Abstract
Introduction:
In the United States, approximately 100,000 individuals of predominantly African ancestry have sickle cell disease (SCD). Patients with SCD present recurrent episodes of acute pain, the hallmark of the disease, and some also develop chronic pain. Currently, the treatment of SCD acute pain only targets its symptoms, rather than underlying mechanisms, and is directed by expert and consensus guidelines.
Areas covered:
While opioids remain the mainstay of therapy for acute pain and are also used to treat SCD-related chronic pain, in some patients, opioids are ineffective or associated with severe undesirable side effects. In those instances, clinicians caring for patients with SCD face the unmet need for effective non-opioid analgesics. Recently, the use of subanesthetic ketamine has been explored as a strategy to meet this need. While definitive evidence of its efficacy is lacking, some information exists suggesting that subanesthetic ketamine improves pain control and may have opioid-sparing effects in SCD-related acute pain. However, ketamine can also yield undesirable psychotomimetic and cardiovascular effects.
Expert Opinion:
After weighing potential risks and benefits, in the absence of better alternatives and in settings where it can be administered safely, ketamine may be a reasonable option for patients with SCD-related acute refractory pain.
Keywords: Ketamine, Sickle cell, Pain, NMDA receptor, Acute
1. Introduction
In the United States, approximately 100,000 individuals of predominantly African ancestry have sickle cell disease (SCD) [1]. From an early age, patients with SCD face a life-long challenge of having recurrent acute pain episodes related to microvaso-occlusion and as they grow older, some will develop chronic pain. In SCD, pain is a complex entity encompassing a broad spectrum of pain syndromes resulting from complications of the disease; patients may present acute or chronic pain, and some have chronic pain punctuated by recurrent episodes of acute pain. The underlying mechanism of pain in SCD is still largely unknown. However, our understanding of pain in sickle cell disease has evolved and while acute pain is the hallmark of the disease and derives from microvaso-occlusion, it is now abundantly clear that many patients, particularly adults, have chronic pain.
Recently, in an effort to facilitate investigations of underlying mechanisms and development of novel therapies and ultimately improve the diagnoses and treatment of SCD-related pain, experts in the field have published diagnostic criteria for acute [2] and chronic [3] SCD pain. Acute pain is the most prominent sickle complication and the main reason why patients with SCD seek medical attention, accounting for over 100,000 hospitalizations and over $600,000 million dollars in yearly costs [4]. The treatment of SCD-related pain mainly targets its symptoms and for the most part is not mechanism-based, but is guided by expert and consensus opinions [5,6]. However, while the guidelines issued by the National Heart, Lung, and Blood Institute (NHLBI) in 2014 [5] and The America Society of Hematology in 2020 [6] recommend the administration of parenteral opioids to patients with SCD presenting with acute pain within 60 minutes of arrival to the emergency department, fewer than 50% of patients receive parenteral opioids within the recommended time-frame [7]. A consequence is that, in many instances, patients with SCD may receive delayed and inadequate treatment when they present with acute pain.
While opioids are the mainstay of therapy for acute pain, both in emergency departments and for hospitalized patients, for some, opioids are ineffective and, with escalating doses, can be associated with severe side effects and refractory and increasing pain, which in some patients can result from tolerance or opioid-induced hyperalgesia [8]. Therefore, clinicians caring for patients with SCD-related acute pain have explored pharmacological therapies to be used as adjunctive to opioids or non-opioid alternatives. Over the last decade, a growing number of clinicians and institutions have adopted the use of subanesthetic ketamine to modulate acute SCD pain given its known analgesic and opioid-sparing effects despite the lack of definitive evidence of its efficacy in SCD-related acute pain [9,10]. Here we discuss the use of ketamine to treat SCD-related acute pain and briefly examine the data that has led to its increased use in hospitalized patients.
2. Ketamine an anesthetic affecting many neurotransmitter systems implicated in acute and chronic pain
A dissociative anesthetic, ketamine has been used since the 1960s and proven safe and effective in many clinical settings including battlefield rescues, emergency departments, and operating rooms [11]. Ketamine’s principal mechanism of action is the non-competitive blockade of the NMDA receptor, a cation channel mostly located in excitatory synapses (Figure). However, ketamine also affects opioidergic, GABAergic, monoaminergic, and muscarinic neurotransmissions, as well as substance P, alpha-amino-3-hydroxyl-5-methyl-4-isoxazole propionate (AMPA), and sigma receptors signaling [11]. The understanding that activation of NMDA receptors has been implicated in the development of central sensitization, inflammatory, nociceptive, and neuropathic pain, as well as of opioid tolerance and opioid-induced hyperalgesia provides the rationale for the use of subanesthetic ketamine for the treatment of acute and chronic pain [12–14].
