Summary
Memory B cells (MBCs) are an essential part of our immunological memory. They respond fast upon re-encountering pathogens and can differentiate into plasma cells that secrete protective antibodies. The focus of this review is on MBCs that lack, or express low levels of, CD21, hereafter referred to as CD21–/low. These cells are expanded in peripheral blood with age and during chronic inflammatory conditions such as viral infections, malaria, common variable immunodeficiency, and autoimmune diseases. CD21–/low MBCs have gained significant attention; they produce disease-specific antibodies/autoantibodies and associate with key disease manifestations in some conditions. These cells can be divided into subsets based on classical B-cell and other markers, e.g. CD11c, FcRL4, and Tbet which, over the years, have become hallmarks to identify these cells. This has resulted in different names including age-associated, autoimmune-associated, atypical, tissue-like, tissue-resident, tissue-restricted, exhausted, or simply CD21–/low B cells. It is however unclear whether the expanded ‘CD21–/low’ cells in one condition are equivalent to those in another, whether they express an identical gene signature and whether they have a similar function. Here, we will discuss these issues with the goal to understand whether the CD21–/low B cells are comparable in different conditions.
Keywords: CD21–/low, memory B cells, exhaustion, age-associated, atypical, autoimmune-associated
CD21-/low memory B cells are expanded with age in healthy individuals and in patients with viral infections and autoimmune conditions and are associated with pathogen-specific antibodies as well as key disease manifestations. Depending on condition, CD21-/low memory B cells have been termed age-associated, autoimmune-associated, atypical, tissue-like, tissue-resident, tissue-restricted, exhausted or simply CD21-/low B cells. CD21-/low memory B cells are often CD27-IgD-CD11c+ and in some conditions they express Tbet, in others FcRL4 and in yet others both.
Graphical Abstract
Graphical Abstract.
Introduction
B-cell populations as defined by classical markers
B cells can be divided into several populations: transitional B cells, the new emigrants from the bone marrow (BM); naïve B cells that have not yet encountered their cognate antigen; germinal centre (GC) B cells that have encountered antigen and are undergoing proliferative expansion and selection in tertiary structures in secondary lymphoid organs; memory B cells (MBCs) and antibody-producing plasma cells, both elements of immunological memory. Under healthy conditions, circulating peripheral blood (PB) B cells are in a resting state, while the B cells in, e.g. tonsils, representing a lymphoid organ continuously exposed to a plethora of foreign antigens, also contain activated, proliferating B cells. The different B-cell populations can be identified based on their expression of various cell surface markers using flow cytometry. Classical markers that are used to define B cell populations are CD27/IgD [1] and CD24/CD38 [2]. Based on the CD27/IgD markers, MBCs are defined as unswitched (UnSw, CD27+IgD+), switched (Sw, CD27+IgD−), or double-negative (DN, CD27−IgD-) (Fig. 1a). Note that Sw MBCs can be IgM+IgD-. Using the CD24/CD38 markers identifies MBCs as CD38-/low. Over the last decade or so it has become clear that limiting MBCs to only these populations is too simplistic [3, 4]. For instance, although most B cells express CD21 (complement receptor 2 (CR2), a small population of MBCs that express low levels of or altogether lack this marker (herein CD21−/low) have been found. These MBCs are of particular interest because of their increase in frequency with age and during chronic infections and autoimmune inflammatory conditions [5–7], and their association with disease manifestations, as discussed below. The CD21−/low has been found to express exceptionally high levels of the typical B-cell markers CD19 and CD20 [8], and to have a large cell size [9]. Depending on the study, CD21−/low MBCs have been divided into subsets using combinations of the classical B-cell markers and markers such as FcRL4, Tbet, and CD11c. The two latter were initially described to be expressed by CD21−/low age-associated B cells (ABCs) in mice [10, 11]. In this review, we will discuss the phenotype, in vitro responses, gene expression patterns, and association of CD21−/low MBCs with clinical parameters. Our aim is to understand whether the CD21−/low MBCs represent a cell population that shares phenotype and function in health, in autoimmune conditions, and during infections, and whether they are functionally exhausted, as previously proposed.
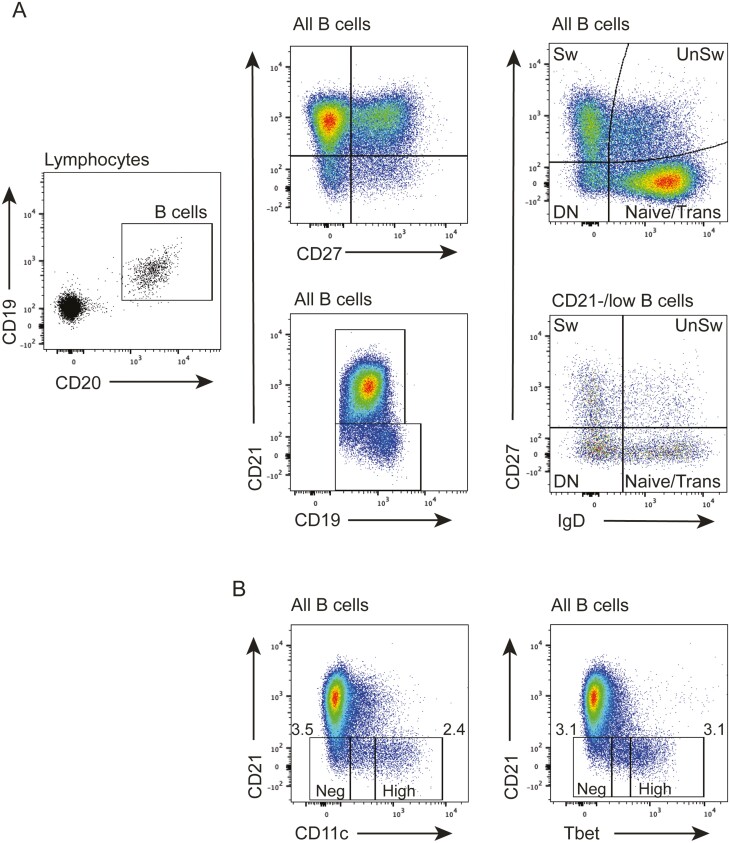
Figure 1:
Gating strategies for human B cell subsets. (A) Peripheral blood lymphocytes were gated on CD19+CD20+ and CD19+CD20+CD21–/low B cells, as indicated. Based on CD27 and IgD expression, the cells can be divided into switched memory (Sw), unswitched memory (UnSw), double negative (DN) memory as well as naive and transitional B cells. (B) B cells show heterogeneous expression of CD11c and Tbet, ranging from negative to high levels, showing that high expression levels are largely found on CD21–/low B cells.
CD21−/low B cells in health
CD21−/low tissue-resident FcRL4+ and tissue-restricted Tbethi B cells
At the beginning of the 21st century, a new family of immunoglobulin (Ig)-like receptors termed IRTA1, 2, 3, 4, and 5 were discovered [12, 13]. IRTA1 (also named FCRH4; Fc receptor homologue 4 or FcRL4; Fc receptor-like protein) was selectively and consistently expressed by B cells located in the tonsils [9], and in Peyer’s patches [14]. The tonsillar FcRH4+ (hereafter FcRL4+) did not express CD21, and despite lacking CD27, the conventional MBC marker, they had already class-switched to IgG or IgA [9] (Table 1, Fig. 2). Typical of MBCs, the FcRL4+ cells expressed Ig V genes that showed signs of somatic hypermutation, in fact on par with the FcRL4− MBCs. In contrast to the FcRL4− cells, those that were FcRL4+ were large in size, consistent with a proliferating population, with an extensive cytoplasm but no rough endoplasmic reticulum, as would be typical of plasmablasts/cells. The FcRL4+ cells were thus considered MBCs and were subsequently found to also express CD11c (αX) integrin and high levels of CD20 [15]. They represented around 10% of tonsillar B cells but were not detected (<0.5%) among PB, BM, or splenic B cells. This led to the conclusion that the FcRL4+ B cells represent a distinct tissue-based population of MBCs. To summarize the phenotype of these tissue-resident CD21−/low MBCs, they are CD27−IgD−CD38−CD11c+FcRL4+. Despite the lack of FcRL4+ B cells in PB from healthy individuals [9], there is a CD21−/low MBC population in PB that constitutes approximately 5% of the B-cell pool, comprising both CD27+ and CD27− as well as switched and unswitched cells [51]. In fact, a recent study showed that in healthy individuals, CD21−/low MBCs are found not only in PB and the tonsils but also in lymph nodes (LNs), spleen, BM, and the thoracic duct [19].
