Abstract
Objective
To evaluate a new protocol of risk stratification and early discharge for children with febrile neutropenia (FN).
Design
Prospective service evaluation from 17 April 2020 to 16 April 2021.
Setting
13 specialist centres in the UK.
Patients
405 children presenting with FN.
Intervention
All children received intravenous antibiotics at presentation. Risk stratification was determined using the Australian-UK-Swiss (AUS) rule and eligibility for homecare assessed using criteria including disease, chemotherapy, presenting features and social factors. Those eligible for homecare could be discharged on oral antibiotics after a period of observation proportional to their risk group.
Main outcome measures
Median duration of admission and of intravenous antibiotics, and percentage of patients with positive blood cultures, significant infection, readmission within 7 days of initial presentation, intensive care unit (ICU) admission, death from infection and death from other causes.
Results
13 centres contributed 729 initial presentations of 405 patients. AUS rule scores were positively correlated with positive blood cultures, significant infection, ICU admission and death. 20% of children were eligible for homecare with oral antibiotics, of which 55% were low risk (AUS 0–1). 46% low-risk homecare eligible patients were discharged by 24 hours vs 2% homecare ineligible. Homecare readmission rates were 14% overall and 16% for low-risk cases (similar to a meta-analysis of previous studies). No child eligible for homecare was admitted to ICU or died.
Conclusions
Use of the AUS rule and homecare criteria allow for safe early outpatient management of children with FN.
Keywords: infectious disease medicine, paediatrics
WHAT IS ALREADY KNOWN ON THIS TOPIC
Febrile neutropenia (FN) is a common emergency presentation for children with cancer, but significant morbidity and mortality is rare.
Reducing length of hospital admissions for those at low risk of complications is important to patients and their families.
Multiple risk stratification rules exist to identify those children at low risk of complications, but none are both sensitive and specific.
WHAT THIS STUDY ADDS
The Australian-UK-Swiss (AUS) rule and homecare criteria identify children that can be safely discharged on oral antibiotics and parental monitoring.
Selected children with low-risk FN episodes require <24 hours of inpatient care.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The Children’s Cancer and Leukaemia Group continues to recommend the AUS-based homecare protocol as a safe way to manage low-risk FN episodes beyond the COVID-19 pandemic.
Future research should focus on identifying barriers to implementing homecare protocols, involving both professionals, patients and parents.
Introduction
The management of febrile neutropenia (FN) is a vital part of paediatric oncology/haematology practice and constitutes a significant proportion of supportive care workload. Within the UK, care of a child with cancer is coordinated by one of 20 principal treatment centres (PTCs), with over 100 paediatric oncology shared care centres (POSCUs) providing defined aspects of care such as initial management of FN closer to the child’s home. Historically, children with FN episodes have been managed with inpatient antibiotic treatment until ‘negative’ blood cultures results were available and the fever has defervesced. A 2017 audit of UK practice found that for patients who were outpatients at presentation of FN, the median duration of admission was 3 days.1
Early in the COVID-19 pandemic, actual and anticipated demands for hospital bed use were higher than those available within some areas.2 When the risks of SARS-CoV2 infection in children with cancer were unknown, many families and professionals were keen to reduce stays, reducing the risk of hospital-acquired infection.
In response, the UK’s Children’s Cancer and Leukaemia Group (CCLG) published new national guidance for the management of FN. They advised using the three-item Australia-UK-Swiss (AUS) rule to risk-stratify patients and identify those who could be safely discharged home after a shorter duration of intravenous antibiotics. The AUS rule was developed in a large multicentre, prospective study, and validated in the international PICNICC+ dataset.3 4 Combined with an associated management pathway including homecare criteria, it was piloted in Melbourne Australia using an ambulatory intravenous antibiotic regimen.5 The programme was safe, resulted in significant reduction in bed occupancy and up to $A12 000 lower healthcare costs per patient.6 The CCLG guidance was based on this programme, but used an oral antibiotic regimen, in line with National Institute for Health and Care Excellence (NICE) guidelines.7 This approach is also supported by the evidence that the majority of clinically significant positive blood cultures in this population are identified within 24 hours of the sample being taken.8
The new CCLG guidance was a significant change in practice, and to provide quality assurance to centres choosing to adopt this approach, we performed a 1-year prospective service evaluation designed to assess safety and efficacy.
