Abstract
The most common type of dementia disease is Alzheimer, which placing a heavy burden on the healthcare system all over the world. At the same time, psoriasis is also one of the most common health problems, as a skin disease. Alzheimer's disease (AD) is more often in patients with psoriasis than in the general people. Several evidence has proved the relation between AD and psoriasis through immune-mediated pathophysiologic processes. This review aims to summary the potential relation between AD and psoriasis, and provide suggestions based on the relationship at the same time. Neurologists, dermatologists should pay attention to the relationship between Alzheimer’s disease and psoriasis. Dermatology and neurology need referral each other when it is necessary.
Keywords: psoriasis, Alzheimer, IL-17, relation, inflammation, genetic
Introduction
Alzheimer’s disease (AD) is a chronic neurodegenerative disease, as the most common cause of dementia in the elderly, affecting approximately 30 million people all over the world in 2015.1,2 As we all know, the pathogenesis of AD remains unclear, but nowadays it is thought that various environmental stimuli, brain inflammation, and genetic susceptibility may cause the disease. More and more evidence has implied that aberrant immune responses, such as T-helper (Th)1/Th17 cells and cytokines, may associated with neurodegeneration in AD through inflammation level.3–5 The hallmark of AD is neurofibrillary tangles and amyloid plaques.6
Psoriasis, characterized by plaques and scales, is an immune-mediated, chronic skin disease.7 It not only causes a tremendous economic burden, but also affects about 2% of the world’s population. As the most common form of psoriasis, plaque psoriasis, accounts for 90% of cases.8 The pathogenesis of psoriasis is considered as immunological, genetic, as well as multifactorial and environmental factors.9 Moreover, psoriasis can also lead to physical impairment and inability to work and decrease quality of life of patients significantly, as a crucial social issue.10 The immune pathways in psoriasis involve NK cells, macrophage cells trigger, dendritic cells, and amplify topical Inflammation. Psoriasis is a Th1/Th17-mediated skin disease involving IL-17 and TNF-α signaling pathways.11,12 The treatment for psoriasis has transferred from traditional strategies to biologics targeting IL-17A and TNF-α which had been demonstrated more effective. A new era of psoriasis treatment has begun when biologic agents became the first-line protocol in treating moderate to severe psoriasis all over the world.13 Biologic agents usually target IL-12, 17, 23, TNF-α, or their receptors.14
Genome-wide association studies (GWASs) have implied that inflammation could affect progression and pathogenesis of AD, as well demonstrated genetic overlap between psoriasis and AD.15 In addition, recent study by Ji Hyun Lee proved psoriasis may be associated with an increased risk of mental health disorders.12 But psoriasis occurs in patients much younger than AD, it may be that psoriasis could influence AD. An important question has been raised concerning about whether psoriasis and AD are linked. Our review aims to discuss the relation between psoriasis and AD. We hope we can give the neurologists and dermatologists a novel perspective on the relationship between AD and psoriasis.
Materials and Methods
N.Y.M searched the articles from the PubMed and Web of science. We selected the articles published in the last five years. A combination of Medical Subject Heading terms and free terms, including “AND” and “OR” Boolean operators, was utilized to retrieve the targeted literatures. The search string was psoriasis AND Alzheimer.