3. Ketamine, an old anesthetic repurposed for pain management
There is some evidence, albeit not definitive, supporting the use of ketamine for acute and chronic pain management. Over the last decades there has been a significant increase in the use of ketamine infusions to treat acute and chronic pain with. However, there is great variability in settings where it is administered, indications, patient selection, monitoring requirements, and treatment parameters. In an effort to address such variability in practice, the American Society of Regional Anesthesia and Pain Medicine, American Academy of Pain Medicine, and American Society of Anesthesiologists issued consensus guidelines providing guidance on patient selection, indications, and contraindications for ketamine administration for the treatment of acute [15] and chronic [16] pain.
For acute pain, the guidelines recommend that ketamine should be considered for the treatment of acute pain as a stand-alone analgesic or opioid adjunct in patients undergoing painful surgery [15]. Additionally, the same guidelines also indicate that patients with opioid-dependence, opioid-tolerance, or who have sleep apnea may benefit from ketamine for the treatment of postoperative pain [15]. Regarding ketamine administration in settings where patients do not receive intensive monitoring, a ketamine bolus up to 0.35 mg/kg, and infusions at subanesthetic doses (no higher than 1 mg/kg per hour) [15]. For chronic pain, there is moderate evidence suggesting that patients with complex regional pain syndrome may have improvement in pain for up to 12 weeks and weak evidence that those with spinal cord injury pain may have some short-term benefit from ketamine administration [16]. Regarding other therapeutic parameters, for patients with chronic pain, there is moderate evidence supporting the use of repeated, frequent, and longer infusions of ketamine at higher doses administered on an outpatient setting [16]. In all settings, ketamine should be avoided in patients with cardiovascular, neurological, endocrinological, metabolic, and psychiatric conditions and in those who are pregnant, given the potential for psychotomimetic, neurological, and hemodynamic side-effects as well as the lack of safety data in pregnancy [16].
4. The use of subanesthetic ketamine to treat SCD-related pain
In 2014, the NHLBI and in 2020, the American Society of Hematology (ASH)[6] issued guidelines providing recommendations and suggestions (conditional recommendations) for the management of acute and chronic pain in patients with SCD. In both guidelines there are strong recommendations for prompt (within 60 minutes of arrival) initiation of pain management in patients with SCD presenting with acute pain. Among pharmacological interventions, the guidelines suggest administration of opioids, according to the patients’ baseline opioid use and prior history of effective doses with the caveat that the recommendation is conditional based on moderate (for adults) and low certainty (for children) in the evidence of its efficacy [5,6]. For patients with SCD hospitalized with acute pain that refractory or not ameliorated by opioids, the 2020 ASH guidelines suggest ketamine at subanesthetic doses (up to 1mg/kg/hour) as an adjuvant to opioids remarking that the suggestion is based on very low certainty in the evidence of its efficacy [6].
An extensive review of ketamine use in acute pain is beyond the scope of this work as two systematic reviews of existing case reports and series describing administration of ketamine to treat SCD acute pain have been recently published [9,10]. Overall, the studies show that there is significant variability on the practice of using ketamine to treat SCD-related pain. Ketamine is administered to SCD patients in different settings including emergency departments, in intensive care units or inpatient wards and at highly variable dose ranges via intranasal or intravenous routes. Furthermore, the timing and indication for initiation, titration, and duration of ketamine infusions is also highly variable, often unclear, and mostly left to clinician’s discretion. It is also important to note that in most reports, ketamine, even at subanesthetic doses, can be associated with undesirable psychotomimetic or hemodynamic effects including confusion, hallucinations, dysphoria, nystagmus, nausea, vomiting, and changes in blood pressure. Nevertheless, despite the fact that the majority of studies are retrospective and lack a control group, most conclude that ketamine does reduce pain scores compared and that, in some cases, it appears to have opioid-sparing effects.
