Abstract
Background and Objective
Systemic lupus erythematosus (SLE) is an autoimmune disease, with hydroxychloroquine being the main therapeutic agent for the treatment of SLE. This research explored the effects of hydroxychloroquine combined with low-dose aspirin on maternal and infant outcomes and cytokines of pregnant women with SLE.
Methods
Ninety pregnant women with SLE were divided into the hydroxychloroquine (HCQ) group (45 cases) and the hydroxychloroquine combined with low-dose aspirin (HCQASP) group (45 cases) by random number table. Patients in the HCQ group were treated with oral administration of hydroxychloroquine, while patients in the HCQASP group were treated with low-dose aspirin based on oral administration of hydroxychloroquine. Pregnancy outcomes, fetal outcomes, and cytokine levels were statistically analyzed.
Results
The HCQASP group had a significantly higher proportion of full-term pregnancies and a significantly lower proportion of hypertension, prematurity, and pregnancy loss than the HCQ group. Neonates in the HCQASP group also had significantly higher birth weights and Apgar scores and a significantly lower proportion of neonatal asphyxia than the HCQ group. After treatment, the HCQASP group had significantly higher interleukin (IL-2) and interferon (IFN)-γ levels and significantly lower IL-4 and IL-10 levels than the HCQ group.
Conclusion
Hydroxychloroquine combined with low-dose aspirin can effectively improve the pregnancy outcomes of pregnant women with SLE by affecting the levels of T helper (Th) 2 and Th1 cytokines.
Key Points
| Hydroxychloroquine combined with low-dose aspirin can effectively improve the pregnancy outcomes of pregnant women with systemic lupus erythematosus by affecting the levels of T helper (Th) 2 and Th1 cytokines. |
| The current findings provide a reference for the clinical improvement of maternal and infant outcomes in pregnant patients with systemic lupus erythematosus. |
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by abnormalities of the immune system [1]. Patients with SLE require long-term or even lifelong medication, while pregnant patients with SLE are prone to adverse pregnancy outcomes [2]. Changes in estrogen and prolactin levels in pregnant women lead to self-reactive cellular dysfunction, making the condition prone to recurrence or worsening [3]. Vasculitis due to maternal activity can cause poor blood supply to the placenta, affecting fetal blood circulation and leading to fetal growth retardation [4]. Therefore, how to control SLE during pregnancy and how to prevent adverse outcomes are common clinical problems that are faced.
Hydroxychloroquine is widely used in rheumatic immune diseases and is the main therapeutic agent for SLE. Hydroxychloroquine can stabilize the disease, reduce relapses, prevent systemic damage, improve the prognosis, and reduce the dosage of hormones and immunosuppressants [5]. Cord blood hydroxychloroquine concentrations are the same as maternal concentrations [6]. Since the introduction of hydroxychloroquine in pregnancy, the effects of hydroxychloroquine on both pregnancy and fetal outcomes have also been investigated.
The physiological hypercoagulable state of pregnancy is crucial in self-protection of the body and may also induce pre-eclampsia, fetal growth restriction, and venous thromboembolism [7]. In pregnant patients with SLE, the effects of pregnancy superimposed on the pathophysiology of SLE may have a higher risk of thrombosis, often with adverse pregnancy outcomes [8]. Aspirin can reduce the degree of platelet activation, promote vasodilation, improve blood supply to the placenta, and prevent microthrombosis [9].
Individual T helper (Th) 1/Th2 balance is one of the critical determinants for histopathology of lupus nephritis. The development of SLE is related to the level of cytokines secreted by Th2 and Th1 cells [10]. Th1 cells mainly secrete interleukin (IL)-2 and interferon (IFN)-γ, mediating cellular immunity and delayed hypersensitivity, while Th2 cells mainly secrete IL-4, IL-5, IL-6 and IL-10, which mainly act on B cells, stimulating B-cell activation and secretion of immunoglobulins, and mediating humoral immunity [11]. Th1 cytokines play an essential role in the development of diffuse proliferative lupus nephritis (DPLN), while Th2 cytokines play an essential role in the development of membranous lupus nephritis (MLN) [12]. Aspirin can effectively regulate the balance of Th1/Th2 cytokines [13].
