Abstract
Occupational chemical hazards in the fire service are hypothesized to play a role in increased cancer risk, and reliable sampling technologies are necessary for conducting firefighter chemical exposure assessments. This study presents the military-style dog tag as a new configuration of silicone passive sampling device to sample individual firefighters’ exposures at one high and one low fire call volume department in the Kansas City, Missouri metropolitan area. The recruited firefighters (n=56) wore separate dog tags to assess on- and off-duty exposures (ndogtags=110), for a total of 30 24h shifts. Using a 63 PAH method (GC-MS/MS), the tags detected 45 unique PAHs, of which 18 have not been previously reported as firefighting exposures. PAH concentrations were higher for on- compared to off-duty tags (0.25<Cohen’s d≤0.80) and for the high compared to the low fire call volume department (0.25≤d<0.70). Using a 1530 analyte screening method (GC-MS), di-n-butyl phthalate, diisobutyl phthalate, guaiacol, and DEET were commonly detected analytes. The number of fire attacks a firefighter participated in was more strongly correlated with PAH concentrations than firefighter rank or years in the fire service. This suggested that quantitative data should be employed for firefighter exposure assessments, rather than surrogate measures. Because several detected analytes are listed as possible carcinogens, future firefighter exposure studies should consider evaluating complex mixtures to assess individual health risks.
Keywords: Passive sampling, firefighter, occupational exposure, personal monitoring, polycyclic aromatic hydrocarbons, phthalates
Graphical Abstract
1. INTRODUCTION
As essential components of the public safety net, career and volunteer firefighters must maintain a high fitness level to respond to emergencies in the communities they serve (Poston et al. 2011). Unfortunately, firefighters appear to have a relatively high incidence of negative health outcomes, including occupationally-related cancers (Daniels et al. 2014; Guidotti 2007; LeMasters et al. 2006; Tsai et al. 2015). Cohort and case-control studies have reported possible to probable associations between firefighting and the following cancers: non-Hodgkin’s lymphoma, multiple myeloma, prostate, brain, bladder, buccal cavity, pharynx, intestine, kidney, liver, gall bladder, lung, mesothelioma, esophagus, skin, testicles, rectal, colon, stomach, leukemia, and melanoma (Daniels et al. 2014; Glass et al. 2016; Guidotti 2007; LeMasters et al. 2006; Pukkala 1995; Tsai et al. 2015). Firefighters have an estimated 10–20% increased risk of developing cancer compared to the general population, although this risk is doubled for specific cancers (e.g. mesothelioma at 100% increased risk) (Daniels et al. 2014; LeMasters et al. 2006). The greater risks for incident cancers and cancer-related mortality among firefighters are hypothesized to be associated with occupational chemical exposures specific to the fire service.
During fire suppression activities, firefighters may be exposed to recognized or probable carcinogens, such as select polycyclic aromatic hydrocarbons (PAHs), diesel fumes, and asbestos (Di(2-ethylhexyl)phthalate (DEHP) 2000; IARC Working Group 2010; Alexander and Baxter 2014; Daniels et al. 2014; Stec et al. 2018). The International Agency for Research on Cancer (IARC) concluded firefighter occupational exposures ought to be classified as “possibly” carcinogenic to humans due to a limited number of studies and evidence (IARC Working Group 2010). However, negative health endpoints beyond cancer also result from occupational exposures and should be included in firefighter exposure health studies. For instance, exposure to PAHs is associated with increased risks of cardiovascular disease and mortality from heart attack (Sobus et al. 2009), in addition to increasing blood pressure and heart rate, and accelerating the progression of atherosclerosis (Guidotti 2007; McClean et al. 2004; Serdar et al. 2012). Mechanistic and epidemiological evidence also associate PAH exposures with airway inflammation and asthma (Al-Daghri et al. 2013; Karimi et al. 2015; and Perera et al. 2009). Such epidemiological studies can be best informed by a detailed characterization of chemical exposures that firefighters experience.
Currently, epidemiological studies associating adverse health outcomes with firefighter exposures to potentially harmful chemicals are almost exclusively based on crude surrogate measures, including job title, department fire call volume, and career length in the fire service (Dahm et al. 2015; Daniels et al. 2015; Daniels et al. 2014). Fire departments and stations may vary by the average number of fire calls per month, types of fires, maintenance of personal protective equipment (PPE), and frequency of situations involving hazardous materials (Dahm et al. 2015; Daniels et al. 2015; Daniels et al. 2014; Stec et al. 2018). Firefighters also may experience occupational exposures inside their fire departments due to cross-contamination of engine cabs and station quarters from contaminated PPE and diesel fumes (LeMasters et al. 2006). With potentially large variability between and within departments, such surrogate measures may produce biased risk estimates (e.g. overestimate or underestimate the exposure quantity and duration) and promote the need for quantitative measurements.
Two methodologies that attempt to overcome the shortcomings inherent in surrogate measures include active air samplers (AAS) and biological samples. Some studies have used AASs to assess firefighter chemical exposures, but the devices are costly, require electricity to function, and complicated to operate (Bohlin et al. 2010). Because stationary samplers may underestimate individual exposures compared to personal samplers (Bohlin et al. 2010; Paulik et al. 2018), AAS backpacks are equipped with a battery pack and pump to continuously sample air and to assess personal chemical exposure (IARC Working Group 2010; Dixon et al. 2018; Strandberg et al. 2018). However, participants’ compliance may be reduced due to concerns about safety and backpack weight (Guidotti 2007; McClean et al. 2012). To overcome compliance issues, some studies favor biological samples, such as blood or urine (Dahm et al. 2015; McClean et al. 2004; Serdar et al. 2012; Sobus et al. 2009). While biological samples integrate all routes of personal chemical exposures, they are subject to significant inter- and intra-individual variability (Guidotti 2007; Liu et al. 2007; Ramirez et al. 2011). Biological samples typically account for short-term exposures (e.g. within 72 h) and there are a limited number of biological metabolites (Koch et al. 2014; Paustenbach and Galbraith 2006). Not every unique chemical has a unique biological metabolite, such that studies using biomonitoring samples may not adequately address exposures to currently unmonitored chemicals.
Passive sampling devices (PSDs) can meet the need for a firefighter personal sampler that sequesters unmonitored chemicals. PSDs function by diffusing and concentrating the bioavailable, or gaseous phase, fraction of volatile and semi-volatile organic compounds (VOCs, SVOCs) into a hydrophobic polymer over time (Anderson and Hillwalker 2008; Anderson et al. 2017; O’Connell et al. 2014; Strandberg et al. 2018). Although higher molecular weight SVOCs predominantly exist on surfaces and in dust (Liagkouridis et al. 2015; Liu et al. 2007; Wei et al. 2018; Weschler and Nazaroff 2012), a sizable fraction of SVOCs remain bioavailable in the gaseous phase (Krol et al. 2011; Liagkouridis et al. 2015; Liu et al. 2007; Ramirez et al. 2011; Wei et al. 2018; Weschler and Nazaroff 2012). The gaseous phase VOC and SVOC fractions are significant to human exposures and subsequent health effects via a combination of inhalation, dermal absorption, and limited ingestion (Krol et al. 2011; Lorber 2008; Schreder et al. 2016; Weschler and Nazaroff 2012; Wilford et al. 2004). Estimates indicate that human uptake of SVOCs by skin absorption can be large, potentially equal to or exceeding intake through inhalation (Weschler and Nazaroff 2012). Furthermore, gaseous phase PAHs, such as fluoranthene and dibenzothiophene, can account for up to 86% of the lifetime cancer risk (i.e. benzo[a]pyrene toxic equivalence factors), based on ambient air concentrations near industrial sites and road tunnels (Ramirez et al. 2011; Samburova et al. 2017). SVOC inhalation exposures during firefighting activities may increase due to high temperatures increasing the gaseous phase fraction (Liu et al. 2007; Ramirez et al. 2011; Tsai et al. 2015). Quantifying the bioavailable SVOC fraction is critical for firefighter exposure assessments, and this need can be met using a new model of personal PSDs.
A recent advancement in PSDs is the personal silicone wristband, derived from commercially available wristbands (O’Connell et al. 2014). Silicone wristbands provide personal exposure assessments to partner with demographic data and health outcomes, leading to the inference of lifestyle and behaviors associated with chemical concentrations (Anderson et al. 2017; Bergmann et al. 2017; Dixon et al. 2019; Dixon et al. 2018; Donald et al. 2016a; Hammel et al. 2016; Hammel et al. 2018; Harley et al. 2019; Kile et al. 2016; Paulik et al. 2018; Vidi et al. 2017). To date, silicone PSDs have been used to measure PAH exposures with pregnant women and occupationally exposed roofers (Dixon et al. 2018; O’Connell et al. 2014); flame retardants with pre-school children, college students, and housecats (Hammel et al. 2016; Hammel et al. 2018; Kile et al. 2016; Poutasse et al. 2019); and pesticides with farmers in developing countries and farmworker children in the US (Bergmann et al. 2017; Dixon et al. 2019; Donald et al. 2016a; Vidi et al. 2017). Because accurate measurements of chemical exposure are a critical component for estimating health effects, wristbands complemented current methods of personal chemical exposure in three separate studies. Hammel et al. 2016 compared wristbands with hand wipes and urine samples for organophosphate esters (OPEs), finding more significant correlations between the urinary metabolites and OPEs in wristbands (rs = 0.5–0.65, p<0.001) than with hand wipes (rs = 0.37–0.46, p<0.05) (Hammel et al. 2016). The follow-up study by Hammel et al. 2018 further correlated polybrominated diphenyl ether concentrations between wristbands and human serum (rs = 0.39–0.57, p<0.05) (Hammel et al. 2018). Similarly, Dixon et al. 2018 compared concentrations of PAHs in wristbands with AAS backpacks and urinary metabolites, finding more significant correlations between the corresponding urinary metabolites and PAHs in wristbands (rs = 0.44–0.76, p<0.04) than with the backpack (rs = 0.44–0.53, p<0.04) (Dixon et al. 2018). Because silicone PSDs can act as a biological mimic for human uptake, this study employed the military-style silicone dog tag as a new configuration, developed with the assistance of firefighter focus groups.
