Abstract
Aging is associated with compromised hippocampal function and reduced adult neurogenesis in the dentate gyrus. Since new neurons have been linked to hippocampal functions, such as cognition, age-related decline in new neuron formation may contribute to impaired hippocampal function. We investigated whether a rewarding experience known to stimulate neurogenesis in young adult rats, namely sexual experience, would restore new neuron production and hippocampal function in middle-aged rats. Sexual experience enhanced the number of newly generated neurons in the dentate gyrus with both single and repeated exposures in middle-aged rats. Following continuous long-term exposure to sexual experience, cognitive function was improved. However, when a prolonged withdrawal period was introduced between the final mating experience and behavioral testing, the improvements in cognitive function were lost despite the presence of more new neurons. Taken together, these results suggest that repeated sexual experience can stimulate adult neurogenesis and restore cognitive function in the middle-aged rat as long as the experience persists throughout the testing period. The extent to which changes in adult neurogenesis underlie those in cognition remain unknown.
Keywords: dentate gyrus, object recognition, middle age, sexual experience
Introduction
In both humans and rodents, aging is associated with a decrement in cognitive function (Bizon et al., 2009; Driscoll et al., 2006; Frick et al., 2003; Lewis et al., 2008; Oler and Markus, 1998; Soei and Daum, 2008). Age-associated changes in cognition are evident as early as mid-life. The hippocampus has been associated with many of the cognitive functions that decline with aging (Geinisman et al., 1986; Smith et al., 2000). While the neural substrates of age-associated changes in cognition remain unknown, changes in hippocampal plasticity may play a role.
The dentate gyrus of the hippocampus is a major site of adult neurogenesis in the mammalian brain ( Leuner and Gould, 2010; McEwen, 2001). Evidence suggests that changes in adult neurogenesis can affect hippocampal functions (Leuner et al., 2006; Shors, 2004) with reports supporting claims that new neurons are involved in certain types of learning and memory (Gould et al., 1999a; Snyder et al., 2009; Gu et al., 2012), anxiety regulation (Leuner and Gould, 2010), and feedback of the stress response (Snyder et al., 2011). Aging is associated with a substantially reduced rate of neurogenesis in the dentate gyrus; this appears to be a general phenomenon among mammalian species, including rats, mice, tree shrews, dogs, marmosets and macaques (Cameron and McKay, 1999; Gould et al., 1999b; Heine et al., 2004; Jin et al., 2003; Kempermann et al., 1998; Kronenberg et al., 2006; Kuhn et al., 1996; Lemaire et al., 2000; Leuner et al., 2007; McDonald and Wojtowicz, 2005; Nacher et al., 2003; Rao et al., 2006; Seki and Arai, 1995; Simon et al., 2005; Siwak-Tapp et al., 2007). Reduced adult neurogenesis becomes evident in mid-life, before the time when an age-associated decline in cognitive function has been reported (Frick et al., 2003; Knuttinen et al., 2001), suggesting that reduced adult neurogenesis may eventually contribute to these problems (Drapeau et al., 2003; Drapeau et al., 2007).
Experience has been shown to alter the rate of adult neurogenesis (Leuner and Gould, 2010). Some studies suggest that rewarding experiences, such as running (Stranahan et al., 2006) and intracranial self-stimulation (Takahashi et al., 2009), increase the number of new neurons in the dentate gyrus. Sexual behavior is known to be rewarding to rodents. Rats will bar press to gain access to a receptive female (Beck and Bialy, 1993; Everitt et al., 1987) and develop conditioned place preferences to locations previously associated with a sexually receptive female (Camacho et al., 2004). Additionally, sexual behavior activates reward centers within the brain (Agmo and Berenfeld, 1990; Damsma et al., 1992), much like those activated by exercise, intracranial self-stimulation, and environmental enrichment (Brene et al., 2007; Garris et al., 1999; Segovia et al., 2010).
In young adult male rats, sexual experience increases cell proliferation and adult neurogenesis in the hippocampus (Leuner et al., 2010) and also exerts anxiolytic effects in many behavioral paradigms (Edinger and Frye, 2007; Fernandez-Guasti et al., 1989; Rodriguez-Manzo et al., 1999; Waldherr and Neumann, 2007), although its potential influence on cognition has not been examined. The possibility that sexual experience during mid-life can restore hippocampal structure and function to young adult levels has not been investigated. Here, we examined this possibility and found that even in older rats, sexual experience increases the numbers of new neurons and enhances cognitive function.
Materials and Methods
Ethics Statement
Procedures were conducted in accordance with Princeton University AALAC (protocol # 1756, approved July 2009) and The National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental Animals
Young adult male and female Sprague Dawley rats (2–3 mo) and middle-aged retired breeder male Sprague-Dawley rats (9–11 mo) (Taconic, Germantown, NY) were provided ad libitum access to food and water, except as stated below (see Behavior) and maintained on a reverse 12:12 light-dark cycle (lights on 1900 hr). The middle-aged retired breeder rats had a long history of sexual experience and were used for this study because they are much more sexually responsive than middle-aged virgin males, which are often completely resistant to mating (unpublished observation). Males (including retired breeders) were housed two per cage and were acclimated to the colony for 5 d before sexual experience began. No obvious fighting was observed between young adult or retired breeder rats. Rats housed together were included in the same experimental group. Females were individually-housed following bilateral ovariectomy (OVX) under Nembutal anesthesia and allowed to recover for one week. Sexual receptivity was induced in OVX rats by subcutaneous injection of estrogen (200 mg/0.2 ml sesame oil) 48 hrs and progesterone (500 mg/0.2 ml sesame oil) 3 hrs before pairing the female with a male.
Sexual Behavior
Male rats were placed in a novel cage with a sexually-receptive female or remained undisturbed in their home cage. Beginning from the first intromission, males were permitted to engage in sexual behavior for 30 min after which they were returned to their home cage. All exposures were monitored and videotaped in the dark under red-light illumination (1300–1600 h). If the rat did not initiate sexual behavior within 30 min, the session was terminated. All digital videos were analyzed for mounts, intromissions, and ejaculations (Leuner et al., 2010).
BrdU Administration and Perfusion
To assess the effects of sexual experience on cell proliferation in middle-aged rats, retired breeder male rats were injected intraperitoneally with 200 mg/kg of the DNA synthesis marker bromodeoxyuridine (BrdU) either directly after removal from the home cage (control) or 30 min after the first intromission (sexual experience). This dose of BrdU was used because it labels a maximal number of cells in the dentate gyrus (Cameron and McKay, 2001). Rats were then perfused 2 hrs after BrdU injection. This post-BrdU survival time is sufficient to label cells in S-phase but not to allow the labeled cells to divide, thus providing a measure of cell proliferation.
