Abstract
Objectives
Pulmonary function impairment and chronic respiratory symptoms after tuberculosis are relatively common in low-income and middle-income countries. We aimed to estimate the impact of post-tuberculosis (post-TB) on pulmonary function.
Methods
This large cross-sectional, population-based study included subjects aged 15 years or older with technically acceptable postbronchodilator spirometry measurements. Post-TB was diagnosed on the basis of radiological evidence and/or medical history. Airflow obstruction was defined as a postbronchodilator forced expiratory volume in 1 s/forced vital capacity ratio below the lower limit of normal of Global Lung Function Initiative (GLI) lung function equations. Small airway dysfunction was diagnosed if at least two of the following indicators were less than 65% of predicted: maximal mid-expiratory flow, forced expiratory flow (FEF) 50% or FEF 75%.
Results
In this population sample (N=8680, mean age: 40.1 years), 610 (7.0% (95% CI 6.5 to 7.6) participants were post-TB. Post-TB subjects had more frequent respiratory symptoms (46.8% vs 28.3%). Among post-TB subjects, 130 (21.3% (95% CI 18.1 to 24.8)) had airflow obstruction; OR of airflow obstruction was significantly associated with post-TB after adjustment for other confounding factors (OR 1.31, 95% CI 1.05 to 1.62). Post-TB was also associated with small airway dysfunction (OR 1.28, 95% CI1.07 to 1.53), which was present in 297 (48.9% (95% CI 33.9 to 53.0)) post-TB subjects.
Conclusions
Our findings support existing knowledge that post-TB is positively associated with pulmonary function impairment and make for frequent respiratory symptoms. Post-TB should be considered as a potentially important cause of airflow obstruction and respiratory symptoms in patients originating from countries with a high burden of tuberculosis.
Keywords: tuberculosis, COPD epidemiology, respiratory infection
Key messages.
What is already known on this topic
Several publications have reported the prevalence of post-tuberculosis (post-TB) sequelae, but the definition of post-TB and studied population are inconsistent. We wished estimate the prevalence of post-TB in low-income areas in China and identify the impact of post-TB on pulmonary function.
What this study adds
Our findings confirmed that post-TB is highly prevalent, is positively associated with airflow obstruction and associated with a high frequency of respiratory symptoms.
How this study might affect research, practice or policy
Better understanding and addressing airflow obstruction and respiratory symptoms after TB is needed; this is most relevant in low-resource, high-burden settings, where healthcare resources and diagnostics means are limited.
Introduction
Tuberculosis (TB) is a major global health problem especially in low-income and middle-income countries; it is among the top 10 causes of death and the leading cause from a single infectious agent. An estimated 10.0 million people developed TB worldwide, leading to an estimated 1.3 million TB deaths among HIV-negative people in 2020.1 China is the third contributor to this worldwide epidemic and accounts for 8.4% of all cases, after India (26%) and Indonesia (8.5%).2 While treatment is available and efficient in non-multidrug-resistant/extensively drug-resistant (MDR/XDR) cases, microbiological cure may not prevent long-term pulmonary complications of TB.
While smoking remains the key risk factor for chronic obstructive pulmonary disease (COPD), a considerable burden of the disease in low-income and middle-income countries cannot be explained by smoking alone. TB and other non-smoking risk factors of COPD such as domestic pollution are of increasing importance.3–6 An association between past TB and airflow obstruction, characteristic of COPD, has been reported in large population-based epidemiological studies.7–10 However, most of these studies included subjects above 40 years of age and are limited to low TB burden regions. There is also some evidence that pulmonary TB is related to persistent symptoms and has a substantial adverse impact on quality of life. A high burden of self-reported symptoms after TB treatment completion has been reported.11–13 Better understanding and addressing of airflow obstruction and respiratory symptoms after TB is needed: this is most relevant in low-resource, high-burden settings, where healthcare resources and diagnostic modalities are limited.
In this study, we aimed to describe the general characteristics, pulmonary function parameters and chronic respiratory symptoms of people with post-TB. We also quantified the association between post-TB status and chronic respiratory symptoms and further characterised the relationship between post-TB and pulmonary function impairment.
Methods
Study design and participants
The present study, conducted in Tibet and Xinjiang Uygur Autonomous Regions, analysed pulmonary health in long-term residents of these areas aged 15 years or older, details of which have been reported elsewhere.14 A multistage stratified sampling procedure was used to select subjects from 13 local regions between June 2015 and August 2016. The proportion of samples from each gender and age group was based on the 2010 census of the Chinese population. A standardised questionnaire covering sociodemographic status, living conditions, respiratory symptoms, history of respiratory diseases and comorbidities, environmental and occupational factors was administered by experienced interviewers at local community health centres. Furthermore, a range of physical measurements were undertaken using a standard protocol, including anthropometry, blood pressure, oxygen saturation by pulse oximetry (SpO2) and lung function. A posterior–anterior chest radiograph was obtained during deep inspiration in a standing position using a radiography unit.
