Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing corona virus disease 2019 (COVID-19) can infect multiple tissues, including endocrine organs, such as the pancreas, adrenal, thyroid, and adipose tissue. The main receptor for SARS-CoV-2, ACE2, is ubiquitously expressed in the cells of the endocrine organs and accordingly, the virus has been detected in various amounts in all endocrine tissues in post-mortem samples from COVID-19 patients. The infection with SARS-CoV-2 may directly lead to organ damage or dysfunction, such as hyperglycaemia or in rare cases, new-onset diabetes. Furthermore, an infection with SARS-CoV-2 may have indirect effects affecting the endocrine system. The exact mechanisms are not yet completely understood and have to be further investigated. Conversely, endocrine diseases may affect the severity of COVID-19 and emphasis has to be laid on reducing the prevalence, or enhance the treatment, of these often non-communicable diseases in the future.
Keywords: ACE2, COVID-19, SARS-CoV-2, endocrine, metabolic, autoimmune
Abbreviations: ACE2, angiotensin-converting enzyme 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; GRP78, glucose related protein 78; Nsps, non-structural proteins; DPP4, dipeptidyl peptidase 4; TMPRSS2, transmembrane serine protease 2; MERS, Middle East Respiratory Syndrome; NRP1, neuropilin 1; HMGB1, high mobility group box 1; HS, heparin sulfate; CTSL, cathepsin L
Introduction
SARS-CoV-2 enters the body via the respiratory system with the lung as the primary target. However, SARS-CoV-2 has a broad tissue tropism making COVID-19 a systemic disease [1]. The main receptor for SARS-CoV-2, the angiotensin-converting enzyme 2 (ACE2) and its co-receptor transmembrane serine protease 2 (TMPRSS2) are ubiquitously expressed in the body including endocrine organs, such as pancreas, thyroid, testis, ovary, adrenal glands and pituitary. Accordingly, either due to direct virus-induced damage or to indirect effects, the endocrine system is highly susceptible to an infection with SARS-CoV-2 [2]. Furthermore, endocrine diseases, in particular, metabolic diseases have been shown to negatively impact on COVID-19 morbidity and mortality. Therefore, in order to prevent severe COVID-19, a well-defined endocrine disease status and specifically good metabolic health, is of utmost importance.
In the current review, we describe the coronavirus SARS-CoV-2 and how it enters human cells via its main receptor, ACE2. We review the expression of ACE2 and other alternative receptors and mediators of cell entry in the endocrine system. Additionally, we discuss infection susceptibility of different endocrine tissues and how this may lead to new-onset of endocrine diseases. Furthermore, we discuss how endocrine diseases may predispose to a more severe course of COVID-19.
Coronaviruses
Coronaviruses belong to the Coronaviridae family in the order Nidovirales [3] and can be subdivided into four subfamilies, termed Alpha-, Beta-, Gamma-, and Delta-coronavirus. The host range of coronaviruses is very broad and spans several mammalian and avian hosts [4]. Coronaviruses are enveloped positive-sense single-stranded RNA viruses containing an RNA genome of approximately 30 kb. This genome encodes varying number of structural and non-structural proteins depending on the virus subtype. Subgenomic RNAs utilize the transcription and translation systems of the host to synthesize four structural proteins: spike (S), membrane (M), envelope (E), and nucleocapsid (N), as well as a polyprotein that is further cleaved into several non-structural proteins (Nsps) involved in genome transcription and replication [5], [6], [7].
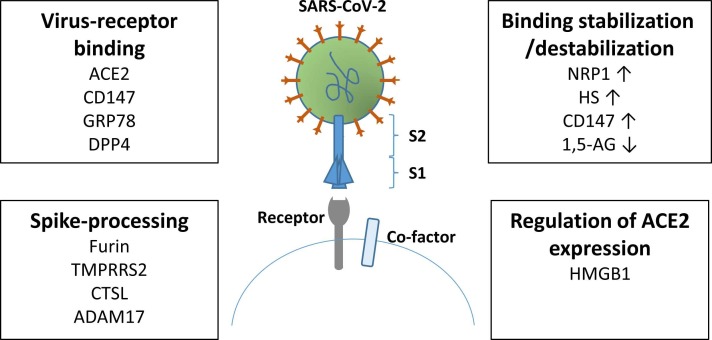
The entry into host cells is mediated by the transmembrane spike glycoprotein forming homotrimers protruding from the viral surface [8]. The spike protein consists of two functional subunits, S1 and S2. S1 is responsible for the binding to the host receptor, whereas S2 is responsible for the fusion of the viral and cellular membranes ( Fig. 1). In many coronaviruses, the spike protein is cleaved between the S1 and S2 subunits, which however, remain covalently bound to each other. This leads to conformational changes promoting membrane fusion with the host. Depending on the virus subtype, different domains within the S1 subunit are responsible for binding to their distinct entry receptors.
Fig. 1.
Factors influencing SARS-CoV-2 entry. When the SARS-CoV-2 spike protein binds to its receptor, the virus is internalized into the host cell. A number of recepors on the host cell have been shown to be involved in direct binding of the SARS-CoV-2 spike protein. However, before that the spike protein is primed by different proteases. The binding between SARS-CoV-2 and its receptor can be stabilized/destabilized. The expression of the main receptor for SARS-CoV-2, ACE2, is regulated by HMGB1.
SARS-CoV-1 and SARS-CoV-2
SARS-CoV-1 and SARS-CoV-2 belong to the Betacoronaviruses (subgenus Sarbecovirus). For SARS-CoV-1, first described in 2003, eight mRNAs encoding 12 different proteins have been described. SARS-CoV-2 has eight mRNAs giving rise to 14 overlapping open reading frames [9]. Furthermore, SARS-CoV-2 harbours a furin-cleavage site in the spike protein, which is not present in SARS-CoV-1 [10]. For priming of the spike protein, SARS-CoV-2 uses the serine protease TMPRSS2 [11].