This study investigated the effects of hydroxychloroquine combined with low-dose aspirin on the change in disease, pregnancy outcomes, and fetal outcomes in pregnant patients with SLE, with the aim of providing effective clinical drug treatment options to reduce disease activity, decrease obstetric complications, and improve poor pregnancy outcomes.
Methods
Participants
Ninety pregnant patients with SLE were recruited. A random number table was used to divide the 90 eligible patients into a control group (n = 45) and an observation group (n = 45). The study was approved by the Medical Ethics Committee of The Fourth Hospital of Shijiazhuang, and all patients agreed to cooperate by signing the informed consent form.
Inclusion criteria: All patients met the SLE classification criteria established by the European Alliance of Associations for Rheumatology/American College of Rheumatology (EULAR/ACR) [2019], and those with suspected SLE were diagnosed with a total score of ≥10; for those with atypical clinical presentation or diagnostic difficulties, rheumatologists were invited, or consulted, to assist in the diagnosis; women in early pregnancy; patients with a stable condition (Systemic Lupus Erythematosus Disease Activity Index [SLEDAI] score of 0–4) for 6 months or more, with no cytotoxic immunosuppressant treatment for 6 months or more, and glucocorticoid use at a prednisone dose of ≤ 10 mg/day (or equivalent); no significant organ damage, renal disease in the inactive phase, negative anti-double-strand DNA (dsDNA) antibodies by immunological examination, and normal serum complement C3 level; and no serious diseases of other systems.
Exclusion criteria: Pregnant patients with other severe disease (e.g., malignancy, trauma, consciousness, mental disorders); patients with a history of smoking and use of drugs that severely affect pregnancy outcome (e.g., cyclophosphamide, methotrexate) before and during pregnancy; patients with a family history of adverse pregnancy outcome; and patients with incomplete clinical data.
The control group (hydroxychloroquine [HCQ]) received hydroxychloroquine tablets at a dose of 0.2 g/day two times daily and placebo. Patients received low-dose prednisone depending on the condition of the SLE or related complications in mid to late pregnancy. Hydroxychloroquine treatment was continued, and placebo was discontinued until the 35th full week of gestation.
In the observation group (hydroxychloroquine combined with low-dose aspirin [HCQASP]), hydroxychloroquine tablets were administered at a dose of 0.2 g/day twice and aspirin enteric tablets at a dose of 50 mg/day. Patients received low-dose prednisone depending on the condition of the SLE or related complications in mid to late pregnancy. Hydroxychloroquine was administered continuously, and aspirin was discontinued until 35 full weeks of pregnancy.
Analysis
The SLEDAI score (≥ 15, severely active; 10–14, moderately active; 5–9, mildly active; 0–4, stable disease) was employed to determine the activity of SLE.
The Apgar score is a common tool used for evaluating newborns immediately after delivery. A healthy neonate scores 10 points, neonates with mild asphyxia score ≤ 7 points, and neonates with severe asphyxia score ≤ 4 points.
Fasting venous blood was collected at the time of enrollment and after treatment (35 full weeks of pregnancy, or delivery or miscarriage), centrifuged to obtain a serum sample for storage in a refrigerator at − 20 °C. Serum cytokine levels were measured by enzyme-linked immunosorbent assay (ELISA). The ELISA kit was purchased from Abcam (Cambridge, UK). Anticardiolipin antibodies (ACAs) and anti-β2-glycoprotein I antibodies (ab2GPI) were also assessed by ELISA (QUANTA Lite).
Statistical Analysis
SPSS 22.0 software (IBM Corporation, Armonk, NY, USA) was used for statistical analysis. Data are expressed as n (%) or mean ± standard deviation. P-values for each group were derived from the Mann–Whitney test or two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests. Fisher’s exact test was used for assessing the distribution of observations or phenomena between the two groups. A p-value <0.05 was considered statistically significant.
Results
Table 1 shows the demographic and clinical characteristics of pregnant patients with SLE. There were no significant differences in age, pre-pregnancy body mass index (BMI), SLE duration, history of abortion, proportion of primipara or multiparous women, ACAs, aβ2GPI antibodies, lupus anticoagulant (LAC), and SLEDAI at admission between the two groups. There was also no significant difference in serum Th1/Th2 cytokine levels between the two groups.
