Abstract
Background/Aims
To provide longer-term data on efficacy, safety, immunogenicity and pharmacokinetics (PK) of ranibizumab biosimilar SB11 compared with the reference ranibizumab (RBZ) in patients with neovascular age-related macular degeneration (nAMD).
Methods
Setting: Multicentre. Design: Randomised, double-masked, parallel-group, phase III equivalence study. Patient population: ≥50 years old participants with nAMD (n=705), one ‘study eye’. Intervention: 1:1 randomisation to monthly intravitreal injection of 0.5 mg SB11 or RBZ. Main outcome measures: Visual efficacy endpoints, safety, immunogenicity and PK up to 52 weeks.
Results
Baseline and disease characteristics were comparable between treatment groups. Of 705 randomised participants (SB11: n=351; RBZ: n=354), 634 participants (89.9%; SB11: n=307; RBZ: n=327) completed the study until week 52. Previously reported equivalence in primary efficacy remained stable up to week 52 and were comparable between SB11 and RBZ. The adjusted treatment difference between SB11 and RBZ in full analysis set at week 52 of change from baseline in best-corrected visual acuity was −0.6 letters (90% CI −2.1 to 0.9) and of change from baseline in central subfield thickness was −14.9 µm (95% CI –25.3 to –4.5). The incidence of ocular treatment-emergent adverse events (TEAEs) (SB11: 32.0% vs RBZ: 29.7%) and serious ocular TEAE (SB11: 2.9% vs RBZ: 2.3%) appeared comparable between treatment groups, and no new safety concerns were observed. The PK and immunogenicity profiles were comparable, with a 4.2% and 5.5% cumulative incidence of antidrug antibodies up to week 52 for SB11 and RBZ, respectively.
Conclusions
Longer-term results of this study further support the biosimilarity established between SB11 and RBZ.
Keywords: retina, neovascularisation, macula, degeneration
Introduction
Neovascular age-related macular degeneration (nAMD) is a leading cause of visual impairment and blindness for people over 50 years of age in many parts of the world. Loss of vision can result in reduced participation in daily activities and increased need to rehabilitative facilities, which can lead to loss in vision-related quality of life or depression, and is associated with a total annual direct cost of more than 575 million dollars.1–3 Repeated intravitreal injections (IVI) of vascular endothelial growth factor-A (VEGF-A) inhibitors such as aflibercept, brolucizumab or ranibizumab (RBZ) are current standard treatments for nAMD.2 4–9 Given the substantial economic burden of these therapies, the introduction of biosimilars could reduce the cost of nAMD treatment and expand patient’s access to anti-VEGF treatments, similarly to the situation following the introduction of antitumour necrosis factor alpha10–12 or other biosimilars13 for various indications. Therefore, the availability of biosimilars could reduce the socioeconomic burden of blindness caused by nAMD.
Biosimilars are inherently different from chemically synthesised generic drugs as biosimilars are derived from living cells and, therefore, typically have bigger molecular size and more complex structures than generic drugs.14 Nevertheless, biosimilars also should be high-quality products that are manufactured through stringently controlled biotechnology processes in facilities that follow Good Manufacturing Practices guidelines. Both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approve biosimilars based on the totality of evidence of a comprehensive comparability exercise that demonstrates that there are no clinically meaningful differences between the biosimilar and the reference product in terms of quality characteristics, biological activity, clinical safety and efficacy.15–19 Therefore, biosimilars may have substantially higher research and development risks and cost and be more complex to manufacture than small molecule generic drugs.20 The development of biosimilars costs over 100 times more expensive and it usually takes 3–4 times longer than generic drugs.20
SB11 is a FDA and EMA approved RBZ biosimilar which demonstrated similarity to the reference product in analytical and non-clinical studies. In a phase III randomised clinical trial in patients with nAMD, equivalence in terms of efficacy has been demonstrated for the primary efficacy endpoints of change from baseline of optical coherence tomography (OCT) central subfield thickness (CST) at week 4 and best-corrected visual acuity (BCVA) at week 8, with additional safety data provided through the 6-month visit.21 This report describes the results through the preplanned 52-week final visit including efficacy, safety, immunogenicity and pharmacokinetics (PK).
Methods
Study design
This randomised, double-masked, parallel-group, multicentre, 52-week phase III clinical trial, registered in clinicaltrials.gov (NCT03150589) and EudraCT (2017-000422-36), evaluated the efficacy and safety of SB11 in comparison with monthly RBZ for treatment of nAMD. The trial was conducted in 75 centres in 9 countries and the trial protocol (SB11-G31-AMD) was reviewed and approved by an Independent Ethics Committee or Institutional Review Board.21 The study adhered to the tenets of Declaration of Helsinki, Good Clinical Practice guidelines and International Council for Harmonization. All patients provided written informed consent prior to entering the study.
The full list of inclusion and exclusion criteria for study participants was previously reported.21 In brief, participants were included if they were ≥50 years of age; had a previously untreated subfoveal choroidal neovascularisation (CNV) lesion secondary to nAMD in the study eye with evidence of activity as documented on OCT by the presence of subretinal fluid or intraretinal fluid and leakage from CNV detected by fluorescein angiography (FA). At baseline visit (day 1), participants were randomised in a 1:1 ratio (randomisation blocks of fixed size=4) by the Interactive Web Response System (IWRS) to receive an IVI of either 0.5 mg SB11 (Samsung Bioepis, Incheon, Republic of Korea) or 0.5 mg RBZ (Lucentis, Genentech, San Francisco, California, USA) in 0.05 mL every 4 weeks from baseline up to week 48. The study was double-masked throughout the study period except staffs from sponsor and clinical research organisation, who were designated per protocol for unmasking after the interim analysis. However, participants, investigators, and other study personnel remained masked throughout the study period. Data described were collected between March 2018 and December 2019.
