Abstract
To achieve the UNAIDS target of diagnosing 95% of all persons living with HIV, enhanced HIV testing services with greater attractional value need to be developed and implemented. We conducted a discrete choice experiment (DCE) to quantify preferences for enhanced HIV testing features across two high-risk populations in the Kilimanjaro Region in northern Tanzania. We designed and fielded a survey with 12 choice tasks to systematically recruited female barworkers and male mountain porters. Key enhanced features included: testing availability on every day of the week, an oral test, integration of a general health check or an examination for sexually transmitted infections (STI) with HIV testing, and provider-assisted confidential partner notification in the event of a positive HIV test result. Across 300 barworkers and 440 porters surveyed, mixed logit analyses of 17,760 choices indicated strong preferences for everyday testing availability, health checks, and STI examinations. Most participants were averse to oral testing and confidential partner notification by providers. Substantial preference heterogeneity was observed within each risk group. Enhancing HIV testing services to include options for everyday testing, general health checks, and STI examinations may increase the appeal of HIV testing offers to high-risk populations.
Keywords: HIV counseling and testing, discrete choice experiment, preference heterogeneity, sub-Saharan Africa, Tanzania
INTRODUCTION
The United Nations General Assembly and UNAIDS have set for 2025 the ambitious ‘95–95-95’ target: diagnosing 95% of all persons living with HIV (PLWH), initiating antiretroviral therapy for 95% of those diagnosed, and achieving viral suppression for 95% of those treated (UNAIDS, 2021; United Nations General Assembly, 2021). To achieve the ‘first 95’ in Eastern and Southern Africa, more than 1.6 million undiagnosed PLWH need to test for HIV, including more than 200,000 in Tanzania (UNAIDS, 2020). Many of these PLWH will be among difficult-to-reach populations who have already bypassed existing testing opportunities because of stigma, privacy concerns, geographical barriers, inconvenient hours of operation, fear of needles, or gendered perceptions of HIV testing services (Chanda et al., 2017; Fay et al., 2011; Kranzer et al., 2014; Okal et al., 2020; Qiao et al., 2018; Risher et al., 2013; Sileo et al., 2018; Strauss et al., 2015; Treves-Kagan et al., 2017; Tun et al., 2018).
Recognizing these hindrances, Tanzania’s National Multisectoral Strategic Framework for HIV and AIDS, which highlights low rates of HIV testing among high-risk persons, calls for a mix of differentiated models of HIV testing to increase testing uptake and ultimately achieve the first 95 target (Tanzania Commission for AIDS, 2018). Across diverse settings in sub-Saharan Africa, multiple strategies have been employed to increase HIV testing, including home-based testing (Sabapathy et al., 2012), provider-initiated testing (Roura et al., 2013; Topp et al., 2012), and work-place testing (Houdmont et al., 2013), but iteratively implementing, evaluating, and optimizing these interventions can be costly and slow.
Well-designed discrete choice experiments (DCEs), which systematically and efficiently evaluate population preferences, offer an opportunity to engage stakeholders in the design and implementation of new HIV testing options. DCEs have been widely used to characterize end-user preferences in various contexts related to HIV, including prevention (Cameron et al., 2013; Newman et al., 2016; Quaife et al., 2018; Terris-Prestholt et al., 2013), testing (Indravudh et al., 2017; Johnson et al., 2010; Ostermann et al., 2014; Ostermann, Njau, et al., 2015; Phillips et al., 2002; Strauss et al., 2018), service delivery (d’Elbee et al., 2018; Kruk et al., 2016; Zanolini et al., 2018), and treatment (Beusterien et al., 2007; Bregigeon-Ronot et al., 2017; Hauber et al., 2009; Mühlbacher et al., 2013; Ostermann, Mühlbacher, et al., 2020). With the goal of designing optimized HIV testing interventions for high-risk populations, this study used a DCE to characterize preferences for expanded testing hours, less invasive specimen sampling using an oral swab, the integration of health screening examinations with HIV testing, and assisted confidential partner notification in the event of a positive test result.
METHODS
This manuscript is part of a study that seeks to evaluate the effect of a preference-informed offer of HIV testing on testing rates of high-risk populations (ClinicalTrials.gov Protocol NCT02714140). The study protocol and methods have been previously published (Ostermann et al., 2021; Ostermann, Njau, et al., 2020), and details of the design of the DCE are presented in the Supplemental Material. Methods pertaining to this study are summarized below.
Ethics approvals
The protocol was approved by the Ethics Review Committee at Kilimanjaro Christian Medical University College (Protocols #273 and #901) and the National Institute for Medical Research (NIMR/HQ/R.8a/Vol. IX/1363 and NIMR/HQ/R.8a/Vol. IX/2603) in Tanzania and by the Institutional Review Boards at Duke University (Duke University Health System IRB, Protocol Pro00075996) and the University of South Carolina (Health Sciences South Carolina IRB, facilitated review, Pro00060760) in the United States. Informed consent was obtained from all participants.
Study sample
The study was conducted in Moshi, the administrative and commercial capital of the Kilimanjaro Region in northern Tanzania. Study participants comprised women employed in randomly selected bars, restaurants and guesthouses serving alcohol to patrons (“female barworkers”) and male porters supporting climbers of nearby Mount Kilimanjaro (“male porters”) who were sequentially approached as they exited Mount Kilimanjaro National Park. We previously characterized these groups as populations at high risk of HIV infection (Ostermann, Njau, et al., 2015). Eligible participants were residents of Moshi, able to read, and ages 18 to 49.
