Abstract
Benzo[a]pyrene [B(a)P], a potent procarcinogen found in combustion products such as diesel exhaust and cigarette smoke, has been recently shown to activate the c-Jun NH2-terminal kinase 1 (JNK1) and induce caspase-3-mediated apoptosis in Hepa1c1c7 cells. However, the molecules of the signaling pathway that control the mitogen-activated protein kinase cascades induced by B(a)P and the interaction between those and apoptosis by B(a)P have not been well defined. We report here that B(a)P promoted Cdc42/Rac1, p21-activated kinase 1 (PAK1), and JNK1 activities in 293T and HeLa cells. Moreover, alpha-PAK-interacting exchange factor (α PIX) mRNA and its protein expression were upregulated by B(a)P. While overexpression of an active mutant of α PIX (ΔCH) facilitated B(a)P-induced activation of Cdc42/Rac1, PAK1, and JNK1, overexpression of mutated αPIX (L383R, L384S), which lacks guanine nucleotide exchange factor activity, SH3 domain-deleted αPIX (Δ SH3), which lacks the ability to bind PAK, kinase-negative PAK1 (K299R), and kinase-negative SEK1 (K220A, K224L) inhibited B(a)P-triggered JNK1 activation. Interestingly, overexpression of αPIX (Δ CH) and a catalytically active mutant PAK1 (T423E) accelerated B(a)P-induced apoptosis in HeLa cells, whereas αPIX (Δ SH3), PAK1 (K299R), and SEK 1 (K220A, K224L) inhibited B(a)P-initiated apoptosis. Finally, a preferential caspase inhibitor, Z-Asp-CH2-DCB, strongly blocked the αPIX (Δ CH)-enhanced apoptosis in cells treated with B(a)P but did not block PAK1/JNK1 activation. Taken together, these results indicate that αPIX plays a crucial role in B(a)P-induced apoptosis through activation of the JNK1 pathway kinases.
Benzo[a]pyrene [B(a)P] is a member of the polycyclic aromatic hydrocarbons (PAHs), compounds that are found in combustion products such as cigarette smoke and diesel exhaust and are thought to be carcinogens capable of tumor initiation, promotion, and progression (38). B(a)P binds to the aryl hydrocarbon receptor as a ligand and induces the expression of cytochrome P450-1A1 (CYP1A1) (40). Subsequently, B(a)P is metabolized by CYP1A1 to electrophilic species, such as anti-7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene [B(a)PDE], which can eventually induce mutations or oxidative DNA damage (12, 20, 33). Via this mechanism, B(a)P and/or other PAHs can induce tumors in the mammary gland, liver, and lungs (15, 16, 18, 31). In addition, B(a)P and/or other PAHs have been shown to induce apoptosis, or programmed cell death, in vitro in Hepa1c1c7 hepatoma cells (27), Daudi human B cells (42), human ectocervical cells (39), and A20.1 murine B- cells (8). Recently, several studies have focused on the association between cellular signaling pathways and apoptosis induced by B(a)P. For example, B(a)P absorbed on carbon black induces the expression and release of tumor necrosis factor alpha, resulting in apoptosis of RAW 264.7 macrophage cells via mitogen-activated protein kinase (MAPK) (11). On the other hand, it has been demonstrated that B(a)P mimics signaling through the insulin-like growth factor-I receptor (IGF-IR) and increases cell survival through phosphatidylinositol 3-kinase (P13-K) activation in human mammary epithelial cells (48). It has also been suggested that c-Jun NH2-terminal kinase 1 (JNK1) activation might be independent of B(a)P-induced apoptosis in Hepa1c1c7 cells (27), although the interaction between the JNK1 signaling pathway and apoptosis induced by B(a)P has not been well elucidated.
Very recently, Kong et al. demonstrated that Rho-21 guanine nucleotide exchange factors (GEFs) are associated with apoptosis; the proto-oncogene Vav, which acts as a specific GEF on Rac and modulates cytoskeletal reorganization and cytokine production in T cells, has been reported to act upstream of caspase activation and regulate peptide-specific cell death in thymocytes (25). In addition, Tiam-1, a proto-oncogene and another GEF for Rac1, has been reported to regulate apoptosis induced by bufalin, a component of the Chinese medicine chan'su, via Rac1, p21-activated protein kinase (PAK) and JNK signaling pathways in HL60 and U937 cells (24). These findings together infer that GEFs in general may be closely involved in apoptosis.
Recently, novel GEFs for Rac1, termed the PAK-interacting exchange factor (PIX) family, have been identified (29). PIX family proteins, which consist of two isoforms (αPIX and βPIX), bind tightly through the N-terminal SH3 domain to a conserved proline-rich PAK sequence located at the C terminus of the p21-binding domain (PBD) and are colocalized with PAK to form activated Cdc42-and Rac1-driven focal complexes (29, 37). αPIX is activated by signaling cascades from the platelet-derived growth factor receptor and EphB2 receptor and from integrin-induced signaling through PI3-kinase, leading to PAK activation (57). It has also been demonstrated that αPIX is involved in Rac-type morphological changes such as lamellipodia formation in PC12 cells and photoreceptor axon guidance in Drosophila through interactions with PAK (19, 36). In addition, PIX has been shown to bind the G-protein-coupled receptor kinase-interacting protein, known as GIT1, leading to focal complex disassembly and stimulation of cell motility (59). Because PAK lies upstream of the JNK pathway (3, 7, 58), we tried to elucidate the possible involvement of αPIX in JNK signaling cascades and their various biological effects including apoptosis on cells in response to B(a)P.
In this study, we show that αPIX mediates signals induced by B(a)P leading to JNK1 kinase activation through Cdc42/Rac1, PAK1, and SEK1 kinases in 293T and HeLa cells. B(a)P also increases the levels of αPIX mRNA and its protein. While B(a)P induces caspase-protease-mediated apoptosis, αPIX accelerates B(a)P-triggered apoptosis through activation of JNK1 pathway kinases. These results indicate that αPIX may play a pivotal role in B(a)P-induced apoptosis through the JNK signaling pathway.
MATERIALS AND METHODS
Materials.
HeLa cells were kindly supplied by the Cell Resource Center of the Biomedical Research Institute of Development, Aging and Cancer, Tohoku University. A fluorogenic substrate for caspase-3 protease (Ac-DEVD-MCA) was purchased from the Peptide Institute (Osaka, Japan). B(a)P was from Sigma Chemical Co. (St. Louis, Mo.). All other chemicals were obtained through standard suppliers.
Plasmids and antibodies.
Plasmid carrying the full-length cDNA of human αPIX was a gift from T. Nagase (KIAA0006). Deletion mutants of αPIX tagged with a myc or hemagglutinin (HA) epitope at the C terminus were generated using a PCR-based technique (46). All constructs were subcloned into the pCS2+ vector for transfection into 293T or HeLa cells and into pGEX2T (Phrmacia) to make bacterial glutathione S-transferase (GST) fusion constructs. pGEX fusion protein of the p21-binding domain (PBD) of PAK1 (pGEX-PBD) was generated by cloning a PCR-amplified fragment of putative Cdc42 and the Rac binding domain of human PAK1 (amino acids 70 to 133) into pGEX2T for affinity precipitation. Amino-terminally myc-tagged wild-type and mutant (K299R, T423E, and H83L H86L) human PAK1 clones and wild-type Cdc42 and Rac1 were kindly provided by Bruce J. Mayer (45). Plasmid encoding wild-type human Bcl-2 was kindly provided by Y. Eguchi and Y. Tsujimoto (44).
The monoclonal antibody for the flag epitope tag was from Sigma. The monoclonal antibodies for the myc epitope tag and HA epitope tag were from Santa Cruz and Berkeley Antibody Co., respectively. The polyclonal antibodies for Cdc42, Rac1, αPIX, and the GST epitope tag were from Santa Cruz.
Cell culture, DNA transfections, and in vitro kinase assay.
293T human embryonal kidney cells (expressing simian virus 40 T antigen) were transfected with a maximum of 20 μg of plasmid DNA per 10-cm-diameter dish by a calcium phosphate coprecipitation method with concurrent treatment with 25 μM chloroquine essentially as described previously (45). Plasmid DNA was transfected into HeLa cells using DMRIE-C reagent (Life Technologies, Gaithersburg, Md.). At 18 to 24 h after transfection, the cells were placed in medium with 0.5% fetal bovine serum (FBS) and incubated for a further 12 h. The cells were treated with B(a)P (Sigma), which was concentrated 1,000-fold in dimethyl sulfoxide (DMSO), prior to lysis or were left untreated. The cells were harvested for immunoprecipitation and kinase assays, which were performed as previously described (45). To purify myc-tagged PAK protein, monoclonal antibody against myc epitope (1 μg) was incubated with 500 μ 1 of cell lysate for 2 h at 4°C and precipitated with protein G-agarose (Boehringer, Mannheim, Germany). To purify GST-JNK protein, glutathione-Sepharose 4B (Amersham Life Science, Piscataway, N.J.) was incubated with 500 μ 1 of cell lysate for 30 min at 4°C. Immunoprecipitates were washed extensively before being used for immunoblotting or for an in vitro kinase assay with myelin basic protein or GST–c-Jun as substrate. The results were visualized with a Bio Imaging Analyzer (BAS1000; Fuji, Tokyo, Japan), and images were quantitated with NIH Image software. The relative activity of PAK/JNK was calculated by adjusting the densitometric value and standardization.
