Abstract
Objective
The objective of the study is to assess systemic immune markers and microbial factors related to periodontitis severity in people living with HIV.
Methods
Eighty people living with HIV (PLWH), who exhibited in the last two viral load measurements < 40 copies/mL, underwent full-mouth periodontal examinations and sub-gingival plaque sampling. Periodontitis was classified according to the CDC-AAP case definition. Inflammation, immune-activation, and immunosenescence markers were assessed, microbiological analyses were performed, and oral care routines and HIV characteristics were noted.
Results
From our group of PLWH, 42.5% and 57.5% suffered from moderate and severe periodontitis, respectively. Oral care habits did not differ between PLWH with moderate and severe periodontitis. Bacterial subgingival plaque loads were higher, and Porphyromonas gingivalis was more prevalent in PLWH with severe periodontitis than with moderate periodontitis (53% vs 7%, respectively). Mean C-reactive protein levels [CRP, 1.6 mg/L versus 0.8 mg/L, p = 0.020] and percentages of senescent CD28-CD57 + CD8 + T-cells in peripheral blood [16.5 versus 8.9, p = 0.035] were higher with severe periodontitis. Infection duration, CD4 count, CD4/CD8 ratio and type of antiretroviral therapy did not differ between both groups.
Conclusions
Periodontitis severity is related to increased prevalence of Porphyromonas gingivalis, elevated CRP levels, and higher frequencies of circulating CD8 + senescent cells in PLWH.
Supplementary information
The online version contains supplementary material available at 10.1007/s00784-022-04758-6.
Keywords: HIV, Periodontitis, Inflammation, Immune senescence, Age-related diseases
Introduction
People with human immunodeficiency virus (HIV) are known to have a higher prevalence of periodontitis compared to the general population [1, 2]. Periodontitis is a chronic disease that is linked to, amongst others, specific oral microorganisms [3]. Periodontitis is also associated with cardiovascular and systemic diseases [4] such as diabetes mellitus and rheumatoid arthritis, as well as with early ageing and age-related diseases [5–7]. In periodontitis, bacteria and bacterial products from the oral biofilm and inflammatory mediators, which are produced locally within the periodontal tissues, enter the blood. This is thought to result in a high inflammatory load reflected by, amongst others, higher levels of CRP [8]. Early periodontitis diagnosis would enable timely therapeutic intervention, reduce tooth loss and, potentially, lower the risk of age-related diseases [9]. Depending on whether a person is undergoing HIV treatment with combination antiretroviral therapy (cART), as well as which periodontal disease classification is used, the prevalence of periodontal diseases in people living with HIV (PLWH) varies between 30 and 100% [2, 10, 11]. PLWH who have periodontitis at the time of HIV diagnosis have a high risk of accelerated periodontal attachment loss over time, compared to seronegative controls [12]. It is not clear whether a HIV infection enhances periodontitis [1, 13].
PLWH are also at a higher risk of developing prematurely age-related diseases, e.g. cardiovascular diseases and diabetes mellitus [14–17]. The driver of this early ageing is believed to be the ongoing systemic immune activation and inflammation. Despite controlled suppression of viral replication under cART, the immune system of PLWH has been shown to age prematurely, leading to immunosenescence [18, 19]. Several studies noted a noticeable relative increase in senescent cell populations in PLWH compared to age-matched controls [20–22], which is linked to systemic immune activation and exhausted CD4+ T-cell function [23]. An increase in senescent cells can, in turn, amplify the inflammation [24, 25]. In PLWH, immune activation and systemic inflammation appear to be caused by the translocation of microbial products from the gut mucosal surfaces to the peripheral blood. Microbial translocation could, potentially, lead to systemic inflammation [26]. Current research is focusing almost exclusively on the large intestine as the site of microbial product translocation to the blood [27–29], while other mucosal sites, such as the oral cavity, have rarely been studied. Periodontitis could also be a potential source of microbial translocation to the blood.
A previous study could not find an association between the microbial translocation and immune senescence markers and the size of the periodontal inflammatory surface area (PISA) in PLWH [30]. A possible explanation for this alleged absence of association could be related to the PISA which reflects the surface area of the bleeding pocket epithelium. It is well-known, however, that periodontal pockets might, in themselves, play an important role since they could serve as an area of microbial translocation [31]. Also, it has not been possible to classify patients into groups according to the bleeding and severity of the periodontitis. Consequently, it is challenging to detect patterns and behaviours amongst patients according to their periodontal status. Hence, assessing periodontitis with a different classification system, which is frequently used in epidemiologic studies, that is based more extensively on pocket depth rather than bleeding pocket epithelium, might give more insight.
Thus, the aim of this study was to investigate which markers related to microbial translocation and immune senescence are related to the severity of the periodontitis in PLWH.
Methods
In a cross-sectional study, PLWH visiting the HIV outpatient clinic of the University Medical Center Groningen (UMCG), the Netherlands, were recruited. Inclusion criteria were age ≥ 18 years, presence of ≥ 6 teeth, use of combination antiretroviral therapy (cART) for ≥ 6 months and the last two viral load measurements yielded < 40 copies/mL. Exclusion criteria were a history of radiation therapy in the head and neck region and an inability to understand spoken or written Dutch or English. The ethics committee of the University Medical Center Groningen (METc number 2014/128) approved this study.
All the participants had to complete a validated health and oral care assessment questionnaire to identify any medical conditions that might be associated with periodontitis, e.g. diabetes and cardiovascular diseases [32–34]. Also, the participants had to complete a questionnaire about their oral health care habits (e.g., brushing frequency, type of toothbrush, interdental cleaning, etc.; additional Table 1). Finally, all the participants were asked if they could indicate with a number how important they rated their dental health on a VAS scale from 0 to 10 (0 indicated not important at all and 10 very important).
