Abstract
β-Catenin is a cytoplasmic protein that participates in the assembly of cell-cell adherens junctions by binding cadherins to the actin cytoskeleton. In addition, it is a key component of the Wnt signaling pathway. Activation of this pathway triggers the accumulation of β-catenin in the nucleus, where it activates the transcription of target genes. Abnormal accumulation of β-catenin is characteristic of various types of cancer and is caused by mutations either in the adenomatous polyposis coli protein, which regulates β-catenin degradation, or in the β-catenin molecule itself. Aberrant accumulation of β-catenin in tumors is often associated with mutational inactivation of the p53 tumor suppressor. Here we show that overexpression of wild-type p53, by either transfection or DNA damage, down-regulates β-catenin in human and mouse cells. This effect was not obtained with transcriptionally inactive p53, including a common tumor-associated p53 mutant. The reduction in β-catenin level was accompanied by inhibition of its transactivation potential. The inhibitory effect of p53 on β-catenin is apparently mediated by the ubiquitin-proteasome system and requires an active glycogen synthase kinase 3β (GSK3β). Mutations in the N terminus of β-catenin which compromise its degradation by the proteasomes, overexpression of dominant-negative ΔF-β-TrCP, or inhibition of GSKβ activity all rendered β-catenin resistant to down-regulation by p53. These findings support the notion that there will be a selective pressure for the loss of wild-type p53 expression in cancers that are driven by excessive accumulation of β-catenin.
β-Catenin plays a dual role in cells as a major structural component of cell-cell adherens junctions and as a pivotal signaling molecule in the Wnt pathway, transmitting transcriptional cues into the nucleus. In adherens junctions, β-catenin bridges between cadherin and the actin cytoskeleton through an interaction with α-catenin (2, 10). Either the nonjunctional pool of β-catenin is degraded by the ubiquitin-proteasome system or, under certain conditions, β-catenin enters the nucleus and, together with lymphoid enhancer factor/T-cell factor transcription factors (9, 34, 56), activates transcription by providing the transactivation domain to this heterodimeric complex (82). The targeting of β-catenin to the proteasome is achieved primarily through its phosphorylation by a multimolecular complex consisting of glycogen synthase kinase 3β (GSK3β), the adenomatous polyposis coli (APC) tumor suppressor protein, and axin (38). The phosphoserine motif in the N terminus of β-catenin (91) is recognized by β-TrCP, an F-box component of the E3 ubiquitin ligase complex SCFβTrCP (29, 41, 46, 71, 88). Activation of the Wnt/wg signaling pathway leads to inhibition of β-catenin degradation by decreasing the ability of GSK3β to phosphorylate β-catenin. This reduces its susceptibility to degradation by the ubiquitin-proteasome system, leading to its accumulation (93).
Studies in recent years have suggested that β-catenin is a potent oncogene product (64), and its accumulation has been implicated in tumorigenesis in a wide variety of human cancers (65, 66, 94). In colorectal cancer (CRC) the increase in β-catenin level is attributed to mutations in APC, which occur in about 80% of such tumors (55, 65). Accumulation of β-catenin can also be triggered by mutations in the β-catenin gene itself, affecting the amino-terminal region of the protein that contains the GSK3β phosphorylation sites (57, 70). Such mutations are frequent in colon cancers retaining a wild-type (wt) APC gene (66) and are also prevalent in melanoma, hepatocellular carcinoma (HCC), and a variety of other tumors (13, 16, 22–24, 36, 42, 43, 54, 70, 83, 87, 89, 95).
The mechanism responsible for β-catenin-associated tumorigenesis is suggested to involve β-catenin- and LEF-1/TCF-activated genes, including genes that control the cell cycle (such as those for cyclin D1 [73, 80] and c-myc [32]), genes that are involved in cell-extracellular matrix interactions (such as those for matrilysin [14], fibronectin [26], and WISP-1 [90]), and genes for various transcription factors, including Tcf-1 (68), c-jun and fra-1 (48), and PPARδ (31). The oncogenic role of β-catenin is also supported by studies showing that introduction of mutant APC, or β-catenin, into transgenic mice results in enhanced tumor formation (25, 27, 63).
Another protein which is implicated in many types of cancer is p53. Mutations in the p53 gene are found in about 50% of human cancers (reviewed in references 45 and 61). Under normal conditions, p53 is most probably latent, owing to its rapid ubiquitination and proteolytic degradation. Mdm2, an oncoprotein possessing E3 ubiquitin ligase activity, plays a major role in this process (5, 61). A variety of conditions can lead to the rapid stabilization and activation of p53. These include damage to DNA or to the mitotic spindle, ribonucleotide depletion, hypoxia, heat shock, and exposure to nitric oxide (4, 35, 45, 61). In addition, p53 is induced by several oncogenic proteins, such as myc, ras, and adenovirus E1A, providing a direct link between oncogenic processes and the tumor suppressor action of p53 (reviewed in references 3, 35, 45, and 61). The activation of p53 by these proteins relies mainly on ARF, a tumor suppressor protein that binds to Mdm2 and suppresses its p53 ubiquitination activity, thereby inhibiting p53 degradation (72). Activated p53 can affect the cell cycle, apoptosis, senescence, DNA repair, cell differentiation, and angiogenesis (35, 76), mostly via its function as a transcription factor that activates a number of target genes and by its interaction with a variety of proteins. Some of the better-studied p53 target genes are those for p21 (WAF1), which is mainly involved in G1 arrest; GADD45 and 14-3-3ς, which contribute to G2 arrest; and BAX, Fas (APO1), PIG3, and KILLER (DR5), which lead to caspase activation and apoptosis (12, 18).
A possible cross talk between p53 and β-catenin is suggested by the observation that cancers accumulating β-catenin (as a result of APC mutations) also exhibit a high frequency of p53 mutations, which was first illustrated by the analysis of human CRC (40). Direct evidence for a cross talk between β-catenin and p53 was recently provided by studies demonstrating that excess β-catenin can induce an accumulation of active p53 (15). This may explain, at least in part, the selective pressure for loss of p53 activity in tumors harboring deregulated β-catenin, such as CRC and HCC.
To elucidate why retention of functional p53 is disadvantageous to such tumors, we studied the effect of p53 on the level and transcriptional activity of β-catenin. We report here that elevated levels of wt p53 down-regulate β-catenin in a variety of cell types. This down-regulation depends on the integrity and functionality of p53 and is not observed with a common tumor-associated p53 mutant. Moreover, this effect of p53 is exerted on wt β-catenin but not on the stable S33Y β-catenin mutant, and it is blocked by the proteasome inhibitor MG132, a dominant-negative component of the E3 ubiquitin ligase complex ΔF-β-TrCP, and by LiCl, which inhibits GSK3β activity. Furthermore, p53 can lead to a decrease in the level of the endogenous wt β-catenin in SW480 cells but not in that of the ΔS45 β-catenin mutant of HCT116 cells. Together with the observations of Damalas et al. (15), our findings outline a negative feedback control involving β-catenin and p53, where excess β-catenin induces the accumulation of p53, while high p53 levels down-regulate β-catenin. Disruption of this feedback loop likely affects tumorigenesis driven by deregulated β-catenin activity and may therefore underlie the high frequency of p53 inactivation observed in CRC, HCC, and probably other types of cancer.
MATERIALS AND METHODS
Cells and transfections.
The HCT116 and SW480 human colon carcinoma cell lines, the human H1299 lung adenocarcinoma cell line, and the 293 adenovirus-transformed human embryo kidney cell line were cultured in Dulbecco's modified Eagle's medium supplemented with 10% bovine calf serum (BCS). wt mouse embryonal fibroblasts (MEF), p53−/− MEF, and double-mutant p53−/− Mdm2−/− MEF (15) were cultured in Dulbecco's modified Eagle's medium plus 10% BCS, 100 μM nonessential amino acid mixture (Biological Industries Israel), and 50 μM β-mercaptoethanol. WI38 normal human embryonic lung fibroblasts were cultured in minimum essential medium with 20% BCS. For transfections, cells were plated to form a 50 to 70% confluent culture in a 30-mm-diameter dish. The SW480 and HCT116 cell lines were transfected using Lipofectamine (GIBCO BRL), while the 293 cells were transfected by the calcium phosphate method. The treatment with 30 mM LiCl in some experiments was overnight, the treatment with 10 μM MG132 was for 4 h, and the treatment with 5 μg of doxorubicin (DOX) per ml or 5 μg of cisplatin per ml was for periods ranging from 2 to 48 h.
Plasmids.
The following expression plasmids encoding various p53 constructs were employed: mouse wt p53 (20), mouse mutant p53Δ13–52 (30), hemagglutinin (HA)-tagged human p53 (HA-p53) (50), human wt p53 (7), and the human R175H mutant p53 (7, 60). The reporter plasmid containing the cyclin G promoter-luciferase (30), the LEF/TCF reporter plasmids TOPFLASH and FOPFLASH (82), the ΔF-β-TrCP-expressing plasmid (71), and the vectors expressing various β-catenin forms (HA–β-catenin, HA-S33Y β-catenin, and vesicular stomatitis virus [VSV]–β-catenin) (73, 74, 93) were as described previously.
Protein analysis.