SARS-CoV-2 has 79.6% homology with SARS-CoV-1 [12], whereas it shares 96.1% and 93.3% of its genome with the bat coronaviruses RmYN02 and RaTG13 [13], respectively, suggesting that a bat virus is the ancestor of SARS-CoV-2.
ACE2-mediated SARS-CoV-2 infection
The canonical receptor for SARS-CoV-2 is ACE2. ACE2 is part of the renin-angiotensin-aldosterone system (RAAS), where it mainly controls the generation of the vasodilator peptide angiotensin 1–7 from the vasoconstrictor peptide. Furthermore, ACE2 cleaves angiotensin I to angiotensin 1–9, which may be converted to angiotensin 1–7 by ACE [14].
ACE2 exists in two forms, a membrane-spanning cellular and an unbound soluble form [14]. Membrane-bound ACE2 (mACE2) constitutes the majority of ACE2; it contains a transmembrane domain anchoring the cleavable N-terminal domain. A membrane-bound protease (secretase) generates soluble ACE2 (sACE2) by enzymatic cleavage of mACE2. sACE2 appears in the circulation in very low concentrations. Both mACE2 and sACE2 are capable of binding the spike protein. Entry of SARS-CoV-2 into cells depends on binding of the spike protein to extracellular domains of cellular mACE2, which might explain why so far the spike protein, and especially the receptor binding domain, is the only known target structure for protective/neutralizing antibodies [15]. After binding to mACE2, the spike proteins are proteolytically activated by host cell proteases *[11], [14], *[16], resulting in fusion of the viral envelope with the plasma membrane or the endosome membrane of the host and viral entry into the cell. Following binding of SARS-CoV-2 to ACE2, the receptor is internalized by the infected cell leading to a distinct downregulation of ACE2 [17].
Alternatively, binding of the SARS-CoV-2 spike protein to ACE2 leads to an uptake of virus particles into endosomes. After an initial furin-cleavage, cathepsin L (CTSL), a pH-sensitive endosomal protease, primes the spike protein by cleaving it into smaller fragments. This leads to fusion of the viral envelope with the endosomal membrane and release of viral proteins and genome. This alternative route of virus entry is not as efficient as the cell membrane fusion. For SARS-CoV-1, cell membrane fusion was reported to be 100–1000 times more efficient than endosome fusion [18]. The mode of entry is dependent on the protease expression in the host cell [19].
Upon release of the viral genome into the host cell, translation of viral proteins and replication of the genome are established using the machinery of the host [20]. Furthermore, non-structural SARS-CoV-2 proteins inhibit the processing of cellular proteins. For example, Nsp1 shuts off host translation [21]. New virus particles are assembled in the cytoplasm and released into the extracellular space via exocytosis [22].
Since the COVID-19 pandemics commenced, SARS-CoV-2 has evolved by acquiring genomic mutations, resulting in a number of variants. Most of these variants, are among others, mutated in the receptor binding domain of the spike protein increasing the binding affinity to the receptor of the host thereby improving transmissibility and infectivity relative to the original strain [23].
ACE2 expression and SARS-CoV-2 infection in endocrine tissues
The cellular levels of ACE2 depend on numerous factors, such as clinical conditions, other RAAS components, and gene polymorphisms [24]. Furthermore, an age-dependent difference of ACE2 expression could account for the strikingly different COVID-19 disease course in adults and children [25], [26]. Upon inflammatory stress, the expression of the anti-inflammatory cytokine interferon (IFN)-alpha and the IFN-α pathway is activated [27]. IFNs in turn upregulate the expression of ACE2 [28], [29].
In the endocrine system, ACE2 is expressed in all organs to varying degrees [30]. However, it is still not clear whether a high or a low expression is beneficial in relation to COVID-19 [31]. An elevated level of ACE2 could potentially increase the susceptibility for infection; however, several studies showed that higher expression of ACE2 has an anti-inflammatory and protective function against severe acute lung failure [32], [33], [34].
Hypothalamus-pituitary-adrenal (HPA)/hypothalamus-pituitary-gonadal (HPG) axes
Both the hypothalamus and the pituitary, that as part of the HPA- and HPG-axes regulate the function of most endocrine glands, express ACE2 [30]. Furthermore, both ACE2 and TMPRSS2 were demonstrated to be expressed in the human adrenal glands [35], [36]. Specifically, a higher level of ACE2 protein expression was detected in stromal cells and in small capillaries of the adrenal glands *[37], [38], [39], whereas the expression in steroid-producing cells was lower [35], [39]. TMPRSS2 was mostly found in adrenocortical cells [35], [38]. Accordingly, SARS-CoV-2 could be detected in the adrenal glands of post-mortem COVID-19 patients [38], [39], [40], where SARS-CoV-2 was mostly found in scattered cell populations of adrenocortical cells and in endothelial cells [38], [39]. Notably, based on histological findings, the pituitary and adrenal glands do not appear predominantly affected in the course of COVID-19 [41]. Nevertheless, although cellular damage due to SARS-CoV-2 infections might not directly lead to adrenal insufficiency, COVID-19 associated haemorrhages and infarctions might, as histopathological studies on COVID-19 adrenal tissues revealed fibrin and microthrombi depositions in adrenal capillaries [39].
The testis has a high constitutive expression of ACE2 due to RAAS functions in Leydig cells [42], [43]. ACE2 and TMPRSS2 are mainly expressed in spermatocytes, spermatids, Sertoli cells, testicular tubuli and interstitium [44], [45]. Testicular mRNA levels of ACE2 and TMPRSS2 are shown to be increased in COVID-19 patients [45]. Accordingly, SARS-CoV-2 could be detected in testis in the seminiferous cells [45], [46].
In the ovaries, co-expression of ACE2 and TMPRSS2 was demonstrated mainly in oocytes and partially in granulosa cells [22]. The abundant expression of ACE2 is correlated with the generation of angiotensin 1–7, which stimulates ovarian follicle growth, oocyte maturation and ovulation [23]. ACE2 expression appears to be lower in the human ovaries than in the testis [44], which may indicate a higher susceptibility of male gonads for SARS-CoV-2 than female gonads.