Table 1:
CD21–/low MBCs; phenotypes, FcRL4 and/or Tbet expression, associations to disease
| Condition | Termed | Location | Phenotype in PB | FcRL4 | Tbet | Proportion in PB (range) | Disease association | Ref |
|---|---|---|---|---|---|---|---|---|
| Health | ||||||||
| Healthy individuals | Tissue-resident | Tonsil | CD27−IgD−CD38− CD11c+ | + | – | ND | NA | [9, 15–18] |
| Healthy individuals | Tissue restricted | PB BM Spleen |
CD27+/−IgD− CD38lowCD11c+ | – | Tbethi | ~3% (1–30)* | NA | [17, 19, 20] |
| CVID | CD21–/low | PB SLO BAL |
CD27−IgD+ IgM+CD38lowCD11c+ | + | Tbethi | CVID: ~14% | Part of classification criteria | [8, 18, 21–23] |
| SLE | CD11hi Tbethi | PB Kidneys |
CD27− CD38lowCD11chi | + | Tbethi | ~10% (1–50) | Disease activity, auto ab, PCs | [16] |
| SLE | DN2 | PB | CD27− IgD- CD11c+ CXCR5- | – | Tbethi | ~20% (2–80) | Disease activity, auto ab, PCs | [24] |
| Established RA | CD21–/low | PB SF |
CD27− IgD- | – | ND | ~10% (2–30) | Joint destruction | [25] |
| Malaria (P. falciparum and P. vivax) |
Atypical | PB | CD27−CD11c+ CXCR5− | +/ - | Tbethi | parasitemia: ~15% (3–21) w/o parasitemia: ~10% (8-12) |
Antigen-specific ab, auto ab to red blood cells, and anaemia | [26–36] |
| HIV | Exhausted, tissue-like | PB | CD27− CD11c+ | + | Tbethi | ~7% (2–22) | HIV-specific IgG | [20, 37–39] |
| Primary Sjögren syndrome | PB | CD27− CD38lowCD11c+ | ND | Tbet+ | ~10% (1–-25) | ND | [40] | |
| Systemic sclerosis | PB | CD38lowCD11c+ | ND | ND | Before treatment: ~5% (1–25) After treatment: ~8% (5–25) |
Disease activity | [41, 42] | |
| Crohn’s disease | PB Gut |
Tbet+ | ND | Tbet+ | Active disease: ~3% (2–4) Quiescent disease: ~2% (1.5–3) |
Disease activity | [43] | |
| Multiple sclerosis | CD21low | PB Cerebrospinal fluid |
CD11c+ | ND | ND | <60 years ~1.5% (0–15) >60 years ~1.5% (0–3) |
ND | [44] |
| HBV | Atypical | PB Liver |
CD27− CD11c+ | ND | Tbethi | ~6% (0–16) | HBV specific IgG | [45] |
| HCV | Tissue-like | PB | CD27−CD11c+ | + | Tbet+ | Non-cirrhosis: ~18% (5–30) Cirrhosis: ~17% (4–70) |
Frequency decreases after treatment | [46, 47] |
| COVID-19 | Atypical/DN2 | PB | CD27−CD11c+ | ND | Tbet+ | Mild infection ~15% (1–40) ICU: ~30% (5–75) |
Disease activity | [48–50] |
Antibody (ab), bone marrow (BM), bronchioalveolar lavage (BAL), cerebrospinal fluid (CSF), common variable immunodeficiency (CVID), double negative 2 (DN2), healthy individuals (HI), hepatitis B virus (HBV), hepatitis C virus (HCV), intensive care unit (ICU), multiple sclerosis (MS), not determined (ND). Not applicable (NA), peripheral blood (PB), rheumatoid arthritis (RA), secondary lymphoid organs (SLO), systemic lupus erythematosus, SLE), Systemic lupus erythematosus disease activity index (SLEDAI), Synovial fluid (SF), Switched.
*This is the general level combining all studies in the table.
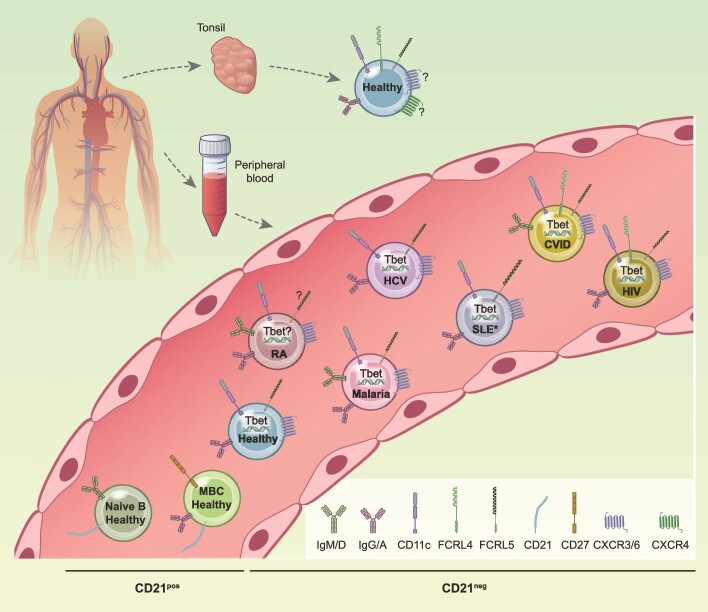
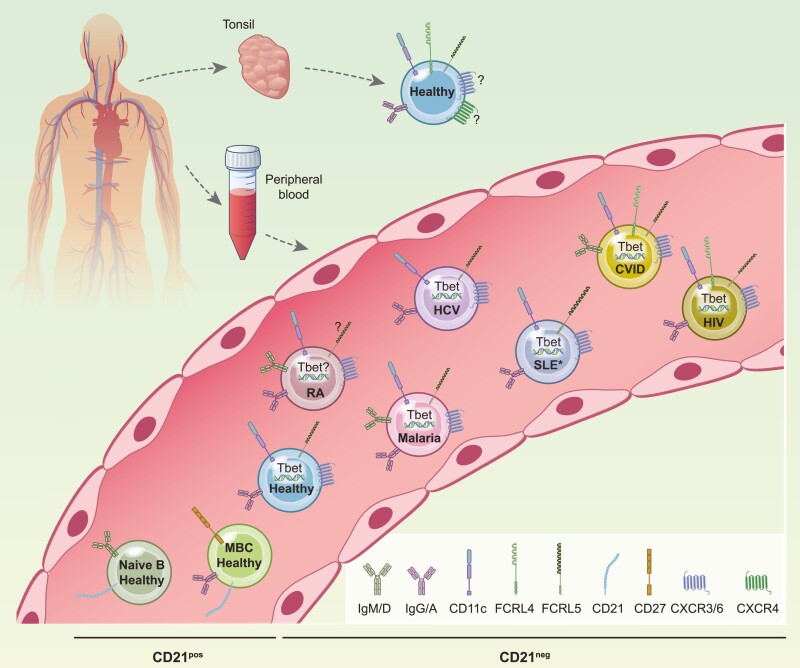
Figure 2:
Cartoon depicting phenotypes of CD21–/low memory B cells (MBCs) in tonsils and peripheral blood (PB). In healthy individuals, CD21–/low MBCs in tonsils are FcRL4+, whereas in PB they are Tbethi. The expression of these two markers in PB CD21–/low MBCs in the indicated conditions is shown. Shown is also expression of additional markers according the key. In some conditions, CD21–/low MBCs express different isotypes. Rheumatoid arthritis (RA), hepatitis C virus (HCV), systemic lupus erythematosus (SLE), common variable immunodeficiency (CVID), human immunodeficiency virus (HIV). *In SLE, CD21–/low MBCs have been shown to be FcRL4+ in one study and FcRL4– in another. ? indicates unclear expression.
In mice, splenic CD21–/low ABCs expand with age [11, 52]. These cells express CD11c as well as Tbet [10], a transcription factor first described in T cells as a master regulator of T helper 1 (TH1) cell development [53]. It was recently reported that CD11c and Tbet are also expressed in human PB CD21–/low B cells [16, 19, 24]. Consistent with these reports, the flow cytometry plots in Fig. 1b show that PB CD21–/low MBCs express Tbet and CD11c, and that the cells can be divided into different subsets based on expression levels. Moreover, CD21–/lowTbethi cells are also present in spleen and BM, constituting on average 3–4% of all B cells in the respective tissue, though proportions varied between donors, from 1% to 10% [19]. Due to the lack of CD21–/lowTbethi MBCs in LNs and thoracic duct, this suggests that these cells do not circulate via the lymphatic system. The CD21–/lowTbethi cells were, therefore, referred to as tissue-restricted MBCs. Among the splenic CD21–/lowTbethi MBCs were antigen-experienced cells that expressed influenza-specific IgG1 on their cell surface [19], suggesting that these cells derive from previous influenza infections. The presence of CD21–/lowTbethi in PB and spleen and their absence in LNs has been confirmed in other studies where it was also found that CD21–/lowTbethi cells are not present in tonsils [17]. To summarize the phenotype of the tissue-restricted CD21–/low MBCs, they are CD27+/–CD38lowCD11c+Tbethi.
The discovery of a novel FcRL4+ MBC population in tonsils led to the analyses of this marker on CD21–/low B-cells expanded in chronic inflammatory conditions. While FcRL4+ MBCs were found in tonsils but not PB in healthy individuals, the situation might be different in disease, which turned out to be the case in common variable immunodeficiency (CVID) and human immunodeficiency virus (HIV). The discovery of CD21–/lowTbethi MBCs in healthy PB revealed that the expression of FcRL4 and Tbethi appears to be mutually exclusive. However, in CVID and HIV CD21–/low MBCs are FcRL4+Tbethi while in other autoimmune conditions and most chronic infections the CD21–/low MBCs are FcRL4-Tbethi, as discussed below.
CD21–/low B cells and associations under chronic inflammatory conditions
Compared with healthy individuals, an expansion of PB CD21–/low B cells has been described in chronic inflammatory conditions. In these disease contexts, CD21–/low B cells have been termed ABCs (by analogy to these cells in mice), atypical (malaria), tissue-like and/or exhausted (HIV), autoimmune-associated or, simply, CD21–/low B cells. In some conditions, not only PB but also additional tissues have been analysed, e.g. spleen, LN, BM, kidneys, and synovial fluid/tissue. The initial studies of CD21–/low MBCs did not assess the expression of Tbet and/or FcRL4. However, based on classical B-cell markers and Ig isotypes, we link the early and more recent studies to aid our understanding of CD21–/low MBCs in chronic inflammatory conditions.