Methods
Overview of the guidelines
FN was defined as per NICE guidelines9 (neutrophils ≤0.5×109/L and either fever ≥38°C or clinical evidence of sepsis).7 It was recommended that all patients (both inpatient and outpatient) with FN received first-line intravenous antibiotics, as per local policy within 1 hour of arrival to hospital or inpatient febrile episode. The patient’s AUS rule score was used to determine the minimum recommended period of intravenous antibiotics and inpatient observation (table 1). After initial inpatient treatment, children who met all eligibility criteria in table 1 could be discharged to a homecare programme.
Table 1.
AUS rule variables and homecare eligibility criteria
| AUS rule | Yes | No | |
| Preceding chemotherapy more intensive than ALL maintenance | 1 | 0 | |
| Total white cell count <0.3×109/L | 1 | 0 | |
| Platelet <50×109/L | 1 | 0 | |
| Minimum observation period (if clinically stable and fulfil homecare criteria) | |||
| AUS=0 | AUS=1 | AUS=2 | AUS=3 |
| 4–8 hours | 4–24 hours | 24 hours | 48 hours |
| Eligibility criteria for homecare | |||
| Disease status: leukaemia/lymphoma in remission (as per last bone marrow aspirate or solid tumour stable/responding (as per oncologist) | |||
| Low-risk disease group: NOT ANY OF—ALL induction, or acute infant leukaemias, acute myeloid leukaemia, postallogeneic haematopoietic stem cell transplant within 3 months or still on immunosupression, congenital immunodeficiency, aplastic anaemia, Down syndrome Centres may have particular local concerns about other specific diagnosis | |||
| No confirmed focus of infection requiring inpatient care* | |||
| No medical complication requiring inpatient care† | |||
| No severe sepsis at FN presentation‡ | |||
| Availability of a 24-hour caregiver | |||
| Good education of patient and carer on reportable symptoms | |||
| Availability of a telephone | |||
| Within 1 hour of treating hospital | |||
| Treating team preference | |||
| No previous history of non-compliance with medical care | |||
Variations may consider excluding ANY patient who has received a fluid bolus, or had a past unplanned admission to ICU. If well, presence of infiltrates on CXR may not be a contraindication to oral antibiotic therapy.
At the time of writing, SARS-CoV2/COVID-19-positive swabs should NOT NECESSARILY be a contraindication to homecare.
*Including, but not limited to, central venous access device site infection, cellulitis, perianal cellulitis or pain, significant pneumonia, infection with multidrug-resistant bacteria.
†Including, but not limited to, pain requiring intravenous analgesia, poor oral intake or excessive loss requiring intravenous hydration; respiratory distress or oxygen requirement.
‡Severe sepsis includes any of (i) altered conscious state, (ii) inotrope requirement, (iii) fluid bolus requirement >40 mL/kg or (iv) respiratory support requirement.
ALL, acute lymphoblastic leukaemia; AUS, Australian-UK-Swiss; CXR, chest X-ray; FN, febrile neutropenia; ICU, intensive care unit.
Homecare involved treatment with oral antibiotics, parents taking their child’s temperature every 4–6 hours when awake and daily clinical reviews via telephone until antibiotics were stopped. Prior to discharge, patients and their families received education about the homecare programme and must have tolerated one dose of oral antibiotics.