To assess whether continuous sexual experience alters the number of newly generated cells in the dentate gyrus of middle-aged rats, retired breeder rats were exposed to a sexually receptive female once daily, for 28 consecutive days. After each bout of sexual experience, during the first 14 days, males were injected intraperitoneally with 50 mg/kg of BrdU, along with controls and perfused following the final sexual experience on day 28. This dose of BrdU, and injection paradigm, was used to label a large number of proliferating cells to obtain a better estimate of the magnitude of the effect over time.
To assess whether discontinuous sexual experience produces an increase in adult neurogenesis, we exposed young adult and retired breeder male rats to a receptive female daily for 14 days. Following the final mating test on Day 14, sexually experienced males and control rats were injected with 200 mg/kg of BrdU and perfused after a 2 week survival time (a time point when the majority of new cells in the dentate gyrus express the mature neuronal marker NeuN, (Cameron and McKay, 2001)).
To assess the effects of both continuous and discontinuous sexual experience on behaviors associated with the hippocampus, rats exposed to either 28d of continuous sexual experience (tested immediately after sexual experience) or 14d of sexual experience followed by 14d of no sexual experience (tested 14d after sexual experience) underwent either a cognitive task associated with the hippocampus, novel object recognition testing. These rats were perfused 30 min after behavioral testing.
For perfusion, rats were anesthetized with an overdose of Nembutal and transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.5.
Behavior
Object Recognition
The novel object recognition test was performed as previously described (Bevins and Besheer, 2006; Dere et al., 2007). This version of the novel object recognition test has been shown to involve the hippocampus in rats (Dere et al., 2007). The testing apparatus consisted of an open-field box made of 1/2" plywood (50 cm3) painted white. During the familiarization phase, rats explored two identical objects (Duplo structures) for 3 min and were returned to their home cages for 3 hrs (Jessberger et al., 2009). During the recognition phase, rats were returned to the testing apparatus, presented with a third copy of the familiar object and a novel object, and allowed to explore them for 3 min. The left/right position of the novel object was counterbalanced between each rat. Object exploration was defined as directing the nose toward the object at 2 cm and/or touching the object with the nose or paws. The following measures were calculated: time exploring familiar and novel object during object recognition test, total time exploring sample objects (total time spent exploring both identical objects during the familiarization phase), and difference score (time spent with the novel object minus time spent with the familiar object during the recognition phase). For comparison purposes, young adult male rats were also tested on novel object recognition.
Immunohistochemistry
Coronal sections (40 Km) were cut throughout the entire rostrocaudal extent of the dentate gyrus on a vibratome into a bath of 0.1 M PBS, pH 7.5.
For BrdU peroxidase staining, a 1:12 series of sections was mounted onto glass slides, dried, and pretreated by heating in 0.1 M citric acid, pH 6.0. Slides were then rinsed in PBS, incubated in trypsin for 10 min, denatured in 2 M HCl:PBS for 30 min, rinsed, and incubated with a mouse monoclonal antibody to BrdU (1:200 with 0.5% Tween 20; Vector, Burlingame, CA, cat. no. VPB209). The next day, slides were rinsed, incubated with biotinylated anti-mouse (1:200; Vector, Burlingame, CA, cat. no. BA2000) for 60 min, rinsed, incubated with avidin–biotin complex (1:100; Vector, cat. no. PK6100) for 60 min, rinsed, and reacted in 0.01% diaminobenzidine with 0.003% H2O2 (Sigma-Aldrich, cat. no. D4293). Slides were counterstained with cresyl violet, dehydrated, cleared with Citrisolv (Fisher Scientific, Fair Lawn, NJ), and coverslipped under Permount (Fisher Scientific).
Double labeling with immunofluorescence for BrdU and the neuronal marker neuronal nuclei (NeuN) was carried out to determine whether the newly-labeled cells were neurons. For double-labeling immunofluorescence of BrdU and NeuN, free-floating sections were rinsed in 0.1 M TBS (pH 7.5), denatured in 2 M HCl:TBS for 30 min, rinsed in TBS, and incubated with rat anti-BrdU (1:200 with 0.5% Tween 20; Accurate, Westbury, NY, cat. no. OBT0030) and mouse anti-NeuN (1:500; Chemicon, Temecula, CA, cat. no. MAB377). Sections were then rinsed, incubated with biotinylated anti-rat (1:250; Chemicon, cat. no. AP183B) for 90 min, rinsed, and incubated for 30 min in the dark with streptavidin-conjugated Alexa 568 (1:1000; Invitrogen) to visualize BrdU and with goat anti-mouse Alexa 488 (1:500; Invitrogen Molecular Probes, cat. no. A11029) to visualize NeuN. Finally, sections were rinsed, mounted onto glass slides, dried, and coverslipped using glycerol in TBS (3:1).
Microscopic Data Analysis
Quantitative analysis was conducted on coded slides to keep group assignments unknown. The numbers of BrdU-labeled cells on every 12th unilateral section throughout the entire rostrocaudal extent of the dentate gyrus (granule cell layer, subgranular zone, and hilus) were counted at 100× with an Olympus BX-50 light microscope by using a modified version of the optical fractionator method (Ngwenya et al., 2005; West et al., 1991). The simplified formula for the estimated total number of labeled cells was: N Q × (1/ssf), which is the total number of labeled cells (N Q ) counted multiplied by the reciprocal of the section sampling fraction (1/ssf or 1/12) (Leuner et al., 2009).
Brightfield photomicrographs were taken with an Olympus U-PMTUC camera attached to the microscope using ImagePro software (Media Cybernetics, Bethesda, MD). Images were cropped and optimized by adjusting brightness and color balance in Adobe Photoshop 7.0 (San Jose, CA).
For purposes of comparison, the density of BrdU-labeled cells was also determined in the subventricular zone (SVZ) of middle-aged rats assessed for cell proliferation (Kuhn et al., 1996; Mirescu et al., 2004). This analysis included a substantial part of the SVZ, but excluded the anterior portion. BrdU-labeled cells in the SVZ present on every 12th unilateral coronal section of the dentate gyrus were counted (1.8 to 4.8 mm from Bregma; (Paxinos and Watson, 1998)) and expressed as the number of cells per mm3. The volume of the analyzed area was determined by using Cavalieri’s principle on video- projected images with cross-sectional area measurements performed using ImagePro software (Gundersen et al., 1999).
