Abstract
The significant and growing global prevalence of diabetes continues to challenge people with diabetes (PwD), healthcare providers, and payers. While maintaining near-normal glucose levels has been shown to prevent or delay the progression of the long-term complications of diabetes, a significant proportion of PwD are not attaining their glycemic goals. During the past 6 years, we have seen tremendous advances in automated insulin delivery (AID) technologies. Numerous randomized controlled trials and real-world studies have shown that the use of AID systems is safe and effective in helping PwD achieve their long-term glycemic goals while reducing hypoglycemia risk. Thus, AID systems have recently become an integral part of diabetes management. However, recommendations for using AID systems in clinical settings have been lacking. Such guided recommendations are critical for AID success and acceptance. All clinicians working with PwD need to become familiar with the available systems in order to eliminate disparities in diabetes quality of care. This report provides much-needed guidance for clinicians who are interested in utilizing AIDs and presents a comprehensive listing of the evidence payers should consider when determining eligibility criteria for AID insurance coverage.
Keywords: automated insulin delivery, closed-loop, type 1 diabetes, consensus recommendations
Graphical Abstract
ESSENTIAL POINTS.
AID therapy increases time in target glucose range with either no increase or a reduction in hypoglycemia compared with other diabetes therapies; AID therapy should therefore be considered for all populations with type 1 diabetes as it increases the likelihood of reaching recommended glycemic targets
Healthcare providers need to be aware of the different AID systems available, their benefits, and their limitations, to be able to advise and support people with diabetes to increase the likelihood that the clinical benefits of AID are realized
Commercially available AID systems still require basic diabetes management skills, including carbohydrate counting, for optimal glycemic control; opportunities to review and refresh these skills, where needed, should be sought
Specific AID training and support for users and healthcare providers are important to maximize clinical benefits of AID therapy
AID therapy is associated with significant improvements in quality of life and reduced burden of diabetes management for people with diabetes and their families
Since clinical outcomes with AID therapy depend on high AID usage, consideration should be given to the usability of available AID systems; optimal AID systems require low user input to achieve excellent glycemic outcomes
There are well documented and multifactorial racial and ethnic disparities in prescribing AID system technologies; healthcare provider preconceptions and unconscious biases about individual, family, and psychological attributes required to use AID technology effectively should be recognized and mitigated to ensure fair and equitable access to AID systems.
Diabetes is a chronic, demanding condition that poses a constant burden both on people with diabetes and on healthcare systems. Only a minority of persons with type 1 diabetes (T1D) meet widely accepted glycemic goals (1), demonstrating that there is an unmet need for better methods to achieve these goals. During the past 6 years, we have seen tremendous advances in automated insulin delivery (AID) technologies. Studies with various AID systems unequivocally demonstrate improvement in glycemic outcomes in people with T1D across all age groups, in all genders, and regardless of diabetes duration, prior insulin delivery modality, or baseline glycated hemoglobin (HbA1c) levels (2–6). Studies have also suggested cost-effectiveness of these systems (7–10). Yet despite the success of AIDs in improving glycemic control, guidance for integrating AID systems into clinical practice is limited. Moreover, as with all new technologies, negotiating insurance coverage for AID has been protracted.
In 2021, the Advanced Technologies & Treatments for Diabetes (ATTD) Congress organized an international panel of clinicians, researchers, and patient advocates with expertise in AID to develop clinical guidelines for initiating AID for individuals with T1D. The panel was divided into 9 working groups to address the various aspects of AID therapy, including evolution of AID; clinical evidence; determining the target population for AID use; initiation of AID; education and training; utilization of AID; AID data reporting; psychological issues/user perspective; and the future of AID. Recommendations from each working group were presented to the full panel and voted upon. This article summarizes the consensus recommendations from the panel.
The purpose of this report is 2-fold: (1) to provide needed guidance to clinicians who are interested in utilizing AID; and (2) to serve as a comprehensive review of evidence for payers to consider, when determining eligibility criteria for AID insurance coverage.
Evolution of AID Systems
Refinements in continuous glucose monitoring (CGM) technologies and dosing algorithms have led to the development of AID systems for the purpose of enhancing glucose management and minimizing burden around insulin delivery. AID systems utilize a sophisticated controller algorithm that continuously adjusts insulin delivery in response to real-time sensor glucose levels, residual insulin action and other inputs, such as meal intake and exercise announcement. The algorithm accommodates variability of insulin requirements between and within individual users. However, despite significant advances in controller algorithms in providing closed-loop insulin delivery between meals, users must still manually announce carbohydrate intake to achieve adequate postprandial insulin coverage. This is needed because current hybrid systems are not physiologic in that they rely on a delayed subcutaneous glucose signal (sensor lag time of 4 to 10 minutes) (11) and delayed subcutaneous insulin delivery into the circulation (peak insulin levels appear 45 to 60 minutes after injection) (12). Therefore, one of the major limitations for fully automated systems is the pharmacokinetics and pharmacodynamics profiles of commercially available insulins.
Currently, all commercially available AID systems are single hormone (insulin only) systems. Dual hormone AID systems, which incorporate other hormones (glucagon, pramlintide) to more closely mimic pancreatic physiology, are under development (13, 14). The addition of glucagon to an AID system may confer additional protection from hypoglycemia. Pramlintide, an analogue of amylin which is co-secreted with insulin from beta-cells, reduces postprandial glucose excursions by slowing gastric emptying and suppressing glucagon secretion.
AID Algorithms
Several types of control algorithms have been developed, including model predictive control (MPC), proportional integral derivative (PID), and fuzzy logic (FL) controllers (15). MPC algorithms use patient-specific model parameters to calculate insulin delivery by minimizing the difference between model-predicted glucose concentrations and target glucose over a prespecified prediction time horizon. Thus, the algorithm adjusts the insulin treatment in order to bring the predicted glucose levels into the target range. PID controllers are reactive, adjusting insulin delivery by assessing glucose excursions from 3 perspectives: the proportional component calculates the deviation of measured glucose level from the target glucose; the integral component calculates the area under the curve between measured and target glucose, the third derivative component takes into account the rate of change of measured glucose, and all together dictate the amount of insulin delivered. Some PID controllers have been modified to also include feedback of a model-predicted insulin profile. A fuzzy logic control algorithm is a clinical approach to the modulation of insulin delivery based on a set of rules that imitate the line of reasoning of diabetes practitioners, which in turn are based on common medical knowledge, experience of diabetes practitioners, and known recommendations.
Hybrid and Fully AID Systems
Current commercially available AID systems require users to manually enter prandial insulin boluses and signal exercise while automatically modulating insulin delivery. Fully AID systems, which obviate the need for carbohydrate counting and manually initiated prandial boluses, are under development at present, but the benefits in reduced user burden come at the expense of glycemic control (16). Use of truly faster insulin analogs within the AID system or glucose-lowering adjuvant therapies may make this approach more feasible in the future (see “The Future of AID: What Will It Look Like?”). Table 1 presents a description of commercially available AID systems. Table 2 presents some of the AID systems that are currently in development or under regulatory review.
Table 1.
Commercially available AID systems
| Medtronic 670G/770G | Medtronic 780G | CamAPS FX | Diabeloop | Control-IQ | Omnipod 5 | |
|---|---|---|---|---|---|---|
| Algorithm and approach | PID algorithm with insulin feedback with adaptive insulin limits Located on pump |
PID algorithm with insulin feedback with adaptive insulin limits and model based auto-corrections Located on pump |
Treat to target adaptive MPC algorithm (interoperable) App on unlocked smartphone |
Treat to target adaptive MPC algorithm App on smartphone /Handheld device |
Treat to range adaptive MPC algorithm (interoperable) Located on pump |
Treat to target adaptive MPC algorithm (interoperable) Located within pod (controlled from the Omnipod 5 controller or a phone App) |
| Target glucose | Fixed target: 120 mg/dL (6.7 mmol/L) Optional activity target: 150 mg/dL (8.3 mmol/L) |
Target: 100 mg/dL (5.6 mmol/L) (default); Customizable: 110 mg/dL (6.1 mmol/L) or 120 mg/dL (6.7 mmol/L) Optional activity target: 150 mg/dL (8.3 mmol/L) |
Target: 104 mg/dL (5.8 mmol/L) (default); Customizable between 80 mg/dL and 200 mg/dL (4.4 mmol/L and 11.0 mmol/L) Optional activity mode |
Target: 110 mg/dL (6.1 mmol/L) (default); Customizable from 100 mg/dL (5.5 mmol/L) to 130 mg/dL (7.2 mmol/L) Zen-mode: (20–40 mg/dL, 0.5–2.2 mmol/L) higher than current target Activity mode (customizable) |
Fixed target range: 112.5–160 mg/dL (6.2–8.9 mmol/L) Intensified overnight target range of 112.5–120 mg/dL (6.2–6.7 mmol/L) Optional activity range 140–160 mg/dL (7.8–8.9 mmol/L) |
Target: customizable between 110 mg/dL and 150 mg/dL (6.1 mmol/L and 8.3 mmol/L) in increments of 10 mg/dL Optional activity target: 150 mg/dL (8.3 mmol/L) |
| Basal insulin delivery | Algorithm driven basal insulin delivery adjusted every 5–10 minutes based on real-time CGM data | |||||
| Automated correction boluses | None. Manual correction boluses targeting 150 mg/dL (8.3 mmol/L) based on control algorithm parameters not programmed sensitivity factors | Automated correction boluses targeting 120 mg/dL (6.7 mmol/L) once automatic basal reaches maximum. Correction boluses based on control algorithm parameters not programmed sensitivity factors |
Automated correction boluses via more aggressive basal rate adjustments Optional use of “Boost” mode (user ability to temporary increase insulin delivery) Manual correction boluses optional based on programmed sensitivity factors |
Automated correction boluses | Automated correction boluses (60% of the calculated correction dose) if glucose predicted to exceed 180 mg/dL (10.0 mmol/L) targeting glucose of 110 mg/dL (6.1 mmol/L) Manual correction boluses optional |
Automated correction boluses via more aggressive basal rate adjustments Manual correction boluses optional |
| Safety parameters | Maximum hourly basal insulin delivery, but not maximum total hourly delivery Maximum 4 h basal insulin delivery Minimum insulin delivery for 2.5 h Maximum bolus amount |
Maximum hourly basal insulin delivery, but not maximum total hourly delivery Maximum 7 h basal insulin delivery Maximum basal delivery in 24 h Maximum bolus amount Minimum insulin delivery for 3–6 h |
Maximum insulin delivery in 24 h Maximum bolus amount Minimum insulin delivery for 1.5 h |
Variable aggressiveness A bolus for a given meal can be modulate by ± 10% increment Alert for rescue carbohydrates |
Maximum insulin delivery in 2 h Maximum insulin delivery in 24 h Maximum bolus amount |
Maximum individual insulin delivery at any given time Maximum bolus amount |
| Settings that can be modified by user/HCP | Insulin to carbohydrate ratio Active insulin time Temp glucose target |
Insulin to carbohydrate ratio Active insulin time Target glucose for algorithm Temp glucose target |
Target system glucose Insulin to carbohydrate ratio Boost or Ease off—more or less aggressive algorithm |
Target system glucose Total daily dose Algorithm treatment reactivity (aggressiveness) Insulin to carbohydrate ratio |
Basal insulin rates Insulin to carbohydrate ratio Insulin sensitivity factor Temp glucose target Sleep mode |
Insulin to carbohydrate ratio Insulin sensitivity factor (user boluses) Active insulin time (user boluses) Target glucose for algorithm Activity glucose target with attenuated insulin delivery |
| Algorithm learning | Based on TDD and an estimate of fasting glucose and the plasma insulin concentration at the time of fasting | Based on TDD and an estimate of fasting glucose and the plasma insulin concentration at the time of fasting | Adapts to day-to-day, prandial and diurnal patterns; independent of programmed basal and sensitivity pump settings | Based on TDD | Based on TDD, updated with each Pod change (every 3 days) | |
| Compatible insulin pump | 670G/770G | 780G | Designed as interoperable controller; currently available with Dana RS, Dana I, mylife YpsoPump | Kaleido patch pump Roche Accu-Chek |
Designated by FDA as interoperable controller; currently available in Tandem t:slim X2 | Designated by FDA as interoperable controller; Omnipod 5 ACE |
| Compatible CGM system | Guardian 3 Duration 7 days Requires calibrations (min 4–6x/d) |
Guardian 3 Duration 7 days Requires calibrations (min 2x/d) CE mark: Guardian 4, duration 7 days, factory calibrated, optional calibration |
Dexcom G6 Duration 10 days Factory calibrated, optional calibration |
Dexcom G6 Duration 10 days Factory calibrated, optional calibration |
Dexcom G6 Duration 10 days Factory calibrated, optional calibration |
Interoperable iCGM currently available: Dexcom G6 Duration 10 days Factory calibrated, optional calibration |
| Data management system | Carelink; manual downloading of pump required for 670G, automated download with 770G | Carelink; automated app compatibility | Diasend; automated download | Diasend; download | t:Connect mobile; automated download | Omnipod Connect; automated download |
| Compatible insulin | Rapid only | Rapid only | Rapid and ultra-rapid | Rapid only | Rapid only | Rapid only |
| Approved indications for use | FDA and CE mark 7 years and upward excluding pregnancy for 670G and 2 years and upward for 770G (FDA only) |
CE mark 7 to 80 years excluding pregnancy |
CE mark 1 year and upward including pregnancy |
CE mark 12–18 years (DBL4T) >18 years (DBLG1) excluding pregnancy |
FDA and CE mark 6 years and upward excluding pregnancy |
FDA cleared 2 years and upward excluding pregnancy |
| Other benefits | Extensive clinical experience (>200,000 users) Robust training and support Remote monitoring capabilities (770G) |
Evidence base from clinical trials Increased usability compared to 670G Remote monitoring capability |
Evidence base from clinical trials Mobile app for remote insulin bolusing Online app updates Remote monitoring capability Online training for HCPs and users |
Remote monitoring capability— YourLoops web-based platform | Extensive clinical experience (>270,000 users) FDA cleared the t:connect mobile app for remote insulin bolusing Online firmware upgrade Online training for HCPs and users |
Evidence base from clinical trials Online firmware upgrade Online training for HCPs and users |
Abbreviations: CGM, continuous glucose monitoring; HCP, health care provider; MPC, model predictive control; PID, proportional integral derivate; TDD, total daily dose.
Table 2.
AID systems under development or regulatory review
| Tidepool Loop | iLet (insulin only) | Inreda (insulin and glucagon) | |
|---|---|---|---|
| Algorithm and approach | MPC algorithm iPhone app |
MPC algorithm Located on pump |
Insulin PID algorithm Located on pump |
| Compatible insulin pump | Omnipod patch pump MiniMed Medtronic |
iLet pump | Inreda pump |
| Compatible CGM system | Dexcom G6 Medtronic Guardian Connect |
Dexcom G6 | Medtronic |
| Regulatory status | FDA regulatory submission made | Not submitted | CE mark |
Abbreviations: MPC, model predictive control; PID, proportional integral derivative.