Discrete choice experiment
As part of an in-person survey, preferences for enhanced HIV testing characteristics were assessed using a DCE. A DCE, a quantitative survey method grounded in random utility theory (McFadden, 1974; Thurston, 1927) and Lancaster’s theory of consumer demand (Lancaster, 1966), simulates real-world choice situations by asking participants to choose between products or services whose characteristics are systematically varied by means of an experimental design. The analysis of participants’ choices provides estimates of individuals’ relative preferences for each characteristic.
Attributes and levels
The product or service characteristics evaluated in a DCE are commonly referred to as attributes and levels. The selection of attributes and levels for this DCE was guided by a survey of HIV testing facilities in the study area and focus group discussions with members of the target population. Two attributes described testing features commonly available in the area, including testing venue (health facility; free-standing HIV testing center; home) and pre-test counseling modalities (one-on-one; in a group; with a partner). Four attributes included enhanced features: testing availability every day of the week (vs. weekdays only), an oral swab to obtain the sample for the HIV test (vs. venipuncture or finger prick), the integration of a general health check or an examination for sexually transmitted infections (STI) with an HIV test (vs. HIV testing only), and assisted confidential partner notification in the event of a positive HIV test result (vs. self-disclosure).
Experimental design
The experimental design of a DCE represents the subset of potential choice tasks that is used to estimate preference parameters with the smallest possible error. Ngene software (ChoiceMetrics, Australia, 2018) version 1.12b was used to select an experimental design that minimized the D-error for a mixed logit model with effects-coded, normally distributed priors (Johnson et al., 2007). Statistical priors were obtained from a pilot study with 236 participants. Participants were randomized across 10 sets of choice tasks, with 12 tasks each. The order of choice tasks was randomized across participants. Each choice task included three testing alternatives; the order of alternatives was randomized within each choice task.
DCE administration
In-person surveys were fielded by trained research staff, in Kiswahili, on iOS devices, using Comet survey software (Selway Labs, Englewood, CO, 2017). Participants initially ranked the levels of each attribute (e.g., venipuncture vs. finger prick vs. oral swab). These data were used to populate a participant-specific comprehension task, followed by 12 choice tasks. Using a best-best preference elicitation approach (Ghijben et al., 2014), participants were asked in each task to first select their most preferred option (or “alternative”) from three testing options presented; then, participants were asked to select their more preferred of the two remaining options. A sample choice task illustrating all enhanced HIV testing characteristics included in the DCE is shown in Figure 1.
Figure 1.
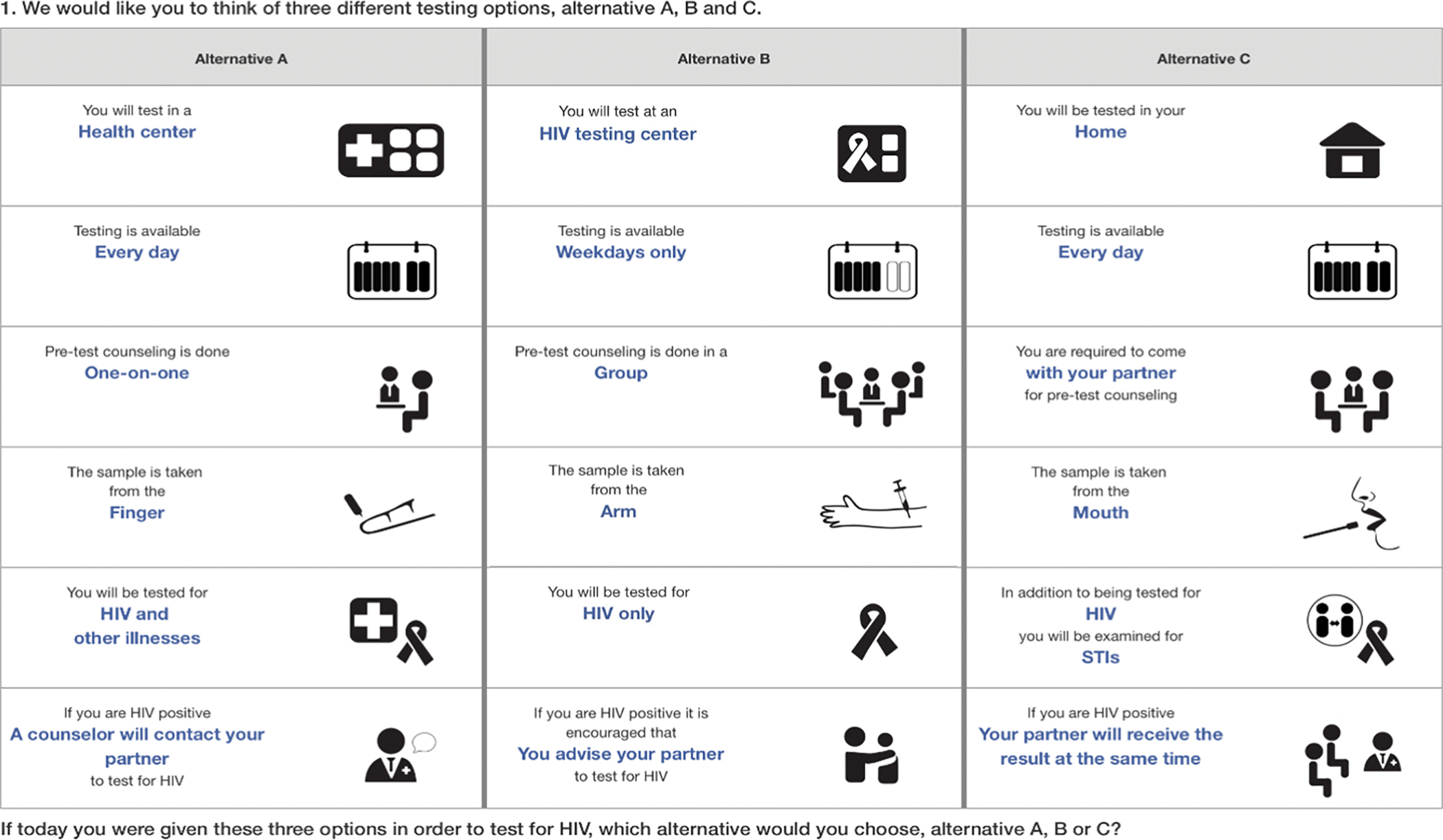
Sample DCE choice task.
