Abstract
Introduction:
The antimicrobial substantivity of Mixture of Doxycycline, Citric acid, and Tween 80 (MTAD), Tetraclean, Tetraclean NA, Q-Mix, 2% Chlorhexidine (CHX) and Octenisept was assessed in human root dentine blocks infected with Enterococcus (E.) faecalis.
Methods and Materials:
A total of 170 dentine tubes were prepared from human maxillary incisors. After crown and apical third removal, cementum was abraded. The remaining center-holed pieces were cut into 4-mm blocks, infected with E. faecalis in Brain Heart Infusion (BHI) broth for 28 days, then randomly divided into 6 experimental groups (n=25) and 2 controls (n=10). At 0, 7, 14, 21 and 28 days, dentine chips were removed from the canals, with sequential round burs with increasing diameters, and collected into freshly prepared BHI broth. After culturing, growing colonies were counted as colony forming units (CFU). Conventional non-parametric tests (Kruskal-Wallis and Mann-Whitney tests) were used to assess intra-group (at different time frames) and inter-group (at each experimental time) differences (P=0.05).
Results:
Tetraclean yielded the lowest CFU counts (P<0.001) at each observation time. Tetraclean NA and Q-Mix showed better (P<0.001) substantivity than 2% CHX and MTAD (except for Q-Mix versus MTAD at 14 days, P=0.21). Conclusions: In this in vitro study, Tetraclean NA and Q-Mix displayed the best antimicrobial substantivity against E. faecalis after Tetraclean in infected human root dentine. Considering the findings of our study and potential drawbacks of antibiotic-based irrigants, free-antibiotic irrigants may represent viable alternative for final rinse in root canal treatment.
Key Words: Antimicrobial Substantivity, Enterococcus faecalis , MTAD, Qmix, Tetraclean
Introduction
Bacteria causing persistent endodontic infections are usually located in areas unaffected by instruments and antimicrobial substances. Elimination of Enterococcus (E.) faecalis which is present up to 74% of asymptomatic and persistent endodontic infections [1-3] is a complicated task requiring the use of various instrumentation techniques, irrigation-activation protocols and intracanal medicaments. The use of a final root canal irrigant that remains active for a prolonged period after its application might be helpful in eliminating the persistent microorganisms and avoid bacterial recolonization. Sodium Hypochlorite (NaOCl) is the main endodontic irrigant [4] but it does not have antimicrobial substantivity i.e. a residual antimicrobial activity for a prolonged period [5]. Thus, other products characterized by their antimicrobial substantivity such as chlorhexidine (CHX) or certain antibiotics (doxycycline) have been included in the formulation of root canal irrigants [6]. More recently, root canal irrigants combining substantivity, lower surface tension and smear layer removal capacity have been introduced [7-12].
Q-Mix (Dentsply Tulsa Dental, Tulsa, OK, USA) is a CHX-based root canal irrigant that also contains EDTA, saline and a detergent, which decreases surface tension. Due to the presence of Ethylenediaminetetraacetic acid (EDTA), Q-Mix can remove the smear layer [13-15]. Two antibiotic-based root canal irrigants, Mixture of Doxycycline, Citric acid, and Tween 80 (MTAD) (BioPure, Dentsply, Tulsa Dental, Tulsa, OK, USA) and Tetraclean (Ogna Laboratori Farmaceutici, Muggiò, Italy) have been developed for smear layer removal and final root canal disinfection [7, 16]. They are mixtures of doxycycline (150 mg/mL-1 vs 50 mg/mL-1, respectively), citric acid and detergents (Tween 80 vs polypropylene glycol and cetrimide respectively) [7, 16]. Tetraclean NA (Ogna Laboratori Farmaceutici, Muggiò, Italy) has been recently introduced without antibiotics being included in its formulation (citric acid, cetrimide and polypropylene glycol) [11, 12]. Octenisept (Schülke and Mayr, Nordersdedt, Germany) is an antiseptic for skin burns, wound disinfection and mouth rinse consisting of phenoxyethanol and octenidine hydrochloride that demonstrated broad spectrum antimicrobial effects covering both gram-positive and gram-negative bacteria, fungi and several viral species [15]. Octenidine has been evaluated against E. faecalis [17] with promising results in disinfecting root canal dentine after different exposure times leading to consider the latter as a potent root canal irrigant [18].
