Abstract
Objective
The relationship between anti-SSA/RO antibodies and pregnancy has been reported previously, and we aim to visualize the rates of maternal and infant outcomes with anti-SSA/RO.
Methods
We systematically searched records from Pubmed, Cochrane, Embase, and Web of Science databases, pooled incidence rates of adverse outcomes of pregnancy, and 95% confidence intervals (CIs) were performed with RStudio.
Results
A total of 890 records comprising 1675 patients and 1920 pregnancies were searched from the electronic databases. For maternal outcomes, the pooled estimate rates were 4% for termination of pregnancy, 5% for spontaneous abortion, 26% for preterm labor, and 50% for cesarean operation. While for fetal outcomes, the pooled estimate rates were 4% for perinatal death, 3% for intrauterine growth retardation, 6% for endocardial fibroelastosis, 6% for dilated cardiomyopathy, 7% for congenital heart block, 12% for congenital heart block recurrence, 19% for cutaneous neonatal lupus erythematosus, 12% for hepatobiliary disease and 16% for hematological manifestations. A subgroup analysis of congenital heart block prevalence was performed, diagnostic method and study region were found to affect heterogeneity to some extent.
Conclusion
Cumulative analysis of data from real-world studies confirmed adverse pregnancy outcomes of women with anti-SSA/RO, serves as a reference and a guide for the diagnosis and subsequent treatment of these women, thereby enhancing maternal and infant health. Additional studies with real-world cohorts are required to validate these results.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12969-023-00803-0.
Keywords: Anti-SSA/RO, NLE, CHB, Pregnancy
Introduction
Anti-SSA/RO, a crucial component of serological markers, is frequently used to diagnose Sjogren’s syndrome and Systemic lupus erythematosus (SLE). The management of pregnancy in women with autoimmune diseases is undoubtedly an integral part of the treatment of the disease. Neonatal Lupus Syndrome (NLE) is a condition associated with anti-SSA/RO antibodies that affect the fetal myocardium during pregnancy and can lead to dilated cardiomyopathy, endocardial fibroelastosis (EFE), congenital heart block (CHB), and skin, liver, and hematological system [1]. The prevailing theory is that maternal anti-SSA/RO antibodies are deposited in the fetal myocardial tissue via passive placental transmission, resulting in subsequent inflammation and fibrosis [2, 3].
In addition to the CHB mouse model constructed by Carús et al. [4], pathological examination of the hearts of 10 children who died of EFE by Nield [5] combined with relevant clinical studies [6] has further validated the association of maternal antibodies with poor pregnancy outcomes. Furthermore, high titers of maternal antibodies were found to be associated with fetal damage in a study that was conducted prospectively by Edgar Jaeggi et al. [7].
However, most existing clinical studies have restrictions such as insufficient participants, narrow scopes, and limited endpoints. Given these premises, we aimed to systematically estimate the rates of maternal and infant outcomes of pregnant women with anti-SSA/RO antibodies.
Materials and methods
Study design
This study adheres to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) statement. This review is registered on PROSPERO (CRD42022384043).
Search strategy
We searched Pubmed, Cochrane, Embase, and Web of Science databases for records up to October 2022, using the terms “anti-RO” or “anti-SSA” and the following combinations “pregnancy” “Pregnant Women” “mothers” “maternal” and “fetal”.
Inclusion and exclusion criteria
Studies that meet the following criteria were included:
A real-world observational study of pregnancy outcomes in anti-SSA/RO-positive pregnant women.
They provided research methods and specific data.
They were published in 2000 or later.
Studies that meet the following criteria were excluded:
Reviews, case reports, or conference abstracts.
Articles not in English.
Articles for which full text was not available were excluded.
Outcomes analyzed
The following maternal endpoints were analyzed: termination of pregnancy, spontaneous abortion, preterm labor, and cesarean operation. The following fetal outcomes were analyzed: perinatal death, intrauterine growth retardation, endocardial fibroelastosis (EFE), dilated cardiomyopathy (DCM), congenital heart block (CHB), congenital heart block recurrence and cutaneous neonatal lupus erythematosus (CNLE).
Data collection and quality assessment
Initial screening was based on titles and abstracts, followed by a full-text reading of studies that met inclusion criteria and ultimate exclusion based on exclusion criteria. Information from the included literature was documented in a specific database, including author, publication date, study population, study type, study region, enrollment time, sample size, number of positives, etc. The Newcastle-Ottawa Scale (NOS), which includes three components: choice of study groups, comparability of cohort and control groups, and determination of outcome, was used to assess the quality of included studies. Three or fewer were regarded as low, four to six as intermediate, and nine or more as an outstanding quality. The aforementioned work was completed independently by 3 authors (XRS, XHS, and YX), and disputes were discussed.
Statistical analysis
Statistical analysis was performed with RStudio (version 2022.07.2) and Review Manager (version 5.4). For non-normally distributed incidence rates, logit transformation was used to make them close to a normal distribution, and normality tests were performed by using the Shapiro-Wilk test. Pooled incidence rates of outcomes and 95% confidence intervals (CIs), were calculated by the random-effect model or fix-effect model. Furthermore, heterogeneity between studies was assessed using I2. Sources of heterogeneity were explored through subgroup analysis. Sensitivity analysis was conducted by omitting each study sequentially to evaluate the stability of the study results.