Table 1.
Patient characteristics of the 80 PLWH with moderate and severe periodontitis
| Total population | People with moderate periodontitis (n = 34) | People with severe periodontitis (n = 46) | p value | ||
|---|---|---|---|---|---|
| Male, N (%) | 69 (86.3) | 27 (79.4) | 42 (91.3) | 0.127 | |
| Mean age in years (SD) | 50.8 (11.4) | 50.6 (11.9) | 51.0 (11.1) | 0.891 | |
| Mean BMI (kg/m2) (SD) | 24.3 (3.0) | 23.9 (3.1) | 24.5 (2.8) | 0.317 | |
| Tobacco use, N (%) | Current smoker | 22 (27.5) | 6 (17.6) | 16 (34.8) | 0.227 |
| Never smoked | 32 (40) | 16 (47.1) | 16 (34.8) | ||
| Former smokers | 26 (32.5) | 12 (35.3) | 14(30.4) | ||
| People with diabetes mellitus, N (%) | 6 (7.5) | 2 (5.9) | 4 (8.7) | 0.637 | |
| History of cardiovascular disease, N (%) | 17 (23.6) | 8 (25.8) | 9 (22.0) | 0.703 | |
Periodontal assessment
The eligible participants underwent full-mouth periodontal examinations by an experienced dental hygienist (H.G.). The periodontium of all the teeth was examined with a periodontal probe (Williams probe 14 W, Hu-Friedy Mfg. Co., LLC, UK). Bleeding on probing (BoP), probing pocket depth (PPD) and clinical attachment level (CAL) were measured at six sites per tooth. The number of missing teeth was recorded. The presence of periodontitis was defined according to the CDC-AAP case definition of periodontitis surveillance for epidemiologic studies. Mild periodontitis was recorded for cases with ≥ 2 interproximal sites with a CAL ≥ 3 mm and ≥ 2 interproximal sites with a PPD ≥ 4 mm (not on the same tooth) or 1 site with a PPD ≥ 5 mm. The participants were classified as having moderate periodontitis in the presence of ≥ 2 interproximal sites with a CAL ≥ 4 mm (not on the same tooth) or ≥ 2 interproximal sites with a PPD ≥ 5 mm, also not on the same tooth. Severe periodontitis was recorded if the participants had ≥ 2 interproximal sites with clinical attachment loss, a CAL ≥ 6 mm, not on the same tooth, and ≥ 1 interproximal site with a PPD ≥ 5 mm [35–37].
Periodontal (sub-gingival) plaque samples were taken from each quadrant. The paper points were inserted to the depth of the pockets from the deepest bleeding pocket in each quadrant of the dentition and consequently left in place for 10 s and pooled in 2 ml of reduced transport fluid. Microbiological analysis of the samples was performed by the Oral Microbiology Laboratory of the UMCG, according to standard culturing protocol [38, 39]. The outcome variables of the microbiological analyses were the presence of the periodontal pathogens Aggregatibacter actinomycetemcomitans (Aa), Porphyromonas gingivalis (Pg), Prevotella intermedia (Pi), Tannerella forsythia (Tf), Parvimonas micra (Pm), Fusobacterium nucleatum (Fn), Campylobacter rectus (Cr), and the total anaerobic viable count (TVC) [40]. Participants who had used antibiotics in the last 3 months were excluded from the microbiological analysis.
Flow cytometric analyses of immune parameters
Venous blood was drawn to measure the absolute numbers of CD3+, CD4+ and CD8+ T-cells and measured using the MultiTest TruCount method with MultiTest reagents directed at CD45/3/4/8 (Becton Dickinson). To assess if periodontitis accelerates immune senescence, a variety of immune parameters identifying naïve (CD45R0−CCR7+ CD28+), senescent (CD28−CD57+) and activated (HLA-DR+ or CD38+) subsets within the CD4+ and CD8+ T-cells were employed. The viral load measurement was performed on EDTA plasma samples using the Abbott Real-Time HIV-1 assay. The participants were classified according to the ‘Revised Surveillance Case Definition for HIV-Infection’ in three stages, based on the CD4 T-lymphocyte count or the presence of opportunistic illness [41]. Additional information was collected about the mode of HIV-transmission, years of HIV-infection, type of cART and current CD4+/CD8+, CD4+ nadir levels.
ELISA assessment of soluble markers
Serum concentrations of the inflammation markers CRP, IL-6 and CXCL-10 and the microbial translocation and inflammation markers sCD14, LPS and sCD163 were assessed with ELISA. IL-6, LPS, CXCL-10 (R&D systems, Minneapolis, MN), sCD-14 and sCD-163 (Thermo Scientific, Waltham, MA) were measured according to the Dynex DS-2 system manufacturer’s protocol. High-sensitivity CRP levels were measured and determined using Cobas (Roche Diagnostics).
Missing data were completed from the patient chart and by searching the database of the national HIV-monitoring Foundation (SHM), which is the executive organization for the registration and monitoring of consenting participants with HIV-infection for care in the 27 Dutch HIV-treatment centres [42].
Statistical analysis
Q–Q plots were used to determine the distribution of the data. The qualitative and quantitative features of PLWH with severe periodontitis and PLWH with moderate periodontitis were compared by means of a Fisher exact/Chi-square test and independent samples t tests or a Mann–Whitney U test, when appropriate. All the analyses were performed using the SPSS software, version 23 (SPSS, Chicago, IL, USA).