Since concerns were expressed regarding antibiotic-based root canal irrigants due to the risk of bacterial resistance, allergy and tooth discoloration [19, 20], antibiotic-free solutions might be more suitable in the future. Therefore, evaluation of antimicrobial substantivity of antibiotic-free root canal irrigants is of prime importance to determine their potential to replace antibiotic based root canal irrigants. To the best of our knowledge, the antimicrobial substantivity of MTAD and Tetraclean was investigated in previous studies with encouraging results [7, 21, 22]. However, this property has not been assessed yet for Q-Mix, Tetraclean NA and Octenisept. Thus, relevant comparison of their antimicrobial substantivity against E. faecalis may lead to modify the clinical strategies for root canal irrigation especially in case of persistent endodontic infections.
The aim of this in vitro study was to assess and compare the antimicrobial substantivity of six root canal irrigants (2% CHX, MTAD, Octenisept, Q-Mix, Tetraclean, Tetraclean NA) against E. faecalis infected human root dentine. The null hypothesis tested was that there would be no difference in residual antimicrobial activity against E. faecalis between the tested disinfecting solutions.
Materials and Methods
The methodology of the present study was based on the procedure previously described by Haapasalo and Ørstavik [23]. Seventy-two extracted maxillary incisors with 14-18 mm root lengths and without fracture, carious lesion and resorption were selected. Teeth were thoroughly cleaned and stored in a 0.5% chloramine T solution for 7 days at 5°C until the experiment.
The crown and the apical third (5 mm) were removed with a rotary diamond saw at 1000 rpm (Isomet Plus precision saw, Buehler, IL, USA) under water-cooling. Cementum was removed by using abrasive paper (180-240 grit) (Ecomet 3, variable-speed grinder-polisher, Buehler, IL, USA), which resulted in a center-holed piece of root dentine with a 6-mm outer diameter. In order to prevent dehydration, all teeth and dentin slices were preserved in vials containing sterile water during the procedure.
Depending on the root length, the remaining piece of each tooth was then sectioned into 2 or 3, 4-mm thick slices with a diamond saw as shown in Figure 1. The root canals of the 4-mm blocks were enlarged (standardized) with an ISO 023 slow speed round bur (Brasseler GmbH & Co., Lemgo, Germany). Each dentine block (n=170) was individually treated in an ultrasonic bath (Bandelin Sonorex Super Digital 10 P, Sigma Aldrich, Saint Louis, MO, USA) with 17% EDTA (pH 7.2) (Ogna Laboratori Farmaceutici, Muggiò, Italy) (4 min) and 5.25% NaOCl (Ogna Laboratori Farmaceutici, Muggiò, Italy) (4 min). The specimens were then placed in glass tubes containing 5 mL Brain Heart Infusion (BHI) broth (Oxoid, Basingstoke, UK) and autoclaved (121ºC, 15 min, 15 psi). Following transfer to yeast extract-glucose broth (YG broth; Yeast Extract, Oxoid, Hampshire, England) (10 g/L glucose) and incubation (at 37°C for 24 h as a test for sterility), the blocks were subjected to ultrasonic treatment for 10 min.
Figure 1.
Schematic view of used dentin tubes
Experimental and control groups
A total of 170 specimens were randomly distributed into 6 experimental and 2 control groups according to the root canal irrigant used after the contamination period as follows: Group 1 (n=25), MTAD; Group 2 (n=25), Tetraclean; Group 3 (n=25), Tetraclean NA; Group 4 (n=25), Q-Mix; Group 5 (n=25), 2% CHX; Group 6 (n=25), Octenisept; Group 7 (n=10), positive control (infected dentine tubes); Group 8 (n=10), negative control (sterile dentine tubes).
