Abstract
Background & Aims:
Portopulmonary hypertension (PoPH) is type of pulmonary arterial hypertension occurring exclusively in those with portal hypertensive liver disease. Liver transplantation (LT) can significantly improve outcomes. Current guidelines counsel against immediate adjustments to targeted therapy post-LT, and suggest routine echocardiography as sufficiently informative to guide therapeutic adjustments. Current practice patterns for adjusting targeted therapy post-LT in PoPH, and how they compare to guidelines, are not well established.
Approach & Results:
We performed an IRB-approved, cross-sectional mixed-methods survey-based study of United States PoPH providers. Anonymized requests to complete the survey were sent through professional networks between 1/20/22 and 4/20/22. Responses were compared between cardiologists and pulmonologists using Fisher’s Exact Tests, at a significance of 0.05. A total of 85 PoPH physicians were included in the final analysis (66% pulmonologists, 34% cardiologists). Following LT, the majority of respondents routinely used a combination of standard cardiopulmonary assessment modalities to guide adjustment of targeted therapy following LT. Most respondents (69%) started by adjusting parenteral prostacyclins with small titrations and frequent reassessments within three months of LT, but some (19.7%) adjusted targeted therapy immediately post-LT.
Conclusions:
Our survey of PoPH providers showed that the majority of respondents favored serial integrated cardiopulmonary testing (including routine right heart catheterization) to guide adjustment of targeted therapy in PoPH after LT, and almost one fifth of respondents weaned therapy immediately after LT. Our study demonstrates heterogeneity in PoPH practice patterns after LT, highlights differences between current practice patterns and the most recent guidelines, emphasizes the need for additional research, and supports a team-based approach to standardize care for these high-risk patients and optimize post-LT outcomes.
Keywords: pulmonary hypertension, prostacyclin, guidelines, right heart catheterization, weaning
Introduction
Portopulmonary hypertension (PoPH) is a particularly lethal form of pulmonary arterial hypertension (PAH) occurring exclusively in those with portal hypertensive liver disease, estimated to afflict between 6–10% of all patients with liver cirrhosis (1–4). Even with the use of targeted pulmonary vasodilator therapy, many PoPH patients will unfortunately succumb to progressive right heart failure (5–7). In select PoPH patients, liver transplantation (LT) can significantly improve morbidity, mortality, and pulmonary vascular disease severity; many PoPH patients are able to decrease or fully discontinue their targeted pulmonary vasodilator therapy following LT (8–10). In the absence of high-quality evidence, PoPH treatment strategies are primarily based on retrospective reviews, case series, and expert opinion, and there exists considerable variability in the approach to targeted therapy in PoPH (5, 11–13). This uncertainty extends to PoPH patients following successful LT: there are no clear recommendations on the optimal process to wean targeted vasodilator therapy, and PoPH can recur or worsen following LT in some patients. The most recent guidelines, published by the International Liver Transplant Society in 2016, recommend serial echocardiography every 4–6 months following LT, suggest adjustment of targeted therapy can be accomplished successfully by monitoring serial echocardiographic examinations, note that right heart catheterization post-LT is not routinely advised unless “clinically indicated”, and counsel against treatment adjustments in the immediate post-LT period (5). As no controlled studies have addressed the process of weaning from targeted vasodilator therapy following LT in PoPH, and the agreement between clinical practice and published guidelines is unknown, the objective of this study was to better understand the practice patterns of PoPH providers following LT. We hypothesized there would be significant discrepancies between current guideline recommendations and actual practice patterns for adjusting targeted vasodilator therapy and monitoring PoPH patients following LT.
Materials and Methods
We performed a cross-sectional mixed-methods survey-based study of a convenience sample of pulmonary vascular disease physicians who manage PoPH patients in the United States. Pulmonary hypertension providers (Physicians with an MD or DO degree) who were members of either the Pulmonary Hypertension Association’s Pulmonary Hypertension Clinician and Researcher’s network (PHCR) (N=126) or the International Society of Heart and Lung Transplantation’s Pulmonary Vascular Disease Interdisciplinary Network (ISHLT-PVD) (N=46) were sent requests to complete the survey. Survey requests were sent between January 20th 2022 and April 20th 2022 via email link. Survey questions included basic demographic information as well as questions concerning physician practices regarding the management of PoPH following LT. Branching logic was used to display selected questions on the basis of prior survey responses as detailed in the survey questions supplement (Supplementary File S1).
Survey responses were anonymized, and only responses from providers confirming practice in the United States were included. Responses are reported by specialty (cardiology versus pulmonology). Responses regarding PoPH management strategies following LT were also separated into low (less than 5), medium (5–10), and high (more than 10 PoPH patients undergoing LT annually) volume providers and compared. Responses were also compared by United Network for Organ Sharing (UNOS) regions categorized by the median MELD at transplant into low (<22, regions 3, 6, 10, 11), medium (22–25, regions 2, 4, 7, 8), and high (>25, regions 1, 5, 9) MELD groups (14).
Descriptive data are reported as number (%). Associations between survey responses and respondent characteristics were assessed using Fisher’s Exact Tests. A two-sided p-value of 0.05 was considered significant. All analyses were performed using R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria). This study conforms to the Declarations of Helsinki and Istanbul, was reviewed and approved by our institutional review board (IRB 2021–0927), and all subjects provided informed consent to participate.
Results
Of the 172 subjects sent requests, a total of 85 (49.4%) PAH physicians who practice in the United States completed the survey and were included in the final analysis (Table 1). Approximately two-thirds of the respondents (65.9%) were pulmonologists, with the remaining one-third (34.1%) cardiologists. Most respondents were experienced pulmonary vascular disease specialists, with two-thirds (67.1%) having cared for PAH patients for at least 10 years. Respondents were well distributed by geographic region. PoPH volume by center was well distributed, with approximately one-third (31.8%) practicing in centers managing 10 or fewer PoPH patients in the past five years, a comparable proportion (37.6%) practicing in centers managing 25–50 PoPH patients in the past five years, and roughly one-tenth (10.6%) practicing in centers caring for more than 50 PoPH patients in the past five years. Individual providers managed a median of 3 PoPH patients currently being evaluated for LT, the minority of which (30.0%) were felt likely to undergo LT. The majority of respondents (83.5%) managed PoPH following LT, with most (70.4%) managing less than 5 PoPH patients after LT annually.