Results
Patients
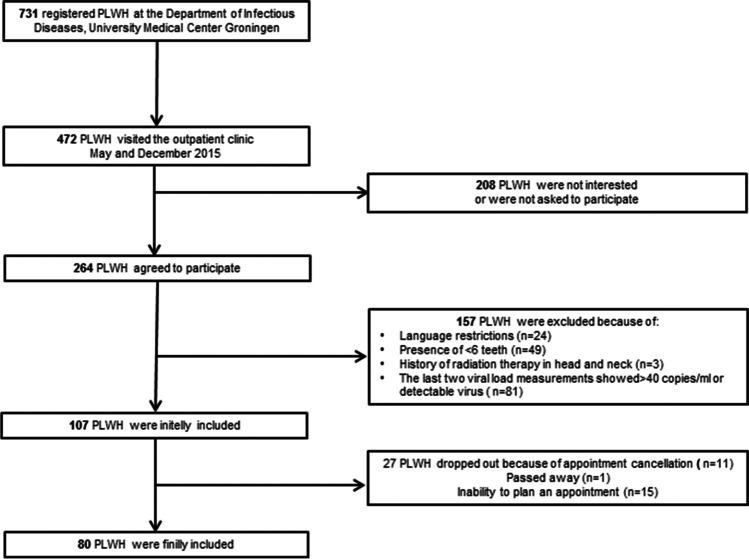
All the included participants’ characteristics are shown in Table 1. From the initial 472 who visited the outpatient clinic, 264 participants accepted the invitation to participate in this study. Of these, 184 participants had to be excluded for several reasons (Fig. 1), resulting in 80 participants being eligible for the study. All the included participants were diagnosed with moderate (42.5%) or severe (57.5%) periodontitis; no one was diagnosed with no periodontitis or mild periodontitis. There were no significant differences between the severe and moderate periodontitis groups regarding gender, age, diabetes, and cardiovascular diseases (Table 1).
Fig. 1.
Flow chart of patient inclusion
Periodontal measurements
The oral characteristics of the participants are described in Table 2. BoP was significantly higher in participants with severe periodontitis (46.7%), compared to moderate periodontitis (34.7%, p = 0.001). None of the reported oral care habits were significantly different between both groups. Pm was detected in all the participants with severe periodontitis and in 89.7% of the group with moderate periodontitis (p = 0.042). Pg was much more prevalent in the participants with severe periodontitis (21 participants, 45.7%) than in participants with moderate periodontitis (two participants, 5.9%, p < 0.001). Aa was only present in 4 participants (8.7%) with severe periodontitis and in none of the participants with moderate periodontitis (p = 0.780). The presence of Fn was not significantly different between the two groups. Pm was present in 95% and Fn in 94% of all the participants, making them the most frequently detected bacteria in both groups.
Table 2.
Oral care factors of the 80 PLWH with moderate and severe periodontitis
| Total population (n = 80) | People with moderate periodontitis (n = 34) | People with severe periodontitis (n = 46) | p value | ||
|---|---|---|---|---|---|
| Mean probing pocket depth, mm (PPD, SD) | 3.1 (0.5) | 2.8 (0.3) | 3.4 (0.5) | 0.000 | |
| Mean cemento-enamel junction, mm (CEJ, SD) | 1.5 (0.3) | 1.4 (0.2) | 1.6 (0.4) | 0.006 | |
| Mean clinical attachment level, mm (CAL, SD) | 4.6 ( 0.7) | 4.2 (0.3) | 5.0 (0.7) | 0.000 | |
| Bleeding on probing, % (BoP, SD) | 41.6 (17.0) | 34.7 ( 14.7) | 46.7 (16.9) | 0.001 | |
| Dentist awareness of HIV infection*, %, N (75) | No | 25 (33.3) | 11 (34.4) | 14 (32.6) | 0.878 |
|
Dental wishes of the patient: Patient wishes to maintain teeth** |
Yes | 75 (96.2) | 32 (97.0) | 43 (95.6) | 0.748 |
| Periodontal/gum diseases treated | Yes | 38 (47.5) | 13 (34.2) | 25 (65.8) | 0.154 |
| Frequency of brushing teeth (per day) | Not daily | 3 (3.8) | 1 (2.9) | 2 (4.3) | 0.339 |
| Daily | 14 (17.5) | 3 (8.8) | 11 (23.9) | ||
| Twice a day | 54 (67.5) | 26 (76.5) | 28 (60.9) | ||
| More than twice a day | 9 (11.3) | 4 (11.8) | 5 (10.9) | ||
| Use of interdental cleaning materials | Yes | 70 (87.5) | 29 (85.3) | 41 (89.1) | 0.608 |
| Did not use antibiotics in the last 3 months | 67 | 29(43.3) | 38 (56.7) | 0.748 | |
| Presence of Aggregatibacter actinomycetemcomitans, N (%) | 4 (5) | 0 (0.0) | 4(8.7) | 0.078 | |
| Presence of Porphyromonas gingivalis, N (%) | 23 (28.8) | 2 ( 5.9) | 21 (45.7) | < 0.001 | |
| Presence of Prevotella intermedia, N (%) | 61 (76.3) | 27 (79.4) | 34(73.9) | 0.568 | |
| Presence of Tannerella forsythia, N (%) | 44 (55.0) | 18 (52.9) | 26 (56.5) | 0.750 | |
| Presence of Parvimonas micra, N (%) | 76 (95.0) | 31 (91.2) | 45 (97.8) | 0.042 | |
| Presence of Fusobacterium nucleatum, N (%) | 76 (95.0) | 32 (94.1) | 44 (95.7) | 0.756 | |
| Presence of Campylobacter rectus, N (%) | 25 (31.3) | 11 (31.0) | 14 (30.4) | 0.885 | |
| Mean bacterial load (SD) | 1.81E + 008 (2.41E + 008) | 9.32 E + 007 (1.77 E + 008) |
2.25 E + 008 (2.51E + 008) |
0.006 | |
| Median VAS regarding importance of dental health (IQR) | 9.0 (8–10) | 9 (8–10) | 9 (8–9) | 0.065 | |
*This question was applicable to only 75 people who stated they had visited a dentist
**Data available for 78 people
Bacterial translocation, inflammation and immunosenescence
Infection duration, CD4 count and CD4/ CD8 ratio, as well as type of cART, were not significantly different between the severe periodontitis and moderate periodontitis groups (Table 3). We did not detect higher LPS or sCD14 levels as markers of bacterial translocation in PLWH with severe periodontitis, nor did we detect an elevation of systemic IL-6, sCD163, CXCL10 as markers of inflammation in PLWH with severe periodontitis. Only the CRP levels were significantly higher in the group with severe periodontitis than in the group with moderate periodontitis [1.6 mg/L (IQR 0.9–3.0) versus 0.8 mg/L (IQR 0.5–2.0), respectively, p = 0.02] (Table 4).