Table 1.
Demographic and clinical characteristics of pregnant patients with systemic lupus erythematosus treated with hydroxychloroquine or hydroxychloroquine combined with low-dose aspirin
| Characteristics | Study group | p value | |
|---|---|---|---|
| HCQ [n = 45] |
HCQASP [n = 45] |
||
| Maternal age, years | 28.3 ± 3.9 | 28.6 ± 4.2 | 0.316 |
| BMI before pregnancy, kg/m2 | 21.8 ± 3.6 | 21.4 ± 3.9 | 0.283 |
| SLE duration, months | 59.4 ± 15.1 | 61.5 ± 16.7 | 0.117 |
| Parity | |||
| Primipara | 34 (75.6) | 31 (68.9) | 0.638 |
| Multipara | 11 (24.4) | 14 (31.1) | |
| History of abortion | |||
| Yes | 10 (22.2) | 12 (26.7) | 0.807 |
| No | 35 (77.8) | 33 (73.3) | |
| ACA | |||
| + | 5 (11.1) | 7 (15.6) | 0.758 |
| − | 40 (88.9) | 38 (84.4) | |
| aβ2GPI | |||
| + | 8 (17.8) | 6 (13.3) | 0.772 |
| − | 37 (82.2) | 39 (86.7) | |
| LAC | |||
| + | 3 (6.7) | 8 (17.8) | 0.197 |
| − | 42 (93.3) | 37 (82.2) | |
| Anti-Ro/SSA antibodies | |||
| + | 9 (20) | 13 (28.9) | 0.462 |
| − | 36 (80) | 32 (71.1) | |
| SLEDAI at admission | 2.47 ± 1.08 | 2.58 ± 1.11 | 0.813 |
| Serum IL-2 at admission | 9.57 ± 3.43 | 9.63 ± 3.87 | 0.998 |
| Serum IFN-γ at admission | 14.69 ± 4.28 | 13.95 ± 3.96 | 0.834 |
| Serum TNF-α at admission | 43.24 ± 12.09 | 39.69 ± 13.42 | 0.568 |
| Serum IL-4 at admission | 22.21 ± 8.35 | 23.46 ± 9.72 | 0.924 |
| Serum IL-6 at admission | 52.76 ± 17.52 | 55.64 ± 16.58 | 0.862 |
| Serum IL-10 at admission | 35.46 ± 14.18 | 38.02 ± 13.05 | 0.828 |
Data are expressed as n (%) or mean ± SD
P-values for each group were derived from the Mann–Whitney test. Fisher’s exact test was used for assessing the distribution of observations or phenomena between the two groups
HCQ hydroxychloroquine, HCQASP hydroxychloroquine combined with low-dose aspirin, BMI body mass index, SLE systemic lupus erythematosus, ACA anticardiolipin antibodies, SLEDAI Systemic Lupus Erythematosus Disease Activity Index, aβ2GPI anti-β2-glycoprotein I antibodies, LAC lupus anticoagulant, SD standard deviation, IL interleukin, IFN interferon, TNF tumor necrosis factor
Table 2 shows the pregnant outcomes of patients. No significant differences were observed in the proportion of gestational diabetes mellitus, SLE recrudesce, pre-eclampsia, vaginal delivery or cesarean section, and premature rupture of membranes between the two groups. Patients in the HCQASP group had a significantly higher proportion of full-term pregnancies and a significantly lower proportion of hypertension, prematurity, and pregnancy loss than those in the HCQ group.
Table 2.
