Abstract
Background and purpose
We evaluate whether non-haemorrhagic imaging markers (NHIM) (white matter hyperintensity patterns, lacunes and enlarged perivascular spaces (EPVS)) can discriminate cerebral amyloid angiopathy (CAA) from hypertensive cerebral small vessel disease (HTN-cSVD) among patients with isolated lobar intracerebral haemorrhage (isolated-LICH).
Methods
In patients with isolated-LICH, four cSVD aetiologic groups were created by incorporating the presence/distribution of NHIM: HTN-cSVD pattern, CAA pattern, mixed NHIM and no NHIM. CAA pattern consisted of patients with any combination of severe centrum semiovale EPVS, lobar lacunes or multiple subcortical spots pattern. HTN-cSVD pattern consisted of any HTN-cSVD markers: severe basal ganglia PVS, deep lacunes or peribasal ganglia white matter hyperintensity pattern. Mixed NHIM consisted of at least one imaging marker from either pattern. Our hypothesis was that patients with HTN-cSVD pattern/mixed NHIM would have a higher frequency of left ventricular hypertrophy (LVH), which is associated with HTN-cSVD.
Results
In 261 patients with isolated-LICH, CAA pattern was diagnosed in 93 patients, HTN-cSVD pattern in 53 patients, mixed NHIM in 19 patients and no NHIM in 96 patients. The frequency of LVH was similar among those with HTN-cSVD pattern and mixed NHIM (50% vs 39%, p=0.418) but was more frequent in HTN-cSVD pattern compared with CAA pattern (50% vs 20%, p<0.001). In a regression model, HTN-cSVD pattern (OR: 7.38; 95% CI 2.84 to 19.20) and mixed NHIM (OR: 4.45; 95% CI 1.25 to 15.90) were found to be independently associated with LVH.
Conclusion
Among patients with isolated-LICH, NHIM may help differentiate HTN-cSVD from CAA, using LVH as a marker for HTN-cSVD.
Keywords: Stroke, hemorrhage, Magnetic Resonance Imaging, cerebrovascular disorders
WHAT IS ALREADY KNOWN ON THIS TOPIC
The Boston criteria for cerebral amyloid angiopathy (CAA) are useful for diagnosing CAA non-invasively using neuroimaging. However, because of the imperfect specificity for possible CAA in the Boston criteria, isolated lobar intracerebral haemorrhage (isolated-LICH) may also be caused by hypertensive cerebral small vessel disease (HTN-cSVD). Currently, there are no validated methods of further discriminating the two aetiologies in patients that present with isolated-LICH and no additional haemorrhagic markers.
WHAT THIS STUDY ADDS
This work demonstrates that in patients with isolated-LICH, the concurrent presence of other non-hemorrhagic imaging markers (NHIM) such as white matter hyperintensity patterns, lacunes and enlarged perivascular spaces may help discriminate CAA from HTN-cSVD.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The results of this study can aid clinicians in understanding the underlying aetiology for a patient that presents with lobar haemorrhage. This study provides the impetus to further characterise the underlying aetiology in patients with isolated-LICH and no NHIM.
Introduction
The creation of the MRI-based Boston criteria for cerebral amyloid angiopathy (CAA) in the late 1990s was a major advance in the field of cerebral small vessel disease (cSVD).1 Strictly lobar location of the intracerebral haemorrhage (ICH) and concomitant lobar cerebral microbleeds (CMBs) provide an accurate diagnosis of CAA in vivo without needing histopathology, as long as rare causes such as mass lesion and gross vascular pathologies are ruled out. Similarly, based on the presence of any CMBs in deep brain areas (including patients with mixed-location ICH/CMBs), a diagnosis of hypertensive cSVD (HTN-cSVD), the other major cause of primary ICH, can be made.2 Given the distinct recurrence risks and treatment options of these cSVD subtypes, the accurate diagnoses of these diseases are critical.
