Abstract
Background
Self-regulation of payment disclosure by pharmaceutical industry trade groups is a major global approach to increasing transparency of financial relationships between drug companies and healthcare professionals and organisations. Nevertheless, little is known about the relative strengths and weaknesses of self-regulation across countries, especially beyond Europe. To address this gap in research and stimulate international policy learning, we compare the UK and Japan, the likely strongest cases of self-regulation of payment disclosure in Europe and Asia, across three dimensions of transparency: disclosure rules, practices, and data.
Results
The UK and Japanese self-regulation of payment disclosure had shared as well unique strengths and weaknesses. The UK and Japanese pharmaceutical industry trade groups declared transparency as the primary goal of payment disclosure, without, however, explaining the link between the two. The rules of payment disclosure in each country provided more insight into some payments but not others. Both trade groups did not reveal the recipients of certain payments by default, and the UK trade group also made the disclosure of some payments conditional on recipient consent. Drug company disclosure practices were more transparent in the UK, allowing for greater availability and accessibility of payment data and insight into underreporting or misreporting of payments by companies. Nevertheless, the share of payments made to named recipients was three times higher in Japan than in the UK, indicating higher transparency of disclosure data.
Conclusions
The UK and Japan performed differently across the three dimensions of transparency, suggesting that any comprehensive analysis of self-regulation of payment disclosure must triangulate analysis of disclosure rules, practices, and data. We found limited evidence to support key claims regarding the strengths of self-regulation, while often finding it inferior to public regulation of payment disclosure. We suggest how the self-regulation of payment disclosure in each country can be enhanced and, in the long run, replaced by public regulation to strengthen the industry’s accountability to the public.
Keywords: Comparative case study, Transparency, Financial conflicts of interest, Payment disclosure, Research and development, Self-regulation, Pharmaceutical industry
Background
Collaborations between pharmaceutical companies and the healthcare sector facilitate drug development and commercialisation, disease awareness, and disseminating information on medicine use [1–5]. They have become ever more pronounced as new specialist drugs, including for genetic diseases and some cancers, proliferated, requiring advice from medical experts and researchers throughout the product life cycle [6–10]. Similarly, decreasing industry investment in in-house drug development has emphasised academic partnerships as sources of innovation [11–14].
However, these collaborations may generate financial conflicts of interest (FCOIs) biasing healthcare practice, research, education, and policy [15–17]. The main global response to these concerns is disclosure of drug company payments – including for consultancies, sponsorships, and grants – to healthcare professionals (HCPs) and organisations (HCOs) [18–20]. Payment disclosure involves different “transparency provisions”: (1) self-regulation, primarily voluntary codes of conduct developed and enforced by industry trade groups; (2) public regulation, typically sunshine legislation mandating drug companies to disclose certain payments to some recipient categories; or (3) their combination [18, 21–25].
With few exceptions [26], research to-date has utilised single-case studies to examine the strengths and weaknesses of public regulation in the US [23, 27, 28] and France [29]; and self-regulation in the UK [30–32], Germany [10, 33], Ireland [34], Australia [18, 35–37], and Japan [38–44]. There have been fewer direct comparisons of countries with public regulation and self-regulation [18, 21, 22, 24, 45, 46]. Compared with public regulation, self-regulation of payment disclosure is described by its proponents as less burdensome, more flexible and responsive to the needs of a globalised industry [10, 25, 45, 47–49]. Strengths of public regulation include mandatory disclosures and more healthcare industries and payments covered by the requirements [18, 22, 45]. Crucially, both are critiqued for low disclosure accessibility, quality, and user-friendliness [21, 24].
Against this background, except for Europe [24, 26, 46], the relative strengths and weaknesses of self-regulation of payment disclosure across countries are poorly understood. Nevertheless, the variability of European cases is low as nearly all national trade groups follow – but rarely exceed – the minimum requirements from the Code of Practice of the European Federation of Pharmaceutical Industries and Associations (EFPIA) [21, 24, 50, 51]. What also underscores the need for comparative analysis of self-regulation of payment disclosure is its global prevalence [36]. Indeed, it is the default approach in countries without public regulation [51] and only two of the largest pharmaceutical markets – the US and France – have comprehensive sunshine legislation, while other known examples have limited scope or exist alongside self-regulation [18, 21, 24]. Self-regulation is also an important policy tool, e.g. in benchmarking [18], with country rankings of disclosure statistics and code compliance becoming an argument against the introduction of sunshine legislation [47, 52, 53].
We seek to compare the strengths and weaknesses of self-regulation of payment disclosure using two crucial case studies: the UK and Japan. While each country has been analysed separately, the focus has been on select payments to HCPs and HCOs [30, 31, 38, 39, 41, 42, 54]. Disparate research designs have further constrained possibilities for systematic analysis of the uniqueness and commonalities relative to other cases, reducing generalisable international policy learning [30, 31, 38]. In addressing these limitations, we use a typology of dimensions of transparency developed in internationally comparative research on payments to patient organisations, an area of industry self-regulation separate from the scope of this article [55]. We examine three such dimensions: (1) the scope of payment disclosure rules established by the industry trade groups; (2) how payments are disclosed in practice; and (3) the insight into industry-healthcare sector financial relationships generated by the payment data.
Japan and the UK have the third and seventh largest pharmaceutical markets worldwide [56], with extensive drug manufacturing and research and development (R&D) capacity [57, 58]. They are likely the best cases of self-regulation of payment disclosure in Europe and Asia, and potentially globally, having been characterised as more comprehensive than another established example of self-regulation, Australia [36, 38].
Since 2015, the Code of Practice of the Association of the British Pharmaceutical Industry (henceforth ABPI Code) has included payments and recipients exceeding EFPIA’s minimum requirements [24, 31, 59]. Despite important shortcomings [30, 31], the transparency of disclosures by companies following the ABPI Code is the highest within the EFPIA ecosystem, especially following its major revision in 2021, which introduced the disclosure of additional payment and recipient categories [21, 24]. Further, while only around two-thirds of individuals receiving payments consent to disclosure [60], this reflects the pattern observed in most European countries where industry interprets payment data as falling under the European privacy laws [26, 30, 47, 52, 53].
As far as we know, since 2011 the Japan Pharmaceutical Manufacturers Association (JPMA) has developed Asia’s most comprehensive standards on interactions with the healthcare sector. Specifically, the “Transparency Guidelines for the Relation between Corporate Activities and Medical Institutions” (henceforth JPMA Transparency Guidelines) [61], focus on payment disclosure, while the “JPMA Code of Practice” (henceforth JPMA Code) [62] covers promotion.
Context of self-regulation of payment disclosure in the UK and Japan
The pharmaceutical industry globally faces similar political and regulatory pressures to enhance transparency [18, 21, 25, 36, 49], potentially resulting in similar approaches to disclosure. In the UK, key manifestations of these pressures have included a parliamentary investigation into the industry’s influence on healthcare provision [63] and a medical product safety review [64, 65]. Controversy has also been caused by the Japanese company Astellas’ illicit marketing, resulting in its temporary suspension from the ABPI [48, 66]. In Japan, several well-publicised scandals have involved kickbacks to increase prescription [41] and data fabrication in clinical trials [44, 67–71]. The ensuing criminal investigations have prompted policy discussions on transparency, leading to the development of the new Clinical Trials Act [44, 72, 73].
As members of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) [74, 75], the ABPI and JPMA subscribe to its Code of Practice [62, 74] and transparency guidelines on fees for services [76], sponsorship of events and meetings [77], and continuing medical education [78]. They also share company members, including the UK-headquartered GSK and AstraZeneca and Japan-headquartered Takeda and Astellas [79–82]. Like other multinationals, their global codes of conduct [49, 51] make similar commitments to transparency [83–86] and payment disclosure [85, 86]. The ABPI and JPMA collaborate [87, 88], including via EFPIA Japan [89], an organisation representing European company interests which contributed to the development of the JPMA Transparency Guidelines [90].
Although the “broad parameters” of payment disclosure are shared internationally, detailed expectations vary across jurisdictions [18, 25]. Indeed, IFPMA expects its member associations to implement codes reflecting national circumstances [49]. Therefore, the ABPI implements EFPIA’s standards [74] that exceed IFPMA’s requirements regarding payment disclosure [51]. In comparison, the JPMA responds, among others, to the expectations of domestic fair trade and marketing organisations [91].
Payment disclosure is also affected by the trade groups’ varying governance structures. As part of EFPIA’s compliance monitoring, the ABPI publishes yearly evaluations of company compliance with its Code [47, 52, 53]. Complaints regarding alleged breaches of the ABPI Code are considered by the Prescription Medicines Code of Practice Authority (PMCPA), a quasi-autonomous body within the ABPI [92, 93]. The PMCPA publishes reports of all complaints and levies “administrative charges” on breaching companies, and in instances of particularly problematic company behaviour, it can rule a breach of §2 of the Code (promotion that “brings discredit to, and reduction of confidence”) and can issue a public reprimand [92]. Contrastingly, because the Transparency Guidelines are voluntary for its member companies, the JPMA does not monitor company adherence or sanction non-compliance [38, 94]. However, the JPMA’s executive board investigates complaints regarding alleged breaches of the JMPA Code, which is distinct to the Transparency Guidelines, and decides on penalties, but does not publish their details systematically [94].
Differences between each country’s “medical economy” are also significant [91, 95, 96]. Although both have publicly funded healthcare offering universal coverage to residents [57], Japan combines physician-owned hospitals and clinics with a sizeable sector of hospitals owned by public authorities and a few not-for-profit hospitals run by charities or foundations [58, 91]. Contrastingly, in the UK, healthcare is predominantly delivered by practice-owning physicians, who are patients’ first point of contact, and salaried staff in publicly-owned National Health Service (NHS) hospitals [97].
To some extent, these health-system level differences entail different patterns of the industry-healthcare sector interactions. Like in other major pharmaceutical markets [91], in the UK and Japan, industry marketing targets physicians, including medical Key Opinion Leaders (KOLs) [63, 94, 98, 99] and hospitals [41, 100]. The JPMA has also sought to regulate financial ties pertinent to Japan, such as extensive funding of physicians’ and university research [91]. Similarly, while attempts at establishing reciprocity and loyalty with physicians have been reported internationally [101–103], their role might be more pronounced in Japan, given the cultural importance of informal gifts, associated with evidence of bribes and kickbacks in medical procurement, prescription, and clinical trials [91].
Lastly, self-regulation belongs to wider mechanisms governing interactions between pharmaceutical companies and the healthcare sector [10, 51, 104]. In Japan, FCOIs are regulated chiefly via the Physicians’ Act, the Medical Service Act, and the Health Insurance Act, which criminalise bribes, kickbacks and gifts to publicly employed physicians [91]. In addition, the Fair Competition Code [105] determines how recipients can spend company payments [91, 105], while the Clinical Trials Act mandates financial disclosure by researchers and research institutions [44, 73]. Although the Clinical Trials Act does not mandate disclosure from drug companies, the study types from the Clinical Trials Act were voluntarily adopted in the JPMA Transparency Guidelines in 2018 in relation to the reporting of R&D payments and expenditure (no changes were introduced to the reporting of non-R&D payments). Nevertheless, FCOI regulation by professional organisations [91] and hospitals [106] has been historically weak, with reports of senior medics seeking to maintain close industry ties [42, 94].
In the UK, the overarching approach to undue influence is covered by the Bribery Act, making bribery a corporate offence [45, 93]. The NHS has voluntary guidance on FCOI disclosure, which is characterised by important loopholes [107, 108]. FCOIs are also self-regulated by the medical profession, but attempts at creating a centralised register for all clinical staff have been inconclusive [109, 110]. The ABPI maintains close contact with government and professional organisations [93], which support industry self-regulation of payment disclosure [64, 111], having issued joint guidelines for HCOs with the ABPI [112]. Finally, freedom of information legislation is increasingly important in gaining insights into FCOIs in the NHS [108].
Methods
We compared the transparency of disclosure rules, practices, and data [55].
We examined disclosure rules which the ABPI and JPMA described in codes, guidelines, and press releases. We identified these documents on trade group websites and by googling “Association of the British Pharmaceutical Industry”, “ABPI”, “Japan Pharmaceutical Manufacturers Association” (日本製薬工業協会 or Nihon Seiyaku Kogyo Kyokai), “JPMA”, together with the terms “transparency” (透明性 or Tomeisei), “disclosure” (公開 or Kokai), “payments” (支払い or Shiharai), and “transfers of value”. We also consulted the 2019 publication “Pharmaceutical Association Code of Practice”, comprising the JPMA Code of Practice, the Transparency Guidelines, and a glossary of relevant regulations.
We considered the JPMA Transparency Guidelines from 2018, both the English [61] and Japanese versions [113] (cited in-text as JTG2018), and the JPMA Code from October 2019, both the English [62] and Japanese [114] versions (cited in-text as JC2019). The Guidelines were further revised in 2022, without, however, introducing changes to payment disclosure. On the other hand, significant changes were being introduced to the ABPI Code at the time of writing and therefore we considered its 2019 version (cited in-text as AC2019) [74] and the newest revision implemented in 2021 (cited in-text as AC2021) [59]. We note any differences between the two, but if no change occurred, we only mention the 2019 version.
Drawing on earlier comparative research [24, 46] and single-case studies of ABPI and JPMA disclosures [30, 31, 38], we compared the disclosure rules in relation to the aim of disclosure, payment recipients, and payments either included or exempted from disclosure.
We examined disclosure practices suggested by to-date analyses of self-regulation [55, 115–117]. First, the availability of disclosures, understood as the share of companies publishing disclosures within companies committing to disclosure rules. Second, accessibility concerned where disclosures were published online. Third, we considered the electronic format of documents with drug company disclosures. Fourth, by evidence of underreporting we meant acknowledged cases of payments which should have been reported but were not. Fifth, evidence of misreporting involved attribution of payments to incorrect recipients.
We analysed disclosure availability, accessibility, and format in 2018 as this was the latest year for which we had collected the Japanese disclosures. In so doing, in the UK, we considered (1) Disclosure UK, the ABPI’s centralised payments database; (2) company “methodological notes” which describe their approaches to disclosure and are published alongside Disclosure UK [30, 31]; and (3) any relevant disclosure guides that we could find on the ABPI website [118]. In Japan, we evaluated disclosure availability, accessibility and format using payment disclosures published on individual company websites [38, 42, 43]. No changes to disclosure availability, accessibility and format have been made by either the ABPI or the JPMA since 2018.
We investigated the evidence of underreporting and misreporting by searching the PMCPA’s open archive for any cases involving breaches of clause 24 of the ABPI Code (“Transfers of Value to Health Professionals and Healthcare Organisations”); we considered the entire period since the current disclosure regulations were introduced in the UK (2015). There was no systematic evidence documenting potential underreporting and misreporting by JPMA members. Contrastingly, because JTG2018 are not mandatory (and involve no penalties for breaches), no platform existed for reporting potential cases of payment misreporting or underreporting.
In examining disclosure data, we summarised company disclosures using the original ABPI and JPMA payment categories, subsequently aggregating them into UK-only, Japan-only, and shared. We converted payment values from sterling and yen to US dollar using the average yearly exchange rates published by the UK Office of National Statistics [119] and the Bank of Japan [120] (1 USD = 0.75 pound sterling and 110.4 yen). The 2021 revision of the ABPI Code broadened the scope of one payment category (“joint working” replaced with “collaborative working”) and further specified the meaning of another (“fees for service and consultancy” replaced with “contracted services”) [59]. These changes did not alter the nature or structure of the disclosure data significantly, allowing for the same approach to data analysis to be applied in future research. The JPMA have made no changes to the reporting of payment categories since 2018. In practice, some companies delayed the reporting of R&D payments using the updated categories which the JPMA introduced voluntarily to JTG2018 to reflect the new Clinical Trials Act. Nevertheless, this delay did not affect the overall reported R&D volume of payments.
Two researchers collected and analysed the data separately for each country, with interpretations agreed by the entire team.
Results
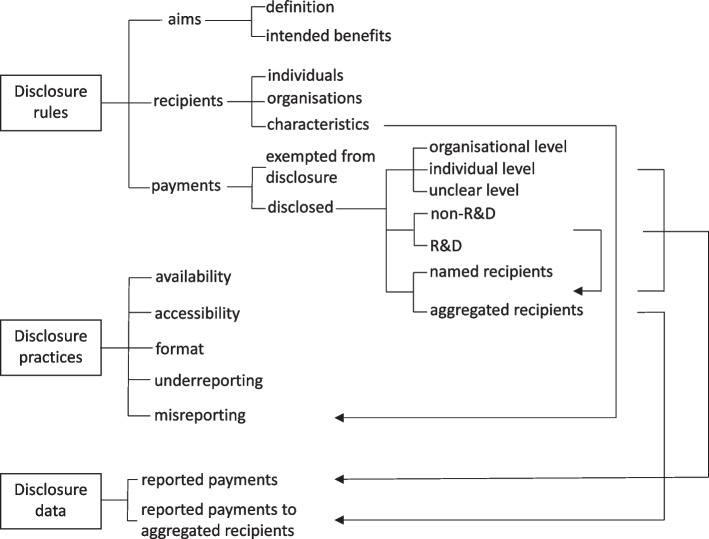
Disclosure rules, practices, and data formed three distinct dimensions of transparency of self-regulated payment disclosure in the UK and Japan. Figure 1 summarises their constituent parts and relationships.
Fig. 1.
Relationships between disclosure rules, practices, and data in pharmaceutical industry self-regulation in the UK and Japan
Disclosure rules
Aim of disclosure
The ABPI (AC2019, Introduction) and JPMA (JTG2018, Chapter 1 – Purpose) identified transparency as the primary aim of disclosure, without, however, defining it.
According to the ABPI, transparency, or “openness”, was achieved by making payments publicly available [111, 121], and, as such, was instrumental in “building and maintaining confidence” in the industry- healthcare sector collaborations (AC2019, Introduction). Its additional benefits included demonstrating ethical behaviour, managing FCOIs in the NHS, and increasing public understanding of industry-healthcare sector collaborations [111, 121]. Likewise, the JPMA viewed transparency as key for addressing FCOIs and demonstrating commitment to ethical standards and scientific progress (JTG2018, Introduction).
Payment recipients
Recipient categories
The ABPI and JPMA distinguished individual- and organisational-level recipients. Both trade groups defined organisational-level recipients similarly, using overlapping examples (Table 1). At the individual level, however, the JPMA (JTG2018, Chapter 3 – Disclosure Recipients) defined HCPs more broadly, referring to medical personnel, while the ABPI Code mentioned professions and roles in decision-making involving medicines (AC2019, Clause 1.4). Nevertheless, in 2018 at least some companies in the UK reported payments to professionals providing treatment or interventions without prescription medicines, such as occupational health specialists, speech and language therapists, and social workers. The JPMA mentioned an additional HCP category associated with “medical operations”, corresponding with ABPI’s non-HCP “other relevant decisions makers” (ORMDs), which companies reporting their payments in Disclosure UK in 2018 interpreted as NHS administrators, managers, and board members (AC2019, Clause 1.5).
Table 1.
Payment recipients subject to disclosure under the JPMA Transparency Guidelines and the ABPI Code
| Comparative recipient categories | JPMA Transparency Guidelines (2018) | ABPI Code (2019 and 2021) | |
|---|---|---|---|
| Level of aggregation | Recipient categories | ||
| Individual | Healthcare professionals | “Medical personnel (physicians, dentists, pharmacists, public health nurses, nurses, and other persons involved in medical and nursing care)” (JTG2018, Chapter 3 – Disclosure Recipients, Medical personnel, etc.) | Anyone “who in the course of their professional activities may administer, purchase, recommend or supply a medicine” (AC2019, Clause 1.4) |
| Other healthcare personnel | “Persons involved in medical operations (officers and employees of medical institutions other than medical personnel, and other persons involved in the selection or purchase of medicines)” (JTG2018, Chapter 3 – Disclosure Recipients, Medical personnel, etc.) | Other Relevant Decision Makers – “particularly includes those with an NHS [National Health Service] role who “who could influence in any way the administration, consumption, prescription, purchase, recommendation, sale, supply or use of any medicine but who are not health professionals” (AC2019, Clause 1.5) | |
| Non-healthcare personnel | “Life science researchers in medicine, pharmacy, science and engineering” (JTG2018, Chapter 3 – Disclosure Recipients, Medical personnel, etc.) | “Individual members of the public, including patients, individual patients not representing a patient organisation and journalists” (AC2021, Clause 4.5) | |
| Organisational | Healthcare organisations | “Medical institutions, research institutions or departments, medical organisations, and foundations.” (JTG2018, Chapter 3 – Disclosure Recipients, (1) medical institution (2) research institution (3) Medical Organizations (4) Foundation, etc.) | “Institutions, organisations or associations that are comprised of health professionals and/or that provide healthcare or conduct research” (AC2019, Clause 19.2), such as a “hospital, clinic, foundation, university or other teaching institution or learned society” (AC2019, Clause 1.9) |
If the recipient categories are the same in the 2019 and 2021 versions of the ABPI Code only the 2019 version is cited. For new recipient categories the 2021 version is cited
Two other individual-level categories did not overlap. From 2023, the ABPI provisions will extend to patients and journalists as members of the public (AC2021, Introduction). Conversely, the JPMA requires the disclosure of payments to life science researchers (JTG2018, Chapter 3 – Disclosure Recipients). While not explicitly mentioned in the ABPI Code, payments reported in Disclosure UK in 2018 suggests that at least some companies voluntarily interpreted scientists, such as biochemists and microbiologists, as HCPs or ORDMs.
