Abstract
Objectives
We assessed real-world weight change and pregnancy outcomes among pregnant women living with HIV who used integrase strand transferase inhibitor (INSTI)-based combined antiretroviral therapy (cART).
Methods
In a retrospective cohort study from 2014–2021 for prevention of perinatal HIV infection, we evaluated changes of weight from the first prenatal visit to near delivery in two groups. The categories of change were: low (< 0.18 kg/week), normal (0.18–0.59 kg/week), and high (>0.59 kg/week). The backbones were lamivudine + tenofovir disoproxil or lamivudine + zidovudine. The comparison groups were women with body mass index (BMI) < 25 vs. BMI ≥ 25 and INSTI-naive vs. INSTI-experienced. Continuous variables were analyzed with a Kruskal-Wallis test and count or categorical data with Chi-squared tests
Results
We enrolled 198 pregnant women. At study entry, 74 had BMI < 25 and 124 had BMI ≥ 25. Excess gestational weight gain was more frequent among women who were INSTI-naive both among the BMI < 25 and the BMI ≥ 25 groups. However, the proportion of participants per weight change category was only significantly different between INSTI-naive women with baseline BMI < 25 and INSTI-experienced with BMI < 25. In particular, INSTI-naives with BMI < 25 had significantly higher rates of excess gestational weight gain (31.6%), than participants with BMI < 25 who conceived in use of INSTI (11.8%), p=0.004. Rates of unfavorable pregnancy outcomes were low and did not differ significantly between groups.
Conclusions
BMI < 25, INSTI-naive participants gained more weight during pregnancy than BMI ≥ 25 participants who conceived in use of INSTI. Rates of adverse pregnancy outcomes did not differ between the groups.
Keywords: Anti-HIV agents, HIV integrase inhibitors, gestational weight gain, pregnancy outcomes
Introduction
Currently, guidelines of developed countries and the WHO recommend integrase strand transferase inhibitor (INSTI)-based combined antiretroviral therapy (cART) as a preferred regimen for the treatment of adults living with HIV and prevention of perinatal HIV acquisition (1–4). INSTI-based cART regimens are potent, well-tolerated with few toxic side effects, have a high barrier to genetic resistance, few drug-drug interactions, low pill burden, and do not need to be taken with food. INSTIs have been investigated in studies such as ADVANCE, which was a Phase III protocol conducted for 96 weeks in non-pregnant adults, the preliminary results of which were published at 48 weeks, that evaluated the efficacy and safety of two INSTI-based regimens (dolutegravir (DTG) + emtricitabine (FTC)/tenofovir alafenamide (TAF) vs. DTG+FTC/tenofovir disoproxil (TDF)) compared with an NNRTI (efavirenz (EFV))-based regimen in a population that was 59% women living with HIV and 99% black (5). At 48 weeks, in the DTG+FTC/TAF group average weight gain was 6kg and 14% of the participants became obese, whereas in the DTG+FTC/TDF group weight gain was 3kg with 7% obesity, and in the EFV-based group weight gain was 1kg with 6% obesity (5). Weight gain was significantly higher in females than males in all three groups and both INSTI arms demonstrated a significant weight gain compared with the EFV arm (5). A systematic review also concluded that, excessive weight gain is a source of concern in the adult population (6). However, there have been few studies of the effects of weight change among pregnant women living with HIV. In pregnant women not living with HIV, excessive weight gain during gestation may be associated with pregnancy complications including hypertensive disorders such as pre-eclampsia, and HELLP (Hemolysis, Elevated Liver enzymes, and Low Platelet count) as well as adverse obstetric outcomes (7).
NICHD P1081 (NCT01618305) is a Phase IV multicenter, randomized, open-label trial comparing HIV virologic response (plasma HIV viral load <200 copies/mL near delivery), tolerability (remaining on study drug through delivery), and safety (maternal and infant adverse event (AE) ≥grade 3) of cART (raltegravir (RAL) vs. EFV + backbone) when initiated during pregnancy (8). A P1081 substudy found that antiretroviral (ARV)-naive pregnant women living with HIV starting on raltegravir-based cART were more likely than those starting EFV to have high rates of weight gain (9). A substudy of IMPAACT 2010 (NCT03048422) showed that women starting DTG-based cART in pregnancy gained more weight antepartum than women starting EFV (10).
One of the gaps in our knowledge about effect of INSTI use on weight change among pregnant women living with HIV is that there is scant data based on real-world experience outside of clinical trials. Real world studies can reveal whether trial findings still apply in settings in which the window for study visits is more flexible or to pregnant women living with HIV who would not have met the eligibility requirements for the P1081 and 2010 trials, such as ART-experienced pregnant women. As of 2020, approximately 1.3 million pregnant women were living with HIV around the world (11), and an increasing percentage of pregnant women living with HIV are conceiving in use of INSTI (12). Thus, it is worthwhile to investigate weight change among pregnant women living with HIV, including those who are treatment-experienced, in a real-world study. The aims of this study were to assess weight change among pregnant women living with HIV at a reference center for prevention of perinatal HIV acquisition in a middle-income country in use of raltegravir- or dolutegravir-based cART and their association, if any, with adverse obstetric and neonatal outcomes.
Materials and Methods
The present study was a retrospective substudy of an established cohort of pregnant women living with HIV. The group involved in the current study also took part in the follow-up of this cohort prospectively. A detailed description of our institution’s cohort has been previously published (13). From October 2014 to October 2021, all pregnant women living with HIV who were referred for prenatal care to our center, which is a national reference for PMTCT in Rio de Janeiro that is one of the epicenters of HIV epidemic in Brazil, and met the study inclusion criteria were included.