Pancreas
Contradictory findings have been reported in the case of the pancreas. Some reports displayed no expression of ACE2 in the insulin-producing β-cells but in pancreatic epithelial cells only [47], [48], [49], whereas others demonstrated the presence of ACE2 in β-cells in a subset of COVID-19 patients [50], [51], *[52], [53]. Several studies have shown that insulin-producing β-cells are permissive to direct infection with SARS-CoV-2. This has been associated with degranulation [51], impaired insulin-secretion [51], [53], de- or transdifferentiation [51], [54], and cell death *[52], [53]. SARS-CoV-2 viral antigens have been described in both endothelial, exocrine and endocrine cells [52]. Patients with a high ACE2-expression in endothelial cells did not show a significantly higher level of SARS-CoV-2 virus particles in these cells, whereas an elevated expression of ACE2 in β-cells could be associated with higher numbers of SARS-CoV-2 viral particles [52]. Thus, it is not yet completely clear whether cells are infected because they have a higher expression of ACE2 or whether the expression of ACE2 is induced because of the infection. As mentioned above, this could be due to induction of IFN-α, which leads to increased expression of ACE2. Nevertheless, since the level of direct endocrine cell infection in the pancreas seems to be limited, this can probably not by itself explain the elevation in new-onset diabetes, which has been observed in a number of cases [55].
Adipose tissue
In adipose tissue, ACE2 is believed to regulate local levels of angiotensin II [56]. Diet and obesity have been shown to affect the expression of ACE2 in adipose tissue [57], as ACE2 levels were decreased after weight loss [58]. Similar to other organs, it remains unclear whether high levels of ACE2 in adipose tissue in relation to SARS-CoV-2 is advantageous or not. It seems that not only the abundance but also the functionality and shedding of the enzyme may be of importance [59].
SARS-CoV-2 could frequently be detected in adipose tissue from post-mortem COVID-19 patients *[27], [46], [60], [61]. Furthermore, it was demonstrated that adipocytes are susceptible to infection with SARS-CoV-2 in vitro [61], [62], [63], [64]. Therefore, it has been suggested that adipose tissue may also act as a reservoir for coronaviruses [65] as previously shown for influenza A [66].
Thyroid
Thyroidal ACE2 expression was detected in ∼90% of deceased COVID-19 patients, whereas normal thyroid tissue from patients without SARS-CoV-2 infection showed no ACE2 protein expression [67]. Results obtained in thyroid specimens from deceased COVID-19 patients showed that thyrocytes can be directly infected by SARS-CoV-2 as the SARS-CoV-2 genome could be detected in follicular cells. No clear correlation between the presence of viral genome and the expression of ACE2, TMPRSS2, and furin could be observed [68].
Other receptors and factors involved in SARS-CoV-2 infection
In addition to ACE2, several other factors were shown to mediate SARS-CoV-2 entry (Fig. 1). The proteases furin, TMPRSS2, and CTSL prime the spike protein for entry. The ADAM17 protease exerts proteolytic activity on ACE2, and the co-receptors neuropilin-1 (NRP1) and heparin sulfate (HS) assist the binding between the spike protein and ACE2. NRP1 is highly abundant in subcutaneous adipose tissue and decreased after weight loss [58]. Of note, all these factors increase the efficiency of viral entry. However, it is still not clear whether a higher or a lower expression of these factors is beneficial for health [31]. Another factor identified to be important for SARS-CoV-2 infection is high mobility group box 1 (HMGB1). The pro-viral HMGB1 regulates the expression of the ACE2 gene and is critical for viral entry [69].
Alternative receptors may also permit cell entry. One of these is CD147, also known as basigin or extracellular matrix metalloprotease inducer, belonging to the immunoglobulin superfamily. Expression of CD147 has been shown to increase the susceptibility to infection with SARS-CoV-2, whereas blockade or knockdown decreased this effect in most studies [70], [71], [72]. CD147 facilitates viral entry via endocytosis. Even though, CD147 does not bind ACE2, silencing of CD147 decreases the levels of ACE2 via a yet unknown mechanism [70]. Glucose related protein 78 (GRP78) promotes viral entry by working itself as a receptor or by stabilizing the binding between the spike protein and ACE2 [73], [74]. Both CD147 and GRP78 are tumour markers [75], [76], indicating why cancer patients have a higher risk of severe COVID-19 [77]. Furthermore, it was recently shown that the expression of GRP78 is increased in the jejunum of rats fed a high fat diet [78].
Dipeptidyl peptidase 4 (DPP4) is a target for several medications for type 2 diabetes and a receptor for the Middle East Respiratory Syndrome (MERS) coronavirus. Whether DPP4 is also an alternative receptor for SARS-CoV-2 is still debated as studies are showing contradictory results [79].
Endocrine diseases and COVID-19
In comparison to the general population, endocrine and in particular metabolic diseases augment the risk of a worse clinical outcome and increased mortality of COVID-19 [80]. Obesity alone accounts for 20% of COVID-19 hospitalizations, whereas obesity in combination with type 2 diabetes and hypertension is responsible for up to 60% of all COVID-19 hospitalizations and also to increased morbidity and mortality [81]. From the onset of the COVID-19 pandemic, it was further speculated whether COVID-19 may lead to new-onset diabetes [55]. Data arise that COVID-19 may lead to the development of type 1 diabetes [82]. In many cases an infection with SARS-CoV-2 led to a deterioration of prediabetes or pre-existing type 2 diabetes mellitus [80]. Most data available on new-onset diabetes in relation to COVID-19 are derived from case-reports, small single-centre clinical series, or non-clinical databases of incident diabetes at the population level. Therefore, to verify true new-onset diabetes in closer temporal relationship with an acute episode of COVID-19, a global registry CoviDIAB of newly diagnosed diabetes was set up in 2020 [83], and now (January 2023) first set of data starts to emerge [84]. Data collected from October 2020 to April 2022 from 61 hospitals in 25 countries reported 102 cases of new-onset diabetes. Among adults, 59% had type 2 diabetes and for the remaining 41%, the subtype was still unknown. Among children, there were two cases of new-onset type 1 diabetes. In 45% of patients with new-onset diabetes, hyperglycaemia persisted after the infection resolved. Further follow-up data beyond 3-months showed remission of diabetes in 18% and persistent diabetes in 82% of the cases. In the coming years, it is essential to follow-up on this and to verify whether new-onset diabetes resulting from infection with SARS-CoV-2, is permanent or not.