Protein levels were monitored by Western blotting. The following antibodies were used. Polyclonal anti-β-catenin and monoclonal antivinculin were from Sigma; monoclonal anti-β-catenin (clone 14c19220) was from Transduction Laboratories. The 14c19220 antibody was generated against amino acids 571 to 781 and recognizes the caspase-cleaved 65-kDa product of β-catenin (11, 81). Monoclonal antibodies against p53 included anti-human p53 DO1 (84), PAb1801 (8), anti-mouse p53 (which cross-reacts with human p53), and PAb421 (28). A polyclonal anti-p53 was kindly provided by Varda Rotter, Weizmann Institute of Science). Monoclonal anti-HA clone 12CA5 way from Boehringer Mannheim), and polyclonal anti-HA Y11 sc-805 was from Santa Cruz Biotechnology. Fractionation of proteins into Triton X-100-soluble and -insoluble fractions was performed as previously described (71), and equal volumes of lysates from the two fractions were analyzed by Western blotting. Western blots were developed using the ECL method (Amersham). Autoradiograms were scanned with a GS-700 imaging densitometer (Bio-Rad Laboratories) using the FotoLook PS 2.07.2 software. The intensity of the bands was quantitated using the NIH image 1.61 software.
Luciferase assay
For transactivation assays, 1 μg of luciferase reporter plasmid was cotransfected with 2 μg of the β-catenin construct and 2 μg of the various p53 constructs, as indicated. A β-galactosidase-expressing vector was included as an internal control for transfection efficiency. After 24 h, the cells were lysed and both luciferase and β-galactosidase activities were determined with enzyme assay kits (Promega). Luminescence was quantitated using a TD-20e luminometer (Turner Design) from duplicate plates. The results represent data from at least five independent experiments.
Immunofluorescence microscopy
Cells were cultured on glass coverslips, fixed with 3% paraformaldehyde in phosphate-buffered saline, and permeabilized with 0.5% Triton X-100. The coverslips were incubated with the primary antibodies as described above. The secondary antibodies were Alexa 488-conjugated goat anti-mouse or anti-rabbit immunoglobulin G (Molecular Probes) and Cy3-conjugated goat anti-mouse or anti-rabbit immunoglobulin G (Jackson ImmunoResearch Laboratories). Images were acquired using the DeltaVision system (Applied Precision) equipped with a Zeiss (Oberkochen, Germany) Axiovert 100 microscope and a Photometrics (Tucson, Ariz.) 300 series scientific-grade cooled charge-coupled device camera (reading 12-bit images), using a 100×, 1.3 numerical-aperture plan-Neofluar objective (Zeiss). For quantitative image processing, the Priism software was employed (37). At least 50 transfected and 50 nontransfected cells were examined in each experiment.
RESULTS
Excess p53 reduces β-catenin protein levels
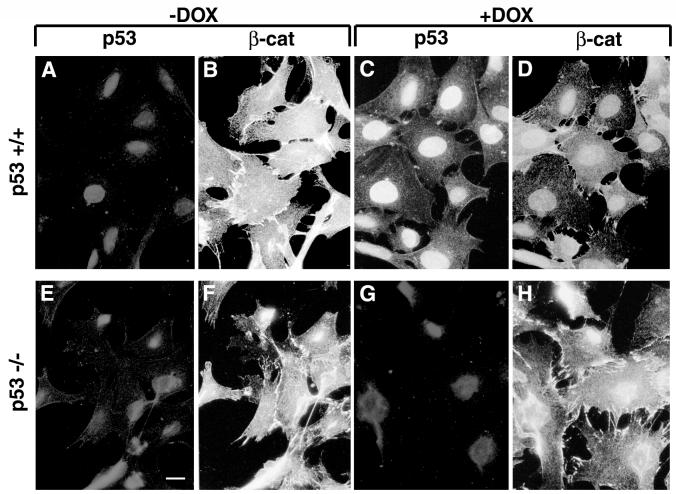
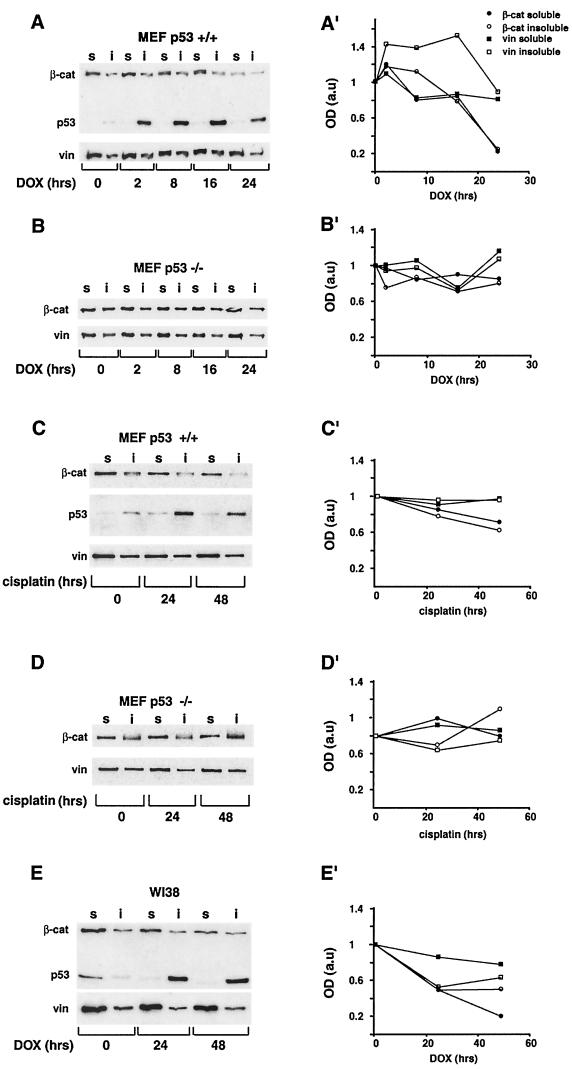
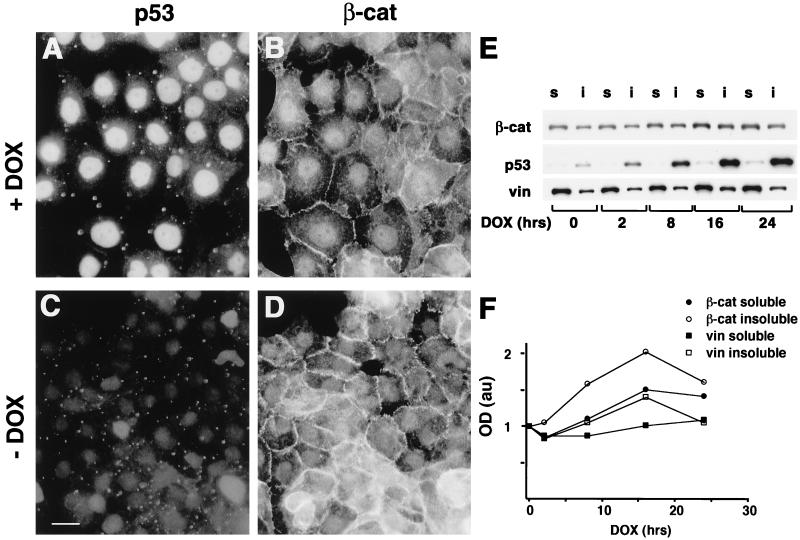
Since recent studies have shown that excess β-catenin can promote the accumulation of p53 (15), we examined the possibility that there is a reciprocal relationship between p53 and β-catenin. We have addressed this question by monitoring the level of endogenous β-catenin in cells in which p53 was induced by DNA damage. MEF from wt mice (p53+/+) and from p53 knockout mice (p53−/−) were treated for 24 h with 5 μg of DOX per ml, which effectively induces p53 stabilization and accumulation (4). DOX treatment elicited a strong nuclear p53 accumulation in the p53+/+ MEF (Fig. 1C; compare to Fig. 1A). β-Catenin was present in the cytoplasm and adherens junctions in both p53+/+ and p53−/− cells before DOX treatment (Fig. 1B and F), but following DOX treatment, adherens junctions were disrupted in both cell types (Fig. 1D and H). However, while in p53−/− MEF the intensity of β-catenin staining remained unchanged and the protein was strongly visible in the cytoplasm and at the edges of the disrupted junctions (Fig. 1H), in p53+/+ MEF β-catenin staining was significantly reduced in the majority of cells (Fig. 1D). During the 24 h of DOX treatment, no morphological signs of apoptosis were observed in these cells (Fig. 1C, D, G, and H and data not shown).To quantify the effect of p53 on β-catenin levels, cell lysates isolated after 0, 2, 8, 16, and 24 h of DOX treatment were immunoblotted with anti-β-catenin antibody. To distinguish between free cytosolic and cytoskeletal or nuclear β-catenin, cells were fractionated into Triton X-100-soluble and Triton X-insoluble pools (Fig. 2A). In p53+/+ MEF, p53 levels peaked at 16 h after DOX treatment and declined at later times (Fig. 2A). This p53 was largely Triton insoluble, reflecting its nuclear accumulation. β-Catenin, in both the Triton X-100-soluble and -insoluble fractions, decreased considerably after 24 h of DOX treatment (Fig. 2A and A′), while no significant decrease in the vinculin level was observed under these conditions (Fig. 2A and A′). In contrast, in p53−/− MEF neither β-catenin nor vinculin levels decreased following DOX treatment (Fig. 2B and B′). When p53 expression was induced by cisplatin treatment of p53+/+ MEF (Fig. 2C), a comparable decrease in β-catenin was observed (Fig. 2C′). Cisplatin did not induce a detectable change in β-catenin levels of p53−/− MEF (Fig. 2D and D′). The effect of p53 on β-catenin was also observed in normal WI38 human embryonic lung fibroblasts (Fig. 2E). p53 was strongly induced in these cells after DOX treatment, and its localization shifted from the Triton X-100-soluble to the Triton-insoluble fraction, probably reflecting its translocation from the cytoplasm to the nucleus. In these cells, treatment with DOX for 48 h induced a fivefold reduction in the Triton X-100-soluble β-catenin (Fig. 2E′), while the Triton X-100-insoluble fraction was only moderately affected (Fig. 2E and E′). In contrast, the levels of vinculin in the Triton X-100-soluble fraction of these cells did not change significantly (Fig. 2E and E′). Immunofluorescence analysis of WI38 cells treated with DOX for 24 or 48 h revealed intact nuclei and well-spread cells (data not shown), implying that the DOX treatment did not induce apoptosis in these cells.