Results
Searched outcomes
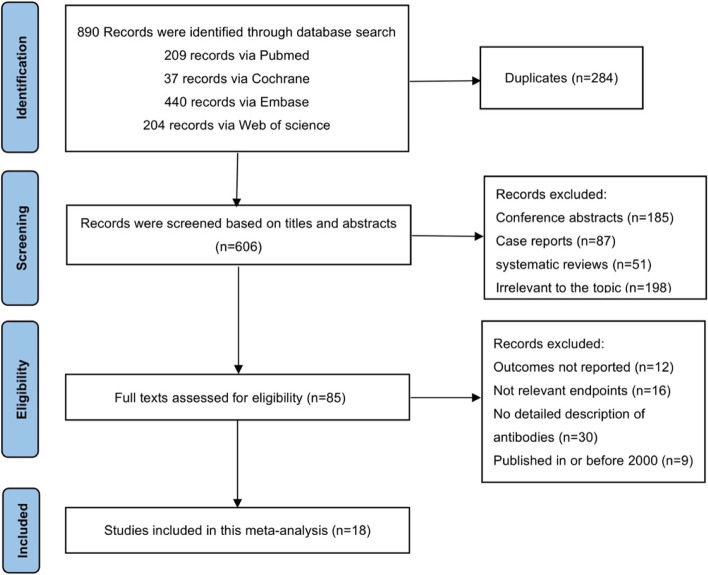
A total of 890 records were searched from the above-mentioned electronic databases. Two hundred eighty-four duplicate records were removed. Five hundred twenty-one studies were screened based on the titles and abstracts. Sixty-seven studies were excluded after reading the full texts. Ultimately, 18 articles that met the screening criteria were included in this meta-analysis as shown in Fig. 1.
Fig. 1.
Flow diagram representing the study selection
Study characteristics
The main characteristics of the included studies were shown in Table 1, including study type, study region, enrollment period, and the number of participants. This analysis encompassed a total number of 1675 patients and 1920 pregnancies. And they were enrolled from the year 1987–2019 and distributed in different regions such as Canada, France, Italy, Sweden, and the USA. As no RCT trials were included in our meta-analysis, we carried out a quality assessment by using the Newcastle-Ottawa scale (NOS). The NOS assessment results for the 20 studies were 5–8.
Table 1.
Main characteristics of the studies included in this analysis
| Study (year) | Country | Study design | Period(y) | Participants (pregnancies) | Maternal outcomes | Fetal outcomes | NOS score |
|---|---|---|---|---|---|---|---|
| Ruffatti 2016 [8] | Italy | retrospective | 2009–2014 | 12(12) | type of delivery, pregnancy complications, gestational period (weeks), preterm labor | 5 min APGAR score, neonatal complications, birth weight, cardiac complications, NICU admission, small for gestational age | ****** |
| Zuppa 2008 [9] | Italy | retrospective | 2004–2005 | 10(10) | type of delivery, gestational period (weeks), preterm labor | live birth, birth weight, hematologic manifestation, echoencephalographic abnormalities, liver manifestations | ****** |
| Morel 2017 [10] | France | – | 2000–2014 | 174(187) | gestational period (weeks), preterm labor | fetal death /perinatal death, birth weight, DCM, EFE, hydrops, Pericardial effusion, congenital heart disease, pacemaker implantation | ***** |
| Cimaz 2003 [11] | Canada | prospective | 1987–2000 | 112(128) | preterm labor | live birth, type of block, skin manifestations, hematologic manifestation, liver manifestations, prolonged QT interval, CHB recurrence, hematological and hepatic manifestations | ****** |
| Boros 2007 [12] | Canada | – | 1999–2004 | 75(87) | – | type of block, hydrocephalus, cutaneous involvement, hematologic and/or liver enzyme abnormalities, hematological and hepatic manifestations | ****** |
| Miniaoui 2022 [13] | France | – | 2000–2019 | 215(215) | terminations of pregnancy, disease-free survival probability at 5 years and 10 years, the probability of developing SLE/SS, the estimated median time to progression | fetal death /perinatal death, live birth | ****** |
| Gladman 2002 [14] | Canada | prospective | 1988–1997 | 105(118) | spontaneous abortion | intrauterine deaths, DCM, type of block, peripheral pulmonary artery stenosis, CHB recurrence | ****** |
| Pisoni 2010 [15] | Italy | prospective | 2004–2008 | 22(24) | terminations of pregnancy | type of block, cutaneous involvement, CHB recurrence | ****** |
| Skog 2015 [16] | Sweden | prospective | 2000–2013 | 155(212) | type of delivery, preterm labor, preeclampsia | fetal death /perinatal death, type of block, CHB recurrence, neonatal complications | ***** |