Other endocrine diseases, such as hypovitaminosis D and adrenal dysfunction may also impact susceptibility and severity of COVID-19 [85], [86], whereas few cases of primary adrenal insufficiency due to COVID-19 have been reported [87]. Despite the fact that many patients experience persistent fatigue after COVID-19 [88], these symptoms are most probably not accounted for by alterations in adrenal function [89]. On the other hand, many patients, who have recovered from COVID-19, might have received extended glucocorticoid treatment for 6 weeks or longer, which may give rise to iatrogenic secondary adrenal insufficiency [90].
Cases of atypical subacute thyroiditis, being negative for thyroid antibodies, have also been reported after COVID-19. Furthermore, autoimmune thyroiditis or Graves’ disease has been described [91]. In addition, thyroid disease has been reported as a consequence of the administration of vaccines against SARS-CoV-2 [92]. Future studies will be needed to assess whether this observed increase in the incidence of Graves‘ disease is sustained for a long period of time. Patients with controlled hypothyroidism and hyperthyroidism do not have a higher prevalence of COVID-19, nor do they have a worse prognosis when infected with the virus [92].
Other endocrine complications that have been reported after COVID-19 are changes in the menstrual cycle in some women. This is most likely not due to the actual infection but rather psychological stress [93]. On the other hand, occasional cases of reduced oocyte quality and ovarian function were reported [44]. In men, COVID-19 may cause a short-term decrease in fertility due to damaged testicular tissue and/or impaired spermatogenesis [94]. Furthermore, in comparison to healthy people, decreased testosterone/LH and FSH/LH ratios were detected in COVID-19 patients [95].
Potential mechanisms of endocrine disease
Most focus in relation to endocrine complications due to COVID-19 has been on the pancreas and the potential risk of developing new-onset diabetes after infection with SARS-CoV-2. However, as mentioned above, SARS-CoV-2 has been detected in all endocrine organs, which may result in dysfunction. Underlying mechanisms leading to endocrine diseases are not yet completely identified but accumulating evidence and theories are emerging ( Fig. 2). First, a direct infection may lead to tissue damage and reduced function. However, as the rate of infection in endocrine tissues often seems to be limited to smaller amounts of cells, other indirect mechanisms may be responsible as well. Since autoantibodies are repeatedly observed in patients with COVID-19 during acute disease, a mechanistic link may be autoimmunity caused by targeting of self-antigens due to impairment in the regulatory T cell response or molecular mimicry [96].
Fig. 2.
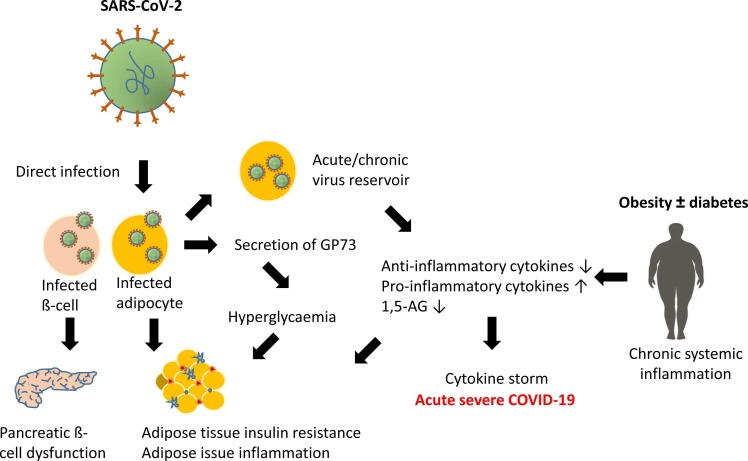
Plausible mechanisms of dysregulation in endocrine diseases and COVID-19. There are several reasons why endocrine and especially metabolic diseases may lead to severe progression of COVID-19. One possible cause is the chronic inflammatory responses in the internal adipose tissue. In adipose tissue with overly enlarged fat cells, there is mass production of pro-inflammatory cytokines. In addition, infiltration of immune cells into the adipose tissue is enhanced. These cells produce inflammatory substances themselves, leading to a chronic inflammatory state. Moreover, these inflammatory substances are strongly suspected of contributing to insulin resistance, a precursor of type 2 diabetes. Infection with SARS-CoV-2 under this condition is associated with a high risk of an immune system overreaction, a so-called cytokine storm, which is a potentially life-threatening immune system derailment. Conversely, direct infection with SARS-CoV-2 can lead to organ damage.
Patients with metabolic diseases, such as diabetes, obesity or non-alcoholic fatty acid liver disease, frequently exhibit a chronic subclinical low-grade inflammation [55], [97]. This leads to a decrease in the anti-inflammatory cytokines IL-10 and IL-1rα and an increase in the expression of the pro-inflammatory cytokines TNF-α, IL-6 and IL-1β, which may be further enhanced during an infection with SARS-CoV-2, thus leading to a cytokine storm. The pro-inflammatory cytokines inhibit insulin signalling [98]. Therefore, in severe COVID-19, the inflammatory response to SARS-CoV-2 may promote insulin resistance and endothelial dysfunction [80]. Furthermore, it has been suggested that the adipose tissue may serve as a virus reservoir that over a prolonged time might give rise to the production of pro-inflammatory cytokines from adipocytes and infiltrating macrophages [65]. In addition, insulin resistance may be worsened by the stress-induced glucogenic factor GP73, which is secreted from SARS-CoV-2-infected cells, and may lead to gluconeogenesis in hepatocytes [99].