Notes: Attributes and levels were introduced individually, prior to the administration of the DCE. After the most preferred option was selected, participants were asked to identify the next best option (“best-best” elicitation format). See Supplemental Material for the DCE survey and information provided to the participant about each attribute and level.
Statistical analysis
DCE choice data were analyzed using gender-specific mixed logit models with effects-coded correlated, normally distributed random coefficients (Hole, 2007). Coefficient estimates from the mixed logit model represent estimates of participants’ average preferences for each attribute level. The estimated standard deviations of these coefficients describe the variation in preferences across participants (“preference heterogeneity”). Individual participants’ choices were combined with information on the distribution of preferences across participants to derive individual-level preference estimates (“posterior betas”) for each attribute level using a method proposed by Revelt and Train (Revelt & Train, 2000; Train, 2003). Statistical analyses were performed using STATA version 16.1 (StataCorp, College Station, Texas).
RESULTS
Between September 2017 and July 2018, 300 female barworkers and 440 male porters were enrolled into the study. The median age among barworkers was 30 (inter-quartile range, IQR, 24 to 35) years, the median age among porters was 31 (IQR 26 to 36) years. One third (32%) of female barworkers and 66% of male porters were married. More than half (58%) of female barworkers and 44% of porters had completed primary school education. The majority of female barworkers (95%) and male porters (80%) had previously tested for HIV, but fewer than half (46% of barworkers; 49% of porters) had tested in the past year.
Mixed logit analyses of 17,760 choices (12 first and 12 second choices times 740 participants) indicate strong preferences for everyday testing availability (vs. weekdays only), for health checks, and for STI examinations (vs. HIV testing only). They also indicate a strong aversion to oral swabs (vs. finger prick or venipuncture), and most participants were averse to provider-assisted confidential partner notification (vs. self-disclosure) in the case of a positive HIV test (Figure 2). Results demonstrate substantial heterogeneity in the extent to which individual participants valued enhanced HIV testing features. Small but statistically significant differences in average preferences were noted between risk groups for each enhanced testing feature, except for everyday testing availability, however, the distributions were similar across groups.
Figure 2.
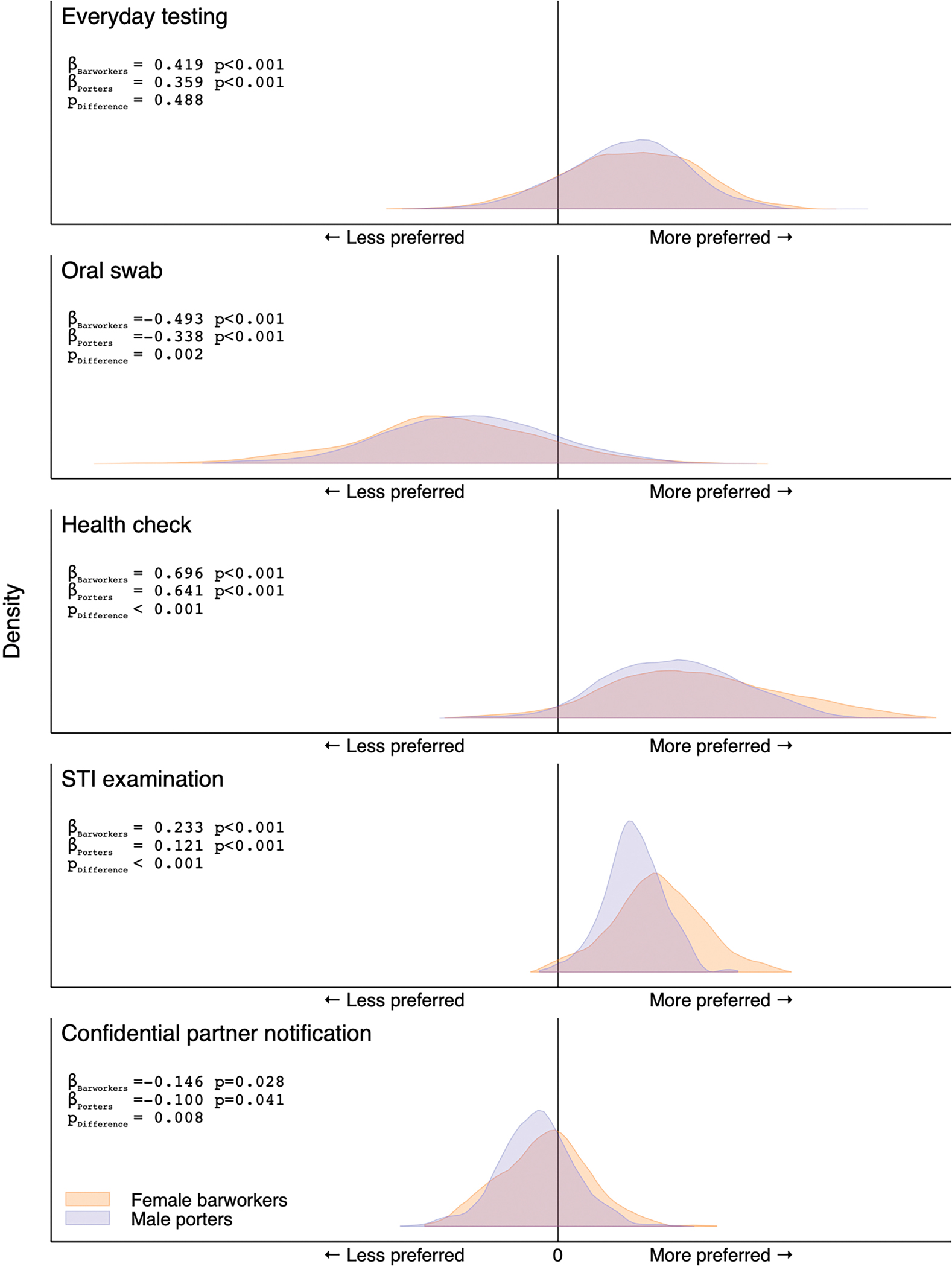
Distribution of preferences for enhanced HIV counseling and testing features among 300 female barworkers and 440 male mountain porters, Tanzania, 2017.