FIG. 1.
Effect of DOX-induced p53 expression on β-catenin organization and level. MEF cells were plated on coverslips, treated with 5 μg of DOX per ml for 24 h, fixed, and double stained for p53 using an anti-mouse p53 polyclonal antibody (A, C, E, and G) and for β-catenin (β-cat) using a monoclonal anti-β-catenin antibody (B, D, F, and H). Bar, 10 μm.
FIG. 2.
Effect of p53 elevation on β-catenin levels in the Triton X-100-soluble and -insoluble fractions of different cell types. Cells were treated with 5 μg of DOX per ml (A and B) or 5 μg of cisplatin per ml (C and D) for the indicated time periods. Proteins were fractionated into Triton X-100-soluble (lanes s) and -insoluble (lanes i) fractions, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and subjected to Western blot analysis using the antibodies indicated in the legend to Fig. 1 and with an antivinculin (vin) antibody. The intensities of the bands from representative gels of MEF p53+/+ (A and C) and MEF p53−/− (B and D) cells were quantified and plotted (A′, C′, B′, and D′, respectively). Extracts from WI38 cells (E and E′) were blotted with the monoclonal anti-β-catenin antibody (β-cat), vinculin (vin), and a mixture of the anti-human p53 antibodies DO1 and 1801 and analyzed as described for MEF cells. a.u., arbitrary units.
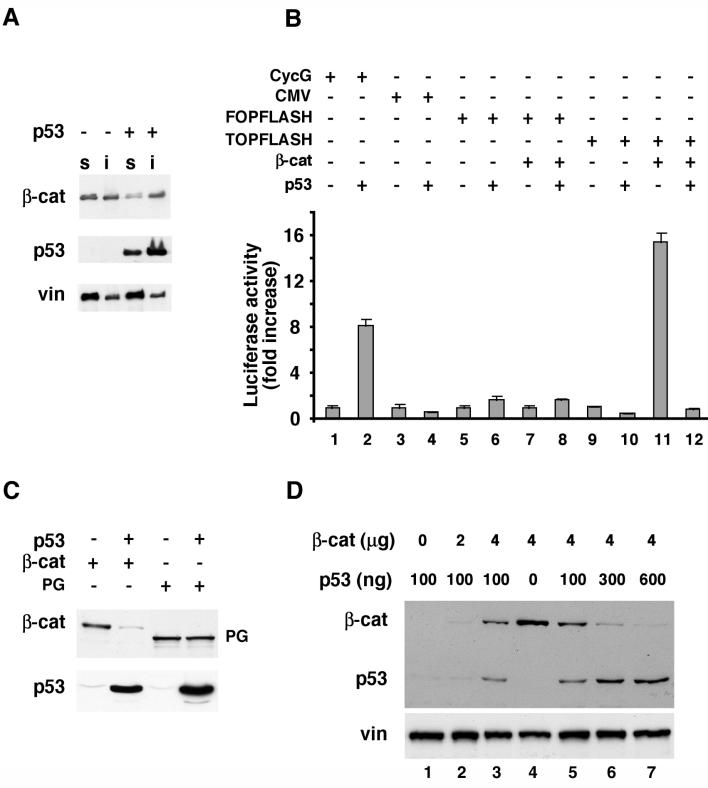
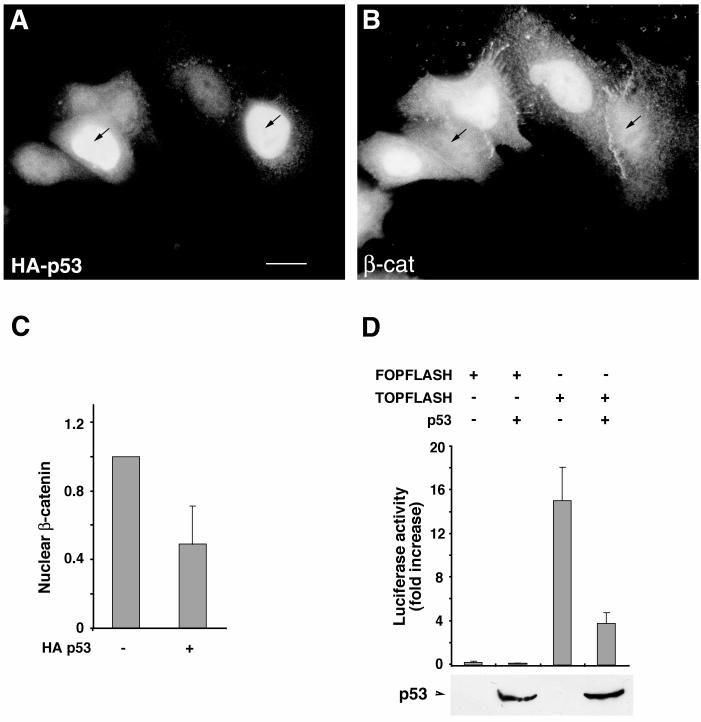
The ability of excess p53 to reduce β-catenin levels was also observed in 293 cells transfected with a p53 expression plasmid (Fig. 3). Transfection of p53 into these cells resulted in a major decrease in the level of endogenous Triton X-100-soluble β-catenin (which is believed to represent the signaling pool of β-catenin) but not in that of vinculin (Fig. 3A). To determine whether p53 can also affect the signaling activity of β-catenin, p53 and β-catenin were cotransfected into 293 cells together with the LEF-1 reporter plasmid TOPFLASH (82) or with a variety of control plasmids. The transfected p53 was transcriptionally active as seen from its ability to strongly activate the p53-responsive cyclin G promoter (Fig. 3B, bars 1 and 2). The cytomegalovirus promoter, a target for nonspecific repression by high levels of p53 (78), was repressed only slightly under these conditions (Fig. 3B, bars 3 and 4), while p53 had no effect on the control FOPFLASH reporter plasmid (Fig. 3B, bars 6 and 8). In contrast, p53 very strongly suppressed the β-catenin-mediated luciferase activity driven from the TOPFLASH reporter (Fig. 3B, bars 11 and 12). Analysis of protein levels revealed that in these cotransfected 293 cells, p53 caused a dramatic reduction in the steady-state β-catenin levels (Fig. 3C). In contrast, the levels of the closely related HA-plakoglobin protein (94), transduced with the same expression vector, were not affected by p53 (Fig. 3C). This specific down-regulation of β-catenin is likely responsible for the inhibitory effect of p53 on β-catenin-dependent transcriptional activity.
FIG. 3.
Reciprocal effects of p53 and β-catenin overexpression on the levels of these proteins and the transcriptional activity of β-catenin. (A) p53 or a control empty vector was transfected into 293 cells. After 20 h, cells were fractionated into Triton X-100-soluble (lanes s) and -insoluble (lanes i) fractions and subjected to Western blot analysis. The positions of β-catenin (β-cat), p53, and vinculin (vin) are marked. (B) Transactivation assays with reporter plasmids expressing luciferase under the transcriptional control of different promoters in the presence of p53 and β-catenin. Bars 1 and 2, cyclin G (CycG) promoter; bars 3 and 4, cytomegalovirus promoter; bars 5 to 8, FOPFLASH; bars 9 to 12, TOPFLASH. Cells were collected 20 h after transfection and subjected to luciferase and β-galactosidase assays. The standard error is indicated. (C) Effect of transfected p53 on the levels of cotransfected HA–β-catenin or HA-plakoglobin (PG) blotted with anti-HA antibody. The positions of β-catenin, plakoglobin, and p53 are indicated. (D) H1299 cells (which are p53 deficient) were transfected with increasing amounts of HA–β-catenin (0 to 4 μg) and a constant amount of p53 (100 ng) (lanes 1 to 3) or with a constant amount of HA–β-catenin (4 μg) and increasing amounts of p53 (0 to 600 ng) (lanes 4 to 7). The levels of HA–β-catenin (β-cat) and p53 were determined by Western blot analysis. The endogenous vinculin served as loading control.
Reciprocal relationship between β-catenin and p53 expression.