Drug treatment.
Logarithmically growing 293T cells and HeLa cells were harvested by trypsinization and seeded in 10 ml of fresh medium in a 10-cm dish. After overnight incubation and subsequent 12-h prestarvation, B(a)P was added from 1,000-fold-concentrated stocks in DMSO with a final concentration of 0.1% DMSO in medium, which had no influence on the cells. After various periods of incubation, floating cells and/or trypsinized adhesive cells were combined and sedimented at 800 × g for 10 min, and then the following assays were performed.
Western blot analysis.
Cells were lysed in a lysis buffer (25 mM Tris-HCl [pH 7.4], 10 mM β -glycerophosphate, 150 mM NaCl, 5 mM disodium EDTA, 10 mM sodium pyrophosphate, 1% Triton X-100, 1 mM Na3VO4, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride (PMSF), 20 μg of aprotinin per ml). The cell extracts were clarified by centrifugation, and the supernatants were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were then subjected to Western blot analysis.
Affinity precipitation.
Affinity precipitation with GST-PBD was as described previously (4, 49), except that 293T and HeLa cells were lysed in lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 20 mM MgCl2, 1 mM Na3VO4, 0.5% Triton X-100, 5 μ g of aprotinin per ml, 1 mM PMSF), and precipitates were washed three times in the same buffer.
RT-PCR analysis.
Total RNA isolated from HeLa cells with or without B(a)P treatment was prepared using ISOGEN (Wako Pure Chemical Industries, Ltd., Osaka, Japan). The RNA samples were reverse transcribed using Superscript reverse transcriptase (Life Technologies, Inc.), oligo (dT) primers (Life Technologies, Inc.), and deoxynucleostide triphosphate as specified by the manufacturer. The synthesized cDNAs were amplified by PCR (30 and 25 cycles for αPIX and β-actin, respectively) with Taq DNA polymerase (Boehringer Mannheim) in the presence of deoxynucleostide triphosphate, an appropriate pair of primers, and [α-32P]dCTP (ICN, High Wycombe, United Kingdom). For PCR amplification of the αPIX gene, we used the forward primer 5′-AGTCCTACTCCCTGAGGAAGAGAAA-3′ and the reverse primer 5′-TGAGGTCTTGCTACTGGACTCGCCT-3′; β-actin primers (forward primer 5′-GGTGAAGGTGTCAGCAGCAG-3′ and the reverse primer 5′-GGCCAAGCAGCGCAGCACAG-3′) were also used as a control. The PCR products were subjected to electrophoresis in an 8% (wt/vol) acrylamide gel, and the results were visualized with a Bio Imaging Analyzer.
Measurement of CPP32-like caspase activity.
After treatment with B(a)P, the cells were collected and lysed for 20 min on ice in lysis buffer containing 10 mM HEPES-KOH (pH 7.4), 2 mM EDTA, 0.1% 3-[(3-cholamidopropyl) dimethyl ammonio]-1-propanesulfonate (CHAPS), 1 mM PMSF, and 5 mM dithiothreitol. After centrifugation, the supernatants were collected as lysates. For measurement of caspase activity, 100 μg of lysate proteins in 50 μ l of lysis buffer were mixed with 50 μ l of 2× reaction buffer containing 40 mM HEPES KOH (pH 7.4), 20% glycerol, 1 mM PMSF, and 4 mM dithiothreitol DTT with 50 μ M fluorogenic substrate acetyl-Tyr-Val-Ala-Asp-4-methylcoumaryl-7-4,6-diamidino-2-phenylindole (Ac-DEVD-MCA) and incubated at 37°C for 90 min. The release of amino-4-methylcoumarin was monitored with a spectrofluorometer (Jasco model FP-777; Japan Spectroscopic Co., Ltd., Tokyo, Japan), using an excitation wavelength of 360 nm and an emission wavelength of 460 nm.
Cell death assays.
After a 12-h prestarvation, HeLa cells were prepared with or without caspase inhibitor (carbobenzoxy-l-Asp-α[(2,6-dichlorobenzoyl)oxy] methane [Z-Asp-CH2-DCB] or carbobenzoxy-Tyr-Val-Ala-Asp-fluoromethyl ketone [Z-DEVD-FMK]) for 90 min prior to addition of B(a)P. After treatment with B(a)P, the cells were harvested by trypsinization and fixed in phosphate-buffered saline with 4% paraformaldehyde for 30 min. Following treatment with RNase (1 mg/ml in 0.1 M phosphate buffer [pH 7.0]), the cells were stained with 1 μ g of 4′,6-diamidino-2-phenylindole (DAPI) per ml for 20 min. Cells were placed on slides, and apoptotic cells with condensed or fragmented nuclei were visualized and counted under a fluorescence microscope.
For the selective apoptosis assay, HeLa cells in 6-cm-diameter dishes were transiently transfected with an enhanced green fluorescent protein (eGFP) marker plasmid (pTJM9; 0.5 μg) together with plasmids coding for the indicated proteins (1.5 μ g) (see Fig. 7). At 18 to 24 h after transfection, cells were placed in medium with 0.5% FBS and incubated for a further 12 h before being subjected to B(a)P treatment. After B(a)P treatment, the cells were harvested and stained with DAPI as described above and the fraction of eGFP-positive cells (transfected) that had condensed and fragmented nuclei was determined under a fluorescence microscope. Expression of vector-encoded proteins was confirmed by immunoblotting by using anti-myc, anti-HA, or anti-Flag antibodies.
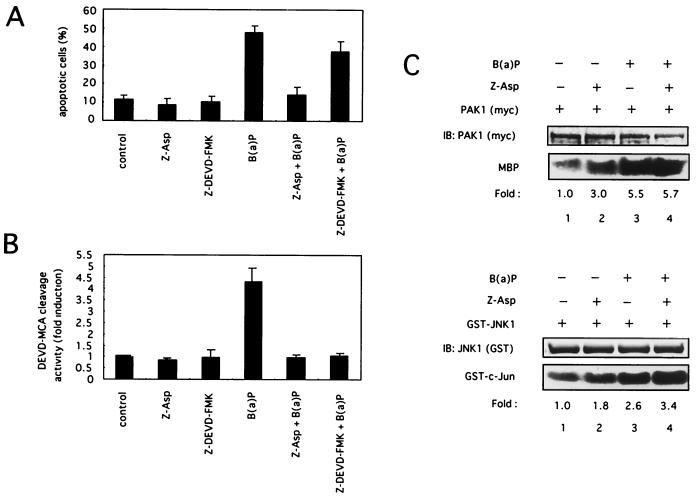
FIG. 7.
Effect of caspase inhibitors on B(a)P-induced apoptosis, caspase-3 protease activation, and PAK1/JNK1 activation. (A and B) Caspase inhibitors decrease B(a)P-induced apoptosis and caspase-3 protease activation. HeLa cells were preincubated with Z-Asp-CH2-DCB (50 μM), Z-DEVD-FMK (50 μ M), or the vehicle (DMSO) for 90 min and then treated with B(a)P (10 μ M). (A) Cells were harvested at the 48-h time point after B(a)P treatment and then stained with DAPI. The apoptotic cells were counted under a fluorescence microscope as in Fig. 5A and B. (B) Cells were collected at the 24-h time point after B(a)P treatment, and the caspase-3 protease activity was measured by proteolytic cleavage of the specific fluorogenic substrate Ac-DEVD-MCA as in Fig. 5C and D. The data shown are the mean and standard deviation of two to four independent experiments. (C) Caspase inhibitors have no effect on PAK1/JNK1 activation by B(a)P. HeLa cells were cotransfected with plasmids encoding myc-tagged PAK1 or GST-tagged JNK1. Then they were treated with 50 μM Z-Asp-CH2-DCB for 90 min or left untreated and then incubated with or without 0.1 μM B(a)P for 90 min. The cells were lysed for immunoprecipitation, and an in vitro kinase assay for PAK1 or JNK1 was performed. Similar results were obtained in three independent experiments. MBP, myelin basic protein.
DNA fragmentation assay.
The DNA fragmentation assay was performed as described previously (27). Briefly, HeLa cells (106) were collected and lysed in buffer containing 40 mM Tris (pH 8.0), 150 mM NaCl, 25 mM EDTA, and 0.5% sodium dodecyl sulfate, DNA was isolated with an equal volume of neutral phenol-chloroform-isoamyl alcohol mixture and precipitated with 0.1 volume of 3 M sodium acetate (pH 5.2)–2 volumes of 100% ethanol at −20°C overnight. The precipitated DNA was dissolved in 50 μ l of 10 mM Tris (pH 8.0)–1 mM EDTA buffer. The DNA fragments were resolved by electrophoresis in a 1.5% agarose gel and stained with ethidium bromide.
RESULTS
B(a)P activates the PAK1/JNK1 signaling pathway.