Despite the growing use of ketamine in sickle pain, it remains unclear what patients benefit most, the best timing and setting, the duration, and the indications for its use. In exploratory cohort studies of hospitalized patients who in total received 181 infusions over an 8-year period [17,18], researchers showed that in children and adolescents with SCD admitted with acute pain, subanesthetic doses of ketamine used as an adjunct to opioids yielded decreases in pain and opioid consumption. In those studies, 54% of ketamine infusions administered to SCD patients produced meaningful reductions in pain and in multivariate analysis, male sex, younger age, pain not located on the chest, and longer infusions were identified as independent predictors of reductions in pain scores, but not of opioid consumption [18]. Most studies report the initiation of ketamine when pain is refractory to opioids, a practice that is in keeping with the 2020 ASH guidelines [6]. Whether timing of ketamine administration impacts on its effect in pain ratings remains unknown. However, a recent study showed that ketamine infusions initiated early (less than 3 days) during hospital admission was associated with greater reductions in pain scores for two days after initiation of the infusion compared with those initiated later (after 3 days) in the hospital course [19]. While this effect only lasted for two days, those findings might suggest that earlier, rather than later, initiation of ketamine, might yield greater benefits to patients with acute pain during hospital admission.
Unlike the known benefits of ketamine in a few chronic pain syndromes, there are very few reports of its use in sickle-related chronic pain. In an outpatient chronic pain clinic, repeated short infusions of ketamine administered to three patients with SCD who had chronic pain did not yield meaningful changes in pain scores or opioid use [20]. Therefore, despite a surge in its use, it remains unproven whether ketamine has a role in the treatment of acute or chronic pain in patients with SCD.
While ketamine has been administered to hospitalized SCD patients for more than a decade [9,10], only two randomized clinical trials of ketamine in acute sickle pain have been published (Table) [21,22]. The first enrolled 240 children and adolescents presenting with acute pain who were randomized to receive ketamine (1mg/kg) or morphine (0.1 mg/kg) intravenously over 10 min in Uganda [21]. The authors showed that while ketamine and morphine yielded similar maximal changes in pain scores, the time to reach these maximal changes was shorter in the ketamine-treated group. While there were no differences in admission rates between the two groups, the ketamine-treated group experienced significantly more side effects including nystagmus and dysphoria [21]. The second trial enrolled 278 adults in Saudi Arabia, randomized patients to receive a single dose of either ketamine (0.3mg/kg) or morphine (0.1mg/kg) upon presentation with acute pain, and used intention-to-treat design [22]. The authors showed that administration of ketamine as the first analgesic for acute pain, yielded similar changes in pain ratings, had an opioid-sparing effect, and did not change hospitalization rates compared to morphine. Together, these two randomized clinical trials of ketamine in acute SCD pain were conducted in emergency departments and examined its effects only over the first 2 hours after presentation to the hospital, thus leaving the question about the efficacy of ketamine in treating hospitalized SCD patients with acute pain unanswered [21,22].
Another consideration lies in the context of recurrent and chronic pain and chronic illness; SCD patients have a high prevalence of psychiatric and psychological comorbidities including depression, disruptive behavior disorder, and anxiety disorder [23–25]. Given the recently demonstrated rapid-onset antidepressant effect of ketamine, the possibility exists that this property of ketamine could contribute to its beneficial effects in patients with SCD with acute pain [26].
5. Expert opinion
For decades, hydroxyurea had been the only therapy shown to reduce the frequency of acute pain episodes in SCD patients. Recently, L-glutamine, an amino acid, and crizanlizumab, a P-selectin antibody, were approved for clinical use by the United States Food and Drug Administration as they decrease the frequency of acute pain episodes. However, these novel therapies have not been shown to have a role in treating SCD-related acute pain. There is a significant unmet need for an effective treatment for acute sickle-pain, particularly when opioids, the mainstay of therapy, are ineffective in providing adequate pain relief. Additionally, in light of the opioid epidemic, in 2017 the Department of Health and Human Services declared the opioid crisis a public health emergency prompting the pursuit of analgesic alternatives to opioids and possibly leading to the observed increase use of ketamine to treat patients with SCD presenting with acute pain episodes.
While the published data indicates that ketamine is often used to treat SCD pain and is reasonably well tolerated, it remains unproven whether ketamine has a beneficial effect in reducing pain and opioid requirements and whether its administration as a sole analgesic or as an adjuvant to opioids is superior to the current standard of care (administration of opioids and/or nonsteroidal anti-inflammatory drugs) is also unknown. Further, if proven effective, the identification of patients who might benefit from ketamine, its optimum dose, timing, and duration of therapy remains to be determined. It is indeed regrettable that there are no registered ongoing or planned randomized clinical trials of ketamine for the treatment of patients with SCD hospitalized with acute pain [27].