| Jaeggi 2010 [7] | Canada | prospective | 2000–2008 | 164(186) | termination of pregnancy | intrauterine death, type of block, endocardial fibroelastosis, hydrops, pericardial effusion, CHB recurrence, hematological and hepatic manifestations | ****** |
| Sonesson 2004 [17] | Sweden | – | 1999–2003 | 24(24) | preterm labor | type of block | ******** |
| Kan 2017 [18] | Canada | retrospective | 2009–2014 | 189(195) | maternal eclampsia | intrauterine death, pacemaker implantation, type of block, endocardial fibroelastosis, pericardial effusion, congenital heart disease, CHB recurrence | ****** |
| Bergman 2012 [19] | Sweden | crosssectional follow-up | 1999–2007 | 48(57) | – | type of block | ***** |
| Gerosa 2007 [20] | Italy | prospective | 2000–2004 | 60(60) | spontaneous abortion, preterm labor | pacemaker implantation,1 min APGAR score, type of block | ******** |
| Motta 2007 [21] | Italy | prospective | 1999–2005 | 43(51) | – | type of block | ****** |
| Chalumeau 2004 [22] | France | – | 1991–2002 | 106(167) | spontaneous abortion, terminations of pregnancy, preterm labor | IUGR,fetal death /perinatal death,live birth,type of block, congenital defects | ******** |
| Brucato 2002 [23] | Italy | prospective | – | 100(126) | type of delivery, ectopic pregnancy, spontaneous abortion, preeclampsia, pregnancy complications, preterm labor | live birth, small for gestational age, type of block, IUGR | ******** |
| Briassouli 2013 [24] | USA | retrospective | – | 61(61) | type of delivery | – | ***** |
Maternal outcomes
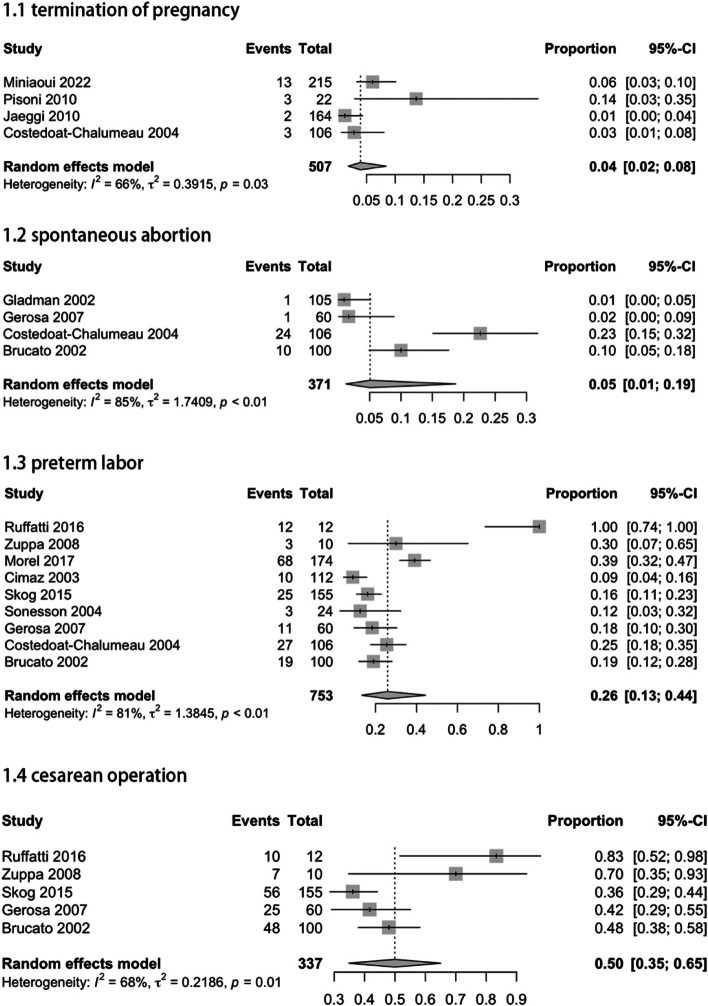
Regarding all 4 studies reporting termination of pregnancy data of women with anti-SSA/Ro positive, the pooled estimate rate for this outcome was 4% (95% CI: 2–8%; I2 = 66%). The 4 studies reporting spontaneous abortion yielded an incidence rate of 5% (95% CI: 1–19%; I2 = 85%). For preterm labor, the combined 9 studies reported an incidence of 26% (95% CI: 13–44%; I2 = 81%). Combining the data from the 5 studies on cesarean operation, the pooled estimate rate was 50% (95% CI: 35–65%; I2 = 68%) (Fig. 2).
Fig. 2.
Adverse maternal outcomes in pregnant women with anti-SSA/RO
Fetal outcomes
Meta-analyses of pooled study data demonstrated that the stillbirth or perinatal death rate was 4% (95% CI: 2–6%; I2 = 76%). Four studies reported IUGR outcomes in pregnant women exposed to anti-SSA/RO antibodies and the pooled data was 3% (95% CI: 0–6%; I2 = 71%). The analysis results showed that the incidence of Endocardial fibroelastosis was 6% (95% CI: 2–17%; I2 = 92%).
Concerning dilated heart disease, the combined 3 reports reported this incidence to be 6% (95% CI: 5–8%; I2 = 85%). Regarding the incidence of CHB, the combined 13 studies reported an incidence of 7% (95% CI: 3–13%; I2 = 87%). There was no difference in the prevalence of CHB between SLE and non-SLE groups based on the mother’s diagnosis (Fig. S1). The 6 studies reporting the recurrence of CHB yielded a recurrence rate of 12% (95% CI: 5–21%; I2 = 0%). Combining the 3 studies on CNLE data, the pooled estimate rate was 19% (95% CI: 14–25%; I2 = 49%). The pooled estimate rate of hepatobiliary disease was 12% (95% CI: 5–30%; I2 = 91%) and the pooled estimate rate of hematological manifestations was 16% (95% CI: 8–32%; I2 = 88%) (Fig. 3).