Comparisons of pregnant outcomes between pregnant patients with systemic lupus erythematosus treated with hydroxychloroquine or hydroxychloroquine combined with low-dose aspirin
| Characteristics | Study group | p value | |
|---|---|---|---|
| HCQ (n = 45) | HCQASP (n = 45) | ||
| Gestational diabetes mellitus | 9 (20%) | 4 (8.9%) | 0.229 |
| Hypertension in pregnancy | 8 (17.8%) | 1 (2.2%) | 0.030 |
| Pre-eclampsia | 5 (11.1%) | 0 (0%) | 0.056 |
| SLE recrudesce | |||
| Mild | 5 (11.1%) | 6 (13.3%) | 0.948 |
| Moderate | 4 (8.9%) | 5 (11.1%) | |
| Severe | 4 (8.9%) | 3 (6.7%) | |
| Full-term | 22 (48.9%) | 34 (75.5%) | 0.031 |
| Prematurity | 15 (33.3%) | 8 (17.8%) | |
| Pregnancy loss | 8 (17.8%) | 3 (6.7%) | |
| Delivery | |||
| Vaginal delivery | 3/37 (8.1%) | 5/42 (11.9%) | 0.717 |
| Caesarian section | 34/37 (91.9%) | 37/42 (88.1%) | |
| Premature rupture of membranes | 6 (13.3%) | 3 (6.7%) | 0.485 |
Data are expressed as n (%)
P-values for each group were derived from the Mann–Whitney test. Fisher’s exact test was used for assessing the distribution of observations or phenomena between the two groups
HCQ hydroxychloroquine, HCQASP hydroxychloroquine combined with low-dose aspirin, SLE systemic lupus erythematosus, SD standard deviation
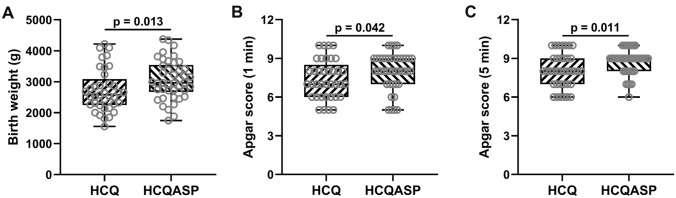
As shown in Table 3, there were no significant differences in fetal survival, fetal growth restriction, and fetal distress between the two groups. Neonates in the HCQASP group had significantly higher birth weights and Apgar scores (1 or 5 min) and a significantly lower proportion of neonatal asphyxia than the HCQ group (Table 3 and Fig. 1a–c).
Table 3.
Comparisons of fetal outcomes between pregnant patients with systemic lupus erythematosus treated with hydroxychloroquine or hydroxychloroquine combined with low-dose aspirin
| Characteristics | Study group | p value | |
|---|---|---|---|
| HCQ [n = 45] |
HCQASP [n = 45] |
||
| Fetal survival | 37 (82.2) | 42 (93.3) | 0.197 |
| Birth weight (kg) | 2.72 ± 0.69 | 3.06 ± 0.64 | 0.013 |
| Apgar score (1 min) | 7.41 ± 1.49 | 8.02 ± 1.39 | 0.042 |
| Apgar score (5 min) | 8.05 ± 1.33 | 8.76 ± 0.96 | 0.011 |
| Fetal growth restriction | 7/37 (18.9) | 4/42 (9.5) | 0.331 |
| Fetal distress | 6/37 (16.2) | 4/42 (9.5) | 0.502 |
| Neonatal asphyxia | 6/37 (16.2) | 1/42 (2.4) | 0.047 |
Data are expressed as n (%) or mean ± SD
P-values for each group were derived from the Mann–Whitney test. Fisher’s exact test was used for assessing the distribution of observations or phenomena between the two groups
HCQ hydroxychloroquine, HCQASP hydroxychloroquine combined with low-dose aspirin, SD standard deviation
Fig. 1.