Common variable immunodeficiency
CVID is one of the more common primary immunodeficiencies. The aetiology is unknown, but an increasing number of different genetic mutations have been discovered. At large, CVID is a B-cell disease and is characterized by low levels of Igs. However, disease manifestations, severity, and expressed Ig isotypes vary between individuals, hence the description ‘variable’. Already in 2002, a group of CVID patients was found to present with an expanded PB CD21–/low B-cell population, in particular patients more likely to have splenomegaly, interstitial lung disease, and other autoimmune complications [8]. The CD21–/low cells were CD27–CD38lowCD11c+ and expressed unmutated IgM and IgD, although this may reflect an inability to class-switch or form functional GCs, depending on the CVID genotype [21]. In addition to FcRL4, the CD21–/low cells expressed FcRL5, another Fc receptor-like protein. The view that there is an expansion of CD21–/lowCD27– B cells in CVID is supported by others [54]. More recently, PB CD21–/low B cells in CVID patients were found to be Tbethi. These CD21-/lowTbethi cells are also present in secondary lymphoid organs (SLOs) and spleen, and in bronchoalveolar lavage (BAL) in CVID patients with interstitial lung disease [17, 18]. Taken together, CD21-/low B cells in CVID are CD27–IgM+IgD+CD38lowCD11c+FcRL5+FcRL4+Tbethi, and depending on the patient-specific genetic defect, CD21–/low B cells are present in spleen, BAL, and SLOs. Therefore, the PB CD21–/low B cells in CVID differ phenotypically from those in healthy individuals in that they are not only Tbethi but also express FcRL4. That the latter is a marker for tissue-residency seemingly does not apply in CVID.
Autoimmune diseases
Autoimmune diseases affect approximately 5–7% of the human population. In most cases the aetiology is unknown, although sex, genetics, and environmental factors are known to play important roles. These diseases are heterogeneous and include a wide range of disorders such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis, and Crohn’s disease. Each diagnosis is based on multiple criteria, which means that there can be considerable variation between individuals.
Systemic lupus erythematosus
SLE is a rheumatic disease that primarily affects younger women and can involve almost every organ in the body including blood, skin, and kidneys. The inflammation is typically mediated by immune complexes that are deposited in e.g. the kidneys, which contributes to the development of nephritis. The proportions of various B-cell populations in SLE patients differ depending on disease manifestation and activity, measured using the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) [55–57]. An expansion of CD21–/low B cells has been described in SLE patients, where this cell population was identified as CD27+/–CD38low expressing IgM or IgG, and the frequency of these cells correlates with disease duration and SLEDAI score [8, 58, 59]. Several groups have further defined the CD21–/low MBCs in PB that are expanded as CD27–CD38lowCD11chiFcRL5+FcRL4+Tbethi, switched or unswitched, or CD27–IgD–CD38lowCD11c+CXCR5–FcRL5+FcRL4–Tbethi (also termed double negative (DN2) cells). In both studies, the frequency of these cells is associated with SLEDAI score and autoantibody levels [16, 24], results supported by others [60, 61]. CD21–/lowCD27–CD38lowCD11chiFcRL5+FcRL4+Tbethi MBCs are also associated with clinical manifestations such as malar rash and nephritis, and CD11c+ B cells have been found in nephritic kidneys [16], although the status of CD21 and CD27 is unclear. CD21–/lowTbethi cells have also been found to associate with increased serum levels of interferon-gamma (IFNγ) as well as interferon-lambda (IFNλ) [60, 61].
There is therefore ample evidence that CD21–/lowTbethi MBCs are expanded in SLE patients, and at frequencies that correlate with both disease activity and serum levels of typical lupus autoantibodies, e.g. anti-dsDNA, anti-Smith antigen and anti-RNP. Furthermore, SLE studies from different laboratories have identified a relatively consistent phenotype, although FcRL4 expression is less clear, the CD21–/low MBCs that associate with disease manifestations are CD27–CD38lowCD11c+CXCR5–FcRL5+Tbethi.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is the most common inflammatory rheumatic disease that leads to synovitis and subsequent joint destruction, most commonly in the hands and feet. During the last decade, it has become clear that B cells are one of the major players in this disease as B-cell depletion with rituximab (anti-CD20 antibodies) can induce remission, particularly in patients positive for antibodies reactive with citrullinated proteins (anti-citrullinated protein antibodies (ACPAs)) and/or Igs, i.e. rheumatoid factor (RF). There are several studies that have described an expansion of CD21–/low B cells in PB from patients with established RA. However, the markers vary, for instance, the expanded CD21–/low B cells have been described as CD27–CD38– [54], CD38– [62], CD27–IgD–IgM– [63]. Although CD21–/low B cells have been shown to express Tbet at the mRNA level [54], it is unclear whether it is all CD21–/low cells or a subset thereof. In ACPA+/RF+ patients with established RA, CD21–/lowCD27–IgD–FcRL4– MBCs are expanded, and the frequencies of these cells correlate with joint destruction [25]. In another study, CD11c+Tbet+ B cells (of unknown CD21 and CD27 status) were found to associate with inflammation and disease activity (disease activity score 28 joints (DAS28)) [64]. Moreover, the frequency of CD21–/low that are CD27+ was increased in female RA patients and was found to correlate with age [11].
In RA, the synovium is the main site of inflammation. The type of local inflammation can vary between patients, with patients stratified into three categories based on immune cell infiltration: pauci-immune, diffuse-myeloid, and lympho-myeloid [65]. Among these, lympho-myeloid synovium harbours a substantial number of infiltrating B and T cells and can be further divided into B cell-rich and B cell-poor based on the number of B cells and B cell-aggregates detected by immunohistochemistry [66]. It has been suggested that B cell-rich synovitis is associated with high disease activity [65]. CD11c+Tbet+ B cells have been found in synovial tissue sections [67], although the CD21 and CD27 status is unclear. In synovial fluid, although B-cell frequencies are low, the vast majority of these are CD21–/low [68], and most of these are CD11c+IgD–, some of which are CD27–FcRL4+ [25]. The presence of FcRL4+ B cells in synovial tissue is supported by other studies [69]. Others have shown a high abundance of CD21–/lowCD11c+Tbet+ MBCs, although the proportions varied (1–90%) between donors [70]. Thus, the expanded CD21–/low MBCs in PB in RA are associated with disease manifestations are CD27–IgD–FcRL4–.
Other autoimmune diseases
CD21–/low B cells are also expanded in patients with other autoimmune diseases. Below, we briefly summarize their phenotypes and associations with clinical manifestations. In patients with systemic sclerosis, a rare but severe rheumatic disease characterized by Raynaud’s phenomenon, inflammation and fibrosis of skin, blood vessels and internal organs, the expanded CD21–/low cells are CD38low, and half of them are CD11c+ and switched [41, 42, 71]. In patients with the highest frequencies of CD21–/lowCD38low, these are associated with disease manifestations both at diagnosis and over time. In patients with primary Sjögren’s syndrome, an inflammatory rheumatic disease characterized by sicca symptoms and an increased risk of developing lymphoproliferative disease, the expanded CD21–/low MBCs have been described as CD27–CD38low, most were IgM+IgD+ and mutated, whereas only a small (approx. 20%) proportion expressed Tbet or CD11c [40, 72]. Further, CD21–/lowCD11c+IgM+ and IgG+ MBCs are expanded in multiple sclerosis (MS), an autoimmune inflammatory disease that engages the brain and spinal cord [44]. The frequency of these cells has been found to be higher in cerebrospinal fluid compared with those in PB. Crohn’s disease is a chronic inflammatory bowel disease that can affect the entire gut. In patients with Crohn’s disease, Tbet+IgG+ MBCs (CD21 status unknown) are expanded in both PB and gut (ileum) and correlate with disease activity [43]. Taken together, in all these diseases a CD21–/low population is expanded, except for Crohn’s where the expanded cells were defined as CD19+Tbet+. The expression (or not) of FcRL4 is unclear.
Acute and chronic infections
Malaria
Malaria is caused by protozoans belonging to the Plasmodium family including P. falciparum and P. vivax. The infection is characterized by high fever and shaking chills; it has variable severity from mild to severe organ manifestations such as cerebral malaria and anaemia. An expansion of PB CD21–/lowCD27–CD11c+, mainly IgG+, MBCs have been described in children persistently exposed to malaria [73] and individuals in malaria-endemic areas [26, 27]. Post-treatment, as patients become free from parasites, they no longer present with high CD21–/low B-cell frequencies [74]. The immune response to P. falciparum infections is dynamic, as observed in acutely infected and previously exposed individuals, where mainly CD21–/low CD11c+ B cells are first expanded and then decrease as the infection resolves [28]. Among CD21–/lowCD11c+CD27–, those that are IgD+ are expanded in the primary response and both IgD+ and IgD– are increased in the recall response. These dynamics of the B-cell response during acute malaria were confirmed in another longitudinal study [29]. Moreover, antigen-specific B cells are largely CD21–/lowCD11c+, and neutralizing IgG antibodies to P. falciparum are produced by CD21–/lowCD27– MBCs [29, 30]. The mutation status of these antibodies is equivalent to antibodies derived from classical MBCs [30, 31]. Most of the PB CD21–/lowCD27– cells are CD11c+CXCR5–FcRL5+FcRL4–Tbethi and switched (IgG1, IgG3) [32, 75]. In some patients with acute malaria caused by P. falciparum or P. vivax, serum levels of autoantibodies against phosphatidylserine (which is exposed on the surface of dying erythrocytes) and red blood cells correlate with PB Tbethi B cells and the development of anaemia [33, 34]. Thus, in malaria, the expansion of CD21–/low MBCs is associated with infection phase and represents cells that give rise to antigen-specific and autoantibodies where the latter can result in anaemia.