The first objective of this study was to evaluate the use and acceptability of the military-style dog tags as a new configuration of personalized silicone PSD. The remaining objectives were to investigate the effects of (1) on- versus off-duty time periods, (2) department fire call volume, and (3) firefighter rank on chemical exposures. Identification and quantification of bioavailable chemical exposures during firefighting activities can provide new opportunities to associate exposures with adverse health outcomes and to assess the effectiveness of innovative PPE to protect first responders and firefighters.
2. MATERIALS AND METHODS
2.1. Materials
Optima-grade solvents were obtained from Fisher Scientific (Pittsburgh, PA, USA). Select analytical standards were purchased as single analytes or composite solutions from Accustandard (New Haven, CT), Sigma-Aldrich (St. Louis, MO), TCI America (Tokyo, Japan), SantaCruz Biotechnology (Dallas, TX), and Chiron (Emeryville, CA). Before use, all laboratory equipment and glassware were washed in an automatic dishwasher with detergent, rinsed with 18 MΩ·cm water, and baked at >300°C for 12 h. Polytetrafluoroethylene (PTFE) storage bags and closures were purchased from Welch Fluorocarbon, Inc. (Dover, NH) for air-tight sampler storage and transport (Donald et al. 2016b).
2.2. Silicone Dog Tag Preparation
The silicone dog tags (Figure 1; 6.0 cm long by 2.5 cm wide by 0.3 cm thick; ~5.4 g; https://24hourwristbands.com, Houston, TX, USA) were prepared as previously reported with minimal modifications (Anderson et al. 2017). Briefly, the dog tags were vacuum oven conditioned at 300°C for 12 h at 0.1 Torr (Vacuum Oven, Blue-M, model no. POM18VC, with Welch Duo-seal pump, model no. 1405). Quality control samples were selected to evaluate for data quality objectives prior to storing the dog tags in sealed metal containers at 4°C (see Section 2.6). Dog tags were transferred to PTFE bags before and after deployment.
Figure 1.
Silicone dog tags (shown in yellow) were worn around the neck underneath firefighting personal protective equipment.
2.3. Firefighter Population and Recruitment
Participant informed consent was obtained in compliance with the procedures approved by the National Development and Research Institutes, Inc. (NDRI) Institutional Review Board (IRB00000634; Oregon State University (OSU) IRB Deferral 8313). Two fire departments in the Kansas City metropolitan area were selected based on the average monthly fire call volume, where “high volume” historically received over 12 fire calls per month and “low volume” fewer than 2 fire calls per month. To ensure the dog tags did not sample bioavailable SVOCs already embedded in current turnout gear, recruited firefighters at each department (nlowvolume=29; nhighvolume=27) were provided with new PPE: turnouts (GXTREME 3.0, Globe Manufacturing Company, LLC, Pittsfield, NH, USA) and hoods (Quest Particle Barrier Hoods, Quest Fire Apparel Inc., Saratoga Springs, NY, USA).
After completing a survey on demographics, occupational history, and current exposures, recruited firefighters wore a dog tag on an elastic necklace during the next 30 on- and off-shift days, acting as their own control for non-occupational exposures. During fire calls, tags were worn over clothing but underneath their turnout gear. The firefighters were instructed to wear the dog tags continuously during all regular activities, including eating, showering, and sleeping, for 30 total on-duty days and 30 total off-duty days. While not being worn, the dog tags were stored in their respective PTFE bags. Sampling occurred from November 2018 to April 2019, and the number of fire attacks a firefighter participated in were recorded. While a “fire call” denoted a fire emergency the department responded to, a “fire attack” was defined as an individual firefighter’s participation during the call. The worn dog tags were returned to OSU in the sealed PTFE bags.
2.4. Dog Tag Extractions
The dog tags underwent post-deployment cleaning to remove particulate matter with two rinses of 18 MΩ·cm and one of isopropanol (O’Connell et al. 2014). The tags were then stored in amber glass jars at −20°C until extraction, as previously described with minimal modifications (Anderson et al. 2017; Dixon et al. 2019; O’Connell et al. 2014; Poutasse et al. 2019). Briefly, deuterated analytes were added as recovery surrogates, with respective average recoveries reported in the Supplementary Material (SM; Table S1). Dog tags were extracted with two 50 mL volumes of ethyl acetate at ambient temperature. Sample extracts were combined and reduced to one mL under nitrogen (Turbo-Vap L, Biotage, Charlotte, NC, USA; RapidVap, LabConco, Kansas City, MO, USA; N-EVAP 111, Organomation Associates, Berlin, MA, USA). Sample extracts were stored at 4°C prior to additional cleanup by solid phase extraction (SPE) (Dixon et al. 2019; Kile et al. 2016; O’Connell et al. 2014). Sample aliquots of 100 uL underwent SPE using acetonitrile (Cleanert S C18, Agela Technologies, Torrance, CA, USA), were solvent exchanged to iso-octane (OA-SYS N-EVAP 111, Organomation Associates, Berlin, MA, USA), and stored at 4°C prior to instrument analysis.
2.5. Instrument Analysis
2.5.2. PAH Method
To investigate documented firefighter exposures, 63 parent and alkylated PAHs were quantitated using an Agilent (Santa Clara, CA) 7890 gas chromatograph (GC) with a 7000 triple-quadruple mass spectrometer (MS/MS) (Anderson et al. 2017; Anderson et al. 2015; Dixon et al. 2018; Donald et al. 2019; Minick and Anderson 2017; Paulik et al. 2018). The instrument parameters, analyte quantification, and target analyte list are given in SM and Table S2, and target analyte concentrations were surrogate-corrected. PAHs were also included in the 1530 screening method (Section 2.5.1).
2.5.1. 1530 Screening Method
The analytical screen of 1530 chemicals used a 6890N GC with a 5975B Mass Selective Detector in full scan mode. The target list included 124 flame retardants, 185 industrial-related chemicals, 98 PAHs, 773 pesticides, 76 personal care products (PCPs), 14 phthalates, and 260 polychlorinated biphenyls (PCBs), dioxins, and furans (Bergmann et al. 2018; Dixon et al. 2019). Details about the semi-quantitative method have been previously reported (Bergmann et al. 2018) and the full analyte list is available online (http://fses.oregonstate.edu/masv-analyte-list). The screening method quantified concentrations within a factor of 2.5 of the true value (Bergmann et al. 2018), and instrument parameters and analyte semi-quantification are given in SM.
2.6. Quality Assurance and Quality Control
To ensure the dog tags met the data quality objectives, quality control accounted for 32% of the study samples analyzed. Quality control samples were dog tag conditioning verification (n=4), trip blanks (n=2), laboratory processing blanks (n=3), post-deployment cleaning blanks (n=2), silicone dialysis blanks (n=2), SPE blanks (n=3), SPE duplicates (n=4), sample overspikes (n=2), instrument solvent blanks (n=22), and continuing calibration verifications (n=14). Sample background correction was conducted using the laboratory processing blanks (see SM). Chemical concentrations in the trip blanks (see SM) were over 10-fold lower than the worn dog tag samples, such that only the laboratory processing blanks were used for background correction. All duplicates and overspikes met data quality objectives at 30–170% of the expected concentrations. All calibration verifications met data quality objectives at ±30% of the true value for 70% of the target analytes (PAH method) or within 2.5 times of the true value for 60% of the target analytes (1530 screening method).
2.7. Statistical Analysis
Statistical analyses were performed using R free software (CRAN R Project version 3.5.2) and SAS statistical software (JMP Pro version 13.0.0; SAS Institute Inc., Cary, NC) for analytes detected in at least one tag. PAH concentrations were converted to moles per gram dog tag (mol/g tag), and analytes from the 1530 screening method were converted to moles per dog tag (mol/tag). Concentrations below the instrument limits of quantitation (LOQs) were substituted with a value equal to LOQ/√2 and below the instrument limits of detections (LODs) with LOD/√2. If target analyte concentrations were approximately log-normally distributed (Kolmogorov’s test, p<0.05), then a log10-transformation was conducted, reassessed for normality, and applied to parametric statistical tests. As an alternative analysis, a modified Kaplan-Meier procedure for non-detected values was applied (Table S3, Table S6).
All chemical categories (e.g. phthalates) and analytes detected in over 50% of the dog tags were investigated for concentration differences between duty shift days (paired t-test) and fire departments (t-test). A subset of chemical categories and analytes (p<0.10, to account for suggestive statistical differences) were selected for investigation with questionnaire variables. With concentration as the response variable, the multivariate regression models were constructed with all occupational-related variables (e.g. years in the fire service) and potential confounders (t-test or ANOVA with Tukey post-hoc test, p<0.10), using a stepwise selection procedure. The final adjusted models were determined using residual analyses, investigating influential observations, and evaluating goodness-of-fit with R-squared values.
3. RESULTS AND DISCUSSION
3.1. Firefighter Compliance
Researchers recruited 56 firefighters who wore PSDs between November 2018 and March 2019, for a total of 30 on- and off-duty days. All firefighters returned their on-duty tags (non-duty=56; 100% compliance), and all but two firefighters returned their off-duty tags (noff-duty=54; 96% compliance). There were 16 dog tags returned to OSU with the PTFE bag partially sealed (85% compliance), which was included as a statistical confounder for select analytes (t-test, p<0.05). All tags detected a minimum of 13 PAHs above the respective LOQs. A summary of firefighter population demographics is given in Table 1.
Table 1.