Table 3.
HIV related characteristics of the 80 PLWH with moderate and severe periodontitis
| N (%) Mean (SD) or median (IQR) |
People with moderate periodontitis (n = 34) | People with Severe periodontitis (n = 46) |
p value | ||
|---|---|---|---|---|---|
| Type of cART | PI-based | 19 (23.8) | 10 (29.4) | 9 (19.6) | 0.379 |
| NNRT-based | 42 (52.5) | 18 (52.9) | 24 (52.2) | ||
| INT-based | 12 (15.0) | 5 (14.7) | 7 (15.2) | ||
| Others | 7 (8.8) | 1 (2.9) | 6 (13.0) | ||
| CDC classification | Stage 1 | 9 (11.3) | 4 (11.8) | 5 (10.9) | 0.987 |
| Stage 2 | 36 (45.0) | 15 (44.1) | 21 (45.7) | ||
| Stage 3 | 35 (43.8) | 15 (44.1) | 20 (43.5) | ||
| CD4+ nadir * | < 200 T-cells/mm3 | 28 (35.0) | 11 (33.34) | 17 (39.5) | 0.692 |
| 200- < 500 T-cells/mm3 | 41 (51.3) | 18 (52.9) | 23(53.5) | ||
| > 500 T-cells/mm3 | 7 (8.8) | 4 (12.1) | 3 (7.0) | ||
| CMV positive | 74 (92.5%) | 31 (91.2%) | 43 (93.5%) | 0.200 | |
| Duration of infection (years) | 9.4 (6–15) | 9.3 (6–14) | 9.5 (7–16) | 0.480# | |
| Duration of cART use | 9.5 (6.1) | 9.3 (5.8) | 9.7 (6.4) | 0.793 | |
| CD4+/CD8+(T-cells/mm3) * | 0.9 (0.6–1.3) | 0.97 (0.8–1.3) | 0.86 (0.5–1.3) | 0.186# | |
| CD4+ nadir (T-cells/mm3) * | 225.0 (123–340) | 218.0 (145–350) | 230.0 (120–320) | 0.593# | |
*CD4+ nadir was not known in 4 people due to incomplete patient charge information+
# Mann–Whitney U test applied due to skewed data distribution
Table 4.
HIV and immunologic related characteristics of the 80 PLWH with moderate and severe periodontitis
| Mean (SD) or median (IQR) | Participants with moderate periodontitis (n = 34) | Participants with severe periodontitis (n = 46) | P value | |
|---|---|---|---|---|
| CD4 + (cells/mm3) | 670.3 (273.7) | 706.7 (274) | 643.3 (273.1) | 0.309 |
| Naive CD4 + T cells (% of CD4 + T cells) | 26.8 (14.3) | 28.6 (15.1) | 25.5 (13.7) | 0.344 |
| CD4 + HLA-DR + T cells (% of CD4 + T cells)* | 14.3 (11–26) | 13.0 (10–21) | 15.0 (12–30) | 0.124# |
| CD4 + CD38 + T cells (% of CD4 + T cells) | 45.1 (15.2) | 47.3 (15.0) | 43.5 (15.2) | 0.266 |
| CD4 + HLA-DR + + CD38 + T cells (% of CD4 + T cells)* | 4.6 (4–8) | 4.7 (3–8) | 4.5 (4–8) | 0.685# |
| CD4 + CD28-CD57 + T cells (% of CD4 + T cells) | 6.6 (6.1) | 5.9 (4.2) | 7.1 (7.2) | 0.331 |
| CD3 + (cells/mm3) | 1428.5 (1165–1785) | 1403.0 (1154–1732) | 1461.0 (1147–1796) | 0.640# |
| CD8 + (cells/mm3) | 695.5 (523–884) | 681.0 (456–808) | 701.0 (559–957) | 0.411# |
| Naive CD8 + T cells (% of CD8 + T cells) | 10.3 (5–19) | 9.8 (5–18) | 10.1 (5–20) | 0.992# |
| CD8 + HLA-DR + T cells (% of CD8 + T cells)* | 22.9 (18–36) | 22.8 (17–33) | 22.9 (18–40) | 0.751# |
| CD8 + CD38 + T cells (% of CD8 + T cells) | 29.6 (19–38) | 28.3 (20–40) | 30 (18–35) | 0.400# |
| CD8 + HLA-DR + + CD38 + T cells (% of CD8 + T cells)* | 8.1 (5–13) | 8.6 (6–13) | 7.7 (5–12) | 0.392 |
| CD8 + CD28-CD57 + T cells (% of CD8 + T cells) | 11.4 (7–23) | 8.9 (6–19) | 16.5 (9–26) | 0.035# |
| CRP (mg /l) | 1.2 (0.6–2.3) | 0.8 (0.5–2.0) | 1.6 (0.9–3.0) | 0.020# |
| IL-6 (pg/ml) | 0.7 (0.0–1.8) | 0.6 (0.0–1.5) | 0.9 (0.0–2.6) | 0.211# |
| sCD14 (µg/ml) | 3.9 (1.3) | 3.6 (1.3) | 4.1 (1.3) | 0.079 |
| sPD-1 (pg/ml) | 204.8 (163–183) | 208.4 (176–260) | 184 (162–250) | 0.243# |
| CXCL10* (pg/ml) | 147 (118–183) | 136.4 (116–173) | 155.1 (118–190) | 0.507# |
| sCD163 (ng/ml) | 34.0 (28–50) | 39.5 (26–57) | 33.8 (29–45) | 0.853# |
| LPS/DPLG70 (ng/ml) | 136 (46.3) | 139.0 (50.3) | 133.7 (43.5) | 0.623 |
*data was not known for 1 person
#Mann–Whitney U test applied due to skewed data distribution
In the participants with severe periodontitis, the median percentage of senescent CD28−CD57+CD8+ T-cells was significantly higher than in those with moderate periodontitis (16.5 versus 8.9%, respectively, p = 0.03). In addition, we found a significant positive correlation between the levels of CD28−CD57+CD8+ T-cells and levels of sCD14 (r = 0.3, p = 0.012), Together, these results suggest that higher levels of senescent CD28−CD57+CD8+ T-cells may be attributed to microbial translocation in severe periodontitis. We found no correlation between the levels of CD28−CD57 + CD8+ T-cells and LPS. Inflammation markers (e.g., CRP and IL-6) were not related to higher levels of CD28−CD57+CD8+ T-cells.
Discussion
We aimed to assess whether systemic immune markers and microbial factors are related to periodontitis severity in PLWH. In this study, we found increased levels of CRP, increased frequencies of CD28−CD57+CD8+ T-cells and a higher prevalence of Pg and Pm in PLWH with severe periodontitis.