Procedures
Pulmonary function tests were measured by trained technicians in all qualified study participants (spirometry) with a MasterScreenTM Pneumo PC spirometer (CareFusion, Yorba Linda, California) according to the American Thoracic Society/European Respiratory Society (ATS/ERS) recommendations by trained technicians.15 The spirometer was calibrated daily using a 3-litre syringe to ensure measured volumes within 3% of syringe volume, before data collection; ambient temperature, humidity and altitude were also recorded daily. Each participant underwent the same procedure two times, before and after receiving a bronchodilator (BD) (400 ug of salbutamol through a 500 mL spacer). The forced expiratory manoeuvres were performed 3–8 times until the forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) were reproducible within 150 mL.16 Acceptability of FVC and FEV1 was scored using an A to F grading system. Performing three acceptable manoeuvres with an FVC variability of 100 mL or less was rated ‘A’, a variability of 100–150 mL was rated ‘B’; variability between 150 and 200 mL was scored ‘C’. A, B or C grades were considered acceptable for analysis. Data were uploaded daily to a database, examined for incoherent data by the study supervisors and by the principal investigator. Quality control, based on the American Thoracic Society/European Respiratory Society criteria, was performed by a field supervisor at the filing centre, and included analysis of flow volume curves for artefacts and appropriate technique. Airflow obstruction was defined as a post-BD FEV1/FVC ratio below the lower limit of normal (LLN) for height, age and sex, based on the reference values from the Global Lung Function Initiative (GLI) lung function equations for a North East Asian population.17 A post-BD FEV1/FVC ratio of <0.70 was also used to define airflow obstruction in a sensitivity analysis based on Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines.18 Spirometric restriction was defined as a post-BD FVC ratio below the LLN for the height, age and sex based on the same reference population with a normal FEV1/FVC ratio. We used three indicators of lung function to assess small airway dysfunction, namely, maximal mid-expiratory flow (MMEF), forced expiratory flow (FEF) at 50% of vital capacity and FEF at 75% of vital capacity. Small airway dysfunction was diagnosed when at least two of these three indicators were below 65% of predicted values.19
Post-TB was defined as having highly suggestive images of pulmonary TB sequalae on chest radiographs and/or a positive medical history of TB.7–9 The diagnosis of post-TB lesions was considered in the presence of discrete linear or reticular fibrotic scars or dense nodules with distinct margins, with or without calcifications, within the upper lobes.20 The radiographs were reviewed by two experienced radiologists who were blinded to the details of the participants, using standard criteria for reporting radiologic abnormalities.21 Discordant findings were resolved by discussion and consensus. A medical history of tuberculosis was defined as a positive answer to the question ‘Has a doctor or other healthcare provider ever told you that you had tuberculosis?’.
Definitions of ever smoker, never smoker, household air pollution (HAP), occupational exposure have been previously reported.14 Self-reported history of physician-diagnosed hypertension, chronic cardiovascular disease of any cause, diabetes mellitus, dyslipidaemia and asthma was also obtained. We defined the chronic respiratory symptoms by their persistence for at least 3 consecutive months during a year based on questionnaires.
Statistical analysis
All analyses were performed with R statistical programme V.4.0.3 (www.r-project. org/). The analyses included a description of the sample, evaluation of mean spirometric results, calculation of the prevalence of airflow obstruction and small airway dysfunction according to history of post-TB. Statistical significance of differences was tested by analysis of variance (ANOVA) or Student’s t test for continuous variables and by χ2 test for categorical variables. Logistic regression models were built to quantify the relationship between post-TB and airflow obstruction. Potential confounders considered were: age, sex, region, educational level, smoking history and pack-years, HAP, occupational exposure and history of asthma. ORs with 95% CIs were determined for three models. Differences with two-sided p<0.05 were considered statistically significant.
Results
Of the 12 991 subjects invited to participate, 11 747 completed the survey questionnaire and performed chest X-rays. Among them, 2503 participants were excluded from this analysis because they could not complete post-BD testing. After excluding 564 participants without reliable spirometric data, the final study sample included 8680 participants (4407 (50.8%) women, 4273 (49.2%) men), with an overall mean age of 40.1 (SD 15.3) years (online supplemental figure E1).
thoraxjnl-2021-218345supp001.pdf (300.6KB, pdf)
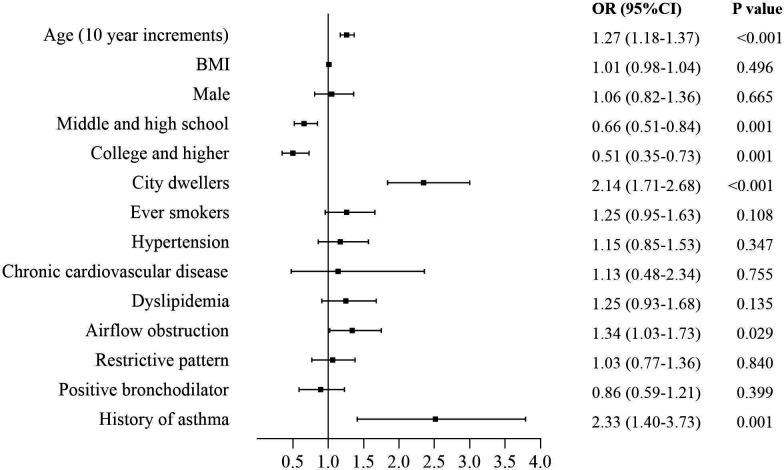
Among the 8680 participants, 610 (7.0%) had evidence of post-TB (table 1). Number of post-TB subjects, as defined by evidence of post-TB on chest radiographs only, was 463 (5.3% (95% CI 4.9% to 5.8%)), while that based on self-reported history of TB only was 98 (1.1% (95% CI 0.9% to 1.4%)). There were 49 (0.6% (95% CI 0.4 to 0.7%)) subjects for whom there was both evidence of post-TB on chest radiographs and a history of TB. Post-TB individuals were more likely to be men, older, ever smokers, city dwellers and have a lower educational level. They reported more comorbidities and had lower oxygen saturation (SpO2). They had a higher frequency of chronic cough, sputum, recurrent wheezing and dyspnoea in daily life when compared with those without ‘post-TB’. Also, they reported a higher rate of at least one of the listed respiratory symptoms (46.8% vs 28.3%). Spirometric indices: when compared with subjects without ‘post-TB’, post-TB participants had lower post-BD values for FEV1/FVC% (mean (SD): 78.4 (12.2) vs 82.4 (10.7), p<0.001), FVC (3.57 (1.02) vs 3.73 (0.96), p<0.001) and FEV1 (2.80 (0.91) vs 3.07 (0.85), p<0.001) and lower MMEF, FEF50 and FEF75 values as % of predicted. Older age, being a city dweller, having a history of asthma and suffering from airflow obstruction significantly increased probability of having a post-TB status, while higher educational level was related with a lower risk (figure 1).