COVID-19
The last few years have been dominated by the COVID-19 pandemic, and much effort has been put into understanding the immunological mechanisms and finding useful strategies to combat the virus. In critically ill COVID-19 patients CD21–/lowCD27– B cells have been observed to be expanded compared with healthy individuals [48]. This observation was confirmed by another study reporting an expansion of CD21–/lowCD27–IgD–CD38–CD11c+Tbet+/hi in the critically ill [49]. The number and proportion of CD21–/lowCD27– cells decreased in patients who recovered but remained expanded in patients who succumbed to the disease [50]. Compared with vaccination-induced MBCs, those induced by the infection showed better antigen-binding capacity and generated more CD21–CD27–CD11c+ MBCs [76].
Human immunodeficiency virus
HIV infects immune cells, preferentially CD4+ T cells, and with antiretroviral therapy, patients live with chronic infection. Although the T cells are the main viral target there are also changes in the peripheral B-cell compartment in these patients [77]. PB CD21–/lowCD27–CD11c+FcRL4+ B cells appear in association with viraemia, are more frequent in viraemic compared with aviraemic patients, produce HIV-specific antibodies, and decrease with treatment [37, 38]. A few years ago, a study found that during HIV infections the specific HIV gp140 response is dominated by expanded PB CD21–/lowCD27–CD11c+Tbethi, switched, B cells [20]. These cells were found to express both FcRL4 and FcRL5 at the mRNA level. More recent work, analysing LNs, showed that HIV-specific B cells of infected individuals were enriched among CD19hiTbethi MBCs and that they expressed mutated Ig genes [39]. The CD21–/low B cells in LNs of HIV patients were Tbethi whereas no such cells were detectable in LNs from healthy individuals. In conclusion, the expanded CD21–/low B cells in PB from HIV patients are TbethiFcRL4+, and hence similar to those in CVID.
Hepatitis B and C
Hepatitis B virus (HBV) can cause chronic infection and cirrhosis, putting the infected individual at high risk of liver cancer and death. HBV infection is also linked to the development of a vascular autoimmune manifestation, polyarteritis nodosa. During chronic HBV infection, PB CD21–/lowCD27–CD11c+FcRL5+Tbethi, largely switched MBCs are expanded and constitute approximately 30% of HB-specific cells, in contrast to HBV-vaccinated patients where the proportion is 10% [45]. Moreover, decreased viral load is associated with decreased proportions of CD21–/low MBCs.
Until recently, hepatitis C virus (HCV) infection was also considered a chronic disease, strongly associated with cirrhosis and hepatocellular cancer. Now, direct-acting antivirals clear the infection within 8–12 weeks in approximately 90% of patients. In HCV-infected individuals, both with and without cirrhosis, there is an expansion of PB CD21–/lowCD27– B cells, of which most lack FcRL4 [46]. Tbet+ B cells are also expanded in HCV-infected individuals; most of these cells were CD21–/lowCD27–CD11c+FcRL5+ and had switched to IgG [47]. Treatment and resolution of infection reduce the levels of the Tbet+ B cells.
Conclusion on phenotype of the expanded CD21–/low MBCs
Taken together, the phenotype that emerges for the expanded PB CD21–/low MBCs can, in most conditions, be defined as CD27–IgD–CD11c+ and largely switched. In malaria and HCV the cells are FcRL4–Tbethi, in CVID and HIV they are FcRL4+Tbethi, and in SLE they are Tbethi while the expression of FcRL4 is unclear. In RA the cells are FcRL4– whereas their Tbet expression is unclear. Therefore, in SLE, malaria and HCV, and likely in RA, but not in CVID and HIV, the expression of FcRL4 and Tbethi is similar to that in healthy individuals. In infectious conditions, the expansion of CD21–/low MBCs is associated with the viral or parasitic load and there are indications that these cells contribute to the production of both disease-specific antibodies and pathogenic autoantibodies. In autoimmune diseases, these cells are associated with key disease manifestations and autoantibodies.
CD21–/low MBCs and exhaustion
A decade ago, the CD21–/low B cells in HIV were proposed to represent a population of exhausted B cells [38]. Exhaustion, or cell exhaustion, is a differentiation state in which cells become unresponsive to stimulation. This phenomenon was first described for T cells in lymphocytic choriomeningitis virus (LCMV) infections and subsequently also in HIV [78]. The conditions that drive exhaustion—chronic antigen exposure and T-cell receptor stimulation—occur in cancer, chronic infection, and autoimmunity. Exhaustion encompasses reduced proliferative potential, increased expression of inhibitory receptor, and enhanced epigenetic enforcement [27, 75, 79, 80]. Upon analysis of the CD21–/lowCD27– B cells in HIV, it was found that these cells responded poorly to BCR activation in vitro and displayed increased expression of inhibitory receptors, e.g. FcRL4 [38]. Similar findings were evident in malaria-infected individuals where the response of CD21–/lowCD27– B cells was poor with respect to proliferation, cytokine production, and production of specific antibodies [27, 75]. However, in malaria the CD21–/low cells are not considered exhausted, as the response of these cells seemingly depends on the type of stimuli, in fact, these cells might even have a beneficial role [81].
In vitro proliferation and differentiation capacity
One of the criteria for considering the CD21–/lowCD27– MBCs in HIV as exhausted was their reduced in vitro proliferative response to signaling through the BCR (anti-Ig), although they did respond to anti-Ig when combined with TLR9 agonist (CpG) and CD40L, on par with classical MBCs [38] (Table 2). Moreover, after polyclonal stimulation (Staphylococcus aureus and CpG) the cells differentiated into plasma cells producing HIV-specific antibodies. In CVID, compared to naïve B cells, the CD21–/low cells show reduced proliferation in response to anti-Ig combined with CD40L and cytokine, whereas they secrete more antibodies after stimulation with CD40L and cytokines [21]. Somewhat similar, in SLE, CD21–/low MBCs isolated as DN2 cells or CD11chi showed a poor proliferative response after activation with TLR7 combined with cytokines or CD3-activated T cells, whereas generation of Ig secreting cells was comparable with classical MBCs [16, 24]. Moreover, the CD21–/low MBCs produced typical SLE autoantibodies. Also in malaria, CD21–/lowCD27– MBCs show a poor proliferative response to anti-Ig combined with anti-CD40, CpG, and cytokines [75]. These stimuli did not give rise to antibody production. However, when stimulated with SEB and autologous Tfh cells, the cells differentiated into plasmablasts and secrete antibodies, although not to the same extent as classical MBCs [29]. In RA, CD21–/low MBCs proliferate and differentiate in response to anti-Ig combined with TLR7 agonist and cytokine, on par with classical MBCs [25]. When it comes to PB CD21–/low MBCs from healthy individuals, although their proliferative response to TLR7 agonist and cytokines was reduced compared to classical MBCs, combining with anti-Ig resulted in a response as efficient as classical MBCs in terms of both proliferation and plasmablast differentiation [51]. The study on tonsillar FcRL4+ (CD21–/low) MBCs found that their proliferative response to anti-Ig was poor whereas CD40L combined with cytokines induced proliferation and generated antibody secreting cells, similar to classical MBCs (FcRL4–) [9]. Together this shows that the CD21–/low MBCs, whether the cells are obtained from healthy individuals or from those with chronic inflammatory conditions, can be induced to differentiate into plasma cells secreting (auto) antibodies, similar to classical MBCs. The proliferative response of the CD21–/low MBCs, however, varies and might depend on their transcriptome, as discussed below, but also their in vivo experience, as the cells are large in size and at least some of them have undergone expansion (Ki-67+) [15, 20, 28] [30, 82].
Table 2:
In vitro activation of CD21–/low MBCs
| Condition | CD21–/low phenotype | Control cells | Proliferation (stimuli) |
ASC/Ig secretion (stimuli) | Ref |
|---|---|---|---|---|---|
| DISEASE (peripheral blood) | |||||
| HIV | CD21– CD27– | CD27+ MBCs | Reduced (anti-Ig+CD40L), similar (anti-Ig + CD40L+ CpG) | Reduced frequency of ASC and increased frequency of HIV-specific ASC (S. aureus + CpG) | [38] |
| CVID | CD21-/low | Naïve B cells | Reduced (anti-Ig+cytokine+CD40L or CpG stimulation) | ND/ increased (cytokine+CD40L) | [21] |
| SLE | CD11chi | Classical MBCs | ND | Similar/ similar (Co-culture with anti-CD3 activated T cells) | [16] |
| SLE | CD21-/low CD11c+ Tbethi (DN2) | Classical MBCs | Reduced (R848 + cytokines) | Similar (R848 + cytokines) | [24] |
| Malaria | CD21–CD27– | Classical MBCs | Reduced (anti-Ig+ anti-CD40+cytokines+CPG) | None/ND (CpG, IL-10, SAC and PWM) | [75] |
| Malaria | CD21– CD27– MBCs | Classical MBCs | ND | Reduced/ ND (SEB + cTfh cells) | [29] |
| Established RA | CD21–/low | Classical MBCs | Similar (anti-Ig+ R848+ cytokine) | Similar/ ND (anti-Ig+ R848+ cytokine) | [25] |
| HEALTH | |||||
| PB | CD21–/low MBCs | Classical MBCs | Reduced (R848+IL2) similar (anti-Ig+ cytokine+ R848) | Similar/ ND (anti-Ig+ cytokine+ R848) | [51] |
| Tonsils | (CD21–/low) FcRL4+ MBCs | Classical FcRL4– MBCs | Reduced (anti-Ig), similar (cytokines+CD40L) | Increased/ND (cytokines + CD40L) | [9] |
Antibody secreting cells (ASC), anti-Ig (anti-Immunoglobulin), common variable immunodeficiency (CVID), double negative 2 (DN2), human immunodeficiency virus (HIV), not determined (n/d), peripheral blood (PB), pokeweed mitogen (PWM), rheumatoid arthritis (RA), Staphylococcus aureus Cowan (SAC), Staphylococcus enterotoxin B (SEB), Systemic lupus erythematosus (SLE), CpG Toll like receptor 9 agonist, R848, Toll like receptor 7/8 agonist.