Contamination with E. faecalis and irrigation procedures
Isolated 24-h colonies of pure cultures of E. faecalis (ATCC® 29212™) (American Type Culture Collection, Manassas, VA, USA) were suspended in 5 mL of BHI broth and the turbidity was adjusted to a 0.5-1 McFarland. Two milliliters of sterile BHI were removed with sterile pipettes and replaced with 2 mL of bacterial inoculum in each bottle except for group 8 under laminar flow to avoid contamination. The bottles were closed and kept at 37°C for 28 days, with the replacement of 1 mL of contaminated BHI for 1 mL of freshly prepared BHI every 2 days. After the contamination period, the bacterial growth was verified using Brown and Brenn staining method (Figure 2). Each specimen was removed from its bottle, and the root canal was irrigated with 5 mL of sterile saline by using sterile plastic syringes and 27-gauge needles and dried with sterile paper points. In order to prevent contact of the irrigants with the outer surface of the specimens, the latter was covered with nail varnish. Thereafter, specimens were fixed at the bottom of wells of 24-well cell culture plates using disinfected sticky wax, which also obliterated the apical surface of the root canal. Finally, disinfecting solutions were delivered into the canal lumen with sterile 3 mL plastic syringes and 27-gauge needles until the dentine tubes were totally filled. Solutions were removed using sterile paper points 10 min after placement into the lumen.
Figure 2.
A microscope view (magnification 400×) of stained dentine tube infected in vitro with E. faecalis for 28 days (Brown and Brenn staining)
Collection of dentine chips
The specimens were then incubated at 37°C for 28 days and fresh BHI added every 2 days. At experimental times of 0, 7, 14, 21 and 28 days, dentine chips were removed from the root canals of all specimens with sequential sterile low-speed (400 rpm) round burs (Brasseler Dental Savannah, GA, USA) with increasing diameters of ISO sizes: 025, 027, 029, 031 and 033, respectively (Figure 1). Each bur removed 0.1 mm of dentine around the canal which was controlled using a digital caliper (Mitutoyo, Tokyo, Japan). The powder dentine samples were immediately collected in separate test tubes containing 3 mL of freshly prepared BHI broth; l00 μL from each test tube was cultured on blood agar. Growing colonies were counted and recorded as colony forming units (CFU).
Statistical analysis
The D’Agostino and Pearson omnibus normality test was used to assess the normal distribution of all data. Non-parametric Kruskal-Wallis test was used to investigate the differences between the experimental groups and the positive control. The differences between groups at each experimental time were evaluated using the Kruskal-Wallis test and the Dunn’s multiple comparison test for independent samples was used to compare all pairs of data at each time frame. The Friedman test for paired observations was used to assess the differences between time frames within each experimental group; the difference between all pairs of data (day 0 vs day 7, day 7 vs day 14, day 14 vs day 21 and day 21 vs day 28) within each group was evaluated by using the Dunn’s multiple comparison test for paired samples. The significance level was set at 0.05.
Results
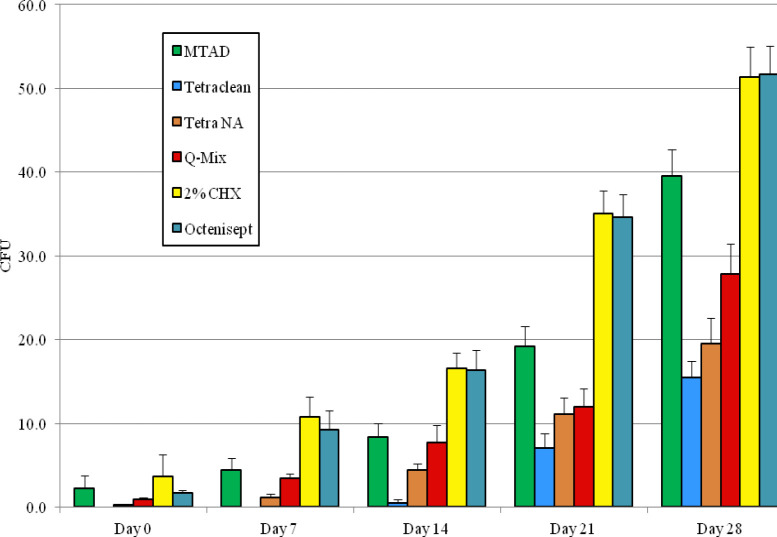
The mean values and standard deviations of the CFU counts for each group at the different time frames are reported in Table 1. Negative and positive samples provided constant mean CFU counts of 0 and 120, respectively. The trend of the mean CFU counts for each group is shown graphically in Figure 3.
Table 1.