The enrolled criteria were cART-naive pregnant woman living with HIV who regularly used INSTI (RAL-based regimen (400 mg twice daily) or DTG (50 mg once daily) plus two analogs: lamivudine + TDF or lamivudine + zidovudine) for at least four weeks between the study entry visit and the near delivery visit (“INSTI naive”) or became pregnant using INSTI for at least six months before conception (“INSTI experienced”). In addition, participants were required to have an entry visit and near delivery weight and height. We excluded women with gestational diabetes as it can cause weight gain independent of cART use. For the purposes of this study, the entry visit was the first prenatal care visit at the center. The comparison groups were women with (body mass index) BMI < 25 vs. BMI ≥ 25 and treatment-naive vs. treatment-experienced. BMI was calculated as weight in kilograms divided height in meters squared.
During medical visits, the accompanying practitioner completed a standardized case report form from which we captured data that were anonymized and inserted into our center’s database in accordance with our standard operating procedures for data quality control and assurance.
These data included sociodemographic characteristics (age, race/ethnicity, marital status, and education level), clinical, immunological, and virological characteristics (HIV viral load at study entry and near delivery, CD4 count [cells/mm3], duration of INSTI use), gestational age at study entry based on ultrasound or the last menses, maternal weight and height measured at prenatal visits, and subsequent prenatal care visits, and the sex, weight, and length of the newborn at birth. In addition, we captured data on the BMI of each study participant at the first visit and near delivery. BMI < 25 at baseline will be referred to hereafter as “underweight or normal weight” and BMI ≥ 25 as “overweight or obese”. For the purposes of this study, the rate of weight gain per week was defined as the weight measured at the near delivery visit (within seven days before) minus the weight at the enrollment visit divided by the number of weeks between the two visits. We hypothesized that women who conceived in use of INSTI-based cART would experience normal weight gain during pregnancy, whereas women who initiated cART during pregnancy would be more likely to experience high weight gain.
Intergrowth-21st tables (14) were used to classify the newborn as small for gestational age (SGA) defined as below the 10th percentile for weight. We also tallied the number of preterm deliveries (≥ 20 and < 37 completed gestational weeks), extremely preterm (≥ 20 and < 34 weeks), low birth weight (<2,500 g), extremely low birth weight (<1,500 g), macrosomia (birth weight >4,000 g), abortion (< 20 weeks), stillbirth, neonatal death (death of live born infants up to 28 days post-partum), and a composite outcome consisting of any one of the aforementioned outcomes (preterm delivery, low or extremely low birth weight, macrosomia, abortion, stillbirth, small for gestational age, or neonatal death). For the purposes of this study, weight gain was the primary outcome measure, and other adverse obstetric and neonatal outcomes were the secondary outcome measures.
Data analysis: We report the median and interquartile range (IQR) for continuous variables. The continuous variables were: maternal age in years, gestational age at baseline weight in weeks, duration of INSTI use in days before baseline weight, duration of INSTI use in days from baseline weight to delivery, CD4 cell count (cells/mm3) at baseline weight, baseline weight (kg) and BMI (kg/m2), and log10 HIV RNA near delivery in copies/mL. The categorical variables were race/ethnicity, marital status, education level, alcohol use before pregnancy, tobacco use before pregnancy, HIV RNA viral load (copies/mL) at enrolment divided into the categories: <200, 200–999, 1000–9,999, and ≥10,000. In accordance with Institute of Medicine guidelines, weight gain was divided into the categories: <0.18kg/week (low), 0.18–0.59 kg/week (normal), and > 0.59 kg/week (high) (15). The count data were the number of cases of abortion, stillbirth, neonatal death, prematurity (between 20 weeks of gestation and <37 weeks), extreme prematurity, low birth weight, extremely low birth weight, macrosomia, and SGA. Continuous variables were analyzed with a Kruskal-Wallis test and count or categorical data with Chi-squared tests in SPSS 19. Figures were produced with GraphPad Prism 9.
To assess the categories of weight gain in the naive and experienced groups, the analysis was conducted separately for the underweight/normal weight and obese/overweight groups. Within each group, we determined the number of naive and experienced participants with low, normal, and high weight gain. We conducted a Chi-squared test to assess whether the number of participants with low, normal, and height weight gain was significantly different between the naive and experienced groups. This work was approved by the local ethics committee and participants provided informed consent.
Results
Between 2014 and 2021, 755 pregnant women living with HIV registered for care at our prenatal clinic. For the analysis of weight gain, 515 did not fulfill the eligibility criteria were excluded as seen in Figure 1. Among them, 393 used a non-INSTI regimen, 96 used an INSTI regimen for less than four weeks between the baseline weight and near delivery, 25 conceived in use of INSTI, but used INSTI for less than six months, and one had gestational diabetes. Thus, the population base of the study was 240 pregnant women living with HIV. The number of evaluable participants for the weight gain analysis after excluding 42 due to missing data was 198. Of these, 58 (29.3%) used DTG and 140 (70.7%) RAL-based regimens. At baseline, 74/198 (37.4%) of the eligible participants were underweight or normal weight and 124/198 (62.6%) were overweight or obese. Among the underweight or normal weight participants, 57/74 (77%) were INSTI-naive whereas 17/74 (23%) were INSTI-experienced. Among the overweight and obese participants, there were 101/124 (81.5%) naive and 23/124 (18.5%) experienced pregnant women.
Figure 1.