Recipient characteristics
The ABPI’s “disclosure template” (AC2019, Clause 24.1) required the reporting of recipients’ address information and “institution name” and, optionally, “location”. Some companies reporting payments in 2018 interpreted “location” as an organisational subunit (e.g., a department within a university). The ABPI Code did not explain the difference between “names” and “locations”, potentially leading to confusion in identifying recipients [31], which could not be addressed by optional – and not published – identification numbers [122]. Further optional characteristics were HCP “speciality” and “role”, but in the absence of a shared list of categories and their descriptors companies used them inconsistently, thereby hindering or preventing reliable aggregation [24, 31]. For example, in the 2018 Disclosure UK database anaesthesiology was referred to variously as “anaesth”, “anaesthesia”, “anesthesiology” [sic], "anaesthiesia" [sic], and “anaesthetics”.
While the JPMA demanded no organisational-level characteristics (besides names), the individual-level characteristics overlapped with those required by the ABPI: affiliation and, if available, organisational sub-units, such as clinics and departments (corresponding with ABPI’s “names” and “locations”) (JTG2018, Chapter 5 – Publication details). Companies also reported recipients’ roles but without specialty and work address. Like in the UK, recipient characteristics were often missing and unstandardised in disclosures.
Disclosed payments
The ABPI mandated the disclosure of payments made for “promotional purposes or otherwise, in connection with the development or sale of [prescription] medicines” (AC2019, Clause 1.10, 24.1–2). In describing disclosed payments, the JPMA did not refer to promotion specifically, instead mentioning collaborations related to research or other activities undertaken by payment recipients, not necessarily in academic settings (JTG2018, Chapter 1 Background of Transparency Guidelines Enforcement; Chapter 4 – Target Payments for public disclosure).
We follow the ABPI’s and JPMA’s distinction between payments unrelated to companies’ research and development (R&D) and payments – or expenditure more broadly – related to R&D. We also compare organisational- and individual-level payments corresponding with the basic recipient categories.
Non-R&D payments
Organisational-level payments
The JPMA and ABPI mandated the disclosure of, respectively, one and four categories of non-R&D payments to HCOs (Table 2). While the JPMA category covered payments to HCOs exclusively, this was the case only for two of the four ABPI categories (donations and grants, and joint working).
Table 2.
Non-R&D payments subject to disclosure under the JPMA Transparency Guidelines and the ABPI Code
| JPMA Transparency Guidelines (2018) | ABPI Code (2019, 2021) | ||||||
|---|---|---|---|---|---|---|---|
| Payments | Recipients | Mode of disclosure | Payment description | Payments | Recipients | Mode of disclosure | Payment description |
| Scholarship donations (B1) | HCOs | Named recipients | Donations to universities and research institutions for research and educational programs (Chapter 5, 3, Publication Subject, B, Academic research support expenses.) | Donations and grants | HCOs | Named recipients or aggregate recipients depending on recipient consent (starting from 2019—only named recipients) | "Medical and educational goods and services which enhance patient care, or benefit the NHS and maintain patient care" (AC2019, Clause 19.1–2). Replaced by donations (“physical items, services or benefits-in-kind)” and grants (“provision of funds”) (AC2021, Clause 1.5, 23.1) |
| General donations (B2) | Donations to non-profit organizations and incorporated foundations to support their operations (Chapter 5, 3, Publication Subject, B, Academic research support expenses.) | ||||||
| Donations to academic and other societies (B3) | Donations to professional medical societies to support their operations (Chapter 5, 3, Publication Subject, B, Academic research support expenses.) | ||||||
| Expenses related to co-sponsored conferences (B4) | Named recipients | Expenses made to host luncheon, seminars and symposiums in conferences hosted by professional medical societies (Chapter 5, 3, Publication Subject, B, Academic research support expenses) | Contributions to costs of events | HCPs and HCOs | Named recipients or aggregate recipients depending on recipient consent (only HCPs) | Organisational-level sponsorship agreements and individual-level registration fees, costs of travel, and accommodation (AC2019, Clause 22.5, 24.2). Replaced by “sponsorship” of events/meetings (organisational-level payments) and “support” of HCPs to attend events/meetings (individual-level payments), both of which can be “professional, promotional, scientific and educational” (AC2021, Clause 1.22–3, 10). Examples include “congresses, conferences, [and] symposia” (AC2021, Clause 1.7) | |
| Expenses for lecture conferences (D1) | HCPs | Aggregate recipients | Expenses to organize conferences and symposiums for drug promotion, such as travel fees, and venue, meal, and reception costs (Chapter 5, 3, Publication Subject, D, Information provision-related expenses.) | ||||
| Lecture fees (C1) | Named recipients | Compensation to physicians for writing endeavours to other healthcare professionals, directly paid to writers (Chapter 5, 3, Publication Subject, C, Manuscript/writing fees, etc) | Fees for service and consultancy | Organisational-level "contracts between companies and institutions, organisations or associations of health professionals" (AC2019, Clause 21) and individual- or organisational-level “fees to consultants …, or to their employers on their behalf, for certain services … such as chairing and speaking at meetings, assistance with training and participation in advisory boards” (AC2019, Clause 23.2) | |||
| Manuscript writing fee/supervising fees (C2) | Compensation to physicians for advice and expertise to promote a particular medical product on the market, directly paid into the pocket of physicians (Chapter 5, 3, Publication Subject, C, Manuscript/writing fees, etc | ||||||
| Consulting/commissioning fees (C3) | Compensation to physicians for speaking, training, and educational endeavours to other healthcare professionals, directly paid to lecturers (Chapter 5, 3, Publication Subject, C, Manuscript/writing fees, etc | ||||||
| Other expenses related to academic research support expenses (B5) | HCOs | Named recipients | No descriptions available | No corresponding ABPI payment categories | |||
| Other Information provision-related expenses (D4) | HCPs | Aggregate recipients | |||||
| Explanation meeting expenses (D2) | Expenses to organize study seminars for drug promotion (Chapter 5, 3, Publication Subject, D, Information provision-related expenses.) | Payments for food and drink below £75 (100$) per head are excluded from disclosure requirements. Higher payments are prohibited (AC2019, Clause 1.10, 22.1–2) | |||||
| Expenses for provision of literature and other products (D3) | Expenses for articles and documents in medicine/pharmacy, and advertising products such as pens and calendars (Chapter 5, 3, Publication Subject, D, Information provision-related expenses.) | “[I]nexpensive notebooks, pens and pencils for use at [company organised scientific] meetings” with value less than £6 per head excluding Value-Added Tax (AC2019, Clause 1.10, 18.3) are not disclosed | |||||
| Other expenses (E) | HCOs and HCPs | Aggregate recipients | Expenses for hospitality, etc. as social courtesy (Chapter 5, 3, Publication Subject, E, Other expenses.) | No corresponding ABPI payment categories | |||
| No corresponding JPMA payment categories | Joint working (replaced by collaborative working) | HCOs | Named recipients or aggregate recipients per company depending on recipient consent (starting from 2019—only named recipients) | "Situations where, for the benefit of patients, one or more pharmaceutical companies and the NHS pool skills, experience and/or resources for the joint development and implementation of patient centred projects and share a commitment to successful delivery" (AC2019, Clause 20). Subsumed under “collaborative working”, covering “initiatives which either enhance patient care or are for the benefit of patients or alternatively benefit the (…) NHS and, as a minimum, maintain patient care” (AC2021, Clause 20.1, 20.4) | |||
| Contracted services (data disclosed starting from 2023) | Members of the public including patients and journalists | Aggregate recipients per company and recipient category | Consultancy “services such as speaking at and chairing meetings, involvement in medical/ scientific studies, clinical trials or training services, writing articles and/or publications, participation at advisory board meetings, and participation in market research where such participation may involve remuneration and/or hospitality” (AC2021, Clause 24.1) | ||||
Unless stated recipient categories from the 2019 ABPI Code are the same in the 2021 version of the Code
HCPs Healthcare professionals, HCOs Healthcare organisations, NHS National Health Service in the UK
The JPMA Category B covered payments for subsidising recipients’ academic activities unrelated to companies’ R&D, allowing recipients to specify the purpose of funding (JTG2018, Chapter 5, 3 – Publication Subject, B Academic research support expenses). Depending on the organisational context, this category was divided into “scholarship donations” to academic institutions, such as universities or hospitals (B1); “general donations” to foundations, and non-governmental organisations, for example, providing ballpoint pens and other items and donations to a university (B2); and “donations to academic or professional societies” made to support their activities, including meetings and lectures (B3). However, if a company event, such as a luncheon or seminar, was held at a conference organised by a professional society, the respective payments would fall under “conference co-sponsoring” (B4).
We link JPMA subcategories B1-B3 to the broader category of “donations, grants and benefits in kind” from the ABPI Code (AC2019, Clause 19.1–2, 24.2), subsequently broken down into “donations” ("physical items, services or benefits in-kind”) and grants (“funds”) (AC2021, Clause 1.5, 23.1). The ABPI category was more comprehensive as it was not limited to education or academic support, also relating to “supporting healthcare” (AC2021, Clause 23.2), and was not restricted to the types of recipients listed by the JPMA. Conversely, we interpret B4 (conference co-sponsoring) as belonging to the ABPI’s broader organisational-level category of “contributions towards the costs of meetings” (AC2019, Clause 22.1, 24.2), most recently renamed as “sponsorship agreements” (AC2021, Clause 1.4, 1.22, 10.11). The ABPI category covered meetings organised by drug companies, HCOs or “other independent organisations” more broadly (AC2021, Clause 1.22). It also included costs of subsistence for participating HCPs (AC2019, Clause 22.1, AC2021, Clause 1.4, 10.1). Contrastingly, the JPMA Code (JC2019, I-2 Medical products Promotional code, Chapter 8 Provision of goods) and the Fair Competition Code (FCC, Criteria for the Operation of Article 4 of the Code, Operational standards regarding examples of restricted offerings) banned payments for travel, attendance or entertainment related to non-promotional (e.g. academic) meetings organised by HCPs.
Unlike the JPMA, the ABPI Code included disclosure of “fees for service and consultancy” to organisations acting on behalf of their employees (AC2019, Clause 23.2, 23.4, 24.2) and contracts with organisations (AC2019, Clause 21, 24.2). In addition, the categories of “joint working” (AC2019, Clause 24.2 and 20), later subsumed under “collaborative working” (AC2021, Clause 20), involved projects combining resources provided by donors and recipients alike and, as such, were not mentioned by the JMPA.
Individual-level payments
The JPMA and ABPI each mandated the disclosure of two categories of non-R&D payments to individual-level recipients (Table 2).
The JPMA “Manuscript/writing fees” (category C) included two specific subcategories – “honorariums for lectures” (C1) and “manuscript writing or supervision” (C2) – and a more general one, “consulting and commissioning” (C3), covering other forms of consultancy, such as advisory boards (JTG, Chapter 5, 3 – Publication Subject, C, manuscript/writing fees, etc.). We consider C1-C3 as matching the ABPI’s individual-level “fees for service and consultancy” (AC2019, Clause 24.2, 23.2–4) or “contracted services” (AC2021, Clause 24), which mentioned “writing articles and/or publications” and “speaking at and chairing meetings” as examples (AC2021, Clause 24.1).
The JPMA also required the disclosure of expenses related to “information provision” (category D) (JTG2018, Chapter 5, 3 – Publication Subject, D, Information provision-related expenses). These payments covered, first, expenses for promotional meetings held by companies, including support for venue, HCPs’ attendance, travel, accommodation, and post-meeting receptions with food and drink (D1). This subcategory overlapped with ABPI’s individual-level “sponsorship” (AC2019, Clause 22.5), subsequently renamed as “support” (AC2021, Clause 1.23), of attendance at meetings/events. The overlap would only be partial because the ABPI category covered not only promotional but also scientific, educational and training meetings/events (AC2019, Clause 22.1).
Second, “explanation meetings” (D2) referred to payments for food and drink provided by company sales representatives during promotional meetings in clinical settings. Under the ABPI Code, these payments would either be excluded from disclosure, if the value of food and drink was below £75 ($100) per head, or prohibited, if the value was above that figure (AC2019, Clause 1.10, 22.1–2).
A final JPMA individual-level subcategory covered costs associated with the provision of materials to assist HCPs with their scientific research (D3). There was no clear corresponding ABPI Code category. Although donations to individuals were prohibited by the ABPI (AC2019, Clause 19.1), it was conceivable that HCPs or ORDMs could claim research materials as “expenses” associated with consultancy services (AC2019, Clause 23.4).
Aggregate disclosure
Following the interpretation of the UK Data Protection Act and European privacy laws by companies following the ABPI Code, all HCPs and ORDMs receiving payments could only be named in drug company disclosures depending on their consent. The value of payments lacking consent to disclosure was aggregated per category (e.g. fees for service and consultancy; support for events participation). However, since late 2021 the ABPI has recommended “legitimate interest” as the preferred lawful basis for disclosure [64]: “a company asserts their transparency commitments over the data rights of the individual HCP” [123], without seeking their explicit agreement before disclosing payments. Nevertheless, companies “must allow individuals to exercise their right to raise objections” [123, 124]. The only exception to this rule were contracted services delivered by members of the public, a category introduced by the newest ABPI Code, and lacking a corresponding JPMA category (AC2021, Clause 24). Unlike payments to HCPs and ORDMs, these payments were aggregated by default (AC2021, Clause 24.6).
In Japan, only the individual recipients of “Manuscript/writing fees” (category C) were named. The JPMA informally advised its member companies to seek consent regarding the publication of their names. However, this was not an explicit rule, and data on the percentage of individuals who refused disclosure was not published. In practice, many companies accepted that HCPs could not receive payments unless they agreed for their names to be disclosed.
Conversely, payments related to “information provision” (category D) were reported as a lump sum per company. Another aggregated category – and lacking a corresponding ABPI Code category – involved expenses for social courtesies (JTG2018, Chapter. 5. E – Other expenses), including expenses for congratulations and condolences, and provision of food and beverages. This was an annual total that was aggregated and did not differentiate costs for either HCPs or HCOs.
R&D payments and expenditure
The JPMA and ABPI disclosure requirements covered payments for clinical trials, such as consultancy fees, which, under the JMPA Transparency guidelines would fall under “clinical trials” and “specific clinical research (AC2019, Clause 23.2; JTG, Chapter 5, 3 – Publication Subject, A, R&D expenses, etc.) (Table 3). In addition, both trade groups’ requirements encompassed non-clinical studies (AC2019, Clause 23.2; JTG, Chapter 5, 3 – Publication Subject, A, R&D expenses, etc.). In practice, JPMA members and companies following the ABPI Code disclosed payments related to both company- and investigator-initiated or sponsored studies [46].
Table 3.
R&D payments subject to disclosure under in the JPMA Transparency Guidelines and the ABPI Code
| JPMA Transparency Guidelines (2018) | ABPI Code (2019, 2021) | ||
|---|---|---|---|
| Research activities | Reported information | Research activities | Reported information |
| Clinical trials, post-marketing clinical trials, adverse drug reaction/infection case reports, and post-marketing surveillance conducted under GCP/GVP/GPSP ordinances | Organisational-level recipients, number of contracts, value of payments (JTG2018, Chapter 5, 3, Publication Subject, A, Research and development expenses, etc) | Clinical trials defined consistent with Directive 2001/20/EC [125] | Total value of all R&D payments per company, without naming recipients or distinguishing organisational and individual recipients |
| Specific clinical research conducted under the Clinical Trials Act [73] | Organisational-level recipients, organisations conducting research, research Identification number recorded in the Japan Registry of Clinical Trials, the name and affiliation of the principal investigator of the research institution, the name of the contract research organization number of contracts, value of funding (JTG2018, Chapter 5, 3, Publication Subject, A, Research and development expenses, etc) | ||
| Research carried out under the Ethical Guidelines for Medical and Health Research involving Human Subjects issued by Ministry of Health, Labour and Welfare [126] | Name of facility to which funds are provided, number of contracts, value of funding (JTG2018, Chapter 5, 3, Publication Subject, A, Research and development expenses, etc) | Prospective non-interventional studies | |
| Non-clinical research, for example basic research, pharmaceutical research | Name of facility receiving funding (JTG2018, Chapter 5, 3, Publication Subject, A, Research and development expenses, etc) | Non-clinical (laboratory) studies following the OECD Principles of Good Laboratory Practice [127] | |
| Other costs | Costs associated with meetings that are not paid to medical institutions, etc. (e.g., venue fees, food and beverage costs, and travel expenses; costs of tests, etc. not paid to medical institutions) (JTG2018, Chapter 5, 3, Publication Subject, A, Research and development expenses, etc) | No equivalent ABPI payment category | - |
GCP Good clinical practice, GVP Good Vigilance Practice, GPSP Good Post-Marketing Surveillance Practices, OECD Organisation for Economic Co-operation and Development, R&D Research and development
Finally, the ABPI Code category of prospective non-interventional studies would fall under the broader JPMA category of medical research involving human subjects, understood as any clinical research complying with the Ministry of Health, Labour and Welfare’s Ethical Guidelines for Medical Research Involving Human Subjects, which had to be followed in the conduct of virtually all medical research, whether interventional, observational, prospective, or retrospective [128].
The ABPI expected aggregate disclosure of R&D payments as a lump sum per company (AC2019, Clause 23.2). Nevertheless, reporting practices suggested that some of the key elements of what is commonly understood to be R&D expenditure [46] were excluded by some companies, for example, honoraria to contract research organisations and payments to study sites [46]. On the other hand, the ABPI allowed companies to decide which costs were considered “subsidiary” to R&D activities, and therefore reported as R&D payments (AC2019, Clause 23.2), or reported as non-R&D payments, and therefore potentially on a name basis.
Contrastingly, the JPMA specified which payments were to be reported as either R&D or non-R&D (categories B – academic research support expenses- and C – lecture, writing or consulting fees), with category A (R&D) covering only research specifically contracted to and conducted by medical institutions (JTG2018, Chapter 5, 3 – Publication Subject, A, Research and development expenses, etc.). The JPMA mandated the disclosure of organisational recipient names and clinical trial numbers. The investigators names were to be disclosed, too, but not their individual fees. Importantly, not only did the JPMA mandate the disclosure of R&D payments (e.g. consultancies paid to clinical trial investigators) but R&D expenditure including “research funds” and “expenses” paid to medical institutions for outsourced research. Minor exceptions from disclosure included loan of equipment and damages paid to clinical trial participants.
Payments explicitly exempted from disclosure
Both industry trade groups listed the same or corresponding payments as excluded from disclosure.
The ABPI and JPMA excluded payments related to drug samples (AC2019, Clause 1.10; JTG2018, Chapter 4 – Target Payments for public disclosure). The ABPI also excluded market research, understood as “collection and analysis of information [on medicines]” in instances “where the company does not know the identity of the participants” (AC2019, Clause 12.2, 23.3). While the JPMA did not use the term “market research”, it would ordinarily fall under subcategories C1-C3 (JTG2018, Chapter 5, 3 – Publication Subject, C, Manuscript/writing fees, etc.). Further, under the ABPI Code companies did not need to report payments regarding “items which are to be passed on to patients and which are part of a formal patient support programme” (AC2019, Clause 18.2), “inexpensive notebooks, pens and pencils for use at those meetings” (AC2019, Clause 18.3) and subsistence below £75 ($100) per head (AC2019, Clause 22.1). These payments would be covered by JPMA’s category D (JTG2018, Chapter 5, 3 – Publication Subject, D, Information provision-related expenses).
Some exemptions were only mentioned by either trade group. While companies following the ABPI Code were not expected to disclose payments regarding over-the-counter medicines and "ordinary course purchases and sales of medicines”, these payments were excluded implicitly by the JPMA by not being mentioned in the Transparency Guidelines (AC2019, Clause 1.10).
The JPMA excluded from disclosure investigational drugs, support for membership fees, advertising fees, and capital for exhibition fees at conferences (JTG2018, Chapter 4 – Target Payments for public disclosure). Although not regulated by the ABPI, some companies reported investigational drugs as R&D payments, while others excluded them from disclosure [46].
Disclosure practices
Availability
In 2018, 122 companies published their payments in Disclosure UK, the ABPI’s online platform for payment disclosure [129]. Among the disclosing companies were 63 of the 67 ABPI members (94%) and 59 non-members disclosing payments voluntarily. Four of the 67 ABPI members (6.0%) – Adaptimmune, Daval International, Sintetica, and Stallergenes Greer – did not submit disclosure reports. As they joined the ABPI towards the end of 2018, it is likely that none of the payments they might have made fell under the disclosure requirements.
In 2018, the JPMA had 72 members, all of which published payment disclosures in accordance with to the JTG2018. However, the total number of disclosing companies was 86 as it included subsidiaries and affiliates. Some of the disclosures were published late, contrary to the JTG2018. As no comprehensive disclosure platform existed, we could not tell whether other companies complied with JTG2018 voluntarily.
An unknown number of pharmaceutical companies did not subscribe to the trade groups’ disclosure codes and therefore did not disclose payments. According to the ABPI [74] and JPMA [130], disclosing companies formed a vast majority, especially of larger ones, but important exceptions included Vertex in the UK and Gilead in Japan.
Accessibility
The ABPI and JPMA leave gathering disclosures to companies. The ABPI collates and integrates company disclosures [131], and subsequently publishes them as Disclosure UK, a centralised database searchable by company and recipient names [132]. Starting from 2023, aggregated payments for services rendered by members of the public will be disclosed on individual company websites, that is, separately from Disclosure UK [59].
JPMA members disclose all payments in disclosure reports published on their websites. The JPMA prohibits companies from requiring registration or entering additional information to access the disclosure data (JTG2018, Chapter 6 – Notes from the perspective of ensuring transparency). However, 28 of the 86 (30.2%) companies required registration to access the 2018 data, apparently breaching the Transparency Guidelines. One possible reason is that companies had insufficient time to comply with the new requirements prohibiting registration, which entered into force in September 21 2018, in time for the data release.
To increase disclosure data accessibility, two non-governmental organisations, Tansa and the Medical Governance Research Institute, collect disclosures made by individual companies in Japan, integrating them into the Money Database [38, 106]. It comprises data from 2016 to 2019 and is searchable by company and recipient names and payment categories [133].
