. [Google Scholar]
- Henry, M. A., Gai F., Enes P., Perez-Jimenez A., and Gasco L... 2018. Effect of partial dietary replacement of fishmeal by yellow mealworm (Tenebrio molitor) larvae meal on the innate immune response and intestinal antioxidant enzymes of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 83:308–313. doi: 10.1016/j.fsi.2018.09.040. [DOI] [PubMed] [Google Scholar]
- Jia, R., Cao L.-P., Du J.-L., He Q., Gu Z.-Y., Jeney G., Xu P., and Yin G.-J... 2020. Effects of high-Fat diet on steatosis, endoplasmic reticulum stress and autophagy in liver of tilapia (Oreochromis niloticus). Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00363. [DOI] [Google Scholar]
- Keppler, D., and Decker K... 1974. Glycogen determination with amyloglucosidase. In: Bergmeyer, H. U., editor. Methods of enzymatic analysis No. 3. New York: Academic Press; p. 1127–1131. [Google Scholar]
- Levene, A. P., Kudo H., Thursz M. R., Anstee Q. M., and Goldin R. D... 2010. Is oil red-O staining and digital image analysis the gold standard for quantifying steatosis in the liver? Hepatology. 51:1859–1859. doi: 10.1002/hep.23551. [DOI] [PubMed] [Google Scholar]
- Li, L. Y., Lv H. B., Jiang Z. Y., Qiao F., Chen L. Q., Zhang M. L., and Du Z. Y... 2020. Peroxisomal proliferator-activated receptor α-b deficiency induces the reprogramming of nutrient metabolism in zebrafish. J. Physiol. 598:4537–4553. doi: 10.1113/jp279814. [DOI] [PubMed] [Google Scholar]
- Librán-Pérez, M., Polakof S., Lopez-Patino M. A., Miguez J. M., and Soengas J. L... 2012. Evidence of a metabolic fatty acid-sensing system in the hypothalamus and Brockmann bodies of rainbow trout: implications in food intake regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302:R1340–1350. doi: 10.1152/ajpregu.00070.2012. [DOI] [PubMed] [Google Scholar]
- Librán-Pérez, M., Figueiredo-Silva A. C., Panserat S., Geurden I., Miguez J. M., Polakof S., and Soengas J. L... 2013a. Response of hepatic lipid and glucose metabolism to a mixture or single fatty acids: Possible presence of fatty acid-sensing mechanisms. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 164:241–248. doi: 10.1016/j.cbpa.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Librán-Pérez, M., Lopez-Patino M. A., Miguez J. M., and Soengas J. L... 2013b. Oleic acid and octanoic acid sensing capacity in rainbow trout Oncorhynchus mykiss is direct in hypothalamus and Brockmann bodies. PLoS One 8:e59507. doi: 10.1371/journal.pone.0059507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastoraki, M., Katsika L., Enes P., Guerreiro I., Kotzamanis Y. P., Gasco L., Chatzifotis S., and Antonopoulou E... 2022. Insect meals in feeds for juvenile gilthead seabream (Sparus aurata): effects on growth, blood chemistry, hepatic metabolic enzymes, body composition and nutrient utilization. Aquaculture. 561:738674. doi: 10.1016/j.aquaculture.2022.738674 [DOI] [Google Scholar]
- Mastoraki, M., Mollá Ferrándiz P., Vardali S. C., Kontodimas D. C., Kotzamanis Y. P., Gasco L., Chatzifotis S., and Antonopoulou E... 2020. A comparative study on the effect of fish meal substitution with three different insect meals on growth, body composition and metabolism of European sea bass (Dicentrarchus labrax L.). Aquaculture. 528:735511. doi: 10.1016/j.aquaculture.2020.735511. [DOI] [Google Scholar]
- Melenchón, F., Larrán A. M., de Mercado E., Hidalgo M. C., Cardenete G., Barroso F. G., Fabrikov D., Lourenço H. M., Pessoa M. F., and Tomás-Almenar C... 2020. Potential use of black soldier fly (Hermetia illucens) and mealworm (Tenebrio molitor) insectmeals in diets for rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 27:491–505. doi: 10.1111/anu.13201. [DOI] [Google Scholar]