Since in a recent study we have shown that excess β-catenin can promote the accumulation of exogenously introduced p53 in H1299 cells (which lack p53) (15), we wished to examine, in the same cellular system, the predicted reciprocal relationship between β-catenin and p53 expression. The results shown in Fig. 3D clearly demonstrate that when a constant level of p53 was cotransfected into H1299 cells with increasing levels of β-catenin, the increase in the expression of p53 was highest when high levels of β-catenin were transfected (Fig. 3D, compare lanes 2 and 3). In contrast, when a constant high level of β-catenin was cotransfected with increasing amounts of p53, a decrease in the level of β-catenin was already apparent with the initial low level of p53 (Fig. 3D, lanes 4 and 5), and a dramatic reduction in β-catenin was seen with the higher concentrations of p53 (Fig. 3D, lanes 4 to 7). Taken together, these results demonstrate the dose-dependent reciprocal relationship between β-catenin and p53 expression in the same cells and that p53 can reduce the levels and transcriptional activity of β-catenin, irrespective of whether β-catenin is expressed from the endogenous gene or from an expression plasmid.
p53 mutants fail to reduce β-catenin levels
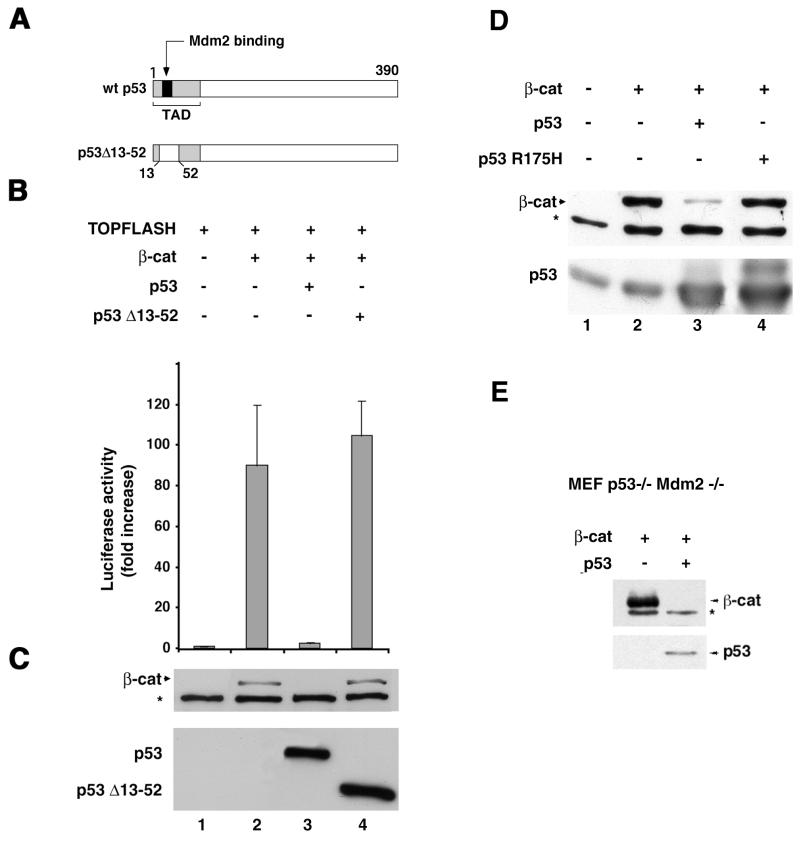
Next, we examined the ability of mutant forms of p53 to down-regulate β-catenin. Unlike wt p53, which strongly reduced the levels and transcriptional activity of β-catenin in 293 cells, the mouse p53 deletion mutant p53Δ13–52, which lacks transactivation and Mdm2 binding activities (the transactivation domain is only partially deleted but is apparently nonfunctional) (Fig. 4A), had no detectable effect on β-catenin level and transcriptional activity (Fig. 4B and C). Importantly, the cancer-associated hot spot human p53 mutant (p53R175H) (7, 60) was also unable to down-regulate β-catenin (Fig 4D). The presence of Mdm2 was apparently not required for the p53-mediated reduction in β-catenin level, since in double-mutant MEF (deficient in both p53 and Mdm2), transfection of p53 was still capable of efficiently decreasing β-catenin expression (Fig. 4E). Hence, the integrity and functionality of p53 are required for its ability to down-regulate β-catenin and are apparently independent of Mdm2.
FIG. 4.
p53 mutants fail to reduce β-catenin (β-cat) levels and transactivation capacity. (A) Schematic representation of the p53Δ13–52 mutant. TAD, transactivation domain. (B) Transactivation in 293 cells, using the TOPFLASH reporter plasmid transfected either alone (bar 1) or with HA–β-catenin (bars 2 to 4) in the presence of wt mouse p53 (bar 3) or the mutant Δ13–52 mouse p53 (bar 4). Cells were harvested 20 h after transfection and subjected to luciferase activity assay. The standard error is indicated. (C) Western blot analysis for β-catenin, p53, and p53Δ13–52 in cell lysates from the experiment in panel B. (D) Human wt p53 or a human p53R175H mutant was cotransfected with HA–β-catenin into 293 cells and subjected to Western blot analysis. (E) p53 can reduce the levels of β-catenin in Mdm2-deficient MEF. p53−/− Mdm2−/− double-mutant MEF were transfected with 5 μg of β-catenin plasmid in the presence or absence of 300 ng of p53 plasmid. The level of the transfected (HA-tagged) β-catenin was determined by Western blot analysis. The positions of β-catenin and p53 are indicated. The asterisks in panels C, D, and E represent a nonspecific band obtained with the anti-HA antibody.
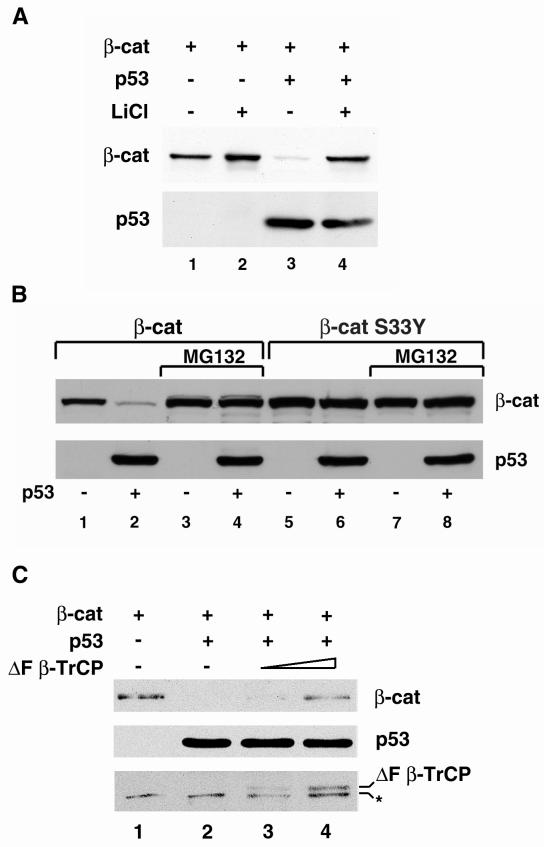
Involvement of GSK3β and the proteasome system in p53-mediated down-regulation of β-catenin.
The targeting of β-catenin to the proteasome is achieved through its phosphorylation by GSK3β in a multiprotein complex, followed by its recognition by β-TrCP, a component of the E3 ubiquitin ligase system (29, 41, 46, 71, 88). To examine whether the reduction in β-catenin level by p53 requires the activity of GSK3β, the effect of p53 on β-catenin in cells treated with LiCl, which inhibits the activity of GSK3β (91), was determined. As shown in Fig. 5A, incubation with LiCl blocked the ability of p53 to lower β-catenin levels (Fig. 5A, compare lanes 3 and 4). The effect of p53 on cotransfected β-catenin was also blocked in the presence of the proteasome inhibitor MG132 (Fig. 5B, lanes 3 and 4), implying that p53 promotes the degradation of β-catenin through the proteasome system. Furthermore, p53 did not reduce the level of the S33Y mutant β-catenin, which is relatively refractory to phosphorylation by GSK3β and hence to proteasomal degradation (21, 29, 46), in either the presence (Fig. 5B, lanes 7 and 8) or in the absence (Fig. 5B, lanes 5 and 6) of MG132. When HA-tagged ubiquitin was cotransfected with β-catenin in the presence or absence of p53, an increase in ubiquitinated β-catenin was detected in immunoprecipitates obtained from cells overexpressing p53 (data not shown). Further support for the notion that p53 overexpression stimulates β-catenin degradation via the classical Wnt pathway was obtained from cotransfection of 293 cells with ΔF-β-TrCP, which binds to serine-phosphorylated β-catenin and blocks its degradation (71). As shown in Fig. 5C, expression of ΔF-β-TrCP partially blocked the p53-induced reduction in β-catenin levels. These results suggest that p53 can promote the turnover of β-catenin by augmenting its β-TrCP-mediated proteasomal degradation and that typical oncogenic mutations of β-catenin (such as the S33Y mutation) can render it resistant to high levels of p53.
FIG. 5.