Format
Disclosure UK is available online and downloadable for further analysis as an Excel file. By contrast, in 2018, none of JPMA members published their data in a directly analysable format, instead choosing PDF (portable document format) files or tables embedded in a webpage.
Evidence of underreporting
The PMCPA, the ABPI’s self-regulatory oversight body, publicised three cases of payment underreporting.
The Japanese company Astellas voluntarily admitted to failing to disclose payments to UK nurses manning patient support lines for a support program and to pharmacies in relation to patient enrolment [134]. This was part of a larger case involving a lack of oversight of, and materials produced for, patient support programs, leading to §2 rulings as the PMCPA considered Astellas had brought discredit upon and reduced confidence in the industry.
Following a complaint by an ex-employee, another Japanese company, Daiichi-Sankyo, was also ruled in breach of §2 and publicly reprimanded in 2019 by the PMCPA for grossly under-reporting its payments to at least 132 HCPs sponsored to attend conferences, including 98 who attended a five-day European Society of Cardiology (ESC) congress in Germany in 2018 [135]. In addition, at least 15 and 28 HCPs were sponsored to attend ESC congresses in 2016 and 2017. The company had paid for travel, accommodation, food, and registration fees for these 132 HCPs, but none of this was disclosed – a total of around $629,000 of which $531,600 was for 2018. As even more underreporting could have occurred, the company stated it did not have complete visibility of the total payments that had not been reported.
Finally, an ex-employee revealed that Indivior, a company that has ratified the ABPI Code, did not disclose any 2017 payments, allegedly because it did not know it had agreed to disclose them since becoming an official “non-member” in 2017 [136]. The PMCPA also ruled a breach of §2.
We could not establish whether any JPMA members had been caught underreporting or not. Notably, there was one company reporting the value of zero in category A (R&D), and one in both category C (Lecture/writing/consulting fees) and category D (information provision expenses). It is impossible to ascertain whether, in fact, no payments were made in these categories or whether payments were made but not reported.
Evidence of misreporting
We identified three PMCPA cases involving payments attributed to incorrect individuals in the UK, all reported by the affected HCPs. All these instances were consistent with the lack unique recipient identifiers, as described above.
Merck Sharp & Dohme reported having made payments to an individual that had received none. Apparently, the company had sponsored another individual with the same name [137]. Amgen made a similar mistake, explaining that it had sponsored another individual with the same name working in the same area [138]. Importantly, Merck Sharp & Dohme made an additional incorrect payment disclosure related to the same individual as in the above case, leading to a breach of §2 ruling by the PMCPA as the company had failed to address the underlying problems [139].
Without procedures for submitting complaints or publishing corrections, establishing whether misreporting had been identified in JMPA members’ disclosures was impossible.
Disclosure data
Payments to corresponding payment categories
In 2018, the 122 companies following the ABPI Code reported payments worth almost 4.5 times less than the 86 JPMA members, including subsidiaries (Table 5 in Appendix). Another key difference was the R&D shares, constituting 74.1% of the total in the UK but only 42.7% in Japan, even though the Japanese reporting also included R&D expenditure more broadly, as explained above.
Companies following the ABPI Code reported $632,985,775 (95.1%) using payment categories corresponding with the JPMA categories, including all individual-level non-R&D payments and R&D payments (Table 5 in Appendix). JMPA members reported $2,589,064,594 (86.6%) using payment categories corresponding with the ABPI categories, including $1,018,619,673.61 (39.3%) for individual-level payments and $1,570,444,920 (60.7%) for organisational-level payments.
Payments to aggregated recipients
Companies following the ABPI Code reported payments worth over $144.3 m to named recipients, representing 21.7% of the total, while the respective figures for JPMA members were $1.7bn and 59.9% (Table 4).
In the UK, the shares of payments to named recipients ranged across the payment categories – from all or nearly all (joint working and grants and donations) to none (R&D) (Table 4). Importantly, as R&D made up almost three-quarters of all reported payments, it constituted practically all aggregated payments (97.7%). The remaining aggregated payments were predominantly non-R&D individual-level payments, representing 42.7% of all non-R&D payments to individuals (Table 6 in Appendix).
Table 4.
Drug company R&D and non-R&D payments disclosed under the JPMA Transparency Guidelines and the ABPI Code (2018)
| JPMA payment categories | Value of payments, ($) | Value of payments –merged JPMA categories ( $) | Value of payments – merged JPMA – named recipients ($) | ABPI payment categories | Total value of payments ($) | Value of payments – named recipients ($) |
|---|---|---|---|---|---|---|
| Research and development (A) | 1,276,805,878.8 | 1,276,805,878.8 (100%) | 1,236,732,890.2 (96.9%) | Research and development | 493,470,880.7 (100%) | 0.0 (0%) |
| Scholarship donations (B1) | 167,048,419.4 | 217,575,581.0 (100%) | 217,575,581.0 (100%) | Donations and Grants | 35,118,661.8 (100%) | 35,082,449.0 (99.9%)1 |
| General donations (B2) | 34,517,905.3 | |||||
| Donations to academic and other societies (B3) | 16,009,256.3 | |||||
| Expenses related to co-sponsored conferences (B4) | 76,063,460.5 | 845,194,783.3 (100%) | 76,063,460.5 (10.0%) | Contributions to costs of events | 55,207,855.0 (100%) | 48,774,249.8 (88.3%) |
| Expenses for lecture conferences (D1) | 769,131,322.9 | |||||
| Lecture fees (C1) | 211,419,118.7 | 248,042,904.90 (100%) | 248,042,904.90 (100%) | Fees for service and consultancy | 74,931,974.0 (100%) | 53,459,921.3 (71.3%) |
| Manuscript writing fee/supervising fees (C2) | 8,973,254.3 | |||||
| Consulting/commissioning fees (C3) | 28,934,542.5 | |||||
| Other (C)2 | 161,435.2 | |||||
| Other expenses related to academic research support expenses (B5) | 11,203,780.9 | 12,621,124.4 (100%) | 11,203,780.9 (88.8%) | No corresponding ABPI payment categories | ||
| Other Information provision-related expenses (D4) | 1,417,343.5 | |||||
| Explanation meeting expenses (D2) | 275,045,094.6 | 275,045,094.6 (100%) | 0.0 (0%) | Excluded from or prohibited by disclosure requirements | ||
| Expenses for provision of literature and other products (D3) | 69,080,312.8 | 69,080,312.8 (100%) | 0.0 (0%) | |||
| Other expenses (E) | 42,814,875.4 | 42,814,875.4 (100%) | 0.0 (0%) | No corresponding ABPI payment categories | ||
| No corresponding JPMA payment categories | Joint working | 7,003,799.0 (100%) | 7,003,799.0 (100%) | |||
| Total | 2,988,626,001.0 (100%) | 2,988,626,001.0 (100%) | 1,791,064,063.3 (59.9%) | Total | 665,733,170.5 (100%) | 144,320,419.0 (21.7%) |
1 Until 2019 ABPI members could consent healthcare organisations before disclosing their payments but few companies chose to do so. Consenting healthcare organisations is currently prohibited
2 Originally, Category C did not have an "Other" item, but one company recorded the amount as "Other”
In Japan, subcategory D1 (promotional meeting expenses) attracted the largest share (64.2%) of all aggregated payments. Of the 86 companies, 46 (53.5%) apparently breached the Transparency Guidelines by aggregating some payments in category A (R&D), with the aggregated amount representing 3.1% of the total payment value (Table 4). In contrast to the UK, aggregated non-R&D payments constituted 96.6% of all aggregated payments.
The values of aggregated payments per company ranged from $0 to over $48 m in the UK and from $0 to $96 m in Japan (Tables 7 and 8 in Appendix). In the UK, 71 of the 122 companies (58.2%) reported at least 50% of their payments in aggregate, but 21 companies (17.2%) reported more than 90% (Table 9 in Appendix). The company levels of aggregated payments were lower in Japan, with 20 of the 86 companies (23.3%) reporting over 50% in aggregate. The group of companies with very high levels of aggregated payments (over 90%) was not found in Japan, with only 2 (2.3%) reporting at least 75% (Table 10 in Appendix).
The UK and Japanese shares of aggregated R&D vs non-R&D payments reported by companies were highly contrasting. Only 8 of the 122 companies following the ABPI Code (6.6%) had over 50% of all their payments constituted by aggregated non-R&D payments (Table 9 in Appendix). However, for R&D payments the corresponding number of companies was 56, representing 45.9% of all companies following the ABPI Code.
In Japan, 66 of the 86 companies (76.7%) reported at least 50% of their non-R&D payments in aggregate (categories D information provision expenses – and E – Other expenses). However, for R&D payments the corresponding number of companies was 3, which constituted 3.5% of all the 86 reporting companies (category A – R&D) (Table 8 in Appendix).
In the UK, aggregated R&D payments were considerably larger than aggregated non-R&D payments, with the value of the third quartile over 11 times larger ($145,195.9 vs $1,615,243.1 – see Table 7 in Appendix). In Japan, the opposite was true; aggregated non-R&D payments were much higher than aggregated R&D payments, with the value of the third quartile almost 60 times higher ($16,872,972.9 vs $288,770.5 – see Table 8 in Appendix).
Discussion
In its current form, the self-regulation of payment disclosure has operated in Japan since 2011 and in the UK since 2015. In Japan, disclosure research has identified widespread FCOIs among clinical trialists [72, 140, 141] and authors of clinical practice guidelines [43, 142, 143], while in the UK – across hospitals [100], commissioning [144], expert advisory [145], and policymaking bodies [32]. However, given the overall volume of payments reported annually in each country – in 2018, close to $3bn in Japan and over $0.6bn in the UK – a significant share of FCOIs affecting healthcare actors is likely to go unnoticed. Our comparative case study of three dimensions of transparency – disclosure rules, practices, and data – sheds light into why this may be the case and how the transparency of payment disclosures could be improved.
The ABPI and JPMA disclosure rules covered a broad range of payment recipients. The ABPI mandated a greater scope of non-R&D payment disclosure. On the one hand, almost all JPMA non-R&D payments were encompassed by the ABPI categories. On the other hand, the ABPI categories often lacked corresponding JPMA categories. Conversely, the JPMA required the disclosure of not only R&D payments but also R&D expenditure. It also mandated disclosure without referring to recipient consent. Shared weaknesses included some payments being exempted from named disclosure, while others – not disclosed altogether.
Companies following the ABPI Code had more transparent disclosure practices. There was certainty about how many disclosures were available; they were more accessible and had an analysable format. The ABPI complaints mechanism provided some insights into possible underreporting and misreporting.
However, JPMA members generated more transparent disclosure data, with the overall share of payments to named recipients almost three times higher, and considerably higher company-level shares compared to the UK.
Implications for self-regulation and public regulation
We use our findings to evaluate claims regarding key advantages self-regulation as the industry’s preferred approach to governing drug promotion, stakeholder interactions and communication, and providing information on medicines [49, 104]. We also compare self-regulation with legally mandated transparency provisions identified in the US and eleven European countries [24].
Corresponding with IFPMA’s declared emphasis on increasing transparency [49, 51, 93], the JPMA and ABPI presented transparency as disclosure’s primary aim. Nevertheless, it was unexplained how transparency was to be achieved; for example, how disclosures were expected to be used, by whom, and to what purpose [20]. Limited attention given to “transparency relations” [146] is also common in European public regulation, as evidenced by a lack of granular and easily interpretable disclosures in many countries [24]. In comparison, public regulation does eliminate non-transparency resulting from the aggregation of certain payments by default [24]. Yet, the evidence from Japan (and Australia [36]) demonstrates that mandating disclosure may not require new sunshine legislation or clarifications of data privacy laws by public authorities, as it has been the case in Europe [18, 21, 24], and can be achieved within self-regulation.
In Japan, individual-level disclosure has arguably increased the industry’s accountability by revealing FCOIs underreported by KOLs [44, 99] and clinical triallists [72, 147] as well as examples of poor FCOI management [142, 143]. Contrastingly, the ABPI only “encourages” companies to observe its new non-mandatory guidance on “legitimate interests”, promising to “further increase the transparency” of non-R&D payments, but without committing explicitly to full disclosure, at least in the short run [64]. Yet, the comparison with Japan demonstrates that, contrary to the European industry’s emphasis on protecting commercial secrecy [46], it is possible to disclose at least organisational-level R&D payments without jeopardising companies’ R&D investments. It also shows the need for rebalancing the UK transparency debate – by focusing on encouraging the full disclosure of a relatively small share of non-R&D payments to individual HCPs [64] it has neglected systemic, non-individualised, and non-itemised disclosure of R&D payments to researchers and research institutions with key roles in drug development and marketing [46].
Reflecting IFPMA’s work on “synchronisation” of national codes [51], important similarities in the ABPI and JPMA disclosure rules were demonstrated by overlapping disclosure goals, recipient and payment categories. Nevertheless, as each trade group often referred to unique industry-supported activities, the extent of overlap was uncertain. Therefore, although the corresponding payment categories attracted around 90% of payments reported in each country, more granular descriptions of disclosures would likely produce lower figures. Furthermore, the emphasis of disclosure requirements differed, with the ABPI prioritising disclosure of non-R&D payments, while the JPMA – R&D payments and expenditure. Overall, while detailed internationally shared standards are a key advantage of self-regulation over national legislation in the EFPIA ecosystem [10, 22, 45], our findings suggest that the same cannot be said about the current state of self-regulation globally.
The assertion that self-regulation is more comprehensive than public regulation [10, 49, 51, 93, 148] received mixed support. The JPMA and ABPI disclosure rules covered prescribers as well as professions and roles shaping prescription through policy decisions [18, 31]. In this respect, self-regulation exceeded the scope of the US Sunshine Act, covering only physicians, nurses, and teaching hospitals [24], as well as disclosure legislation in several European countries, such as Estonia [149], Hungary [150] or Latvia [151], focusing on personnel involved in medicine prescription, supply, or distribution. Further, while the JPMA covered life science researchers, only Danish disclosure legislation included disclosure of payments to select researchers, i.e., bioanalysts and pharmacoeconomists [152].
Nevertheless, self-regulation missed some key areas of concern [153]. Unlike in some countries with public regulation, the ABPI and JPMA did not cover payments related to over-the-counter medicines [21], medical devices, or veterinary products [24, 36]. Also excluded from disclosure were organisations targeted by the industry’s lobbying [32, 103, 154–156], which are sometimes covered by public regulation, such as the Ministry of Health (Portugal) [157] and media organisations (France) [158].
The scope of payments covered by the ABPI and JPMA was broader than in some European countries with public regulation, such as Greece [159] and Hungary [150], concentrating on event delivery or participation. Notably, R&D payment disclosure mandated by the JPMA was significantly closer to the most comprehensive French and US disclosure requirements than self-regulation in Europe (R&D payments always aggregated) or Australia (R&D payments not disclosed) [18, 36, 46]. Contrastingly, payments for food and beverage were covered by public regulation in France and the US [18], but excluded from disclosure in the UK below the threshold of $100 (£75) and disclosed in the aggregate in Japan. This is an important gap in disclosure as data disclosed under the US Sunshine Act demonstrates that payments for food and beverage are widespread [160–162] and associated with increased prescription of promoted drugs [163, 164]. Finally, US disclosure requirements also include ownership or investment interests (e.g. royalties, licenses) held by physicians or their immediate family members – about 12% of reported payments amounts in the USA – but these are not covered by the ABPI and JPMA [165].
There was mixed evidence for the claim that self-regulation is more detailed than public regulation [51]. The disclosure categories used by the ABPI and JPMA were broad and the reporting did not distinguish their constituent recipients or drug company activities, making them difficult to interpret [166]. However, only two countries with public regulation – the US and France – use comprehensive sets of specific payment categories [18]; others either disclose few specific payments (Latvia [151], Lithuania [167, 168]) or use broad categories such as “professional affiliation” (Denmark [169]) or a “value, good or right” (Portugal [157]), without itemising them in the disclosure databases [24]. In addition, consistent with earlier research [30, 31], we identified examples of ambiguous or unstandardised reporting, potentially preventable by more detailed disclosure guidelines.
We found problematic the claim that self-regulation often involves higher levels of compliance than public regulation [49, 93, 170]. The cases of ABPI members not disclosing payments demonstrate that companies may start or stop following self-regulation at any point, creating potential gaps and uncertainties in reporting. Further, while the proponents of self-regulation would expect effective scrutiny of companies’ conduct from HCPs [93], the fact that only two HCPs complained about payment misreporting suggests, more than anything, that relatively few recipients check their disclosure records in sufficient detail, potentially making the PMCPA complaints-based system insufficient to reveal this practice systematically. This interpretation is supported by frequent reporting inaccuracies associated with the absence of recipient identifiers and categories [24, 31]. Similarly, the cases of underreporting considered by the PMCPA indicate that only current or ex-employees – and not, for example, competitor companies – may have sufficient knowledge of relevant internal practices, therefore limiting the pool of potential complainants [48, 92, 93, 171].
Key examples of noncompliance in Japan included delayed disclosure publication, some companies creating barriers to disclosure access, and reporting R&D payments in the aggregate [44, 106, 143]. However, consistent with earlier concerns about difficulties in measuring companies’ compliance with the JPMA standards [91], revealing underreporting and misreporting was practically impossible without publicly available disclosure standards (“methodological notes”) used by individual companies and a complaints mechanism overseen by the trade group. Just like in the UK, misreporting might be caused by ambiguities in disclosure data. Also reflecting earlier research [54, 99], underreporting could result from the relatively narrow JPMA payment categories, encompassing fewer industry activities than the ABPI categories. Notably, two of the three UK cases of underreporting involved Japanese companies. The PMCPA characterised one of them, Astellas, as displaying “multiple organisational and cultural failings” and a corporate culture that prioritised “the bottom line” over compliance obligations and ethical norms [48].
Contrastingly, the only available evaluation of the US Open Payments database that we were aware of demonstrated little evidence of non-compliance with the reporting standards [172], suggesting the superiority of legally binding requirements associated with potential penalties over self-regulatory standards [21, 24, 46].
Limitations
Our study has important limitations. As pharmaceutical industry self-regulation has not been mapped in Asia [51], we might have missed important comparators for the UK as the strongest case of self-regulation in Europe [24]. Further, because we compared only two cases, any strengths and weaknesses identified were only relative to the other case. Therefore, considering another comparator might have revealed disclosure aims, recipients or payments missing from disclosure. In addition, as the recipient and payment categories were unique, and often described only in general terms, we could only ascertain their “correspondence” but not “equivalence”. Consequently, the comparisons across the payment categories require cautious interpretation.
We analysed similarities and differences between the two cases descriptively, without explaining the underlying mechanisms. For example, we did not consider the company and trade group and cultures more broadly, which may be an important factor behind the varying disclosure practices [26, 51]. One key aspect of such cultures worth in-depth investigation concerns the mechanisms for collecting and checking the quality and accuracy of disclosure data.
Future research on varieties of self-regulation might employ mixed methods to examine the two-way relationship between self-regulation at the global, trade group, and company levels, as well as other transparency provisions and the “medical economy” more broadly [48, 66, 91]. Of particular importance would be to trace the development of the different forms of regulation over time [104].
Conclusions and policy recommendations
Our study indicates that measuring the transparency of payment disclosure associated with self-regulation should triangulate disclosure rules, practices, and data, given the possible inconsistences and important relationships between these three dimensions.
Overall, our comparative analysis of payment disclosure in the UK and Japan offered limited evidence to support key claims concerning the strengths of self-regulation. Despite its important advantages, self-regulation seemed inferior to public regulation, which was consistent with findings from Europe and Australia [24, 36, 46].
Our comparison highlights the priority areas for improvement for both the ABPI and JPMA.
Eliminating exemptions from disclosure.
Removing aggregate disclosure by default.
Introducing more granular disclosure of payments and recipients.
Increasing the richness of disclosure data by expanding recipient characteristics, following the example of the Australian pharmaceutical industry trade group [173].
Introducing additional standards to prevent underreporting (mandatory declarations that no payments were made) and misreporting (mandatory recipient identifiers).
Creating stronger compliance monitoring of disclosure practices and data [51], including regular independent audits to complement the reactive, complaints-based systems [48, 63, 66, 153].
Introducing public rewards and sanctions specific to disclosure standards, e.g. highlighting top performers and underperformers across different disclosure practices and data [148].
We identified several priority areas for the JPMA.
Extending the list of non-R&D payment categories (e.g. a broader range of consultancy-related payments, following the example of the ABPI Code) to enhance the scope of disclosure.
Mandating companies to publish their internal disclosure standards using the example of “methodological notes” introduced by the EFPIA and ABPI.
Creating a searchable and downloadable payments database for all companies subscribing to the JPMA Transparency Guidelines.
Making the Transparency Guidelines mandatory for JPMA members, including sanctions for non-compliance.
Increasing the transparency of the complaints process by establishing an arms-length body investigating and publishing details of alleged breaches of standards, potentially modelled on the PMCPA.
Finally, we propose the following priorities for the ABPI.
Making the receipt of payments conditional on agreeing for their publication, which seems to be the established practice followed by the JPMA members.
Expanding and standardising the scope of internal disclosure standards described in company methodological notes [46, 51].
Our findings reinforce earlier calls for replacing industry self-regulation with sunshine legislation made in the UK and Japan [38, 65] and correspond with the UK government’s ongoing work on expanding or replacing Disclosure UK with legislation [174]. Nevertheless, as such, public regulation not a panacea. Any legislation should be premised on a comprehensive international evaluation of disclosure rules, practices and data [24, 46, 55, 175], including their unintended consequences [23, 176, 177]. One structured way of embedding the perspectives of HCPs, HCOs, patients, and members of the public into the policy proposals would involve developing a “theory of change” [178] capturing multifaceted relationships between disclosure, transparency, as well as the economic power of the industry and the state’s regulatory power [18–20]. In so doing, it is important to consider a range of potential goals of transparency, such as increasing the industry’s accountability to the public revealing potential FCOIs [21, 24, 26, 55, 106] and protecting the integrity of healthcare research or policymaking [20, 146, 179].
