Abstract
Following a proliferative phase of variable duration, most normal somatic cells enter a growth arrest state known as replicative senescence. In addition to telomere shortening, a variety of environmental insults and signaling imbalances can elicit phenotypes closely resembling senescence. We used p53−/− and p21−/− human fibroblast cell strains constructed by gene targeting to investigate the involvement of the Arf-Mdm2-p53-p21 pathway in natural as well as premature senescence states. We propose that in cell types that upregulate p21 during replicative exhaustion, such as normal human fibroblasts, p53, p21, and Rb act sequentially and constitute the major pathway for establishing growth arrest and that the telomere-initiated signal enters this pathway at the level of p53. Our results also revealed a number of significant differences between human and rodent fibroblasts in the regulation of senescence pathways.
When propagated in culture, normal somatic cells withdraw from the cell cycle after a finite number of divisions and enter an irreversible arrest designated replicative senescence (24). Compared to other proliferative declines, senescence is unique in that it is triggered by the accumulation of cell divisions. The theory that the attrition of telomeres constitutes the molecular counting mechanism for elapsed cell division has gained widespread acceptance (13, 27), although the existence of additional “clocks” has not been ruled out (55). It has been suggested that replicative senescence mechanisms operating in normal somatic cells may have evolved as a defense against the development of neoplasia in long-lived species (2, 72).
A number of phenotypic traits have been associated with senescence (16). Cells become enlarged, acquire a flattened and irregular shape (5), and exhibit increased acidic β-galactosidase activity (SA-β-gal) (20). Senescence is also accompanied by a number of characteristic changes in gene expression (37, 62). Among these, the upregulation of the cyclin-dependent kinase inhibitors (CKIs) p21Cip1/Waf1 (p21) and p16Ink4a (p16) appears to be functionally relevant for the establishment and maintenance of the senescent state (10, 23, 28, 61, 64).
Recent results with mouse embryo fibroblast (MEF) cells derived from the telomerase RNA knockout mouse indicate that the onset of senescence does not correlate with telomere length (7). It is likely, however, that replicative senescence of human cells is at least partially triggered by telomere attrition (63, 72). The main support for this hypothesis comes from the observation that expression of the telomerase reverse transcriptase catalytic subunit (TERT) is sufficient to cause immortalization of some (8) but not all (32, 42) human cell types.
In addition to telomere shortening, a diverse spectrum of signals can cause phenotypes closely resembling senescence: DNA damage (57), oxidative stress (14), histone deacetylase inhibitors (48), methylation inhibitors (71), and expression of some activated oncogenes, such as Ras, Raf, or MEK (59, 75). Some conditions, such as prolonged culture under hyperoxic conditions, can accelerate the rate of telomere attrition (73), but most of the “premature senescence” states occur rapidly and appear to be independent of telomere length (45, 69). Premature senescence states are accompanied by many of the markers associated with replicative exhaustion of normal cells, such as SA-β-gal activity, induction of p21 and/or p16, and others (19, 59).
The tumor suppressor p19ARF (p14ARF in human cells) has emerged as an interesting candidate for linking transformation and senescence responses. Arf is the second protein, in addition to p16, expressed from the INK4a locus, but bears no homology to p16 or any other CKI (26). Arf has been shown to neutralize the ability of Mdm2 to promote p53 degradation, leading to the stabilization and accumulation of p53 (53, 74). Ectopic expression of Arf causes growth arrest with the hallmarks of premature senescence (19, 31). Expression of a variety of proliferation-promoting proteins, such as E1a (17), c-Myc (76), v-Ras (50), v-Abl (54), and E2F-1 (4), upregulates Arf and induces p53 stabilization. Among these, Ras and E2F-1 cause premature senescence, while c-Myc and E1a trigger apoptosis. However, activation of p53 in response to DNA damage appears to be independent of Arf (31, 65).
Arf was shown to be upregulated in aging MEFs (76), and its inactivation elicits immortalization of these cells (31). Upregulation of Arf has also been reported in senescent human fibroblasts (19). However, the expression levels of p53 and Mdm2, the downstream targets of Arf, do not appear to change significantly under the same conditions (67, 68). We have previously knocked out the p21 and p53 genes in normal human fibroblasts (10, 11). In this communication, we use these reagents to perform a genetic epistasis analysis of the Arf-Mdm2-p53-p21 pathway in premature as well as natural senescence states. In summary, loss of either p21 or p53 abrogated the ability of Arf to induce premature senescence, whereas oncogenic Ras [Ha-(G12V)Ras, hereafter referred to simply as Ras] was capable of causing growth arrest in both p21−/− and p53−/− cells. Ectopic expression of Arf increased p53 and p21 protein levels, but had no effect on p16. Ras induced both p21 and p16, but did not upregulate Arf, indicating that induction of p21 is Arf independent. Arf was not upregulated in senescence elicited by replicative exhaustion, arguing that it does not mediate signaling initiated by telomere erosion.
MATERIALS AND METHODS
Cell lines and culture conditions.
LF1 is a normal human diploid fibroblast cell strain derived from embryonic lung (10). p21−/− (10) and p53−/− (11) derivatives of LF1 were constructed by gene targeting. Where indicated, p21−/− and p53−/− cells were immortalized by the expression of hTERT as described previously (69). Human fibroblasts (LF1 or WI-38) were cultured in Ham's F-10 medium supplemented with 15% fetal bovine serum (FBS) in an atmosphere of 2% O2, 5% CO2, and 93% N2 (70). Cell lines U2OS (ATCC HTB 96) and HeLa (ATCC CCL2) were cultured in McCoy's medium and Dulbecco's modified Eagle's medium (DMEM), respectively. Serum supplementation was with 10% FBS in each case. Retrovirus vectors were packaged in the cell line PA317 (ATCC CRL 9078), which was cultured in DMEM supplemented with 10% FBS (69). U2OS, HeLa, and PA317 cells were cultured in air adjusted to 5% total CO2 content. For immortalization, cells were infected with hTERT-expressing retrovirus, and the derived clones were tested for telomerase activity by using the telomeric repeat amplification protocol (TRAP) assay and passaged for at least 30 population doublings (PDs) to ensure that they were immortalized.
Retroviral vectors.
Retroviral vectors of the pBabe series (46) and the vector pWZL-blasticidin were obtained from J. Morgenstern (Millenium Pharmaceuticals). pBabe puro Ha-(G12V)Ras, Arf cDNA, and hTERT cDNA were obtained from S. Lowe (59), Y. Xiong (74), and R. Weinberg (41), respectively. Retroviral vectors were transfected into PA317 cells, and viral supernatants were collected as described previously (69).
Immunoblotting and histochemical methods.
Exponentially growing cells were harvested directly into Laemmli sample buffer and subjected to immunoblotting analysis by standard methods (70). Equivalent loading of lanes was determined by running pilot gels loaded with various dilutions of extracts and analyzing images of Coomassie-stained gels on the Gel-Doc (Bio-Rad) digital gel documentation system and Molecular Analysis software package (36, 70). SA-β-gal activity was detected as described previously (70). Bromodeoxyuridine (BrdU) labeling was performed for 24 h, and labeled cells were detected as described previously (70). The sources of antibodies were as follows: Amersham, actin (N-350); Biosource, Arf (AHZ0472); Calbiotech, Mdm2 (Ab-1), pan-Ras (Ab-3); NeoMarkers, Arf (Ab-2); PharMingen, Rb (G3-245); Santa Cruz, p16 (H-156; sc-759), p21 (C-19; sc-397), p53 (FL-393; sc-6243).
RT-PCR analysis.