Table 1:
Survey Respondent Characteristics (N=85)
| Characteristic | Frequency (%) or Median (IQR) |
|---|---|
| Specialty | |
| Pulmonology | 56 (65.9%) |
| Cardiology | 29 (34.1%) |
| Provider Age (years) | |
| 31–40 | 14 (16.5%) |
| 41–50 | 33 (38.8%) |
| 51–60 | 23 (27.1%) |
| 61–70 | 10 (11.8%) |
| 71+ | 3 (3.5%) |
| Don’t wish to Disclose | 2 (2.4%) |
| Years of PAH Practice | |
| Less than 5 years | 9 (10.6%) |
| 5–10 years | 19 (22.4%) |
| 10–20 years | 34 (40.0%) |
| More than 20 years | 23 (27.1%) |
| UNOS Region of Practice | |
| Region 1 (CT, MA, ME, NH, RI, Eastern VT) | 6 (7.1%) |
| Region 2 (DE, DC, MD, NJ, PA, WV, Northern VA) | 8 (9.4%) |
| Region 3 (AL, AR, FL, GA, LA, MS, Puerto Rico) | 5 (5.9%) |
| Region 4 (OK, TX) | 7 (8.2%) |
| Region 5 (AZ, CA, NV, NM, UT) | 12 (14.1%) |
| Region 6 (AK, HI, ID, MT, OR, WA) | 1 (1.2%) |
| Region 7 (IL, MN, ND, SD, WI) | 5 (5.9%) |
| Region 8 (CO, IA, KS, MO, NE, WY) | 5 (5.9%) |
| Region 9 (NY, Western VT) | 5 (5.9%) |
| Region 10 (IN, MI, OH) | 12 (14.1%) |
| Region 11 (KY, NC, SC, TN, VA) | 5 (5.9%) |
| Number of PoPH patients managed in past 5 years | |
| 0–10 patients | 27 (31.8%) |
| 10–25 patients | 17 (20.0%) |
| 25–50 patients | 32 (37.6%) |
| 50–75 patients | 5 (5.9%) |
| 75–100 patients | 2 (2.4%) |
| More than 100 patients | 2 (2.4%) |
| Number of PoPH patients currently evaluated for LT | 3 (1 to 8) |
| Percentage of PoPH patients likely to undergo LT | 30.0% (0.5% to 55.0%) |
| Manages PoPH after Liver Transplant | 71 (83.5%) |
| Number of PoPH patients undergoing LT managed annually* | |
| Less than 5 | 50 (70.4%) |
| 5–10 | 15 (21.1%) |
| More than 10 | 6 (8.5%) |
Abbreviations: PoPH = Portopulmonary Hypertension, LT = Liver Transplantation, UNOS = United Network for Organ Sharing
Among those respondents who reported managing PoPH patients after LT (N=71)
There was consistency in managing PoPH patients being evaluated for LT between cardiologists and pulmonologists (Figure 1, Figure 2, Supplementary Table S2). Although all targeted therapy drug categories were used, most respondents favored a combination of Phosphodiesterase-5 Inhibitors (PDE-5i, including sildenafil and tadalafil), Endothelin Receptor Antagonists (ERA, including bosentan, ambrisentan, and macitentan), and parenteral prostacyclin medications (including parenteral treprostinil and epoprostenol) as the primary management strategy for PoPH patients, including those awaiting LT. There was a higher proportion of PDE-5i and ERA use by cardiologists versus pulmonologists in the management of all PoPH (90.1% versus 75.6% and 81.8% versus 49.0% respectively), and when optimizing PoPH patients for LT ( 86.4% versus 81.6% and 68.2% versus 42.9% respectively). In contrast to this, pulmonologists were more likely to utilize inhaled prostacyclin therapy in the management of all PoPH (16.3% versus 4.5%) and when optimizing PoPH patients for LT (18.4% versus 9.1%). Parenteral prostacyclin therapy was prioritized by both cardiologists and pulmonologist to manage PoPH patients awaiting LT (59.2% of PoPH patients awaiting LT, versus 40.8% of all PoPH patients), with the majority of respondents favoring intravenous parenteral prostacyclin administration over the sub-cutaneous route (62.0% versus 29.6%).
Figure 1:
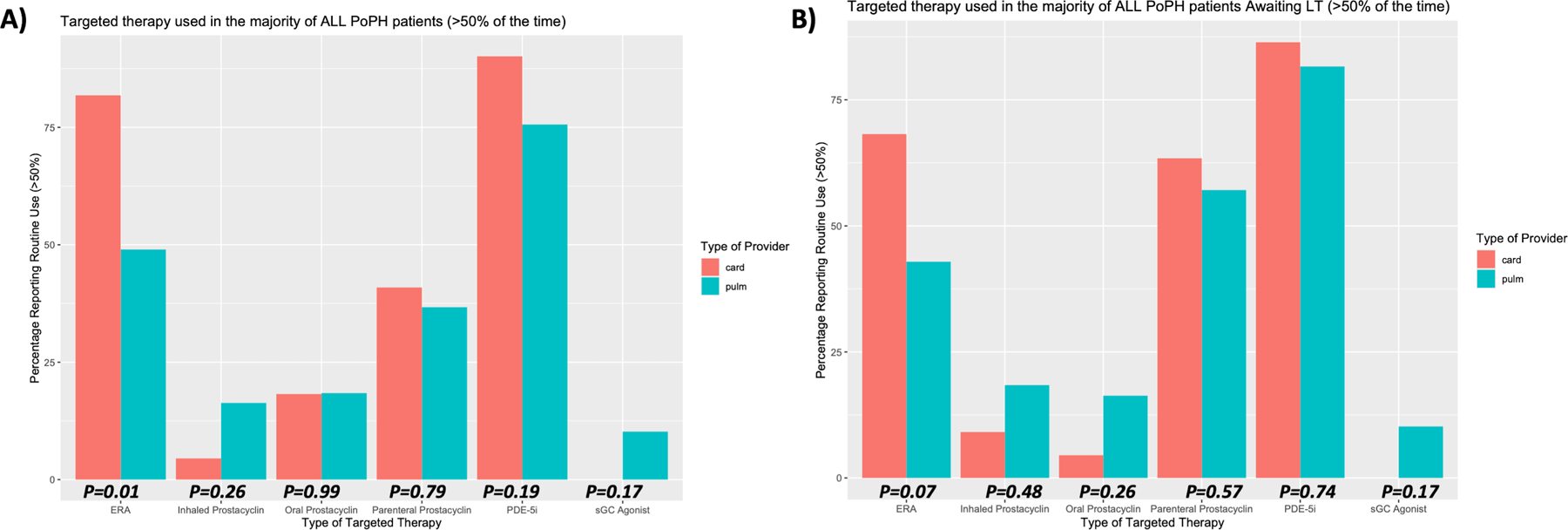
Targeted Therapy Used in PoPH Patients Stratified by Provider Specialty
Attitudes regarding type of targeted PAH therapy routinely used in all PoPH patients, and in those PoPH patients currently being evaluated for liver transplantation. Responses are stratified by provider specialty (Cardiology or Pulmonology), and p-values shown are computed using Fisher’s Exact Test between groups.