Given the evidence of the limited impact of sunshine legislation on reducing FCOIs or encouraging their understanding by the public [23], any new legislation should be complemented by educational campaigns enhancing the understanding and scrutiny of disclosure rules, practices and data [180]. Any sunshine legislation should be integrated – including via shared databases – with FCOI evaluation undertaken by HCOs such as hospitals, commissioning bodies, regulators, and professional organisations. No less important are solutions addressing the underlying problem of financial dependency of healthcare actors on drug company funding [18, 117, 181]. Some of the available options include FCOI management policies such as limiting or banning certain forms of financial relationships [18, 20, 26, 182].
Acknowledgements
The authors would like to thank Erika Yamashita and Ayumu Kawamoto for their constructive opinions on this study.
Abbreviations
- ABPI
Association of the British Pharmaceutical industry
- EFPIA
European Federation of Pharmaceutical Manufacturers and Associations
- Fair Competition Code
FCC in Japan
- GCP
Good clinical practice
- GVP
Good Vigilance Practice
- GPSP
Good Post-Marketing Surveillance Practices
- HCO
Healthcare organisation
- HCP
Healthcare professional
- IFPMA
International Federation of Pharmaceutical Manufacturers and Associations
- JPMA
Japan Pharmaceutical Manufacturers Association
- OECD
Organisation for Economic Co-operation and Development
- R&D
Research and development
Appendix
Table 5.
Distribution of payments across corresponding and non-corresponding JPMA and ABPI payment categories (2018)
| Payment categories | Organisational-level payments ($) | Individual-level payments ($) | Unclear if organisational- or individual-level payments ($) | Total ($) | |||
|---|---|---|---|---|---|---|---|
| ABPI payment categories with corresponding JPMA categories | Donations and grants | 35,118,661.84 | Fee for Service and consultancy | 49,188,377.90 | Research and development | 493,470,880.71 | 632,985,775.44 (95.1%) |
| Contribution to costs of events | 39,095,432.05 | Contribution to costs of Events | 16,112,422.94 | ||||
| Total | 74,214,093.89 | Total | 65,300,800.84 | Total | 493,470,880.71 | ||
| ABPI payment categories without corresponding JPMA categories | Fee for service and consultancy | 25,743,596.09 | 32,747,395.07 (4.9%) | ||||
| Joint working | 7,003,798.98 | ||||||
| Total | 32,747,395.07 | ||||||
| Total – ABPI payment categories | 665,733,170.53 (100.0%) | ||||||
| JPMA payment categories with corresponding ABPI categories | Scholarship donations (B1) | 167,048,419.36 | Lecture fees (C1) | 211,419,118.67 | 2,589,064,593.79 (86.6%) | ||
| General donations (B2) | 34,517,905.28 | Manuscript writing fee/supervising fees (C2) | 8,973,254.30 | ||||
| Donations to academic and other societies (B3) | 16,009,256.31 | Consulting/commissioning fees (C3), | 28,934,543.36 | ||||
| Expenses related to co-sponsored conferences (B4) | 76,063,460.47 | Others (C) | 161,435.24 | ||||
| Research and development (A) | 1,276,805,878.76 | Expenses for lecture conferences (D1) | 769,131,322.85 | ||||
| Total | 1,570,444,920.18 | Total | 1,018,619,673.61 | ||||
| JPMA payment categories without corresponding ABPI categories | Other expenses related to academic research support expenses (B5) | 11,203,780.92 | Explanation meeting expenses (D2) | 275,045,094.56 | Other expenses (E) | 42,814,875.43 | 399,561,407.22 (13.4%) |
| Expenses for provision of literature and other products (D3) | 69,080,312.81 | ||||||
| Other Information provision-related expenses (D4) | 1,417,343.50 | ||||||
| Total | 11,203,780.92 | Total | 345,542,750.87 | Total | 42,814,875.43 | ||
| Total – JPMA payment categories | 2,988,626,001.01 (100.0%) | ||||||
Table 6.
Payments to individual- and organisational-level recipients under the JPMA Transparency Guidelines and the ABPI Code (2018)
| Comparative JPMA-ABPI recipient categories | JPMA Transparency Guidelines | ABPI Code | |||||
|---|---|---|---|---|---|---|---|
| Recipients | Recipient categories | JPMA recipient categories | Total value of payments ($) | Value of payments to named recipients ($) | ABPI recipient categories | Total value of payments ($) | Value of payments to named recipients ($) |
| Individual level | Healthcare professionals | Medical personnel | 1,364,162,424.48 (100%) | 249,488,350.76 (18.3%) | Healthcare professionals | 65,300,800.84 (100.0%) | 37,406,101.85 (57.3%) |
| Other healthcare personnel | Persons involved in medical operations | Other relevant decision makers | |||||
| Non-healthcare professions and roles | Life science researchers | No equivalent category | |||||
| Organisational level | Healthcare organisations | Healthcare organisations | 1,581,648,701.10 (100%) | 1,541,575,712.50 (97.5%) | Healthcare organisations | 106,961,488.98 (100.0%) | 106,914,317.19 (99.9%) |
| Unclear if individual or organisational level | No relevant JPMA recipient categories | 42,814,875.43 (100%) | 0 (0%) | Recipients of research and development payments- | 493,470,880.71 (100.0%) | 0 (0%) | |
| Total | 2,988,626,001.01 (100%), | 1,791,064,063.27 (59.9%) | 665,733,170.5 (100.0%) | 144,320,419.0 (21.7%) | |||
Table 7.
Descriptive statistics for aggregated company-level payments reported by companies following the ABPI Code (2018)
| Payment categories | Minimum ($) | Median (IQR) ($) | Maximum ($) |
|---|---|---|---|
| All aggregated payments | |||
| Value | 0.0 | 164,675.9 (10,975.9—1,674,936.9) | 48,451,656.3 |
| Share of all payments | 0.0% | 60.2% (19.2%—80.4%) | 100.0% |
| Aggregated non-R&D payments | |||
| Value | 0.0 | 35,417.6 (1,700.9—145,195.9) | 1,468,816.7 |
| Share of all payments | 0.0% | 5.5% (1.1%—16.4%) | 100.0% |
| Share of all aggregated payments | 0.0% | 20.1% (3.8%—100%) | 100% |
| Aggregated non-R&D payments to HCPs | |||
| Value | 0.0 | 34,623.5 (1,700.9—145,195.9) | 1,468,816.7 |
| Share of payments to HCPs | 0.0% | 49.7% (14.7%—77.1%) | 100.0% |
| Share of aggregated non-R&D payments | 0.0% | 100.0% (100.0%—100.0%) | 100.0% |
| R&D payments | |||
| Value | 0.0 | 92,443.7 (0—1,615,243.1) | 47,430,167.7 |
| Share of all payments | 0.0% | 43.6% (0 – 73.3%) | 100.0% |
| Share of all aggregated payments | 0.0% | 79.9% (0%—96.2%) | 100.0% |
Aggregated payments Payments without named recipients, HCPs Healthcare professionals, HCOs Healthcare organisations, R&D Research and development
Table 8.
Descriptive statistics for aggregated company-level payments reported by JPMA member companies (2018)
| Minimum | Median (IQR) ($) | Maximum | |
|---|---|---|---|
| All aggregated payments (category A, D, and E) | |||
| Value | 0.0 | 6,346,032.8 (1,215,424.8– 17,573,059.3) | 96,159,420.3 |
| Share of all payments | 0.0% | 38.1% (29.1% – 49.1%) | 83.1% |
| Aggregated non-R&D payments (category D and E) | |||
| Value | 0.0 | 6,325,825.0 (1,001,734.5– 16,872,972.9) | 96,096,014.5 |
| Share of all payments | 0% | 36.5% (25.9%– 46.0%) | 83.1% |
| Share of all aggregated payments | 0% | 99.9% (94.0%– 100%) | 100% |
| Aggregated non-R&D payments to HCPs (category D) | |||
| Value | 0.0 | 5,949,062.3 (879,128.7– 15,754,217.5) | 94,574,275.4 |
| Share of all payments | 0% | 34.5% (23.8%– 44.2%) | 80.9% |
| Share of all aggregated non-R&D payments | 0% | 94.9% (91.2%– 97.9%) | 100% |
| Aggregated non-R&D payments (category E) | |||
| Value | 0.0 | 221,831.0 (24,419.9– 683,182.9) | 3,456,984.4 |
| Share of all payments | 0.0% | 1.4% (0.4% – 2.6%) | 23.8% |
| Share of all aggregated non-R&D payments | 0.0% | 4.5% (2.0% – 8.6%) | 100% |
| Aggregated R&D payments (category A) | |||
| Value | 0.0 | 978.8 (0.0– 288,770.5) | 7,237,318.8 |
| Share of all payments | 0.0% | 0.0% (0.0%– 1.5%) | 41.4% |
| Share of all aggregated payments | 0.0% | 0.1% (0.0%– 5.2%) | 92.9% |
Category A of R&D is further subdivided into separate detailed categories of public disclosures. Depending on the company, there was sometimes a mix of categories that were published with named information and categories that were published in aggregate. This table aggregates the latter
Category D is expenses related to information provision
Category E is defined as other non-R&D expenses that cannot be classified in any other category. It is described as including costs for hospitality as a social ritual
Aggregated payments Payments without named recipients, HCPs Healthcare professionals, HCOs Healthcare organisations, R&D Research and development
Table 9.
Aggregated payments – company-level data reported by companies following the ABPI Code (2018)
| Company name recorded in Disclosure UK | Standardised international name recorded on drug company website | Value of aggregated payments, ($) | Aggregated payments—share of all payments, (%) | Value of aggregated non-R&D payments to HCOs and HCPs ($) | Aggregated non-R&D payments—share of all payments | Aggregated non-R&D payments—share of aggregated payments | Value of aggregated HCPs payments ($) | Aggregated HCPs payments – share of payments to HCPs | Aggregated HCPs payments – share of aggregated non-R&D payments to HCPs and HCOs | Value of R&D payments ($) | R&D—share of all payments | R&D—share of all undisclosed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Martindale Pharma | Martindale Pharma | 10,224.80 | 100.00% | 10,224.80 | 100.00% | 100.00% | 10,224.80 | 100.00% | 100.00% | 0.00% | 0.00% | |
| Avexis EU Ltd | Avexis EU | 5,121.90 | 100.00% | 5,121.90 | 100.00% | 100.00% | 5,121.90 | 100.00% | 100.00% | 0.00% | 0.00% | |
| Clinuvel (UK) Ltd | Clinuvel | 1,367.10 | 100.00% | 0.00% | 0.00% | N/A | N/A | 1,367.10 | 100.00% | 100.00% | ||
| Biotest UK | Biotest | 9,585,976.60 | 99.60% | 28,135.10 | 0.30% | 0.30% | 28,135.10 | 77.00% | 100.00% | 9,557,841.50 | 99.30% | 99.70% |
| Clovis Oncology UK Ltd | Clovis Oncology | 975,763.80 | 99.40% | 225,033.50 | 22.90% | 23.10% | 225,033.50 | 97.40% | 100.00% | 750,730 | 76.50% | 76.90% |
| Bluebird Bio UK Limited | Bluebird Bio | 1,046,010.10 | 98.30% | 0.00% | 0.00% | 0.00% | N/A | 1,046,010 | 98.30% | 100.00% | ||
| Sunovion Pharmaceuticals Europe Ltd | Sunovion Pharmaceuticals Europe | 997,565.00 | 97.80% | 348,327.50 | 34.20% | 34.90% | 348,327.50 | 100.00% | 100.00% | 649,237 | 63.70% | 65.10% |
| Allergan Ltd | Allergan | 36,889,921.10 | 97.40% | 935,807.80 | 2.50% | 2.50% | 935,807.80 | 70.30% | 100.00% | 35,954,113.30 | 95.00% | 97.50% |
| Eisai Ltd | Eisai | 5,838,665.00 | 96.10% | 176,061.60 | 2.90% | 3.00% | 176,061.60 | 60.90% | 100.00% | 5,662,603.30 | 93.20% | 97.00% |
| Indivior UK Ltd | Indivior | 865,474.10 | 96.10% | 74,798.90 | 8.30% | 8.60% | 74,798.90 | 80.20% | 100.00% | 790,675 | 87.80% | 91.40% |
| Tesaro UK Ltd | Tesaro | 916,484.40 | 95.70% | 136,050.50 | 14.20% | 14.80% | 136,050.50 | 100.00% | 100.00% | 780,434 | 81.50% | 85.20% |
| Otsuka Pharmaceuticals UK Ltd | Otsuka Pharmaceuticals | 2,223,454.80 | 95.20% | 186,453.80 | 8.00% | 8.40% | 186,453.80 | 80.60% | 100.00% | 2,037,001.00 | 87.20% | 91.60% |
| AstraZeneca | AstraZeneca | 64,712,032.10 | 94.60% | 1,364,300.20 | 2.00% | 2.10% | 1,364,300.20 | 47.10% | 100.00% | 63,347,731.90 | 92.60% | 97.90% |
| Alcon UK Ltd | Alcon | 1,300,368.30 | 94.50% | 498,527.60 | 36.20% | 38.30% | 498,527.60 | 100.00% | 100.00% | 801,840.70 | 58.30% | 61.70% |
| Bristol-Myers Squibb Pharmaceuticals Ltd | Bristol-Myers Squibb | 43,015,683.90 | 94.20% | 682,542.40 | 1.50% | 1.60% | 682,542.40 | 46.70% | 100.00% | 42,333,141.50 | 92.70% | 98.40% |
| Servier Laboratories Ltd | Servier Laboratories | 9,416,038.10 | 94.10% | 548,154.30 | 5.50% | 5.80% | 548,154.30 | 73.00% | 100.00% | 8,867,883.80 | 88.60% | 94.20% |
| Celgene Ltd | Celgene | 20,684,739 | 92.80% | 107,653 | 0.50% | 0.50% | 107,653 | 12.90% | 100.00% | 20,577,086 | 92.40% | 99.50% |
| Diurnal | Diurnal | 2,166,212 | 92.70% | 0.00% | 0.00% | 0.00% | N/A | 2,166,212 | 92.70% | 100.00% | ||
| GW Pharmaceuticals plc | GW Pharmaceuticals | 1,143,354.20 | 91.80% | 7,797.40 | 0.60% | 0.70% | 7,797.40 | 14.10% | 100.00% | 1,135,556.80 | 91.20% | 99.30% |
| Akcea Therapeutics UK | Akcea Therapeutics | 266,954.30 | 90.90% | 34,389.60 | 11.70% | 12.90% | 34,389.60 | 56.20% | 100.00% | 232,565 | 79.20% | 87.10% |
| Syner-med | Syner-Med | 57,872.30 | 90.10% | 0.00% | 0.00% | N/A | N/A | 57,872 | 90.10% | 100.00% | ||
| Takeda UK Ltd | Takeda | 12,866,306.90 | 88.40% | 481,291.60 | 3.30% | 3.70% | 481,291.60 | 100.00% | 100.00% | 12,385,015.30 | 85.10% | 96.30% |
| Octapharma Ltd | Octapharma | 706,412.60 | 87.70% | 59,525.40 | 7.40% | 8.40% | 59,525.40 | 72.90% | 100.00% | 646,887 | 80.30% | 91.60% |
| Roche Products Limited | Roche | 32,860,281.40 | 87.60% | 1,302,844.20 | 3.50% | 4.00% | 1,302,844.20 | 43.40% | 100.00% | 31,557,437.10 | 84.20% | 96.00% |
| Merz Pharma UK Ltd | Merz Pharma | 3,809,689.40 | 85.70% | 514,001.80 | 11.60% | 13.50% | 514,001.80 | 55.30% | 100.00% | 3,295,687.60 | 74.10% | 86.50% |
| Janssen-Cilag Ltd | Janssen Pharmaceuticals | 28,864,738.90 | 84.40% | 1,707,603.50 | 5.00% | 5.90% | 1,707,603.50 | 55.20% | 100.00% | 27,157,135.40 | 79.40% | 94.10% |
| Biogen Idec Ltd | Biogen | 17,022,394.10 | 83.90% | 534,532.30 | 2.60% | 3.10% | 534,532.30 | 72.10% | 100.00% | 16,487,861.80 | 81.30% | 96.90% |
| Shionogi Limited | Shionogi | 223,033.00 | 83.70% | 44,076.60 | 16.50% | 19.80% | 44,076.60 | 100.00% | 100.00% | 178,956 | 67.20% | 80.20% |
| Merck Sharp & Dohme Ltd | Merck Sharp & Dohme | 27,875,141.40 | 83.30% | 78,327.80 | 0.20% | 0.30% | 78,327.80 | 3.70% | 100.00% | 27,796,813.70 | 83.10% | 99.70% |
| Shire Pharmaceuticals Ltd | Shire Pharmaceuticals | 3,533,626.60 | 80.60% | 595,532.80 | 13.60% | 16.90% | 595,532.80 | 100.00% | 100.00% | 2,938,093.80 | 67.10% | 83.10% |