Notes: Results of gender-specific mixed logit models with effects-coded correlated random coefficients. Distributions represent kernel densities of individual-level preference estimates for 300 female barworkers (blue) and 440 male mountain porters (orange). The x-axes represent preferences for (to the right of the black line) or against (left of the black line): (a) everyday testing relative to testing on weekdays only, (b) oral swab relative to the estimated more preferred of either venipuncture or finger prick; (c) a complementary health check or (d) a complementary STI examination relative to HIV testing only; (e) confidential assisted partner notification in the event of a positive test result relative to self-disclosure. Effects-coded (mean) coefficient estimates from gender-specific mixed logit models are indicated by ßBarworkers and ßPorters. The statistical significance of differences in the distribution of individual-level preference estimates between risk groups, as evaluated by Student’s t-tests, is indicated by pDifference.
DISCUSSION
On average, members of these two high-risk groups strongly preferred HIV testing options that were combined with health examinations and available on weekends; in contrast, most participants demonstrated an aversion to oral testing and provider-assisted confidential partner notification in the event of a positive HIV test. While we observed statistically significant differences in mean preferences between male and female high-risk groups, the overall distributions of preferences were strikingly similar. These findings can inform the design of new HIV testing strategies aimed at achieving the “first 95” target of the 95–95-95 UNAIDS goal.
Across sub-Saharan Africa, modern preference elicitation studies for HIV testing, including DCEs, have focused preference assessments largely around cost, location of and distance to services, privacy, antiretroviral therapy availability, self-testing, and incentives (Beckham et al., 2020; Korte et al., 2019; Ostermann, Brown, et al., 2015; Ostermann et al., 2014; Ostermann, Njau, et al., 2015; Schaffer et al., 2020; Strauss et al., 2018; Uzochukwu et al., 2011). Relatively few studies have examined the attractional value of enhancements to HIV testing services, such as integrating general health examinations and STI testing. Our findings are in keeping with the only other preference study we could identify that examined this attribute. Using a DCE limited to a rural sample of Ugandan men, Schaffer et al. found that the offer of multi-disease testing (i.e., tuberculosis, malaria, hypertension, and diabetes) at the time of HIV testing was significantly associated with increased predicted uptake (Schaffer et al., 2020). This preference for undergoing HIV testing in the context of broader health screening is supported by observational data from a large mobile- and home-testing campaign in Kenya and Uganda, which offered additional services such as hypertension and diabetes screening and malaria testing (Chamie et al., 2016).
In our study, oral testing was less preferred by both high-risk populations — a finding that conforms with our preference research conducted in 2012–2014 in this region (Ostermann et al., 2014; Ostermann, Njau, et al., 2015), but contrasts with findings from two other studies in sub-Saharan Africa. In a Ugandan DCE conducted among pregnant women and their male partners, oral testing was preferred over finger pricks; but not for the subgroup who had tested previously for HIV (Korte et al., 2019). Similarly, among long distance truck drivers in Kenya, there was overall indifference to oral vs. finger-prick testing, but those who had never tested were more likely to prefer oral testing (Strauss et al., 2018). One potential explanation for the differences across these three countries is differential familiarity with oral testing as an accurate, governmentally-approved, HIV testing option (Unitaid-World Health Organization, 2018). Oral testing, including self-testing (Ekouevi et al., 2020; Hlongwa et al., 2020; Njau et al., 2019), had not been approved for public use by regulatory authorities in Tanzania at the time of this study, and as such may be subject to misperceptions about accuracy (Njau et al., 2014). A comprehensive information campaign may be required for oral testing to be widely accepted as an enhanced HIV testing strategy in Tanzania.
Assisted partner notification services have been recommended by the World Health Organization since 2016 (World Health Organization, 2016), and individual-level randomized trials have demonstrated that, when implemented for HIV-positive testers, this approach increases both HIV testing uptake and identification of HIV infections among sexual partners as compared with passive referrals (Brown et al., 2011; Cherutich et al., 2017; Dalal et al., 2017; Rosenberg et al., 2015). Our data suggest that, for persons who are considering an HIV test, presenting an HIV testing offer that, a priori, includes assisted confidential partner notification, on average, is of limited utility. The extent to which the credibility of confidentiality assurances vs. other considerations factor into testing preferences and decisions warrants further exploration.