Total RNA was isolated from subconfluent cultures by using Trizol reagent (Life Technologies). RNA yields were determined by absorbance measurements, and RNA integrity was verified by agarose gel electrophoresis. Two micrograms of total RNA was reverse transcribed with the SuperScript reverse transcription-PCR (RT-PCR) kit (Life Technologies). One microliter of the reverse-transcribed product was used to program hot-start PCRs. The linear range of amplification for each target and batch of reverse-transcribed product was determined empirically in pilot time course experiments with 4-cycle intervals (47). The β-actin primers were purchased from Clontech, and the Arf primers were GAACATGGTGCGCAGGTTCT and CCTCAGCCAGGTCCACGGG. PCR products were electrophoresed on 1.1% agarose gels containing 0.5 μg of ethidium bromide per ml and analyzed by using the Gel-Doc (Bio-Rad) digital gel documentation system and the Molecular Analysis software package.
Northern hybridization.
Twenty micrograms of total RNA was electrophoresed on a 1.2% formaldehyde–agarose gel and transferred to Zeta-Probe membrane (Bio-Rad). Probes were prepared by PCR amplification from plasmid templates in the presence of 100 μCi of [α-32P]dCTP. The exon 1β INK4a (Arf) probe was made with the primers GTGCGCAGGTTCTTGGTGACC and CTGGTCTTCTAGGAAGCGGCT. The p21 probe was made to exon 3 of p21 with the primers CGGCTGATCTTCTCCAAGAGG and GAACAGTACAGGGTGTGGTCC. Hybridization and washes were performed with the Northern Max kit (Ambion). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe was labeled by random priming of a gel-purified cDNA fragment and hybridized to the same blots after stripping the membranes in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) at 98°C for 2 h.
RNase protection.
The template for the in vitro transcription of the Arf probe was generated by PCR from a P1 clone containing the whole INK4a genomic locus (12). The PCR primers were CTGCTCACCTCTGGTGCCA and GGATCCTAATACGACTCACTATAGGGAGGCCGGACTTTTCGAGGGCCT. The 5′ primer was internal to exon 1β, and the 3′ primer was located in the intron between exons 1β and 2. The template for the synthesis of the control actin probe was synthesized in the same manner from a genomic actin plasmid clone. The primer sequences were TCCCGAGGAGCACCCCGT and GGATCCTAATACGACTCACTATAGGGAGGGGTAGCGGGCCACTCACCT. The 5′ primer was internal to exon 2, and the 3′ primer was located in the intron between exons 2 and 3. The 3′ primer in both cases included the T7 promoter sequence on its 5′ end. The PCR-amplified fragments were gel purified with the Qiagen gel extraction kit and used directly as templates for T7 polymerase transcription as described previously (38). Probes were purified on 5% polyacrylamide–urea gels. Full-length probes were cut out and eluted overnight at 37°C in a mixture of 0.5 M ammonium acetate, 1 mM EDTA, and 0.2% SDS. Five micrograms of total RNA prepared as outlined above was hybridized with both probes by using the HybSpeed RNase protection kit (Ambion). Reactions were displayed on 5% polyacrylamide–urea gels, which were subsequently dried and autoradiographed.
RESULTS
Ectopic expression of Arf elicits premature senescence in a p21-dependent manner.
Human papillomavirus (HPV) E6 antagonized premature senescence by Arf in WI-38 human fibroblasts (19). Since a number of p53 targets, such as p21, 14–3-3δ, and gadd45 have been implicated in growth control, we sought to further define the downstream Arf effector(s). Nonimmortalized p21+/+ and p21−/− LF1 human fibroblasts were infected with a retrovirus vector expressing the Arf cDNA (or a control empty vector), and pooled drug-resistant cells after 5 days of selection were either stained for SA-β-gal activity (Fig. 1) or labeled with BrdU for 24 h (Fig. 2). Arf expression in normal (p21+/+) cells induced a characteristic enlarged and flattened cellular morphology accompanied by SA-β-gal activity (Fig. 1A and B) as well as a strong inhibition of BrdU incorporation (Fig. 2A). In contrast, p21−/− cells did not display senescent phenotypes (Fig. 1C and D) and continued to incorporate BrdU at levels similar to those seen in empty vector-infected cells (Fig. 2A). Both Arf and empty vector-infected p21−/− cells continued to proliferate at the same rate, and single cells formed microcolonies with equivalent efficiencies when monitored by time-lapse photography (69). In addition to investigating the effects of Arf on mid-passage p21+/+ cells (Fig. 1A and B and 2A), we infected p21+/− cells (parental cells of the p21−/− cell line) at late passage (three to four passages prior to senescence) with Arf retrovirus and observed a clear induction of the premature senescence phenotype.
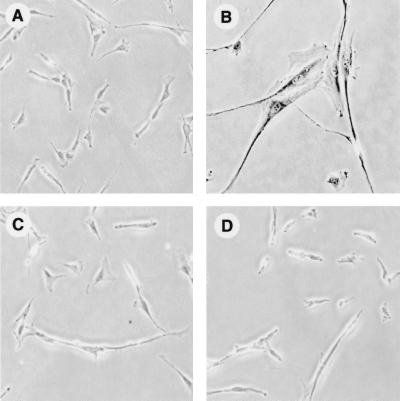
FIG. 1.
Histochemical staining of Arf-expressing p21−/− and p21+/+ cells for SA-β-gal activity. Cells were infected with pBabe-puro-Arf or empty pBabe-puro vectors, selected with puromycin for 5 days, stained for SA-β-gal activity, and photographed. (A) p21+/+ cells, pBabe-puro. (B) p21+/+ cells, pBabe-puro-Arf. (C) p21−/− cells, pBabe-puro. (D) p21−/− cells, pBabe-puro-Arf. All panels are shown at equal magnification.
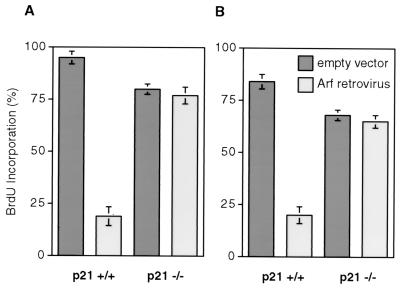
FIG. 2.
BrdU incorporation assays of Arf-expressing p21−/− and p21+/+ cells. (A) Nonimmortalized p21+/+ and p21−/− cells. (B) hTERT-immortalized p21+/+ and p21−/− cells. Cells were treated as indicated in Fig. 1. At the end of the drug selection, cells were labeled for 24 h with BrdU and subsequently processed for histochemical in situ detection of BrdU incorporation. Since hTERT-immortalized cells are puromycin resistant, the pWZL-Blast vector was used in panel B. Cells were scored microscopically in random fields; a minimum of 200 cells were scored for each determination. The percent means and standard deviations of BrdU-positive cells are presented.
We and others (45, 69) have shown that Ras can elicit premature senescence in cells that have been engineered to express telomerase activity. To investigate the effect of telomerase activity on Arf-induced premature senescence, we infected hTERT-immortalized p21+/+ and p21−/− cells with Arf-expressing or control empty retrovirus vectors. The presence of telomerase did not reduce the inhibition of BrdU uptake by Arf in p21+/+ cells or affect the BrdU uptake in p21−/− cells (Fig. 2B). Similarly, telomerase did not alter the other effects (or lack thereof) of Arf on nonimmortalized p21+/+ or p21−/− cells discussed above. Therefore, both oncogenic Ras and Arf can induce a premature senescent phenotype in a telomerase-independent manner.