Firefighter demographics are presented for the high and low call volume departments. The term “operational firefighter” includes firefighters (n=22; 39%), firefighter/paramedics (n=8; 14%), and firefighter/driver operators (n=5; 9%). Due to rounding, not all percentages total 100.
| Firefighter Variable | High Call Volume | Low Call Volume |
|---|---|---|
| Age | 37.4±7.98 | 38.8±7.09 |
| Years in fire service | 13.7±9.72 | 13.4±6.83 |
| Rank | ||
| Operational firefighter | 18 (62%) | 17 (63%) |
| Captain | 7 (24%) | 7 (26%) |
| Chief | 4 (14%) | 3 (11%) |
| Number of fire attacks | ||
| Overall fire calls | 112 | 38 |
| Minimum | 0 | 0 |
| Median | 13 | 7 |
| Maximum | 25 | 16 |
| Education | ||
| High school graduate | 1 (3%) | 1 (4%) |
| College classes | 23 (79%) | 16 (59%) |
| College graduate | 4 (14%) | 10 (37%) |
| Advanced degree | 1 (3%) | 0 (0%) |
| Minority Status | ||
| Caucasian male | 26 (90%) | 26 (96%) |
| Caucasian female | 0 (0%) | 1 (4%) |
| Non-Caucasian male | 3 (10%) | 0 (0%) |
3.2. Chemical Detections
3.2.1. Chemical Detections: PAH Quantitative Method
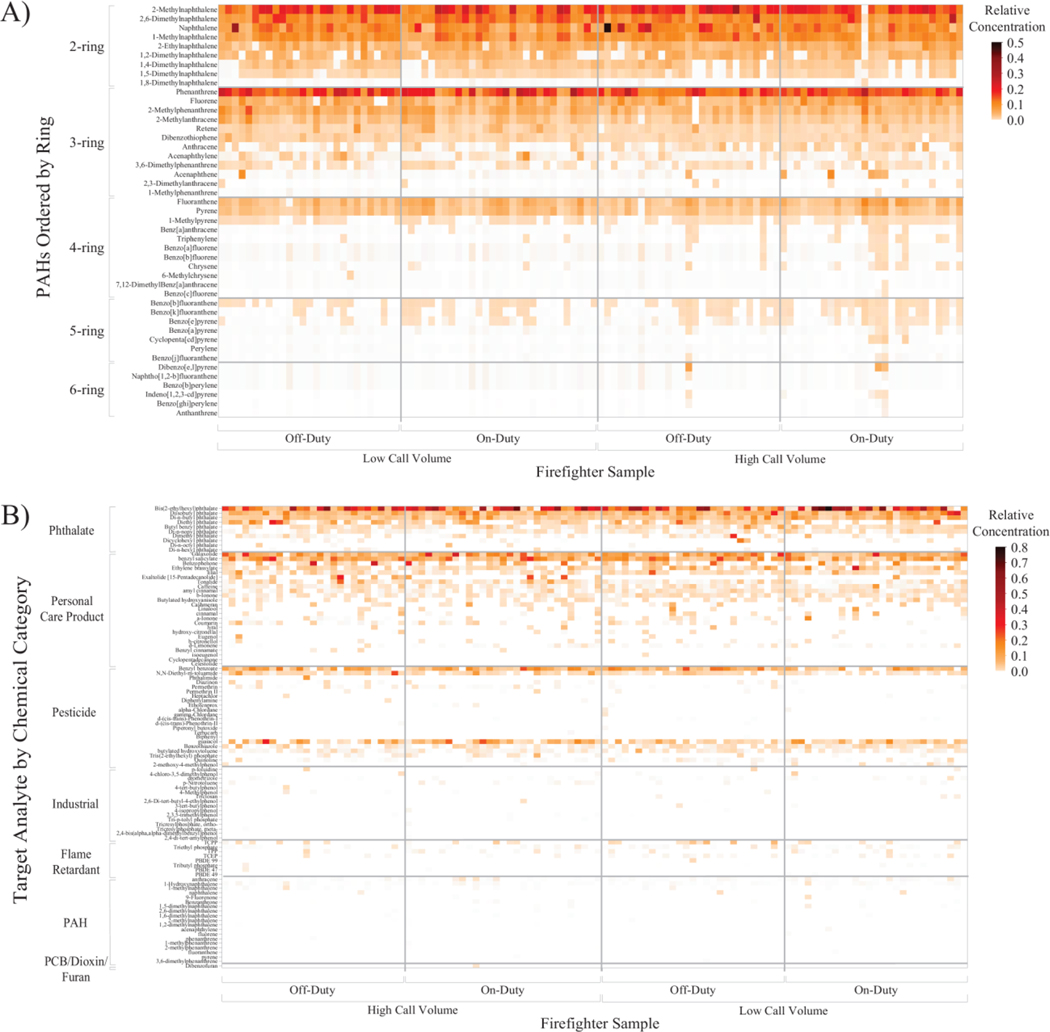
Out of 110 dog tags, a total of 45 PAHs were detected at least once and 21 PAHs were detected in over 50% of the samples (Table S2, Figure S1A). No two dog tags had identical PAH exposure profiles, as visually represented in Figure 2A.
Figure 2.
The heat maps display each firefighter dog tag sample by fire department and by duty shift in conjunction with their exposure profiles for the A) PAH method and B) 1530 screening method. PAHs were grouped by the number of fused aromatic rings, and the screening method analytes were grouped by chemical category. No two tags had the same chemical exposure profile.
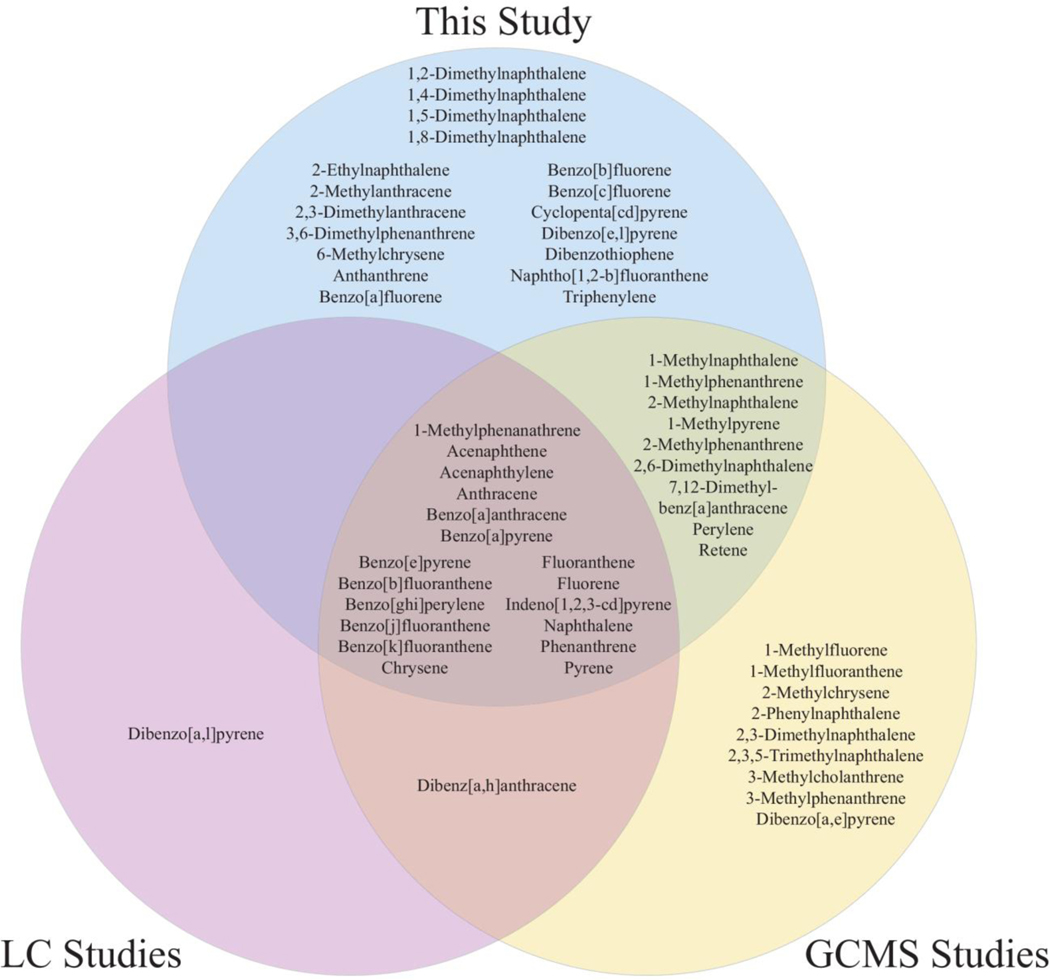
To the authors’ knowledge, this study is the first to identify personal firefighter exposures to 18 unique PAHs (Figure 3, Table S2). Notably, six of these previously unidentified PAHs were detected in over 75% of the dog tags: 2-ethylnaphthalene, 1,4-dimethylnaphthalene, 1,5-dimethylnaphthalene, 1,2-dimethylnaphthalene, dibenzothiophene, and 2-methylanthracene (Table S2, Figure S1A). Although firefighter PAH exposures have been documented using PSDs, AASs, swatch samples, skin wipe samples, and dust samples (Alexander and Baxter 2014; Fabian et al. 2011; Fent et al. 2014; Fent et al. 2018; Kirk and Logan 2015; Oliveira et al. 2017; Shen et al. 2015; Sjostrom et al. 2019; Stec et al. 2018; Strandberg et al. 2018; Wingfors et al. 2018), these results indicate a need to explore exposures and toxicities beyond the EPA’s 16 priority PAHs (Andersson and Achten 2015).
Figure 3.
Compared to earlier studies examining personal firefighter exposures, the dog tag samples identified 18 PAHs previously unassociated with firefighting using gas chromatography triple quadruple mass spectrometry (Anderson et al. 2015). Studies using gas chromatography mass spectrometry (GCMS) included (Alexander and Baxter 2014; Fabian et al. 2011; Shen et al. 2018; Sjostrom et al. 2019; Stec et al. 2018; Strandberg et al. 2018; Wingfors et al. 2018). Studies using liquid chromatography (LC) included (Fent et al. 2014; Fent et al. 2018; Oliveira et al. 2017).