In the absence of CMBs or cortical superficial siderosis (cSS), isolated lobar intracerebral haemorrhage (isolated-LICH), the ‘possible’ category of the modified Boston criteria for CAA, carries a 62% specificity for diagnosing CAA using pathology as the gold standard.3 However, in 26 patients that were diagnosed as having isolated-LICH, only 10 were found to have CAA confirmed by pathology. Therefore, isolated-LICH may be due to causes other than CAA—namely, HTN-cSVD.
Over the past 5 years, several MRI-based non-haemorrhagic imaging markers (NHIM), such as white matter hyperintensity (WMH) patterns, lacunes and enlarged perivascular spaces (EPVS), have emerged as cSVD biomarkers.4 Furthermore, the specific distributions and topography of these NHIM have been associated with the presence of either HTN-cSVD or CAA.5–7 Herein, we aimed to determine whether these NHIM can be used to improve the ability to differentiate cSVD subtypes in patients with isolated-LICH. Because there are no validated radiopathological criteria to diagnose HTN-cSVD, we used the presence of left ventricular hypertrophy (LVH), which has been demonstrated to be a surrogate marker for HTN-cSVD.8 We hypothesised that patients with isolated-LICH with the HTN-cSVD NHIM pattern have a higher frequency of LVH than patients with CAA pattern or patients with no NHIM. Given that the presence of mixed-location ICH/CMBs reflects severe HTN-cSVD,2 9 we also hypothesised that patients with mixed NHIM will have a higher frequency of LVH than patients with CAA pattern or patients with no NHIM.
Methods
Clinical and demographic variables
For this study, we analysed patient data from consecutive patients that presented to Massachusetts General Hospital with non-traumatic spontaneous ICH from 2003 to 2019. As described elsewhere, vascular malformations and other potential ICH aetiologies were exonerated prior to inclusion in this database such that the presumed cause of ICH in all patients was cSVD.5 Demographic information such as vascular risk factors (hypertension, diabetes mellitus and hyperlipidaemia), alcohol usage, smoking history, atrial fibrillation, coronary artery disease, prior stroke, dementia history and prehospital antithrombotic therapies were entered in the database. The admission systolic blood pressure and diastolic blood pressure, Glasgow Coma Scale scores and laboratory values, including white blood cell counts, platelet counts, haemoglobin levels, serum creatinine levels and international normalised ratios, were recorded. Treatments such as extraventricular drain placement and surgical evacuation were collected as well. In addition, clinical outcomes such as length of stay, discharge modified Rankin scores10 and in-hospital death were determined. Only patients who received at least one MRI study with a haemosiderin-sensitive MRI sequence were included in the analysis.
The presence of LVH (an imaging marker for hypertensive end-organ damage11 and hence HTN-cSVD2 8 12 13) was determined by screening transthoracic echocardiography reports within 1 year of the event. LVH was defined according to the American Society of Echocardiography guidelines using left ventricular mass indexed to body surface area (with a left ventricular mass index cut-off of >95 g/m2 for women and >115 g/m2 for men).14 In patients that did not receive a transthoracic echocardiography study, admission ECGs were reviewed for the presence of LVH using the Sokolow-Lyon criteria (ie, amplitude sum of S wave in V1+R wave in V5 or V6 is ≥3.5 mV).15 Electrocardiographic criteria carries a high specificity for LVH based on echocardiography measurements,16 and both methods have been used simultaneously in prior reports.17 18 Lastly, pathology reports from patients that received haematoma evacuation or autopsy were reviewed for a diagnosis of CAA or HTN-cSVD.
