Abstract
The damaged-DNA binding protein DDB consists of two subunits, DDB1 (127 kDa) and DDB2 (48 kDa). Mutations in the DDB2 subunit have been detected in patients suffering from the repair deficiency disease xeroderma pigmentosum (group E). In addition, recent studies suggested a role for DDB2 in global genomic repair. DDB2 also exhibits transcriptional activity. We showed that expression of DDB1 and DDB2 stimulated the activity of the cell cycle regulatory transcription factor E2F1. Here we show that DDB2 is a cell cycle-regulated protein. It is present at a low level in growth-arrested primary fibroblasts, and after release the level peaks at the G1/S boundary. The cell cycle regulation of DDB2 involves posttranscriptional mechanisms. Moreover, we find that an inhibitor of 26S proteasome increases the level of DDB2, suggesting that it is regulated by the ubiquitin-proteasome pathway. Our previous study indicated that the cullin family protein Cul-4A associates with the DDB2 subunit. Because cullins are involved in the ubiquitin-proteasome pathway, we investigated the role of Cul-4A in regulating DDB2. Here we show that DDB2 is a specific target of Cul-4A. Coexpression of Cul-4A, but not Cul-1 or other highly related cullins, increases the ubiquitination and the decay rate of DDB2. A naturally occurring mutant of DDB2 (2RO), which does not bind Cul-4A, is not affected by coexpression of Cul-4A. Studies presented here identify a specific function of the Cul-4A gene, which is amplified and overexpressed in breast cancers.
The DDB2 subunit (also referred to as p48 or p48DDB) of the damaged-DNA binding protein DDB is mutated in xeroderma pigmentosum (group E, XP-E). Several naturally occurring mutants of DDB2 have been identified and characterized (36, 37, 41). These mutants are deficient in damaged-DNA recognition (17, 36, 37). One of these mutants, referred to as 2RO, also fails to associate with the DDB1 subunit (also referred to as p127 or p127DDB) (42); a patient harboring this mutation developed malignant skin cancer and died at the age of 14 (7). Another mutant, 82TO, is able to associate with the DDB1 subunit but is impaired in its ability to stimulate nuclear localization of DDB1 (42). A patient harboring the 82TO mutation exhibited severe sun sensitivity (25). In addition, a nonsense mutation in the DDB2 gene was correlated with the development of multiple skin neoplasia (18a). The incidence of tumors in patients with DDB2 mutations suggests a role for DDB2 in the pathway of tumor suppression. The damaged-DNA binding function of DDB2 suggested a role in DNA repair, which was supported by several lines of evidence. First, fibroblasts, isolated from the XP-E patients harboring mutations in the DDB2 gene, exhibited reduced DNA repair activity (17, 21, 41). In addition, microinjection of the wild-type protein could complement the deficiency in cells harboring mutant DDB2 (21, 41). Recent studies suggested that DDB is involved in global genomic repair (17, 18, 47). Furthermore, it was shown that DDB2 is downstream of p53 and that its mRNA level is increased by p53 (18).
DDB2 also associates with cell cycle-regulatory proteins. We showed that DDB2 could interact with the cell cycle transcription factor E2F1 (12, 42). Moreover, in conjunction with the DDB1 subunit, DDB2 cooperates with E2F1 to stimulate transcription from E2F-regulated promoters in transient-transfection assays. While DDB1 has been implicated in transcription from other promoters (26), the transcriptional stimulatory function of DDB2 is specific for E2F-regulated promoters (12). The DDB1 subunit of DDB is also a target of viral proteins. The hepatitis B virus X protein interacts with DDB1, and this interaction is important for the establishment of infection and the life cycle of the virus (27, 34a, 45, 46). The V protein encoded by the paramyxovirus simian virus 5 also associates with DDB1, and this interaction is correlated with a retardation of cell cycle progression (28, 29). We showed that DDB2 binds to the cullin family member Cul-4A (43). Binding to Cul-4A is interesting because a recent study indicated that the Cul-4A gene is overexpressed and amplified in breast cancer (3).
Cullins represent a family of proteins that are components of E3 ubiquitin ligases (see reference 51 for a review). The E3 ubiquitin ligases are believed to be involved in selecting target proteins for ubiquitination. Cullins associate with the ubiquitin-conjugating enzyme E2 and with the target proteins to enhance selective ubiquitination. Caenorhabditis elegans encodes five cullins: Cul-1, Cul-2, Cul-3, Cul-4, and Cul-5 (24). Mammalian cells express six cullins, since they encode two distinct but highly homologous cullin 4 proteins, Cul-4A and Cul-4B (24). Cullins are also found in yeast. The yeast cdc53 gene product, which is involved in the ubiquitination of p40sic and G1 cyclins, is related to Cul-1 (10, 33, 50). In addition, the APC2 gene product, which is involved in the ubiquitination of the mitotic cyclins in yeast and humans, is homologous to the cullin proteins (53). The five C. elegans cullins and the six human cullins possess extensive sequence homology, suggesting that they use similar mechanisms to ubiquitinate target proteins. All six human cullins were shown to be modified by NEDD8, a small ubiquitin-like polypeptide (14). For Cul-1, the NEDD8 modification has been correlated with nuclear localization (11). In addition, the cullins associate with a Ring finger domain containing small polypeptide ROC1 or Rbx1 (23, 40). Apc2, which is involved in the ubiquitination of cyclin B, associates with a ring finger-containing protein, Apc11. The interaction with the Ring finger-containing protein is essential for the ubiquitin-ligase activity (references 48 and 51 and references therein). Despite the similarities, there are also differences in the mechanism by which cullins select targets. For example, Cul-1 associates with the target proteins through F-box-containing proteins whereas Cul-2 does not bind the F-box-containing proteins (2, 51). Instead it is believed that the SOCS box-containing proteins serve as functional partners of Cul-2 (22, 30). For the other cullins, it is unclear how they select the targets or substrates for ubiquitination.
Cul-1 is the best-characterized cullin with regard to function. Several target proteins have been identified for Cul-1. Interestingly, many of the Cul-1 targets are cell cycle regulatory proteins. For example, the stability of the cell cycle-inhibitory proteins p27Kip1 and p21Cip1 is regulated by Cul-1 (references 51 and 52 and references therein). A recent study indicated that the c-Myc protein induces the expression of Cul-1 to then cause proteolysis of p27Kip1 (39). Other studies have shown that cyclin A (34, 51), cyclin E (6, 44, 49), cyclin D (52), and E2F1 (32) are also targeted for ubiquitination by the Cul-1 pathway. The Skp1 protein bridges the interaction between Cul-1 and the F-box-containing proteins, which associates with the target proteins (see reference 51 for a review). There are multiple F-box proteins, which is consistent with the observation that Cul-1 is involved in the ubiquitination of several proteins. Very little is known about the function of Cul-2. A recent study indicated that the hypoxia-induced transcription factor is a target of ubiquitination by Cul-2 and that it also involves the VHL tumor suppressor protein (19, 30, 39). The only known target for Cul-3 is cyclin E. Cul-3 associates with cyclin E and induces its ubiquitination (44). It is unclear whether the interaction between Cul-3 and cyclin E is mediated by another protein.
Here we show that Cul-4A targets DDB2 for ubiquitination. DDB2 is a cell cycle-regulated protein. Its level peaks at the G1/S boundary and decreases in S phase. We did not detect any decrease in the mRNA levels of DDB2. On the other hand, addition of a proteasome inhibitor increased the level of the DDB2 protein, suggesting that DDB2 is regulated by the ubiquitin-proteasome pathway. We also show that DDB2 is a specific target of Cul-4A. Cul-4B, which has 82% sequence identity to Cul-4A, failed to induce the ubiquitination of DDB2. The basis of the specificity lies in the N-terminal unique region of Cul-4A. The results provide an insight into the cell cycle regulation of DDB2 and establish a specific cellular function of Cul-4A.
