Abstract
Background
Recognition of the role of vitamin D in immune function has led to interest in its relationship with SARS-CoV-2 infection. Although clinical studies to date have had conflicting results, many individuals currently take high doses of vitamin D to prevent infection.
Objective
The goal of this study was to investigate the relationship between serum 25-hydroxyvitamin D (25OHD) and vitamin D supplement use with incident SARS-CoV-2 infection.
Methods
In this prospective cohort study, 250 health care workers were enrolled at a single institution and observed for 15 mo. Participants completed questionnaires every 3 mo regarding new SARS-CoV-2 infection, vaccination, and supplement use. Serum was drawn at baseline, 6, and 12 mo for 25OHD and SARS-CoV-2 nucleocapsid antibodies.
Results
The mean age of the participants was 40 y, BMI 26 kg/m2, 71% were Caucasian, and 78% female. Over 15 mo, 56 participants (22%) developed incident SARS-CoV-2 infections. At baseline, ∼50% reported using vitamin D supplements (mean daily dose 2250 units). Mean serum 25OHD was 38 ng/mL. Baseline 25OHD did not predict incident SARS-CoV-2 infection (OR: 0.98; 95% CI: 0.80, 1.20). Neither the use of vitamin D supplements (OR: 1.18; 95% CI: 0.65, 2.14) or supplement dose was associated with incident infection (OR: 1.01 per 100-units increase; 95% CI: 0.99, 1.02).
Conclusion
In this prospective study of health care workers, neither serum 25OHD nor the use of vitamin D supplements was associated with the incident SARS-CoV-2 infection. Our findings argue against the common practice of consuming high-dose vitamin D supplements for the presumed prevention of COVID-19.
Keywords: Vitamin D, SARS-CoV-2 infection, COVID-19
Introduction
The COVID-19 pandemic, caused by the SARS-CoV-2, has resulted in morbidity and mortality throughout the world that are unprecedented in modern times [1]. Given the continued high prevalence of COVID-19, it is important to identify modifiable factors that decrease the risk and severity of infection.
Vitamin D plays a key role in both innate and adaptive immunity [2, 3]; for this reason, there has been a great deal of interest in the link between vitamin D and susceptibility to, and severity of, infection with SARS-CoV-2 [4, 5]. Vitamin D has been reported to regulate innate immunity by increased production of peptides such as cathelidicin and β-defensin-2, subsequently blocking the viral entry into cells and suppressing replication [6, 7]. Vitamin D also promotes autophagy and the generation of nitric oxide, both important in the cellular response to infection [8, 9]. The biologically active metabolite of Vitamin D, 1,25 dihydroxy vitamin D, exerts anti-inflammatory action on the adaptive immune system via the nuclear vitamin D receptor expressed in antigen-presenting cells and activated T cells [10]. Vitamin D may be particularly important in resistance to respiratory infections, because it also helps to maintain the physical epithelial barrier [11, 12]. In some clinical studies, vitamin D supplementation lowered the risk of respiratory infections [[13], [14], [15]]; however, this finding is not uniform [16]. Vitamin D also influences the proliferation of immune cells, their maturation to plasma cells, and production of immunoglobulins. An inverse relationship between serum 25-hydroxy vitamin D (25OHD) and total immunoglobulin levels has been reported, suggesting that low 25OHD may be linked to immune dysregulation [17, 18]. Vitamin D may block cellular entry of SARS-CoV-2 as vitamin D-inducible peptide human β defensin has been shown to bind to the SARS-CoV-2-receptor-binding domain, angiotensin-converting enzyme 2 (ACE2) [19]. Vitamin D may also lower the risk of COVID-19 related cytokine storm by lowering interleukin-6 levels [20, 21]. For the above reasons, it has been hypothesized that vitamin D may protect against SARS-CoV-2 infection and disease severity by blocking cellular entry of the virus, promoting viral clearance, and reducing the systemic inflammatory response [22].
Although the threshold that denotes vitamin D deficiency is debated in the field, most organizations and studies have defined deficiency as either below 20 or 30 ng/mL [23, 24]. According to these definitions, vitamin D deficiency is prevalent, with estimates of at least 40% in the general population [25, 26]. Serum 25OHD levels have been shown to be even lower among health care workers, particularly those working in hospitals [27]. As vitamin D deficiency is easily modifiable, identification of low vitamin D as a risk factor for COVID-19 would directly foster simple repletion strategies to reduce the risk of infection. Since the onset of the pandemic, the use of supplements believed to improve immunity has burgeoned [28]. Despite a lack of evidence, a recent study found that nearly 70% of health care workers reported recommending vitamin D supplements to their patients for the prevention of COVID-19, regardless of the patients’ baseline 25OHD level [29]. Given the widespread use of vitamin D supplements for COVID-19 prevention as well as increasing evidence of potential harms of high-dose vitamin D [[30], [31], [32]], it is critical to investigate whether and to what extent vitamin D status and supplement use relate to infection risk among healthy individuals.
