Abstract
The etiology of central precocious puberty (CPP) is multiple and heterogeneous, including congenital and acquired causes that can be associated with structural or functional brain alterations. All causes of CPP culminate in the premature pulsatile secretion of hypothalamic GnRH and, consequently, in the premature reactivation of hypothalamic-pituitary-gonadal axis. The activation of excitatory factors or suppression of inhibitory factors during childhood represent the 2 major mechanisms of CPP, revealing a delicate balance of these opposing neuronal pathways. Hypothalamic hamartoma (HH) is the most well-known congenital cause of CPP with central nervous system abnormalities. Several mechanisms by which hamartoma causes CPP have been proposed, including an anatomical connection to the anterior hypothalamus, autonomous neuroendocrine activity in GnRH neurons, trophic factors secreted by HH, and mechanical pressure applied to the hypothalamus. The importance of genetic and/or epigenetic factors in the underlying mechanisms of CPP has grown significantly in the last decade, as demonstrated by the evidence of genetic abnormalities in hypothalamic structural lesions (eg, hamartomas, gliomas), syndromic disorders associated with CPP (Temple, Prader-Willi, Silver-Russell, and Rett syndromes), and isolated CPP from monogenic defects (MKRN3 and DLK1 loss-of-function mutations). Genetic and epigenetic discoveries involving the etiology of CPP have had influence on the diagnosis and familial counseling providing bases for potential prevention of premature sexual development and new treatment targets in the future. Global preventive actions inducing healthy lifestyle habits and less exposure to endocrine-disrupting chemicals during the lifespan are desirable because they are potentially associated with CPP.
Keywords: gonadotropin-releasing hormone, central precocious puberty, kisspeptins, MKRN3, DLK1, hypothalamic hamartoma, endocrine-disrupting chemicals
Graphical Abstract
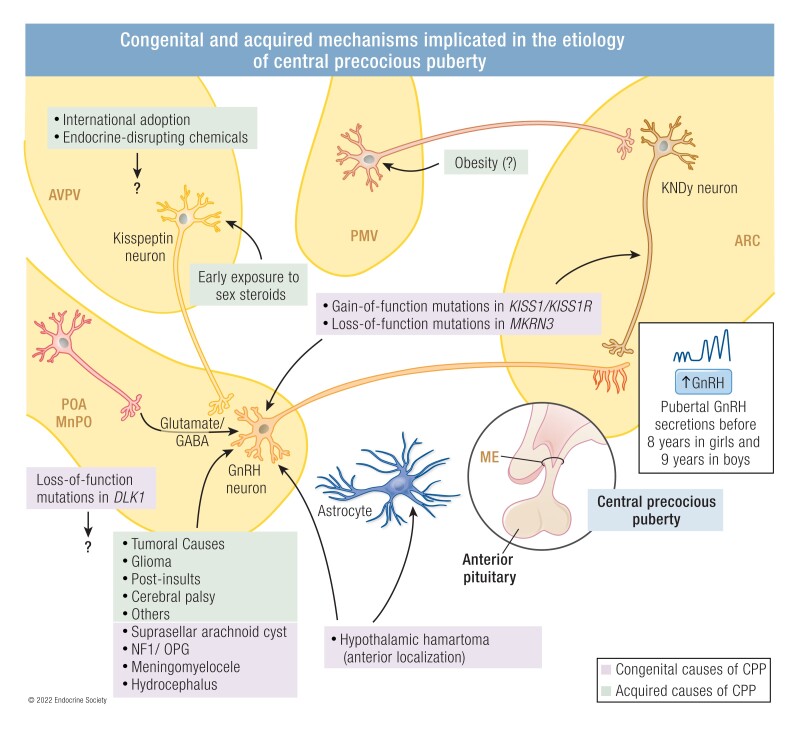
Congenital and acquired mechanisms implicated in the etiology of central precocious puberty. ARC, arcuate nucleus; AVPV, anteroventral periventricular nucleus; GABA, gamma-aminobutyric acid; ME, median eminence; MnPO, median preoptic nucleus; PMV, ventral premammillary nucleus; POA, preoptic area; OPG: optic pathways glioma.
ESSENTIAL POINTS.
Central precocious puberty (CPP) results from the premature reactivation of pulsatile hypothalamic GnRH secretion, which can be caused by congenital or acquired disorders with or without central nervous system (CNS) abnormalities.
Hypothalamic hamartoma (HH) is the most well-known congenital cause of CPP with CNS abnormalities and its anatomical connection to the anterior hypothalamus, autonomous neuroendocrine activity in GnRH neurons or trophic factors secreted by HH (such as TGF-α), and mechanical pressure applied to the hypothalamus are the main potential mechanisms of HH-related CPP.
The identification of monogenic causes of familial CPP, mainly represented by loss-of-function mutations in the maternally imprinted MKRN3 gene, highlighted novel congenital causes of CPP without CNS abnormalities.
Loss-of-function mutations in DLK1, an antiadipogenic factor, in familial CPP were associated with unfavorable metabolic outcome in women, indicating a potential link between the reproductive and metabolic systems.
Acquired causes of CPP with CNS abnormalities such as neoplastic lesions, hydrocephalus, neonatal infections, traumatic brain injury, cranial irradiation, and encephalopathies can disrupt the regulatory mechanisms of GnRH release and lead to CPP by poorly established mechanisms.
CPP can be associated with multiple anomalies, including growth, metabolic and neurocognitive defects, characterizing syndromic forms of CPP.
Endocrine-disrupting chemicals (EDC) have a potential effect in pubertal development through central and peripheral mechanisms and their possible role in human pubertal timing needs to be investigated by well-designed longitudinal studies considering time, duration, dose, and combination of EDC exposure.
Precocious puberty is a prevalent endocrine disorder that affects children worldwide (1, 2). Classically, it is defined as the development of secondary sexual characteristics before the age of 8 years in girls and 9 years in boys, and it has a clear female predominance (3, 4). The premature reactivation of pulsatile hypothalamic GnRH secretion leads to central precocious puberty (CPP), the most common mechanism of precocious sexual development (3).
The incidence of CPP is 10- to 20-fold higher in females and quite variable in distinct geographical regions, varying from 0.217 to 26.28 per 10 000 girls and 0.02 to 0.9 per 10 000 boys (1, 5–7). These epidemiological data were mainly based on national patient registries, national insurance claims data, and tertiary care centers. A Spanish epidemiological study showed an estimated CPP global prevalence of 19 per 100 000 individuals (girls, 37; boys, 0.46) (8). The annual incidence ranged between 0.02 and 1.07 new cases per 100 000 individuals, with a remarkable increase from 2000 onwards among girls for the period analyzed (1997-2009) (8). A more recent study involving a very large group of children (8596) with premature sexual development suggested that the annual incidence of CPP has substantially increased in Denmark throughout the past 20 years (1998-2017) reaching 9.2 per 10 000 girls and 0.9 per 10 000 boys (1). Similarly, epidemiological longitudinal studies demonstrated a significant increase of CPP in children from Korea (6, 7). The first Korean study (12 351 children) evidenced an increased incidence of CPP from 0.33 per 10 000 girls to 5.04 per 10 000 girls and from 0.03 per 10 000 boys to 0.12 per 10 000 boys between 2004 to 2010 (6). From 2008 to 2014, the incidence of CPP increased from 8.94 per 10 000 girls to 41.53 per 10 000 girls and from 0.16 per 10 000 boys to 1.47 per 10 000 boys (7).
The clinical hallmarks of CPP include progressive breast development in girls and increase of testes volume in boys and reflect GnRH and gonadotropin-stimulated sex steroid actions (gonadarche) (3, 9). Accelerated growth velocity (>6 cm/y) and advanced bone age (higher than 1 year or 2 SD score of chronological age) represent common features of progressive CPP. Hormonal findings confirming diagnosis of CPP include pubertal basal or GnRH-stimulated LH levels (3). The presence of family history or CPP associated with multiple anomalies can suggest a genetic etiology (3). The prevalence of familial CPP was 27.5% in a large cohort of 153 children with idiopathic CPP (10). In the recent past, magnetic resonance imaging (MRI) of the central nervous system (CNS) was the only approach in the investigation of the etiology of CPP, searching mainly for congenital or acquired anatomical alterations. More recently, genetic studies were added to the algorithm of etiology investigation of familial or sporadic CPP in children without CNS anatomical lesions (3).
Comprehension of the mechanisms underlying CPP expanded significantly in the past 2 decades, by using refined clinical approaches, updated genetic studies, and neuroimaging (11). Distinct genetic approaches ranging from genome-wide association studies, linkage analysis, comparative genomic hybridization, and whole-exome and genome sequencing have allowed for the identification of new players implicated in not only variations within normal puberty timing, but also within the etiology of progressive CPP (12, 13). Monogenic causes of familial CPP, including syndromic and nonsyndromic forms, have helped reveal novel signaling pathways implicated with normal and abnormal pubertal timing in humans (14–16). The genetic discoveries involving the etiology of CPP have had an important influence on the diagnosis (more precise and earlier) and familial counseling and can provide the bases for potential new treatment targets in the future. Additionally, the involvement of metabolic alterations (overweight/obesity, metabolic syndrome) and endocrine-disrupting chemicals (EDC) exposure were added to our current knowledge on the etiology of CPP (17).
Several factors could influence the timing and tempo of puberty, including genetics, lifestyle, nutrition, and environmental exposures (11, 18, 19); however, the mechanisms underlying the increasing trend in the incidence of CPP are uncertain. The growing influence of nutritional status (overweight or obesity) has been highlighted as a major influence on premature pubertal development, especially in girls (20). Other potential mechanisms include prenatal and postnatal exposure to endocrine disruptors, international adoption, use of electronic devices, and psychosocial influences (21–24). An increased incidence of precocious and accelerated puberty was demonstrated in a small cohort of Italian girls during and after lockdown for the coronavirus disease 2019 (23, 25). This was potentially related to weight gain, more frequent use of electronic devices, and stress (25). Notably, earlier age at puberty has been associated with a higher risk of metabolic, oncologic (estrogen-dependent cancer in women in adulthood), and cardiovascular disorders in adulthood (26). It has been hypothesized that the increasing prevalence and progression of CPP may also represent an adaptive mechanism to escape from ectopic adiposity in girls, especially in those who had higher abdominal fat distribution (27).
Recognition of the causes of CPP has contributed to our understanding of the potential mechanisms responsible for normal and abnormal pubertal timing, allowing the development of novel strategies, including prophylactic intervention, diet alterations, and behavior targeted therapies, increased surveillance, and delivery of personalized care. Here, we present a comprehensive review on the multiple known CPP etiologies, exploring the status of the studies aimed at untangling the underlying mechanisms implicated in the premature hypothalamic GnRH secretion.
Neurobiological Basis of Puberty
The hypothalamus is the main control center for different physiological functions, including growth, metabolism, and puberty/reproduction. It is responsible for the pulsatile GnRH secretion into the hypophyseal portal blood system that in turn controls the release of gonadotropins, LH and FSH, from the anterior pituitary to drive gonadal maturation and function. These gonadotropins participate in the maturation and functioning of the gonads (ie, testis and ovary) that are responsible for the synthesis of sex hormones (28), thus promoting the development of secondary sex characteristics (9) and the production of gametes. What signals the activation of this axis to incite pubertal onset has been a long-standing enigma in neuroendocrinology. Clinical observations of pubertal phenotypes lead to the identification of specific allelic variants in genes coding factors such as kisspeptins, neurokinin-B (NKB), and leptin that were later experimentally shown to be key players in mammalian puberty (29).
The KISS1 gene encodes the kisspeptins that bind to and activate their specific receptor KISS1R (previously known as GPR54) (30). The identification of loss-of-function mutations in KISS1R, a G-protein coupled receptor, in families with congenital hypogonadotropic hypogonadism was a critical discovery in the field of neuroendocrine regulation of reproduction (30, 31). Analyses of the reproductive and hormonal phenotypes of Kiss1r null mice and humans revealed that hypothalamic GnRH synthesis and neuronal migration, and pituitary responsiveness to GnRH, were preserved, suggesting that this system is an upstream regulator of GnRH release (32). Kisspeptins were originally identified as metastasis suppressor peptides (33). Physiological and pharmacological studies have shown that the kisspeptins ligand/receptor system is an essential part of the excitatory network that regulates GnRH secretion. Later, very rare inactivating KISS1 mutations were also identified in patients with congenital hypogonadotropic hypogonadism, underscoring the importance of kisspeptins for puberty and reproduction in humans (34).
Multiple human, animal, and in vitro studies have shown that GnRH secretion requires the stimulatory action of kisspeptins (35–37). KISS1 and KISS1R are broadly expressed in human and animal tissues, with high expression in the placenta and several brain areas, including hypothalamus. In the hypothalamus, kisspeptins is expressed in the arcuate nucleus (ARC) of male and female rodents, and in the anteroventral periventricular nucleus/periventricular nucleus continuum (AVPV) of female rodents. Kisspeptins neurons in the ARC coexpress the neuropeptides, NKB (encoded by TAC3 gene) and dynorphin A (called KNDy neurons) (38, 39). In these KNDy neurons, the coordinated action of NKB (stimulatory) and dynorphin (inhibitory) controls release of kisspeptins to effect pulsatile GnRH and LH secretion (40). By contrast, kisspeptins neurons in the AVPV/periventricular nucleus continuum are involved in the positive feedback of sex steroids, leading to the preovulatory LH surge in females mice (41–43). Administration of kisspeptins results in increases in plasma LH concentrations in healthy men (36, 44), and in women kisspeptins also induce LH release, although the response varies across the menstrual cycle (45). Kisspeptins stimulate gonadotropin release less potently but in a more physiologically effective way than current treatments with GnRH analogues (35).
Hypothalamic kisspeptins neurons serve as the nodal regulatory center of reproductive function (46). As stated previously, kisspeptin expression, synthesis, and release are tightly regulated by metabolic cues at multiple levels (47). Agouti-related protein and pro-opiomelanocortin neurons are critical components of neuroendocrine circuits regulating activity of kisspeptins neurons (48), as well as additional hypothalamic centers that have also been identified as regulators of these neurons (47, 49). Although peripheral signals transmit fundamental metabolic information to achieve successful reproduction, kisspeptins neurons do not seem to be first-order responders for the main metabolic cues, such as leptin, insulin, and ghrelin (47).
The coordination of energy intake and expenditure is a complex process that is orchestrated by specific neuronal populations in the hypothalamus and influenced by both peripheral and central signals that ultimately regulate body weight. Glial cells have been recognized as important protagonists in this neuroendocrine process (50). Metabolic hormones, such as leptin and ghrelin, and specific nutrients relay information regarding nutritional status to hypothalamic neuronal circuits to determine appetite and energy expenditure. Some of these metabolic and nutritional factors, as well as neuropeptides controlling energy homeostasis, also participate in the control of puberty (29). The interaction of these 2 physiological axes is clearly observed at the 2 extremes of body energy status: chronic energy deficiency, such as malnutrition or anorexia, which can be accompanied by delayed puberty or lack of pubertal progression, whereas the opposite condition of energy excess, as seen in obesity, has been associated with early pubertal onset (17, 51). This phenomenon of nutritional effects on pubertal development has been extensively documented in girls (52), but it may also occur in boys (53).
There is a clear relationship between nutrition, the timing of pubertal onset and its progression, and linear growth (54). Nutritional status plays an important role in regulating growth, and excess body weight/adiposity early in life (11, 54). Deardorff et al (55) demonstrated the relationship between childhood overweight and obesity and pubertal onset among Mexican-American boys and girls by using data from the Center for the Health Assessment of Mothers and Children of Salinas (55). This study investigated the association between body mass index (BMI) at age 5 years and multiple markers of pubertal onset in 336 Mexican-American children. No association between these factors was observed in boys, but it found significantly earlier thelarche in overweight and obese girls, menarche in overweight girls, and pubarche in obese girls, as compared to normal-weight girls (55). Martos-Moreno et al (56), analyzing data from the Madrid Cohort of Pediatric Obesity, also demonstrated a strong relationship between sex, ethnicity, growth, nutrition, and puberty. Ethnicity is one of the main determinants of adipose tissue distribution with increased trunk body fat accumulation in Latino children with obesity, which is directly related to the development of metabolic derangement (57). The age of menarche, which occurs approximately 2 years after the onset of thelarche, correlates positively with bone age and negatively with the amount of growth that remains to occur (58). The connection between metabolic status and puberty has not only been found in clinical studies, but it has also been clearly demonstrated in preclinical studies employing animal models of early under- or overnutrition where pubertal timing is delayed or advanced, respectively (17). These studies provided a valuable tool to discern the mechanisms involved in pubertal disorders caused by metabolic distress (59).
The cellular energy sensors mammalian target of rapamycin (mTOR), AMP-activated protein kinase, and the sirtuin, are also involved in the metabolic regulation of puberty (17). In the hypothalamus, mTOR and AMP-activated protein kinase signaling operate in a reciprocal manner to promote or repress puberty, respectively, via activation or inhibition of Kiss1 neurons in the arcuate nucleus depending on energy status (60, 61).
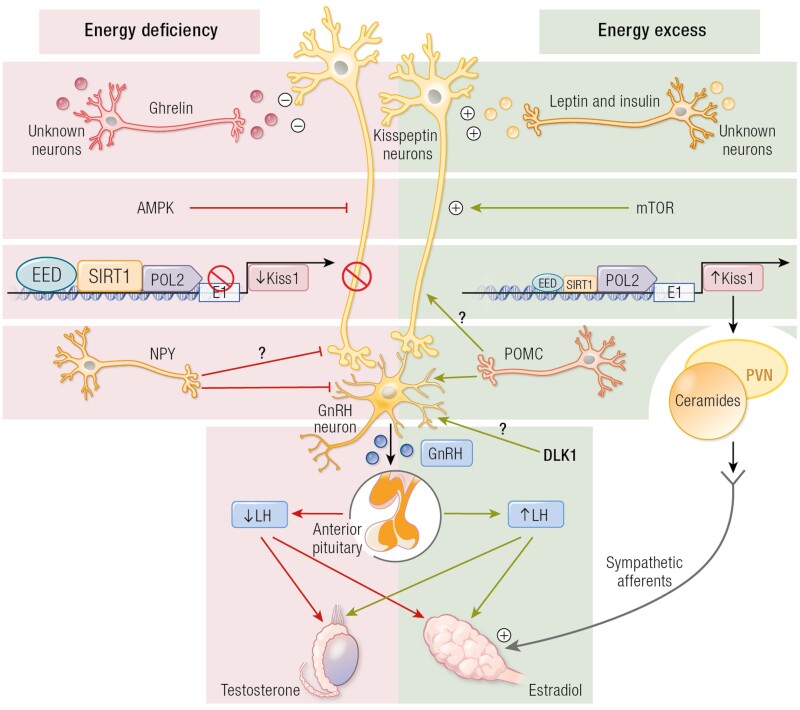
In addition, brain ceramides have been proposed as potential mediators in the control of energy balance in adult rodents. They mediate the orexigenic actions of ghrelin and oppose the anorectic effects of leptin, as well as control brown fat activity (62). Heras et al (62) demonstrated that female rats with early-onset overweight not only presented advanced puberty, but that this was associated with an enhancement of hypothalamic ceramide content and that central pharmacological activation of ceramide signaling mimicked the advancement of puberty caused by obesity (62). These data clearly support the concept of a tight relationship between energy homeostasis and puberty, with a putative role of cellular energy sensors and metabolic mediators in the control of puberty (Fig. 1).
Figure 1.
Schematic representation of the putative role of cellular energy sensors and metabolic mediators in the control of puberty. In conditions of energy insufficiency, hypothalamic activation of AMP-activated protein kinase (AMPK), together with persistence of the repressive action of SIRT1 at the Kiss1 promoter, leads to reduced Kiss1 expression and delayed puberty. By contrast, in conditions of energy sufficiency and timely removal of SIRT1 from the Kiss1 promoter, together with the presumable activation of mammalian target of rapamycin (mTOR), allows increased Kiss1 expression and the normal occurrence of puberty. At extreme conditions of energy excess (eg, early-onset obesity), precocious removal of SIRT1 from the Kiss1 promoter causes a change in the chromatin landscape that accelerates the rise in Kiss1 expression, leading to early puberty. In addition, obesity presumably also causes the activation of an alternative pathway, involving kisspeptins innervation of the paraventricular nucleus (PVN) and ceramide (CER) synthesis at this site, which apparently contribute to the precocious activation of the ovary in obesity via direct sympathetic inputs. ARC, arcuate nucleus; Dyn, dynorphin; GnRH, gonadotrophin-releasing hormone; Kp, kisspeptins; mTOR, mammalian target of rapamycin; NKB, neurokinin B; SIRT1, Sirtuin; SNS, sympathetic nervous system.
Menarche is an important and specific marker of pubertal timing in females. Perry et al (12) described that menarche signals are enriched in imprinted regions, with 3 loci, MKRN3/MAGEL2, Delta-like Noncanonical Notch Ligand 1 (DLK1)/WDR25, and KCNK9, showing parent-of-origin specific associations concordant with known parental expression patterns. Indeed, loss-of-function mutations in MKRN3 and DLK1 have been associated with CPP in several families, as described in the following section (14, 63). Subsequent studies in a normal population using expanded genomic analysis identified a rare variant in the MKRN3 5′ untranslated region (UTR) (rs530324840) and another variant in the MKRN3 locus (rs530324840), which had significant associations with the age of menarche (12).