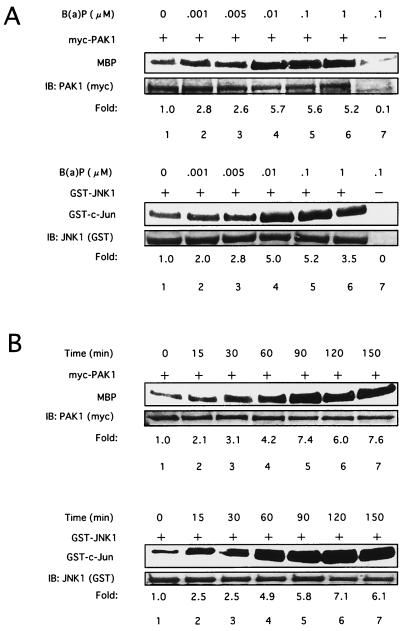
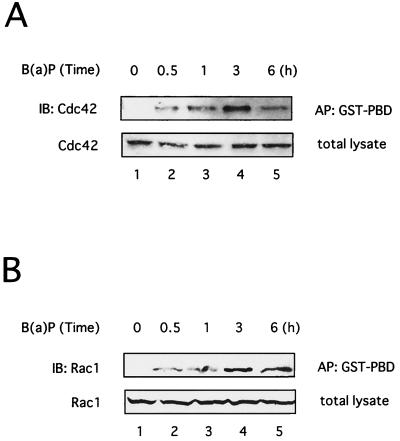
We initially investigated whether JNK1 was activated following B(a)P treatment in 293T cells as reported in Hepa1c1c7 cells (27). As shown in Fig. 1A, JNK1 activation was observed with 1 nM B(a)P, and this activity was maintained at least to 1 μ M B(a)P. In addition, JNK1 activation increased from 15 min after treatment with 0.1 μ M B(a)P in a time-dependent manner, and activity was maintained at least through 150 min (Fig. 1B). Since PAK lies upstream of the JNK1 pathway (3, 7, 58), we next examined whether PAK1 was activated in response to B(a)P. As shown in Fig. 1, PAK1 was also activated by B(a)P in a time- and dose-dependent manner, which coincided with the B(a)P-induced JNK1 activation. Strong activation of PAK1 and JNK1 was also detected in 293T cells after treatment with 10 μ M B(a)P as well as 0.1 and 1 μ M B(a)P (data not shown). Western blot analysis confirmed equivalent amounts of the corresponding kinase proteins (Fig. 1, lower panels). We further examined Cdc42 or Rac1 activation induced by B(a)P in vivo by affinity precipitation of GTP-bound Cdc42 or Rac1 with GST-PBD as previously described (4), because Cdc42/Rac1 interacts with PAK and stimulates PAK activity (3, 7, 58). B(a)P treatment resulted in an increase in the amount of activated Cdc42 and Rac1 that precipitated with GST-PBD in 293T cells in a time-dependent manner, reaching a maximum at 3 h after treatment with 0.1 μM B(a)P (Fig. 2B). B(a)P also increased the amount of activated Cdc42 and Rac1 in a dose-dependent manner (data not shown). Similar results were obtained with HeLa cells on treatment with B(a)P under the same experimental conditions as in the experiments in Fig. 1 and 2 (data not shown). These results indicated that B(a)P induced the activation of Cdc42/Rac1 and PAK1 as well as JNK1 in a time- and dose-dependent manner.
FIG. 1.
B(a)P induces PAK1 and JNK1 activities. Dose-dependent (A) and time-dependent (B) activation of PAK1 or JNK1 induced by B(a)P is shown. 293T cells were transfected with plasmids encoding Myc-tagged PAK1 or GST-tagged JNK1. At 48 h later, the cells were lysed for immunoprecipitation and an in vitro kinase assay for PAK1 or JNK1 was performed with recombinant myelin basic protein or GST–c-Jun as substrates, respectively. Before making cell lysates, transfected cells were treated with B(a)P at different concentrations (A) or for different times (B) as indicated above the lanes. The number at the bottom indicates the relative PAK1 or JNK1 kinase activity as a densitometric fold increase over the activity present in cells without B(a)P treatment (lane 1). Similar results were obtained in three independent experiments. MBP, myelin basic protein.
FIG. 2.
B(a)P induces the activation of Cdc42 and Rac1. 293T cells were cotransfected with plasmids encoding wild-type Cdc42 (A) or Rac1 (B). The cells were treated with 0.1 μM B(a)P for the indicated times and lysed for affinity precipitation (AP) with immobilized GST fusion protein of the p21-binding domain of PAK1 (GST-PBD) as described in Materials and Methods. Precipitated GTP-bound p21 was detected by immunoblotting (IB) with anti-Cdc42 (A) or Rac1 (B) antibody. Similar results were seen in three independent experiments.
α PIX mediates the signal initiated by B(a)P, leading to the activation of the JNK1 pathway kinases.
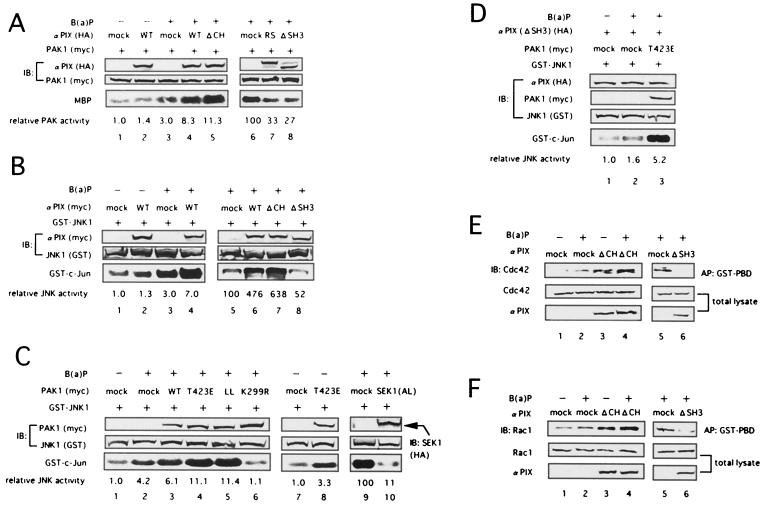
Human PIX family proteins bind tightly to PAK and are colocalized with PAK to form activated Cdc42- and Rac1-driven focal complexes (29, 37), leading to PAK activation through Cdc42/Rac1 (2, 13, 57). We therefore next studied the effect of αPIX on B(a)P-induced PAK1 activation. In our experiment, PAK1 kinase activity was measured after B(a)P treatment in 293T cells coexpressing PAK1 with wild-type human αPIX [αPIX (WT)] (WT) or various mutants of human αPIX. We performed the experiments under conditions ensuring that PAK1 or JNK1 was weakly activated by stimulation with B(a)P in the absence of αPIX (Fig. 3A and B, lanes 1 and 3; Fig 3C, lanes 1 and 2). Without B(a)P stimulation, αPIX (WT) did not adequately activate PAK1 (Fig. 3A, lane 2). However, in response to B(a)P stimulation, wild-type as well as N-terminally deleted αPIX (ΔCH), an active mutant of αPIX which has been reported to potently activate PAK1 in response to PDGF or EphB2 stimulation (57), enhanced the kinase activity of PAK1 (lanes 4 and 5). By contrast, mutated αPIX (L383R, L384S), which lacks GEF activity, and SH3 domain-deleted αPIX (ΔSH3), which lacks the ability to bind to PAK, suppressed B(a)P-induced PAK1 activation (lanes 7 and 8). These results indicate that αPIX mediates the signals triggered by B(a)P stimulation, leading to PAK1 activation. It has also been reported that Rac mediates signals to the MEKK-SEK-JNK pathway via PAK (53). Therefore, we further examined whether αPIX was involved in the activation of the JNK1 kinase induced by B(a)P. In cells coexpressing GST-JNK1 with αPIX (WT) or mutated αPIX, the kinase activity of JNK1 was measured after B(a)P stimulation. As shown in Fig. 3B, while both αPIX (WT) and αPIX (ΔCH) enhanced the JNK1 activation by B(a)P (lanes 4, 6, and 7), αPIX (ΔSH3) and αPIX (L383R, L384S) inhibited the kinase activity of JNK1 in cells treated with B(a)P (lane 8 and data not shown). These results suggest that αPIX is involved in JNK1 activation induced by B(a)P. Moreover, we evaluated whether the signals initiated by B(a)P were transduced through PAK1 to JNK1. As shown in Fig. 3C, PAK1 (WT) modestly accelerated the kinase activity of JNK1 in cells treated with B(a)P compared with mock vector (lane 3). On the other hand, we detected much higher JNK1 kinase activity with a constitutively active mutant, PAK1 (T423E), and PAK1 (H83L, H86L), which lacks the ability to bind Cdc42/Rac1, in cells treated with B(a)P than with a mock vector (Fig. 3C, lanes 4 and 5). In addition, we found that PAK1 (T423E) and PAK1 (H83L, H86L) effectively enhanced JNK1 kinase activity in the absence of B(a)P (Fig. 3C, lane 8, and data not shown). In contrast, kinase-negative PAK1 (K299R) inhibited JNK1 activation by B(a)P (lane 6). These findings show that PAK1 acts as an upstream mediator of JNK1 in response to B(a)P. In addition, coexpression of kinase-negative SEK1 (K220A, K224L), which is one of the MAPK kinases and lies upstream of JNK1 (14), also strongly suppressed B(a)P-induced JNK1 activation (lane 10). We furthermore showed that PAK1 (T423E) could also restore B(a)P-triggered JNK1 activation in cells expressing αPIX (ΔSH3) (Fig. 3D, lane 3). Next, we examined the effect of αPIX on Cdc42/Rac1 activation by B(a)P. As shown in Fig. 3E and F, αPIX (ΔCH) and αPIX (WT) enhanced B(a)P-induced activation of Cdc42 and Rac1 (lanes 4 and data not shown), which was detected by the increased amount of GTP-bound Cdc42 or Rac1 in vivo as shown in Fig. 2. By contrast, αPIX (ΔSH3) and αPIX (L383R, L384S) blocked the activation of Cdc42/Rac1 by B(a)P (lanes 6 and data not shown). These findings indicate that αPIX promotes Cdc42/Rac1 activity in response to B(a)P. Similar results were obtained with HeLa cells under the same experimental conditions as in the experiment in Fig. 3 (data not shown). Taken together, these results strongly suggest that αPIX plays a crucial role in the Cdc42/Rac1-PAK1-SEK1-JNK1 signaling pathway initiated by B(a)P.