A further explanation for the increased severity and mortality of COVID-19 in patients with metabolic dysregulations is the serum level of the glucose-like metabolite 1,5-anhydro-D-glucitol (1,5-AG), which is decreased in patients with diabetes. This factor binds directly to the SARS-CoV-2 spike protein and inhibits cellular entry [100]. This could explain why patients with diabetes are more prone to an infection with SARS-CoV-2.
Prevention
The most effective way to prevent severe COVID-19 is still vaccination despite the high amount of breakthrough infections, which are even higher in people with metabolic diseases [80]. Furthermore, in the light of the corona pandemic with a higher morbidity and mortality for those patients with endocrine diseases, the importance of an early diagnosis of these underlying diseases for preventing long-term complications after COVID-19 is emphasized. Blood glucose levels should be well controlled as patients with uncontrolled or poorly controlled blood glucose levels were shown to experience a worse disease course than those with normoglycaemia [101]. One way of achieving this would be more digitalization. A recent study with a digital health application that aimed to improve glycaemic control in patients with type 2 diabetes resulted in significant HbA1c reduction as well as a positive effect on metabolic parameters, indicating that such an application may be effective in supporting patient diabetes management by motivating patients to adopt healthier lifestyles and improving their self-management [102]. Preferentially, non-communicable metabolic diseases should be avoided from the first state via nutrition programs and physical exercise.
However, not only for metabolic diseases, but also for other endocrine diseases, a well-defined endocrine disease status is of utmost importance in order to prevent severe COVID-19.
Summary
The main receptor for SARS-CoV-2, ACE2, and other factors important for viral entry are ubiquitously expressed in endocrine organs. Accordingly, either due to direct virus-induced damage or to indirect effects, the endocrine system is highly affected by an infection with SARS-CoV-2. Consequently, there appears to be a vicious cycle between endocrine diseases and COVID-19. On one hand, endocrine complications, in particular metabolic diseases, may predispose for severe COVID-19 and mortality. On the other hand, COVID-19 may lead to onset of endocrine dysfunctions. The exact mechanisms behind this interplay are incompletely understood, but different hypotheses and evidence are accumulating. The prevalence of non-communicable endocrine diseases should preferably be decreased or patients should be well-treated to avoid severe COVID-19 courses.
Practice points
-
•
ACE2 and other factors responsible for infection of SARS-CoV-2 are ubiquitously expressed in cells of the endocrine system.
-
•
SARS-CoV-2 viral RNA have been detected in all endocrine organs.
-
•
Endocrine diseases, in particular metabolic diseases, may worsen the disease course of COVID-19.
-
•
COVID-19 may lead to new-onset diabetes.
-
•
Glucocorticoid treatment may lead to adrenal insufficiency.
Research agenda
-
•
Further investigation is warranted to confirm mechanisms of viral interference with glucose metabolism and/or glucocorticoid balance.
-
•
Underlying pathophysiology of new-onset diabetes caused by COVID-19 is not completely understood. Therefore, further investigation and collection of data is necessary.
-
•
It remains unclear whether new-onset diabetes due to infection with SARS-CoV-2 is permanent. Follow-up studies in this regard are very important.
-
•
Further studies are required to investigate the mechanisms behind the interplay between the endocrine system and COVID-19.
-
•
In the future, emphasis should be placed on avoiding the vicious circle between a viral infection and endocrine diseases. It is important to screen at an early stage and new strategies should be explored to reduce the prevalence of these diseases in the population.
Funding
This study was supported by the German Research Foundation (DFG, project no. 314061271 and project no. 288034826).
Declaration of competing interest
The authors declare no competing interest.
References
- [1].Stein S.R., Ramelli S.C., Grazioli A., et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612:758–763. doi: 10.1038/s41586-022-05542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puig-Domingo M., Yildiz B.O., Giustina A., et al. COVID-19 and endocrine and metabolic diseases. An updated statement from the European Society of Endocrinology. Endocrine. 2021;72:301–316. doi: 10.1007/s12020-021-02734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lefkowitz E.J., Dempsey D.M., Hendrickson R.C., et al. Virus taxonomy: the database of the International Committee on Taxonomy of Viruses (ICTV) Nucleic Acids Res. 2018;46:D708–D717. doi: 10.1093/nar/gkx932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamkiewicz K., Esquivel Gomez L.R., Kühnert D. In: Viral Fitness and Evolution: Population Dynamics and Adaptive Mechanisms. Domingo E., Schuster P., Elena S.F., editors. Springer International Publishing; Cham: 2023. Genome Structure, Life Cycle, and Taxonomy of Coronaviruses and the Evolution of SARS-CoV-2; pp. 305–339. [Google Scholar]
- 5.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison A.G., Lin T., Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020;41:1100–1115. doi: 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michel C.J., Mayer C., Poch O., et al. Characterization of accessory genes in coronavirus genomes. Virol J. 2020;17:131. doi: 10.1186/s12985-020-01402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Adv Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan W., Zheng Y., Zeng X., et al. Structural biology of SARS-CoV-2: open the door for novel therapies. Signal Transduct Target Ther. 2022;7:26. doi: 10.1038/s41392-022-00884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peacock T.P., Goldhill D.H., Zhou J., et al. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nat Microbiol. 2021;6:899–909. doi: 10.1038/s41564-021-00908-w. [DOI] [PubMed] [Google Scholar]