In mice, MKRN3 expression is higher in the hypothalamus compared with cortical brain, liver, and testis before sexual maturation, and expression in the hypothalamus and brain declines with pubertal development, as in rats and female nonhuman primates (64). The decline of MKRN3 expression before sexual maturation in mice is independent of changes in sex steroids and leptin, indicating that MKRN3 regulation is upstream in the HPG axis (64, 65). MKRN3 is expressed in KNDy neurons and inhibits transcription of 2 important GnRH stimulators, KISS1 and TAC3, in vitro (64). Hypothalamic kisspeptins mRNA levels are maximum around the time of puberty in both male and female rats (66). Similarly, Tac3 expression also increases during pubertal development in the ARC in mouse, with the time course of the increase strikingly parallel to the decrease in MKRN3 expression (14, 67). Notably, MKRN3 did not alter promoter activity of prodynorphin (PDYN), the gene encoding the inhibitory peptide dynorphin, which is coexpressed in KNDy neurons. Taken together, these data point to an important role of MKRN3 in KNDy neurons, and in association with MKRN3 loss-of-function variants in precocious pubertal development in humans, indicates that MKRN3 is a component of the inhibitory network suppressing the HPG axis during childhood (14, 64).
MKRN3 is an E3 ubiquitin ligase and undergoes autoubiquitination (64). MKRN3 regulates GnRH1 transcription through ubiquitination of methyl-CpG-DNA binding protein 3, disrupting its binding to the GnRH1 promoter (68). In addition, ubiquitination of poly A-binding proteins by MRKN3 leads to shortening of the poly A tail length of GnRH1 mRNA, compromising the formation of the translation initiation complex (69). These data indicate that MKRN3 can control both transcriptional and posttranscriptional switches of pubertal initiation.
Little is known about specific genes that regulate male puberty. In boys, some BMI-increasing alleles have been shown to associate with earlier, and others with delayed, sexual development; these genetic results mimic the controversy in epidemiological studies, some of which show opposing correlations between prepubertal BMI and male puberty (70). Although the mechanisms regulating pubertal onset may largely be shared in males and females, the relationship between body mass and pubertal timing in boys may be more complex than previously thought and requires further genetic studies.
Ethnic Influences on Human Puberty
The timing of normal pubertal onset is proposed to be influenced by ethnicity, especially in girls. The first study designed to analyze this possibility was performed by Harlan et al (71) and published in 1980; indeed, despite the grand ethnic diversity found in this country, as well as others, the possible ethnic influence on puberty has only been seriously addressed in recent decades. The data of this seminal study (71) suggested that Afro-American girls enter puberty earlier and reach menarche at a younger age than Caucasian and Hispanic girls. It was not until 2002 that it was shown that non-Hispanic Black girls and boys were shown to mature earlier than Mexican American or non-Hispanic White children (72). The authors of this later study indicated that the national reference data in the United States for the timing of sexual maturation should take this information into consideration to appropriately interpretate the normal age of sexual maturation in US children. Despite few confirmatory studies (73, 74), it is generally accepted that African American girls enter pubertal development before Caucasian and Hispanic girls (75).
Unfortunately, there are limited data available regarding pubertal timing that takes ethnical diversity throughout the world into consideration. However, most pediatric endocrinologists would agree that the reported ethnical differences regarding pubertal timing are derived from a mixture of genetic, social, and environmental factors that are heterogenous within and between populations (75, 76), as well as the interaction between these different elements.
A recent systematic review and meta-analysis found that age at pubertal onset, with thelarche assessed by physical or clinical examination of the breast, decreased by a mean of almost 3 months per decade from 1977 to 2013 (77). Thus, in function of the criteria employed, the diagnosis of CPP will clearly be affected as well as the subsequent treatment (78).
Multiple Etiologies of CPP
CPP can result from congenital (genetic and nongenetic causes) or acquired CNS lesions (3). Patients with CPP can be classified as familial or sporadic cases, and as syndromic or nonsyndromic forms (3, 11). If the underlying causes are not identified, CPP is classified as idiopathic, the most prevalent form in the female sex (3). According to epidemiological studies, the prevalence of CPP is up to 23 girls for every boy (79). Progress in the genetic investigation of patients with familial or sporadic CPP, presently classified as idiopathic, will reduce the prevalence of the idiopathic form in both sexes in the future.
A refined etiological classification of several CPP causes is suggested in this review, aiming to explore the underlying putative mechanisms of the premature reactivation of the HPG axis (Table 1). We have divided the current causes into 2 major groups: congenital or acquired causes. Both groups are then divided into 2 other branches, characterized by the presence (or absence) of structural brain lesions. Another fundamental component of this classification involves the recognition of signs of syndromic features associated with CPP, characterized by a more complex developmental phenotype in the congenital causes (Table 2). The identification of familial CPP or gene alterations can be relevant in the diagnosis of a congenital disease, such as monogenic or syndromic CPP with distinct inheritable patterns that would promote an active surveillance of new cases in the same family.
Table 1.
Etiology of CPP: congenital and acquired causes with or without CNS lesions
| 1–Congenital | 2–Acquired |
|---|---|
| 1A–with CNS lesions | 2A–with CNS lesions |
| • Hypothalamic hamartoma (HH) | • Tumoral causes |
| • Neurofibromatosis type 1 (NF1) | Astrocytoma, ependymoma, pinealoma, hypothalamic or optic pathways glioma, craniopharyngioma, dysgerminoma (non-hCG secreting), meningioma |
| • Arachnoid cysts | • Post-insults |
| • Meningomyelocele/Chiari malformation type 2 | Neonatal infections, granulomatosis disease, cerebrovascular accidents, hydrocephalus, traumatic brain injury, cranial irradiation, encephalopathies |
| Other conditions: | • Cerebral palsy |
| • Hydrocephalus and/or meningomyelocele | |
| • Chiari type 1 malformation | |
| • Septo-optic dysplasia | |
| • Tuberous sclerosis complex | |
| • Duplication of the pituitary gland | |
| • Other rare syndromic forms of CPP | |
| 1B–without CNS lesions | 2B–without CNS lesions |
| • Gain-of-function mutations in the genes encoding kisspeptin (KISS1/KISS1R) | • Endocrine-disrupting chemicals |
| • Loss-of-function mutations in Makorin ring finger 3 (MKRN3) | • International adoption |
| • Loss-of-function mutations in delta-like-homolog type 1 (DLK1) | • Early exposure to sex steroids |
| • Syndromic CPP |
Table 2.
Genetic and epigenetic syndromes associated with central precocious puberty
| Disorder (OMIM) | Critical region | Main molecular diagnosis | Prevalence of CPP | Other main clinical features | Putative mechanism(s) or gene involved in CPP |
|---|---|---|---|---|---|
| Syndromic CPP without CNS lesions | |||||
| Temple syndrome (616222) | 14q32.2 | 1. UPD(14)mat 2. DLK1/MEG3:IG-DMR hypomethylation 3. 14q32.2 paternal deletion |
80%-90% | Prenatal and postnatal growth failure, hypotonia, small hands and/or feet, obesity, motor delay | DLK1 |
| Prader-Willi syndrome (176270) | 15q11-q13 | 1. 15q11-q13 paternal deletion 2. UPD(15)mat |
4% | Hypotonia, obesity, growth failure, cognitive disabilities, hypogonadism | MKRN3 |
| Silver-Russell syndrome (180860) |
11p15.5 | IGF2/H19:IG-DMR hypomethylation | Overall: 5-15% UPD(7)mat: likely higher prevalence |
Prenatal and postnatal growth retardation, relative macrocephaly, prominent forehead, body asymmetry, feeding difficulties | 11p15.5 defects: not established |
| Not established (chromosome 7) | UPD(7)mat | UPD(7)mat: possible imprinted or recessive factors to be elucidated | |||
| Williams-Beuren syndrome (194050) |
7q11.23 | Hemizygous 7q11.23 deletion | 3%-18% | Distinct face, cardiovascular disease, short stature, intellectual disability, hypersociability | Contiguous gene syndrome CPP mechanism remains unclear |
| Xp22.33 deletion (SHOX region) |
Xp22.33 | Xp22.33 deletion with pseudo-autosomal dominant inheritance, involving SHOX | Rare cases | SHOX phenotypes: body disproportion, short stature, Madelung deformity | CPP mechanism remains unclear |
| Xp11.23-p.11.22 duplication syndrome (300881) |
Xp11.23-p11.22 | Xp11.23-p11.22 duplication with X-linked dominant inheritance | Females: 70% Males: 11% |
Intellectual disability, speech delay, electroencephalogram abnormalities, excessive weight, skeletal anomalies | Contiguous gene syndrome. CPP mechanism remains unclear |
|
MECP2 defects (300005) |
Xq28 | Defects with X-linked dominant inheritance: 1. MECP2 loss-of-function mutations 2. Xq28 duplication involving MECP2 |
Rare cases of atypical Rett syndrome | Neurodevelopmental phenotypes, intellectual disability, autism | MECP2 |
| X-linked intellectual developmental disorder Snijders Blok type
(300958) |
Xp11.4 | X-linked dominant de novo mutations in DDX3X affecting females | Females: 13% | Intellectual disability, developmental delay, hypotonia, behavior problems, movement disorders, skin abnormalities | DDX3X |
| Kabuki syndrome (147920) |
12q13.12 | Loss-of-function mutations in KMT2D | Premature thelarche: 40% CPP: uncommon |
Neurodevelopmental phenotypes, typical distinct face, short stature, multiple anomalies | Possible downregulation of estrogenic receptor activation |
| Mucopolysaccharidosis type IIIA or Sanfilippo disease (252900) | 17q25.3 | Homozygous or compound heterozygous mutations in SGSH | Very rare (5 boys) | Severe neurologic deterioration, visceromegaly, skeletal abnormalities | Possible accumulation of glycosaminoglycans triggering GnRH |
| Rare cases of distinct copy number variants: 1p36 deletion (14), 9p distal deletion (15), and 9q34.3 duplication (including NOTCH1) (16). | |||||
| Syndromic CPP with CNS lesions | |||||
| Pallister-Hall syndrome (146510) |
7p.14.1 | Heterozygous pathogenic variant in GLI3 | Unknown | HH, mesoaxial polydactyly, panhypopituitarism, imperforate anus and other visceral anomalies | Hypothalamic hamartoma |
| Neurofibromatosis type 1 (162200) |
17q11.2 | Heterozygous pathogenic variant in NF1 | 72% | Multiple café au lait spots, axillary and inguinal freckling, multiple cutaneous neurofibromas, iris Lisch nodules, and choroidal freckling, learning disabilities, optic nerve and other central nervous system gliomas, malignant peripheral nerve sheath tumors, scoliosis, tibial dysplasia, and vasculopathy | Optic pathway glioma or hamartoma |
| Tuberous sclerosis complex (191100 and 613254) | 9q34 16p13 |
Loss-of-function mutation in TSC1 Loss-of-function mutation in TSC2 |
Rare | Seizures, intellectual disability, and facial angiofibromas | Giant cell astrocytoma or hypothalamic hamartoma |
Congenital Etiology of CPP
Congenital Causes With CNS Lesions
The most common congenital cause of CPP associated with CNS lesions in both sexes is hypothalamic hamartoma (HH), which is implicated in early HPG axis reactivation by several putative mechanisms. A systematic review and meta-analysis including 15 studies and 1853 girls identified 9% of CPP patients with CNS alterations in MRI, with HH representing 40% of these lesions (80).
The overall prevalence of intracranial pathology (congenital and acquired) associated with CPP range from 0% to 24.3% in girls, and up to 74% in boys, with congenital causes representing the greatest prevalence in both sexes (80–82). If considering only girls aged <6 years, the prevalence of intracranial pathology increases up to 29% (80). The prevalence of CNS lesions in boys with CPP was revised in distinct ethnical groups. A Turkish study including 100 boys with CPP identified only 26% with CNS lesions, indicating that the number of idiopathic male CPP cases is increasing over time (83). In addition, Chinese and Taiwan studies demonstrated a low prevalence of lesions in CPP male cohorts (16.3% and 7%, respectively) (84, 85). More recently, a high prevalence (40%) of monogenic causes (loss-of-function MKRN3 mutations) were found in 20 boys with familial CPP without CNS lesions, suggesting the importance of genetic analysis in males (86).
There is a lack of consensus whether some congenital abnormalities, such as Rathke cleft cysts, Chiari malformation, and pineal and arachnoid cysts, are definitive causes of CPP. Some investigators considered them as incidental findings (87), whereas others as potential causes of CPP based on epidemiological evidence (88). CNS lesions with questionable relationship with CPP have been counted in the prevalence data of some studies, impairing the estimation of the real prevalence of pathological CNS lesions associated with CPP (80, 89, 90).
Prevalence of incidental findings in MRI studies of patients with CPP are quite variable (ranging from 2.7% to 9.6%) and include distinct conditions, such as pineal cysts, pituitary microadenomas, pituitary enlargement, pituitary asymmetry, absent septum pellucidum, variation of perivascular space (normal), nonspecific white matter lesion, and hyperintense thalamic lesions (87). Of note, pineal cysts, classified as an incidental finding in CPP patients in several studies (80, 87, 89), are usually asymptomatic with a prevalence of 1.9% in the childhood population (88).
Hypothalamic Hamartoma
HH are rare nonneoplastic benign lesions constituted of ectopic hypothalamic tissue typically located at the base of the cranium, under the third ventricle, in proximity to the tuber cinereum and the mammillary bodies (91–93). They are usually small lesions, measuring between 0.5 and 2 cm in diameter, which as a rule remain unchanged over time. HH can occur as an isolated lesion or as part of a syndrome. Clinically, HH can be asymptomatic and if symptomatic both endocrine and neurological manifestations can be present with a heterogeneous spectrum. In this hypothalamic malformation, CPP manifests at a very early chronological age (mean age of 2.5 years in girls and 3.7 years in boys) (91).
Neurological symptoms associated with HH characteristically include gelastic (brief spells of laughter) or dacrystic (crying) seizures, usually presenting during infancy, with later occurrence of different seizure types, such as focal and generalized seizures associated with an epileptic encephalopathy. Developmental delay, loss of acquired developmental milestones, and behavioral disturbances frequently accompany the more severe scenario (94).
The anatomy of the HH as revealed by MRI is predictive of the clinical syndrome and it is appropriate to consider 2 recognized clinicopathological subtypes: HH that are functionally connected to the pituitary stalk and tuber cinereum can cause CPP, whereas those functionally connected to the region of the mammillary bodies and limbic circuit result in epilepsy (91).
The potential mechanisms for the formation of HH as well as the CPP caused by them are not completely known (95). Considered as a model for studying the onset of puberty, HH can accelerate sexual development by producing bioactive substances that mimic a cascade of events that trigger the onset of puberty (96). It has also been suggested that HH that manifest CPP contains intracellular signaling networks and transcription factors essential for pulsatile secretion of GnRH. Both the presence of GnRH-secreting neurons within the HH, as regulatory neurons connected to GnRH neurons in the HH or to hypothalamic neuronal networks, including astrocytic and ependymal cells in the HH tissue, may be necessary for the physiopathological condition of HH-related CPP (95, 96). It is postulated that CNS developmental abnormalities that result in the formation of HH originate from sporadic defects that affect genes of morphogenic pathways involved in the embryonic development of the ventral hypothalamus and third ventricle (97).
Through immunohistochemical studies, the detection of the presence of GnRH-secreting neurons in some HH led to the concept that HH advances puberty by functioning as an ectopic GnRH-releasing pulse generator (95, 96). The study of 2 HH associated with CPP in females revealed that neither of them contained GnRH neurons, but astroglial cells expressing TGF-α and its erbB-1 receptor (96). TGF-α is a member of the epidermal growth factor family that mediates the facilitating effect that glial cells exert on GnRH neurons. Therefore, some HH can induce CPP, not through the secretion of GnRH, but through the synthesis of trophic factors, such as TGF-α, capable of activating a normal neuronal network of GnRH (96, 97). Interestingly, most of the resected HH tissue from subjects with or without a history of CPP did not express kisspeptins or mRNA of KISS1R (98).
The GnRH-secreting neurons in the mediobasal hypothalamus receive abundant GABAergic innervation (91, 99) and those with a wide projection in HH tissue are innervated by GABAergic interneurons. In contrast to the classical inhibitory GABAergic effect on mature GnRH neurons, in immature cells GABA promotes GnRH release, as demonstrated by electrophysiological and pharmacological studies in animal models (100). This suggests the hypothesis that the pulsatile release of GnRH could be related to the GABA excitatory activity in HH (100–102). A schematic representation of the potential mechanisms of CPP caused by HH is presented in Fig. 2.
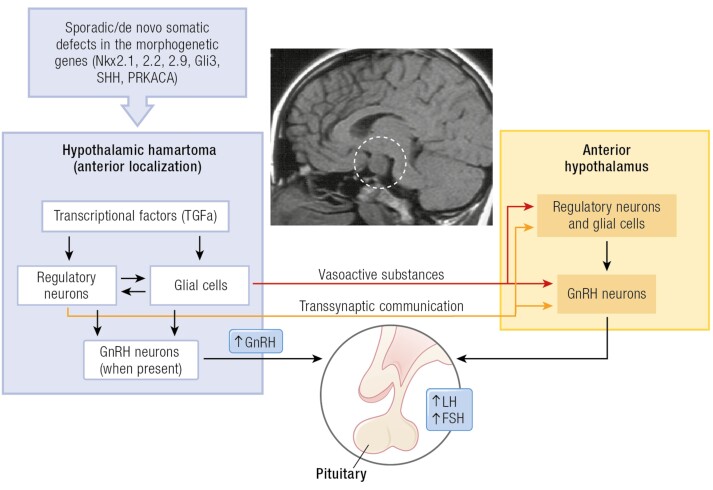
Figure 2.
Schematic pathophysiology of hypothalamic hamartoma-related CPP. Sagittal MRI view revealing a large parahypothalamic hamartoma (dotted white circle) attached to anterior hypothalamus as cause of CPP. The HH formation may be determined by sporadic/de novo somatic mutations in genes required for hypothalamic morphogenesis. Several genes, most of them encoding proteins of the Sonic hedgehog (SHH) pathway, have been implicated in the development of syndromic and nonsyndromic hypothalamic hamartoma (HH). The underlying mechanisms leading the ability of HHs to activate GnRH secretion and induce CPP are multiple, including autonomous secretion of GnRH by HH, trans synaptic activation, via myelinated fibers, connecting the HH to the hypothalamus, and the secretion of glial products and bioactive substances (TGF-α and downstream factors) capable of stimulating a neuronal network involved in the GnRH secretion. In addition, the effect of the mechanical pressure that HH could apply to the hypothalamus could represent another potential mechanism. There is evidence that the anatomical position (anterior localization) of HH has a pivotal role for the occurrence of CPP.
Considering that HH is the consequence of a defect in the normal embryonic hypothalamic developmental process, that germline mutations in GLI3 constitute the genetic basis of Pallister-Hall syndrome, which includes HH, and that Gli3 is a regulatory protein of the Sonic Hedgehog (SHH) morphogenetic pathway, it was hypothesized that defects in genes encoding other proteins of the SHH pathway, such as PRKACA, SMO, CREBBP, and GLI2, could be the genetic basis of nonsyndromic HH (103). The SHH signaling pathway plays an essential role during vertebrate embryonic development and in tumorigenesis (104).
Using a candidate gene study approach, Saitsu et al (105) studied peripheral leukocyte and HH tissue DNA of 18 patients with HH (5 patients with additional syndromic features). Inactivating somatic mutations in 2 genes, GLI3 and OFD1, were identified in Pallister-Hall syndrome and type I orofacial-digital syndrome, respectively (105). In this study, 2 other candidate genes, UBR5 and ZNF263, were proposed (105).
The multicenter study by Hildebrand et al (103) included 38 cases with HH and gelastic epilepsy, of whom 24 had intellectual deficit and 15 had CPP without other syndromic signs. Fourteen somatic defects (4 nonsense mutations, 2 frameshift mutations, 1 insertion, and 7 copy number variants [CNVs] or loss of heterozygosity) in genes involved in the regulation of the SHH pathway were identified in 14 of 38 (37%) patients (103). Four patients with somatic point mutations in GLI3, 1 patient with a CNV in GLI3 and 3 patients with somatic mutations in PRKACA represented the most relevant findings of this study. These data implicate defects in the SHH pathway in the pathogenesis of HH associated with epileptic syndrome. Furthermore, PRKACA is currently associated with the pathogenesis of HH (103). However, the patients who manifested CPP were not analyzed separately, and consequently the potential mechanisms involved in this endocrine manifestation were not discussed. Recently, novel pathogenic germline or somatic variants in DYNC2H1, KIAA0556, and PTPN11 genes were identified in patients with HH-related epilepsy, with or without CPP, suggesting that disruption of the SHH signaling pathway associated with cilia or the RAS/MAPK pathway may lead to the development of HH (106).
Neurofibromatosis type 1 (NF1)
NF1 is an autosomal dominant multisystemic neurocutaneous disorder characterized by increased risk of benign and malignant tumor formation affecting primarily skin, bone, and the CNS. NF1 is caused by loss-of-function mutations in the NF1 tumor suppressor gene located on chromosome 17q11.2 (107, 108), which encodes the neurofibromin protein. Comprising more than 2800 amino acids (~220 KDa), neurofibromin contains a small domain (280–300 amino acids) that is structurally and functionally similar to a family of proteins that function as negative RAS regulators (109). Because increased RAS activation is associated with numerous human cancers (110), individuals with NF1 are predisposed to a range of tumors affecting the central and peripheral nervous systems, including optic pathways glioma (OPG), which are a source of significant morbidity in this population (111).