Figure 2:
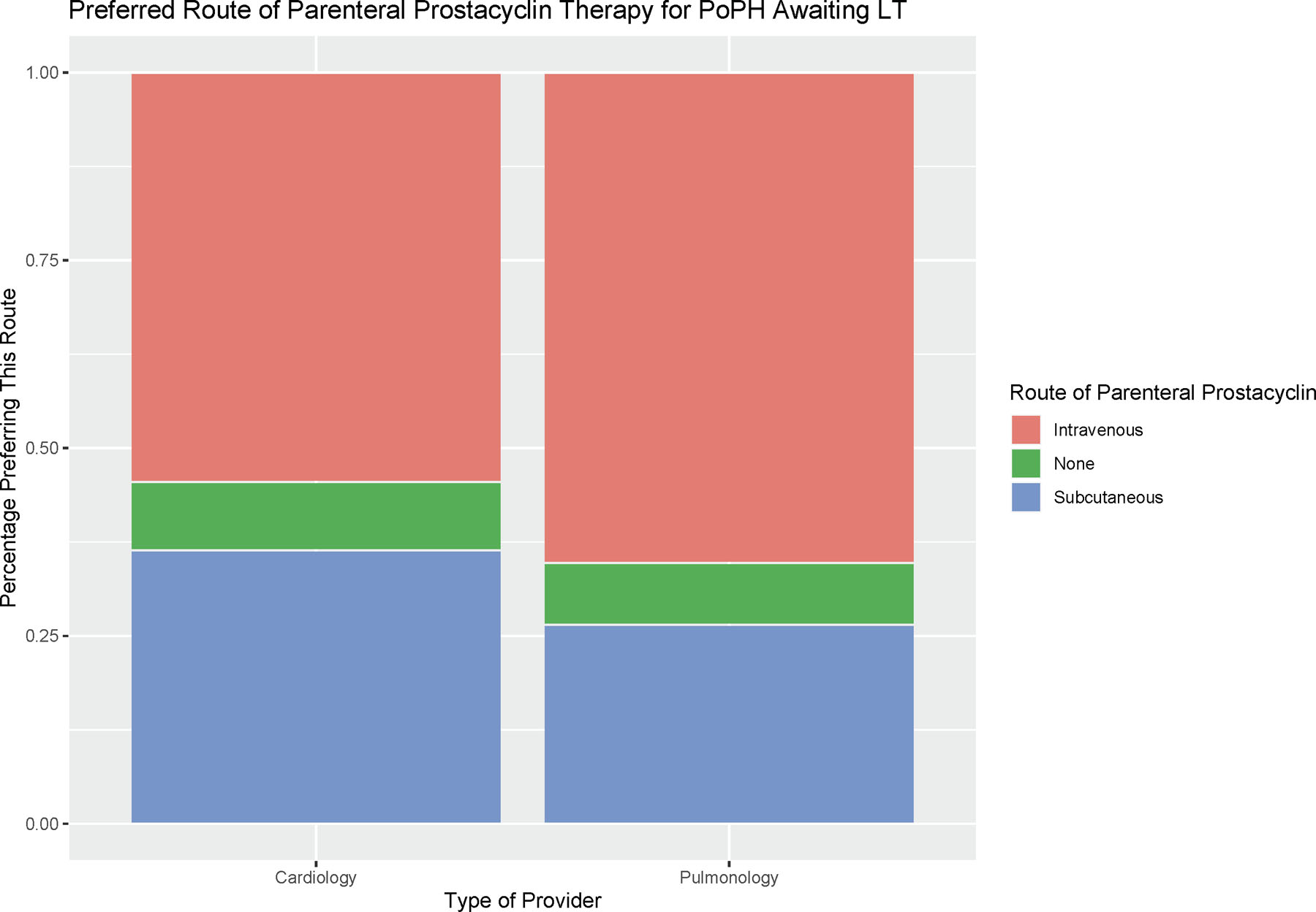
Preferred Route of Parenteral Prostacyclin Therapy for PoPH Awaiting LT
Preferred route of parenteral prostacyclin therapy (intravenous, sub-cutaneous, or no preference) are reported for cardiology and pulmonology providers.
The majority of respondents used a combination of standard cardiopulmonary assessment modalities (echocardiography, right heart catheterization, sub-maximal exercise testing, and circulating natriuretic peptide levels) to guide adjustment of targeted therapy following LT (Table 2, Supplementary Table S2, Figure 3). All cardiology respondents reported routinely using right heart catheterization to guide adjustment of PoPH therapy after LT, a practice pattern shared by the majority (90.0%) of pulmonologists. Echocardiography was broadly popular with all respondents (96% of pulmonologists and 95.5% of cardiologists). Pulmonologists more frequently employed six-minute walk testing to guide adjustment of PoPH therapy after LT (91.8% versus 72.7%), whereas cardiologists were more likely to use natriuretic peptide assays (95.5% versus 85.7%). Echocardiography was most frequently employed every three or six months to guide treatment adjustments. Six-minute walk testing and natriuretic peptide assays (BNP and NTproBNP) were often performed with each clinic visit, and most respondents indicating testing occurred routinely within the first three months following LT. Right heart catheterization was typically performed routinely between six months and one year after LT, although it is important to note that some respondents (7.1% of pulmonologists and 9.1% of cardiologists) indicated cardiac catheterization decisions are frequently informed by findings on transthoracic echocardiography, and thus may occur more or less frequently. A small minority of respondents (N=4, 5.6%) utilized either cardiopulmonary exercise testing or cardiac magnetic resonance imaging to guide adjustment of PoPH targeted therapy after LT.
Table 2:
Post LT Monitoring Questions and Responses (N=71)
| Survey Question | Pulmonologists (N=49) | Cardiologist (N=22) |
|---|---|---|
| Frequency of Echocardiography to guide adjustment of targeted therapy in PoPH after LT | ||
| Every 1–2 months | 2 (4.2%) | 2 (9.5%) |
| Every 3 months | 19 (40.4%) | 10 (47.6%) |
| Every 4–5 months | 3 (6.4%) | 3 (14.3%) |
| Every 6 months | 17 (36.2%) | 6 (28.6%) |
| Every 7–12 months | 5 (10.6%) | 0 (0.0%) |
| As Needed | 1 (2.1%) | 0 (0.0%) |
| Frequency of RHC to guide adjustment of targeted therapy in PoPH after LT | ||
| Every 1–2 months | 0 (0.0%) | 2 (9.1%) |
| Every 3 months | 6 (13.6%) | 2 (9.1%) |
| Every 4–5 months | 2 (4.5%) | 2 (9.1%) |
| Every 6 months | 13 (29.5%) | 7 (31.8%) |
| Every 7–12 months | 12 (27.2%) | 5 (22.7%) |
| Based on Echocardiogram | 3 (6.8%) | 2 (9.1%) |
| As Needed | 8 (18.2%) | 2 (9.1%) |
| Frequency of six-minute walk test to guide adjustment of targeted therapy in PoPH after LT | ||
| Every 1–2 months | 4 (8.9%) | 2 (12.5%) |
| Every 3 months | 30 (66.7%) | 8 (50.0%) |
| Every 4–5 months | 1 (2.2%) | 2 (12.5%) |
| Every 6 months | 8 (17.8%) | 4 (25.0%) |
| Every 7–12 months | 2 (4.4%) | 0 (0.0%) |
| As Needed | 0 (0.0%) | 0 (0.0%) |
| Frequency of BNP/NTproBNP to guide adjustment of targeted therapy in PoPH after LT | ||
| Every 1–2 months | 5 (11.9%) | 3 (14.3%) |
| Every 3 months | 24 (57.1%) | 13 (61.9%) |
| Every 4–5 months | 1 (2.4%) | 3 (14.3%) |