Alzheimer’s Disease
As the most frequent cause of dementia, Alzheimer’s disease is nearly responsible for 50–75% of all its cases. It will rob people’s ability to live independently finally, as the most common cause of dementia. During decades of research, there is still no prescription to slow the progression of AD, to say nothing of a curable therapy, the pathogenic mechanism exists still unclear.16 The hyperphosphorylated tau in the brain and accumulation of beta-amyloid peptides (Ab) are the most possible hypothesis of pathogenesis for AD. Nevertheless, this hypothesis has a problem, AD was allowed as to be treated based on irreversible stages instead of one that can be cured. We need to find earlier-stage targets of AD, and then that action could be taken before irreversible damage occurs.17
Moreover, according to the state of the art of dementia research: new frontiers which published in Alzheimer’s Disease International World Alzheimer Report 2018, especially after the age of 80 years, women are more likely to get Alzheimer’s disease than are men. A higher tau load may influence women, though it has a similar amyloid β burden.18,19 From a study of twins, the risk of Alzheimer’s disease is mainly dependent on heritable factors for nearly 60–80%.20 A substantial part of Alzheimer’s disease can be explained by the common APOE ε4 allele, but cannot completely account for the heritability of it.21,22 To identify novel genetic variants in Alzheimer’s disease, the number of Alzheimer’s disease-associated risk alleles were increased to more than 40 through large genome-wide association studies.23 Compared with noncarriers, an estimated 2 times decreased lifetime risk of Alzheimer’s disease was found in carriers of the protective APOE ε2 allele. In other words, homozygous APOE ε2 allele carriers have an exceptionally low likelihood of Alzheimer’s disease.24,25
The preclinical phase of Alzheimer’s disease was called as the cellular phase by basic scientists. Before cognitive impairment is observed, the insidious progression of the disease was drive by alterations in microglia, neurons, and astroglia.26 In this cellular disease landscape, alterations in the vessels, dysfunction of the glymphatic system, ageing, and neuroinflammation act upstream or in parallel to accumulating amyloid β.27–30 Though the central in the study of Alzheimer’s disease is cellular pathology, there are still great progress in understanding the preceding biochemical phase of this disease. The study about function on purified γ-secretase complexes proved aggregation-prone, and premature release of longer amyloid β peptides led for clinical mutations in presenilins destabilise the γ-secretase–APP interactions.31 These insights may support potential therapeutic approaches to tackle amyloid β in Alzheimer’s disease.
It is clear that the blood–brain barrier, the glymphatic, the vasculature and other clearance systems of the brain, the potentially the gastrointestinal microbiome, and the peripheral immune system affect the clinical development of the disease.32,33 Blood–brain barrier integrity is also affected by vascular pathology. In the past, the pathophysiology and genetic basis of Alzheimer’s disease have substantial progress. If it keeps up this pace, multimodal treatment and very early identification of patients will become a reality in the future.
Psoriasis
Psoriasis is a disfiguring chronic and common skin condition. In the past 100 years, our understanding of this disease has improved fast, and then more effective therapies have also been developed. One hundred years ago, psoriasis was recognized as a remitting and relapsing disease in dermatology. It was seen only can temporary remission, but not cure after treatment.34 But nowadays, it has been an outdated approach for treating psoriasis as an isolated skin disease. Psoriasis is now believed as a systemic immune-mediated inflammatory disease associated with several comorbidities, such as cardiovascular disease, psoriatic arthritis and mood disorders.
During the past report, there are five types of psoriasis in patients such as guttate (droplet) or eruptive psoriasis, which is characterised by scaly teardrop-shaped spots, plaque psoriasis (also known as psoriasis vulgaris), pustular psoriasis, which can either generalised pustular psoriasis (a rare and serious form of psoriasis), or take the form of palmoplantar pustulosis (pustular psoriasis of the palms and soles), erythrodermic psoriasis, which is a rare but very serious complication of psoriasis, and plaque psoriasis (also known as psoriasis vulgaris).
Diagnosis of psoriasis is usually made on clinical findings, at the same time skin biopsy is seldom used to diagnose psoriasis. People usually use the Psoriasis Area and Severity Index (PASI) score to quantify disease severity of infiltration, scaling, thickness, the extent of lesions and erythema in patients with widespread disease.35 Psoriasis can be triggered by infections, it is not contagious. The physician in dermatology should enquire more about patient’s history for up to 30% of patients in psoriasis develop psoriatic arthritis.36
The function of immune system in psoriasis is now widely accepted by all over the world.37 Genome-wide scans for psoriasis have identified predominantly immune-related genes, providing a relation between genetics and immunity.38 The dysregulated interactions in the immune system with resident cutaneous cell types can influence psoriatic skin lesions. Several highly specific therapies have been set by research of the immunopathogenesis in psoriasis. Psoriasis is mainly a T-cell-mediated and dendritic cell disease which has complex feedback loops from keratinocytes, vascular endothelial cells, neutrophilic granulocytes, antigen-presenting cells, and the cutaneous nervous system. Interest in the interleukin-23/Th17 axis in psoriasis is rising now, it has generated several novel targeted therapies.39 As a subset of T-lymphocytes expressing interleukin 17, Th17 cells are distinct from the classical Th17 cells, which play a predominant role in the pathogenesis for psoriasis and other inflammatory disorders as well.40 Almost every cutaneous cell type with complex dysregulation, especially cytokine production and proliferation by epidermal keratinocytes, is affected by the interleukin-23/Th17 axis pathway and TNFα pathway.