Outcomes assessment
Endpoints
Details of the primary endpoints of change from baseline in BCVA at week 8 and in CST at week 4 were previously reported.21 Prespecified secondary efficacy endpoints included longer-term assessment of change from baseline in BCVA and the proportions of participants who lost fewer than 15 letters or who gained 15 or more letters in BCVA from baseline at weeks 24 and 52. Additional secondary endpoints included change from baseline in CST and in central retinal lesion thickness (CRLT) (a manual measurement of the distance between the internal limiting membrane and base of retinal pigment epithelium, inclusive of any observable CNV or scar tissue, measured at the fovea but not including pigment epithelial detachment) as well as change from baseline in total CNV size and proportion of participants with active CNV leakage on fundus photography or FA at weeks 24 and 52 based on assessment by central reading centre personnel. As a secondary outcome to confirm biosimilarity, total CNV size and total lesion size including total area of any classic or occult CNV and any features that could obscure the boundaries of classic or occult CNV was measured by FA, following previously published definitions,21 22 just as how it was done in the reference product’s pivotal studies such as MARINA23 24 and ANCHOR.25 26
Prespecified exploratory endpoints included the proportion of participants without intraretinal or subretinal fluid and the change from baseline in subscale scores and composite score of the National Eye Institute 25-Item Visual Function Questionnaire (NEI VFQ-25) at weeks 24 and 52.
Safety
Safety evaluation at each visit included collection of ocular and non-ocular adverse events (AEs) and serious adverse events (SAEs) through physical and full ophthalmic examinations (including but not limited to external examination of the eye, slit lamp examination, intraocular pressure(IOP) measurements, routine screening for eyelid/pupil and so on). All AEs were recorded from the time the participant signed the written informed consent until week 52 or an earlier termination visit. The AEs that emerged during the treatment with an investigational product (treatment-emergent adverse events (TEAEs)) were analysed for the purpose of safety analyses, accounting for its severity and causality. Adverse events of special interest (AESIs) also were collected up to week 52 using classification criteria previously reported.21
Immunogenicity
Immunogenicity analyses were performed in all participants on blood samples collected prior to IVI injection of the study drug at weeks 0, 4, 8, 16, 24, 36 and at any time during the visit at weeks 1 and 52 or early termination visit, using same immunoassays as previously reported.21 If a participant had an unresolved AE that was possibly related to antidrug antibodies (ADAs), the participant was asked to return for blood sampling until antibody titres returned to baseline or stabilised to an acceptable level. Overall ADA results were determined as positive for a participant with treatment-induced or treatment-boosted ADAs, where treatment-induced ADAs indicates at least one positive result after predose of week 0 for participants with negative ADAs at predose of week 0, and treatment-boosted ADAs indicates at least one positive result with higher titre level compared with titre at week 0 for participants with positive ADAs at predose of week 0.
Pharmacokinetics
Serum samples for the measurements of drug concentration were taken predose (trough serum concentration (Ctrough)) and 24–72 hours postdose (close to maximum serum concentration (Cmax)) at weeks 0, 4, 8, 16, 24, 36 and at any time during the visit at weeks 1 and 52. If the fellow eye received RBZ due to nAMD during the study period, all concentrations measured after fellow eye treatment were excluded from summary statistics.
Statistical analysis
Sample size
For the primary endpoint of change from baseline in BCVA, a sample size of 352 participants per treatment group was required to achieve an overall 10% significance level and 80% power to establish equivalence. The calculation was based on the predefined equivalence margin of −3 and 3 letters,21 and an assumed 5% loss of randomised participants.
For the primary endpoint of change from baseline in CST, a sample size of 323 participants per treatment group was required to achieve an overall 5% significance level and 80% power to establish equivalence. The calculation was based on historical data from the MARINA23 and PIER27 studies, the predefined equivalence margin of −36 and 36 µm,21 and an assumed 10% loss from the full analysis set (FAS). Therefore, an overall sample size of 704 was chosen to allow for enough power to detect equivalence with both primary endpoints.
Analysis sets
The FAS included all randomised participants, excluding one inadvertently randomised participant who did not receive the study drug. The safety set (SAF) consisted of all participants who received at least one study drug administration during the study period after randomisation. The PK analysis set (PKS) included participants who had at least one PK sample analysed.
Efficacy endpoints analysis
Details on primary endpoint analysis have been reported previously.21
Secondary endpoint analysis of changes from baseline in BCVA and in CST in the FAS at week 52 was performed using analysis of covariance (ANCOVA) model with the baseline BCVA or CST as a covariate and region (country) and treatment group as factors. For the analysis of proportion of participants who lost fewer than 15 letters or gained 15 letters or more compared with baseline in BCVA, and for the proportion of participants with active CNV leakage in the FAS, the adjusted risk difference between the two treatment groups was calculated using a Cochran-Mantel-Haenszel test with 95% CIs with stratification by region (country). For the analysis of change from baseline in CRLT and CNV size in the FAS, inferential statistics were based on ANCOVA model with the baseline CRLT or CNV size as a covariate and region (country) and treatment as fixed factors, respectively. All secondary endpoint analyses were performed based on available data; no missing data were imputed.
Exploratory efficacy and other endpoints analysis
For the exploratory endpoints, the proportion of participants without the presence of intraretinal or subretinal fluid in the FAS was evaluated using OCT and was summarised by treatment group and visit. The change from baseline in subscale and composite scores of NEI VFQ-25 were calculated in the FAS and was summarised by treatment groups and visits, excluding those participants who received RBZ in the fellow eye due to nAMD during the study period after randomisation. Safety, immunogenicity and PK outcomes were summarised descriptively by treatment groups and time points.
Results
Participant disposition, demographics and baseline characteristics
Among 1095 participants screened from March 2018 to November 2018, 705 participants were randomised to receive either SB11 (N=351) or RBZ (N=354). One ineligible participant was randomised to SB11 group by error of the study coordinator inadvertently clicking the IWRS randomisation button and was subsequently discontinued before administering the study drug. Accordingly, 704 participants received at least one IVI of SB11 (N=350) or RBZ (N=354). A total of 634 (89.9%) participants completed the 52 weeks of the study (SB11: n=307; RBZ: n=327); for those who did not complete the study, reasons for discontinuation are indicated (figure 1). Baseline demographic and disease characteristics appeared comparable between the treatment groups.21
Figure 1.