| Every 6 months | 9 (21.4%) | 1 (4.8%) |
| Every 7–12 months | 2 (4.8%) | 0 (0.0%) |
| As Needed | 1 (2.4%) | 1 (4.8%) |
Abbreviations: PoPH = Portopulmonary Hypertension, LT = Liver Transplant, RHC = Right Heart Catheterization, BNP = Brain Natriuretic Peptide, NTproBNP = N-Terminal prohormone of Brain Natriuretic Peptide
Figure 3:
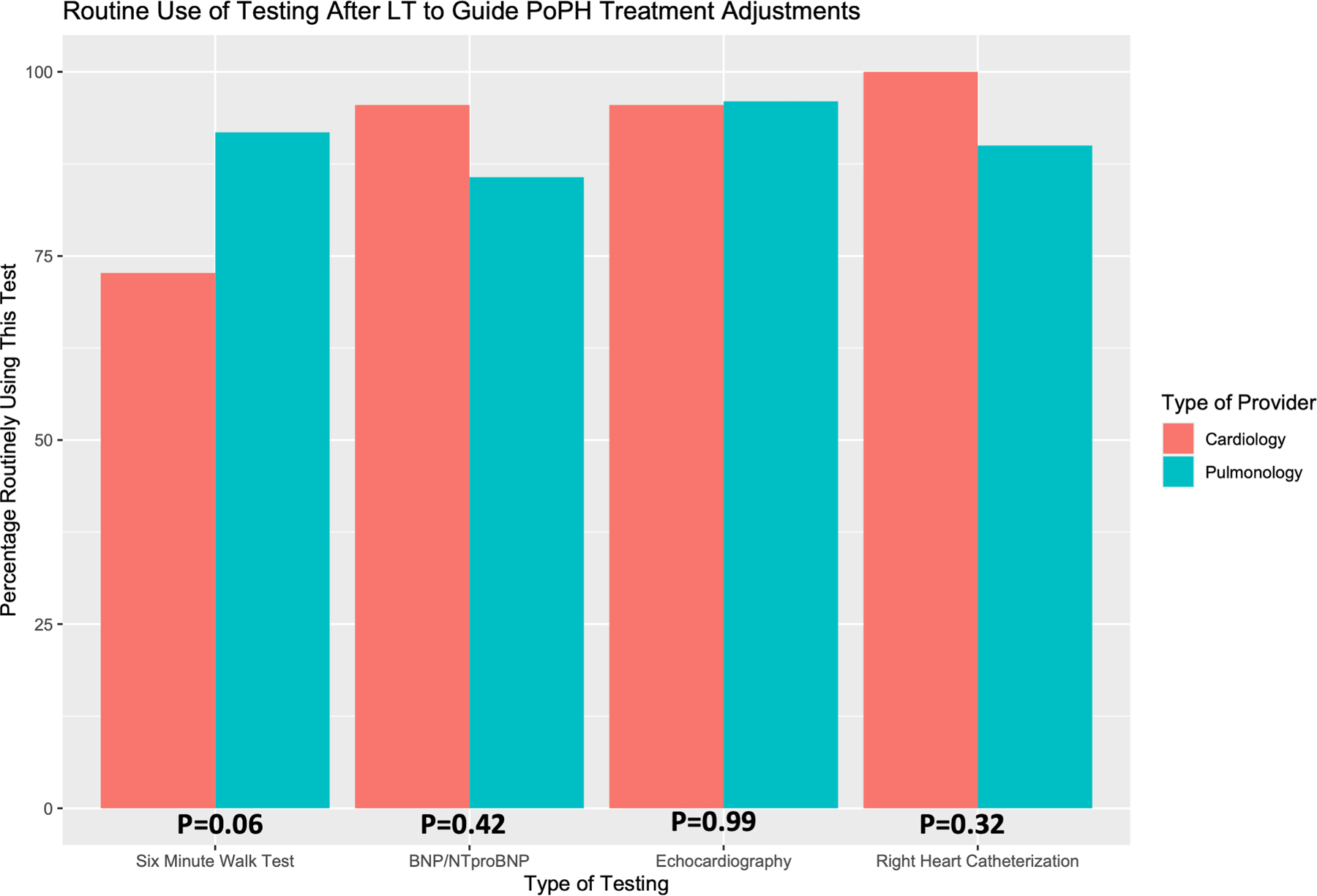
Routine Use of Cardiopulmonary Testing after LT to Guide Adjustment of PoPH Targeted Therapy
Attitudes regarding routine use of various types of cardiopulmonary testing after LT to inform adjustments to PoPH targeted therapy. Responses are stratified by provider specialty (Cardiology or Pulmonology), and p-values shown are computed using Fisher’s Exact Test between groups.
When comparing PoPH management strategies post-LT, there was very little variation stratified by provider volume of PoPH patients or regional median MELD at transplant (Supplementary Table S3). However, high volume PoPH providers (those caring for ten or more PoPH patients undergoing LT annually) were more likely to routinely utilize all cardiopulmonary testing modalities to guide treatment adjustment following LT as compared to their counterparts caring for fewer PoPH patients undergoing LT annually.
Following LT, respondents reported that it was common to attempt adjusting/weaning targeted therapy, with roughly half of all respondents (50.7%) always attempting targeted therapy de-escalation following LT (Table 3, Figure 4). Pulmonologists most often waited until at least three months after LT to attempt adjusting targeted PoPH therapy (N=33, 67.3%). Cardiologists on the other hand were more likely to adjust targeted therapy in PoPH sooner, regardless of treatment modality (oral, inhaled, or parenteral), with roughly half of all cardiology respondents (N=12, 54.5%) adjusting targeted therapy either immediately after LT or within the first two months following LT, and an additional third (N=7, 31.8%) adjusting therapy at three months. In general, respondents reported attempting to adjust parenteral prostacyclin therapy first (N= 49, 69.0%) and adjusting oral phosphodiesterase-5 inhibitor therapy last (N=51, 77.5%). When adjusting parenteral prostacyclin therapy (epoprostenol and treprostinil), the majority of cardiology and pulmonology respondents employed a similar approach, making small adjustments to parenteral therapy (titrating down by 25–50%, N=48, 67.6%) and evaluating response soon after treatment changes (within 1–2 months, N=42, 61.8%). Most respondents felt it would take on average between four and twelve months before oral targeted therapy could be discontinued in PoPH patients following LT (N=40, 56.3%), with a slightly quicker rate of parenteral therapy discontinuation (between three and six months, N=37, 52.1%), and with the caveat that not all PoPH patients would be able to discontinue targeted therapy following LT. There were no significant differences in PoPH management post-LT by regional median MELD at transplant (Supplementary Table S4). High-volume PoPH providers were more likely to always attempt to adjust targeted therapy in PoPH following LT relative to their counterparts practicing at medium or low-volume centers (83.3% versus 53.3% and 42% respectively), and compared to low volume providers were more likely to make small adjustments to parenteral prostacyclins (83.3% versus 58.0%) with rapid reassessment (83.3% versus 62.0%).