- [11].Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(271–80) doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu R., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H., Chen X., Hu T., et al. A novel bat coronavirus closely related to SARS-CoV-2 contains natural insertions at the S1/S2 cleavage site of the spike protein. Curr Biol. 2020;30(2196–203) doi: 10.1016/j.cub.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oz M., Lorke D.E. Multifunctional angiotensin converting enzyme 2, the SARS-CoV-2 entry receptor, and critical appraisal of its role in acute lung injury. Biomed Pharm. 2021;136 doi: 10.1016/j.biopha.2020.111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang K.A., Chen X., Mohapatra A., et al. Structural basis for a conserved neutralization epitope on the receptor-binding domain of SARS-CoV-2. Nat Commun. 2023;14:311. doi: 10.1038/s41467-023-35949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Walls A.C., Park Y.J., Tortorici M.A., et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(281–92) doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y., Zhu Q., Fox D.M. SARS-CoV-2 down-regulates ACE2 through lysosomal degradation. Mol Biol Cell. 2022;33:ar147. doi: 10.1091/mbc.E22-02-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuyama S., Ujike M., Morikawa S., et al. Protease-mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proc Natl Acad Sci USA. 2005;102:12543–12547. doi: 10.1073/pnas.0503203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padmanabhan P., Desikan R., Dixit N.M. Targeting TMPRSS2 and Cathepsin B/L together may be synergistic against SARS-CoV-2 infection. PLoS Comput Biol. 2020;16 doi: 10.1371/journal.pcbi.1008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].V'Kovski P., Kratzel A., Steiner S., et al. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schubert K., Karousis E.D., Jomaa A., et al. SARS-CoV-2 Nsp1 binds the ribosomal mRNA channel to inhibit translation. Nat Struct Mol Biol. 2020;27:959–966. doi: 10.1038/s41594-020-0511-8. [DOI] [PubMed] [Google Scholar]
- 22.Eymieux S., Uzbekov R., Rouillé Y., et al. Secretory vesicles are the principal means of SARS-CoV-2 egress. Cells. 2021:10. doi: 10.3390/cells10082047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J., Zhang H.X., Zhang J. Investigation on the interaction mechanism of different SARS-CoV-2 spike variants with hACE2: insights from molecular dynamics simulations. Phys Chem Chem Phys. 2023 doi: 10.1039/d2cp04349a. [DOI] [PubMed] [Google Scholar]
- 24.Coto E., Avanzas P., Gomez J. The renin-angiotensin-aldosterone system and coronavirus disease 2019. Eur Cardiol. 2021;16 doi: 10.15420/ecr.2020.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dioguardi M., Cazzolla A.P., Arena C., et al. Innate immunity in children and the role of ACE2 expression in SARS-CoV-2 infection. Pedia Rep. 2021;13:363–382. doi: 10.3390/pediatric13030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.South A.M., Brady T.M., Flynn J.T. ACE2 (Angiotensin-Converting Enzyme 2), COVID-19, and ACE inhibitor and Ang II (Angiotensin II) receptor blocker use during the pandemic: the pediatric perspective. Hypertension. 2020;76:16–22. doi: 10.1161/HYPERTENSIONAHA.120.15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Basolo A., Poma A.M., Bonuccelli D., et al. Adipose tissue in COVID-19: detection of SARS-CoV-2 in adipocytes and activation of the interferon-alpha response. J Endocrinol Invest. 2022;45:1021–1029. doi: 10.1007/s40618-022-01742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennighausen L., Lee H.K. Activation of the SARS-CoV-2 receptor Ace2 through JAK/STAT-dependent enhancers during pregnancy. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.108199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sajuthi S.P., DeFord P., Li Y., et al. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat Commun. 2020;11:5139. doi: 10.1038/s41467-020-18781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lazartigues E., Qadir M.M.F., Mauvais-Jarvis F. Endocrine Significance of SARS-CoV-2's Reliance on ACE2. Endocrinology. 2020:161. doi: 10.1210/endocr/bqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oz M., Lorke D.E., Kabbani N. A comprehensive guide to the pharmacologic regulation of angiotensin converting enzyme 2 (ACE2), the SARS-CoV-2 entry receptor. Pharm Ther. 2021;221 doi: 10.1016/j.pharmthera.2020.107750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imai Y., Kuba K., Rao S., et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigues Prestes T.R., Rocha N.P., Miranda A.S., et al. The anti-inflammatory potential of ACE2/Angiotensin-(1-7)/Mas receptor axis: evidence from basic and clinical research. Curr Drug Targets. 2017;18:1301–1313. doi: 10.2174/1389450117666160727142401. [DOI] [PubMed] [Google Scholar]
- 34.Verdecchia P., Cavallini C., Spanevello A., et al. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao Y., Xu B., Guan W., et al. The adrenal cortex, an underestimated site of SARS-CoV-2 infection. Front Endocrinol. 2020;593179:11. doi: 10.3389/fendo.2020.593179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou L., Niu Z., Jiang X., et al. SARS-CoV-2 targets by the pscRNA profiling of ACE2. TMPRSS2 Furin Proteases Isc. 2020;23 doi: 10.1016/j.isci.2020.101744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hikmet F., Mear L., Edvinsson A., et al. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16 doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanczkowski W., Evert K., Stadtmuller M., et al. COVID-19 targets human adrenal glands. Lancet Diabetes Endocrinol. 2022;10:13–16. doi: 10.1016/S2213-8587(21)00291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paul T., Ledderose S., Bartsch H., et al. Adrenal tropism of SARS-CoV-2 and adrenal findings in a post-mortem case series of patients with severe fatal COVID-19. Nat Commun. 2022;13:1589. doi: 10.1038/s41467-022-29145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong D.W.L., Klinkhammer B.M., Djudjaj S., et al. Multisystemic cellular tropism of SARS-CoV-2 in autopsies of COVID-19 patients. Cells. 2021:10. doi: 10.3390/cells10081900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fitzek A., Gerling M., Puschel K., et al. Post-mortem histopathology of pituitary and adrenals of COVID-19 patients. Leg Med. 2022;102045:57. doi: 10.1016/j.legalmed.2022.102045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edenfield R.C., Easley C.A. Implications of testicular ACE2 and the renin-angiotensin system for SARS-CoV-2 on testis function. Nat Rev Urol. 2022;19:116–127. doi: 10.1038/s41585-021-00542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verma S., Saksena S., Sadri-Ardekani H. ACE2 receptor expression in testes: implications in coronavirus disease 2019 pathogenesis†. Biol Reprod. 