MATERIALS AND METHODS
Cell culture.
HeLa cells were grown in spinner culture using suspension-minimal essential medium (S-MEM; GIBCO BRL) plus 5% calf serum. Monolayer cultures of human primary fibroblasts (IMR-90), HeLa calls, and human 293 cells were maintained in Dulbecco modified Eagle medium containing 10% fetal bovine serum.
Cell synchronization and cell cycle analysis.
HeLa cells were arrested at the G1/S boundary by the thymidine-aphidicolin double-block method described by Heintz et al. (13). Human primary fibroblasts were grown to 70% confluence and then maintained in medium without serum for 72 h. The synchronized cells were stimulated with 10% fetal bovine serum. The cells were harvested at different time points to assay the levels of various proteins and RNA.
RNase protection assay.
Antisense RNA probes were generated by in vitro transcription of linearized templates by using T3 RNA polymerase in the presence of [32P]UTP. DDB2 probe was synthesized from a XhoI-linearized Bluescript II KS(+) template containing a HindIII-EcoRI fragment of the DDB2 cDNA. Cyclophilin probe was synthesized using T3 RNA polymerase from a BamHI-linearized pTRI-cyclophilin-human antisense control template (Ambion) in which the cyclophilin fragment has been inserted into the KpnI-EcoRI sites of an Ambion pTRIPLEscript vector. Total RNA from the cells was isolated by using Trizol (GIBCO BRL) as specified by the manufacturer. To detect the mRNA levels of DDB2 and cyclophilin, antisense RNA probes were hybridized with 20 or 10 μg of total RNA, respectively. Hybridization was performed for 16 h at 45°C in 5 μl of hybridization buffer containing 80% formamide, 40 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES, pH 6.4), 400 mM sodium acetate, and 1 mM EDTA. Next day, 330 μl of the RNase I buffer (Promega) containing 10 U of RNase I (Promega) was added to the hybridization mix, and the digestion was allowed to continue for 1 h at 37°C. Following the digestion, 40 μl of ammonium acetate was added to the samples and the protected mRNAs were precipitated with ethanol, resuspended in formamide dye, denatured at 100°C for 3 min, and loaded onto a 4.5% denaturing sequencing gel. Protected bands were visualized by autoradiography.
Expression plasmids.
The cullin cDNAs were subcloned in pcDNA3.1/V5-His vector (Invitrogen) in frame with the V5-epitope. The cDNAs were amplified by PCR. The following primers were used to carry out the PCR: for Cul-1, upstream primer, CCCGGATCCCACCATGTCGTCAACCCGGAGC; downstream primer, CCCGCGGCCGCGCCAAGTAACTGTAGGTGTC; for Cul-2, upstream primer, CCCGGATCCCACCATGTCTTTGAAACCAAGA; downstream primer, CCCGGGCCCCGCGACGTAGCTGTATTCATC; for Cul-3, upstream primer, CCCGGTACCCACCATGTCGAATCTTAGCAAA; downstream primer, CCCGGGCCCTGCTACATATGTGTATACTTT; for Cul-4B, upstream primer, CCCGGATCCACCATGATTGATCCGGATTTTGCA; downstream primer, CCCGGGCCCTGCAATATAGTTGTACTGGTT; and for Cul-5, upstream primer, GGGGGATCCCACCATGGCGACGTCTAATCTGTTA; downstream primer, GGGTCTAGATGCCATATATATGAAAGTGTT. The mouse Cul-4A cDNA was subcloned in two steps. The XhoI-XbaI fragment encoding the first 300 residues of Cul-4A was subcloned in the same sites of the pcDNA3.1/V5-His vector. The remaining part of the cDNA (starting from the XbaI site at position 797 relative to the first coding ATG) was amplified by PCR (upstream primer, GGGTCTAGAAGAGGAAGCAGA; downstream primer, GGGTCTAGATGCCACGTAGTGGTACTG) and subsequently subcloned into the same vector at the XbaI site. The Cul4A deletion mutant was generated by PCR amplification of the mouse Cul4A cDNA from position 156 relative to the first coding ATG up to the 3′ end of the cDNA (upstream primer, GGGCTCGAGGCCATGGGGCTGCCTGACAACTACACT; downstream primer, CCGGGCCCTGCCACGTAGTGGTACTG) and subcloned into the pcDNA3.1/V5-His vector at the XhoI and ApaI sites. All cullin clones were confirmed by DNA sequencing.
Transfection in mammalian cells.
Transient transfections were carried out by the calcium phosphate coprecipitation method as previously described (12). The total concentration of the DNA for transfection was maintained at 20 μg/100-mm-diameter plate by adding empty vector DNA.
Preparation of nuclear extracts.
Cytosolic and nuclear extracts were prepared from the synchronized cells at various times of the cell cycle by the method described by Dignam et al. (8). Briefly, the harvested cells were washed with phosphate-buffered saline (PBS) and suspended in 2 volumes of hypotonic buffer, and membranes were disrupted by 30 strokes of a Kontes 2-ml tissue grinder. The nuclei were pelleted by centrifugation at 3,000 × g for 5 min. The nuclear pellet was extracted with high-salt buffer (0.5 M KCl). Extracted nuclear proteins were obtained by centrifugation at 13,000 × g for 10 min.
Immunoprecipitation and Western blot analysis.
Cells were harvested after DNA transfection. The harvested cells were washed twice with PBS, and cell extracts were prepared by incubation in a lysis buffer (PBS containing 10 mM CHAPS, 2 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 1 mM sodium vanadate, 1 mM sodium fluoride, 5 μg of aprotinin per ml, and 5 μg of leupeptin per ml) for 30 min at 4°C. For immunoprecipitation of the ubiquitinated proteins, cells harvested 40 h posttransfection were treated with MG 132 (final concentration, 10 mM) for 5 h and N-ethylmaleimide (5 μM) was included in the lysis buffer instead of dithiothreitol. The extracts (1 mg) were subjected to immunoprecipitation either with V5 antibody (2 h with primary antibody and 2 h with protein G beads at 4°C) or with agarose beads covalently linked to T7 antibodies for 2 h at 4°C. The immunoprecipitates were extensively washed with the lysis buffer, and the bound proteins were eluted with gel-loading buffer at room temperature for 10 min, boiled, and subjected to Western blot assay. Western blot analyses were performed by using anti-rabbit and anti-mouse Fab fragments conjugated to horseradish peroxidase (Amersham) and ECL Western blot detection reagents (Amersham) as specified by the manufacturer. V5 antibodies were purchased from Invitrogen, and T7 antibodies were obtained from Novagen. Antibodies against cyclin E, cyclin A, cdk2, and ubiquitin were from Santa Cruz Biotechnology, and those against α-tubulin were from Neomarkers.
RESULTS
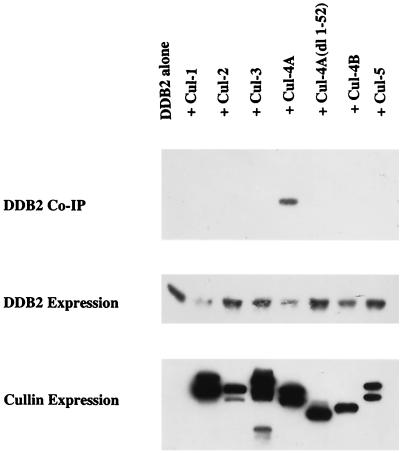
Cul-4A associates with DDB2 depending on its N-terminal unique sequences.