FIG. 3.
αPIX enhances the activation of Cdc42/Rac1, PAK1, and JNK1 induced by B(a)P. (A and B) 293T cells were transfected with plasmids encoding myc-tagged PAK1 (A) or GST-tagged JNK1 (B) and HA- or myc-tagged wild-type (WT) or mutated forms of αPIX as indicated above the lanes. (C) 293T cells were cotransfected with plasmids encoding GST-tagged JNK1 and myc-tagged wild-type (WT) or mutated forms of PAK1 or HA-tagged dominant-negative SEK1 (AL) as indicated above the lanes. (D) 293T cells were cotransfected with plasmids encoding GST-tagged JNK1, HA-tagged α PIX (ΔSH3), and myc-tagged PAK1 (T423E) as indicated above the lanes. Mock vector pCS+ DNA was transfected to adjust the total amount of transfected DNA per dish. At 48 h later, the cells were lysed for immunoprecipitation, and an in vitro kinase assay for PAK1 (A) or JNK1 (B to D) was performed as described in the Lagend to Fig. 1. Before making cell lysates, transfected cells were treated with (+) or 0.1 μM B(a)P for 90 min or left untreated (−). The number at the bottom (lanes 1 to 5 in panel A, lanes 1 to 4 in panel B, lanes 1 to 8 in panel C, lanes 1 to 3 in panel D) indicates the relative PAK1 (A) or JNK1 (B to D) kinase activity as the fold increase over the activity present in cells without B(a)P treatment (lane 1 in panels A to D and lane 7 in panel C), and the other number at the bottom (lanes 6 to 8 in panel A, lanes 5 to 8 in panel B, lanes 9 and 10 in panel C) indicates the relative PAK1 (A) or JNK1 (B and C) activity as the percentage of the activity present in cells with B(a)P treatment (lane 6 in panel A, lane 5 in panel B, and lane 9 in panel C). The expression levels of αPIX, PAK1, SEK1, and JNK1 in each cell lysate were shown at the center. (E and F) 293T cells were cotransfected with plasmids encoding αPIX (ΔCH) or αPIX (ΔSH3) and wild-type Cdc42 (E) or Rac1 (F). Mock vector pCS2+ DNA was transfected to adjust the total amount of transfected DNA per dish. Cells were treated (+) with 0.1 μM B(a)P for 3 h or left untreated (−) and lysed for affinity precipitation with GST-PBD. Precipitated GTP-bound p21 was detected by immunoblotting with anti-Cdc42 (E) or Racl (F) antibody. The expression levels of αPIX (ΔCH), αPIX (Δ SH3), Cdc42, and Racl in each cell lysate are shown at the bottom. Similar results were obtained in three independent experiments. Abbreviations: MBP, myelin basic protein; αPIX (RS), αPIX (L383R, L384S); PAK1 (LL), PAK1 (H83L, H86L); SEK1 (AL), SEK1 (K220A, K224L).
B(a)P induces overexpression of αPIX.
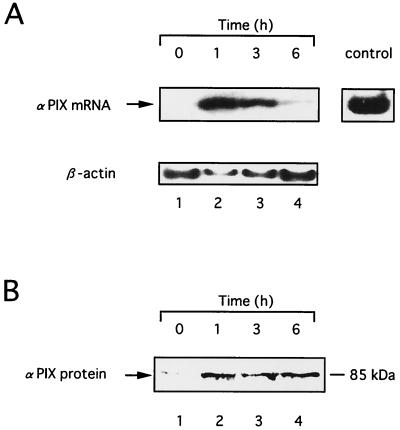
B(a)P binds to AhR, leading to the induction of CYP1A1 and other cytochrome P450 species, which in turn metabolize B(a)P to nucleophilic species such as B(a)PDE (12, 33, 40). It has also been reported that B(a)P causes induction of the c-Ha-ras and c-myc proto-oncogenes in rat aortic smooth muscle cells through a transcriptional mechanism (6, 41). Therefore we next investigated the effect of B(a)P on the induction of αPIX. To examine the upregulation of αPIX due to B(a)P, we monitored the level of expression of αPIX mRNA in HeLa cells. We used reverse transcription-PCR (RT-PCR), with β-actin as an internal control for semiquantitative analysis of mRNA expression. When HeLa cells were treated with 0.1 μ M B(a)P, the level of expression of αPIX mRNA was increased after B(a)P treatment, reaching a maximum at 1 h, and decreased thereafter (Fig. 4A). We also examined the effect of B(a)P on the expression of the αPIX protein in HeLa cells. As shown in Fig. 4B, the level of αPIX protein was also elevated 1 h after B(a)P treatment and the elevated expression of αPIX protein was maintained at least through to 6 h.
FIG. 4.
αPIX is upregulated in HeLa cells treated with B(a)P. HeLa cells were deprived of serum for 24 h and then treated with 0.1 μ M B(a)P for the indicated hours. (A) RT-PCR analysis of the expression of αPIX mRNA. The total RNA was extracted, and expression of αPIX mRNA was assessed by RT-PCR as described in Materials and Methods (top). Expression of β-actin mRNA was measured in each sample (bottom). (B) Western blot analysis of expression of αPIX protein. Immunoblotting was performed with polyclonal antibodies against human αPIX. Similar results were seen in three independent experiments.
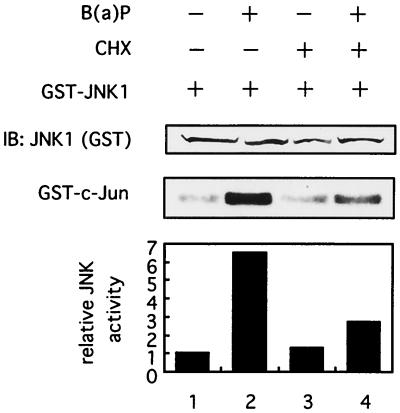
Cycloheximide inhibits B(a)P-induced JNK activation.
Next, we examined the effect of an inhibitor of protein synthesis, cycloheximide, on B(a)P-induced JNK1 activity to investigate a possible mechanism of activation of JNK1 pathway kinases induced by B(a)P. As shown in Fig. 5, cycloheximide, which effectively inhibited an increase in the expression of αPIX protein induced by B(a)P (data not shown), significantly blocked the B(a)P-induced JNK1 activation (lane 4). These findings may suggest that some other proteins, besides αPIX, overexpressed by B(a)P may be involved in the JNK1 activation triggered by B(a)P.
FIG. 5.
Cycloheximide inhibits B(a)P-induced activation of JNK1. 293T cells were transfected with plasmids encoding GST-tagged JNK1. At 48 h later, the cells were lysed for immunoprecipitation and an in vitro kinase assay for JNK1 was performed. Prior to making cell lysates, transfected cells were preincubated with (+) or without (−) 1 μg of cycloheximide (CHX) per ml for 1 h and then treated with 0.1 μM B(a)P for 3 h or left untreated as indicated above the lanes. Similar results were seen in three independent experiments.
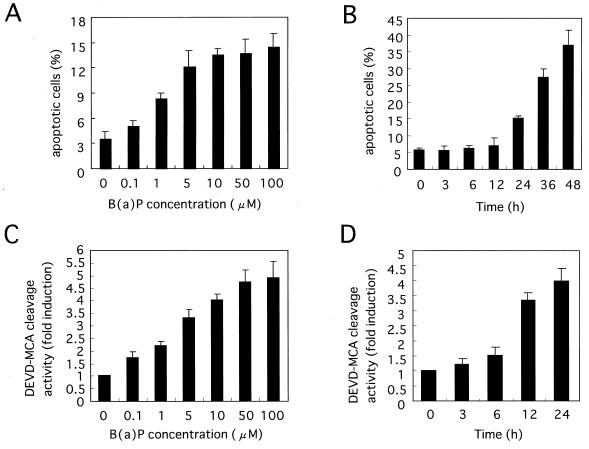
B(a)P induces apoptosis in HeLa cells.