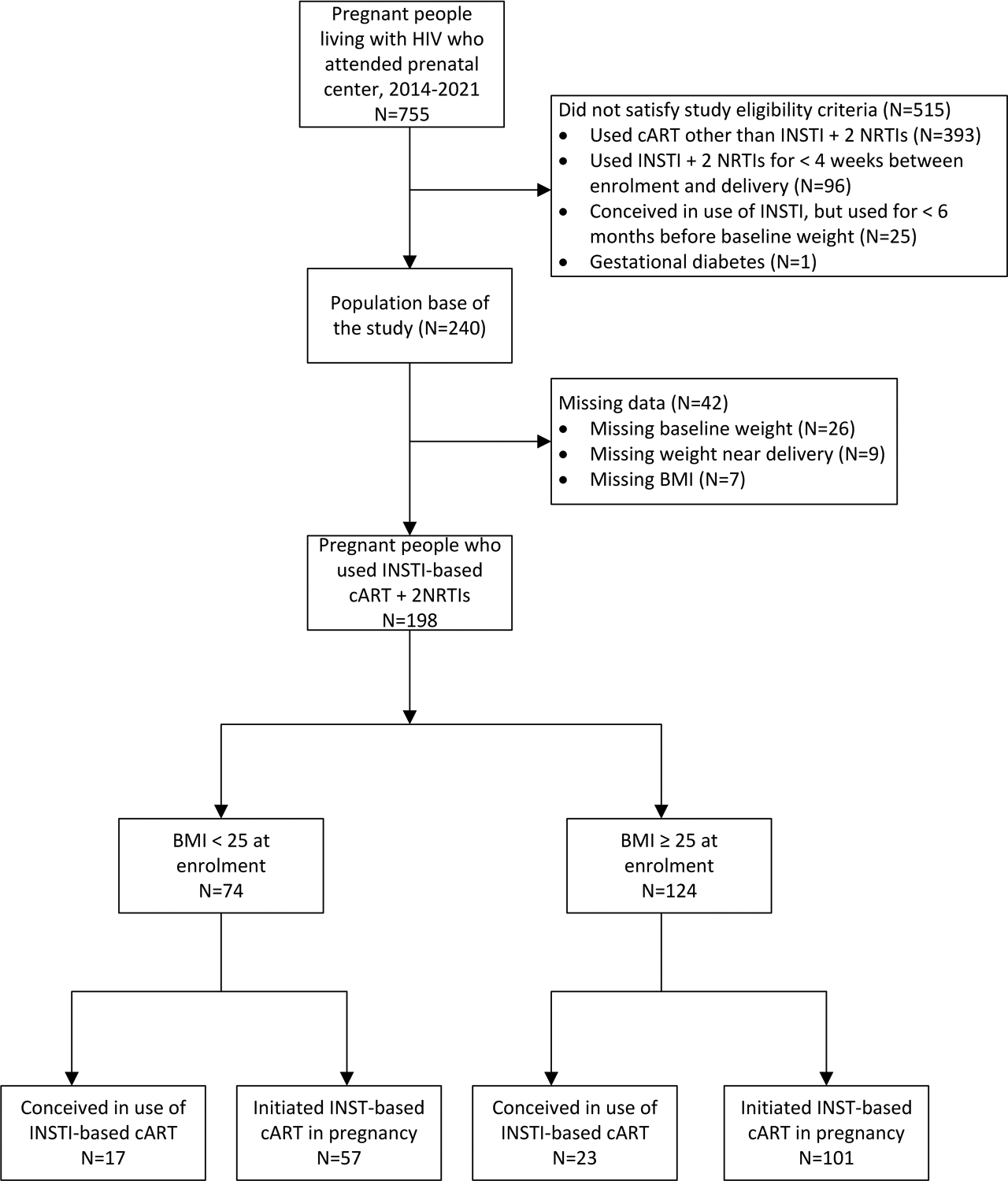
Flow chart of participants enrolled in the weight gain analysis (n = 198 final participants).
The median age of the 198 study participants was 28 years (IQR: 22–33 years). Maternal age did not differ significantly based on BMI or treatment experience, as seen in Table 1. The majority of the participants self-identified as non-white. Among 74 underweight or normal weight participants, sociodemographic characteristics differed somewhat between the naive (N=57) and experienced (N=17) groups. In the experienced group, 12/17 (70.6%) participants were married versus 21/57 (36.8%) in the naive group (p=0.049). With respect to educational attainment, 13/17 (76.5%) of the experienced participants had begun or completed secondary school, whereas 18/57 (31.6%) had reached this education level in the naive group (p=0.02). Regarding clinical characteristics, the treatment-experienced participants had their baseline weight measured at a median gestational age of 14 week versus a median of 20 weeks for the naive participants (p=0.02). Among the overweight or obese participants, the median weight at study entry of the treatment-experienced participants was 84.6 kg versus 74.6 kg for the INSTI-naive group (p=0.013). Among the experienced participants, the median duration of INSTI use from initiation to delivery was 619 days in the BMI < 25 group and 696 days in the BMI ≥ 25 group (Figure 2). Among the treatment-naive participants, the median duration of INSTI use from initiation through delivery was 140 days for those with those with BMI < 25 versus 141 days for those with BMI ≥ 25. Among the underweight/normal weight participants who were treatment- experienced, at the first prenatal visit HIV RNA viral load was 0 copies/mL and CD4 count was 497 cells/mm3, whereas those who were treatment-naive had a median HIV RNA of 4.1 log10 copies/mL at study entry and CD4 cell count of 369 cells/mm3. With respect to the overweight/obese participants, the treatment-experienced group had HIV RNA viral load at study entry of 0 copies and CD4 count of 596 cells/mm3, whereas the naive participants had 3.8 log10 copies/mL of HIV RNA and median CD4 count of 433 cells/mm3.
Table 1.