Table 3:
Post LT Treatment Adjustment Questions and Responses (N=71)
| Survey Question | Pulmonologists (N=49) | Cardiologist (N=22) | p-value |
|---|---|---|---|
| How frequently do you adjust targeted therapy in PoPH patients after LT? | 0.50 | ||
| Less than 10% of the time | 3 (6.1%) | 0 (0.0%) | |
| 10–25% of the time | 5 (10.2%) | 0 (0.0%) | |
| 25–50% of the time | 10 (20.4%) | 5 (22.7%) | |
| 50–75% of the time | 9 (18.4%) | 5 (22.7%) | |
| I always try to adjust targeted therapy in PoPH after LT | 22 (44.9%) | 12 (54.5%) | |
| How soon after LT do you adjust targeted therapy in PoPH patients after LT? | 0.10 | ||
| Immediately after LT | 6 (12.2%) | 8 (36.4%) | |
| 1–2 months after LT | 9 (18.4%) | 4 (18.2%) | |
| 3 months after LT | 24 (49.0%) | 7 (31.8%) | |
| 4–5 months after LT | 2 (4.1%) | 0 (0.0%) | |
| 6 months after LT | 7 (14.3%) | 1 (4.5%) | |
| Other Frequency | 1 (2.0%) | 2 (9.1%) | |
| When adjusting targeted therapy in PoPH after LT, which therapy is adjusted first? | 0.44 | ||
| Oral PDE-5i | 4 (8.2%) | 4 (18.2%) | |
| Oral sGC agonist | 0 (0.0%) | 0 (0.0%) | |
| Oral ERA | 5 (10.2%) | 2 (9.1%) | |
| Oral prostacyclin class | 4 (8.2%) | 0 (0.0%) | |
| Inhaled prostacyclin class | 3 (6.1%) | 0 (0.0%) | |
| Parenteral prostacyclin class | 33 (67.3%) | 16 (72.7%) | |
| When adjusting targeted therapy in PoPH after LT, which therapy is adjusted last? | 0.65 | ||
| Oral PDE-5i | 33 (67.3%) | 18 (81.8%) | |
| Oral sGC agonist | 1 (2.0%) | 0 (0.0%) | |
| Oral ERA | 10 (20.4%) | 2 (9.1%) | |
| Oral prostacyclin class | 0 (0.0%) | 0 (0.0%) | |
| Parenteral prostacyclin class | 5 (10.2%) | 2 (9.1%) | |
| When adjusting parenteral prostacyclin therapy, what strategy do you use? | 0.90 | ||
| Titrate by 25% and assess | 26 (53.1%) | 10 (45.5%) | |
| Titrate by 50% and assess | 7 (14.3%) | 5 (22.7%) | |
| Titrate by 75% and assess | 1 (2.0%) | 1 (4.5%) | |
| Titrate off (100%) and assess | 4 (8.2%) | 1 (4.5%) | |
| Other titration | 9 (18.4%) | 4 (18.2%) | |
| Do not use parenteral agents | 2 (4.1%) | 1 (4.5%) | |
| When adjusting parenteral prostacyclin therapy, how long do you titrate before reassessing?* | 0.82 | ||
| Over 1 month | 14 (29.8%) | 8 (38.1%) | |
| Over 1–2 months | 13 (27.7%) | 7 (33.3%) | |
| Over 3 months | 9 (19.1%) | 2 (9.5%) | |
| Over 3–6 months | 5 (10.6%) | 1 (4.8%) | |
| Other timeframe for titration | 6 (12.8%) | 3 (14.3%) | |
| On average, how long does it take to fully discontinue oral/inhaled targeted therapy after LT? | 0.35 | ||
| 1 month after LT | 0 (0.0%) | 1 (4.5%) | |
| 2 months after LT | 3 (6.1%) | 1 (4.5%) | |
| 3 months after LT | 5 (10.2%) | 5 (22.7%) | |
| 4–6 months after LT | 12 (24.5%) | 5 (22.7%) | |
| 7–12 months after LT | 19 (38.8%) | 4 (18.2%) | |
| Greater than 1 year after LT | 7 (14.3%) | 4 (18.2%) | |
| Other | 3 (6.1%) | 2 (9.1%) | |
| On average, how long does it take to fully discontinue parenteral targeted therapy after LT? | 0.20 | ||
| 1 month after LT | 2 (4.1%) | 5 (22.7%) | |
| 2 months after LT | 3 (6.1%) | 1 (4.5%) | |
| 3 months after LT | 15 (30.6%) | 5 (22.7%) | |
| 4–6 months after LT | 11 (22.4%) | 6 (27.3%) | |
| 7–12 months after LT | 10 (20.4%) | 1 (4.5%) | |
| Greater than 1 year after LT | 3 (6.1%) | 2 (9.1%) | |
| Other | 5 (10.2%) | 2 (9.1%) | |
Abbreviations: PoPH = Portopulmonary Hypertension, LT = Liver Transplant, PDE-5i = Phosphodiesterase 5 Inhibitors, ERA = Endothelin Receptor Antagonist, sGC = Soluble Guanylate Cyclase
P-values computed using Fisher’s Exact Test for categorical variables between groups
Among respondents using parenteral prostacyclin therapy (N=47 pulmonologists, N=21 cardiologists)
Figure 4:
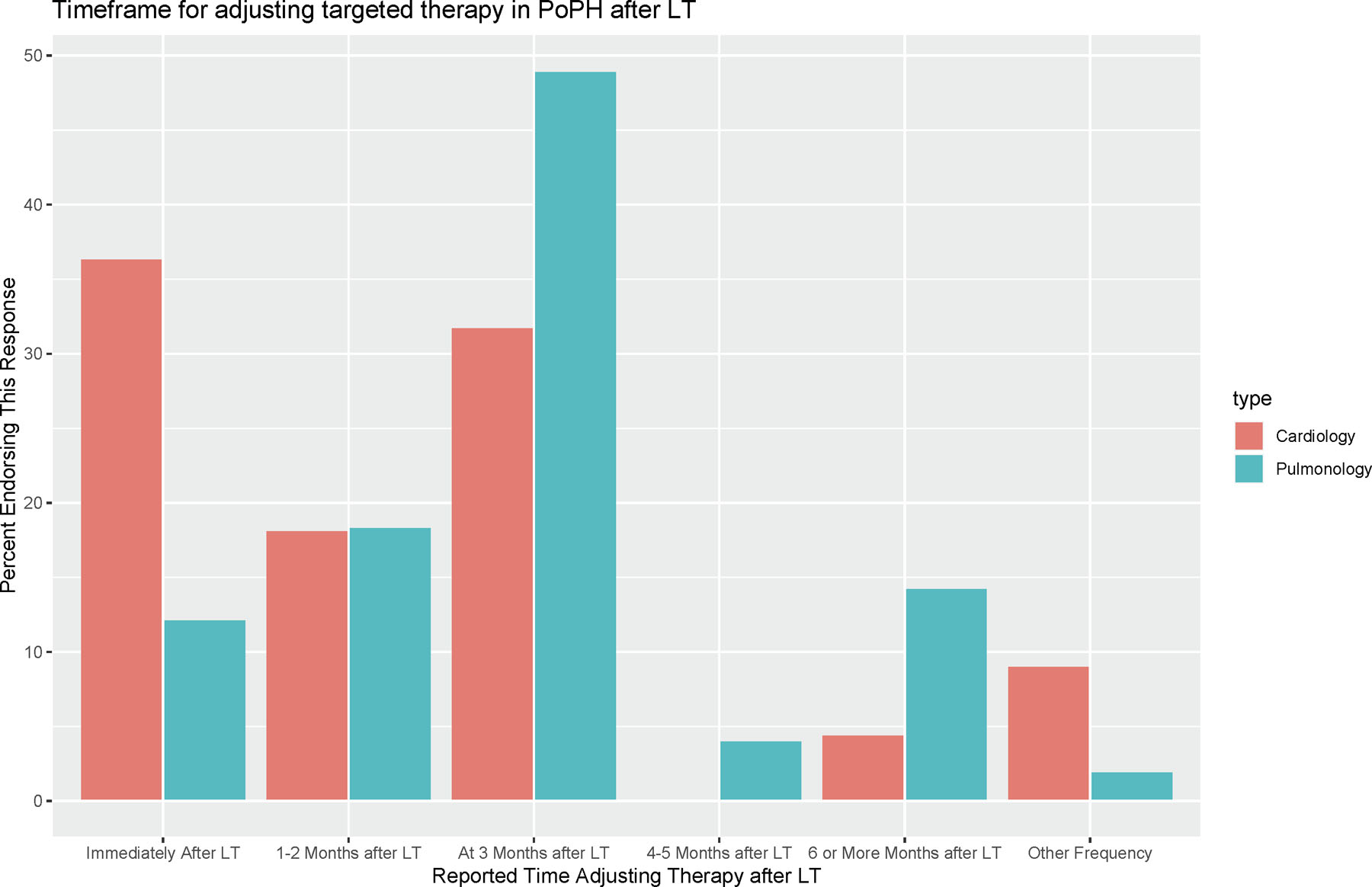
Timeframe for Adjusting Targeted Therapy in PoPH after LT
Attitudes regarding when to adjust targeted therapy in PoPH following LT. Responses are stratified by provider specialty (Cardiology or Pulmonology).