Imaging analysis
ICH location on CT was categorised as either lobar, deep, cerebellar, intraventricular or mixed by two neurologists (RWR and ASD). The presence of perihaematomal oedema, subarachnoid haemorrhage and intraventricular extension were recorded. MRIs of the brain were analysed by a neurologist (ASD) blinded to clinical information for cSVD markers (as outlined in the STRIVE criteria4 19), including WMHs (scored by the Fazekas scale20 and classified using validated patterns5), EPVS (graded using a 4-point scale6), lacunes,7 21 22 CMBs23 and cSS.24 Severe EPVS were considered to be a score >2.6
Regarding WMH patterns, two established patterns were reviewed on brain MRI fluid-attenuated inversion recovery (FLAIR) sequences: multiple subcortical spots WMH pattern and peribasal ganglia WMH pattern. Multiple subcortical spots WMH pattern has been associated with CAA and refers to the presence of >10 small circles or spots of WMHs in the subcortical white matter.5 Peribasal ganglia WMH pattern has been associated with HTN-cSVD and is defined as the presence of WMHs encircling the basal ganglia.5 Lacunes were considered to be round, fluid-filled cavities ranging from 3 mm to 15 mm in diameter.19 They are often seen with a central hypointensity on T1-weighted images with a rim of surrounding hyperintensity on FLAIR.4 FLAIR, T2-weighted images and T1-weighted images were reviewed to ensure that those structures identified as lacunes were compatible with the aforementioned definition and were not additional mimics such as EPVS or WMHs. Lacunes were further separated into lobar lacunes, which are associated with CAA, and deep lacunes, which are associated with HTN-cSVD.21 Lacunes are considered lobar when found in the frontal, parietal, temporal, insular or occipital lobes, whereas deep lacunes are found in the internal capsule, thalamus or basal ganglia.7
The diagnosis of possible and probable CAA was made based on the ICH distribution and the presence of strictly lobar CMBs or cSS as outlined in the modified Boston criteria.25 Patients with strictly deep ICH/CMBs or mixed-location ICH/CMBs were diagnosed with HTN-cSVD.2 9 Available neuropathology results among isolated-LICH patients (n=10) were compared with the aetiologic classification by NHIM but were not incorporated into the final radiologic diagnosis because of their small numbers. The patients with possible CAA (isolated-LICH) were extracted from the full ICH database for analysis in this study. These patients were categorised into four cSVD subgroups using the presence and distribution of NHIM: HTN-cSVD pattern, CAA pattern, mixed NHIM and no NHIM (figure 1). The HTN-cSVD pattern was defined as any number of strict HTN-cSVD features: severe basal ganglia PVS, deep lacunes or peribasal ganglia WMH pattern. The CAA pattern consisted of patients with any number of strict CAA features: severe centrum semiovale (CSO) EPVS, lobar lacunes or multiple subcortical spots WMH hyperintensity pattern. The mixed NHIM group was defined as the presence of at least one imaging finding from the CAA pattern and HTN-cSVD pattern. The no NHIM group did not have any of the aforementioned biomarkers.
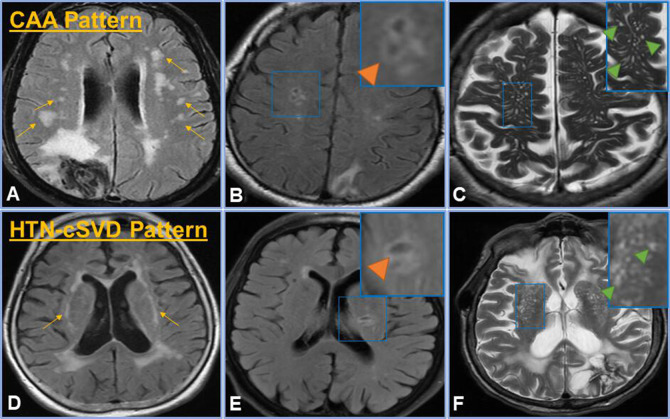
Figure 1.
cSVD groups. CAA pattern was defined by any number of CAA features ((A) multiple subcortical spots pattern on FLAIR (yellow arrows), (B) lobar lacunes on FLAIR (inset, orange arrow) and (C) severe CSO EPVS as seen on T2 (inset, green arrows)), whereas HTN-cSVD pattern includes one or more of the features in the bottom panel, which are classically attributed to HTN-cSVD ((D) peribasal ganglia WMH on FLAIR (yellow arrows), (E) deep lacunes on FLAIR (inset, orange arrow) and (F) severe basal ganglia EPVS as seen on T2 (inset, green arrows)). The mixed NHIM group included at least one imaging finding from both the CAA pattern and HTN-cSVD pattern. No NHIM had none of these imaging characteristics.CAA, cerebral amyloid angiopathy; CSO, centrum semiovale; cSVD, cerebral small vessel disease; EPVS, enlarged perivascular spaces; FLAIR, fluid-attenuated inversion recovery; HTN-cSVD, hyperintensive cerebral small vessel disease; NHIM, non-haemorrhagic imaging markers; WMH, white matter hyperintensities.