Baseline demographic and clinical characteristics of pregnant women living with HIV in use of INSTI, 2014–2021 (N=198)*. In the table below, p-values < 0.05 are bolded.
| Baseline BMI < 25 | Baseline BMI ≥ 25 | |||||
|---|---|---|---|---|---|---|
| Experienced (N=17) | Naive (N=57) | Experienced (N=23) | Naive (N=101) | |||
| Demographic characteristics at enrolment | Median (IQR) | Median (IQR) | p | Median (IQR) | Median (IQR) | p |
| Median age in years (IQR) | 26 (20.5–31.5) | 24 (18.5–29.5) | 0.052 | 29 (22.5–35.5) | 28 (22–34) | 0.257 |
| Missing, N(%) | 0 | 0 | 1 (4.3%) | 0 | ||
| Ethnicity | N (%) | N (%) | p | N (%) | N (%) | P |
| White, N (%) | 7 (43.8%) | 13 (22.8%) | 0.124 | 5 (21.7%) | 27 (26.5%) | 0.885 |
| Non-white, N (%) | 9 (52.9%) | 44 (77.2%) | 17 (73.9%) | 54 (53.5%) | ||
| Missing, N(%) | 1 (5.9%) | 0 | 1 (4.3%) | 0 | ||
| Marital status | N (%) | N (%) | p | N (%) | N (%) | p |
| Single, N (%) | 4 (23.5%) | 30 (52.6%) | 0.049 | 6 (26.1%) | 42 (43.8%) | 0.124 |
| Married/stable union, N (%) | 12 (70.6%) | 21 (36.8%) | 14 (60.9%) | 52 (51.5%) | ||
| Divorced/widowed, N (%) | 0 | 0 | 1 (4.3%) | 2 (2%) | ||
| Missing (N, %) | 1 (5.9%) | 6 (10.5%) | 2 (8.7%) | 5 (5%) | ||
| Education (years) | N (%) | N (%) | p | N (%) | N (%) | p |
| Illiterate, N (%) | 0 | 1 (1.8%) | 0.02 | 0 | 2 (2.1%) | 0.956 |
| Some or all primary school, N (%) | 3 (17.6%) | 27 (47.4%) | 8 (34.8%) | 32 (31.6%) | ||
| Some or all secondary school, N (%) | 13 (76.5%) | 18 (31.6%) | 12 (52.2%) | 58 (57.4%) | ||
| Some or all college, N (%) | 0 | 3 (5.3%) | 1 (4.3%) | 4 (4%) | ||
| Missing, N (%) | 1 (5.9%) | 8 (14%) | 2 (8.7%) | 5 (5%) | ||
| Clinical characteristics | N (%) | N (%) | p | N (%) | N (%) | p |
| Median gestational age at baseline weight in weeks (IQR) | 14 (9.1–18.9) | 20 (14.8–25.3) | 0.02 | 19.5 (14–25) | 19 (13–25) | 0.492 |
| Missing N (%) | 1 (5.9%) | 4 (7%) | 3 (13%) | 7 (6.9%) | ||
| Median (IQR) | Median (IQR) | p | Median (IQR) | Median (IQR) | P | |
| Baseline weight (kg) | 56 (51.1–61) | 55.5 (51.2–59.8) | 0.832 | 84.6 (72.9–96.3) | 74.6 (66.1–83.1) | 0.013 |
| Missing, N (%) | 0 | 0 | ||||
| Baseline BMI (kg/m 2) | 23.1 (20–26.2) | 22.7 (21.2–24.1) | 0.883 | 31.6 (29.5–33.7) | 29.1 (25.7–32.3) | 0.135 |
| Missing, N (%) | 0 | 0 | 0 | 0 | ||
| INSTI use before baseline weight (days) | 542 (261–823) | 0 (0–0) | NA* | 600 (374–827) | 0 (0–0) | NA* |
| Missing N (%) | 0 | 0 | 0 | 0 | ||
| INSTI use from baseline to near delivery (weeks) | 21 (15–27) | 18 (13.5–22.5) | 0.386 | 17 (10.2–23.9) | 17 (10.4–23.7) | 0.775 |
| Missing N (%) | 0 | 0 | 0 | 1 (1%) | ||
| INSTI use from initiation to delivery (days) | 619 (330–906) | 140 (106–174) | <0.001 | 696 (521–872) | 141 (100–182) | <0.001 |
| Missing, N (%) | 1 (5.9%) | 4 (7%) | 3 (13%) | 5 (5%) | ||
| Virologic and immunological characteristics | Median (IQR) | Median (IQR) | p | Median (IQR) | Median (IQR) | p |
| CD4 count (cells/mm3) at baseline (IQR) | 497 (323–671) | 369 (199–539) | 0.075 | 596 (417–775) | 433 (276–591) | 0.004 |
| Missing, N (%) | 1 (5.9%) | 1 (1.8%) | 1 (4.4%) | 2 (2%) | ||
| Log10 HIV RNA copies/mL at baseline (IQR) | 0 (0–1.3) | 4.1 (3.5–4.7) | <0.001 | 0 (0–0.8) | 3.8 (3.2–4.4) | <0.001 |
| Missing, N (%) | 0 | 1 (1.8%) | 1 (4.4%) | 3 (3%) | ||
| HIV viral load (copies/mL) at enrollment copies/mL | N (%) | N (%) | p | N (%) | N (%) | p |
| <200 | 12 (70.6%) | 2 (3.6%) | <0.001 | 18 (78.3%) | 12 (12.1%) | <0.001 |
| 200–999 | 2 (11.8%) | 8 (143%) | 1 (4.4%) | 10 (10.1%) | ||
| 1,000–9,999 | 1 (5.9%) | 15 (26.8%) | 2 (8.7%) | 35 (35.4%) | ||
| ≥10,000 | 2 (11.8%) | 31 (55.4%) | 1 (4.4%) | 42 (42.4%) | ||
| Missing, N (%) | 0 | 1 (1.8%) | 1 (4.4%) | 2 (2%) | ||
“NA” = “not applicable”. The statistical test could not be performed because the variable was zero for all participants in one group.