CPP has been reported primarily in NF1 children with OPG with an estimated prevalence of 3% (112). This observation is consistent with the theory that lesions located close to the hypothalamus interfere with tonic CNS inhibition of the HPG axis, resulting in the premature onset of puberty. In several studies, CPP developed exclusively in those patients with NF1 who had OPG involving the optic chiasm (113–117). However, CPP in the NF1 population in the absence of OPG has been described by some authors (118). It was hypothesized that mild cerebral abnormalities, undetectable with MRI, such as slow-growing hamartomas, may lead to CPP in NF1 children without OPG (112). Considering that neurofibromin is part of a signal transduction chain extending from extracellular signals to transcriptional regulation in the nucleus, an abnormal signal transmission pathway could represent a potential underlying mechanism of tumorigenesis (119). Almost all NF1-OPGs) are benign pilocytic astrocytomas (World Health Organization grade I astrocytomas) that can arise anywhere along the optic pathway, including the optic nerves, optic chiasm, optic tracts, and optic radiations (111, 120). However, in individuals with NF1, the majority (75%–85%) of OPGs are located within the optic nerve and chiasm (prechiasmal or anterior optic pathway), with a smaller proportion of tumors located in the optic tracts and radiations (postchiasmal or posterior optic pathway). NF1-OPGs occur most frequently in young children (median age at diagnosis, 4.5 years) (115, 121), with rare cases described in older adolescents (122, 123). Notably, some patients may have both conditions during follow-up, evolving from precocious to delayed/absent puberty (124).
The latter evidence suggests that continued monitoring of individuals with NF1 into adulthood for the development of OPGs and for progression of known OPGs is warranted.
Other CNS Conditions
Other congenital lesions associated with CPP are suprasellar arachnoid cysts, hydrocephalus, tuberous sclerosis, septo-optic dysplasia, Chiari II malformations, and myelomeningocele. Intracranial arachnoid cysts are benign, nongenetic developmental cavities that contain clear secretions similar in nature to cerebrospinal fluid and are completely situated within the arachnoid membrane. Arachnoid cysts are relatively rare, usually congenital, but may also arise after an infection, trauma, or hemorrhage (88). Suprasellar arachnoid cysts account for approximately 10% of all arachnoid cysts and occur almost exclusively in children, somewhat more frequently in boys than in girls. Neurological and visual field abnormalities have been reported in 25% to 85% of patients with suprasellar arachnoid cysts, as well as diverse endocrinological disorders, most notable CPP in 10% to 40% of these children (88, 125, 126). Arachnoid cysts may cause a wide spectrum of endocrinological disorders such as deficiencies of GH, TSH-releasing hormone, and ACTH, but stimulates the HPG axis leading to CPP. These hormone disorders are all due to the proximity of the cyst to the hypothalamic-pituitary area, but their direct mechanisms are unknown (88, 127).
Meningomyelocele, the commonest type of spina bifida, occurs because of abnormal development of the neural tube and manifests as failure of the complete fusion of posterior arches of the spinal column, leading to dysplastic growth of the spinal cord and meninges. Children with meningomyelocele have an increased incidence of CPP (128). Epidemiological studies showed that the prevalence of CPP in girls with meningomyelocele is around 50% and ranges from 10% to 30% in boys (129–131). Although the exact causative mechanism of CPP in children with meningomyelocele is unknown, several studies demonstrated an association with hydrocephalus, which may alter HPG axis function. Additionally, evidence shows that increased perinatal intracranial pressure and brainstem malformations, such as Chiari II malformations, which involves both the cerebellum and brain stem tissue pushing into the foramen magnum, are influential prognostic factors for the development of CPP (128).
Type I Chiari malformation is a disorder characterized by a displacement of the cerebellar tonsils through the foramen magnum into the upper cervical spinal canal without myelomeningocele. Patients with type II Chiari malformation with meningomyelocele can frequently present with CPP, whereas very rare reports show an association between type I Chiari malformation and early puberty (132). The development of CPP in patients with type I Chiari malformation was associated with an increase in peri- and postnatal intracranial pressure because of impaired cerebrospinal fluid circulation or compression and distortion of the hypothalamus (133).
Septo-optic dysplasia is a heterogeneous congenital condition defined by the presence of 2 or more features of the triad composed by optic nerve hypoplasia, multiple hypothalamic pituitary deficits, and midline brain defects. The midline brain developmental insult in this congenital disorder starts early (between the fifth and eighth gestational weeks) damaging the arrival of GnRH neurons in the hypothalamus that normally occurs by week 13. Pathogenic variants in the genes HESX1, SOX2, SOX3, and OTX2 that encode essential factors for normal forebrain and pituitary development were identified in patients with septo-optic dysplasia (134). Regarding pubertal development, patients with this dysplasia can more frequently have delayed puberty. However, CPP has been described in 7% of a cohort of 171 patients with septo-optic dysplasia (135). A potential mechanism to explain the premature HPG activation includes the abnormal hypothalamic-pituitary anatomy that may alter the normal suppression of GnRH neurons, leading to earlier onset of pituitary gonadotropin secretion (135, 136).
Tuberous sclerosis complex (TSC) is a relatively rare autosomal dominant neurocutaneous disorder secondary to mutations in the TSC1 or TSC2 tumor suppressor genes, which code for the tumor suppressor proteins hamartin and tuberin, respectively (137). The classic triad of this complex disease includes seizures, intellectual disability, and facial angiofibroma. In addition, patients with TSC are at risk of developing multiple benign and malignant tumors in various organ systems (skin, brain/nervous system, kidneys, heart, and lung), resulting in increased morbidity and mortality. Tuberous sclerosis is exceptionally revealed by CPP, and rare cases have been described associated with CNS tumors, such as giant cell astrocytoma or hypothalamic hamartoma and periventricular calcified lesions (138, 139).
Duplication of the pituitary gland is an extremely rare developmental anomaly (140). It may be associated with other midline malformations such as facial anomalies (median cleft lip, median cleft face syndromes, and hypertelorism), vertebral malformations, nasopharyngeal teratoma, and other CNS abnormalities, such as the agenesis of the corpus callosum, posterior fossa abnormalities, the absence of the olfactory bulbs and tracts, the absence of the anterior commissure, and anatomic variations of the circle of Willis. When all these anomalies are present, they are called duplication of pituitary gland-plus syndrome. The hypothalamus and pituitary involvement may be clinically associated with pubertal disorders, such as precocious or delayed puberty (141). The exact mechanism responsible for the early increase in frequency and amplitude of GnRH pulses causing precocious puberty in these patients is still unknown. Burke et al (142) proposed that the same development disorder leading to pituitary duplication may also contribute to the precocious secretion of GnRH from nuclear derangement and failure of regulation of GnRH neurons.
Congenital Causes Without CNS lesions
Gain-of-function Mutations in the Genes Encoding Kisspeptins (KISS1-KISS1R)
The important role of the kisspeptins system in GnRH regulation and the identification of mutations in KISS1 and KISS1R associated with congenital hypogonadotropic hypogonadism encouraged researchers to investigate if activation of this system would result in CPP. However, only 1 rare variant in KISS1 and 1 in KISS1R have been reported in patients with CPP. The heterozygous activating mutation of KISS1R (p.Arg386Pro) was identified in association with CPP in 2008 (143). This mutation was identified in an adopted girl who had progressive thelarche from birth, suggesting early, persistent, and slightly increased estrogen secretion. Accelerated growth, skeletal maturation, and progression of breast development were noted at age 7 years. In vitro, the p.Arg386Pro mutation, located in the C-terminal tail of the receptor, led to prolonged activation of intracellular signaling pathways by kisspeptins, resulting in higher and more sustained inositol phosphate accumulation from decreasing KISS1R degradation (143, 144). Subsequently, 1 rare kisspeptins variant, p.Pro74Ser, was identified in the heterozygous state in a boy who developed sporadic CPP at age 1 year, with high concentrations of basal LH and testosterone (145). In vitro studies showed that the capacity to stimulate signal transduction was significantly greater for p.Pro74Ser than for the wild type, suggesting that this variant might be more resistant to degradation, resulting in greater kisspeptin bioavailability (145). His mother and maternal grandmother, both of whom had normal pubertal development, also carried the p.Pro74Ser mutation in the heterozygous state, suggesting incomplete, sex-dependent penetrance. Rhie et al (146) identified polymorphisms that were more frequently seen in patients with CPP and large populational analysis (147) identified a variant close to KISS1 associated with age at menarche in the normal population without CPP but the association of these variants with pubertal onset still needs to be validated. Although mutations in KISS1 and KISS1R are rarely associated with CPP, this system plays a crucial role in GnRH regulation and has a great potential for target therapies.
Loss-of-function Mutations in Makorin Ring Finger 3 (MKRN3)
Despite years of research trying to identify a genetic cause of CPP using a candidate gene approach, only rare mutations had been identified (143, 145, 148, 149). In 2013, an unbiased approach using exome sequencing analysis in multiple families with CPP identified the association of mutations in MKRN3 with familial CPP (14). In this first report of mutations of MKRN3, 4 deleterious mutations—3 frameshift and 1 missense—were detected in 5 of 15 families (33%) with several members with CPP. After this first report of MKRN3 mutations in familial cases of CPP, mutations in MKRN3 were also identified in patients without a known family history of CPP (150). MKRN3 mutations are now the most commonly known genetic defect associated with CPP, with an overall frequency of ~10%; the frequency of mutations in MKRN3 is higher in cases of familial CPP, ~33% to 46% (14, 151, 152).
MKRN3 is a member of the Makorin protein family (153, 154). The MKRN3 protein has a centrally located RING finger motif (C3HC4), 2 amino-terminal C3H zinc finger motifs followed by a Makorin zinc finger motif unique to the Makorin protein family, and a carboxy-terminal C3H zinc finger motif (Fig. 3) (153). C3H zinc-finger motifs have been implicated in mRNA binding, whereas the RING zinc-finger motif is responsible for E3 ubiquitin ligase activity (155). These several domains suggest that MKRN3 has multiple actions. MKRN3 is highly conserved among species, and the mouse and human MKRN3 amino acid sequences share 69% identity and 82% similarity (153). Mice and humans usually do not have conserved UTRs, yet the MKRN3 3′-UTR has 90% identity between these 2 species, suggesting a functional significance to this region of the MKRN3 gene (153). Indeed, the conserved sequence includes 2 ATTTA motifs, a binding site for miR-30, which is a repressor microRNA. miR-30 is expressed in KNDy neurons and is the first element shown to regulate MKRN3 expression (156).
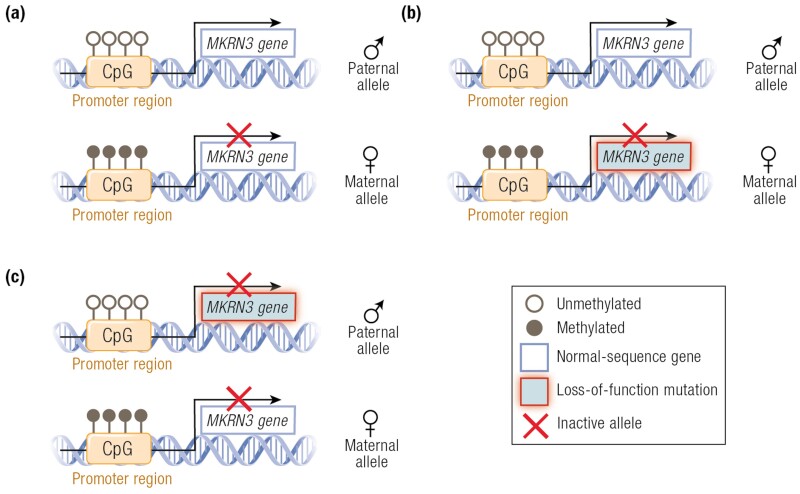
Figure 3.
Representation of the 2 autosomal alleles of the imprinted MKRN3 gene and distinct genotypes of affected and unaffected CPP individuals. (A) Normal pattern of imprinting of MKRN3 gene, with silencing of the maternal allele (by methylation of its promoter region) and monoallelic expression of the paternal allele. (B) The genotype of an individual who inherited a maternal loss-of-function mutation. The individual will not present with CPP because the paternal allele is normally expressed. (C) The genotype of an individual who inherited a paternal loss-of-function mutation. The individual will present with CPP because both alleles are inactive: the paternal is mutated and the maternal is silenced. Adapted from Canton et al (157).
MKRN3 also interacts with and suppresses the activity of Nptx1, a secreted protein important for neuron development (158). The RING finger domain of MKRN3 is essential for binding with and for polyubiquitination of Nptx1 during puberty initiation (158). Missense mutations in MKRN3 identified in patients with CPP, encoding p.Cys340Gly and p.Arg365Ser, located in the RING finger domain, and p.Phe417Ile and p.His420Gln, located in the C-terminal zinc finger domain, resulted in loss of the ability to inhibit GnRH1 promoter activity (68). The 2 RING finger MKRN3 mutants also lost the ability to inhibit KISS1 and TAC3 promoter activity (64). These data not only confirm that these mutations result in loss of function of MKRN3 but also implicate the RING finger ubiquitin ligase domain as an important functional domain of MKRN3.
MKRN3 mutations in association with CPP have been described in more than 150 patients from many countries (13–15, 86, 150, 152, 159–176). However, MKRN3 mutations were not identified in a subgroup of 260 Korean girls with familial CPP (172) and a lower frequency of mutations in familial cases was also described in other series from Italy and Turkey (8.7% and 5.3%, respectively) (170, 176).
MKRN3 is located on the long arm of chromosome 15, in a region with a cluster of imprinted genes. Some of these genes are expressed only from the paternally inherited allele and are associated with Prader-Willi syndrome (PWS), whereas others are only maternally expressed and associated with Angelman syndrome (153, 154). MKRN3 is included in the cluster of genes with exclusive paternal allele expression. The schematic representation of the 2 autosomal alleles of the imprinted MKRN3 gene and distinct genotypes of affected and nonaffected CPP individuals is shown in Fig. 3 (157). Studies have shown that MKRN3 deletion is neither required nor responsible for the PWS phenotype, with several cases of PWS presenting without deletion of MKRN3 (177–179). Moreover, the inclusion of MKRN3 within the deletion causing PWS does not predict the pubertal phenotype because most patients with PWS with deletions including MKRN3 do not develop CPP (177, 180, 181). Kanber et al (178) reported a patient presenting with signs of PWS—obesity, high pain threshold, and developmental delay—with an unbalanced translocation resulting in the deletion of MKRN3, MAGEL2, and NDN genes. After further phenotypic evaluation, the patient was determined to have CPP but not PWS. To date, at least 16 additional cases have been reported with PWS and CPP (182–184). The early pubertal onset in these patients is likely from loss of MKRN3. It is important to highlight that the CPP phenotype may not be documented in patients with PWS, who have other clinical features that can obscure detection of early pubertal development, especially in boys given the subtlety of the first signs of pubertal onset. It is also worth noting that hypogonadism, either primary or secondary, is a major feature of PWS. Even with early reactivation of GnRH secretion, the lack of precocious pubertal development in these patients may be due to the associated inability to secrete gonadotropins or sex steroids due to concomitant hypogonadism.
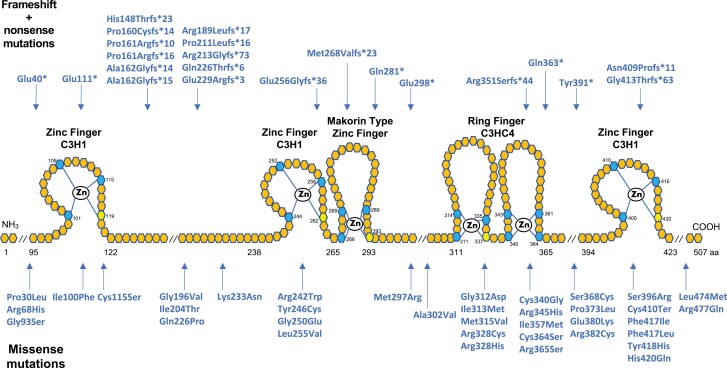
To date, 59 inactivating mutations in the coding sequence of MKRN3 have been described, including 6 nonsense, 16 frameshift, and 37 missense mutations (Fig. 4). Missense mutations account for 63% of MKRN3 mutations, frameshift for 27%, and nonsense for 10%. Of the 59 mutations, 15 were found within sequences encoding the 3 C3H1 zinc finger motifs, which have RNA binding activity, and 2 mutations were found in the Makorin type zinc finger domain. Twelve mutations were found in the RING finger C3HC4 domain, responsible for E3 ubiquitin ligase activity. Of these, 10 were missense mutations. Thirty (51%) mutations were outside of these domains: 11 were frameshift, 3 nonsense, and 16 missense mutations. Notably, the 11 frameshift mutations occurred between the first 2 C3H1 domains, highlighting an area susceptible to pathogenic frameshift mutations.
Figure 4.
Schematic representation of MKRN3 protein structure and location of loss-of-function mutations identified in patients with CPP. Hexagons represent individual amino acids, and corresponding numbers indicate amino acid positions. Top row mutations are frameshift and nonsense, whereas bottom row are missense mutations. Blue and yellow hexagons represent key cysteine and histidine amino acids, respectively, necessary for zinc ion interaction. RING finger C3HC4 is a protein-binding domain responsible for ubiquitin ligase activity. Zinc finger C3H1 are RNA binding domains. Makorin type Zinc finger is a specific Cys–His domain identified in the proteins of the makorin family. Notably, 15 mutations (27%) were detected between the first 2 C3H1 domains, 11 of which are frameshift. Mutations also tend to cluster within the C3HC4 RING finger domain (20%), the vast majority of which are missense.
The most prevalent MKRN3 mutation, identified in at least 34 patients, is an indel variant affecting a poly-C region at cDNA positions 475-481 (7 cytosines), which results in a frameshift mutation with a premature stop codon (150, 161). The resulting amino acid change is either at Pro160, Pro161, or Ala162 (159). Figure 4 shows the varying nomenclatures describing the mutations reported in this hotspot region (159).
Two unrelated girls with nonsyndromic CPP were found to have heterozygous whole gene deletion of MKRN3. One had a 584-kb deletion (GRCh37/hg19 chr 15:23 798 088-24 382 443) at 15q11.2 involving MKRN3, MAGEL2, and NDN genes (185). Consistent with the imprinted and paternal expression of MKRN3, both deletions were confirmed to be paternally inherited. The 2 patients had no family history of CPP and had no symptoms suggestive of PWS. One patient had a BMI in the 75th percentile; the other patient, with a larger deletion, had a BMI in the 99th percentile for her age and both had a family history of obesity. Given the prevalence of obesity, it is difficult to draw conclusions about the relevance of the deletion to this phenotype. An increase in obesity in patients with nonsyndromic CPP from MKRN3 mutations has not been detected, compared with those with CPP without MKRN3 mutations (151, 159). Premature ovarian failure was reported in the grandmother of an individual with CPP harboring an MKRN3 mutation (168). Additional dysmorphisms were rare and included esotropia detected in 2 affected siblings, and clinodactyly and lumbar hyper lordosis in 2 unrelated girls, one of whom also exhibited a high-arched palate and dental abnormalities (150, 168). All these findings could have been incidental.
Patients with CPP carrying loss-of-function mutations in MKRN3 exhibited typical clinical and hormonal features of premature activation of the HPG axis, including early pubertal signs, such as breast development, testis enlargement, accelerated linear growth, advanced bone age, and elevated basal and/or GnRH-stimulated LH levels. Most studies reported no significant differences in clinical and laboratory features of patients with CPP with or without MKRN3 mutation (151, 186). However, Simon et al (152) described that girls with MKRN3 mutations were younger at puberty onset than those without MKRN3 mutations. Seraphim et al (159) showed an earlier age at diagnosis in female patients with CPP associated with MKRN3 mutations, when compared with girls with idiopathic CPP. The earlier identification of pubertal onset likely resulted from family awareness of the diagnosis of CPP because of the higher frequency of familial cases in the cohort (159). Because CPP is significantly more frequent in girls, the number of boys with MKRN3 mutations is smaller, making it difficult to draw associations. Bessa et al (86) showed a higher frequency of MKRN3 mutations in boys with CPP and no CNS lesions than in girls. It has been proposed that boys have a smaller advance in the timing of puberty onset compared with girls (173, 176). However, this may be due to difficulties in identifying testicular enlargement, the first clinical evident sign of pubertal onset in boys. In fact, most boys included in these studies were diagnosed based on family history, many later in their pubertal development, highlighting the challenges of diagnosis of CPP in boys. Two studies with large patient sample sizes described higher basal FSH levels at the time of the diagnosis in girls with CPP with MKRN3 mutations compared with CPP girls without MKRN3 mutations (150, 159). It is unclear why higher FSH levels were found in patients with CPP and MKRN3 mutations; the difference might be attributable to the impact of different frequencies of pulsatile GnRH release on gonadotropin secretion (150).
In a study by Seraphim et al (159), significantly greater bone age advancement and higher basal LH levels were found in patients harboring frameshift, stop codon, and promoter region mutations in MKRN3, compared with those harboring missense MKRN3 mutations. The response to GnRH analogue (leuprolide acetate) treatment in patients with CPP with MKRN3 mutations was assessed in 11 girls treated for a mean time of 2.9 years. These girls reached expected family height similarly to girls with CPP without MKRN3 mutations, demonstrating the efficacy of GnRH analogues in preserving genetic adult height potential, regardless of the etiology of CPP (186). This study also showed that the prevalence of metabolic and reproductive disorders was similar in patients with CPP because of MKRN3 mutations compared to those with idiopathic CPP.