This study is the first to evaluate preferences for readily-implementable, enhanced testing features targeting high-risk populations in sub-Saharan Africa. The strengths of this study include its size (the largest DCE eliciting HIV testing preferences among specific high-risk populations in sub-Saharan Africa), rigorous sampling and analytic approaches, and a focus on policy-relevant options developed from extensive qualitative work (Njau et al., 2014) and prior DCEs (Ostermann et al., 2014; Ostermann, Njau, et al., 2015). Limitations include potential hypothetical bias, which is implicit in stated preference surveys (Quaife et al., 2018), that these populations were predominantly experienced HIV-testers, and uncertain generalizability of findings to other high-risk populations. Further, because the DCE did not include an opt-out alternative – a decision motivated by high rates of prior HIV testing and concerns about social desirability bias stemming from decades-long efforts to promote HIV testing – the results do not allow for direct inferences about the impact of enhanced features on testing uptake. Finally, this analysis focuses on the distribution of population preferences estimated using standard mixed logit methods and as such highlights general policy implications; reasons for the variation in preferences could not be explored. In a separate latent class analysis of the same data, we describe distinct profiles of preferences that could be used to design testing options for specific sub-populations (Ostermann et al., 2021).
In conclusion, this DCE suggests that both male and female high-risk groups, on average, similarly preferred enhanced HIV testing options that included everyday testing availability and both general and STI health checks. Oral testing was less-preferred compared to more invasive, blood-based sampling methods, and most participants preferred self-disclosure over assisted confidential partner notification. We observed substantial preference heterogeneity for these enhanced HIV testing features among both high-risk groups. However, their strikingly similar distributions across these groups do not support the implementation of differentiating HIV testing models based on group membership. These findings will guide further work evaluating uptake of DCE-informed HIV testing options (NCT02714140) as part of an overall strategy assessing the utility of DCEs for designing novel HIV testing interventions in sub-Saharan Africa.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to the study participants and to the study research assistants, Martha Masaki, Beatrice Mandao, Elizabeth Mbuya, Honoratha Israel, Yombya Madukwa, Mohamed Mcharo, Upendo Nnko, Stephen Sikumbili, Edward Singo, Blandina Zenze, Leonia Rugalabamu, Suzan Kitomari, Stanny Komu, and Beldad Mmari, for input on study procedures and study implementation.
The authors thank the staff of the Kilimanjaro Clinical Research Institute, especially Professor Blandina Mmbaga, Dr. Aisa Shayo, and Zuhura Lintu, the University of South Carolina’s Arnold School of Public Health, especially the Department of Health Services Policy & Management and the Center for Health Care Quality, the Duke Global Health Institute and Duke University’s Center for Health Policy and Inequalities Research, for administrative support; and members of the Duke Center for AIDS Research and the study’s Scientific Advisory Board for feedback on study feasibility, design, analytic methods, and implementation.
Finally, the authors acknowledge Dr. Credianus Mgimba (Regional Medical Officer, Kilimanjaro Region), Dr. Best Magoma (former Regional Medical Officer, Kilimanjaro Region), Dr. Eligy Mosille (Regional AIDS Control Coordinator, Kilimanjaro Region), Ms. Dafrosa Itemba (Director, Tanzania Women Research Foundation), and members of the Moshi District Council administration, for their support of the study’s development and implementation.
FUNDING
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health in the United States under Award Numbers R01MH106388 and R21MH96631 and by the Duke University Center for AIDS Research (CFAR), an NIH funded program (P30AI064518). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DECLARATION OF INTEREST STATEMENT
No potential competing interest was reported by the authors.
REFERENCES
- Beckham SW, Crossnohere NL, Gross M, & Bridges JFP (2020). Eliciting Preferences for HIV Prevention Technologies: A Systematic Review. Patient. 10.1007/s40271-020-00486-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beusterien KM, Dziekan K, Schrader S, Flood E, Flood R, Shearer A, & Davis EA (2007). Patient preferences among third agent HIV medications: a US and German perspective. AIDS Care, 19(8), 982–988. 10.1080/09540120701294278 [DOI] [PubMed] [Google Scholar]
- Bregigeon-Ronot S, Cheret A, Cabie A, Prazuck T, Volny-Anne A, Ali S, Bottomley C, Finkielsztejn L, Philippe C, & Parienti JJ (2017). Evaluating patient preference and satisfaction for human immunodeficiency virus therapy in France. Patient Prefer Adherence, 11, 1159–1169. 10.2147/PPA.S130276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LB, Miller WC, Kamanga G, Nyirenda N, Mmodzi P, Pettifor A, Dominik RC, Kaufman JS, Mapanje C, Martinson F, Cohen MS, & Hoffman IF (2011). HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr, 56(5), 437–442. 10.1097/qai.0b013e318202bf7d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MP, Newman PA, Roungprakhon S, & Scarpa R (2013). The marginal willingness-to-pay for attributes of a hypothetical HIV vaccine. Vaccine, 31(36), 3712–3717. https://www.sciencedirect.com/science/article/pii/S0264410X13007068?via%3Dihub [DOI] [PubMed] [Google Scholar]