Mean (SD) of CFU counts for the study groups at the different time intervals investigated. The results of the Kruskal-Wallis (K-W) test for within-group comparison (last column) and for between-group comparison at each time interval (bottom row) all revealed a highly significant difference
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | P -value (K-W test) | |
|---|---|---|---|---|---|---|
| MTAD | 2.20 (1.56) a,A | 4.43 (1.40) a,B | 8.32 (1.72) a,C | 19.22 (2.41) a,D | 39.49 (3.19) a,E | P<0.001 |
| Tetraclean | 0.00 (0.00) b,A | 0.00 (0.00) b,A | 0.52 (0.36) b,B | 7.09 (1.73) b,C | 15.53 (1.91) b,D | P<0.001 |
| Tetra NA | 0.24 (0.13) c,A | 1.12 (0.49) c,B | 4.41 (0.79) c,C | 11.13 (1.88) c,d,D | 19.52 (3.11) c,E | P<0.001 |
| Q-Mix | 0.95 (0.23) d,A | 3.44 (0.56) d,B | 7.65 (2.18) a,C | 11.99 (2.15) d,D | 27.88 (3.52) d,E | P<0.001 |
| 2% CHX | 3.68 (2.60) e,A | 10.77 (2.40) e,B | 16.58 (1.89) d,C | 35.10 (2.71) e,D | 51.33 (3.62) e,E | P<0.001 |
| Octenisept | 1.64 (0.33) a,A | 9.23 (2.33) f, e,B | 16.36 (2.37) d,C | 34.65 (2.67) f,e,D | 51.66 (3.39) e,E | P<0.001 |
| P -value (K-W test) | P<0.001 | P<0.001 | P<0.001 | P<0.001 | P<0.001 |
Different superscript lower-case and upper-case letters indicate statistically significant differences (P<0.05) among groups at each time interval (columns) and within groups at different time intervals (rows) respectively (P=0.05)
Figure 3.
Mean (SD) of CFU counts for all study groups at the different time intervals
Not all data followed a Gaussian distribution according to the D’Agostino and Pearson omnibus normality test, so all the comparisons were performed using non-parametric tests. The results of the Friedman test for within-group comparison at the different time frames and the Kruskal-Wallis test for between-group comparison at each time interval revealed significant differences (Table 1).
The most effective irrigant at each experimental time was Tetraclean; in fact, the CFU counts of the group treated with Tetraclean resulted significantly lower than any other group at each observation time (P<0.001 in all cases).
The irrigants showing the lower activity against E. faecalis were Octenisept and 2% CHX. CFU counts on day 7, 14, 21 and 28 were not significantly different between these two groups, but they were significantly higher than any other group at each experimental time, except at Day 0 when the CFU counts of Octenisept and MTAD groups were not statistically different (P=0.16).
Tetraclean NA and Q-Mix provided CFU counts significantly lower (P<0.001) than 2% CHX, MTAD and Octenisept groups (except for Q-Mix vs MTAD at 14 days) (P=0.21) contrary to the data in Table 1. CFU counts of Tetraclean NA group were significantly lower than Q-Mix (except for 21 days) (P=0.09) at each observation time (P<0.001).
Discussion
Several strategies have been recommended to assess the presence and eradication of bacteria within dentinal tubules after different disinfection procedures [22-25]. Dentine block models have been reported to allow bacterial penetration into dentinal tubules for up to 500 µm from the main root canal [1]. By forcing E. faecalis into dentinal tubules with serial centrifugation, Ma et al. [25] overcame the difficulty of obtaining an in vitro homogenous bacterial invasion and the standardization of samples by culturing. Nonetheless, the potential influence of centrifugation forces on bacterial resistance to disinfecting agents (changes in DNA expression, auto/co-aggregation and zeta potentials) and of time and gradualness in their increasing (possible bacterial death) on the quality of in vitro intratubular infection needs to be clarified.
In the present study, the positive control group showed viable bacteria at all experimental times, which indicated the reliability of the method, confirming the quality of intratubular infection before treatment. The CFU counts of the positive group were not added in Figure 3 since they were off scale respect to other groups. After the contamination period, histological evaluation was selected to assess the penetration depth of E. faecalis within the tubules.
Since the present study aimed to assess substantivity of combinational products including EDTA, viability staining and confocal laser scanning microscopy (CLSM) was not appropriate to measure bacterial killing. Indeed, EDTA may affect the bacterial cell wall and allows the red viability stain to penetrate into E. faecalis even though they are not killed, leading to false-positive results [26]. Further reliable comparative experiments could not be performed with EDTA only or with NaOCl/EDTA by using a dentine infection model with viability stain and CLSM. Therefore, we decided to use powdered dentine on plates for CFU counting to evaluate the disinfection into the dentinal tubules at different layer depths.