Figure 2.
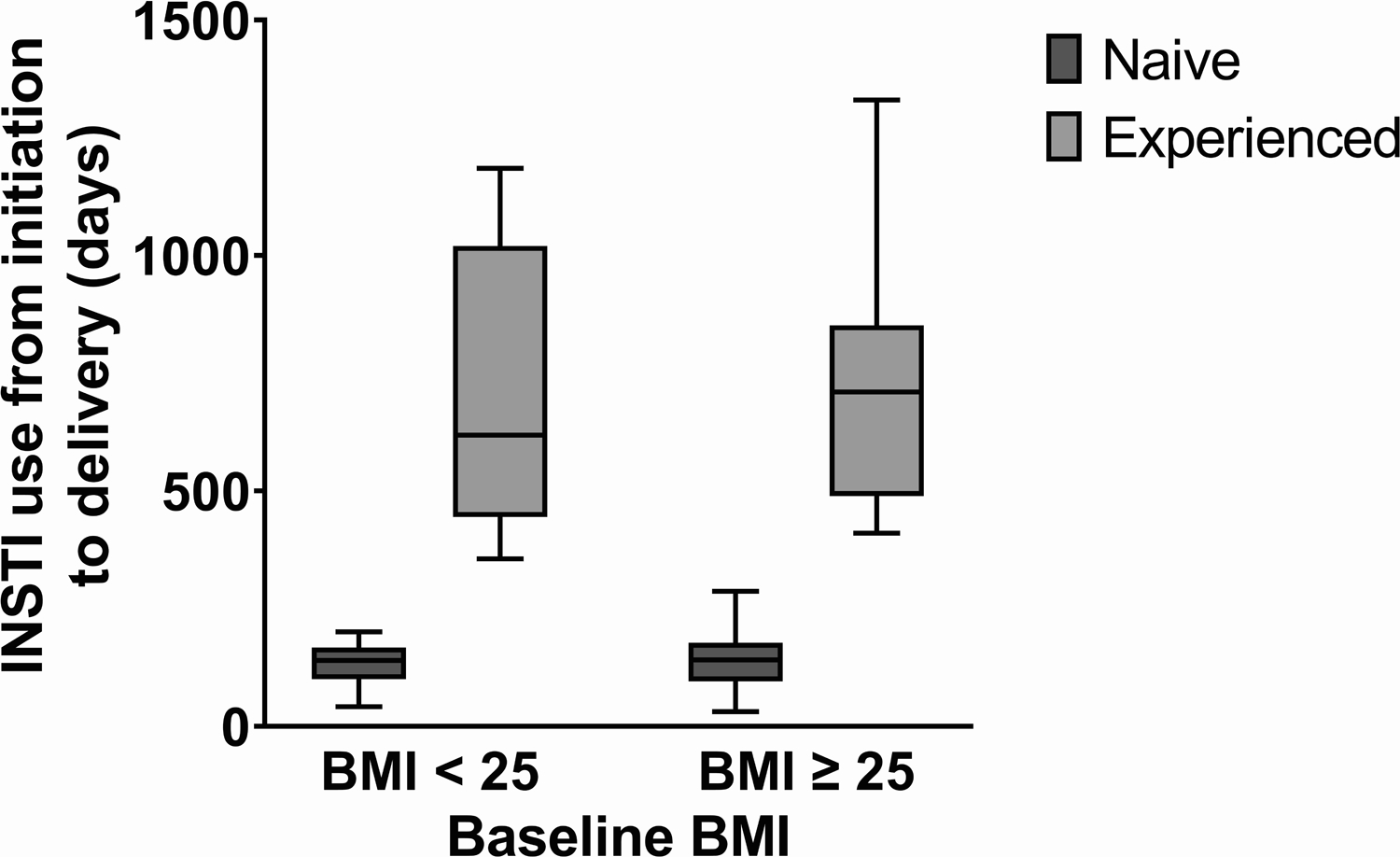
Duration of INSTI use by treatment-experience and BMI (body mass index).
Near delivery, median HIV RNA was undetectable for all groups, as seen in Table 2A. Among the participants who were overweight or obese at baseline, median weight near delivery was 90 kg in the treatment-experienced group and 82.3 kg in the naive group (p=0.026). In the baseline BMI < 25 subgroup, the proportion of participants experiencing low, normal, or high weight gain was significantly different between the treatment-naive and treatment-experienced groups, as seen in Table 2B and Figure 3. Among the participants who were treatment-experienced 2/17 (11.8%) had excess gestational weight gain, whereas in the group of treatment-naive participants, 18/57 (31.6%) had excess gestational weight gain (p=0.004).
Table 2A.