The more elevated CRP levels in the severe periodontitis participants compared to the moderate periodontitis participants are in line with the results of the Paraskevas et al. meta-analysis [8]. This meta-analysis showed that in a population of non-HIV participants with periodontitis, a high inflammatory load was reflected by higher levels of CRP. A small increase in CRP levels is commonly regarded as an indication of low-grade inflammation, which periodontitis, per definition, is [8]. Elevated CRP levels are considered to be a risk factor for developing cardiovascular diseases in the general population. As a matter of fact, the relative risk of developing cardiovascular diseases falls into three major categories, i.e. low, average and high, based on the systemic CRP levels. Specifically, CRP levels of < 1.0, 1.0 to 3.0 and > 3.0 mg/L correspond to low, average and high risk, respectively [43]. In our study, the PLWH with severe periodontitis had a median CRP of 1.6 mg/L and so, according to the aforementioned categorization, would have an average relative risk of cardiovascular diseases, while the PLWH with moderate periodontitis, with a CRP of 0.8 mg/L, would have a low relative risk. The higher CRP levels in the group with severe periodontitis could be attributed to more periodontal inflammation. Periodontal treatment by dental professionals predictably reduces periodontal inflammation and probing pocket depths and thus periodontitis severity [44]. Consequently, professional periodontal therapy could decrease the CRP levels and potentially lower the risk of cardiovascular disease [45].
We found higher percentages of senescent CD28−CD57+CD8+ T-cells in the severe periodontitis group. While it is known that older adults are characterized by a proportional accumulation of senescent CD28−CD57+CD8+ T-cells compared to younger adults, in our study, the mean age did not differ between the PLWH with severe and moderate periodontitis; thus, age is presumably not a contributing factor here. An increase in CD28−CD57+CD8+ T-cells has also been observed in individuals with other chronic viral infections [46]. CD28−CD57+CD8+ T-cells are believed to approach end-stage senescence [46]. Moreover, in general, nearly all HIV-infected people are also infected with cytomegalovirus (CMV) [47, 48]. Several studies demonstrated that a persistent CMV infection, possibly acquired prior to HIV, also plays a substantial role in accelerating immunosenescence [47, 49]. Since almost all the participants in our cohort (92.5%) were infected with CMV, CMV infection alone is not likely to have been responsible for the difference in the proportions of senescent CD8+ T-cells between the study groups. Next to CMV, inflammation is well known to accelerate immunosenescence and to contribute to higher percentages of CD28−CD57+ CD8+ T-cells [46, 50]. We did not find a significant relationship between senescent CD4+ T-cells and periodontitis severity. Another study showed that senescent CD4+ T-cells decline after starting a successful cART, while the percentage of senescent CD8+ T-cells does not decrease [51]. The fact that all our study subjects had been on cART for a mean period of 9.5 years might explain the link found between CD8+ T-cells and periodontitis severity.
Another finding is the positive relationship between the presence of the oral bacteria Pm and Pg and severe periodontitis. Although Pm is part of the normal commensal flora, it is significantly more present in patients with severe/moderate periodontitis than in patients with good periodontal health, gingivitis and/or mild periodontitis [52, 53]. Similarly, in the general population, the prevalence of Pg is significantly higher in subjects with severe periodontitis than in subjects with good gingival health or various degrees of gingivitis [54, 55]. Pg can produce a large amount of butyric acid as a metabolite, which might collapse the homeostasis in the periodontal tissues, and so contribute to the progression of periodontitis [56]. Pg could also induce HIV-1 reactivation since butyric acid has been found to be responsible for reactivating latent HIV virus [57, 58]. The levels of butyric acid were, however, not investigated in our study’s participants. Consequently, any reactivation in those individuals due to the presence of butyric acid, or due to the translocation of microbial products from the blood, should be interpreted with caution.