Recent evidence suggests that in addition to its carcinogenic effect, B(a)P may induce apoptosis in vitro (27, 42). We therefore investigated the effect of B(a)P on inducing apoptosis in HeLa cells. Since B(a)P by itself is a poor inducer of cell death in HeLa cells (data not shown), prestarvation was carried out, in which cells were placed in medium with 0.5% FBS and incubated for a further 12 h before B(a)P treatment, and then various apoptosis assays were performed. When HeLa cells were treated with 10 μM B(a)P for 48 h, numerous cells which showed morphological changes such as membrane blebbing, nuclear condensation, and the formation of small apoptotic bodies characteristic of apoptosis were observed (data not shown). Furthermore, to confirm whether B(a)P-initiated cell death was indeed apoptosis, a DNA fragmentation assay was performed and genomic DNA digestion by B(a)P was detected in HeLa cells (data not shown). These findings indicated that B(a)P could induce apoptosis in HeLa cells as well as in Hepa1c1c7 and Daudi human B cells. Furthermore, to quantify B(a)P-induced cell death, we counted apoptotic cells with condensed or fragmented nuclei under a fluorescence microscope after staining them with DAPI, as described in Materials and Methods. As shown in Fig. 6A, the percentage of apoptotic cells increased in a dose-dependent manner at B(a)P concentrations ranging from 0.1 to 100 μM. The percentage of apoptotic cells also began to increase in a time-dependent manner from 24 h after 10 μM B(a)P treatment (Fig. 6B). Moreover, the DNA fragmentation assay demonstrated that B(a)P induced the increase in the extent of the typical DNA ladder in a time- and dose-dependent manner, which coincided with the percentage of apoptotic cells (data not shown). As reported previously, caspase family proteases, especially caspase-3, play critical roles in the apoptotic process (30, 34, 50). It has also been reported that caspase-3, but not caspase-1, plays a critical role in B(a)P-induced apoptosis in Hepa1c1c7 cells (27). To determine whether caspase-3 was involved in B(a)P-initiated apoptosis in HeLa cells as well as in Hepa1c1c7 cells, a fluorogenic assay of caspase-3 protease activity was performed. As shown in Fig. 6C, the activation of caspase-3 induced by B(a)P was also detected in a dose-dependent manner. In addition, the activation of caspase-3 began to increase from 12 h to at least 24 h after B(a)P treatment (Fig. 6D). This activation of caspase-3 followed the activation of JNK1 signaling pathway kinases (Fig. 1) and preceded the occurrence of apoptosis induced by B(a)P (Fig. 6B).
FIG. 6.
B(a)P induces apoptosis and activates caspase-3 protease in HeLa cells. (A and B) Dose-dependent (A) and time-dependent (B) apoptosis induced by B(a)P. HeLa cells were incubated with different concentrations of B(a)P for 24 h (A) or 10 μM B(a)P for different times (B). After the cells were harvested and stained with DAPI, the apoptotic cells with condensed or fragmented nuclei were visualized and counted under a fluorescence microscope as described in Materials and Methods. (C and D) B(a)P activates caspase-3 protease in a dose-dependent (C) and time-dependent (D) manner. HeLa cells were treated with B(a)P as in panels A and B, respectively. Then cell lysates were prepared and caspase-3 protease activity was measured by proteolytic cleavage of the specific fluorogenic substrate Ac-DEVD-MCA. Similar results were obtained in three independent experiments. The data shown are the mean and standard deviation for two to four independent experiments.
Effect of caspase inhibitors on B(a)P-induced PAK1/JNK1 activation and apoptosis.
Since two pathways, the caspase protease cascade and the JNK1 signaling pathway, were activated in response to B(a)P, we next examined a possible regulatory association between these pathways, especially during apoptosis induced by B(a)P. In this study, Z-Asp-CH2-DCB and Z-DEVD-FMK were used as a preferential caspase inhibitor and caspase-3-specific inhibitor, respectively. HeLa cells were pretreated for 90 min with the caspase inhibitors before being subjected to B(a)P treatment, and then caspase-3 activation and the percentage of apoptotic cells were measured as described above. Fig. 7A and B show that Z-Asp-CH2-DCB completely inhibited the caspase-3 activation and apoptosis induced by B(a)P. Interestingly, while Z-DEVD-FMK completely inhibited the B(a)P-induced caspase-3 activation, Z-DEVD-FMK reduced the apoptotic cell percentage by only approximately 20% (48% versus 39%), suggesting that caspases other than caspase-3 might be predominantly involved in apoptosis by B(a)P in HeLa cells. These results may explain why the caspase-3 activation by B(a)P was mild in HeLa cells (Fig. 6C and D). In contrast, Z-Asp-CH2-DCB did not prevent the B(a)P-initiated PAK1 and JNK1 kinase activation in HeLa cells at all (Fig. 7C). Interestingly, we found that treatment of cells with Z-Asp-CH2-DCB induced PAK1 activation and, to a lesser extent JNK1 activation in the absence of B(a)P. Although the detailed mechanism is unknown, Z-Asp-CH2-DCB might activate the PAK1 and JNK1 kinases by acting as a stress, because JNK1 pathway kinases are important mediators of stress signals. Z-DEVD-FMK also failed to inhibit PAK1 and JNK1 activation by B(a)P (data not shown). These results indicate that caspases inhibited by Z-Asp-CH2-DCB are important mediators of B(a)P-induced apoptosis and suggest that PAK1/JNK1 kinases could lie upstream of caspase proteases in a single cascade pathway or that PAK1/JNK1 and caspases could participate in independent parallel pathways.
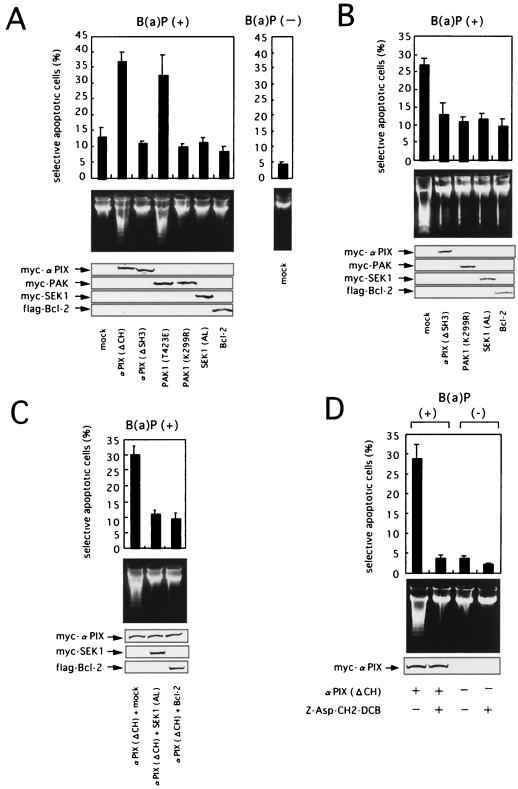
Effects of αPIX on B(a)P-induced apoptosis.
Many reports have demonstrated that JNK activation contributes to the induction of apoptosis in response to various stimuli, such as UV, gamma, or ionizing radiation; oxidative stress; heat; tumor necrosis factor; withdrawal of nerve growth factor; and ceramide (9, 21, 22, 52, 54, 55). In addition, GEFs, such as Tiam-1 and Vav, regulate apoptotic cell death (24, 25).
We therefore investigated a possible functional role of αPIX and the downstream JNK1 pathway kinases in B(a)P-triggered apoptosis. As indicated in Fig. 8, in this study marker plasmid expressing eGFP was transiently cotransfected into HeLa cells together with expression plasmids for either mutated forms of αPIX or other mutant JNK pathway kinases as well as a mock vector (negative control) or Bcl-2, a potent antiapoptotic protein (positive control). Expression of vector-encoded proteins was confirmed by immunoblotting (Fig. 8, bottom panels). HeLa cells were prestarved in medium with 0.5% FBS for a further 12 h after transfection and then treated with 10 μM B(a)P for 24 or 36 h or left untreated to evaluate apoptosis in eGFP-positive cells by selective apoptotic assay, as described in Materials and Methods. When the cells were treated with 10 μM B(a)P for 24 h, overexpression of αPIX (ΔCH) led to a significant increase in the number of apoptotic cells showing chromatin condensation and nuclear fragmentation compared the number detected after treatment with a mock vector (Fig. 8A, top). Overexpression of PAK1 (T423E) also greatly promoted B(a)P-induced apoptosis. By contrast, after treatment with B(a)P for 24 h, overexpression of αPIX (ΔSH3) and Bcl-2 slightly inhibited B(a)P-induced apoptosis compared with the effect of the empty vector. In addition, overexpression of PAK1 (K299R) or SEK1 (K220A, K224L) also slightly reduced apoptotic cell death by B(a)P. At 36 h after treatment with 10 μM B(a)P, the inhibition of B(a)P-induced apoptosis was more marked in cells overexpressing αPIX (ΔSH3), PAK1 (K299R), or SEK1 (K220A, K224L) as well as Bcl-2 (Fig. 8B, top). Likewise, overexpression of mutated αPIX (L383R, L384S) also efficiently reduced the percentage of apoptotic HeLa cells after B(a)P treatment (data not shown). The effect of overexpressing mutant proteins on B(a)P-induced cell death was not the result of differential overexpression of mutant proteins, because similar levels of mutant proteins were detected by immunoblotting (Fig. 8, bottom). Since cleavage of genomic DNA into oligonucleosomal fragments is a hallmark of apoptosis, we next examined the extent of B(a)P-induced DNA fragmentation. As shown in Fig. 8A center, overexpression of αPIX (Δ CH) or PAK1 (T423E) enhanced ladder formation by fragmented DNA. By contrast, overexpression of αPIX (ΔSH3), PAK1 (K299R), or SEK1 (K220A, K224L), as well as Bcl-2, decreased the extent of DNA laddering (Fig. 8B, center). SEK1 (K220A, K224L), Bcl-2, or the mock vector was transiently transfected into HeLa cells together with αPIX (ΔCH) and eGFP to determine whether SEK1 (K220A, K224L) could attenuate the αPIX (ΔCH)-enhanced apoptosis in cells treated with B(a)P. After treatment with 10 μM B(a)P for 24 h, the percentage of apoptotic cells overexpressing SEK1 (K220A, K224L) as well as Bcl-2 was strongly suppressed, compared with the percentage after treatment with the mock vector (Fig. 8C).