Table 1.
Characteristics of participants according to history of post-TB
| Variables | Post-TB (n=610) | Non post-TB (n=8070) | P value |
| Male | 328 (53.8%) | 3945 (48.9%) | 0.022 |
| Age, years | 49.31 (15.21) | 39.49 (15.17) | <0.001 |
| City dweller | 256 (42.1%) | 2580 (32.1%) | <0.001 |
| Education level | |||
| Primary school and lower | 362 (59.4%) | 3512 (43.5%) | <0.001 |
| Middle and high school | 180 (29.5%) | 3225 (40.0%) | |
| College and higher | 68 (11.1%) | 1333 (16.5%) | |
| Smoking status | |||
| Never smoker | 405 (66.4%) | 5866 (72.7%) | <0.001 |
| Ever smoker | 205 (33.6%) | 2201 (27.3%) | |
| Comorbidities | |||
| Hypertension | 97 (16.3%) | 636 (8.0%) | <0.001 |
| Chronic cardiovascular disease | 11 (1.8%) | 66 (0.8%) | 0.022 |
| Diabetes mellitus | 14 (2.7%) | 128 (2.1%) | 0.303 |
| Dyslipidaemia | 70 (13.4%) | 478 (7.9%) | <0.001 |
| History of asthma | 32 (5.3%) | 118 (1.5%) | <0.001 |
| Respiratory symptoms | |||
| Frequent cough | 109 (18.0%) | 757 (9.5%) | <0.001 |
| Sputum | 110 (18.2%) | 750 (9.4%) | <0.001 |
| Recurrent wheezing | 54 (8.9%) | 310 (3.9%) | <0.001 |
| Dyspnoea in daily life | 198 (32.7%) | 1570 (19.6%) | <0.001 |
| At least one of the symptoms | 284 (46.8%) | 2259 (28.3%) | <0.001 |
| Lung function parameters | |||
| FVC post-BD, L | 3.57 (1.02) | 3.73 (0.96) | <0.001 |
| FVC post-BD % pred | 106.20 (25.55) | 104.19 (22.33) | 0.060 |
| FEV1 post-BD, L | 2.80 (0.91) | 3.07 (0.85) | <0.001 |
| FEV1 post-BD % pred | 100.55 (28.49) | 101.47 (23.60) | 0.437 |
| FEV1/FVC post-BD, % | 78.40 (12.18) | 82.38 (10.72) | <0.001 |
| MMEF post-BD % pred | 68.95 (35.07) | 78.90 (57.69) | <0.001 |
| FEF50% post-BD % pred | 83.52 (36.17) | 88.48 (31.06) | 0.001 |
| FEF75% post-BD % pred | 85.01 (58.02) | 92.33 (47.12) | 0.003 |
| Oxyhemoglobin saturation (SpO2), % | 91.39 (6.39) | 93.45 (5.25) | <0.0001 |
Data are expressed as number (%) or mean (SD).
FEF, forced expiratory flow; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; MMEF, maximum mid-expiratory flow; post-BD, post-bronchodilator.
Figure 1.
Forest plot showing OR for post-TB participants. Each square represents an OR. The horizontal lines indicate 95% CIs. BMI, body mass index; TB, tuberculosis.
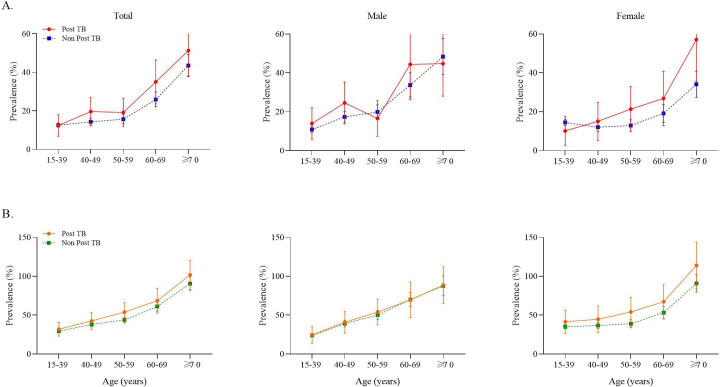
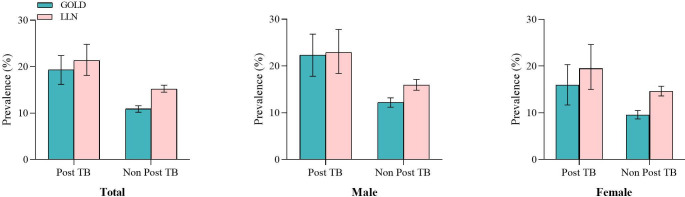
Overall prevalence of airflow obstruction in post-TB subjects (21.3% (95% CI 18.1% to 24.8%) was significantly higher than in ‘non-post-TB’ subjects (15.2% (95% CI 14.5% to 16.0%)). This was also the case for small airway dysfunction (48.9% (297 of 607) vs 37.5% (2993 of 7987)). Standardised prevalence of these conditions increased steadily with age (p<0.001 for airflow obstruction and small airway dysfunction) (figure 2). Using the GOLD threshold of a fixed FEV1/FVC ratio <0.7, prevalence of airflow obstruction in post-TB subjects (all: 19.3% (95% CI 16.2 to 22.4); men: 22.3% (95% CI 17.8 to 26.8); women: 16.0% (95% CI 11.7 to 20.3)) was also significantly higher than in ‘non-post-TB’ participants (all: 10.9% (95% CI 10.2 to 11.6); men: 12.2% (95% CI 11.2 to 13.2); women: 9.6% (95% CI 8.7 to 10.5)) (figure 3).