Fig. 3.
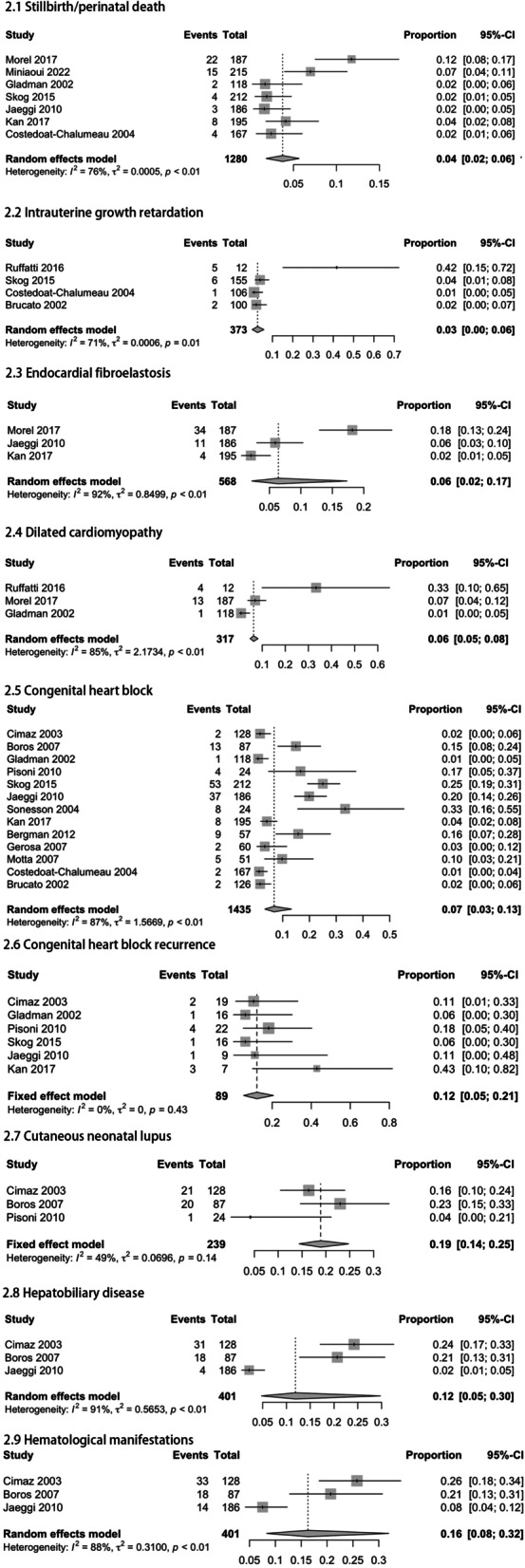
Adverse fetal outcomes observed in pregnant women with anti-SSA/RO
Subgroup analysis
Due to the large heterogeneity of study outcomes in this paper and given the small number of articles included for the remaining outcomes, we further performed a subgroup analysis of CHB prevalence, grouped according to study region, publication time, and diagnostic method. By calculating I2, we could conclude that all of them (Figs. S2, S3 and S4) affected heterogeneity to some extent.
Sensitivity analysis and publication bias analysis
We conducted sensitivity analysis by omitting each study sequentially and no significant changes were observed, indicating that the results of the meta-analysis were relatively stable (Fig. S5). As most of the studies included in this paper did not have a control group and were mainly observational studies on prevalence outcomes with no or little publication bias, no correlation analysis was conducted.
Discussion
In this article, we studied the incidence of various pregnancy outcomes for both mother and baby in anti-SSA/RO-positive pregnant women. Stillbirth and perinatal mortality both occurred in 4% of pregnancies, whereas intrauterine growth restriction occurred in 6% of pregnancies, pregnancy termination occurred in 4% of pregnancies, and 5% of pregnancies ended in spontaneous abortion. CHB is the major cause of these unfavorable pregnancies, and our findings show a prevalence of 7% of anti-SSA/RO-associated CHB. Previous prospective studies in women with anti-SSA/RO antibodies have shown a risk of cardiac NL of approximately 2% if the mother didn’t have a previously affected pregnancy [11, 25, 26]. However, in the study by Buyon [27], mothers with a previous CHB pregnancy outcome were two to three times more likely to have another pregnancy that remained CHB than those mothers with anti-SSA/RO antibodies who had never had an affected child, which may explain the difference in our analysis. A further pooling of data from six articles suggested a CHB recurrence rate of 12% would further support this conclusion.
A large US cohort study [28] showed that maternal health status, steroid regimen, antibody level, and disease severity of the first affected child did not predict recurrence, and the relatively high recurrence rate suggests that mothers with previously affected children should be targeted for monitoring and treatment. In addition to focusing on the subsequent births of these particular mothers, given the familial aggregation of autoimmune diseases [29], we need to further assess the future health of those siblings of children with CHB who are currently temporarily healthy. In their study, Johannes Mofors and colleagues evaluated the risk of disease in the siblings of children diagnosed with CHB [30]. They discovered that these children’s siblings had an increased risk of autoimmune disease; however, the authors concluded that this finding may be biased because siblings of patients diagnosed with chronic CHB may undergo medical screening more frequently than the general population.