The suggested oral antibiotic regimen was ciprofloxacin plus co-amoxiclav (or clarithromycin if allergic to penicillin). Antibiotics could be stopped if that patient was clinically well; had been apyrexial (temperature <38°C) for at least 24 hours; had negative blood cultures and the absence of a need for rationalising and/or continuation of antibiotics. Reasons for further medical review and/or admission are given in online supplemental table 1.
archdischild-2021-323254supp001.pdf (530.8KB, pdf)
The CCLG low-risk FN programme modified the implementation toolkit from Melbourne.10 The guidance provided centres and clinicians with the option to adapt the recommendations based on local practice and service pressures. Children with homecare eligible episodes were not mandated to be sent home after the recommended period of observation and treating team discretion was included in the eligibility criteria.
Service evaluation
A service evaluation plan was developed prior to the introduction of the guideline and publicised via the CCLG website.11 Pseudo-anonymised episode data relating to predefined outcomes was contemporaneously collected via Qualtrics software (online supplemental file). Centres were encouraged to submit data at discharge or 7 days of presentation, whichever was sooner. A separate data collection form was submitted at representation.
The Paediatric Oncology Trainees Group and individual clinical leads were used to identify local data collection leads. Centres were encouraged to submit data from the date they implemented the new guidance, but implementation of the guideline, and participation in the service evaluation, was at the discretion of individual centres.
Teams were asked to contact the protocol team urgently regarding any serious adverse events related to infection (ICU admission or death) during this period. Stopping criteria were identified prior to implementation of the protocol (online supplemental table 2).
Data were collected on initial episodes from 17 April 2020 to 16 April 2021. Data submission was closed on 7 May 2021.
Analysis
The following prespecified core outcomes were analysed monthly, stratified by AUS rule score: median duration from start of FN episode (attendance at hospital if outpatient, onset of signs/symptoms if inpatient) to discharge, percentages of patients with any positive blood cultures, significant infection requiring intravenous antibiotics, representation within 7 days of initial episode, readmission within 7 days of initial episode, ICU admission, death from infection and death from other causes during that episode. The percentage of cases eligible for homecare as per table 1 and stratified by centre was calculated. Fisher’s exact test was used to determine if the percentage of homecare eligible patients with an AUS rule 0–1 subsequently readmitted was significantly above 15% (expected percentage from systematic review of similar approaches).12 Duration of intravenous antibiotics was added to the required dataset 6 weeks after the start of the project, and included in subsequent analyses. Hypothesis tests were considered statistically significant if p<0.05.
Analyses were performed using a custom script in the R statistical environment using the ‘dplyr’, ‘knitr’, ‘readxl’, ‘janitor’, ‘scales’, ‘reshape2’, ‘ggplot2’, ‘qwraps2’ and ‘ggalluvial’ packages. The script was run 13 times over the year and all outputs shared with the study authors and contributors.
Prior to final analysis, data were assessed for inconsistencies and local centres contacted to provide clarification for selected episodes. If the inconsistency could not be resolved, the relevant outcome for the episode was omitted from analysis.
Results
Thirteen centres (5 PTCs and 8 POSCUs) contributed 729 initial FN episodes in 405 patients (range 3–203 episodes/centre). Six hundred and six episodes (83%) were from PTCs. There were a further 79 episodes within 7 days of an initial episode, 64 of which were representations following discharge after the initial episode and 15 where the child remained an inpatient after an episode resolved and then developed further FN symptoms. Not all centres contributed cases throughout the whole study either because they did not initially implement the protocol and/or stopped submitting cases (online supplemental figure 1); 97% episodes had data submitted for all the initial prespecified core outcomes.
The most common underlying diagnosis was acute lymphoblastic leukaemia (ALL) (online supplemental table 3). The diagnostic label of some patients changed during the study due to treatment decisions (eg, ALL where the patient then went on to have haematopoietic stem cell transplant (HSCT)). There were significant differences in the cohorts from different centres with respect to diagnosis and AUS score distribution (online supplemental tables 4 and 5, Fisher’s exact test p<1×10−7 for both).
The core outcomes for all patients are summarised in table 2. AUS rule scores were positively correlated with percentages of positive blood cultures, significant infections and ICU admission and median duration of intravenous antibiotics.
Table 2.