Expression of inhibitory receptors
As discussed above, the CD21–/low MBCs in HIV express FcRL4 [38], which has been considered to be an inhibitory receptor due to its immunoreceptor tyrosine-based inhibitory motifs (ITIMs). Indeed, signalling via FcRL4 in the context of BCR activation is inhibitory; however, in the context of Toll-like receptor (TLR) agonists it is activating [83]. Like FcRL4, FcRL5 is a member of the Fc receptor-like proteins and has immunomodulatory potential [84], and is also expressed on CD21–/low MBCs in HIV [20]. Unlike FcRL4, which is a receptor for IgA, FcRL5 binds IgG [85]. FcRL5 has both inhibitory (ITIMs) and activating motifs (ITAMs), implying inhibitory and stimulatory potential. Recent work suggests that FcRL5 is part of the BCR co-receptor complex, together with CD21, and amplifies the BCR response [84]. Hence, under these conditions FcRL5 has activating properties. However, in the absence of CD21, FcRL5 acts as an inhibitory receptor and reduces the response to BCR stimulation. This does not necessarily mean that the expression of FcRL5 is synonymous with unresponsiveness, as this may depend on stimuli, although this is less clear. In the context of CD21–/low MBCs, in SLE, malaria, HCV, and RA they lack FcRL4, whereas in CVID and HIV they express this receptor. FcRL5 is expressed on CD21–/low MBCs in most conditions. As the function of FcRL4 and FcRL5 is seemingly dependent on the stimuli, expression of these receptors is not necessarily a sign of exhaustion.
Chemokine receptor-expression
Cells in PB that express CXCR3 and/or CXCR6 migrate towards sites of inflammation and/or extralymphatic sites. By contrast, expression of CXCR4 indicates migration to BM, spleen and LN, and expression of CXCR5 to spleen and LNs. These chemokine receptors can also be an indication of where cells reside. A pattern of CXCR3/CXCR6 expression and a lack of CXCR4 and CXCR5 has emerged for CD21–/low MBCs, which appears to be independent of condition and of whether the cells express FcRL4, FcRL5, and/or Tbet (Table 1). This said, this does not mean that all CD21–/low MBCs that are for instance Tbethi express CXCR3. Rather, expression of these receptors is seemingly dependent on disease dynamics, as exemplified in malaria [28]. Here, the proportion of CXCR3 positive CD21–/lowCD27–CD11c+ MBCs increased from around 20% to 70% during the first month after primary infection. Nevertheless, this suggests that the CD21–/low MBCs in PB that express CXCR3/CXCR6 are destined for inflammatory sites.
Gene expression in CD21–/low MBCs
Several laboratories have analysed genes expressed by the CD21–/low B cells to understand their fate, for instance, whether they are on a path to plasma cells, as they in all conditions can differentiate into antibody secreting cells in vitro. In general, these studies corroborated the expression (or lack) of identifying phenotypic markers at the mRNA level, e.g. expression of ITGAX (CD11c), TBX21 (Tbet), and/or FCRL4, FCRL5, and CXCR3/6, but lack of CR2, CD27, and CXCR4/5. Below we discuss genes that are shared, and others that characterize the CD21–/low MBCs in different conditions.
Analysing sorted CD27–IgD– PB B cells from healthy individuals by single-cell RNA sequencing (sc-RNAseq) identified four clusters (DN1-4), of which DN2 likely represented the CD21–/lowCD27–IgD– MBCs, expressing ITGAX, whereas DN1 and 4 showed an expression pattern similar to classical MBCs [3]. Other genes expressed in DN2 included TBX21, although not all cells were positive, FCRL5 and TNFRSF1B but not CXCR5, IL4R, IGHM or IGHD. The expression or lack of these genes is confirmed in another sc-RNAseq study [86]. Genes indicative of plasma cell precursors were not discussed, presumably not expressed. Analysing tonsillar CD21–/lowFcRL4+ MBCs detected AICDA (AID) but not genes typical of plasma cells, e.g. PRDM1 (BLIMP1), IRF4, and XBP1 [9, 15], which indicated that neither of these cells are plasma cell precursors.
In HIV, antigen-specific PB CD21–/lowCD27– IgG+ MBCs were found to express AICDA and TNFRSF1B [87], which has been confirmed by others in sc-RNAseq data [35]. The latter study also detected high levels of SYK and a lack of IL4R. Isolating CD19hi HIV-specific MBCs and, by inference, CD21–/lowTbet+FCRL4+, from LNs enabled the identification of AICDA [39]. High levels of SYK mRNA have also been detected in PB CD21–/low MBCs in CVID [88]. Analysis of PB CD21–/lowCD27– MBCs as bulk or the corresponding population in sc-RNAseq data from patients in a malaria-endemic area found high levels of AICDA, SYK, TNFRSF1B as well as IL21R, and IFNγ signature [35, 89]. In the sc-RNAseq study the expression of plasma cell genes, e.g. PRDM1 was prominent in the ‘activated MBC’ but not in the ‘ABC’ (CD21–/low) cluster. Another study followed the dynamics of malaria-specific Tbet-expressing CD21–/lowCD27– B-cell responses to acute malaria infection [29]. It demonstrated that IL21R was expressed in healthy individuals in malaria-endemic areas before the start of the malaria season and down-regulated a week after treatment of the first acute malaria episode (in convalescence), while TLR7, TLR9, PRDM1, and CD38 showed the opposite pattern. These findings indicate that at convalescence the CD21–/lowCD27– are plasma cell precursors.
In SLE, isolated PB CD11chi and, by inference, CD21–/lowTbethi MBCs were found to express high levels of AICDA, TNFRSF1B, SYK, IL21R as well as TLR9 and TLR7, and low IL4R [16]. Pathway analyses found an enrichment of IL-21-inducible genes. Moreover, the cells expressed genes indicative of plasma cell precursors, e.g. PRDM1 and XBP1 [16]. Similar gene expression patterns were found in another SLE study of PB CD21–/low DN2 (CD27–IgD–CD11c+CXCR5–Tbethi) MBCs, for instance, PRDM1 and XBP1 but also IRF4 [24]. Moreover, these cells expressed elevated levels of IFNLR1 (IFNλ receptor 1), supported by others [61], as well as an enrichment of IFN-regulated genes. Thus, in SLE the CD21–/low MBCs are seemingly bound to become plasma cells.
Taken together, among the genes expressed by CD21–/low MBCs in healthy individuals and individuals with inflammatory conditions seemingly have in common is AICDA. This encodes the AID enzyme that is involved in somatic hypermutation and class-switch recombination, events that take place inside GCs or extrafollicularly [90, 91]. Expression of this gene in circulating cells is, at least to us, rather surprising considering that the activity of this enzyme introduces mutations, albeit at specific sites. The CD21–/low cells also share expression of TNFRSF1B, encoding the high affinity TNF receptor that is involved in, for instance, cell survival. Another gene that is shared, at least in most inflammatory conditions, is SYK, which encodes a tyrosine kinase involved in BCR signalling. Also shared in some conditions is TLR7/9 expression, which is consistent with the finding that in several conditions the cells can be activated with TLR agonists in combination with, e.g. cytokines. In this context, the expression of cytokine receptors such as IL21R and the lack of IL4R, together with the detection of genes regulated by the IL21R, as well as by IFN and IFNγ, are consistent with studies in mice [92]. Here, it was shown that TLR activation in combination with IL21 or IFNγ promotes Tbet expression in CD21−/low B cells and that this expression is inhibited by IL4, whereas CD11c expression is linked to IL21. Genes that might distinguish the CD21−/low MBCs in the various conditions are those linked to plasma cell differentiation, e.g. PRDM1, XBP1, and IRF4, expressed in PB in SLE but not in tonsils in healthy individuals; the status of these genes in the other human conditions is less clear. In this context, we find the study in malaria noteworthy [29], where it was demonstrated how gene expression varied over the course of the disease and in convalescence. It would seem that IL21 and IFNγ are important early in the infection, while a week after acute infection the cells take on a plasma cell precursor signature. This recalls the importance of considering also disease duration and immunosuppressive treatments that can have an impact on the B cells, parameters that may affect the read-out, and even our understanding of the CD21–/low MBCs.
Ontogeny of CD21–/low memory B cells
In mice, ABCs are thought to develop from mature, naïve (follicular) B cells based on the observation that after the transfer of such cells into congenic hosts, cells with an ABC phenotype were found to be expanded [52]. ABCs express MBC markers indicative of different MBC populations [93], although it is unclear whether they are generated directly from naïve cells or via ‘classic’ MBCs. Nevertheless, the generation of ABCs is dependent on T cells and TLR7 [11, 94], and CD11c+Tbet+ B cells develop in a T-cell dependent manner outside GCs [95].
In humans, generation/maintenance of CD21–/low B cells is dependent on T cells, IL21R and Tbet while the importance of TLR is less clear [17, 86]. The CD21–/low cells are antigen-experienced MBCs in the majority of conditions, as the cells are isotype-switched and express BCRs that have undergone somatic hypermutation. As in mice, it is unclear whether these cells are generated directly from naïve B cells, although there are indications that they develop from naïve B cells via MBCs [3]. This could indicate that the cells are formed in GCs; however, class switching and somatic hypermutation can also take place extrafollicularly [91]. In fact, in LN from HIV viraemic patients, CD21–/lowTbethi MBCs accumulated outside GCs [39], although this does not necessarily mean that HIV is representative of all conditions with an expanded Tbet population.