In a recent study, we observed an endogenous interaction between Cul-4A and DDB2 (43). However, it was not clear whether the other members of the cullin family could also bind DDB2. Cul-4B is particularly interesting because it has extensive sequence identity to Cul-4A. Human Cul-4B (also known as KIAA0695) has 82% sequence identity to mouse Cul-4A. The mouse Cul-4A cDNA clone was characterized by Ohta et al. (40), and it encodes a 759-amino-acid aa polypeptide. The published human Cul-4A cDNA (3), however, encodes a 659-aa polypeptide which lacks 100 N-terminal residues encoded by the mouse clone. It is possible that these are products of differential splicing because the human clone has 95% identity to the mouse clone. The human clone produced a protein that is much smaller than the major form of Cul-4A in HeLa cells and human primary fibroblasts and that failed to bind DDB2 (data not shown). The size of the protein encoded by the mouse clone is very similar (about 85 kDa) to that of the endogenous Cul-4A protein in human cells. Moreover, the protein encoded by the mouse cDNA clone, like the endogenous human Cul-4A protein, bound DDB2. Therefore, in this study we used the mouse clone to characterize the function of the mammalian Cul-4A.
To compare the cullins, we subcloned the cullin cDNAs in a mammalian expression vector in frame with the V5 epitope tag. The V5-tagged cullin cDNA clones were transfected into human 293 cells along with a plasmid expressing T7 epitope-tagged DDB2. An aliquot of the transfected cell extract was analyzed for expression of the DDB2 and cullin proteins using T7 and V5 antibodies in Western blot assays (Fig. 1, lower panels). To assay for an interaction between the cullins and DDB2, the extracts of the transfected cells were immunoprecipitated with V5 antibody to immunoprecipitate the cullins and the immunoprecipitates were subjected to Western blot analysis using the T7 antibody to detect DDB2. We observed that only Cul-4A could bind DDB2, since there was no detectable coprecipitation of DDB2 with the other cullins. Because of the variations in expression of DDB2 and the cullins, this experiment was performed several times using two or three cullins at one time (results not shown). Consistently, only Cul-4A exhibited binding to DDB2. The main difference between Cul-4A and Cul-4B lies in their N-terminal region. Because Cul-4B failed to coprecipitate DDB2, we suspected that the N-terminal sequences of Cul-4A might be critical for binding to DDB2. A deletion mutant of Cul-4A lacking the N-terminal 52 residues was generated. This mutant did not show any interaction with DDB2 [Fig. 1, lane Cul-4A(dl 1–52)], suggesting that the N-terminal unique region in the Cul-4A protein is essential for binding to DDB2. This result also explained why Cul-4B, a highly homologous cullin, is unable to bind DDB2. It is noteworthy that Cul-4B and the deletion mutant of Cul-4A, unlike the other cullins, migrated as a single band (Fig. 1). It is possible that these two polypeptides failed to undergo NEDD8 modification, which might also explain why they did not bind DDB2. Therefore, we cannot rule out the possibility that the N-terminal sequences of Cul-4A are important for its modification by NEDD8 and that the NEDD8 modification of Cul-4A supports its binding to DDB2. In any event, of the five cullins that migrated as double bands, only Cul-4A was able to associate with DDB2.
FIG. 1.
DDB2 specifically binds Cul-4A. Plasmids expressing V5-tagged cullins (19 μg of cullin 1, 2, 3, or 5; 3 μg of the wild-type or mutant cullin 4A; or 6 μg of cullin 4B DNA) were transfected in human 293 cells along with a plasmid expressing T7-tagged DDB2 (1 μg). Total DNA was made up to 20 μg with empty V5 vector where necessary. The transfected cells were washed with PBS, and cell extracts were prepared as described in Materials and Methods. The extracts were subjected to immunoprecipitation with V5 antibody. The immunoprecipitates were eluted with gel-loading buffer at room temperature for 10 min, boiled, and subjected to a Western blot assay. The blot was probed with T7 antibody to assay for the presence of coimmuoprecipitated DDB2.
DDB2 is a cell cycle-regulated protein, and its level is regulated by the ubiquitin-proteasome pathway.
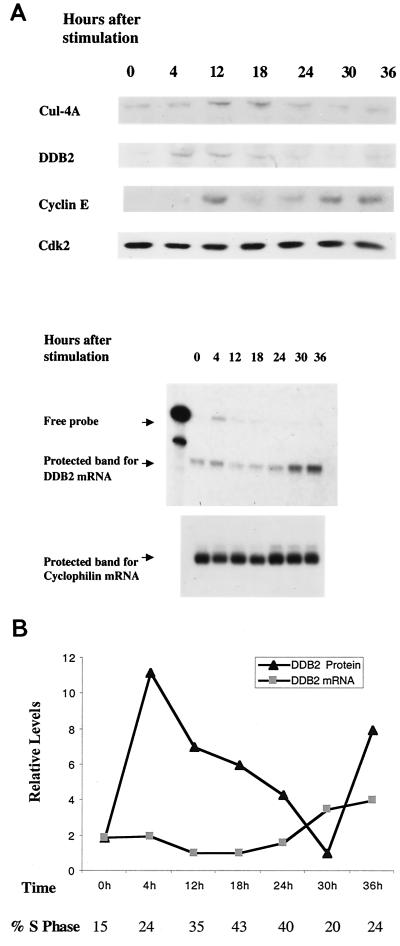
Because DDB2 could associate with the cell cycle transcription factor E2F1 (12, 42), we sought to investigate whether DDB2 is also a cell cycle-regulated protein. We used synchronized primary human fibroblasts for this purpose. Young primary fibroblasts are synchronized to the G0/G1 phases of the cell cycle by being maintained in medium containing no serum for 72 h. The cells were then stimulated by replenishing the medium with 10% fetal bovine serum. They were harvested at different time points after serum stimulation and used for both protein and RNA analyses (Fig. 2). DDB2 is mainly a nuclear protein and is undetectable in the cytosol (31, 42). To improve the analysis, we prepared nuclear extracts by the procedure of Dignam et al. (8). The extracts were subjected to Western blot analysis using peptide antibodies specific for DDB2 and Cul-4A. In addition, relevant parts of the Western blot were probed for cyclin E and cdk2. The level of cdk2 does not change significantly during the cell cycle, and therefore it also serves as a loading control. Results of this analysis indicate that DDB2 is a cell cycle-regulated protein (Fig. 2). Its level is low in growth-arrested cells but increases after serum stimulation and peaks at the G1/S boundary, as indicated by the level of cyclin E and flow cytometry. Moreover, there was a clear decline in the level of DDB2 in cells enriched for S phase. To determine whether the change in the DDB2 protein level is a result of an altered mRNA level, total RNA from the synchronized cells was analyzed for DDB2-mRNA by an RNase protection assay. The RNase protection analysis did not reveal any significant change in the steady-state level of the DDB2 mRNA during cell cycle progression (Fig. 2A, lower panel). The band intensities for the DDB2 mRNA and protein were quantified by densitometric scanning, and the relative intensities are shown in Fig. 2B. The results suggest that DDB2 is regulated during the cell cycle at least partly by a posttranscriptional mechanism.
FIG. 2.
DDB2 is regulated during the cell cycle by a posttranscriptional mechanism. (A) Human primary fibroblasts were synchronized by serum starvation for 72 h followed by stimulation with 10% FBS. At the indicated time points, cells were harvested and nuclear extracts were prepared and subjected to Western blot analysis (with 250 μg of nuclear extract) for DDB2, Cul-4A, cyclin E, and cdk2. The Lower panel shows the changes in the levels of DDB2 and cyclophilin mRNA at various time points of the cell cycle. Total RNA from the cells was subjected to RNase protection assays using antisense RNA probes corresponding to DDB2 and cyclophilin as described in Materials and Methods. (B) Plot showing the relative levels of the DDB2 protein and mRNA during the progression of the cell cycle. Band intensities were determined by densitometric scanning of the films corresponding to the Western blot and RNase protection assays. After normalization, the relative intensities were plotted.