We also analyzed the prevalence of CHB according to whether the mother had SLE or not. After integrating the data from three studies, there was no significant difference between the two groups, suggesting that the pregnancy outcome of mothers with positive anti-SSA/RO antibodies was not related to the type of autoimmune disease, but closely related to their antibodies. Miniaoui [13] followed 157 asymptomatic mothers for 11 years and found that the probability of asymptomatic mothers developing SLE was 12.7% and that of asymptomatic mothers developing SS was 28.1%.
We discovered skin lesions in roughly 19% of the babies born to moms with antibody positivity. In contrast to cardiac NLE, CNLE most commonly manifests as a transient, reversible cutaneous lesion that is analogous to subacute cutaneous lupus erythematosus. The rash most commonly appears in the periorbital and spectacle-like areas of the face. However, in some patients, it can also be seen in the vulvar triangle and on the soles of the feet as erythematous dusky patches [31]. The skin lesions often emerge for the first time at the age of 12 weeks and last until the age of 6 months [1], which is roughly the same time as autoantibodies are eliminated from the infant’s serum. According to the findings of a study conducted by Izmirly, fetuses who have had previous cutaneous lesions are at a higher risk of future recurrent NLE, in particular cardiac lesions, somewhere between 6 and 10 times more frequently than other children without previous affected conditions [32]. In addition, we found that 16% of the infants developed hematological abnormalities mainly characterized by three-line reduction, and 12% of the infants developed liver diseases mainly characterized by liver enzyme abnormalities. These abnormalities mostly disappeared by the first year of life, with no clinical manifestations.
The antibody-mediated myocardial injury usually progresses gradually, starting with first-degree AVB to irreversible second and third-degree AVB, and possibly even EFE and DCM. The latter two both had an incidence of about 6% according to our analysis. Given the high mortality rate of these two diseases [33], most fetuses may be born dead. Considering the small number of studies we included and the fact that echocardiography is a highly subjective diagnostic modality, the data require further validation.
However, no similar cases have been reported in the maternal heart, and we believe that this antibody damage is unique to the fetal stage of development. Considering such a low incidence, there is evidence that the pathogenesis may involve more than antibodies derived from mothers. In recent years hypotheses have also emerged regarding cumulative genetic transmission [34], fetal MHC [35, 36] cardiac manifestations of allograft rejection and graft-versus-host disease [37], Type-I IFN system activation [38], and the protective role of β2-GPI has also emerged [39].
Currently, routine antenatal testing does not detect autoimmune antibodies, and the majority of women who are positive for anti-SSA/RO antibodies have no clinical manifestations.
Most CHB cannot be diagnosed early and 70% of them die in utero [40]. Among live births, the average 10-year survival rate is 86% [3]. In current clinical practice, most fetal screening starts at 13–16 weeks. However, a review by Norbert Gleicher [6] suggests that the associated pathological changes begin early in the second trimester. This reminds us that anti-SSA/RO antibodies are IgG. From the 13th week [41], IgG antibodies are transferred from the mother to the fetus via the placenta, and combined with the timing of the formation of the anatomical function of the cardiac conduction system, damage to cardiac conduction tissue occurs as early as approximately 8–13 weeks of gestational age [42]. It is very crucial to start prenatal monitoring. Previously, abnormalities were primarily detected during a routine fetal ultrasound. However, Carolina Llanos et al. [43] found that some histologically definite EFE were not detected on echocardiography after the autopsy of 18 antibody-positive fetuses, and therefore fetal echocardiography may not be a sensitive enough diagnostic tool. The study has shown [19] that some infants with no cardiac manifestations before or after birth were diagnosed with CHB during follow-up, which may also serve as a side-effect validation of the limitations of the available diagnostic tools.
Common clinical treatments currently available include hydroxychloroquine, steroids, plasmapheresis, and intravenous gamma globulin (IV-Ig). Our study found that in maternal outcomes, the incidence of preterm birth reached a staggering 26%. With regard to treatment in terms of preterm birth, we found that in a retrospective cohort study [44], the rate of preterm birth was significantly lower in SLE pregnant women exposed to hydroxychloroquine than in those not exposed. Although this study was limited to patients with a diagnosis of SLE, the results also suggest some protective effect of hydroxychloroquine in the treatment of preterm labor, considering that 40% of SLE patients have anti-SSA/RO antibodies [3]. Among the above treatment regimens, which is more effective in reducing the rate of preterm birth and which is more effective in improving maternal antibody titers among different treatments is still unknown. Moreover, this paper did not stratify according to antibody titers, so further relevant studies are needed in the future to achieve more targeted prevention.
This is the first meta-analysis of maternal and infant outcomes in women with anti-SSA/RO antibodies. The results of our subgroup analysis suggested that diagnostic methods, regions of studies, and study period might be sources of heterogeneity. Because some studies employed only one certain diagnostic method and others used both echocardiography and ECG as screening tools, which we believe influenced disease detection rates to some extent. Echocardiography is a highly subjective diagnostic modality that is influenced by the physician’s experience and expertise so regional differences and publication time could also affect the diagnosis of the disease. In addition, we believe the following factors may also affect heterogeneity: 1. The inclusion of patients with varying treatment regimens may affect the results of the analysis; 2. Medication adherence, follow-up time, etc. Consequently, subgroup analysis may not adequately account for all the heterogeneity. Due to the absence of a control group, a meta-analysis of pooled prevalence typically has greater heterogeneity. All the literature used in this study met the inclusion criteria and the data from each study conformed to a normal distribution. Despite the greater heterogeneity, it is hoped that the combined data in this paper will be available to all researchers.