Blocking of GSK3β activity, polyubiquitination, and proteasomal degradation inhibit the effect of p53 on β-catenin (β-cat). (A) wt HA–β-catenin was transfected into 293 cells with p53 (lanes 3 and 4) or without p53 (lanes 1 and 2), and half of the cultures were treated overnight with 30 mM LiCl (lanes 2 and 4) before harvesting of the cells and determination of the levels of HA–β-catenin and p53 by Western blot analysis. (B) wt β-catenin (lanes 1 to 4) or the HA-S33Y β-catenin mutant (lanes 5 to 8) was cotransfected with p53 into 293 cells. After 16 h, MG132 (25 μM) was added to the indicated samples (lanes 3, 4, 7, and 8). The cells were harvested 4 h later and subjected to Western blot analysis. The upper panel shows the levels of β-catenin (wt or S33Y mutant). The lower panel shows the transfected p53. (C) VSV-tagged β-catenin (1 μg) was transfected alone (lane 1) or cotransfected with p53 (1 μg) (lane 2) and increasing concentrations (2 μg [lane 3] and 4 μg [lane 4]) of the dominant-negative HA-tagged ΔF-β-TrCP. The transfected wt β-catenin was detected by anti-VSV antibody, while ΔF-β-TrCP expression was monitored with an anti-HA tag antibody. The asterisk indicates a nonspecific band obtained with the anti-HA antibody.
p53 can repress wt but not mutant β-catenin in colon cancer cell lines.
The SW480 CRC cell line expresses a truncated APC that is deficient in its ability to promote β-catenin degradation (58). Nevertheless, these cells contain phosphorylated β-catenin that coprecipitates with the E3 ubiquitin ligase component β-TrCP (29), suggesting that the proteasomal degradation pathway responsible for β-catenin turnover is only partially attenuated in these cells. The endogenous p53 in SW480 cells has a mutation of Arg to His at position 273 and another mutation of Pro to Ser at position 309 (1). Transfection of wt p53 into these cells led to a substantial drop in nuclear β-catenin levels (Fig. 6A and B). Computerized quantitation of the immunofluorescence images revealed about a twofold decrease in nuclear β-catenin levels (Fig. 6C) and a fourfold reduction in β-catenin-mediated transactivation in these cells (Fig. 6D).
FIG. 6.
wt p53 down-regulates β-catenin in SW480 CRC cells. (A and B) SW480 cells were transfected with HA-p53 (4 μg) and immunostained for HA (A) and β-catenin (β-cat) (B) 20 h after transfection. The arrows point to the p53-transfected cells. (C) Computerized quantitation of nuclear β-catenin in control, nontransfected cells and in p53-transfected cells. (D) Transactivation in SW480 cells cotransfected with p53 and either TOPFLASH or FOPFLASH reporter plasmid. Cells were harvested 20 h after transfection and subjected to luciferase assay and Western blot analysis for the transfected HA-p53. The position of HA-p53 on the Western blot is indicated. Bar, 10 μm.
The effect of p53 on mutant β-catenin was also examined in the HCT116 CRC cell line. These cells express mutant β-catenin with a deletion of serine 45 (ΔS45) and contain wt APC (57) and wt p53 (19). Treatment of HCT116 cells with DOX for 24 h led to the nuclear accumulation of p53 (Fig. 7A [compare to Fig. 7C]), but no significant change in the β-catenin staining pattern or disruption of adherens junctions was observed in these cells (Fig. 7B [compare to Fig. 7D]). The analysis of the Triton X-100-soluble and -insoluble fractions from these cells also failed to reveal a significant change in β-catenin levels (Fig. 7E and F). Consistent with these observations, transfection of p53 into HCT116 cells also had no effect on the levels of the endogenous mutant β-catenin (data not shown).
FIG. 7.
p53 fails to affect the mutant β-catenin ΔS45 of HCT116 CRC cells. (A to D) HCT116 cells were cultured on coverslips for 24 h in either the presence (A and B) or the absence (C and D) of 5 μg of DOX per ml. After fixation, the cells were double immunostained for p53 using a mixture of DO1 and 1801 antibodies (A and C) and for β-catenin (β-cat) (B and D) using a polyclonal anti-β-catenin antibody. Bar, 10 μm. (E) HCT116 cells were treated with 5 μg of DOX per ml for the indicated times and fractionated into Triton X-100-soluble (lanes s) and -insoluble (lanes i) fractions. The lysates were subjected to Western blot analysis. The positions of β-catenin, p53, and vinculin (vin) (as a control) are indicated. (F) Quantitative analysis of the intensities of the bands shown in panel E. au, arbitrary units.
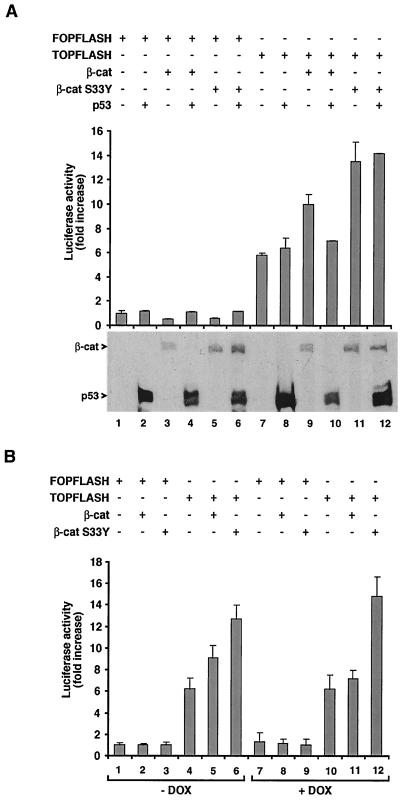
The presence of deregulated mutant β-catenin in HCT116 cells results in constitutive β-catenin-mediated transcriptional activity (Fig. 8A, lane 7). In contrast to the case in SW480 cells, excess wt p53 failed to inhibit the transcriptional activity of the endogenous mutant (ΔS45) β-catenin in HCT116 cells (Fig. 8A, lane 8 [compare to lane 7]). However, p53 repressed transactivation driven by excess transfected wt β-catenin (Fig. 8A, lanes 9 and 10). This inhibition was accompanied by a reduction in the level of the transfected wt β-catenin (Fig. 8A, lanes 9 and 10). In contrast, transfection of the more stable tumor-associated mutant β-catenin S33Y resulted in augmented transactivation that was resistant to wt p53 coexpression (Fig. 8A, lanes 11 and 12). We also examined the ability of the endogenous wt p53 in HCT116 cells to affect β-catenin-mediated transactivation by inducing p53 expression with DOX. The results demonstrate that increasing endogenous p53 levels by this approach led to the inhibition of transactivation driven by the transfected wt β-catenin (Fig. 8B, compare bars 5 and 10 to bar 4) but not by the mutant endogenous ΔS45 β-catenin or the transfected mutant β-catenin S33Y, which were resistant to elevated p53 (Fig, 8B, compare bars 4 to 10 and 6 to 12). These results suggest that the cellular machinery necessary for the inhibitory effect of p53 on β-catenin is intact in HCT116 cells and that the inability of wt p53 to down-regulate the endogenous β-catenin (ΔS45) or the transfected S33Y is due to the N-terminal mutations in the serine motifs that regulate β-catenin degradation.
FIG. 8.
p53 does not inhibit transactivation by the ΔS45 β-catenin of HCT116 cells and of transfected S33Y but inhibits the activity of wt β-catenin in these cells. (A) HCT116 cells were transfected with FOPFLASH (lanes 1 to 5) or TOPFLASH (lanes 7 to 12), together with p53 (lanes 2, 4, 6, 8, 10, and 12), HA–β-catenin (lanes 3, 4, 9, and 10), or p53 and HA-S33Y β-catenin (lanes 5, 6, 11, and 12). Luciferase activity was determined 20 h after transfection, and Western blot analyses of the cell lysates for the expression of β-catenin (β-cat) (wt) and the mutant (S33Y) and for p53 were performed. (B) HCT116 cells were transfected with wt and S33Y β-catenin and either treated with DOX (to elevate p53 levels) as described for Fig. 7 (bars 7 to 12) or left untreated (bars 1 to 6). Transactivation driven by the FOPFLASH and TOPFLASH reporter plasmids was determined. The standard error is shown.
Taken together, the results imply that mutations within the GSK3β phosphorylation domain of β-catenin that render it more resistant to proteasomal degradation also make β-catenin more resistant to down-regulation by activated p53. Importantly, such β-catenin mutations are prevalent in a variety of human cancers.
DISCUSSION
This study demonstrated that high levels of functional p53 could down-regulate the amount and transcriptional activity of β-catenin. This effect was eliminated by blocking the activities of components that regulate the turnover of β-catenin, such as GSK3β, a dominant-negative form of β-TrCP, and the proteasomal system. p53 therefore most probably exerted its effect by accelerating the degradation of β-catenin by the proteasome. While p53 could down-regulate wt β-catenin in the presence of wt or even mutant APC, it failed to affect mutants of β-catenin that are resistant to the APC-GSK3β-axin-mediated degradation. It is unlikely that the basal low levels of p53 in nonstressed cells are sufficient to cause β-catenin down-regulation. Rather, it is conceivable that this inhibitory effect is exerted only by high levels of activated p53, as seen in cells exposed to extensive genotoxic stress (Fig. 2). Constitutive activity of β-catenin can exert both proliferative and antiapoptotic effects (62). Hence, the down-regulation of β-catenin by activated p53 is likely to contribute to the antiproliferative effects of p53 and possibly also facilitates p53-mediated apoptosis. While in our experiments p53 activation was attained by treatment of cultured cells with DNA-damaging agents, it is conceivable that a similar situation may pertain also in emerging colorectal tumors with multiple genomic aberrations, which is typical of late stages in colorectal carcinogenesis.