| Pharmasure Ltd | Pharmasure | 188,530.60 | 80.30% | 71,118.00 | 30.30% | 37.70% | 71,118.00 | 100.00% | 100.00% | 117,413 | 50.00% | 62.30% |
| Pharma Mar SA | PharmaMar | 123,647.10 | 80.30% | 9,384.20 | 6.10% | 7.60% | 9,384.20 | 40.70% | 100.00% | 114,263 | 74.20% | 92.40% |
| Amicus Therapeutics | Amicus Therapeutics | 213,547.90 | 80.10% | 105,430.20 | 39.50% | 49.40% | 105,430.20 | 70.30% | 100.00% | 108,118 | 40.50% | 50.60% |
| PTC Therapeutics Limited | PTC Therapeutics | 821,220.80 | 79.70% | 19,591.00 | 1.90% | 2.40% | 19,591.00 | 22.10% | 100.00% | 801,630 | 77.80% | 97.60% |
| LEO Laboratories Ltd | LEO Pharma | 2,138,828.80 | 79.00% | 429,337.00 | 15.90% | 20.10% | 429,337.00 | 80.80% | 100.00% | 1,709,491.80 | 63.20% | 79.90% |
| Astellas Pharma Ltd | Astellas Pharma | 2,435,505.40 | 78.70% | 94,330.90 | 3.00% | 3.90% | 94,330.90 | 44.50% | 100.00% | 2,341,174.60 | 75.70% | 96.10% |
| Bayer Plc | Bayer | 31,660,582.20 | 78.50% | 1,961,751.60 | 4.90% | 6.20% | 1,961,751.60 | 49.70% | 100.00% | 29,698,830.60 | 73.60% | 93.80% |
| Mitsubishi Tanabe Pharma Europe Ltd | Mitsubishi Tanabe Pharma | 619,928.00 | 78.30% | 0.00% | 0.00% | 0.00% | N/A | 619,928 | 78.30% | 100.00% | ||
| Bial Pharma UK Ltd | Bial Pharma | 530,965.80 | 78.00% | 32,811.70 | 4.80% | 6.20% | 32,811.70 | 18.80% | 100.00% | 498,154 | 73.20% | 93.80% |
| Alimera Sciences Limited | Alimera Sciences | 167,083.00 | 77.80% | 28,539.50 | 13.30% | 17.10% | 28,539.50 | 69.50% | 100.00% | 138,543 | 64.50% | 82.90% |
| Intercept Pharma | Intercept Pharma | 740,818.70 | 77.70% | 15,623.30 | 1.60% | 2.10% | 15,623.30 | 47.20% | 100.00% | 725,195 | 76.10% | 97.90% |
| Eli Lilly & Company Ltd | Eli Lilly | 8,954,564.90 | 77.50% | 1,260,978.70 | 10.90% | 14.10% | 1,260,978.70 | 56.10% | 100.00% | 7,693,586.20 | 66.50% | 85.90% |
| Boehringer Ingelheim Ltd | Boehringer Ingelheim | 6,504,936.90 | 77.20% | 1,189,117.10 | 14.10% | 18.30% | 1,189,117.10 | 61.40% | 100.00% | 5,315,819.80 | 63.10% | 81.70% |
| AbbVie Limited | AbbVie | 15,016,637.00 | 75.80% | 1,105,735.20 | 5.60% | 7.40% | 1,105,735.20 | 32.30% | 100.00% | 13,910,901.70 | 70.20% | 92.60% |
| Santen UK Limited | Santen Pharmaceutical | 2,277,818.70 | 75.00% | 123,464.50 | 4.10% | 5.40% | 123,464.50 | 37.10% | 100.00% | 2,154,354.20 | 70.90% | 94.60% |
| Jazz Pharma | Jazz Pharmaceuticals | 672,669.40 | 74.70% | 251,931.40 | 28.00% | 37.50% | 251,931.40 | 80.20% | 100.00% | 420,738 | 46.70% | 62.50% |
| Alexion Pharma UK Ltd | Alexion Pharmaceuticals | 819,412.00 | 73.40% | 616,953.00 | 55.30% | 75.30% | 616,953.00 | 93.10% | 100.00% | 202,459 | 18.10% | 24.70% |
| Daiichi Sankyo UK Ltd | Daiichi Sankyo | 1,390,606.60 | 72.50% | 53,465.40 | 2.80% | 3.80% | 53,465.40 | 14.30% | 100.00% | 1,337,141.30 | 69.70% | 96.20% |
| Almirall Ltd | Almirall | 1,134,393.40 | 72.40% | 345,675.70 | 22.00% | 30.50% | 345,675.70 | 77.30% | 100.00% | 788,717.70 | 50.30% | 69.50% |
| Merck Serono Ltd | Merck Serono | 4,205,087.10 | 71.40% | 665,812.60 | 11.30% | 15.80% | 665,812.60 | 53.50% | 100.00% | 3,539,274.60 | 60.10% | 84.20% |
| BioMarin Europe Ltd | BioMarin Pharmaceutical | 3,118,921.30 | 71.00% | 93,463.50 | 2.10% | 3.00% | 46,291.70 | 8.60% | 49.50% | 3,025,457.80 | 68.90% | 97.00% |
| IQVIA IES UK Limited | IQVIA | 23,373.00 | 70.00% | 23,373.00 | 70.00% | 100.00% | 23,373.00 | 70.00% | 100.00% | 0.00% | 0.00% | |
| Novo Nordisk Limited | Novo Nordisk | 9,570,827.10 | 69.50% | 1,142,592.40 | 8.30% | 11.90% | 1,142,592.40 | 45.30% | 100.00% | 8,428,234.60 | 61.20% | 88.10% |
| Dr Falk Pharma UK Ltd | Dr Falk Pharma | 155,851.20 | 69.10% | 155,851.20 | 69.10% | 100.00% | 155,851.20 | 70.40% | 100.00% | 0.00% | 0.00% | |
| Ever Pharma UK Ltd | EVER Pharma | 13,770.00 | 68.90% | 13,770.00 | 68.90% | 100.00% | 13,770.00 | 68.90% | 100.00% | 0.00% | 0.00% | |
| Leadiant Biosciences Limited | Leadiant Biosciences | 64,093.20 | 68.30% | 3,189.80 | 3.40% | 5.00% | 3,189.80 | 21.40% | 100.00% | 60,903 | 64.90% | 95.00% |
| ApoPharma Inc | ApoPharma | 117,871.70 | 66.60% | 0.00% | 0.00% | 0.00% | N/A | 117,872 | 66.60% | 100.00% | ||
| Sandoz Ltd | Sandoz | 1,672,523.40 | 64.80% | 15,926.50 | 0.60% | 1.00% | 15,926.50 | 5.10% | 100.00% | 1,656,596.90 | 64.10% | 99.00% |
| Amgen Ltd | Amgen | 6,676,709.70 | 62.00% | 216,333.20 | 2.00% | 3.20% | 216,333.20 | 20.70% | 100.00% | 6,460,376.40 | 60.00% | 96.80% |
| Novartis Pharmaceuticals UK Ltd | Novartis | 18,771,370.10 | 61.90% | 688,181.20 | 2.30% | 3.70% | 688,181.20 | 15.10% | 100.00% | 18,083,188.90 | 59.60% | 96.30% |
| Profile Pharma Ltd | Profile Pharma | 832,647.10 | 60.30% | 31,287.10 | 2.30% | 3.80% | 31,287.10 | 56.50% | 100.00% | 801,360 | 58.00% | 96.20% |
| Pfizer Ltd | Pfizer | 23,184,710.00 | 60.20% | 84,336.10 | 0.20% | 0.40% | 84,336.10 | 1.70% | 100.00% | 23,100,373.80 | 60.00% | 99.60% |
| GlaxoSmithKline plc | GlaxoSmithKline | 22,745,157.20 | 59.50% | 0.00% | 0.00% | 0.00% | N/A | 22,745,157.20 | 59.50% | 100.00% | ||
| Gilead | Gilead | 6,505,440.80 | 58.50% | 1,212,679.50 | 10.90% | 18.60% | 1,212,679.50 | 70.20% | 100.00% | 5,292,761.30 | 47.60% | 81.40% |
| Sanofi Aventis | Sanofi | 8,015,216.90 | 57.20% | 1,354,442.30 | 9.70% | 16.90% | 1,354,442.30 | 46.70% | 100.00% | 6,660,774.60 | 47.60% | 83.10% |
| Recordati Pharmaceuticals Ltd | Recordati Pharmaceuticals | 46,194.60 | 56.30% | 46,194.60 | 56.30% | 100.00% | 46,194.60 | 57.20% | 100.00% | 0.00% | 0.00% | |
| Mundipharma International Limited | Mundipharma | 146,645.70 | 54.70% | 146,645.70 | 54.70% | 100.00% | 146,645.70 | 77.50% | 100.00% | 0.00% | 0.00% | |
| Bausch & Lomb UK Ltd | Bausch + Lomb | 93,492.00 | 54.50% | 0.00% | 0.00% | 0.00% | N/A | 93,492 | 54.50% | 100.00% | ||
| Teva UK Limited | Teva | 2,342,104.30 | 54.10% | 136,427.60 | 3.20% | 5.80% | 136,427.60 | 100.00% | 100.00% | 2,205,676.70 | 50.90% | 94.20% |
| Concordia International Rx UK Ltd | Concordia International | 64,728.20 | 51.30% | 24,762.00 | 19.60% | 38.30% | 24,762.00 | 98.10% | 100.00% | 39,966 | 31.70% | 61.70% |
| HRA Pharma UK & Ireland Ltd | HRA Pharma | 95,128.00 | 50.00% | 0.00% | 0.00% | 0.00% | N/A | 95,128 | 50.00% | 100.00% | ||
| Aguettant Ltd | Aguettant | 21,565.70 | 47.40% | 21,565.70 | 47.40% | 100.00% | 21,565.70 | 50.00% | 100.00% | 0.00% | 0.00% | |
| Actelion Pharmaceuticals UK Ltd | Actelion Pharmaceuticals | 849,107.70 | 45.10% | 535,930.70 | 28.40% | 63.10% | 535,930.70 | 82.90% | 100.00% | 313,177 | 16.60% | 36.90% |
| Ipsen Developments Ltd | Ipsen | 299,426.90 | 44.10% | 110,836.70 | 16.30% | 37.00% | 110,836.70 | 32.60% | 100.00% | 188,590 | 27.80% | 63.00% |
| A. Menarini Farmaceutica Internazionale S.r.l | A. Menarini Farmaceutica Internazionale | 128,098.00 | 44.10% | 94,690.70 | 32.60% | 73.90% | 94,690.70 | 50.30% | 100.00% | 33,407 | 11.50% | 26.10% |
| Grunenthal Ltd | Grunenthal | 262,966.20 | 41.60% | 107,925.20 | 17.10% | 41.00% | 107,925.20 | 49.60% | 100.00% | 155,041 | 24.50% | 59.00% |
| Britannia Pharmaceuticals | Britannia Pharmaceuticals | 74,980.50 | 39.80% | 74,833.00 | 39.70% | 99.80% | 74,833.00 | 86.00% | 100.00% | 147 | 0.10% | 0.20% |
| Guerbet Laboratories Ltd | Guerbet Laboratories | 103,659.40 | 39.50% | 31,494.20 | 12.00% | 30.40% | 31,494.20 | 64.90% | 100.00% | 72,165 | 27.50% | 69.60% |
| Alnylam Pharmaceuticals UK & Ireland | Alnylam Pharmaceuticals | 426,896.40 | 38.60% | 92,292.60 | 8.40% | 21.60% | 92,292.60 | 57.90% | 100.00% | 334,604 | 30.30% | 78.40% |
| Accretio | Accretio | 794.7 | 35.60% | 794.7 | 35.60% | 100.00% | 794.7 | 100.00% | 100.00% | 0.00% | 0.00% | |
| Chugai Pharma UK | Chugai Pharmaceutical | 217,303.10 | 35.40% | 88,239.20 | 14.40% | 40.60% | 88,239.20 | 45.10% | 100.00% | 129,064 | 21.00% | 59.40% |
| Dermal | Dermal Laboratories | 72,110.20 | 34.50% | 17,527.10 | 8.40% | 24.30% | 17,527.10 | 13.60% | 100.00% | 54,583 | 26.10% | 75.70% |
| Gedeon Richter (UK) Ltd | Gedeon Richter | 114,012.80 | 33.00% | 114,012.80 | 33.00% | 100.00% | 114,012.80 | 80.20% | 100.00% | 0.00% | 0.00% | |
| GE Healthcare Limited | GE Healthcare | 201,164.10 | 32.60% | 152,954.20 | 24.80% | 76.00% | 152,954.20 | 100.00% | 100.00% | 48,210 | 7.80% | 24.00% |
| CSL Behring | CSL Behring | 222,579.20 | 31.50% | 118,769.70 | 16.80% | 53.40% | 118,769.70 | 32.10% | 100.00% | 103,810 | 14.70% | 46.60% |
| Lundbeck Ltd | Lundbeck | 248,286.50 | 31.40% | 86,423.80 | 10.90% | 34.80% | 86,423.80 | 16.00% | 100.00% | 161,863 | 20.50% | 65.20% |
| Chiesi Ltd | Chiesi | 2,032,217.40 | 28.70% | 628,223.90 | 8.90% | 30.90% | 628,223.90 | 45.60% | 100.00% | 1,403,993.40 | 19.80% | 69.10% |
| Besins Healthcare (UK) Ltd | Besins Healthcare | 99,987.00 | 27.70% | 99,987.00 | 27.70% | 100.00% | 99,987.00 | 87.00% | 100.00% | 0.00% | 0.00% | |
| Napp Pharmaceuticals Ltd | Napp Pharmaceuticals | 774,726.20 | 24.40% | 774,726.20 | 24.40% | 100.00% | 774,726.20 | 62.80% | 100.00% | 0.00% | 0.00% | |
| Fresenius-Kabi Ltd | Fresenius Kabi | 48,412.80 | 23.90% | 48,412.80 | 23.90% | 100.00% | 48,412.80 | 23.90% | 100.00% | 0.00% | 0.00% | |
| Norgine | Norgine | 66,774.70 | 20.50% | 33,266.10 | 10.20% | 49.80% | 33,266.10 | 18.40% | 100.00% | 33,509 | 10.30% | 50.20% |
| Fresenius Medical Care (UK) Ltd | Fresenius Medical Care | 20,644.90 | 20.20% | 20,644.90 | 20.20% | 100.00% | 20,644.90 | 46.80% | 100.00% | 0.00% | 0.00% | |
| Orion Pharma (UK) Ltd | Orion Pharma | 95,822.50 | 16.10% | 5,845.40 | 1.00% | 6.10% | 5,845.40 | 26.10% | 100.00% | 89,977 | 15.10% | 93.90% |
| Proveca Ltd | Proveca | 7,116.20 | 14.10% | 7,116.20 | 14.10% | 100.00% | 7,116.20 | 100.00% | 100.00% | 0.00% | 0.00% | |
| RB | Reckitt Benckiser | 5,947.80 | 13.80% | 2,436.10 | 5.70% | 41.00% | 2,436.10 | 6.20% | 100.00% | 3,511.70 | 8.20% | 59.00% |
| Sobi Ltd | Sobi | 92,529.20 | 13.40% | 92,529.20 | 13.40% | 100.00% | 92,529.20 | 100.00% | 100.00% | 0.00% | 0.00% | |
| ALK-Abello Ltd | ALK-Abello | 14,955.80 | 11.90% | 3,909.10 | 3.10% | 26.10% | 3,909.10 | 7.70% | 100.00% | 11,046.70 | 8.80% | 73.90% |
| Ferring Pharma | Ferring Pharmaceuticals | 102,525.80 | 9.10% | 67,058.40 | 6.00% | 65.40% | 67,058.40 | 9.20% | 100.00% | 35,467 | 3.20% | 34.60% |
| Orphan Europe UK Ltd | Orphan Europe | 1,714.90 | 6.40% | 1,714.90 | 6.40% | 100.00% | 1,714.90 | 50.00% | 100.00% | 0.00% | 0.00% | |
| Consilient Health Ltd | Consilient Health | 19,993.20 | 6.10% | 19,993.20 | 6.10% | 100.00% | 19,993.20 | 32.70% | 100.00% | 0.00% | 0.00% | |
| Aegerion Pharmaceuticals Limited | Aegerion Pharmaceuticals | 1,702.90 | 4.20% | 1,702.90 | 4.20% | 100.00% | 1,702.90 | 100.00% | 100.00% | 0.00% | 0.00% | |
| Accord-UK Ltd | Accord Healthcare | 8,332.00 | 4.00% | 8,332.00 | 4.00% | 100.00% | 8,332.00 | 37.90% | 100.00% | 0.00% | 0.00% | |
| Colonis Pharma Ltd | Colonis | 534.2 | 3.20% | 534.2 | 3.20% | 100.00% | 534.2 | 66.70% | 100.00% | 0.00% | 0.00% | |
| Baxter Healthcare Ltd | Baxter Healthcare | 11,800.90 | 2.00% | 11,800.90 | 2.00% | 100.00% | 11,800.90 | 3.80% | 100.00% | 0.00% | 0.00% | |
| Lincoln Medical | Lincoln Medical | 357.2 | 2.00% | 357.2 | 2.00% | 100.00% | 357.2 | 50.00% | 100.00% | 0.00% | 0.00% | |
| Alliance Pharmaceuticals Ltd | Alliance Pharma | 2,285.20 | 1.70% | 2,285.20 | 1.70% | 100.00% | 2,285.20 | 18.80% | 100.00% | 0.00% | 0.00% | |
| Pierre Fabre Ltd | Pierre Fabre | 1,202.00 | 1.40% | 1,202.00 | 1.40% | 100.00% | 1,202.00 | 2.70% | 100.00% | 0.00% | 0.00% | |
| Flynn Pharma | Flynn Pharma | 4,101.60 | 1.30% | 2,231.10 | 0.70% | 54.40% | 2,231.10 | 1.70% | 100.00% | 1,870.50 | 0.60% | 45.60% |
| Rosemont Pharmaceuticals Ltd | Rosemont Pharmaceuticals | 601 | 1.20% | 601 | 1.20% | 100.00% | 601 | 11.70% | 100.00% | 0.00% | 0.00% | |
| Bracco UK Ltd | Bracco | 555.4 | 0.40% | 555.4 | 0.40% | 100.00% | 555.4 | 0.60% | 100.00% | 0.00% | 0.00% | |
| Seqirus | Seqirus | 492.7 | 0.40% | 492.7 | 0.40% | 100.00% | 492.7 | 0.40% | 100.00% | 0.00% | 0.00% | |
| Bio Products Laboratory Ltd | Bio Products Laboratory | 0.00% | 0.00% | N/A | 0.00% | N/A | 0.00% | N/A | ||||
| Galen Ltd | Galen | 0.00% | 0.00% | N/A | 0.00% | N/A | 0.00% | N/A | ||||
| PARI Medical Ltd | PARI Medical | 0.00% | 0.00% | N/A | 0.00% | N/A | 0.00% | N/A | ||||
| Shield TX UK Ltd | Shield Therapeutics | 0.00% | 0.00% | N/A | 0.00% | N/A | 0.00% | N/A | ||||
| STD Pharmaceutical Products Ltd | STD Pharmaceutical Products | 0.00% | 0.00% | N/A | N/A | N/A | 0.00% | N/A | ||||
| Stirling Anglian Pharmaceuticals Ltd | Stirling Anglian Pharmaceuticals | 0.00% | 0.00% | N/A | N/A | N/A | 0.00% | N/A | ||||
| Thea Pharmaceuticals Ltd | Thea Pharmaceuticals | 0.00% | 0.00% | N/A | N/A | N/A | 0.00% | N/A | ||||
| Tillotts Pharma UK Ltd | Tillotts Pharma | 0.00% | 0.00% | N/A | N/A | N/A | 0.00% | N/A | ||||
| UCB Pharma Ltd | UCB Pharma | 0.00% | 0.00% | N/A | N/A | N/A | 0.00% | N/A | ||||
| Veriton Pharma | Veriton Pharma | 0.00% | 0.00% | N/A | N/A | N/A | 0.00% | N/A | ||||
| Vifor Pharma Group | Vifor Pharma | 0.00% | 0.00% | N/A | N/A | N/A | 0.00% | N/A |
The companies are sorted descendingly based on the overall share of aggregated payments
Actelion was purchased by Janssen in June 2017 and is now known as Janssen Pulmonary Hypertension
Akcea Therapeutics became a wholly owned subsidiary of Ionis Pharmaceuticals in 2021
Aegerion was acquired by Amryt Pharma in September 2019
Allergan was acquired by AbbVie in 2020 and is now known as Allergan Aesthetics
Avexis EU changed its name to Novartis Gene Therapy EU in 2020
Profile Pharma changed its name to Zambon in February 2022
Lincoln Medical chanegd name to Bioprojet UK in January 2021
Tesaro was acquired by GSK in 2019
Martindale Pharma became an Ethypharm Group Company in 2017
Sunovion Pharmaceuticals changed its name to CNX Therapeutics in 2021
Aggregated payments Payments without named recipients, R&D Research and development
Table 10.