CONSORT diagram of participants’ flow through the trial. (A) One participant was excluded from both full analysis set and safety set, because this participant was misrandomised and discontinued from the study before first dosing. (B) One participant was initially randomised to receive SB11 but incorrectly received the injection to the fellow eye, while RBZ was injected to the study eye until week 20 (Study day 141) of the study. This participant was discontinued from the study (Study day 164) primarily due to protocol deviation but later included in the RBZ treatment group in the safety set. IP, investigational product; RBZ, reference ranibizumab.
Efficacy
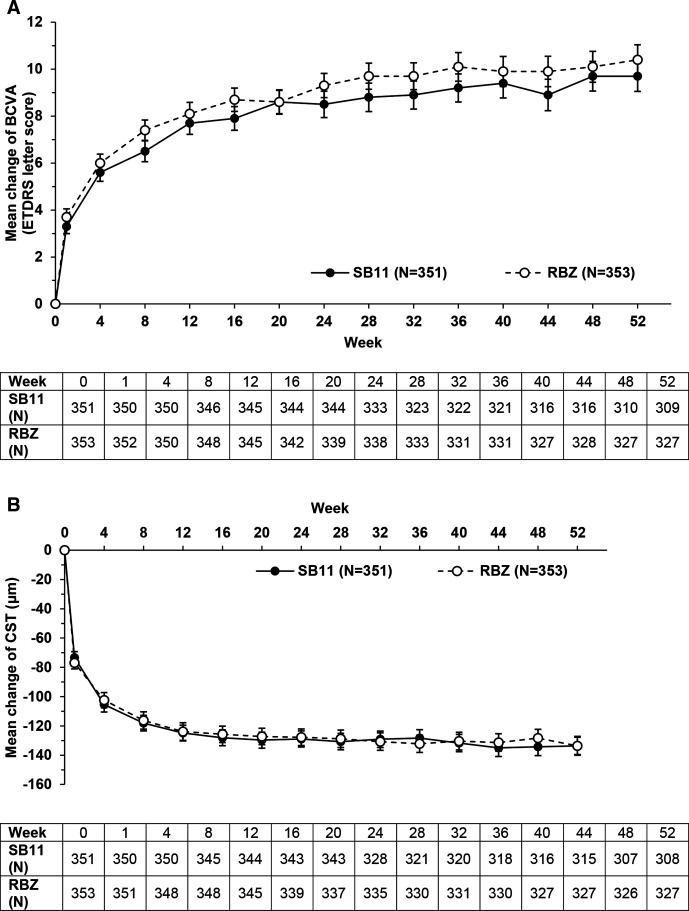
Results of the two primary efficacy endpoint analyses were previously reported.21 Secondary efficacy endpoints were evaluated in the interim analysis at week 2421 and at the final analysis at week 52. Final study results for change from baseline in BCVA and in CST in the FAS showed that the previously reported improvements in the primary efficacy outcomes remained stable and appeared comparable between treatment groups at all time points up to week 52. Specifically, change from baseline in BCVA was 9.8 letters for SB11 and 10.4 letters for RBZ at week 52 (adjusted treatment difference (SE): −0.6 (0.9); 90% CI −2.1 to –0.9) (figure 2A, table 1, online supplemental table 1). Change from baseline in CST was −140.0 µm for SB11 and −125.1 µm for RBZ at week 52 (adjusted treatment difference (SE): −14.9 (5.3); 95% CI –25.3 to –4.5) (figure 2B, table 1, online supplemental table 2).
Figure 2.
(A) Change from baseline in BCVA at each timepoint through week 52 in the full analysis set. Mean change±SE from baseline through week 52 in BCVA in the full analysis set. The table reports the number of participants per timepoint. The complete list of values and participants per timepoint is reported in online supplemental table 1. Mean (SD) change from baseline in BCVA for participants who completed week 52 of the study: SB11 (n=309), 9.7 (11.4) letters; RBZ (n=327), 10.4 (11.5) letters. Circles represent mean and error bars represent SE at each timepoint. (B) Change from baseline in CST at each timepoint through week 52 in the full analysis set. Mean change±SE from baseline through week 52 in CST in the FAS. The table reports the number of participants per timepoint. The complete list of values and participants per timepoint is reported in online supplemental table 2. Mean (SD) change from baseline in CST for participants who completed week 52 of the study: SB11 (n=308), –133.6 (103.9) μm; RBZ (n=327), –128.4 (116.1) μm. Circles represent mean and error bars represent SE at each timepoint. BCVA, best-corrected visual acuity (ETDRS letter score); CST, central subfield thickness; ETDRS, early treatment diabetic retinopathy study; RBZ, reference ranibizumab.
Table 1.
Secondary efficacy endpoints measurements at week 52
| Endpoint at week 52 (analysis set) |
Treatment | n | Change from baseline, least square mean (SE) | Difference (SB11-RBZ) |
||
| Mean | SE | 95% CI (90% CI for BCVA) |
||||
| BCVA (letters)* (FAS) |
SB11 (N=351) | 309 | 9.8 (0.8) | −0.6 | 0.9 | −2.1 to 0.9 |
| RBZ (N=353) | 327 | 10.4 (0.7) | ||||
| CST (μm)† (FAS) |
SB11 (N=351) | 308 | −140.0 (4.5) | −14.9 | 5.3 | –25.3 to –4.5 |
| RBZ (N=353) | 327 | −125.1 (4.3) | ||||
| CRLT (μm)‡ (FAS) |
SB11 (N=351) | 308 | −161 (5.1) | −11.5 | 6.0 | −23.2 to 0.1 |
| RBZ (N=353) | 327 | −149.5 (4.9) | ||||
| CNV size (mm2)§ (FAS) |
SB11 (N=351) | 303 | −5.2 (0.3) | −0.6 | 0.3 | −1.2 to 0.1 |
| RBZ (N=353) | 313 | −4.6 (0.3) | ||||
| Endpoint at week 52 (analysis set) |
Treatment | n’ | Responders, n (%) | Adjusted difference (SB11-RBZ) |
|
| Adjusted difference (%) | 95% CI | ||||
| Participants who lost <15 letters in BCVA compared with baseline¶ (FAS) |
SB11 (N=351) | 309 | 299 (96.8) | −1.2 | −3.8 to 1.3 |
| RBZ (N=353) | 327 | 320 (97.9) | |||
| Participants who gained ≥15 letters in BCVA compared with baseline¶ (FAS) |
SB11 (N=351) | 309 | 107 (34.6) | −3.2 | −10.5 to 4.2 |
| RBZ (N=353) | 327 | 123 (37.6) | |||
| Participants with active CNV leakage¶ (FAS) |
SB11 (N=351) | 303 | 158 (52.1) | −7.4 | −15.0 to 0.2 |
| RBZ (N=353) | 313 | 185 (59.1) | |||
*Inferential statistics were based on analysis of covariance model with the baseline BCVA as a covariate and region (country) and treatment as fixed factors.