- Melenchón, F., de Mercado E., Pula H. J., Cardenete G., Barroso F. G., Fabrikov D., Lourenço H. M., Pessoa M. F., Lagos L., Weththasinghe P.,. et al. 2022. Fishmeal dietary replacement up to 50%: a comparative study of two insect meals for rainbow trout (Oncorhynchus mykiss). Animals. 12:179. doi: 10.3390/ani12020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, S. 1968. Amino acid analysis: aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J. Biol. Chem. 243:6281–6283. doi: 10.1016/S0021-9258(18)94488-1. [DOI] [PubMed] [Google Scholar]
- Nogales-Mérida, S., Gobbi P., Józefiak D., Mazurkiewicz J., Dudek K., Rawski M., Kierończyk B., and Józefiak A... 2018. Insect meals in fish nutrition. Rev. Aquac doi: 10.1111/raq.12281. [DOI] [Google Scholar]
- NRC. 2011. Nutrient requirements of fish and shrimp. 1st ed. Washington, DC: Natl. Acad. Press. [Google Scholar]
- Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:45e45–45e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippinato, L., Gasco L., Di Vita G., and Mancuso T... 2020. Current scenario in the European edible-insect industry: a preliminary study. J. Insects Food Feed. 6:371–381. doi: 10.3920/jiff2020.0008. [DOI] [Google Scholar]
- Polakof, S., Míguez, J.M., Moon, T.W., and Soengas J.L... 2007a. Evidence for the presence of a glucosensor in hypothalamus, hindbrain, and Brockmann bodies of rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292:R1657–R1666. doi: 10.1152/ajpregu.00525.2006. [DOI] [PubMed] [Google Scholar]
- Polakof, S., Míguez, J.M., and Soengas J.L... 2007b. In vitro evidences for glucosensing capacity and mechanisms in hypothalamus, hindbrain, and Brockmann bodies of rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293:R1410–R1420. doi: 10.1152/ajpregu.00283.2007. [DOI] [PubMed] [Google Scholar]
- Polakof, S., Míguez, J.M., and Soengas J.L... 2008a. Changes in food intake and glucosensing function of hypothalamus and hindbrain in rainbow trout subjected to hyperglycemic or hypoglycemic conditions. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 194:829–839. doi: 10.1007/s00359008-0354-y. [DOI] [PubMed] [Google Scholar]
- Polakof, S., Míguez, J.M., and Soengas J.L... 2008b. Dietary carbohydrates induce changes in glucosensing capacity and food intake in rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295:R478–R489. doi: 10.1152/ajpregu.00176.2008. [DOI] [PubMed] [Google Scholar]
- Polakof, S., Panserat, S., Plagnes-Juan, E., and Soengas J.L... 2008c. Altered dietary carbohydrates significantly affect gene expression of the major glucosensing components in Brockmannn bodies and hypothalamus of rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295:R1077–R1088. doi: 10.1152/ajpregu.90476.2008. [DOI] [PubMed] [Google Scholar]
- Polakof, S., Panserat S., Soengas J. L., and Moon T. W... 2012. Glucose metabolism in fish: a review. J. Comp. Physiol. B: Biochem. Syst. Environ. Physiol. 182:1015–1045. doi: 10.1007/s00360-012-0658-7. [DOI] [PubMed] [Google Scholar]
- Randazzo, B., Zarantoniello M., Gioacchini G., Cardinaletti G., Belloni A., Giorgini E., Faccenda F., Cerri R., Tibaldi E., and Olivotto I... 2021. Physiological response of rainbow trout (Oncorhynchus mykiss) to graded levels of Hermetia illucens or poultry by-product meals as single or combined substitute ingredients to dietary plant proteins. Aquaculture. 538:736550. doi: 10.1016/j.aquaculture.2021.736550. [DOI] [Google Scholar]
- Ray, A.K., and Ringø E... 2014. The gastrointestinal tract of fish. In: Merrifield, D. and Ringø E., editor. Aquaculture nutrition: gut health, probiotics and prebiotics. Oxford: John Wiley & Sons, Ltd; p. 1–13. [Google Scholar]