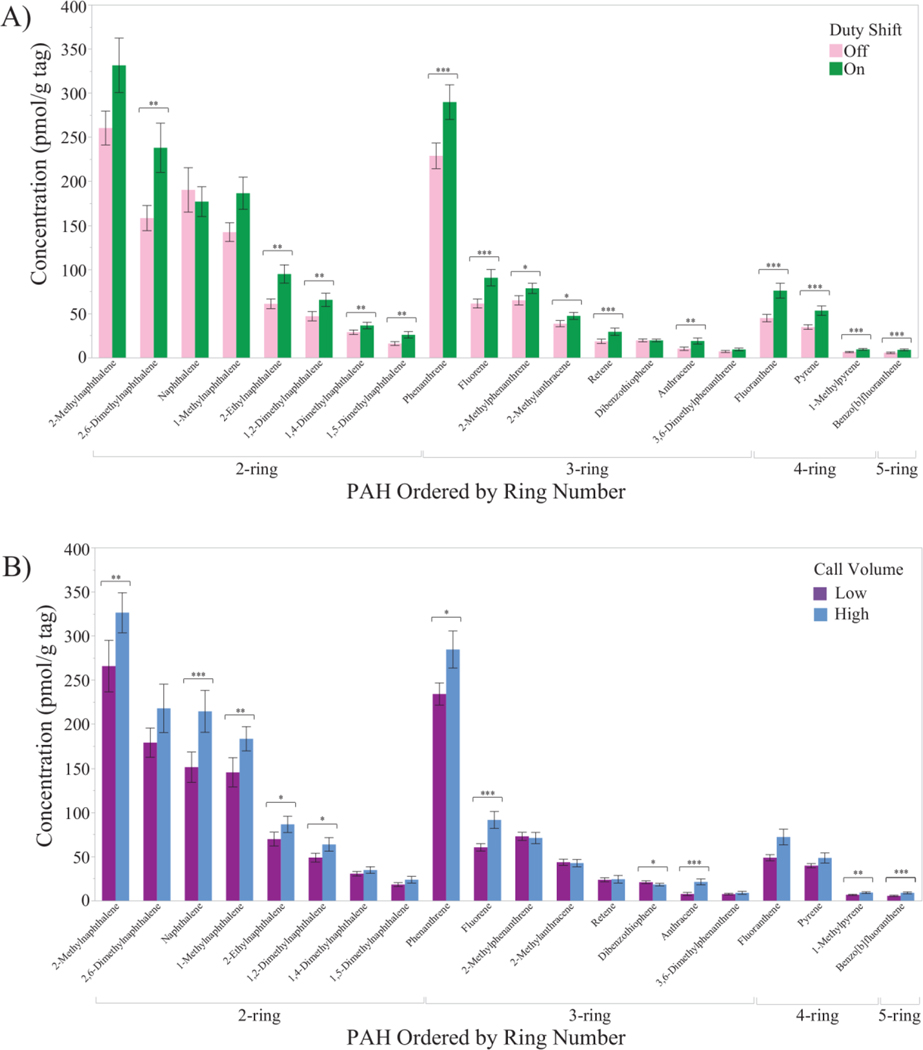
Table S3 compares on- and off-duty paired PAH tag concentrations (paired t-test) and low and high call volume department PAH tag concentrations (t-test). Figure 4A presents graphical representations of differences between paired duty status tags, while differences between fire departments are shown in Figures 4B.
Figure 4.
Bar graphs compare the PAH concentrations, ordered by the number of aromatic rings, between (A) on- and off-duty paired dog tags and (B) high and low call volume departments. Generally, occupational PAH concentrations were higher than non-occupational concentrations, and higher at the high compared to the low call volume department. Bold*: p<0.10. Bold**: p<0.05. Bold***: p<0.01 (2-sided p-value).
The firefighter dog tags illustrated occupational PAH exposures, similar to a previous study where silicone wristbands were worn by roofers (n=8) applying hot mopping-grade asphalt (t=40 h) (O’Connell et al. 2014). The roofer study used a 33 PAH method (Allan et al. 2012), where 23 PAHs were detected. If the same 33 PAH method had been applied to the firefighter study, then 27 PAHs would have been detected, where the four additional target analytes were 9-methylanthracene, 3,6-dimethylphenanthrene, 6-methylchrysene, and indeno[1,2,3-c,d]pyrene. Indeno[1,2,3-c,d]pyrene is listed as an EPA priority PAH (Andersson and Achten 2015) and has been previously identified as a firefighter exposure (Strandberg et al. 2018). Because the silicone PSDs were worn for different lengths of time, direct concentration comparisons were not available. In both studies, samplers were worn underneath PPE, such that silicone PSDs could evaluate the PPE effectiveness when considering bioavailable PAH exposures.
3.2.2. Chemical Detections: 1530 Screening Method
Out of 110 dog tags, 101 unique chemicals were detected at least once (Figure 2B, Figure S2): 10 phthalates, 25 personal care products (PCPs), 19 PAHs, 15 pesticides, 22 industrial-related chemicals, nine flame retardants, and one PCB/dioxin/furan. No two dog tags had the same chemical exposure profile (Figure 2B), consistent with previous studies (Dixon et al. 2018; Donald et al. 2016a).
The on-duty firefighter dog tags had a mean of 25.5 chemical detections, ranging from a minimum of 18 and a maximum of 35. This was a larger mean number of detections compared to the 20 mean detections from 262 silicone wristbands worn on three continents (Dixon et al. 2019). In North America alone (n=163), the mean number of total detections was 22.5, although these wristbands were worn during both occupational and non-occupational time periods and represent a wide demographic range.
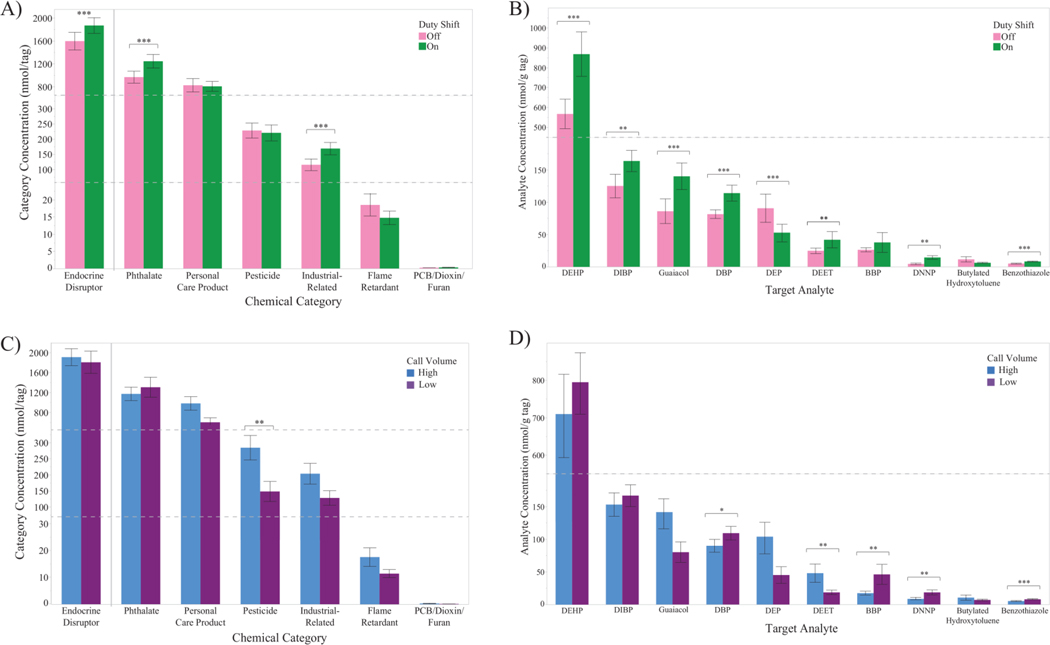
Twenty-one target analytes were detected in over 50% of the samples, of which 11 were considered potential endocrine-disrupting chemicals (Table S5; http://fses.oregonstate.edu/masv-analyte-list, accessed August 2, 2019). Phthalates and PCPs were the most commonly detected categories and at the largest relative concentrations. This article does not examine PCP chemicals because of a focus on chemical exposures likely related to the occupation of firefighting. The 10 target analytes categorized as phthalates, industrial-related chemicals, and pesticides were selected for further investigation (Table S6). Table S6 includes comparisons of on- and off-duty paired dog tag concentrations (paired t-test) and of low and high call volume department dog tag concentrations (t-test). Figure 5A–D present graphical representations of both categorical and single analyte data. Differences between duty shift dog tags are shown in Figures 5A and 5B, while differences between fire departments are shown in Figures 5C and 5D.
Figure 5.
Bar graphs compare the chemical concentrations (A, B) between on- and off-duty paired dog tags for A) summed chemical categories and B) target analytes and (C, D) between high and low call volume departments for A) summed chemical categories and B) target analytes. Bold*: p<0.10. Bold**: p<0.05. Bold***: p<0.01 (two-sided p-value). Abbreviations: DEHP – di(2-ethylhexyl) phthalate, DIBP – diisobutyl phthalate, DBP – di-n-butyl phthalate, DEP – diethyl phthalate, DEET – N,N-dietyl-m-toluamide, BBP – butyl benzyl phthalate, DNNP – di-n-nonyl phthalate.
3.3. Chemical Concentrations and Firefighter Variables: PAH Quantitative Method
Out of 21 PAHs displayed in Figure 4A, 20 demonstrated higher concentrations for on- compared to off-duty tags, of which 13 were statistically significant (Figure 4A, Table S3; paired t-test, 0.25<Cohen’s d≤0.70, p<0.05). Between fire departments, 15 of the 20 PAHs demonstrated higher concentrations at the high compared to low call volume department and seven were statistically significant (Figure 4B, Table S3; t-test, d>0.15, p<0.05).
If Table S3 indicated statistically significant differences, then multivariate models were constructed for those PAHs to investigate associations with variables in the questionnaire (Figure 6A–C, Table S4; see Section 2.7). In the multivariate models, firefighter rank and years in the fire service were considered as surrogate measurements compared to silicone dog tag chemical data. A total of 19 PAHs were included in Figure 6A–C, with all 110 dog tag samples.