Virologic, maternal, and obstetric outcomes of pregnant women living with HIV in use of INSTI, 2014–2021 (N=198). In the table below, p-values < 0.05 are bolded.*
| Baseline BMI < 25 | Baseline BMI ≥ 25 | |||||
|---|---|---|---|---|---|---|
| Conceived in use of cART (N=17) | Initiated cART in pregnancy (N=57) | Conceived in use of CART (N=23) | Initiated CART in pregnancy (N=101) | |||
| Median (IQR) | Median (IQR) | p | Median (IQR) | Median (IQR) | p | |
| Log10 HIV RNA near delivery copies/mL | 0 (0–0.8) | 0 (0–0) | 0.16 | 0 (0–0) | 0 (0–0.8) | 0.145 |
| Missing, N (%) | 1 (5.9%) | 1 (1.8%) | 2 (8.7%) | 3 (3%) | ||
| Weight near delivery (kg) | 66.8 (60.2–73.5) | 63.7 (56.8–70.6) | 0.748 | 90 (76.8–103.3) | 82.3 (74.7–89.9) | 0.026 |
| Missing, N (%) | 0 | 0 | 0 | 0 | ||
| Obstetric and neonatal outcomes | N (%) | N (%) | p | N (%) | N (%) | P |
| Abortion/stillbirth/neonatal death, N (%) | 0 | 0 | NA* | 0 | 0 | NA |
| Missing, N (%) | 0 | 0 | 1 (4.4%) | 0 | ||
| Prematurity < 37 weeks, N (%) | 2 (11.8%) | 3 (5.3%) | 0.349 | 3 (13%) | 7 (6.9%) | 0.34 |
| Missing, N (%) | 0 | 0 | 0 | 0 | ||
| Prematurity < 35 weeks, N (%) | 1 (5.9%) | 0 | 0.065 | 1 (4.3%) | 2 (2%) | 0.463 |
| Missing, N (%) | 0 | 0 | 0 | 0 | ||
| Low birth weight (<2500 g), N (%) | 3 (17.6%) | 10 (17.5%) | 0.992 | 2 (8.7%) | 8 (7.9%) | 0.902 |
| Missing, N (%) | 0 | 0 | 0 | 0 | ||
| Extremely low birth weight (<1500 g), N (%) | 1 (5.9%) | 0 | 0.065 | 0 | 1 (1%) | 0.632 |
| Missing, N (%) | 0 | 0 | 0 | 0 | ||
| Small for gestational age, N (%) | 1 (5.9%) | 8 (14%) | 0.367 | 0 | 8 (7.9%) | 0.163 |
| Missing, N (%) | 0 | 0 | 0 | 0 | ||
| Macrosomia, N (%) | 0 | 0 | NA* | 0 | 0 | NA* |
| Missing, N (%) | 0 | 0 | 0 | 0 | ||
| Composite adverse neonatal outcome, N (%) | 4 (23.5%) | 15 (26.3%) | 0.817 | 3 (13%) | 17 (16.8%) | 0.656 |
| Missing, N (%) | 0 | 0 | 0 | 0 | ||
“NA” = “not applicable”. The statistical test could not be performed because the variable was zero for all participants in one group.
Table 2B.
Percent of naive and experienced participants by weight change category and baseline BMI. In the table below, p-values < 0.05 are bolded.
| Baseline BMI < 25 | Baseline BMI ≥ 25 | |||||
|---|---|---|---|---|---|---|
| Conceived in use of cART (N=17) | Initiated cART in pregnancy (N=57) | Conceived in use of CART (N=23) | Initiated CART in pregnancy (N=101) | |||
| Weight gain category (kg/week) | N (%) | N (%) | p | N (%) | N(%) | p |
| Low (<0.18), N (%) | 5/17 (29.4%) | 2/57 (3.5%) | 0.004 | 10 (43.5%) | 30 (29.7%) | 0.429 |
| Normal (>=0.18 and <=0.59), N (%) | 10/17 (58.8%) | 37/57 (64.9%) | 10 (43.%) | 52 (51.5%) | ||
| High (>0.59), N (%) | 2/17 (11.8%) | 18/57 (31.6%) | 3 (13%) | 19 (18.8%) | ||
Figure 3.
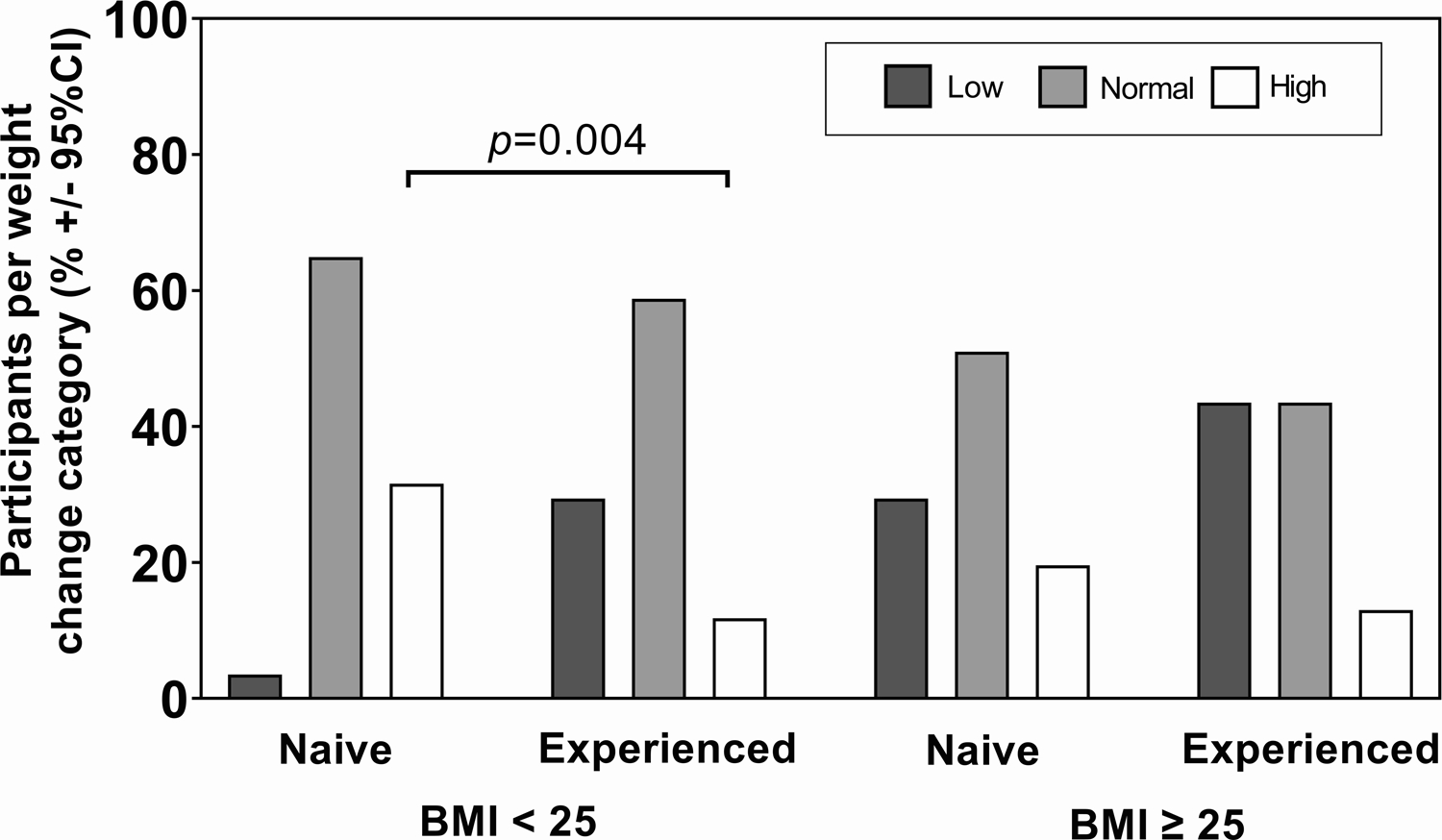
Weight change among pregnant women living with HIV in use of INSTI, 2014–2021 (N=199). Percent of naive and experienced participants by weight change category and baseline BMI. The categories of change were: low (< 0.18 kg/week), normal (0.18–0.59 kg/week), and high (>0.59 kg/week).