When asked to provide comments, respondents expressed similar concerns regarding the heterogenous management strategies to prepare PoPH patients for LT, the variable post-LT courses in these patients, and the need for a flexible and individualized treatment plan following LT (Supplementary Table S5). The unclear relationship between pulmonary vascular disease and LT in PoPH, the presence of refractory or worsening PoPH following LT, and the risk of de-novo PAH/PoPH after LT were all mentioned by respondents as factors complicating the adjustment of targeted PoPH therapy after LT.
Discussion
We report the results from our multidisciplinary survey of PoPH provider practice patterns following LT. We found that, compared to all PoPH patients, respondents specifically favored (intravenous) parenteral prostacyclin therapy in PoPH patients specifically awaiting LT. The majority of respondents used integrated cardiopulmonary assessments (echocardiography, right heart catheterization, sub-maximal exercise testing, and natriuretic peptide levels) to guide targeted therapy adjustment following LT. Although respondents tended to adjust targeted therapy early (the majority within three months of LT), and parenteral prostacyclin therapy was typically the first targeted therapy to be weaned, only slight adjustments (decreasing dose by 25–50%) were favored by most respondents, accompanied by timely (within 1–2 months) assessments of cardiopulmonary response to down-titration. Full liberation from PoPH targeted therapy after LT was felt to take at least a year, and respondents noted some PoPH patients either worsen or are never able to fully discontinue targeted therapy after LT. There were several notable differences in approach to PoPH targeted therapy after LT between cardiologists and pulmonologists and across provider PoPH volume.
As expected, given the limited evidence from the only large prospective randomized controlled trial of PoPH targeted therapy (PORTICO), and reflecting the clinical equipoise regarding management strategies for PoPH in the most recent guidelines and a recent survey of provider practice patterns, all classes of targeted therapy were employed by respondents to manage hemodynamic disease in PoPH (1, 5, 11, 15–16). Curiously, we observed a greater frequency of inhaled prostacyclin use amongst pulmonologists relative to cardiologists in managing PoPH patients, including those awaiting LT. We suspect this reflects a greater comfort level for inhaled therapy amongst pulmonary specialists, who are probably more likely than their cardiology counterparts to see patients with coexisting pulmonary parenchymal disease, and consequently may be more accustomed to prescribing inhaled prostacyclin therapeutics (17). Perhaps unsurprisingly, given its status as the most potent targeted therapy preferred for high-risk PAH patients, parenteral (specifically intravenous) prostacyclin medications were more frequently used by respondents when managing PoPH patients in the context of potential LT. This result aligns with the near-universal use of parenteral prostacyclin therapy in managing PoPH reported by Dubrock and colleagues in their previous survey of PoPH practice patterns in LT candidates (11). We suspect this preference for intravenous prostacyclin therapy reflects published reports suggesting an association between aggressive parenteral prostacyclin therapy and improved PoPH outcomes prior to LT, or possibly the ability to more readily achieve and maintain optimal hemodynamics to qualify for MELD exception points in PoPH patients potentially suitable for LT with parenteral prostacyclin therapy, but we did not ask respondents specifically why they favored a given modality of targeted therapy for PoPH (5, 8, 18–20). It remains to be seen if this observed treatment preference will affect guidelines for managing PoPH prior to LT, and neither the 2016 International Liver Transplant Society guidelines nor the guidelines emerging from the 6th World Symposium on PAH espouse a preference for any specific modality of targeted therapy in PoPH awaiting LT (5, 15).
Integrated cardiopulmonary assessments were favored by the majority of respondents to guide adjustment of targeted therapy in PoPH after LT, with routine use of all testing modalities reported by the overwhelming majority (>75%) of all respondents. An integrated approach to cardiopulmonary testing to guide PoPH treatment adjustments following LT was also favored by providers at high-volume PoPH centers relative to low-volume ones. This practice is contrasted with the 2016 International Liver Transplant Society guidelines (which suggest routine echocardiography alone is sufficient to guide adjustment of targeted therapy in PoPH following LT, and counsels against routine serial cardiac catheterization unless otherwise clinically indicated); more closely approximating the algorithm for adjusting targeted therapy in PAH favored by the 6th World Symposium in PAH and the 2015 ERS/ESC PAH guidelines, which incorporate routine right heart catheterization every six to twelve months, with serial 3-month walk testing and natriuretic peptide assessments (5, 15, 21). Of note, cardiology respondents were more likely to routinely rely on invasive hemodynamics to guide adjustment of PoPH targeted therapy after LT, and pulmonology respondents used sub-maximal exercise testing more often. Although this discrepancy may reflect specialty bias (cardiologists frequently perform right heart catheterizations themselves, and pulmonologists are responsible for interpreting six-minute walk testing), we did not ask respondents to detail cardiopulmonary testing modality preferences and only surveyed a small cohort of PoPH providers, and thus cannot comment on the true frequency or potential underpinnings of these observed preferences.