Statistical analysis
Continuous variables were reported as mean (SD) or median (IQR) based on the normality of the distribution, and categorical variables were reported as per cent and count. The t-test or non-parametric Wilcoxon rank-sum test was used to identify differences between continuous variables, and Fisher’s Exact test was used to identify differences in categorical variables. Vascular risk factors, creatinine (which has also been associated with HTN-cSVD2) and the presence of LVH was compared between each of the four cSVD subgroups using the appropriate univariate tests. Lastly, a logistic regression was constructed to determine the factors that predicted LVH (as a surrogate for HTN-cSVD). The variables (determined a priori) included in this model were age, sex, vascular risk factors, coronary artery disease, atrial fibrillation, creatinine, HTN-cSVD pattern, CAA pattern and mixed NHIM. Two-tailed p values <0.05 were considered to be statistically significant.
Statistical analyses were performed with SPSS for Windows, V.23.0. Unpublished data are available upon reasonable request from the corresponding author.
Results
Seventeen hundred and ninety one patients with ICH were included in the study, of which 1289 (72%) received an MRI of the brain (figure 2). HTN-cSVD was diagnosed in 631 (49%) patients, probable CAA was diagnosed in 396 (31%) patients and isolated-LICH (possible CAA) was diagnosed in 261 (20%) patients. The baseline characteristics of patients with isolated-LICH are shown in table 1. The mean age of the cohort was 73 (±12) years and 147 (56%) were females. Hypertension was the most frequent risk factor identified and was found in 193 (74%) patients. Coronary artery disease was found in 38 (15%) patients and atrial fibrillation was found in 43 (16%) patients. Ninety-four (36%) patients were on antiplatelet therapy at admission, and 31 (12%) patients were on anticoagulation at admission. The average systolic blood pressure during admission was 162 mm Hg (±29 mm Hg) and the average diastolic blood pressure during admission was 85 mm Hg (±16 mm Hg).
Figure 2.
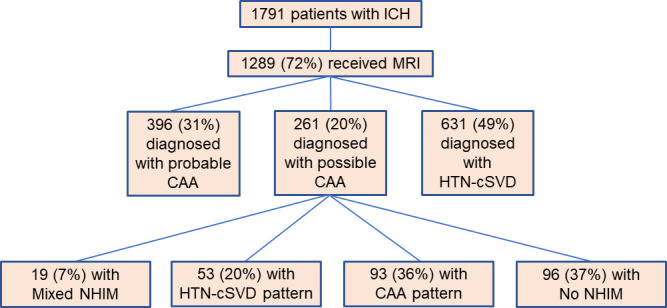
Participant selection methodology. The above schematic depicts the total number of patients included in the study (n=1791). Of these, 1289 (72%) received an MRI scan of the brain. From this cohort, patients were diagnosed with either probable CAA, possible CAA (isolated lobar ICH) or HTN-cSVD. Lastly, the patients with isolated lobar ICH were further stratified into patients with HTN-cSVD pattern, CAA pattern, mixed NHIM or no NHIM. CAA, cerebral amyloid angiopathy; HTN-cSVD, hyperintensive cerebral amyloid angiopathy; ICH, intracerebral haemorrhage; NHIM, non-haemorrhagic imaging markers.
Table 1.