We began this prospective study in May of 2020, early in the COVID-19 pandemic, to investigate the relationship between vitamin D status and supplemented use with incident SARS-CoV-2 infection in a cohort of health care workers. Based upon data linking vitamin D deficiency to the risk of other respiratory infections, we hypothesized that individuals with low 25OHD levels would have an increased incidence of infection with SARS-CoV-2.
Methods
Study population
We recruited 250 health care workers at our institution and observed them for up to 15 mo between May 2020 to April 2022. This study was approved by the institutional review board at the Hospital for Special Surgery. Informed consent was signed by all the participants. Individuals over age 18 were eligible for enrollment, regardless of whether their job involved direct patient care. We excluded participants with a previous established diagnosis of COVID-19 (clinical infection, positive viral PCR, or antibodies) or any respiratory illness with new-onset fever, cough, or dyspnea within 14 d of enrollment. (enrollment flowchart in supplementary file). Most participants (96%) were enrolled between May and November 2020 before COVID-19 vaccines became available in the United States. One participant received the COVID-19 vaccine before enrollment.
Study design
In this prospective cohort study, participants completed questionnaires every 3 mo regarding new SARS-CoV-2 infection, vaccination, medical history, and supplement use, including vitamin D2, vitamin D3, and multivitamins. We considered that vitamin D from food sources would have a minimal contribution to the overall intake and therefore did not have a specific questionnaire to collect this data to minimize the participant burden. Serum samples were obtained at baseline, 6 mo, and 12-month follow-up for measurement of 25OHD and SARS-CoV-2 nucleocapsid antibodies. As 25OHD does not vary by recency of food intake or have a diurnal variation, random blood samples were obtained for serum 25OHD and not required to be performed fasting or at a specific time of day [33]. Serum 25OHD was measured by chemiluminescent microparticle immunoassay (Abbott Diagnostics, Abbott Park, IL). Anti-nucleocapsid antibodies to SARS-CoV-2 were measured by chemiluminescent microparticle immunoassay (Abbott Diagnostics, Abbott Park, IL). IgA, IgM, and IgG were measured by quantitative rate nephelometry (IMMAGE Immunochemistry System, Beckman Coulter).
Statistical analysis
All analyses were carried out in SAS v 13.2. P values < 0.05 were considered statistically significant. Data that were not normally distributed (immunoglobulins) were log-transformed before analysis. Associations between serum 25OHD and age, BMI, and immunoglobulins were assessed using Spearman rank correlations. ORs were calculated to estimate the likelihood of SARS-Cov-2 infection as a function of baseline serum 25OHD and vitamin D supplement use when levels were dichotomized (25OHD level <30 ng/dL or ≥30 ng/dL and supplement use yes/no). Logistic regression was used to calculate the likelihood of SARS-Cov-2 infection for each 10 ng/mL decrease in baseline 25OHD level and 100 IU decrease in baseline vitamin D supplement dosage. T-tests were used to assess distributional differences in person characteristics when classified by baseline vitamin D level <30 ng/dL or ≥30 ng/dL.
Sample size calculations were performed assuming 90% power and two-tailed alpha of 1% to detect a difference in rates of SARS-CoV-2 infection between individual baseline serum 25OHD less than and levels ≥30 ng/mL. From the literature available at the time the study was designed, we assumed an overall infection rate of 25% and that ∼40% of participants would have serum 25OHD levels <30 ng/mL [25, 26]. According to our calculations, 250 participants, with a relative risk of infection of 2.00, would imply that 35% of those with 25OHD <30 ng/mL and 18% of those with 25OHD ≥30 ng/mL would have incident infections.
Results
Baseline characteristics of the participants
A total of 250 participants were enrolled in this study. The baseline characteristics of the study population are detailed in Table 1 . Mean age was 40 y, and 78% were female. Most participants (71%) were White, 12% were African American, 12% Asian, and 5% identified as from another racial group; 17% were Hispanic. The mean BMI was 26 kg/m2. Most participants (85%) had never smoked. Chronic medical conditions reported by the participants included asthma (11%), hypertension (10%), and diabetes mellitus (4%).
TABLE 1.
Baseline characteristics of the study population
| Overall | |
|---|---|
| Age (y) mean ± SD) | 40 ± 13 |
| Race (White, n, %) | 174, 71% |
| Sex (Female, n, %) | 193, 78% |
| BMI (kg/m2, mean ± SD) | 26 ± 6 |
| Tobacco use (never, n, %) | 213, 86% |
| Alcohol use (yes, n, %) | 188, 75% |
| Calcium supplement use (yes, n, %) | 71, 28% |
| Calcium supplement dose (mg/d, mean ± SD) | 434 ± 471 |
| Vitamin D supplement use (n, %) | 124, 50% |
| Vitamin D supplement dose (IU/d, mean ± SD) | 2250 ± 1776 |
Serum 25OHD levels and vitamin D supplement use
At baseline, the mean 25OHD was 38 ± 15 ng/mL. Twenty-eight percent of participants had serum 25OHD levels <30 ng/mL. Participants with 25OHD <30 ng/mL were younger (37 vs. 41, P = 0.01) and had higher BMIs than participants with higher 25OHD levels (28 ± 7 vs 25 ± 5, P =0.01). Half of the participants reported using vitamin D supplements at baseline, with an average dose of 2250 IU per day (range from 250 IU to 7742 IU). The daily dose of vitamin D was ≥2000 IU in 26% of the participants and ≥4000 IU in 12% participants. Baseline vitamin D supplement users averaged 43 ± 13 y old, whereas supplement non-users averaged 37 ± 12 y of age (P < 0.0001). A greater frequency of supplement use was reported by participants with 25OHD ≥30 ng/mL (57% compared with 29% in participants with baseline 25OHD<30 ng/mL (P < 0.0001).Variability in 25OHD levels over the study duration was <5%.