- Chamie G, Clark TD, Kabami J, Kadede K, Ssemmondo E, Steinfeld R, Lavoy G, Kwarisiima D, Sang N, Jain V, Thirumurthy H, Liegler T, Balzer LB, Petersen ML, Cohen CR, Bukusi EA, Kamya MR, Havlir DV, & Charlebois ED (2016). A hybrid mobile approach for population-wide HIV testing in rural east Africa: an observational study. The Lancet HIV, 3(3), e111–e119. 10.1016/s2352-3018(15)00251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda MM, Perez-Brumer AG, Ortblad KF, Mwale M, Chongo S, Kamungoma N, Kanchele C, Fullem A, Barresi L, Barnighausen T, & Oldenburg CE (2017). Barriers and Facilitators to HIV Testing Among Zambian Female Sex Workers in Three Transit Hubs. AIDS Patient Care STDS, 31(7), 290–296. 10.1089/apc.2017.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherutich P, Golden MR, Wamuti B, Richardson BA, Asbjornsdottir KH, Otieno FA, Ng’ang’a A, Mutiti PM, Macharia P, Sambai B, Dunbar M, Bukusi D, Farquhar C, & a, P. S. S. G. (2017). Assisted partner services for HIV in Kenya: a cluster randomised controlled trial. Lancet HIV, 4(2), e74–e82. 10.1016/S2352-3018(16)30214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Elbee M, Indravudh PP, Mwenge L, Kumwenda MM, Simwinga M, Choko AT, Hensen B, Neuman M, Ong JJ, Sibanda EL, Johnson CC, Hatzold K, Cowan FM, Ayles H, Corbett EL, & Terris-Prestholt F (2018). Preferences for linkage to HIV care services following a reactive self-test: discrete choice experiments in Malawi and Zambia. AIDS, 32(14), 2043–2049. 10.1097/qad.0000000000001918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal S, Johnson C, Fonner V, Kennedy CE, Siegfried N, Figueroa C, & Baggaley R (2017). Improving HIV test uptake and case finding with assisted partner notification services. AIDS, 31(13), 1867–1876. 10.1097/QAD.0000000000001555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekouevi DK, Bitty-Anderson AM, Gbeasor-Komlanvi FA, Coffie AP, & Eholie SP (2020). HIV self-testing: The key to unlock the first 90 in West and Central Africa. Int J Infect Dis, 95, 162–166. 10.1016/j.ijid.2020.02.016 [DOI] [PubMed] [Google Scholar]
- Fay H, Baral SD, Trapence G, Motimedi F, Umar E, Iipinge S, Dausab F, Wirtz A, & Beyrer C (2011). Stigma, health care access, and HIV knowledge among men who have sex with men in Malawi, Namibia, and Botswana. AIDS Behav, 15(6), 1088–1097. 10.1007/s10461-010-9861-2 [DOI] [PubMed] [Google Scholar]
- Ghijben P, Lancsar E, & Zavarsek S (2014). Preferences for oral anticoagulants in atrial fibrillation: a best-best discrete choice experiment. Pharmacoeconomics, 32(11), 1115–1127. 10.1007/s40273-014-0188-0 [DOI] [PubMed] [Google Scholar]
- Hauber AB, Mohamed AF, Watson ME, Johnson FR, & Hernandez JE (2009). Benefits, risk, and uncertainty: preferences of antiretroviral-naive African Americans for HIV treatments. AIDS Patient Care STDS, 23(1), 29–34. 10.1089/apc.2008.0064 [DOI] [PubMed] [Google Scholar]
- Hlongwa M, Mashamba-Thompson T, Makhunga S, Muraraneza C, & Hlongwana K (2020). Men’s perspectives on HIV self-testing in sub-Saharan Africa: a systematic review and meta-synthesis. BMC Public Health, 20(1), 66. 10.1186/s12889-020-8184-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hole A (2007). Fitting mixed logit models by using maximum simulated likelihood. The Stata Journal, 7, 388–401. [Google Scholar]
- Houdmont J, Munir F, & Grey M (2013). Acceptance of repeat worksite HIV voluntary counselling and testing in a rural South African factory. AIDS Care, 25(9), 1199–1202. 10.1080/09540121.2013.764388 [DOI] [PubMed] [Google Scholar]
- Indravudh PP, Sibanda EL, d’Elbee M, Kumwenda MK, Ringwald B, Maringwa G, Simwinga M, Nyirenda LJ, Johnson CC, Hatzold K, Terris-Prestholt F, & Taegtmeyer M (2017). ‘I will choose when to test, where I want to test’: investigating young people’s preferences for HIV self-testing in Malawi and Zimbabwe. AIDS, 31 Suppl 3, S203–S212. 10.1097/QAD.0000000000001516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson FR, Kanninen B, Bingham M, & Ozdemir S (2007). Experimental design for stated choice studies. In Kanninen B (Ed.), Valuing envronmental amenities using stated choice studies: A common sense approach to theory and practic. Springer. [Google Scholar]
- Johnson FR, Ozdemir S, & Phillips KA (2010). Effects of simplifying choice tasks on estimates of taste heterogeneity in stated-choice surveys. Social Science & Medicine, 70(2), 183–190. http://linkinghub.elsevier.com/retrieve/pii/S0277953609006972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte JE, Strauss M, Ba A, Buregyeya E, Matovu JK, Kisa R, Musoke W, Chemusto H, Vrana-Diaz CJ, Malek AM, Wanyenze RK, & George G (2019). HIV testing preferences among pregnant women attending antenatal care and their male partners: a discrete choice experiment in Uganda. Afr J AIDS Res, 18(4), 332–340. 10.2989/16085906.2019.1686032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzer K, Meghji J, Bandason T, Dauya E, Mungofa S, Busza J, Hatzold K, Kidia K, Mujuru H, & Ferrand RA (2014). Barriers to provider-initiated testing and counselling for children in a high HIV prevalence setting: a mixed methods study. PLoS Med, 11(5), e1001649. 10.1371/journal.pmed.1001649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk ME, Yamey G, Angell SY, Beith A, Cotlear D, Guanais F, Jacobs L, Saxenian H, Victora C, & Goosby E (2016). Transforming Global Health by Improving the Science of Scale-Up. PLoS Biol, 14(3), e1002360. 10.1371/journal.pbio.1002360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster KJ (1966). A New Approach to Consumer Theory. Journal of Political Economy, 74(2), 132–157. [Google Scholar]