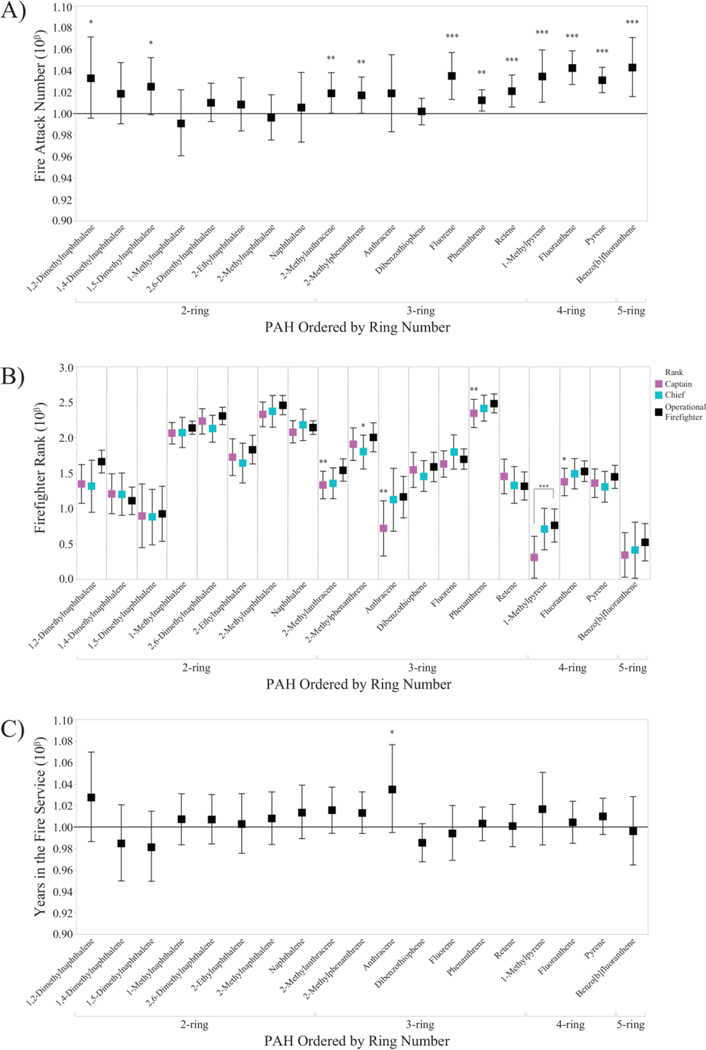
Figure 6.
Exponentiated β parameter coefficients from the 19 PAH models are shown for occupation-related variables of A) number of fire attacks during the sampling period, B) firefighter rank, and C) years spent in the fire service. In Figures 6A and 6C, a positive change in PAH concentration was represented by the solid line at 10β=1.0. Bold*: p<0.10. Bold**: p<0.05. Bold***: p<0.01.
To our knowledge, this is the first paper to associate personalized firefighter bioavailable PAH exposures with questionnaire data. Generally, PAH concentrations were most strongly correlated with the number of fire attacks a firefighter participated in during the sampling period, compared to firefighter rank or number of years in the fire service (Figure 6A–C, Table S4). For instance, with every additional fire-related emergency, the median pyrene tag concentrations increased by 3% (95% CI: 1.01, 1.06). When examining the median number of fire calls per participant by department (low=7, high=13), this represented a difference of over 25% in pyrene concentrations by department. Although this trend was less pronounced for LMW PAHs, such as naphthalene, this correlation must be considered in the context of chronic PAH exposures during a firefighter’s full career. Fire departments may wish to consider implementing additional decontamination procedures for especially active firefighters. For instance, field decontamination using wet soap, water, and scrubbing reduced PAH concentrations on used turnout jackets by a median of 85% (Fent et al. 2017), such that thorough decontamination procedures may help reduce personal PAH exposures.
Compared to fire attack numbers, firefighter rank (Figure 6B, Table S4) was less positively correlated with PAH dog tag concentrations. Compared to captains and chiefs, operational firefighters generally experienced higher concentrations, although only four PAHs were significant out of 19 PAHs investigated (Dunnett’s, p<0.05). Because captains and chiefs have more administrative roles than operational firefighters (Firefighter Ranks 2016), they are less likely to enter a burning structure and therefore less likely to experience high PAH exposures during a fire event. The number of fire attacks and rank were not correlated (ANOVA, p>0.05).
Similarly, years spent in the fire service (Figure 6C, Table S4) was less positively correlated with PAH concentrations than fire attack numbers. However, a small, non-significant trend was observed for 14 out of 19 PAHs, where an increasing number of years was correlated with increasing concentration. For instance, with every additional year in the fire service, anthracene concentrations increased by 3% (95% CI: 0.995, 1.08). This trend may be related to an increased willingness to take risks, or an increased comfort level, during a fire-related emergency. During a fire event, more experienced firefighters may spend more time on the fireground or within a burning structure, and therefore spend more time in locations with higher PAH concentrations, compared to less experienced firefighters. As a result, PAH exposures may inadvertently increase with the number of years spent in the fire service. The fire attacks numbers and fire service years were not correlated (ANOVA, p>0.05). Because rank and fire service years were less predictive of PAH dog tag concentrations than fire attack numbers, quantitative data, should be employed for exposure assessments, rather than surrogate measures.
Notably, naphthalene concentrations were not statistically significant based on fire attack number, rank, or years in the fire service (Figure 6A–C), although naphthalene has been a highly prevalent PAH in previous firefighter exposure studies (Fent et al. 2018; Strandberg et al. 2018). Naphthalene, while predominantly in the gaseous phase, has many different sources (e.g. vehicle exhaust) and is one of the most volatile PAHs in the analytical method (Anderson et al. 2015). We have previously demonstrated that naphthalene will reach equilibrium between the environment and the silicone passive sampling device within 48 h (Anderson et al. 2017; Dixon et al. 2018). Comparatively, higher molecular weight PAHs (e.g. pyrene) may reach equilibrium over several weeks. If a firefighter does not respond to a fire-related emergency on the final on-duty sampling day, then the naphthalene concentrations in the silicone dog tag could equilibrate to the fire department’s indoor environment, but would still be indicative of body burden (Dixon et al. 2018; Hammel et al. 2016; Hammel et al. 2018). In this study, naphthalene concentrations were not statistically different (t-test, p>0.10) between firefighters who did and did not engage with a fire on the last day of on-duty sampling. However, during firefighting, naphthalene may not be the most toxicologically important PAH, compared to higher molecular weight PAHs, when examining adverse health outcomes (see Section 3.5) (Chang et al. 2019; Geier et al. 2018; Ramirez et al. 2011; Samburova et al. 2014).
3.4. Chemical Concentrations and Firefighter Variables: 1530 Screening Method
If Table S6 indicated statistically significant differences between concentrations of on- and off-duty tags or the high and low call volume departments, then multivariate models were constructed for categorical and analyte data to investigate associations with variables in the questionnaire (Figure 7A–C, Table S7; see Section 2.7).
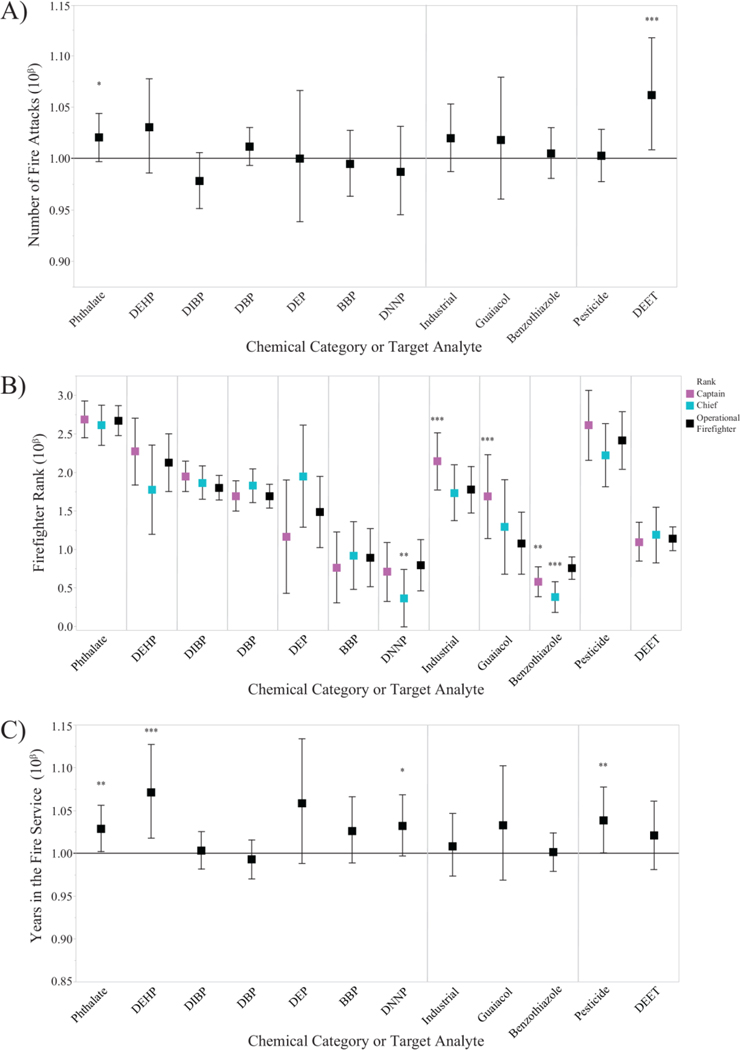
Figure 7.
Exponentiated β parameter coefficients from the 12 screening method models are shown for occupation-related variables of A) number of fire attacks during the sampling period, B) firefighter rank, and C) years spent in the fire service. In Figures 7A and 7C, a positive change in chemical concentration was represented by the solid line at 10β=1.0. Bold*: p<0.10. Bold**: p<0.05. Bold***: p<0.01.
3.4.1. Phthalates
Summed phthalate concentrations were higher for on- compared to off-duty tags, as well as for di(2-ethylhexyl) phthalate (DEHP), diisobutyl phthalate (DIBP), di-n-butyl phthalate (DBP), and di-n-nonyl phthalate (DNNP) (Figure 5A, Figure 5B, Table S6; paired t-test, d>0.25, p<0.05). Diethyl phthalate (DEP) concentrations were lower in on- compared to off-duty tags (Figure 5B, Table S6; paired t-test, d>0.40, p<0.05).
Summed phthalate concentrations between call volume departments were comparable (Figure 5C, Table S6; t-test, r<0.10, p>0.10), but individual phthalate concentrations differed. Concentrations of butyl benzyl phthalate (BBP) and di-n-nonyl phthalate (DNNP) were lower at the high compared to low call volume department (Figure 5D, Table S6; t-test, d>0.40, p<0.05).
This is the first paper to associate individualized firefighter phthalate exposures with questionnaire data. No overall trends were observed for the number of fire attacks and firefighter rank (Figure 7A and 7B). However, phthalate concentrations were slightly higher with increasing years spent in the fire service (Figure 7C, Table S7), which was consistent with the PAH results (Figure 6C). Because of this consistency, we again suggest that this trend may be linked to an increased willingness to take risks, or increased comfort level, during fire calls (Section 3.3). Sources for phthalates include firefighter turnout gear, gloves, and hoods (Alexander 2012; Alexander and Baxter 2014), such that more experienced firefighters may spend more time in their turnout gear on the fireground than less experienced firefighters. Consequently, increasing years in the fire service may increase both phthalate and PAH exposures.