Interoperability and Intraoperability
The ability of components of an AID system (CGM, insulin pump, and algorithm) to communicate accurately and interact effectively with each other is critical for achieving optimal glycemic control. This can come in the form of intra- or interoperability. Intraoperability describes the exchange of data and interaction within the same system provided by the same manufacturer. Interoperability facilitates the exchange of data and interaction of different AID system components, offering users increased choice and flexibility for a personalized AID system. However, this depends on commercial agreements between device manufacturers.
Summary of Clinical Evidence
Clinical evidence supporting the efficacy and safety of AID systems has grown over the last 5 years with the introduction of multiple commercially available, and soon to become available, AID systems. As of March 2022, the U.S. Food & Drug Administration (FDA) has approved the Medtronic 670G/770G (4, 17, 18), the Control-IQ (2, 19, 20), and recently cleared the first tubeless AID system, the Insulet Omnipod 5 (21). Conformite Europeenne (CE) approval has been granted to the Medtronic 780G (5, 22, 23); CamAPS FX (6); Diabeloop (24, 25); Inreda (26); Control-IQ, and Medtronic 670G. Some systems are currently under FDA review, including the Medtronic 780G (5, 22, 23) and Tidepool Loop (27).
Randomized Controlled Trials
Randomized controlled trials (RCTs) and single-arm studies with interventions of 3 months or longer, including children as young as 2 years and adults up to 75 years of age with T1D have been conducted (Tables 3 and 4). Some RCTs provide separate analyses for adolescents and adults allowing evaluation in specific age groups. Study designs vary from single-arm trials without a concurrent comparator to parallel-group studies and crossover randomized trials. The lack of a control group in single-arm studies limits the ability to determine how much of this achievement is attributed to AID use, as opposed to a study effect. Furthermore, some of the populations studied differ in baseline time in range (TIR; 70–180 mg/dL). Lower baseline TIR was found to be associated with a greater improvement in TIR on AID (33). These differences in study design impair the ability to do cross-study comparisons.
Table 3.
Randomized controlled trials for commercially available AID systems
| AID system (author & publication year) | Study design (type, duration, comparison group) | Study population (number of participants & age, mean baseline HbA1c) | Glycemic outcomesa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ΔMean sensor glucose | ΔTIR 70–180 mg/dL | ΔTBR < 70 mg/dL | ΔTBR < 54 mg/dL | ΔTAR > 250 mg/dL (or 300 or 180 mg/dL) | ΔHbA1c | ||||
| Children/Adolescents | |||||||||
| AHCL vs 670G Bergenstal et al, 2021 (23) |
Crossover trial, 2 13-week periods, comparison of AHCL vs 670Gb and vs baselinec | N = 113, 14–29 yo, T1D, baseline mean HbA1c: 7.9%, TIR: 57% | −7 mg/dLb −7 mg/dL: 670G −14 mg/dL: AHCL |
+4%b +6%: 670G +10%: AHCL |
0%b −0.1%: 670G −0.2%: AHCL |
−0.04%b +0.04%: 670G 0%: AHCL |
−1%b −3%: 670G −4%: AHCL |
−0.2%b −0.3%: 670G −0.5%:AHCL |
|
| AHCL Collyns et al, 2021 (5) |
Crossover trial, 2 4-week periods, comparison of AHCL vs PLGS | N = 33, 7–21 yo, (N = 14, 14–21 yo, N = 19, 7–13 yo), T1D, baseline mean HbA1c, TIR: NA | −13 mg/dL: 14–21 yo −9 mg/dL: 7–13 yo |
+14%: 14–21 yo +12%: 7–13 yo |
−0.4%: 14–21 yo −0.7%: 7–13 yo |
−0.1%: 14–21 yo −0.2%: 7–13 yo |
−14%: 14–21 yo −11%: 7–13 yo (T > 300 mg/dL) |
NA | |
| Control-IQ Isganaitis et al, 2020 (3) |
6-mo randomized trial, comparing CIQ with SAP | N = 63, 14–24 yo, T1D, baseline mean HbA1c: 8.1%, TIR: 52% | −18 mg/dL | +13% | −0.7% | −0.09% | −8% | −0.30% | |
| Control-IQ Breton et al, 2020 (20) |
16-week randomized trial, comparing CIQ with SAP | N = 101, 6–13 yo, T1D, baseline mean HbA1c: 7.7%, TIR: 53% | −13 mg/dL | +11% | −0.40% | −0.07% | −6% | −0.40% | |
| CamAPS FX Ware et al, 2022 (28) |
4-mo randomized trial, comparing CamAPS FX with SAP | N = 74, 1–7 yo, T1D, baseline mean HbA1c: 7.3%, TIR: NA | −13 mg/dL | +9% | +0.07% | +0.02% | −1% (T > 300 mg/dL) | −0.4% | |
| Adults | |||||||||
| 670G McAuley et al, 2020 (4) |
6-mo randomized trial comparing 670G with MDI/CSII | N = 120, ≥ 25 yo, T1D, baseline mean HbA1c: 7.4%, TIR: 55% | −13 mg/dL | +15% | −2.0% Median |
−0.6% Median |
−2.9% Median |
−0.4% | |
| Control-IQ Brown et al, 2019 (2) |
6-mo randomized trial, comparing CIQ with SAP | N = 168, 14–71 yo, T1D, baseline mean HbA1c: 7.4%, TIR: 61% | All the group | −13 mg/dL | +11% | −0.9% | −0.1% | −5.3% | −0.33% |
| N = 105, 25–71 yo | +10% | −2.2% | |||||||
| CamAPS, FX Tauschmann et al, 2018 (6) |
3-mo randomized trial, comparing CamAPS FX algorithm with SAP | N = 86, ≥ 6 yo, T1D, baseline mean HbA1c: 8.3%d, TIR: NA | All the group | −15 mg/dL | +11% | −0.8% | −0.1% (<50 mg/dL) | −1.4% (T > 300 mg/dL) | −0.36% |
| N = 44, ≥ 22 yo | +10% | −0.5% (<63 mg/dL) | −0.3% | ||||||
| CamAPS FX Boughton et al, 2022 (29) |
4-mo randomized trial, comparing CamAPS FX with SAP | N = 37, 60 yo and older, T1D, baseline mean HbA1c: 7.4%, TIR: 70% | −13 mg/dL | +9% | −0.1% | −0.0% | −0.7% (T > 300 mg/dL) | −0.2% | |
| Diabeloop. Benhamou et al, 2019 (24) |
Crossover trial, 2 12-week periods, comparing Diabeloop with SAP | N = 68, ≥ 18 yo, T1D, baseline mean HbA1c: 7.6%, TIR: NA | −9 mg/dL | +9% | −2.4% | −0.5% (<50 mg/dL) | −4.3% | −0.15% | |
Abbreviations: AHCL, advanced hybrid AID; AID, automated insulin delivery; CSII, continuous subcutaneous insulin delivery; HbA1c, glycated hemoglobin; MDI, multiple daily injections; mo, month; SAP, sensor-augmented pump, TAR, time above range (>180 mg/dL [>10.0 mmol/L], >250 mg/dL [13.9 mmol/L); TBR, time below range (<70 mg/dL [<3.9 mmol/L], <54 mg/dL [<3.0 mmol/L); TIR, time in range (70–180 mg/dL [3.9–10 mmol/L]), yo, years old.
Reported glycemic metrics are mean differences between groups for randomized trial.
Comparison between 2 AIDs.
Glycemic metrics estimated from reported means in each group
Differences reported from rank tests.
Table 4.
Single-arm studies for commercially available AID systems
| AID system (author & publication year) | Study design (type, duration, comparison group) | Study population (number of participants & age, mean baseline HbA1c) | Glycemic outcomesa | |||||
|---|---|---|---|---|---|---|---|---|
| ΔMean sensor glucose | ΔTIR 70–180 mg/dL | ΔTBR < 70 mg/dL | ΔTBR < 54 mg/dL | ΔTAR > 250 mg/dL (or 300 or 180 mg/dL) | ΔHbA1c | |||
| Children/Adolescents | ||||||||
| 670G Bergenstal et al, 2016 (17) Garg et al, 2017 (30) |
3-mo single-arm study | N = 30, 14–21 yo, T1D, baseline mean HbA1c: 7.7%, TIR: 60% | −5 mg/dL | +7% | −1.5% | −0.2% | −1% (T > 300 mg/dL) | −0.6% |
| 780G Carlson et al, 2022 (22) |
3-mo single-arm study | N = 39, 14–21 yo, T1D, baseline mean HbA1c: 7.5%, TIR: 62% | −6 mg/dL | +6% | −1% | −0.3% | −1.6% | −0.5% |
| 670G Forlenza et al, 2019 (18) |
3-mo single-arm study | N = 105, 7–13 yo, T1D, baseline mean HbA1c: 7.9%, TIR: 56% | −7 mg/dL | +9% | −1.7% | −0.5% | −3% | −0.4% |
| 670G Forlenza et al, 2022 (31) |
3-mo single-arm study | N = 46, 2–7 yo, T1D, baseline mean HbA1c: 8.0%, TIR: 56% | −12 mg/dL | +8% | −0.1% | 0% | −4% | −0.5% |
| Omnipod 5b Brown et al, 2021 (21) |
3-mo single-arm study | N = 112, 6–13 yo, T1D, baseline mean HbA1c: 7.7%, TIR: 53% | −23 mg/dL | +16% | −0.4% | −0.1% | −9% | −0.7% |
| Omnipod 5b Sherr et al, 2022 (32) |
3-mo single-arm study | N = 80, 2–6 yo, T1D, baseline mean HbA1c: 7.4%, TIR: 57% | −14 mg/dL | +11% | −0.3% | +0.1% | −6% | −0.6% |
| Adults | ||||||||
| 670G Bergenstal et al, 2016 (17) | 3-mo single-arm study | N = 94, 22–75 yo, T1D, baseline mean HbA1c: 7.3%, TIR: 69% | +2 mg/dL | +5% | −3% | −0.5% (<50 mg/dL) | −0.5% (T > 300 mg/dL) | −0.5% |
| 780G Carlson et al, 2022 (22) |
3-mo single-arm study | N = 118, 22–75 yo, T1D, baseline mean HbA1c: 7.5%, TIR: 71% | −4 mg/dL | +4% | −0.9% | −0.3% | −1% | −0.5% |
| Omnipod 5 Brown et al, 2021 (21) |
3-mo single-arm study | N = 129, 14–70 yo, T1D, baseline mean HbA1c: 7.2%, TIR: 65% | −8 mg/dL | +9% | −1.6% | −0.4% | −4% | −0.4% |
Abbreviations: TAR, time above range (>180 mg/dL [>10.0 mmol/L], >250 mg/dL [13.9 mmol/L); TBR, time below range (<70 mg/dL [<3.9 mmol/L], <54 mg/dL [<3.0 mmol/L); TIR, time in range (70–180 mg/dL [3.9–10 mmol/L]).
Reported glycemic metrics are mean change from baseline to follow-up for single-arm studies (comparison of study period with baseline).
Omnipod 5 is expected to be commercially available during 2022.
In general, all the AID systems have uniformly demonstrated an increase in TIR and a reduction in mean glucose, time in hyperglycemia, and HbA1c. Overall improvement in glycemic control was similar across all age groups and was evident during both day and night. Yet even with AID use, TIR improves more overnight than during the day. TIR increased by 9% to 16% for most systems while HbA1c levels decreased by 0.3% to 0.5%, with either no change or a reduction in time in hypoglycemia. The greatest improvement in glycemic control is seen in those who have the lowest baseline TIR or highest HbA1c (33, 34). The effect on hypoglycemia has varied, also depending on the comparison group features and the amount of hypoglycemia present at baseline. In some studies, use of AID has been shown to reduce hypoglycemia even when compared to sensor-augmented pump (SAP) therapy with predictive low glucose suspend (PLGS) (5, 35). Of note, AID use resulted in reduced rates of both hypoglycemia and hyperglycemia, thus increasing TIR. This contradicts the paradigm that improving glycemic control necessarily leads to an increase in hypoglycemia (36).
Real-World Studies
Real-world data are now also available, shedding light on true AID acceptance and performance. It is reassuring to find that outcomes are similar to those of the pivotal studies in the means of TIR and time below range (TBR), with a modest reduction in HbA1c of 0.3% to 0.4% (35, 37–40). (Table 5). Current data also supports improved quality of life and users’ reported outcomes (42–44). However, several publications on real-world use of the Medtronic 670G revealed that approximately one-third of youth starting on the 670G system discontinue use within 1 year (45, 46). Recent studies showed increased use of auto-mode on Medtronic’s Advanced Hybrid AID compared to 670G (86% vs 75%, respectively) (23) and the real-world data of the use of Tandem’s Control-IQ which reported 94% use of auto-mode (35).
Table 5.
Key real-world studies
| Closed-loop system (author & publication year) | Study design (type, duration, comparison group) | Study population (number of participants & age, mean baseline HbA1c) | Number of participants by age category | Glycemic outcomes (start to end of study) | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔMean sensor glucose | ΔTIR 70–180 mg/dL | ΔTBR < 70 mg/dL | ΔTBR < 54 mg/dL | ΔTAR > 250 mg/dL | ΔHbA1c | ||||
| 670G Stone MP et al, 2018 (37) |
3-mo retrospective, CareLink system data comparing baseline | N = 3141, >7 yo, T1D, no baseline HbA1c | N = 2066, 22–60 yo N = 649, ≥ 60 yo |
−7 mg/dL −6 mg/dL |
+8% +6% |
−0.7% −0.4% |
−0.1% | −2.7% | |
| N = 105, 7–13 yo N = 244, 14–21 yo |
−17 mg/dL −10 mg/dL |
+11% +8% |
+0.5% −0.3% |
||||||
| 670G Akturk et al, 2019 (38) |
6-mo retrospective single-center study comparing study period with baseline SAP use | N = 127, 21–68 yo, T1D, baseline mean HbA1c: 7.6% | −12 mg/dL | +11% | −1% | −0.2% | −0.5% | −0.4% | |
| 780G Da Silva et al, 2022 (40) |
2-mo retrospective, CareLink system data comparing study period with baseline | N = 812, T1D, baseline mean estimated HbA1c: 7.2% | No data | −15.7 mg/dL | +12% | −0.3% | −0.1% | −4.2% | −0.4% |
| Control-IQ Breton & Kovatchev 2021 (35) |
12-mo retrospective, real-world observational study, comparing study period with baseline (PLGS)** | N = 9010, 6–91 yo, T1D or T2D, baseline mean estimated HbA1c: 7.3% (N = 7813 T1D) |
N = 5616, 19–63 yo N = 1773, >63 yo |
−13 mg/dL −12 mg/dL |
+10% +9% |
−0.8% 0% |
+0.1% 0% |
−3% −2% |
−0.3% GMI for all group |
| N = 716, 6–13 yo N = 905, 14–18 yo |
−15.5 mg/dL −13 mg/dL |
+12% +12% |
+0.1% +0.1% |
+0.1% +0.1% |
−5% −6% |
||||
| Control-IQ Messer et al, 2021 (41) |
6-mo prospective, real-world single-center comparing study period with baseline | N = 191, children and adolescents with T1D, baseline mean HbA1c: 7.6% | −12.5 mg/dL | +9.4% | −0.4% | 0% | −4.3% | −0.3% GMI | |
| Loop Open Source Lum et al, 2021 (27) |
6-mo prospective, real-world observational study comparison of study period with baseline** | N = 558, 1–71 yo, T1D, baseline mean HbA1c: 6.8% | −10 mg/dL | +7% | −0.2% | −0.05% | −2% | −0.3% | |
**Time in range change from baseline estimated from median.
Abbreviations: GMI, glucose management index; HbA1c, glycated hemoglobin; mo, month; yo, years old.