Conclusion
In conclusion, this meta-analysis further clarifies the impact of anti-SSA/RO antibodies on maternal and infant outcomes during pregnancy. Therefore, it serves as a reference and a guide for the diagnosis and subsequent treatment of these women, thereby enhancing maternal and infant health. Additional studies with real-world cohorts are required to validate these results.
Supplementary Information
Additional file 1: Fig. S1. The prevalence of CHB between SLE and non-SLE groups. Fig. S2. Subgroup analysis of different types of diagnostic method. Fig. S3. Subgroup analysis based on regions of studies. Fig. S4. Subgroup analysis based on publication time. Fig. S5. Sensitivity analysis.
Abbreviations
- NLE
Neonatal lupus syndrome
- EFE
Endocardial fibroelastosis
- CHB
Congenital heart block
- DCM
Dilated cardiomyopathy
- CNLE
Cutaneous neonatal lupus erythematosus
Authors’ contributions
XRS and MJW were responsible for the study design; XRS, XHS, and YX searched, selected the articles, and extracted data; XC, TR, and JC guided the application of statistical software; XRS and XHS interpreted and analyzed data; XRS wrote the manuscript; MJW, JW, and TC supervised the whole process. All authors approved the final version of the manuscript and agreed to be responsible for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (82101887), Suzhou Minsheng science and technology project (SYS2019043 and SYS2020108), and Beijing Health Alliance Charitable Foundation (B21046ES).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiangrui Sheng, Xiaohui Song and Yue Xiong contributed equally to this work.
Contributor Information
Jing Cao, Email: 1009014752@qq.com.
Tao Cheng, Email: chengtao0526@126.com.
Mingjun Wang, Email: 81489895@qq.com.
References
- 1.Lee LA, Weston WL. New findings in neonatal lupus syndrome. Am J Dis Child. 1984;138(3):233–236. doi: 10.1001/archpedi.1984.02140410013006. [DOI] [PubMed] [Google Scholar]
- 2.Horsfall AC, Venables PJ, Taylor PV, Maini RN. Ro and La antigens and maternal anti-La idiotype on the surface of myocardial fibres in congenital heart block. J Autoimmun. 1991;4(1):165–176. doi: 10.1016/0896-8411(91)90015-5. [DOI] [PubMed] [Google Scholar]
- 3.Wainwright B, Bhan R, Trad C, Cohen R, Saxena A, Buyon J, et al. Autoimmune-mediated congenital heart block. Best Pract Res Clin Obstet Gynaecol. 2020;64:41–51. doi: 10.1016/j.bpobgyn.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Miranda-Carús ME, Boutjdir M, Tseng CE, DiDonato F, Chan EK, Buyon JP. Induction of antibodies reactive with SSA/Ro-SSB/La and development of congenital heart block in a murine model. J Immunol. 1998;161(11):5886–5892. [PubMed] [Google Scholar]
- 5.Nield LE, Silverman ED, Taylor GP, Smallhorn JF, Mullen JBM, Silverman NH, et al. Maternal anti-Ro and anti-La antibody-associated endocardial fibroelastosis. Circulation. 2002;105(7):843–848. doi: 10.1161/hc0702.104182. [DOI] [PubMed] [Google Scholar]
- 6.Gleicher N, Elkayam U. Preventing congenital neonatal heart block in offspring of mothers with anti-SSA/Ro and SSB/La antibodies: a review of published literature and registered clinical trials. Autoimmun Rev. 2013;12(11):1039–1045. doi: 10.1016/j.autrev.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Jaeggi E, Laskin C, Hamilton R, Kingdom J, Silverman E. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus a prospective study of 186 antibody-exposed fetuses and infants. J Am Coll Cardiol. 2010;55(24):2778–2784. doi: 10.1016/j.jacc.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 8.Ruffatti A, Cerutti A, Favaro M, Del Ross T, Calligaro A, Hoxha A, et al. Plasmapheresis, intravenous immunoglobulins and bethametasone - a combined protocol to treat autoimmune congenital heart block: a prospective cohort study. Clin Exp Rheumatol. 2016;34(4):706–713. [PubMed] [Google Scholar]
- 9.Zuppa AA, Fracchiolla A, Cota F, Gallini F, Savarese I, D'Andrea V, et al. Infants born to mothers with anti-SSA/Ro autoantibodies: neonatal outcome and follow-up. Clin Pediatr (Phila) 2008;47(3):231–236. doi: 10.1177/0009922807307264. [DOI] [PubMed] [Google Scholar]
- 10.Morel N, Lévesque K, Maltret A, Baron G, Hamidou M, Orquevaux P, et al. Incidence, risk factors, and mortality of neonatal and late-onset dilated cardiomyopathy associated with cardiac neonatal lupus. Int J Cardiol. 2017;248:263–269. doi: 10.1016/j.ijcard.2017.07.100. [DOI] [PubMed] [Google Scholar]
- 11.Cimaz R, Spence DL, Hornberger L, Silverman ED. Incidence and spectrum of neonatal lupus erythematosus: a prospective study of infants born to mothers with anti-Ro autoantibodies. J Pediatr. 2003;142(6):678–683. doi: 10.1067/mpd.2003.233. [DOI] [PubMed] [Google Scholar]