Figure 2.
Standardised prevalence of airflow obstruction and small airway dysfunction in subjects based on history of post-TB. (A) Standardised prevalence of airflow obstruction. (B) Standardised prevalence of small airway dysfunction. Dots represent mean prevalence and error bars represent 95% CI. TB, tuberculosis.
Figure 3.
Prevalence of airflow obstruction based on two different diagnostic criteria for airway obstruction (GOLD and LLN) according to history of post-TB. Bars represent mean prevalence and error bars represent 95% CI. TB, tuberculosis.
Table 2 presents the characteristics and spirometric results of participants according to post-TB and airflow obstruction status. Subjects with airflow obstruction and a history of post-TB had lower post-BD spirometric values, a higher proportion of GOLD III-IV stages (online supplemental figure E2), more respiratory symptoms, a more frequent history of asthma, a lower SpO2 and a higher percentage of subjects with a chronic obstructive pulmonary disease assessment test (CAT) score ≥10 compared with ‘non-post-TB’ participants. Importantly, post-TB subjects without airflow obstruction actually reported more respiratory symptoms (46.0% vs 29.5%, p<0.001) and had a lower SpO2 (91.0% (0.2) vs 94.4% (0.1), p<0.001) and a lower FVC (absolute value and % predicted) than ‘non-post-TB’ subjects with airflow obstruction.
Table 2.
Characteristics of participants according to AO and post-TB status
| Variables | P-TB (+)/AO (+) (n=130) | P-TB (−)/AO (+) (n=1230) | P-TB (+)/AO (−) (n=480) | P-TB (−)/AO (−) (n=6840) | P value* | P value† |
| Male | 75 (57.7%) | 627 (51.0%) | 253 (52.7%) | 3318 (48.5%) | 0.519 | 0.075 |
| Age group, years | 53.90 (14.66) | 42.34 (16.74) | 48.07 (15.13) | 38.98 (14.81) | <0.001 | <0.001 |
| City dwellers | 53 (40.8%) | 280 (22.8%) | 203 (42.5%) | 2300 (33.8%) | <0.001 | <0.001 |
| Education level | ||||||
| Primary school and lower | 93 (71.5%) | 576 (46.8%) | 269 (56.0%) | 2936 (42.9%) | <0.001 | <0.001 |
| Middle and high school | 30 (23.1%) | 517 (42.0%) | 150 (31.3%) | 2708 (39.6%) | ||
| College and higher | 7 (5.4%) | 137 (11.2%) | 61 (12.7%) | 1196 (17.5%) | ||
| Smoking status | ||||||
| Never smoker | 85 (65.4%) | 857 (69.7%) | 320 (66.7%) | 5009 (73.3%) | 0.595 | 0.002 |
| Ever smoker | 45 (34.6%) | 373 (30.3%) | 160 (33.3%) | 1828 (26.7%) | ||
| History of asthma | 16 (12.3%) | 28 (2.3%) | 16 (3.3%) | 90 (1.3%) | 0.428 | 0.005 |
| Respiratory symptoms | ||||||
| Frequent cough | 30 (23.3%) | 131 (10.7%) | 79 (16.5%) | 626 (9.3%) | 0.001 | <0.001 |
| Sputum | 31 (24.0%) | 125 (10.2%) | 79 (16.6%) | 625 (9.2%) | <0.001 | <0.001 |
| Recurrent wheezing | 18 (14.0%) | 65 (5.3%) | 36 (7.5%) | 245 (3.6%) | 0.079 | <0.001 |
| Dyspnoea in daily life | 48 (37.2%) | 257 (20.9%) | 150 (31.4%) | 1313 (19.4%) | <0.001 | <0.001 |
| At least one of the symptoms | 64 (49.6%) | 362 (29.5%) | 220 (46.0%) | 1897 (28.1%) | <0.001 | <0.001 |
| CAT score‡ | ||||||
| <10 | 22 (17.1%) | 322 (26.3%) | ||||
| ≥10 | 107 (82.9%) | 904 (73.7%) | ||||
| Lung function parameters | ||||||
| FVC post-BD, L | 3.53 (1.11) | 3.85 (1.02) | 3.58 (0.99) | 3.71 (0.94) | <0.001 | 0.004 |
| FVC post-BD % pred | 106.52 (28.16) | 108.47 (24.20) | 106.11 (24.83) | 103.42 (21.89) | 0.052 | 0.012 |
| FEV1 post-BD, L | 2.17 (0.85) | 2.48 (0.79) | 2.98 (0.85) | 3.17 (0.82) | <0.001 | <0.001 |
| FEV1 post-BD % pred | 79.41 (26.39) | 83.14 (21.53) | 106.28 (26.27) | 104.77 (22.42) | <0.001 | 0.157 |
| FEV1/FVC post-BD, % | 60.60 (10.44) | 64.15 (9.99) | 83.22 (7.08) | 85.66 (6.86) | <0.001 | <0.001 |
| MMEF post-BD % pred | 34.59 (17.63) | 45.21 (73.41) | 77.95 (32.87) | 84.60 (52.50) | <0.001 | 0.013 |
| FEF50% post-BD % pred | 40.74 (18.44) | 47.01 (17.64) | 94.84 (30.86) | 95.53 (27.09) | <0.001 | 0.587 |
| FEF75% post-BD % pred | 41.87 (34.86) | 48.82 (25.95) | 96.58 (57.56) | 99.72 (45.9) | <0.001 | 0.147 |
| SpO2, % | 92.24 (6.60) | 94.58 (4.68) | 91.17 (6.32) | 93.25 (5.31) | <0.001 | <0.001 |
Data are expressed as number (%) or mean (SD).