Phthalates have also been associated with a wide variety of consumer products, such as medical products and automotive parts (Alexander and Baxter 2014; Radke et al. 2018; Ventrice et al. 2013). Manufacturers frequently add phthalates as plasticizers to increase longevity, although select phthalates are banned from use in childcare products in Europe due to health concerns (Ventrice et al. 2013). The US EPA has classified multiple phthalates as probable carcinogens (Di(2-ethylhexyl)phthalate (DEHP) 2000; Ventrice et al. 2013; Zarean et al. 2016), prompting a need to consider phthalate exposures in conjunction with other possible carcinogens.
3.4.2. Industrial-Related
Summed industrial-related concentrations were higher for on- compared to off-duty tags, as well as guaiacol and benzothiazole concentrations (Figure 5A, Figure 5B, Table S6; paired t-test, d>0.55, p<0.01). Industrial-related concentrations were comparable between departments, although benzothiazole concentrations were statistically lower at the high call volume department (Figure 5D, Table S6; t-test, r>0.55, p<0.01). Because the concentrations of only two target analytes from the industrial-related category (i.e. guaiacol and benzothiazole) were modeled with questionnaire data, overall trends were not examined.
To our knowledge, this is the first documented occurrence of bioavailable guaiacol and benzothiazole exposures among structural firefighters. Guaiacol, a major component of wood smoke and creosote (Edye and Richards 1991), is categorized as a probable human carcinogen by IARC (Toxicological Profile for Wood Creosote, Coal Tar Creosote, Coal Tar, Coal Tar Pitch, and Coal Tar Pitch Volatiles 2002). Among wildland firefighters, urinary metabolites of guaiacol have been documented (Neitzel et al. 2009) and guaiacol has been recommended as a measure of wood smoke exposure. Although the number of fire attacks and years in the fire service were not associated with tag concentrations (Figure 7A and 7B), guaiacol concentrations were higher from dog tags worn by captains (10β=1.69, 95% CI: 1.14, 2.23) compared to operational firefighters (10β=1.08, 95% CI: 0.68, 1.48) (Figure 7B, Table S7).
Benzothiazole and its derivatives are typically manufactured for a variety of industrial applications, such as corrosion inhibitors to increase product performance (e.g. tires) and as azo dyes for consumer products (Asimakopoulos et al. 2013; Donald et al. 2019; Wang et al. 2013). Benzothiazole also occurs naturally as a volatile organic constituent in black tea leaves (Vitzthum et al. 1975) and a flavor compound by fungi (Gallois et al. 1990). Interestingly, derivatives of benzothiazole (e.g. 2-arylbenzothiazole) are used as a basis for various pharmacological agents and have emerged as promising anti-cancer therapeutics in drug discovery (Weekes and Westwell 2009). With this background information, the frequent detection of benzothiazole (n=81 dog tags, 74%) served as a reminder that chemical exposures may not necessarily lead to adverse health outcomes and that individual mixture components of complex firefighter chemical exposures may have significant toxicological implications (see Section 3.5). Although the number of fire attacks and years in the fire service were not significant variables (Figure 7A and 7B), higher median benzothiazole concentrations were associated with dog tags worn by operational firefighters compared to captains and chiefs (Figure 7B, Table S7).
3.4.3. Pesticides
Summed pesticide concentrations were similar between on- and off-duty tags (Figure 5A, Table S6), but N,N-diethyl-m-toluamide concentrations were higher for on- compared to off-duty dog tags (Figure 5B, Table S6; paired t-test, d>0.25, p<0.05). Combined pesticide concentrations were higher at the high compared to low call volume department, potentially driven by N,N-diethyl-m-toluamide concentrations (Figure 5C, Figure 5D, Table S6, t-test, d>0.35, p<0.05).
N,N-diethyl-m-toluamide is commonly known as the insect repellant DEET, and the number of fire attacks was statistically associated with increasing concentrations (Figure 7A; 6%, 95% CI: 1.01, 1.12). DEET may be commonly present at building or car fires, even during winter months. Although DEET is not currently classifiable as a human carcinogen according to the US EPA (Keith et al. 2017), future cancer risk assessments of firefighter chemical exposures may need to consider the mixture effects of personal pesticide use with occupational chemicals.
3.5. Toxicological Implications
As demonstrated in this study, firefighters are exposed to complex chemical mixtures while both on- and off-duty; however, toxicological studies and cancer risk assessments typically focus on single chemicals (Altenburger et al. 2013; Carpenter et al. 2002). Compared to single component toxicological tests, chemicals may have different toxicities in a mixture, which could result in additive, synergistic, or antagonistic effects (Carpenter et al. 2002). When evaluating chemicals individually, researchers may inadvertently over- or underestimate firefighter health risks (Aylward et al. 2015; Stevenson et al. 2015). In this study, silicone dog tag data provided data on potential representative mixtures of PAHs and phthalates, which could be applied to future toxicological experiments. Real-world individualized chemical mixtures may inform toxicological experiments and provide a broader context for future firefighter risk assessments.
3.6. Limitations
There were several limitations associated with this study. First, the firefighters were non-random recruitments and not necessarily representative of the wider US firefighter population. Second, the small sample size limited the ability to assess for potential confounders. Third, firefighters did not keep daily logs of their activities and potential exposures during the study period. However, given the evidence presented in this study, daily logs and personal diaries may be less useful than quantitative data for exposure assessments. Fourth, the silicone dog tags sample both SVOCs and VOCs in the bioavailable phase, which combines inhalation, dermal, and some ingestion exposure pathways. The chemical exposures associated with a specific exposure pathway cannot currently be isolated with this study design. Although chemicals concentrations in the dog tags demonstrated potential exposure, they should not be considered equivalent to internal dose.
4. CONCLUSIONS
This is the first study to apply military-style silicone dog tags as a personal PSDs to investigate occupational and non-occupational firefighter bioavailable chemical exposures at multiple fire departments. The dog tags detected 18 PAHs that have not been previously reported as firefighter exposures, and several commonly detected analytes besides PAHs (e.g. phthalates, DEHP) are currently classified as possible carcinogens. When detected, the majority of chemicals had higher tag concentrations when worn on- compared to off-duty and from the high compared to the low call volume department. The number of fire attacks were positively associated with increasing PAH dog tag concentrations, demonstrating that quantitative data provides a more accurate picture of firefighter exposures compared to crude surrogate measurements. For future cancer and non-cancer risk assessments, researchers ought to consider the complexity of firefighter chemical exposures, as demonstrated in this study, to fully evaluate occupational health risks.
Supplementary Material
Acknowledgements
The authors give special thanks to the participating firefighters at the Raytown Fire Protection District and Southern Platte Fire Protection District. Special thanks is also given to the Institutional Review Boards, the OSU Superfund Research Program Core D for chemistry support, Brittany Hollerbach, Hannah Kelley, Richard Scott, Clarisa Caballero-Ignacio, Michael Barton, Jessica Scotten, Holly Dixon, Peter Hoffman, Kaci Graber, Caoilinn Haggerty, and Teresa Valdez.
Funding Sources
Funding is provided by the Federal Emergency Management Agency through the Assistance to Firefighters Grant Program (EMW-2016-FP-000754).
Abbreviations
- BBP
Butyl benzyl phthalate
- DBP
Di-n-butyl phthalate
- DEET
N,n-diethyl-m-toluamide
- DEHP
Di(2-ethylhexyl) phthalate
- DEP
Diethyl phthalate
- DIBP
Diisobutyl phthalate
- DNNP
Di-n-nonyl phthalate
- GC
Gas chromatograph
- LOQ
Limit of quantitation
- MS
Mass spectrometer
- PAH
Polycyclic aromatic hydrocarbon
- PCB
Polychlorinated biphenyl
- PPE
Personal protective equipment
- PSD
Passive sampling device
- PTFE
Polytetrafluoroethylene
Footnotes
Conflict of Interest
Kim A. Anderson, an author of this research, discloses a financial interest in MyExposome, Inc., which is marketing products related to the research being reported. The terms of this arrangement have been reviewed and approved by OSU in accordance with its policy on research conflicts of interest. The authors have no other disclosures.