2020;103:449–451. doi: 10.1093/biolre/ioaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bechmann N., Maccio U., Kotb R., et al. COVID-19 infections in gonads: consequences on fertility? Horm Metab Res. 2022;54:549–555. doi: 10.1055/a-1891-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma X., Guan C., Chen R., et al. Pathological and molecular examinations of postmortem testis biopsies reveal SARS-CoV-2 infection in the testis and spermatogenesis damage in COVID-19 patients. Cell Mol Immunol. 2021;18:487–489. doi: 10.1038/s41423-020-00604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poma A.M., Bonuccelli D., Giannini R., et al. COVID-19 autopsy cases: detection of virus in endocrine tissues. J Endocrinol Invest. 2022;45:209–214. doi: 10.1007/s40618-021-01628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coate K.C., Cha J., Shrestha S., et al. SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in beta cells. Cell Metab. 2020;32(1028–40) doi: 10.1016/j.cmet.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kusmartseva I., Wu W., Syed F., et al. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. 2020;32(1041–51) doi: 10.1016/j.cmet.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu F., Long X., Zhang B., et al. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol. 2020;18(2128–30) doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fignani D., Licata G., Brusco N., et al. SARS-CoV-2 receptor angiotensin I-Converting enzyme type 2 (ACE2) is expressed in human pancreatic beta-cells and in the human pancreas microvasculature. Front Endocrinol. 2020;596898:11. doi: 10.3389/fendo.2020.596898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muller J.A., Gross R., Conzelmann C., et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3:149–165. doi: 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
- [52].Steenblock C., Richter S., Berger I., et al. Viral infiltration of pancreatic islets in patients with COVID-19. Nat Commun. 2021;12:3534. doi: 10.1038/s41467-021-23886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu C.T., Lidsky P.V., Xiao Y., et al. SARS-CoV-2 infects human pancreatic beta cells and elicits beta cell impairment. Cell Metab. 2021;33(1565–76) doi: 10.1016/j.cmet.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang X., Uhl S., Zhang T., et al. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metab. 2021;33(1577–91) doi: 10.1016/j.cmet.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steenblock C., Hassanein M., Khan E.G., et al. Diabetes and COVID-19: short- and long-term consequences. Horm Metab Res. 2022;54:503–509. doi: 10.1055/a-1878-9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupte M., Boustany-Kari C.M., Bharadwaj K., et al. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2008;295:R781–R788. doi: 10.1152/ajpregu.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gomez-Zorita S., Milton-Laskibar I., Garcia-Arellano L., et al. An overview of adipose tissue ACE2 modulation by diet and obesity. potential implications in COVID-19 infection and severity. Int J Mol Sci. 2021:22. doi: 10.3390/ijms22157975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soll D., Beer F., Spranger L., et al. Effects of weight loss on adipose and muscular neuropilin 1 mRNA expression in obesity: potential implication in SARS-CoV-2 infections? Obes Facts. 2022;15:90–98. doi: 10.1159/000520419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamthe D.D., Sarangkar S.D., Dalvi M.S., et al. Angiotensin converting enzyme 2 level and its significance in COVID-19 and other diseases patients. Eur J Clin Invest. 2023;53 doi: 10.1111/eci.13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez-Colon G.J., Ratnasiri K., Chen H., et al. SARS-CoV-2 infection drives an inflammatory response in human adipose tissue through infection of adipocytes and macrophages. Sci Transl Med. 2022;14:eabm9151. doi: 10.1126/scitranslmed.abm9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saccon T.D., Mousovich-Neto F., Ludwig R.G., et al. SARS-CoV-2 infects adipose tissue in a fat depot- and viral lineage-dependent manner. Nat Commun. 2022;13:5722. doi: 10.1038/s41467-022-33218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colleluori G., Graciotti L., Pesaresi M., et al. Visceral fat inflammation and fat embolism are associated with lung's lipidic hyaline membranes in subjects with COVID-19. Int J Obes. 2022;46:1009–1017. doi: 10.1038/s41366-022-01071-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reiterer M., Rajan M., Gómez-Banoy N., et al. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab. 2021;33(2174–88) doi: 10.1016/j.cmet.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zickler M., Stanelle-Bertram S., Ehret S., et al. Replication of SARS-CoV-2 in adipose tissue determines organ and systemic lipid metabolism in hamsters and humans. Cell Metab. 2022;34:1–2. doi: 10.1016/j.cmet.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ryan P.M., Caplice N.M. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity. 2020;28:1191–1194. doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maier H.E., Lopez R., Sanchez N., et al. Obesity increases the duration of influenza a virus shedding in adults. J Infect Dis. 2018;218:1378–1382. doi: 10.1093/infdis/jiy370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koehler V.F., Knosel T., Hasmann S.E., et al. Thyroidal ACE2 protein expression and thyroid function tests in patients with COVID-19: results from a retrospective case series and a prospective cohort study. Thyroid. 2022 doi: 10.1089/thy.2022.0229. [DOI] [PubMed] [Google Scholar]
- 68.Macedo S., Pestana A., Santos L., et al. Detection of SARS-CoV-2 infection in thyroid follicular cells from a COVID-19 autopsy series. Eur Thyroid J. 2022:11. doi: 10.1530/ETJ-22-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei J., Alfajaro M.M., DeWeirdt P.C., et al. Genome-wide CRISPR screens reveal host factors critical for SARS-CoV-2 infection. Cell. 2021;184(76–91) doi: 10.1016/j.cell.2020.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fenizia C., Galbiati S., Vanetti C., et al. SARS-CoV-2 entry: at the crossroads of CD147 and ACE2. Cells. 2021:10. doi: 10.3390/cells10061434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geng J., Chen L., Yuan Y., et al. CD147 antibody specifically and effectively inhibits infection and cytokine storm of SARS-CoV-2 and its variants delta, alpha, beta, and gamma. Signal Transduct Target Ther. 2021;6:347. doi: 10.1038/s41392-021-00760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang K., Chen W., Zhang Z., et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5:283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carlos A.J., Ha D.P., Yeh D.W., et al. The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection. J Biol Chem. 2021;296 doi: 10.1016/j.jbc.2021.100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ha D.P., Van Krieken R., Carlos A.J., et al. The stress-inducible molecular chaperone GRP78 as potential therapeutic target for coronavirus infection. J Infect. 