FIG. 8.
αPIX and the JNK pathway kinases facilitate the apoptotic cell death induced by B(a)P. (A) αPIX (ΔCH) and PAK1 (T423E) accelerate B(a)P-induced apoptotic cell death. HeLa cells were cotransfected with an eGFP expression vector and test plasmids as indicated. At 24 h posttransfection, the cells were placed in medium with 0.5% FBS and incubated for a further 12 h. They were then treated with 10 μ M B(a)P for 24 h or left untreated and were subjected to analysis. (Top) Quantitation of apoptotic cell death by using nuclear morphology. (Center) DNA integrity of transfected cells. Total DNA from the transfected samples was isolated, and an equal amount of DNA from each was separated on a 1.5% agarose gel. (Bottom) Expression levels of myc-tagged αPIX and PAK1, HA-tagged SEK1, and Flag-tagged Bcl-2 proteins as determined by immunoblot analysis. (B) αPIX (ΔSH3), PAK1 (K299R) and SEK1 (AL) inhibit apoptotic cell death induced by B(a)P. HeLa cells were cotransfected with an eGFP expression vector and test plasmids as indicated. At 36 h posttransfection, the cells were treated with 10μ M B(a)P for 36 h and subjected to analysis. (C) SEK1 (AL) blocks apoptosis accelerated by αPIX (ΔCH) in cells treated with B(a)P. HeLa cells were cotransfected with an eGFP expression vector and plasmids encoding myc-tagged αPIX (ΔCH) and HA-tagged SEK1 (AL) or Flag-tagged Bcl-2 as indicated. The total amount of transfected DNA was made constant by adding vector pCS2+ DNA. At 36 h posttransfection, the cells were treated with 10 μM B(a)P for 24 h and subjected to analysis. (D) The caspase inhibitor Z-Asp-CH2-DCB inhibits apoptosis accelerated by αPIX (Δ CH) in cells treated with B(a)P. HeLa cells were cotransfected with an eGFP expression vector and plasmids encoding myc-tagged αPIX (Δ CH) or mock vector as indicated. At 36 h posttransfection, the cells were preincubated with Z-Asp-CH2-DCB (50 μM) or vehicle (DMSO) for 90 min and treated with 10 μ M B(a)P for 24 h. Then the cells were harvested and subjected to analysis. Similar results were obtained in three independent experiments. The data shown are the mean and standard deviation for three independent experiments. SEK1 (AL), SEK1 (K220A, K224L).
Finally, we confirmed that Z-Asp-CH2-DCB, which did not prevent the B(a)P-initiated PAK1 and JNK1 kinase activation at all (Fig. 7C), greatly prevented apoptosis accelerated by αPIX (ΔCH) in HeLa cells exposed to 10 μ M B(a)P for 24 h (Fig. 8D). These results verified that the death signal induced by B(a)P is mediated, at least in part, via the αPIX-Cdc42/Rac1-PAK1-SEK1-JNK1 signaling pathway in HeLa cells and that αPIX is involved in B(a)P-triggered apoptosis at a step upstream of caspase activation.
DISCUSSION
B(a)P has been known as a carcinogen that affects cells by either a direct interaction with DNA or formation of hydroxy radicals and superoxides, leading to tumor initiation, promotion, and progression (12, 20, 33, 38, 40). On the other hand, a few recent studies have demonstrated that B(a)P induces apoptotic cell death and have focused on its association with cellular signaling pathways, especially the MAPK cascades and apoptosis (8, 27, 39, 42). For example, Lei et al. reported that B(a)P induces JNK kinase activation in Hepa1c1c7 cells during apoptosis (27), although the interaction between the JNK pathway and apoptosis induced by B(a)P has not been well elucidated.
The MAPK cascades occur in a variety of biological phenomena, including apoptotic cell death, cellular proliferation, differentiation, and transformation. Many reports have demonstrated that JNK activation contributes to the induction of apoptosis (9, 21, 22, 52, 54, 55). On the other hand, quite a few reports have demonstrated that the activation of JNK can be either nonapoptotic or antiapoptotic depending on the various cell biological settings (28, 35, 56). These findings appear to suggest that the JNK pathway kinases, such as SEK1 and JNK1, have bidirectional functions (cell proliferation and differentiation or programmed cell death) and that the actual function of the kinases depends on cosignals provided by different stimuli, cell types, and environmental conditions (10).
Therefore, in an attempt to identify the mediators of the JNK activation induced by B(a)P on the JNK signaling pathway and the interaction between the JNK pathway and the apoptotic cascade, we especially focused on the commitment of αPIX. αPIX, a recently identified GEF for Cdc42/Rac1, has been reported to bind tightly to PAK and to be colocalized with PAK to form activated Cdc42- and Rac1-driven focal complexes, leading to PAK activation (29, 37), and to be involved in various biological effects, including Rac-type morphological changes such as lamellipodia formation and photoreceptor axon guidance (19, 36).
In the present study, we found that Cdc42/Rac1, PAK1, and JNK1 activity increased in a dose- and time-dependent manner in 293T and HeLa cells treated with B(a)P (Fig. 1 and 2 and data not shown). We also confirmed that the B(a)P-induced activation of Cdc42/Rac1, PAK1, and JNK1 kinases was maintained for at least 6 h with parallel time kinetics (Fig. 2 and data not shown). To study the role of αPIX in the JNK pathway triggered by B(a)P, αPIX was overexpressed in 293T and HeLa cells. αPIX (ΔCH), an active form of αPIX, significantly increased Cdc42/Rac1, PAK1, and JNK1 activation in cells treated with B(a)P (Fig. 3A, B, E, and F). More importantly, αPIX (L383R, L384S), which lacks GEF activity, and αPIX (ΔSH3), which abolishes the ability to bind to PAK, clearly blocked B(a)P-initiated Cdc42/Rac1, PAK1, and JNK1 activity (Fig. 3A, B, E, and F), demonstrating that αPIX lies on the B(a)P-triggered JNK1 signaling pathway and plays a pivotal role in the activation of JNK pathway kinases induced by B(a)P. In addition, constitutively active mutants of PAK1, PAK1 (T423E) and PAK1 (H83L, H86L), enhanced the B(a)P-initiated JNK1 activation, whereas the kinase-negative mutant of PAK1, PAK1 (K299R), or of SEK1, SEK1 (K220A, K224L), significantly inhibited the JNK1 activity induced by B(a)P (Fig. 3C), suggesting that PAK1 and SEK1 may also lie on the B(a)P-induced JNK1 signaling cascade. Moreover, we found evidence that B(a)P triggers an increase in both αPIX mRNA and protein expression (Fig. 4). With respect to the mechanism of activation of JNK1 pathway kinases induced by B(a)P, the following possibilities can be considered. One possibility is that B(a)P may primarily cause an increase in expression of αPIX protein itself, a key regulatory factor in this pathway, thus activating the Cdc42/Rac1-PAK1 signaling pathway. In fact, we found that an inhibitor of protein synthesis, cycloheximide, which effectively inhibited an increase in the expression of αPIX protein (data not shown), significantly blocked the B(a)P-induced JNK1 activation (Fig. 5). We can deduce from these findings that some other proteins besides αPIX that are upregulated by B(a)P may be involved in the JNK1 activation by B(a)P. This hypothetical mechanism may explain why the maximal activation of B(a)P-induced JNK1 pathway kinases requires 3 to 6 h. Our observation that the overexpression of αPIX protein induced by B(a)P required 1 h to appear and it maintained at least for another 5 h (Fig. 4B) is also consistent. Another possibility is that B(a)P may activate the JNK1 pathway through the mitogen receptors, such as IGF-IR, because some reports have demonstrated that PAHs mimic antigen and mitogen receptor signaling by protein tyrosine kinases (1, 26, 32). In addition, it has been reported that B(a)P can mimic signaling through the IGF-IR and significantly increase the activity of PI3-K via insulin receptor substrate 1 and Shc (48). Via this mechanism, B(a)P may be able to activate the Cdc42/Rac1-PAK1-SEK1-JNK1 signaling pathway, since Cdc42/Rac are activated by PI3-K (17, 47). Since we previously reported that αPIX is activated by direct association with the p85 regulatory subunit of PI3-K, leading to the activation of Cdc42/Rac1-PAK1 pathway (57), B(a)P may activate the Cdc42/Rac1-JNK1 pathway through αPIX. However, the possibility that B(a)P may induce the overexpression of some proteins, which enhances αPIX activity, is not excluded. It is more likely that other unknown mechanisms, together with those mentioned above, may synergistically cause the B(a)P-induced activation of the JNK1 pathway. This complicated mechanism could explain why exogenous overexpression of αPIX does not simply mimic the actions of B(a)P and give rise to a marked stimulation of the JNK1 pathway kinases. Again, it is possible that the Cdc42/Rac1-PAK1-SEK1-JNK1 pathway might also have been activated through another pathway that does not involve αPIX. Furthermore, with respect to the mechanism of upregulation of αPIX protein expression by B(a)P, the following possibilities can be considered. One is that B(a)P activates the Cdc42/Rac1-PAK1-SEK1-JNK1 signaling pathway almost instantly, and thus αPIX would probably be among the genes controlled by transcription factors phosphorylated by activated JNK (23). Positive feedback, including the activated αPIX, would further enhance signals through Cdc42/Rac1 to JNK1. Another possible mechanism is that expression of αPIX might be increased by some unknown signaling pathway(s) other than the PAK1-SEK1-JNK1-transcription factor pathway. Taken together, these findings demonstrate that αPIX is, at least in part, functionally involved in JNK1 activation via activation of the downstream kinase cascade Cdc42/Rac1-PAK1-SEK1 pathway.