Altogether, the data gathered provide solid evidence for the safety and efficacy of AID system use for a broad age range of PwD. Rates of acute complications such as severe hypoglycemia (SH) and diabetic ketoacidosis (DKA) were low. Of note, almost all pivotal trials exclude (or have very few) participants with a recent history of DKA or SH, thereby substantially lowering the risk of such complications. Real-world observational trials show lower rates of DKA/SH than those published in the US T1D Exchange Registry (1). Several studies also suggested improved quality of life, reduced diabetes burden, reduced fear of hypoglycemia and a return to restful sleep for PwD and family (44, 47–53), while few studies failed to find improvements in patient-reported outcomes (43, 54) (see “Psychological Issues and PwD Perspectives on AID Systems”).
Knowledge Gaps
Cost-effectiveness studies of AID systems are scarce. However, an analysis of the MiniMed 670G AID system vs continuous subcutaneous insulin delivery (CSII), showed that the higher acquisition costs of the AID system were offset by clinical benefits, reduced complication costs, and quality of life improvements, which represented an overall cost-effective treatment option for people with T1D (8). Similar results were reported for the MiniMed 670G AID system vs multiple daily injections (MDI) and intermittent scanned CGM (isCGM) (10). Additional data on other systems will be valuable.
Another knowledge gap is the use of AID systems in special populations. Data are accumulating on AID use in young children (< 6 years) with T1D (55–57). Several feasibility studies describe AID use in other populations, such as pregnant women with T1D (58, 59) and people with T2D (60, 61). To support AID implementation in these populations, larger and longer randomized controlled studies are needed. In addition, both RCTs and real-world studies lack racial and ethnic diversity, thereby limiting universal AID adoption (62).
Target Populations for AID Therapy
Selecting the people who will benefit most from AID system use is essential to optimize both efficacy and safety of treatment. Table 6 presents graded evidence-based recommendations for individuals who should be considered for AID system use (American Diabetes Association [ADA] evidence-grading system) (87).
Table 6.
Summary of recommendations: target populations
|
AID should be considered for all people with T1D, especially those experiencing suboptimal glycemia, problematic hypoglycemia, and/or significant glycemic variability. AID use can be particularly useful in persons at moderate to high risk for frequent and/or severe hypoglycemia (74, 88) and hypoglycemia unawareness (75, 76). Furthermore, small initial studies reported an improvement in hypoglycemia awareness with the use of AID systems (76, 77).
Additionally, lifestyle and quality of life issues should be considered when determining treatment options. As previously mentioned, evidence from numerous RCTs and real-world studies support the safety and efficacy of use of AID systems in young, school-aged pediatric populations and in adolescent/adult populations (2, 3, 5, 17, 18, 20, 23, 30, 35, 46, 56, 57, 63–67, 70–73, 89, 90). Although some studies included children from the age of 1 year, and adult populations older than 65 years (2, 4, 23, 27, 29, 35, 37, 68, 69, 89, 91), additional research is required to truly estimate the impact of AID in these age groups.
AID use can be beneficial in pregnant women (58, 60, 78–81), but the glucose targets needed during pregnancy are lower than most commercially available AID systems currently offer. The benefits of AID have also been demonstrated in insulin-naïve users with T2D in outpatient (60) and inpatient, noncritical care settings (61, 92) and in people on hemodialysis (82, 84) or with gastroparesis (83). However, additional studies are needed to confirm safety and efficacy for these populations.
Each candidate for AID use should be evaluated by their healthcare provider, to determine their ability to manage intensive insulin therapy. Factors to consider include proficiency in mealtime insulin dosing, motivation, willingness to participate in formal device training, manual dexterity/visual status, and financial/insurance status.
Initiating AID System Use
Table 7 presents general recommendations for initiating AID use in PwD.
Table 7.
Summary of recommendations: initiating AID use
|
|---|
|
|
|
|
|
|
|
The Optimal Time to Initiate AID
Early initiation of diabetes technologies in recently diagnosed PwD has been shown to improve and sustain long-term glycemic control, and thereby perhaps reduce the risk of diabetes-related complications (93–95). Moreover, tight glycemic control from disease onset in people with T1D may help to preserve beta-cell function (96). There are no definitive data to support the benefit of early initiation of AID systems on long-term metabolic control or beta-cell preservation (97). Studies are underway to examine the safety and efficacy of early AID adoption in adults and children newly diagnosed with T1D (98, 99). Likely benefits of early initiation include long-term glycemic control, long-term device acceptance, durable use, and a particular benefit for preschool age (100).
Setting Up the AID System
AID settings should be selected according to individualized glycemic targets, based on recently acquired CGM metrics (101). In poorly controlled individuals, using the highest system glucose target possible for the first few weeks is suggested. When determining the settings, the healthcare provider should use conservative estimates to ensure prevention of hypoglycemia. The information needed for initiating the AID system and the parameters that affect automated insulin delivery differ widely across different AID systems. Clinical judgment should be used where programmed regimens do not result in optimal glycemic outcomes. Requirements for initiating AID for specific systems are provided in Table 8.
Table 8.
Recommendation for preparation and initiation of AID system
| Medtronic 670G/770G/780G | Tandem Control-IQ | CamAPS FX | Insulet OP5 | |
|---|---|---|---|---|
| Preparation before starting AID |
|
|||
| Information needed to start AID | TDD from 3–7 days of using the Medtronic AID pump | Body weight and TDD, basal profile, CHO:I ratios, CF’s | Body weight and TDD | Basal profile, CHO:I ratios, CF’s, AIT |
| Recommended initial settings |
|
|
|
|
| Glucose targets |
|
|
|
|
| AID adaptivity |
|
|
|
|
| Autocorrection |
|
|
|
|
| Modifiable factors to optimize AID |
|
|
|
|
| Exercise |
|
|
|
|
| Reverse correction if below target |
|
|
|
|
| Extended bolus |
|
|
|
|
| Use of CGM trend in bolus calculation |
|
|
|
|
Abbreviations: AIT, active insulin time; CF, correction factor; CGM, continuous glucose monitor; ICR, insulin to carb ratio; TDD, total daily insulin dose.
Education, Training, and Support
A rigorous, comprehensive, consistent, and structured education curriculum for AID must be of high priority for all AID systems and must be individualized for each PwD. The following recommendations present the essential elements that should be considered in providing education and training to individuals who are initiating AID. Table 9 presents recommendations for patient training and education.
Table 9.
Summary of recommendations for training and education
|
|
|
|
|
|
Abbreviations: AID, automated insulin delivery; CGM, continuous glucose monitoring; CL, closed loop; PwD, people with diabetes.
Training for AID Systems
It is important to emphasize that transition to AID systems should be individualized. In general, persons who are CGM naïve will benefit from several days of CGM use before commencing AID. This period can be used for education on alarms, trend arrows, and data interpretation for optimization of insulin therapy, which may allow for better starting parameters for AID transition. CGM user engagement requires insertion of sensors, replacing sensors when failures occur, and knowledge on how to troubleshoot sensor failures. This education is vital to avoid burnout, frustration, and to optimize successful device use. Those who are naïve to CSII therapy should follow existing protocols for switching from MDI to CSII therapy, considering 1 to 2 weeks of SAP therapy before commencing AID. Education for CSII-related eventualities such as alternating pump insertion sites, replacing infusion sets, and how to troubleshoot pump occlusions are advised. Pump users should consider using SAP mode for several days if switching from a different pump brand, allowing for adaptation both to the user interface and to the bolus calculator that may require insulin dose adjustments. It is recommended that PwD and care partners demonstrate understanding of the AID system features, how to use them, and how to troubleshoot. Initial training can be successful when delivered face-to-face, by videoconference (107–110), and with supporting roles for e-learning, video, simulation apps, and combined approaches. Where applicable, industry should continue their essential role in certifying trainers to provide initial device training.
Emphasize Choice and Personal Reasons
PwD should have the opportunity to assess the full benefit and burden of available AID systems to decide if and which device is most suitable for them. Educational support, personal resources, age/licensing/availability/insurance, and personal preferences need to be considered, and unbiased sources should be heavily utilized by PwD (eg, clinical educators, non-commercial entities such as JDRF, ADA, Association of Diabetes Care & Education Specialists [ADCES], Diabeteswise.org, or BDCPantherDiabetes.org).
Prioritize Comprehensive Education
PwD must be trained and assessed for proficiency on general diabetes management, carbohydrate counting, insulin pump use, and CGM use in order to use an AID system safely. We recommend the creation and use of a universal pre-AID checklist or framework to comprehensively review essential education. AID training is not just technical and cannot be separated from the overall management of diabetes. It is actually adding on the tip of the pyramid of education. The base is the core diabetes knowledge and management education, CGM basics, insulin pump basics, and on the top is the AID basics education. Table 10 presents a comprehensive pre-AID education checklist.
Table 10.
Pre-AID comprehensive education checklist
| Core diabetes knowledge |
|---|
| ȃInsulin action time |
| ȃBlood glucose and blood ketone testing |
| ȃImportance of proper nutrition |
| ȃImportance of physical activity |
| ȃTreating hypoglycemia |
| ȃCarbohydrate counting |
| ȃChecking in witd psychological concerns around diabetes and diabetes care |
| Insulin pump basics |
| ȃSet changes and site rotation |
| ȃConnecting and disconnecting infusion set or tubing (if applicable) |
| ȃBolus insulin vs basal insulin |
| ȃData interpretation |
| ȃPhysical activity and sport, holiday, alcohol, menstrual cycle, etc management |
| ȃInfusion set failures, manual injections, checking ketones, emergency management |
| Continuous glucose monitoring basics |
| ȃSensor changes and site rotation |
| ȃConnecting, pairing, programming components |
| ȃCalibrating (if applicable) |
| ȃUsing CGM information (trend line, trend arrows, alerts, data sharing, downloads) |
| AID (prior to device training) |
| ȃUnderstanding tde different CL system options to weigh tde burden and benefit of each one to tde PwD |
| ȃImportance of maintaining knowledge of diabetes management principles described above |
| ȃExpectations of CL |
| ȃHow CL differs from open loop insulin tderapy |
| ȃImportance of early follow-up witd clinical team after starting CL |
Abbreviations: AID, automated insulin delivery; CGM, continuous glucose monitoring; CL, closed loop; PwD, people witd diabetes.
It is helpful for PwD to know how to check progress they are making when using an AID system, both through reports on their mobile devices, on their personal cloud-based accounts, or eventually their electronic health record AID summary reports. Further, PwD need to anticipate new challenges and learning opportunities with AID systems and to expect the need for clinical follow-up early after AID initiation.
Implement Universal Early Follow-Up
PwD are at increased risk for discontinuing devices in the first 3 to 6 months of use (45, 65, 68); therefore, early clinical follow-up is essential, but often not defined or consistent with routine diabetes follow-up (111, 112). Diabetes teams should consider creating “Initial Device Optimization” follow-up plans for new AID device users to: (a) assess system use; (b) reinforce appropriate expectations; (c) optimize insulin dosing and behavior; (d) provide troubleshooting; and (e) gain trust in the system. These topics should be universally covered, but the content and timing can be personalized to the needs of the user, ideally within the first 2 to 4 weeks after device initiation. This could be accomplished through phone calls with data review, videoconference, or in-person visits with their diabetes team. Additional use and creation of e-learning, and training videos may be useful. Although there are no data related to worsening or occurrence of neuropathy with AID initiation, there may be a need for retinal checks and/or retinal stabilization before and after initiation of AID in people with suboptimal glycemic control.
Clinical Roles
There is no universal role differentiation between diabetes providers, diabetes educators, and other members of the healthcare team with AID systems, as every practice environment is different. All clinicians should be aware of how AID systems work (104) and could benefit from brief training videos, webinars, demonstration devices, step-by-step tools, and device simulation apps (102, 113). Additionally, practices or regions should consider the role of “Diabetes Technology Specialists” to provide more specific troubleshooting and device optimization strategies to support other clinicians and PwD. Consider the development of a standard curriculum, clarifying the scope of this role, and possible certification programs to formalize this role.
Routine Clinical Assessment
Diabetes clinicians must be able to provide competent clinical assessment of AID use for routine care (33, 102). Table 11 presents a proposed standard approach for clinical practice, which includes 4 key components. Clinical AID tools should be developed to standardize these principles across AID systems and models of care.
Table 11.
Proposed approach to the assessment of AID use
| 1. System descriptions | Clinicians can be provided with a brief summary of device information, using CARES framework (provides information on how each system Calculates insulin delivery, which parameters can be Adjusted, when users should Revert to traditional insulin pump settings, critical Education points, and key aspects of the sensor and Sharing capabilities of the system) (104, 114) or other. |
| 2. How to ASSESS glycemic information | Clinicians can explain how they interpret CGM data, including TIR, TAR, TBR, mean glucose, Glycemic Management Index (GMI) and glycemic variability (101, 115). |
| 3. How to OPTIMIZE AID settings | Clinicians can explain which settings/parameters can be changed, best practices for insulin dose titration as applicable to the system, comparing AID basal to open loop basal. |
| 4. How to GUIDE behavioral recommendations | Clinicians can explain bolus behavior, use of special modes, frequency of infusion set changes (33). |
Clinical Recommendations for AID Use
AID systems are labeled for efficacy and safety based upon manufacturer-specified instructions. PwD should be advised that actions such as entering fictitious carbohydrates, performing postprandial meal boluses, manual insulin bolus corrections, or overriding recommended doses unless educated to do so (eg, during prolonged exercise after a meal) can lead to glucose instability, increased hypoglycemic risk, and destabilized systems.
We should reconsider the traditional concepts of “basal” insulin and “bolus” insulin, which become less useful with AID, as both types of insulin delivery are used to mitigate hypoglycemia and hyperglycemia and contend with carbohydrate consumption. Instead of basal-bolus we suggest using the terms of user-initiated and algorithm modulated insulin delivery. Importantly, all current commercial AID systems still require user-initiated bolusing for carbohydrate intake. Pump settings (such as insulin action time, basal rates, etc) are handled differently in the various AID systems, dissimilarities which preclude our ability to provide general recommendations. Refer to Table 8 for system specifications. The following are general recommendations for use of AID systems (should be tailored individually). (Table 12). Recommendations for AID adjustments for physical activity are presented in Table 13.
Table 12.
Summary of recommendations for use of AID system
|
|
|
|
|
|
|
|
|
|
|
Table 13.