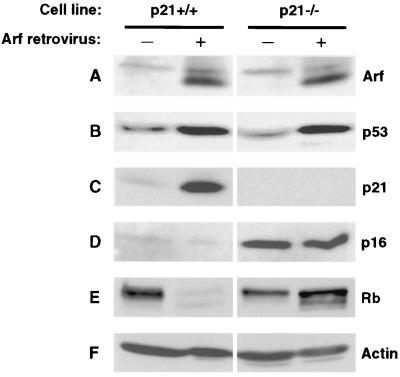
In subsequent experiments Arf-infected cells were analyzed by immunoblotting. First, we confirmed the expression of exogenously introduced Arf in both p21+/+ and p21−/− cells (Fig. 3A). Ectopic expression of Arf resulted in upregulation of p53 (Fig. 3B) in both p21+/+ and p21−/− cells. Arf caused a strong increase in p21 expression in p21+/+ cells (Fig. 3C), while p21−/− cells remained completely negative. Interestingly, Arf elicited no change in p16 expression in either p21+/+ or p21−/− cells (Fig. 3D). The marked difference in p16 expression between empty vector-infected p21+/+ and p21−/− cells is due to the fact that we used early-passage cultures of LF1 (p21+/+) cells, which express low levels of p16, whereas, due to the two cycles of gene targeting necessary to generate p21−/− cells, these cultures were of significantly more advanced age. The upregulation of p16 during the extended-life span phase of p21−/− cells has been previously noted (10). p21−/− cells proliferate somewhat more slowly than young p21+/+ cells and, despite the increased levels of p16, contain significant amounts of hyperphosphorylated Rb in both the absence and presence of ectopic Arf (Fig. 3E). In contrast, in young p21+/+ cells, Arf caused a shift of Rb to the hypophosphorylated form, which correlated with the induction of the premature senescence phenotype. Since p16 was expressed at very low levels under these conditions, the strong upregulation of p21 is apparently sufficient to elicit the observed inhibition of growth. It should be noted that the overall decrease in Rb protein levels that occurred concomitantly with the shift to hypophosphorylated status has been observed previously and occurs in a variety of nonproliferative states, including quiescence (59). Taken together, these results show that Arf induces p21 but not p16 expression, that p21 is necessary for the establishment of the premature senescence phenotype, and that this process is independent of hTERT expression.
FIG. 3.
Immunoblot analysis of Arf-expressing p21+/+ and p21−/− cells. Exponentially growing cells were infected with pBabe-puro-Arf or empty pBabe-puro viruses, and puromycin-resistant pools of cells were selected as indicated in Fig. 1. For each infection, 12 10-cm-diameter dishes were harvested, pooled, and processed for immunoblotting. (A) Arf. (B) p53. (C) p21. (D) p16. (E) Rb. (F) Actin.
Oncogenic Ras can elicit premature senescence in both p21+/+ and p21−/− cells.
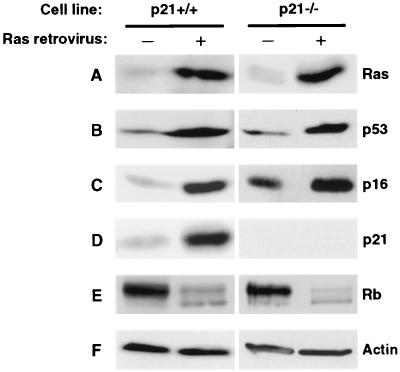
It has been reported that loss of p53, but not of p21, allowed MEFs to bypass Ras-induced growth arrest (51). In order to investigate the role of p21 in this process in human cells, we introduced Ras into both p21+/+ and p21−/− fibroblasts. A senescent phenotype was observed in drug-resistant pools derived from both cell lines after infection with a Ras retrovirus vector, but not a control empty vector. In subsequent experiments, the drug-resistant pools were further analyzed by immunoblotting. First, expression of the exogenously introduced Ras was confirmed in both p21+/+ and p21−/− cells (Fig. 4A). Consistent with previous reports (22, 35, 59), ectopic expression of Ras resulted in the upregulation of p53 and p16 in p21+/+ cells (Fig. 4B and C). The same result was observed in p21−/− cells. Interestingly, Ras was capable of upregulating not only the basal levels of p16 in normal early-passage (p21+/+) cells, but also further increased the elevated p16 levels found in p21−/− cells. Given the elevated basal levels of p16 in aged (p21−/−) cells, the Ras-elicited increase in p16 expression was of smaller magnitude than that observed in young (p21+/+) cells; however, the upregulation in p21−/− cells was consistent and reproducibly observed in several independent experiments. As expected, p21 expression was strongly upregulated in p21+/+, but not p21−/− cells (Fig. 4D). In contrast to the effects of Arf, Ras caused a shift of Rb to the hypophosphorylated form, not only in normal (p21+/+) cells, but also in p21−/− cells (Fig. 4E). Taken together, these results show that Ras upregulates both p21 and p16 expression and that the absence of p21 is not sufficient to bypass the induction of premature senescence. The correlation of the shift to a hypophosphorylated Rb status with growth arrest in response to either Arf or Ras expression implicates Rb as an important and common downstream effector of premature senescence, although clearly Arf and Ras can reach this target by different pathways.
FIG. 4.
Immunoblot analysis of Ras-expressing p21+/+ and p21−/− cells. Exponentially growing cells were infected with pBabe-puro-Ha-Ras(G12) or empty pBabe-puro viruses and processed as indicated in Fig. 3. (A) Ras. (B) p53. (C) p16. (D) p21. (E) Rb. (F) Actin.
Both Arf and Ras upregulate p21 in a p53-dependent manner.
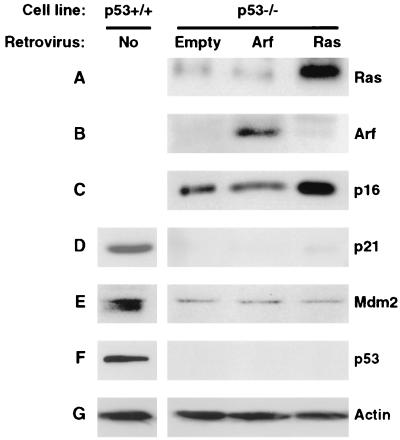
The requirement for p53 in both Arf- and Ras-induced premature senescence has been investigated by interfering with the stability and/or activity of endogenous p53 protein by the ectopic expression of HPV E6, Mdm2, or dominant-defective p53 proteins (19, 59). We have previously constructed gene-targeted p53−/− derivatives of LF1 cells (11) and were thus in position to investigate this issue by using complete genetic ablation of p53. In agreement with previous indications (19, 58), no growth arrest was observed in p53−/− cells after ectopic expression of Arf, while a typical premature senescent phenotype was elicited by the introduction of Ras. In subsequent experiments, the drug-resistant pools were further analyzed by immunoblotting. First, expression of the exogenously introduced Arf and Ras proteins was confirmed (Fig. 5A and B). As was observed in p21−/− cells, expression of Ras upregulated p16, whereas the expression of Arf did not (Fig. 5C). Likewise, the basal level of p16 was significantly elevated in p53−/− cells relative to early-passage normal (p53+/+) LF1 cells; since the p53−/− cells were immortalized during their extended-life span phase, this result is consistent with elevated p16 expression observed in p21−/− cells and further indicates that the age-dependent upregulation of p16 is independent of both p21 and p53 expression. No upregulation of p21 was observed in p53−/− cells in response to either Arf or Ras expression (Fig. 5D). Interestingly, the basal levels of both the p21 and Mdm2 proteins were significantly depressed in p53−/− cells compared to those in normal early-passage (p53+/+) LF1 cells, indicating that p53 is required not only for induced expression, but also for basal expression of these proteins (Fig. 5D and E). These results are in agreement with data from p53−/− derivatives of Li Fraumeni fibroblasts for p21 (6, 34) and p53−/− MEFs for Mdm2 (44). In summary, we have shown that loss of p53 function abrogates Arf- but not Ras-induced premature senescence, and that the upregulation of p21 is in both cases p53 dependent.