Baseline characteristics of patients with isolated-LICH (n=261)
| Age (years), mean±SD | 73 (±12) |
| Female sex | 147 (56) |
| Race | |
| White | 226 (87) |
| Black | 16 (6) |
| Asian | 10 (4) |
| Hispanic | 8 (3) |
| Other | 1 (0) |
| Vascular risk factors | |
| Hypertension | 193 (74) |
| Hyperlipidaemia | 131 (50) |
| Diabetes | 40 (15) |
| Coronary artery disease | 38 (15) |
| Atrial fibrillation | 43 (16) |
| Prior stroke | 43 (16) |
| Substance use | |
| Smoking history | 66 (25) |
| Alcohol abuse | 60 (23) |
| Dementia | 23 (8) |
| Antithrombotic therapies | |
| None | 136 (52) |
| Antiplatelet | 94 (36) |
| Anticoagulant | 31 (12) |
| Glasgow Coma Scale score, median (IQR) | 15 (13, 15) |
| Systolic blood pressure (mm Hg), mean±SD | 162 (±29) |
| Diastolic blood pressure (mm Hg), mean±SD | 85 (±16) |
| Laboratory values, mean±SD | |
| White blood cell count (×103/µL), mean±SD | 9.8 (±4.3) |
| Haemoglobin (g/dL), mean±SD | 13.6 (±1.8) |
| Platelets (×103/µL), mean±SD | 232 (±67) |
| SCr (mg/dL), mean±SD | 0.97 (±0.44) |
| International normalised ratio, mean±SD | 1.3 (±0.8) |
| LVH | 59 (23) |
| CT findings | |
| Presence of perihaematoma oedema | 236 (90) |
| Presence of subarachnoid haemorrhage | 84 (32) |
| Presence of intraventricular extension | 86 (33) |
| Haemosiderin-sensitive MRI sequence | |
| GRE | 182 (70) |
| SWI/SWAN | 73 (28) |
| Other | 6 (2) |
| Treatments | |
| Extraventricular drain placement | 28 (11) |
| Surgical evacuation | 30 (12) |
| Outcomes | |
| Length of stay, median (IQR) | 7 (4, 12) |
| Poor outcome | 120 (46) |
| In-hospital mortality | 93 (36) |
This table shows the baseline characteristics of patients with isolated-LICH. Data are counts (n) and percentages (%), means and SD, or medians and IQRs. Poor outcome was considered to be a discharge modified Rankin Scale score >2.
GRE, gradient echo sequences; LICH, lobar intracerebral haemorrhage; LVH, left ventricular hypertrophy; SCr, serum creatinine; SWAN, susceptibility-weighted angiography; SWI, susceptibility weighted imaging.
Of the patients with isolated-LICH, the CAA pattern was diagnosed in 93 (36%) patients, HTN-cSVD pattern in 53 (20%) patients, mixed NHIM in 19 (7%) patients and no NHIM in 96 (37%) patients. The number of biomarkers found in each cSVD group is shown in figure 3, and the prevalence of the individual NHIM for each cSVD group is shown in figure 4. Greater than 75% of patients with the CAA pattern and HTN-cSVD pattern had only one NHIM, while approximately 80% of patients in the mixed NHIM group had at least 2 or 3 NHIM.
Figure 3.
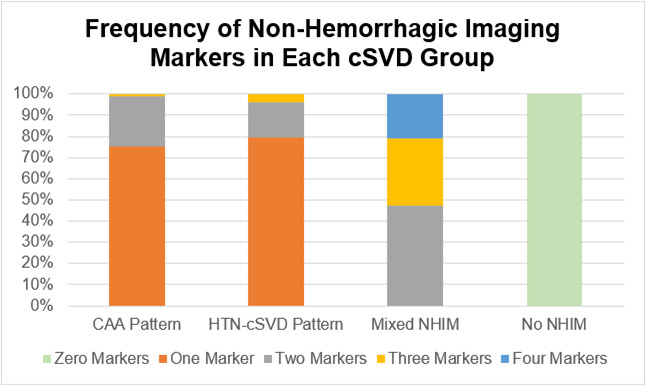
Frequency of NHIM in cSVD group. This figure depicts the frequency of haemorrhagic markers found in each subgroup of isolated-LICH. The majority of patients with the CAA pattern and the HTN-cSVD pattern have one marker (with the maximum being three markers). Patients with mixed NHIM can have up to four markers, and patients with no NHIM will have zero markers (by definition). CAA, cerebral amyloid angiopathy; cSVD, cerebral small vessel disease; HTN-cSVD, hyperintensive cerebral small vessel disease; LICH, lobar intracerebral haemorrhage; NHIM, non-haemorrhagic imaging markers.