Comparisons of (a) birth weight, (b) Apgar score (1 min), and (c) Apgar score (5 min) between pregnant patients with SLE treated with HCQ (n = 37) or HCQASP (n = 42). P-values were derived from the Mann–Whitney test. SLE systemic lupus erythematosus, HCQ hydroxychloroquine, HCQASP hydroxychloroquine combined with low-dose aspirin
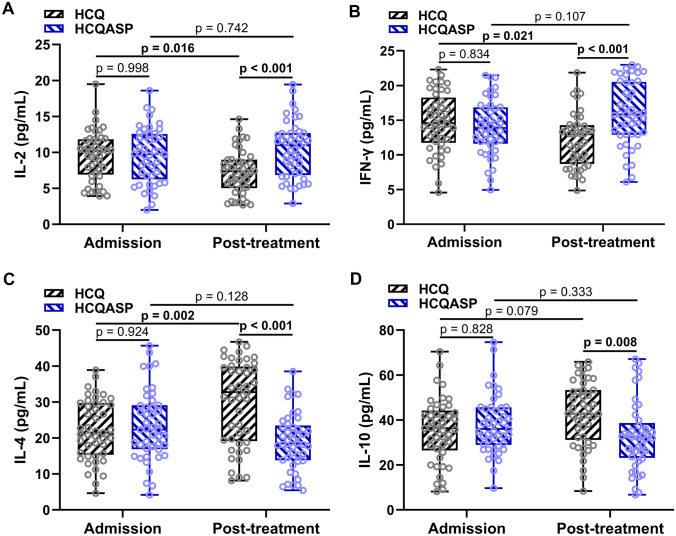
In the HCQ group, the IL-2 and IFN-γ levels were significantly decreased and the IL-4 level was significantly increased after treatment (Fig. 2a–d). However, in the HCQASP group, no cytokines showed a significantly altered level after treatment (Fig. 2a–d). After treatment, patients in the HCQASP group had significantly higher IL-2 and IFN-γ levels and significantly lower IL-4 and IL-10 levels than those in the HCQ group (Fig. 2a–d).
Fig. 2.
Comparisons of (a) serum IL-2, (b) IFN-γ, (c) IL-4, and (d) IL-10 levels at admission and after treatment between pregnant patients with SLE treated with HCQ (n = 45) or HCQASP (n = 45). P-values were derived from two-way ANOVA followed by Tukey’s multiple comparison tests. IL interleukin, IFN interferon, SLE systemic lupus erythematosus, HCQ hydroxychloroquine, HCQASP hydroxychloroquine combined with low-dose aspirin, ANOVA analysis of variance
Discussion
Epidemiological studies on SLE have shown that patients have significant sex, age, race, and regional differences. The incidence and prevalence are significantly higher in female patients than in male patients [14]. Factors contributing to sex differences include immune regulation by sex hormones, genetic coding on the X chromosome, epigenetic modifications, and pregnancy-specific immune phenomena [15]. SLE and pregnancy are mutually unfavorable factors [16]. The 30–40% increase in blood volume during pregnancy increases the burden on the heart and kidneys, making it easier to induce SLE pathogenesis [8]. SLE patients need to take medication long-term or even lifelong, and the pros and cons of medication use during pregnancy must be weighed up carefully.
The ‘2020 Chinese Guidelines for the Diagnosis and Treatment of Systemic Lupus Erythematosus’ published by the Chinese Rheumatology Association recommended hydroxychloroquine as the main treatment for SLE [17]. Hydroxychloroquine can stabilize the disease, reduce relapses, prevent systemic damage, improve the prognosis of lupus nephritis, and reduce the dosage of hormones and immunosuppressants [5]. It has been found that the cord blood hydroxychloroquine concentration is the same as the maternal concentration, i.e. maternal and fetal exposure to similar hydroxychloroquine concentrations during pregnancy [6]. The clinical efficacy and safety of hydroxychloroquine are high, and studies suggest that hydroxychloroquine is well tolerated in pregnant women and less toxic to the fetus, which significantly improves the prognosis of the newborn [18].
Patients with SLE often have a hypercoagulable state of blood and an imbalance in the body’s coagulation-fibrinolytic system, which further progresses to form microthrombi, leading to damage to various systems [19]. Patients with SLE in combination with pregnancy may be at higher risk of thrombosis with the effects of pregnancy superimposed on the pathophysiology of SLE, and often have adverse pregnancy outcomes [20]. In SLE patients, the enhanced intravascular coagulation, reduced placental blood flow, and decreased perfusion initiates a coagulation cascade that accelerates platelet activation, thereby increasing thromboxane and decreasing prostacyclin, which may increase the chance of placental thrombophilia [21, 22]. Aspirin, a cyclooxygenase (COX)-1) inhibitor, can adjust the balance of platelets, thromboxane A2 (TAX2), and prostacyclin to reduce platelet activation, promote vasodilation, improve placental blood supply, prevent microthrombosis, and reduce adverse pregnancy outcomes and obstetric complications [9]. The protective effect of low-dose acetylsalicylic acid (LDASA) in pre-eclampsia has been demonstrated in non-autoimmune, high-risk obstetric patients and patients with SLE with prior renal involvement and/or antiphospholipid antibodies (aPL) [23]. In pregnant patients with SLE without renal involvement, and who were aPL-negative, there is a low risk of severe obstetric complications, such as early pre-eclampsia. LDASA treatment does not provide a statistically significant advantage over these complications [23]. The 2020 ACR Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases suggests treatment with LDASA in all pregnant women with SLE [24].