2020;81:452–482. doi: 10.1016/j.jinf.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fu J., Song B., Du J., et al. Impact of BSG/CD147 gene expression on diagnostic, prognostic and therapeutic strategies towards malignant cancers and possible susceptibility to SARS-CoV-2. Mol Biol Rep. 2022:1–13. doi: 10.1007/s11033-022-08231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ge R., Kao C. Cell surface GRP78 as a death receptor and an anticancer drug target. Cancers. 2019:11. doi: 10.3390/cancers11111787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xia P., Dubrovska A. Tumor markers as an entry for SARS-CoV-2 infection? FEBS J. 2020;287:3677–3680. doi: 10.1111/febs.15499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Angelini G., Castagneto-Gissey L., Salinari S., et al. Upper gut heat shock proteins HSP70 and GRP78 promote insulin resistance, hyperglycemia, and non-alcoholic steatohepatitis. Nat Commun. 2022;13:7715. doi: 10.1038/s41467-022-35310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rangu R., Wander P.L., Barrow B.M., et al. Going viral in the islet: mediators of SARS-CoV-2 entry beyond ACE2. J Mol Endocrinol. 2022;69:R63–R79. doi: 10.1530/JME-21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steenblock C., Schwarz P.E.H., Ludwig B., et al. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9:786–798. doi: 10.1016/S2213-8587(21)00244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Hearn M., Liu J., Cudhea F., et al. Coronavirus disease 2019 hospitalizations attributable to cardiometabolic conditions in the United States: a comparative risk assessment analysis. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rahmati M., Keshvari M., Mirnasuri S., et al. The global impact of COVID-19 pandemic on the incidence of pediatric new-onset type 1 diabetes and ketoacidosis: a systematic review and meta-analysis. J Med Virol. 2022;94:5112–5127. doi: 10.1002/jmv.27996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rubino F., Amiel S.A., Zimmet P., et al. New-onset diabetes in Covid-19. N Engl J Med. 2020;383:789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rubino F., McIntyre R., Chai Z., et al. New-Onset Diabetes and COVID-19: Evidence from a Global Clinical Registry. 2023.
- 85.Chifu I., Detomas M., Dischinger U., et al. Management of patients with glucocorticoid-related diseases and COVID-19. Front Endocrinol. 2021;705214:12. doi: 10.3389/fendo.2021.705214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karampela I., Vallianou N., Magkos F., et al. Obesity, hypovitaminosis D, and COVID-19: the bermuda triangle in public health. Curr Obes Rep. 2022;11:116–125. doi: 10.1007/s13679-022-00471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jensterle M., Herman R., Janez A., et al. The relationship between COVID-19 and hypothalamic-pituitary-adrenal axis: a large spectrum from glucocorticoid insufficiency to excess-The CAPISCO international expert panel. Int J Mol Sci. 2022;23:7326. doi: 10.3390/ijms23137326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steenblock C., Walther R., Tselmin S., et al. Post COVID and apheresis - where are we standing? Horm Metab Res. 2022;54:715–720. doi: 10.1055/a-1945-9694. [DOI] [PubMed] [Google Scholar]
- 89.Clarke S.A., Phylactou M., Patel B., et al. Normal adrenal and thyroid function in patients who survive COVID-19 infection. J Clin Endocrinol Metab. 2021;106:2208–2220. doi: 10.1210/clinem/dgab349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kanczkowski W., Beuschlein F., Bornstein S.R. Is there a role for the adrenal glands in long COVID? Nat Rev Endocrinol. 2022;18:451–452. doi: 10.1038/s41574-022-00700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jimenez-Blanco S., Pla-Peris B., Marazuela M. COVID-19: a cause of recurrent Graves' hyperthyroidism? J Endocrinol Invest. 2021;44:387–388. doi: 10.1007/s40618-020-01440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rossetti C.L., Cazarin J., Hecht F., et al. COVID-19 and thyroid function: what do we know so far? Front Endocrinol. 2022;1041676:13. doi: 10.3389/fendo.2022.1041676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Phelan N., Behan L.A., Owens L. The impact of the COVID-19 pandemic on women's reproductive health. Front Endocrinol. 2021;642755:12. doi: 10.3389/fendo.2021.642755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Achua J.K., Chu K.Y., Ibrahim E., et al. Histopathology and ultrastructural findings of fatal COVID-19 infections on testis. World J Mens Health. 2021;39:65–74. doi: 10.5534/wjmh.200170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma L., Xie W., Li D., et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2021;93 doi: 10.1002/jmv.26259. 456-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Choutka J., Jansari V., Hornig M., et al. Unexplained post-acute infection syndromes. Nat Med. 2022;28 doi: 10.1038/s41591-022-01810-6. 911-23. [DOI] [PubMed] [Google Scholar]
- 97.Steenblock C., Hassanein M., Khan E.G., et al. Obesity and COVID-19: what are the consequences? Horm Metab Res. 2022;54:496–502. doi: 10.1055/a-1878-9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boucher J., Kleinridders A., Kahn C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014:6. doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wan L., Gao Q., Deng Y., et al. GP73 is a glucogenic hormone contributing to SARS-CoV-2-induced hyperglycemia. Nat Metab. 2022;4:29–43. doi: 10.1038/s42255-021-00508-2. [DOI] [PubMed] [Google Scholar]
- 100.Tong L., Xiao X., Li M., et al. A glucose-like metabolite deficient in diabetes inhibits cellular entry of SARS-CoV-2. Nat Metab. 2022;4:547–558. doi: 10.1038/s42255-022-00567-z. [DOI] [PubMed] [Google Scholar]
- 101.Laurenzi A., Caretto A., Molinari C., et al. No evidence of long-term disruption of glycometabolic control after SARS-CoV-2 infection. J Clin Endocrinol Metab. 2022;107:e1009–e1019. doi: 10.1210/clinem/dgab792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bretschneider M.P., Klasek J., Karbanova M., et al. Impact of a digital lifestyle intervention on diabetes self-management: a pilot study. Nutrients. 2022:14. doi: 10.3390/nu14091810. [DOI] [PMC free article] [PubMed] [Google Scholar]