- 12.Boros CA, Spence D, Blaser S, Silverman ED. Hydrocephalus and macrocephaly: new manifestations of neonatal lupus erythematosus. Arthritis Rheum. 2007;57(2):261–266. doi: 10.1002/art.22543. [DOI] [PubMed] [Google Scholar]
- 13.Miniaoui I, Morel N, Lévesque K, Maltret A, Driessen M, Masseau A, et al. Health outcomes of 215 mothers of children with autoimmune congenital heart block: analysis of the French neonatal lupus syndrome registry. J Rheumatol. 2022;49(10):1124–1130. doi: 10.3899/jrheum.210703. [DOI] [PubMed] [Google Scholar]
- 14.Gladman G, Silverman ED, Yuk L, Luy L, Boutin C, Laskin C, et al. Fetal echocardiographic screening of pregnancies of mothers with anti-Ro and/or anti-La antibodies. Am J Perinatol. 2002;19(2):73–80. doi: 10.1055/s-2002-23555. [DOI] [PubMed] [Google Scholar]
- 15.Pisoni CN, Brucato A, Ruffatti A, Espinosa G, Cervera R, Belmonte-Serrano M, et al. Failure of intravenous immunoglobulin to prevent congenital heart block: findings of a multicenter, prospective, observational study. Arthritis Rheum. 2010;62(4):1147–1152. doi: 10.1002/art.27350. [DOI] [PubMed] [Google Scholar]
- 16.Skog A, Lagnefeldt L, Conner P, Wahren-Herlenius M, Sonesson S-E. Outcome in 212 anti-Ro/SSA-positive pregnancies and population-based incidence of congenital heart block. Acta Obstet Gynecol Scand. 2016;95(1):98–105. doi: 10.1111/aogs.12785. [DOI] [PubMed] [Google Scholar]
- 17.Sonesson S-E, Salomonsson S, Jacobsson L-A, Bremme K, Wahren-Herlenius M. Signs of first-degree heart block occur in one-third of fetuses of pregnant women with anti-SSA/Ro 52-kd antibodies. Arthritis Rheum. 2004;50(4):1253–1261. doi: 10.1002/art.20126. [DOI] [PubMed] [Google Scholar]
- 18.Kan N, Silverman ED, Kingdom J, Dutil N, Laskin C, Jaeggi E. Serial echocardiography for immune-mediated heart disease in the fetus: results of a risk-based prospective surveillance strategy. Prenat Diagn. 2017;37(4):375–382. doi: 10.1002/pd.5021. [DOI] [PubMed] [Google Scholar]
- 19.Bergman G, Eliasson H, Mohlkert L-A, Wahren-Herlenius M, Sonesson S-E. Progression to first-degree heart block in preschool children exposed in utero to maternal anti-SSA/Ro52 autoantibodies. Acta Paediatr. 2012;101(5):488–493. doi: 10.1111/j.1651-2227.2011.02563.x. [DOI] [PubMed] [Google Scholar]
- 20.Gerosa M, Cimaz R, Stramba-Badiale M, Goulene K, Meregalli E, Trespidi L, et al. Electrocardiographic abnormalities in infants born from mothers with autoimmune diseases--a multicentre prospective study. Rheumatology (Oxford) 2007;46(8):1285–1289. doi: 10.1093/rheumatology/kem073. [DOI] [PubMed] [Google Scholar]
- 21.Motta M, Rodriguez-Perez C, Tincani A, Lojacono A, Chirico G. Outcome of infants from mothers with anti-SSA/Ro antibodies. J Perinatol. 2007;27(5):278–283. doi: 10.1038/sj.jp.7211688. [DOI] [PubMed] [Google Scholar]
- 22.Costedoat-Chalumeau N, Amoura Z, Lupoglazoff J-M, Huong DLT, Denjoy I, Vauthier D, et al. Outcome of pregnancies in patients with anti-SSA/Ro antibodies: a study of 165 pregnancies, with special focus on electrocardiographic variations in the children and comparison with a control group. Arthritis Rheum. 2004;50(10):3187–3194. doi: 10.1002/art.20554. [DOI] [PubMed] [Google Scholar]
- 23.Brucato A, Doria A, Frassi M, Castellino G, Franceschini F, Faden D, et al. Pregnancy outcome in 100 women with autoimmune diseases and anti-Ro/SSA antibodies: a prospective controlled study. Lupus. 2002;11(11):716–721. doi: 10.1191/0961203302lu252oa. [DOI] [PubMed] [Google Scholar]
- 24.Briassouli P, Halushka MK, Reed JH, Molad Y, Fox-Talbot K, Buyon L, et al. A central role of plasmin in cardiac injury initiated by fetal exposure to maternal anti-Ro autoantibodies. Rheumatology (Oxford) 2013;52(8):1448–1453. doi: 10.1093/rheumatology/ket156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brucato A, Frassi M, Franceschini F, Cimaz R, Faden D, Pisoni MP, et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum. 2001;44(8):1832–1835. doi: 10.1002/1529-0131(200108)44:8<1832::AID-ART320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 26.Brucato A. Prevention of congenital heart block in children of SSA-positive mothers. Rheumatology (Oxford) 2008;47(Suppl 3):iii35–iiii7. doi: 10.1093/rheumatology/ken153. [DOI] [PubMed] [Google Scholar]