Aggregated payments – company-level data reported by companies following the JPMA Code
| Company name | Standardised International Name recorded on drug company website | Value of aggregated payments (A & D & E) ($) | Aggregated payments—share of all payments (%) | Value of aggregated non-R&D payments (category D&E) ($) | Aggregated non-R&D payments (category D&E)—share of all payments (%) | Aggregated non-R&D payments (category D&E)—share of aggregated payments (%) | Value of aggregated non-R&D payments to HCPs (Category D—HCPs) ($) | Aggregated non-R&D payments to HCPs (Category D—HCPs)—share of all payments | Aggregated non-R&D payments to HCPs (Category D—HCPs)—share of aggregated non-R&D payments | Value of Aggregated payments (Category E—HCPs & HCOs) | Aggregated payments (Category E—HCPs & HCOs)—share of all payments | Aggregated payments (Category E—HCPs & HCOs)—share of aggregated payments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EA Pharma Co., Ltd., | EA Pharma | 17,413,922 | 83.14% | 17,413,922.10 | 83.14% | 100.00% | 16,934,873.19 | 80.85% | 97.25% | 479,048.91 | 2.29% | 2.75% |
| CELGENE CORPORATION, | Celgene | 14,623,785 | 78.99% | 13,238,120.06 | 71.51% | 90.52% | 9,781,135.70 | 52.83% | 73.89% | 3,456,984.36 | 18.67% | 26.11% |
| Shire Japan KK, | Shire Pharmaceuticals | 2,966,023 | 72.39% | 2,790,852.82 | 68.11% | 94.09% | 2,776,982.95 | 67.78% | 99.50% | 13,869.87 | 0.34% | 0.50% |
| Kracie Pharma, Ltd | Kracie Pharma | 3,582,579 | 71.79% | 3,581,678.14 | 71.77% | 99.97% | 3,262,506.69 | 65.38% | 91.09% | 319,171.45 | 6.40% | 8.91% |
| Mylan EPD G.K | Mylan | 6,520,184 | 70.40% | 6,520,183.79 | 70.40% | 100.00% | 6,326,172.06 | 68.30% | 97.02% | 194,011.73 | 2.09% | 2.98% |
| TOA EIYO LTD, | Toa Eiyo | 3,508,745 | 69.79% | 3,435,278.29 | 68.33% | 97.91% | 2,627,617.59 | 52.27% | 76.49% | 807,660.70 | 16.07% | 23.51% |
| Taisho Toyama Pharmaceutical Co Ltd | Taisho Toyama Pharmaceutical | 16,597,647 | 68.98% | 16,597,120.42 | 68.97% | 100.00% | 15,342,534.76 | 63.76% | 92.44% | 1,254,585.66 | 5.21% | 7.56% |
| Otsuka Pharmaceutical Factory, Inc | Otsuka Pharmaceutical Factory | 6,884,704 | 66.78% | 6,877,315.57 | 66.71% | 99.89% | 6,592,638.08 | 63.95% | 95.86% | 284,677.49 | 2.76% | 4.14% |
| NIHON PHARMACEUTICAL CO., LTD., | Nihon Pharmaceutical | 3,759,253 | 64.89% | 2,509,810.90 | 43.32% | 66.76% | 2,362,612.60 | 40.78% | 94.14% | 147,198.30 | 2.54% | 5.86% |
| Maruishi Pharmaceutical Co., Ltd., | Maruishi Pharmaceutical | 2,343,468 | 63.02% | 2,303,006.16 | 61.93% | 98.27% | 2,289,985.76 | 61.58% | 99.43% | 13,020.40 | 0.35% | 0.57% |
| KOWA PHARMACEUTICAL COMPANY LTD | Kowa Pharmaceuticals | 15,077,379 | 62.98% | 15,077,379.35 | 62.98% | 100.00% | 13,062,422.28 | 54.57% | 86.64% | 2,014,957.07 | 8.42% | 13.36% |
| TSUMURA & CO., | Tsumura | 18,435,580 | 61.85% | 18,435,580.08 | 61.85% | 100.00% | 18,054,929.32 | 60.57% | 97.94% | 380,650.76 | 1.28% | 2.06% |
| Meiji Seika Pharma Co., Ltd., | Meiji Seika Pharma | 14,380,059 | 60.27% | 9,283,129.91 | 38.91% | 64.56% | 8,217,558.56 | 34.44% | 88.52% | 1,065,571.35 | 4.47% | 11.48% |
| Kissei Pharmaceutical Co., Ltd., | Kissei Pharmaceutical | 14,024,145 | 60.13% | 12,360,144.23 | 53.00% | 88.13% | 10,361,946.51 | 44.43% | 83.83% | 1,998,197.72 | 8.57% | 16.17% |
| Biofermin Pharmaceutical Co., Ltd | Biofermin Pharmaceutical | 275,192 | 58.48% | 275,192.21 | 58.48% | 100.00% | 252,229.91 | 53.60% | 91.66% | 22,962.30 | 4.88% | 8.34% |
| Pfizer Japan Inc., | Pfizer | 58,456,238 | 54.88% | 58,088,767.18 | 54.53% | 99.37% | 57,599,771.22 | 54.07% | 99.16% | 488,995.96 | 0.46% | 0.84% |
| FUJIFILM Toyama Chemical Co., Ltd | FUJIFILM Toyama Chemical | 2,118,662 | 53.82% | 1,874,576.16 | 47.62% | 88.48% | 937,288.08 | 23.81% | 50.00% | 937,288.08 | 23.81% | 50.00% |
| Takeda Pharmaceutical Company Limited., | Takeda | 63,042,432 | 53.71% | 63,042,432.07 | 53.71% | 100.00% | 62,259,597.55 | 53.04% | 98.76% | 782,834.52 | 0.67% | 1.24% |
| KYORIN Pharmaceutical Co., Ltd., | Kyorin Pharmaceutical | 10,720,914 | 52.77% | 10,628,868.04 | 52.32% | 99.14% | 10,024,752.71 | 49.35% | 94.32% | 604,115.33 | 2.97% | 5.68% |
| Bristol-Myers Squibb K.K, | Bristol-Myers Squibb | 29,971,754 | 50.18% | 29,388,030.74 | 49.20% | 98.05% | 29,023,493.46 | 48.59% | 98.76% | 364,537.28 | 0.61% | 1.24% |
| AYUMI Pharmaceutical Corporation, | AYUMI Pharmaceutical | 2,023,069 | 49.73% | 2,023,069.23 | 49.73% | 100.00% | 1,977,970.68 | 48.62% | 97.77% | 45,098.55 | 1.11% | 2.23% |
| Santen Pharmaceutical Co., Ltd., | Santen Pharmaceutical | 10,179,708 | 49.29% | 9,969,835.96 | 48.27% | 97.94% | 9,642,725.64 | 46.69% | 96.72% | 327,110.32 | 1.58% | 3.28% |
| BEE BRAND MEDICO DENTAL.Co.,Ltd | Bee Brand Medico Dental | 19,795 | 48.66% | 19,622.78 | 48.24% | 99.13% | 18,918.67 | 46.51% | 96.41% | 704.11 | 1.73% | 3.59% |
| Wakamoto Pharmaceutical Co., Ltd., | Wakamoto Pharmaceutical | 2,244,556 | 48.06% | 312,400.00 | 6.69% | 13.92% | 296,499.89 | 6.35% | 94.91% | 15,900.11 | 0.34% | 5.09% |
| Novartis Pharma K.K., | Novartis | 46,059,783 | 46.80% | 44,474,637.68 | 45.19% | 96.56% | 43,949,275.36 | 44.66% | 98.82% | 525,362.32 | 0.53% | 1.18% |
| DAIICHI SANKYO COMPANY, LIMITED., | Daiichi Sankyo | 96,159,420 | 46.36% | 96,096,014.49 | 46.33% | 99.93% | 94,574,275.36 | 45.60% | 98.42% | 1,521,739.13 | 0.73% | 1.58% |
| Eisai Co., Ltd., | Eisai | 42,690,217 | 45.55% | 35,452,898.55 | 37.83% | 83.05% | 33,958,333.33 | 36.24% | 95.78% | 1,494,565.22 | 1.59% | 4.22% |
| Torii Pharmaceutical Co., Ltd., | Torii Pharmaceutical | 7,665,201 | 45.31% | 7,199,367.81 | 42.55% | 93.92% | 6,397,904.66 | 37.81% | 88.87% | 801,463.15 | 4.74% | 11.13% |
| TEIJIN PHARMA LIMITED., | Teijin Pharma | 12,028,670 | 45.15% | 12,028,670.28 | 45.15% | 100.00% | 11,579,067.71 | 43.46% | 96.26% | 449,602.57 | 1.69% | 3.74% |
| Kyowa Hakko Kirin Company, Limited, | Kyowa Kirin | 32,108,834 | 44.71% | 32,108,834.48 | 44.71% | 100.00% | 30,100,019.44 | 41.92% | 93.74% | 2,008,815.04 | 2.80% | 6.26% |
| ZERIA Pharmaceutical Co., Ltd., | Zeria Pharmaceutical | 3,308,774 | 44.55% | 3,308,774.35 | 44.55% | 100.00% | 3,108,254.69 | 41.85% | 93.94% | 200,519.66 | 2.70% | 6.06% |
| Otsuka Pharmaceutical Co., Ltd., | Otsuka Pharmaceuticals | 48,269,344 | 44.15% | 48,269,344.24 | 44.15% | 100.00% | 45,835,694.77 | 41.92% | 94.96% | 2,433,649.47 | 2.23% | 5.04% |
| MOCHIDA PHARMACEUTICAL CO., LTD., | Mochida Pharmaceutical | 11,897,575 | 44.02% | 11,897,575.13 | 44.02% | 100.00% | 11,197,795.26 | 41.43% | 94.12% | 699,779.87 | 2.59% | 5.88% |
| POLA-Pharma., | Pola Pharma | 1,184,913 | 43.94% | 1,183,855.95 | 43.90% | 99.91% | 1,103,215.32 | 40.91% | 93.19% | 80,640.63 | 2.99% | 6.81% |
| MSD K.K., | Merck Sharp & Dohme | 46,779,000 | 43.24% | 46,779,000.07 | 43.24% | 100.00% | 46,145,657.57 | 42.66% | 98.65% | 633,342.50 | 0.59% | 1.35% |
| Fuso Pharmaceutical Industries, Ltd., | Fuso Pharmaceutical Industries | 1,001,214 | 43.02% | 1,001,213.99 | 43.02% | 100.00% | 859,742.25 | 36.94% | 85.87% | 141,471.74 | 6.08% | 14.13% |
| Janssen Pharmaceutical K.K., | Janssen Pharmaceuticals | 17,626,105 | 42.36% | 13,342,653.98 | 32.06% | 75.70% | 13,201,639.49 | 31.73% | 98.94% | 141,014.49 | 0.34% | 1.06% |
| Astellas Pharma Inc., | Astellas Pharma | 45,407,609 | 42.29% | 42,110,507.24 | 39.22% | 92.74% | 42,074,275.36 | 39.19% | 99.91% | 36,231.88 | 0.03% | 0.09% |
| Alfresa Pharma Corporation | Alfresa Pharma | 789,557 | 40.88% | 789,557.18 | 40.88% | 100.00% | 760,764.52 | 39.39% | 96.35% | 28,792.66 | 1.49% | 3.65% |
| Sanofi K.K., | Sanofi | 21,834,459 | 39.34% | 21,834,459.30 | 39.34% | 100.00% | 21,785,766.29 | 39.25% | 99.78% | 48,693.01 | 0.09% | 0.22% |
| Bayer Yakuhin, Ltd., | Bayer | 26,629,090 | 39.10% | 26,629,090.02 | 39.10% | 100.00% | 25,870,326.41 | 37.99% | 97.15% | 758,763.61 | 1.11% | 2.85% |
| Eli Lilly Japan K.K., | Eli Lilly | 47,449,666 | 38.45% | 46,777,425.98 | 37.90% | 98.58% | 46,414,707.45 | 37.61% | 99.22% | 362,718.53 | 0.29% | 0.78% |
| TAIHO PHARMACEUTICAL CO., LTD., | Taiho Pharmaceutical | 19,423,820 | 38.35% | 16,964,923.77 | 33.50% | 87.34% | 15,584,041.93 | 30.77% | 91.86% | 1,380,881.84 | 2.73% | 8.14% |
| Sumitomo Dainippon Pharma Co., Ltd., | Sumitomo Dainippon Pharma | 23,679,188 | 37.88% | 20,925,766.86 | 33.48% | 88.37% | 19,537,706.79 | 31.26% | 93.37% | 1,388,060.07 | 2.22% | 6.63% |
| Nippon Boehringer Ingelheim Co., Ltd., | Boehringer Ingelheim | 41,288,043 | 37.61% | 41,288,043.48 | 37.61% | 100.00% | 41,089,311.60 | 37.42% | 99.52% | 198,731.88 | 0.18% | 0.48% |
| Maruho Co., Ltd., | Maruho | 14,461,866 | 37.54% | 14,461,865.66 | 37.54% | 100.00% | 14,040,456.29 | 36.45% | 97.09% | 421,409.37 | 1.09% | 2.91% |
| TOYAMA CHEMICAL CO., LTD., | Toyama Chemical | 939,531 | 36.74% | 66,948.24 | 2.62% | 7.13% | 63,923.69 | 2.50% | 95.48% | 3,024.55 | 0.12% | 4.52% |
| Nippon Chemiphar Co., Ltd., | Nippon Chemiphar | 868,502 | 36.57% | 868,501.90 | 36.57% | 100.00% | 649,543.15 | 27.35% | 74.79% | 218,958.75 | 9.22% | 25.21% |
| ASAHI KASEI PHARMA CORPORATION, | Asahi Kasei Pharma | 15,323,641 | 36.42% | 15,323,641.30 | 36.42% | 100.00% | 14,504,673.91 | 34.47% | 94.66% | 818,967.39 | 1.95% | 5.34% |
| Nippon Zoki Pharmaceutical Cp., Ltd., | Nippon Zoki | 2,623,265 | 36.32% | 2,621,573.48 | 36.29% | 99.94% | 2,567,429.69 | 35.54% | 97.93% | 54,143.79 | 0.75% | 2.07% |
| Yakult Honsha Company, Limited., | Yakult Honsha | 3,967,391 | 36.20% | 3,958,333.33 | 36.12% | 99.77% | 3,894,927.53 | 35.54% | 98.40% | 63,405.80 | 0.58% | 1.60% |
| ASKA Pharmaceutical Co., Ltd | ASKA Pharmaceutical | 4,306,431 | 34.17% | 4,306,431.16 | 34.17% | 100.00% | 4,052,943.84 | 32.16% | 94.11% | 253,487.32 | 2.01% | 5.89% |
| Hisamitsu Pharmaceutical Co., Inc., | Hisamitsu Pharmaceutical | 10,572,249 | 33.96% | 10,572,248.75 | 33.96% | 100.00% | 9,938,856.72 | 31.93% | 94.01% | 633,392.03 | 2.03% | 5.99% |
| Novo Nordisk Pharma Ltd., | Novo Nordisk | 10,852,882 | 33.89% | 10,852,882.05 | 33.89% | 100.00% | 10,741,762.68 | 33.55% | 98.98% | 111,119.37 | 0.35% | 1.02% |
| FUJIFILM RI Pharma, Co, Ltd | FUJIFILM RI Pharma | 1,306,961 | 33.37% | 1,003,295.82 | 25.62% | 76.77% | 983,575.85 | 25.11% | 98.03% | 19,719.97 | 0.50% | 1.97% |
| Mitsubishi Tanabe Pharma Corporation, | Mitsubishi Tanabe Pharma | 36,521,739 | 33.17% | 36,521,739.14 | 33.17% | 100.00% | 34,048,913.05 | 30.92% | 93.23% | 2,472,826.09 | 2.25% | 6.77% |
| KM Biologics Co., Ltd | KM Biologics | 921,668 | 32.45% | 460,786.59 | 16.22% | 49.99% | 458,519.94 | 16.14% | 99.51% | 2,266.65 | 0.08% | 0.49% |
| AbbVie GK, | AbbVie | 16,981,345 | 32.39% | 16,562,567.82 | 31.59% | 97.53% | 15,810,942.72 | 30.16% | 95.46% | 751,625.10 | 1.43% | 4.54% |
| TERUMO CORPORATION, | Terumo Corporation | 6,923,109 | 32.34% | 6,917,581.52 | 32.31% | 99.92% | 6,290,380.43 | 29.38% | 90.93% | 627,201.09 | 2.93% | 9.07% |
| GlaxoSmithKline K.K., | GlaxoSmithKline | 13,906,467 | 31.85% | 13,906,467.39 | 31.85% | 100.00% | 13,751,005.43 | 31.49% | 98.88% | 155,461.96 | 0.36% | 1.12% |
| Merck Serono Co., Ltd., | Merck Serono | 2,937,777 | 30.99% | 2,937,776.53 | 30.99% | 100.00% | 2,937,776.53 | 30.99% | 100.00% | 0.00 | 0.00% | 0.00% |
| EN Otsuka Pharmaceutical Co., Ltd | EN Otsuka Pharmaceutical Company | 71,257 | 30.58% | 71,257.32 | 30.58% | 100.00% | 66,079.05 | 28.36% | 92.73% | 5,178.27 | 2.22% | 7.27% |
| Nippon Kayaku Co., Ltd | Nippon Kayaku | 2,812,512 | 30.34% | 2,812,512.05 | 30.34% | 100.00% | 2,587,808.83 | 27.92% | 92.01% | 224,703.22 | 2.42% | 7.99% |
| Chugai Pharmaceutical Co., Ltd., | Chugai Pharmaceutical | 41,071,879 | 29.35% | 41,071,879.46 | 29.35% | 100.00% | 39,528,055.44 | 28.25% | 96.24% | 1,543,824.02 | 1.10% | 3.76% |
| Asahi Kasei Medical Co.,Ltd | Asahi Kasei Medical | 468,089 | 29.07% | 444,990.95 | 27.64% | 95.07% | 294,257.25 | 18.28% | 66.13% | 150,733.70 | 9.36% | 33.87% |
| Kaken Pharmaceutical Co., Ltd., | Kaken Pharmaceutical | 6,171,882 | 28.28% | 6,131,466.27 | 28.09% | 99.35% | 5,607,744.07 | 25.69% | 91.46% | 523,722.20 | 2.40% | 8.54% |
| AstraZeneca K.K., | AstraZeneca | 22,176,141 | 28.23% | 22,176,140.67 | 28.23% | 100.00% | 22,169,956.30 | 28.22% | 99.97% | 6,184.37 | 0.01% | 0.03% |
| UCB Japan Co., Ltd., | UCB Pharma | 3,123,206 | 27.10% | 2,975,907.14 | 25.82% | 95.28% | 2,906,786.32 | 25.22% | 97.68% | 69,120.82 | 0.60% | 2.32% |
| NIPPON SHINYAKU CO., LTD., | Nippon Shinyaku | 5,801,649 | 26.29% | 5,776,574.34 | 26.18% | 99.57% | 5,277,143.88 | 23.91% | 91.35% | 499,430.46 | 2.26% | 8.65% |
| Senju Pharmaceutical Co., Ltd | Senju Pharmaceutical | 5,148,426 | 25.33% | 5,126,615.46 | 25.22% | 99.58% | 4,822,323.58 | 23.73% | 94.06% | 304,291.88 | 1.50% | 5.94% |
| Shionogi & Co,. Ltd., | Shionogi | 10,643,116 | 23.68% | 9,981,884.05 | 22.21% | 93.79% | 9,673,913.04 | 21.52% | 96.91% | 307,971.01 | 0.69% | 3.09% |
| ONO PHARMACEUTICAL CO., LTD., | Ono Pharmaceutical | 36,116,877 | 22.99% | 36,116,877.29 | 22.99% | 100.00% | 35,193,555.22 | 22.40% | 97.44% | 923,322.07 | 0.59% | 2.56% |
| Biogen Japan, Ltd | Biogen | 2,661,488 | 22.84% | 2,661,487.57 | 22.84% | 100.00% | 2,578,023.89 | 22.12% | 96.86% | 83,463.68 | 0.72% | 3.14% |
| Minophagen Pharmaceutical Co., | Minophagen Pharmaceutical | 117,047 | 22.23% | 102,494.47 | 19.47% | 87.57% | 98,727.13 | 18.75% | 96.32% | 3,767.34 | 0.72% | 3.68% |
| Toray Industries, Inc., | Toray Industries | 671,091 | 21.99% | 317,852.06 | 10.41% | 47.36% | 266,172.20 | 8.72% | 83.74% | 51,679.86 | 1.69% | 16.26% |
| SANWA KAGAKU KENKYUSHO CO., LTD., | Sanwa Kagaku Kenkyusho | 3,373,423 | 18.57% | 3,373,423.43 | 18.57% | 100.00% | 3,303,505.77 | 18.18% | 97.93% | 69,917.66 | 0.38% | 2.07% |
| Kyoto Pharmaceutical Industries, Ltd | Kyoto Pharmaceutical Industries | 7,541 | 11.82% | 7,540.93 | 11.82% | 100.00% | 0.00 | 0.00% | 0.00% | 7,540.93 | 11.82% | 100.00% |
| Fujimoto Pharmaceutical Corporation, | Fujimoto Pharmaceutical Corporation | 194,381 | 11.76% | 194,325.65 | 11.75% | 99.97% | 175,697.58 | 10.63% | 90.41% | 18,628.07 | 1.13% | 9.59% |
| Taisho Pharmaceutical Co., Ltd., | Taisho Pharmaceutical | 837,051 | 7.50% | 77,541.10 | 0.69% | 9.26% | 69,191.95 | 0.62% | 89.23% | 8,349.15 | 0.07% | 10.77% |
| The Research Foundation for Microbial Diseases of Osaka University | The Research Foundation For Microbial Diseases of Osaka University | 73,024 | 1.36% | 69,122.31 | 1.29% | 94.66% | 55,076.16 | 1.03% | 79.68% | 14,046.15 | 0.26% | 20.32% |
| Teikoku Seiyaku Co., Ltd., | Teikoku Seiyaku | 34,602 | 0.83% | 34,602.49 | 0.83% | 100.00% | 27,826.58 | 0.67% | 80.42% | 6,775.91 | 0.16% | 19.58% |
| SEIKAGAKU CORPORATION, | Seikagaku Corporation | 51,634 | 0.41% | 43,530.90 | 0.35% | 84.31% | 39,760.64 | 0.32% | 91.34% | 3,770.26 | 0.03% | 8.66% |
| Kowa Company. Ltd., | Kowa Company | 50,991 | 0.26% | 49,825.33 | 0.25% | 97.71% | 41,897.97 | 0.21% | 84.09% | 7,927.36 | 0.04% | 15.91% |
| Japan Tobacco Inc., | Japan Tobacco | 19,526 | 0.08% | 19,526.21 | 0.08% | 100.00% | 0.00 | 0.00% | 0.00% | 19,526.21 | 0.08% | 100.00% |
| Otsuka Holdings Co., Ltd | Otsuka Holdings | 0 | 0.00% | 0.00 | 0.00% | 0.00% | 0.00 | 0.00% | 0.00% | 0.00 | 0.00% | 0.00% |
| Alcon Pharmaceuticals Ltd | Alcon | 0 | 0.00% | 0.00 | 0.00% | 0.00% | 0.00 | 0.00% | 0.00% | 0.00 | 0.00% | 0.00% |
Category A covers expenses related to Research and Development. Category D is expenses related to information provision. Category E is defined as other non-R&D expenses that cannot be classified in any other category. It is described as including costs for hospitality as a social ritual
Company name in 2018, respectively
Toray consists of the sum of the Pharmaceuticals and Medical Products Divisions
Merck Serono Co., Ltd. changed its name to Merck Biopharma Co., Ltd. in April 2019
Sumitomo Dainippon Pharma Co., Ltd. changed its name to Sumitomo Pharma Co., Ltd. in April 2022
Japan tobacco withdrew from JPMA in March 2018
KOWA PHARMACEUTICAL COMPANY LTD. was merged into KOWA in April 2019
Taisho Toyama Pharmaceutical Co Ltd. changed its name to Taisho Pharma Co., Ltd. in April 2019
FUJIFILM Toyama Chemical Co., Ltd. was formed by the merger of FUJIFILM RI Pharma, Co, Ltd and TOYAMA CHEMICAL CO., LTD, in October 2018
Authors’ contributions
PO conceived the paper, designed the study, acquired, analysed, and interpreted the data and drafted the manuscript. HS conceived the paper, designed the study, acquired, analysed, and interpreted the data and drafted the manuscript. ER conceived the paper, designed the study, acquired, analysed, and interpreted the data and drafted the manuscript. SM conceived the paper, designed the study, acquired, analysed and interpreted the data and drafted the manuscript. AO conceived the paper, designed the study, acquired, analysed, and interpreted the data and drafted the manuscript. All authors have approved the final submitted version of the manuscript. All authors have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
This study (SM as PI and PO as Co-I) was supported by the grant ‘Following the money: cross-national study of pharmaceutical industry payments to medical associations and patient organisations’, awarded by The Swedish Research Council (VR), no. 2020–01822. The funder had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the articles; and in the decision to submit it for publication.
Availability of data and materials
The documentary materials supporting the analysis of disclosure rules in the article are cited throughout the Findings. The analysis of disclosure practices and data was based on the Japanese Money Database https://db.tansajp.org/ as well as the Disclosure UK database https://search.disclosureuk.org.uk/ and the repository of drug company methodological notes managed by the Association of the British Pharmaceutical Industry: https://www.abpi.org.uk/reputation/disclosure-uk/methodological-notes-by-company-and-year/.
Declarations
Ethics approval and consent to participate
No ethics approval or consent to participate were needed as the study relied on publicly available data aggregated at the organisational or country level. The ethical implications of this study article were approved via a peer ethics review process at the Department of Social and Policy Sciences, University of Bath in February 2020.
Consent for publication
Not applicable.