The management of psoriasis over the past 100 years should be celebrated as an outstanding example of successful translational research. The best approach to care patients in psoriasis is an individualised one. We should pay attention to improve the physical symptoms of the rash and try our best to increase the quality of patients’ life, empower patients to live well.
The Relation Between Psoriasis and Alzheimer
As we all know, Alzheimer’s Disease and psoriasis are both inflammatory disorders, and their relation has been investigated.
An increased risk for psoriasis patients to develop Alzheimer’s Disease has been demonstrated by previous studies, with a risk ratio ranging from 1.10 to 1.25.41,42 The risk also increases among dementia patients developing psoriasis.43 But there is a study proved psoriasis had a surprisingly protective function for Alzheimer’s Disease in 2008 (HR=0.54).44 Furthermore, the risk of developing non-vascular dementia and vascular dementia in psoriasis patients was higher than those without psoriasis (RR=1.41, RR=1.13, respectively) according to a recent meta-analysis.45 The link between psoriasis and Alzheimer’s disease was investigated in Korea by a population-based case-control cohort study, a slightly but significantly increasing level was found of Alzheimer’s disease in psoriasis patients (HR=1.09) than those without it.46 One study revealed that psoriasis patients had a higher risk of mild cognitive impairment, such as verbal memory, executive function and visuospatial function.47
Pathogenesis
The psoriasis’ and Alzheimer’s disease’s mechanisms is complicated and remain unclear. In psoriasis, dendritic cells and activated T cells accumulated producing cytokines such as IL-23 and TNFα, and these cytokines will act on keratinocytes. TNF-α can also play a vital role in the pathogenesis of Alzheimer’s disease, exacerbating tau and Aβ pathologies in vivo.48 In patients with Alzheimer’s disease TNF blocking agents can improve the cognitive function. During a case-control study, patients in psoriasis treated with TNF blocking agents such as adalimumab, infliximab and etanercept have a lower possibility for developing Alzheimer’s disease (OR =0.47) than those patients without such treatment.49
One of the most important axis is IL-12/23 axis in psoriasis development, and IL-12/ IL-23 common subunit p40 targeted by monoclonal antibodies are widely used in treating psoriasis.50 The importance of IL-23/IL-12A axis has been verified in Alzheimer’s disease about the pathogenesis of age-associated inflammation from recent reviews.51,52 At the same time, we can find p40 level in cerebrospinal fluid was increased from the mouse model of Alzheimer’s disease, and the blockage of p40 improved cognitive deficits and led to fewer Aβ plaques.3
Genetic
Genetic can also be a evidence in the link between psoriasis and Alzheimer’s disease. The strongest genetic risk factor is Apolipoprotein E (APOE) for Alzheimer’s disease. APOE, the main cholesterol carrier, can greatly affect tau phosphorylation and Aβ deposition and is related to other neurodegenerative disorders and cardiovascular diseases as well.53 In addition, APOE genotypes can also be an independent risk factor for the severity and onset of psoriasis in many studies.54–56 During a meta-analysis with seven studies, including 966 psoriasis patients and 1086 controls, it revealed that people with ε2 allele could increase the risk, whereas ε3/ε3 genotype or ε3 allele had a decreased possibility to develop psoriasis.56 In a study about the genetic overlap between their two diseases, two pleiotropic loci and eight polymorphisms were found associated with Alzheimer’s disease and psoriasis.15
During a study from Shougang Liu, their work proposed that STFs (ZFPM2, HLX, ANHX, PPARG and ZNF415) mediate the metabolic disorders and initiation of chronic inflammation and increase the risk of developing Alzheimer’s Disease in the psoriasis population. ZNF384, potential transcription factor, may modulate STFs ZNF415, ANHX, HLX and PPARG, thus subsequently metabolic syndromes and triggering hyperinflammatory states. A potential therapeutic method is targeting ZNF384 for treating AD and plaque psoriasis.57
Clinical
From a study Miri Kim in Korea about a nationwide population-based cohort study which discussed the increased risk of Alzheimer’s disease in patients with psoriasis. The conclusion proved that compared to elderly (≥65 years) patients (HR 1.30 vs 1.08, p < 0.0001), middle-aged (40–64 years) was more pronounced. Of course, the incidence of events and absolute number were high above 65 years and low under 65. But the people who develop psoriasis at younger ages (40–60 years) are more likely to develop AD than age-matched individuals who are not diagnosed with psoriasis. The patients more than 40 years with psoriasis should pay attention and even MRI screen for Alzheimer.