FIG. 5.
Immunoblot analysis of Arf- or Ras-expressing p53−/− cells. Exponentially growing cells were infected with pBabe-bleo-Arf, pBabe-bleo-Ras, or empty pBabe-bleo viruses, and bleomycin-resistant pools were selected and processed as indicated in Fig. 3. Uninfected p53+/+ cells were included as controls for the immunoblotting detection of p21, Mdm2, and p53. To facilitate this analysis, p53−/− cells immortalized by the expression of hTERT were used. (A) Ras. (B) Arf. (C) p16. (D) p21. (E) Mdm2. (F) p53. (G) Actin.
Ectopic expression of Ras does not upregulate Arf.
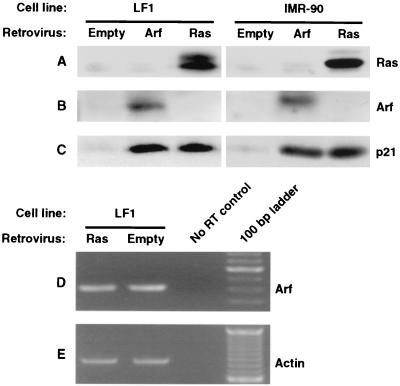
One explanation for the observed p53 dependence of p21 induction by Ras would be that Ras upregulates Arf and thus activates the Arf-Mdm2-p53-p21 pathway. This hypothesis is consistent with observations that in MEFs, Arf is upregulated in response to diverse oncogenic stimuli, including Ras (50). Unfortunately, in spite of repeated attempts, we did not succeed in ablating Arf expression in normal human fibroblasts by using antisense methods (data not shown). We therefore asked next whether the ectopic expression of Ras could induce the expression of Arf (Fig. 6). This analysis was hampered by the fact that the basal levels of Arf protein are so low as to be undetectable by immunoblotting in LF1 or IMR-90 fibroblasts (Fig. 6B). Expression of Ras likewise did not result in the expression of detectable levels of Arf protein. This situation was not remedied by testing several antibodies or by combining immunoprecipitation of large amounts of crude extract with subsequent immunoblotting. Ectopically expressed Arf was readily detected in both cell lines by simple immunoblotting, as was the upregulation of p21 by either Arf or Ras (Fig. 6C). Similar results were obtained with WI-38 fibroblasts. We next used RT-PCR as a more sensitive method of detection. Arf mRNA was clearly detectable, and quantitative PCR using an actin standard failed to reveal any upregulation by the ectopic expression of Ras (Fig. 6D and E). The fidelity of our method of RT-PCR quantification of Arf mRNA was supported by Northern hybridization and RNase protection methods of detection (see below and Fig. 8 and 9). We therefore conclude that ectopic expression of Ras does not elicit upregulation of Arf expression in normal human fibroblasts.
FIG. 6.
Absence of Arf induction by Ras in normal human fibroblasts. Exponentially growing cells were infected with pBabe-puro-Arf, pBabe-puro-Ras, or empty pBabe-puro viruses and processed as indicated in Fig. 3. (A, B, and C) Immunoblotting analysis of Ras, Arf, and p21, respectively. Two different cell strains of normal human fibroblasts were used: LF1 (left panels) and IMR-90 (right panels). (D and E) RT-PCR analysis of Arf expression in LF1 cells. Quantitative RT-PCR was performed as indicated in Materials and Methods. In the experiment shown, the Arf (D) and β-actin (E) reactions were amplified for 20 and 32 cycles, respectively.
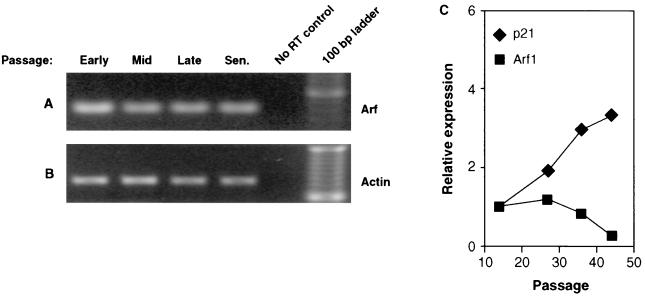
FIG. 8.
Absence of Arf induction during replicative aging of normal human fibroblasts. (A and B) RT-PCR analysis of Arf expression in LF1 cells. In the experiment shown, the Arf (A) and β-actin (B) reactions were amplified for 20 and 32 cycles, respectively. (C) Northern hybridization analysis of Arf and p21 expression in LF1 cells. Data were quantified by PhosphorImager analysis and normalized to a GAPDH signal. Northern hybridization was performed on two separate occasions with samples prepared from cells that were independently passaged into senescence. Both experiments yielded consistent results; one representative experiment is shown. Cells were grown in a 2% O2 atmosphere.
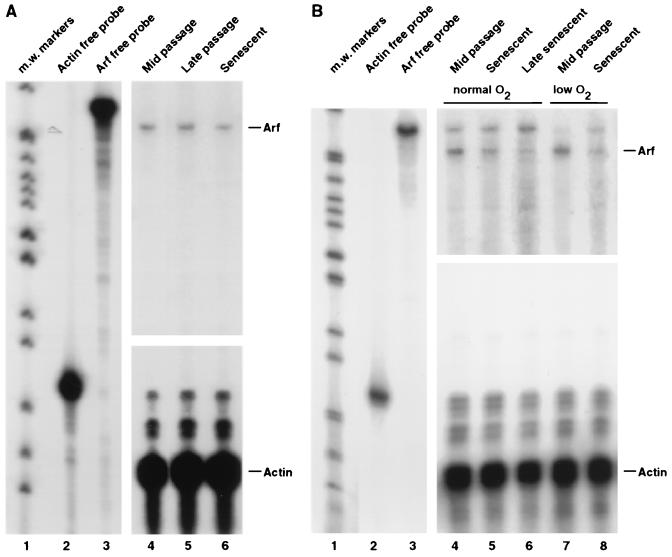
FIG. 9.
Absence of Arf induction during replicative aging of normal human fibroblasts. (A) RNase protection analysis of LF1 cells. (B) RNase protection analysis of WI-38 cells grown under normoxic (lanes 4, 5, and 6) and hypoxic (lanes 7 and 8) conditions. m.w., molecular weight.
Arf is not induced in senescence elicited by replicative exhaustion.
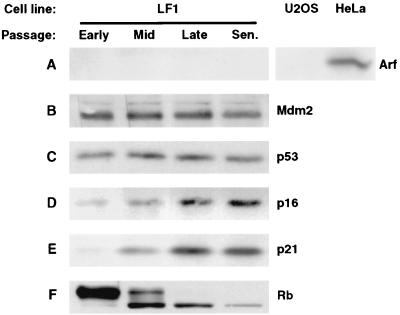
Normal LF1 fibroblasts were serially passaged until they entered senescence, and protein and RNA samples were collected at several stages. The expression of Arf protein was not detected at any time by immunoblotting analysis (Fig. 7A) or by immunoprecipitation followed by immunoblotting. The expression levels of Mdm2 and p53 proteins, the downstream targets of Arf, likewise did not change at any time during the passaging regimen or in senescent cultures (Fig. 7B and C). The absence of changes in Mdm2 and p53 expression in senescence has also been noted by others (67, 68). In contrast, we readily observed upregulation of p16 and p21, as well as a shift of Rb to the hypophosphorylated form in late-passage and senescent cells (Fig. 7D, E, and F). The changes in p16 and p21 expression and Rb phosphorylation status have been previously observed by several groups and validate that characteristic age-dependent changes were indeed taking place in our cultures.