In the present study, the 2% CHX substantivity remained for at least 28 days on human dentine after 10 min of exposure. This finding is in accordance with previous studies, which demonstrated that CHX substantivity could be maintained 28 days with only 5 min of exposure [27]. The correlation between the microbial viability and the presence of 2% CHX on dentine after a 5 min exposure has been evaluated in vitro [27, 28]. 2% CHX solution was detected for 48 h and 7 days, and its presence was associated with a low percentage of viable cells in E. faecalis biofilms [29]. However, on day 30, the CHX effect over the microbial cells was equivalent to the control (saline) and CHX amount was considered absent. The CHX antimicrobial activity slowed down considerably after 10 min exposure when tested on young and mature E. faecalis biofilms by using a dentine infection model and CLSM [30]. Additional efforts are necessary to better understand how CHX binds to mineralized and demineralized dentine in order to maximize its retention and effectiveness and optimize its clinical use [31]. Therefore, a 28 days observation and a 10 min exposure have been selected to better investigate kinematic of substantivity of the experimental solutions even if this exposure time was not acceptable from a clinical point of view. Further investigations are also needed to determine at which level chelating agents use influence the physical and chemical properties of dentine [32] and the adhesion and sealing ability of the root canal sealers [33].
The demineralizing effects of chemical solutions are beneficial permitting deeper penetration of antimicrobial agents and better smear layer removal. However, the use of chelating or decalcifying agents in combinational products for more than 3-5 min contact times could promote erosion in the dentinal surface and not be clinically acceptable. Poggio et al. [9] evaluated decalcifying capability of different irrigating solutions and showed that for all irrigating solutions, the maximum amount of Ca2+ extracted from root canal dentine was reached after 10 min contact time except for citric acid-based agents (Tetraclean and Tetraclean NA) which induced a higher and still increasing calcium release even after 10 min contact time. Since the aim was to better understand substantivity of the tested solutions over time, clinically acceptable 1-5 min exposures were not considered in the present study.
Residual antibacterial activity of CHX and Octenisept resulted comparable except in the first sampling that showed better results for Octenisept. Lin et al. [34] suggested that the limited antibacterial effect of CHX in the first h, was related to the dentine absorption time and stated that CHX antibacterial capability increased after reaching the saturation point. Q-Mix demonstrated efficient initial activity (0.79% residual bacterial load at day 0) and better substantivity against E. faecalis than 2% CHX at all experimental periods. The presence of a detergent in Q-Mix formula increases its wettability enhancing its dentinal tubule penetration depth. The nature of the CHX-dentine interaction may also explain the findings obtained for Q-Mix group. The presence of collagen is an important factor enhancing the substantivity of CHX [35] and impacting the adhesion of E. faecalis to dentine [36]. EDTA increases the dentine porosity [37] and allows CHX to be trapped within the collagen network [31, 35] and to bind to the collagen matrix and underlying mineralized matrix (negatively charged) [38].
Stojicic et al. [8] evaluated the efficacy of Q-Mix, 2% CHX, and MTAD against E. faecalis and mixed plaque bacteria, in planktonic and biofilm culture. In biofilm experiments, Q-Mix killed up to 12 times more biofilm bacteria than 2% CHX and MTAD after 1 and 3 min [8], which is in accordance with the findings of the present study.
BioPure MTAD and Tetraclean are mixtures of doxycycline, citric acid and surfactants with different antibiotic concentration and type of detergent [7, 16]. Substantivity of doxycycline and MTAD has been demonstrated for up to 4 weeks [22, 27]. In contrast to the findings of the present study, Khademi et al. [27] found that antibacterial substantivity of 2% CHX was significantly greater than doxycycline. The antibiotic concentration in MTAD formula may improve substantivity and the detergent may increase doxycycline penetration depth into dentinal tubules. Doxycycline 100 mg mL-1 and MTAD showed more effective substantivity than NaOCl at 28 days against planktonic status of E. faecalis in bovine root dentine [22, 27]. However, when a “biofilm method” was used, the efficacy of BioPure MTAD regarding root canal disinfection seemed lower [7, 16]. A study using E. faecalis biofilm revealed that BioPure MTAD was less effective than NaOCl and Tetraclean against biofilm bacteria [7].