- Rimoldi, S., Benedito-Palos L., Terova G., and Pérez-Sánchez J... 2015. Wide-targeted gene expression infers tissue-specific molecular signatures of lipid metabolism in fed and fasted fish. Rev. Fish Biol. Fish. 26:93–108. doi: 10.1007/s11160-015-9408-8. [DOI] [Google Scholar]
- Riva, G., Villanova M., Cima L., Ghimenton C., Bronzoni C., Colombari R., Crestani M., Sina S., Brunelli M., D’Errico A.,. et al. 2018. Oil Red O is a useful tool to assess donor liver steatosis on frozen sections during transplantation. Transplant. Proc. 50:3539–3543. doi: 10.1016/j.transproceed.2018.06.013. [DOI] [PubMed] [Google Scholar]
- Skiba-Cassy, S., Geurden I., Panserat S., and Seiliez I... 2016. Dietary methionine imbalance alters the transcriptional regulation of genes involved in glucose, lipid and amino acid metabolism in the liver of rainbow trout (Oncorhynchus mykiss). Aquaculture. 454:56–65. doi: 10.1016/j.aquaculture.2015.12.015. [DOI] [Google Scholar]
- Smith, P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., and Klenk D. C... 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tran, H. Q., Nguyen T. T., Prokešová M., Gebauer T., Doan H. V., and Stejskal V... 2022. Systematic review and meta-analysis of production performance of aquaculture species fed dietary insect meals. Rev. Aquac. doi: 10.1111/raq.12666. [DOI] [Google Scholar]
- Viegas, I., Caballero-Solares A., Rito J., Giralt M., Pardal M. A., Meton I., Jones J. G., and Baanante I. V... 2014. Expressional regulation of key hepatic enzymes of intermediary metabolism in European seabass (Dicentrarchus labrax) during food deprivation and refeeding. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 174:38–44. doi: 10.1016/j.cbpa.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Viegas, I., Rito J., Gonzalez J. D., Jarak I., Carvalho R. A., Meton I., Pardal M. A., Baanante I. V., and Jones J. G... 2013. Effects of food-deprivation and refeeding on the regulation and sources of blood glucose appearance in European seabass (Dicentrarchus labrax L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 166:399–405. doi: 10.1016/j.cbpa.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Viegas, I., Rito J., Jarak I., Leston S., Caballero-Solares A., Meton I., Pardal M. A., Baanante I. V., and Jones J. G... 2015. Contribution of dietary starch to hepatic and systemic carbohydrate fluxes in European seabass (Dicentrarchus labrax L.). Br. J. Nutr. 113:1345–1354. doi: 10.1017/S0007114515000574. [DOI] [PubMed] [Google Scholar]
- Weil, C., Lefèvre F., and Bugeon J... 2013. Characteristics and metabolism of different adipose tissues in fish. Rev. Fish Biol. Fish. 23:157–173. doi: 10.1007/s11160-012-9288-0. [DOI] [Google Scholar]
- Weththasinghe, P., Lagos L., Cortes M., Hansen J. O., and Overland M... 2021. Dietary inclusion of black soldier fly (Hermetia Illucens) larvae meal and paste improved gut health but had minor effects on skin mucus proteome and immune response in Atlantic salmon (Salmo Salar). Front. Immunol. 12:599530. doi: 10.3389/fimmu.2021.599530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarantoniello, M., Randazzo B., Gioacchini G., Truzzi C., Giorgini E., Riolo P., Gioia G., Bertolucci C., Osimani A., Cardinaletti G.,. et al. 2020. Zebrafish (Danio rerio) physiological and behavioural responses to insect-based diets: a multidisciplinary approach. Sci. Rep. 10:10648. doi: 10.1038/s41598-020-67740-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Lu R., Qin C., and Nie G... 2020. Precision nutritional regulation and aquaculture. Aquac. Rep. 18. doi: 10.1016/j.aqrep.2020.100496. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
None of the data were deposited in an official repository. The data that support the study findings are available from the authors upon request.