FIG. 7.
Immunoblot analysis of normal human fibroblasts during replicative aging. Human fibroblasts (LF1 cell line) were serially subcultured from early passage until they acquired the senescent phenotype. Protein extracts were prepared at the indicated times: early passage, p14; mid-passage, p28; late passage, p37; senescence, p44. U2OS and HeLa cells were included as negative and positive controls for the immunoblotting detection of Arf. (A) Arf. (B) Mdm2. (C) p53. (D) p16. (E) p21. (F) Rb.
Because Arf protein expression was below the level of detection, we sought to rigorously examine Arf gene expression by using three different methods of mRNA quantification. First, we performed quantitative RT-PCR on RNA extracted from cells at various passages (Fig. 8A and B). This analysis revealed no significant changes in Arf mRNA expression. Second, we determined Arf mRNA abundance by Northern hybridization with a probe directed against exon 1β of the INK4a locus that does not detect the p16 mRNA (Fig. 8C). This analysis showed Arf mRNA expressed at a relatively constant level in early-, mid-, and late-passage cells and declining somewhat in senescence. The data were normalized against GAPDH mRNA expression; as a further internal control, we examined the expression of p21 mRNA, which showed a typical age-dependent induction. Finally, we examined Arf mRNA expression by RNase protection with a probe directed against exon 1β (Fig. 9A). RNase protection was used because it is more sensitive than Northern hybridization, and we were thus able to achieve a significantly better signal/noise ratio. These experiments likewise showed Arf mRNA expression at a constant level in growing cells, with a slight downturn in senescent cells. In summary, the analysis of Arf expression produced consistent results by three distinct methods of mRNA quantification on samples prepared from cells that were passaged independently into senescence on two separate occasions.
One group has reported that Arf mRNA expression, quantified by RT-PCR, becomes upregulated in senescence by a factor of six- to sevenfold (19). We therefore sought to further investigate possible causes for this discrepancy. One possibility is that Arf expression may be cell line specific. Dimri et al. (19) used WI-38 fibroblasts, while we used the LF1 cell strain (10). We therefore passaged WI-38 cells into senescence and examined Arf mRNA expression by RNase protection (Fig. 9B, lanes 7 and 8); no Arf mRNA upregulation was apparent in these experiments. Another possibility is culture conditions: we routinely passage our cells under hypoxic (2% O2) conditions, while Dimri et al. (19) used normoxic conditions. However, WI-38 cells passaged into senescence under either hypoxic or normoxic conditions did not show increased expression of Arf mRNA (Fig. 9B, lanes 4, 5, 7, and 8). Yet another possibility is that changes in Arf expression may be transient; this was suggested by studies showing that p21 mRNA is upregulated in late-passage and early-senescent cells, but becomes downregulated as cells are allowed to persist in the senescent state (64). This explanation was considered unlikely, because previous experiments (Fig. 8C) showed that Arf expression was clearly dropping at times that p21 expression was still increasing. We nevertheless examined Arf mRNA expression in WI-38 cells at late passage and in early, mid-, and late senescence and found no upregulation at any time. We did, however, obtain evidence that the downregulation of Arf we observed previously occurs in mid- to late senescence (Fig. 9B, lanes 5 and 6).
DISCUSSION
The role of p21 and p16 in natural and premature senescence states.
The fact that the ectopic expression of either p21 or p16 can elicit a premature senescence-like state (40) indicates that either protein, if expressed at sufficient levels, can limit proliferation. It is therefore of interest to understand what constitutes biologically relevant levels of expression, the manner in which expression is regulated, and the functional interplay between the two responses under any physiological condition. The results reported here are indicative of significant differences in senescence signaling pathways between human and rodent fibroblasts. In the future, it will be important to thoroughly explore tissue-specific as well as well as species-specific differences in both replicative and induced senescence pathways. Provocative tissue-specific differences in human senescence pathways have already begun to emerge (21).
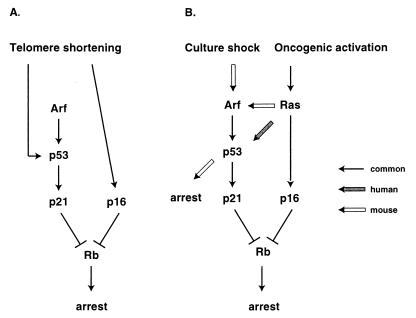
p21−/− human fibroblasts bypassed senescence and entered into an extended life span phase of growth (10), whereas p21−/− MEFs did not show a significant deviation from wild-type cells in a 3T3 passaging regimen (51). In agreement, the upregulation of p21 in aging and senescent human fibroblasts is well documented (1, 64, 66), whereas rodent fibroblasts do not display this response (39, 51). Given that p53−/− MEFs undergo spontaneous immortalization at high frequency (29), it would appear that a p53 target other than p21 is the relevant downstream effector of senescence in rodent cells (Fig. 10).
FIG. 10.
Summary of p53-p21 and p16-Rb senescence pathways in human and rodent fibroblasts. (A) Replicative senescence. Only human-specific pathways are shown, since the existence of a non-crisis arrest due to telomere attrition has not been convincingly demonstrated in the mouse. (B) Induced (or premature) senescence states. For simplicity, only Ras-mediated oncogenic activation and Arf-mediated “culture shock” (63) are shown, although a number of other effectors and/or stimuli are known to be capable of inducing senescence-like arrest. Common and human- and mouse-specific pathways are shown.
A similar situation applies to some premature senescence states: neither Ras nor Arf caused growth arrest in p53−/− MEFs, but could do so in p21−/− MEFs (25, 31, 51). These results argue that in rodent cells, Ras and Arf can trigger a p53-dependent growth arrest pathway that is independent of p21. In contrast, in human fibroblasts, p53 and p21 appear to be acting in a linear pathway, with p21 being the relevant downstream effector: both p53−/− and p21−/− cells escaped senescence due to replicative exhaustion (10; A. Dutriaux and J. Sedivy, unpublished observations), both p53−/− and p21−/− cells were arrested by Ras, and neither p53−/− nor p21−/− cells were arrested by Arf (this communication).
While interference with the p16-Rb pathway contributes to bypass of senescence in human cells (61), loss of Rb in MEFs promoted growth, but did not elicit immortalization (58, 76). Likewise, Ras did not arrest p53−/− MEFs, in spite of the observation that p16 was upregulated under these conditions (59). These results indicate that the upregulation of p16 elicited by either aging or activation of Ras is not sufficient to significantly reduce proliferation in MEFs.
Growth arrest mediated by Ras or Raf requires activation of MEK (35, 75), and recent evidence suggests that the ensuing activation of the Ets1 and Ets2 transcription factors is responsible for upregulating the p16 promoter (49). In human fibroblasts, relatively high levels of p16 can be tolerated without complete abrogation of proliferation, as evidenced by the growth of p21−/− cells during their extended-life span phase, albeit at slower rates than presenescent p21+/+ cells (10). The fact that Ras could cause arrest of p21−/− cells (this communication) suggests that either the further upregulation of p16 elicited by Ras may become growth limiting or yet another Ras effector is responsible for the arrest. Two lines of evidence suggest that the high levels of p16 found in p21−/− cells may be limiting for growth: expression of a p16-insensitive cyclin D1-Cdk4 fusion protein (33) notably accelerated proliferation (W. Wei and J. Sedivy, unpublished observations), and spontaneous loss of p16 expression has been observed in p21−/− cells, indicating that such loss provides the cells with a selective advantage (A. Smogorzewska and T. De Lange, personal communication). It has been reported that inhibition of growth by ectopic p16 requires inhibition of cyclin E-Cdk2 complexes by redistribution of p21 and/or p27 from the pool normally bound by cyclin D-Cdk4/6 complexes (30, 43). Since normal human fibroblasts (such as LF1) express abundant p27, this mechanism is likely still operative in p21−/− cells. In agreement with this interpretation, infection of p21−/− cells with a p16-expressing retrovirus vector elicited a senescent phenotype (W. Wei and J. Sedivy, unpublished observations), indicating that p16, if expressed at sufficient levels, can cause growth arrest in the absence of p21.