- McFadden D (1974). Conditional Logit Analysis of Qualitative Choice Behavior. In Zarembka P (Ed.), Frontiers in Econometrics. Academic Press. [Google Scholar]
- Mühlbacher AC, Stoll M, Mahlich J, & Nübling M (2013). Patient preferences for HIV/AIDS therapy - a discrete choice experiment. Health Econ Rev, 3(1), 14. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3662594/pdf/2191-1991-3-14.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PA, Cameron MP, Roungprakhon S, Tepjan S, & Scarpa R (2016). Acceptability and Preferences for Hypothetical Rectal Microbicides among a Community Sample of Young Men Who Have Sex with Men and Transgender Women in Thailand: A Discrete Choice Experiment. AIDS Behav, 20(11), 2588–2601. 10.1007/s10461-015-1258-9 [DOI] [PubMed] [Google Scholar]
- Njau B, Covin C, Lisasi E, Damian D, Mushi D, Boulle A, & Mathews C (2019). A systematic review of qualitative evidence on factors enabling and deterring uptake of HIV self-testing in Africa. BMC Public Health, 19(1), 1289. 10.1186/s12889-019-7685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njau B, Ostermann J, Brown D, Muhlbacher A, Reddy E, & Thielman N (2014). HIV testing preferences in Tanzania: a qualitative exploration of the importance of confidentiality, accessibility, and quality of service. BMC Public Health, 14, 838. 10.1186/1471-2458-14-838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okal J, Lango D, Matheka J, Obare F, Ngunu-Gituathi C, Mugambi M, & Sarna A (2020). “It is always better for a man to know his HIV status” - A qualitative study exploring the context, barriers and facilitators of HIV testing among men in Nairobi, Kenya. PLoS One, 15(4), e0231645. 10.1371/journal.pone.0231645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J, Brown DS, Muhlbacher A, Njau B, & Thielman N (2015). Would you test for 5000 Shillings? HIV risk and willingness to accept HIV testing in Tanzania. Health Econ Rev, 5(1), 60. 10.1186/s13561-015-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J, Flaherty BP, Brown DS, Njau B, Hobbie AM, Mtuy T, Masnick M, Mühlbacher AC, & Thielman NM (2021). What factors influence HIV testing? Modeling preference heterogeneity using latent classes and class-independent random effects. Journal of Choice Modelling, 100305. 10.1016/j.jocm.2021.100305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J, Mühlbacher A, Brown DS, Regier DA, Hobbie A, Weinhold A, Alshareef N, Derrick C, & Thielman NM (2020). Heterogeneous Patient Preferences for Modern Antiretroviral Therapy: Results of a Discrete Choice Experiment. Value Health, 23(7), 851–861. 10.1016/j.jval.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J, Njau B, Brown DS, Muhlbacher A, & Thielman N (2014). Heterogeneous HIV testing preferences in an urban setting in Tanzania: results from a discrete choice experiment. PLoS One, 9(3), e92100. 10.1371/journal.pone.0092100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J, Njau B, Hobbie A, Mtuy T, Masaki ML, Shayo A, van Zwetselaar M, Masnick M, Flaherty B, Brown DS, Muhlbacher AC, & Thielman NM (2020). Using discrete choice experiments to design interventions for heterogeneous preferences: protocol for a pragmatic randomised controlled trial of a preference-informed, heterogeneity-focused, HIV testing offer for high-risk populations. BMJ Open, 10(11), e039313. 10.1136/bmjopen-2020-039313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J, Njau B, Mtuy T, Brown DS, Muhlbacher A, & Thielman N (2015). One size does not fit all: HIV testing preferences differ among high-risk groups in Northern Tanzania. AIDS Care, 27(5), 595–603. 10.1080/09540121.2014.998612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Maddala T, & Johnson FR (2002). Measuring preferences for health care interventions using conjoint analysis: an application to HIV testing. Health Serv Res, 37(6), 1681–1705. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1464051/pdf/hesr_01115r.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao S, Zhang Y, Li X, & Menon JA (2018). Facilitators and barriers for HIV-testing in Zambia: A systematic review of multi-level factors. PLoS One, 13(2), e0192327. 10.1371/journal.pone.0192327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaife M, Terris-Prestholt F, Di Tanna GL, & Vickerman P (2018). How well do discrete choice experiments predict health choices? A systematic review and meta-analysis of external validity. Eur J Health Econ, 19(8), 1053–1066. 10.1007/s10198-018-0954-6 [DOI] [PubMed] [Google Scholar]
- Revelt D, & Train K (2000). Customer-specific taste parameters and mixed logit: Households’ choice of electricity supplier.