REFERENCES
- Al-Daghri NM, Alokail MS, Abd-Alrahman SH, Draz HM, Yakout SM, Clerici M. 2013. Polycyclic Aromatic Hydrocarbon Exposure and Pediatric Asthma in Children: A Case–Control Study. Environ Health 12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander BM. 2012. Contamination of Firefighter Personal Protective Gear:University of Cincinnati. [Google Scholar]
- Alexander BM, Baxter CS. 2014. Plasticizer Contamination of Firefighter Personal Protective Clothing: A Potential Factor in Increased Health Risks in Firefighters. J Occup Environ Hyg 11:D43–48. [DOI] [PubMed] [Google Scholar]
- Allan SE, Smith BW, Anderson KA. 2012. Impact of the Deepwater Horizon Oil Spill on Bioavailable Polycyclic Aromatic Hydrocarbons in Gulf of Mexico Coastal Waters. Environ Sci Technol 46:2033–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenburger R, Backhaus T, Boedeker W, Faust M, Scholze M. 2013. Simplifying Complexity: Mixture Toxicity Assessment in the Last 20 Years. Environ Toxicol Chem 32:1685–1687. [DOI] [PubMed] [Google Scholar]
- Anderson K, Hillwalker W. 2008. Bioavailability. In: Ecotoxicology Vol. 1, (Jorgensen SE, Fath BD, eds). Oxford:Elsevier, 348–357. [Google Scholar]
- Anderson KA, Szelewski MJ, Wilson G, Quimby BD, Hoffman PD. 2015. Modified Ion Source Triple Quadrupole Mass Spectrometer Gas Chromatograph for Polycyclic Aromatic Hydrocarbon Analyses. J Chromatogr 1419:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KA, Points III GL, Donald CE, Dixon HM, Scott RP, Wilson G, Tidwell LG, Hoffman PD, Herbstman JB, O’Connell SG. 2017. Preparation and Performance Features of Wristband Samplers and Considerations for Chemical Exposure Assessment. J Expo Sci Environ Epidemiol 27:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JT, Achten C. 2015. Time to Say Goodbye to the 16 EPA PAHs? Toward an Up-to-Date Use of PACs for Environmental Purposes. Polycycl Aromat Compd 35:330–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimakopoulos AG, Wang L, Thomaidis NS, Kannan K. 2013. Benzotriazoles and Benzothiazoles in Human Urine from Several Countries: A Perspective on Occurrence, Biotransformation, and Human Exposure. Environ Int 59:274–281. [DOI] [PubMed] [Google Scholar]
- Aylward LL, Seiber JN, Hays SM. 2015. California Biomonitoring Data: Comparison to NHANES and Interpretation in a Risk Assessment Context. Regul Toxicol Pharmacol 73:875–884. [DOI] [PubMed] [Google Scholar]
- Bergmann AJ, North PE, Vasquez L, Bello H, Ruiz MdCG, Anderson KA. 2017. Multi-Class Chemical Exposure in Rural Peru using Silicone Wristbands. J Expo Sci Environ Epidemiol 27:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann AJ, Points GL, Scott RP, Wilson G, Anderson KA. 2018. Development of Quantitative Screen for 1550 Chemicals with GC-MS. Anal Bioanal Chem 410:3101–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlin P, Jones KC, Levin JO, Lindahl R, Strandberg B. 2010. Field Evaluation of a Passive Personal Air Sampler for Screening of PAH Exposure in Workplaces. J Environ Monit 12:1437–1444. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, Arcaro K, Spink DC. 2002. Understanding the Human Health Effects of Chemical Mixtures. Environ Health Perspect 110:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Siddens LK, Heine LK, Sampson DA, Yu Z, Fischer KA, Löhr CV, Tilton SC. 2019. Comparative Mechanisms of PAH Toxicity by Benzo[a]pyrene and Dibenzo[def,p]chrysene in Primary Human Bronchial Epithelial Cells Cultured at Air-Liquid Interface. Toxicol Appl Pharmacol 379:114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm MM, Bertke S, Allee S, Daniels RD. 2015. Creation of a Retrospective Job-Exposure Matrix Using Surrogate Measures of Exposure for a Cohort of US Career Firefighters from San Francisco, Chicago and Philadelphia. Occup Environ Med 72:670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RD, Kubale TL, Yiin JH, Dahm MM, Hales TR, Baris D, Zahm SH, Beaumont JJ, Waters KM, Pinkerton LE. 2014. Mortality and Cancer Incidence in a Pooled Cohort of US Firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occup Environ Med 71:388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RD, Bertke S, Dahm MM, Yiin JH, Kubale TL, Hales TR, Baris D, Zahm SH, Beaumont JJ, Waters KM, Pinkerton LE. 2015. Exposure-Response Relationships for Select Cancer and Non-Cancer Health Outcomes in a Cohort of US Firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occup Environ Med 72:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di(2-ethylhexyl)phthalate (DEHP). 2000.US Environmental Protection Agency. [Google Scholar]
- Dixon HM, Scott RP, Holmes D, Calero L, Kincl LD, Waters KM, Camann DE, Calafat AM, Herbstman JB, Anderson KA. 2018. Silicone Wristbands Compared with Traditional Polycyclic Aromatic Hydrocarbon Exposure Assessment Methods. Anal Bioanal Chem 410:3059–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon HM, Armstrong G, Barton M, Bergmann AJ, Bondy M, Halbleib ML, Hamilton W, Haynes E, Herbstman J, Hoffman P, Jepson P, Kile ML, Kincl L, Laurienti PJ, North P, Paulik LB, Petrosino J, Points GL, Poutasse CM, Rohlman D, Scott RP, Smith B, Tidwell LG, Walker C, Waters KM, Anderson KA. 2019. Discovery of Common Chemical Exposures Across Three Continents using Silicone Wristbands. R Soc Open Sci 6:181836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald CE, Scott RP, Blaustein KL, Halbleib ML, Sarr M, Jepson PC, Anderson KA. 2016a. Silicone Wristbands Detect Individuals’ Pesticide Exposures in West Africa. R Soc Open Sci 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald CE, Elie MR, Smith BW, Hoffman PD, Anderson KA. 2016b. Transport Stability of Pesticides and PAHs Sequestered in Polyethylene Passive Sampling Devices. Environ Sci Pollut Res 23:12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald CE, Scott RP, Wilson G, Hoffman PD, Anderson KA. 2019. Artificial Turf: Chemical Flux and Development of Silicone Wristband Partitioning Coefficients. Air Qual Atmos Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edye LA, Richards GN. 1991. Analysis of condensates from wood smoke. Components derived from polysaccharides and lignins. Environ Sci Technol 25:1133–1137. [Google Scholar]
- Fabian TZ, Borgerson JL, Gandhi PD, Baxter CS, Ross CS, Lockey JE, Dalton JM. 2011. Characterization of Firefighter Smoke Exposure. Fire Technol 50:993–1019. [Google Scholar]
- Fent KW, Eisenberg J, Snawder J, Sammons D, Pleil JD, Stiegel MA, Mueller C, Horn GP, Dalton J. 2014. Systemic Exposure to PAHs and Benzene in Firefighters Suppressing Controlled Structure Fires. Ann Occup Hyg 58:830–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent KW, Alexander B, Roberts J, Robertson S, Toennis C, Sammons D, Bertke S, Kerber S, Smith D, Horn G. 2017. Contamination of Firefighter Personal Protective Equipment and Skin and the Effectiveness of Decontamination Procedures. J Occup Env Hyg 14:801–814. [DOI] [PubMed] [Google Scholar]
- Fent KW, Evans DE, Babik K, Striley C, Bertke S, Kerber S, Smith D, Horn GP. 2018. Airborne contaminants during controlled residential fires. J Occup Env Hyg 15:399–412. [DOI] [PubMed] [Google Scholar]
- Firefighter Ranks. 2016. Firefighting 101. [Google Scholar]
- Gallois A, Gross B, Langlois D, Spinnler H-E, Brunerie P. 1990. Influence of Culture Conditions on Production of Flavour Compounds by 29 Ligninolytic Basidiomycetes. Mycol Res 94:494–504. [Google Scholar]
- Geier MC, Minick DJ, Truong L, Tilton S, Pande P, Anderson KA, Teeguardan J, Tanguay RL. 2018. Systematic Developmental Neurotoxicity Assessment of a Representative PAH Superfund Mixture Using Zebrafish. Toxicol Appl Pharmacol 354:115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass D, Pircher S, Del Monaco A, Vander Hoorn S, Sim M. 2016. Mortality and Cancer Incidence in a Cohort of Male Paid Australian Firefighters. Occup Environ Med 73:761–771. [DOI] [PubMed] [Google Scholar]
- Guidotti TL. 2007. Evaluating Causality for Occupational Cancers: The Example of Firefighters. Occup Med-Oxf 57:466–471. [DOI] [PubMed] [Google Scholar]
- Hammel SC, Hoffman K, Webster TF, Anderson KA, Stapleton HM. 2016. Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ Sci Technol 50:4483–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel SC, Phillips AL, Hoffman K, Stapleton HM. 2018. Evaluating the Use of Silicone Wristbands To Measure Personal Exposure to Brominated Flame Retardants. Environ Sci Technol 52:11875–11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Parra KL, Camacho J, Bradman A, Nolan JE, Lessard C, Anderson KA, Poutasse CM, Scott RP, Lazaro G. 2019. Determinants of Pesticide Concentrations in Silicone Wristbands Worn by Latina Adolescent Girls in a California Farmworker Community: The COSECHA Youth Participatory Action Study. Sci Total Environ 652:1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. 2010. IARC monographs on the evaluation of carcinogenic risks to humans 98:9–764. [PMC free article] [PubMed] [Google Scholar]
- Karimi P, Peters KO, Bidad K, Strickland PT. 2015. Polycyclic Aromatic Hydrocarbons and Childhood Asthma. Eur J Epidemiol 30:91–101. [DOI] [PubMed] [Google Scholar]
- Keith S, Harper C, Ashizawa A, Williams RL, Llados F, Coley C, Carlson-Lynch H. 2017. Toxicological Profile for DEET (N,n-diethyl-meta-toluamide). [PubMed] [Google Scholar]
- Kile ML, Scott RP, O’Connell SG, Lipscomb S, MacDonald M, McClelland M, Anderson KA. 2016. Using Silicone Wristbands to Evaluate Preschool Children’s Exposure to Flame Retardants. Environ Res 147:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk KM, Logan MB. 2015. Firefighting Instructors’ Exposures to Polycyclic Aromatic Hydrocarbons During Live Fire Training Scenarios. J Occup Env Hyg 12:227–234. [DOI] [PubMed] [Google Scholar]