The exact molecular mechanism responsible for the down-regulation of β-catenin by p53 remains to be elucidated. So far, we have been unable to demonstrate a direct protein-protein interaction between p53 and β-catenin (data not shown), suggesting that additional proteins are likely to play a role in mediating β-catenin down-regulation. p53 is a transcription factor, which is capable of direct sequence-specific transcription activation and indirect, albeit target gene selective, transcriptional repression. We have shown that p53 mutants deficient in transcriptional activity, owing either to deletion of the transactivation domain (p53Δ13–52) or to a more global conformational change affecting both the DNA binding and the transactivation domains (p53R175H), failed to reduce β-catenin levels. These findings support the view that the down-modulation of β-catenin depends on the transcriptional activities of p53. For example, the decrease in β-catenin might be mediated by the action of the products of one or several p53 target genes whose expression is induced in cells exposed to high levels of activated p53. One interesting possibility is suggested by studies showing that APC mRNA is induced by excess p53 in several cell types (59). Such induction of APC expression by p53 may contribute to enhanced β-catenin degradation in cells carrying a functional APC gene and may account for part of the observed reduction in β-catenin protein following p53 elevation. This observation cannot, however, explain the ability of p53 to reduce β-catenin expression in SW480 CRC cells, which harbor a mutant APC gene and are devoid of functional APC protein.
Another potential contributor to the inhibitory effect of p53 on β-catenin is the Dickkopf protein, whose gene has recently been reported to be subject to transcriptional activation by p53 (85). Dickkopf-1 blocks low-density lipoprotein (LDL) receptor-related protein 6-mediated Wnt/β-catenin signaling by directly interacting with LDL receptor-related protein 6 (49). In addition to transactivation, transcriptional repression by p53 may also play a role in suppressing β-catenin. One potential mediator of such an effect may be presenilin 1, whose expression is repressed by p53 (69). Since presenilin 1 can stabilize β-catenin (92), its down-regulation by p53 might lead to destabilization of β-catenin. It is conceivable that additional p53-regulated genes may also play a role in the down-regulation of β-catenin, and p53 might turn on a coordinated transcriptional program designed to inhibit β-catenin-mediated signaling.
While this paper was under revision, two studies were published suggesting that Siah-1, a transcriptional target of p53, can down-regulate β-catenin. Liu et al. (47) and Matsuzawa and Reed (51) reported that a p53-inducible gene product(Siah-1) can mediate the down-regulation of β-catenin by a novel degradation pathway mediated by SIP, Ebi, and the carboxy terminus of APC. This novel pathway may provide an additional means by which p53 can inhibit the accumulation of β-catenin, especially of the oncogenic β-catenin mutants with mutations in the GSK phosphorylation sites, since this pathway operates independently of GSK3β (47). It is of note that whereas overexpression of Siah-1 in SW480 cells was unable to inhibit β-catenin signaling (47), transfection of p53 into these cells did cause a marked reduction in the level of nuclear β-catenin and LEF-dependent transcription (Fig. 6). Taken together, these observations imply that p53 can block β-catenin signaling through multiple downstream effectors, with Siah-1 being just one of those. Minimally, the data argue that there are at least two major alternative pathways for p53-induced suppression of β-catenin: a canonical cascade involving components of the Wnt pathway and a second mechanism involving Siah-1, SIP, and Ebi. Both pathways are probably turned on simultaneously when p53 levels become sufficiently elevated, and they may synergize to achieve a more effective response. This could be particularly important when the target is an abundant protein such as β-catenin. The relative contribution of each of the two pathways to the elimination of β-catenin signaling probably varies with cell type and cell context and may be modulated by additional genetic alterations that occur in the course of tumorigenesis. Furthermore, the existence of multiple parallel pathways for down-regulating β-catenin by activated p53 may provide a fail-safe mechanism in case one of the components along either of the pathways becomes inactivated.
Alternatively, p53 and β-catenin may compete for a common interacting molecule required for β-catenin stabilization. A possible candidate is the p300 (also called CBP) transcriptional coactivator, since both p53 and β-catenin can bind to p300 (6, 33, 79) and a recent study has shown that p53 and β-catenin compete for p300 binding (53). A similar competition for p300 binding between p53 and NF-κB, resulting in the inhibition of the transcriptional activity of each of these proteins by an excess of the other, has been described (86). Our preliminary results indicate that overexpression of p300 can protect wt β-catenin from down-regulation by p53 (data not shown). It is thus tempting to speculate that when p300 is present in limited amounts, increased p53 levels can displace the endogenous β-catenin from its complex with p300 and render it more susceptible to ubiquitination and degradation.
A simple explanation that needs to be ruled out is that the reduction in β-catenin protein is merely a consequence of p53-mediated apoptosis. Such a possibility may rely on previous observations that β-catenin is subject to cleavage by caspases, mainly caspases 3, 6, and 8, in cells undergoing apoptosis (11, 81). We believe that this is an unlikely explanation for the effects of p53 on β-catenin for several reasons. First, no morphological changes suggestive of apoptosis were noted in any of the cell types used in this study when the cells were harvested for biochemical and immunohistochemical analysis (Fig. 1, 6, and 7 and data not shown). In addition, using the same antibody which was employed to demonstrate caspase-mediated β-catenin cleavage (11, 81), we did not detect cleaved forms of β-catenin in extracts of cells exposed to high p53 activity (Fig. 1A, C, and E and 7E and data not shown). Hence, we suggest that the effect of p53 on β-catenin most probably is exerted through the pathway responsible for the normal proteasomal degradation of β-catenin.
A previous study has shown that excess deregulated β-catenin can lead to the induction of active p53 (15) and that this is also achieved through changes in the rate of proteasomal degradation of the affected protein (p53). In the present study, we demonstrate a reciprocal relationship between p53 and β-catenin in the same cell system by comparing the levels of the two proteins under conditions in which one protein was in excess over the other (Fig. 3D). It is clear from these results that increasing levels of β-catenin augment the levels of p53 when a constant amount of p53 is cotransduced into the same cells. On the other hand, when high levels of β-catenin are transfected, elevation of p53 results in a specific, dose-dependent down-regulation of β-catenin expression (Fig. 3D). Taken together, these studies delineate an autoregulatory loop in which excess β-catenin induces p53 activation, which in turn leads to down-regulation of β-catenin levels and activity. This control loop might serve as an effective means for curbing the potential oncogenic effects of deregulated β-catenin. Its disruption may unleash the oncogenic activity of β-catenin, thereby contributing to tumor progression. The disruption of such autoregulation may occur through mutations in the p53 gene that will render it unable to repress β-catenin activity or by inactivation of the tumor suppressor ARF, which stabilizes p53 (72). It is noteworthy that the ability to repress β-catenin is lost in the p53R175H mutant, which is frequently seen in human CRC where β-catenin is deregulated through the loss of functional APC (39, 60). This loop may also be disrupted by mutations in β-catenin that render it resistant to the inhibitory effect of p53, as is the case with the S33Y β-catenin mutant. In this regard, it is of interest that while p53 mutations predominate in the major class of CRC (involving β-catenin deregulation through APC gene inactivation), they are significantly less frequent in CRC carrying direct stabilizing mutations within β-catenin itself (17, 44, 52, 75, 77). Moreover, in a recent study employing N-myc transgenic mice, the majority of liver tumors arising on a p53+/− background displayed either a β-catenin mutation or a loss of the remaining p53 allele (67). This further suggests that these two mutational events often tend to be mutually exclusive during tumorigenesis. Our findings offer an appealing explanation for these observations by predicting that early mutations in β-catenin will render it refractory to down-regulation by p53, thereby significantly reducing the pressure for subsequent mutational inactivation of p53. Conversely, the predominance of p53 mutations in the major class of CRC, which are associated with APC inactivation, suggests that the ability of wt p53 to restrain the deregulated wt β-catenin is a key component of its tumor suppressor function and a major cause for the elimination of p53 function in such tumors.
ACKNOWLEDGMENTS
This study was supported by grants from the German-Israeli Foundation for Scientific Research and Development, the Cooperation Program in Cancer Research between the German Cancer Research Center (DKFZ) and the Israeli Ministry of Science and Arts (IMOSA), CaP CURE, The Israel Science Foundation, The Crown Endowment Fund for Immunological Research, The M. D. Moross Institute for Cancer Research, NIH (grant RO1 CA-40099), and the German-Israel Project Cooperation (DIP). B.G. holds the E. Neter Chair of Cell and Tumor Biology, and A.B.-Z. holds the Lunenfeld-Kunin Chair in Genetics and Cell Biology.
We thank G. Del Sal and J. Zhurinsky for illuminating discussions.