Concluding remarks
In all chronic inflammatory conditions discussed above, CD21–/low MBCs have been found to be expanded. In most conditions these cells, or subsets thereof, associated with clinical parameter(s), e.g. in SLE with disease activity, in RA with joint destruction and in malaria with anaemia. Based on the chemokine-receptor expression the PB CD21–/low MBCs in chronic inflammatory conditions would be expected to migrate to sites of inflammation. Indeed, in SLE they are found in kidneys, in RA in inflamed joints, in HIV in LNs, and in Crohn’s disease in the inflamed intestine. The function of CD21–/low MBCs in SLE may well be as plasma cells, as the expressed genes suggest that they are precursors of these cells, and the CD21–/lowCD11c+Tbethi subset correlates with both plasma cell frequencies and autoantibody levels. In malaria, the CD21–/lowCD11c+Tbethi correlate with antibodies against red blood cells, and anaemia. Here, plasma cell genes are expressed in convalescence but not the acute phase, indicating that the CD21–/low cells might have different functions at different time points. In most other conditions expression of plasma cell genes has not been reported, either because they were not tested for or they were not detected, except for the study on tonsillar FcRL4+ cells where these genes were not expressed. Therefore, the CD21–/low MBCs in the other conditions are seemingly not precursors of plasma cells, suggesting that they have an, as yet unknown function(s). Whether this unknown function is the same as that of PB in health is currently unknown, but a possibility. Thus, this argues that the CD21–/low B cells are not comparable in the different conditions.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- ABCs
age-associated B cells
- ACPA
anti-citrullinated protein antibodies
- AID/AICDA
activation-induced cytidine deaminase
- BAL
bronchoalveolar lavage
- BM
bone marrow
- BCR
B cell receptor
- BLIMP
B lymphocyte-induced maturation protein
- CD
cluster of differentiation
- CVID
common variable immunodeficiency
- CR
complement receptor
- CXCR
chemokine receptor
- DAS28
disease activity score 28 joints
- DN
double negative
- dsDNA
double-stranded DNA
- GC
germinal centre
- FCRH
Fc receptor homologue
- FcRL
Fc receptor-like
- HBV
hepatitis B virus
- HB
hepatitis B
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IFN
interferon
- IFNLR
interferon lambda receptor
- IG/Ig
immunoglobulin
- IL
interleukin
- IL4R
interleukin 4 receptor
- IRTA
Immune receptor translocation-associated protein
- IRF4
interferon regulatory factor
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- ITAM
immunoreceptor tyrosine-based activation motif
- ITGAX
CD11c/integrin αX
- LCMV
lymphocytic choriomeningitis virus
- LN
lymph node
- MBCs
memory B cells
- MS
multiple sclerosis
- PB
peripheral blood
- P. falciparum
Plasmodium falciparum
- PRDM
PR domain zinc finger protein
- RF
rheumatoid factor
- RNP
ribonucleoproteins
- sc-RNAseq
single-cell RNA sequencing
- SLE
systemic lupus erythematosus
- SLEDAI
Systemic Lupus Erythematosus Disease Activity Index
- SLO
secondary lymphoid organs
- RA
rheumatoid arthritis
- Sw
switched
- Tbet
T-box expressed in T cells
- TBX
T-box transcription factor
- TLR
toll like receptor
- TNF
tumor necrosis factor
- TNFRSF
tumour necrosis factor receptor superfamily
- UnSw
unswitched
- XBP1
x-box binding protein.
Contributor Information
Inger Gjertsson, Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg 40530, Sweden.
Sarah McGrath, Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg 40530, Sweden.
Kristoffer Grimstad, Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg 40530, Sweden; School of Bioscience, University of Skövde, Skövde 54128, Sweden.
Charlotte A Jonsson, Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg 40530, Sweden.
Alessandro Camponeschi, Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg 40530, Sweden.
Katrin Thorarinsdottir, Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg 40530, Sweden.
Inga-Lill Mårtensson, Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg 40530, Sweden.
Future prospects
In autoimmunity, the CD21–/low MBCs often correlate to disease-specific manifestations, and in chronic infections they contribute to production of disease-specific neutralizing antibodies. Although the knowledge of these cells has exploded over recent years, many questions still remain. For instance, how are the CD21–/low MBCs generated? What is the function of the CD21–/low MBCs in the different conditions? What are their function in the inflamed tissue? Do the cells change function during the course of a disease? Are these cells pathogenic or do they have a protective potential? Can they provide an inhibitory or stimulatory treatment target?
Animal research
Not applicable.
Conflict of interests
The authors declare no competing interests.
Funding
Funding has been received from: the Swedish Research Council 2018-03128 and 2021-01150 (ILM), 2016-00288 (IG), the Patient Association for Rheumatic Diseases R-94129 (ILM), R-940945 (IG), the Swedish Cancer Foundation 19 0464 (ILM), the Childhood Cancer Foundation PR2018-0170 (ILM), and the ALF agreement between the Swedish government and the county councils ALFGBG-965435 (IG), ALFGBG-277797 (ILM).
Data availability
Not applicable.
Author Contributions
All authors have contributed scientifically and intellectually to the manuscript. SM has produced and analysed the flow cytometry data shown in Fig. 1, AC has produced Fig. 2. IG and ILM has edited and finalised the manuscript. All authors have read and approved the final manuscript.
Permission to reproduce (for relevant content)
Not applicable.
Clinical trial registration
Not applicable.
References
- 1. Klein U, Rajewsky K, Küppers R.. Human immunoglobulin (Ig)M IgD peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. . J Exp Med 1998, 188, 1679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sanz I, Wei C, Lee FE, Anolik J.. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol 2008, 20, 67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stewart A, Ng JC, Wallis G, Tsioligka V, Fraternali F, Dunn-Walters DK.. Single-cell transcriptomic analyses define distinct peripheral B cell subsets and discrete development pathways. Front Immunol 2021, 12, 602539. doi: 10.3389/fimmu.2021.602539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weisel F, Shlomchik M.. Memory B cells of mice and humans. Annu Rev Immunol 2017, 35, 255–84. doi: 10.1146/annurev-immunol-041015-055531. [DOI] [PubMed] [Google Scholar]
- 5. Phalke S, Marrack P.. Age (autoimmunity) associated B cells (ABCs) and their relatives. Curr Opin Immunol 2018, 55, 75–80. doi: 10.1016/j.coi.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 6. Cancro MP. Age-associated B cells. Annu Rev Immunol 2020, 38, 315–40. doi: 10.1146/annurev-immunol-092419-031130. [DOI] [PubMed] [Google Scholar]
- 7. Gao X, Cockburn IA.. The development and function of CD11c(+) atypical B cells - insights from single cell analysis. Front Immunol 2022, 13, 979060. doi: 10.3389/fimmu.2022.979060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Warnatz K, Wehr C, Dräger R, Schmidt S, Eibel H, Schlesier M, et al. Expansion of CD19(hi)CD21(lo/neg) B cells in common variable immunodeficiency (CVID) patients with autoimmune cytopenia. Immunobiology 2002, 206, 502–13. [DOI] [PubMed] [Google Scholar]
- 9. Ehrhardt GR, Hsu JT, Gartland L, Leu CM, Zhang S, Davis RS, et al. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J Exp Med 2005, 202, 783–91. doi: 10.1084/jem.20050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P.. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proc Natl Acad Sci USA 2013, 110, E3216–24. doi: 10.1073/pnas.1312348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c⁺ B-cell population is important for the development of autoimmunity. Blood 2011, 118, 1305–15. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller I, Hatzivassiliou G, Cattoretti G, Mendelsohn C, Dalla-Favera R.. IRTAs: a new family of immunoglobulinlike receptors differentially expressed in B cells. Blood 2002, 99, 2662–9. doi: 10.1182/blood.v99.8.2662. [DOI] [PubMed] [Google Scholar]
- 13. Hatzivassiliou G, Miller I, Takizawa J, Palanisamy N, Rao PH, Iida S, et al. IRTA1 and IRTA2, novel immunoglobulin superfamily receptors expressed in B cells and involved in chromosome 1q21 abnormalities in B cell malignancy. Immunity 2001, 14, 277–89. doi: 10.1016/s1074-7613(01)00109-1. [DOI] [PubMed] [Google Scholar]
- 14. Falini B, Tiacci E, Pucciarini A, Bigerna B, Kurth J, Hatzivassiliou G, et al. Expression of the IRTA1 receptor identifies intraepithelial and subepithelial marginal zone B cells of the mucosa-associated lymphoid tissue (MALT). Blood 2003, 102, 3684–92. doi: 10.1182/blood-2003-03-0750. [DOI] [PubMed] [Google Scholar]
- 15. Ehrhardt GR, Hijikata A, Kitamura H, Ohara O, Wang JY, Cooper MD.. Discriminating gene expression profiles of memory B cell subpopulations. J Exp Med 2008, 205, 1807–17. doi: 10.1084/jem.20072682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE. Nat Commun 2018, 9, 1758. doi: 10.1038/s41467-018-03750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Keller B, Strohmeier V, Harder I, Unger S, Payne KJ, Andrieux G, et al. The expansion of human T-bet(high)CD21(low) B cells is T cell dependent. Sci Immunol 2021, 6, eabh0891. doi: 10.1126/sciimmunol.abh0891. [DOI] [PubMed] [Google Scholar]