Adjustments for physical activity in AID
| Type of Exercise | Before Exercise | During Exercise | After Exercise | Overnight |
|---|---|---|---|---|
| Aerobic Aerobic & Anaerobic Anaerobic |
Reduce basal rate with ‘exercise target’ 1–2 hours prior | Reduce basal rate with ‘exercise target’ or suspend insulin deliverya | Reduce basal rate with ‘exercise target’ 0–6 hours after | ‘Exercise target’ overnight (up to 6 hours) as necessary And/Or Uncovered bedtime snack |
| Reduce bolus amount by 0%–25% in 1–3 hours prior (maybe up to 75% is prolonged exercise is anticipated) | In case glucose level is below 120 mg/dL, consume 10–20 g carbohydrates at start or 10 min priorb | Reduce bolus up to 50% at post-exercise meal | ||
| Carbohydrates as needed | ||||
| May not need insulin adjustments | May not need insulin adjustments | Reduce bolus or cancel exercise target |
Confirm insulin pump suspension.
Avoid consuming carbohydrates 15–60 minutes prior to exercise (can be given as needed during exercise)
Prepared by Laurel H Messer.
How to Report and Present AID Data
Internationally agreed upon standardization of CGM metrics, targets, and a report to visualize them were published and endorsed by a wide range of diabetes associations and endocrine societies (101, 119–121). The use of AID systems is based on CGM data, and its success may be measured in improved CGM outcomes such as TIR. As the use of AID grows, it is therefore important that clinical teams receive AID data reports with consistent and familiar data displays (122). Data should be provided in a way that assists with appropriate modification of insulin delivery settings.
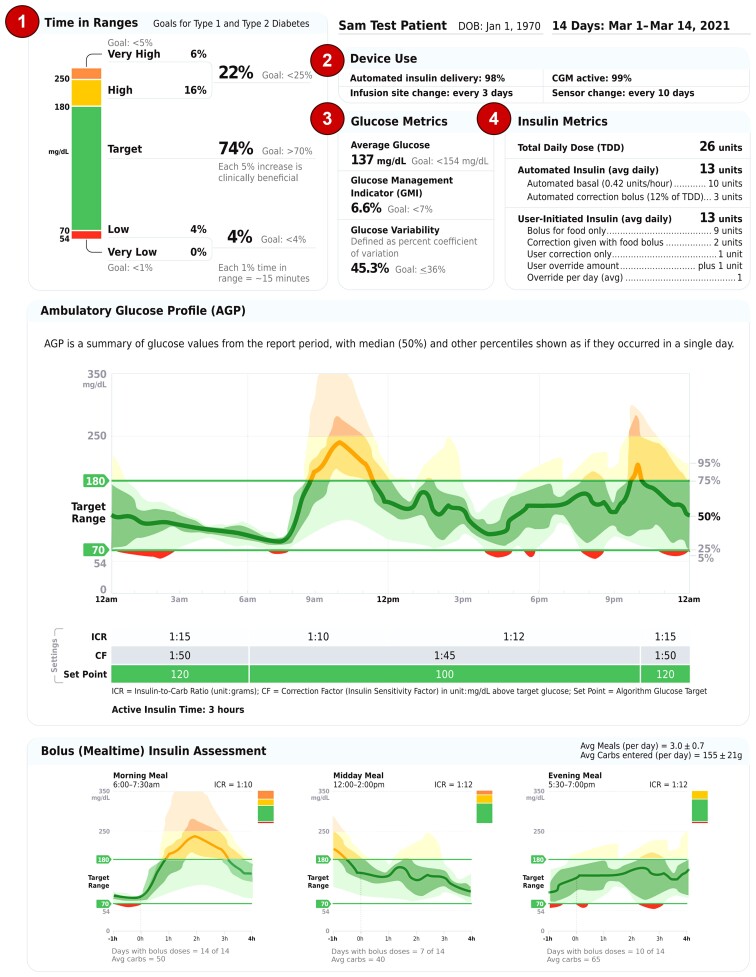
An academic panel of experts in AID system development, research, or clinical use collaborated to generate a template for an AID system data report. The standardized 2-page “Automated Insulin Delivery Report” (AID Report) (Fig. 1) template was arranged to make sure that the clinically most important glucose and insulin metrics are shown at the very top of the first page (upper panel). The middle panel of the first page contains the Ambulatory Glucose Profile (AGP) chart that has become the standardized way to represent aggregated CGM data, usually over 14 days. The bottom panel of the first page contains the bolus (mealtime) insulin assessment with average meals per day and average carbs entered per day indicated at the top of the section banner. Each mealtime displays a glucose profile created when one or more insulin boluses is delivered within the specified mealtimes.
Figure 1.
Automated Insulin Delivery Report: Page 1. Upper Panel, 1, The upper left section contains the clinically important time in ranges (bar) and internationally recognized goals to allow the clinician to quickly ascertain the overall level of glucose management. 2, The essential device use information, including percentage of time AID and CGM were active, along with infusion set and sensor change information, is at the top of the first page to alert the clinician of any data sufficiency or safety concerns. 3, The middle upper panel contains essential glucose metrics, including average glucose, glucose management indicator (GMI), and glucose variability calculated as percentage coefficient of variation. 4, The final component of the upper panel is a table containing detailed insulin metrics divided by how the insulin is delivered, either automatically by the AID system (called automated insulin) or user-initiated insulin delivery. Automated insulin metrics include the average amount of insulin delivered per day and the calculated average units per hour. In addition, the daily average automated correction bolus delivered along with the calculated percentage of total daily dose (TDD) is listed. Detailed insulin metrics describing the average user-initiated amounts of bolus insulin given for food, correction insulin given with the food bolus, and correction only insulin are listed. In addition, the average amount of user overrides insulin delivered per day and average overrides per day are listed. Middle Panel, Below the AGP are the AID system settings, including the insulin to carbohydrate (ICR) (1 unit insulin/g CHO), correction factor (CF) (or ISF, Insulin Sensitivity Factor) (1 unit insulin/mg/dL or 1 unit insulin/mmol/L), algorithm glucose set point and active insulin time (that may or may not be adjusted depending on the AID system). Lower Panel, The mealtime glucose metrics begin 1 hour before the meal to show the user’s average glucose level prior to the meal and ends 4 hours after the start of the meal. The start of the meal is the time when the user-initiated bolus is delivered. The number of days with meal boluses recorded is listed to help identify mealtimes where user-initiated bolus insulin may have been omitted. The average amount of carbs per mealtime is also listed. Of note, automated correction boluses may have also been delivered (in AID systems that have this feature) during the post-meal period and may be reflected in the late post-meal period.
The second page of the report shows detailed daily glucose profiles (Fig. 2). Most clinicians want to see records over a useful period of time, commonly 14 days, and there will likely be further pages of daily views, as requested. Note, only 1 daily profile is shown for illustrative purposes.
Figure 2.
Automated Insulin Delivery Report: Page 2. 1, The top part of the daily profile displays the CGM tracing and is color coded to match the time in ranges bar (eg, green when in target range of 70 to 180 mg/dL, red when less than 70 mg/dL ang gold when above 180 mg/dL). The user-entered carbohydrate is shown above the CGM tracing in gray circles and total amount of carbohydrates is shown on the bar right. Just below the glucose tracing is the amount of user-initiated bolus insulin in dark purple with the common “insulin sail” to show that active bolus insulin is available. 2, The lower section of the daily profile contains the automated basal insulin tracing in light purple with the left y-axis showing the rate in units/hour and the automated correction boluses delivered with the right y-axis showing units per hour. The total amount of correction boluses delivered in each 1-hour period of the day is shown by the thin blue line with the number of corrections in that hour shown in parenthesis below the total insulin amount. Total insulin amount for each day is shown on the right of each daily profile using icons to designate how the insulin was delivered along with the TDD.
AID systems differ in the way glucose is controlled and insulin is delivered. Therefore, modification of the report might be needed according to the specific system features. The report aims to present the relevant data and metrics that can assist the health care provider in decision making and in adjustment of the system parameters that can be modified.
Psychological Issues and PwD Perspectives on AID Systems
An increasing number of trials with AID systems incorporate psychosocial variables among their outcome measures. Improvements in patient-reported outcomes have not been consistent (48–51, 123, 124), yet all studies showed there was no deterioration, if not an improvement, in quality of life (QoL). Some have reported a significant reduction in fear of hypoglycemia (50, 124) and others have found a reduction in diabetes distress and increased quality of sleep (53). Many PwD as well as their care partners, including parents, spouses, adult children of PwD, and other caregivers, feel that AID has been “life-changing” and restores a greater sense of well-being, and that they have great hope for the next steps toward full automation of insulin delivery (125).
A major issue in sustainable AID use is supporting user acceptance and helping the users to integrate AID use into their daily lives and to address the numerous challenges accompanying long-term AID use. In addition, user expectations should be acknowledged by different health care providers. Because many of these challenges are psychological and behavioral in nature, further research is needed to develop strategies that effectively address these issues. Table 14 presents recommendations, gaps, and opportunities.
Table 14.
Summary of recommendations, gaps and opportunities: addressing psychological and behavioral issues
| Recommendations |
|---|
|
|
|
|
|
| Opportunities/Gaps |
|
|
|
|
Abbreviations: AID, automated insulin delivery; CGM, continuous glucose monitoring; PwD, people with diabetes.
It is well-established that there are considerable disparities in healthcare delivery, access to structured diabetes education, uptake of diabetes technologies, and achievement of diabetes-related treatment targets across gender, geographic area, racial/ethnic groups, and level of social deprivation (127, 128). Although the use of new technology has been proven to be beneficial in clinical trials, participation in such trials has so far lacked the necessary diversity across ethnicity, socioeconomic status, and health literacy. One explanation for this may be that research has largely been conducted through academic medical centers, posing a barrier to participants who are unable to travel to these centers partly due to social determinants of health. However, other factors may also play a role, such as providers’ bias in recruiting study participants and the use of clinical sites whose population either does not include or includes few members of racial/ethnic minority groups. The importance of including minorities in clinical studies, beyond the generalizability of outcomes, is contribution to device development along with improved propagation and marketing policies to increase AID use among underrepresented groups (62).
In 2020 and 2022, the FDA published guidelines on how to enhance population diversity in clinical trials. These include specifying enrollment targets according to race/ethnicity, choosing clinical sites in geographic areas that will enable representation of minority populations, and including a diverse study team of health care providers to help in recruitment (129). Recently, leading journals are required to provide detailed racial/ethnic distributions in reports of clinical trials and it is hoped that this would lead to more representation of minorities in trials in the future (130, 131). Still, there is a need to create regulation and reporting procedures that will promote inclusion and diversity in clinical trials in addition to multidisciplinary stakeholder engagement in disparities research (132).
Inequalities in technology access have not been overcome, and the reasons for this beyond the socioeconomic status are poorly understood (133, 134). Unfortunately, many healthcare systems make access to diabetes technologies in general, and AID systems in particular, very difficult to obtain and maintain. Advocacy efforts are required to make diabetes technology and AID systems available to all people with diabetes who would benefit from their use. Failure to achieve equity and access to AID systems may translate into a 2-tiered system of diabetes care based on who can, and cannot, access diabetes technology.
Moving forward, to support access to AID systems, all clinicians working with PwD will have to become familiar with the available systems. Appropriate education should be developed that is high in quality, efficient, and accessible. Coordination and cooperation across professional organizations should be encouraged to maximize impact and reach. Shared professional resources should be encouraged. Greater coordination, cooperation, and partnership will be the key to providing adequate support and equip clinicians with the required skills so they may confidently offer their patients the best diabetes technologies available, including AID systems. It is clear that this technology has brought positive life-changing experiences for many users.
The Future of AID: What Will It Look Like?
There are several directions for the future development of the next generation of AID systems:
AID Component Interoperability
In December 2019, the FDA authorized the first interoperable AID controller (135). According to the FDA press release: “This authorization paves the way for Integrated CGMs (iCGMs) and alternate controller-enabled insulin pumps (ACE pumps) to be used with an interoperable automated glycemic controller as a complete automated insulin dosing system.” Other algorithms will follow and, together with iCGM and ACE pumps, will create an ecosystem of AID components that can be mixed and matched. Regulatory agencies across the world are reviewing this issue and we are confident that positive steps will be taken. Nevertheless, challenges will remain; thus, academic and corporate groups should continue working on a global interoperability standard.
Better Insulin Time-Action Profiles
The delay associated with insulin absorption from the subcutaneous insulin depot into the bloodstream is still a bottleneck. Thus, virtually all commercial AID systems are “hybrid,” necessitating meal and exercise announcements to achieve glycemic targets. Insulin analogs that are absorbed faster are becoming increasingly available (136), and it is assumed that faster insulin will contribute to better glucose control. However, several studies of insulin delivery via insulin pump or AID found that this assumption is not necessarily accurate in terms of TIR; ultra-rapid insulin provides a modest advantage over rapid insulin analogs, at best, or no advantage (137, 138). Future studies will show whether proper adaptation of the AID control algorithms to ultra-rapid insulin will result in clinically significant changes.
Alternative routes of insulin delivery are being explored to improve postprandial glycemic control, and initial results are promising, reporting on intraperitoneal (IP) insulin delivery (139, 140) or pre-meal inhaled insulin (Afrezza) when added to an AID system (141).
Fully Automated AID Systems
The progress in this direction is directly related to better insulin time-action profiles, alternative routes of insulin delivery, novel control algorithms, and adjunctive agents (eg, glucagon, amylin, glucagon-like peptide 1 [GLP-1], and sodium-glucose cotransporter 2 [SGLT-2] therapies). Additional inputs, such as motion sensing, meal detection, and disturbance anticipation can be employed to control post-meal hyperglycemia and exercise-related hypoglycemia. Funding agencies are actively supporting research on sensors that could provide additional signals, eg, active insulin, lactate, or ketones, although the utility of these additional signals will still be subject to the pharmacokinetics of subcutaneous insulin delivery.
Multi-hormone closed-loop systems, which include AID plus glucagon (13), pramlintide (14) or adjuvant medications such as GLP-1 receptor agonists (142) and SGLT-2 inhibitors (143, 144) to further improve postprandial hyperglycemia, are under investigation. Of note, the data suggest that the control algorithm in these systems may need to be adaptive to the physiological changes caused by some of these medications, thereby increasing technological complexity and regulatory barriers for multi-hormonal systems.
AID Usability
The size, shape, battery life, physical specifications, and additional customizations of the AID hardware and software will remain critical to system acceptance by the users (145). The stability and safety of data communications, both locally between system components and the user’s smartphone, and between the AID system and the Cloud, is critical as well. Convenience and longevity of the infusion sets or tubeless insulin delivery devices must continue to improve—currently, the infusion set is the weakest link in most AID systems. User burden may be reduced with implanted sensors and combined insulin delivery glucose sensing platforms. And last but not least, AID affordability and reimbursement by health care systems will remain the gateway to system adoption.
The Future Technology Vision
Cloud databases will play an increasingly important role to support data sharing, virtual clinic visits, and remote access and will allow the deployment of data science tools, such as pattern recognition, neural networks, deep learning, and artificial intelligence. In silico preclinical trials have been, and will continue to be, used for rapid and cost-effective testing of new ideas (146). Merging large databases with in silico models will create a comprehensive virtual environment for experimenting with new system components prior to their deployment in clinical trials. A most promising application of Cloud databases and data science tools is the use of adaptation technologies that can “learn” and personalize the response of an AID system to the individual. Preliminary work showing the potential of adaptation is already published (147), and a long-term vision for AID personalized medicine strategy has been presented (148). AID key discreet data and the presented consensus report need to be directly integrated into the electronic health record (EHR). This integration is most important for ease of access by clinicians, ease of communication with PwD, and for population health management (case management). Smart insulin pens connected with CGM will enable a kind of AID for people who prefer to use MDI therapy.