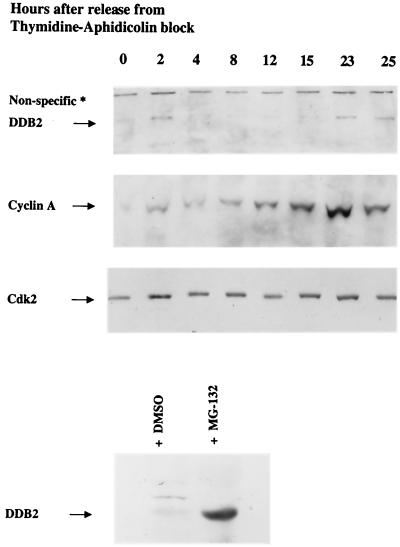
Cell cycle regulation of DDB2 was also observed in HeLa cells. HeLa cells were synchronized at the G1/S phase boundary by a double thymidine-aphidicolin block (13). After release from the block, the cells were harvested at different time points and nuclear extracts were analyzed for DDB2. Immediately after release from the block, there was an increase in the level of DDB2 (Fig. 3). The increase in the level of DDB2 was followed by a decrease in the level during S phase. Later, at the 23-h time point (Fig. 3) there was a second peak of both DDB2 and cyclin A, which corresponded to a new cycle. These results are consistent with what we observed in primary fibroblasts. Because DDB2 associates with Cul-4A, which is believed to be a component of E3 ubiquitin ligase, we sought to determine whether the level of DDB2 protein is regulated by the ubiquitin-proteasome pathway. HeLa cells were treated for 5 h with MG 132, which is a specific inhibitor of the 26S proteasome. Nuclear extracts were prepared from MG 132-treated or dimethyl sulfoxide treated cells. Equal amounts of the extracts were subjected to Western blot assays for the DDB2 protein. As can be seen in Fig. 3 (lower panel), treatment with MG 132 increased the level of DDB2, suggesting that it is a target of the ubiquitin-proteasome pathway.
FIG. 3.
DDB2 accumulates in the nucleus at the G1/S boundary in synchronized population of HeLa cells, and its level is regulated by the ubiquitin-proteasome pathway. HeLa cells were arrested at the G1/S boundary by a thymidine-aphidicolin double block. The cells were harvested at indicated time points, and nuclear extracts were prepared as described in Materials and Methods and analyzed for the levels of DDB2 (100 μg of extract), cyclin A (50 μg), and cdk2 (50 μg) by a Western blot assay. The lower panel shows the effect of proteasome inhibitor on the level of DDB2. For this, HeLa suspension culture cells were treated with either MG 132 (10 μM) or equal volume of dimethyl sulfoxide for 5 h. Cells were harvested, and 100 μg of nuclear extracts was analyzed for DDB2.
Coexpression of Cul-4A specifically increases the decay rate of the DDB2 polypeptide.
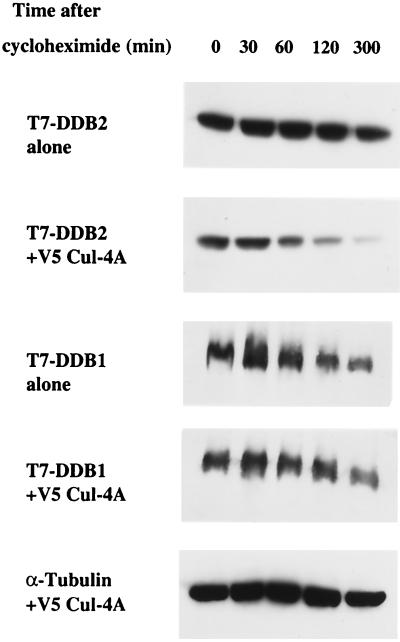
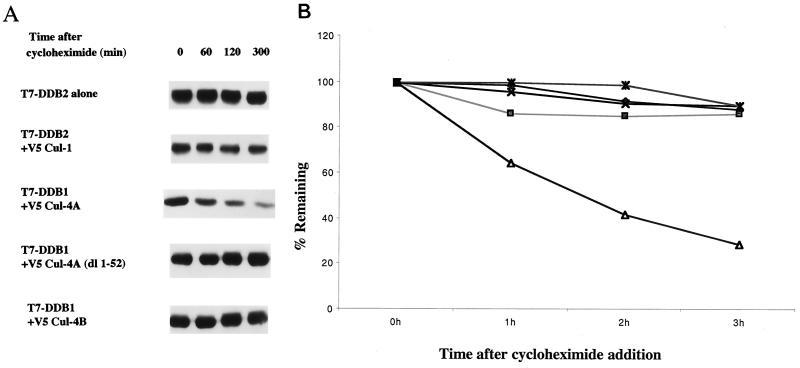
Cul-4A belongs to a family of E3 ubiquitin ligases, and it associates with DDB2. Moreover, we consistently observed that the expression of Cul-4A reduced the steady-state levels of the DDB2 subunit, whereas the steady-state levels of the DDB1 subunit were essentially unaltered in the same experiments (data not shown). Therefore, we sought to determine whether Cul-4A expression increases the decay rate of DDB2. A Cul-4A expression plasmid was cotransfected with plasmids that express T7 epitope-tagged DDB1 and DDB2 into HeLa cells. Five plates were transfected for each set of experiments. To compensate for the variations in the transfection efficiencies in different plates, cells from the same set were harvested by trypsinization at 16 h after transfection, pooled, and divided equally among five plates. At 24 h after replating, the cells were treated with cycloheximide (20 μg/ml). At various time points following the cycloheximide addition, the cells were harvested for analysis of the DDB proteins. Equal amounts of the transfected cell extracts were subjected to Western blot analysis. The blots were probed with T7 antibody, which detects both DDB1 and DDB2 expressed by the transfected plasmids. During these analyses, we observed that the DDB2 polypeptide decayed at a much higher rate in cells that were also transfected with Cul-4A than in cells that were cotransfected with empty vectors (Fig. 4). The DDB1 polypeptide, however, did not exhibit any change in the decay rate by the coexpression of Cul-4A (Fig. 4). These results are consistent with the notion that Cul-4A specifically reduces the half-life of the DDB2 polypeptide. To further investigate the specificity, we compared Cul-1, Cul-4A, Cul-4B, and the deletion mutant Cul-4A (dl 1–52) for their ability to enhance the decay rate of DDB2 (Fig. 5). Cul-1, Cul-4B, and Cul-4A (dl 1–52) had very little effect on the decay rate of DDB2. Cul-4A, on the other hand, reduced the half-life of DDB2 from much greater than 3 h to less than 2 h (Fig. 5B). This is consistent with the notion that DDB2 is targeted for proteolysis specifically by Cul-4A.
FIG. 4.
Coexpression of Cul-4A specifically reduces the half-life of DDB2. Five plates (10-cm-diameter dish) of HeLa cells were transfected with plasmids that express T7 epitope-tagged DDB1 and DDB2 in the presence or absence of a plasmid expressing V5-tagged Cul-4A. At 16 h posttransfection, the cells were trypsinized and the cells from the same set were pooled and replated on five plates. After 24 h, cycloheximide was added to the medium at a final concentration of 20 μg/ml and the cells were harvested at the indicated time points. Total-cell extracts were prepared as described in Materials and Methods. Then 100-μg portions of the transfected cell extracts were analyzed by Western blot assays. The blot was probed with antibodies against the T7 epitope.
FIG. 5.