Our study has some limitations. The study is explorative in nature, so we cannot exclude that the relationship between severe periodontitis with percentages of senescent CD28−CD57+CD8+ T-cells and the prevalence of Pg is a chance finding. Due to the explorative character of the study, a calculation of the minimum sample size was not performed, and this may have, in part, influenced the findings. This is why our results should be seen as hypothesis generating, not as definitive findings. Another limitation of our study is that only subjects with severe and moderate periodontitis were compared. Therefore, in future studies, the results should be confirmed in subjects with mild periodontitis and in subjects with non-HIV periodontal diseases. Next, as with any periodontal screening instrument, the one used herein has its limitations too. The CDC-AAP case definition classification used by epidemiologic studies to survey periodontitis [35–37] is based on PPD and CAL. PLWH are known to have more permanent gingival or periodontal tissue loss compared to non-HIV periodontal patients because of necrotizing ulcerative gingivitis or necrotizing ulcerative periodontitis in the past [13]. These conditions are usually seen in patients with a compromised immune system. The severity of periodontitis in patients with irreversible attachment loss, possibly obtained before starting cART, might be overestimated by this classification system. Nevertheless, the CDC-AAP case definition classification is the best method as the measurements are extensive, and, as it is commonly used, the results can be compared. An early diagnosis of periodontitis in PLWH might allow for a timely therapeutic intervention by oral health professionals. Consequently, less periodontal inflammation could have a positive effect on the oral health and thereby could probably reduce the risk of age-related diseases.
Conclusion
In our study, we observed that severe periodontitis in PLWH is related to higher levels of CRP, higher levels of Pg and higher proportions of senescent CD8 + T-cells. This might suggest that severe periodontitis in PLWH could contribute to a higher risk of developing age-related inflammatory conditions such as cardiovascular and systemic diseases.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Prof. dr. A.M.H. Boots for critically reading the manuscript.
Author contribution
HG: Conceptualization (supporting; resources; project administration; methodology (supporting); formal analysis (equal); writing, original draft (equal); writing review and editing (lead). KD: Methodology (lead); writing, original draft (equal); formal analysis (equal); writing, review and editing (equal). AV: Writing, original draft (supporting); writing, review and editing (equal); visualization. FS: Writing, original draft (supporting); writing, review and editing (equal); supervision. WB: Funding acquisition; conceptualization (lead); methodology (supporting); writing, original draft (supporting); writing, review and editing (equal).
Funding
This work was supported by the University Medical Center Groningen as a Healthy Ageing Pilot Project (HAP-2013–2-182).
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
W.B reports a GSK reimbursement to the institution for inclusion in a trial other than the submitted work. For the remaining authors, none were declared.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hester Groenewegen, Email: h.groenewegen@umcg.nl.
Konstantina Delli, Email: k.delli@umcg.nl.
Arjan Vissink, Email: a.vissink@umcg.nl.
Frederik K. L. Spijkervet, Email: f.k.l.spijkervet@umcg.nl
Wouter F. W. Bierman, Email: w.f.w.bierman@umcg.nl
References
- 1.Polvora TLS, Nobre AVV, Tirapelli C, Taba M, Jr, Macedo LD, Santana RC, et al. Relationship between human immunodeficiency virus (HIV-1) infection and chronic periodontitis. Expert Rev Clin Immunol. 2018;14(4):315–327. doi: 10.1080/1744666X.2018.1459571. [DOI] [PubMed] [Google Scholar]
- 2.Groenewegen H, Bierman WFW, Delli K, Dijkstra PU, Nesse W, Vissink A, et al. Severe periodontitis is more common in HIV- infected patients. J Infect. 2019;78(3):171–177. doi: 10.1016/j.jinf.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol. 1994;2000(5):78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 4.Tonetti MS, Van Dyke TE. working group 1 of the joint EFP/AAP workshop. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. 2013;84(4 Suppl):S24–9. doi: 10.1902/jop.2013.1340019. [DOI] [PubMed] [Google Scholar]
- 5.Nesse W, Linde A, Abbas F, Spijkervet FK, Dijkstra PU, de Brabander EC, et al. Dose-response relationship between periodontal inflamed surface area and HbA1c in type 2 diabetics. J Clin Periodontol. 2009;36(4):295–300. doi: 10.1111/j.1600-051X.2009.01377.x. [DOI] [PubMed] [Google Scholar]
- 6.Ziukaite L, Slot DE, Van der Weijden FA. Prevalence of diabetes mellitus in people clinically diagnosed with periodontitis: a systematic review and meta-analysis of epidemiologic studies. J Clin Periodontol. 2018;45(6):650–662. doi: 10.1111/jcpe.12839. [DOI] [PubMed] [Google Scholar]
- 7.Kapila YL. Oral health’s inextricable connection to systemic health: special populations bring to bear multimodal relationships and factors connecting periodontal disease to systemic diseases and conditions. Periodontol 2000. 2021;87(1):11–16. doi: 10.1111/prd.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008;35(4):277–290. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 9.Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2000. 2012;60(1):15–39. doi: 10.1111/j.1600-0757.2011.00425.x. [DOI] [PubMed] [Google Scholar]