†Inferential statistics were based on analysis of covariance model with the baseline CST as a covariate and region (country) and treatment as fixed factors.
‡Inferential statistics were based on analysis of covariance model with the baseline CRLT as a covariate and region (country) and treatment as fixed factors.
§Inferential statistics were based on analysis of covariance model with the baseline total CNV size as a covariate and region (country) and treatment group as fixed factors.
¶The adjusted difference and its 95% CI were analysed by a stratified Cochran-Mantel-Haenszel test with region (country) as a factor.
BCVA, best-corrected visual acuity (letter score); CNV, choroidal neovascularisation; CRLT, central retinal lesion thickness; CST, central subfield thickness; FAS, full analysis set; n, number of participants with available data at week 52; N, total number of participants; n’, number of participants with available assessment results at week 52 (percentages are based on n’); RBZ, reference ranibizumab.
bjophthalmol-2021-319637supp001.pdf (491.8KB, pdf)
The proportions of participants who lost fewer than 15 letters in BCVA (table 1, (online supplemental figure 1A) and gained 15 or more letters in BCVA (table 1, (online supplemental figure 1B) as well as the change from baseline in CRLT, CNV size and the proportion of participants with active CNV leakage in the FAS (table 1, online supplemental figure 1C) were maintained through week 52, with further improvements from the week 24 results,21 and were comparable between treatment groups at all timepoints. The analysis of exploratory endpoints showed that the proportion of participants without intraretinal or subretinal fluid in the FAS increased over time (online supplemental figure 2, online supplemental table 3) and were comparable between the treatment groups at week 52 (SB11: 84.4%; RBZ: 81.0%). Quality of life as assessed by NEI VFQ-25 score also improved over time, with mean change from baseline to week 52 (SB11: 4.54; RBZ: 6.47) appearing comparable between treatment groups (online supplemental table 4).
Safety
Exposure was similar between the SB11 (N=350) and RBZ (N=354) groups, with a mean number of study drug administrations (SD) of 12.2 (2.2) vs 12.4 (2.1) and a median duration of study drug exposure (min, max) of 337 days (1, 358) vs 337 days (1, 361), respectively. The incidence of AEs, including TEAEs, SAEs and TEAEs leading to study drug discontinuation and death was comparable between treatment groups, with most TEAEs of mild or moderate intensity and not related to the study drug (table 2). The incidence of ocular TEAEs in the study eye that led to study drug discontinuation was similar between two groups (SB11: n=7; 2.0% vs RBZ: n=4; 1.1%). Although there was a small numerical difference, there was no statistical or clinically relevant difference between the two groups identified (Difference (SE): −0.009 (0.009); 90% CI −0.027 to 0.009; p=0.328). There were no myocardial infarction or stroke events. There was one study participant with death of unknown cause in the SB11 treatment group and two participants with death of unknown cause in the RBZ treatment group. Ocular TEAEs in the study eye which occurred in ≥5% of participants were ‘Intraocular pressure increased’ (SB11: n=23; 6.6% vs RBZ: n=26; 7.3%) and ‘Conjunctival haemorrhage’ (SB11: n=16; 4.6% vs RBZ: n=18; 5.1%). No definitive differences in the overall incidence of AESIs between treatment groups were identified. ‘Intraocular pressure increase’ occurred in 3 (0.9%) and 6 (1.7%) participants, while death was reported for 1 (0.3%) and 2 (0.6%) participants within the SB11 and RBZ groups, respectively. Furthermore, 2 (0.6%) and 4 (1.1%) participants in the SB11 group and none in the RBZ group experienced ‘Endophthalmitis’ or ‘Eye disorders’ which included iridocyclitis (n=3; 0.9%), uveitis (n=1; 0.3%) or vitritis (n=1; 0.3%). The number of participants with cells and flare in the anterior chamber and vitreous cells were low with no notable difference between treatment groups.
Table 2.