In summary, MKRN3 is the first gene with loss-of-function mutations identified in humans with an inhibitory effect on GnRH secretion. It is also the first imprinted gene associated with non-syndromic CPP.
Loss-of-function Mutations in DLK1
DLK1, also known as preadipocyte factor 1 or fetal antigen 1 is a key element of the Notch signaling pathway and several other intracellular pathways. It is widely expressed in different tissues during embryonic development, including adipocytes and muscular tissue; however, at postnatal life in humans, the expression is highest in endocrine glands, mainly in adrenals, pituitary, pancreas, and gonads (187).
In 2017, Dauber et al (63) described a complex defect of DLK1 (~14-kb deletion encompassing the first exon and 269-bp duplication) in a large multigenerational family with CPP. The DLK1 mutation was identified in all 5 patients through an innovative approach, including linkage analysis followed by whole-genome sequencing (63). The CPP phenotype was only expressed when the mutant gene was inherited from the father, in an inheritance compatible with the imprinting pattern of DLK1 (ie, the maternal allele is silenced). It is notable that DLK1 is located in a cluster of imprinted genes at chromosome 14q32.2, which has been associated with Temple syndrome (188, 189).
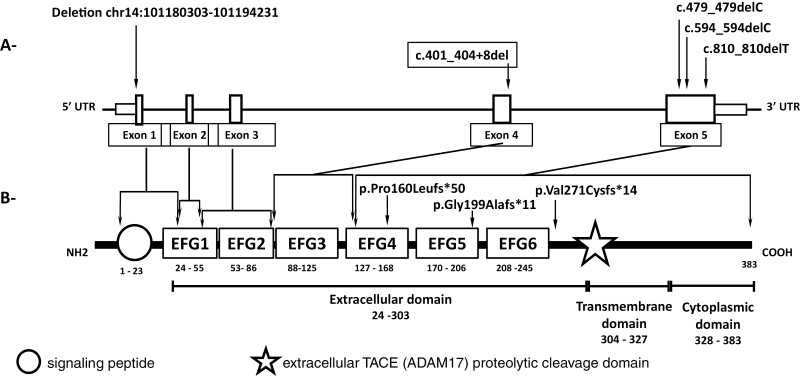
Temple syndrome has several distinct clinical features, such as short stature, small hands and feet, truncal obesity, and, interestingly, early onset of puberty, which has been described in 80% to 90% of affected subjects (189, 190). Notably, the deletion of DLK1 described by Dauber et al (63) led to CPP and a higher prevalence of metabolic syndrome, without the other syndromic features. Confirming the importance of this monogenic cause of CPP, Gomes et al (191) performed DLK1 sequencing analysis in 60 patients with either CPP or a history of precocious menarche. Three distinct heterozygous frameshift mutations in exon 5 of DLK1 (p.Gly199Alafs*11, p.Val271Cysfs*14, and p.Pro160Leufs*50) were identified in 5 patients from 3 unrelated families. The metabolic phenotype became more evident as the number of affected women with DLK1 deficiency increased: overall the group had more obesity/overweight (60%), insulin resistance (70%), type 2 diabetes (30%), and high cholesterol levels (50%) when compared with a paired idiopathic CPP cohort of 20 females (191). It is noteworthy that the extracellular soluble portion of DLK1 can be quantified in the serum, using a commercially available soluble DLK1 ELISA. In both studies, serum DLK1 levels were measured in the affected subjects and in controls, and were undetectable in all affected subjects, confirming the loss of gene function (63, 191). In 2020, a collaborative study between Brazilian and Spanish centers identified a novel heterozygous de novo deletion in exon 4 of DLK1 (c.401_404 + 8del) in a girl with sporadic CPP who had undetectable serum DLK1 levels (192). The schematic representation of the human DLK1 gene and protein is shown in Fig. 5.
Figure 5.
Schematic representation of the human DLK1 gene (A) and protein (B), respectively. (A) Human DLK1 gene (transcript ENST00000341267.9): clear blue boxes indicate the coding sequences (5 exons) of the gene. The localization of the allelic variants identified in familial CPP (63, 191) are indicated by arrows.(B) Human DLK1 protein structure (P80370): the orange circle indicates the signaling peptide; dark blue boxes indicate the six EGF-like repeats. Blue star indicates the extracellular TACE (ADAM17) proteolytic cleavage domain. Purple boxes indicate the region of the protein affected by the mutation in DLK1. The numbers represent the amino acid positions of the indicated domain. EGF: epidermal growth fator. Adapted from Montenegro et al. (192).
Notch signaling is a highly preserved signaling pathway that consists in the interaction of a notch receptor and a Notch ligand (193). In mammals there are 4 types of notch receptors (ie, NOTCH1, NOTCH2, NOTCH3, and NOTCH4), all of which have a single transmembrane passage. When the extracellular domain interacts with a notch ligand from another cell (ie, trans interaction), a series of enzymatic cleavages (ADAM/TACE and γ-secretase) results in the release of the intracellular domain, which acts in the nucleus of the receiving cell (the cell with the NOTCH receptor). The canonical ligands are characterized by a DSL domain (delta, serrate, and lag2); however, a large number of noncanonical ligands exist, among which is the DLK1 (193). Although most ligands elicit the series of activating events previously described, DLK1 has an inhibitory role on notch signaling. It usually interacts with NOTCH receptors in “cis” (that is, the receptor and the DLK1 are in the same cell, and not in different cells) or through its cleaved extracellular domain in an almost paracrine way. These types of interaction do not generate the force necessary to expose the NOTCH receptor extracellular domain to cleavage. Therefore, the DLK1 represents an inhibitor of Notch signaling (159).
Beyond the identification of loss-of-function mutations in DLK1 in patients with familial CPP, further evidence has pointed to a role of DLK1 in the regulation of pubertal timing. First, genome-wide association studies had implicated DLK1 as 1 of the 3 imprinted loci associated with the age at menarche in a large cohort of healthy women of European ancestry (147). Second, albeit a widespread expression during embryonic development, postnatal Dlk1 mRNA and protein expression is restricted to some endocrine tissues such as the pituitary, adrenal gland, pancreas, testes, prostate, ovaries, and a subset of neurons in the central nervous system (194). Immunohistochemistry in the mouse hypothalamus revealed neuronal Dlk1 expression in the suprachiasmatic, supraoptic, paraventricular, lateral, dorsomedial, and arcuate nuclei (195). Indeed, Dlk1- producing neurons are particularly abundant in the hypothalamic arcuate nucleus, a main site for the neuroendocrine control of puberty, especially for harboring kisspeptin neurons (196). Moreover, Dauber et al (63) demonstrated Dlk1 mRNA expression in immortalized AVPV- and arcuate-specific neuronal kisspeptins cell lines. Third, 2 Dlk1 mRNA isoforms have been described in the hypothalamus: 1 translated into the full-length protein and a second form that produces a shorter protein of 30 kDa composed only of the extracellular domain of Dlk1 (195). Interestingly, the 30-kDa soluble and biologically active form predominates in the hypothalamus (195). In addition, Dlk1 has been shown to act as an inhibitor of Notch signaling, and Rbpjκ-dependent Notch signaling regulates progenitor maintenance and differentiation of hypothalamic arcuate neurons (197). Biehl et al (198) have shown that manipulating Rbpjκ-dependent Notch signaling affects kisspeptin neuronal development. Although the precise mechanisms by which Dlk1 blocks puberty remain unknown, taken together these data advocate for a potential function of Dlk1 on the regulation of the reproductive axis (198).
It is well documented that pubertal activation of the reproductive axis and maintenance of fertility are critically dependent on the body’s energy reserves and metabolic status (199). DLK1 has been implicated in several aspects of energy metabolism, including its function as an inhibitor of adipogenesis by preventing the preadipocyte proliferation and differentiation into mature adipocytes (200) and its association with insulin resistance in both rodents and humans (201–203). The presence of DLK1 protein in most orexigenic Agouti-related protein and NPY neurons and only to a lesser extent in anorexigenic cocaine- and amphetamine-regulated transcript containing neurons and the DLK1 mRNA regulation by nutritional challenges strengthen its role in hypothalamic control of body weight, possibly through actions on the development and synaptic plasticity of neurons in the arcuate (196, 204). In addition, DLK1 is involved in beige fat biogenesis and adaptive thermogenesis (205). Moreover, Wermter et al (206) analyzed a large cohort of trio families (2 parents and 1 obese child) from European descent and identified a synonymous polymorphism within the DLK1 gene in association with childhood and adolescent obesity.
Likewise, Dlk1-deficient mice have an accelerated weight gain that occurs at later ages and is associated with fat deposition, dyslipidemia, and enlarged fatty liver (207). Conversely, mice overexpressing Dlk1 were shown to have limited accumulation of adipose tissue and reduced liver steatosis during postnatal life, even when challenged with a high-fat diet (208). Intriguingly, more recent data from humans exhibited a positive correlation of DLK1 circulating levels with BMI, fat content, and insulin resistance (200, 209). In agreement with these findings, Dlk1-deficient mice exhibit protection against insulin resistance and obesity induced by a high-fat diet. These results parallel the data regarding leptin in humans, whereas congenital leptin deficiency leads to early-onset severe obesity, patients with acquired obesity have higher levels of leptin because of leptin resistance (210). Either way, acting as a gatekeeper or as an enhancer of adipogenesis in different contexts, the evidence so far has unveiled the importance of Dlk1 for this differentiation process and suggest a possible role of Dlk1 as a metabolic regulator of reproduction.
Syndromic CPP Without CNS Lesions
CPP without CNS lesions is mostly frequently described as an isolated entity, but it may also present combined with other signs and symptoms, encompassing a syndromic form (16). To date, few studies have contributed to identifying patients with syndromic disorders among large CPP cohorts (16, 211). In this setting, a promising translational study investigated 36 selected patients with CPP associated with multiple anomalies through (epi)genetic studies (16). Rare genetic abnormalities were identified in 12 (33%) of them, including genetic defects in loci known to be involved with CPP (14q32.2 and 7q11.23; further discussed in a later section) or candidate chromosomal regions or genes.
CPP has been demonstrated to be part of the phenotypic spectrum of rare genetic syndromes caused by distinct defined molecular mechanisms, such as epigenetic defects, CNVs, and gene mutations (16, 211). Recently, the premature activation of the reproductive axis has been described as a possible component of imprinting disorders, a group of congenital diseases caused by disturbances in imprinted genes affecting growth, development, and metabolism (212). Temple syndrome (OMIM 616222) is a rare imprinting disorder marked by precocious puberty in 80% to 90% of cases. Additionally, patients characteristically present with prenatal and postnatal growth failure, hypotonia, small hands and/or feet, and obesity (16, 189). It is caused by the disruption of the chromosome 14q32.2, a chromosomal region carrying a cluster of imprinted genes, including DLK1 and its primary imprinting control center, the DLK1/MEG3:intergenic-differentially methylated region (DLK1/MEG3:IG-DMR) (213). Three main 14q32.2 molecular abnormalities can underlie Temple syndrome phenotype: maternal uniparental disomy of chromosome 14, hypomethylation of the DLK1/MEG3:IG-DMR on the paternal allele (epimutation), and paternal deletion of the DLK1/MEG3 domain (189, 213). These 3 mechanisms harbor in common the lack of expression of the paternal copy of DLK1 gene and clinically may manifest CPP (16, 189, 213). Interestingly, barely detectable levels of serum DLK1 were measured in Temple syndrome patients from epimutations and deletions at chromosome 14q32.2 (212). Based on these lines of evidence, it has been postulated that DLK1 deficiency is probably the leading cause of premature pubertal development in Temple syndrome patients (16).
PWS (OMIM 176270) is a classic imprinting disorder, mostly characterized by hypotonia, obesity, growth failure, cognitive disabilities, and hypogonadism, but has also been associated with precocious puberty albeit infrequently (about 4%) (179). PWS occurs from an absence of the paternally expressed imprinted genes at chromosome 15q11-q13. The MKRN3 gene, the most prevalent factor associated with familial CPP, is located at the boundary of this critical region (179). Meader et al (214) investigated paternal deletions in this region and they postulated that the low frequency of early puberty in Prader-Willi syndrome patients was most likely from concomitant disturbances leading to hypogonadism (214).
Silver-Russell syndrome (OMIM 180860) is a clinically and (epi)genetically heterogeneous imprinting disorder, mainly characterized by prenatal and postnatal growth retardation. The most common mechanisms are hypomethylation of the IGF2/H19:IG-DMR and maternal uniparental disomy of chromosome 7 (UPD(7)mat) (215). Interestingly, precocious puberty was described in some of the first cases reported in the historical cohorts of Silver-Russell syndrome (216). More frequently, patients develop early puberty, characterized by age at puberty onset at the younger limit of the normal range (212, 215). Children with UPD(7)mat are likely to develop puberty at younger ages (215), possibly from still unknown pubertal influencing factors located in this chromosome. Interestingly, another well-known chromosome 7 abnormality (Williams-Beuren syndrome) can present with premature sexual development. Williams-Beuren syndrome (also called Williams syndrome; OMIM 194050) is a multisystem disorder caused by hemizygous 7q11.23 deletion, leading to a contiguous gene syndrome with clinical heterogeneity (217, 218). The main clinical features are distinct craniofacial appearance, cardiovascular disease, short stature, intellectual disability, and hypersociability (218). Affected children may present an early puberty (up to 50%) or CPP (3%-8%) (217–219). Syndromic girls were reported to have menarche approximately 2 years earlier than control girls (217, 219). Putative genotype-phenotype correlations for genes within the 7q11.23 deletion have been described (218). However, the exact gene or mechanism involved in this premature puberty phenotype remains unknown. Remarkably, an enriched signal for association with age at menarche within the critical region of Williams syndrome was identified in a large genome-wide association study, increasing the likelihood for this chromosome to carry a potential pubertal influencing factor (12).
Different studies have associated CPP with other rare CNVs, such as 1p36 deletion (220), 9p distal deletion (221), and 9q34.3 duplication (including NOTCH1 gene) (222). Two distinct CNVs syndromes involving the X chromosome were shown in association with a premature puberty phenotype (16, 223). Canton et al (16) identified three familial CPP cases carrying Xp22.33 deletions, involving the pseudoautosomal region 1 (SHOX gene region). The pedigree analysis revealed that early puberty cosegregated with SHOX phenotypes (body disproportion, short stature, and Madelung deformity) in a dominant pattern of inheritance. The exact mechanism involved in the pubertal phenotype of these cases remains to be elucidated (16).
The rare Xp11.23-p.11.22 duplication syndrome (OMIM 300881) is a contiguous gene syndrome with an X-linked dominant inheritance, mainly characterized by intellectual disability, speech delay, electroencephalogram abnormalities, overweight, skeletal anomalies, and early puberty (223). Since the first report by Giorda et al (223), precocious pubertal development has been described in familial and sporadic cases. Evidence from 3 studies showed precocious puberty in around 11% of males and 70% of females, the latter presenting age at menarche from 8 to 9 years (223–225).
The putative role of genes on the X chromosome in the genetic architecture of human pubertal timing may also be suspected by the description of rare cases of defects in the X-linked gene methyl-CpG-binding protein 2 (MECP2) presenting with precocious puberty (226–228). Loss-of-function mutations in MECP2 are associated with neurodevelopmental disorders, mainly with Rett syndrome (OMIM 312750), an X-linked dominant disorder occurring mostly in females (226). Remarkably, CPP has been described in atypical or rare cases of girls with Rett syndrome (226, 227). A boy with severe intellectual disability and CPP was described with an Xq28 duplication including MECP2 (228). Interestingly, precocious puberty was also described in up to 13% of girls with dominant de novo mutations in the X-linked gene dead-box helicase 3 X-linked (chromosome Xp11.4), which causes an X-linked intellectual development disorder (OMIM 300958) (229).
Autism spectrum disorder is also a CNS disorder that has been associated with X-linked genes defects and sporadically with CPP (229, 230). A cohort study of health care data from Florida (USA) compared children with or without autism spectrum disorder. Notably, the autism group had a higher risk of CPP (adjusted hazard ratio, 4.64) (230).
Epigenetics Role in CPP
Epigenetics is defined as the study of mitotically and/or meiotically heritable changes in gene function that cannot be explained by changes in DNA sequence. The epigenetic marks encompass maintained chemical additions to DNA and histones that play a key role in regulating chromatin and/or gene expression and lead to gene silencing or activation (231). Epigenetic information in mammals can be transmitted by the following main mechanisms: mitotically stable DNA methylation; posttranslational modifications of histone proteins; chromatin remodeling and noncoding RNAs (such as miRNAs and long noncoding RNAs) (232, 233). The loss of effectiveness of epigenetic regulation has been associated with distinct human diseases (122, 233).
In the past decade, growing evidence has demonstrated the participation of epigenetic mechanisms in the control of puberty, indicating its potential modulating role in human pubertal timing (12, 14). Indeed, the identification of disease-causing inactivating mutations in imprinted genes in several families with CPP has suggested a role for epigenetic mechanisms in this hypothalamic disorder (14, 63). Since the first report by Abreu et al (14), inactivating mutations in the imprinted gene MKRN3 have been described as the most common cause of monogenic CPP. More recently, loss-of-function mutations in the DLK1 gene were described as a rarer cause of CPP (63, 191). Interestingly, both genes are paternally expressed from the imprinting of the maternal allele, leading to its silencing (see sections on MKRN3 and DLK1 mutations). Genomic imprinting is an epigenetic process of gene silencing established through DNA methylation of promoter or regulatory regions by which selected genes are expressed in a parental-specific manner, leading to their monoallelic expression (233). Based on this, Canton et al (16) investigated imprinting defects of MKRN3 and DLK1 through methylation-specific analysis in a subset of patients with idiopathic CPP associated with multiple anomalies (n = 36). Three patients were identified with hypomethylation at the DLK1 imprinting control region, an epigenetic mechanism associated with Temple syndrome, which may be clinically subtle among CPP cohorts (see section on syndromic CPP). No DNA methylation defects were identified in the MKRN3 promoter region (16). Similarly, in an Asian study, no abnormality of MKRN3 methylation was detected in 19 patients with CPP (234). These data suggested that MKRN3 methylation defects are not a common cause of CPP, whereas DLK1 hypomethylation may be considered in patients with sporadic CPP resembling Temple syndrome.
Genome-wide DNA methylation patterns associated with pubertal timing were investigated in CPP and healthy girls. Bessa et al (235) evaluated the methylome profiling in leukocytes of 10 CPP girls and 33 healthy prepubertal and pubertal girls. A widespread pattern of DNA hypermethylation was identified in girls with both normal and abnormal puberty, indicating that the pubertal process in humans is associated with specific changes in epigenetically driven regulatory control. There was an enrichment of changes in methylation of zinc-finger genes, including hypomethylation at the promoter of ZFP57 in human peripheral leukocytes and, also in the hypothalamic region of female rhesus. This elevated hypothalamic ZFP57 expression during peripubertal development indicated potential enhanced repression of downstream ZFP57 target genes (235).
In a study of 34 healthy American girls, a hypermethylation pattern in leukocyte DNA was demonstrated during pubertal development (236). In addition, 3 more studies analyzed longitudinally genome-wide changes in leukocyte DNA methylation of healthy girls and boys during pubertal development, identifying several differentially methylated genomic regions related to normal puberty (237–239). Almstrup et al (237) identified the most significant region for both sexes in the promoter region of Thyroid Hormone Receptor Interactor 6 (TRIP6), located at 7q22. Measurements of circulating levels of TRIP6 in healthy pre-, mid-, and postpubertal healthy Danish children evidenced a significant increase as individuals progressed through puberty. Therefore, the methylation of the promoter of TRIP6 was potentially associated with pubertal development (237). However, little is known about the functional role of human TRIP6.
Several important animal studies have corroborated the emerging role of epigenetic mechanisms in pubertal timing (240). There is an emerging concept that a switch from epigenetic repression to activation within arcuate kisspeptin neurons is a core mechanism underlying the initiation of puberty (232). Lomniczi et al (241) identified an epigenetic mechanism of transcriptional repression that regulated the initiation of female puberty in rats. The principal contributors to this mechanism of repression were silencers of the Polycomb group (PcG) of proteins, which could affect the gene network that is involved in the stimulatory control of puberty, including Kiss1 and glutamatergic receptors genes (242). The kdm6b enzyme was identified as a central node of this network by erasing the PcG-dependent histone modification of H3K27me3. It was suggested that Kdm6b repression could be a PcG mechanism to modulate puberty-activating gene networks (232, 242). Zinc-finger factors have also been implicated as an epigenetic modulator of puberty through processes of transcriptional repression (243). In studies using monkeys and rodents, GATAD1, a zinc finger protein decreasing in peripuberty, was identified as a key player for the repression of Kiss1 and Tac3 in the arcuate neurons and restraining puberty (243). The Trithorax group (TrxG) of proteins is a complex of transcriptional activators (232). Notably, both PcG and TrxG are chromatin proteins known to have mutually antagonistic activity. TrxG proteins are known to antagonize PcG proteins by implementing methylation of histone marks at H3K4 (232). Animal studies evaluating interactions with the Kiss1 promoter have shown that, before initiation of puberty, as the PcG-mediated gene silencing declines, the TrxG activation increases, configuring a fine-tuned dual process of epigenetic regulation of pubertal timing (232).