Competing interests
Dr Saito received personal fees from TAIHO Pharmaceutical Co. Ltd outside the scope of the submitted work. Dr Ozaki received personal fees from Medical Network Systems outside the scope of the submitted work. Dr. Ozieranski’s former PhD student was supported by a grant from Sigma Pharmaceuticals, a UK pharmacy wholesaler and distributor (not a pharmaceutical company). The PhD work funded by Sigma Pharmaceuticals is unrelated to the subject of this paper. Dr Mulinari’s partner is employed by ICON, a global Contract Research Organization whose customers include many pharmaceutical companies. Ms Rickard reports no conflicting interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Said M, Zerhouni E. The role of public–private partnerships in addressing the biomedical innovation challenge. Nat Rev Drug Discov. 2014;13(11):789–790. doi: 10.1038/nrd4438. [DOI] [PubMed] [Google Scholar]
- 2.Yu HWH. Bridging the translational gap: collaborative drug development and dispelling the stigma of commercialization. Drug Discov Today. 2016;21(2):299–305. doi: 10.1016/j.drudis.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Reichman M, Simpson PB. Open innovation in early drug discovery: roadmaps and roadblocks. Drug Discov Today. 2016;21(5):779–788. doi: 10.1016/j.drudis.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Twombly JM, Fälting J, Giorgetti M, Maroney AC, Osswald G. How partnership should work to bring innovative medicines to patients. Drug Discov Today. 2020;25(6):965–968. doi: 10.1016/j.drudis.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Cheng F, Ma Y, Uzzi B, Loscalzo J. Importance of scientific collaboration in contemporary drug discovery and development: a detailed network analysis. BMC Biol. 2020;18(1):138. doi: 10.1186/s12915-020-00868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbasi J. Public-Private Partnership Speeds Investigator Access to Cancer Drugs. JAMA. 2017;317(8):797–797. doi: 10.1001/jama.2017.1089. [DOI] [PubMed] [Google Scholar]
- 7.Baker RG, Hoos AX, Adam SJ, Wholley D, Doroshow JH, Lowy DR, Tabak LA, Collins FS. The Partnership for Accelerating Cancer Therapies. Cancer J. 2018;24(3):111–114. doi: 10.1097/PPO.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bérubé G. How to utilize academic research efforts in cancer drug discovery. Expert Opin Drug Discov. 2019;14(4):331–334. doi: 10.1080/17460441.2019.1582637. [DOI] [PubMed] [Google Scholar]
- 9.Berry SA, Coughlin CR, McCandless S, McCarter R, Seminara J, Yudkoff M, LeMons C. Developing interactions with industry in rare diseases: lessonslearned and continuing challenges. Genet Med. 2020;22(1):219–226. doi: 10.1038/s41436-019-0616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buske K, Jentzsch S, Bechter C. Is Self-Regulation Sufficient? Case of the German Transparency Code. Adm Sci. 2016;6(1):3. doi: 10.3390/admsci6010003. [DOI] [Google Scholar]
- 11.Busfield J. Documenting the financialisation of the pharmaceutical industry. Soc Sci Med. 2020;258:113096. doi: 10.1016/j.socscimed.2020.113096. [DOI] [PubMed] [Google Scholar]
- 12.Nayak RK, Avorn J, Kesselheim AS. Public sector financial support for late stage discovery of new drugs in the United States: cohort study. BMJ. 2019;367:l5766. doi: 10.1136/bmj.l5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naci H, Carter AW, Mossialos E. Why the drug development pipeline is not delivering better medicines. BMJ Br Med J. 2015;351:h5542. doi: 10.1136/bmj.h5542. [DOI] [PubMed] [Google Scholar]
- 14.Lazonick W, Hopkins M, Jacobson K, Sakinç ME, Tulum Ö. US pharma’s financialized business model. Institute for New Economic Thinking Working Paper Series 2017(60). https://www.ineteconomics.org/uploads/papers/WP_60-Lazonick-et-al-US-Pharma-Business-Model.pdf.
- 15.Lo B, Field MJ. Conflict of Interest in Medical Research, Education, and Practice. 2009. https://www.nap.edu/catalog/12598/conflict-of-interest-in-medical-research-education-and-practice [PubMed]
- 16.Moynihan R, Bero L, Hill S, Johansson M, Lexchin J, Macdonald H, Mintzes B, Pearson C, Rodwin MA, Stavdal A, et al. Pathways to independence: towards producing and using trustworthy evidence. BMJ. 2019;367:l6576. doi: 10.1136/bmj.l6576. [DOI] [PubMed] [Google Scholar]
- 17.Rodwin MA. Conflict of interest in the pharmaceutical sector: a guide for public management. Depaul J Health Care L. 2019;21:1. [Google Scholar]
- 18.Grundy Q, Habibi R, Shnier A, Mayes C, Lipworth W. Decoding disclosure: Comparing conflict of interest policy among the United States, France, and Australia. Health Policy. 2018;122(5):509–518. doi: 10.1016/j.healthpol.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Grundy Q, Mazzarello S, Bero L. A comparison of policy provisions for managing “financial” and “non-financial” interests across health-related research organizations: A qualitative content analysis. Account Res. 2020;27(4):212–237. doi: 10.1080/08989621.2020.1748015. [DOI] [PubMed] [Google Scholar]
- 20.Grundy Q, Parker L, Wong A, Fusire T, Dimancesco D, Tisocki K, Walkowiak H, Vian T, Kohler J. Disclosure, transparency, and accountability: a qualitative survey of public sector pharmaceutical committee conflict of interest policies in the World Health Organization South-East Asia Region. Glob Health. 2022;18(1):33. doi: 10.1186/s12992-022-00822-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabbri A. Santos Al, Mezinska S, Mulinari S, Mintzes B: Sunshine Policies and Murky Shadows in Europe: Disclosure of Pharmaceutical Industry Payments to Health Professionals in Nine European Countries. Int J Health Policy Manag. 2018;7(6):504–509. doi: 10.15171/ijhpm.2018.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mental Health Europe. Sunshine and transparency laws and regulations and codes across Europe. https://mhe-sme.org/wp-content/uploads/2017/09/Mapping-of-Sunshine-Laws-in-Europe.pdf
- 23.Lexchin J, Fugh-Berman A. A Ray of Sunshine: Transparency in Physician-Industry Relationships Is Not Enough. J Gen Intern Med. 2021;36(10):3194–3198. doi: 10.1007/s11606-021-06657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozieranski P, Martinon L, Jachiet P-A, Mulinari S. Accessibility and quality of drug company disclosures of payments to healthcare professionals and organisations in 37 countries: a European policy review. BMJ Open. 2021;11(12):e053138. doi: 10.1136/bmjopen-2021-053138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.EFPIA . Annex 2 European federation of pharmaceutical industries and associations. In: Mental Health Europe, editor. Shedding light on transparent cooperation in healthcare: the way forward for sunshine and transparency laws across europe. 2019. [Google Scholar]
- 26.Mulinari S, Martinon L, Jachiet P-A, Ozieranski P. Pharmaceutical industry self-regulation and non-transparency: country and company level analysis of payments to healthcare professionals in seven European countries. Health Policy. 2021;125(7):915–922. doi: 10.1016/j.healthpol.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Adashi EY, Cohen IG: Enforcement of the Physician Payments Sunshine Act: Trust and Verify. JAMA. 2021. 10.1001/jama.2021.13156. [DOI] [PubMed]
- 28.Thacker PD. A few tiny steps towards transparency: how the Sunshine Act shone light on industry’s influence in medicine. BMJ. 2020;370:m3229. doi: 10.1136/bmj.m3229. [DOI] [PubMed] [Google Scholar]
- 29.Goupil B, Balusson F, Naudet F, Esvan M, Bastian B, Chapron A, Frouard P. Association between gifts from pharmaceutical companies to French general practitioners and their drug prescribing patterns in 2016: retrospective study using the French Transparency in Healthcare and National Health Data System databases. BMJ. 2019;367:l6015. doi: 10.1136/bmj.l6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulinari S, Ozieranski P. Disclosure of payments by pharmaceutical companies to healthcare professionals in the UK: analysis of the Association of the British Pharmaceutical Industry’s Disclosure UK database, 2015 and 2016 cohorts. BMJ Open. 2018;8(10):e023094. doi: 10.1136/bmjopen-2018-023094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozieranski P, Csanadi M, Rickard E, Tchilingirian J, Mulinari S. Analysis of Pharmaceutical Industry Payments to UK Health Care Organizations in 2015. JAMA Netw Open. 2019;2(6):e196253. doi: 10.1001/jamanetworkopen.2019.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rickard E, Ozieranski P. A hidden web of policy influence: The pharmaceutical industry’s engagement with UK’s All-Party Parliamentary Groups. PLoS ONE. 2021;16(6):e0252551. doi: 10.1371/journal.pone.0252551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoll M, Hubenschmid L, Koch C, Lieb K. Voluntary disclosures of payments from pharmaceutical companies to healthcare professionals in Germany: a descriptive study of disclosures in 2015 and 2016. BMJ Open. 2020;10(9):e037395. doi: 10.1136/bmjopen-2020-037395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriarty F, Larkin J, Fahey T. Payments reported by the pharmaceutical industry in Ireland from 2015 to 2019: an observational study. Health Policy. 2021;125(10):1297–1304. doi: 10.1016/j.healthpol.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Fabbri A, Grundy Q, Mintzes B, Swandari S, Moynihan R, Walkom E, Bero LA. A cross-sectional analysis of pharmaceutical industry-funded events for health professionals in Australia. BMJ Open. 2017;7(6):e016701. doi: 10.1136/bmjopen-2017-016701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parker L, Karanges EA, Bero L. Changes in the type and amount of spending disclosed by Australian pharmaceutical companies: an observational study. BMJ Open. 2019;9(2):e024928. doi: 10.1136/bmjopen-2018-024928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson J, Moynihan R, Walkom E, Bero L, Henry D. Mandatory Disclosure of Pharmaceutical Industry-Funded Events for Health Professionals. PLoS Med. 2009;6(11):e1000128. doi: 10.1371/journal.pmed.1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozaki A, Saito H, Senoo Y, Sawano T, Shimada Y, Kobashi Y, Yamamoto K, Suzuki Y, Tanimoto T. Overview and transparency of non-research payments to healthcare organizations and healthcare professionals from pharmaceutical companies in Japan: Analysis of payment data in 2016. Health Policy. 2020;124(7):727–735. doi: 10.1016/j.healthpol.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Saito H, Tani Y, Ozaki A, Sawano T, Shimada Y, Yamamoto K, Tanimoto T. Financial ties between authors of the clinical practice guidelines and pharmaceutical companies: an example from Japan. Clin Microbiol Infect. 2019;25(11):1304–1306. doi: 10.1016/j.cmi.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 40.Sawano T, Ozaki A, Saito H, Shimada Y, Tanimoto T. Pharmaceutical Company Payments to Japanese Government Drug Regulation Committee Members. Clin Pharmacol Ther. 2020;108(5):1049–1054. doi: 10.1002/cpt.1892. [DOI] [PubMed] [Google Scholar]
- 41.Ozaki A, Murayama A, Harada K, Saito H, Sawano T, Tanimoto T, Ozieranski P. How Do Institutional Conflicts of Interest Between Pharmaceutical Companies and the Healthcare Sector Become Corrupt? A Case Study of Scholarship Donations Between Department of Clinical Anesthesiology, Mie University, and Ono Pharmaceutical in Japan. Front Public Health. 1875;2022:9. doi: 10.3389/fpubh.2021.762637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozaki A, Saito H, Onoue Y, Sawano T, Shimada Y, Somekawa Y, Tsuji A, Tanimoto T. Pharmaceutical payments to certified oncology specialists in Japan in 2016: a retrospective observational cross-sectional analysis. BMJ Open. 2019;9(9):e028805. doi: 10.1136/bmjopen-2018-028805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito H, Ozaki A, Sawano T, Shimada Y, Tanimoto T. Evaluation of Pharmaceutical Company Payments and Conflict of Interest Disclosures Among Oncology Clinical Practice Guideline Authors in Japan. JAMA Netw Open. 2019;2(4):e192834–e192834. doi: 10.1001/jamanetworkopen.2019.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawano T, Ozaki A, Saito H, Shimada Y, Tanimoto T. Payments From Pharmaceutical Companies to Authors Involved in the Valsartan Scandal in Japan. JAMA Netw Open. 2019;2(5):e193817. doi: 10.1001/jamanetworkopen.2019.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mental Health Europe. Shedding light on transparent cooperation in healthcare: the way forward for sunshine and transparency laws across Europe [https://mhe-sme.org/wp-content/uploads/2019/01/MHE-SHEDDING-LIGHT-REPORT-Final.pdf]
- 46.Ozieranski P, Martinon L, Jachiet P-A, Mulinari S: Tip of The Iceberg? Country- and Company-Level Analysis of Drug Company Payments for Research and Development in Europe. Int J Health Policy Manag. 2022. https://www.ijhpm.com/article_4227.html. [DOI] [PMC free article] [PubMed]
- 47.EFPIA. Codes Committee Activities Report. 2018. https://www.efpia.eu/media/554642/efpia-code-report-2018.pdf
- 48.Mulinari S, Davis C, Ozieranski P: Failure of Responsive Regulation? Pharmaceutical Marketing, Corporate Impression Management and Off-Label Promotion of Enzalutamide in Europe. J White Collar Corp Crime 2020:2631309X2097047. https://journals.sagepub.com/doi/full/10.1177/2631309X20970477.
- 49.Shaw B, Whitney P. Ethics and compliance in global pharmaceutical industry marketing and promotion: The role of the IFPMA and self-regulation. Pharm Policy Law. 2016;18:199–206. [Google Scholar]
- 50.Ozieranski P, Martinon L, Jachiet P-A, Mulinari S. Accessibility and quality of drug company disclosures of payments to healthcare professionals and organisations in 37 countries: A European policy review. BMJ Open. 2021;11(12):e053138. doi: 10.1136/bmjopen-2021-053138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Francer J, Izquierdo JZ, Music T, Narsai K, Nikidis C, Simmonds H, Woods P. Ethical pharmaceutical promotion and communications worldwide: codes and regulations. Philos Ethics Humanit Med. 2014;9(1):7. doi: 10.1186/1747-5341-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.EFPIA. Report on Ethics & Compliance Activities. 2019. https://www.efpia.eu/media/554639/efpia-code-report-2019.pdf
- 53.EFPIA Report on Ethics & Compliance Activities. 2020. https://www.efpia.eu/media/602865/efpia-code-report-2020-20210629.pdf
- 54.Kusumi E, Murayama A, Kamamoto S, Kawashima M, Yoshida M, Saito H, Sawano T, Yamashita E, Tanimoto T, Ozaki A. Pharmaceutical payments to Japanese certified hematologists: a retrospective analysis of personal payments from pharmaceutical companies between 2016 and 2019. Blood Cancer J. 2022;12(4):54. doi: 10.1038/s41408-022-00656-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pashley D, Ozieranski P, Mulinari S. Disclosure of Pharmaceutical Industry Funding of Patient Organisations in Nordic Countries: Can Industry Self-Regulation Deliver on its Transparency Promise? Int J Health Serv. 2022;52(3):347–362. doi: 10.1177/00207314221083871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.IQVIA. Top 10 Pharmaceutical Markets Worldwide. 2019. https://www.iqvia.com/-/media/iqvia/pdfs/canada/2019-trends/top10worldwidesales_en_19.pdf?la=en&hash=5B6D9922E053B42D9F2A1FD7A1883A87
- 57.Sasaki T, Izawa M, Okada Y. Current Trends in Health Insurance Systems: OECD Countries vs. Japan. Neurol Med Chir. 2015;55(4):267–275. doi: 10.2176/nmc.ra.2014-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsuda S. Health Policy in Japan - Current Situation and Future Challenges. JMA J. 2019;2(1):1–10. doi: 10.31662/jmaj.2018-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.ABPI. ABPI Code of Practice. https://www.pmcpa.org.uk/media/3292/2021-abpi-code-of-practice-technical-release.pdf
- 60.ABPI. Increased percentage of healthcare professionals agreeing to be named on Disclosure UK in 2020. https://www.abpi.org.uk/media/news/2021/june/increased-percentage-of-healthcare-professionals-agreeing-to-be-named-on-disclosure-uk-in-2020/
- 61.JPMA. Transparency Guideline for the Relation between Corporate Activities and Medical Institutions. https://www.jpma.or.jp/english/code/transparency_guideline/eki4g60000003klk-att/transparency_gl_intro_2018.pdf
- 62.JPMA. JPMA Code of Practice. https://www.jpma.or.jp/english/code/practice/eki4g60000003js0-att/code_practice201910.pdf
- 63.House of Commons Health Committee: The Influence of the Pharmaceutical Industry. In. London; 2005. https://publications.parliament.uk/pa/cm200405/cmselect/cmhealth/42/42.pdf.
- 64.ABPI. ABPI champions use of ‘Legitimate Interests’ to boost transparency. https://www.abpi.org.uk/media/news/2021/december/abpi-champions-use-of-legitimate-interests-to-boost-transparency/
- 65.Cumberlege J. First Do No Harm. The Independent Medicines and Medical Devices Safety Review. https://www.immdsreview.org.uk/downloads/IMMDSReview_Web.pdf [DOI] [PubMed]
- 66.Cohen D, Mulinari S, Ozieranski P. The whistleblowing drama behind Astellas’s suspension from the ABPI. BMJ. 2019;366:l4353. doi: 10.1136/bmj.l4353. [DOI] [PubMed] [Google Scholar]
- 67.Shiraishi J, Sawada T, Kimura S, Yamada H, Matsubara H, for the KHSG Retraction: Enhanced Cardiovascular Protective Effects of Valsartan in High-Risk Hypertensive Patients With Left Ventricular Hypertrophy; Sub-Analysis of the KYOTO HEART Study. Circ J. 2011;75(4):806–814. doi: 10.1253/circj.CJ-11-0059. [DOI] [PubMed] [Google Scholar]
- 68.Shimokawa H. Urgent Announcement From the Editor-in-Chief Concerning Article Retractions. Circ J. 2013;77(2):552a. doi: 10.1253/circj.CJ-66-0058. [DOI] [PubMed] [Google Scholar]
- 69.The Lancet Editors Retraction—Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study. Lancet. 2013;382(9895):843. doi: 10.1016/S0140-6736(13)61847-4. [DOI] [PubMed] [Google Scholar]
- 70.Yui Y. Concerns about the Jikei Heart Study. Lancet. 2012;379(9824):e48. doi: 10.1016/S0140-6736(12)60599-6. [DOI] [PubMed] [Google Scholar]
- 71.Johnson M. Takeda Pharmaceutical Acknowledges "Inappropriate" Drug Marketing. https://www.pharmaceuticalonline.com/doc/takeda-pharmaceutical-acknowledges-inappropriate-drug-marketing-0001
- 72.Ozaki A, Takita M, Tanimoto T. A call for improved transparency in financial aspects of clinical trials: a case study of the CREATE-X trial in the New England Journal of Medicine. Invest New Drugs. 2018;36(3):517–522. doi: 10.1007/s10637-018-0577-x. [DOI] [PubMed] [Google Scholar]
- 73.Japan Government. Act No. 16 of April 14, 2017. https://www.mhlw.go.jp/file/06-Seisakujouhou-10800000-Iseikyoku/0000213334.pdf
- 74.PMCPA. Code of practice for the pharmaceutical industry. 2019. https://www.pmcpa.org.uk/the-code/previous-abpi-codes-of-practice/codes-of-practice-pdfs-2019-2006/
- 75.IFPMA. Who we are. https://www.ifpma.org/who-we-are/our-membership/full-members/associations/#!/.
- 76.IFPMA . IFPMA Note for Guidance on Fees for Services. 2020. [Google Scholar]
- 77.IFPMA Note for Guidance on Sponsorship of Events and Meetings. https://www.ifpma.org/wp-content/uploads/2020/07/IFPMA-Note-for-Guidance-on-Sponsorship-of-Events-and-Meetings-2020-update.pdf
- 78.IFPMA Note for Guidance on Continuing Medical Education. https://www.ifpma.org/wp-content/uploads/2020/07/IFPMA_Note_for_Guidance_Medical-Education.pdf
- 79.Sagonowsky E. The top 20 pharma companies by 2020 revenue. https://www.fiercepharma.com/special-report/top-20-pharma-companies-by-2020-revenue
- 80.JPMA. Member Companies. https://www.jpma.or.jp/english/about/member/index.html
- 81.ABPI. ABPI Members list. https://www.abpi.org.uk/membership2/abpi-members-list/
- 82.IFPMA. Who we are - Full members – companies. https://www.ifpma.org/who-we-are/our-membership/full-members/companies/#!/
- 83.AstraZeneca. AstraZeneca Code of Ethics - Values, Behaviours, and Policies. https://www.astrazeneca.com/content/dam/az/PDF/Sustainability/Code_of_Ethics_English.pdf
- 84.GSK. Our code of conduct. https://www.gsk.com/media/4800/english-code-of-conduct.pdf
- 85.Astellas . Astellas Group Code of Conduct. 2019. [Google Scholar]
- 86.Takeda. Living Our Values Every Day. Global Code of Conduct. https://www.takeda.com/49d3ff/siteassets/system/who-we-are/corporate-governance/compliance/coc_digital-booklet_external.pdf
- 87.Pharma Japan. JPMA Exchanges Opinions with ABPI. https://pj.jiho.jp/article/p-1300861919321
- 88.EFPIA. Joint Industry Statement: ABPI, EFPIA, Farmindustria, IMC, IFPMA, JPMA, LEEM, PhRMA, vfa. UN Climate Change Conference (COP26): Biopharmaceutical Industry Actions to Tackle Climate Change. https://www.efpia.eu/news-events/the-efpia-view/statements-press-releases/joint-industry-statement-abpi-efpia-farmindustria-imc-ifpma-jpma-leem-phrma-vfa/
- 89.EFPIA Japan. https://www.efpia.eu/about-us/efpia-japan/
- 90.EFPIA Japan. About Enactment of “JPMA Transparency Guideline” - EFPIA Japan Chairman Statement. http://efpia.jp/link/100301Chairman_Dr_Kato_Comment_release_E.pdf
- 91.Rodwin MA. Conflicts of interest and the future of medicine : the United States, France, and Japan. New York and Oxford: Oxford University Press; 2011. [Google Scholar]
- 92.Zetterqvist AV, Merlo J, Mulinari S. Complaints, Complainants, and Rulings Regarding Drug Promotion in the United Kingdom and Sweden 2004–2012: A Quantitative and Qualitative Study of Pharmaceutical Industry Self-Regulation. PLoS Med. 2015;12(2):e1001785. doi: 10.1371/journal.pmed.1001785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Comité de Déontovigilance des Entreprises du Médicament . Self-Regulation: Ethics Surveillance and Monitoring Systems in the Pharmaceutical Industry in Europe. 2013. [Google Scholar]
- 94.Saito H, Ozaki A, Sawano T, Shimada Y, Yamamoto K, Suzuki Y, Tanimoto T. Pharmaceutical Company Payments to the Professors of Orthopaedic Surgery Departments in Japan. JBJS. 2020;102(9):e39. doi: 10.2106/JBJS.19.01005. [DOI] [PubMed] [Google Scholar]
- 95.Rodwin MA, Okamoto A. Physicians’ conflicts of interest in Japan and the United States: lessons for the United States. (0361–6878 (Print)). https://read.dukeupress.edu/jhppl/article-abstract/25/2/343/13200/Physicians-Conflicts-of-Interest-in-Japanand-the?redirectedFrom=fulltext. [DOI] [PubMed]
- 96.Rodwin MA. Reforming pharmaceutical industry-physician financial relationships: lessons from the United States, France, and Japan. 2011;39(4):662–70. [DOI] [PubMed]
- 97.World Health Organization . Regional Office for E, European Observatory on Health S, Policies, Cylus J, Richardson E, Findley L, Longley M, O’Neill C, Steel D: United Kingdom: health system review. Copenhagen: World Health Organization. Regional Office for Europe; 2015. [PubMed] [Google Scholar]
- 98.Saito S, Mukohara K, Bito S. Japanese Practicing Physicians’ Relationships with Pharmaceutical Representatives: A National Survey. PLoS ONE. 2010;5(8):e12193. doi: 10.1371/journal.pone.0012193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saito H, Ozaki A, Kobayashi Y, Sawano T, Tanimoto T. Pharmaceutical Company Payments to Executive Board Members of Professional Medical Associations in Japan. JAMA Intern Med. 2019;179(4):578–580. doi: 10.1001/jamainternmed.2018.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moberly T. NHS joint working with industry is out of public sight. BMJ. 2019;364:l1353. doi: 10.1136/bmj.l1353. [DOI] [PubMed] [Google Scholar]
- 101.Angell M: The Truth About the Drug Companies: How They Deceive Us and What to Do About It. New York: Random House Publishing Group; 2004.