Discussion
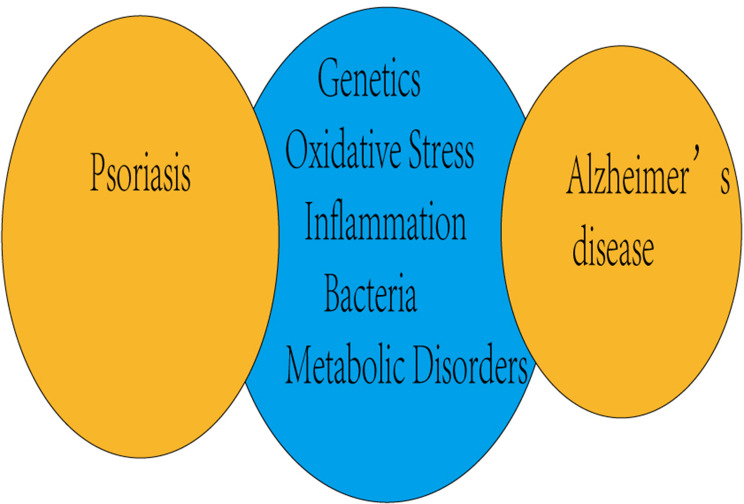
This paper provided a systematic analysis of the relation between Psoriasis and Alzheimer (Figure 1). Thus far, few studies have investigated about the potential association in psoriasis and Alzheimer’s Disease. Most observational studies have been cited previously in this review prove that diagnosed psoriasis was associated with subsequent Alzheimer’s Disease.
Figure 1.
Main common factors associated with psoriasis and neurodegenerative diseases.
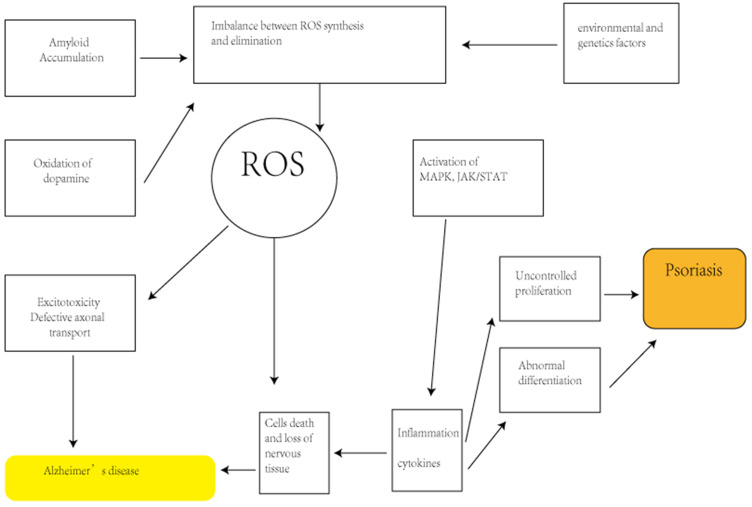
Psoriasis is famous for its great comorbidity associated with different body system and organs. As a result, it is essential to analyze the potential connection between this skin disease and neurodegenerative diseases. This possible common background includes similar inflammatory cytokines, potential pathogenesis, oxidative stress phenomenon (Figure 2) and shared genetic factors. Psoriasis may increase the risk of Alzheimer’s disease.
Figure 2.
The role of oxidative stress in pathogenesis of psoriasis and neurodegenerative diseases.
Of course, although we still cannot determine the correlation between Alzheimer’s disease and psoriasis clearly, this review may have some clinical applications in the future. Dermatologists and neurologists should be aware of the association between psoriasis and Alzheimer’s disease. If dermatologists observe AD signs or symptoms in patients with psoriasis, neurology consultation or referral is needed. When neurologists receive patients with Alzheimer’s disease, attention should also be paid to whether they have related skin symptoms. Dermatology consultation or referral is needed if necessary.
Undeniably, this review has several limitations. Firstly, we only discussed the relation between psoriasis and Alzheimer’s disease. Many other skin diseases which related to inflammation may also be associated with Alzheimer’s disease but are not summarized in our review. Secondly, mechanisms of the association between psoriasis and AD have not been well illustrated. And the pathogenesis between the two diseases need be illustrated more clearly. We need further studies about the mechanisms and pathogenesis of the correlation between psoriasis and AD. Longer follow-up, prospective and larger studies are warranted. To complete these contributions mostly rely on the close cooperation among neurologists and dermatologists.58
In conclusion, our review has supported that Alzheimer’s disease was associated with an increased risk of psoriasis. And then, the patients with psoriasis more than 40 years old need pay attention to the AD. However, large-scale stratified research which based on both psoriasis treatment status and severity is still required for the clearer risk of subsequent AD and its subtypes as well.