A study in mice evidenced that a miRNA switch could regulate the rise in hypothalamic GnRH production before puberty, with miR-200 and miR-155 being identified as 2 essential components of this process, regulating transcriptional factors of GnRH, and interfering in the correct initiation of puberty (244). Subsequently, an experimental study in rats demonstrated an increase in hypothalamic miR30-b expression during postnatal maturation that was associated with a concomitant Mkrn3 decrease. Functional in vitro analyses demonstrated a strong repressive action of miR-30b on Mkrn3 3′UTR and consequently reduction of Mkrn3 expression (156). Interestingly, circulating relative miR30-b levels was assessed in 26 boys with constitutional delay of growth and puberty (245). The miR30-b levels increased during puberty in these boys, appearing to be related to the HPG axis activity (245). Changes in mir30-b and other miRNAs causing CPP in humans are still to be unmasked.
Acquired Etiology of CPP
Acquired Causes With CNS Lesions
Different acquired conditions affecting the hypothalamus or brain, such as tumors, cranial irradiation, infection, trauma, and hydrocephalus, have been recognized as classical causes of premature GnRH secretion and, therefore, CPP (3, 9). However, the precise mechanism is not completely understood in the great majority of these conditions. The mechanisms by which distinct neoplastic or nonneoplastic lesions result in CPP remain hypothetical (246). Most evidence suggests that tumor sites near the hypothalamic-pituitary region disrupt the neuroendocrine balance with consequent early secretion of GnRH (9, 246). Increased intracranial pressure represents another possible explanation of organic CPP (246).
The main risk factors for CPP from CNS abnormalities include a younger age and male sex (246). Chalumeau et al (247) recognized 3 predictors of CNS lesions in girls, including age <6 years, elevated basal estradiol levels (>110 pmol/L), and absence of pubic hair.
Tumoral Causes
The presence of CNS tumors in CPP is relatively uncommon (248). In a systematic review including 15 studies and 1853 girls with CPP, the incidence of CNS tumors was 1.6% (80). These tumors included astrocytomas, gliomas, craniopharyngiomas, suprasellar tumors, hypothalamic tumors, pontine tumor, pinealoma, and fourth ventricular tumor (80). A recent study involving brain MRI of 770 girls with CPP revealed 13.5% with CNS lesions, with only 2 tumors (0.25%) being identified, including 1 low-grade glioma and 1 meningioma (89). The clinical presentation of CNS tumors is related to the tumor expansion velocity, its location, and the age of the child. Clinical findings can be quite varied, ranging from signs of increased intracranial pressure to localizing neurological signs and symptoms. Infratentorial tumors can occur in the posterior fossa (medulloblastomas, cerebellar astrocytomas, gliomas, and ependymomas) and their clinical presentations consist in signs of increased intracranial pressure, headache, emesis, weight loss, ataxia, and neuropathy. If CPP presents in this setting, it is most likely secondary to increased intracranial pressure causing interference in the hypothalamic region. Supratentorial tumors (craniopharyngiomas, gliomas, germinomas, pineal tumors, ependymomas) manifest with visual disturbances, signs of increased intracranial pressure, macrocephaly, and neuroendocrine dysfunction such as growth failure or diabetes insipidus (9).
Low-grade gliomas represent more than 40% of CNS tumors (124). Juvenile pilocytic astrocytoma is the most common lesion type with unpredictable growth and the possibility of spontaneous involution, late-onset progression, or leptomeningeal metastases can occur (124). Ten to 16% are linked to NF1 and although 50% to 60% of pediatric low-grade gliomas involve the cerebellum, cerebral hemispheres or brainstem, 30% to 50% affect the optic nerves, chiasm, and tracts, the hypothalamus, and suprasellar midline (124). Interestingly, precocious puberty has not been described in optic glioma, astrocytoma, or ependymoma localized outside the suprasellar area. As mentioned previously, hypothalamic astrocytes can secrete neuroactive substances that stimulate the release of GnRH (249) and they accelerate GnRH neuronal phenotypic differentiation (250). Therefore, in children with this type of tumor, CPP might be caused by tumor location, and/or tumor cell type (246).
Pineal tumors include germ-cell tumors, pineal parenchymal tumors, and glial tumors. These lesions comprise as much as 7% of CNS tumors in childhood (246, 251). The pineal gland is located near the cerebral aqueduct; therefore, tumors rising in this location may also produce obstructive hydrocephalus and CPP. In this context, precocious puberty may occur either by tumor-induced hydrocephalus resulting in CPP, or through hCG secretion from germ-cell secreting tumors originating a GnRH-independent or peripheral form of precocious puberty (246). In fact, all tumoral lesions arising in locations potentially related to obstructive hydrocephalus can evolve with CPP.
Other Conditions
Several insults, such as neonatal infections, cerebrovascular accidents, hydrocephalus, traumatic brain injury, cranial irradiation, and encephalopathies, can be associated with CPP (11). An increased pressure on the hypothalamic-pituitary area with loss of normal childhood hypothalamic inhibition of pituitary gonadotropins could be one of the factors responsible for CPP. Cranial radiation largely used for the treatment of primary CNS tumors can result in CPP through direct effects on GnRH secretion (246, 252). Radiation therapy for childhood cancers represents a cause of early reactivation of GnRH secretion (253). Lower doses (18-24 Gray) of radiotherapy were associated with precocious puberty in girls, whereas doses higher than 25 Gray affected both sexes. Notably, younger age at radiotherapy confers a higher risk of CPP (253, 254). This modality of treatment might cause damage to GABAergic neurons, which could lead to premature activation of GnRH neurons (253, 255).
Children affected by neurodevelopmental disability could experience early pubertal changes at least 20 times more frequently than the general population (256). Limited data about CPP among children affected by cerebral palsy are available (257); however, a multicenter, cross-sectional study including 207 children (84 girls and 123 boys aged 3-18 years; 71% White) with cerebral palsy of moderate to severe motor impairment demonstrated that puberty begins earlier but ends later in children with cerebral palsy, compared with children in the general population (256, 257). In addition, menarche occurs later in girls with cerebral palsy, with more advanced sexual maturation being associated with more body fat in girls but less body fat in boys (257). These clinical observations suggest that early activation of the HPG axis in patients with cerebral palsy is due to a loss of hypothalamic inhibition of GnRH secretion during childhood (256, 257). Moreover, severe brain damage and antiepileptic drugs may affect some neurotransmitter pathways involved in gonadotropin control (258). Notably, most patients with cerebral palsy need antiepileptic drugs treatment with a potential interference in pubertal timing.
Acquired Causes of CPP Without CNS Lesions
Endocrine-disrupting Chemicals
EDCs are chemical compounds (natural or synthetic) that may alter endocrine and homeostatic systems by affecting hormone secretion or their targets (259, 260). The sources of substances known as EDCs may include drugs, pesticides, fungicides, plasticizers, industrial products, cosmetics, heavy metals, and naturally botanical chemicals. The comprehension of the mechanisms of action and the consequences of exposure to EDCs must consider key aspects, such as age at exposure, latency from exposure, mixture of compounds, nontraditional dose-response dynamics, and possible epigenetic effects (259). Nevertheless, several studies have provided evidence of the effects of EDCs on endocrine systems, including the male and female reproductive axis (259, 260). In the past several decades, there was a populational secular trend for earlier age at pubertal development in girls and boys, as well as increasing rates of incidence and prevalence of CPP in both sexes (2, 21). These trends have been related to multiple possible factors, including environmental factors such as the exposure to EDCs (2, 21, 261). The categories of EDCs that have been associated with CPP include the following: bisphenols, phthalates, pesticides, heavy metals, essential oils, pharmaceuticals, parabens, flame retardants, and phytoestrogens (21, 259–261).
Most studies in humans linking EDC exposure to early pubertal development had retrospective or cross-sectional designs (21, 261), not identifying potential causal mechanisms. Causal mechanistic links between exposure and pubertal phenotypes have been proposed mainly by experimental animal models (21, 260). Real-world methodological difficulties of human studies include differences among the pubertal milestones evaluated (age of thelarche, pubarche, or menarche), exposure to several compounds at the same time, differences in exposure dose and duration, and differences in exposure routes. EDCs are known as hormonally active substances that function by binding to specific receptors and altering hormonal synthesis, action, and metabolism (259, 261). Many EDCs are known to present estrogenic or antiandrogenic actions. Notably, until recently, the main putative actions of EDCs were estrogenic or antiandrogenic activities, indicating a peripheral mechanism for association with pubertal outcomes (260). However, current data have suggested EDC actions on the developing brain, including the GnRH neurons. The hypothalamic GnRH network is regulated by organizational processes occurring in fetal and early postnatal life, both critical windows of development, when fetuses and neonates are vulnerable to environmental exposures, especially to EDCs (21, 260). Similarly, the timing of puberty onset is triggered by the reactivation of the HPG axis through a process regulated by genetic, epigenetic, and environmental factors, indicating that it may also be susceptible to disturbance by EDCs. Therefore, growing evidence has related these critical periods of exposure to EDCs with pubertal development disorders, based on the assumption that the neuroendocrine control of reproduction responds to environmental disruption (21, 260, 261). Nevertheless, there is no compelling current evidence that EDCs cause an up or downregulation in the GnRH and kisspeptins system (260, 261). Table 3 summarizes the main EDCs and their putative mechanisms of action associated with CPP.
Table 3.
Endocrine-disruptors chemicals and putative mechanisms of action associated with central precocious puberty
| Category | Chemicals related with pubertal disorders | Main sources | Putative mechanisms involved in pubertal disorders, especially in CPP | Possible molecular targets in GnRH network in rodent models (259, 260, 261) |
|---|---|---|---|---|
| Bisphenols | Bisphenol A | Plasticizers, drink and food containers, building materials | - Estrogenic activity by binding to estrogen receptors - Impairment of kisspeptin neurons maturation at the arcuate nucleus |
- At hypothalamic nuclei: estrogen receptor-∞, estrogen receptor-ß, kisspeptin,
GABA, glutamate, RFamide-related peptide 3 - Effects on DNA methylation (Dnmt1, Dnmt3a, Dnmt3b, Esr1) - Effects on histone posttranslational modifications |
| Phthalates | Phthalates compounds | Plasticizers, consumer products (especially cosmetics), food packing, toys | - Estrogenic activity by binding to estrogen receptors - Antiandrogenic activity - Increased kisspeptin activity |
At hypothalamic nuclei: estrogen receptor-∞, estrogen receptor-ß, kisspeptin |
| Pesticides | Persistent (DDT and DDE) and nonpersistent | Agricultural applications | - Estrogenic activity by binding to estrogen receptors - Antiandrogenic activity by altering sex steroid synthesis enzymes |
- At hypothalamic nuclei: estrogen receptor-∞, estrogen receptor-ß, glutamate
- Effects on DNA methylation (Esr1) |
| Heavy metal | Mercury | Diet-delivered, especially in fish and seafoods | Accumulation in the hypothalamus, the pituitary, and the gonads | |
| Essential oil | Lavender oil components | Lavender-fragranced products | Premature breast development: estrogenic and antiandrogenic properties of components of lavender essential oil | |
| Pharmaceutical | Paracetamol | Analgesics and antipyretics | - Inhibition of prostaglandins signalling possibly disrupting physiological
roles of prostaglandins in the development of the brain and the gonads - Increase of estrogen production - Inhibition of androgen production |
|
| Parabens | Parabens | Consumer products (cosmetics and personal care) | Estrogenic activity | At hypothalamic nuclei: estrogen receptor-∞ |
| Flame retardants | Polybrominated diphenyl ethers (PBDE) | Plastics, textiles, furniture | Estrogenic and androgenic properties | |
| Polychlorinated biphenyl (PCB) | Mixtures | Oils, additives | Action on estrogen receptors and neurotransmitter receptors | - At hypothalamic nuclei: estrogen receptor-∞, estrogen receptor-ß, kisspeptin
- Effects on miRNAs |
| Phytoestrogens | Genistein | Soybean, oats | Estrogenic properties | At hypothalamic nuclei: estrogen receptor-ß, kisspeptin |
| Dioxins | Dioxin | Industrialized products | Antiandrogenic activity |
A systematic review and meta-analysis evaluated the current evidence on the timing of pubertal onset in girls and boys following prenatal or postnatal exposures to xenobiotic EDCs (262). The authors demonstrated that postnatal exposure to phthalates may be associated with earlier thelarche and later pubarche. However, no consistent evidence for associations between timing of pubertal onset and exposures to any of the studied xenobiotic EDCs was found (262).
Epigenetic marks are considered players for the transmission of environmental information to physiological systems, such as the GnRH network (21, 260). Recently, epigenetic mechanisms have been hypothesized as potential targets for EDC actions in the brain, especially during development (263, 264). Animal studies have shown that exposure to distinct EDCs in critical periods of development may have disrupting effects on DNA methylation, histone posttranslational modifications, and noncoding RNA (260, 265). However, whether the effects of EDCs targeting epigenetic mechanisms modulators of pubertal timing would manifest as premature sexual development in humans is still an open field. Remarkably, regarding CPP, the body of current evidence shows a significant variability among different populations in the responsiveness to the exposure of EDCs (266). Besides differences in routes, doses, and times of exposure, the individual genetic background may also be considered as an additional factor modulating the responsiveness to EDCs exposures.
International Adoption
Various authors from different countries have analyzed a possible increase in the clinical cases of CPP among children emanating from international adoption; however, more information is clearly required. Considering the current state of knowledge, there are 4 elements that must be rigorously evaluated to confirm this working hypothesis: (1) the lack of epidemiological data in the international literature regarding CPP; (2) the information regarding the incidence of CPP among children coming from national or international adoption is only partial; (3) the available data regarding the precise chronological age in children undergoing international adoption is often deficient because of uncertainty regarding the patient’s date of birth; and (4) there is enormous disagreement among clinicians as to whether the actual criteria for defining precocious puberty are appropriate.
According to The Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat that monitors population policies worldwide, approximately 260 000 international adoptions occur each year (267). International adoptions substantially increased during the past 2 decades in Western countries (268, 269), with the United States, France, Spain, and Italy having the highest number of international adoptions (1). This increased risk of CPP in internationally adopted children compared with the local population (270–274) is of great importance and the possible underlying mechanisms should be elucidated.
Nearly 60% of immigrants to Spain are from Latin America and there is a high percentage of adoptions from the same countries (8). These adopted children had more than 5 times more risk to develop CPP, all of whom were girls, with no increased risk found in those who immigrated to Spain from Latin America with their families (8). This difference in risk was not explainable by sex, genetics, or specific environmental factors; however, the BMI tended to be lower in adopted children compared with immigrants from Latin American countries (8). This may indicate increased nutritional deficiencies in adopted children (270, 274), which could modify their response to a nutritionally enriched environment. Furthermore, immigrants live with their families and most likely have less affective deprivation compared with adopted subjects (274). In this study (8), 83.8% of the children adopted abroad arrived before 6 years of age lessening the possibility of discordance in the chronological age of these children.
In a Danish cohort of children who were diagnosed with CPP between 1998 and 2017, the annual incidence of CPP and normal variants of early puberty was found to increase substantially during the past 20 years (8), with CPP increasing in immigrant girls, but with no observed in boys (1). In a French cohort, a much higher incidence of CPP was found in girls compared with boys in sporadic and familial forms, as well as in adopted children (275). In a Korean study based on a national registry, the incidence and prevalence rates of CPP were very high (7); however, adoptees from Korea were not found to have an increased risk of precocious puberty compared with that of their country of adoption. Thus, the effect of origin might be explained, at least in part, by genetic factors linked to ethnicity or environmental factors in different countries.
It is suggested that the older the child is at adoption, the higher the risk of early puberty, with very young children at adoption having little or no increased risk of early puberty (274). Although the mechanisms underlying the relationship between international adoption and CPP remain unknown, ethnical, emotional, and environmental factors may be involved. Follow-up programs for these children starting at the time of their arrival to host countries would be of great utility. In addition, adequate nutrition should be established, avoiding excess weight gain (9) because exogenous obesity is common in occidental countries and an increased BMI is associated with early puberty (276). Indeed, girls with a BMI greater than or equal to the 85th percentile have the highest risk of both early pubertal onset and earlier age of menarche among Black, Hispanic, and Caucasian girls (277). It is possible that these adopted children have experienced various degrees of malnutrition, emotional abandonment, and illnesses, including important infectious processes, with little or no clinical information in many cases (22) that could influence the possibility of experiencing early pubertal onset or precocious puberty.
Early Exposure to Sex Steroids
Peripheral or gonadotropin-independent precocious puberty is caused by excessive sex hormones produced autonomously in the gonads or adrenal glands, β-human chorionic gonadotropin-secreting tumors, or exposure to exogenous sex hormones. It is well known that long-term exposure to sex steroids because of a GnRH-independent process can lead to CPP. This transition from peripheral to central precocious puberty usually occurs after initiation of treatment for the underlying peripheral disease, particularly in patients with significantly advanced bone age (>10 years). Once the negative feedback effect of pubertal sex steroid concentrations is removed, the hypothalamic-pituitary-gonadal (HPG) axis can subsequently be activated. In this situation, GnRH analogs are frequently necessary as adjuvant therapy in progressive CPP in combination with therapies for peripheral precocious puberty.
The development of CPP after prolonged androgen exposure has been documented in several disorders, including congenital adrenal hyperplasia, Leydig cell tumors, familial male-limited precocious puberty, and androgen-secreting adrenocortical tumors (278, 279). A retrospective study that included 63 patients with virilizing adrenocortical tumors demonstrated that 37 of them had puberty and 10 patients manifested CPP or early fast puberty (278). Tall stature and older age at diagnosis of the adrenal tumors were associated with higher risk of CPP. This study reinforces the importance of close follow-up after surgery to identify and treat consequences of early exposure to androgen excess.
Notably, CPP can be the first clinical manifestation of peripheral precocious puberty that may have a late diagnosis. This presentation was demonstrated in nonclassical congenital adrenal hyperplasia, familial male-limited precocious puberty, and McCune Albright syndrome patients (280). Nonclassical congenital adrenal hyperplasia was diagnosed in approximately 5% of girls who initially presented CPP (280).
The mechanisms that trigger secondary HPG axis reactivation are not well understood to date and the temporal correlation between skeletal maturation and HPG axis maturation may be a clue. A positive feedback mechanism from sex steroids (estrogens and androgens as well as their precursors or metabolites) on kisspeptins neurons or other key central stimulatory factors that expressed estrogen and androgen receptors could induce hypothalamic GnRH secretion leading subsequently to CPP. However, this hypothesis still needs to be demonstrated in experimental studies.
Perspectives and Conclusions
The etiology of CPP is multiple and heterogeneous, including diverse congenital and acquired causes that can be associated with different lesions or functional alterations of CNS. The importance of genetic and/or epigenetic factors in the underlying mechanisms of CPP, such as brain anomalies or tumors (hamartomas, gliomas), syndromic forms (Temple syndrome, PWS, Rett syndrome) and imprinting disorders leading to familial CPP (MKRN3 and DLK1 loss-of-function mutations) has grown significantly in the last decade. Indeed, the previously known high prevalence of idiopathic CPP in girls (90%) and boys (50%) should be reduced substantially when an updated genetic strategy of investigation in familial and sporadic cases with CPP is established.
The assessment of novel serum proteins (MKRN3, DLK1, KISS1, neurokinin B) through immunoassays could represent an innovative approach for CPP etiology investigation. The discoveries involving the role of these neuropeptides in determining premature reactivation of HPG axis, or even as biochemical screening for further mutational gene analysis in selected CPP patients, are open fields to be explored (11, 63, 192, 281–283).
Exposure to EDC either prenatally or postnatally during childhood has been proposed as possible mechanisms for the occurrence of CPP (21, 260). However, the effects of EDC are difficult to determine because they are compound specific, sexually dimorphic, and time exposure dependent (260).
Despite the successful outcome after CPP treatment with long-acting GnRH analogs, the recent genetic discoveries involving the etiology of CPP may provide the bases for potential new target treatments in specific and selected subgroups.
Girls with early or precocious abnormal puberty have longer chronic estrogen exposure than girls with normal pubertal development. They have potentially an increased subsequent risk of obesity, type 2 diabetes mellitus, cardiovascular disease, breast cancer, and other malignancies (284, 285). In addition, the early sexual development is also associated with more frequent risk-taking behaviors (286).
Because CPP has been linked to changes in body weight and obesity, exposure to EDC, and stressful life events, preventive measures to avoid these conditions, such as healthy living habits, are highly recommended.
The identification of the major causative factors (genetic or environmental players) involved in the regulation of the hypothalamic neural network that modulate GnRH secretion will positively influence prevention, early and precise diagnosis, and better outcomes of children who experienced precocious sexual development.
Acknowledgments
We thank John C. Magnotto for his assistance editing Fig. 4 and the references.
Abbreviations
- ARC
arcuate nucleus
- AVPV
anteroventral periventricular nucleus
- BMI
body mass index
- CNS
central nervous system
- CNV
copy number variation
- CPP
central precocious puberty
- DLK1
Delta Like Non-Canonical Notch Ligand 1
- EDC
endocrine-disrupting chemical
- HH
hypothalamic hamartoma
- HPG
hypothalamic-pituitary-gonadal
- MECP2
methyl-CpG-binding protein 2
- MKRN3
Makorin ring finger 3
- MRI
magnetic resonance imaging
- mTOR
mammalian target of rapamycin
- NF1
neurofibromatosis type 1
- NKB
neurokinin B
- OPG
optic pathways glioma
- PcG
Polycomb group
- PWS
Prader-Willi syndrome
- SHH
Sonic Hedgehog pathway
- TRIP6
Thyroid Hormone Receptor Interactor 6
- TrxG
Trithorax group
- TSC
tuberous sclerosis complex
- UPD(7)mat
uniparental disomy of chromosome
- UTR
untranslated region
Contributor Information
Vinicius N Brito, Discipline of Endocrinology & Metabolism, Department of Internal Medicine, University of Sao Paulo Medical School, University of Sao Paulo, Sao Paulo 01246 903, Brazil.