The relationship between p16 and p21.
In human fibroblasts, the upregulation of p16 in either natural or premature senescence states appears to be independent of the Arf-Mdm2-p53-p21 pathway, since knockout of either p21 or p53 did not prevent the upregulation of p16 in aging cells and the ectopic expression of Arf did not upregulate p16 (this communication). The upregulation of p21 in aging cells is likely dependent on a signal initiated by telomere shortening, since introduction of hTERT into presenescent fibroblasts blocked the upregulation of p21 (44; Wei and Sedivy, unpublished). On the other hand, introduction of hTERT did not reduce the high levels of p16 in p21−/− or p53−/− cells in their extended-life span phase. The failure of hTERT to prevent the aging-related upregulation of p16 has also been reported in other cell types (32). These results argue that in addition to being insensitive to the activation of the p53-p21 pathway, the regulation of p16 expression does not respond in an obvious way to the elimination of the telomere-initiated senescence signal.
In human fibroblasts, p21 appears to be the primary determinant of senescence, since p21−/− cells are defective in this response (10), whereas expression of the p16-insensitive cyclin D1-Cdk4 fusion protein did not elicit significant life span extension in normal fibroblasts (Wei and Sedivy, unpublished). Since biochemically p21 is a potent inhibitor of both Cdk4/6 and Cdk2 kinases, it has the ability, if expressed at sufficient levels, to block the phosphorylation of Rb and thus to cause growth arrest even in the complete absence of p16. p21 is therefore likely to be a critical mediator of replicative senescence in human cell types that upregulate its expression as part of their natural senescence program (10). Some cell types, however, do not appear to upregulate p21 in senescence (9, 18). The reasons behind these differences are currently not understood.
The role of Arf in natural and premature senescence states.
In contrast to senescence elicited by replicative exhaustion, the premature senescence states triggered by either Arf or Ras proceed unimpeded in hTERT-immortalized cell lines (69; this communication). Arf has been reported to be upregulated in senescent human fibroblasts (19), but in a careful reexamination, we have failed to find any evidence for this upregulation. The downstream effectors p21 and p16 appear to be shared between premature and natural senescence states, although the manner in which they are engaged can vary. Thus, Arf appears to induce solely the Mdm2-p53-p21 pathway in human fibroblasts, while Ras induced independently both p21 and p16 and perhaps another growth inhibitory pathway yet to be defined.
Both the regulation of the Arf gene and its downstream effectors appear to differ between human and rodent fibroblasts. First, Arf was upregulated in aging and senescent MEFs (76), while its expression remained unchanged under the same conditions in human fibroblasts and even showed downregulation in late-senescent cells (this communication). Second, ectopic expression of Arf arrested p21−/− MEFs (25), while it could not arrest p21−/− human fibroblasts (this communication). Therefore, the growth inhibitory activity of Arf appears to be mediated solely by the Arf-Mdm2-p53-p21 pathway in human fibroblasts. Third, Ras upregulated Arf in rodent cells (50, 56), but it could not do so in human fibroblasts (this communication). Recent evidence in human cells indicates that the effector pathway linking Ras to Arf-independent but p53-dependent induction of p21 may proceed through induction of the PML protein and subsequent phosphorylation of p53 on Ser15 (22). In rodent cells, Ras promotes the recruitment of p53 into PML bodies where it is acetylated by p300/CBP (52); to what extent this mechanism functions in human cells is not known.
Premature senescence states, such as those elicited by Arf or Ras, appear to result from imbalances in signaling networks that regulate growth and proliferation and may have evolved as natural defense mechanisms against neoplastic transformation. Mounting evidence that the senescence response observed in MEFs is not a telomere length-dependent event (7) have led to proposals that it may be caused by environmental factors, such as in vitro culture conditions (63, 72). Senescence in rodent cells could then be more appropriately likened to premature senescence states in human cells. This line of reasoning agrees with our observations that Arf, the expression of which is known to respond to signaling imbalances (60), but not DNA damage (65) or oxidative stress (15), is upregulated in senescent rodent cultures but not human cultures.
Pathways of natural senescence.
It is widely assumed that in human cells, critically shortened telomere ends can initiate a signaling cascade that eventually results in the upregulation of p21 and/or p16. Although much of this pathway remains to be elucidated, the experiments reported here have begun to delineate the downstream components. Genetic analysis has implicated p21 as the key regulator, with p53 as the immediate upstream activator and Rb as the probable downstream target. The placement of Rb at the bottom of this regulatory hierarchy is consistent with our observations that all senescence states, either natural or premature, were correlated with the presence of hypophosphorylated Rb. The failure to detect changes in the expression of Arf or Mdm2 in senescent human fibroblasts makes it unlikely that these proteins are involved in transmitting the telomere-initiated signal. We therefore propose that the telomere signal enters the p53-p21-Rb pathway at the level of p53.
What is known about signaling components upstream of p53? Several groups have documented that the levels of p53 protein do not rise in senescence (67, 68), a result we have corroborated here. This is in contrast to the response elicited by most forms of DNA damage, which result in a significant accumulation of p53 protein caused by its stabilization. Likewise, the phosphorylation of p53 elicited by DNA damage and senescence involves overlapping but distinct sites (67), indicating that telomere shortening and DNA damage are not identical physiological states. Although p53 does not accumulate in senescence, it becomes activated as a transcriptional activator (3). One mechanism of activation may involve ADP ribosylation by the enzyme poly(ADP-ribose) polymerase (PARP) and the ATM pathway (67), while the other may involve acetylation by p300/CBP and the PML pathway (22, 52). Further work is needed to delineate these responses and link them with the signals elicited by critically shortened telomeres.
ACKNOWLEDGMENTS
We gratefully acknowledge J. Morgenstern for pWZL-Blast retrovirus vector, S. Lowe for Ha-(G12V)Ras cDNA, D. Sidransky for a P1 clone of the genomic INK4a locus, R. Weinberg for hTERT cDNA, Y. Xiong for Arf cDNA, and D. DiMaio for helpful comments on the manuscript.
This work was supported by NIH grant AG16694 to J.M.S.