In the present study, Tetraclean caused an immediate drop to undetectable CFU counts yielding mean values equal to 0 at Day 0 and Day 7. Its low surface tension (29.1 mJ/m2) [8] improving its wettability, cetrimide and the bacteriostatic activity of doxycycline may explain our results.
The solutions containing cetrimide (Tetraclean and Tetraclean NA) exerted the highest substantivity. Since its cationic nature, cetrimide interacts with dentine, resulting in an action close to that of CHX, and is effective against many gram-positive and gram-negative bacteria. Cetrimide showed also substantivity when combined with CHX [39]. However, when used alone, 0.2% cetrimide exerted residual antimicrobial activity for 24 h against E. faecalis biofilm, comparable with 2% CHX [39] with no difference in mineralized and demineralized dentine [37]. Pappen et al. [40] showed that modifications of MTAD formula, where Tween 80 was replaced by cetrimide, were more effective against E. faecalis and polymicrobial biofilm than MTAD.
Tetraclean NA and Q-Mix showed the best substantivity after Tetraclean. The highest regrowth was recorded for Tetraclean NA at day 21 and for Q-Mix at day 28 with no significant difference at day 21. Q-Mix resulted as effective as 6% NaOCl against E. faecalis within dentinal tubules [25]. A previous study showed that Tetraclean NA maintained its antimicrobial activity on 60-day E. faecalis mature biofilms, 20 min and 72 h after dentine contact [10]. Under the same experimental conditions, Hypoclean, a 5.25% NaOCl modified with lowered surface tension, yielded better antibacterial activity than Tetraclean NA and Hypoclean/Tetraclean NA combination at each experimental time. Thus, further studies are required to clarify the potential antibacterial efficacy of Tetraclean NA.
Conclusion
Within the conditions of this study, Tetraclean solution displayed the best antimicrobial substantivity against E. faecalis into dentine tubules following by Q-Mix and Tetraclean NA. Therefore, the null hypothesis was rejected. Considering the findings of our study and the potential drawbacks of antibiotic-based irrigants, Q-Mix and Tetraclean NA may represent viable alternative. Further studies regarding antibacterial substantivity of free-antibiotic irrigants should be performed in order to confirm our encouraging results and to determine their scope and usefulness.
Acknowledgment
The authors wish to express their gratitude to Dr. Luciano Giardino and Dr. Paolo Savadori for histological processing and staining of dentine specimens.
Conflict of Interest:
‘None declared’.
References
- 1.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32(2):93–8. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 2.Mohammadi Z, Jafarzadeh H, Shalavi S, Palazzi F. Recent Advances in Root Canal Disinfection: A Review. Iran Endod J. 2017;12(4):402–6. doi: 10.22037/iej.v12i4.17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohammadi Z, Shalavi S, Giardino L, Palazzi F, Asgary S. Impact of Ultrasonic Activation on the Effectiveness of Sodium Hypochlorite: A Review. Iran Endod J. 2015;10(4):216–20. doi: 10.7508/iej.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammadi Z, Yaripour S, Shalavi S, Palazzi F, Asgary S. Root Canal Irrigants and Dentin Bonding: An Update. Iran Endod J. 2017;12(2):131–6. doi: 10.22037/iej.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenstein G, Polson A. The role of local drug delivery in the management of periodontal diseases: a comprehensive review. J Periodontol. 1998;69(5):507–20. doi: 10.1902/jop.1998.69.5.507. [DOI] [PubMed] [Google Scholar]
- 6.Mohammadi Z, Giardino L, Palazzi F, Asgary S. Agonistic and Antagonistic Interactions between Chlorhexidine and Other Endodontic Agents: A Critical Review. Iran Endod J. 2015;10(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Giardino L, Ambu E, Savoldi E, Rimondini R, Cassanelli C, Debbia EA. Comparative evaluation of antimicrobial efficacy of sodium hypochlorite, MTAD, and Tetraclean against Enterococcus faecalis biofilm. J Endod. 2007;33(7):852–5. doi: 10.1016/j.joen.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Stojicic S, Shen Y, Qian W, Johnson B, Haapasalo M. Antibacterial and smear layer removal ability of a novel irrigant, QMiX. Int Endod J. 2012;45(4):363–71. doi: 10.1111/j.1365-2591.2011.01985.x. [DOI] [PubMed] [Google Scholar]