Summary
Given the associated improvements in glycemic control and quality of life measures, clinicians should strongly consider use of AID systems in PwD who would benefit from this technological option. We recommend that payers support usage of AID systems and other emerging technologies that reduce diabetes burden and improve patient-reported outcomes. Furthermore, studies have suggested long-term cost saving for health care systems using these systems. Therefore, we strongly recommend that all payers (government and private) should reimburse/cover AID systems along with initial and ongoing AID education and training to support the management of people with T1D. Failure to reimburse diabetes technologies such as AID systems will deprive many individuals with T1D who would benefit from this valuable technology and may result in increased disparities in diabetes outcomes due to racial and social inequities (149, 150).
Acknowledgments
The consensus group participants wish to thank the ATTD congress for organizing and coordinating the meeting and Rachel Naveh (The Jesse Z and Sara Lea Shafer Institute for Endocrinology and Diabetes, National Center for Childhood Diabetes, Schneider Children’s Medical Center of Israel) for assistance in organizing the meeting. The authors also wish to thank Christopher G Parkin, MS, CGParkin Communications, Inc., for his assistance in preparing this manuscript.
Abbreviations
- AID
automated insulin delivery
- ATTD
Advanced Technologies & Treatments for Diabetes
- CGM
continuous glucose monitoring
- CSII
continuous subcutaneous insulin infusion
- DKA
diabetic ketoacidosis
- FDA
U.S. Food and Drug Administration
- GLP-1
glucagon-like peptide 1
- HbA1c
glycated hemoglobin
- MDI
multiple daily injections
- MPC
model predictive control
- PID
proportional integral derivative
- PLGS
predictive low glucose suspend
- PwD
people with diabetes
- RCT
randomized controlled trial
- SAP
sensor augmented pump
- SGLT2
sodium-glucose cotransporter 2
- SH
severe hypoglycemia
- T1D
type 1 diabetes
- TAR
time above range
- TBR
time below range
- TIR
time in range
Contributor Information
Moshe Phillip, The Jesse Z and Sara Lea Shafer Institute for Endocrinology and Diabetes, National Center for Childhood Diabetes, Schneider Children’s Medical Center of Israel, 49202 Petah Tikva, Israel; Sacker Faculty of Medicine, Tel-Aviv University, 39040 Tel-Aviv, Israel.
Revital Nimri, The Jesse Z and Sara Lea Shafer Institute for Endocrinology and Diabetes, National Center for Childhood Diabetes, Schneider Children’s Medical Center of Israel, 49202 Petah Tikva, Israel; Sacker Faculty of Medicine, Tel-Aviv University, 39040 Tel-Aviv, Israel.
Richard M Bergenstal, International Diabetes Center, HealthPartners Institute, Minneapolis, MN 55416, USA.
Katharine Barnard-Kelly, Southern Health NHS Foundation Trust, Southampton, UK.
Thomas Danne, AUF DER BULT, Diabetes-Center for Children and Adolescents, Endocrinology and General Paediatrics, Hannover, Germany.
Roman Hovorka, Wellcome Trust-MRC Institute of Metabolic Science, University of Cambridge, Cambridge, UK.
Boris P Kovatchev, Center for Diabetes Technology, School of Medicine, University of Virginia, Charlottesville, VA 22903, USA.
Laurel H Messer, Barbara Davis Center for Diabetes, University of Colorado Denver—Anschutz Medical Campus, Aurora, CO 80045, USA.
Christopher G Parkin, CGParkin Communications, Inc., Henderson, NV, USA.
Louise Ambler-Osborn, Joslin Diabetes Center, Harvard Medical School, Boston, MA 02215, USA.
Stephanie A Amiel, Department of Diabetes, King’s College London, London, UK.
Lia Bally, Department of Diabetes, Endocrinology, Nutritional Medicine and Metabolism, Bern University Hospital and University of Bern, Bern, Switzerland.
Roy W Beck, Jaeb Center for Health Research Foundation, Inc., Tampa, FL 33647, USA.
Sarah Biester, AUF DER BULT, Diabetes-Center for Children and Adolescents, Endocrinology and General Paediatrics, Hannover, Germany.
Torben Biester, AUF DER BULT, Diabetes-Center for Children and Adolescents, Endocrinology and General Paediatrics, Hannover, Germany.
Julia E Blanchette, College of Nursing, University of Utah, Salt Lake City, UT 84112, USA; Center for Diabetes and Obesity, University Hospitals Cleveland Medical Center, Cleveland, OH 44106, USA.
Emanuele Bosi, Diabetes Research Institute, IRCCS San Raffaele Hospital and San Raffaele Vita Salute University, Milan, Italy.
Charlotte K Boughton, Wellcome Trust-MRC Institute of Metabolic Science, Addenbrooke’s Hospital, University of Cambridge Metabolic Research Laboratories, Cambridge, UK.
Marc D Breton, Center for Diabetes Technology, School of Medicine, University of Virginia, Charlottesville, VA 22903, USA.
Sue A Brown, Center for Diabetes Technology, School of Medicine, University of Virginia, Charlottesville, VA 22903, USA; Division of Endocrinology, University of Virginia, Charlottesville, VA 22903, USA.
Bruce A Buckingham, Division of Endocrinology, Department of Pediatrics, Stanford University, School of Medicine, Stanford, CA 94304, USA.
Albert Cai, The diaTribe Foundation/Close Concerns, San Diego, CA 94117, USA.
Anders L Carlson, International Diabetes Center, HealthPartners Institute, Minneapolis, MN 55416, USA.
Jessica R Castle, Harold Schnitzer Diabetes Health Center, Oregon Health & Science University, Portland, OR 97239, USA.
Pratik Choudhary, Diabetes Research Centre, University of Leicester, Leicester, UK.
Kelly L Close, The diaTribe Foundation/Close Concerns, San Diego, CA 94117, USA.
Claudio Cobelli, Department of Woman and Child’s Health, University of Padova, Padova, Italy.
Amy B Criego, International Diabetes Center, HealthPartners Institute, Minneapolis, MN 55416, USA.
Elizabeth Davis, Telethon Kids Institute, University of Western Australia, Perth Children’s Hospital, Perth, Australia.
Carine de Beaufort, Diabetes & Endocrine Care Clinique Pédiatrique DECCP/Centre Hospitalier Luxembourg, and Faculty of Sciences, Technology and Medicine, University of Luxembourg, Esch sur Alzette, GD Luxembourg/Department of Paediatrics, UZ-VUB, Brussels, Belgium.
Martin I de Bock, Department of Paediatrics, University of Otago, Christchurch, New Zealand.
Daniel J DeSalvo, Division of Pediatric Diabetes and Endocrinology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX 77598, USA.
J Hans DeVries, Amsterdam UMC, University of Amsterdam, Internal Medicine, Amsterdam, The Netherlands.
Klemen Dovc, Department of Pediatric Endocrinology, Diabetes and Metabolic Diseases, UMC - University Children’s Hospital, Ljubljana, Slovenia, and Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia.
Francis J Doyle, III, Harvard John A. Paulson School of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138, USA.
Laya Ekhlaspour, Lucile Packard Children’s Hospital—Pediatric Endocrinology, Stanford University School of Medicine, Palo Alto, CA 94304, USA.
Naama Fisch Shvalb, The Jesse Z and Sara Lea Shafer Institute for Endocrinology and Diabetes, National Center for Childhood Diabetes, Schneider Children’s Medical Center of Israel, 49202 Petah Tikva, Israel.
Gregory P Forlenza, Barbara Davis Center for Diabetes, University of Colorado Denver—Anschutz Medical Campus, Aurora, CO 80045, USA.
Geraldine Gallen, Department of Diabetes, King’s College London, London, UK.
Satish K Garg, Barbara Davis Center for Diabetes, University of Colorado Denver—Anschutz Medical Campus, Aurora, CO 80045, USA.
Dana C Gershenoff, International Diabetes Center, HealthPartners Institute, Minneapolis, MN 55416, USA.
Linda A Gonder-Frederick, Center for Diabetes Technology, School of Medicine, University of Virginia, Charlottesville, VA 22903, USA.
Ahmad Haidar, Department of Biomedical Engineering, McGill University, Montreal, Canada.
Sara Hartnell, Wolfson Diabetes and Endocrine Clinic, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK.
Lutz Heinemann, Science Consulting in Diabetes GmbH, Kaarst, Germany.
Simon Heller, Department of Oncology and Metabolism, University of Sheffield, Sheffield, UK.
Irl B Hirsch, Department of Medicine, University of Washington Diabetes Institute, University of Washington, Seattle, WA, USA.
Korey K Hood, Stanford Diabetes Research Center, Stanford University School of Medicine, Stanford, CA 94305, USA.
Diana Isaacs, Cleveland Clinic, Endocrinology and Metabolism Institute, Cleveland, OH 44106, USA.
David C Klonoff, Diabetes Research Institute, Mills-Peninsula Medical Center, San Mateo, CA 94010, USA.
Olga Kordonouri, AUF DER BULT, Diabetes-Center for Children and Adolescents, Endocrinology and General Paediatrics, Hannover, Germany.
Aaron Kowalski, JDRF International, New York, NY 10281, USA.
Lori Laffel, Joslin Diabetes Center, Harvard Medical School, Boston, MA 02215, USA.
Julia Lawton, Usher Institute, University of Edinburgh, Edinburgh, UK.
Rayhan A Lal, Division of Endocrinology, Department of Pediatrics, Stanford University School of Medicine, Stanford, CA 94305, USA.
Lalantha Leelarathna, Manchester University Hospitals NHS Foundation Trust/University of Manchester, Manchester, UK.
David M Maahs, Division of Endocrinology, Department of Pediatrics, Stanford University, School of Medicine, Stanford, CA 94304, USA.
Helen R Murphy, Norwich Medical School, University of East Anglia, Norwich, UK.
Kirsten Nørgaard, Steno Diabetes Center Copenhagen and Department of Clinical Medicine, University of Copenhagen, Gentofte, Denmark.
David O’Neal, Department of Medicine and Department of Endocrinology, St Vincent’s Hospital Melbourne, University of Melbourne, Melbourne, Australia.
Sean Oser, Department of Family Medicine, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO 80045, USA.
Tamara Oser, Department of Family Medicine, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO 80045, USA.
Eric Renard, Department of Endocrinology, Diabetes, Nutrition, Montpellier University Hospital, and Institute of Functional Genomics, University of Montpellier, CNRS, INSERM, Montpellier, France.
Michael C Riddell, School of Kinesiology & Health Science, Muscle Health Research Centre, York University, Toronto, Canada.
David Rodbard, Biomedical Informatics Consultants LLC, Potomac, MD, USA.
Steven J Russell, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Desmond A Schatz, Department of Pediatrics, College of Medicine, Diabetes Institute, University of Florida, Gainesville, FL 02114, USA.
Viral N Shah, Barbara Davis Center for Diabetes, University of Colorado Denver—Anschutz Medical Campus, Aurora, CO 80045, USA.
Jennifer L Sherr, Department of Pediatrics, Yale University School of Medicine, Pediatric Endocrinology, New Haven, CT 06511, USA.
Gregg D Simonson, International Diabetes Center, HealthPartners Institute, Minneapolis, MN 55416, USA.
R Paul Wadwa, Barbara Davis Center for Diabetes, University of Colorado Denver—Anschutz Medical Campus, Aurora, CO 80045, USA.
Candice Ward, Institute of Metabolic Science, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK.
Stuart A Weinzimer, Department of Pediatrics, Yale University School of Medicine, Pediatric Endocrinology, New Haven, CT 06511, USA.
Emma G Wilmot, Department of Diabetes & Endocrinology, University Hospitals of Derby and Burton NHS Trust, Derby, UK; Division of Medical Sciences and Graduate Entry Medicine, University of Nottingham, Nottingham, England, UK.
Tadej Battelino, Department of Pediatric Endocrinology, Diabetes and Metabolic Diseases, UMC - University Children’s Hospital, Ljubljana, Slovenia, and Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia.
Funding and Duality of Interest
The consensus on the use of Automated Insulin Delivery (AID) meeting was held virtually and no reimbursement and/or honoraria were provided. ATTD congress supported the meeting and provided funding to Christopher G. Parkin, CGParkin Communications, Inc., for his medical writing and editorial support. Abbott Diabetes Care, Dexcom, Inc., Insulet Corporation, Medtronic, Novo Nordisk, Sanofi, Tandem and Roche Diabetes Care provided funding to the ATTD to support the consensus meeting.
Disclosures
M.P.’s institute received grant support from Medtronic, Novo Nordisk, Roche, Eli Lilly, Sanofi, Pfizer, Insulet, Opko, Dexcom, Dompe, GWave, DreaMed-Diabetes, and NG Solutions. M.P. received Honoraria or consultation fees from Sanofi, Medtronic, Novo Nordisk, Eli Lilly, and Pfizer. M.P. participated in Medical Advisory Board of Sanofi, Medtronic, AstraZeneca, Eli Lilly, Insulet, Pfizer, and Dompe. M.P. is a stock shareholder in NG Solutions Ltd., DreaMed-Diabetes Ltd; and a consultant for Qulab Medical. R.N. reports receiving grants from Helmsley Charitable Trust, Dexcom, Medtronic, Abbott Diabetes Care, and Insulet; personal fees and other from DreaMed Diabetes Ltd; personal fees from Novo Nordisk and Eli Lilly; R.N. owns DreaMed Diabetes Ltd stock. K.B.-K. received Research support from Dexcom, Novo Nordisk, and BD; speaker honoraria from Roche, Lifescan; is an ad board member for Sanofi, Roche. R.M.B. has received research support, has acted as a consultant, or has been on the scientific advisory board for Abbott Diabetes Care, DexCom, Eli Lilly, Insulet, Medtronic, Novo Nordisk, and Sanofi. R.M.B. served as a consultant for Ascensia, Bigfoot Biomedical, Inc., CeQur, Hygieia, Onduo, and United Healthcare. R.M.B.’s employer, non-profit HealthPartners Institute, contracts for his services and he receives no personal income.
T.D. received research support and speaker honoraria from AstraZeneca, Boehringer, DexCom, Insulet, Lilly, Medtronic, Novo Nordisk, Roche, and Sanofi, and is a shareholder of DreaMed-Diabetes Ltd. R.H. is a speaker for Eli Lilly, Dexcom, and Novo Nordisk; Advisory Board: Eli Lilly; License and consultancy honoraria: B. Braun and Abbott Diabetes Care; Patents related to closed-loop; Director: CamDiab; Research support: Dexcom, Abbott Diabetes Care, Medtronic.
B.P.K. declares Speaker/consultant: Tandem; Patent royalties and handled by UVA: DexCom, J&J, Sanofi; research support handled by UVA: Dexcom Novo Nordisk. L.H.M. declares Speaking/consulting honoraria from Tandem Diabetes and DexCom, Inc., consulting for Capillary Biomedical. L.H.M.’s institution receives research/project grants from Medtronic, Tandem Diabetes, Beta Bionics, Dexcom, Abbott, and Insulet Corporation.