In this research, patients with SLE also had no renal involvement. Comparison of pregnancy outcomes between the HCQ and HCQASP groups showed no significant difference in gestational diabetes mellitus and pre-eclampsia, but combination therapy with aspirin significantly reduced the rate of hypertension in pregnancy. There were no significant differences in the recurrence of SLE during pregnancy or in the activity (SLEDAI score) at the time of SLE recurrence. However, combination therapy with aspirin significantly improved pregnancy outcomes, increasing the proportion of full-term deliveries and reducing prematurity and pregnancy loss. Finally, no significant differences were found in the occurrence of premature rupture of membranes and the mode of delivery was also not significantly different.
We also compared fetal outcomes in the two groups. Thirty-seven fetuses in the HCQ group survived and 42 in the HCQASP group, but no significant differences were observed. Comparing the weight and Apgar score of the neonates in both groups, the combination of aspirin treatment significantly improved both their weight and Apgar score. In addition, there was a significant difference between the two groups in neonatal asphyxia, but not in fetal distress and fetal growth restriction.
The development of SLE is related to the Th2 and Th1 cytokine levels [11]. Estrogen and prolactin during pregnancy induce a shift of T1 cells towards T2 cells, which leads to an increased immune action of T2 cells in pregnant women, resulting in a cellular immune imbalance of the T1/T2 type [25, 26]. Usually, during the onset of SLE, T2 cells become the main guide, further aggravating the disease in SLE patients [10]. Elevated estrogen levels during pregnancy affect the phagocytic function of macrophages, leading to less easy clearance of immune complexes, which in turn accelerates the degree of SLE activity [27]. Compared with healthy subjects, SLE patients had significantly lower IL-2 and IFN-γ blood levels and significantly higher tumor necrosis factor (TNF)-α, IL-4, IL-6, and IL-10 levels [11]. Changes in Th1/Th2 cytokines are closely related to SLE disease activity. Aspirin can effectively regulate the balance of Th1/Th2 cytokines [13]. It can be speculated that hydroxychloroquine combined with aspirin may improve the disease by decreasing Th2 cytokines and increasing Th1 cytokines, which is in line with the correlation between the increase of Th2 cytokines and the decrease of Th1 cytokines and the disease activity of SLE.
SLE and pregnancy are mutually unfavorable factors. The patients we recruited were in a stable phase of disease at the time of recruitment (early pregnancy), and as the gestational weeks progressed, the patients would move from a stable phase to an active phase of disease. The patients’ serum IL-2 and IFN-γ levels decreased (Th1 cytokines), while their IL-4 and IL-10 levels increased (Th2 cytokines). We found this trend in both the HCQ and HCQASP groups, but aspirin supplementation significantly improved this trend compared with single HCQ dosing.
Conclusions
The present work in pregnant patients with SLE compared the differences in the effects of hydroxychloroquine alone and hydroxychloroquine combined with low-dose aspirin on pregnancy outcomes, and the effects on serum Th1/Th2 cytokines. Hydroxychloroquine combined with low-dose aspirin can effectively improve the pregnancy outcomes of pregnant women with SLE by affecting the levels of Th2 and Th1 cytokines. The results provide a reference for the clinical improvement of maternal and infant outcomes in pregnant patients with SLE.
Declarations
Funding
This study was supported by the Shijiazhuang Science and Technology Research and Development Plan (201200773).
Conflicts of interest
Na Zhang, Hong-Xia Zhang, Yu-Wei Li, and Yuan Li declare that they have no conflicts of interest in relation to this work.