*P value difference between post-TB without airflow obstruction and airflow obstruction without post-TB.
†P value difference between normal population and post-TB without airflow obstruction.
‡CAT score difference for p<0.001.
AO, airflow obstruction; CAT, chronic obstruction pulmonary disease assessment test; FEF, forced expiratory flow; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; MMEF, maximum mid-expiratory flow; post-BD, post-bronchodilator; P-TB, post-tuberculosis; P-TB(−)/AO(+), airflow obstruction without post-TB; P-TB(−)/AO(−), normal population without airflow obstruction and post-TB; P-TB(+)/AO(−), post-TB without airflow obstruction; P-TB(+)/AO(+), airflow obstruction with post-TB; SpO2, oxyhemoglobin saturation.
Subjects with a restrictive pattern: online supplemental table E1 shows the demographic characteristics of participants according to their lung function (presence of a restrictive pattern vs normal) and post-TB status. Post-TB participants with normal spirometry had nearly two times as much respiratory symptoms as those without a history of post-TB and had lower pre-BD and post-BD (% predicted) FEV1/FVC% and MMEF. Post-TB subjects with a restrictive pattern had more respiratory symptoms, lower SpO2 and more severe lung function indices. Prevalence of airflow obstruction, restrictive pattern and small airway dysfunction according to post-TB status is shown in online supplemental figure E3.
When compared with ‘non-post-TB’ participants, having a post-TB status was associated with an OR (95% CI) of 1.31 (1.05 to 1.62) for airflow obstruction and of 1.28 (1.07 to 1.53) for small airway dysfunction after adjusting for gender, age, region, education, smoking status, exposure to HAP, occupation and history of asthma. In a subgroup analysis, being a post-TB never smoker was independently associated with airflow obstruction (1.37 (1.04 to 1.78], p=0.023) and (1.47 (1.18 to 1.83), p<0.001) small airway dysfunction after adjusting for above-mentioned variables (table 3). The effect sizes for potential confounders are provided in online supplemental tables E2 and E3. Post-TB was significantly associated with respiratory symptoms after adjusting for gender, age, region and education, smoking status, exposure to HAP and occupation (online supplemental table E4).
Table 3.
Adjusted associations for airflow obstruction according to history of post-TB in participants aged ≥15 years
| Non-post-TB | Post-TB | P value | |
| Overall subjects | |||
| Airflow obstruction | 1230 | 130 | |
| Crude OR (95% CI) | 1.00 (ref) | 1.50 (1.22 to 1.83) | <0.001 |
| Adjusted OR (95% CI)* | 1.00 (ref) | 1.31 (1.05 to 1.62) | 0.013 |
| Restrictive pattern | 1374 | 99 | |
| Crude OR (95% CI) | 1.00 (ref) | 0.94 (0.75 to 1.17) | 0.613 |
| Adjusted OR (95% CI)* | 1.00 (ref) | 1.02 (0.80 to 1.28) | 0.865 |
| Small airway dysfunction† | 2993 | 297 | |
| Crude OR (95% CI) | 1.00 (ref) | 1.59 (1.35 to 1.88) | <0.001 |
| Adjusted OR (95% CI)* | 1.00 (ref) | 1.28 (1.07 to 1.53) | 0.006 |
| Never smokers | |||
| Airflow obstruction | 857 | 85 | |
| Crude OR (95% CI) | 1.00 (ref) | 1.55 (1.20 to 1.98) | <0.001 |
| Adjusted OR (95% CI)‡ | 1.00 (ref) | 1.37 (1.04 to 1.78) | 0.023 |
| Restrictive pattern | 1005 | 65 | |
| Crude OR (95% CI) | 1.00 (ref) | 0.92 (0.69 to 1.20) | 0.575 |
| Adjusted OR (95% CI)‡ | 1.00 (ref) | 0.98 (0.73 to 1.30) | 0.904 |
| Small airway dysfunction | 2143 | 205 | |
| Crude OR (95% CI) | 1.00 (ref) | 1.78 (1.45 to 2.18) | <0.001 |
| Adjusted OR (95% CI)‡ | 1.00 (ref) | 1.47 (1.18 to 1.83) | 0.001 |
*Adjusted OR: adjustments for age, sex, region and education plus history of asthma, and exposure to HAP and occupation and smoking status.
†Adjusted OR: adjusted OR except for smoking status.
‡Assessed in small airway dysfunction with data missing for 86 participants.
HAP, household air pollution; TB, tuberculosis.