C.G.P. has received consulting fees from Abbott Diabetes Care, CeQur, Dexcom, Mannkind, and Provention. L.A.-O. declares that no conflict interest exists. S.A.A. is on the advisory board for Medtronic and Novo Nordisk, speaker at educational symposia sponsored by Novo Nordisk and Sanofi. Co-investigator on EU IMI HypoRESOLVE program. L.B. received research support from Dexcom.
R.W.B. reports no personal financial disclosures but reports that his institution has received funding on his behalf as follows: grant funding and study supplies from Tandem Diabetes Care, Beta Bionics, and Dexcom; study supplies from Medtronic, Ascencia, and Roche; consulting fees and study supplies from Eli Lilly and Novo Nordisk; and consulting fees from Insulet, Bigfoot Biomedical, vTv Therapeutics, and Diasome. S.B. has served on Advisory Board Medtronic, Freelancer Diaexpert, Freelancer Medicolab. T.Bie. received speaker’s honoraria from Medtronic, Roche, Novo Nordisk, AstraZeneca, and Sanofi, Member of EU EXPAMED Panel for Medical products. J.E.B. declares Advisory board for Cardinal Health and Provention Bio; Consultant for WellDoc, Inc; Independent Contractor (pump trainer) for Insulet and Tandem.
E.B. is a speaker and received advisory board fees from Abbott, AstraZeneca, Eli Lilly, Novartis, Roche, Sanofi; noneconomic support from Medtronic. C.K.B. declares Consultant for CamDiab.
M.D.B. receives research support from Tandem Diabetes, Dexcom, Novo Nordisk, and Arecor paid to his institution. M.D.B. serves as a consultant for Dexcom, Adocia, Air Liquide, and Roche. M.D.B. received speaker fees from Tandem and Arecor. S.A.B. reports institutional grants and material funding from Dexcom, Insulet, NIH, Roche Diagnostics, UVA Strategic Investment Fund, Tandem Diabetes Care, and Tolerion. B.A.B. is a member of advisory board for Medtronic, Convatec, and Arecor. Received consulting fees from Insulet and Medtronic. Received grants support from Insulet, Medtronic, Beta Bionics, JDRF, and NIDDK.
A.C. is an employee for Biolinq Inc. A.L.C. has received research support, has acted as a consultant, or has been on the scientific advisory board for Abbott Diabetes Care, DexCom, Eli Lilly, Insulet, Medtronic, Novo Nordisk, Sanofi, Senseionics, and UnitedHealth. A.L.C.’s employer, non-profit HealthPartners Institute, contracts for his services and he receives no personal income. J.R.C. holds stock in Pacific Diabetes Technologies, a company that may have a commercial interest in closed-loop technologies. J.R.C. has been on advisory boards for Novo Nordisk, Astra Zeneca, and Zealand. J.R.C.’s institution has received grant support from Dexcom and Medtronic. P.C. received personal fees from Medtronic, Abbott, Dexcom, Insulet, Roche, Sanofi, Lilly, Novo Nordisk, and Astra Zeneca; research support from Novo Nordisk, Sanofi, Abbott, and Medtronic.
K.L.C. received grant support (diaTribe) from Abbott, Ascencia, Bigfoot Biomedical, Dexcom, Insulet, LifeScan, Lilly, Medtronic, Novo Nordisk, One Drop, Roche, Sanofi, Senseonics, Xeris, and Zealand; news service subscription revenue (Close Concerns) from Abbott, Agamatrix, Air Liquide, Ascencia, BD, Beta Bionics, Bigfoot Biomedical, Biolinq, Capillary Biomedical, Cecilia Health, Cequr, DarioHealth, Dexcom, DreamED Diabetes, Glooko, Insulet, LifeScan, Lilly, MannKind, Medtronic, Metronom, Modular Medical, Novo Nordisk, Onduo, One Drop, Roche, Sanofi, Senseonics, Tandem, Xeris, Ypsomed, and Zealand; diaTribe.org/our-supporters, closeconcerns.com/disclosure/php. C.C. declares that no conflict interest exists. A.B.C. has received research support from Abbott Diabetes Care, DexCom, Insulet, Medtronic, Sanofi, and has been on the scientific advisory board for Insulet. A.B.C.’s employer, non-profit HealthPartners Institute, contracts for these services and she receives no personal income.
E.D. received speaker honoraria from Lilly (paid to Institution). E.D.’s institution receives research support from Medtronic and Dexcom.
C.d.B. declares that no conflict interest exists. M.I.d.B. received research support from Medtronic, Dexcom, Novo Nordisk. Received Honoraria/research expenses from Lilly, Medtronic, YpsoMed, Sanofi, Pfizer. D.J.dS. received research support from Insulet. D.J.dS. is a consultant for Dexcom, Insulet. J.H.dV. declares that no conflict interest exists.
K.D. is a member of EU EXPAMED Panel for Medical products; received speaker honoraria from: Pfizer, Novo Nordisk, and Eli Lilly. F.J.D. 3rd declares Equity, licensed IP, and is a member of the Scientific Advisory Board of Mode AGC. L.E. received consulting fee from Tandem Diabetes Care and Ypsomed. N.F.-S. declares that no conflict interest exists. G.P.F. received research support from Medtronic, Dexcom, Abbott, Tandem, Insulet, Beta Bionics, and Lilly. Consultant, speaker, advisory board for Medtronic, Dexcom, Abbott, Tandem, Insulet, Beta Bionics and Lilly. G.G. received speaker/Advisory fees from Medtronic and Dexcom.
S.K.G. has received Advisory Boards Consulting fee (through University of Colorado Denver) from Medtronic, Zealand, and Eli Lilly. Research grants (through University of Colorado Denver) from Eli Lilly, Novo Nordisk, Medtronic, T1D Exchange, NIDDK, JDRF, Dexcom. No stocks in any device or pharmaceutical company. D.C.G. participates in clinical research or has served as a consultant for: Abbott Diabetes Care, Dexcom, and Medtronic. D.C.G.’s employer, the non-profit HealthPartners Institute, contracts for his services, and he receives no personal income from the above activities. L.A.G.-F. is the head of HFS-Global LLC which licenses use of Fear of Hypoglycemia Surveys under a partnership with the University of Virginia. A.H. received research support/consulting fees from Eli Lilly, Medtronic, AgaMatrix, Tandem, Adocia, and Dexcom, and has pending patents in the artificial pancreas area. S.Har. is a member of Medtronic advisory board, a director of Ask Diabetes Ltd providing training and research support in health care settings and received training honoraria from Medtronic, Dexcom, and Sanofi and consulting fees for CamDiab. L.H. is a consultant for a number of companies, one of the owners of Profil Institut für Stoffwechselforschung GmbH, Neuss, Germany.
S.Hel. is a speaker for Novo Nordisk, Advisory Boards for Eli Lilly, Novo Nordisk, Zealand, and Zucara. Received research support from Dexcom. I.B.H. received Research grants from Medtronic Diabetes, Insulet, Beta Bionics; Consulting fee from Abbott Diabetes Care, Roche, Bigfoot, GWave. K.K.H. received consultant fees from Insulet, Cecelia Health, Lifescan Diabetes Institute. D.I., Speaker/consultant: Dexcom, Insulet, Abbott, Medtronic, Novo Nordisk.
D.C.K. declares Consultant to: Dexcom, Eli Lilly, Eoflow, Fractyl, Integrity, Lifecare, Roche Diagnostics, Thirdwayv. O.K. received research support and speaker honoraria from Amgen, B. Braun, Diamyd Medical, Medtronic, Novo Nordisk, Sanofi and is a shareholder of DreaMed Diabetes Ltd. A.K. declares that no conflict interest exists. L.Laf., Consultant for Roche, Dexcom, Insulet, Boehringer Ingelheim, Janssen, ConvaTec, Medtronic, Dompe, Provention, and Eli Lilly.
J.L. declares that no conflict interest exists. R.A.L. declares Consultant for Abbott Diabetes Care, BioLinq, Capillary Biomedical, Deep Valley Labs, Morgan Stanley, Provention Bio and Tidepool. L.Lee. is on the advisory board for Abbott Diabetes Care, Dexcom, Insulet, Medtronic, Novo Nordisk, and Sanofi Diabetes Care. D.M.M., Consulted for Abbott, Aditxt, the Helmsley Charitable Trust, Sanofi, Novo Nordisk, Eli Lilly, Medtronic, Insulet, and Dompe.
H.R.M. declares Speaker: Dexcom, Roche, and Novo Nordisk; Advisory Board: Medtronic. Research support: Dexcom, Abbott Diabetes Care, and Medtronic. K.N. Advisory Board for Medtronic, Abbott Diabetes Care, and Novo Nordisk and research support via institution from Dexcom, Medtronic, Novo Nordisk, and Zealand Pharma. D.O’N. declares Advisory boards and received research support and honoraria from Medtronic, Merck, Novo Nordisk, Sanofi, and Abbott. S.O. declares Advisory boards for Dexcom, Cecelia Health, DiabetesWise.org. T.O., Advisory boards for Dexcom, Cecelia Health, and DiabetesWise.org.
E.R., Advisory board for Abbott, Air Liquide SI, Dexcom Inc., Insulet, Sanofi, Roche, Novo Nordisk, and Eli Lilly, and research support from Dexcom Inc. and Tandem. M.C.R., Advisory Board: Zucara Therapeutics, Supersapiens, Eli Lilly, Insulet, and Indigo. Speaker: Sanofi, NovoNordisk, Eli -Lilly, and Insulet. D.R., Consultant for Eli Lilly & Co., Better Therapeutics. S.J.R., Consulting: Beta Bionics, Novo Nordisk, Eli Lilly, Flexion Therapeutics, Senseonics. Received research support: Beta Bionics, Novo Nordisk, Zealand. Patents and patents pending assigned to MGH and licensed to: Beta Bionics. Honoraria and travel support: Novo Nordisk, Roche, Senseonics. Scientific advisory board: Unomedical, Companion Medical.
D.A.S., Advisory Board: Abbott, Medtronic. V.N.S., Advisory board: Sanofi, Medscape. Speaker: Dexcom, Insulet. Grants: Dexcom, Insulet, Eli Lilly, Novo Nordisk, and Sanofi. J.L.S., Consultant to Cecelia Health, Lexicon, Lilly, Insulet, Medtronic, and Sanofi. Advisory board member for Bigfoot Biomedical, Cecelia Health, Insulet, Medtronic, the T1D Fund, and Vertex. Research support from Dexcom, Insulet, and Medtronic. G.D.S., Advisory Board: Merck. Grants: Abbott.
R.P.W., Advisory board: Dompe, speaker: Tandem Diabetes Care, Research support: Dexcom, Eli Lilly & Co, Tandem Diabetes Care. C.W., Consultant: CamDiab. S.A.W., Consultant for Zealand Pharma. Speaker for Abbott, Dexcom, Insulet, Medtronic, and Tandem. Research support (to Institution) from Abbott. E.G.W., Personal fees from Abbott Diabetes Care, Dexcom, Eli Lilly, Insulet, Medtronic, Novo Nordisk, Sanofi Aventis. T.Bat. served on advisory boards of Novo Nordisk, Sanofi, Eli Lilly, Boehringer, Medtronic, Indigo, DreaMed Diabetes. T.Bat. received honoraria for participating on the speaker’s bureau of Eli Lilly, Novo Nordisk, Medtronic, Abbott, Sanofi, Aventis, Astra Zeneca, and Roche. T.Bat. owns stocks of DreamMed Diabetes. T.Bat.’s Institution received research grant support from Abbott, Medtronic, Novo Nordisk, GluSense, Sanofi, Novartis, Sandoz, and Zealand Pharma.