Ethics approval
This study was approved by the Medical Ethics Committee of the Fourth Hospital of Shijiazhuang, and all patients agreed to cooperate by signing the informed consent form.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Data relating to this study can be obtained upon reasonable request to the corresponding author.
Code Availability
Not applicable.
Authors’ Contributions
NZ, H-XZ, Y-WL, and YL collected and analyzed the data and wrote the manuscript. YL conceived the idea for the study and obtained the funding.
Footnotes
Na Zhang and Hong-Xia Zhang contributed equally to this work.
References
- 1.Kiriakidou M, Ching CL. Systemic lupus erythematosus. Ann Intern Med. 2020;172:81–96. doi: 10.7326/AITC202006020. [DOI] [PubMed] [Google Scholar]
- 2.Moroni G, Ponticelli C. Pregnancy in women with systemic lupus erythematosus (SLE) Eur J Intern Med. 2016;32:7–12. doi: 10.1016/j.ejim.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Shopit A, Wang J. Biochemical and clinical predictors in pregnant women with antiphospholipid syndrome and systemic lupus erythematosus: comprehensive update. Arch Gynecol Obstet. 2021;304:1153–1160. doi: 10.1007/s00404-021-06178-5. [DOI] [PubMed] [Google Scholar]
- 4.Pagnoux C, Mahendira D, Laskin CA. Fertility and pregnancy in vasculitis. Best Pract Res Clin Rheumatol. 2013;27:79–94. doi: 10.1016/j.berh.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Ponticelli C, Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE) Expert Opin Drug Saf. 2017;16:411–419. doi: 10.1080/14740338.2017.1269168. [DOI] [PubMed] [Google Scholar]
- 6.Costedoat-Chalumeau N, Amoura Z, Duhaut P, Huong DL, Sebbough D, Wechsler B, Vauthier D, Denjoy I, Lupoglazoff JM, Piette JC. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases: a study of one hundred thirty-three cases compared with a control group. Arthritis Rheum. 2003;48:3207–3211. doi: 10.1002/art.11304. [DOI] [PubMed] [Google Scholar]
- 7.Sousa Gomes M, Guimaraes M, Montenegro N. Thrombolysis in pregnancy: a literature review. J Matern Fetal Neonatal Med. 2019;32:2418–2428. doi: 10.1080/14767058.2018.1434141. [DOI] [PubMed] [Google Scholar]
- 8.Jiang M, Wang Y, Fu Q, Lin S, Wu J, Di W. Preeclampsia risk prediction model for Chinese pregnant patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2020;72:1602–1610. doi: 10.1002/acr.24265. [DOI] [PubMed] [Google Scholar]
- 9.Lecarpentier E, Haddad B. Aspirin for the prevention of placenta-mediated complications in pregnant women with chronic hypertension. J Gynecol Obstet Hum Reprod. 2020;49:101845. doi: 10.1016/j.jogoh.2020.101845. [DOI] [PubMed] [Google Scholar]
- 10.Muhammad Yusoff F, Wong KK, Mohd Redzwan N. Th1, Th2, and Th17 cytokines in systemic lupus erythematosus. Autoimmunity. 2020;53:8–20. doi: 10.1080/08916934.2019.1693545. [DOI] [PubMed] [Google Scholar]
- 11.Talaat RM, Mohamed SF, Bassyouni IH, Raouf AA. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: correlation with disease activity. Cytokine. 2015;72:146–153. doi: 10.1016/j.cyto.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Miyake K, Akahoshi M, Nakashima H. Th subset balance in lupus nephritis. J Biomed Biotechnol. 2011;2011:980286. doi: 10.1155/2011/980286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M, Zhang P, Yu S, Zhou G, Lv J, Nallapothula D, Guo C, Wang Q, Singh RR. Heparin and aspirin combination therapy restores T-cell phenotype in pregnant patients with antiphospholipid syndrome-related recurrent pregnancy loss. Clin Immunol. 2019;208:108259. doi: 10.1016/j.clim.2019.108259. [DOI] [PubMed] [Google Scholar]