In our institutions, we do use subanesthetic ketamine to treat patients with SCD hospitalized with acute pain when the pain is refractory despite increasing doses of opioid, when there are significant undesirable opioid-related side-effects, or when there is a suspicion of opioid-induced hyperalgesia. We do acknowledge that while some studies suggest that subanesthetic ketamine has the potential to improve pain control and have opioid-sparing effects, definitive evidence demonstrating its beneficial effect is lacking. We are also circumspect about the fact that after single doses or during infusions, ketamine can yield undesirable psychotomimetic (dysphoria and hallucinations) and cardiovascular (hypertension, tachycardia) effects. Therefore, it is imperative that we balance the potential benefits and risks of using ketamine either as a sole analgesic or as an adjuvant therapy to treat SCD-related pain. In conclusion, ketamine may or may not be the way forward for pain management in patients with SCD. In the absence of better alternatives, however, and in settings where it can be administered safely, adding subanesthetic ketamine to the ongoing therapeutic approach may be a reasonable and useful option to treat refractory pain in patients with SCD hospitalized with acute pain.
Figure 1:
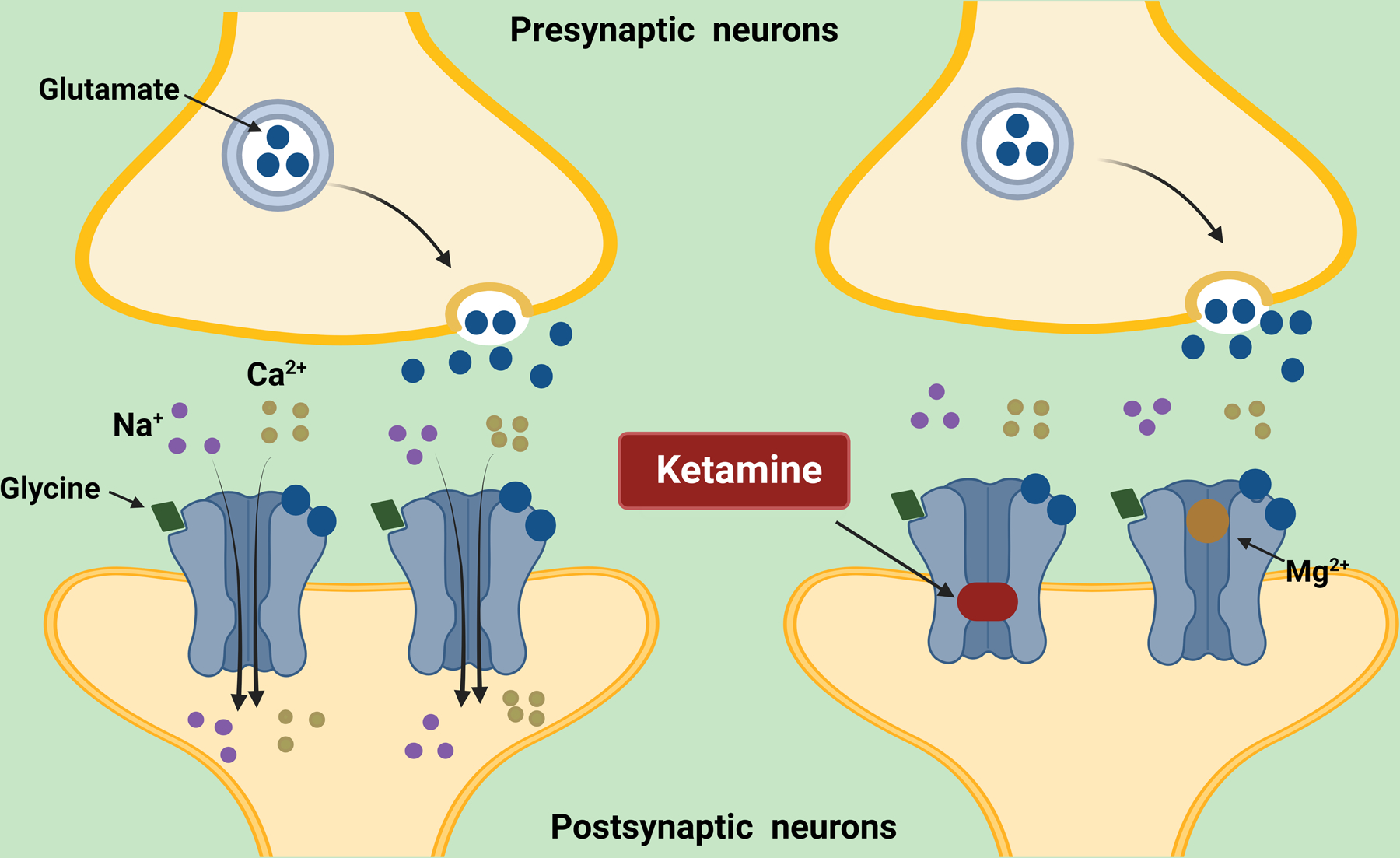
Ketamine, a dissociative anesthetic, is a non-competitive antagonist of the glutamate N-methyl-D-aspartate (NMDA) receptor, a cation channel mostly located in excitatory synapses. As an antagonist of the NMDA receptor, ketamine blocks the open channel and occludes the flow of cations. As activation of NMDA receptors has been implicated in the development of central sensitization, inflammatory, nociceptive, and neuropathic pain, as well as of opioid tolerance and opioid-induced hyperalgesia. These findings provide mechanisms that justify the pursuit of the use of subanesthetic ketamine doses (up to 1 mg/kg/hour) to treat acute and chronic pain including pain associated with sickle cell disease. Created with BioRender.com
Table 1.