Discussion
To the best of our knowledge, this is the first study with a rigorous sampling design to estimate the burden of post-TB and assess the association with chronic airflow obstruction and respiratory symptoms in China. Our findings suggest that socioeconomic status, respiratory symptoms, comorbidities and lung function parameters were worse in participants who had post-TB compared with ‘non post-TB’ participants, and even worse in those with post-TB and airflow obstruction. Our data show that 7.0% of residents had post-TB: in this group, 21.3% had airflow obstruction and 48.9% small airway dysfunction. Post-TB was associated with an increased odds of airflow obstruction and small airway dysfunction after adjustment for confounding factors. The magnitude of the OR between TB and airflow obstruction was much higher among never smokers, which suggests that post-TB may be a major risk factor per se especially among never smokers. We also filled in the age gap by providing data for subjects aged below 40 years old, since most previous reports of an association between airflow obstruction and post-TB included subjects aged over 40.6 8–10
Prior publications have reported the prevalence of post-TB sequelae, but the related burden remains undetermined.8–10 22 23 The reasons may be inconsistency in definition, heterogeneity of populations studied and a lack of adequate control for confounders. The findings from our study indicate that the burden of post-TB among residents aged over 15 years is substantial. In our study, the proportion of chronic respiratory symptoms in post-TB cases was nearly two times that of non-affected individuals: this can have a negative impact on quality of life. Subjects with Post-TB also had more chronic comorbidities. Also, all lung parameters were significantly decreased in Post-TB subjects, even when a BD was used. We confirm that up to half of Post-TB people suffer from chronic and clinically relevant pulmonary function impairment and, in a majority of patients with airflow obstruction, obstruction was moderate to severe.24–27 In most cases, pulmonary function impairment remains undiagnosed and untreated, possibly resulting in an increased mortality compared with the standard population.28 29 Currently, there is much to do regarding the long-term medical and socioeconomic consequences of chronic lung function abnormalities after post-TB.30
There were also few studies reported post-TB was associated with a higher risk of airflow obstruction. The study from 13 geographically diverse, low-resource settings recruiting participants reported the adjusted odds of 3.78 times higher among those with previous tuberculosis disease than those without a history of tuberculosis disease.6 Data from Korea National Health and Nutrition Examination Survey reported that airflow obstruction was associated with both a history and TB lesions on chest X-ray (OR 4.47, 95% CI 3.07 to 6.51) after adjustment for some confounders.7 The finding from Burden of Obstructive Lung Disease (BOLD) results showed that a self-reported history of tuberculosis was associated with airflow obstruction (adjusted OR 2.51, 95% CI 1.83 to 3.42).8 A cross-sectional analysis of the Guangzhou Biobank Cohort Study reported prior TB remained independently associated with an increased risk of airflow obstruction (OR: 1.37; 95% CI 1.13 to 1.67).9 TheLatin American Project for the Investigation of Obstructive Lung Disease (PLATINO) study found participants with a medical history of tuberculosis were 2.3 times more likely to present airflow obstruction than those without such a diagnosis.10 However, these studies included participants aged over 35 years old, incorporated no post-BD testing, used different definitions of post-TB. In the present study with a rigorous sampling design and strictly quality control, we found a nearly 1.31 time increase in risk of airflow obstruction in post-TB subjects after adjusting for confounders, and a stronger association in never smokers (OR: 1.37; 95% CI 1.04 to 1.78). Besides, airflow obstruction was present in 21.3% of post-TB cases, which are consistent with a recent meta-analysis reporting a pooled prevalence of COPD in patients with post pulmonary TB of 21% (95% CI: 16% to 25%)5 and another study from low- income and middle- income countries reporting COPD was more common (25.7%) among those with previous tuberculosis than those without a history of tuberculosis.6 Therefore, for vast majority of patients after TB, the development of adjuvant interventions to prevent or to suspend further deterioration of lung function in individuals with post-TB could be an essential tool.
Small airway dysfunction is characterised by premature airway closure and air trapping, regional heterogeneity and exaggerated volume dependence of airflow limitation. A study emphasised that small airway dysfunction preceded both the spirometric evidence of COPD and detection of emphysema by CT and considered as a precursor of COPD and asthma.31 The mechanisms are potential involvement of vasculature within the bronchovascular bundle in and around small airways after TB.32 Health professionals should possibly play a more active role in the detection of small airway dysfunction after pulmonary TB. We still found that post-TB subjects with a spirometric restrictive pattern had frequent sputum, recurrent wheezing, lower oxygen saturation and severe pulmonary lung function indices. It is proposed that a chronic inflammatory response and long-term anatomic alterations induced by pulmonary tuberculosis are the main pathological basis for airflow obstruction and restriction pattern.33–35 Chronic residual or recurrent inflammation-induced narrowing of airways, peribronchial fibrosis, extensive fibrosis and stiffening of the lung parenchyma after tuberculosis contribute to the functional findings reported.36 37 Indeed, there was an increased odds for small airway dysfunction in post-TB subjects studied.
Our study has important public health implications. Improving detection and treatment of TB should be implemented in China, particularly in areas with poor economic and health conditions. The objectives are not only effectively controlling of TB per se but also decreasing its long-term impacts on lung function, respiratory symptoms and quality of life through professional management, early pulmonary function and chest imaging. The importance of the interrelationship between post-TB and chronic airflow obstruction has been clearly illustrated in our study, especially among never smokers. Further studies are required to better determine the role of inhaled or even systemic steroids in patients with TB in whom airway obstruction is detected.