Core outcomes stratified by AUS score
| AUS: 0 (n=72) | AUS: 1 (n=230) | AUS: 2 (n=243) | AUS: 3 (n=186) | |
| Duration of intravenous antibiotics (days) n; median (IQR) |
69; 3 (1, 5) | 209; 3 (2, 6) | 216; 4 (3, 8) | 169; 7 (3, 11) |
| Duration from episode to discharge (hours) n; median (IQR) |
71; 72 (28, 228) | 226; 96 (52, 192) | 238; 120 (72, 240) | 178; 192 (78, 336) |
| Outcomes | ||||
| Positive blood culture (excl contaminants) | 12 (17%) | 36/229 (16%) | 49 (20%) | 57 (31%) |
| Significant infection requiring intravenous antibiotics | 13 (18%) | 29/229 (13%) | 56 (23%) | 57 (31%) |
| Representation within 7 days | 5 (7%) | 25 (11%) | 12 (5%) | 17 (9%) |
| Readmission within 7 days | 5 (7%) | 22 (10%) | 11 (5%) | 16 (9%) |
| ICU admission | 0 (0%) | 2/229 (1%) | 4 (2%) | 8 (4%) |
| Death from infection | 0 (0%) | 0/229 (0%) | 0 (0%) | 2 (1%) |
| Death from other cause | 0 (0%) | 0/229 (0%) | 3 (1%) | 2 (1%) |
All data are count (%), unless otherwise specified.
AUS, Australian-UK-Swiss; ICU, intensive care unit.
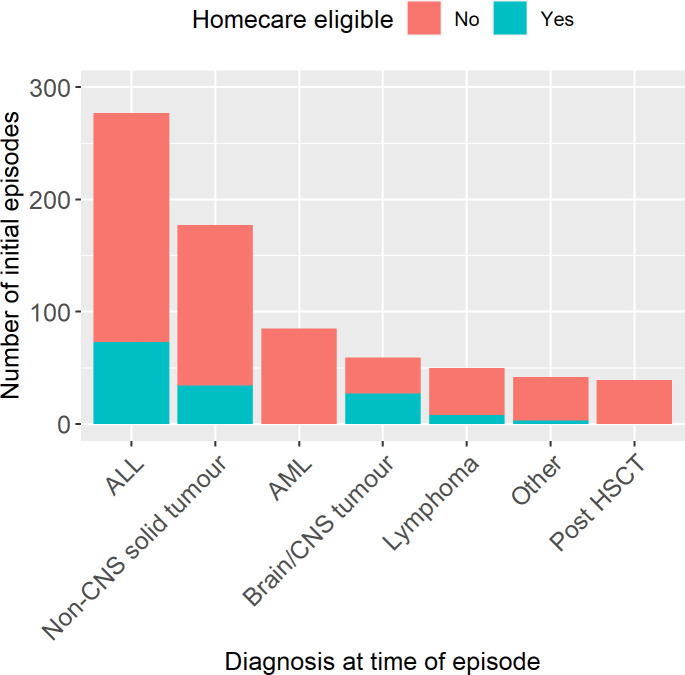
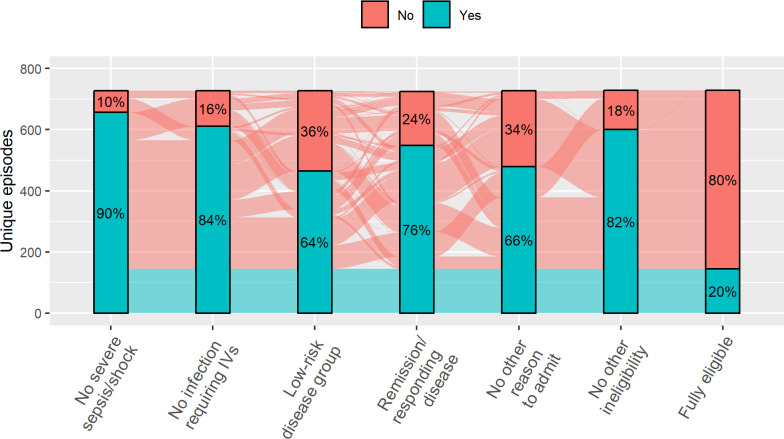
A total of 145 initial episodes (20%) were eligible for homecare with oral antibiotics (range 0%–42%/centre) (online supplemental figure 2). As some disease groups were excluded from homecare (see table 1), the underlying diagnoses of children with a homecare-eligible episode were different to those ineligible (figure 1). Some episodes had multiple reasons recorded for ineligibility while others just one (figure 2). Importantly, there were cases where each of the five criteria were the only reason for ineligibility (ie, non-redundancy of eligibility criteria). Four centres specified additional diagnoses that were considered high risk. These included Philadelphia-positive ALL (two centres), high-risk neuroblastoma during COJEC induction (three centres), postautologous HSCT (one centre) and all sarcomas (two centres). Clinician preference was a reason for ineligibility in 71 initial episodes; in 38 (5.2%) of these it was the only reason for ineligibility.