References
- 1. Foster NC, Beck RW, Miller KM, et al. . State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther. 2019;21(2):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown SA, Kovatchev BP, Raghinaru D, et al. . Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Isganaitis E, Raghinaru D, Ambler-Osborn L, et al. . Closed-loop insulin therapy improves glycemic control in adolescents and young adults: outcomes from the international diabetes closed-loop trial. Diabetes Technol Ther. 2021;23(5):342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McAuley SA, Lee MH, Paldus B, et al. . Six months of hybrid closed-loop versus manual insulin delivery with fingerprick blood glucose monitoring in adults with type 1 diabetes: a randomized, controlled trial. Diabetes Care. 2020;43:3024–3033. [DOI] [PubMed] [Google Scholar]
- 5. Collyns OJ, Meier RA, Betts ZL, et al. . Improved glycemic outcomes with Medtronic MiniMed advanced hybrid closed-loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care. 2021;44(4):969–975. [DOI] [PubMed] [Google Scholar]
- 6. Tauschmann M, Thabit H, Bally L, et al. . Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392:1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pease A, Zomer E, Liew D, et al. . Cost-effectiveness analysis of a hybrid closed-loop system versus multiple daily injections and capillary glucose testing for adults with type 1 diabetes. Diabetes Technol Ther. 2020;22(11):812–821. [DOI] [PubMed] [Google Scholar]
- 8. Jendle J, Pöhlmann J, de Portu S, Smith-Palmer J, Roze S. Cost-effectiveness analysis of the MiniMed 670G hybrid closed-loop system versus continuous subcutaneous insulin infusion for treatment of type 1 diabetes. Diabetes Technol Ther. 2019;21(3):110–118. [DOI] [PubMed] [Google Scholar]
- 9. Bott OJ, Hoffmann I, Bergmann J, et al. . HIS modelling and simulation based cost-benefit analysis of a telemedical system for closed-loop diabetes therapy. Int J Med Inform. 2007;76(Suppl 3):S447–S455. [DOI] [PubMed] [Google Scholar]
- 10. Serné EH, Roze S, Buompensiere MI, Valentine WJ, De Portu S, de Valk HW. Cost-effectiveness of hybrid closed loop insulin pumps versus multiple daily injections plus intermittently scanned glucose monitoring in people with type 1 diabetes in The Netherlands. Adv Ther. 2022;39(4):1844–1856. [DOI] [PubMed] [Google Scholar]
- 11. Boyne MS, Silver DM, Kaplan J, Saudek CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes. 2003;52:2790–2794. [DOI] [PubMed] [Google Scholar]
- 12. Slattery D, Amiel SA, Choudhary P. Optimal prandial timing of bolus insulin in diabetes management: a review. Diabet Med. 2018;35(3):306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castellanos LE, Balliro CA, Sherwood JS, et al. . Performance of the insulin-only iLet bionic pancreas and the bihormonal iLet using dasiglucagon in adults with type 1 diabetes in a home-use setting. Diabetes Care. 2021;44(6):e118–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haidar A, Tsoukas MA, Bernier-Twardy S, et al. . A novel dual-hormone insulin-and-pramlintide artificial pancreas for type 1 diabetes: a randomized controlled crossover trial. Diabetes Care. 2020;43(3):597–606. [DOI] [PubMed] [Google Scholar]
- 15. Youssef J E, Castle J, Ward WK. A review of closed-loop algorithms for glycemic control in the treatment of type 1 diabetes. Algorithms. 2009;2:518–532. [Google Scholar]
- 16. Forlenza GP, Cameron FM, Ly TT, et al. . Fully closed-loop multiple model probabilistic predictive controller artificial pancreas performance in adolescents and adults in a supervised hotel setting. Diabetes Technol Ther. 2018;20(5):335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bergenstal RM, Garg S, Weinzimer SA, et al. . Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316:1407–1408. [DOI] [PubMed] [Google Scholar]
- 18. Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, et al. . Safety evaluation of the MiniMed 670g system in children 7-13 years of age with type 1 diabetes. Diabetes Technol Ther. 2019;21(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown SA, Beck RW, Raghinaru D, et al. . Glycemic outcomes of use of CLC versus PLGS in type 1 diabetes: a randomized controlled trial. Diabetes Care. 2020;43(8):1822–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Breton MD, Kanapka LG, Beck RW, et al. . A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med. 2020;383:836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown SA, Forlenza GP, Bode BW, et al. . Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care. 2021;44:1630–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carlson AL, Sherr JL, Shulman DI, et al. . Safety and glycemic outcomes during the MiniMed™ Advanced Hybrid Closed-Loop system pivotal trial in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2022;24(3):178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bergenstal RM, Nimri R, Beck RW, et al. . A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet. 2021;397(10270):208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benhamou P-Y, Franc S, Reznik Y, et al. . Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digital Health. 2019;1:e17–e25. [DOI] [PubMed] [Google Scholar]
- 25. Kariyawasam D, Morin C, Casteels K, et al. . Hybrid closed-loop insulin delivery versus sensor-augmented pump therapy in children aged 6-12 years: a randomised, controlled, cross-over, non-inferiority trial. Lancet Digit Health. 2022;4:e158–e168. [DOI] [PubMed] [Google Scholar]
- 26. Blauw H, Onvlee AJ, Klaassen M, van Bon AC, DeVries JH. Fully closed loop glucose control with a bihormonal artificial pancreas in adults with type 1 diabetes: an outpatient, randomized, crossover trial. Diabetes Care. 2021;44(3):836–838. [DOI] [PubMed] [Google Scholar]
- 27. Lum JW, Bailey RJ, Barnes-Lomen V, et al. A real-world prospective study of the safety and effectiveness of the loop open source automated insulin delivery system. Diabetes Technol Ther. 2021;23(5):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ware J, Allen JM, Boughton CK, et al. Randomized trial of closed-loop control in very young children with type 1 diabetes. N Engl J Med. 2022;386(3):209-219. [DOI] [PubMed]
- 29. Boughton CK, Hartnell S, Thabit H, et al. . Hybrid closed-loop glucose control with sensor augmented pump therapy in older adults with type 1 diabetes: an open-label multicentre, multinational, randomised, crossover study. Lancet Healthy Longevity. 2022:e135–e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garg SK, Weinzimer SA, Tamborlane WV, et al. . Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Forlenza GP, Ekhlaspour L, DiMeglio LA, et al. . Glycemic outcomes of children 2-6 years of age with type 1 diabetes during the pediatric MiniMed™ 670G system trial. Pediatr Diabetes. 2022;23(3):324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sherr JL, Bode BW, Forlenza GP, et al. Safety and glycemic outcomes with a tubeless automated insulin delivery system in very young children with type 1 diabetes: a single-arm multicenter clinical trial. Diabetes Care. 2022;45(8):1907-1910. [DOI] [PMC free article] [PubMed]
- 33. Schoelwer MJ, Kanapka LG, et al. . Predictors of time-in-range (70-180 mg/dL) achieved using a closed-loop control system. Diabetes Technol Ther. 2021;23(7):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ekhlaspour L, Town M, Raghinaru D, Lum JW, Brown SA, Buckingham BA. Glycemic outcomes in baseline hemoglobin A1C subgroups in the international diabetes closed-loop trial. Diabetes Technol Ther. 2022;24(8):588–591. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Breton MDS, Kovatchev BP. One year real-world use of the control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther. 2021;23(9):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications of insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 37. Stone MP, Agrawal P, Chen X, et al. . Retrospective analysis of 3-month real-world glucose data after the MiniMed 670G system commercial launch. Diabetes Technol Ther. 2018;20(10):689–692. [DOI] [PubMed] [Google Scholar]
- 38. Akturk HK, Giordano D, Champakanath A, et al. . Long-term real-life glycaemic outcomes with a hybrid closed-loop system compared with sensor-augmented pump therapy in patients with type 1 diabetes. Diabetes Obes Metab. 2020;22(4):583–589. [DOI] [PubMed] [Google Scholar]
- 39. Beato-Víbora PI, Gallego-Gamero F, Ambrojo-López A, Gil-Poch E, Martín-Romo I, Arroyo-Díez FJ. Rapid improvement in time in range after the implementation of an advanced hybrid closed-loop system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2021;23(9):609–615. [DOI] [PubMed] [Google Scholar]
- 40. Da Silva J, Lepore G, Battelino T, et al. . Real-world performance of the MiniMed 780G system: first Report of outcomes from 4120 Users. Diabetes Technol Ther. 2022;24(2):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Messer LH, Berget C, Pyle L, et al. . Real-world use of a new hybrid closed loop improves glycemic control in youth with type 1 diabetes. Diabetes Technol Ther. 2021;23:837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hood, KK, Garcia-Willingham N, Hanes S, et al.Lived experience of CamAPS FX closed loop system in youth with type 1 diabetes and their parents. Diabetes Obes Metab. 2022. doi: doi: 10.1111/dom.14815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cobry EC, Hamburger E, Jaser SS. Impact of the hybrid closed-loop system on sleep and quality of life in youth with type 1 diabetes and their parents. Diabetes Technol Ther. 2020;22(11):794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abraham MB, de Bock M, Smith GJ, et al. . Effect of a hybrid closed-loop system on glycemic and psychosocial outcomes in children and adolescents with type 1 diabetes: a randomized clinical trial. JAMA Pediatr. 2021;175:1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Messer LH, Berget C, Vigers T, et al. . Real world hybrid closed-loop discontinuation: predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes. 2020;21:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Berget C, Messer LH, Vigers T, et al. . Six months of hybrid closed loop in the real-world: an evaluation of children and young adults using the 670G system. Pediatr Diabetes. 2020;21(2):310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bergenstal RM, Gal RL, Connor CG, et al. . Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95–102. [DOI] [PubMed] [Google Scholar]
- 48. Ziegler C, Liberman A, Nimri R, et al. . Reduced worries of hypoglycaemia, high satisfaction, and increased perceived ease of use after experiencing four nights of MD-logic artificial pancreas at home (DREAM4). J Diabetes Res. 2015;2015:590308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cobry EC, Kanapka LG, Cengiz E, et al. . Health-related quality of life and treatment satisfaction in parents and children with type 1 diabetes using closed-loop control. Diabetes Technol Ther. 2021;23(6):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bisio A, Brown SA, McFadden R, et al. . Sleep and diabetes-specific psycho-behavioral outcomes of a new automated insulin delivery system in young children with type 1 diabetes and their parents. Pediatr Diabetes. 2021;22(3):495–502. [DOI] [PubMed] [Google Scholar]
- 51. Wheeler BJ, Collyns OJ, Meier RA, et al. . Improved technology satisfaction and sleep quality with Medtronic MiniMed Advanced Hybrid Closed-Loop delivery compared to predictive low glucose suspend in people with type 1 diabetes in a randomized crossover trial. Acta Diabetol. 2021;59(1):31–37. [DOI] [PubMed] [Google Scholar]
- 52. Kudva YC, Laffel LM, Brown SA, et al. . Patient-reported outcomes in a randomized trial of closed-loop control: the pivotal international diabetes closed-loop trial. Diabetes Technol Ther. 2021;23:673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cobry EC, Bisio A, Wadwa RP, Breton MD. Improvements in parental sleep, fear of hypoglycemia, and diabetes distress with use of an advanced hybrid closed loop system. Diabetes Care. 2022;45(5):1292–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barnard KD, Wysocki T, Ully V, et al. . Closing the loop in adults, children and adolescents with sub-ptimally controlled type 1 diabetes under free living conditions: a psychosocial substudy. J Diabetes Sci Technol. 2017;11:1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tauschmann M, Allen JM, Nagl K, et al. . Home use of day-and-night hybrid closed-loop insulin delivery in very young children: a multicenter, 3-week, randomized trial. Diabetes Care. 2019;42:594–600. [DOI] [PubMed] [Google Scholar]
- 56. Ekhlaspour L, Schoelwer MJ, Forlenza GP, et al. . Safety and performance of the Tandem t:slim X2 with control-IQ automated insulin delivery system in toddlers and preschoolers. Diabetes Technol Ther. 2021;23(5):384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Salehi P, Roberts AJ, Kim GJ. Efficacy and safety of real-life usage of MiniMed 670G automode in children with type 1 diabetes less than 7 years old. Diabetes Technol Ther. 2019;21(8):448–451. [DOI] [PubMed] [Google Scholar]
- 58. Stewart ZA, Wilinska ME, Hartnell S, et al. . Closed-loop insulin delivery during pregnancy in women with type 1 diabetes. N Engl J Med. 2016;375(7):644–654. [DOI] [PubMed] [Google Scholar]
- 59. Stewart ZA, Wilinska ME, Hartnell S, et al. . Day-and-night closed-loop insulin delivery in a broad population of pregnant women with type 1 diabetes: a randomized controlled crossover trial. Diabetes Care. 2018;41(7):1391–1399. [DOI] [PubMed] [Google Scholar]
- 60. Kumareswaran K, Thabit H, Leelarathna L, et al. . Feasibility of closed-loop insulin delivery in type 2 diabetes: a randomized controlled study. Diabetes Care. 2014;37(5):1198–1203. [DOI] [PubMed] [Google Scholar]
- 61. Thabit H, Hartnell S, Allen JM, et al. . Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial. Lancet Diabetes Endocrinol. 2017;5:117–124. [DOI] [PubMed] [Google Scholar]
- 62. Akturk HK, Agarwal S, Hoffecker L, Shah VN. Inequity in racial-ethnic representation in randomized controlled trials of diabetes technologies in type 1 diabetes: critical need for new standards. Diabetes Care. 2021;44(6):e121–e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dovc K, Battelino T. Closed-loop insulin delivery systems in children and adolescents with type 1 diabetes. Expert Opin Drug Deliv. 2020;17(2):157–166. [DOI] [PubMed] [Google Scholar]
- 64. Sherr JL, Buckingham BA, Forlenza GP, et al. . Safety and performance of the Omnipod hybrid closed-loop system in adults, adolescents, and children with type 1 diabetes over 5 days under free-living conditions. Diabetes Technol Ther. 2020;22(3):174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lal RA, Basina M, Maahs DM, et al. . One year clinical experience of the first commercial hybrid closed-loop system. Diabetes Care. 2019;42(12):2190–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Braune K, O'Donnell S, Cleal B, et al. . Real-world use of Do-It-Yourself artificial pancreas systems in children and adolescents with type 1 diabetes: online survey and analysis of self-reported clinical outcomes. JMIR Mhealth Uhealth. 2019;7(7):e14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Varimo T, Pulkkinen MA, Hakonen E, et al. . First year on commercial hydrid closed-loop system - experience on 111 children and adolescents with type 1 diabetes. Pediatr Diabetes. 2021;22(6):909–915. [DOI] [PubMed] [Google Scholar]
- 68. Berget C, Akturk HK, Messer LH, et al. . Real world performance of hybrid closed loop in youth, young adults, adults and older adults with type 1 diabetes: identifying a clinical target for hybrid closed loop use. Diabetes Obes Metab. 2021;23(9):2048–2057. [DOI] [PubMed] [Google Scholar]
- 69. McAuley SA, Trawley S, Vogrin S, et al. . Closed-loop insulin delivery versus sensor-augmented pump therapy in older adults with type 1 diabetes (ORACL): a randomized, crossover trial. Diabetes Care. 2022;45:381–390. [DOI] [PubMed] [Google Scholar]
- 70. Fuchs J, Hovorka R. Benefits and challenges of current closed-loop technologies in children and young people with type 1 diabetes. Front Pediatr. 2021;9:679484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fuchs J, Allen JM, Boughton CK, et al. . Assessing the efficacy, safety and utility of closed-loop insulin delivery compared with sensor-augmented pump therapy in very young children with type 1 diabetes (KidsAP02 study): an open-label, multicentre, multinational, randomised cross-over study protocol. BMJ Open. 2021;11(2):e042790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Petruzelkova L, Jiranova P, Soupal J, et al. . Pre-school and school-aged children benefit from the switch from a sensor-augmented pump to an AndroidAPS hybrid closed loop: a retrospective analysis. Pediatr Diabetes. 2021;22(4):594–604. [DOI] [PubMed] [Google Scholar]
- 73. Dovc K, Boughton C, Tauschmann M, et al. . Young children have higher variability of insulin requirements: observations during hybrid closed-loop insulin delivery. Diabetes Care. 2019;42(7):1344–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Anderson SM, Buckingham BA, Breton MD, et al. . Hybrid closed-loop control is safe and effective for people with type 1 diabetes who are at moderate to high risk for hypoglycemia. Diabetes Technol Ther. 2019;21(6):356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Abitbol A, Rabasa-Lhoret R, Messier V, et al. . Overnight glucose control with dual- and single-hormone artificial pancreas in type 1 diabetes with hypoglycemia unawareness: a randomized controlled trial. Diabetes Technol Ther. 2018;20(3):189–196. [DOI] [PubMed] [Google Scholar]
- 76. Malone SK, Peleckis AJ, Grunin L, et al. . Characterizing glycemic control and sleep in adults with long-standing type 1 diabetes and hypoglycemia unawareness initiating hybrid closed loop insulin delivery. J Diabetes Res. 2021;2021:6611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Burckhardt MA, Abraham MB, Dart J, et al. . Impact of hybrid closed loop therapy on hypoglycemia awareness in individuals with type 1 diabetes and impaired hypoglycemia awareness. Diabetes Technol Ther. 2021;23:482–490. [DOI] [PubMed] [Google Scholar]
- 78. Polsky S, Akturk HK. Case series of a hybrid closed-loop system used in pregnancies in clinical practice. Diabetes Metab Res Rev. 2020;36(3):e3248. [DOI] [PubMed] [Google Scholar]
- 79. Farrington C, Stewart Z, Hovorka R, Murphy H. Women’s experiences of day-and-night closed-loop insulin delivery during type 1 diabetes pregnancy. J Diabetes Sci Technol. 2018;12(6):1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Farrington C, Stewart ZA, Barnard K, Hovorka R, Murphy HR. Experiences of closed-loop insulin delivery among pregnant women with Type 1 diabetes. Diabet Med. 2017;34(10):1461–1469. [DOI] [PubMed] [Google Scholar]
- 81. Stewart ZA, Yamamoto JM, Wilinska ME, et al. . Adaptability of closed loop during labor, delivery, and postpartum: a secondary analysis of data from two randomized crossover trials in type 1 diabetes pregnancy. Diabetes Technol Ther. 2018;20(7):501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bally L, Gubler P, Thabit H, et al. . Fully closed-loop insulin delivery improves glucose control of inpatients with type 2 diabetes receiving hemodialysis. Kidney Int. 2019;96(3):593–596. [DOI] [PubMed] [Google Scholar]
- 83. Kaur H, Schneider N, Pyle L, et al. . Efficacy of hybrid closed-loop system in adults with type 1 diabetes and gastroparesis. Diabetes Technol Ther. 2019;21(12):736–739. [DOI] [PubMed] [Google Scholar]
- 84. Boughton CK, Tripyla A, Hartnell S, et al. . Fully automated closed-loop glucose control compared with standard insulin therapy in adults with type 2 diabetes requiring dialysis: an open-label, randomized crossover trial. Nat Med. 2021;27:1471–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sherwood JS, Jafri RZ, Balliro CA, et al. . Automated glycemic control with the bionic pancreas in cystic fibrosis-related diabetes: A pilot study. J Cyst Fibros. 2020;19(1):159–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Scully KJ, Palani G, Zheng H, Moheet A, Putman MS. The effect of Control IQ™ hybrid closed loop technology on glycemic control in adolescents and adults with cystic fibrosis related diabetes. Diabetes Technol Ther. 2022;24(6):446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. American Diabetes Association . ADA evidence-grading system for “Standards of Medical Care in Diabetes”. Diabetes Care. 2021;44(Suppl 1):S221–S222. [DOI] [PubMed] [Google Scholar]
- 88. Kovatchev BP, Kollar L, Anderson SM, et al. . Evening and overnight closed-loop control versus 24/7 continuous closed-loop control for type 1 diabetes: a randomised crossover trial. Lancet Digit Health. 2020;2(2):e64–e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Carlson AL, Bode BW, Brazg RL, et al. . 97-LB: Safety and Glycemic Outcomes of the MiniMed Advanced Hybrid Closed-Loop (AHCL) System in Subjects with T1D American Diabetes Association 80th Scientific Sessions. June 12-16, 2020; Virtual.