Following LT, most respondents reported adjusting targeted therapy early, typically in the first three months, with timely (within 1–2 months) reassessment of disease status using integrated cardiopulmonary testing. This algorithm, centered around routine and careful weaning of PoPH targeted therapy following LT, was also favored by providers at high-volume PoPH centers relative to respondents practicing at low-volume centers. We were surprised to find the routine weaning of targeted therapy in most or all PoPH patients after LT was supported by the majority of respondents (67.6%), contrasting this with roughly half of all respondents (51.5%) surveyed by DuBrock and colleagues who endorsed weaning therapy “often” or “nearly always” in PoPH following LT (15). Although this shift in practice patterns over time was interesting to see, and may represent an increased comfort level amongst specialists with weaning targeted therapy in PoPH or a greater weight placed on the beneficial hemodynamic effects of LT in PoPH in some patients (impressions supported by the additional anecdotal comments supplied by respondents in our survey), we did not ask respondents to justify or explain their response to this specific question and would not be able to draw further conclusions. The majority of all respondents started by gradually weaning parenteral prostacyclin therapy, an approach supported by the 2016 International Liver Transplant Society guidelines (5). Given that parenteral prostacyclin therapy is the most labor-intensive and complicated targeted therapy available in PAH, and there are unique infectious risks of indwelling intravenous catheters in the immunosuppressed post-transplant patient, it is not surprising that this was the first medication to be weaned (26). Adjusting targeted therapy was by no means universal, however, and a small minority of respondents rarely adjusted targeted therapy in PoPH after LT. At the other extreme, almost one fifth of all respondents (N=14, 19.7%) reported titrating targeted PAH therapy immediately after LT, with gradual titration and comprehensive cardiopulmonary evaluation (including echocardiography, right heart catheterization, sub-maximal exercise testing, and natriuretic peptide level assessments) within 1–2 months after titration to assess response. The underlying drivers of this practice pattern adjusting targeted therapy directly after LT are unclear, and the widespread adoption of this approach stands in contrast to the 2016 International Liver Transplant Society guidelines advising against major changes to targeted therapy immediately following LT (5).
As the response to LT in PoPH ranges from significant worsening of pulmonary vascular disease requiring an escalation in targeted therapy, to a complete resolution of hemodynamic disease and cessation of all targeted therapy following LT, and inappropriate adjustment of targeted therapy may be associated with considerable morbidity and mortality, it is perhaps unsurprising that the majority of respondents favor serial routine integrated cardiopulmonary testing assessments to help guide adjustment of targeted therapy in PoPH after LT (9, 20, 22–25). This aligns with the practice patterns observed by DuBrock and colleagues, who noted the majority of PoPH providers surveyed favored using a combination of symptoms, echocardiography, and right heart catheterization to guide weaning of targeted therapy following LT (15). We suspect the inability to predict post-LT outcomes in PoPH patients, coupled with our incomplete understanding of PoPH pathophysiology and how portal hypertensive liver disease drives pulmonary vascular remodeling in some patients, injects a high degree of uncertainty into the management of PoPH following LT, favoring the expensive and invasive “real-world” approach to monitoring seen in our survey despite more limited echocardiography-centric societal recommendations. Additionally, we saw that cardiologists were more likely to titrate therapy immediately after LT, and tended to adjust targeted therapy earlier than pulmonologists. Although we cannot be certain, we suspect this may in part reflect the more frequent use of routine right heart catheterization by cardiologists when adjusting PoPH therapy. With invasive hemodynamics more readily available, cardiologists may feel more comfortable with an earlier and more aggressive titration strategy of targeted PoPH therapy after LT than pulmonologists. By a similar token, providers at high-volume PoPH centers were more likely to always attempt weaning targeted therapy in PoPH after LT (favoring early parenteral prostacyclin titration with frequent reassessments), and more apt to integrate information from multiple cardiopulmonary testing modalities when considering therapeutic adjustments when compared to practitioners at low-volume PoPH centers. Taken together, these practice patterns suggest a prevailing belief amongst PoPH specialists that a greater degree of clinical risk stratification (achieved via an integrated cardiopulmonary assessment) may help facilitate early, frequent, and safe weaning of targeted therapy in PoPH. What remains to be determined are if differences truly exist between sub-specialty PoPH providers in approaching therapeutic adjustments after LT, the rationale underpinning practice variations in weaning therapy after LT, and most importantly if a specific weaning algorithm (timing in relation to LT, choice of medication to wean, etc.) can influence relevant post-transplant outcomes (morbidity, mortality, graft function) in this patient population. A crucial unanswered question is if more cardiopulmonary testing in PoPH results in improved outcomes and more effective/quicker weaning of targeted therapy post-LT, or instead confers a “false sense of security”, increasing costs and procedural risks without providing tangible clinical benefit. Several respondents’ comments referenced this conundrum, noting that while adjustment and weaning of targeted therapy after LT was a highly variable and individualized process, some fortunate patients can come off parenteral prostacyclin and targeted therapy immediately following LT, and aggressive weaning of targeted therapy with careful and frequent clinical and cardiopulmonary assessments may be both safe and effective. Although our work is important in highlighting these practice pattern variations in managing PoPH following LT, further research supported by international liver transplant and pulmonary vascular disease organizations is needed to fully disentangle this complex relationship and answer these remaining questions. Only through such collaborative multicenter efforts, such as prospective longitudinal cohort studies and international registry investigations, can we begin to elucidate the effects of post-LT management on outcomes in PoPH, optimize post-transplant management to be cost-effective while reducing variation, accumulate strong evidence to inform updates to societal guidelines, and ultimately enhance post-LT outcomes in PoPH.
There are several limitations to our study. First, our results may not reflect the attitudes and practice patterns of other providers involved in the management of PoPH patients undergoing LT, such as hepatologists, anesthesiologists, and transplant surgeons. Pursuit of LT, although guided by a national transplant allocation system (MELD prioritization), could also be influenced by individual provider preferences, and may have introduced selection bias into our survey of post-LT management (18). Due to the nature of the study, underlying biases (recall, response) may have affected our results. We collected data from providers through the PHCR and ISHLD-PVD networks and did not survey providers who were not members of these two networks, potentially introducing selection bias into our results. We did not collect data from non-responders and cannot comment on if these factors impacted response rates and results. Our study is limited to physician providers (MD or DO) practicing in the United States caring for adult PoPH patients, and cannot be generalized to other practitioners, other geographic regions, or the pediatric population. We observed a response rate of 49.4%, and our data may not represent all providers managing PoPH after LT. However, the number of unique respondents (N=85) is comparable to the known number of Certified Centers of Care specializing in PAH (N=83) as credentialed by the Pulmonary Hypertension Association in 2022, and the number of cardiology and pulmonology providers who practiced at centers performing more than 50 liver transplantations annually as of 2017 (N=73), and we believe our results capture the practice patterns and attitudes of a considerable proportion of practicing US-based PoPH specialists (11, 27).
In summary, the results of this multidisciplinary survey highlight a heterogenous approach to PoPH after LT, and we observed notable differences between the reported practice patterns regarding management of PoPH after LT and the most recently published guidelines regarding management of PoPH following LT (5, 15). Specifically, respondents used parenteral (intravenous) prostacyclin therapy more frequently when managing potential LT candidates with PoPH, almost one fifth of respondents weaned targeted therapy immediately after LT, and respondents generally favored serial integrated cardiopulmonary testing (including routine right heart catheterization) to guide adjustment of targeted therapy in PoPH following LT. Our results highlight the lack of high-quality, multicenter outcomes research on LT in PoPH and emphasize the need for further investigation into the relationship between PoPH management strategies and post-transplant outcomes, updated guidance regarding management strategies of PoPH after LT, and a team-based approach to LT in PoPH in order to optimize post-LT outcomes.