Summary of all adverse events up to week 52 in the safety set
| SB11 (N=350) n (%) |
RBZ (N=354) n (%) |
|
| TEAEs | ||
| Any TEAE | 255 (72.9) | 256 (72.3) |
| Ocular TEAEs in the study eye | 112 (32.0) | 105 (29.7) |
| Ocular TEAEs in the fellow eye | 92 (26.3) | 77 (21.8) |
| Non-ocular TEAEs | 194 (55.4) | 205 (57.9) |
| Serious TEAE | 50 (14.3) | 51 (14.4) |
| TEAEs by severity | ||
| Mild TEAEs | 117 (33.4) | 122 (34.5) |
| Moderate TEAEs | 106 (30.3) | 107 (30.2) |
| Severe TEAEs | 32 (9.1) | 27 (7.6) |
| TEAEs by relatedness | ||
| Related TEAEs | 21 (6.0) | 10 (2.8) |
| Not related TEAEs | 234 (66.9) | 246 (69.5) |
| SAEs (by relatedness) | ||
| Any SAE | 52 (14.9) | 52 (14.7) |
| Related SAEs | 6 (1.7) | 3 (0.8) |
| Not related SAEs | 46 (13.1) | 49 (13.8) |
| Serious ocular AE in the study eye (by preferred term) | ||
| Any ocular SAE in the study eye | 10 (2.9) | 8 (2.3) |
| Visual acuity reduced | 2 (0.6) | 1 (0.3) |
| Endophthalmitis | 2 (0.6) | 0 (0.0) |
| Cataract | 2 (0.6) | 0 (0.0) |
| Iridocyclitis | 1 (0.3) | 0 (0.0) |
| Macular oedema | 1 (0.3) | 1 (0.3) |
| Retinal haemorrhage | 1 (0.3) | 1 (0.3) |
| Retinal pigment epithelial tear | 1 (0.3) | 0 (0.0) |
| Subretinal fluid | 1 (0.3) | 1 (0.3) |
| Uveitis | 1 (0.3) | 0 (0.0) |
| Vitritis | 1 (0.3) | 0 (0.0) |
| Cataract subcapsular | 0 (0.0) | 1 (0.3) |
| Macular degeneration | 0 (0.0) | 2 (0.6) |
| Retinal artery occlusion | 0 (0.0) | 1 (0.3) |
| Serious ocular AE in the fellow eye (by preferred term) | ||
| Any ocular SAE in the fellow eye | 3 (0.9) | 2 (0.6) |
| Retinal haemorrhage | 2 (0.6) | 0 (0.0) |
| Age-related macular degeneration | 1 (0.3) | 0 (0.0) |
| Vitreous haemorrhage | 1 (0.3) | 0 (0.0) |
| Choroidal neovascularisation | 0 (0.0) | 1 (0.3) |
| Retinal artery occlusion | 0 (0.0) | 1 (0.3) |
| Serious non-ocular AE (Occurrence ≥0.5% in either treatment group) (by preferred term) | ||
| Any non-ocular SAE | 41 (11.7) | 42 (11.9) |
| Atrial fibrillation | 4 (1.1) | 3 (0.8) |
| Cardiac failure congestive | 2 (0.6) | 2 (0.6) |
| Pancreatitis acute | 0 (0.0) | 2 (0.6) |
| Cystitis | 0 (0.0) | 2 (0.6) |
| Femoral neck fracture | 1 (0.3) | 2 (0.6) |
| Acute kidney injury | 3 (0.9) | 1 (0.3) |
| Chronic obstructive pulmonary disease | 2 (0.6) | 0 (0.0) |
| Hypertension | 3 (0.9) | 0 (0.0) |
| AESI* | 8 (2.3) | 8 (2.3) |
| TEAEs leading to IP discontinuation | ||
| Any TEAEs leading to IP discontinuation | 9 (2.6) | 5 (1.4) |
| Ocular TEAEs in the study eye leading to IP discontinuation | 7 (2.0) | 4 (1.1) |
| Ocular TEAEs in the fellow eye leading to IP discontinuation | 0 (0.0) | 0 (0.0) |
| Non-ocular TEAEs leading to IP discontinuation | 2 (0.6) | 1 (0.3) |
| Deaths | 2 (0.6) | 4 (1.1) |
Percentages are based on the number of participants in the safety set.
Adverse events were coded to System Organ Class and preferred term using medical dictionary for regulatory activities (MedDRA) coding dictionary Version 20.1.
If a participant had multiple events with different severity (or causality), then the participant was counted only once at the worst severity (or worst causality, ie, related) for the number of participants (N).
*Adverse events of special interest were collected using six different categories: category 1, any case of new onset intraocular pressure of >21 mm Hg that does not respond to treatment, except the transient pressure rise observed within an hour after intravitreal injection of IP; category 2, any case of intraocular pressure ≥35 mm Hg, at any time, that required treatment; category 3, any case of intraocular infection such as endophthalmitis; category 4, any case of intraocular inflammation such as iritis, vitritis and iridocyclitis; category 5, Iatrogenic traumatic cataract; category 6, arterial thromboembolic events defined as non-fatal stroke, non-fatal myocardial infarction or vascular death (including deaths of unknown as cause).
AE, adverse event; AESI, adverse event of special interest; IP, investigational product; n, number of participants with event; N, total number of participants; RBZ, reference ranibizumab; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
Immunogenicity and pharmacokinetics
Immunogenicity analyses were performed in the SAF, including total of 330 participants treated with SB11 and 327 participants treated with RBZ with available overall ADAs results. The overall cumulative incidence of ADAs up to week 52 was low (SB11: 14/330; 4.2% vs RBZ: 18/327; 5.5%), and most antibodies were non-neutralising (table 3). No statistical or clinically relevant differences in the incidence of ADAs and neutralising antibodies (NAbs) were observed between treatment groups at any of the timepoints (table 3). The incidence of TEAEs by overall ADAs result up to week 52 appeared comparable between two treatment groups (ADAs positive subgroup: SB11: 78.6%, RBZ: 72.2%; ADAs negative subgroup: SB11: 72.1%, RBZ: 72.4%).
Table 3.
Incidence of ADA and NAbs at weeks 24–52 in the safety set
| Timepoint | Parameter | Result | SB11 (n=350) n/n’ (%) |
RBZ (n=354) n/n’ (%) |
P value* |
| Overall cumulative incidence of ADA | |||||
| Up to week 52 |
ADA | Positive | 14/330 (4.2) | 18/327 (5.5) | – |
| Negative | 312/330 (94.5) | 308/327 (94.2) | – | ||
| Inconclusive† | 4/330 (1.2) | 1/327 (0.3) | – | ||
| Incidence of ADA and NAb at each visit | |||||
| Week 24 | ADA | Positive | 7/294 (2.4) | 2/290 (0.7) | 0.18‡ |
| NAb | Positive | 0/7 (0.0) | 1/2 (50.0) | – | |
| Week 36 | ADA | Positive | 8/270 (3.0) | 5/274 (1.8) | 0.38§ |
| NAb | Positive | 2/8 (25.0) | 0/5 (0.0) | – | |
| Week 52 | ADA | Positive | 9/257 (3.5) | 12/267 (4.5) | 0.56§ |
| NAb | Positive | 1/9 (11.1) | 0/12 (0.0) | – | |
*P values calculated by using χ² or Fisher’s exact test to compare the distribution of positivity between treatment groups.
†The result was considered inconclusive in cases of positive ADA result at baseline and either negative ADA result or positive ADA result with equal or lower titre at the subsequent timepoint.
‡Two-sided p value was estimated based on Fisher’s exact test, if expected frequency in one cell was less than 5.