Incident SARS-CoV-2 infection
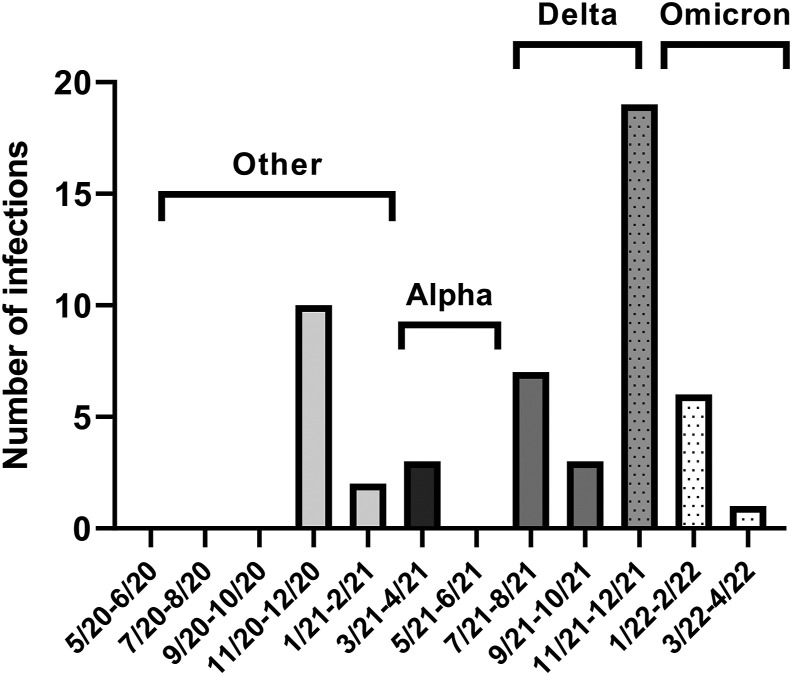
Fifty-six participants (22%) became infected with SARS-CoV-2 during the study follow-up period (Figure 1 ). Among the infected participants, 55% were symptomatic; the most common symptoms were cough, sore throat, and loss of taste or smell. Nine participants (16%) sought medical care for COVID-19 symptoms. None of the participants were admitted to the hospital for COVID-19. One participant had positive antibodies at baseline, and 2 participants had SARS-CoV-2 infection twice. As described above, 249 of 250 participants were not vaccinated at study entry; however, all participants were vaccinated over the course of the study as this was a requirement of continued employment at our institution. The mean time between study enrollment and vaccination was 5 mo. Of the total number of SARS-CoV-2 infections, 41 were breakthrough infections that occurred after participants had been fully vaccinated.
FIGURE 1.
Incidence of SARS-CoV-2 infections during the study follow-up. Predominant variants of each time period are denoted.
Serum 25OHD and SARS-CoV-2 infection
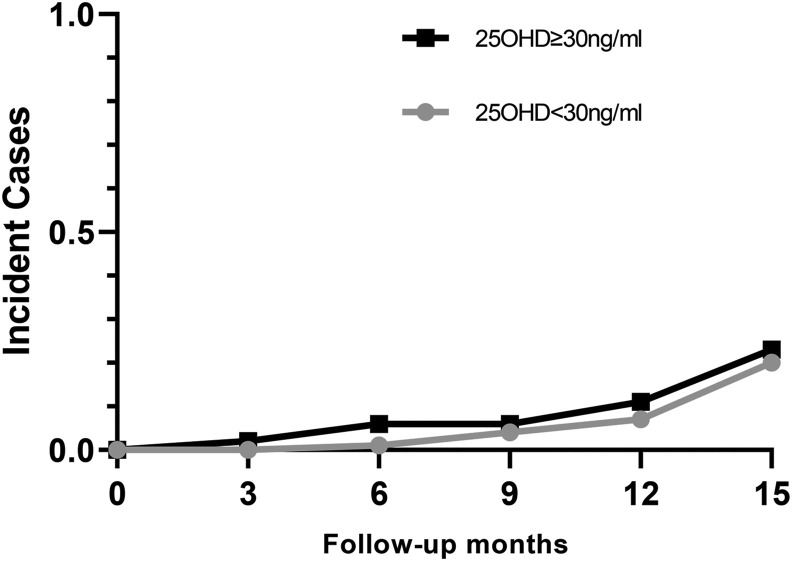
Baseline serum 25OHD did not predict SARS-CoV-2 infection, either when assessed using serum 25OHD as a continuous variable, (OR: 0.98; 95% CI: 0.80, 1.20), or using 30 ng/mL as a threshold, (OR: 1.02, 95% CI: 0.83, 1.25; Figure 2 ). Additional cut-points in increments of 10 ng/mL between 10 and 70 ng/mL were also investigated, and no significant relationship between 25OHD and incident infection was observed. Further, serum 25OHD did not predict breakthrough SARS-CoV-2 infection after full vaccination (OR: 0.99; 95% CI: 0.97, 1.02). Among the participants who were infected, there was no association between the presence of symptoms and baseline 25OHD level. The relationship between 25OHD and incident infection was further examined in the subgroups according to age, sex, race and ethnicity, BMI, and use of supplemental vitamin D. We found no evidence of a relationship between baseline serum 25OHD and incident infection among any of these subgroups. Furthermore, a multivariate model that adjusted for age, sex, BMI, race, ethnicity, and season was performed. There was no evidence of a relationship between serum 25OHD and infection. Analyses were similarly unchanged when we excluded the 1 participant who was vaccinated before study entry, and the 1 participant who was found to have had positive antibodies at baseline when the serum was later analyzed in a batch.