(A) The decay rate of DDB2 is specifically enhanced by the expression of Cul-4A. Five plates of HeLa cells were transfected with plasmids that express T7 epitope-tagged DDB2 alone or with plasmids expressing V5-tagged Cul-4A or V5-Cul-1 or V5-Cul-4A (dl 1–52) or Cul-4B. At 16 h posttransfection, the cells from same set were trypsinized, pooled, and replated on five plates. After 24 h, the cells were treated with cycloheximide (20 μg/ml) and harvested at the indicated time points. Then 100-μg portions of the transfected cell extracts were analyzed by Western blot assays. The blot was probed with antibodies against the T7 epitope. (B) Plot showing the decay rate of DDB2 in the presence of different cullins. The band intensities were determined by densitometric scanning. For each plot, the band intensity corresponding to the zero hour time point was taken as 100% and the relative intensities of the bands at various time points were plotted. Symbols: ⧫, DDB2; ■, DDB2 + Cul-1; ▵, DDB2 + Cul4A; ×, DDB2 + Cul-4B; ∗, DDB2 + Cul-4A (dl 1–52).
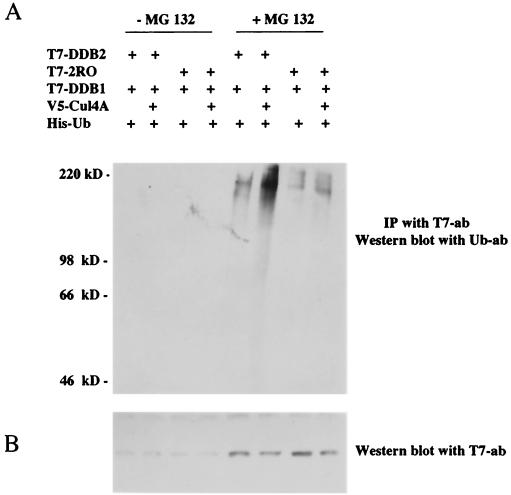
Cul-4A induces ubiquitination of DDB2.
An increase in the decay rate of DDB2 by Cul-4A also predicts that DDB2 is its ubiquitination target because Cul-4A is an E3 ubiquitin ligase. We investigated whether Cul-4A induces ubiquitination of DDB2. HeLa cells were transfected with plasmids expressing Cul-4A, T7-tagged DDB2, or a naturally occurring mutant of DDB2 (2RO) that does not bind Cul-4A (43), along with a plasmid expressing His-tagged ubiquitin. The transfection mixture also contained the plasmid expressing T7-tagged DDB1. At 40 h after transfection, one set of the transfected cells was treated for 5 h with MG 132, which is a specific inhibitor of the 26S proteasome. For many proteins, the polyubiquitinated forms are rapidly degraded by the 26S proteasome and inhibition of the proteasome activity is necessary to detect the polyubiquitinated forms. Extracts of the transfected cells were immunoprecipitated with T7 antibody to immunoprecipitate the DDB polypeptides. The immunoprecipitates were thoroughly washed and subjected to Western blot analysis. The blot was probed with a monoclonal antibody against ubiquitin (see Materials and Methods). As can be seen in Fig. 6, ubiquitinated DDB was detected only in MG 132-treated cells, and that clearly depended on the expression of Cul-4A. A mutant DDB2 (2RO) which does not bind Cul-4A did not exhibit any significant increase in ubiquitination by the coexpression of Cul-4A. The mutant DDB2 was also cotransfected with T7-DDB1. The lack of increase in ubiquitination, therefore, also confirms the notion that DDB1 is not ubiquitinated by Cul-4A.
FIG. 6.
(A) Cul-4A induces ubiqitination of DDB2. HeLa cells were transfected with plasmids expressing T7-DDB2 (4 μg), T7-DDB1 (6 μg), T7-2RO (4 μg), His-tagged ubiquitin (U6) (1 μg), and V5-Cul-4A (9 μg) in the indicated combinations. At 40 h after transfection, the cells were treated with MG 132 for 5 h. The cells were washed and lysed as described in Materials and Methods. Equal amount of extracts (1 mg) were immunoprecipitated (IP) with T7 antibodies (ab) covalently linked to agarose beads, and the immunoprecipitates were subjected to Western blot assay. The blot was probed with monoclonal antibodies against ubiquitin. (B) Western blot of the extracts (100 μg) probed with T7 antibody (ab) to assay for the expression levels of DDB2 in the above extracts.
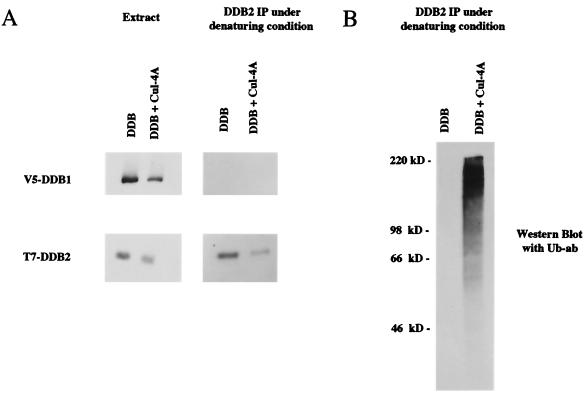
To further confirm that DDB2 itself, but not a DDB2-associated protein, is ubiquitinated by Cul-4A, the immunoprecipitation was carried out after treating the extracts with sodium dodecyl sulfate (SDS). HeLa cells were transfected with plasmids expressing T7-DDB2 and V5-DDB1 in the presence and absence of the Cul-4A expression plasmid. Transfected cells were treated with MG 132 for 5 h before being harvested for protein extraction. Extracts were treated with 2% SDS at 37°C for 10 min. The SDS-treated extracts were diluted 10-fold and then subjected to immunoprecipitation with T7 antibody. The SDS treatment disrupted the interaction between the two DDB subunits, since we did not detect any coprecipitation of DDB1 with DDB2 (Fig. 7A). In the absence of SDS treatment, DDB1 always coprecipitates with DDB2 (42). During DDB2 purification, DDB1 is the only protein that copurified with it (20), suggesting that DDB1 is the most tightly associated protein; therefore, the disruption of the DDB1-DDB2 interaction serves as a good reference. When aliquots of the same immunoprecipitates were probed for the ubiquitinated protein by Western blot assays with the ubiquitin antibody, we could easily detect ubiquitinated proteins coprecipitating with DDB2 (Fig. 7B). Taken together, this result confirms the notion that DDB2 itself is ubiquitinated by Cul-4A.
FIG. 7.
DDB2 itself is ubiquitinated by Cul-4A. HeLa cells were transfected with plasmids expressing T7-tagged DDB2 and V5-tagged DDB1 in the presence and absence of the Cul-4A expression plasmid. At 40 h after transfection, the cells were treated with MG 132 for 5 h. Total-cell extracts were prepared as described in Materials and Methods. Equal amount of the transfected cell extracts were treated with 2% SDS (final concentration) at 37°C for 10 min to disrupt protein-protein interactions. The SDS-treated extracts were diluted 10-fold and then subjected to immunoprecipitation (IP) with T7 antibody covalently linked to agarose beads. The immunoprecipitates were subjected to Western blot assay by using monoclonal antibodies against T7 for DDB2 and V5 for DDB1 (A) and ubiquitin (Ub ab) for ubiquitinated proteins (B).