§Two-sided p value was estimated based on χ² test, if expected frequency in all cells was greater or equal to 5.
ADA, antidrug antibody; N, total number of participants; n, number of participants with the specified assessment result; n’, number of participants with available assessment results at each visit (percentages are based on n’); NA, not available; NAb, neutralising antibody; RBZ, reference ranibizumab.
The overall cumulative incidence of ADA results was determined as positive for a participant with treatment-induced or treatment-boosted ADA up to the relevant timepoint, that is, at least one positive result for participants with negative ADA at baseline or at least one positive result with higher titre compared with baseline.
PK analyses were performed on 54 participants in the PKS (SB11: n=25; RBZ: n=29). The mean serum concentrations appeared comparable between SB11 and RBZ up to week 52 (online supplemental figure 3, online supplemental table 5). Only three participants in the PKS had positive ADA results; therefore, the impact of immunogenicity on PK could not be assessed confidently. Only one of these three cases in the RBZ group was ADAs positive at week 36, and serum concentration at week 36 was similar to concentrations measured at other time points. The other two cases in SB11 group were ADAs positive at week 52 only, so no PK data were available for comparison with earlier visits.
Discussion
To our knowledge, this is the first manuscript that has reported Phase III, week 52 follow-up data of ranibizumab biosimilar for the treatment of nAMD. This week 52 analysis of the phase III study shows that SB11 and its reference RBZ have comparable efficacy, safety, immunogenicity and PK profiles up to week 52. These data demonstrate no clinically meaningful differences between SB11 and RBZ. The similarity of all primary and secondary efficacy endpoints was maintained at all timepoints up to week 52, confirming the comparable longer-term efficacy of SB11 and RBZ. Moreover, final 52-week results remained in line with results from previously reported studies with RBZ for nAMD, including MARINA,23 ANCHOR,25 CATT28 and HARBOR.29
The safety profile of SB11 appears to be consistent with the known RBZ profile; no new safety concerns were identified during the study period. TEAEs were mostly mild or moderate in intensity, and the majority of events was not related to the study treatment. There was no difference in incidence of ocular TEAEs in the study eye identified that led to study drug discontinuation, although there was a small numerical, but not a statistically or clinically relevant difference. A much larger number of study participants from a much larger case series would be needed to determine if these small numerical differences were due to chance or truly represented a small difference in ocular TEAEs. The incidence of intraocular inflammation (iridocyclitis, uveitis and vitritis) observed with SB11 was low compared with that reported for RBZ,23 29–31 although it was higher numerically in the SB11 group compared with the RBZ group.
The incidence of ADAs and NAbs with SB11 is comparable with RBZ, and the cumulative incidence of ADAs at week 52 is within the known 1%–9% range for RBZ.30 Much larger studies would be needed to determine if these small numerical differences truly represented small differences in immunogenicity results. PK analyses showed comparable PK profiles and furthermore, systemic concentrations of both investigational drugs up to 52 weeks are below the 11–27 ng/mL RBZ concentration range necessary to inhibit the biological activity of VEGF-A by 50%, thus limiting the potential for unintended effects due to systemic VEGF-A inhibition.31 Additionally, absorption of IVI anti-VEGF product into systemic circulation is very limited and sampling of vitreous fluid was not planned for this study, indicating that systemic PK data have limitations in their interpretation and were evaluated in this study primarily to support the overall assessment of the safety profiles of two products.32
Experience has shown that biosimilars in other fields of medicine can contribute to reducing healthcare costs and improve patient’s access to therapies with approved labels.10–13 33 34 Future research is warranted to investigate the contribution of RBZ biosimilars to reducing undertreatment in nAMD35–39 and improving the visual outcomes in the clinical practice setting.
SB11 is a ranibizumab biosimilar approved by FDA and EMA for nAMD, retinal vein occlusion and myopic choroidal neovascularization.17 An economical component may be associated not only with the selection of a drug, but also the initiation and duration of an effective treatment. With SB11 and a number of other biosimilars under development, the availability of biosimilars may have a major role in ophthalmology,40 like the introduction of biosimilars (eg, SB2 as a biosimilar of infliximab) in other therapeutic areas.10–13 In summary, the longer-term results of SB11 support the previously reported efficacy, safety, immunogenicity and PK compared with RBZ in participants with nAMD, supporting its use as a safe and effective RBZ biosimilar.
Acknowledgments
Medical writing support was provided by Daniela Kenzelmann Broz and Suzanne Einmahl (SFL Regulatory Affairs & Scientific Communications, Basel, Switzerland) and funded by Samsung Bioepis Co., Ltd., Incheon, Republic of Korea.
Footnotes
Presented at: Meeting presentations: Bressler NM, Woo SJ, et al. Phase III RCT comparing SB11 (ranibizumab biosimilar) with ranibizumab in neovascular AMD (nAMD): 1-year results. American Academy of Ophthalmology 2020. Abstract # 30064285 (Poster on demand #PO393).
Contributors: NMB: conceptualisation, methodology, writing—original draft, writing—review and editing, project administration, supervision. MV: conceptualisation, investigation, writing—review and editing, resources, validation, funding acquisition. JH: conceptualisation, investigation, writing—review and editing, resources, validation, funding acquisition. JE: conceptualisation, investigation, writing—review and editing, resources, validation, funding acquisition. DZ: conceptualisation, investigation, writing—review and editing, resources, validation, funding acquisition. JS: conceptualisation, investigation, writing—review and editing, resources, validation, funding acquisition. AV: conceptualisation, investigation, writing—review and editing, resources, validation, funding acquisition. AP: conceptualisation, investigation, writing—review and editing, resources, validation, funding acquisition. GV: conceptualisation, investigation, writing—review and editing, resources, validation, funding acquisition. JL: conceptualisation, investigation, writing—review and editing, resources, validation, funding acquisition. VM: conceptualisation, investigation, writing—review and editing, resources, validation, funding acquisition. YHY: conceptualisation, investigation, writing—review and editing, resources, validation. TP: conceptualisation, investigation, writing—review and editing, resources, validation, funding acquisition. TK: conceptualisation, formal analysis, validation, writing—review and editing, data curation. DS: conceptualisation, methodology, writing—review and editing, project administration, supervision. IO: conceptualisation, methodology, writing—original draft, writing—review and editing, project administration. HJ: methodology, writing—review and editing, resources, validation. MYK: conceptualisation, writing—original draft, writing—review and editing, validation, visualisation, project administration. SJW: conceptualisation, methodology, investigation, writing—original draft, writing—review and editing, funding acquisition, project administration, supervision.