FIGURE 2.
Incident cases of SARS-CoV-2 infection according to baseline serum 25OHD. Participants with 25OHD <30 ng/mL shown in grey and those with 25OHD ≥30 ng/mL shown in black. Baseline 25OHD did not predict SARS-CoV-2 infection (OR: 1.02; 95% CI: 0.83, 1.25).
Vitamin D supplement use and SARS-CoV-2 infection
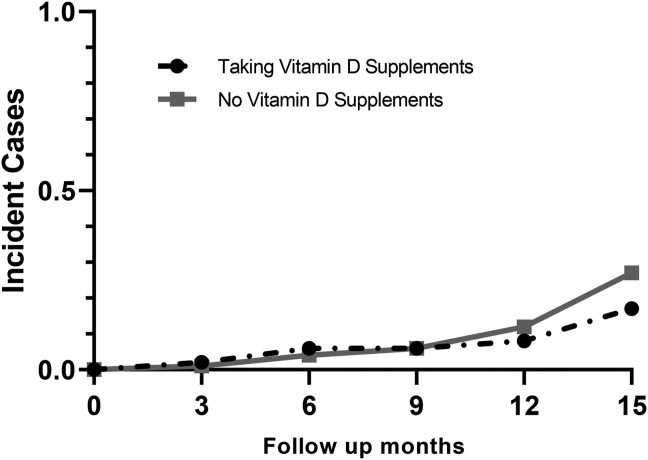
The use of vitamin D supplements at baseline was not associated with SARS-CoV-2 infection. Supplement use was evaluated both as a categorical variable (OR: 1.18; 95% CI: 0.65, 2.14; Figure 3 ) and according to dose (OR: 1.007 per 100-IU increase; 95% CI: 0.99, 1.02). Moreover, the dose of vitamin D supplements did not relate to the incident infection when calculated in thresholds as <2000 (OR: 1.48; 95% CI: 0.74, 2.96) and 4000 IU daily (OR: 1.37; 95% CI: 0.50, 3.79).
FIGURE 3.
Incident cases of SARS-CoV-2 infection according to vitamin D supplement use. Participants who reported using vitamin D supplements shown with the dashed black line and those who reported no use shown with the solid grey line. Use of vitamin D supplements was not associated with incident SARS-CoV-2 infection (OR: 1.18; 95% CI: 0.65, 2.14).
Vitamin D level, circulating antibodies, and COVID-19 infection
At baseline, IgG, IgA, and IgM were within the normal range: IgG 1063 ± 299 (ref, 751–1560 mg/dL), IgA 212 ± 103 (ref, 82–453 mg/dL) and IgM 115 ± 63 (ref, 46–304 mg/dL). There was no association between baseline 25OHD level and IgG, IgA, and IgM levels. Furthermore, baseline levels of immunoglobulins were not related to the incident infection.
Discussion
This prospective study investigated the relationship between serum 25OHD, vitamin D supplement use, and incident SARS-CoV-2 infection among healthy individuals observed over multiple waves of viral variants in the United States. We found no association between baseline serum 25OHD and incident SARS-CoV-2 infection overall or with breakthrough infections after full vaccination. Further, we found no association between the use of vitamin D supplements, or supplement dose and incident SARS-CoV-2 infection.
Previous work investigating the relationship between serum 25OHD and SARS-Cov-2 infection has yielded conflicting results. Most previous studies with positive findings were retrospective [22, [34], [35], [36], [37], [38], [39]], raising the possibility that their findings may have been influenced by selection bias or confounding. Many studies have focused on hospitalized patients who had more severe infections [22, [34], [35], [36], [37], [38], [39]]. Low 25OHD in these populations may reflect poor general health or chronic disease burden, rather than being an independent risk factor for infection. A recent meta-analysis found an increased risk of SARS-CoV-2 infection in subjects with lower 25OHD [40]. However, the studies included were retrospective and heterogeneous in terms of study design, enrollment period, geographic area, and confounding risk factors [40]. Consistent with the findings of our longitudinal study, large cross-sectional studies have not found evidence of a relationship [41, 42]. A UK Biobank study, which included 417,342 individuals found that baseline serum 25OHD was not associated with SARS-CoV-2 infection after adjusting for confounding factors [43]. In a US cohort, low 25OHD levels were not independently associated with the risk of seropositivity [42]. Interestingly, a recent investigation using a national cohort of 158,835 patients with confirmed COVID-19 found that vitamin D treatment was paradoxically associated with greater odds of extended hospitalization, mechanical ventilation/extracorporeal membrane oxygenation, hospice referral, or death among patients with severe disease [44]. However, it is possible that vitamin D may have been used as a treatment more frequently for those patients who were most severely ill.