REFERENCES
- 1.Alcorta D A, Xiong Y, Phelps D, Hannon G, Beach D, Barrett J C. Involvement of the cyclin-dependent kinase inhibitor p16(INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci USA. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artandi S E, DePinho R A. Mice without telomerase: what can they teach us about human cancer? Nat Med. 2000;6:852–855. doi: 10.1038/78595. [DOI] [PubMed] [Google Scholar]
- 3.Atadja P, Wong H, Garkavtsev I, Veillette C, Riabowol K. Increased activity of p53 in senescing fibroblasts. Proc Natl Acad Sci USA. 1995;92:8348–8352. doi: 10.1073/pnas.92.18.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates S, Phillips A C, Clark P A, Stott F, Peters G, Ludwig R L, Vousden K H. p14ARF links the tumour suppressors RB and p53. Nature. 1998;395:124–125. doi: 10.1038/25867. [DOI] [PubMed] [Google Scholar]
- 5.Bayreuther K, Rodemann H P, Hommel R, Dittmann K, Albiez M, Francz P I. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc Natl Acad Sci USA. 1988;85:5112–5116. doi: 10.1073/pnas.85.14.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bean L J H, Stark G R. Phosphorylation of serines 15 and 37 is necessary for efficient accumulation of p53 following irradiation with UV. Oncogene. 2001;20:1076–1084. doi: 10.1038/sj.onc.1204204. [DOI] [PubMed] [Google Scholar]
- 7.Blasco M A, Lee H W, Hande M P, Samper E, Lansdorp P M, Depinho R A, Greider C W. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 8.Bodnar A G, Quellette M, Frolkis M, Holt S E, Chiu C P, Morin G B, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 9.Brenner A J, Stampfer M R, Aldaz C M. Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation. Oncogene. 1998;17:199–205. doi: 10.1038/sj.onc.1201919. [DOI] [PubMed] [Google Scholar]
- 10.Brown J P, Wei W, Sedivy J M. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- 11.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown J P, Sedivy J M, Kinzler K W, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 12.Cairns P, Polasik T J, Eby Y, Tokino K, Califano J, Merlo A, Mao L, Herath J, Jenkins R, Westra W, Rutter J L, Buckler A, Gabrielson E, Tockman M, Cho K R, Hedrick L, Bova G S, Issacs W, Koch W, Schwab D, Sidransky D. Frequency of homologous deletion at p16/CDKN2 in primary human tumours. Nat Genet. 1995;11:210–212. doi: 10.1038/ng1095-210. [DOI] [PubMed] [Google Scholar]
- 13.Campisi J. Cancer, aging and cellular senescence. In Vivo. 2000;14:183–188. [PubMed] [Google Scholar]
- 14.Chen Q M, Bartholomew J C, Campisi J, Acosta M, Reagan J D, Ames B N. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J. 1998;332:43–50. doi: 10.1042/bj3320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chien M, Rinker-Schaeffer C, Stadler W M. A G2/M growth arrest response to low-dose intermittent H2O2 in normal uroepithelial cells. Int J Oncol. 2000;17:425–432. doi: 10.3892/ijo.17.3.425. [DOI] [PubMed] [Google Scholar]
- 16.Cristofalo V J, Pignolo R J. Molecular markers of senescence in fibroblast-like cultures. Exp Gerontol. 1996;31:111–123. doi: 10.1016/0531-5565(95)02018-7. [DOI] [PubMed] [Google Scholar]
- 17.de Stanchina E, McCurrach M E, Zindy F, Shieh S-Y, Ferbeyre G, Samuelson A V, Prives C, Roussel M F, Sherr C J, Lowe S W. E1A signaling to p53 involves the p19ARF tumor suppressor. Genes Dev. 1998;12:2434–2442. doi: 10.1101/gad.12.15.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickson M A, Hahn W C, Ino Y, Ronfard V, Wu J Y, Weinberg R A, Louis D N, Li F P, Rheinwald J G. Human keratinocytes that express hTERT and also bypass a p16INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimri G P, Itahana K, Acosta M, Campisi J. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14ARF tumor suppressor. Mol Cell Biol. 2000;20:273–285. doi: 10.1128/mcb.20.1.273-285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimri G P, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano E E, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farwell D G, Shera K A, Koop J I, Bonnet G A, Matthews C P, Reuther G W, Coltrera M D, McDougall J K, Klingelhutz A J. Genetic and epigenetic changes in human epithelial cells immortalized by telomerase. Am J Pathol. 2000;156:1537–1547. doi: 10.1016/S0002-9440(10)65025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferbeyre G, de Stanchina E, Querido E, Baptiste N, Prives C, Lowe S W. PML is induced by oncogenic ras and promotes premature senescence. Genes Dev. 2000;15:2015–2027. [PMC free article] [PubMed] [Google Scholar]
- 23.Gire V, Wynford-Thomas D. Reinitiation of DNA synthesis and cell division in senescent human fibroblasts by microinjection of anti-p53 antibodies. Mol Cell Biol. 1998;18:1611–1621. doi: 10.1128/mcb.18.3.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990;249:1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- 25.Groth A, Weber J D, Willumens B M, Sherr C J, Roussel M F. Oncogenic Ras induces p19ARF and growth arrest in mouse embryo fibroblasts lacking p21Cip1 and p27Kip1 without activating cyclin D-dependent kinases. J Biol Chem. 2000;275:27473–27480. doi: 10.1074/jbc.M003417200. [DOI] [PubMed] [Google Scholar]
- 26.Haber D A. Splicing into senescence: the curious case of p16 and p19ARF. Cell. 1997;91:555–558. doi: 10.1016/s0092-8674(00)80441-9. [DOI] [PubMed] [Google Scholar]
- 27.Harley C B, Futcher A B, Greider C W. Telomeres shorten during aging of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 28.Huschtscha L I, Reddel R R. p16INK4a and the control of cellular proliferative life span. Carcinogenesis. 1999;20:921–926. doi: 10.1093/carcin/20.6.921. [DOI] [PubMed] [Google Scholar]
- 29.Jacks T. Lessons from the p53 mutant mouse. J Cancer Res Clin Oncol. 1996;122:319–327. doi: 10.1007/BF01220798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang H, Chou H S, Zhu L. Requirement of cyclin E-Cdk2 inhibition in p16INK4a-mediated growth suppression. Mol Cell Biol. 1998;18:5284–5290. doi: 10.1128/mcb.18.9.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamijo T, Zindy F, Roussel M F, Quelle D E, Downing J R, Ashmun R A, Grosveld G, Sherr C J. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 32.Kiyono T, Foster S A, Koop J I, McDougall J K, Galloway D A, Klingelhutz A J. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 33.Konstantinidis A K, Radhakrishnan R, Gu F, Rao R N, Yeh W K. Purification, characterization, and kinetic mechanism of cyclin D1·CDK4: a major target for cell cycle regulation. J Biol Chem. 1998;273:26506–26515. doi: 10.1074/jbc.273.41.26506. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Jenkins C W, Nichols M A, Xiong Y. Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene. 1994;9:2261–2268. [PubMed] [Google Scholar]
- 35.Lin A W, Barradas M, Stone J C, Aelst L V, Serrano M, Lowe S W. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu K K, Bazarov A V, Yoon L S, Sedivy J M. Isolation of temperature-sensitive mutations in the c-raf-1 catalytic domain and expression of conditionally active and dominant-negative forms of Raf-1 in cultured mammalian cells. Cell Growth Differ. 1998;9:367–380. [PubMed] [Google Scholar]
- 37.Ly D H, Lockhart D J, Lerner R A, Schultz P G. Mitotic misregulation and human aging. Science. 2000;287:2486–2492. doi: 10.1126/science.287.5462.2486. [DOI] [PubMed] [Google Scholar]
- 38.Mateyak M K, Obaya A J, Adachi S, Sedivy J M. Phenotypes of c-Myc-deficient rat fibroblasts isolated by targeted homologous recombination. Cell Growth Differ. 1997;8:1039–1048. [PubMed] [Google Scholar]
- 39.Mazars G R, Jat P S. Expression of p24, a novel p21Waf1/Cip1/Sdi1-related protein, correlates with measurement of the finite proliferative potential of rodent embryo fibroblasts. Proc Natl Acad Sci USA. 1997;94:151–156. doi: 10.1073/pnas.94.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McConnell B B, Starborg M, Brookes S, Peters G. Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts. Curr Biol. 1998;8:351–356. doi: 10.1016/s0960-9822(98)70137-x. [DOI] [PubMed] [Google Scholar]