With respect to obstetric and neonatal outcomes, there were no stillbirths or neonatal deaths. Among underweight and normal weight participants, the rate of preterm delivery was 2/17 (11.8%) for those who were INSTI-experienced and 3/57 (5.3%) for the INSTI-naive (p=0.349). In the overweight and obese group, the prematurity rate (<37 weeks) was 3/23 (13%) among INSTI-experienced participants and 7/101 (6.9%) for those who were INSTI-naive (p=0.34). Regarding low birth weight (< 2500 g), the frequency among the underweight and normal weight participants who were INSTI-experienced was 3/17 (17.6%) versus 10/57 (17.5%) in the INSTI-experienced (p=0.992). Within the group of participants who were overweight or obese, the INSTI-experienced mothers had a preterm delivery rate (< 35 weeks) of 1/23 (4.3%) versus 2/101 (2%) for those who were INSTI-naive (p=0.499). There were no cases of macrosomia in the cohort.
Discussion
In this real world study, excess gestational weight gain was more frequent among women who were INSTI-naive both among the BMI < 25 and the BMI ≥ 25 groups. However, the proportion of participants per weight change category was only significantly different between INSTI-naive and experienced women with baseline BMI < 25. In particular, the baseline BMI < 25 group had the highest rates of excess gestational weight gain (31.6%), followed by INSTI-naive participants who were overweight or obese at baseline (18.8%). INSTI-experienced participants had the lowest rates of excess gestational weight gain, at approximately 12%, irrespective of baseline BMI. These rankings of excess weight gain confirm our initial hypothesis that women who started cART during pregnancy would experience higher rates of gestational weight gain than INSTI-experienced women. The present results provide real-world confirmation of gestational weight gain associated with the use of INSTI-based regimens, and therefore supports the findings of studies such as the NICHD P1081 and IMPAACT 2010 trials (9, 10).
Although reviewing all of the mechanisms through which INSTIs may lead to weight gain is beyond the scope of this study, we will mention some mechanisms that are the topic of ongoing research. These include damaging adipocytes and altering appetite or the gut microbiome (16, 17). Furthermore, it has long been known that beginning cART is accompanied by weight gain, a “return to health effect” that is more pronounced in individuals with low CD4 cell counts and high HIV RNA viral load (18). Our participants who were starting INSTI-based regimens had significantly lower baseline CD4 cell counts and higher HIV RNA viral load than those who were INSTI-experienced. We can conjecture that the return to health effect was more pronounced in naive than experienced participants. The higher frequency of excess gestational weight gain in treatment-naive participants may have also been multifactorial involving a return health together with the effects of INSTIs on appetite, fat deposits, and intestinal flora (16).
We found that in pregnant women who used INSTI rates of neonatal outcomes including SGA, low birth weight, and macrosomia did not differ significantly by treatment-experience or baseline BMI. This is similar to the findings of a cohort study of 333 pregnant women living with HIV in Atlanta, Georgia, USA, in which those with excessive gestational weight gain did not have higher rates of obstetric and neonatal complications (19). In addition, the IMPAACT 2010 study found that in 643 participants in 9 countries, excess gestational weight gain was not associated with adverse pregnancy outcomes (10). An analysis of the Tsepamo cohort in Botswana included 4,467 treatment-experienced pregnant women measured baseline weight and weight gain in the second trimester (20). Although BMI was not measured, the study found that excess weight gain was associated with macrosomia. As noted above, in our cohort, there were no cases of macrosomia. Evaluating all of these studies together, the available evidence is compatible with a protective or neutral effect of INSTI-associated gestational weight gain on adverse maternal and neonatal outcomes.
Our results indicated that excess gestational weight gain was most frequent in underweight or normal weight women who started INSTI-based regimens. This finding could inform education about a healthy lifestyle for pregnant women living with HIV. HIV management of underweight or normal weight pregnant women who are starting INSTI could prioritize education about the benefits of diet and physical activity, which are safe and effective for reducing gestational weight gain (21).