Participants in our study were observed through multiple waves of viral variants in New York. Many of the infections that we observed were during the Omicron wave, which may have been a reflection of the high infectivity of this viral strain but also may have been related to waning antibody levels from the time since vaccination. Although it is conceivable that our sample size was too small to detect a relationship between 25OHD and infection risk, the observed rate of infection we observed was greater than we predicted in our a previousi power calculation, suggesting that the null finding of our study was unlikely to be due to a small sample size. Although we investigated several cut-points for 25OHD, it is conceivable that a non-linear relationship exists between serum 25OHD and incident infection which we were not able to detect in our analyses.
We did not find that use of vitamin D supplements lowered the risk of incident SARS-CoV-2 infection nor did we find a relationship between the dosage of supplements and incident infection. In our study, about half of the participants took vitamin D supplements at baseline. Our findings are consistent with those of a recent meta-analysis that included 1 randomized controlled trial (RCT) and 3 cohort studies in which vitamin D supplementation did not significantly reduce the risk of COVID-19 [45]. In a study from the United Kingdom Biobank, the use of vitamin D supplements was associated with a 34% reduced risk of COVID-19. As only individuals who had clinical COVID-19 tests were included, these findings may not have reflected relationships with asymptomatic disease in the general population. Furthermore, the information about vitamin D supplement use was collected a median of 10 y before the COVID-19 tests, so may not have reflected participants’ supplementation use at the time of testing [46].
A few early RCTs among non-vaccinated health care workers investigated the relationship between vitamin D supplement use and the risk of SARS-CoV-2 infection with conflicting results. The findings of these trials are limited by their short durations, focus on high doses of vitamin D, and high rates of drop-out and cross-over between groups. In 1 study of frontline Mexican health care workers, rates of SARS-CoV-2 infection were lower in those who received 4000 IU of vitamin D daily for 30 d than placebo. However, the drop-out rate in this study was nearly 50%, making it challenging to generalize these results [47]. In contrast, an RCT conducted in Russia found no difference in SARS-CoV-2 morbidity among health care workers who received high - dose vitamin D supplements (50,000 units per week for 2 wk followed by 5,000 units per d) versus low - dose vitamin D supplements (2000 units per d) for 3 mo [48].
Two large RCTs studying vitamin D supplementation and COVID-19 infection have recently been published. A phase 3 pragmatic randomised controlled trial (CORONAVIT) RCT randomized 2958 participants with 25OHD below 75 nmol/L (∼30 ng/mL) to 800 IU or 3200 IU daily for 6 mo. The investigators found no association between vitamin D supplement use or dose and SARS-CoV-2 infections [49]. A large RCT from Norway similarly found no effect of vitamin D supplementation for 6 mo given as cod liver oil (400 IU per d) on incident infections [50]. Both studies relied on self-reports of infection. Although our study was observational, our null findings are similar to those of the above RCTs. Our study extends those findings by including a more racially and ethnically diverse population, as most participants in those trials were White. In addition, we observed subjects for over a year, through multiple waves of the pandemic, whereas these trials had a shorter duration of follow-up. Finally, we were able to detect both symptomatic and asymptomatic infections with our use of antibodies to detect seroconversion among our participants and confirm that the participants did not have a history of asymptomatic infection before study entry.
Despite a lack of data to support the role of vitamin D in COVID-19 prevention, the fear of contracting infection and easy access to over-the-counter supplements has led to high rates of supplement use to boost immunity across the worldwide [28]. Although many health care practitioners recommend prophylactic vitamin D supplements to their patients for the prevention of COVID-19 [29], our results suggest that there is not sufficient evidence for this practice. Further, mounting evidence of the risks of high-dose vitamin D supplementation suggests that there may be risks associated with this practice [30, 32, 51].