Figure 1.
Diagnosis at time of febrile neutropenia episode. ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; CNS central nervous system; HSCT, haematopoietic stem cell transplant. N=729.
Figure 2.
Reasons for non-eligibility. Bars summarise percentage meeting each eligibility criteria. Flows between bars represent individual episodes coloured by overall eligibility. Flow widths are proportional to the number of episodes. N=729.
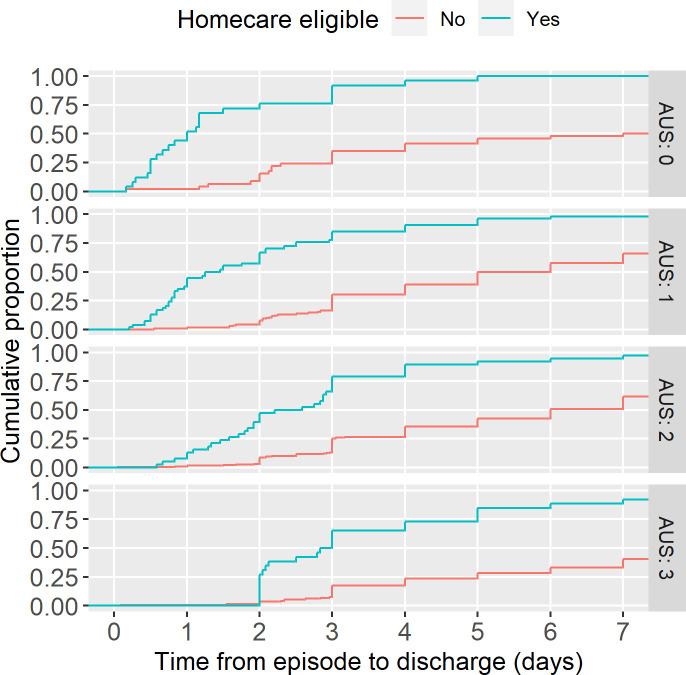
Duration from the start of FN episode to discharge was significantly lower in those eligible for homecare (p<0.001 Mann-Whitney U test). Median duration of admission stratified by AUS rule score was 24, 32, 58 and 70 hours for AUS 0–3 respectively in those eligible compared with 72, 96, 120 and 192 hours median duration overall (figure 3). Forty-eight per cent low-risk (AUS 0–1) homecare eligible episodes were discharged within 24 hours, compared with 2% AUS 0–1 but homecare ineligible.
Figure 3.
Duration of admission. Empirical cumulative distribution function for length of stay from time of febrile neutropenia episode to hospital discharge facetted by Australian-UK-Swiss (AUS) score. Data only for those alive at time of discharge and censored beyond 7 days as centres were asked to submit episodes after 7 days of inpatient stay. N=722
Those eligible for homecare were more likely to be low risk compared with those that were not (54% vs 37%, respectively, χ2 test p=0.0003).