- 102.Lakoff A. The anxieties of globalization: antidepressant sales and economic crisis in Argentina. Soc Stud Sci. 2004;34(2):247–269. doi: 10.1177/0306312704042624. [DOI] [PubMed] [Google Scholar]
- 103.Ozieranski P, King LP. Governing drug reimbursement policy in Poland: The role of the state, civil society, and the private sector. Theory Soc. 2017;46(6):577–610. doi: 10.1007/s11186-017-9300-8. [DOI] [Google Scholar]
- 104.Mulinari S. Regulating Pharmaceutical Industry Marketing: Development, Enforcement, and Outcome of Marketing Rules. Sociol Compass. 2016;10(1):74–86. doi: 10.1111/soc4.12335. [DOI] [Google Scholar]
- 105.Ethical Drug Manufacturing and Marketing Fair Trade Council. Notices and fair competition rules for the pharmaceutical industry,Enforcement Regulations, Operation Standards. http://www.iyakuhin-koutorikyo.org/index.php?action_download=true&kiji_type=1&file_type=2&file_id=2355
- 106.Kobashi Y, Watanabe M, Kimura H, Higuchi A, Ozaki A. Are Pharmaceutical Company Payments Incentivising Malpractice in Japanese Physicians? Int J Health Policy Manag. 2019;8(10):627–628. doi: 10.15171/ijhpm.2019.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heath I, Adlington K. Conflicts of interest within England’s NHS. BMJ. 2017;357:j1590. doi: 10.1136/bmj.j1590. [DOI] [PubMed] [Google Scholar]
- 108.Feldman HR, DeVito NJ, Mendel J, Carroll DE, Goldacre B. A cross-sectional study of all clinicians’ conflict of interest disclosures to NHS hospital employers in England 2015–2016. BMJ Open. 2018;8(3):e019952. doi: 10.1136/bmjopen-2017-019952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rimmer A. Nine in 10 professional organisations say doctors should have to register their financial interests. BMJ. 2021;373:n933. doi: 10.1136/bmj.n933. [DOI] [PubMed] [Google Scholar]
- 110.Coombes R. Doctors’ duty to declare their interests should be enforced, says GMC. BMJ. 2021;373:n1329. doi: 10.1136/bmj.n1329. [DOI] [PubMed] [Google Scholar]
- 111.ABPI. Healthcare professionals: Step up to Disclosure UK. https://www.abpi.org.uk/media/xgfh53z1/disclosure-uk-hcp-leaflet-2020.pdf
- 112.Department of Health, NHS England, ABPI. Moving beyond sponsorship. Joint working between the nhs and pharmaceutical industry. https://www.networks.nhs.uk/nhs-networks/joint-working-nhs-pharmaceutical/documents/joint%20working%20toolkit%20dh.abpi.pdf
- 113.企業活動と医療機関等の関係の 透明性ガイドラインについて(解説). https://www.jpma.or.jp/basis/tomeisei/aboutguide/lofurc0000001g37-att/181018.pdf
- 114.製薬協コード・オブ・プラクティス. https://www.jpma.or.jp/basis/code/lofurc0000001dqt-att/code2.pdf
- 115.Rickard E, Ozieranski P, Mulinari S. Evaluating the transparency of pharmaceutical company disclosure of payments to patient organisations in the UK. Health Policy. 2019;123(12):1244–1250. doi: 10.1016/j.healthpol.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 116.Ozieranski P, Csanadi M, Rickard E, Mulinari S. Under-reported relationship: a comparative study of pharmaceutical industry and patient organisation payment disclosures in the UK (2012–2016). BMJ Open. 2020. 10.1136/bmjopen-2020-037351. [DOI] [PMC free article] [PubMed]
- 117.Ozieranski P, Pitter JG, Rickard E, Mulinari S, Csanadi M. A ‘patient–industry complex’? Investigating the financial dependency of UK patient organisations on drug company funding. Sociology of Health & Illness. 2021;n/a(n/a). 10.1111/1467-9566.13409. [DOI] [PubMed]
- 118.ABPI. Guide: How to raise queries about Disclosure UK data. https://www.abpi.org.uk/publications/guide-to-raising-queries-about-the-information-on-disclosure-uk-for-hcps-and-hcos
- 119.Office of National Statistics. Average Sterling exchange rate: US Dollar. https://www.ons.gov.uk/economy/nationalaccounts/balanceofpayments/timeseries/auss/mret
- 120.Bank of Japan. BOJ’s main statistical data. https://www.stat-search.boj.or.jp/index_en.html
- 121.ABPI . Disclosure UK: Factsheet for HCPs and HCOs. 2021. [Google Scholar]
- 122.ABPI. Mandatory template for the 2021 ABPI Code – Agreed. https://www.pmcpa.org.uk/the-code/mandatory-template-for-the-2021-abpi-code-agreed/
- 123.ABPI. Disclosure UK | What is Legitimate Interests? https://www.abpi.org.uk/media/xlxffupt/abpi-legitimate-interests-factsheet-december-2021.pdf
- 124.ABPI. I’m a HCP or ORDM. https://www.abpi.org.uk/reputation/disclosure-uk/about-disclosure-uk/i-m-a-hcp-or-ordm/
- 125.Directive of the European Parliament and of the Council on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. https://health.ec.europa.eu/system/files/2016-11/dir_2001_20_en_0.pdf [PubMed]
- 126.Ministry of Health LaW . Ethical guidelines for medical and health research involving human subjects. 2021. [Google Scholar]
- 127.OECD. OECD Principles on Good Laboratory Practice. https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/mc/chem(98)17&doclanguage=en
- 128.Eba J, Nakamura K. Overview of the ethical guidelines for medical and biological research involving human subjects in Japan. Jpn J Clin Oncol. 2022;52(6):539–544. doi: 10.1093/jjco/hyac034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.ABPI. Welcome to Disclosure UK. https://search.disclosureuk.org.uk/
- 130.JPMA. Data BOOK 2021. https://www.jpma.or.jp/news_room/issue/databook/2021_en/lofurc0000004we3-att/DB2021_en_full.pdf
- 131.ABPI. Disclosure UK: Frequently Asked Questions. https://www.abpi.org.uk/media/noxp5ivl/disclosure-uk-faqs-2021.pdf
- 132.ABPI. Disclosure UK. https://www.abpi.org.uk/our-ethics/disclosure-uk/
- 133.Medical Governance Research Institute. Yen for Docs Database. https://yenfordocs.jp/en
- 134.PMCPA. Case no. AUTH/2883/10/16 - Voluntary admission by Astellas UK. Patient support programmes. https://www.pmcpa.org.uk/cases/completed-cases/auth28831016-voluntary-admission-by-astellas-uk/
- 135.PMCPA. Case no. AUTH/3285/12/19 - Ex-Employee v Daiichi-Sankyo. https://www.pmcpa.org.uk/cases/completed-cases/auth32851219-ex-employee-v-daiichi-sankyo/
- 136.PMCPA. Case no. AUTH/3138/12/18 - Ex-employee v Indivior. https://www.pmcpa.org.uk/cases/completed-cases/auth31381218-ex-employee-v-indivior/
- 137.PMCPA. Case no. AUTH/3141/12/18 - Health Professional v Merck Sharp & Dohme.https://www.pmcpa.org.uk/cases/completed-cases/auth31411218-health-professional-v-merck-sharp-dohme/
- 138.PMCPA. Case no. AUTH/3140/12/18 - Health professional v Amgen.https://www.pmcpa.org.uk/cases/completed-cases/auth31401218-health-professional-v-amgen/
- 139.PMCPA. Case no. AUTH/3236/8/19 - Health professional v Merck Sharp & Dohme.https://www.pmcpa.org.uk/cases/completed-cases/auth3236819-health-professional-v-merck-sharp-dohme/
- 140.Ozaki A. Conflict of Interest and the CREATE-X Trial in the New England Journal of Medicine. Sci Eng Ethics. 2018;24(6):1809–1811. doi: 10.1007/s11948-017-9966-3. [DOI] [PubMed] [Google Scholar]
- 141.Ozaki A, Saito H, Sawano T, Shimada Y, Tanimoto T. Accuracy of post-publication Financial Conflict of Interest corrections in medical research: A secondary analysis of pharmaceutical company payments to the authors of the CREATE-X trial report in the New England Journal of Medicine. Bioethics. 2021;35(7):704–713. doi: 10.1111/bioe.12854. [DOI] [PubMed] [Google Scholar]
- 142.Hashimoto T, Murayama A, Mamada H, Saito H, Tanimoto T, Ozaki A. Evaluation of financial conflicts of interest and drug statements in the coronavirus disease 2019 clinical practice guideline in Japan. Clin Microbiol Infect. 2022;28(3):460–462. doi: 10.1016/j.cmi.2021.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kida F, Murayama A, Saito H, Ozaki A, Shimada Y, Tanimoto T. Pharmaceutical company payments to authors of the Japanese Clinical Practice Guidelines for Hepatitis C treatment. Liver Int. 2021;41(3):464–469. doi: 10.1111/liv.14761. [DOI] [PubMed] [Google Scholar]
- 144.Moberly T. CCGs fail to declare pharma funding. BMJ. 2018;360:j5911. doi: 10.1136/bmj.j5911. [DOI] [PubMed] [Google Scholar]
- 145.Mandeville KL, Barker R, Packham A, Sowerby C, Yarrow K, Patrick H. Financial interests of patient organisations contributing to technology assessment at England’s National Institute for Health and Care Excellence: policy review. BMJ. 2019;364:k5300. doi: 10.1136/bmj.k5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fierlbeck K. Transparency, Pharmaceuticals, and the Problem of Policy Change. In: Fierlbeck K, Graham J, Herder M, editors. Transparency, Power, and Influence in the Pharmaceutical Industry: Policy Gain or Confidence Game? University of Toronto Press; 2021. pp. 13–62. [Google Scholar]
- 147.Ozaki A, Saito H, Sawano T, Shimada Y, Tanimoto T. Accuracy of post-publication Financial Conflict of Interest corrections in medical research: A secondary analysis of pharmaceutical company payments to the authors of the CREATE-X trial report in the New England Journal of Medicine. Bioethics. 2021;n/a(n/a). https://onlinelibrary.wiley.com/doi/full/10.1111/bioe.12854. [DOI] [PubMed]
- 148.Dukes G, Braithwaite J, Moloney JP. Positive regulation: The complementary role of supports and sanctions. In: Dukes G, Braithwaite J, Moloney JP, editors. Pharmaceuticals, corporate crime and public health. Edward Elgar Publishing; 2014. [Google Scholar]
- 149.Republic of Estonia: Medicinal Products Act. 2005. (2013). https://www.riigiteataja.ee/en/eli/ee/525112013005/consolide/current.
- 150.Republic of Hungary: Act XCVIII of 2006 on the General Provisions Relating to the Reliable and Economically Feasible Supply of Medicinal Products and Medical Aids and on the Distribution of Medicinal Products. 2006. 2011. https://net.jogtar.hu/getpdf?docid=a0600098.tv&targetdate=&printTitle=Act+XCVIII+of+2006&dbnum=62&getdoc=1.
- 151.Republic of Latvia. Cabinet Regulation No. 378 Adopted 17 May 2011. Procedures for Advertising Medicinal Products and Procedures by Which a Medicinal Product Manufacturer is Entitled to Give Free Samples of Medicinal Products to Physicians. https://likumi.lv/ta/en/en/id/230392-procedures-for-advertising-medicinal-products-and-procedures-by-which-a-medicinal-product-manufacturer-is-entitled-to-give-free-samples-of-medicinal-products-to-physicians
- 152.Danish Medicines Agency. Økonomisk støtte (får dækket udgifter) til sundhedspersoners fagrelevante aktiviteter i udlandet eller internationale fagrelevante kongresser og konferencer i Danmark. https://laegemiddelstyrelsen.dk/da/godkendelse/fag-og-sundhedspersoners-oekonomiske-stoette-fra-virksomheder/
- 153.Lexchin J. Voluntary Self-Regulatory Codes: What Should We Expect? Am J Bioeth. 2003;3(3):49–50. doi: 10.1162/15265160360706570. [DOI] [PubMed] [Google Scholar]
- 154.Ozieranski P, McKee M, King L. Pharmaceutical Lobbying under Postcommunism: Universal or Country-Specific Methods of Securing State Drug Reimbursment in Poland. Health Econ Pol’y L. 2012;7:175. doi: 10.1017/S1744133111000168. [DOI] [PubMed] [Google Scholar]
- 155.Ozieranski P, McKee M, King L. The politics of health technology assessment in Poland. Health Policy. 2012;108(2):178–193. doi: 10.1016/j.healthpol.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 156.Mulinari S, Davis C. The will of Congress? Permissive regulation and the strategic use of labeling for the anti-influenza drug Relenza. Soc Stud Sci. 2019;50(1):145–169. doi: 10.1177/0306312719890015. [DOI] [PubMed] [Google Scholar]
- 157.Republic of Portugal. Decreto-Lei n.º 5/2017 de 6 de janeiro 2017. https://extranet.infarmed.pt/pmro/documentacao/Circulares/Decreto-Lei_5_2017.pdf
- 158.Transparence Santé. Bénéficiaires. https://www.transparence.sante.gouv.fr/pages/beneficiaires/
- 159.Republic of Greece: Law 4316/2014 (A ‘270). 2014. https://www-forin-gr.translate.goog/laws/law/3345/nomos-4316-2014?_x_tr_sl=el&_x_tr_tl=en&_x_tr_hl=en&_x_tr_pto=sc#!/?article=66.
- 160.Kesselheim AS, Woloshin S, Lu Z, Tessema FA, Ross KM, Schwartz LM. Internal Medicine Physicians’ Financial Relationships with Industry: An Updated National Estimate. J Gen Intern Med. 2019;34(2):195–197. doi: 10.1007/s11606-018-4688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pakanati KC, Siddamreddy S, Nalleballe K, Thombre V, Sheng S, Veerapaneni K, Yadala S, Kapoor N, Dandu V, Avula A, et al. Industry payments to hospitalist physicians: a 5-year analysis of the Open Payments programme from 2014 to 2018. Intern Med J. 2020;50(12):1547–1550. doi: 10.1111/imj.15116. [DOI] [PubMed] [Google Scholar]
- 162.Elsamadicy AA, Freedman IG, Koo AB, Reeves BC, Havlik J, David WB, Hong CS, Kolb L, Laurans M, Matouk CC, et al. Characteristics of Reported Industry Payments to Neurosurgeons: A 5-Year Open Payments Database Study. World Neurosurg. 2021;145:e90–e99. doi: 10.1016/j.wneu.2020.09.137. [DOI] [PubMed] [Google Scholar]
- 163.DeJong C, Aguilar T, Tseng C-W, Lin GA, Boscardin WJ, Dudley RA. Pharmaceutical Industry-Sponsored Meals and Physician Prescribing Patterns for Medicare Beneficiaries. JAMA Intern Med. 2016;176(8):1114–1122. doi: 10.1001/jamainternmed.2016.2765. [DOI] [PubMed] [Google Scholar]
- 164.Ornstein C, Tigas M, Grochowski Jones R. Now There’s Proof: Docs Who Get Company Cash Tend to Prescribe More Brand-Name Meds. https://www.propublica.org/article/doctors-who-take-company-cash-tend-to-prescribe-more-brand-name-drugs
- 165.Open Payments. The facts about Open Payments data. https://openpaymentsdata.cms.gov/summary
- 166.Adlington K, Godlee F. Disclosure UK: transparency should no longer be an optional extra. BMJ. 2016;354:i3730. doi: 10.1136/bmj.i3730. [DOI] [PubMed] [Google Scholar]
- 167.Republic of Lithuania. Pharmacy law no. Amendment of articles x-709 2, 51 and supplementation of the law by article 511.https://e-seimas.lrs.lt/portal/legalAct/lt/TAP/584c3770f16d11e993e1a0efdbde7def
- 168.Republic of Lithuania. Dėl informacijos apie perleistų verčių gavėjams perleistas vertes, juridinių asmenų duomenų bei sveikatos priežiūros ir (ar) farmacijos specialistų asmens duomenų teikimo valstybinei vaistų kontrolės tarnybai prie lietuvos respublikos sveikatos apsaugos ministerijos ir informacijos apie perleistas vertes bei sveikatos priežiūros ir (ar) farmacijos specialistų asmens duomenų skelbimo tvarkos aprašo patvirtinimo 2020 m. Birželio 23 d. Nr. V-1537, vilnius. https://www.e-tar.lt/portal/lt/legalAct/05a540a0b6ea11eab9d9cd0c85e0b745
- 169.Danish Medicines Agency. Sundhedspersoners tilknytning til virksomheder. https://laegemiddelstyrelsen.dk/da/godkendelse/sundhedspersoners-tilknytning-til-virksomheder/
- 170.Osborn JE. Can I Tell You the Truth-A Comparative Perspective on Regulating Off-Label Scientific and Medical Information. Yale J Health Policy Law Ethics. 2010;10:299. [PubMed] [Google Scholar]
- 171.Vilhelmsson A, Davis C, Mulinari S. Pharmaceutical Industry Off-label Promotion and Self-regulation: A Document Analysis of Off-label Promotion Rulings by the United Kingdom Prescription Medicines Code of Practice Authority 2003–2012. PLOS Med. 2016;13(1):e1001945. doi: 10.1371/journal.pmed.1001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.US Office Office of Inspector General. Open Payments Data: Review of Accuracy, Precision, and Consistency in Reporting. https://oig.hhs.gov/oei/reports/oei-03-15-00220.pdf
- 173.Karanges EA, Nangla C, Parker L, Fabbri A, Farquhar C, Bero L. Pharmaceutical industry payments and assisted reproduction in Australia: a retrospective observational study. BMJ Open. 2021;11(9):e049710. doi: 10.1136/bmjopen-2021-049710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.United Kingdom Government. Government response to the Report of the Independent Medicines and Medical Devices Safety Review. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1005847/IMMDS_Review_-_Government_response_-_220721.pdf
- 175.Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, Blomberg N, Boiten J-W, da Silva Santos LB, Bourne PE, et al. The FAIR Guiding Principles for scientific data management and stewardship. Scientific Data. 2016;3(1):160018. doi: 10.1038/sdata.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kanter GP, Carpenter D, Lehmann L, Mello MM. Effect of the public disclosure of industry payments information on patients: results from a population-based natural experiment. BMJ Open. 2019;9(2):e024020. doi: 10.1136/bmjopen-2018-024020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mulinari S, Ozieranski P. Capitalizing on transparency: Commercial surveillance and pharmaceutical marketing after the Physician Sunshine Act. Big Data Soc. 2022;9(1):20539517211069631. doi: 10.1177/20539517211069631. [DOI] [Google Scholar]
- 178.ActKnowledge. Theory of Change: The Basics. https://www.actknowledge.org/akresources/actknowledge-publications/
- 179.Hood C. Accountability and Transparency: Siamese Twins, Matching Parts, Awkward Couple? West Eur Polit. 2010;33(5):989–1009. doi: 10.1080/01402382.2010.486122. [DOI] [Google Scholar]
- 180.Wolfe S. Mandatory disclosure of all pharmaceutical and medical device companies’ payments to healthcare providers: learning from the USA. Drug Ther Bull. 2022;60(4):52. doi: 10.1136/dtb.2021.000061. [DOI] [PubMed] [Google Scholar]
- 181.Saghy E, Mulinari S, Ozieranski P. Drug company payments to General Practices in England: Cross-sectional and social network analysis. PLOS ONE. 2021;16(12):e0261077. doi: 10.1371/journal.pone.0261077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.King M, Bearman PS. Gifts and influence: Conflict of interest policies and prescribing of psychotropic medications in the United States. Soc Sci Med. 2017;172:153–162. doi: 10.1016/j.socscimed.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The documentary materials supporting the analysis of disclosure rules in the article are cited throughout the Findings. The analysis of disclosure practices and data was based on the Japanese Money Database https://db.tansajp.org/ as well as the Disclosure UK database https://search.disclosureuk.org.uk/ and the repository of drug company methodological notes managed by the Association of the British Pharmaceutical Industry: https://www.abpi.org.uk/reputation/disclosure-uk/methodological-notes-by-company-and-year/.