Supplementary Material
Support:
AJ is supported by the NIH (2UL1TR001425-05A1) and the Parker B. Francis Foundation (2021 Fellow), and receives research support from United Therapeutics. JME receives research support from Johnson and Johnson/Actelion, Reata, United Therapeutics, Liquidia, Phase Bio, Complexa, Goassamer Bio, and Bayer, and serves on the consulting and advisory board for Altavant, United Therapeutics, Liquidia, Bayer, and Goassamer Bio. EJK, SAS, and JME have no relevant support disclosures.
Abbreviations
- LT
Liver Transplantation
- MELD
Model for End-Stage Liver Disease
- PAH
Pulmonary Arterial Hypertension
- PoPH
Portopulmonary Hypertension
Footnotes
Conflicts of Interest – EK and SAS have no financial relationships to disclose.
References
- 1.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014;59:1144–65. [DOI] [PubMed] [Google Scholar]
- 3.Krowka MJ. Hepatopulmonary syndrome and portopulmonary hypertension: the pulmonary vascular enigmas of liver disease. Clin Liver Dis 2020;15(S1):S13:S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawut SM, Krowka MJ, Trotter JF, Roberts KE, Benza RL, Badesch DB, et al. Clinical risk factors for portopulmonary hypertension. Hepatology 2008;48(1):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krowka M, Fallon MB, Kawut SM, Fuhrmann V, Heimbach JK, Ramsay MA, et al. International liver transplant society practice guidelines: diagnosis and management of hepatopulmonary syndrome and portopulmonary hypertension. Transplantation 2016;100:1440–52. [DOI] [PubMed] [Google Scholar]
- 6.Krowka MJ, Miller DP, Barst RJ, Taichman D, Dweik RA, Badesch DB, et al. Portopulmonary hypertension: a report from the US-based REVEAL registry. Chest 2012;141(4):906–915. [DOI] [PubMed] [Google Scholar]
- 7.DuBrock HM, Burger CD, Bartolome SD, Feldman JP, Ivy DD, Rosenzweig EB, et al. Health disparities and treatment approaches in portopulmonary hypertension and idiopathic pulmonary arterial hypertension: an analysis of the Pulmonary Hypertension Association Registry. Pulm Circ 2021;11(3):20458940211020913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DuBrock HM, Runo JR, Sadd CJ, Burger CD, Cartin-Ceba R, Rosen CB, et al. Outcomes of liver transplantation in treated portopulmonary hypertension patients with a mean pulmonary arterial pressure >= 35mmHg. Transplant Direct 2020;6(12):e630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savale L, Sattler C, Coilly A, Conti F, Renard S, Francoz C, et al. Long-term outcome in liver transplantation candidates with portopulmonary hypertension. Hepatology 2017;65(5):1683–1692. [DOI] [PubMed] [Google Scholar]
- 10.Cartin-Ceba R, Burger C, Swanson K, Vargas H, Aqel B, Keaveny AP, et al. Clinical outcomes after liver transplantation in patients with portopulmonary hypertension. Transplantation 2021;105(10):2283–2290. [DOI] [PubMed] [Google Scholar]
- 11.DuBrock HM, Salgia RJ, Sussman NL, Bartolome SD, Kadry Z, Mulligan DC, et al. Portopulmonary hypertension: a survey of practice patterns and provider attitudes. Transplant Direct 2019;5(6):e456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas C, Glinskii V, de Jesus Perez V, Sahay S . Portopulmonary hypertension: from bench to bedside. Front Med (Lausanne) 2020;7:569413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savale L, Guimas M, Ebstein N, Fertin M, Jevnikar M, Renard S, et al. Portopulmonary hypertension in the current era of pulmonary hypertension management. J Hepatol 2020;73(1):130–139. [DOI] [PubMed] [Google Scholar]
- 14.Lai JC, Roberts JP, Vittinghoff E, Terrault NA, Feng S. Patient, center and geographic characteristics of nationally placed livers. Am J Transplant 2012;12(4):947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galie N, Channick RN, Frantz, RP, Grunig E, Jing ZC, Moiseeva O, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019;53:1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sitbon O, Bosch J, Cottreel E, Csonka D, de Groote P, Hoeper MM, et al. Macitentan for the treatment of portopulmonary hypertension (PORTICO): a multicentre, randomised, double-blind, placebo-controlled, phase 4 trial. Lancet Respir Med 2019;7(7):594–604. [DOI] [PubMed] [Google Scholar]
- 17.Waxman A, Restrepo-Jaramillo R, Thenappan T, Ravichandran A, Engel P, Bajwa A, et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med 2021;384:325–334. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg DS, Batra S, Sahay S, Kawut SM, Fallon MB. MELD exceptions for portopulmonary hypertension: current policy and future implementation. Am J Transplant 2014;14(9):2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awdish RLA, Cajigas HR. Early initiation of prostacyclin in portopulmonary hypertension: 10 years of a transplant center’s experience. Lung 2013;191(6):593–600. [DOI] [PubMed] [Google Scholar]
- 20.Hollatz TJ, Musat A, Westphal S, Decker C, D’Alessandro AM, Keevil J, et al. Treatment with sildenafil and treprostinil allows successful liver transplantation of patients with moderate to severe portopulmonary hypertension. Liver Transpl 2012;18(6):686–695. [DOI] [PubMed] [Google Scholar]
- 21.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2015;46:903–975. [DOI] [PubMed] [Google Scholar]
- 22.Koneru B, Ahmed S, Weisse AB, Grant GP, McKim KA. Resolution of pulmonary hypertension of cirrhosis after liver transplantation. Transplantation 1994;58(10):1133–1135. [PubMed] [Google Scholar]
- 23.Koch DG, Caplan M, Reuben A. Pulmonary hypertension after liver transplantation: case presentation and review of the literature. Liver Transpl 2009;15(4):407–412. doi: 10.1002/lt.21713. [DOI] [PubMed] [Google Scholar]
- 24.Rafanan AL, Maurer J, Mehta AC, Schilz R. Progressive portopulmonary hypertension after liver transplantation treated with epoprostenol. Chest 2000;118(5):1497–1500. doi: 10.1378/chest.118.5.1497. [DOI] [PubMed] [Google Scholar]
- 25.Hemnes AR, Robbins IM. Sildenafil monotherapy in portopulmonary hypertension can facilitate liver transplantation. Liver Transpl 2009;15(1):15–19. doi: 10.1002/lt.21479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitterman N, Poms A, Miller DP, Lombardi S, Farber HW, Barst RJ . Bloodstream infections in patients with pulmonary arterial hypertension treated with intravenous prostanoids: insights from the REVEAL registry. Mayo Clin Proc 2012;87(9):825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Find a PH Care Center, 2020. [Internet]. Pulmonary Hypertension Association, 8401 Colesville Road, Suite 200, Silver Spring, MD 20910 Published 2020, Accessed 1 February 2022. Available from: https://phassociation/org/phcarecenters/accredited-centers/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