- 9.Poggio C, Dagna A, Vinci A, Beltrami R, Cucca L, Giardino L. Decalcifying capability of irrigating solutions on root canal dentin mineral content. Contemp Clin Dent. 2015;6(2):201–5. doi: 10.4103/0976-237X.156046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giardino L, Estrela C, Generali L, Mohammadi Z, Asgary S. The in vitro Effect of Irrigants with Low Surface Tension on Enterococcus faecalis. Iran Endod J. 2015;10(3):174–8. doi: 10.7508/iej.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souza MA, Montagner A, Lana DL, Vidal CM, Farina AP, Cecchin D. Comparative evaluation of the retaining of QMix and chlorhexidine formulations on human dentin: a chemical analysis. Clin Oral Investig. 2017;21(3):873–8. doi: 10.1007/s00784-016-1837-9. [DOI] [PubMed] [Google Scholar]
- 12.Nourzadeh M, Amini A, Fakoor F, Raoof M, Sharififar F. Comparative Antimicrobial Efficacy of Eucalyptus Galbie and Myrtus Communis L Extracts, Chlorhexidine and Sodium Hypochlorite against Enterococcus Faecalis. Iran Endod J. 2017;12(2):205–10. doi: 10.22037/iej.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagzap JB, Patil SS, Gade VJ, Chandhok DJ, Upagade MA, Thakur DA. Effectiveness of Three Different Irrigants - 17% Ethylenediaminetetraacetic Acid, Q-MIX, and Phytic Acid in Smear Layer Removal: A Comparative Scanning Electron Microscope Study. Contemp Clin Dent. 2017;8(3):459–63. doi: 10.4103/ccd.ccd_524_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farhad Mollashahi N, Saberi E, Karkehabadi H. Evaluation of Cytotoxic Effects of Various Endodontic Irrigation Solutions on the Survival of Stem Cell of Human Apical Papilla. Iran Endod J. 2016;11(4):293–7. doi: 10.22037/iej.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammadi Z. An update on the antibiotic-based root canal irrigation solutions. Iran Endod J. 2008;3(2):1–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Dunavant TR, Regan JD, Glickman GN, Solomon ES, Honeyman AL. Comparative evaluation of endodontic irrigants against Enterococcus faecalis biofilms. J Endod. 2006;32(6):527–31. doi: 10.1016/j.joen.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Sedlock DM, Bailey DM. Microbicidal activity of octenidine hydrochloride, a new alkanediylbis[pyridine] germicidal agent. Antimicrob Agents Chemother. 1985;28(6):786–90. doi: 10.1128/aac.28.6.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tandjung L, Waltimo T, Hauser I, Heide P, Decker EM, Weiger R. Octenidine in root canal and dentine disinfection ex vivo. Int Endod J. 2007;40(11):845–51. doi: 10.1111/j.1365-2591.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 19.Krishna BV, Gibb AP. Use of octenidine dihydrochloride in meticillin-resistant Staphylococcus aureus decolonisation regimens: a literature review. J Hosp Infect. 2010;74(3):199–203. doi: 10.1016/j.jhin.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Mohammadi Z, Jafarzadeh H, Shalavi S, Yaripour S, Sharifi F, Kinoshita JI. A Review on Triple Antibiotic Paste as a Suitable Material Used in Regenerative Endodontics. Iran Endod J. 2018;13(1):1–6. doi: 10.22037/iej.v13i1.17941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tay FR, Mazzoni A, Pashley DH, Day TE, Ngoh EC, Breschi L. Potential iatrogenic tetracycline staining of endodontically treated teeth via NaOCl/MTAD irrigation: a preliminary report. J Endod. 2006;32(4):354–8. doi: 10.1016/j.joen.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Mohammadi Z, Giardino L, Mombeinipour A. Antibacterial substantivity of a new antibiotic-based endodontic irrigation solution. Aust Endod J. 2012;38(1):26–30. doi: 10.1111/j.1747-4477.2010.00263.x. [DOI] [PubMed] [Google Scholar]