Funding: Planning, conduct and analysis of the study was funded by Samsung Bioepis Co., Ltd., Incheon, Republic of Korea.
Competing interests: NB reported receiving grants from Samsung Bioepis to Johns Hopkins University during the conduct of the study and receiving grants from Bayer, Biogen, F. Hoffman-LaRoche, Novartis, Regeneron outside the submitted work. JS is a consultant for Bayer and Zeiss and received lecture fee from Bayer. AV received grants from Novartis, Bayer, Opthtotec/Iveric Bio, Samsung Bioepis, Amgen, Qilu, Chengdu Kanghong, Roche, Mylan, Receptos, Shire, Panoptica, Xbrain, Formycon, Genentech, Bioeq, Allergan, Thrombogenics, Regeneron, Alcon and Clearside Biomedical and is a consultant for and an advisory board member of Novartis, Bayer, Allergan, Bausch & Lomb, Medicontour and Zeiss. AP is a consultant for Roche, Bayer and Novartis received travel grants from Novartis and his company has received investigator fees from Samsung Bioepis, Roche, Iveric Bio, Allergan and Chengdu Kanghong. GV is a consultant for Alcon and Novartis, received travel grants from Novartis and Medicontur and his department has been involved in the conduct of several studies sponsored by Samsung Bioepis, Allergan, Chengdu Kanghong, Xbrane Biopharma, Thrombogenics, Amgen, Qilu, F. Hoffmann-La Roche, Bayer, Ophtotec, Novartis and Regeneron. YHY is a consultant for Alcon, Allergan Bayer and Roche, is Board Member of Allergan, Bayer and Roche, received grants from Allergan, Samsung Bioepis, Bayer, Novartis and Roche and received lecture fee from Allergan, Bayer and Roche. TP received travel grants from Alcon, Novartis and Bausch & Lomb and his department has been involved in the conduct of several studies sponsored by Mylan, Samsung Bioepis, Xbrane Biopharma, Kanghong Pharmaceuticals, F. Hoffmann-La Roche, Allergan, Bayer and Ophtotec. SJW is a consultant for Samsung Bioepis, Janssen, Allergan, Novartis, Curacle, Novelty Nobility, Alteogen, Philophos, Panols Bioscience, is equity owner of Retimark and Panolos Bioscience, is Board Member of Novartis and Novelty Nobility, received grants from Samsung Bioepis, Novelty Nobility, Novartis, Abbvie, Alteogen and Curacle and received lecture fee from Novartis, Bayer, Allergan, Abbvie, Alcon and Taejoon. Inkyung Oh, Hansol Jeong and Mercy Yeeun Kim are employees of Samsung Bioepis.
Provenance and peer review: Not commissioned; internally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. How to access data: To gain access, data requestors must enter into a data access agreement with Samsung Bioepis. Additional Information Who can access the data: Upon request, and subject to certain criteria, conditions and exceptions, Samsung Bioepis will provide access to individual de-identified participant data. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply. Types of analyses: The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply. Mechanisms of data availability: Data may be made available with a signed data access agreement.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Friedman DS, O'Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564–72. 10.1001/archopht.122.4.564 [DOI] [PubMed] [Google Scholar]
- 2. Mitchell P, Liew G, Gopinath B, et al. Age-Related macular degeneration. Lancet 2018;392:1147–59. 10.1016/S0140-6736(18)31550-2 [DOI] [PubMed] [Google Scholar]
- 3. Lim LS, Mitchell P, Seddon JM, et al. Age-Related macular degeneration. The Lancet 2012;379:1728–38. 10.1016/S0140-6736(12)60282-7 [DOI] [PubMed] [Google Scholar]
- 4. Agarwal A, Aggarwal K, Gupta V. Management of neovascular age-related macular degeneration: a review on landmark randomized controlled trials. Middle East Afr J Ophthalmol 2016;23:27–37. 10.4103/0974-9233.173133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bakri SJ, Thorne JE, Ho AC, et al. Safety and efficacy of anti-vascular endothelial growth factor therapies for neovascular age-related macular degeneration: a report by the American Academy of ophthalmology. Ophthalmology 2019;126:55–63. 10.1016/j.ophtha.2018.07.028 [DOI] [PubMed] [Google Scholar]
- 6. Grisanti S, Tatar O. The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog Retin Eye Res 2008;27:372–90. 10.1016/j.preteyeres.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 7. Hussain RM, Ciulla TA. Emerging vascular endothelial growth factor antagonists to treat neovascular age-related macular degeneration. Expert Opin Emerg Drugs 2017;22:235–46. 10.1080/14728214.2017.1362390 [DOI] [PubMed] [Google Scholar]
- 8. Sacconi R, Giuffrè C, Corbelli E, et al. Emerging therapies in the management of macular edema: a review. F1000Res 2019;8. 10.12688/f1000research.19198.1. [Epub ahead of print: 12 08 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shao J, Choudhary MM, Schachat AP. Neovascular age-related macular degeneration. Dev Ophthalmol 2016;55:125–36. 10.1159/000438969 [DOI] [PubMed] [Google Scholar]
- 10. Jensen TB, Kim SC, Jimenez-Solem E, et al. Shift from adalimumab Originator to biosimilars in Denmark. JAMA Intern Med 2020;180:902–3. 10.1001/jamainternmed.2020.0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peyrin-Biroulet L, Danese S, Cummings F, et al. Anti-Tnf biosimilars in Crohn's disease: a patient-centric interdisciplinary approach. Expert Rev Gastroenterol Hepatol 2019;13:731–8. 10.1080/17474124.2019.1645595 [DOI] [PubMed] [Google Scholar]
- 12. Dörner T, Strand V, Cornes P, et al. The changing landscape of biosimilars in rheumatology. Ann Rheum Dis 2016;75:974–82. 10.1136/annrheumdis-2016-209166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. European Federation of Pharmaceutical Industries and Associations (EFPIA) . Promoting safe and effective biosimilars can lead to more affordable biologic medicines. Available: https://www.efpia.eu/publications/data-center/medicines-costs-in-context/biosimilars/ [Accessed 24 Mar 2020].