There are some limitations to our study. First, the cross-sectional design of this study cannot formally establish the temporal sequence and causal relationship between post-TB and airflow obstruction. Longitudinal studies are needed in order to determine for how long and how often lung function impairment persists after TB. Second, ATS/ERS recommendations define the presence of a restrictive pulmonary disorder as having a total lung capacity (TLC) below the fifth percentile of predicted value. However, performing plethysmography or helium dilution measurements in a large study population such as ours is unrealistic. Using FVC as a surrogate for TLC is most often accepted in studies on restrictive pulmonary disorders. Third, the FEF50% predicted value was automatically reported from our spirometer and derived from the European Community for Steel and Coal report in 1993.38 The equation is more appropriate for adults aged 18–70 years, so there may be a bias for older or younger people. Fourth, bronchiectasis has been shown to be associated with a history of TB and may be associated with small airway dysfunction.39 However, chest CT was rarely available because of our resource-limited setting. Furthermore, a history of bronchiectasis was self-reported by only 14 participants precluding any further analysis. Finally, it is important to note that our findings relate to post-TB defined by self-reported history and radiological changes on chest radiographs. The extent of TB lesions on chest radiographs could not be evaluated due to limited availability of CT scans, and the definition of radiologic evidence may lack sensitivity and underestimate post-TB status. However, in a TB-prevalent region, presence of fibrotic scars or calcified nodules in the upper lobes is usually secondary to healed TB and is, thus, considered as specific.
In conclusion, post-TB status was associated with pulmonary function impairment, including airflow obstruction and small airway dysfunction, as well as with chronic respiratory symptoms. We also found that association between post-TB and pulmonary impairment was stronger among never smokers. Strategies must be developed in terms of research, prevention, earlier detection of functional impairment and management of post-TB patients as a way of preventing chronic pulmonary sequelae and disability.
Acknowledgments
We acknowledge the following investigators for their continuous support, assistance and cooperation: Wang Miao from Beijing Anzhen Hospital, HongSheng Zhang, Xiaomeng Li, Xiaoming Tan, and Aonan Li from Beijing Hospital, Zengwu Wang, Linfeng Zhang, and Xin Wang from Fu Wai Hospital, Yundai Chen, and Bin Feng from Chinese PLA General Hospital, Sinan Wu and Wenquan Liu form China-Japan Friendship Hospital.
Footnotes
ZX and TS contributed equally.
Contributors: YG, CW and TS conceived and designed the study. YG and CW supervised the study. ZX did the statistical analysis. All authors contributed to acquisition, analysis or interpretation of data. YG and ZX drafted the manuscript. All authors revised the report and approved the final version before submission. As a guarantor, YG accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This work was supported by the Ministry of Science and Technology of China (2018YFC1315101); Beijing Hospital Clinical Research 121 Project (BJ-2018-199).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Institutional Review Board and Ethics Committee of Beijing Hospital (2013BJYYEC-042C-01). Participants gave informed consent to participate in the study before taking part.
References
- 1. World Health Organization . Global tuberculosis report 2021, 2021. [Google Scholar]
- 2. Chakaya J, Khan M, Ntoumi F, et al. Global Tuberculosis Report 2020 - Reflections on the Global TB burden, treatment and prevention efforts. Int J Infect Dis 2021;113 Suppl 1:S7–12. 10.1016/j.ijid.2021.02.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Matteis S, Jarvis D, Darnton A, et al. The occupations at increased risk of COPD: analysis of lifetime job-histories in the population-based UK Biobank cohort. Eur Respir J 2019;54:1900186. 10.1183/13993003.00186-2019 [DOI] [PubMed] [Google Scholar]
- 4. Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. The Lancet 2009;374:733–43. 10.1016/S0140-6736(09)61303-9 [DOI] [PubMed] [Google Scholar]
- 5. Fan H, Wu F, Liu J, et al. Pulmonary tuberculosis as a risk factor for chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ann Transl Med 2021;9:390–90. 10.21037/atm-20-4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamenar K, Hossen S, Gupte AN, et al. Previous tuberculosis disease as a risk factor for chronic obstructive pulmonary disease: a cross-sectional analysis of multicountry, population-based studies. Thorax 2022;77:1088–97. 10.1136/thoraxjnl-2020-216500 [DOI] [PubMed] [Google Scholar]
- 7. Choi CJ, Choi WS, Lee SY, et al. The definition of past tuberculosis affects the magnitude of association between pulmonary tuberculosis and respiratory dysfunction: Korea National health and nutrition examination survey, 2008-2012. J Korean Med Sci 2017;32:789–95. 10.3346/jkms.2017.32.5.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amaral AFS, Coton S, Kato B, et al. Tuberculosis associates with both airflow obstruction and low lung function: BOLD results. Eur Respir J 2015;46:1104–12. 10.1183/13993003.02325-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lam K-bongH, Jiang CQ, Jordan RE, et al. Prior TB, smoking, and airflow obstruction: a cross-sectional analysis of the Guangzhou Biobank cohort study. Chest 2010;137:593–600. 10.1378/chest.09-1435 [DOI] [PubMed] [Google Scholar]
- 10. Menezes AMB, Hallal PC, Perez-Padilla R, et al. Tuberculosis and airflow obstruction: evidence from the PLATINO study in Latin America. Eur Respir J 2007;30:1180–5. 10.1183/09031936.00083507 [DOI] [PubMed] [Google Scholar]
- 11. Allwood BW, Stolbrink M, Baines N, et al. Persistent chronic respiratory symptoms despite TB cure is poorly correlated with lung function. Int J Tuberc Lung Dis 2021;25:262–70. 10.5588/ijtld.20.0906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Osman M, Welte A, Dunbar R, et al. Morbidity and mortality up to 5 years post tuberculosis treatment in South Africa: a pilot study. Int J Infect Dis 2019;85:57–63. 10.1016/j.ijid.2019.05.024 [DOI] [PubMed] [Google Scholar]
- 13. Meghji J, Lesosky M, Joekes E, et al. Patient outcomes associated with post-tuberculosis lung damage in Malawi: a prospective cohort study. Thorax 2020;75:269–78. 10.1136/thoraxjnl-2019-213808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo Y, Xing Z, Shan G, et al. Prevalence and risk factors for COPD at high altitude: a large cross-sectional survey of subjects living between 2,100–4,700 M above sea level. Front Med 2020;7:1–10. 10.3389/fmed.2020.581763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller MR, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38. 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 16. Enright P. FEV1 and FVC repeatability goals when performing spirometry. Prim Care Respir J 2010;19:194–94. 10.4104/pcrj.2010.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-Ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324–43. 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the gold science Committee report 2019. Eur Respir J 2019;53:1900164. 10.1183/13993003.00164-2019 [DOI] [PubMed] [Google Scholar]
- 19. Xiao D, Chen Z, Wu S, et al. Prevalence and risk factors of small airway dysfunction, and association with smoking, in China: findings from a national cross-sectional study. Lancet Respir Med 2020;8:1081–93. 10.1016/S2213-2600(20)30155-7 [DOI] [PubMed] [Google Scholar]
- 20. Thumerelle C, Pouessel G, Errera S. Radiologic manifestations of pulmonary tuberculosis. Archives de Pédiatrie 2005;12:S132–6. [DOI] [PubMed] [Google Scholar]
- 21. State. TUSoADo . Instruction to panel for completing chest X-ray and classification worksheet (DS-3024). Available: http://wwwcdcgov/ncidod/dq/dsforms/3024htm [Accessed 26 Nov 2009].