A novel contribution of the present study is that to our knowledge it is the first in a low or middle income country that investigated BMI in pregnant women living with HIV, in addition to raw weight. A new insight that emerged from this was that BMI influenced the magnitude of gestational weight change, with participants with low or normal BMI gaining more weight during pregnancy than those with higher BMI. Another strength of the study is that participants received care at a single HIV referral institution that where HIV management adhered strictly to national guidelines, data was captured using standardized forms, all physicians received the same training, and there was an established routine of caring for pregnant women living with HIV. Among the weaknesses of the study was the small sample size that limits its generalizability, and the imbalance in size between the naive and experienced groups. In addition, as tenofovir may also be associated with weight gain, TDF may have also influenced weight gain in the study participants. Furthermore, pre-pregnancy weight was not available.
In summary, pregnant women living with HIV who started an INSTI-based regimen during pregnancy with BMI < 25 showed greater gestational weight gain compared to those who were INSTI-experienced. This cohort had no adverse neonatal outcomes that were significantly different based on treatment-experience or baseline BMI. To this extent, our findings are compatible with other studies that have concluded that insufficient gestational weight gain, but not necessarily excess gestational weight gain, is associated with adverse maternal and neonatal outcomes.
Acknowledgements
We thank the pregnant women who participated in the study; all the staff of the Infectious Diseases Department, the Neonatology Department, and the Maternity-Fetal Department at the Hospital Federal dos Servidores do Estado; and the Brazilian Ministry of Health. Funding: This research did not receive any specific grant funding.
Funding statement:
This work received no specific funding.
Footnotes
Ethics approval statement: The study was approved by the local IRB.
Patient consent statement: Patients provided informed consent.
Conflict of interests disclosure: The authors declare no conflicts of interest.
Data availability statement:
The data that support the findings of this study are openly available in GitHub at https://doi.org/10.5281/zenodo.6941822, reference number v1.0.0.
References
- 1.World Health Organization. Updated Recommendations on HIV Prevention, Infant Diagnosis, Antiretroviral Initiation and Monitoring. Geneva: WHO; 2021. [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents Panel Members and Consultants. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Washington, DC: U. S. Department of Health and Human Services; 2021. [Google Scholar]
- 3.European AIDS Clinical Society. Guidelines. Version 11.0. Brussels, Belgium: EACS; 2021. [Google Scholar]
- 4.British HIV Association. BHIVA guidelines on antiretroviral treatment for adults living with HIV-1 2022 - consultation version. London: BHIVA; 2022. [Google Scholar]
- 5.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. New England Journal of Medicine. 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 6.Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clinical Infectious Diseases. 2020; 71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voerman E, Santos S, Inskip H, et al. Association of gestational weight gain with adverse maternal and infant outcomes. -Journal of the American Medical Association. 2019; 321:1702–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.João EC, Morrison RL, Shapiro DE, et al. Raltegravir versus efavirenz in antiretroviral-naive pregnant women living with HIV (NICHD P1081): an open-label, randomised, controlled, phase 4 trial. The Lancet HIV. 2020; 7:e322–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutinho CM, Warshaw M, Duarte G, et al. Weight gain with raltegravir vs. efavirenz during pregnancy. Poster Abstract 288. Conference on Retroviruses and Opportunistic Infections Abstracts eBook. Denver, 2022: 273–4. [Google Scholar]
- 10.Hoffman RM, Ziemba L, Brummel S, et al. Antenatal weight gain and adverse pregnancy outcomes in IMPAACT 2010. Oral Abstracts No. 176. Conference on Retroviruses and Opportunistic Infections Abstract eBook. Boston, USA, 2021: 57–8. [Google Scholar]
- 11.WHO. Global HIV Programme data and statistics. https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics. WHO; 2022. [Google Scholar]
- 12.Rasi V, Peters H, Sconza R, et al. Trends in antiretroviral use in pregnancy in the UK and Ireland, 2008–2018. Hiv Medicine. 2022; 23:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benamor Teixeira ML, Fuller TL, Fragoso Da Silveira Gouvêa MI, et al. Efficacy of three antiretroviral regimens initiated during pregnancy: clinical experience in Rio de Janeiro. Antimicrobial Agents and Chemotherapy. 2020; 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stirnemann J, Villar J, Salomon LJ, et al. International estimated fetal weight standards of the INTERGROWTH-21st Project. Ultrasound in Obstetrics & Gynecology. 2017; 49:478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. Washington, DC; 2009. [PubMed] [Google Scholar]
- 16.Andrikopoulou M, Panigrahi SK, Jaconia GD, Gyamfi-Bannerman C, Smiley RM and Page-Wilson G. Pregnancy-specific adaptations in leptin and melanocortin neuropeptides in early human gestation. The Journal of Clinical Endocrinology & Metabolism. 2021; 106:e5156–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai R, Lv S, Wu H and Dai L. Effects of different integrase strand transfer inhibitors on body weight in patients with HIV/AIDS: a network meta-analysis. BMC Infectious Diseases. 2022; 22:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant PM, Kitch D, McComsey GA, et al. Long-term body composition changes in antiretroviral-treated HIV-infected individuals. Aids. 2016; 30:2805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph NT, Satten GA, Williams RE, et al. The effect of antiretroviral therapy for the treatment of Human Immunodeficiency Virus (HIV)-1 in pregnancy on gestational weight gain. Clinical Infectious Diseases. 2021;ciab994. [DOI] [PubMed] [Google Scholar]
- 20.Zash R, Caniglia EC, Diseko M, et al. Maternal weight and birth outcomes among women on antiretroviral treatment from conception in a birth surveillance study in Botswana. Journal of the International Aids Society. 2021; 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kominiarek MA and Peaceman AM. Gestational weight gain. American Journal of Obstetrics and Gynecology. 2017; 217:642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in GitHub at https://doi.org/10.5281/zenodo.6941822, reference number v1.0.0.