Sixty-four patients represented within 7 days of the initial episode (23 homecare eligible, 41 ineligible and therefore received standard care prior to representation). A further 15 patients remained inpatients and had another FN episode within 7 days and were excluded from analysis. Reasons for representation are described in online supplemental table 6 and were not significantly different between those eligible for homecare and those that were not (Fisher’s exact test p=0.111); 23/145 (14%) homecare eligible episodes represented within 7 days, of which, 20 (90%) were readmitted. Readmission rates were 16% in the low-risk group (AUS 0–1), which was not different to the expected rate of 15% based on a meta-analysis of previous studies of ambulatory/oral regimens for low-risk patients (binomial exact test p=0.754).7
Overall, 14 FN episodes resulted in admission to ICU and 7 children died during that inpatient episode (2 of infection, 5 of other causes). No child eligible for homecare with AUS score 0–1 went to ICU or died within 7 days of the episode. No representation within 7 days of discharge resulted in ICU admission or death.
There were 172 (21%) FN episodes with at least one documented positive blood culture (excluding contaminants as defined by the treating team). One hundred ninety organisms were identified, of which 39% were Gram-negative organisms (58% enterobacterales, 15% Pseudomonas, 23% others and 4% unspecified), and 61% were Gram-positive (25% coagulase-negative staphylococci (CoNS) and 12% viridans streptococci) (online supplemental table 7). In children eligible for homecare, there were a total of 15 positive blood cultures (10 at initial presentation, 5 at representation); 5/15 (33%) were Gram-negative, but 8/15 (53%) were CoNS.
None of the criteria for stopping the project were met. A sensitivity analysis with one episode per patient, selected at random, did not show important differences in the core outcomes (online supplemental table 8).
Discussion
This service evaluation assessed new national guidance developed responsively at the outbreak of the COVID-19 pandemic, to use the AUS rule as a means of safely determining which patients with FN can be managed with homecare. The direct impact of COVID-19 infection in children with cancer has been described elsewhere.13 14
Twenty per cent of episodes were eligible for homecare, none of which resulted in ICU admission or death. This adds to the growing data suggesting that there is a group of patients with FN who can be safely managed at home.
The percentage of episodes identified as low risk (AUS 0–1) was 41.4% in this study, compared with 44% in the Australian and European PICNICC datasets. There was a greater chance of significant infection with higher AUS values, showing consistency of the rule’s performance across time points and geographical locations.3 4 The rate of readmission following early discharge was consistent with that described within a meta-analysis of previously published data,12 an Australian implementation study5 and with the rate of readmission reported in the CCLG’s 2017 audit of FN within the UK (where patients were discharged after a longer period of hospital admission).1
A previous study found parents desire shorter courses of intravenous antibiotics and are willing to accept a chance of readmission.15 16 It was shown parents view the use of risk stratification positively and would like the communication of their child’s risk to then come to a shared decision about preferred place of care for the FN episode. Data from Australia collected during the COVID-19 pandemic (after the implementation of the new UK guidance) found that parents and professionals were even more supportive of home-based care of low-risk FN based on the AUS rule than before.17
We found variation in the percentage of children eligible for homecare in the different centres. This could be due to differences in the patient cohorts (impacting on the percentage meeting eligibility criteria) and clinician’s anxiety about early discharge.15 16 The duration of inpatient stay for some AUS 0–1 homecare episodes was longer than recommended. This may represent logistical barriers to discharge or uncertainty/anxiety from the treating team about the new protocol. The latter is supported by our finding that 5% of episodes were ineligible for homecare due to treating team preference alone, despite an extensive list of other reasons for ineligibility.
Within this dataset, most representations after discharge with FN (in both homecare eligible and ineligible episodes) resulted in readmission to hospital, although this was not required by the guideline. This may reflect caution of healthcare professionals who are used to managing this population as inpatients. Only around a quarter of these episodes had a significant infection requiring antibiotics, and none resulted in ICU admission or death.