Next, we examined the role of αPIX in B(a)P-induced apoptotic cell death. In the present study, while we found that B(a)P induced caspase-3 activation and subsequent apoptosis in HeLa cells treated with B(a)P in a dose- and time-dependent manner (Fig. 6), other caspases may be predominantly involved in B(a)P-induced apoptosis (Fig. 7A and B). Interestingly, overexpression of αPIX (ΔCH) induced a significant increase in B(a)P-induced apoptotic cell death (Fig. 8A) while overexpression of αPIX (ΔSH3) and αPIX (L383R, L384S) greatly inhibited B(a)P-triggered apoptosis (Fig. 8B and data not shown), demonstrating that αPIX plays a crucial role in apoptosis triggered by B(a)P. In addition, overexpression of PAK1 (T423E) promoted B(a)P-induced apoptosis (Fig. 8A) whereas overexpression of PAK1 (K299R) and SEK1 (K220A, K224L) greatly suppressed B(a)P-triggered apoptosis. Likewise, overexpression of dominant negative Cdc42 or Rac1 (Cdc42- or Rac1-T17N, respectively), which inhibited B(a)P-induced PAK1/JNK1 activation, also suppressed apoptotic cell death by B(a)P (data not shown). Moreover, overexpression of SEK1 (K220A, K224L) significantly blocked the apoptotic cell death enhanced by coexpressing αPIX (ΔCH) in HeLa cells treated with B(a)P (Fig. 8C). These observations strongly indicate that α PIX plays a crucial role in B(a)P-initiated apoptosis via regulation of the JNK pathway kinases in HeLa cells. Our findings showed that B(a)P-induced apoptosis followed the activation of the JNK pathway kinases and subsequent caspase proteases and that Z-Asp-CH2-DCB could completely prevent the apoptotic cell death facilitated by αPIX (Δ CH) in HeLa cells treated with B(a)P, but not by the JNK pathway kinases (Fig. 7C and 8D), demonstrating that the JNK pathway lies upstream of the caspase proteases. Figure 9 illustrates our model of the B(a)P-induced apoptotic cascade via activation of JNK pathway kinases in HeLa cells. The targets for JNK in induction of apoptosis most probably involve transcriptionally regulated gene products, such as c-Jun, whose activation has been reported to be sufficient to initiate apoptotic cell death (5). This possibility remains to be confirmed. By contrast, it has recently been reported that PAK1 protects against apoptosis induced by interleukin-3 deprivation in FL5.12 lymphoid progenitor cells (43). However, our observations clearly indicate that PAK1 contributes to apoptosis, but not to cell survival, induced by B(a)P stimulation. Consistent with our observations, Thomas et al. recently reported that PAK1 and JNK1 might play a role downstream of Cdc42 in transducing the p53-dependent apoptotic signal via phosphorylation of Bcl-2 (51). PAK1 may very well have bidirectional functions for the regulation of cell survival and programmed cell death as well as some other JNK pathway kinases, including SEK1 and JNK1 (10).
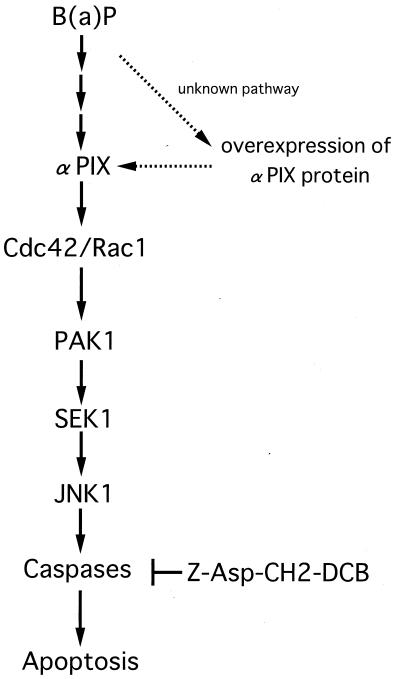
FIG. 9.
Model for the B(a)P-induced apoptotic signaling pathway, which is mediated by αPIX. B(a)P activates Cdc42/Rac1 via αPIX. Activated Cdc42/Rac1 leads to activation of PAK1, which induces an increase in JNK1 activity destined to undergo an apoptotic response in HeLa cells.
In summary, our findings suggest that αPIX plays an important role in B(a)P-induced apoptotic cell death via the activation of the Cdc42/Rac1-PAK1-SEK1-JNK1 signaling pathway.
ACKNOWLEDGMENTS
We thank Bruce J. Mayer, Department of Genetics and Developmental Biology, University of Connecticut Health Center, Farmington, Yutaka Eguchi and Yoshihide Tsujimoto, Osaka University Graduate School of Medicine, and Takahiro Nagase, Kazusa DNA Research Institute, for providing us with plasmids used in these experiments. Technical support by Research Equipment Center, Hamamatsu University School of Medicine, is acknowledged.
This work is supported by grant-in-aids from the Ministry of Education, Science, Culture, Sports, and Technology of Japan (B,C-2:12218215), the Ministry of Health and Welfare for Comprehensive 10-Year Strategy for Cancer Control, and the Smoking Research Foundation.
REFERENCES
- 1.Archuleta M M, Schieven G L, Ledbetter J A, Deanin G G, Burchiel S W. 7,12-Dimethylbenz[a] anthracene activates protein-tyrosine kinases Fyn and Lck in the HPB-ALL human T-cell line and increases tyrosine phosphorylation of phospholipase C-gamma 1, formation of inositol 1,4,5-trisphosphate, and mobilization of intracellular calcium. Proc Natl Acad Sci USA. 1993;90:6105–6109. doi: 10.1073/pnas.90.13.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagrodia S, Bailey D, Lenard Z, Hart M, Guan J L, Premont R T, Taylor S J, Cerione R A. A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J Biol Chem. 1999;274:22393–22400. doi: 10.1074/jbc.274.32.22393. [DOI] [PubMed] [Google Scholar]
- 3.Bagrodia S, Derijard B, Davis R J, Cerione R A. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 4.Bagrodia S, Taylor S J, Jordon K A, Van Aelst L, Cerione R A. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- 5.Bossy-Wetzel E, Bakiri L, Yaniv M. Induction of apoptosis by the transcription factor c-Jun. EMBO J. 1997;16:1695–1709. doi: 10.1093/emboj/16.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bral C M, Ramos K S. Identification of benzo[a]pyrene-inducible cis-acting elements within c-Ha-ras transcriptional regulatory sequences. Mol Pharmacol. 1997;52:974–982. doi: 10.1124/mol.52.6.974. [DOI] [PubMed] [Google Scholar]
- 7.Brown J L, Stowers L, Baer M, Trejo J, Coughlin S, Chant J. Human Ste20 homologue hPAK1 links GTPases to the JNK MAP kinase pathway. Curr Biol. 1996;6:598–605. doi: 10.1016/s0960-9822(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 8.Burchiel S W, Davis D A, Ray S D, Barton S L. DMBA induces programmed cell death (apoptosis) in the A20.1 murine B cell lymphoma. Fundam, Appl. Toxicol. 1993;21:120–124. doi: 10.1006/faat.1993.1080. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y R, Wang X, Templeton D, Davis R J, Tan T H. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Seimiya H, Naito M, Mashima T, Kizaki A, Dan S, Imaizumi M, Ichijo H, Miyazono K, Tsuruo T. ASK1 mediates apoptotic cell death induced by genotoxic stress. Oncogene. 1999;18:173–180. doi: 10.1038/sj.onc.1202276. [DOI] [PubMed] [Google Scholar]
- 11.Chin B Y, Choi M E, Burdick M D, Strieter R M, Risby T H, Choi A M. Induction of apoptosis by particulate matter: role of TNF-alpha and MAPK. Am J Physiol. 1998;275:L942–L949. doi: 10.1152/ajplung.1998.275.5.L942. [DOI] [PubMed] [Google Scholar]
- 12.Conney A H. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- 13.Daniels R H, Zenke F T, Bokoch G M. alphaPix stimulates p21-activated kinase activity through exchange factor-dependent and -independent mechanisms. J Biol Chem. 1999;274:6047–6050. doi: 10.1074/jbc.274.10.6047. [DOI] [PubMed] [Google Scholar]
- 14.Derijard B, Raingeaud J, Barrett T, Wu I H, Han J, Ulevitch R J, Davis R J. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 15.Eldridge S R, Gould M N, Butterworth B E. Genotoxicity of environmental agents in human mammary epithelial cells. Cancer Res. 1992;52:5617–5621. [PubMed] [Google Scholar]
- 16.Ethier S P, Ullrich R L. Induction of mammary tumors in virgin female BALB/c mice by single low doses of 7,12-dimethylbenz[a]anthracene. JNCI. 1982;69:1199–1203. [PubMed] [Google Scholar]
- 17.Hawkins P T, Eguinoa A, Qiu R G, Stokoe D, Cooke F T, Walters R, Wennstrom S, Claesson-Welsh L, Evans T, Symons M, Stephens L. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 18.Heidelberger C. Chemical carcinogenesis. Annu Rev Biochem. 1975;44:79–121. doi: 10.1146/annurev.bi.44.070175.000455. [DOI] [PubMed] [Google Scholar]