Immunofluorescence analyses were carried out with a Zeiss Axiovert confocal microscope with Argon 458/488 and HeNe 543 lasers and 510LSM software. For the NeuN analysis, the percentage of BrdU+ cells that were NeuN+ was determined from 25 randomly selected BrdU-labeled cells in the dentate gyrus. Optical stacks of 1-lm thick sections were obtained through all putatively double-labeled cells. To verify double labeling throughout their extent, cells were examined in orthogonal planes.
Statistics
All data were analyzed using unpaired Student’s t-tests and ANOVA followed by Bonferroni post-hoc analysis. Welch’s correction for unequal variance was applied when necessary. Pearson correlations were performed, where appropriate.
Results
Middle-age retired breeder rats readily engage in sexual behavior
Like young adult virgins, middle-aged retired breeders reliably, but not always, engaged in sexual behavior when exposed to a sexually receptive female. For the single sexual experience study, only rats which copulated (approximately 60%) were included in the BrdU analysis. For the 28 d and 14d on/14d off sexual experience studies, all rats copulated within the first two days of exposure to a sexually receptive female. The rate of copulation was 80% over the 28d period and 71% over the 14d period. Among rats in the continuous sexual experience group (28d), the latency to mount, intromit, and ejaculate did not significantly differ. Additionally, no significant difference in the frequency of each behavior was observed (p > 0.05, for all comparisons; Table 1). Among rats in the discontinuous sexual experience group (14d on/14d off), which received one BrdU injection after completing 14d of exposure to a sexually-receptive female, no differences in the latency to mount and number of mounts were observed across days (p > 0.05, for all comparisons). However, significant differences were observed in the latency to intromit (F(2, 42) = 10.4, p < 0.001) and the number of intromissions (F(2, 42) = 13.0, p < 0.0001), as well as the latency to ejaculate (F(2, 42) =10.0, p < 0.001) and the number of ejaculations (F(2, 42) = 8.0, p = 0.001) (Table 2). A positive correlation was observed between the average latency to intromit over the 14d paradigm and the number of days each retired breeder copulated with the sexually receptive female (r2 = 0.97, p < 0.0001).
Table 1.
Sexual behavior of middle-aged male rats exposed to a receptive female on day 1, 7, and 14.
| Day of sexual experience | |||
|---|---|---|---|
| 1 | 7 | 14 | |
| Latency to Mount | 94.6 ± 25.8 | 179.6 ± 82.9 | 256.2 ± 123.9 |
| Number of Mounts | 29.5 ± 3.8 | 21.4 ± 4.5 | 31.3 ± 4.3 |
| Latency to Intromit | 511.5 ± 124.6* | 1196.0 ± 166.5*** | 495.4 ± 141.5 |
| Number of Intromissions | 24.3 ± 3.0** | 20.9 ± 5.7*** | 49.5 ± 6.1 |
| Latency to Ejaculate | 815.3 ± 125.6* | 1335.0 ± 125.5*** | 665.9 ± 114.5 |
| Number of Ejaculations | 2.0 ± 0.3* | 0.8 ± 0.2*** | 1.7 ± 0.2 |
| % copulation | 81.8 ± 8.4 | 59.1 ± 10.7 | 81.8 ± 8.4 |
Table 2.
Sexual behavior of middle-aged male rats exposed to repeated BrdU injections.
| Day of sexual experience | |||
|---|---|---|---|
| 1 | 14 | 28 | |
| Latency to Mount (sec) | 49.1 ± 9.8 | 146.9 ± 62.0 | 72.9 ± 53.5 |
| Number of Mounts | 24.33 ± 4.80 | 30.3± 5.4 | 21.9 ± 4.7 |
| Latency to Intromit (sec) | 424.4 ± 192.0 | 648.3 ± 172.5 | 297.7 ± 146.4 |
| Number of Intromissions | 26.1 ± 4.7 | 19.3 ± 6.4 | 41.2 ± 7.9 |
| Latency to Ejaculate (sec) | 841.7 ± 187.4 | 1164.0± 230.1 | 867.8 ± 176.0 |
| Number of Ejaculations | 2.2 ± 0.5 | 0.9 ± 0.4 | 1.3 ± 0.3 |
| % copulation | 83.3 ± 11.2 | 83.3 ± 11.2 | 91.7 ± 8.3 |
Sexual experience increases cell proliferation and neurogenesis in the middle-aged hippocampus
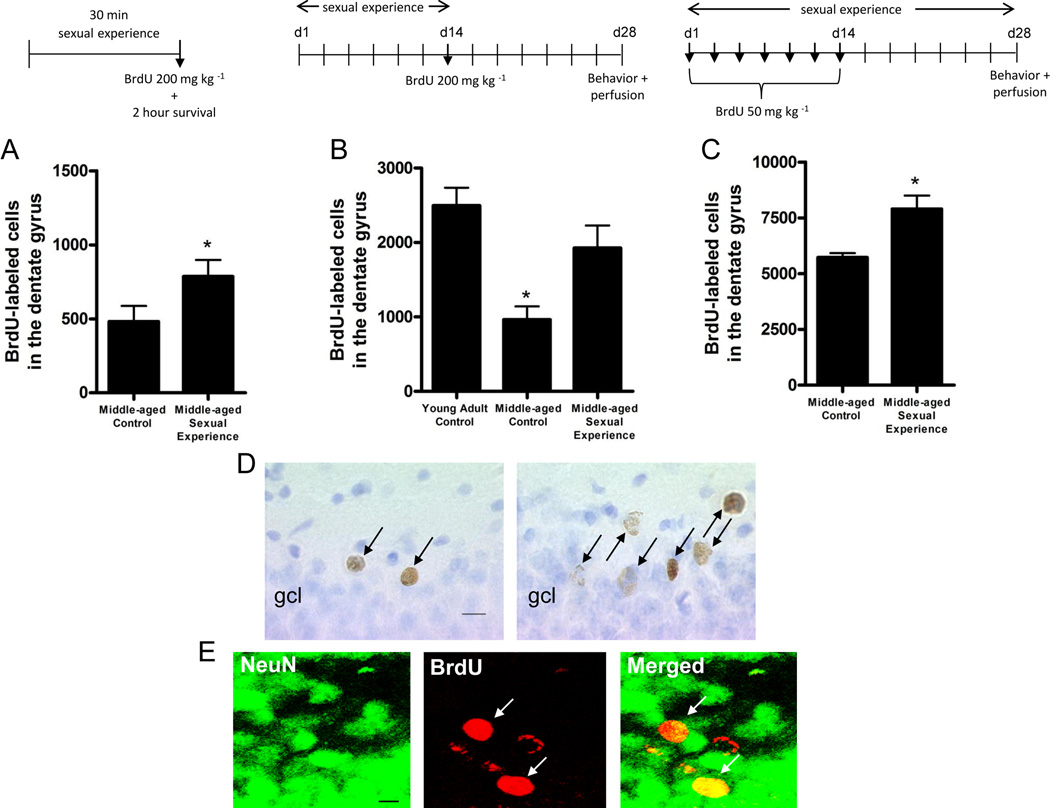
A single mating session enhanced cell proliferation in the dentate gyrus of middle-aged retired breeders, compared to age-matched controls. Retired breeder rats with 30 min of sexual experience, followed by a single injection of BrdU, and perfused after 2 hrs exhibited an increase in the number of BrdU-labeled cells in the dentate gyrus compared to control retired breeders (t(20) = 1.98, p < 0.05; Figure 1). It should be noted that the controls in the present study remained confined to their home cages, except for routine cage changes. Previous studies in our lab suggest that naïve home cage controls serve as a suitable control group, as no differences in BrdU-labeled cells exist between naive rats and those exposed to a non-receptive females (Leuner et al., 2010). The number of BrdU-labeled cells did not correlate with any measure of sexual behavior during the single mating session, including latencies to mount, intromit and ejaculate and numbers of mounts, intromissions and ejaculations (p > 0.05, for each comparison). No significant difference was observed in the density of BrdU-labeled cells in the subventricular zone between controls and middle-aged rats with sexual experience (Control: 136.6 ± 26.5, Sexual Experience: 176.7 ± 51.0, p > 0.05).