Ana P M Canton, Discipline of Endocrinology & Metabolism, Department of Internal Medicine, University of Sao Paulo Medical School, University of Sao Paulo, Sao Paulo 01246 903, Brazil.
Carlos Eduardo Seraphim, Discipline of Endocrinology & Metabolism, Department of Internal Medicine, University of Sao Paulo Medical School, University of Sao Paulo, Sao Paulo 01246 903, Brazil.
Ana Paula Abreu, Division of Endocrinology, Diabetes and Hypertension, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Delanie B Macedo, Discipline of Endocrinology & Metabolism, Department of Internal Medicine, University of Sao Paulo Medical School, University of Sao Paulo, Sao Paulo 01246 903, Brazil; Division of Endocrinology, Diabetes and Hypertension, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA; Núcleo de Atenção Médica Integrada, Centro de Ciências da Saúde, Universidade de Fortaleza, Fortaleza 60811 905, Brazil.
Berenice B Mendonca, Discipline of Endocrinology & Metabolism, Department of Internal Medicine, University of Sao Paulo Medical School, University of Sao Paulo, Sao Paulo 01246 903, Brazil.
Ursula B Kaiser, Division of Endocrinology, Diabetes and Hypertension, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Jesús Argente, Hospital Infantil Universitario Niño Jesús, Department of Endocrinology and Department of Pediatrics, Universidad Autónoma de Madrid, Spanish PUBERE Registry, CIBER of Obesity and Nutrition (CIBEROBN), Instituto de Salud Carlos III, IMDEA Institute, Madrid 28009, Spain.
Ana Claudia Latronico, Discipline of Endocrinology & Metabolism, Department of Internal Medicine, University of Sao Paulo Medical School, University of Sao Paulo, Sao Paulo 01246 903, Brazil.
Financial Support
V.N.B. and A.C.L are supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP #2019/27631-7). A.C.L. is supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq # 303183/2020-9). C.E.S. is supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq #142362/2019-0). A.P.A. is supported by National Institute of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development grants R00HD 091381, R21 HD098684, and R01 HD082314. U.B.K. is supported by NIH R01 HD082314, R21 HD098684, R37 HD019938, and U54 AG062322. B.B.M. is supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP # 19/26780-9) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq #307571/2021-1). J.A. is supported by the Spanish Ministry of Science and Innovation with the help of European FEDER funding (FIS PI19/00166), and the Network Center for Biomedical Research on Obesity and Nutrition (CIBEROBN) Instituto Carlos III, Madrid, Spain. Ana P.M. Canton A.P.M.C is supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP #2022/00719-4).
Disclosures
The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Bräuner EV, Busch AS, Eckert-Lind C, Koch T, Hickey M, Juul A. Trends in the incidence of central precocious puberty and normal variant puberty among children in Denmark, 1998 to 2017. JAMA Netw Open. 2020;3(10):e2015665e2015665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24(5):668–693. [DOI] [PubMed] [Google Scholar]
- 3. Latronico AC, Brito VN, Carel JC. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016;4(3):265–274. [DOI] [PubMed] [Google Scholar]
- 4. Carel JC, Léger J. Clinical practice. Precocious puberty. N Engl J Med. 2008;358(22):2366–2377. [DOI] [PubMed] [Google Scholar]
- 5. Sørensen K, Mouritsen A, Aksglaede L, Hagen CP, Mogensen SS, Juul A. Recent secular trends in pubertal timing: implications for evaluation and diagnosis of precocious puberty. Horm Res Paediatr. 2012;77(3):137–145. [DOI] [PubMed] [Google Scholar]
- 6. Kim SH, Huh K, Won S, Lee KW, Park MJ. A significant increase in the incidence of central precocious puberty among Korean girls from 2004 to 2010. PLoS One. 2015;10(11):e0141844e0141844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim YJ, Kwon A, Jung MK, et al. Incidence and prevalence of central precocious puberty in Korea: an epidemiologic study based on a national database. J Pediatr. 2019;208:221–228. [DOI] [PubMed] [Google Scholar]
- 8. Soriano-Guillén L, Corripio R, Labarta JI, et al. Central precocious puberty in children living in Spain: incidence, prevalence, and influence of adoption and immigration. J Clin Endocrinol Metab. 2010;95(9):4305–4313. [DOI] [PubMed] [Google Scholar]
- 9. Soriano-Guillén L, Argente J. Central precocious puberty, functional and tumor-related. Best Pract Res Clin Endocrinol Metab. 2019;33(3):101262. [DOI] [PubMed] [Google Scholar]
- 10. de Vries L, Kauschansky A, Shohat M, Phillip M. Familial central precocious puberty suggests autosomal dominant inheritance. J Clin Endocrinol Metab. 2004;89(4):1794–1800. [DOI] [PubMed] [Google Scholar]
- 11. Maione L, Bouvattier C, Kaiser UB. Central precocious puberty: recent advances in understanding the aetiology and in the clinical approach. Clin Endocrinol. 2021;95(4):542–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perry JR, Day F, Elks CE, et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature. 2014;514(7520):92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abreu AP, Macedo DB, Brito VN, Kaiser UB, Latronico AC. A new pathway in the control of the initiation of puberty: the MKRN3 gene. J Mol Endocrinol. 2015;54(3):R131–R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abreu AP, Dauber A, Macedo DB, et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368(26):2467–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stecchini MF, Macedo DB, Reis AC, et al. Time course of central precocious puberty development caused by an MKRN3 gene mutation: a prismatic case. Horm Res Paediatr. 2016;86(2):126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Canton APM, Krepischi ACV, Montenegro LR, et al. Insights from the genetic characterization of central precocious puberty associated with multiple anomalies. Hum Reprod. 2021;36(2):506–518. [DOI] [PubMed] [Google Scholar]
- 17. Vazquez MJ, Velasco I, Tena-Sempere M. Novel mechanisms for the metabolic control of puberty: implications for pubertal alterations in early-onset obesity and malnutrition. J Endocrinol. 2019;242(2):R51–R65. [DOI] [PubMed] [Google Scholar]
- 18. Soriano-Guillén L, Tena-Sempere M, Seraphim CE, Latronico AC, Argente J. Precocious sexual maturation: unravelling the mechanisms of pubertal onset through clinical observations. J Neuroendocrinol. 2021;34(2):e12979. [DOI] [PubMed] [Google Scholar]
- 19. Day FR, Perry JR, Ong KK. Genetic regulation of puberty timing in humans. Neuroendocrinology. 2015;102(4):247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martos-Moreno GA, Chowen JA, Argente J. Metabolic signals in human puberty: effects of over and undernutrition. Mol Cell Endocrinol. 2010;324(1–2):70–81. [DOI] [PubMed] [Google Scholar]
- 21. Lopez-Rodriguez D, Franssen D, Heger S, Parent AS. Endocrine-disrupting chemicals and their effects on puberty. Best Pract Res Clin Endocrinol Metab. 2021;35(5):101579. [DOI] [PubMed] [Google Scholar]
- 22. Stagi S, Papacciuoli V, Boiro D, et al. Auxological and endocrinological features in internationally adopted children. Ital J Pediatr. 2020;46(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verzani M, Bizzarri C, Chioma L, Bottaro G, Pedicelli S, Cappa M. “Impact of COVID-19 pandemic lockdown on early onset of puberty: experience of an Italian tertiary center”. Ital J Pediatr. 2021;47(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pham HT, DiLalla LF, Corley RP, Dorn LD, Berenbaum SA. Family environmental antecedents of pubertal timing in girls and boys: a review and open questions. Horm Behav. 2022;138:105101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stagi S, De Masi S, Bencini E, et al. Increased incidence of precocious and accelerated puberty in females during and after the Italian lockdown for the coronavirus 2019 (COVID-19) pandemic. Ital J Pediatr. 2020;46(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Day FR, Elks CE, Murray A, Ong KK, Perry JR. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci Rep. 2015;5:11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Zegher F, Ibáňez L. On the rising incidence of early breast development: puberty as an adaptive escape from ectopic adiposity in mismatch girls. Eur J Endocrinol. 2021;185(1):L1–L2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tena-Sempere M. Deciphering puberty: novel partners, novel mechanisms. Eur J Endocrinol. 2012;167(6):733–747. [DOI] [PubMed] [Google Scholar]
- 29. Avendano MS, Vazquez MJ, Tena-Sempere M. Disentangling puberty: novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Hum Reprod Update. 2017;23(6):737–763. [DOI] [PubMed] [Google Scholar]
- 30. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 31. De Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tena-Sempere M. The roles of kisspeptins and G protein-coupled receptor-54 in pubertal development. Curr Opin Pediatr. 2006;18(4):442–447. [DOI] [PubMed] [Google Scholar]
- 33. Lee JH, Miele ME, Hicks DJ, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88(23):1731–1737. [DOI] [PubMed] [Google Scholar]
- 34. Topaloglu AK, Tello JA, Kotan LD, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366(7):629–635. [DOI] [PubMed] [Google Scholar]
- 35. Jayasena CN, Abbara A, Narayanaswamy S, et al. Direct comparison of the effects of intravenous kisspeptin-10, kisspeptin-54 and GnRH on gonadotrophin secretion in healthy men. Hum Reprod. 2015;30(8):1934–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dhillo WS, Chaudhri OB, Patterson M, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90(12):6609–6615. [DOI] [PubMed] [Google Scholar]
- 37. Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update. 2014;20(4):485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. [DOI] [PubMed] [Google Scholar]
- 39. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Clarkson J, Han SY, Piet R, et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci USA. 2017;114(47):E10216–E10E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang C, Bosch MA, Qiu J, Rønnekleiv OK, Kelly MJ. 17β-Estradiol increases persistent Na+ current and excitability of AVPV/PeN Kiss1 neurons in female mice. Mol Endocrinol. 2015;29(4):518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Overgaard A, Tena-Sempere M, Franceschini I, Desroziers E, Simonneaux V, Mikkelsen JD. Comparative analysis of kisspeptin-immunoreactivity reveals genuine differences in the hypothalamic Kiss1 systems between rats and mice. Peptides. 2013;45:85–90. doi: 10.1016/j.peptides.2013.04.013 [DOI] [PubMed] [Google Scholar]
- 43. Wang L, Suzanne. Differential roles of hypothalamic AVPV and arcuate kisspeptin neurons in estradiol feedback regulation of female reproduction. Neuroendocrinology. 2020;110(3–4):172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. George JT, Veldhuis JD, Roseweir AK, et al. Kisspeptin-10 is a potent stimulator of LH and increases pulse frequency in men. J Clin Endocrinol Metab. 2011;96(8):E1228–E1E36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chan Y-M, Butler JP, Sidhoum VF, Pinnell NE, Seminara SB. Kisspeptin administration to women: a window into endogenous kisspeptin secretion and GnRH responsiveness across the menstrual cycle. J Clin Endocrinol Metab. 2012;97(8):E1458–E1E67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dhillo WS. Kisspeptin, neurokinin B and new players in reproduction. Semin Reprod Med. 2019;37(04):153–154. [DOI] [PubMed] [Google Scholar]
- 47. Navarro VM. Metabolic regulation of kisspeptin — the link between energy balance and reproduction. Nat Rev Endocrinol. 2020;16(8):407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. True C, Verma S, Grove KL, Smith MS. Cocaine- and amphetamine-regulated transcript is a potent stimulator of gnrh and kisspeptin cells and may contribute to negative energy balance-induced reproductive inhibition in females. Endocrinology. 2013;154(8):2821–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Navarro VM, Kaiser UB. Metabolic influences on neuroendocrine regulation of reproduction. Curr Opin Endocrinol Diabetes Obes. 2013;20(4):335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fuente-Martín E, García-Cáceres C, Argente-Arizón P, et al. Ghrelin regulates glucose and glutamate transporters in hypothalamic astrocytes. Sci Rep. 2016;6:23673. doi: 10.1038/srep23673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hill JW, Elias CF. Neuroanatomical framework of the metabolic control of reproduction. Physiol Rev. 2018;98(4):2349–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reinehr T, Roth CL. Is there a causal relationship between obesity and puberty? Lancet Child Adolesc Health. 2019;3(1):44–54. [DOI] [PubMed] [Google Scholar]
- 53. De Leonibus C, Marcovecchio ML, Chiavaroli V, de Giorgis T, Chiarelli F, Mohn A. Timing of puberty and physical growth in obese children: a longitudinal study in boys and girls. Pediatr Obes. 2014;9(4):292–299. [DOI] [PubMed] [Google Scholar]
- 54. Marcovecchio ML, Chiarelli F. Obesity and growth during childhood and puberty. World Rev Nutr Diet. 2013;106:135–141. doi: 10.1159/000342545 [DOI] [PubMed] [Google Scholar]
- 55. Deardorff J, Reeves JW, Hyland C, et al. Childhood overweight and obesity and pubertal onset among Mexican American boys and girls in the CHAMACOS longitudinal study. Am J Epidemiol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martos-Moreno G, Martínez-Villanueva J, González-Leal R, Chowen JA, Argente J. Sex, puberty, and ethnicity have a strong influence on growth and metabolic comorbidities in children and adolescents with obesity: report on 1300 patients (the Madrid Cohort). Pediatr Obes. 2019;14(12):e12565. [DOI] [PubMed] [Google Scholar]
- 57. Martos-Moreno G, Martínez-Villanueva J, González-Leal R, et al. Ethnicity strongly influences body fat distribution determining serum adipokine profile and metabolic derangement in childhood obesity. Front Pediatr. 2020;8:551103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. L SG, K S, J A. Precocious puberty. In: Sarafoglou K, HG, Roth R, eds. New York, NY: Editorial Mc Graw Hill; 2017:643–661. [Google Scholar]
- 59. Chehab FF. 20 years of leptin: leptin and reproduction: past milestones, present undertakings, and future endeavors. J Endocrinol. 2014;223(1):T37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roa J, Garcia-Galiano D, Varela L, et al. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology. 2009;150(11):5016–5026. [DOI] [PubMed] [Google Scholar]
- 61. Vazquez MJ, Toro CA, Castellano JM, et al. SIRT1 mediates obesity- and nutrient-dependent perturbation of pubertal timing by epigenetically controlling Kiss1 expression. Nat Commun. 2018;9(1):4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Heras V, Castellano JM, Fernandois D, et al. Central ceramide signaling mediates obesity-induced precocious puberty. Cell Metab. 2020;32(6):951–966.e8. [DOI] [PubMed] [Google Scholar]
- 63. Dauber A, Cunha-Silva M, Macedo DB, et al. Paternally inherited DLK1 deletion associated with familial central precocious puberty. J Clin Endocrinol Metab. 2017;102(5):1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Abreu AP, Toro CA, Song YB, et al. MKRN3 inhibits the reproductive axis through actions in kisspeptin-expressing neurons. J Clin Invest. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Roberts SA, Abreu AP, Navarro VM, et al. The peripubertal decline in makorin ring finger protein 3 expression is independent of leptin action. J Endocr Soc. 2020;4(7):bvaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Navarro VM, Castellano JM, FernáNdez-FernáNdez R, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145(10): 4565–4574. [DOI] [PubMed] [Google Scholar]
- 67. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102(6):2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li C, Lu W, Yang L, et al. MKRN3 regulates the epigenetic switch of mammalian puberty via ubiquitination of MBD3. Natl Sci Rev. 2020;7(3):671–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li C, Han T, Li Q, et al. MKRN3-mediated ubiquitination of Poly(A)-binding proteins modulates the stability and translation of GNRH1 mRNA in mammalian puberty. Nucleic Acids Res. 2021;49(7):3796–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cousminer DL, Stergiakouli E, Berry DJ, et al. Genome-wide association study of sexual maturation in males and females highlights a role for body mass and menarche loci in male puberty. Hum Mol Genet. 2014;23(16):4452–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Harlan WR, Harlan EA, Grillo GP. Secondary sex characteristics of girls 12 to 17 years of age: the U.S. Health Examination Survey. J Pediatr. 1980;96(6):1074–1078. [DOI] [PubMed] [Google Scholar]
- 72. Sun SS, Schubert CM, Chumlea WC, et al. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110(5):911–919. [DOI] [PubMed] [Google Scholar]
- 73. Herman-Giddens ME, Slora EJ, Wasserman RC, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99(4):505–512. [DOI] [PubMed] [Google Scholar]
- 74. Chumlea WC, Schubert CM, Roche AF, et al. Age at menarche and racial comparisons in US girls. Pediatrics. 2003;111(1):110–113. [DOI] [PubMed] [Google Scholar]
- 75. Ramnitz MS, Lodish MB. Racial disparities in pubertal development. Semin Reprod Med. 2013;31(5):333–339. [DOI] [PubMed] [Google Scholar]
- 76. Styne DM. Puberty, obesity and ethnicity. Trends Endocrinol Metab. 2004;15(10):472–478. [DOI] [PubMed] [Google Scholar]
- 77. Eckert-Lind C, Busch AS, Petersen JH, et al. Worldwide secular trends in age at pubertal onset assessed by breast development among girls: a systematic review and meta-analysis. JAMA Pediatr. 2020;174(4):e195881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Soriano-Guillén L, Argente J. [Central precocious puberty: epidemiology, etiology, diagnosis and treatment]. An Pediatr. 2011;74(5):336.e1–. e13. [DOI] [PubMed] [Google Scholar]
- 79. Brito VN, Spinola-Castro AM, Kochi C, Kopacek C, Silva PC, Guerra-Júnior G. Central precocious puberty: revisiting the diagnosis and therapeutic management. Arch Endocrinol Metab. 2016;60(2):163–172. [DOI] [PubMed] [Google Scholar]
- 80. Cantas-Orsdemir S, Garb JL, Allen HF. Prevalence of cranial MRI findings in girls with central precocious puberty: a systematic review and meta-analysis. J Pediatr Endocrinol Metab. 2018;31(7):701–710. [DOI] [PubMed] [Google Scholar]
- 81. Chiu CF, Wang CJ, Chen YP, Lo FS. Pathological and incidental findings in 403 Taiwanese girls with central precocious puberty at initial diagnosis. Front Endocrinol. 2020;11:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Choi KH, Chung SJ, Kang MJ, et al. Boys with precocious or early puberty: incidence of pathological brain magnetic resonance imaging findings and factors related to newly developed brain lesions. Ann Pediatr Endocrinol Metab. 2013;18(4):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Alikasifoglu A, Vuralli D, Gonc EN, Ozon A, Kandemir N. Changing etiological trends in male precocious puberty: evaluation of 100 cases with central precocious puberty over the last decade. Horm Res Paediatr. 2015;83(5):340–344. [DOI] [PubMed] [Google Scholar]
- 84. Wang J, Zhan S, Yuan J, et al. The incidence of brain lesions in central precocious puberty: the main cause for Chinese boys was idiopathic. Clin Endocrinol. 2021;95(2):303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yoon JS, So CH, Lee HS, Lim JS, Hwang JS. The prevalence of brain abnormalities in boys with central precocious puberty may be overestimated. PLoS One. 2018;13(4):e0195209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bessa DS, Macedo DB, Brito VN, et al. High frequency of MKRN3 mutations in male central precocious puberty previously classified as idiopathic. Neuroendocrinology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mogensen SS, Aksglaede L, Mouritsen A, et al. Pathological and incidental findings on brain MRI in a single-center study of 229 consecutive girls with early or precocious puberty. PLoS One. 2012;7(1):e29829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Savas Erdeve S, Ocal G, Berberoglu M, et al. The endocrine spectrum of intracranial cysts in childhood and review of the literature. J Pediatr Endocrinol Metab. 2011;24(11–12):867–875. [DOI] [PubMed] [Google Scholar]
- 89. Helvacıoğlu D, Demircioğlu Turan S, Güran T, et al. Cranial MRI abnormalities and long-term follow-up of the lesions in 770 girls with central precocious puberty. J Clin Endocrinol Metab. 2021;106(7):e2557–e2e66. [DOI] [PubMed] [Google Scholar]
- 90. Higuchi Y, Hasegawa K, Kubo T, Tanaka H, Tsukahara H. The clinical course of Rathke’s cleft cysts in pediatric patients: impact on growth and pubertal development. Clin Pediatr Endocrinol. 2022;31(1):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Harrison VS, Oatman O, Kerrigan JF. Hypothalamic hamartoma with epilepsy: review of endocrine comorbidity. Epilepsia. 2017;58(Suppl 2):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ramos CO, Latronico AC, Cukier P, et al. Long-term outcomes of patients with central precocious puberty due to hypothalamic hamartoma after GnRHa treatment: anthropometric, metabolic, and reproductive aspects. Neuroendocrinology. 2018;106(3):203–210. [DOI] [PubMed] [Google Scholar]