Acknowledgments
We apologize to the many authors whose studies are important but could not be cited due to space limitation.
Funding Statement
There is no funding to report.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Lond Engl. 2016;388(10053):1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfsgruber S, Molinuevo JL, Wagner M, et al. Prevalence of abnormal Alzheimer’s disease biomarkers in patients with subjective cognitive decline: cross-sectional comparison of three European memory clinic samples. Alzheimers Res Ther. 2019;11(1):8. doi: 10.1186/s13195-018-0463-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vom Berg J, Prokop S, Miller KR, et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat Med. 2012;18(12):1812–1819. doi: 10.1038/nm.2965 [DOI] [PubMed] [Google Scholar]
- 4.Griffin WST. Neuroinflammatory cytokine signaling and Alzheimer’s disease. N Engl J Med. 2013;368(8):770–771. doi: 10.1056/NEJMcibr1214546 [DOI] [PubMed] [Google Scholar]
- 5.Tan MS, Yu JT, Jiang T, Zhu XC, Guan HS, Tan L. IL12/23 p40 inhibition ameliorates Alzheimer’s disease-associated neuropathology and spatial memory in SAMP8 mice. J Alzheimers Dis JAD. 2014;38(3):633–646. doi: 10.3233/JAD-131148 [DOI] [PubMed] [Google Scholar]
- 6.Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25(1):59–70. doi: 10.1111/ene.13439 [DOI] [PubMed] [Google Scholar]
- 7.Boehncke WH, Schön MP. Psoriasis. Lancet Lond Engl. 2015;386(9997):983–994. doi: 10.1016/S0140-6736(14)61909-7 [DOI] [PubMed] [Google Scholar]
- 8.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet Lond Engl. 2007;370(9583):263–271. doi: 10.1016/S0140-6736(07)61128-3 [DOI] [PubMed] [Google Scholar]
- 9.Baran A, Nowowiejska J, Kamiński TW, Krahel JA, Flisiak I. Circulating MAdCAM-1 and ITGB7 in patients with plaque psoriasis and eruptive lichen planus-preliminary data. Biology. 2021;10(11):1129. doi: 10.3390/biology10111129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulat V, Šitum M, Delaš Aždajić M, Lovrić I, Dediol I. Study on the impact of psoriasis on quality of life: psychological, social and financial implications. Psychiatr Danub. 2020;32(Suppl 4):553–561. [PubMed] [Google Scholar]
- 11.Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet Lond Engl. 2021;397(10281):1301–1315. doi: 10.1016/S0140-6736(20)32549-6 [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Han K, Gee HY. The incidence rates and risk factors of Parkinson disease in patients with psoriasis: a nationwide population-based cohort study. J Am Acad Dermatol. 2020;83(6):1688–1695. doi: 10.1016/j.jaad.2019.07.012 [DOI] [PubMed] [Google Scholar]
- 13.Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primer. 2016;24(2):16082. doi: 10.1038/nrdp.2016.82 [DOI] [PubMed] [Google Scholar]
- 14.Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475. doi: 10.3390/ijms20061475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokoyama JS, Wang Y, Schork AJ, et al. Association between genetic traits for immune-mediated diseases and Alzheimer disease. JAMA Neurol. 2016;73(6):691–697. doi: 10.1001/jamaneurol.2016.0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodson R. Alzheimer’s disease. Nature. 2018;559(7715):S1. doi: 10.1038/d41586-018-05717-6 [DOI] [PubMed] [Google Scholar]
- 17.Karlawish J, Jack CR, Rocca WA, Snyder HM, Carrillo MC. Alzheimer’s disease: the next frontier-special report 2017. Alzheimers Dement J Alzheimers Assoc. 2017;13(4):374–380. doi: 10.1016/j.jalz.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 18.Buckley RF, Mormino EC, Rabin JS, et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 2019;76(5):542–551. doi: 10.1001/jamaneurol.2018.4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babapour Mofrad R, Tijms BM, Scheltens P, et al. Sex differences in CSF biomarkers vary by Alzheimer disease stage and APOE ε4 genotype. Neurology. 2020;95(17):e2378–88. doi: 10.1212/WNL.0000000000010629 [DOI] [PubMed] [Google Scholar]
- 20.Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63(2):168–174. doi: 10.1001/archpsyc.63.2.168 [DOI] [PubMed] [Google Scholar]