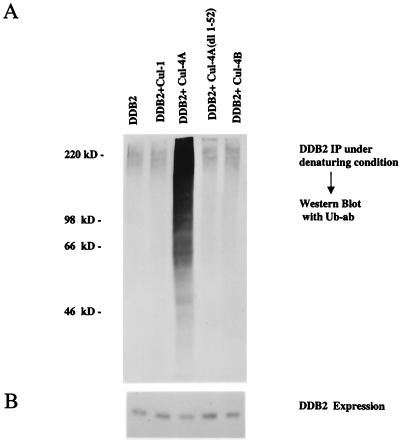
To investigate the specificity of Cul-4A-mediated ubiquitination of DDB2, we compared Cul-1, Cul-4A, Cul-4B, and the deletion mutant Cul-4A (dl 1–52) for their ability to ubiquitinate DDB2. Plasmids expressing these cullins were transfected into HeLa cells along with T7-DDB2 and His-tagged ubiquitin expression plasmids. Transfected cells were harvested after treatment with MG 132. The extracts were treated with 2% SDS before immunoprecipitation, as in the previous experiment. They were then diluted and subjected to immunoprecipitation with T7 antibody, and the immunoprecipitates were probed for ubiquitinated DDB2 by using the monoclonal antibody against ubiquitin. These experiments demonstrated that the ubiquitination, like the binding, is specific for Cul-4A, since there was no detectable increase in DDB2 ubiquitination by the expression of the other cullins (Fig. 8). Taken together, these results suggest that DDB2 is a specific target for ubiquitination by Cul-4A.
FIG. 8.
DDB2 is a specific ubiquitination target of Cul-4A. (A) Cells were transfected with plasmids expressing T7-DDB2 or His-tagged ubiquitin in combination with empty vector, V5-Cul-1, V5-Cul-4A, V5-Cul-4A(dl 1–52), or V5-Cul-4B. At 40 h after transfection, the cells were treated with MG 132 for 5 h. Total-cell extracts were prepared as described in Materials and Methods. Equal amount of extracts were treated with 2% SDS at 37°C for 10 min. The SDS-treated extracts were diluted 10-fold and then subjected to immunoprecipitation (IP) with T7 antibodies and Western blot assay with monoclonal antibodies against ubiquitin (Ub-ab). (B) Level of expression of T7-DDB2 in the extracts. Transfected cell extracts (100 μg) were probed with T7- antibody in a Western blot to assay for DDB2 expression.
DISCUSSION
Several lines of evidence suggested a role for DDB2 in DNA repair (1, 5, 9, 15–18, 20, 36, 37). DDB2 in combination with DDB1 functions as a damaged-DNA recognition factor. It was also shown that DDB2 is a p53-inducible gene. Using an inducible system, it was shown that an increase in the p53 activity correlated with increased expression of the DDB2-mRNA (18). In our cell cycle experiments with growth-arrested primary human fibroblasts, we did not detect any significant change in the steady-state level of the DDB2 mRNA during a 24-h serum stimulation period, which approximately corresponded to one round of the cell cycle. However, there was some increase in DDB2 mRNA following 30 h of serum stimulation. The protein level of DDB2, however, exhibited a significant change during the first 24 h. A significant increase was detected during the early G1 phase, and the level remained high until the G1/S boundary, after which the level dropped to an undetectable level. A similar result was obtained with HeLa cells that were synchronized at the G1/S boundary by a double thymidine-aphidicolin block. There was an increase in the protein level of DDB2 immediately after release, followed by a decrease as the cells progressed through the S phase. These observations clearly suggest that DDB2 functions during early G1 to the G1/S boundary. This is also the period when the checkpoint proteins are expected to scan the genome for DNA damage. DDB2 is essential for the damaged-DNA binding activity of DDB. Therefore, the DDB2 expression profile during the cell cycle will be consistent with a checkpoint function of this protein.
The cell cycle changes in the expression of DDB2 will also be consistent with a role of DDB2 in progression into the S phase. Our previous studies indicated that DDB2 in conjunction with DDB1 could function as a transcriptional partner of E2F1 in transient-transfection assays. We also observed evidence for an endogenous interaction between DDB2 and E2F1. E2F1 is a member of the E2F family of transcription factors (E2Fs), which are believed to play a role in progression into the S phase. E2Fs play a critical role in the expression of genes that are essential for entry and progression through the S phase (35). The E2F-regulated genes are expressed between mid-G1 and early S phase (35), and the DDB2 protein is also expressed at a higher level during that time. However, it is also possible that DDB2 associates with a specific member of the E2F family (such as E2F1) to carry out a function distinct from transcription of the cell cycle genes. A role for the DDB-E2F1 complex in DNA repair cannot be ruled out, and that would also be consistent with the tumor suppression function of E2F1 (35).
Results described here also identify at least one function of the mammalian Cul-4A gene. Lower organisms such as C. elegans carry one cullin 4 gene, while mammalian cells contain genes encoding both Cul-4A and Cul-4B. It is noteworthy that DDB2 homologous sequence was not found in lower organisms, including C. elegans and Drosophila melanogaster (54). DDB1, on the other hand, is highly conserved (54). It will be interesting to determine whether the divergence of the cullin 4 gene and the appearance of the DDB2 gene occurred at about the same time during evolution. The absence of these genes in lower eukaryotes also suggests that Cul-4A and DDB2 function in the complex regulatory circuits that are typically found in higher organisms. By coexpressing all six mammalian cullins with DDB2, we observed that only Cul-4A could coimmunoprecipitate DDB2. Moreover, only Cul-4A increased the ubiquitination and the decay rate of DDB2. The specificity is interesting when Cul-4A and Cul-4B are compared because they have 82% sequence identity. These two cullins diverge mainly in the sequences in their N termini. We show that the N-terminal sequences of Cul-4A are critical for binding to DDB2. The sequence in the N-terminal region of Cul-4A is not found in any other cullins, and that explains why DDB2 coimmunoprecipitated only with Cul-4A. It is, however, also possible that Cul-4B and the N-terminal deletion mutant of Cul-4A lack proper modification (such as NEDD8 conjugation), and this might be responsible for their inactivity. Further studies of NEDD8 modification and its effect on DDB2 binding are necessary to resolve this matter. Nevertheless, the results are consistent with a substrate-specific E3 ligase activity of Cul-4A.
A recent screen on a genomewide scale for human genes that are upregulated at the G1/S boundary, using high-density oligonucleotide arrays, showed that Cul-4A mRNA is cell cycle regulated and is more abundant in cells at the G1/S boundary (4). We observed a modest increase in the nuclear levels of the Cul-4A protein at the G1/S boundary (Fig. 2). Therefore, it is likely that Cul-4A functions at the G1/S boundary. It is noteworthy that the increase in the level of Cul-4A also somewhat correlates with the decrease in the level of DDB2, which is consistent with our observation that Cul-4A increases ubiquitination and the decay rate of DDB2. Unlike DDB2, Cul-4A is detectable in quiescent cells. It is possible that Cul-4A is there to ensure a low level of DDB2 or that there are other substrates that are targeted by Cul-4A in quiescent cells. Clearly, further work is necessary to establish these possibilities. Cul-4A has also been implicated in tumorigenesis. The Cul-4A gene was shown to be amplified in 16% of breast cancers and overexpressed in 47% of breast cancers (3). The same study also indicated the possibility of overexpression of Cul-4A in other cancers. This is interesting because mutations in DDB2 correlated with malignancies of the skin (7, 18a). Our model is that overexpression of Cul-4A would eliminate DDB2 more efficiently by the ubiquitin-proteasome pathway, and the net result would be similar to DDB2 mutations. The model predicts that overexpression of Cul-4A might be sufficient for an oncogenic phenotype. The model also predicts that DDB2 is a critical target of Cul-4A. The specificity of the Cul-4A–DDB2 interaction would support that notion. It is quite likely that the elimination of the DNA repair, damaged-DNA recognition, or apoptotic function of DDB2 by Cul-4A overexpression would predispose cells for the accumulation of mutations.
ACKNOWLEDGMENTS
We thank Y. Xiong (University of North Carolina at Chapel Hill) and R. Stearman (National Institute of Health) for the cullin cDNA clones. We also thank the Kazusa DNA Research Institute (Japan) for the cullin-4B clone, which is designated KIAA0695. We thank D. Bohmann (European Molecular Biology Laboratory) for the His-Ub expression plasmid.
This work is supported by a grant (CA77637 and CA88863) from the NCI to P.R.