Our work had several limitations. This was a single-center study. Vaccination became available mid-way during our study and may have modified the relationship between vitamin D status and infection. It is possible that the study included an entirely unvaccinated population, we might have found different results. However, it is the strength of our study that we included a relatively long follow-up of our patients both before and after vaccination. Although we did screen participants for asymptomatic infections through the development of new nucleocapsid antibodies, we did not perform polymerase chain reaction tests for surveillance and only measured antibody levels every 6 mo. As a result, we may have missed patients whose antibodies waned within this period. As the timing of vaccination as well as most breakthrough infections occurred during a similar timeframe for our participants, the lack of variability limited our ability to test the relationships between vitamin D status, COVID waves, and timing of vaccination. Further, although we observed that 25OHD levels were relatively stable throughout the study follow-up, we were only able to detect fluctuations in levels every 6 mo. We did not collect data on several factors that are known to influence 25OHD, including sun exposure. However, the summed contributions of these many factors are reflected in the baseline and follow-up 25OHD levels of our cohort. Another limitation is that we did not perform liquid chromatography/tandem mass spectrometry (MS) for 25OHD measurements. Previous work has demonstrated high variability between different 25OHD assays [52]. While MS could measure 25OHD2 independently, our chemiluminescent measurement did not and may have detected 25OHD2 less well than 25OHD3, leading to an underestimation of the total 25OHD in those patients using ergocalciferol supplements [53]. In addition, the observed prevalence of vitamin D deficiency (28%) was lower than we assumed in our sample size calculation (40%), it is possible therefore hat the results might be different in populations with more severe vitamin D deficiency. Finally, participants in our cohort were relatively young and in good general health, it is uncertain whether our findings in this cohort are generalizable to an older population with more chronic illness [54]. None of our participants developed severe infections requiring hospitalization. As a result, we were not able to evaluate the relationships between disease severity, serum 25OHD, and vitamin supplement use in our cohort.
In conclusion, our study found that neither vitamin D status nor the use of vitamin D supplements was associated with the incident SARS-CoV-2 infection in a cohort of individuals in good general health. Our results suggest that 25OHD is related to COVID-19 infection in previous retrospective and cross-sectional work because it is a marker for poor health rather than an independent risk factor for infection. These findings argue against the common practice of high-dose vitamin D supplements for the presumed prevention of COVID-19.
Acknowledgments
The authors’ responsibilities were as follows—AS, AM, TL, DJM, JWN, EMS designed research; SC, AH, AD, AK, CZ, KH, KV, MS, EMS conducted research; YL, GD, DJM, JWN, EMS analyzed data; DJM performed statistical analysis; YL, SC, GD, DJM, JWN, EMS wrote paper; EMS had primary responsibility for the final content; and all authors have read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.03.001.
Data Availability
Data described in the manuscript will be made available upon reasonable request.
Funding
This work was funded by a COVID-19 research grant from the Hospital for Special Surgery.
Author disclosures
The authors report no conflicts of interest. The JWN reports research support from Radius Pharmaceuticals. EMS reports research support from Novartis and Radius Pharmaceuticals.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time, Lancet. Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aranow C. Vitamin D and the immune system. J. Investig. Med. 2011;59:881–886. doi: 10.2310/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen C.J., Adams J.S., Bikle D.D., Black D.M., Demay M.B., Manson J.E., et al. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr. Rev. 2012;33:456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilezikian J.P., Bikle D., Hewison M., Lazaretti-Castro M., Formenti A.M., Gupta A., et al. Mechanisms in endocrinology: vitamin D and COVID-19. Eur. J. Endocrinol. 2020;183:R133–R147. doi: 10.1530/EJE-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandeira L., Lazaretti-Castro M., Binkley N. Clinical aspects of SARS-CoV-2 infection and vitamin. Rev. Endocr. Metab. Disord. 2022;23:287–291. doi: 10.1007/s11154-021-09683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuk J.M., Shin D.M., Lee H.M., Yang C.S., Jin H.S., Kim K.K., et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell. Host. Microbe. 2009;6:231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Wang T.T., Dabbas B., Laperriere D., Bitton A.J., Soualhine H., Tavera-Mendoza L.E., et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J. Biol. Chem. 2010;285:2227–2231. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uberti F., Lattuada D., Morsanuto V., Nava U., Bolis G., Vacca G., et al. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J. Clin. Endocrinol. Metab. 2014;99:1367–1374. doi: 10.1210/jc.2013-2103. [DOI] [PubMed] [Google Scholar]
- 9.Mushegian A.A. Autophagy and vitamin D. Sci. Signal. 2017;21:10. doi: 10.1126/scisignal.aan2526. [DOI] [PubMed] [Google Scholar]