It is not possible to draw conclusions about total duration of stay for all episodes as duration was capped at 7 days. This was a deliberate decision: at the time of the launch of the new guidance, the focus was on quick turnaround of safety data on early discharge to key stakeholders.
The rate of bacteraemia found in this study is higher than that seen in the AUS rule derivation study,4 with differences in the handling of contaminants a likely contributor. The criteria for submission of blood culture results was ‘any positive blood cultures (excluding contaminants)’. No further definition of a contaminant was detailed. Contributors were not always directly involved in the care of the patient during the episode and so blood culture results submitted may not have been clinically significant. This ‘overcalling’ of infections further supports this approach as being safe.
Strengths and limitations
The main limitation is some relevant episodes may not have been submitted. The impact of this reporting bias was minimised by using a standardised entry form requiring information easily gained from medical notes, encouraging early data collection and circulating regular interim analyses to encourage submission. Consistent with this, most centres had a consistent number of cases over time.
We did not ask whether children were discharged to homecare, only whether they were eligible. It is therefore plausible that some notionally homecare eligible patients discharged after 36–48 hours were a standard discharge after confirmation of negative blood cultures.
Our study included only a minority of UK centres, perhaps reflecting differences in priorities and capacity to implement significant changes during a pandemic, or other barriers to implementation that we hope to identify in future studies.
Finally, we did not collect whether episodes had started as inpatients or outpatients so direct comparison to previous CCLG audits of purely outpatient episodes is not possible.
Conclusions
Using the AUS rule and homecare criteria identifies children who can be safely discharged on oral antibiotics. Further research in reducing therapy for children with FN should focus on whether this is safe for a greater proportion of children, the best timing for discharge and how to support centres in implementing homecare regimens. Our findings have been shared within the CCLG supportive care group; we anticipate more centres will implement the guidance and a follow-up evaluation is planned.
Acknowledgments
We would like to thank the following clinicians who contributed to the data collection within this service evaluation: Sara Farah, Miriam Sager, Marwa Deghedy, Angela Gilbert, Catriona Boyd, Anna Capsomidis, Nicola Bloxham, Lucy Shimwell, Ayesha Fathani, Sarah Farndon, Vickyanne Carruthers.
Footnotes
Twitter: @DrTomJackson, @jessica_bate, @ashleysgamble, @drbobphillips, @drjessmorgan
Contributors: GMH, BP, JB, RGG, SS, PA, AB-H, BP and JEM were involved in the development of the CCLG FN guidelines. TJJ, RN, BP and JEM collected the data. TJJ analysed the data. TJJ, RN and JEM drafted the manuscript and all other authors contributed revisions. TJJ acts as the guarantor
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Requests to access pseudoanonymised data shall be addressed to TJJ.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This was a service evaluation of a protocol developed in response to anticipated COVID-19 resource constraints and therefore REC approval was not required. Local centres were advised to comply with Caldicott principles for information governance and data were pseudoanonymised by the direct care team.
References
- 1. Morgan J, Phillips B, on behalf of the CCLG Supportive Care Group . ‘Winter 2017’ CCLG Audit of NICE CG151 Neutropenic Sepsis in Children and Young Adults, 2017. Available: https://www.cclg.org.uk/write/MediaUploads/Member%20area/FN_Audit_report_2017.pdf
- 2. Mateen BA, Wilde H, Dennis JM, et al. Hospital bed capacity and usage across secondary healthcare providers in England during the first wave of the COVID-19 pandemic: a descriptive analysis. BMJ Open 2021;11:e042945. 10.1136/bmjopen-2020-042945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phillips B, Morgan JE. Meta-analytic validation of new 'AUS' febrile neutropenia risk score. Pediatr Blood Cancer 2021;68:e28580. 10.1002/pbc.28580 [DOI] [PubMed] [Google Scholar]
- 4. Haeusler GM, Phillips R, Slavin MA, et al. Re-evaluating and recalibrating predictors of bacterial infection in children with cancer and febrile neutropenia. EClinicalMedicine 2020;23:100394. 10.1016/j.eclinm.2020.100394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haeusler GM, Gaynor L, Teh B, et al. Home-Based care of low-risk febrile neutropenia in children-an implementation study in a tertiary paediatric Hospital. Support Care Cancer 2021;29:1609–17. 10.1007/s00520-020-05654-z [DOI] [PubMed] [Google Scholar]
- 6. Tew M, De Abreu Lourenco R, Gordon JR, et al. Cost-effectiveness of home-based care of febrile neutropenia in children with cancer. Pediatr Blood Cancer 2022;69:e29469. 10.1002/pbc.29469 [DOI] [PubMed] [Google Scholar]
- 7. NICE . CG151 neutropenic sepsis: full guideline. Available: http://www.nice.org.uk/ [Accessed 25 Sep 2013].