Randomized clinical trials of ketamine in patients with sickle cell disease presenting with acute pain crisis
| Reference |
Patient Number |
Age* (Years) |
Setting |
Ketamine Dose Route |
Comparison group |
Ketamine Effects |
Adverse events (incidence) † |
|---|---|---|---|---|---|---|---|
| Lubega et al., 2018 [21] |
240 |
11.8±3.5 |
Emergency Department |
1mg/kg Intravenous Sole analgesic |
Morphine 0.1mg/kg |
Reduction in pain scores similar to morphine Shorter time to maximal change in pain scores |
Nystagmus (15%) Dysphoria (11.3%) Salivation (2.5%) |
| Alshahrani et al., 2022 [22] |
278 |
29.4±8.1 |
Emergency Department |
0.3mg/kg Intravenous (Intention-to-treat analysis) |
Morphine 0.1mg/kg |
Reduction in pain scores similar to morphine Opioid-sparing |
Dizziness (1.7%), nausea (1.4%), Vomiting (1.4%) |
Age is shown as mean ± standard deviation.
The incidence of adverse events reflects that in the ketamine-treated groups.
Article highlights.
In sickle cell disease (SCD), pain is a complex entity and, in some cases, underlying mechanisms are incompletely understood.
Opioids are the mainstay of therapy for acute pain but in some SCD patients, opioids are ineffective and can be associated with refractory and inadequately treated pain.
Subanesthetic ketamine has been explored as a strategy to treat refractory SCD-related pain based on retrospective case reports and series suggesting that ketamine improves pain control and has opioid-sparing effects.
While definitive evidence of its efficacy is lacking, there has been a surge on the use of subanesthetic ketamine for the treatment of acute sickle pain.
In settings where it can be administered safely, ketamine may be a reasonable option for patients with SCD-related acute refractory pain.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health Clinical Center and National Heart Lung and Blood Institute, National Institutes of Health (Grant numbers 1ZIACL090052–05, 1ZIACL090053–05, and 1ZIACL090054–05).
Footnotes
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010. Apr;38(4 Suppl):S512–21. [DOI] [PubMed] [Google Scholar]
- 2.Field JJ, Ballas SK, Campbell CM, et al. AAAPT Diagnostic Criteria for Acute Sickle Cell Disease Pain. J Pain 2019. Jul;20(7):746–759. [DOI] [PubMed] [Google Scholar]
- 3. Dampier C, Palermo TM, Darbari DS, et al. AAPT Diagnostic Criteria for Chronic Sickle Cell Disease Pain. J Pain 2017. May;18(5):490–498. ** Of considerable interest, an evidence-based classification system for sickle cell-related chronic pain.