REFERENCES
- 1.Abramic M, Levine A S, Protic M. Purification of an ultraviolet-inducible, damage-specific DNA-binding protein from primate cells. J Biol Chem. 1991;266:22439–22500. [PubMed] [Google Scholar]
- 2.Bai C, Sen P, Mathias N, Hofmann K, Goebl M, Harper J W, Elledge S J. SKP1 connects cell cycle regulation to ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- 3.Chen L-C, Manjeshwar S, Lu Y, Moore D, Ljung B-M, Kuo W-L, Dairkee S H, Wernick M, Colins C, Smith H S. The human homologue for the Caenorhabditis elegans Cul-4 gene is amplified and overexpressed in primary breast cancers. Cancer Res. 1998;58:3677–3683. [PubMed] [Google Scholar]
- 4.Cho R J, Huang M, Campbell M J, Dong H, Steinmetz L, Sapinoso L, Hampton G, Elledge S J, Davis R W, Lockhart D J. Transcriptional regulation and function during the human cell cycle. Nat Genet. 2001;27:48–54. doi: 10.1038/83751. [DOI] [PubMed] [Google Scholar]
- 5.Chu G, Chang E. Xeroderma pigmentosum group E cells lack a nuclear factor that binds to damaged DNA. Science. 1988;242:564–567. doi: 10.1126/science.3175673. [DOI] [PubMed] [Google Scholar]
- 6.Dealy M J, Nguyen K V T, Lo J, Gstaiger M, Krek W, Elson D, Arbeit J, Kipreos E, Johnson R S. Loss of Cul1 results in early embryonic lethality and dysregulation of cyclin E. Nat Genet. 1999;23:245–248. doi: 10.1038/13886. [DOI] [PubMed] [Google Scholar]
- 7.de Weerd-Kastelein E A, Keijzer W, Bootsma D. A third complementation group in xeroderma pigmentosum. Mutat Res. 1974;22:87–91. doi: 10.1016/0027-5107(74)90013-x. [DOI] [PubMed] [Google Scholar]
- 8.Dignam J D, Lebovitz R M, Roeder R. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dualan R, Brody T, Keeney S, Nichols A F, Admon A, Linn S. Chromosomal localization and cDNA cloning of the genes (DDB1 and DDB2) for the p127 and p48 subunits of a human damage-specific DNA binding protein. Genomics. 1995;29:62–69. doi: 10.1006/geno.1995.1215. [DOI] [PubMed] [Google Scholar]
- 10.Feldman R M R, Correll C C, Kaplan K B, Deshaies R J. A complex of Cdc4p, Skp1p, and Cdc53p/Culin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sicp. Cell. 1997;91:221–230. doi: 10.1016/s0092-8674(00)80404-3. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa M, Zhang Y, McCarville J, Ohta T, Xiong Y. The CUL1 C-terminal sequence and ROC1 are required for efficient nuclear accumulation, NEDD8 modification, and ubiquitin ligase activity of CUL1. Mol Cell Biol. 2000;20:8185–8197. doi: 10.1128/mcb.20.21.8185-8197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes S, Shiyanov P, Chen X, Raychaudhuri P. DDB, a putative DNA repair protein, can function as a transcriptional partner of E2F1. Mol Cell Biol. 1998;18:240–249. doi: 10.1128/mcb.18.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heintz N, Sive H L, Roeder R G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during HeLa cell cycle. Mol Cell Biol. 1983;3:539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hori T, Osaka F, Chiba T, Miyamoto C, Okabayashi K, Shimbara N, Kato S, Tanaka K. Covalent modification of all members of human cullin family proteins by NEDD8. Oncogene. 1999;18:6829–6834. doi: 10.1038/sj.onc.1203093. [DOI] [PubMed] [Google Scholar]
- 15.Hwang B J, Chu G. Purification and characterization of a human protein that binds to damaged DNA. Biochemistry. 1993;32:1657–1666. doi: 10.1021/bi00057a033. [DOI] [PubMed] [Google Scholar]
- 16.Hwang B J, Liao J C, Chu G. Isolation of a cDNA encoding a UV-damaged DNA binding factor defective in xeroderma pigmentosum group E cells. Mutat Res. 1996;362:105–117. doi: 10.1016/0921-8777(95)00040-2. [DOI] [PubMed] [Google Scholar]
- 17.Hwang B J, Toering S, Francke U, Chu G. p48 activates a UV-damaged-DNA binding factor and is defective in xeroderma pigmentosum group E cells that lack binding activity. Mol Cell Biol. 1998;18:4391–4399. doi: 10.1128/mcb.18.7.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang B J, Ford J M, Hanawalt P C, Chu G. Expression of p48 xeroderma pigmentosum gene is p53-dependent and is involved in global genomic repair. Proc Natl Acad Sci USA. 1998;96:424–428. doi: 10.1073/pnas.96.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Itoh T, Mori T, Ohkubo H, Yazaizumi M. A newly identified patient with clinical xeroderma pigmentosum phenotype has a non-sense mutation in the DDB2 gene and incomplete repair in (6–4) photoproducts. J Investig Dermatol. 1999;113:251–257. doi: 10.1046/j.1523-1747.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaelin W G, Iliopoulos O, Lonergan K M, Ohh M. Functions of the von Hippel-Lindau tumor suppressor protein. J Intern Med. 1998;243:535–539. doi: 10.1046/j.1365-2796.1998.00335.x. [DOI] [PubMed] [Google Scholar]
- 20.Keeney S, Chang G J, Linn S. Characterization of a human DNA damage binding protein implicated in xeroderma pigmentosum E. J Biol Chem. 1993;268:21293–21300. [PubMed] [Google Scholar]
- 21.Keeney S, Eker A P, Brody T, Vermeulen W, Bootsma D, Hoeijmakers J H, Linn S. Correction of the DNA repair defect in xeroderma pigmentosum group E by injection of a DNA damage-binding protein. Proc Natl Acad Sci USA. 1994;91:4053–4056. doi: 10.1073/pnas.91.9.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamura T, Sato S, Haque D, Liu L, Kaelin W G, Jr, Conway R C, Conway J W. The Elongin BC complex interacts with conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat family. Genes Dev. 1998;12:3872–3881. doi: 10.1101/gad.12.24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamura T, Koepp D M, Conrad M N, Skowyra D, Moreland R J, Iliopoulos O, Lane W S, Kaelin W G, Jr, Elledge S J, Conaway R C. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science. 1999;284:657–661. doi: 10.1126/science.284.5414.657. [DOI] [PubMed] [Google Scholar]
- 24.Kipreos E T, Lander L E, Wing J P, He W W, Hedgecock E M. cul-1 is required for cell cycle exit in C. elegans and identifies a novel gene family. Cell. 1996;85:829–839. doi: 10.1016/s0092-8674(00)81267-2. [DOI] [PubMed] [Google Scholar]
- 25.Kondo S J, Fukuro S, Mamada A, Kawada A, Satoh Y. Assignment of three patients with xeroderma pigmentosum to complementation group E and their characteristic. J Investig Dermat. 1988;90:152–157. doi: 10.1111/1523-1747.ep12462130. [DOI] [PubMed] [Google Scholar]
- 26.Krishnamoorthy R R, Lee T H, Butel J S, Das H K. Apolipoprotein B gene regulatory factor-2 (BRF-2) is structurally and immunologically highly related to hepatitis B virus X associated protein-1 (XAP-1) Biochemistry. 1997;36:960–969. doi: 10.1021/bi961407c. [DOI] [PubMed] [Google Scholar]