Figure 4.
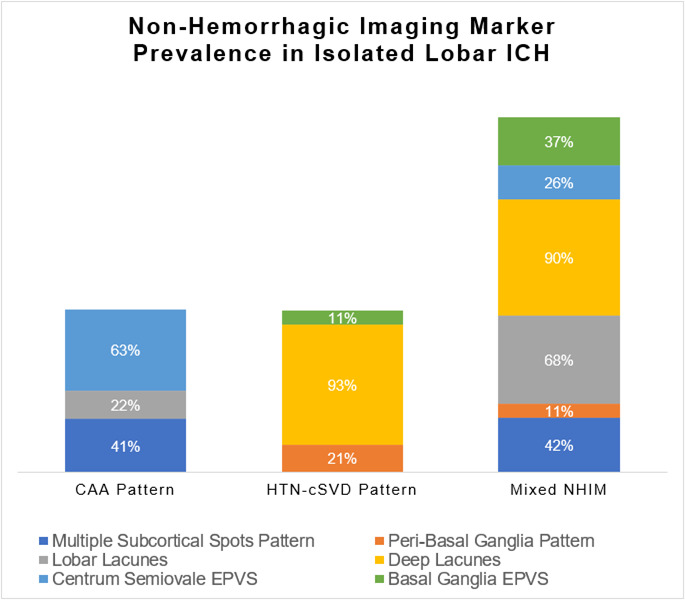
NHIM prevalence in isolated lobar -ICH. This figure depicts the prevalence of each NHIM found in the subgroups of isolated lobar -ICH. Multiple subcortical spots patterns were the most common feature in the CAA pattern, whereas deep lacunes were the most common feature in both the HTN-cSVD pattern and the mixed NHIM pattern. CAA, cerebral amyloid angiopathy; EPVS, enlarged perivascular spaces; HTN-sCVD, hypertensive cerebral small vessel disease; ICH, intracerebral haemorrhage; NHIM, non-haemorrhagic imaging markers.
To determine whether the burden of vascular risk factors was greater in patients with HTN-cSVD pattern, the frequency of vascular risk factors was compared among each cSVD subgroup (as listed in table 2). There were no differences in hypertension, hyperlipidaemia, diabetes, coronary disease or atrial fibrillation between each subgroup. In addition, the admission blood pressure did not differ between patients with the HTN-cSVD pattern compared with the CAA pattern (166±35 mm Hg vs 160±29 mm Hg, p=0.312). Notably, patients with mixed NHIM and HTN-cSVD pattern were older than those patients with no NHIM (p=0.037 and p=0.010, respectively). There was a trend toward higher creatinine levels in patients with HTN-cSVD pattern compared with CAA pattern (1.00±0.32 mg/dL vs 0.96±0.25 mg/dL, p=0.072). Serum creatinine was similar between patients with the HTN-cSVD pattern and patients with mixed NHIM (1.00±0.32 mg/dL vs 0.99±0.33 mg/dL, p=0.926).
Table 2.
Comparison of risk factors among single LICH groups
| CAA pattern (n=93) | HTN-cSVD pattern (n=53) | Mixed NHIM (n=19) | No NHIM (n=96) | |
| Age (years), mean±SD | 72.5±10.6 | 78.7±10.3* | 80.4±8.9† | 68.1±13.7‡ |
| Female sex | 49 (53) | 31 (59) | 14 (74) | 53 (55) |
| Hypertension | 71 (76) | 40 (76) | 17 (90) | 65 (68) |
| Hyperlipiaemia | 47 (51) | 30 (57) | 7 (37) | 47 (49) |
| Diabetes | 13 (14) | 9 (17) | 3 (16) | 15 (16) |
| Coronary disease | 12 (13) | 11 (21) | 2 (11) | 13 (14) |
| Atrial fibrillation | 18 (19) | 11 (21) | 4 (21) | 10 (10) |
| SCr (mg/dL), mean±SD | 0.96±0.25 | 1.00±0.32 | 0.99±0.33 | 0.97±0.63 |
| LVH | 17 (20)§ | 25 (50)¶ | 7 (39)** | 10 (12)†† |
The table above shows the patient demographics, frequency of vascular risk factors, and markers of HTN-cSVD (SCr and LVH) among the various subgroups of single LICH. Data are counts (n) and percentages (%) unless specified otherwise. Of note, p<0.05 when * is compared with ‡, † is compared with ‡, § is compared with ¶, ¶ is compared with ** and ** is compared with ††.