REFERENCES
- 1.Abarzua P, LoSardo J E, Gubler M L, Neri A. Microinjection of monoclonal antibody PAb421 into human SW480 colorectal carcinoma cells restores the transcription activation function to mutant p53. Cancer Res. 1995;55:3490–3494. [PubMed] [Google Scholar]
- 2.Adams C, Nelson W. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr Opin Cell Biol. 1998;10:572–577. doi: 10.1016/s0955-0674(98)80031-8. [DOI] [PubMed] [Google Scholar]
- 3.Albrechtsen N, Dornreiter I, Grosse F, Kim E, Wiesmuller L, Deppert W. Maintenance of genomic integrity by p53: complementary roles for activated and non-activated p53. Oncogene. 1999;18:7706–7717. doi: 10.1038/sj.onc.1202952. [DOI] [PubMed] [Google Scholar]
- 4.Ashcroft M, Taya Y, Vousden K H. Stress signals utilize multiple pathways to stabilize p53. Mol Cell Biol. 2000;20:3224–3233. doi: 10.1128/mcb.20.9.3224-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashcroft M, Vousden K H. Regulation of p53 stability. Oncogene. 1999;18:7637–7643. doi: 10.1038/sj.onc.1203012. [DOI] [PubMed] [Google Scholar]
- 6.Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 7.Baker S J, Fearon E R, Nigro J M, Hamilton S R, Preisinger A C, Jessup J M, vanTuinen P, Ledbetter D H, Barker D F, Nakamura Y, White R, Vogelstein B. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989;244:217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- 8.Banks L, Matlashewski G, Crawford L. Isolation of human-p53-specific monoclonal antibodies and their use in the studies of human p53 expression. Eur J Biochem. 1986;159:529–534. doi: 10.1111/j.1432-1033.1986.tb09919.x. [DOI] [PubMed] [Google Scholar]
- 9.Behrens J, von Kries J, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Ze'ev A, Geiger B. Differential molecular interactions of beta-catenin and plakoglobin in adhesion, signaling and cancer. Curr Opin Cell Biol. 1998;10:629–639. doi: 10.1016/s0955-0674(98)80039-2. [DOI] [PubMed] [Google Scholar]
- 11.Brancolini C, Lazarevic D, Rodriguez J, Schneider C. Dismantling cell-cell contacts during apoptosis is coupled to a caspase-dependent proteolytic cleavage of beta-catenin. J Cell Biol. 1997;139:759–771. doi: 10.1083/jcb.139.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns T F, El-Deiry W S. The p53 pathway and apoptosis. J Cell Physiol. 1999;181:231–239. doi: 10.1002/(SICI)1097-4652(199911)181:2<231::AID-JCP5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Chan E, Gat U, McNiff J, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- 14.Crawford H, Fingleton B, Rudolph-Owen L, Goss K, Rubinfeld B, Polakis P, Matrisian L. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- 15.Damalas A, Ben-Ze'ev A, Simcha I, Shtutman M, Leal J, Zhurinsky J, Geiger B, Oren M. Excess β-catenin promotes accumulation of transcriptionally active p53. EMBO J. 1999;18:3054–3063. doi: 10.1093/emboj/18.11.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de La Coste A, Romagnolo B, Billuart P, Renard C A, Buendia M A, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, Perret C. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delattre O, Olschwang S, Law D J, Melot T, Remvikos Y, Salmon R J, Sastre X, Validire P, Feinberg A P, Thomas G. Multiple genetic alterations in distal and proximal colorectal cancer. Lancet. 1989;2:353–356. doi: 10.1016/s0140-6736(89)90537-0. [DOI] [PubMed] [Google Scholar]
- 18.el-Deiry W S. Regulation of p53 downstream genes. Semin Cancer Biol. 1998;8:345–357. doi: 10.1006/scbi.1998.0097. [DOI] [PubMed] [Google Scholar]
- 19.el-Deiry W S, Harper J W, O'Connor P M, Velculescu V E, Canman C E, Jackman J, Pietenpol J A, Burrell M, Hill D E, Wang Y, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 20.Eliyahu D, Michalovitz D, Eliyahu S, Pinhasi-Kimhi O, Oren M. Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci USA. 1989;86:8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs S Y, Chen A, Xiong Y, Pan Z Q, Ronai Z. HOS, a human homolog of Slimb, forms an SCF complex with Skp1 and Cullin1 and targets the phosphorylation-dependent degradation of IkappaB and beta-catenin. Oncogene. 1999;18:2039–2046. doi: 10.1038/sj.onc.1202760. [DOI] [PubMed] [Google Scholar]
- 22.Fukuchi T, Sakamoto M, Tsuda H, Maruyama K, Nozawa S, Hirohashi S. Beta-catenin mutation in carcinoma of the uterine endometrium. Cancer Res. 1998;58:3526–3528. [PubMed] [Google Scholar]
- 23.Gamallo C, Palacios J, Moreno G, Calvo de Mora J, Suarez A, Armas A. Beta-catenin expression pattern in stage I and II ovarian carcinomas: relationship with beta-catenin gene mutations, clinicopathological features, and clinical outcome. Am J Pathol. 1999;155:527–536. doi: 10.1016/s0002-9440(10)65148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Rostan G, Tallini G, Herrero A, D'Aquila T G, Carcangiu M L, Rimm D L. Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res. 1999;59:1811–1815. [PubMed] [Google Scholar]
- 25.Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 26.Gradl D, Kuhl M, Wedlich D. The Wnt/Wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol. 1999;19:5576–5587. doi: 10.1128/mcb.19.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo M M. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harlow E D, Crawford L V, Pim D C, Williamson N M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981;39:861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hart M, Concordet J, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, Polakis P. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol. 1999;9:207–210. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- 30.Haupt Y, Barak Y, Oren M. Cell type-specific inhibition of p53-mediated apoptosis by mdm2. EMBO J. 1996;15:1596–1606. [PMC free article] [PubMed] [Google Scholar]
- 31.He T, Chan T, Vogelstein B, Kinzler K. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He T, Sparks A, Rago C, Hermeking H, Zawel L, da Costa L, Morin P, Vogelstein B, Kinzler K. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 33.Hecht A, Vleminckx K, Stemmler M, van Roy F, Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 2000;19:1839–1850. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann B, Kemler R. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- 35.Janus F, Albrechtsen N, Dornreiter I, Wiesmuller L, Grosse F, Deppert W. The dual role model for p53 in maintaining genomic integrity. Cell Mol Life Sci. 1999;55:12–27. doi: 10.1007/s000180050266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeng Y M, Wu M Z, Mao T L, Chang M H, Hsu H C. Somatic mutations of beta-catenin play a crucial role in the tumorigenesis of sporadic hepatoblastoma. Cancer Lett. 2000;152:45–51. doi: 10.1016/s0304-3835(99)00433-4. [DOI] [PubMed] [Google Scholar]
- 37.Kam Z, Jones M, Chen O H, Agad D, Sedat J W. Design and construction of an optimal illumination system for quantitative wide-field multi-dimensional microscopy. Bioimaging. 1993;1:71–81. [Google Scholar]
- 38.Kikuchi A. Regulation of beta-catenin signaling in the Wnt pathway. Biochem Biophys Res Commun. 2000;268:243–248. doi: 10.1006/bbrc.1999.1860. [DOI] [PubMed] [Google Scholar]
- 39.Kikuchi-Yanoshita R, Konishi M, Ito S, Seki M, Tanaka K, Maeda Y, Iino H, Fukayama M, Koike. Mori M T, et al. Genetic changes of both p53 alleles associated with the conversion from colorectal adenoma to early carcinoma in familial adenomatous polyposis and non-familial adenomatous polyposis patients. Cancer Res. 1992;52:3965–3971. [PubMed] [Google Scholar]
- 40.Kinzler K W, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 41.Kitagawa M, Hatakeyama S, Shirane M, Matsumoto M, Ishida N, Hattori K, Nakamichi I, Kikuchi A, Nakayama K, Nakayama K. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J. 1999;18:2401–2410. doi: 10.1093/emboj/18.9.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koch A, Denkhaus D, Albrecht S, Leuschner I, von Schweinitz D, Pietsch T. Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res. 1999;59:269–273. [PubMed] [Google Scholar]
- 43.Koesters R, Ridder R, Kopp-Schneider A, Betts D, Adams V, Niggli F, Briner J, von Knebel Doeberitz M. Mutational activation of the beta-catenin proto-oncogene is a common event in the development of Wilms' tumors. Cancer Res. 1999;59:3880–3882. [PubMed] [Google Scholar]
- 44.Laurent-Puig P, Blons H, Cugnenc P H. Sequence of molecular genetic events in colorectal tumorigenesis. Eur J Cancer Prev. 1999;9(Suppl. 1):S39–S47. [PubMed] [Google Scholar]
- 45.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 46.Liu C, Kato Y, Zhang Z, Do V, Yankner B, He X. Beta-Trcp couples beta-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci USA. 1999;96:6273–6278. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J, Stevens J, Rote C, Yost H, Hu Y, Neufeld K, White R, Matsunami N. Siah-1 mediates a novel beta-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol Cell. 2001;7:927–936. doi: 10.1016/s1097-2765(01)00241-6. [DOI] [PubMed] [Google Scholar]