- 18. Unger S, Seidl M, van Schouwenburg P, Rakhmanov M, Bulashevska A, Frede N, et al. The T(H)1 phenotype of follicular helper T cells indicate an IFN-γ-associated immune dysregulation in patients with CD21low common variable immunodeficiency. J Allergy Clin Immunol 2018, 141, 730–40. doi: 10.1016/j.jaci.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 19. Johnson JL, Rosenthal RL, Knox JJ, Myles A, Naradikian MS, Madej J, et al. The transcription factor T-bet resolves memory B cell subsets with distinct tissue distributions and antibody specificities in mice and humans. Immunity 2020, 52, 842–855.e6. doi: 10.1016/j.immuni.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knox JJ, Buggert M, Kardava L, Seaton KE, Eller MA, Canaday DH, et al. T-bet+ B cells are induced by human viral infections and dominate the HIV gp140 response. JCI insight 2017, 2, e92943. doi: 10.1172/jci.insight.92943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rakhmanov M, Keller B, Gutenberger S, Foerster C, Hoenig M, Driessen G, et al. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci USA 2009, 106, 13451–6. doi: 10.1073/pnas.0901984106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friedmann D, Unger S, Keller B, Rakhmanov M, Goldacker S, Zissel G, et al. Bronchoalveolar Lavage fluid reflects a T(H)1-CD21(low) B-Cell interaction in CVID-related interstitial lung disease. Front Immunol 2020, 11, 616832. doi: 10.3389/fimmu.2020.616832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood 2008, 111, 77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 24. Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, et al. Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 2018, 49, 725–739.e6. doi: 10.1016/j.immuni.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thorarinsdottir K, Camponeschi A, Jonsson C, Granhagen Onnheim K, Nilsson J, Forslind K, et al. CD21(-/low) B cells associate with joint damage in rheumatoid arthritis patients. Scand J Immunol 2019, 90, e12792. doi: 10.1111/sji.12792. [DOI] [PubMed] [Google Scholar]
- 26. Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, et al. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol (Baltimore, Md: 1950) 2009, 183, 2176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sullivan RT, Kim CC, Fontana MF, Feeney ME, Jagannathan P, Boyle MJ, et al. FCRL5 delineates functionally impaired memory B cells associated with plasmodium falciparum exposure. PLoS Pathog 2015, 11, e1004894. doi: 10.1371/journal.ppat.1004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sundling C, Rönnberg C, Yman V, Asghar M, Jahnmatz P, Lakshmikanth T, et al. B cell profiling in malaria reveals expansion and remodelling of CD11c+ B cell subsets. JCI Insight 2019, 4, e126492. doi: 10.1172/jci.insight.126492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hopp CS, Skinner J, Anzick SL, Tipton CM, Peterson ME, Li S, et al. Atypical B cells up-regulate costimulatory molecules during malaria and secrete antibodies with T follicular helper cell support. Sci Immunol 2022, 7, eabn1250. doi: 10.1126/sciimmunol.abn1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muellenbeck MF, Ueberheide B, Amulic B, Epp A, Fenyo D, Busse CE, et al. Atypical and classical memory B cells produce Plasmodium falciparum neutralizing antibodies. J Exp Med 2013, 210, 389–99. doi: 10.1084/jem.20121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zinocker S, Schindler CE, Skinner J, Rogosch T, Waisberg M, Schickel JN, et al. The V gene repertoires of classical and atypical memory B cells in malaria-susceptible West African children. J Immunol (Baltimore, Md: 1950) 2015, 194, 929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Obeng-Adjei N, Portugal S, Holla P, Li S, Sohn H, Ambegaonkar A, et al. Malaria-induced interferon-γ drives the expansion of Tbethi atypical memory B cells. PLoS Pathog 2017, 13, e1006576. doi: 10.1371/journal.ppat.1006576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rivera-Correa J, Yasnot-Acosta MF, Tovar NC, Velasco-Pareja MC, Easton A, Rodriguez A.. Atypical memory B-cells and autoantibodies correlate with anemia during Plasmodium vivax complicated infections. PLoS NeglTrop Dis 2020, 14, e0008466. doi: 10.1371/journal.pntd.0008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rivera-Correa J, Mackroth MS, Jacobs T, Schulze Zur Wiesch J, Rolling T, Rodriguez A.. Atypical memory B-cells are associated with Plasmodium falciparum anemia through anti-phosphatidylserine antibodies. Elife 2019, 8, e48309. doi: 10.7554/eLife.48309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holla P, Dizon B, Ambegaonkar AA, Rogel N, Goldschmidt E, Boddapati AK, et al. Shared transcriptional profiles of atypical B cells suggest common drivers of expansion and function in malaria, HIV, and autoimmunity. Sci Adv 2021, 7, eabg8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Portugal S, Obeng-Adjei N, Moir S, Crompton PD, Pierce SK.. Atypical memory B cells in human chronic infectious diseases: an interim report. Cell Immunol 2017, 321, 18–25. doi: 10.1016/j.cellimm.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moir S, Malaspina A, Pickeral OK, Donoghue ET, Vasquez J, Miller NJ, et al. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med 2004, 200, 587–99. [PubMed] [Google Scholar]
- 38. Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med 2008, 205, 1797–805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Austin JW, Buckner CM, Kardava L, Wang W, Zhang X, Melson VA, et al. Overexpression of T-bet in HIV infection is associated with accumulation of B cells outside germinal centers and poor affinity maturation. Sci Transl Med 2019, 11, eaax0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saadoun D, Terrier B, Bannock J, Vazquez T, Massad C, Kang I, et al. Expansion of autoreactive unresponsive CD21-/low B cells in Sjogren’s syndrome-associated lymphoproliferation. Arthritis Rheum 2013, 65, 1085–96. doi: 10.1002/art.37828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Visentini M, Pellicano C, Leodori G, Marrapodi R, Colantuono S, Gigante A, et al. CD21(low) B cells are predictive markers of new digital ulcers in systemic sclerosis. Clin Exp Immunol 2021, 205, 128–34. doi: 10.1111/cei.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marrapodi R, Pellicano C, Radicchio G, Leodori G, Colantuono S, Iacolare A, et al. CD21(low) B cells in systemic sclerosis: a possible marker of vascular complications. . Clin Immunol 2020, 213, 108364. doi: 10.1016/j.clim.2020.108364. [DOI] [PubMed] [Google Scholar]
- 43. Wang Z, Wang Z, Wang J, Diao Y, Qian X, Zhu N.. T-bet-expressing B cells are positively associated with Crohn’s disease activity and support Th1 inflammation. DNA Cell Biol 2016, 35, 628–35. doi: 10.1089/dna.2016.3304. [DOI] [PubMed] [Google Scholar]
- 44. Claes N, Fraussen J, Vanheusden M, Hellings N, Stinissen P, Van Wijmeersch B, et al. Age-associated B cells with proinflammatory characteristics are expanded in a proportion of multiple sclerosis patients. J Immunol (Baltimore, Md: 1950) 2016, 197, 4576–83. [DOI] [PubMed] [Google Scholar]
- 45. Burton AR, Pallett LJ, McCoy LE, Suveizdyte K, Amin OE, Swadling L, et al. Circulating and intrahepatic antiviral B cells are defective in hepatitis B, 2018, 128, 4588–603. doi: 10.1172/JCI121960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Doi H, Tanoue S, Kaplan DE.. Peripheral CD27-CD21- B-cells represent an exhausted lymphocyte population in hepatitis C cirrhosis. Clin Immunol 2014, 150, 184–91. doi: 10.1016/j.clim.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang LY, Li Y, Kaplan DE.. Hepatitis C viraemia reversibly maintains subset of antigen-specific T-bet+ tissue-like memory B cells. J Viral Hepat 2017, 24, 389–96. doi: 10.1111/jvh.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wildner NH, Ahmadi P, Schulte S, Brauneck F, Kohsar M, Lütgehetmann M, et al. B cell analysis in SARS-CoV-2 versus malaria: increased frequencies of plasmablasts and atypical memory B cells in COVID-19. J Leukoc Biol 2021, 109, 77–90. doi: 10.1002/JLB.5COVA620-370RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Woodruff MC, Ramonell RP, Nguyen DC, Cashman KS, Saini AS, Haddad NS, et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol 2020, 21, 1506–16. doi: 10.1038/s41590-020-00814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oliviero B, Varchetta S, Mele D, Mantovani S, Cerino A, Perotti CG, et al. Expansion of atypical memory B cells is a prominent feature of COVID-19. Cell Mol Immunol 2020, 17, 1101–3. doi: 10.1038/s41423-020-00542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thorarinsdottir K, Camponeschi A, Cavallini N, Grimsholm O, Jacobsson L, Gjertsson I, et al. CD21(-/low) B cells in human blood are memory cells. Clin Exp Immunol 2016, 185, 252–62. doi: 10.1111/cei.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP.. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood 2011, 118, 1294–304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol 2005, 5, 521–31. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 54. Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, et al. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood 2010, 115, 5026–36. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, et al. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol (Baltimore, Md: 1950) 2007, 178, 6624–33. [DOI] [PubMed] [Google Scholar]
- 56. Dolff S, Wilde B, Patschan S, Dürig J, Specker C, Philipp T, et al. Peripheral circulating activated b-cell populations are associated with nephritis and disease activity in patients with systemic lupus erythematosus. Scand J Immunol 2007, 66, 584–90. doi: 10.1111/j.1365-3083.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- 57. Jacobi AM, Reiter K, Mackay M, Aranow C, Hiepe F, Radbruch A, et al. Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: delineation by expression of CD27, IgD, and CD95. Arthritis Rheum 2008, 58, 1762–73. doi: 10.1002/art.23498. [DOI] [PubMed] [Google Scholar]