- 21.Bellenguez C, Charbonnier C, Grenier-Boley B, et al. Contribution to Alzheimer’s disease risk of rare variants in TREM2, SORL1, and ABCA7 in 1779 cases and 1273 controls. Neurobiol Aging. 2017;59:220.e1–220.e9. doi: 10.1016/j.neurobiolaging.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 22.Ridge PG, Mukherjee S, Crane PK, Kauwe JSK; Alzheimer’s Disease Genetics Consortium. Alzheimer’s disease: analyzing the missing heritability. PLoS One. 2013;8(11):e79771. doi: 10.1371/journal.pone.0079771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen IE, Savage JE, Watanabe K, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet. 2019;51(3):404–413. doi: 10.1038/s41588-018-0311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genin E, Hannequin D, Wallon D, et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry. 2011;16(9):903–907. doi: 10.1038/mp.2011.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiman EM, Arboleda-Velasquez JF, Quiroz YT, et al. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5000-person neuropathological study. Nat Commun. 2020;11(1):667. doi: 10.1038/s41467-019-14279-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Strooper B, Karran E. The cellular phase of Alzheimer’s disease. Cell. 2016;164(4):603–615. doi: 10.1016/j.cell.2015.12.056 [DOI] [PubMed] [Google Scholar]
- 27.Venegas C, Kumar S, Franklin BS, et al. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature. 2017;552(7685):355–361. doi: 10.1038/nature25158 [DOI] [PubMed] [Google Scholar]
- 28.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14(3):133–150. doi: 10.1038/nrneurol.2017.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Da Mesquita S, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560(7717):185–191. doi: 10.1038/s41586-018-0368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu T, Aron L, Zullo J, et al. REST and stress resistance in ageing and Alzheimer’s disease. Nature. 2014;507(7493):448–454. doi: 10.1038/nature13163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szaruga M, Munteanu B, Lismont S, et al. Alzheimer’s-causing mutations shift Aβ length by destabilizing γ-secretase-Aβn interactions. Cell. 2017;170(3):443–456.e14. doi: 10.1016/j.cell.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 32.Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270–276. doi: 10.1038/s41591-018-0297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gate D, Saligrama N, Leventhal O, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature. 2020;577(7790):399–404. doi: 10.1038/s41586-019-1895-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fry L. Psoriasis. Br J Dermatol. 1988;119(4):445–461. doi: 10.1111/j.1365-2133.1988.tb03248.x [DOI] [PubMed] [Google Scholar]
- 35.Schmitt J, Wozel G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatol Basel Switz. 2005;210(3):194–199. doi: 10.1159/000083509 [DOI] [PubMed] [Google Scholar]
- 36.Henes JC, Ziupa E, Eisfelder M, et al. High prevalence of psoriatic arthritis in dermatological patients with psoriasis: a cross-sectional study. Rheumatol Int. 2014;34(2):227–234. doi: 10.1007/s00296-013-2876-z [DOI] [PubMed] [Google Scholar]
- 37.Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33(1–2):45–56. doi: 10.1007/s12016-007-0039-2 [DOI] [PubMed] [Google Scholar]
- 38.Elder JT. Genome-wide association scan yields new insights into the immunopathogenesis of psoriasis. Genes Immun. 2009;10(3):201–209. doi: 10.1038/gene.2009.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129(6):1339–1350. doi: 10.1038/jid.2009.59 [DOI] [PubMed] [Google Scholar]
- 40.Neurath MF. IL-23: a master regulator in Crohn disease. Nat Med. 2007;13(1):26–28. doi: 10.1038/nm0107-26 [DOI] [PubMed] [Google Scholar]
- 41.Orrell KA, Vakharia PP, Hagstrom EL, Brieva J, West DP, Nardone B. Prevalence of chronic hepatitis B and C in psoriasis patients: a cross-sectional study in a large US population. J Am Acad Dermatol. 2017;77(3):572–573. doi: 10.1016/j.jaad.2017.05.020 [DOI] [PubMed] [Google Scholar]