- 27.Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31(7):1658–1666. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 28.Llanos C, Izmirly PM, Katholi M, Clancy RM, Friedman DM, Kim MY, et al. Recurrence rates of cardiac manifestations associated with neonatal lupus and maternal/fetal risk factors. Arthritis Rheum. 2009;60(10):3091–3097. doi: 10.1002/art.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo C-F, Grainge MJ, Valdes AM, See L-C, Luo S-F, Yu K-H, et al. Familial aggregation of systemic lupus erythematosus and Coaggregation of autoimmune diseases in affected families. JAMA Intern Med. 2015;175(9):1518–1526. doi: 10.1001/jamainternmed.2015.3528. [DOI] [PubMed] [Google Scholar]
- 30.Mofors J, Eliasson H, Ambrosi A, Salomonsson S, Skog A, Fored M, et al. Comorbidity and long-term outcome in patients with congenital heart block and their siblings exposed to Ro/SSA autoantibodies in utero. Ann Rheum Dis. 2019;78(5):696–703. doi: 10.1136/annrheumdis-2018-214406. [DOI] [PubMed] [Google Scholar]
- 31.Laurinaviciene R, Christesen HT, Bygum A. New aspects in the clinical spectrum of neonatal lupus. Eur J Pediatr. 2012;171(5):801–805. doi: 10.1007/s00431-011-1618-z. [DOI] [PubMed] [Google Scholar]
- 32.Izmirly PM, Llanos C, Lee LA, Askanase A, Kim MY, Buyon JP. Cutaneous manifestations of neonatal lupus and risk of subsequent congenital heart block. Arthritis Rheum. 2010;62(4):1153–1157. doi: 10.1002/art.27333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izmirly PM, Saxena A, Kim MY, Wang D, Sahl SK, Llanos C, et al. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation. 2011;124(18):1927–1935. doi: 10.1161/CIRCULATIONAHA.111.033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saxena A, McDonnell E, Ramos PS, Sajuthi S, Marion MC, Langefeld CD, et al. Preferential transmission of genetic risk variants of candidate loci at 6p21 from asymptomatic grandparents to mothers of children with neonatal lupus. Arthritis Rheum. 2012;64(3):931–939. doi: 10.1002/art.33366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strandberg LS, Ambrosi A, Jagodic M, Dzikaite V, Janson P, Khademi M, et al. Maternal MHC regulates generation of pathogenic antibodies and fetal MHC-encoded genes determine susceptibility in congenital heart block. J Immunol. 2010;185(6):3574–3582. doi: 10.4049/jimmunol.1001396. [DOI] [PubMed] [Google Scholar]
- 36.Lundvig DMS, Immenschuh S, Wagener FADTG. Heme oxygenase, inflammation, and fibrosis: the good, the bad, and the ugly? Front Pharmacol. 2012;3:81. doi: 10.3389/fphar.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gleicher N, Elkayam U. Peripartum cardiomyopathy, an autoimmune manifestation of allograft rejection? Autoimmun Rev. 2009;8(5):384–387. doi: 10.1016/j.autrev.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Clancy RM, Markham AJ, Jackson T, Rasmussen SE, Blumenberg M, Buyon JP. Cardiac fibroblast transcriptome analyses support a role for interferogenic, profibrotic, and inflammatory genes in anti-SSA/Ro-associated congenital heart block. Am J Physiol Heart Circ Physiol. 2017;313(3):H631–HH40. doi: 10.1152/ajpheart.00256.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reed JH, Clancy RM, Purcell AW, Kim MY, Gordon TP, Buyon JP. β2-glycoprotein I and protection from anti-SSA/Ro60-associated cardiac manifestations of neonatal lupus. J Immunol. 2011;187(1):520–526. doi: 10.4049/jimmunol.1100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brito-Zerón P, Izmirly PM, Ramos-Casals M, Buyon JP, Khamashta MA. The clinical spectrum of autoimmune congenital heart block. Nat Rev Rheumatol. 2015;11(5):301–312. doi: 10.1038/nrrheum.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rein AJJT, Mevorach D, Perles Z, Gavri S, Nadjari M, Nir A, et al. Early diagnosis and treatment of atrioventricular block in the fetus exposed to maternal anti-SSA/Ro-SSB/La antibodies: a prospective, observational, fetal kinetocardiogram-based study. Circulation. 2009;119(14):1867–1872. doi: 10.1161/CIRCULATIONAHA.108.773143. [DOI] [PubMed] [Google Scholar]
- 43.Llanos C, Friedman DM, Saxena A, Izmirly PM, Tseng C-E, Dische R, et al. Anatomical and pathological findings in hearts from fetuses and infants with cardiac manifestations of neonatal lupus. Rheumatology (Oxford) 2012;51(6):1086–1092. doi: 10.1093/rheumatology/ker515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Do SC, Rizk NM, Druzin ML, Simard JF. Does hydroxychloroquine protect against preeclampsia and preterm delivery in systemic lupus erythematosus pregnancies? Am J Perinatol. 2020;37(9):873–880. doi: 10.1055/s-0039-3402752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. The prevalence of CHB between SLE and non-SLE groups. Fig. S2. Subgroup analysis of different types of diagnostic method. Fig. S3. Subgroup analysis based on regions of studies. Fig. S4. Subgroup analysis based on publication time. Fig. S5. Sensitivity analysis.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.