Figure 1.
Sexual experience increases the production of new granule cells in the dentate gyrus of the hippocampus. (A) A brief exposure to a sexually-receptive female increases the number of newly-labeled cells in the dentate gyrus of middle-aged retired breeder rats (n = 11/group). (B) Daily sexual experience, for 14d, followed by a 14d survival time, restored adult neurogenesis to young adult levels in the middle-aged retired breeder rat (n = 7–12/group). (C) Continuous sexual experience for 28d produced a large number of newly-labeled cells in the dentate gyrus of the middle-aged rat (n = 8–9/group). (D) Continuous exposure to a sexually-receptive female increased the number of newly-labeled cells in the dentate gyrus of middle-aged retired breeder rats. Arrows point to BrdU-labeled cells. gcl = granule cell layer. Scale bar = 10 µm. (E) Most BrdU labeled cells expressed the neuronal markers NeuN, with no difference in the proportion of BrdU-labeled cells expressing NeuN between groups. Arrows point to BrdU-labeled cells. Yellow indicates colocalization of BrdU and NeuN. Scale bar = 10 µm. Bars represent mean ± SEM. * indicates p < 0.05.
A similar increase in BrdU-labeled cells in the dentate gyrus was observed in middle-aged retired breeders with 14d on/14d off and 28d of sexual experience (Figure1). Male retired breeders exposed to a sexually-receptive female for 14 consecutive days, injected with BrdU, followed by 14d without sexual experience displayed more BrdU-labeled cells compared to middle-aged controls (t(30) = 2.29, p < 0.05, unpaired t-test with Welch’s correction; Figure 1), and did not differ from young adult control rats. At this time point, most BrdU-labeled cells expressed the neuronal marker NeuN in both groups (Control: 88.0 ± 7.3%, Sexual Experience: 76.2 ± 4.9%; Figure 1). There was no difference in the proportion of BrdU-labeled cells expressing NeuN between groups (p >0.05, for each comparison) suggesting that the mating-induced increase in BrdU-labeled cells represents an increase in adult neurogenesis. The number of BrdU-labeled cells that survived 2 wks post injection correlated positively with the total number of intromissions on the last day of sexual experience (Day 14) (r2 = 0.34, p < 0.05). Other measures of sexual behavior were not correlated with the number of BrdU-labeled cells 14d after mating. Male retired breeders also exposed to a receptive female for 28 consecutive days displayed significantly more BrdU labeled cells compared to controls (t(9) = 3.42, p < 0.05, unpaired t-test with Welch’s correction; Figure 1). The number of BrdU-labeled cells that survived 4 wks post injection did not significantly correlate with any measure of sexual behavior (p > 0.05, for all comparisons).
Sexual experience alters novel object recognition
Young adult control males showed evidence of object recognition during the testing phase but middle-aged control males from either experiment (14d on/14d off or 28d) did not. Exposure to sexual experience for 14d followed by a 14d survival time did not alter novel object recognition in middle-aged rats (Figure 2) but sexual experience for 28d did such that middle-aged rats performed similarly to the young adults (Figure 3). In the 14d group, time spent exploring the familiar and novel objects was similar between controls and sexually-experienced middle-aged retired breeders (p > 0.05, Figure 2), with no difference in sample object exploration, which serves as a general indication of exploratory behavior (Figure 2). In addition, difference scores were identical between groups (Control: 0.5 ± 0.03, Sexual Experience: 0.5 ± 0.03, p > 0.05). By contrast, in the 28d group, while time spent exploring the familiar object did not significantly differ across days (p > 0.05), time spent exploring the novel object differed between middle-aged controls and sexually experienced males (F(2, 22) = 8.84, p < 0.05; Figure 3). No difference in sample object exploration was observed, suggesting no difference in general exploratory behavior (p > 0.05; Figure 3). The performance of middle-aged rats exposed to 28d of continuous sexual experience was similar to that of young adult controls (p > 0.05; Figure 3). [It should be noted that the amount of time spent exploring objects by the 14d on/ 14d off rats was considerably higher than that observed in the 28d rats, regardless of whether rats had sexual experience or not. This difference may be due to the fact that the 14d on/14d off rats had a single injection of BrdU whereas the 28d rats received 14 injections of BrdU on each of the first 14d of the experiment. Repeated injections may have produced differences in exploratory behavior between studies as a result of injection stress.]
Figure 2.