- 19.Hing H, Xiao J, Harden N, Lim L, Zipursky S L. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell. 1999;97:853–863. doi: 10.1016/s0092-8674(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 20.Huberman E, Sachs L, Yang S K, Gelboin V. Identification of mutagenic metabolites of benzo(a)pyrene in mammalian cells. Proc Natl Acad Sci USA. 1976;73:607–611. doi: 10.1073/pnas.73.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 22.Johnson N L, Gardner A M, Diener K M, Lange-Carter C A, Gleavy J, Jarpe M B, Minden A, Karin M, Zon L I, Johnson G L. Signal transduction pathways regulated by mitogen-activated/extracellular response kinase kinase kinase induce cell death. J Biol Chem. 1996;271:3229–3237. doi: 10.1074/jbc.271.6.3229. [DOI] [PubMed] [Google Scholar]
- 23.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 24.Kawazoe N, Watabe M, Masuda Y, Nakajo S, Nakaya K. Tiam1 is involved in the regulation of bufalin-induced apoptosis in human leukemia cells. Oncogene. 1999;18:2413–2421. doi: 10.1038/sj.onc.1202555. [DOI] [PubMed] [Google Scholar]
- 25.Kong Y Y, Fischer K D, Bachmann M F, Mariathasan S, Kozieradzki I, Nghiem M P, Bouchard D, Bernstein A, Ohashi P S, Penninger J M. Vav regulates peptide-specific apoptosis in thymocytes. J Exp Med. 1998;188:2099–2111. doi: 10.1084/jem.188.11.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieger J A, Born J L, Burchiel S W. Persistence of calcium elevation in the HPB-ALL human T cell line correlates with immunosuppressive properties of polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 1994;127:268–274. doi: 10.1006/taap.1994.1161. [DOI] [PubMed] [Google Scholar]
- 27.Lei W, Yu R, Mandlekar S, Kong A N. Induction of apoptosis and activation of interleukin 1beta-converting enzyme/Ced-3 protease (caspase-3) and c-Jun NH2-terminal kinase 1 by benzo(a)pyrene. Cancer Res. 1998;58:2102–2106. [PubMed] [Google Scholar]
- 28.Lenczowski J M, Dominguez L, Eder A M, King L B, Zacharchuk C M, Ashwell J D. Lack of a role for Jun kinase and AP-1 in Fas-induced apoptosis. Mol Cell Biol. 1997;17:170–181. doi: 10.1128/mcb.17.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manser E, Loo T H, Koh C G, Zhao Z S, Chen X Q, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- 30.Martin S J, Green D R. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- 31.Miller J A. Carcinogenesis by chemicals: an overview. Clowes Memorial Lecture. Cancer Res. 1970;30:559–571. [PubMed] [Google Scholar]
- 32.Mounho B J, Burchiel S W. Alterations in human B cell calcium homeostasis by polycyclic aromatic hydrocarbons: possible associations with cytochrome P450 metabolism and increased protein tyrosine phosphorylation. Toxicol Appl Pharmacol. 1998;149:80–89. doi: 10.1006/taap.1997.8345. [DOI] [PubMed] [Google Scholar]
- 33.Nebert D W, Gonzalez F J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, Munday N A, Raju S M, Smulson M E, Yamin T T, Yu V L, Miller D K. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 35.Nishina H, Fischer K D, Radvanyi L, Shahinian A, Hakem R, Rubie E A, Bernstein A, Mak T W, Woodgett J R, Penninger J M. Stress-signalling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature. 1997;385:350–353. doi: 10.1038/385350a0. [DOI] [PubMed] [Google Scholar]
- 36.Obermeier A, Ahmed S, Manser E, Yen S C, Hall C, Lim L. PAK promotes morphological changes by acting upstream of Rac. EMBO J. 1998;17:4328–4339. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh W K, Yoo J C, Jo D, Song Y H, Kim M G, Park D. Cloning of a SH3 domain-containing proline-rich protein, p85SPR, and its localization in focal adhesion. Biochem Biophys Res Commun. 1997;235:794–798. doi: 10.1006/bbrc.1997.6875. [DOI] [PubMed] [Google Scholar]
- 38.Pitot H C, Dragan Y P. Chemical carcinogenesis. In: Klassen C D, editor. Casarett and Doull's toxicology. New York, N.Y: McGraw-Hill Book Co.; 1996. pp. 201–267. [Google Scholar]
- 39.Rorke E A, Sizemore N, Mukhtar H, Couch L H, Howard P C. Polycyclic aromatic hydrocarbons enhance terminal cell death of human ectocervical cells. Int J Oncol. 1998;13:557–563. doi: 10.3892/ijo.13.3.557. [DOI] [PubMed] [Google Scholar]
- 40.Rowlands J C, Gustafsson J A. Aryl hydrocarbon receptor-mediated signal transduction. Crit Rev Toxicol. 1997;27:109–134. doi: 10.3109/10408449709021615. [DOI] [PubMed] [Google Scholar]
- 41.Sadhu D N, Merchant M, Safe S H, Ramos K S. Modulation of protooncogene expression in rat aortic smooth muscle cells by benzo[a]pyrene. Arch Biochem Biophys. 1993;300:124–131. doi: 10.1006/abbi.1993.1017. [DOI] [PubMed] [Google Scholar]
- 42.Salas V M, Burchiel S W. Apoptosis in Daudi human B cells in response to benzo[a]pyrene and benzo[a]pyrene-7,8-dihydrodiol. Toxicol Appl Pharmacol. 1998;151:367–376. doi: 10.1006/taap.1998.8455. [DOI] [PubMed] [Google Scholar]
- 43.Schurmann A, Mooney A F, Sanders L C, Sells M A, Wang H G, Reed J C, Bokoch G M. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol Cell Biol. 2000;20:453–461. doi: 10.1128/mcb.20.2.453-461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimizu S, Eguchi Y, Kosaka H, Kamiike W, Matsuda H, Tsujimoto Y. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature. 1995;374:811–813. doi: 10.1038/374811a0. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka M, Gupta R, Mayer B J. Differential inhibition of signaling pathways by dominant-negative SH2/SH3 adapter proteins. Mol Cell Biol. 1995;15:6829–6837. doi: 10.1128/mcb.15.12.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka M, Lu W, Gupta R, Mayer B J. Expression of mutated Nck SH2/SH3 adaptor respecifies mesodermal cell fate in Xenopus laevis development. Proc Natl Acad Sci USA. 1997;94:4493–4498. doi: 10.1073/pnas.94.9.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang Y, Yu J, Field J. Signals from the Ras, Rac, and Rho GTPases converge on the Pak protein kinase in Rat-1 fibroblasts. Mol Cell Biol. 1999;19:1881–1891. doi: 10.1128/mcb.19.3.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tannheimer S L, Ethier S P, Caldwell K K, Burchiel S W. Benzo[a]pyrene- and TCDD-induced alterations in tyrosine phosphorylation and insulin-like growth factor signaling pathways in the MCF-10A human mammary epithelial cell line. Carcinogenesis. 1998;19:1291–1297. doi: 10.1093/carcin/19.7.1291. [DOI] [PubMed] [Google Scholar]
- 49.Taylor S J, Shalloway D. Cell cycle-dependent activation of Ras. Curr Biol. 1996;6:1621–1627. doi: 10.1016/s0960-9822(02)70785-9. [DOI] [PubMed] [Google Scholar]
- 50.Tewari M, Quan L T, O'Rourke K, Desnoyers S, Zeng Z, Beidler D R, Poirier G G, Salvesen G S, Dixit V M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 51.Thomas A, Giesler T, White E. p53 mediates bcl-2 phosphorylation and apoptosis via activation of the Cdc42/JNK1 pathway. Oncogene. 2000;19:5259–5269. doi: 10.1038/sj.onc.1203895. [DOI] [PubMed] [Google Scholar]
- 52.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimovitz-Friedman A, Fuks Z, Kolesnick R N. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 53.Vojtek A B, Cooper J A. Rho family members: activators of MAP kinase cascades. Cell. 1995;82:527–529. doi: 10.1016/0092-8674(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 54.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 55.Yang D D, Kuan C Y, Whitmarsh A J, Rincon M, Zheng T S, Davis R J, Rakic P, Flavell R A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 56.Yeh W C, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa J L, Ferrick D, Hum B, Iscove N, Ohashi P, Rothe M, Goeddel D V, Mak T W. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 57.Yoshii S, Tanaka M, Otsuki Y, Wang D Y, Guo R J, Zhu Y, Takeda R, Hanai H, Kaneko E, Sugimura H. alphaPIX nucleotide exchange factor is activated by interaction with phosphatidylinositol 3-kinase. Oncogene. 1999;18:5680–5690. doi: 10.1038/sj.onc.1202936. [DOI] [PubMed] [Google Scholar]
- 58.Zhang S, Han J, Sells M A, Chernoff J, Knaus U G, Ulevitch R J, Bokoch G M. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J Biol Chem. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]
- 59.Zhao Z S, Manser E, Loo T H, Lim L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol Cell Biol. 2000;20:6354–6363. doi: 10.1128/mcb.20.17.6354-6363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]