- 42.Leisner MZ, Riis JL, Schwartz S, Iversen L, Østergaard SD, Olsen MS. Psoriasis and risk of mental disorders in Denmark. JAMA Dermatol. 2019;155(6):745–747. doi: 10.1001/jamadermatol.2019.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin CC, Lin HC, Chiu HW. Association between psoriasis and dementia: a population-based case-control study. Am J Clin Dermatol. 2019;20(3):457–463. doi: 10.1007/s40257-018-00420-8 [DOI] [PubMed] [Google Scholar]
- 44.Pezzolo E, Mutlu U, Vernooij MW, et al. Psoriasis is not associated with cognition, brain imaging markers, and risk for dementia: the Rotterdam Study. J Am Acad Dermatol. 2021;85(3):671–680. doi: 10.1016/j.jaad.2018.07.046 [DOI] [PubMed] [Google Scholar]
- 45.Liu L, Chen ST, Li HJ, et al. Association between psoriasis and dementia: current evidence. Front Aging Neurosci. 2020;12:570992. doi: 10.3389/fnagi.2020.570992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim M, Park HE, Lee SH, Han K, Lee JH. Increased risk of Alzheimer’s disease in patients with psoriasis: a nationwide population-based cohort study. Sci Rep. 2020;10(1):6454. doi: 10.1038/s41598-020-63550-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gisondi P, Sala F, Alessandrini F, et al. Mild cognitive impairment in patients with moderate to severe chronic plaque psoriasis. Dermatol Basel Switz. 2014;228(1):78–85. doi: 10.1159/000357220 [DOI] [PubMed] [Google Scholar]
- 48.Decourt B, Lahiri DK, Sabbagh MN. Targeting tumor necrosis factor alpha for Alzheimer’s disease. Curr Alzheimer Res. 2017;14(4):412–425. doi: 10.2174/1567205013666160930110551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou M, Xu R, Kaelber DC, Gurney ME. Tumor Necrosis Factor (TNF) blocking agents are associated with lower risk for Alzheimer’s disease in patients with rheumatoid arthritis and psoriasis. PLoS One. 2020;15(3):e0229819. doi: 10.1371/journal.pone.0229819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rich P, Bourcier M, Sofen H, et al. Ustekinumab improves nail disease in patients with moderate-to-severe psoriasis: results from Phoenix 1. Br J Dermatol. 2014;170(2):398–407. doi: 10.1111/bjd.12632 [DOI] [PubMed] [Google Scholar]
- 51.Mohammadi Shahrokhi V, Ravari A, Mirzaei T, Zare-Bidaki M, Asadikaram G, Arababadi MK. IL-17A and IL-23: plausible risk factors to induce age-associated inflammation in Alzheimer’s disease. Immunol Invest. 2018;47(8):812–822. doi: 10.1080/08820139.2018.1504300 [DOI] [PubMed] [Google Scholar]
- 52.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16(6):358–372. doi: 10.1038/nrn3880 [DOI] [PubMed] [Google Scholar]
- 53.Belloy ME, Napolioni V, Greicius MD, Quarter A. Century of APOE and Alzheimer’s disease: progress to date and the path forward. Neuron. 2019;101(5):820–838. doi: 10.1016/j.neuron.2019.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campalani E, Allen MH, Fairhurst D, et al. Apolipoprotein E gene polymorphisms are associated with psoriasis but do not determine disease response to Acitretin. Br J Dermatol. 2006;154(2):345–352. doi: 10.1111/j.1365-2133.2005.06950.x [DOI] [PubMed] [Google Scholar]
- 55.Al Harthi F, Huraib GB, Zouman A, Arfin M, Tariq M, Al-Asmari A. Apolipoprotein E gene polymorphism and serum lipid profile in Saudi patients with psoriasis. Dis Markers. 2014;2014:239645. doi: 10.1155/2014/239645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han Y, Liu T, Lu L. Apolipoprotein E gene polymorphism in psoriasis: a meta-analysis. Arch Med Res. 2013;44(1):46–53. doi: 10.1016/j.arcmed.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 57.Liu S, Yuan X, Su H, Liu F, Zhuang Z, Chen Y. ZNF384: a potential therapeutic target for psoriasis and Alzheimer’s disease through inflammation and metabolism. Front Immunol. 2022;13:892368. doi: 10.3389/fimmu.2022.892368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neau JP, Godeneche G, Mathis S, Guillet G. Neurodermatology. Handb Clin Neurol. 2014;121:1561–1594. [DOI] [PubMed] [Google Scholar]