- 93. de Brito VN, Latronico AC, Arnhold IJP, et al. Treatment of gonadotropin dependent precocious puberty due to hypothalamic hamartoma with gonadotropin releasing hormone agonist depot. Arch Dis Child. 1999;80(3):231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cukier P, Castro LH, Banaskiwitz N, et al. The benign spectrum of hypothalamic hamartomas: infrequent epilepsy and normal cognition in patients presenting with central precocious puberty. Seizure. 2013;22(1):28–32. [DOI] [PubMed] [Google Scholar]
- 95. Jung H, Ojeda SR. Pathogenesis of precocious puberty in hypothalamic hamartoma. Horm Res. 2002;57(Suppl 2):31–34. [DOI] [PubMed] [Google Scholar]
- 96. Jung H, Carmel P, Schwartz MS, et al. Some hypothalamic hamartomas contain transforming growth factor alpha, a puberty-inducing growth factor, but not luteinizing hormone-releasing hormone neurons. J Clin Endocrinol Metab. 1999;84(12):4695–4701. [DOI] [PubMed] [Google Scholar]
- 97. Jung H, Parent AS, Ojeda SR. Hypothalamic hamartoma: a paradigm/model for studying the onset of puberty. Endocr Dev. 2005;8:81–93. [DOI] [PubMed] [Google Scholar]
- 98. Chan YM, Fenoglio-Simeone KA, Paraschos S, et al. Central precocious puberty due to hypothalamic hamartomas correlates with anatomic features but not with expression of GnRH, TGFalpha, or KISS1. Horm Res Paediatr. 2010;73(5):312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sullivan SD, Moenter SM. Gamma-aminobutyric acid neurons integrate and rapidly transmit permissive and inhibitory metabolic cues to gonadotropin-releasing hormone neurons. Endocrinology. 2004;145(3):1194–1202. [DOI] [PubMed] [Google Scholar]
- 100. Kerrigan JF, Parsons A, Tsang C, Simeone K, Coons S, Wu J. Hypothalamic hamartoma: neuropathology and epileptogenesis. Epilepsia. 2017;58(Suppl 2):22–31. [DOI] [PubMed] [Google Scholar]
- 101. Wu J, Gao M, Shen JX, Qiu SF, Kerrigan JF. Mechanisms of intrinsic epileptogenesis in human gelastic seizures with hypothalamic hamartoma. CNS Neurosci Ther. 2015;21(2):104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Han SK, Abraham IM, Herbison AE. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology. 2002;143(4):1459–1466. [DOI] [PubMed] [Google Scholar]
- 103. Hildebrand MS, Griffin NG, Damiano JA, et al. Mutations of the sonic hedgehog pathway underlie hypothalamic hamartoma with gelastic epilepsy. Am J Hum Genet. 2016;99(2):423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Carballo GB, Honorato JR, de Lopes GPF, Spohr TCLS. A highlight on Sonic hedgehog pathway. Cell Commun Signal. 2018;16(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Saitsu H, Sonoda M, Higashijima T, et al. Somatic mutations in GLI3 and OFD1 involved in sonic hedgehog signaling cause hypothalamic hamartoma. Ann Clin Transl Neurol. 2016;3(5):356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fujita A, Higashijima T, Shirozu H, et al. Pathogenic variants of DYNC2H1, KIAA0556, and PTPN11 associated with hypothalamic hamartoma. Neurology. 2019;93(3):e237–ee51. [DOI] [PubMed] [Google Scholar]
- 107. Cawthon RM, O’Connell P, Buchberg AM, et al. Identification and characterization of transcripts from the neurofibromatosis 1 region: the sequence and genomic structure of EVI2 and mapping of other transcripts. Genomics. 1990;7(4):555–565. [DOI] [PubMed] [Google Scholar]
- 108. Viskochil D, Buchberg AM, Xu G, et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62(1):187–192. [DOI] [PubMed] [Google Scholar]
- 109. Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356(6371):713–715. [DOI] [PubMed] [Google Scholar]
- 110. Imperial R, Toor OM, Hussain A, Subramanian J, Masood A. Comprehensive pancancer genomic analysis reveals (RTK)-RAS-RAF-MEK as a key dysregulated pathway in cancer: its clinical implications. Semin Cancer Biol. 2019;54:14–28. [DOI] [PubMed] [Google Scholar]
- 111. Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61(3):189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bizzarri C, Bottaro G. Endocrine implications of neurofibromatosis 1 in childhood. Horm Res Paediatr. 2015;83(4):232–241. [DOI] [PubMed] [Google Scholar]
- 113. Habiby R, Silverman B, Listernick R, Charrow J. Precocious puberty in children with neurofibromatosis type 1. J Pediatr. 1995;126(3):364–367. [DOI] [PubMed] [Google Scholar]
- 114. Brauner R, Malandry F, Rappaport R, et al. Growth and endocrine disorders in optic glioma. Eur J Pediatr. 1990;149(12):825–828. [DOI] [PubMed] [Google Scholar]
- 115. Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr. 1994;125(1):63–66. [DOI] [PubMed] [Google Scholar]
- 116. Virdis R, Street ME, Bandello MA, et al. Growth and pubertal disorders in neurofibromatosis type 1. J Pediatr Endocrinol Metab. 2003;16(Suppl 2):289–292. [PubMed] [Google Scholar]
- 117. Cnossen MH, Stam EN, Cooiman LC, et al. Endocrinologic disorders and optic pathway gliomas in children with neurofibromatosis type 1. Pediatrics. 1997;100(4):667–670. [DOI] [PubMed] [Google Scholar]
- 118. Zacharin M. Precocious puberty in two children with neurofibromatosis type I in the absence of optic chiasmal glioma. J Pediatr. 1997;130(1):155–157. [DOI] [PubMed] [Google Scholar]
- 119. Hegedus B, Yeh TH, Lee DY, Emnett RJ, Li J, Gutmann DH. Neurofibromin regulates somatic growth through the hypothalamic-pituitary axis. Hum Mol Genet. 2008;17(19):2956–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Guillamo JS, Créange A, Kalifa C, et al. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain. 2003;126(Pt 1):152–160. [DOI] [PubMed] [Google Scholar]
- 121. Prada CE, Hufnagel RB, Hummel TR, et al. The use of magnetic resonance imaging screening for optic pathway gliomas in children with neurofibromatosis type 1. J Pediatr. 2015;167(4):851–856.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Listernick R, Ferner RE, Piersall L, Sharif S, Gutmann DH, Charrow J. Late-onset optic pathway tumors in children with neurofibromatosis 1. Neurology. 2004;63(10):1944–1946. [DOI] [PubMed] [Google Scholar]
- 123. Chong AL, Pole JD, Scheinemann K, et al. Optic pathway gliomas in adolescence--time to challenge treatment choices? Neuro Oncol. 2013;15(3):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gan HW, Phipps K, Aquilina K, Gaze MN, Hayward R, Spoudeas HA. Neuroendocrine morbidity after pediatric optic gliomas: a longitudinal analysis of 166 children over 30 years. J Clin Endocrinol Metab. 2015;100(10):3787–3799. [DOI] [PubMed] [Google Scholar]
- 125. Starzyk J, Kwiatkowski S, Urbanowicz W, et al. Suprasellar arachnoidal cyst as a cause of precocious puberty--report of three patients and literature overview. J Pediatr Endocrinol Metab. 2003;16(3):447–455. [DOI] [PubMed] [Google Scholar]
- 126. Adan L, Bussières L, Dinand V, Zerah M, Pierre-Kahn A, Brauner R. Growth, puberty and hypothalamic-pituitary function in children with suprasellar arachnoid cyst. Eur J Pediatr. 2000;159(5):348–355. [DOI] [PubMed] [Google Scholar]
- 127. Mohn A, Schoof E, Fahlbusch R, Wenzel D, Dörr HG. The endocrine spectrum of arachnoid cysts in childhood. Pediatr Neurosurg. 1999;31(6):316–321. [DOI] [PubMed] [Google Scholar]
- 128. Almutlaq N, O’Neil J, Fuqua JS. Central precocious puberty in spina bifida children: guidelines for the care of people with spina bifida. J Pediatr Rehabil Med. 2020;13(4):557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Proos LA, Dahl M, Ahlsten G, Tuvemo T, Gustafsson J. Increased perinatal intracranial pressure and prediction of early puberty in girls with myelomeningocele. Arch Dis Child. 1996;75(1):42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dahl M, Proos LA, Ahlsten G, Tuvemo T, Gustafsson J. Increased intracranial pressure perinatally predicts early puberty in girls with myelomeningocele. Eur J Pediatr Surg. 1996;6(Suppl 1):41–42. [PubMed] [Google Scholar]
- 131. Proos LA, Tuvemo T, Ahlsten G, Gustafsson J, Dahl M. Increased perinatal intracranial pressure and brainstem dysfunction predict early puberty in boys with myelomeningocele. Acta Paediatr. 2011;100(10):1368–1372. [DOI] [PubMed] [Google Scholar]
- 132. Stagi S, Bindi G, Galluzzi F, La Cauza F, Salti R. Precocious, early and fast puberty in males with Chiari I malformation. J Pediatr Endocrinol Metab. 2004;17(8):1137–1140. [DOI] [PubMed] [Google Scholar]
- 133. Pucarelli I, Accardo F, Tarani L, Demiraj V, Segni M, Pasquino AM. Precocious puberty in two girls with Chiari I malformation: a contribution to a larger use of brain MRI in the diagnosis of central precocious puberty. Minerva Pediatr. 2010;62(3):315–317. [PubMed] [Google Scholar]
- 134. McCabe MJ, Alatzoglou KS, Dattani MT. Septo-optic dysplasia and other midline defects: the role of transcription factors: HESX1 and beyond. Best Pract Res Clin Endocrinol Metab. 2011;25(1):115–124. [DOI] [PubMed] [Google Scholar]
- 135. Cerbone M, Güemes M, Wade A, Improda N, Dattani M. Endocrine morbidity in midline brain defects: differences between septo-optic dysplasia and related disorders. EClinicalMedicine. 2020;19:100224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hanna CE, Mandel SH, LaFranchi SH. Puberty in the syndrome of septo-optic dysplasia. Am J Dis Child. 1989;143(2):186–189. [DOI] [PubMed] [Google Scholar]
- 137. Wang MX, Segaran N, Bhalla S, et al. Tuberous sclerosis: current update. Radiographics. 2021;210103. [DOI] [PubMed] [Google Scholar]
- 138. Gunatilake S, De Silva DG. Laughing seizures due to a midline intraventricular neoplasm in tuberous sclerosis. Arch Dis Child. 1995;72(5):443–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. de Cornulier M, David A, Cohen JY. [Precocious puberty revealing Bourneville tuberous sclerosis]. Arch Fr Pediatr. 1993;50(5):421–423. [PubMed] [Google Scholar]
- 140. Prezioso G, Petraroli M, Bergonzani M, et al. Duplication of the pituitary gland (DPG)-plus syndrome associated with midline anomalies and precocious puberty: a case report and review of the literature. Front Endocrinol. 2021;12:685888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. de Penna GC, Pimenta MP, Drummond JB, et al. Duplication of the hypophysis associated with precocious puberty: presentation of two cases and review of pituitary embryogenesis. Arq Bras Endocrinol Metabol. 2005;49(2):323–327. [DOI] [PubMed] [Google Scholar]
- 142. Burke M, Zinkovsky S, Abrantes MA, Riley W. Duplication of the hypophysis. Pediatr Neurosurg. 2000;33(2):95–99. [DOI] [PubMed] [Google Scholar]
- 143. Teles MG, Bianco SDC, Brito VN, et al. AGPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358(7):709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bianco SDC, Vandepas L, Correa-Medina M, et al. KISS1R intracellular trafficking and degradation: effect of the Arg386Pro disease-associated mutation. Endocrinology. 2011;152(4):1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Silveira LG, Noel SD, Silveira-Neto AP, et al. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab. 2010;95(5):2276–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Rhie YJ, Lee KH, Ko JM, Lee WJ, Kim JH, Kim HS. KISS1 gene polymorphisms in Korean girls with central precocious puberty. J Korean Med Sci. 2014;29(8):1120–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Day FR, Thompson DJ, Helgason H, et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat Genet. 2017;49(6):834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Tommiska J, Sørensen K, Aksglaede L, et al. LIN28B, LIN28A, KISS1, and KISS1R in idiopathic central precocious puberty. BMC Res Notes. 2011;4(1):363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Krstevska-Konstantinova M, Jovanovska J, Tasic VB, et al. Mutational analysis of KISS1 and KISS1R in idiopathic central precocious puberty. J Pediatr Endocrinol Metab. 2014;27(1–2):199–201. [DOI] [PubMed] [Google Scholar]
- 150. Macedo DB, Abreu AP, Reis AC, et al. Central precocious puberty that appears to be sporadic caused by paternally inherited mutations in the imprinted gene makorin ring finger 3. J Clin Endocrinol Metab. 2014;99(6):E1097–E1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Valadares LP, Meireles CG, De Toledo IP, et al. MKRN3 mutations in central precocious puberty: a systematic review and meta-analysis. J Endocr Soc. 2019;3(5):979–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Simon D, Ba I, Mekhail N, et al. Mutations in the maternally imprinted gene MKRN3 are common in familial central precocious puberty. Eur J Endocrinol. 2016;174(1):1–8. [DOI] [PubMed] [Google Scholar]
- 153. Jong MTC, Carey AH, Caldwell KA, et al. Imprinting of a RING zinc-finger encoding gene in the mouse chromosome region homologous to the Prader-Willi syndrome genetic region. Hum Mol Genet. 1999;8(5):795–803. [DOI] [PubMed] [Google Scholar]
- 154. Jong MT, Gray TA, Ji Y, et al. A novel imprinted gene, encoding a RING zinc-finger protein, and overlapping antisense transcript in the Prader-Willi syndrome critical region. Hum Mol Genet. 1999;8(5):783–793. [DOI] [PubMed] [Google Scholar]
- 155. Bohne A, Darras A, D’Cotta H, Baroiller JF, Galiana-Arnoux D, Volff JN. The vertebrate makorin ubiquitin ligase gene family has been shaped by large-scale duplication and retroposition from an ancestral gonad-specific, maternal-effect gene. BMC Genomics. 2010;11:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Heras V, Sangiao-Alvarellos S, Manfredi-Lozano M, et al. Hypothalamic miR-30 regulates puberty onset via repression of the puberty-suppressing factor, Mkrn3. PLoS Biol. 2019;17(11):e3000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Canton APM, Seraphim CE, Brito VN, Latronico AC. Pioneering studies on monogenic central precocious puberty. Arch Endocrinol Metab. 2019;63(4):438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Liu H, Kong X, Chen F. Mkrn3 functions as a novel ubiquitin E3 ligase to inhibit Nptx1 during puberty initiation. Oncotarget. 2017;8(49):85102–85109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Seraphim CE, Canton APM, Montenegro L, et al. Genotype-phenotype correlations in central precocious puberty caused by MKRN3 mutations. J Clin Endocrinol Metab. 2021;106(4):1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Macedo DB, Franca MM, Montenegro LR, et al. Central precocious puberty caused by a heterozygous deletion in the MKRN3 promoter region. Neuroendocrinology. 2018;107(2):127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Dimitrova-Mladenova MS, Stefanova EM, Glushkova M, et al. Males with paternally inherited MKRN3 mutations may be asymptomatic. J Pediatr. 2016;179:263–265. [DOI] [PubMed] [Google Scholar]
- 162. Neocleous V, Shammas C, Phelan MM, Nicolaou S, Phylactou LA, Skordis N. In silico analysis of a novelMKRN3missense mutation in familial central precocious puberty. Clin Endocrinol. 2016;84(1):80–84. [DOI] [PubMed] [Google Scholar]
- 163. Christoforidis A, Skordis N, Fanis P, et al. A novel MKRN3 nonsense mutation causing familial central precocious puberty. Endocrine. 2017;56(2):446–449. [DOI] [PubMed] [Google Scholar]
- 164. Känsäkoski J, Raivio T, Juul A, Tommiska J. A missense mutation in MKRN3 in a Danish girl with central precocious puberty and her brother with early puberty. Pediatr Res. 2015;78(6):709–711. [DOI] [PubMed] [Google Scholar]
- 165. Schreiner F, Gohlke B, Hamm M, Korsch E, Woelfle J. MKRN3 mutations in familial central precocious puberty. Hormone Res Pediatr. 2014;82(2):122–126. [DOI] [PubMed] [Google Scholar]
- 166. Settas N, Dacou-Voutetakis C, Karantza M, Kanaka-Gantenbein C, Chrousos GP, Voutetakis A. Central precocious puberty in a girl and early puberty in her brother caused by a novel mutation in the MKRN3 gene. J Clin Endocrinol Metab. 2014;99(4):E647–EE51. [DOI] [PubMed] [Google Scholar]
- 167. De Vries L, Gat-Yablonski G, Dror N, Singer A, Phillip M. A novel MKRN3 missense mutation causing familial precocious puberty. Human Reprod. 2014;29(12):2838–2843. [DOI] [PubMed] [Google Scholar]
- 168. Grandone A, Cantelmi G, Cirillo G, et al. A case of familial central precocious puberty caused by a novel mutation in the makorin RING finger protein 3 gene. BMC Endocr Disord. 2015;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Grandone A, Cirillo G, Sasso M, et al. MKRN3 levels in girls with central precocious puberty and correlation with sexual hormone levels: a pilot study. Endocrine. 2018;59(1):203–208. [DOI] [PubMed] [Google Scholar]
- 170. Grandone A, Capristo C, Cirillo G, Sasso M, et al. Molecular screening of MKRN3, DLK1, and KCNK9 genes in girls with idiopathic central precocious puberty. Hormone Res Pediatr. 2017;88(3–4):194–200. [DOI] [PubMed] [Google Scholar]
- 171. Nishioka J, Shima H, Fukami M, et al. The first Japanese case of central precocious puberty with a novel MKRN3 mutation. Hum Genome Var. 2017;4(1):17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Lee H, Jin HS, Shim Y, et al. Low frequency of MKRN3 mutations in central precocious puberty among Korean girls. Horm Metab Res. 2015;48(02):118–122. [DOI] [PubMed] [Google Scholar]
- 173. Ortiz-Cabrera NV, Riveiro-Álvarez R, López-Martínez MÁ, et al. Clinical exome sequencing reveals MKRN3 pathogenic variants in familial and nonfamilial idiopathic central precocious puberty. Hormone Res Pediatr. 2017;87(2):88–94. [DOI] [PubMed] [Google Scholar]
- 174. Lin W-D, Wang C-H, Tsai F-J. Genetic screening of the makorin ring finger 3 gene in girls with idiopathic central precocious puberty. Clin Chem Lab Med. 2016;54(3):e93–ee6. [DOI] [PubMed] [Google Scholar]
- 175. Simsek E, Demiral M, Ceylaner S, Kırel B. Two frameshift mutations in MKRN3 in Turkish patients with familial central precocious puberty. Hormone Res Pediatr. 2017;87(6):405–411. [DOI] [PubMed] [Google Scholar]
- 176. Aycan Z, Savaş-Erdeve S, Çetinkaya S, et al. Investigation of MKRN3 mutation in patients with familial central precocious puberty. J Clin Res Pediatr Endocrinol. 2018;10(3):223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Angulo MA, Butler MG, Cataletto ME. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest. 2015;38(12):1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Kanber D, Giltay J, Wieczorek D, et al. A paternal deletion of MKRN3, MAGEL2 and NDN does not result in Prader–Willi syndrome. Eur J Hum Genet. 2009;17(5):582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14(1):10–26. [DOI] [PubMed] [Google Scholar]
- 180. Costa RA, Ferreira IR, Cintra HA, Gomes LHF, Guida LC. Genotype-phenotype relationships and endocrine findings in Prader-Willi syndrome. Front Endocrinol. 2019;10:864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Maina EN, Webb T, Soni S, et al. Analysis of candidate imprinted genes in PWS subjects with atypical genetics: a possible inactivating mutation in the SNURF/SNRPN minimal promoter. J Hum Genet. 2007;52(4):297–307. [DOI] [PubMed] [Google Scholar]
- 182. Lee HS, Hwang JS. Central precocious puberty in a girl with Prader-Willi syndrome. J Pediatr Endocrinol Metab. 2013;26(11–12):1201–1204. [DOI] [PubMed] [Google Scholar]
- 183. Ludwig NG, Radaeli RF, Silva MMX, et al. A boy with Prader-Willi syndrome: unmasking precocious puberty during growth hormone replacement therapy. Arch Endocrinol Metab. 2016;60(6):596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Wu M-L, Li J, Ding Y, et al. Endocrine and metabolic features of female children with Prader-Willi syndrome: an analysis of 4 cases. Chin J Contemp Pediatr. 2017;19(5):514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Meader BN, Albano A, Sekizkardes H, Delaney A. Heterozygous deletions in MKRN3 cause central precocious puberty without Prader-Willi syndrome. J Clin Endocrinol Metab. 2020;105(8):2732–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ramos CO, Macedo DB, Canton APM, et al. Outcomes of patients with central precocious puberty due to loss-of-function mutations in the MKRN3 gene after treatment with gonadotropin-releasing hormone analog. Neuroendocrinology. 2020;110(7–8):705–713. [DOI] [PubMed] [Google Scholar]
- 187. Macedo DB, Kaiser UB. DLK1, notch signaling and the timing of puberty. Semin Reprod Med. 2019;37(4):174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Temple IK, Cockwell A, Hassold T, Pettay D, Jacobs P. Maternal uniparental disomy for chromosome 14. J Med Genet. 1991;28(8):511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Kagami M, Nagasaki K, Kosaki R, et al. Temple syndrome: comprehensive molecular and clinical findings in 32 Japanese patients. Genet Med. 2017;19(12):1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Ioannides Y, Lokulo-Sodipe K, Mackay DJ, Davies JH, Temple IK. Temple syndrome: improving the recognition of an underdiagnosed chromosome 14 imprinting disorder: an analysis of 51 published cases. J Med Genet. 2014;51(8):495–501. [DOI] [PubMed] [Google Scholar]