- 14. Triplitt C, Hinnen D, Valentine V. How similar are biosimilars? what do clinicians need to know about Biosimilar and follow-on insulins? Clin Diabetes 2017;35:209–16. 10.2337/cd16-0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bui LA, Hurst S, Finch GL, et al. Key considerations in the preclinical development of biosimilars. Drug Discov Today 2015;20 Suppl 1:3–15. 10.1016/j.drudis.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 16. European Medicines Agency (EMA) . Guideline on similar biological medicinal product - Rev 1. Available: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-rev1_en.pdf [Accessed 24 Mar 2020].
- 17. United States Federal Drug Administration (US FDA) . Scientific considerations in demonstrating Biosimilarity to a reference product. Available: https://www.fda.gov/media/82647/download [Accessed 24 Mar 2020].
- 18. European Medicines Agency (EMA) . Biosimilars in the EU - Information guide for healthcare professionals. Available: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf [Accessed 24 Mar 2020].
- 19. United States Federal Drug Administration (US FDA) . Biosimilar development, review, and approval. Available: https://www.fda.gov/drugs/biosimilars/biosimilar-development-review-and-approval [Accessed 24 Mar 2020].
- 20. Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits 2013;6:469–78. [PMC free article] [PubMed] [Google Scholar]
- 21. Woo SJ, Veith M, Hamouz J, et al. Efficacy and safety of a proposed ranibizumab Biosimilar product vs a reference ranibizumab product for patients with neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol 2021;139:68–76. 10.1001/jamaophthalmol.2020.5053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barbazetto I, Burdan A, Bressler NM, et al. Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin: fluorescein angiographic guidelines for evaluation and treatment--TAP and VIP report No. 2. Arch Ophthalmol 2003;121:1253–68. 10.1001/archopht.121.9.1253 [DOI] [PubMed] [Google Scholar]
- 23. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31. 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 24. Kaiser PK, Blodi BA, Shapiro H, et al. Angiographic and optical coherence tomographic results of the marina study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology 2007;114:1868–75. 10.1016/j.ophtha.2007.04.030 [DOI] [PubMed] [Google Scholar]
- 25. Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the anchor study. Ophthalmology 2009;116:57–65. 10.1016/j.ophtha.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 26. Sadda SR, Stoller G, Boyer DS, et al. Anatomical benefit from ranibizumab treatment of predominantly classic neovascular age-related macular degeneration in the 2-year anchor study. Retina 2010;30:1390–9. 10.1097/IAE.0b013e3181e44599 [DOI] [PubMed] [Google Scholar]
- 27. Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 1. Am J Ophthalmol 2008;145:239–48. 10.1016/j.ajo.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 28. CATT Research Group, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897–908. 10.1056/NEJMoa1102673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 Mg or 2.0 Mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2013;120:1046–56. 10.1016/j.ophtha.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 30. Lucentis prescribing information. Available: https://www.gene.com/download/pdf/lucentis_prescribing.pdf [Accessed 2 Apr 2020].
- 31. Lucentis - Summary of Product Characteristics. Available: https://www.ema.europa.eu/en/documents/product-information/lucentis-epar-product-information_en.pdf [Accessed 8 Dec 2020].
- 32. Bressler NM, Ranibizumab B. Biosimilar ranibizumab (SB11) vs reference Ranibizumab-Diving deeper for safety and Efficacy-Reply. JAMA Ophthalmol 2021;139:678–9. 10.1001/jamaophthalmol.2021.1043 [DOI] [PubMed] [Google Scholar]
- 33. Dutta B, Huys I, Vulto AG, et al. Identifying key benefits in European Off-Patent biologics and Biosimilar markets: it is not only about price! BioDrugs 2020;34:159–70. 10.1007/s40259-019-00395-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jensen TB, Bartels D, Sædder EA, et al. The Danish model for the quick and safe implementation of infliximab and etanercept biosimilars. Eur J Clin Pharmacol 2020;76:35–40. 10.1007/s00228-019-02765-3 [DOI] [PubMed] [Google Scholar]
- 35. Chong V. Ranibizumab for the treatment of wet AMD: a summary of real-world studies. Eye 2016;30:270–86. 10.1038/eye.2015.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holz FG, Bandello F, Gillies M, et al. Safety of ranibizumab in routine clinical practice: 1-year retrospective pooled analysis of four European neovascular AMD registries within the luminous programme. Br J Ophthalmol 2013;97:1161–7. 10.1136/bjophthalmol-2013-303232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holz FG, Figueroa MS, Bandello F. Ranibizumab treatment in treatment-naive neovascular age-related macular degeneration: results from luminous, a global real-world study. Retina 2019. 10.1097/iae.0000000000002670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol 2015;99:220–6. 10.1136/bjophthalmol-2014-305327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kiss S, Campbell J, Almony A, et al. Management and outcomes for neovascular age-related macular degeneration: analysis of United States electronic health records. Ophthalmology 2020;127:1179–88. 10.1016/j.ophtha.2020.02.027 [DOI] [PubMed] [Google Scholar]
- 40. Sharma A, Reddy P, Kuppermann BD, et al. Biosimilars in ophthalmology: "Is there a big change on the horizon?". Clinical ophthalmology 2018;12:2137–43. 10.2147/OPTH.S180393 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjophthalmol-2021-319637supp001.pdf (491.8KB, pdf)
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. How to access data: To gain access, data requestors must enter into a data access agreement with Samsung Bioepis. Additional Information Who can access the data: Upon request, and subject to certain criteria, conditions and exceptions, Samsung Bioepis will provide access to individual de-identified participant data. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply. Types of analyses: The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply. Mechanisms of data availability: Data may be made available with a signed data access agreement.