- 10.Alpagot T, Konopka K, Bhattacharyya M, Gebremedhin S, Duzgunes N. The association between gingival crevicular fluid TGF-beta 1 levels and periodontal status in HIV-1(+) patients. J Periodontol. 2008;79(1):123–130. doi: 10.1902/jop.2008.070312. [DOI] [PubMed] [Google Scholar]
- 11.Kroidl A, Schaeben A, Oette M, Wettstein M, Herfordt A, Haussinger D. Prevalence of oral lesions and periodontal diseases in HIV-infected patients on antiretroviral therapy. Eur J Med Res. 2005;10(10):448–453. [PubMed] [Google Scholar]
- 12.Yin MT, Dobkin JF, Grbic JT. Epidemiology, pathogenesis, and management of human immunodeficiency virus infection in patients with periodontal disease. Periodontol 2000. 2007;44:55–81. doi: 10.1111/j.1600-0757.2007.00205.x. [DOI] [PubMed] [Google Scholar]
- 13.Goncalves LS, Goncalves BM, Fontes TV. Periodontal disease in HIV-infected adults in the HAART era: clinical, immunological, and microbiological aspects. Arch Oral Biol. 2013;58(10):1385–1396. doi: 10.1016/j.archoralbio.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation. 2019;140(2):e98–e124. doi: 10.1161/CIR.0000000000000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noubissi EC, Katte JC, Sobngwi E. Diabetes and HIV. Curr Diab Rep. 2018;18(11):1–8. doi: 10.1007/s11892-018-1076-3. [DOI] [PubMed] [Google Scholar]
- 16.Martínez A, Kuraji R, Kapila YL. The human oral virome: shedding light on the dark matter. Periodontol 2000. 2021;87(1):282–298. doi: 10.1111/prd.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teles F, Collman RG, Mominkhan D, Wang Y. Viruses, periodontitis, and comorbidities. Periodontol 2000. 2022;89(1):190–206. doi: 10.1111/prd.12435. [DOI] [PubMed] [Google Scholar]
- 18.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrando-Martínez S, Ruiz-Mateos E, Romero-Sánchez MC, Muñoz-Fernández MÁ, Viciana P, Genebat M, et al. HIV infection-related premature immunosenescence: high rates of immune exhaustion after short time of infection. Curr HIV Res. 2011;9(5):289–294. doi: 10.2174/157016211797636008. [DOI] [PubMed] [Google Scholar]
- 20.Desai S, Landay A. Early immune senescence in HIV disease. Curr HIV/AIDS Rep. 2010;7(1):4–10. doi: 10.1007/s11904-009-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dock JN, Effros RB. Role of CD8 T cell replicative senescence in human aging and in HIV-mediated immunosenescence. Aging Dis. 2011;2(5):382–397. [PMC free article] [PubMed] [Google Scholar]
- 22.Warren JA, Clutton G, Goonetilleke N. Harnessing CD8(+) T cells under HIV antiretroviral therapy. Front Immunol. 2019;26(10):291. doi: 10.3389/fimmu.2019.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sokoya T, Steel HC, Nieuwoudt M, Rossouw TM. HIV as a cause of immune activation and immunosenescence. Mediators Inflamm. 2017;2017:6825493. doi: 10.1155/2017/6825493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci. 2014;69(7):833–842. doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erlandson KM, Ng DK, Jacobson LP, Margolick JB, Dobs AS, Palella FJ, et al. Inflammation, immune activation, immunosenescence, and hormonal biomarkers in the frailty-related phenotype of men with or at risk for HIV infection. J Infect Dis. 2017;215(2):228–237. doi: 10.1093/infdis/jiw523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gnoni M, Beas R, Raghuram A, Díaz-Pardavé C, Riva-Moscoso A, Príncipe-Meneses FS, et al. Potential role of intermittent fasting on decreasing cardiovascular disease in human immunodeficiency virus patients receiving antiretroviral therapy. World J Exp Med. 2021;11(5):66–78. doi: 10.5493/wjem.v11.i5.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zevin AS, McKinnon L, Burgener A, Klatt NR. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS. 2016;11(2):182–190. doi: 10.1097/COH.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blum FC, Hardy BL, Bishop-Lilly KA, Frey KG, Hamilton T, Whitney JB, et al. Microbial dysbiosis during simian immunodeficiency virus infection is partially reverted with combination anti-retroviral therapy. Sci Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-63196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mak G, Zaunders JJ, Bailey M, Seddiki N, Rogers G, Leong L, et al. Preservation of gastrointestinal mucosal barrier function and microbiome in patients with controlled HIV infection. Front Immunol. 2021;31(12):688886. doi: 10.3389/fimmu.2021.688886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groenewegen H, Borjas-Howard JF, Delli K, Meijer K, Vissink A, Spijkervet FKL, et al. Association of periodontitis with markers of immunologic and haemostatic state in people living with HIV. J Infect. 2021;82(3):e17–e19. doi: 10.1016/j.jinf.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Serra eSF, Casarin RCV, Nicolela Junior EL, Passos HM, Sallum AW, Gonçalves RB. Microbial diversity similarities in periodontal pockets and atheromatous plaques of cardiovascular disease patients. PLoS ONE. 2014;9(10):1–7. doi: 10.1371/journal.pone.0109761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Jong KJ, Borgmeijer-Hoelen A, Abraham-Inpijn L. Validity of a risk-related patient-administered medical questionnaire for dental patients. Oral Surg Oral Med Oral Pathol. 1991;72(5):527–533. doi: 10.1016/0030-4220(91)90488-X. [DOI] [PubMed] [Google Scholar]
- 33.de Jong KJ, Oosting J, Abraham-Inpijn L. Medical risk classification of dental patients in the Netherlands. J Public Health Dent. 1993;53(4):219–222. doi: 10.1111/j.1752-7325.1993.tb02707.x. [DOI] [PubMed] [Google Scholar]