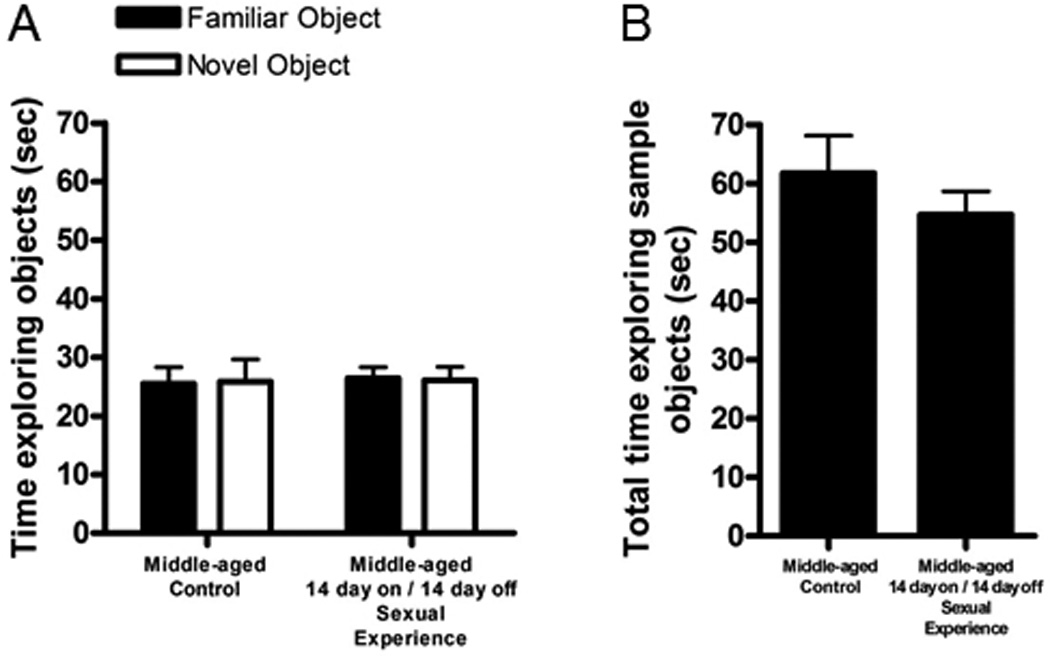
Repeated, but discontinuous sexual behavior, does not alter object recognition memory among middle-aged retired breeder rats. (A) Time exploring the familiar and novel objects was similar between middle-aged controls and retired breeder males with discontinuous sexual experience. (B)Time exploring the sample objects was similar between middle-aged controls and retired breeder males with discontinuous sexual experience (n = 9–11/group). * indicates p > 0.05.
Figure 3.

Continuous, and prolonged, sexual experience restores object recognition in the middle-aged rat. (A) Continuous sexual experience positively influenced recognition memory on the novel object preference test in the middle-aged rat. Retired breeder rats with 4 wks of sexual experience spent more time exploring the novel object than the familiar object, compared to middle-aged controls. No difference was observed between young adult controls and middle-aged retired breeders with sexual experience (n = 4–6/group). (B) Total time exploring the sample objects did not differ between groups. * indicates p < 0.05. Bars represent mean ± SEM.
Discussion
Here we have shown that single and repeated sexual experience increase cell proliferation and adult neurogenesis in the dentate gyrus of middle-aged rats. Since cell proliferation and adult neurogenesis are known to drop precipitously by midlife (Kuhn et al., 1996; van Praag et al., 2005), these findings suggest that sexual experience may partially restore adult neurogenesis to young adult levels. Continuous daily exposure (28d) to sexual experience had beneficial effects on cognitive behavior, restoring object recognition in middle-aged rats to young adult levels. However, repeated but discontinuous sexual experience (14d of sexual experience followed by 14d rest) produced no benefits on object recognition. Instead, sexual experience followed by a comparable time with no such experience prevented any improvements in novel object recognition.
Rats are highly motivated to engage in sexual behavior (Beck and Bialy, 1993; Camacho et al., 2004; Camacho et al., 2009; Everitt et al., 1987; Everitt and Stacey, 1987; Kruger et al., 2005) raising the possibility that the rewarding component of mating is responsible for increased neurogenesis and enhanced cognition. Indeed, other experiences associated with reward, such as running and intracranial self-stimulation, are associated with enhanced neurogenesis and improved cognition (Leuner and Gould, 2010; Stranahan et al., 2006; Takahashi et al., 2009). Evidence suggests that a ceiling may exist in the degree to which reward may enhance neurogenesis. That is, providing a food reward does not further enhance neurogenesis associated with running (Klaus et al., 2009) but whether this is due to the rewarding aspects of running remains unknown. It is important to note that sexual experience can be considered a form of enrichment and also increases physical activity. Both environmental enrichment and running have been shown to stimulate adult neurogenesis and enhance cognition (Leuner and Gould, 2010), raising the possibility that mating has similar effects because it shares characteristics with these other experiences. However, some data suggest that the enriching and physically activating aspects of mating cannot account for the effects on adult neurogenesis. First, environmental enrichment increases neurogenesis by enhancing cell survival, not cell proliferation (reviewed in van Praag et al., 2000). This stands in contrast to our findings that sexual experience increases cell proliferation. Second, physical activity seems to require a longer period of time for the induction of increased cell proliferation than we observed for sexual experience (several days ? Van der Borght et al., 2009 compared to 30 min -present study). Future studies will be needed to determine whether there is overlap in the mechanisms that drive changes in adult neurogenesis and cognition among mating, environmental enrichment and physical activity.
Sexual experience produces substantial elevations in circulating glucocorticoids in both naïve and sexually-experienced young adult rats (Bonilla-Jaime et al., 2006; Leuner et al., 2010; Szechtman et al., 1974) along with enhanced adult neurogenesis in young adults (Leuner et al., 2010; Spritzer et al., 2009). These findings are unexpected given that elevated glucocorticoids are associated with growth suppression in the hippocampus (reviewed in Schoenfeld and Gould, 2011). The effects of sexual experience on circulating glucocorticoid levels were not measured in this study, due to the possible confound of age-related elevations in serum corticosterone (Cameron and McKay, 1999). However, the role of glucocorticoids in the observed results should be further explored. Sexual experience shares many other characteristics with running, including elevated glucocorticoid levels (Brown et al., 2007; Droste et al., 2007) and increased neuronal growth (Eadie et al., 2005; Stranahan et al., 2006; Stranahan et al., 2007; van Praag et al., 1999). Sexual experience and running effects resemble those of intracranial self-stimulation – increased glucocorticoid levels (Burgess et al., 1993; Terry and Martin, 1978) and enhanced adult neurogenesis (Takahashi et al., 2009). While prolonged elevated corticosteroid levels are typically associated with reduced cognitive abilities (Plaschke et al., 2006; Wuppen et al., 2010), previous studies have shown that for young adults, sexual experience decreases anxiety-like behavior in the open field task, elevated plus maze, the light-dark transition task, and novelty suppressed feeding test (Edinger and Frye, 2007; Fernandez-Guasti et al., 1989; Rodriguez-Manzo et al., 1999; Waldherr and Neumann, 2007). No previous studies, however, have examined the influence of sexual experience on learning and memory in male rodents of any age – our study demonstrates a significant improvement in object recognition in older male rats following continuous sexual experience. Taken together, these findings suggest that sexual experience can override the potentially suppressive effects of elevated glucocorticoids on adult neurogenesis in the middle-aged rodent, and possibly on behaviors related to the hippocampus, like cognitive function (Schoenfeld and Gould, 2011).