- 191. Gomes LG, Cunha-Silva M, Crespo RP, et al. DLK1 is a novel link between reproduction and metabolism. J Clin Endocrinol Metab. 2019;104(6):2112–2120. [DOI] [PubMed] [Google Scholar]
- 192. Montenegro L, Labarta JI, Piovesan M, et al. Novel genetic and biochemical findings of DLK1 in children with central precocious puberty: a Brazilian-Spanish study. J Clin Endocrinol Metab. 2020;105(10). [DOI] [PubMed] [Google Scholar]
- 193. D’Souza B, Meloty-Kapella L, Weinmaster G. Canonical and non-canonical Notch ligands. Curr Top Dev Biol. 2010;92:73–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Falix FA, Aronson DC, Lamers WH, Gaemers IC. Possible roles of DLK1 in the Notch pathway during development and disease. Biochim Biophys Acta. 2012;1822(6):988–995. [DOI] [PubMed] [Google Scholar]
- 195. Villanueva C, Jacquier S, de Roux N. DLK1 is a somato-dendritic protein expressed in hypothalamic arginine-vasopressin and oxytocin neurons. PLoS One. 2012;7(4):e36134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Persson-Augner D, Lee YW, Tovar S, Dieguez C, Meister B. Delta-like 1 homologue (DLK1) protein in neurons of the arcuate nucleus that control weight homeostasis and effect of fasting on hypothalamic DLK1 mRNA. Neuroendocrinology. 2014;100(2–3):209–220. [DOI] [PubMed] [Google Scholar]
- 197. Aujla PK, Naratadam GT, Xu L, Raetzman LT. Notch/Rbpjκ signaling regulates progenitor maintenance and differentiation of hypothalamic arcuate neurons. Development. 2013;140(17):3511–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Biehl MJ, Raetzman LT. Rbpj-κ mediated Notch signaling plays a critical role in development of hypothalamic Kisspeptin neurons. Dev Biol. 2015;406(2):235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Roa J, Tena-Sempere M. Connecting metabolism and reproduction: roles of central energy sensors and key molecular mediators. Mol Cell Endocrinol. 2014;397(1–2):4–14. [DOI] [PubMed] [Google Scholar]
- 200. Traustadóttir G, Lagoni LV, Ankerstjerne LBS, Bisgaard HC, Jensen CH, Andersen DC. The imprinted gene Delta like non-canonical Notch ligand 1 (Dlk1) is conserved in mammals, and serves a growth modulatory role during tissue development and regeneration through Notch dependent and independent mechanisms. Cytokine Growth Factor Rev. 2019;46:17–27. [DOI] [PubMed] [Google Scholar]
- 201. Villena JA, Choi CS, Wang Y, et al. Resistance to high-fat diet-induced obesity but exacerbated insulin resistance in mice overexpressing preadipocyte factor-1 (Pref-1): a new model of partial lipodystrophy. Diabetes. 2008;57(12):3258–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Kavalkova P, Touskova V, Roubicek T, et al. Serum preadipocyte factor-1 concentrations in females with obesity and type 2 diabetes mellitus: the influence of very low calorie diet, acute hyperinsulinemia, and fenofibrate treatment. Horm Metab Res. 2013;45(11):820–826. [DOI] [PubMed] [Google Scholar]
- 203. Abdallah BM, Ditzel N, Laborda J, Karsenty G, Kassem M. DLK1 regulates whole-body glucose metabolism: a negative feedback regulation of the osteocalcin-insulin loop. Diabetes. 2015;64(9):3069–3080. [DOI] [PubMed] [Google Scholar]
- 204. Meister B, Perez-Manso M, Daraio T. Delta-like 1 homologue is a hypothalamus-enriched protein that is present in orexin-containing neurones of the lateral hypothalamic area. J Neuroendocrinol. 2013;25(7):617–625. [DOI] [PubMed] [Google Scholar]
- 205. Rhee M, Kim JW, Lee MW, Yoon KH, Lee SH. Preadipocyte factor 1 regulates adipose tissue browning via TNF-α-converting enzyme-mediated cleavage. Metabolism. 2019;101:153977. [DOI] [PubMed] [Google Scholar]
- 206. Wermter AK, Scherag A, Meyre D, et al. Preferential reciprocal transfer of paternal/maternal DLK1 alleles to obese children: first evidence of polar overdominance in humans. Eur J Hum Genet. 2008;16(9):1126–1134. [DOI] [PubMed] [Google Scholar]
- 207. Moon YS, Smas CM, Lee K, et al. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol Cell Biol. 2002;22(15):5585–5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Charalambous M, Da Rocha ST, Radford EJ, et al. DLK1/PREF1 regulates nutrient metabolism and protects from steatosis. Proc Natl Acad Sci USA. 2014;111(45):16088–16093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Jensen CH, Kosmina R, Rydén M, et al. The imprinted gene Delta like non-canonical notch ligand 1 (Dlk1) associates with obesity and triggers insulin resistance through inhibition of skeletal muscle glucose uptake. EBioMedicine. 2019;46:368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Seraphim CE, Argente J, Latronico AC. Delta-like 1 homolog genetics and its emerging role in human puberty. Curr Opin Endocr Metab Res. 2020:22– 28. [Google Scholar]
- 211. Wannes S, Elmaleh-Bergès M, Simon D, et al. High prevalence of syndromic disorders in patients with non-isolated central precocious puberty. Eur J Endocrinol. 2018;179(6):373–380. [DOI] [PubMed] [Google Scholar]
- 212. Abi Habib W, Brioude F, Azzi S, et al. Transcriptional profiling of the DLK1/MEG3 domain explains clinical overlap between imprinting disorders. Sci Adv. 2019;5(2):eaau9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Geoffron S, Abi Habib W, Chantot-Bastaraud S, et al. Chromosome 14q32.2 imprinted region disruption as an alternative molecular diagnosis of Silver-Russell Syndrome. J Clin Endocrinol Metab. 2018;103(7):2436–2446. [DOI] [PubMed] [Google Scholar]
- 214. Meader BN, Albano A, Sekizkardes H, Delaney A. Heterozygous deletions in MKRN3 cause central precocious puberty without Prader-Willi syndrome. J Clin Endocrinol Metab. 2020;105(8):2732–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Wakeling EL, Brioude F, Lokulo-Sodipe O, et al. Diagnosis and management of Silver-Russell syndrome: first international consensus statement. Nat Rev Endocrinol. 2017;13(2):105–124. [DOI] [PubMed] [Google Scholar]
- 216. Wollmann HA, Kirchner T, Enders H, Preece MA, Ranke MB. Growth and symptoms in Silver-Russell syndrome: review on the basis of 386 patients. Eur J Pediatr. 1995;154(12):958–968. [DOI] [PubMed] [Google Scholar]
- 217. Pober BR. Williams-Beuren syndrome. N Engl J Med. 2010;362(3):239–252. [DOI] [PubMed] [Google Scholar]
- 218. Kozel BA, Barak B, Kim CA, et al. Williams syndrome. Nat Rev Dis Primers. 2021;7(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Partsch CJ, Japing I, Siebert R, et al. Central precocious puberty in girls with Williams syndrome. J Pediatr. 2002;141(3):441–444. [DOI] [PubMed] [Google Scholar]
- 220. Kurosawa K, Kawame H, Okamoto N, et al. Epilepsy and neurological findings in 11 individuals with 1p36 deletion syndrome. Brain Dev. 2005;27(5):378–382. [DOI] [PubMed] [Google Scholar]
- 221. Cisternino M, Della Mina E, Losa L, et al. Idiopathic central precocious puberty associated with 11 mb de novo distal deletion of the chromosome 9 short arm. Case Rep Genet. 2013;2013:978087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Giannakopoulos A, Fryssira H, Tzetis M, Xaidara A, Kanaka-Gantenbein C. Central precocious puberty in a boy with 22q13 deletion syndrome and NOTCH-1 gene duplication. J Pediatr Endocrinol Metab. 2016;29(11):1307–1311. [DOI] [PubMed] [Google Scholar]
- 223. Giorda R, Bonaglia MC, Beri S, et al. Complex segmental duplications mediate a recurrent dup(X)(p11.22-p11.23) associated with mental retardation, speech delay, and EEG anomalies in males and females. Am J Hum Genet. 2009;85(3):394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Nizon M, Andrieux J, Rooryck C, et al. Phenotype-genotype correlations in 17 new patients with an Xp11.23p11.22 microduplication and review of the literature. Am J Med Genet A. 2015;167A(1):111– 122. [DOI] [PubMed] [Google Scholar]
- 225. Grams SE, Argiropoulos B, Lines M, et al. Genotype-phenotype characterization in 13 individuals with chromosome Xp11.22 duplications. Am J Med Genet A. 2016;170A(4):967–977. [DOI] [PubMed] [Google Scholar]
- 226. Baş VN, Çetinkaya S, Ağladıoğlu SY, Aksoy A, Gülpınar B, Aycan Z. Report of the first case of precocious puberty in Rett syndrome. J Pediatr Endocrinol Metab. 2013;26(9–10):937–939. [DOI] [PubMed] [Google Scholar]
- 227. Bernstein U, Demuth S, Puk O, Eichhorn B, Schulz S. Novel MECP2 mutation c.1162_1172del; p.Pro388* in two patients with symptoms of atypical Rett syndrome. Mol Syndromol. 2019;10(4):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Tsuji-Hosokawa A, Matsuda N, Kurosawa K, Kashimada K, Morio T. A case of MECP2 duplication syndrome with gonadotropin-dependent precocious puberty. Horm Res Paediatr. 2017;87(4):271–276. [DOI] [PubMed] [Google Scholar]
- 229. Snijders Blok L, Madsen E, Juusola J, et al. Mutations in DDX3X are a common cause of unexplained intellectual disability with gender-specific effects on wnt signaling. Am J Hum Genet. 2015;97(2):343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Geier DA, Geier MR. A Longitudinal cohort study of precocious puberty and autism spectrum disorder. Horm Res Paediatr. 2021;94(5–6):219–228. [DOI] [PubMed] [Google Scholar]
- 231. Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2012;13(2):97–109. [DOI] [PubMed] [Google Scholar]
- 232. Lomniczi A, Ojeda SR. The emerging role of epigenetics in the regulation of female puberty. Endocr Dev. 2016;29:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Feinberg AP. The key role of epigenetics in human disease prevention and mitigation. N Engl J Med. 2018;378(14):1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Suzuki E, Shima H, Kagami M, et al. (Epi)genetic defects of MKRN3 are rare in Asian patients with central precocious puberty. Hum Genome Var. 2019;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Bessa DS, Maschietto M, Aylwin CF, et al. Methylome profiling of healthy and central precocious puberty girls. Clin Epigenetics. 2018;10(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Chen S, Mukherjee N, Janjanam VD, et al. Consistency and variability of DNA methylation in women during puberty, young adulthood, and pregnancy. Genet Epigenet. 2017;9:1179237X–17721540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Almstrup K, Lindhardt Johansen M, Busch AS, et al. Pubertal development in healthy children is mirrored by DNA methylation patterns in peripheral blood. Sci Rep. 2016;6:28657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Thompson EE, Nicodemus-Johnson J, Kim KW, et al. Global DNA methylation changes spanning puberty are near predicted estrogen-responsive genes and enriched for genes involved in endocrine and immune processes. Clin Epigenetics. 2018; 10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Chen S, Refaey H, Mukherjee N, et al. Age at onset of different pubertal signs in boys and girls and differential DNA methylation at age 10 and 18 years: an epigenome-wide follow-up study. Hum Reprod Open. 2020;2020(2):hoaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Vazquez MJ, Daza-Dueñas S, Tena-Sempere M. Emerging roles of epigenetics in the control of reproductive function: focus on central neuroendocrine mechanisms. J Endocr Soc. 2021;5(11):bvab152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Lomniczi A, Loche A, Castellano JM, et al. Epigenetic control of female puberty. Nat Neurosci. 2013;16(3):281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Wright H, Aylwin CF, Toro CA, Ojeda SR, Lomniczi A. Polycomb represses a gene network controlling puberty via modulation of histone demethylase Kdm6b expression. Sci Rep. 2021;11(1):1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Lomniczi A, Wright H, Castellano JM, et al. Epigenetic regulation of puberty via Zinc finger protein-mediated transcriptional repression. Nat Commun. 2015;6:10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Messina A, Langlet F, Chachlaki K, et al. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nat Neurosci. 2016;19(6):835–844. [DOI] [PubMed] [Google Scholar]
- 245. Varimo T, Wang Y, Miettinen PJ, Vaaralahti K, Hero M, Raivio T. Circulating miR-30b levels increase during male puberty. Eur J Endocrinol. 2021;184(5):K11–KK4. [DOI] [PubMed] [Google Scholar]
- 246. Stephen MD, Zage PE, Waguespack SG. Gonadotropin-dependent precocious puberty: neoplastic causes and endocrine considerations. Int J Pediatr Endocrinol. 2011;2011(1):184502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Chalumeau M, Chemaitilly W, Trivin C, Adan L, Bréart G, Brauner R. Central precocious puberty in girls: an evidence-based diagnosis tree to predict central nervous system abnormalities. Pediatrics. 2002;109(1):61–67. [DOI] [PubMed] [Google Scholar]
- 248. Wilne S, Collier J, Kennedy C, Koller K, Grundy R, Walker D. Presentation of childhood CNS tumours: a systematic review and meta-analysis. Lancet Oncol. 2007;8(8):685–695. [DOI] [PubMed] [Google Scholar]
- 249. Ma YJ, Berg-von der Emde K, Rage F, Wetsel WC, Ojeda SR. Hypothalamic astrocytes respond to transforming growth factor-alpha with the secretion of neuroactive substances that stimulate the release of luteinizing hormone-releasing hormone. Endocrinology. 1997;138(1):19–25. [DOI] [PubMed] [Google Scholar]
- 250. Marchetti B. Cross-talk signals in the CNS: role of neurotrophic and hormonal factors, adhesion molecules and intercellular signaling agents in luteinizing hormone-releasing hormone (LHRH)-astroglial interactive network. Front Biosci. 1997;2:d88–125. [DOI] [PubMed] [Google Scholar]
- 251. D’Andrea AD, Packer RJ, Rorke LB, et al. Pineocytomas of childhood. A reappraisal of natural history and response to therapy. Cancer. 1987;59(7):1353–1357. [DOI] [PubMed] [Google Scholar]
- 252. Chemaitilly W, Merchant TE, Li Z, et al. Central precocious puberty following the diagnosis and treatment of paediatric cancer and central nervous system tumours: presentation and long-term outcomes. Clin Endocrinol. 2016;84(3):361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Rose SR, Horne VE, Howell J, et al. Late endocrine effects of childhood cancer. Nat Rev Endocrinol. 2016;12(6):319–336. [DOI] [PubMed] [Google Scholar]
- 254. Haller MJ, Schatz DA. Endocrine complications of childhood cancer therapy: evaluation and management. Pediatr Endocrinol Rev. 2007;4(3):196–204. [PubMed] [Google Scholar]
- 255. Oberfield SE, Soranno D, Nirenberg A, et al. Age at onset of puberty following high-dose central nervous system radiation therapy. Arch Pediatr Adolesc Med. 1996;150(6):589–592. [DOI] [PubMed] [Google Scholar]
- 256. Bruzzi P, Messina MF, Bartoli A, et al. Central precocious puberty and response to GnRHa therapy in children with cerebral palsy and moderate to severe motor impairment: data from a longitudinal, case-control, multicentre, Italian study. Int J Endocrinol. 2017;2017:4807163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Worley G, Houlihan CM, Herman-Giddens ME, et al. Secondary sexual characteristics in children with cerebral palsy and moderate to severe motor impairment: a cross-sectional survey. Pediatrics. 2002;110(5):897–902. [DOI] [PubMed] [Google Scholar]
- 258. Svalheim S, Sveberg L, Mochol M, Taubøll E. Interactions between antiepileptic drugs and hormones. Seizure. 2015;28:12–17. [DOI] [PubMed] [Google Scholar]
- 259. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Lopez-Rodriguez D, Franssen D, Bakker J, Lomniczi A, Parent AS. Cellular and molecular features of EDC exposure: consequences for the GnRH network. Nat Rev Endocrinol. 2021;17(2):83–96. [DOI] [PubMed] [Google Scholar]
- 261. Gore AC, Chappell VA, Fenton SE, et al. EDC-2: the Endocrine Society’s Second Scientific Statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36(6):E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Uldbjerg CS, Koch T, Lim YH, et al. Prenatal and postnatal exposures to endocrine disrupting chemicals and timing of pubertal onset in girls and boys: a systematic review and meta-analysis. Hum Reprod Update. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Kundakovic M, Gudsnuk K, Franks B, et al. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci USA. 2013;110(24):9956–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Cheong A, Johnson SA, Howald EC, et al. Gene expression and DNA methylation changes in the hypothalamus and hippocampus of adult rats developmentally exposed to bisphenol A or ethinyl estradiol: a CLARITY-BPA consortium study. Epigenetics. 2018;13(7):704–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Ernst A, Brix N, Lauridsen LLB, et al. Acetaminophen (Paracetamol) exposure during pregnancy and pubertal development in boys and girls from a nationwide puberty cohort. Am J Epidemiol. 2019;188(1):34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401(6755):763–764. [DOI] [PubMed] [Google Scholar]
- 267. Division . UNDoEaSAP. Child Adoption: Trends and Policies. V. Levels and Trends in Child Adoptions. New York: United Nations Publications; 2009. [Google Scholar]
- 268. Dawood F, Serwint JR. International adoption. Pediatr Rev. 2008;29(8):292–294. [DOI] [PubMed] [Google Scholar]
- 269. Teilmann G, Petersen JH, Gormsen M, Damgaard K, Skakkebaek NE, Jensen TK. Early puberty in internationally adopted girls: hormonal and clinical markers of puberty in 276 girls examined biannually over two years. Horm Res. 2009;72(4):236–246. [DOI] [PubMed] [Google Scholar]
- 270. Virdis R, Street ME, Zampolli M, et al. Precocious puberty in girls adopted from developing countries. Arch Dis Child. 1998;78(2):152–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Baron S, Battin J, David A, Limal JM. [Precocious puberty in children adopted from foreign countries]. Arch Pediatr. 2000;7(8):809–816. [DOI] [PubMed] [Google Scholar]
- 272. Krstevska-Konstantinova M, Charlier C, Craen M, et al. Sexual precocity after immigration from developing countries to Belgium: evidence of previous exposure to organochlorine pesticides. Hum Reprod. 2001;16(5):1020–1026. [DOI] [PubMed] [Google Scholar]
- 273. Kempers MJ, Otten BJ. Idiopathic precocious puberty versus puberty in adopted children; auxological response to gonadotrophin-releasing hormone agonist treatment and final height. Eur J Endocrinol. 2002;147(5):609–616. [DOI] [PubMed] [Google Scholar]
- 274. Teilmann G, Pedersen CB, Skakkebaek NE, Jensen TK. Increased risk of precocious puberty in internationally adopted children in Denmark. Pediatrics. 2006;118(2):e391–e399. [DOI] [PubMed] [Google Scholar]
- 275. Harbulot C, Lessim S, Simon D, et al. Prevalence and clinical characteristics of isolated forms of central precocious puberty: a cohort study at a single academic center. Eur J Endocrinol. 2021;184(2):243–251. [DOI] [PubMed] [Google Scholar]
- 276. Mastrangelo A, Martos-Moreno G, García A, et al. Insulin resistance in prepubertal obese children correlates with sex-dependent early onset metabolomic alterations. Int J Obes. 2016;40(10):1494–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Hiatt RA, Stewart SL, Deardorff J, et al. Childhood socioeconomic status and menarche: a prospective study. J Adolesc Health. 2021;69(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Stecchini MF, Braid Z, More CB, et al. Gonadotropin-dependent pubertal disorders are common in patients with virilizing adrenocortical tumors in childhood. Endocr Connect. 2019;8(5):579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Almeida MQ, Brito VN, Lins TS, et al. Long-term treatment of familial male-limited precocious puberty (testotoxicosis) with cyproterone acetate or ketoconazole. Clin Endocrinol. 2008;69(1):93–98. [DOI] [PubMed] [Google Scholar]
- 280. Neeman B, Bello R, Lazar L, Phillip M, de Vries L. Central precocious puberty as a presenting sign of nonclassical congenital adrenal hyperplasia: clinical characteristics. J Clin Endocrinol Metab. 2019;104(7):2695–2700. [DOI] [PubMed] [Google Scholar]
- 281. Li M, Chen Y, Liao B, Tang J, Zhong J, Lan D. The role of kisspeptin and MKRN3 in the diagnosis of central precocious puberty in girls. Endocr Connect. 2021;10(9): 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Kang MJ, Oh YJ, Shim YS, Baek JW, Yang S, Hwang IT. The usefulness of circulating levels of leptin, kisspeptin, and neurokinin B in obese girls with precocious puberty. Gynecol Endocrinol. 2018;34(7):627–630. [DOI] [PubMed] [Google Scholar]
- 283. Hagen CP, Sørensen K, Mieritz MG, Johannsen TH, Almstrup K, Juul A. Circulating MKRN3 levels decline prior to pubertal onset and through puberty: a longitudinal study of healthy girls. J Clin Endocrinol Metab. 2015;100(5):1920–1926. [DOI] [PubMed] [Google Scholar]
- 284. Lakshman R, Forouhi NG, Sharp SJ, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94(12):4953–4960. [DOI] [PubMed] [Google Scholar]
- 285. Prentice P, Viner RM. Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int J Obes. 2013;37(8):1036–1043. [DOI] [PubMed] [Google Scholar]
- 286. Roberts C. Psychosocial dimensions of early-onset puberty and its treatment. Lancet Diabetes Endocrinol. 2016;4(3):195–197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.