- 8. Haeusler GM, De Abreu Lourenco R, Clark H, et al. Diagnostic yield of initial and consecutive blood cultures in children with cancer and febrile neutropenia. J Pediatric Infect Dis Soc 2021;10:125–30. 10.1093/jpids/piaa029 [DOI] [PubMed] [Google Scholar]
- 9. Children’s Cancer and Leukaemia Group (CCLG) . Managing Febrile Neutropenia in the UK in 2020 - Proposed New Management Pathway v.1.01. Available: https://www.cclg.org.uk/write/MediaUploads/Member%20area/COVID19/2020_CCLG_FN_program_-_Guidance-Protocol_FINAL.pdf [Accessed 5 Jun 2021].
- 10. Haeusler G. Paediatric low risk febrile neutropenia toolkit. Available: https://cancerandinfections.org/kids-low-risk-toolkit [Accessed 25 Jun 2021].
- 11. Children’s Cancer and Leukaemia Group (CCLG) . Managing Febrile Neutropenia in the UK - Service Evaluation v. 1, 2020. Available: https://www.cclg.org.uk/write/MediaUploads/Member%20area/COVID19/2020_CCLG_FN_program_-_Service_evaluation_FINAL.pdf [Accessed 5 Jun 2021].
- 12. Morgan JE, Cleminson J, Atkin K, et al. Systematic review of reduced therapy regimens for children with low risk febrile neutropenia. Support Care Cancer 2016;24:2651–60. 10.1007/s00520-016-3074-9 [DOI] [PubMed] [Google Scholar]
- 13. Millen GC, Arnold R, Cazier J-B. COVID-19 in children with haematological malignancies. Archives of Disease in Childhood. Published online 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mukkada S, Bhakta N, Chantada GL, et al. Global characteristics and outcomes of SARS-CoV-2 infection in children and adolescents with cancer (GRCCC): a cohort study. Lancet Oncol 2021;22:1416–26. 10.1016/S1470-2045(21)00454-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morgan JE, Phillips B, Stewart LA, et al. Quest for certainty regarding early discharge in paediatric low-risk febrile neutropenia: a multicentre qualitative focus group discussion study involving patients, parents and healthcare professionals in the UK. BMJ Open 2018;8:e020324. 10.1136/bmjopen-2017-020324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morgan JE, Phillips RS, Stewart LA, et al. Sharing roles and control in pediatric low risk febrile neutropenia: a multicenter focus group discussion study involving patients, parents, and health care professionals. J Pediatr Hematol Oncol 2020;42:337–44. 10.1097/MPH.0000000000001827 [DOI] [PubMed] [Google Scholar]
- 17. Haeusler GM, De Abreu Lourenco R, Bakos C, et al. Managing low-risk febrile neutropenia in children in the time of COVID-19: what matters to parents and clinicians. J Paediatr Child Health 2021;57:826–34. 10.1111/jpc.15330 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
archdischild-2021-323254supp001.pdf (530.8KB, pdf)
Data Availability Statement
Data are available on reasonable request. Requests to access pseudoanonymised data shall be addressed to TJJ.