- 34.de Jong KJ, Abraham-Inpijn L. A risk-related patient-administered medical questionnaire for dental practice. Int Dent J. 1994;44(5):471–479. [PubMed] [Google Scholar]
- 35.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83(12):1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holtfreter B, Albandar JM, Dietrich T, Dye BA, Eaton KA, Eke PI, et al. Standards for reporting chronic periodontitis prevalence and severity in epidemiologic studies: proposed standards from the Joint EU/USA Periodontal Epidemiology Working Group. J Clin Periodontol. 2015;42(5):407–412. doi: 10.1111/jcpe.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(Suppl 7S):1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 38.van Winkelhoff AJ, van Steenbergen TJ, Kippuw N, De Graaff J. Further characterization of Bacteroides endodontalis, an asaccharolytic black-pigmented Bacteroides species from the oral S cavity. J Clin Microbiol. 1985;22(1):75–79. doi: 10.1128/jcm.22.1.75-79.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Steenbergen TJ, Petit MD, Tijhof CJ, van Winkelhoff AJ, van der Velden U, de Graaff J. Survival in transport media of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia in human subgingival samples. Oral Microbiol Immunol. 1993;8(6):370–374. doi: 10.1111/j.1399-302X.1993.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 40.Zambon JJ. Periodontal diseases: microbial factors. Ann Periodontol. 1996;1(1):879–925. doi: 10.1902/annals.1996.1.1.879. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention (CDC) Revised surveillance case definition for HIV infection–United States 2014. MMWR Recomm Rep. 2014;63(03):1–10. [PubMed] [Google Scholar]
- 42.van Sighem A, Wit FW, Smit C, Matser A, Reiss P (2019) Monitoring report 2019: human immunodeficiency virus (HIV) infection in the Netherlands. Available at: https://www.hiv-monitoring.nl/application/files/4115/7616/1682/HIV_Monitoring_Report_2019_update_dec_2019.pdf. Accessed 14 Apr 2021
- 43.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 44.Cobb CM. Clinical significance of non-surgical periodontal therapy: an evidence-based perspective of scaling and root planing. J Clin Periodontol. 2002;29(Suppl 2):6–16. [PubMed] [Google Scholar]
- 45.Demmer R, Papapanou PN. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol. 2010;2000(53):28–44. doi: 10.1111/j.1600-0757.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28- and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134(1):17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Remis RS, Liu J, Loutfy MR, Tharao W, Rebbapragada A, Huibner S, et al. Prevalence of sexually transmitted viral and bacterial infections in HIV-positive and HIV-negative men who have sex with men in Toronto. PLoS ONE. 2016;11(7):e0158090. doi: 10.1371/journal.pone.0158090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grønborg HL, Jespersen S, Egedal JH, Correia FG, Medina C, Krarup H, et al. Prevalence and clinical characteristics of CMV coinfection among HIV infected individuals in Guinea-Bissau: a cross-sectional study. Trop Med Int Health. 2018;23(8):896–904. doi: 10.1111/tmi.13082. [DOI] [PubMed] [Google Scholar]
- 49.Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol. 2012;24(4):501–506. doi: 10.1016/j.coi.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214(2):231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 51.Kelesidis T, Moser C, Stein JH, Brown TT, Tran TT, Ribaudo HJ, et al. Changes in markers of T-cell senescence and exhaustion with atazanavir-, raltegravir-, and darunavir-based initial antiviral therapy: ACTG 5260s. J Infect Dis. 2016;214(5):748–752. doi: 10.1093/infdis/jiw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy EC, Frick IM. Gram-positive anaerobic cocci–commensals and opportunistic pathogens. FEMS Microbiol Rev. 2013;37(4):520–553. doi: 10.1111/1574-6976.12005. [DOI] [PubMed] [Google Scholar]
- 53.Curtis MA, Diaz PI, Van Dyke TE. The role of the microbiota in periodontal disease. Periodontol 2000. 2020;83(1):14–25. doi: 10.1111/prd.12296. [DOI] [PubMed] [Google Scholar]
- 54.Amaliya A, Laine ML, Delanghe JR, Loos BG, Van Wijk AJ, Van der Velden U. Java project on periodontal diseases: periodontal bone loss in relation to environmental and systemic conditions. J Clin Periodontol. 2015;42(4):325–332. doi: 10.1111/jcpe.12381. [DOI] [PubMed] [Google Scholar]
- 55.van Winkelhoff AJ, Loos BG, van der Reijden WA, van der Velden U. Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J Clin Periodontol. 2002;29(11):1023–1028. doi: 10.1034/j.1600-051X.2002.291107.x. [DOI] [PubMed] [Google Scholar]
- 56.Shirasugi M, Nakagawa M, Nishioka K, Yamamoto T, Nakaya T, Kanamura N. Relationship between periodontal disease and butyric acid produced by periodontopathic bacteria. Inflamm Regen. 2018;38(1):1–5. doi: 10.1186/s41232-018-0081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imai K, Ochiai K, Okamoto T. Reactivation of latent HIV-1 infection by the periodontopathic bacterium Porphyromonas gingivalis involves histone modification. J Immunol. 2009;182(6):3688–3695. doi: 10.4049/jimmunol.0802906. [DOI] [PubMed] [Google Scholar]
- 58.Imai K, Ochiai K. Role of histone modification on transcriptional regulation and HIV-1 gene expression: possible mechanisms of periodontal diseases in AIDS progression. J Oral Sci. 2011;53(1):1–13. doi: 10.2334/josnusd.53.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.