- 22. Jung J-W, Choi J-C, Shin J-W, et al. Pulmonary impairment in tuberculosis survivors: the Korean National health and nutrition examination survey 2008-2012. PLoS One 2015;10:e0141230–12. 10.1371/journal.pone.0141230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caballero A, Torres-Duque CA, Jaramillo C, et al. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study). Chest 2008;133:343–9. 10.1378/chest.07-1361 [DOI] [PubMed] [Google Scholar]
- 24. Allwood BW, van der Zalm MM, Amaral AFS, et al. Post-tuberculosis lung health: perspectives from the first International Symposium. Int J Tuberc Lung Dis 2020;24:820–8. 10.5588/ijtld.20.0067 [DOI] [PubMed] [Google Scholar]
- 25. Ravimohan S, Kornfeld H, Weissman D, et al. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev 2018;27:170077. 10.1183/16000617.0077-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chushkin MI, Ots ON. Impaired pulmonary function after treatment for tuberculosis: the end of the disease? J Bras Pneumol 2017;43:38–43. 10.1590/s1806-37562016000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harries AD, Ade S, Burney P, et al. Successfully treated but not fit for purpose: Paying attention to chronic lung impairment after TB treatment. int j tuberc lung dis 2016;20:1010–4. 10.5588/ijtld.16.0277 [DOI] [PubMed] [Google Scholar]
- 28. Ranzani OT, Rodrigues LC, Bombarda S, et al. Long-Term survival and cause-specific mortality of patients newly diagnosed with tuberculosis in São Paulo state, Brazil, 2010-15: a population-based, longitudinal study. Lancet Infect Dis 2020;20:123–32. 10.1016/S1473-3099(19)30518-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miller TL, McNabb SJN, Hilsenrath P, et al. Personal and societal health quality lost to tuberculosis. PLoS One 2009;4:e5080–7. 10.1371/journal.pone.0005080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hanania NA, Celli BR, Donohue JF, et al. Bronchodilator reversibility in COPD. Chest 2011;140:1055–63. 10.1378/chest.10-2974 [DOI] [PubMed] [Google Scholar]
- 31. Konstantinos Katsoulis K, Kostikas K, Kontakiotis T. Techniques for assessing small airways function: possible applications in asthma and COPD. Respir Med 2016;119:e2–9. 10.1016/j.rmed.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 32. Allwood BW, Rigby J, Griffith-Richards S, et al. Histologically confirmed tuberculosis-associated obstructive pulmonary disease. Int J Tuberc Lung Dis 2019;23:552–4. 10.5588/ijtld.18.0722 [DOI] [PubMed] [Google Scholar]
- 33. Oh JY, Lee YS, Min KH, et al. Difference in systemic inflammation and predictors of acute exacerbation between smoking-associated COPD and tuberculosis-associated COPD. Int J Chron Obstruct Pulmon Dis 2018;13:3381–7. 10.2147/COPD.S177371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guiedem E, Ikomey GM, Nkenfou C, et al. Chronic obstructive pulmonary disease (COPD): neutrophils, macrophages and lymphocytes in patients with anterior tuberculosis compared to tobacco related COPD. BMC Res Notes 2018;11:1–5. 10.1186/s13104-018-3309-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Allwood BW, Maasdorp E, Kim GJ, et al. Transition from restrictive to obstructive lung function impairment during treatment and follow-up of active tuberculosis. Int J Chron Obstruct Pulmon Dis 2020;15:1039–47. 10.2147/COPD.S219731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jin J, Li S, Yu W, et al. Emphysema and bronchiectasis in COPD patients with previous pulmonary tuberculosis: computed tomography features and clinical implications. Int J Chron Obstruct Pulmon Dis 2018;13:375–84. 10.2147/COPD.S152447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration 2013;86:76–85. 10.1159/000350917 [DOI] [PubMed] [Google Scholar]
- 38. Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party standardization of lung function tests, European community for steel and coal. official statement of the European respiratory Society. Eur Respir J Suppl 1993;16:5–40. [PubMed] [Google Scholar]
- 39. Byrne AL, Marais BJ, Mitnick CD, et al. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis 2015;32:138–46. 10.1016/j.ijid.2014.12.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2021-218345supp001.pdf (300.6KB, pdf)
Data Availability Statement
No data are available.