Regarding the mechanism by which rewarding experiences may prevent the negative effects of elevated glucocorticoids, there are a number of obvious candidates including, but not limited to dopamine, endogenous opiates, and oxytocin. With regard to the latter possibility, oxytocin is particularly intriguing with respect to sexual experience. Oxytocin is released in the hippocampus during mating (Waldherr and Neumann, 2007), enhances synaptic plasticity (Monks et al., 2003; Theodosis et al., 1986), and has recently been shown to enhance cell proliferation and neurogenesis in the hippocampus even in the presence of elevated glucocorticoids (Leuner et al., 2011). However, the extent to which oxytocin may mediate the effects of sexual experience (and possibly those of other rewarding experiences) on the aging brain remains unknown. Another possibility for the enhancement of adult neurogenesis following sexual experience in middle-aged rats is that mating-induced increases in testosterone, stimulate cell proliferation and adult neurogenesis. Testosterone levels increase with sexual experience in experienced male rats (Bonilla-Jaime et al., 2006) – this hormone has been associated with increased adult neurogenesis (Spritzer and Galea, 2007). Despite producing opposite effects on object recognition, both continuous and discontinuous repeated sexual experience produced an increase in the number of new neurons in the hippocampus. This pattern of findings raises the possibility that increasing the pool of new neurons is not causally linked to changes in cognitive function following sexual experience. While this conclusion may be correct, it remains possible that new neurons participate in recognition memory but that their activation is altered in rats which experience a period of withdrawal from an established pattern of sexual experience. For example, new neurons generated during a period of sexual experience may be active only under conditions of repeated reward. An additional possibility is that changes in behavior could be linked to changes in a different population of new neurons than the ones presently measured in this study, namely younger neurons. These new neurons would be up to 2 weeks of age at the time of behavioral testing – a time when neuronal activation can occur (Snyder et al., 2009). Future studies will be necessary to determine whether new neurons in the hippocampus are important for recognition memory following repeated sexual experience and whether the activation of these new neurons plays a significant role.
Previous studies have shown that discriminating between familiar and novel objects using a 3 hr inter-trial delay is dependent on the hippocampus in young adult rats (Ainge et al., 2006; Hammond et al., 2004; Jessberger et al., 2009), but that older rats can only perform the hippocampus independent version of this task (with a shorter delay) (Burke et al., 2010; Leite et al., 2011; Terry et al., 2011). Along these lines, we found that young adult controls, but not middle-aged controls, were able to distinguish between the novel and familiar object following a 3 hr delay. Among middle-aged rats subjected to continuous mating experience, object recognition memory improved to a level similar to young adult controls in the hippocampus-dependent version of the onetrial object recognition task. That is, middle-aged rats with continuous sexually experience (28 days) spent significantly more time exploring the novel object compared to the familiar one. By contrast, discontinuous sexual experience (14 days on, 14 days off) did not exhibit any beneficial effects of continuous sexual experience on cognition.
Living in an enriched environment, where rodents have the ability to run on a wheel and interact with novel objects in a general way, as well as running itself leads to increased cognitive ability (Burghardt et al., 2004; Cao et al., 2004; Griffin et al., 2009; Lambert et al., 2005; Leasure and Jones, 2008; O'Callaghan et al., 2007; Trejo et al., 2008), with most of these studies testing cognitive function immediately after exposure to the enriched environment or running wheel. Positive effects of enriched environment living and running on cognitive abilities have also been observed in aged rodents (Kempermann et al., 2002; Kronenberg et al., 2006; Leasure and Jones, 2008; van Praag et al., 2005). While the control retired breeders in our study had an adult life-time exposure to sexual experience, the stimulatory effects of continuous sexual experience were not present once the mating stopped after 14d, resulting in these retired breeder rats showing no change in object recognition compared to age-matched controls. These findings raise the question of whether the beneficial effects of sexual experience require the near term presence of the mating stimuli or whether the detrimental influence of a “withdrawal” period may be blocking the positive influence of prior mating. For example, new neurons generated during the period of mating, as well as other mechanisms that support learning, may be rendered inactive due to the engagement of mechanisms associated with reward withdrawal. In this potential scenario, the substrate for enhanced cognition would exist but remain dormant until the blocking effects of withdrawal were overcome.
While it is common to see an age-induced reduction in adult neurogenesis in laboratory animals, not all aged animals demonstrate a loss in cognitive function (Bizon et al., 2004), an observation that is also made in humans. In humans, intellectually and physically active lifestyles are thought to protect from cognitive impairment (Middleton et al., 2010; Treiber et al., 2011). However, it remains unclear whether such beneficial effects last significantly beyond the time of their occurrence. Some evidence suggests that a higher level of education protects against age-related cognitive decline (Valenzuela et al., 2011), but it is not clear whether formal education builds a fortress of protection or rather, a foundation which engenders a lifelong propensity to seek out cognitively enriching experiences. Although the results of the present study showing that laboratory rats with a very narrow range of opportunities over a lifetime require continuous exposure to rewarding experience in order to reap persistent beneficial effects on cognition, it is not clear how these findings translate to humans with a lifetime of complex experiences. At present, it is not fully understood why a subpopulation of aging animals are able to retain cognitive function despite a reduction is adult hippocampal neurogenesis. Clearly, a complex interaction between environment, age, and cognitive function exists and should be further investigated. It should also be noted that no evidence suggests that sexual experience has beneficial effects on hippocampal structure and function in humans. Since humans demonstrate a much greater range in what a given individual finds rewarding than do laboratory rodents, it seems prudent to translate the results of the present study only in the broadest possible context. That is, our findings may suggest that any experience, providing it is interpreted as rewarding to the individual, may stimulate neuronal growth and prevent cognitive decline in the aging population. The mechanisms which promote these changes and the extent to which each of these effects is causally linked to the others remain the subject of future experimentation.