- 23.Haapasalo M, Orstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66(8):1375–9. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 24.Mohammadi Z, Shalavi S, Soltani MK, Asgary S. A review of the properties and applications of ozone in endodontics: an update. Iran Endod J. 2013;8(2):40–3. [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Wang Z, Shen Y, Haapasalo M. A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J Endod. 2011;37(10):1380–5. doi: 10.1016/j.joen.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Shen Y, Haapasalo M. Effectiveness of endodontic disinfecting solutions against young and old Enterococcus faecalis biofilms in dentin canals. J Endod. 2012;38(10):1376–9. doi: 10.1016/j.joen.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 27.Khademi AA, Mohammadi Z, Havaee A. Evaluation of the antibacterial substantivity of several intra-canal agents. Aust Endod J. 2006;32(3):112–5. doi: 10.1111/j.1747-4477.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 28.Mohammadi Z, Asgary S. A comparative study of antifungal activity of endodontic irrigants. Iran Endod J. 2015;10(2):144–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Bottcher DE, Sehnem NT, Montagner F, Fatturi Parolo CC, Grecca FS. Evaluation of the Effect of Enterococcus faecalis Biofilm on the 2% Chlorhexidine Substantivity: An In Vitro Study. J Endod. 2015;41(8):1364–70. doi: 10.1016/j.joen.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Du T, Wang Z, Shen Y, Ma J, Cao Y, Haapasalo M. Effect of long-term exposure to endodontic disinfecting solutions on young and old Enterococcus faecalis biofilms in dentin canals. J Endod. 2014;40(4):509–14. doi: 10.1016/j.joen.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 31.Carrilho MR, Carvalho RM, Sousa EN, Nicolau J, Breschi L, Mazzoni A, Tjaderhane L, Tay FR, Agee K, Pashley DH. Substantivity of chlorhexidine to human dentin. Dent Mater. 2010;26(8):779–85. doi: 10.1016/j.dental.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panighi M, G'Sell C. Influence of calcium concentration on the dentin wettability by an adhesive. J Biomed Mater Res. 1992;26(8):1081–9. doi: 10.1002/jbm.820260809. [DOI] [PubMed] [Google Scholar]
- 33.Rotstein I, Dankner E, Goldman A, Heling I, Stabholz A, Zalkind M. Histochemical analysis of dental hard tissues following bleaching. J Endod. 1996;22(1):23–5. doi: 10.1016/S0099-2399(96)80231-7. [DOI] [PubMed] [Google Scholar]
- 34.Lin S, Zuckerman O, Weiss EI, Mazor Y, Fuss Z. Antibacterial efficacy of a new chlorhexidine slow release device to disinfect dentinal tubules. J Endod. 2003;29(6):416–8. doi: 10.1097/00004770-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Uchiyama T, Carrilho M, Agee KA, Mazzoni A, Breschi L, Carvalho RM, Tjaderhane L, Looney S, Wimmer C, Tezvergil-Mutluay A, Tay FR, Pashley DH. Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent Mater. 2010;26(8):771–8. doi: 10.1016/j.dental.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kishen A, Sum CP, Mathew S, Lim CT. Influence of irrigation regimens on the adherence of Enterococcus faecalis to root canal dentin. J Endod. 2008;34(7):850–4. doi: 10.1016/j.joen.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 37.van der Graaf ER, ten Bosch JJ. The uptake of water by freeze-dried human dentine sections. Arch Oral Biol. 1990;35(9):731–9. doi: 10.1016/0003-9969(90)90096-s. [DOI] [PubMed] [Google Scholar]
- 38.Misra DN. Interaction of chlorhexidine digluconate with and adsorption of chlorhexidine on hydroxyapatite. J Biomed Mater Res. 1994;28(11):1375–81. doi: 10.1002/jbm.820281116. [DOI] [PubMed] [Google Scholar]
- 39.Baca P, Mendoza-Llamas ML, Arias-Moliz MT, Gonzalez-Rodriguez MP, Ferrer-Luque CM. Residual effectiveness of final irrigation regimens on Enteroccus faecalis-infected root canals. J Endod. 2011;37(8):1121–3. doi: 10.1016/j.joen.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Pappen FG, Shen Y, Qian W, Leonardo MR, Giardino L, Haapasalo M. In vitro antibacterial action of Tetraclean, MTAD and five experimental irrigation solutions. Int Endod J. 2010;43(6):528–35. doi: 10.1111/j.1365-2591.2010.01712.x. [DOI] [PubMed] [Google Scholar]