- 27.Lee T-H, Elledge S J, Butel J S. Hepatitis B virus X protein interacts with a probable cellular DNA repair protein. J Virol. 1995;69:1107–1114. doi: 10.1128/jvi.69.2.1107-1114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin G Y, Paterson R G, Richardson C D, Lamb R A. The V protein of the paramyxovirus SV5 interacts with damage-specific DNA binding protein. Virology. 1998;249:189–200. doi: 10.1006/viro.1998.9317. [DOI] [PubMed] [Google Scholar]
- 29.Lin G Y, Lamb R A. The paramyxovirus simian virus 5V protein slows progression of the cell cycle. J Virol. 2000;74:9152–9166. doi: 10.1128/jvi.74.19.9152-9166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lisztwan J, Imbert G, Wirbelauer C, Gstaiger M, Krek W. The Von-Hippel Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 1999;13:1822–1833. doi: 10.1101/gad.13.14.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, Nichols A F, Graham J A, Dualan R, Abbas A, Linn S. Nuclear transport of human DDB protein induced by ultraviolet light. J Biol Chem. 2000;275:21429–21434. doi: 10.1074/jbc.M000961200. [DOI] [PubMed] [Google Scholar]
- 32.Marti A, Wirbelauer C, Scheffner M, Krek W. Interaction between ubiquitin-protein ligase SCF-skp2 and E2F1 underlies the regulation of E2F1 degradation. Nat Cell Biol. 1999;1:14–20. doi: 10.1038/8984. [DOI] [PubMed] [Google Scholar]
- 33.Mathias N, Johnson S J, Winey M, Adams A E M, Goetsch L, Pringle J R, Byers B, Goebl M G. Cdc53p acts in concert with Cdc4p and Cdc34p to control the G1-to-S phase transition and identifies a conserved family of proteins. Mol Cell Biol. 1996;16:6634–6643. doi: 10.1128/mcb.16.12.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michel J, Xiong Y. Human Cul-1, but not other cullin family members, selectively interacts with Skp1 to form a complex with Skp2 and cyclin A. Cell Growth Differ. 1998;9:439–445. [PubMed] [Google Scholar]
- 34a.Nag, A., A. Datta, K. Yoo, D. Bhattacharyya, A. Chakrabortty, X. Wang, B. L. Slagle, R. H. Costa, and P. Raychandhuri. DDB2 induces nuclear accumulation of the hepatitis B virus X protein independently of binding to DDB1. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 35.Nevins J R. Towards an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998;9:585–593. [PubMed] [Google Scholar]
- 36.Nichols A F, Ong P, Linn S. Mutations specific to the xeroderma pigmentosum group E Ddb- phenotype. J Biol Chem. 1996;271:24317–24320. doi: 10.1074/jbc.271.40.24317. [DOI] [PubMed] [Google Scholar]
- 37.Nichols A F, Itoh T, Graham J A, Liu W, Yamaizumi M, Linn S. Human damage-specific DNA-binding protein p48. J Biol Chem. 2000;275:21422–21428. doi: 10.1074/jbc.M000960200. [DOI] [PubMed] [Google Scholar]
- 38.O'Hagan R C, Ohh M, David G, de Alboran I M, Alt F W, Kaelin W G, Jr, DePinho R A. Myc-enhanced expression of Cul1 promotes ubiquirin-dependent proteolysis and cell cycle progression. Genes Dev. 2000;14:2185–2191. doi: 10.1101/gad.827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohh M, Park C W, Ivan M, Hoffman M A, Kim T Y, Huang L E, Pavletic N, Chau V, Kaelin W G., Jr Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 40.Ohta T, Michel J J, Schottelius A J, Xiong Y. ROC1, a homolog of APC11, represents a family of cullin partners with an associated ubiquitin ligase activity. Mol Cell. 1999;3:535–541. doi: 10.1016/s1097-2765(00)80482-7. [DOI] [PubMed] [Google Scholar]
- 41.Otrin V R, Kuraoka I, Nardo T, McLenigan M, Eker A P M, Stefanini M, Levine A S, Wood R D. Relationship of the xeroderma pigmentosum group E DNA repair defect to the chromatin and DNA binding proteins UV-DDB and replication protein A. Mol Cell Biol. 1998;18:3182–3190. doi: 10.1128/mcb.18.6.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiyanov P, Hayes S A, Donepudi M, Nichols A F, Linn S, Slagle B L, Raychaudhuri P. The naturally occurring mutants of DDB are impaired in stimulating nuclear import of the p125 subunit and E2F1-activated transcription. Mol Cell Biol. 1999;19:4935–4943. doi: 10.1128/mcb.19.7.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiyanov P, Nag A, Raychaudhuri P. Cullin-4A associates with the UV-damaged DNA-binding protein DDB. J Biol Chem. 1999;274:35309–35312. doi: 10.1074/jbc.274.50.35309. [DOI] [PubMed] [Google Scholar]
- 44.Singer J D, Gurian-West M, Clurman B, Roberts J M. Cullin-3 targets cyclin E for ubiquitination and controls S phase in mammalian cells. Genes Dev. 1999;13:2375–2387. doi: 10.1101/gad.13.18.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sitterlin D, Bergarnetti F, Tiollais P, Tennant B C, Transy C. Correct binding of viral X protein to UVDDB-p127 cellular protein is critical for efficient infection by hepatitis B viruses. Oncogene. 2000;19:4427–4431. doi: 10.1038/sj.onc.1203770. [DOI] [PubMed] [Google Scholar]
- 46.Sitterlin D, Bergarnetti F, Transy C. UVDDB p127-binding modulates activities and intracellular distribution of hepatitis B virus X protein. Oncogene. 2000;19:4417–4426. doi: 10.1038/sj.onc.1203771. [DOI] [PubMed] [Google Scholar]
- 47.Tang J Y, Hwang B J, Ford J M, Hanawalt P C, Chu G. Xeroderma pigmentosum p48 gene enhances global genomic repair and suppresses UV-induced mutagenesis. Mol Cell. 2000;5:737–744. doi: 10.1016/s1097-2765(00)80252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tyers M, Willems A R. One ring to rule a superfamily of E3 ubiquitin ligases. Science. 1999;284:601. doi: 10.1126/science.284.5414.601. , 603–604. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Penfold S, Tang X, Hattori N, Riley P, Harper J W, Cross J C, Tyers M. Deletion of the Cul1 gene in mice causes arrest in early embryogenesis and accumulation of cyclin E. Curr Biol. 1999;9:1191–1194. doi: 10.1016/S0960-9822(00)80024-X. [DOI] [PubMed] [Google Scholar]
- 50.Willems A R, Lanker S, Patton E E, Craig K L, Nason T F, Mathias N, Kobayashi R, Wittenberg C, Tyers M. Cdc53 targets phosphorylated G1 cyclins for degradation by the ubiquitin proteolytic pathway. Cell. 1996;86:453–463. doi: 10.1016/s0092-8674(00)80118-x. [DOI] [PubMed] [Google Scholar]
- 51.Winston J T, Chu C, Harper J W. Culprits in the degradation of cyclin E apprehended. Genes Dev. 1999;13:2751–2757. doi: 10.1101/gad.13.21.2751. [DOI] [PubMed] [Google Scholar]
- 52.Yu Z-K, Gervais J L M, Zhang H. Human Cul1 associates with the SKP1/SKP2 complex and regulates p21CIP1/WAF1 and cyclin D proteins. Proc Natl Acad Sci USA. 1998;95:11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zachariae W, Shevchenko A, Andrews P D, Ciosk R, Galova M, Stark M J, Mann M, Nasmyth K. Mass spectrometric analysis of the anaphase-promoting complex from yeast: identification of a subunit related to Cullins. Science. 1998;279:1216–121. doi: 10.1126/science.279.5354.1216. [DOI] [PubMed] [Google Scholar]
- 54.Zolezzi F, Linn S. Studies of the murine DDB1 and DDB2 genes. Gene. 2000;245:151–159. doi: 10.1016/s0378-1119(00)00022-6. [DOI] [PubMed] [Google Scholar]