Acknowledgments
Acknowledgement of Support:
This study was supported by the National Institutes of Health National Institute on Aging (F32 AG033461 to ERG) and National Institute on Mental Health (MH0597405 to EG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Agmo A, Berenfeld R. Reinforcing properties of ejaculation in the male rat: role of opioids and dopamine. Behav Neurosci. 1990;104(1):177–182. doi: 10.1037//0735-7044.104.1.177. [DOI] [PubMed] [Google Scholar]
- Ainge JA, Heron-Maxwell C, Theofilas P, Wright P, de Hoz L, Wood ER. The role of the hippocampus in object recognition in rats: examination of the influence of task parameters and lesion size. Behav Brain Res. 2006;167(1):183–195. doi: 10.1016/j.bbr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Beck J, Bialy M. The role of sexual reward in the temporal patterning of copulatory behaviour in male rats. Acta Neurobiol Exp (Wars) 1993;53(3):451–456. [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial nonmatching-to-sample learning task to study 'recognition memory'. Nat Protoc. 2006;1(3):1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol Aging. 2009;30(4):646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3(4):227–234. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- Bonilla-Jaime H, Vazquez-Palacios G, Arteaga-Silva M, Retana-Marquez S. Hormonal responses to different sexually related conditions in male rats. Horm Behav. 2006;49(3):376–382. doi: 10.1016/j.yhbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Brene S, Bjornebekk A, Aberg E, Mathe AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiol Behav. 2007;92(1–2):136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Johnson MS, Armstrong CJ, Lynch JM, Caruso NM, Ehlers LB, Fleshner M, Spencer RL, Moore RL. Short-term treadmill running in the rat: what kind of stressor is it? J Appl Physiol. 2007;103(6):1979–1985. doi: 10.1152/japplphysiol.00706.2007. [DOI] [PubMed] [Google Scholar]
- Burgess ML, Davis JM, Wilson SP, Borg TK, Burgess WA, Buggy J. Effects of intracranial self-stimulation on selected physiological variables in rats. Am J Physiol. 1993;264(1 Pt 2):R149–R155. doi: 10.1152/ajpregu.1993.264.1.R149. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. 2004;1019(1–2):84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124(5):559–573. doi: 10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho F, Sandoval C, Paredes RG. Sexual experience and conditioned place preference in male rats. Pharmacol Biochem Behav. 2004;78(3):419–425. doi: 10.1016/j.pbb.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Camacho FJ, Portillo W, Quintero-Enriquez O, Paredes RG. Reward value of intromissions and morphine in male rats evaluated by conditioned place preference. Physiol Behav. 2009;98(5):602–607. doi: 10.1016/j.physbeh.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2(10):894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435(4):406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36(8):827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106(1):181–191. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31(5):673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100(24):14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Montaron MF, Aguerre S, Abrous DN. Learning-induced survival of new neurons depends on the cognitive status of aged rats. J Neurosci. 2007;27(22):6037–6044. doi: 10.1523/JNEUROSCI.1031-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, Sutherland RJ. The aging hippocampus: a multi-level analysis in the rat. Neuroscience. 2006;139(4):1173–1185. doi: 10.1016/j.neuroscience.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Droste SK, Chandramohan Y, Hill LE, Linthorst AC, Reul JM. Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenocortical axis mainly at the adrenal level. Neuroendocrinology. 2007;86(1):26–37. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486(1):39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Sexual experience of male rats influences anxiety-like behavior and androgen levels. Physiol Behav. 2007;92(3):443–453. doi: 10.1016/j.physbeh.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Fray P, Kostarczyk E, Taylor S, Stacey P. Studies of instrumental behavior with sexual reinforcement in male rats (Rattus norvegicus): I. Control by brief visual stimuli paired with a receptive female. J Comp Psychol. 1987;101(4):395–406. [PubMed] [Google Scholar]
- Everitt BJ, Stacey P. Studies of instrumental behavior with sexual reinforcement in male rats (Rattus norvegicus): II. Effects of preoptic area lesions, castration, and testosterone. J Comp Psychol. 1987;101(4):407–419. [PubMed] [Google Scholar]
- Fernandez-Guasti A, Roldan-Roldan G, Saldivar A. Reduction in anxiety after ejaculation in the rat. Behav Brain Res. 1989;32(1):23–29. doi: 10.1016/s0166-4328(89)80068-3. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stearns NA, Pan JY, Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learn Mem. 2003;10(3):187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Kilpatrick M, Bunin MA, Michael D, Walker QD, Wightman RM. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature. 1999;398(6722):67–69. doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, Ge S. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat Neurosci. 2012;15(12):1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F. Loss of perforated synapses in the dentate gyrus: morphological substrate of memory deficit in aged rats. Proc Natl Acad Sci U S A. 1986;83(9):3027–3031. doi: 10.1073/pnas.83.9.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999a;2(3):260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A. 1999b;96(9):5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampal dependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus. 2009;19(10):973–980. doi: 10.1002/hipo.20631. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193(Pt 3):199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82(1):26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging. 2004;25(3):361–375. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr., Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16(2):147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2(3):175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52(2):135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18(9):3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus F, Hauser T, Slomianka L, Lipp HP, Amrein I. A reward increases running wheel performance without changing cell proliferation, neuronal differentiation or cell death in the dentate gyrus of C57BL/6 mice. Behav Brain Res. 2009;204(1):175–181. doi: 10.1016/j.bbr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Knuttinen MG, Gamelli AE, Weiss C, Power JM, Disterhoft JF. Age-related effects on eyeblink conditioning in the F344 × BN F1 hybrid rat. Neurobiol Aging. 2001;22(1):1–18. doi: 10.1016/s0197-4580(00)00194-9. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27(10):1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kruger TH, Hartmann U, Schedlowski M. Prolactinergic and dopaminergic mechanisms underlying sexual arousal and orgasm in humans. World J Urol. 2005;23(2):130–138. doi: 10.1007/s00345-004-0496-7. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert TJ, Fernandez SM, Frick KM. Different types of environmental enrichment have discrepant effects on spatial memory and synaptophysin levels in female mice. Neurobiol Learn Mem. 2005;83(3):206–216. doi: 10.1016/j.nlm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156(3):456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Leite MR, Wilhelm EA, Jesse CR, Brandao R, Nogueira CW. Protective effect of caffeine and a selective A2A receptor antagonist on impairment of memory and oxidative stress of aged rats. Exp Gerontol. 