- 48.Mann B, Gelos M, Siedow A, Hanski M, Gratchev A, Ilyas M, Bodmer W, Moyer M, Riecken E, Buhr H. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci USA. 1999;96:1603–1608. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 50.Marin M C, Jost C A, Irwin M S, DeCaprio J A, Caput D, Kaelin W G., Jr Viral oncoproteins discriminate between p53 and the p53 homolog p73. Mol Cell Biol. 1998;18:6316–6324. doi: 10.1128/mcb.18.11.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuzawa S-I, Reed J. Siah-1, SIP, and Ebi collaborate in a novel pathway for beta-catenin degradation linked to p53 responses. Mol Cell. 2001;7:915–926. doi: 10.1016/s1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- 52.Mirabelli-Primdahl L, Gryfe R, Kim H, Millar A, Luceri C, Dale D, Holowaty E, Bapat B, Gallinger S, Redston M. Beta-catenin mutations are specific for colorectal carcinomas with microsatellite instability but occur in endometrial carcinomas irrespective of mutator pathway. Cancer Res. 1999;59:3346–3351. [PubMed] [Google Scholar]
- 53.Miyagishi M, Fujii R, Hatta M, Yoshida E, Araya N, Nagafuchi A, Ishihara S, Nakajima T, Fukamizu A. Regulation of lef-mediated transcription and p53-dependent pathway by associating beta-catenin with CBP/p300. J Biol Chem. 2000;275:35170–35175. doi: 10.1074/jbc.C000258200. [DOI] [PubMed] [Google Scholar]
- 54.Miyoshi Y, Iwao K, Nawa G, Yoshikawa H, Ochi T, Nakamura Y. Frequent mutations in the beta-catenin gene in desmoid tumors from patients without familial adenomatous polyposis. Oncol Res. 1998;10:591–594. [PubMed] [Google Scholar]
- 55.Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T, Nakamura Y. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1:229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- 56.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 57.Morin P, Sparks A, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 58.Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Narayan S, Jaiswal A S. Activation of adenomatous polyposis coli (APC) gene expression by the DNA-alkylating agent N-methyl-N′-nitro-N-nitrosoguanidine requires p53. J Biol Chem. 1997;272:30619–30622. doi: 10.1074/jbc.272.49.30619. [DOI] [PubMed] [Google Scholar]
- 60.Nigro J M, Baker S J, Preisinger A C, Jessup J M, Hostetter R, Cleary K, Bigner S H, Davidson N, Baylin S, Devilee P, Glover T, Collins F S, Weston A, Modali R, Harris C C, Vogelstein B. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989;342:705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- 61.Oren M. Regulation of the p53 tumor suppressor protein. J Biol Chem. 1999;274:36031–36034. doi: 10.1074/jbc.274.51.36031. [DOI] [PubMed] [Google Scholar]
- 62.Orford K, Orford C, Byers S. Exogenous expression of beta-catenin regulates contact inhibition, anchorage independent growth, anoikis, and radiation-induced cell cycle arrest. J Cell Biol. 1999;146:855–867. doi: 10.1083/jcb.146.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M. Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc Natl Acad Sci USA. 1995;92:4482–4486. doi: 10.1073/pnas.92.10.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peifer M. Beta-catenin as oncogene: the smoking gun. Science. 1997;275:1752–1753. doi: 10.1126/science.275.5307.1752. [DOI] [PubMed] [Google Scholar]
- 65.Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 66.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 67.Renard C A, Fourel G, Bralet M P, Degott C, De La Coste A, Perret C, Tiollais P, Buendia M A. Hepatocellular carcinoma in WHV/N-myc2 transgenic mice: oncogenic mutations of beta-catenin and synergistic effect of p53 null alleles. Oncogene. 2000;19:2678–2686. doi: 10.1038/sj.onc.1203617. [DOI] [PubMed] [Google Scholar]
- 68.Roose J, Clevers H. TCF transcription factors: molecular switches in carcinogenesis. Biochim Biophys Acta. 1999;1424:M23–M37. doi: 10.1016/s0304-419x(99)00026-8. [DOI] [PubMed] [Google Scholar]
- 69.Roperch J P, Alvaro V, Prieur S, Tuynder M, Nemani M, Lethrosne F, Piouffre L, Gendron M C, Israeli D, Dausset J, Oren M, Amson R, Telerman A. Inhibition of presenilin 1 expression is promoted by p53 and p21WAF-1 and results in apoptosis and tumor suppression. Nat Med. 1998;4:835–838. doi: 10.1038/nm0798-835. [DOI] [PubMed] [Google Scholar]
- 70.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 71.Sadot E, Simcha I, Iwai K, Ciechanover A, Geiger B, Ben-Ze'ev A. Differential interaction of plakoglobin and beta-catenin with the ubiquitin-proteasome system. Oncogene. 2000;19:1992–2001. doi: 10.1038/sj.onc.1203519. [DOI] [PubMed] [Google Scholar]
- 72.Sherr C J, Weber J D. The ARF/p53 pathway. Curr Opin Genet Dev. 2000;10:94–99. doi: 10.1016/s0959-437x(99)00038-6. [DOI] [PubMed] [Google Scholar]
- 73.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simcha I, Shtutman M, Salomon D, Zhurinsky J, Sadot E, Geiger B, Ben-Ze'ev A. Differential nuclear translocation and transactivation potential of beta-catenin and plakoglobin. J Cell Biol. 1998;141:1433–1448. doi: 10.1083/jcb.141.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simms L A, Radford-Smith G, Biden K G, Buttenshaw R, Cummings M, Jass J R, Young J, Meltzer S J, Leggett B A. Reciprocal relationship between the tumor suppressors p53 and BAX in primary colorectal cancers. Oncogene. 1998;17:2003–2008. doi: 10.1038/sj.onc.1202109. [DOI] [PubMed] [Google Scholar]
- 76.Sionov R V, Haupt Y. The cellular response to p53: the decision between life and death. Oncogene. 1999;18:6145–6157. doi: 10.1038/sj.onc.1203130. [DOI] [PubMed] [Google Scholar]
- 77.Sparks A, Morin P, Vogelstein B, Kinzler K. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–1134. [PubMed] [Google Scholar]
- 78.Subler M A, Martin D W, Deb S. Inhibition of viral and cellular promoters by human wild-type p53. J Virol. 1992;66:4757–4762. doi: 10.1128/jvi.66.8.4757-4762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takemaru K, Moon R. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol. 2000;149:249–254. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 81.Van de Craen M, Berx G, Van den Brande I, Fiers W, Declercq W, Vandenabeele P. Proteolytic cleavage of beta-catenin by caspases: an in vitro analysis. FEBS Lett. 1999;458:167–170. doi: 10.1016/s0014-5793(99)01153-9. [DOI] [PubMed] [Google Scholar]
- 82.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 83.Voeller H, Truica C, Gelmann E. Beta-catenin mutations in human prostate cancer. Cancer Res. 1998;58:2520–2523. [PubMed] [Google Scholar]
- 84.Vojtesek B, Bartek J, Midgley C A, Lane D P. An immunochemical analysis of the human nuclear phosphoprotein p53. New monoclonal antibodies and epitope mapping using recombinant p53. J Immunol Methods. 1992;151:237–244. doi: 10.1016/0022-1759(92)90122-a. [DOI] [PubMed] [Google Scholar]
- 85.Wang J, Shou J, Chen X. Dickkopf-1, an inhibitor of the Wnt signaling pathway, is induced by p53. Oncogene. 2000;19:1843–1848. doi: 10.1038/sj.onc.1203503. [DOI] [PubMed] [Google Scholar]
- 86.Webster G A, Perkins N D. Transcriptional cross talk between NF-kappaB and p53. Mol Cell Biol. 1999;19:3485–3495. doi: 10.1128/mcb.19.5.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei Y, Fabre M, Branchereau S, Gauthier F, Perilongo G, Buendia M A. Activation of beta-catenin in epithelial and mesenchymal hepatoblastomas. Oncogene. 2000;19:498–504. doi: 10.1038/sj.onc.1203356. [DOI] [PubMed] [Google Scholar]
- 88.Winston J, Strack P, Beer-Romero P, Chu C, Elledge S, Harper J. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wright K, Wilson P, Morland S, Campbell I, Walsh M, Hurst T, Ward B, Cummings M, Chenevix-Trench G. Beta-catenin mutation and expression analysis in ovarian cancer: exon 3 mutations and nuclear translocation in 16% of endometrioid tumours. Int J Cancer. 1999;82:625–629. doi: 10.1002/(sici)1097-0215(19990827)82:5<625::aid-ijc1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 90.Xu L, Corcoran R B, Welsh J W, Pennica D, Levine A J. WISP-1 is a Wnt-1- and beta-catenin-responsive oncogene. Genes Dev. 2000;14:585–595. [PMC free article] [PubMed] [Google Scholar]
- 91.Yost C, Torres M, Miller J, Huang E, Kimelman D, Moon R. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Z, Hartmann H, Do V, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, Sommer B, van de Wetering M, Clevers H, Saftig P, De Strooper B, He X, Yankner B. Destabilization of beta-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature. 1998;395:698–702. doi: 10.1038/27208. [DOI] [PubMed] [Google Scholar]
- 93.Zhurinsky J, Shtutman M, Ben-Ze'ev A. Differential mechanisms of LEF/TCF family-dependent transcriptional activation by beta-catenin and plakoglobin. Mol Cell Biol. 2000;20:4238–4252. doi: 10.1128/mcb.20.12.4238-4252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhurinsky J, Shtutman M, Ben-Ze'ev A. Plakoglobin and β-catenin: protein interactions, regulation and biological roles. J Cell Sci. 2000;113:3127–3139. doi: 10.1242/jcs.113.18.3127. [DOI] [PubMed] [Google Scholar]
- 95.Zurawel R, Chiappa S, Allen C, Raffel C. Sporadic medulloblastomas contain oncogenic beta-catenin mutations. Cancer Res. 1998;58:896–899. [PubMed] [Google Scholar]