- 58. Wehr C, Eibel H, Masilamani M, Illges H, Schlesier M, Peter HH, et al. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol 2004, 113, 161–71. doi: 10.1016/j.clim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 59. Kosalka J, Jakiela B, Musial J.. Changes of memory B- and T-cell subsets in lupus nephritis patients. Folia Histochem Cytobiol 2016, 54, 32–41. doi: 10.5603/FHC.a2016.0005. [DOI] [PubMed] [Google Scholar]
- 60. Zumaquero E, Stone SL, Scharer CD, Jenks SA, Nellore A, Mousseau B, et al. IFNgamma induces epigenetic programming of human T-bet(hi) B cells and promotes TLR7/8 and IL-21 induced differentiation. Elife 2019, 8, e41641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barnas JL, Albrecht J, Meednu N, Alzamareh DF, Baker C, McDavid A, et al. B cell activation and plasma cell differentiation are promoted by IFN-λ in systemic lupus erythematosus. J Immunol (Baltimore, Md: 1950) 2021, 207, 2660–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mccomish J, Mundy J, Sullivan T, Proudman SM, Hissaria P.. Changes in peripheral blood B cell subsets at diagnosis and after treatment with disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis: correlation with clinical and laboratory parameters. Int J Rheum Dis 2015, 18, 421–32. doi: 10.1111/1756-185X.12325. [DOI] [PubMed] [Google Scholar]
- 63. Rincón-Arévalo H, Rojas M, Vanegas-García A, Muñoz-Vahos C, Orejuela-Erazo J, Vásquez G, et al. Atypical phenotype and response of B cells in patients with seropositive rheumatoid arthritis. Clin Exp Immunol 2021, 204, 221–38. doi: 10.1111/cei.13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bao W, Xie M, Ye Y.. Age-associated B cells indicate disease activity in rheumatoid arthritis. Cell Immunol 2022, 377, 104533. doi: 10.1016/j.cellimm.2022.104533. [DOI] [PubMed] [Google Scholar]
- 65. Humby F, Lewis M, Ramamoorthi N, Hackney JA, Barnes MR, Bombardieri M, et al. Synovial cellular and molecular signatures stratify clinical response to csDMARD therapy and predict radiographic progression in early rheumatoid arthritis patients. Ann Rheum Dis 2019, 78, 761–72. doi: 10.1136/annrheumdis-2018-214539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rivellese F, Humby F, Bugatti S, Fossati-Jimack L, Rizvi H, Lucchesi D, et al. B cell synovitis and clinical phenotypes in rheumatoid arthritis: relationship to disease stages and drug exposure. Arthritis Rheumatol 2020, 72, 714–25. doi: 10.1002/art.41184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang F, Wei K, Slowikowski K, Fonseka CY, Rao DA, Kelly S, et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol 2019, 20, 928–42. doi: 10.1038/s41590-019-0378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Illges H, Braun M, Peter HH, Melchers I.. Reduced expression of the complement receptor type 2 (CR2, CD21) by synovial fluid B and T lymphocytes. Clin Exp Immunol 2000, 122, 270–6. doi: 10.1046/j.1365-2249.2000.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Amara K, Clay E, Yeo L, Ramskold D, Spengler J, Sippl N, et al. B cells expressing the IgA receptor FcRL4 participate in the autoimmune response in patients with rheumatoid arthritis. J Autoimmun 2017, 81, 34–43. doi: 10.1016/j.jaut.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nakayama T, Yoshimura M, Higashioka K, Miyawaki K, Ota Y, Ayano M, et al. Type 1 helper T cells generate CXCL9/10-producing T-bet(+) effector B cells potentially involved in the pathogenesis of rheumatoid arthritis. Cell Immunol 2021, 360, 104263. doi: 10.1016/j.cellimm.2020.104263. [DOI] [PubMed] [Google Scholar]
- 71. Visentini M, Cagliuso M, Conti V, Carbonari M, Cibati M, Siciliano G, et al. Clonal B cells of HCV-associated mixed cryoglobulinemia patients contain exhausted marginal zone-like and CD21 low cells overexpressing Stra13. Eur J Immunol 2012, 42, 1468–76. doi: 10.1002/eji.201142313. [DOI] [PubMed] [Google Scholar]
- 72. Wilbrink R, Spoorenberg A, Arends S, van der Geest KSM, Brouwer E, Bootsma H, et al. CD27(-)CD38(low)CD21(low) B-Cells are increased in axial spondyloarthritis. Front Immunol 2021, 12, 686273. doi: 10.3389/fimmu.2021.686273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Illingworth J, Butler NS, Roetynck S, Mwacharo J, Pierce SK, Bejon P, et al. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol 2013, 190, 1038–47. doi: 10.4049/jimmunol.1202438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ayieko C, Maue AC, Jura WG, Noland GS, Ayodo G, Rochford R, et al. Changes in B cell populations and merozoite surface protein-1-specific memory B cell responses after prolonged absence of detectable P. falciparum infection. PLoS One 2013, 8, e67230. doi: 10.1371/journal.pone.0067230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Portugal S, Tipton CM, Sohn H, Kone Y, Wang J, Li S, et al. Malaria-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. Elife 2015, 4, e07218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pape KA, Dileepan T, Kabage AJ, Kozysa D, Batres R, Evert C, et al. High-affinity memory B cells induced by SARS-CoV-2 infection produce more plasmablasts and atypical memory B cells than those primed by mRNA vaccines. Cell Rep 2021, 37, 109823. doi: 10.1016/j.celrep.2021.109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Moir S, Malaspina A, Ogwaro KM, Donoghue ET, Hallahan CW, Ehler LA, et al. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci USA 2001, 98, 10362–7. doi: 10.1073/pnas.181347898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wherry EJ, Kurachi M.. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015; 15:486-99. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 2012, 338, 1220–5. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, et al. Defining ‘T cell exhaustion’. Nat Rev Immunol 2019, 19, 665–74. doi: 10.1038/s41577-019-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Holla P, Ambegaonkar A, Sohn H, Pierce SK.. Exhaustion may not be in the human B cell vocabulary, at least not in malaria. Immunol Rev 2019, 292, 139–48. doi: 10.1111/imr.12809. [DOI] [PubMed] [Google Scholar]
- 82. Rincon-Arevalo H, Wiedemann A, Stefanski AL, Lettau M, Szelinski F, Fuchs S, et al. Deep phenotyping of CD11c(+) B cells in systemic autoimmunity and controls. Front Immunol 2021, 12, 635615. doi: 10.3389/fimmu.2021.635615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rostamzadeh D, Kazemi T, Amirghofran Z, Shabani M.. Update on Fc receptor-like (FCRL) family: new immunoregulatory players in health and diseases. Expert Opin Ther Targets 2018, 22, 487–502. doi: 10.1080/14728222.2018.1472768. [DOI] [PubMed] [Google Scholar]
- 84. Franco A, Kraus Z, Li H, Seibert N, Dement-Brown J, Tolnay M.. CD21 and FCRL5 form a receptor complex with robust B-cell activating capacity. Int Immunol 2018, 30, 569–78. doi: 10.1093/intimm/dxy052. [DOI] [PubMed] [Google Scholar]
- 85. Franco A, Damdinsuren B, Ise T, Dement-Brown J, Li H, Nagata S, et al. Human Fc receptor-like 5 binds intact IgG via mechanisms distinct from those of Fc receptors. J Immunol (Baltimore, Md: 1950) 2013, 190, 5739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yang R, Avery DT, Jackson KJL, Ogishi M, Benhsaien I, Du L, et al. Human T-bet governs the generation of a distinct subset of CD11c(high)CD21(low) B cells. Sci Immunol 2022, 7, eabq3277. doi: 10.1126/sciimmunol.abq3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kardava L, Moir S, Shah N, Wang W, Wilson R, Buckner CM, et al. Abnormal B cell memory subsets dominate HIV-specific responses in infected individuals. J Clin Invest 2014, 124, 3252–62. doi: 10.1172/JCI74351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Keller B, Stumpf I, Strohmeier V, Usadel S, Verhoeyen E, Eibel H, et al. High SYK expression drives constitutive activation of CD21(low) B cells. J Immunol (Baltimore, Md: 1950) 2017, 198, 4285–92. [DOI] [PubMed] [Google Scholar]
- 89. Sutton HJ, Aye R, Idris AH, Vistein R, Nduati E, Kai O, et al. Atypical B cells are part of an alternative lineage of B cells that participates in responses to vaccination and infection in humans. Cell Rep 2021, 34, 108684. doi: 10.1016/j.celrep.2020.108684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Roco JA, Mesin L, Binder SC, Nefzger C, Gonzalez-Figueroa P, Canete PF, et al. Class-switch recombination occurs infrequently in germinal centers. Immunity 2019, 51, 337–350.e7. doi: 10.1016/j.immuni.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Elsner RA, Shlomchik MJ.. Germinal center and extrafollicular B cell responses in vaccination, immunity, and autoimmunity. Immunity 2020, 53, 1136–50. doi: 10.1016/j.immuni.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Naradikian MS, Myles A, Beiting DP, Roberts KJ, Dawson L, Herati RS, et al. Cutting edge: IL-4, IL-21, and IFN-gamma interact to govern T-bet and CD11c expression in TLR-activated B cells. J Immunol (Baltimore, Md: 1950) 2016, 197, 1023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Aranburu A, Hook N, Gerasimcik N, Corleis B, Ren W, Camponeschi A, et al. Age-associated B cells expanded in autoimmune mice are memory cells sharing H-CDR3-selected repertoires. Eur J Immunol 2018, 48, 509–21. doi: 10.1002/eji.201747127. [DOI] [PubMed] [Google Scholar]
- 94. Russell Knode LM, Naradikian MS, Myles A, Scholz JL, Hao Y, Liu D, et al. Age-associated B cells express a diverse repertoire of VH and Vkappa genes with somatic hypermutation. J Immunol (Baltimore, Md: 1950) 2017, 198, 1921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Song W, Antao OQ, Condiff E, Sanchez GM, Chernova I, Zembrzuski K, et al. Development of Tbet- and CD11c-expressing B cells in a viral infection requires T follicular helper cells outside of germinal centers. Immunity 2022, 55, 290–307. doi: 10.1016/j.immuni.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.