2011;46(4):309–315. doi: 10.1016/j.exger.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97(20):11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Caponiti JM, Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus. 2012;22(4):861–868. doi: 10.1002/hipo.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Thymidine analog methods for studies of adult neurogenesis are not equally sensitive. J Comp Neurol. 2009;517(2):123–133. doi: 10.1002/cne.22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Sexual experience promotes adult neurogenesis in the hippocampus despite an initial elevation in stress hormones. PLoS One. 2010;5(7):e11597. doi: 10.1371/journal.pone.0011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E. Structural plasticity and hippocampal function. Annu Rev Psychol. 2010;1:111–140. doi: 10.1146/annurev.psych.093008.100359. C1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16(3):216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc Natl Acad Sci U S A. 2007;104(43):17169–17173. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MC, Orr PT, Frick KM. Differential effects of acute progesterone administration on spatial and object memory in middle-aged and aged female C57BL/6 mice. Horm Behav. 2008;54(3):455–462. doi: 10.1016/j.yhbeh.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald HY, Wojtowicz JM. Dynamics of neurogenesis in the dentate gyrus of adult rats. Neurosci Lett. 2005;385(1):70–75. doi: 10.1016/j.neulet.2005.05.022. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- Middleton LE, Barnes DE, Lui LY, Yaffe K. Physical activity over the life course and its association with cognitive performance and impairment in old age. J Am Geriatr Soc. 2010;58(7):1322–1326. doi: 10.1111/j.1532-5415.2010.02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7(8):841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Monks DA, Lonstein JS, Breedlove SM. Got milk? Oxytocin triggers hippocampal plasticity. Nat Neurosci. 2003;6(4):327–328. doi: 10.1038/nn0403-327. [DOI] [PubMed] [Google Scholar]
- Nacher J, Alonso-Llosa G, Rosell DR, McEwen BS. NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol Aging. 2003;24(2):273–284. doi: 10.1016/s0197-4580(02)00096-9. [DOI] [PubMed] [Google Scholar]
- Ngwenya LB, Peters A, Rosene DL. Light and electron microscopic immunohistochemical detection of bromodeoxyuridine-labeled cells in the brain: different fixation and processing protocols. J Histochem Cytochem. 2005;53(7):821–832. doi: 10.1369/jhc.4A6605.2005. [DOI] [PubMed] [Google Scholar]
- O'Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176(2):362–366. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Oler JA, Markus EJ. Age-related deficits on the radial maze and in fear conditioning: hippocampal processing and consolidation. Hippocampus. 1998;8(4):402–415. doi: 10.1002/(SICI)1098-1063(1998)8:4<402::AID-HIPO8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Plaschke K, Feindt J, Djuric Z, Heiland S, Autschbach F, Lewicka S, Martin E, Bardenheuer HJ, Nawroth PP, Bierhaus A. Chronic corticosterone-induced deterioration in rat behaviour is not paralleled by changes in hippocampal NFkappaB-activation. Stress. 2006;9(2):97–106. doi: 10.1080/10253890600691551. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006;5(6):545–5558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzo G, Lopez-Rubalcava C, Fernandez-Guasti A. Anxiolytic-Like effect of ejaculation under various sexual behavior conditions in the male rat. Physiol Behav. 1999;67(5):651–657. doi: 10.1016/s0031-9384(99)00119-5. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233(1):12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia G, Del Arco A, De Blas M, Garrido P, Mora F. Environmental enrichment increases the in vivo extracellular concentration of dopamine in the nucleus accumbens: a microdialysis study. J Neural Transm. 2010;117(10):1123–1130. doi: 10.1007/s00702-010-0447-y. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6(18):2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Memory traces of trace memories: neurogenesis, synaptogenesis and awareness. Trends Neurosci. 2004;27(5):250–256. doi: 10.1016/j.tins.2004.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M, Czeh B, Fuchs E. Age-dependent susceptibility of adult hippocampal cell proliferation to chronic psychosocial stress. Brain Res. 2005;1049(2):244–248. doi: 10.1016/j.brainres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol Learn Mem. 2007;88(2):249–259. doi: 10.1016/j.nlm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20(17):6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29(46):14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soei E, Daum I. Course of relational and non-relational recognition memory across the adult lifespan. Learn Mem. 2008;15(1):21–28. doi: 10.1101/lm.757508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Galea LA. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67(10):1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Weinberg A, Viau V, Galea LAM. Prior sexual experience increases hippocampal cell proliferation and decreases risk assessment behavior in response to acute predator odor stress in the male rat. Behav Brain Res. 2009;200(1):106–112. doi: 10.1016/j.bbr.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9(4):526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szechtman H, Lambrou PJ, Caggiula AR, Redgate ES. Plasma corticosterone levels during sexual behavior in male rats. Horm Behav. 1974;5(2):191–200. doi: 10.1016/0018-506x(74)90043-9. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Zhu Y, Hata T, Shimizu-Okabe C, Suzuki K, Nakahara D. Intracranial self-stimulation enhances neurogenesis in hippocampus of adult mice and rats. Neuroscience. 2009;158(2):402–411. doi: 10.1016/j.neuroscience.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr., Kutiyanawalla A, Pillai A. Age-dependent alterations in nerve growth factor (NGF)-related proteins, sortilin, and learning and memory in rats. Physiol Behav. 2011;102(2):149–157. doi: 10.1016/j.physbeh.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry LC, Martin JB. Hypothalamic-pituitary responses to intracranial self stimulation in the rat. Brain Res. 1978;157(1):89–104. doi: 10.1016/0006-8993(78)90998-8. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Montagnese C, Rodriguez F, Vincent JD, Poulain DA. Oxytocin induces morphological plasticity in the adult hypothalamo-neurohypophysial system. Nature. 1986;322(6081):738–740. doi: 10.1038/322738a0. [DOI] [PubMed] [Google Scholar]
- Treiber KA, Carlson MC, Corcoran C, Norton MC, Breitner JC, Piercy KW, Deberard MS, Stein D, Foley B, Welsh-Bohmer KA. Cognitive stimulation and cognitive and functional decline in Alzheimer's disease: the cache county dementia progression study. J Gerontol B Psychol Sci Soc Sci. 2011;66(4):416–425. doi: 10.1093/geronb/gbr023. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37(2):402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Valenzuela M, Brayne C, Sachdev P, Wilcock G, Matthews F. Cognitive lifestyle and long-term risk of dementia and survival after diagnosis in a multicenter population-based cohort. Am J Epidemiol. 2011;173(9):1004–1012. doi: 10.1093/aje/kwq476. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci U S A. 2007;104(42):16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231(4):482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wuppen K, Oesterle D, Lewicka S, Kopitz J, Plaschke K. A subchronic applicationperiod of glucocorticoids leads to rat cognitive dysfunction whereas physostigmine induces a mild neuroprotection. J Neural Transm. 2010;117(9):1055–1065. doi: 10.1007/s00702-010-0441-4. [DOI] [PubMed] [Google Scholar]