- Risher K, Adams D, Sithole B, Ketende S, Kennedy C, Mnisi Z, Mabusa X, & Baral SD (2013). Sexual stigma and discrimination as barriers to seeking appropriate healthcare among men who have sex with men in Swaziland. J Int AIDS Soc, 16(3 Suppl 2), 18715. 10.7448/IAS.16.3.18715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg NE, Mtande TK, Saidi F, Stanley C, Jere E, Paile L, Kumwenda K, Mofolo I, Ng’ambi W, Miller WC, Hoffman I, & Hosseinipour M (2015). Recruiting male partners for couple HIV testing and counselling in Malawi’s option B+ programme: an unblinded randomised controlled trial. Lancet HIV, 2(11), e483–491. 10.1016/S2352-3018(15)00182-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura M, Watson-Jones D, Kahawita TM, Ferguson L, & Ross DA (2013). Provider-initiated testing and counselling programmes in sub-Saharan Africa: a systematic review of their operational implementation. AIDS, 27(4), 617–626. http://graphics.tx.ovid.com/ovftpdfs/FPDDNCDCPBBAHF00/fs047/ovft/live/gv024/00002030/00002030-201302200-00014.pdf [DOI] [PubMed] [Google Scholar]
- Sabapathy K, Van den Bergh R, Fidler S, Hayes R, & Ford N (2012). Uptake of Home-Based Voluntary HIV Testing in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. PLoS Med, 9(12), e1001351. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3514284/pdf/pmed.1001351.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer EM, Gonzalez JM, Wheeler SB, Kwarisiima D, Chamie G, & Thirumurthy H (2020). Promoting HIV Testing by Men: A Discrete Choice Experiment to Elicit Preferences and Predict Uptake of Community-based Testing in Uganda. Appl Health Econ Health Policy, 18(3), 413–432. 10.1007/s40258-019-00549-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sileo KM, Fielding-Miller R, Dworkin SL, & Fleming PJ (2018). What Role Do Masculine Norms Play in Men’s HIV Testing in Sub-Saharan Africa?: A Scoping Review. AIDS Behav, 22(8), 2468–2479. 10.1007/s10461-018-2160-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss M, George G, Lansdell E, Mantell JE, Govender K, Romo M, Odhiambo J, Mwai E, Nyaga EN, & Kelvin EA (2018). HIV testing preferences among long distance truck drivers in Kenya: a discrete choice experiment. AIDS Care, 30(1), 72–80. 10.1080/09540121.2017.1367086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss M, Rhodes B, & George G (2015). A qualitative analysis of the barriers and facilitators of HIV counselling and testing perceived by adolescents in South Africa. BMC Health Serv Res, 15, 250. 10.1186/s12913-015-0922-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzania Commission for AIDS. (2018). Tanzania National Multisectoral Strategic Framework for HIV and AIDS 2018/19 to 2022/23. P. M. s. O. The United Republic of Tanzania. https://www.tacaids.go.tz/phocadownload/NMSF_IV2018.pdf [Google Scholar]
- Terris-Prestholt F, Hanson K, MacPhail C, Vickerman P, Rees H, & Watts C (2013). How much demand for New HIV prevention technologies can we really expect? Results from a discrete choice experiment in South Africa. PLoS One, 8(12), e83193. 10.1371/journal.pone.0083193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston LL (1927). A Law of Comparative Judgments. Psychological Review, 34, 273–286. 10.1037/h0070288 [DOI] [Google Scholar]
- Topp SM, Li MS, Chipukuma JM, Chiko MM, Matongo E, Bolton-Moore C, & Reid SE (2012). Does provider-initiated counselling and testing (PITC) strengthen early diagnosis and treatment initiation? Results from an analysis of an urban cohort of HIV-positive patients in Lusaka, Zambia. Journal of the International AIDS Society, 15(2), 17352. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3494161/pdf/JIAS-15-17352.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Train KE (2003). Individual-Level Parameters (Discrete choice methods with simulation (pp. 262–283). Cambridge university press. [Google Scholar]
- Treves-Kagan S, El Ayadi AM, Pettifor A, MacPhail C, Twine R, Maman S, Peacock D, Kahn K, & Lippman SA (2017). Gender, HIV Testing and Stigma: The Association of HIV Testing Behaviors and Community-Level and Individual-Level Stigma in Rural South Africa Differ for Men and Women. AIDS Behav, 21(9), 2579–2588. 10.1007/s10461-016-1671-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun W, Vu L, Dirisu O, Sekoni A, Shoyemi E, Njab J, Ogunsola S, & Adebajo S (2018). Uptake of HIV self-testing and linkage to treatment among men who have sex with men (MSM) in Nigeria: A pilot programme using key opinion leaders to reach MSM. J Int AIDS Soc, 21 Suppl 5, e25124. 10.1002/jia2.25124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. (2020). UNAIDS Data 2020 (https://www.unaids.org/en/resources/documents/2020/unaids-data
- UNAIDS. (2021, Januay 25, 2021). 2025 AIDS Targets. Retrieved June 22, 2021 from https://www.unaids.org/en/topics/2025_target_setting
- Unitaid-World Health Organization. (2018). Market and technology landscape: HIV rapid diagnostic tests for self-testing, 4th edition (https://unitaid.org/assets/HIV-Rapid-Diagnostic-Tests-for-Self-Testing_Landscape-Report_4th-edition_July-2018.pdf
- United Nations General Assembly. (2021). Political Declaration on HIV and AIDS: Ending Inequalities and Getting on Track to End AIDS by 2030 (Political declaration. https://www.unaids.org/sites/default/files/media_asset/2021_political-declaration-on-hiv-and-aids_en.pdf [Google Scholar]
- Uzochukwu B, Uguru N, Ezeoke U, Onwujekwe O, & Sibeudu T (2011). Voluntary counseling and testing (VCT) for HIV/AIDS: a study of the knowledge, awareness and willingness to pay for VCT among students in tertiary institutions in Enugu State Nigeria. Health Policy, 99(3), 277–284. 10.1016/j.healthpol.2010.11.007 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2016). Guidelines on HIV Self-Testing and Partner Notification (https://www.who.int/hiv/pub/vct/hiv-self-testing-guidelines/en/
- Zanolini A, Chipungu J, Vinikoor MJ, Bosomprah S, Mafwenko M, Holmes CB, & Thirumurthy H (2018). HIV Self-Testing in Lusaka Province, Zambia: Acceptability, Comprehension of Testing Instructions, and Individual Preferences for Self-Test Kit Distribution in a Population-Based Sample of Adolescents and Adults. AIDS Res Hum Retroviruses, 34(3), 254–260. 10.1089/AID.2017.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