- 10.van Etten E., Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J. Steroid Biochem. Mol. Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y.Y., Liu T.J., Fu J.H., Xu W., Wu L.L., Hou A.N., et al. Vitamin D/VDR signaling attenuates lipopolysaccharideinduced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol. Med. Rep. 2016;13:1186–1194. doi: 10.3892/mmr.2015.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol. Cell. Endocrinol. 2010;321:103–111. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manaseki-Holland S., Qader G., Isaq Masher M., Bruce J., Zulf Mughal M., Chandramohan D., et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop. Med. Int. Health. 2010;15:1148–1155. doi: 10.1111/j.1365-3156.2010.02578.x. [DOI] [PubMed] [Google Scholar]
- 14.Camargo C.A., Jr., Ganmaa D., Frazier A.L., Kirchberg F.F., Stuart J.J., Kleinman K., et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics. 2012 Sep;130:e561–e567. doi: 10.1542/peds.2011-3029. [DOI] [PubMed] [Google Scholar]
- 15.Jolliffe D.A., Camargo C.A., Jr., Sluyter J.D., Aglipay M., Aloia J.F., Ganmaa D., et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet. Diabetes. Endocrinol. 2021;9:276–292. doi: 10.1016/S2213-8587(21)00051-6. [DOI] [PubMed] [Google Scholar]
- 16.Pham H., Waterhouse M., Baxter C., Duarte Romero B., McLeod D.S.A., Armstrong B.K., et al. The effect of vitamin D supplementation on acute respiratory tract infection in older Australian adults: an analysis of data from the D-Health Trial. Lancet. Diabetes. Endocrinol. 2021;9:69–81. doi: 10.1016/S2213-8587(20)30380-6. [DOI] [PubMed] [Google Scholar]
- 17.Chen S., Sims G.P., Chen X.X., Gu Y.Y., Chen S., Lipsky P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 18.Pincikova T., Nilsson K., Moen I.E., Karpati F., Fluge G., Hollsing A., et al. Inverse relation between vitamin D and serum total immunoglobulin G in the Scandinavian Cystic Fibrosis Nutritional Study. Eur. J. Clin. Nutr. 2011;65:102–109. doi: 10.1038/ejcn.2010.194. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L., Ghosh S.K., Basavarajappa S.C., Chen Y., Shrestha P., Penfield J., et al. HBD-2 binds SARS-CoV-2 RBD and blocks viral entry: strategy to combat COVID-19. iScience. 2022;25 doi: 10.1016/j.isci.2022.103856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roffe-Vazquez D.N., Huerta-Delgado A.S., Castillo E.C., Villarreal-Calderon J.R., Gonzalez-Gil A.M., Enriquez C., et al. Correlation of vitamin D with inflammatory cytokines, atherosclerotic parameters, and lifestyle factors in the setting of heart failure: a 12-month follow-up study. Int. J. Mol. Sci. 2019;19:20. doi: 10.3390/ijms20225811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. Hlh Across Speciality Collaboration UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meltzer D.O., Best T.J., Zhang H., Vokes T., Arora V., Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA. Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bilezikian J.P., Formenti A.M., Adler R.A., Binkley N., Bouillon R., Lazaretti-Castro M., et al. Vitamin D: dosing, levels, form, and route of administration: does one approach fit all? Rev. Endocr. Metab. Disord. 2021;22:1201–1218. doi: 10.1007/s11154-021-09693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao L., Carson J.L., Schlussel Y., Noveck H., Shapses S.A. Vitamin D deficiency is associated with reduced mobility after hip fracture surgery: a prospective study. Am. J. Clin. Nutr. 2020;112:613–618. doi: 10.1093/ajcn/nqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forrest K.Y., Stuhldreher W.L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Rosen C.J. Clinical practice. Vitamin D insufficiency. N. Engl. J. Med. 2011;364:248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 27.Sowah D., Fan X., Dennett L., Hagtvedt R., Straube S. Vitamin D levels and deficiency with different occupations: a systematic review. B.M.C. Public. Health. 2017;17:519. doi: 10.1186/s12889-017-4436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayosanmi O.S., Alli B.Y., Akingbule O.A., Alaga A.H., Perepelkin J., Marjorie D., et al. Prevalence and correlates of self-medication practices for prevention and treatment of COVID-19: a systematic review. Antibiotics (Basel) 2022;11 doi: 10.3390/antibiotics11060808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Shatnawi S.F., Khasawneh R.A., Alhamad H. Healthcare providers' perspectives toward the integration of over the counter supplements during COVID-19 pandemic: a cross-sectional study from Jordan. Inquiry. 2022;59 doi: 10.1177/00469580221095825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burt L.A., Billington E.O., Rose M.S., Kremer R., Hanley D.A., Boyd S.K. Adverse effects of high-dose vitamin D supplementation on volumetric bone density are greater in females than males. J. Bone Miner. Res. 2022;35:2404–2414. doi: 10.1002/jbmr.4152. [DOI] [PubMed] [Google Scholar]
- 31.Bouillon R., Manousaki D., Rosen C., Trajanoska K., Rivadeneira F., Richards J.B. The health effects of vitamin D supplementation: evidence from human studies. Nat. Rev. Endocrinol. 2022;18:96–110. doi: 10.1038/s41574-021-00593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders K.M., Stuart A.L., Williamson E.J., Simpson J.A., Kotowicz M.A., Young D., Nicholson G.C. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 33.Jones K.S., Redmond J., Fulford A.J., Jarjou L., Zhou B., Prentice A., et al. Diurnal rhythms of vitamin D binding protein and total and free vitamin D metabolites. J. Steroid. Biochem. Mol. Biol. 2017;172:130–135. doi: 10.1016/j.jsbmb.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernández J.L., Nan D., Fernandez-Ayala M., García-Unzueta M., Hernández-Hernández M.A., López-Hoyos M., et al. Vitamin D Status in hospitalized patients with SARS-CoV-2 infection. J. Clin. Endocrinol. Metab. 2021;106 doi: 10.1210/clinem/dgaa733. e1343–e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdollahi A., Kamali Sarvestani H., Rafat Z., Ghaderkhani S., Mahmoudi-Aliabadi M., Jafarzadeh B., et al. The association between the level of serum 25(OH) vitamin D, obesity, and underlying diseases with the risk of developing COVID-19 infection: a case-control study of hospitalized patients in Tehran, Iran. J. Med. Virol. 2021;93:2359–2364. doi: 10.1002/jmv.26726. [DOI] [PubMed] [Google Scholar]