- 90. Thabit H, Tauschmann M, Allen JM, et al. . Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373(22):2129–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bisio A, Gonder-Frederick L, McFadden R, et al. . The impact of a recently approved automated insulin delivery system on glycemic, sleep, and psychosocial outcomes in older adults with type 1 diabetes: a pilot study. J Diabetes Sci Technol. 2022;16(3):663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bally L, Thabit H, Hartnellet S, et al. . Closed-loop insulin delivery for glycemic control in noncritical care. N Engl J Med. 2018;379:547–556. [DOI] [PubMed] [Google Scholar]
- 93. Mulinacci G, Alonso GT, Snell-Bergeon JK, Shah VN. Glycemic outcomes with early initiation of continuous glucose monitoring system in recently diagnosed patients with type 1 diabetes. Diabetes Technol Ther. 2019;21(1):6–10. [DOI] [PubMed] [Google Scholar]
- 94. Kamrath C, Tittel SR, Kapellen TM, et al. . Early versus delayed insulin pump therapy in children with newly diagnosed type 1 diabetes: results from the multicentre, prospective diabetes follow-up DPV registry. Lancet Child Adolesc Health. 2021;5(1):17–25. [DOI] [PubMed] [Google Scholar]
- 95. Champakanath A, Akturk HK, Alonso GT, Snell-Bergeon JK, Shah VN. Continuous glucose monitoring initiation within first year of type 1 diabetes diagnosis is associated with improved glycemic outcomes: 7-year follow-up study. Diabetes Care. 2022;45:750–753. [DOI] [PubMed] [Google Scholar]
- 96. Shah SC, Malone JI, Simpson NE. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med. 1989;320(9):550–554. [DOI] [PubMed] [Google Scholar]
- 97. Buckingham B, Beck RW, Ruedy KJ, et al. . Effectiveness of early intensive therapy on β-cell preservation in type 1 diabetes. Diabetes Care. 2013;36:4030–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. University of Cambridge . Closed Loop From Onset in Type 1 Diabetes (CLOuD). https://clinicaltrials.gov/ct2/show/NCT02871089. Accessed September 22, 2021.
- 99. Jaeb Center for Health Research. Hybrid Closed Loop . Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes (CLVer). https://clinicaltrials.gov/ct2/show/NCT04233034. Accessed September 22, 2021.
- 100. Patton SR, Noser AE, Youngkin EM, Majidi S, Clements MA. Early initiation of diabetes devices relates to improved glycemic control in children with recent-onset type 1 diabetes mellitus. Diabetes Technol Ther. 2019;21(7):379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Battelino T, Danne T, Bergenstal RM, et al. . Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kimbell B, Rankin D, Ashcroft NL, et al. . What training, support, and resourcing do health professionals need to support people using a closed-loop system? A qualitative interview study with health professionals involved in the closed loop from onset in type 1 diabetes (CLOuD) trial. Diabetes Technol Ther. 2020;22:468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Messer LH. Why expectations will determine the future of artificial pancreas. Diabetes Technol Ther. 2018;20:S265–S268. [DOI] [PubMed] [Google Scholar]
- 104. Boughton CK, Hartnell S, Allen JM, Fuchs J, Hovorka R. Training and support for hybrid closed-loop therapy. J Diabetes Sci Technol. 2022;16(1):218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cescon M, DeSalvo DJ, Ly TT, et al. . Early detection of infusion set failure during insulin pump therapy in type 1 diabetes. J Diabetes Sci Technol. 2016;10:1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Boughton CK, Hartnell S, Allen JM, Hovorka R. The importance of prandial insulin bolus timing with hybrid closed-loop systems. Diabet Med. 2019;36:1716–1717. [DOI] [PubMed] [Google Scholar]
- 107. Pinsker JE, Harsimran Singh H, Molly McElwee Malloy MM, et al. . A virtual training program for the Tandem t:slim X2 insulin pump: implementation and outcomes. Diabetes Technol Ther. 2021;23(6):467–470. [DOI] [PubMed] [Google Scholar]
- 108. Vigersky RA, Velado K, Zhong A, Agrawal P, Cordero TL. The effectiveness of virtual training on the MiniMed™ 670G system in people with type 1 diabetes during the COVID-19 pandemic. Diabetes Technol Ther. 2021;23:104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gómez AM, Henao D, Parra D, et al. . Virtual training on the hybrid close loop system in people with type 1 diabetes (T1D) during the COVID-19 pandemic. Diabetes Metab Syndr. 2021;15(1):243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Petrovski G, Campbell J, Almajali D, Al Khalaf F, Hussain K. Successful initiation of hybrid closed-Loop system using virtual pump training program in a teenager with type 1 diabetes previously treated with multiple daily injections. J Diabetes Sci Technol. 2021;15(6):1394–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Berget C, Thomas SE, Messer LH, et al. . A clinical training program for hybrid closed loop therapy in a pediatric diabetes clinic. J Diabetes Sci Technol. 2020;14:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Messer LH, Berget C, Ernst A, et al. . Initiating hybrid closed loop: a program evaluation of an educator-led control-IQ follow-up at a large pediatric clinic. Pediatr Diabetes. 2021;22(4):586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Levine BJ, Close KL, Dalton D, et al. . Enhancing resources for healthcare professionals caring for people on intensive insulin therapy: summary from a national workshop. Diabetes Res Clin Pract. 2020;164:108169. [DOI] [PubMed] [Google Scholar]
- 114. Messer LH, Berget C, Forlenza GP. A clinical guide to advanced diabetes devices and closed-loop systems using the CARES paradigm. Diabetes Technol Ther. 2019;21:462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ekhlaspour L, Tabatabai I, Buckingham B. A review of continuous glucose monitoring data interpretation in the age of automated insulin delivery. J Diabetes Sci Technol. 2019;13:645–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Messer LH, Forlenza GP, Sherr JL, et al. . Optimizing hybrid closed-loop therapy in adolescents and emerging adults using the MiniMed 670G system. Diabetes Care. 2018;41(4):789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. O’Malley G, Messer LH, Levy C, et al. . Clinical management and pump parameter adjustment of the control-IQ closed-loop control system: results from a 6-month multicenter randomized clinical trial. Diabetes Technol Ther. 2021;23(4):245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lehmann V, Zueger T, Zeder A, et al. . Lower daily carbohydrate intake is associated with improved glycemic control in adults with type 1 diabetes using a hybrid closed-loop system. Diabetes Care. 2020;43(12):3102–3105. [DOI] [PubMed] [Google Scholar]
- 119. Danne T, Nimri R, Battelino T, et al. . International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Holt RIG, DeVries JH, Hess-Fischl A, et al. . The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2021;44:2589–2625. [DOI] [PubMed] [Google Scholar]
- 121. American Diabetes Association . 6. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(Suppl 1):S83–S96. [DOI] [PubMed] [Google Scholar]
- 122. Shah VN, Garg SK. Standardized hybrid closed-loop system reporting. Diabetes Technol Ther. 2021;23(5):323–331. [DOI] [PubMed] [Google Scholar]
- 123. Weissberg-Benchell J, Hessler D, Polonsky WH, Fisher L. Psychosocial impact of the bionic pancreas during summer camp. J Diabetes Sci Technol. 2016;10(4):840–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Weissberg-Benchell J, Hessler D, Fisher L, Russell SJ, Polonsky WH. Impact of an automated bihormonal delivery system on psychosocial outcomes in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(12):723–729. [DOI] [PubMed] [Google Scholar]
- 125. Lawton J, Blackburn M, Rankin D, et al. . Participants’ experiences of, and views about, daytime use of a day-and-night hybrid closed-loop system in real life settings: longitudinal qualitative study. Diabetes Technol Ther. 2019;21(3):119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gonder-Frederick LA, Grabman JH, Kovatchev B, et al. . Is psychological stress a factor for incorporation into future closed-loop systems?. J Diabetes Sci Technol. 2016;10(3):640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Oser SM, Oser TK. Diabetes technologies: we are all in this together. Clinical Diabetes. 2020;38(2):188–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Barnard-Kelly KD, Chernavsky D. Social inequalities and diabetes: a commentary. Diabetes Ther. 2020;11:803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. U.S. Food and Drug Administration . Diversity Plans to Improve Enrollment of Participants From Underrepresented Racial and Ethnic Populations in Clinical Trials; Draft Guidance for Industry, April 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diversity-plans-improve-enrollment-participants-underrepresented-racial-and-ethnic-populations. Accessed June 14, 2022.
- 130.Editors, Rubin E. Striving for diversity in research studies. N Engl J Med. 2021;385(15):1429–1430. [DOI] [PubMed] [Google Scholar]
- 131. Flanagin A, Frey T, Christiansen SL, AMA Manual of Style Committee . Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA. 2021;326(7):621–627. [DOI] [PubMed] [Google Scholar]
- 132. Agarwal S, Crespo-Ramos G, Leung SL, et al. . Solutions to address inequity in diabetes technology use in type 1 diabetes: results from multidisciplinary stakeholder co-creation workshops. Diabetes Technol Ther. 2022;24(6):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Agarwal S, Kanapka LG, Raymond JK, et al. . Racial-ethnic inequity in young adults with type 1 diabetes. J Clin Endocrinol Metab. 2020;105(8):e2960–e2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Addala A, Auzanneau M, Miller K, et al. . A decade of disparities in diabetes technology use and HbA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care. 2021;44(1):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. U.S. Food & Drug Administration . FDA authorizes first interoperable, automated insulin dosing controller designed to allow more choices for patients looking to customize their individual diabetes management device system. https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-interoperable-automated-insulin-dosing-controller-designed-allow-more-choices. Accessed May 4, 2021.
- 136. Svehlikova E, Mursic I, Augustin T, et al. . Pharmacokinetics and pharmacodynamics of three different formulations of insulin aspart: a randomized, double-blind, crossover study in men with type 1 diabetes. Diabetes Care. 2021;44(2):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Dovc K, Piona C, Yeşiltepe Mutlu G, et al. . Faster compared with standard insulin aspart during day-and-night fully closed-loop insulin therapy in type 1 diabetes: a double-blind randomized crossover trial. Diabetes Care. 2020;43(1):29–36. [DOI] [PubMed] [Google Scholar]
- 138. Boughton CK, Hartnell S, Thabit H, et al. . Hybrid closed-loop glucose control with faster insulin aspart compared with standard insulin aspart in adults with type 1 diabetes: a double-blind, multicentre, multinational, randomized, crossover study. Diabetes Obes Metab. 2021;23(6):1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Dassau E, Renard E, Place J, et al. . Intraperitoneal insulin delivery provides superior glycaemic regulation to subcutaneous insulin delivery in model predictive control-based fully-automated artificial pancreas in patients with type 1 diabetes: a pilot study. Diabetes Obes Metab. 2017;19:1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Presti J L, Galderisi A, Doyle FJ, et al. . Intraperitoneal insulin delivery: evidence of a physiological route for artificial pancreas from compartmental modeling. J Diabetes Sci Technol. Published online ahead of print February 10,2022:19322968221076559. doi: 10.1177/19322968221076559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Galderisi A, Cohen N, Calhoun P, et al. . Effect of Afrezza on glucose dynamics during HCL treatment. Diabetes Care. 2020; 43(9):2146–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sherr JL, Patel NS, Michaud CI, et al. . Mitigating meal-related glycemic excursions in an insulin-sparing manner during closed-loop insulin delivery: the beneficial effects of adjunctive pramlintide and liraglutide. Diabetes Care. 2016;39:1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Biester T, Muller I, von dem Berge T, et al. . Add-on therapy with dapagliflozin under full closed loop control improves time in range in adolescents and young adults with type 1 diabetes: the DAPADream study. Diabetes Obes Metab. 2021;23:599–608. [DOI] [PubMed] [Google Scholar]
- 144. Haidar A, Yale JF, Lovblom LE, et al. . Reducing the need for carbohydrate counting in type 1 diabetes using closed-loop automated insulin delivery (artificial pancreas) and empagliflozin: a randomized, controlled, non-inferiority, crossover pilot trial. Diabetes Obes Metab. 2021;23:1272–1281. [DOI] [PubMed] [Google Scholar]
- 145. Commissariat PV, Roethke LC, Finnegan JL, et al. . Youth and parent preferences for an ideal AP system: it is all about reducing burden. Pediatr Diabetes. 2021;22(7):1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Viceconti M, Cobelli C, Haddad T, Himes A, Kovatchev BP, Palmer M. In silico assessment of biomedical products: the conundrum of rare but not so rare events in two case studies. Proc Inst Mech Eng H. 2017;231:455–466. [DOI] [PubMed] [Google Scholar]
- 147. Messori M, Kropff J, Del Favero S, et al. . for the AP@home consortium. Individually adaptive artificial pancreas in subjects with type 1 diabetes: a one-month proof-of-concept trial in free-living conditions. Diabetes Technol Ther. 2017;19:560–571. [Google Scholar]
- 148. Shi D, Dassau E, Doyle FJ III. Multivariate learning framework for long-term adaptation in the artificial pancreas. Bioeng Transl Med. 2018;4:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lipman TH, Hawkes CP. Racial and socioeconomic disparities in pediatric type 1 diabetes: time for a paradigm shift in approach. Diabetes Care. 2021;44(1):14–16. [DOI] [PubMed] [Google Scholar]
- 150. Walker AF, Hood KK, Gurka MJ, et al. . Barriers to technology use and endocrinology care for underserved communities with type 1 diabetes. Diabetes Care. 2021;44(7):1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]