- 41.Meyerson M, Counter C M, Eaton E N, Ellisen L W, Steiner P, Caddle S D, Ziaugra L, Beijersbergen R L, Davidoff M J, Liu Q, Bacchetti S, Haber D A, Weinberg R A. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 42.Migliaccio M, Amacker M, Just T, Reichenbach P, Valmori D, Cerottini J C, Romero P, Nabholz M. Ectopic human telomerase catalytic subunit expression maintains telomere length but is not sufficient for CD8(+) T lymphocyte immortalization. J Immunol. 2000;165:4978–4984. doi: 10.4049/jimmunol.165.9.4978. [DOI] [PubMed] [Google Scholar]
- 43.Mitra J, Dai C Y, Somasundaram K, El-Deiry W S, Satymoorthy K, Herlyn M, Enders G H. Induction of p21WAF1/CIP1 and inhibition of Cdk2 mediated by the tumor suppressor p16INK4a. Mol Cell Biol. 1999;19:3916–3928. doi: 10.1128/mcb.19.5.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Modestou M, Puig-Antich V, Korgaonkar C, Eapen A, Quelle D E. The alternative reading frame tumor suppressor inhibits growth through p21-dependent and p21-independent pathways. Cancer Res. 2001;61:3145–3150. [PubMed] [Google Scholar]
- 45.Morales C P, Holt S E, Ouellette M, Kaur K J, Yan Y, Wilson K S, White M A, Wright W E, Shay J W. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat Genet. 1999;21:115–118. doi: 10.1038/5063. [DOI] [PubMed] [Google Scholar]
- 46.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nanayama H, Yokoi H, Fujita J. Quantification of mRNA by non-radioactive RT-PCR and CCD imaging system. Nucleic Acids Res. 1992;18:4939. doi: 10.1093/nar/20.18.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogryzko V V, Hirai T H, Russanova V R, Barbie D A, Howard B H. Human fibroblast commitment to a senescence-like state in response to histone deacetylase inhibitors is cell cycle dependent. Mol Cell Biol. 1996;16:5210–5218. doi: 10.1128/mcb.16.9.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohtani N, Zobedee Z, Huot T J G, Stinson J A, Sugimoto M, Ohashi Y, Sharrocks A D, Peters G, Hara E. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature. 2001;409:1067–1070. doi: 10.1038/35059131. [DOI] [PubMed] [Google Scholar]
- 50.Palmero I, Pantoja C, Serrano M. p19ARF links the tumour suppressor p53 to Ras. Nature. 1998;395:125–126. doi: 10.1038/25870. [DOI] [PubMed] [Google Scholar]
- 51.Pantoja C, Serrano M. Murine fibroblasts lacking p21 undergo senescence and are resistant to transformation by oncogenic Ras. Oncogene. 1999;18:4974–4982. doi: 10.1038/sj.onc.1202880. [DOI] [PubMed] [Google Scholar]
- 52.Pearson M, Carbone R, Sebastiani C, Cioce M, Fagioli M, Saito S, Higashimoto Y, Appella E, Minucci S, Pandolfi P P, Pelicci P G. PML regulates p53 acetylation and premature senescence by oncogenic Ras. Nature. 2000;406:207–210. doi: 10.1038/35018127. [DOI] [PubMed] [Google Scholar]
- 53.Pomerantz J, Schreiber-Agus N, Liegeois N J, Silverman A, Alland L, Chin L, Potes J, Chen K, Orlow I, Lee H W, Cordon-Cardo C, DePinho R A. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- 54.Radfar A, Unnikrishnan I, Lee H W, Depinho R A, Rosenberg N. p19Arf induces p53-dependent apoptosis during Abelson virus-mediated pre-B cell transformation. Proc Natl Acad Sci USA. 1998;95:13194–13199. doi: 10.1073/pnas.95.22.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reddel R R. A reassessment of the telomere hypothesis of senescence. Bioessays. 1998;20:977–984. doi: 10.1002/(SICI)1521-1878(199812)20:12<977::AID-BIES3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 56.Ries S, Biederer C, Woods D, Shifman O, Shirasawa S, Sasazuki T, McMahon M, Oren M, McCormick F. Opposite effects of Ras on p53: transcriptional activation of mdm2 and induction of p19ARF. Cell. 2000;103:321–330. doi: 10.1016/s0092-8674(00)00123-9. [DOI] [PubMed] [Google Scholar]
- 57.Robles S J, Adami G R. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998;16:1113–1123. doi: 10.1038/sj.onc.1201862. [DOI] [PubMed] [Google Scholar]
- 58.Sage J, Mulligan G J, Attardi L D, Miller A, Chen S Q, Williams B, Theodorou E, Jacks T. Targeted disruption of the three Rb-related genes leads to loss of G1 control and immortalization. Genes Dev. 2000;14:3037–3050. doi: 10.1101/gad.843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serrano M, Lin A W, McCurrach M E, Beach D, Lowe S W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 60.Sharpless N E, DePinho R A. The INK4a/ARF locus and its two gene products. Curr Opin Genet Dev. 1999;9:22–30. doi: 10.1016/s0959-437x(99)80004-5. [DOI] [PubMed] [Google Scholar]
- 61.Shay J W, Pereira-Smith O M, Wright W E. A role for both Rb and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991;196:33–39. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- 62.Shelton D N, Chang E, Whittier P S, Choi D, Funk W D. Microarray analysis of replicative senescence. Curr Biol. 1999;9:939–945. doi: 10.1016/s0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- 63.Sherr C J, DePinho R A. Cellular senescence: mitotic clock or culture clock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 64.Stein G H, Drullinger L F, Soulard A, Dulic V. Differential role for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19:2109–2117. doi: 10.1128/mcb.19.3.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stott F J, Bates S, James M C, McConnell B B, Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden K H, Peters G. The alternative product from the human CDKN2A locus, p14ARF, participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998;17:5001–5014. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tahara H, Sato E, Noda A, Ide T. Increase in the expression level of p21sdi1/cip1/waf1 with increasing division age in both normal and SV40-transformed human fibroblasts. Oncogene. 1995;10:835–840. [PubMed] [Google Scholar]
- 67.Vaziri H, West M D, Allsopp R C, Davison T S, Wu Y, Arrowsmith C H, Poirier G G, Benchimol S. ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly (ADP-ribose) polymerase. EMBO J. 1997;16:6018–6033. doi: 10.1093/emboj/16.19.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Webley K, Bond J A, Jones C J, Blaydes J P, Craig A, Hupp T, Wynford-Thomas D. Posttranslational modifications of p53 in replicative senescence overlapping but distinct from those induced by DNA damage. Mol Cell Biol. 2000;20:2803–2808. doi: 10.1128/mcb.20.8.2803-2808.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei S, Wei W, Sedivy J M. Expression of catalytically active telomerase does not prevent premature senescence caused by overexpression of oncogenic Ha-Ras in normal human fibroblasts. Cancer Res. 1999;59:1539–1543. [PubMed] [Google Scholar]
- 70.Wei W, Sedivy J M. Differentiation between senescence (M1) and crisis (M2) in human fibroblast cultures. Exp Cell Res. 1999;253:519–522. doi: 10.1006/excr.1999.4665. [DOI] [PubMed] [Google Scholar]
- 71.Weller E M, Poot M, Hoehn H. Induction of replicative senescence by 5-azacytidine: fundamental cell kinetic differences between human diploid fibroblasts and NIH-3T3 cells. Cell Prolif. 1993;26:45–54. doi: 10.1111/j.1365-2184.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 72.Wright W E, Shay J W. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nat Med. 2000;6:849–851. doi: 10.1038/78592. [DOI] [PubMed] [Google Scholar]
- 73.Zglinicki T V, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y, Xiong Y, Yarbrough W G. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725–734. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]
- 75.Zhu J, Woods D, MacMahon M, Bishop J M. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zindy F, Eischen C M, Randle D H, Kamijo T, Cleveland J L, Sherr C J, Roussel M F. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]