- Koch HM, Aylward LL, Hays SM, Smolders R, Moos RK, Cocker J, Jones K, Warren N, Levy L, Bevan R. 2014. Inter-and Intra-Individual Variation in Urinary Biomarker Concentrations Over a 6-Day Sampling Period. Part 2: Personal Care Product Ingredients. Toxicol Lett 231:261–269. [DOI] [PubMed] [Google Scholar]
- Krol S, Zabiegala B, Namiesnik J. 2011. Monitoring and Analytics of Semivolatile Organic Compounds (SVOCs) in Indoor Air. Anal Bioanal Chem 400:1751–1769. [DOI] [PubMed] [Google Scholar]
- LeMasters GK, Genaidy AM, Succop P, Deddens J, Sobeih T, Barriera-Viruet H, Dunning K, Lockey J. 2006. Cancer Risk Among Firefighters: A Review and Meta-Analysis of 32 Studies. J Occup Environ Med 48:1189–1202. [DOI] [PubMed] [Google Scholar]
- Liagkouridis L, Cousins AP, Cousins IT. 2015. Physical-Chemical Properties and Evaluative Fate Modelling of ‘Emerging’ and ‘Novel’ Brominated and Organophosphorus Flame Retardants in the Indoor and Outdoor Environment. Sci Total Environ 524:416–426. [DOI] [PubMed] [Google Scholar]
- Liu YN, Tao S, Dou H, Zhang TW, Zhang XL, Dawson R. 2007. Exposure of Traffic Police to Polycyclic Aromatic Hydrocarbons in Beijing, China. Chemosphere 66:1922–1928. [DOI] [PubMed] [Google Scholar]
- Lorber M. 2008. Exposure of Americans to Polybrominated Diphenyl Ethers. J Expo Sci Environ Epidemiol 18:2–19. [DOI] [PubMed] [Google Scholar]
- McClean MD, Rinehart RD, Ngo L, Eisen EA, Kelsey KT, Wiencke JK, Herrick RF. 2004. Urinary 1-Hydroxypyrene and Polycyclic Aromatic Hydrocarbon Exposure Among Asphalt Paving Workers. Ann Occup Hyg 48:565–578. [DOI] [PubMed] [Google Scholar]
- McClean MD, Osborn LV, Snawder JE, Olsen LD, Kriech AJ, Sjodin A, Li Z, Smith JP, Sammons DL, Herrick RF, Cavallari JM. 2012. Using Urinary Biomarkers of Polycyclic Aromatic Compound Exposure to Guide Exposure-Reduction Strategies Among Asphalt Paving Workers. Ann Occup Hyg 56:1013–1024. [DOI] [PubMed] [Google Scholar]
- Minick DJ, Anderson KA. 2017. Diffusive Flux of PAHs Across Sediment-Water and Water-Air Interfaces at Urban Superfund Sites. Environ Toxicol Chem 36:2281–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neitzel R, Naeher LP, Paulsen M, Dunn K, Stock A, Simpson CD. 2009. Biological Monitoring of Smoke Exposure Among Wildland Firefighters: A Pilot Study Comparing Urinary Methoxyphenols with Personal Exposures to Carbon Monoxide, Particular Matter, and Levoglucosan. J Expo Sci Environ Epidemiol 19:349–358. [DOI] [PubMed] [Google Scholar]
- O’Connell SG, Kincl LD, Anderson KA. 2014. Silicone Wristbands as Personal Passive Samplers. Environ Sci Technol 48:3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira M, Slezakova K, Alves MJ, Fernandes A, Teixeira JP, Delerue-Matos C, Pereira MDC, Morais S. 2017. Polycyclic Aromatic Hydrocarbons at Fire Stations: Firefighters’ Exposure Monitoring and Biomonitoring, and Assessment of the Contribution to Total Internal Dose. J Hazard Mater 323:184–194. [DOI] [PubMed] [Google Scholar]
- Paulik LB, Hobbie KA, Rohlman D, Smith BW, Scott RP, Kincl L, Haynes EN, Anderson KA. 2018. Environmental and Individual PAH Exposures Near Rural Natural Gas Extraction. Environ Pollut 241:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paustenbach D and Galbraith D. 2006. Biomonitoring: Is Body Burden Relevant to Public Health? Regul Toxicol Pharm 44:249–261. [DOI] [PubMed] [Google Scholar]
- Perera F, Tang WY, Herbstman J, Tang D, Levin L, Miller R, Ho SM. Relation of DNA Methylation of 5′-CpG Island of ACSL3 to Transplacental Exposure to Airborne Polycyclic Aromatic Hydrocarbons and Childhood Asthma. 2009. PLoS One 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston WSC, Haddock CK, Jahnke SA, Jitnarin N, Tuley BC, Kales SN. 2011. The Prevalence of Overweight, Obesity, and Substandard Fitness in a Population-Based Firefighter Cohort. J Occup Environ Med 53:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutasse CM, Herbstman JB, Peterson ME, Gordon JM, Soboroff PH, Holmes D, Gonzalez D, Tidwell LG, Anderson KA. 2019. Silicone Pet Tags Associate Tris(1,3-Dichloro-2-Isopropyl) Phosphate Exposures with Feline Hyperthyroidism. Environ Sci Technol 53:9203–9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukkala EI. 1995. Cancer Risk by Social Class and Occupation: A Survey of 109,000 Cancer Cases Among Finns of Working Age:Karger Medical and Scientific Publishers. [Google Scholar]
- Radke EG, Braun JM, Meeker JD, Cooper GS. 2018. Phthalate Exposure and Male Reproductive Outcomes: A Systematic Review of the Human Epidemiological Evidence. Environ Int 121:764–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez N, Cuadras A, Rovira E, Marce RM, Borrull F. 2011. Risk Assessment Related to Atmospheric Polycyclic Aromatic Hydrocarbons in Gas and Particle Phases near Industrial Sites. Environ Health Perspect 119:1110–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samburova V, Zielinska B, Khlystov A. 2017. Do 16 Polycyclic Aromatic Hydrocarbons Represent PAH Air Toxicity? Toxics 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreder ED, Uding N, La Guardia MJ. 2016. Inhalation a Significant Exposure Route for Chlorinated Organophosphate Flame Retardants. Chemosphere 150:499–504. [DOI] [PubMed] [Google Scholar]
- Serdar B, Lee D, Dou ZH. 2012. Biomarkers of Exposure to Polycyclic Aromatic Hydrocarbons (PAHs) and DNA Damage: A Cross-Sectional Pilot Study Among Roofers in South Florida. BMJ Open 2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Whitehead TP, McNeel S, Brown FR, Dhaliwal J, Das R, Israel L, Park JS, Petreas M. 2015. High Levels of Polybrominated Diphenyl Ethers in Vacuum Cleaner Dust from California Fire Stations. Environ Sci Technol 49:4988–4994. [DOI] [PubMed] [Google Scholar]
- Shen B, Whitehead TP, Gill R, Dhaliwal J, Brown FR, Petreas M, Patton S, Hammond SK. 2018. Organophosphate Flame Retardants in Dust Collected from United States Fire Stations. Environ Int 112:41–48. [DOI] [PubMed] [Google Scholar]
- Sjostrom M, Julander A, Strandberg B, Lewne M, Bigert C. 2019. Airborne and Dermal Exposure to Polycyclic Aromatic Hydrocarbons, Volatile Organic Compounds, and Particles among Firefighters and Police Investigators. Ann Work Expo Health 63:533–545. [DOI] [PubMed] [Google Scholar]
- Sobus JR, McClean MD, Herrick RF, Waidyanatha S, Onyemauwa F, Kupper LL, Rappaport SM. 2009. Investigation of PAH Biomarkers in the Urine of Workers Exposed to Hot Asphalt. Ann Occup Hyg 53:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec AA, Dickens KE, Salden M, Hewitt FE, Watts DP, Houldsworth PE, Martin FL. 2018. Occupational Exposure to Polycyclic Aromatic Hydrocarbons and Elevated Cancer Incidence in Firefighters. Sci Rep 8:2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M, Alexander B, Baxter CS, Leung YK. 2015. Evaluating Endocrine Disruption Activity of Deposits on Firefighting Gear Using a Sensitive and High Throughput Screening Method. J Occup Environ Med 57:e153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg B, Julander A, Sjostrom M, Lewne M, Akdeva HK, Bigert C. 2018. Evaluation of Polyurethane Foam Passive Air Sampler (PUF) as a Tool for Occupational PAH Measurements. Chemosphere 190:35–42. [DOI] [PubMed] [Google Scholar]
- Toxicological Profile for Wood Creosote, Coal Tar Creosote, Coal Tar, Coal Tar Pitch, and Coal Tar Pitch Volatiles. 2002.US Department of Health and Human Services. [Google Scholar]
- Tsai RJ, Luckhaupt SE, Schumacher P, Cress RD, Deapen DM, Calvert GM. 2015. Risk of Cancer Among Firefighters in California, 1988–2007. Am J Ind Med 58:715–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventrice P, Ventrice D, Russo E, De Sarro G. 2013. Phthalates: European Regulation, Chemistry, Pharmacokinetic and Related Toxicity. Environ Toxicol Pharmacol 36:88–96. [DOI] [PubMed] [Google Scholar]
- Vidi PA, Anderson KA, Chen HY, Anderson R, Salvador-Moreno N, Mora DC, Poutasse C, Laurienti PJ, Daniel SS, Arcury TA. 2017. Personal Samplers of Bioavailable Pesticides Integrated with a Hair Follicle Assay of DNA Damage to Assess Environmental Exposures and their Associated Risks in Children. Mutat Res 822:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitzthum OG, Werkhoff P, Hubert P. 1975. New Volatile Constituents of Black Tea Aroma. J Agric Food Chem 23:999–1003. [Google Scholar]
- Wang L, Asimakopoulos AG, Moon H-B, Nakata H, Kannan K. 2013. Benzotriazole, Benzothiazole, and Benzophenone Compounds in Indoor Dust from the United States and East Asian Countries. Environ Sci Technol 47:4752–4759. [DOI] [PubMed] [Google Scholar]
- Weekes AA, Westwell A. 2009. 2-Arylbenzothiazole as a Privileged Scaffold in Drug Discovery. Curr Med Chem 16:2430–2440. [DOI] [PubMed] [Google Scholar]
- Wei W, Mandin C, Ramalho O. 2018. Influence of Indoor Environmental Factors on Mass Transfer Parameters and Concentrations of Semi-Volatile Organic Compounds. Chemosphere 195:223–235. [DOI] [PubMed] [Google Scholar]
- Weschler CJ, Nazaroff WW. 2012. SVOC Exposure Indoors: Fresh Look at Dermal Pathways. Indoor Air 22:356–377. [DOI] [PubMed] [Google Scholar]
- Wilford BH, Harner T, Zhu JP, Shoeib M, Jones KC. 2004. Passive Sampling Survey of Polybrominated Diphenyl Ether Flame Retardants in Indoor and Outdoor Air in Ottawa, Canada: Implications for Sources and Exposure. Environ Sci Technol 38:5312–5318. [DOI] [PubMed] [Google Scholar]
- Wingfors H, Nyholm JR, Magnusson R, Wijkmark CH. 2018. Impact of Fire Suit Ensembles on Firefighter PAH Exposures as Assessed by Skin Deposition and Urinary Biomarkers. Ann Work Expo Health 62:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarean M, Keikha M, Poursafa P, Khalighinejad P, Amin M, Kelishadi R. 2016. A Systematic Review on the Adverse Health Effects of Di-2-Ethylhexyl Phthalate. Environ Sci Pollut Res Int 23:24642–24693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.