- 36.Ye K., Tang F., Liao X., Shaw B.A., Deng M., Huang G., et al. Does serum vitamin D level affect COVID-19 infection and its severity?-A case-control study. J. Am. Coll. Nutr. 2021;40:724–731. doi: 10.1080/07315724.2020.1826005. [DOI] [PubMed] [Google Scholar]
- 37.Merzon E., Tworowski D., Gorohovski A., Vinker S., Golan Cohen A., Green I., et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. F.E.B.S. Journal. 2020;287:3693–3702. doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Im J.H., Je Y.S., Baek J., Chung M.H., Kwon H.Y., Lee J.S. Nutritional status of patients with COVID-19. Int. J. Infect. Dis. 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaufman H.W., Niles J.K., Kroll M.H., Bi C., Holick M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Ecclesiis O., Gavioli C., Martinoli C., Raimondi S., Chiocca S., Miccolo C., Bossi P., et al. Vitamin D and SARS-CoV2 infection, severity and mortality: a systematic review and meta-analysis. PLoS One. 2022;17 doi: 10.1371/journal.pone.0268396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hastie C.E., Pell J.P., Sattar N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur. J. Nutr. 2021;60:545–548. doi: 10.1007/s00394-020-02372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y., Tong C.H., Bare L.A., Devlin J.J. Assessment of the association of vitamin D level with SARS-CoV-2 seropositivity among working-age adults. JAMA. Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hastie C.E., Mackay D.F., Ho F., Celis-Morales C.A., Katikireddi S.V., Niedzwiedz C.L., et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes. Metab. Syndr. 2020;14:561–565. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fairfield K.M., Murray K.A., Anzalone A.J., Beasley W., Khodaverdi M., Hodder S.L., et al. Association of vitamin D prescribing and clinical outcomes in adults hospitalized with COVID-19. Nutrients. 2022;26:14. doi: 10.3390/nu14153073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosseini B., El Abd A., Ducharme F.M. Effects of vitamin D supplementation on COVID-19 related outcomes: a systematic review and meta-analysis. Nutrients. 2022;14:10. doi: 10.3390/nu14102134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma H., Zhou T., Heianza Y., Qi L. Habitual use of vitamin D supplements and risk of coronavirus disease 2019 (COVID-19) infection: a prospective study in UK Biobank. Am. J. Clin. Nutr. 2021;113:1275–1281. doi: 10.1093/ajcn/nqaa381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villasis-Keever M.A., López-Alarcón M.G., Miranda-Novales G., Zurita-Cruz J.N., Barrada-Vázquez A.S., González-Ibarra J., et al. Efficacy and safety of vitamin D supplementation to prevent COVID-19 in frontline healthcare workers. A randomized clinical trial. Arch. Med. Res. 2022;53:423–430. doi: 10.1016/j.arcmed.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karonova T.L., Chernikova A.T., Golovatyuk K.A., Bykova E.S., Grant W.B., Kalinina O.V., et al. Vitamin D intake may reduce SARS-CoV-2 infection morbidity in health care workers. Nutrients. 2022;14:505. doi: 10.3390/nu14030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jolliffe D.A., Holt H., Greenig M., Talaei M., Perdek N., Pfeffer P., et al. Vitamin D Supplements for Prevention of Covid-19 or other acute respiratory infections: a Phase 3 Randomized Controlled Trial (CORONAVIT) BMJ. 2022;378 doi: 10.1136/bmj-2022-071230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brunvoll S.H., Nygaard A.B., Ellingjord-Dale M., Holland P., Istre M.S., Kalleberg K.T., et al. Prevention of covid-19 and other acute respiratory infections with cod liver oil supplementation, a low dose vitamin D supplement: quadruple blinded, randomised placebo controlled trial. BMJ. 2022;378 doi: 10.1136/bmj-2022-071245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith H., Anderson F., Raphael H., Maslin P., Crozier S., Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women--a population-based, randomized, double-blind, placebo-controlled trial. Rheumatol. 2007;46:1852–1857. doi: 10.1093/rheumatology/kem240. [DOI] [PubMed] [Google Scholar]
- 52.Sempos C.T., Heijboer A.C., Bikle D.D., Bollerslev J., Bouillon R., Brannon P.M., et al. Vitamin D assays and the definition of hypovitaminosis D: results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018;84:2194–2207. doi: 10.1111/bcp.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atef S.H. Vitamin D assays in clinical laboratory: past, present and future challenges. J. Steroid. Biochem. Mol. Biol. 2018;175:136–137. doi: 10.1016/j.jsbmb.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Buckley J.P., Keil A.P., McGrath L.J., Edwards J.K. Evolving methods for inference in the presence of healthy worker survivor bias. Epidemiology. 2015;26:204–212. doi: 10.1097/EDE.0000000000000217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript will be made available upon reasonable request.