- 4.Fingar KR, Owens PL, Reid LD, et al. Characteristics of Inpatient Hospital Stays Involving Sickle Cell Disease, 2000–2016: Statistical Brief #251. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. [PubMed] [Google Scholar]
- 5. National Heart L, and Blood Institute. Evidence-Based Management of Sickle Cell Disease: Expert Panel Report 2014. [9.12.2022]. Available from: https://www.nhlbi.nih.gov/resources/evidence-based-management-sickle-cell-disease-expert-0. ** Of considerable interest, guidelines from the National Heart L, and Blood Institute for pain management in patients with sickle cell disease
- 6.Brandow AM, Carroll CP, Creary S, et al. American Society of Hematology 2020 guidelines for sickle cell disease: management of acute and chronic pain. Blood Adv 2020. Jun 23;4(12):2656–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rees CA, Brousseau DC, Ahmad FA, et al. Adherence to NHLBI guidelines for the emergent management of vaso-occlusive episodes in children with sickle cell disease: A multicenter perspective. Am J Hematol 2022. Aug 22. * Of interest, a study demonstrating the low adherence to the NHLBI guidelines
- 8.Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet 2019. Apr 13;393(10180):1558–1568. [DOI] [PubMed] [Google Scholar]
- 9.Alshahrani MS, Alghamdi MA. Ketamine for Sickle Cell Vaso-Occlusive Crises: A Systematic Review. Saudi J Med Med Sci 2021. Jan-Apr;9(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris EM, Vilk E, Heeney MM, et al. A systematic review of ketamine for the management of vaso-occlusive pain in sickle cell disease. Pediatr Blood Cancer 2021. Jul;68(7):e28989. [DOI] [PubMed] [Google Scholar]
- 11.Potter DE, Choudhury M. Ketamine: repurposing and redefining a multifaceted drug. Drug Discov Today 2014. Dec;19(12):1848–54. [DOI] [PubMed] [Google Scholar]
- 12.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009. Sep;10(9):895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreutzwiser D, Tawfic QA. Expanding Role of NMDA Receptor Antagonists in the Management of Pain. CNS Drugs 2019. Apr;33(4):347–374. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Maher DP, Cohen SP. Emerging concepts on the use of ketamine for chronic pain. Expert Rev Clin Pharmacol 2020. Feb;13(2):135–146. [DOI] [PubMed] [Google Scholar]
- 15. Schwenk ES, Viscusi ER, Buvanendran A, et al. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med 2018. Jul;43(5):456–466. * Of interest, guidelines for the use of ketamine for the treatment of acute pain.
- 16. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med 2018. Jul;43(5):521–546. * Of interest, guidelines for the use of ketamine for the treatment of chronic pain.
- 17.Sheehy KA, Lippold C, Rice AL, et al. Subanesthetic ketamine for pain management in hospitalized children, adolescents, and young adults: a single-center cohort study. J Pain Res 2017;10:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nobrega R, Sheehy KA, Lippold C, et al. Patient characteristics affect the response to ketamine and opioids during the treatment of vaso-occlusive episode-related pain in sickle cell disease. Pediatr Res 2018. Feb;83(2):445–454. [DOI] [PubMed] [Google Scholar]
- 19.Kenney MO, Becerra B, Mallikarjunan A, et al. Early Initiation of Sub-anesthetic Ketamine Infusion in Adults with Vaso-occlusive Crises is Associated with Greater Reduction in Sickle Cell Pain Intensity: A Single Center’s Experience. Pain Med 2022. Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheehy KA, Muller EA, Lippold C, et al. Subanesthetic ketamine infusions for the treatment of children and adolescents with chronic pain: a longitudinal study [journal article]. BMC Pediatrics 2015;15(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubega FA, DeSilva MS, Munube D, et al. Low dose ketamine versus morphine for acute severe vaso occlusive pain in children: a randomized controlled trial. Scand J Pain 2018. Jan 26;18(1):19–27. [DOI] [PubMed] [Google Scholar]
- 22.Alshahrani MS, AlSulaibikh AH, ElTahan MR, et al. Ketamine administration for acute painful sickle cell crisis: A randomized controlled trial. Acad Emerg Med 2022. Feb;29(2):150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myrvik MP, Burks LM, Hoffman RG, et al. Mental health disorders influence admission rates for pain in children with sickle cell disease. Pediatr Blood Cancer 2013. Jul;60(7):1211–4. [DOI] [PubMed] [Google Scholar]
- 24.Bakri MH, Ismail EA, Elsedfy GO, et al. Behavioral impact of sickle cell disease in young children with repeated hospitalization. Saudi J Anaesth 2014. Oct;8(4):504–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lance EI, Cannon AD, Shapiro BK, et al. Co-Occurrence of Neurodevelopmental Disorders in Pediatric Sickle Cell Disease. J Dev Behav Pediatr 2021. Aug 1;42(6):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 2010. Aug;67(8):793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinicaltrials.gov. 2022. Available from: https://clinicaltrials.gov/ct2/results?term=sickle+cell+and+ketamine&Search=Search