- 14.Gergianaki I, Bortoluzzi A, Bertsias G. Update on the epidemiology, risk factors, and disease outcomes of systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2018;32:188–205. doi: 10.1016/j.berh.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Margery-Muir AA, Bundell C, Nelson D, Groth DM, Wetherall JD. Gender balance in patients with systemic lupus erythematosus. Autoimmun Rev. 2017;16:258–268. doi: 10.1016/j.autrev.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Ponticelli C, Moroni G. Immunosuppression in pregnant women with systemic lupus erythematosus. Expert Rev Clin Immunol. 2015;11:549–552. doi: 10.1586/1744666X.2015.1033404. [DOI] [PubMed] [Google Scholar]
- 17.Chinese Rheumatology Assocation, National Clinical Research Center for Dermatologic and Immunologic Diseases, Chinese Systemic Lupus Erythematosus Treatment and Research Group Chinese guidelines for the diagnosis and treatment of systemic lupus erythematosus [in Chinese] Zhonghua Nei Ke Za Zhi. 2020;2020(59):172–185. doi: 10.3760/cma.j.issn.0578-1426.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Abd Rahman R, Min Tun K, Kamisan Atan I, Mohamed Said MS, Mustafar R, Zainuddin AA. New benefits of hydroxychloroquine in pregnant women with systemic lupus erythematosus: a retrospective study in a tertiary centre. Rev Bras Ginecol Obstet. 2020;42:705–711. doi: 10.1055/s-0040-1715140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira KS, Cicarini WB, Alves LCV, Loures CMG, Campos FMF, Santos LID, da Silva MVF, Guimaraes TMD, Toledo V, Reis EA, Neiva CLS, Consoli RV, de Padua PM, Carvalho MDG. Clin Chim Acta. 2019;490:107–112. doi: 10.1016/j.cca.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Bundhun PK, Soogund MZS, Huang F. Arterial/venous thrombosis, fetal loss and stillbirth in pregnant women with systemic lupus erythematosus versus primary and secondary antiphospholipid syndrome: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18:212. doi: 10.1186/s12884-018-1850-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patrono C, Ciabattoni G, Remuzzi G, Gotti E, Bombardieri S, Di Munno O, Tartarelli G, Cinotti GA, Simonetti BM, Pierucci A. Functional significance of renal prostacyclin and thromboxane A2 production in patients with systemic lupus erythematosus. J Clin Invest. 1985;76:1011–1018. doi: 10.1172/JCI112053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostensen M, Clowse M. Pathogenesis of pregnancy complications in systemic lupus erythematosus. Curr Opin Rheumatol. 2013;25:591–596. doi: 10.1097/BOR.0b013e328363ebf7. [DOI] [PubMed] [Google Scholar]
- 23.Tani C, Zucchi D, Haase I, Gerosa M, Larosa M, Cavagna L, et al. Impact of low-dose acetylsalicylic acid on pregnancy outcome in systemic lupus erythematosus: results from a multicentre study. Lupus Sci Med. 2022;9:e000714. doi: 10.1136/lupus-2022-000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, et al. 2020 American College of Rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Care Res (Hoboken). 2020;72:461–488. doi: 10.1002/acr.24130. [DOI] [PubMed] [Google Scholar]
- 25.Aris A, Lambert F, Bessette P, Moutquin JM. Maternal circulating interferon-gamma and interleukin-6 as biomarkers of Th1/Th2 immune status throughout pregnancy. J Obstet Gynaecol Res. 2008;34:7–11. doi: 10.1111/j.1447-0756.2007.00676.x. [DOI] [PubMed] [Google Scholar]
- 26.Piccinni MP, Raghupathy R, Saito S, Szekeres-Bartho J. Cytokines, hormones and cellular regulatory mechanisms favoring successful reproduction. Front Immunol. 2021;12:717808. doi: 10.3389/fimmu.2021.717808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjorkander S, Heidari-Hamedani G, Bremme K, Gunnarsson I, Holmlund U. Peripheral monocyte expression of the chemokine receptors CCR2, CCR5 and CXCR3 is altered at parturition in healthy women and in women with systemic lupus erythematosus. Scand J Immunol. 2013;77:200–212. doi: 10.1111/sji.12021. [DOI] [PubMed] [Google Scholar]