CAA, cerebral amyloid angiopathy; HTN-cSVD, hypertensive cerebral small vessel disease; LICH, lobar intracerebral haemorrhage; LVH, left ventricular hypertrophy; NHIM, non-haemorrhagic imaging markers; SCr, serum creatinine; SCr, serum creatinine.
The frequency of LVH was then compared between each cSVD subgroup. LVH was more frequent in patients with the HTN-cSVD pattern compared with those with the CAA pattern (50% vs 20%, p<0.001) but similar between patients with the HTN-cSVD pattern and patients with mixed NHIM (50% vs 39%, p=0.418). There was a trend toward more LVH among patients with mixed NHIM compared with those with the CAA pattern (39% vs 20%, p=0.080). Patients with no NHIM had a lower frequency of LVH than patients with the HTN-cSVD pattern (12% vs 50%, p<0.001) and patients with mixed NHIM (12% vs 39%, p=0.005). Patients with the CAA pattern and no NHIM had a similar frequency of LVH (20% vs 12%, p=0.161). In the multivariable model (table 3), creatinine levels (OR: 2.20; 95% CI 1.04 to 4.68; p=0.040), HTN-cSVD pattern (OR: 7.38; 95% CI 2.84 to 19.20; p<0.001) and mixed NHIM (OR: 4.45; 95% CI 1.25 to 15.90; p=0.022) were found to be independent predictors of LVH.
Table 3.
Associations with LVH
| Unadjusted | Adjusted | |||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.03 (1.00 to 1.05) | 0.038 | 1.01 (0.98 to 1.04) | 0.724 |
| Sex | 1.07 (0.59 to 1.93) | 0.828 | 0.98 (0.48 to 1.99) | 0.957 |
| Hypertension | 0.81 (0.39 to 1.66) | 0.561 | 0.93 (0.40 to 2.19) | 0.872 |
| Hyperlipidaemia | 0.87 (0.49 to 1.58) | 0.654 | 0.69 (0.33 to 1.44) | 0.324 |
| Diabetes | 0.99 (0.44 to 2.24) | 0.975 | 0.93 (0.36 to 2.42) | 0.881 |
| Coronary artery disease | 1.50 (0.70 to 3.21) | 0.293 | 1.17 (0.45 to 3.03) | 0.752 |
| Atrial fibrillation | 2.10 (1.02 to 4.33) | 0.044 | 1.72 (0.74 to 4.02) | 0.210 |
| Creatinine | 2.22 (1.04 to 4.76) | 0.040 | 2.20 (1.04 to 4.68) | 0.040 |
| CAA pattern | 1.55 (0.82 to 2.94) | 0.179 | 1.90 (0.78 to 4.66) | 0.161 |
| HTN-cSVD pattern | 4.53 (2.32 to 8.83) | <0.001 | 7.38 (2.84 to 19.20) | <0.001 |
| Mixed NHIM | 2.06 (0.76 to 5.57) | 0.157 | 4.45 (1.25 to 15.90) | 0.019 |
This table shows univariate and multivariable associations between predictor variables and LVH.
CAA, cerebral amyloid angiopathy; HTN-cSVD, hypertensive cerebral small vessel disease; LVH, left ventricular hypertrophy; NHIM, non-haemorrhagic imaging markers.
To further validate our results, we compared our cSVD classification to available neuropathological data. Twenty seven (10%) patients with isolated-LICH received either haematoma evacuation, a biopsy or postmortem autopsy evaluation. Seventeen of these 27 evaluations were non-diagnostic. Based on pathology review, two patients were found to have hypertensive arteriopathy and eight patients were found to have CAA. Both patients with hypertensive arteriopathy were classified as HTN-cSVD pattern using our NHIM classification. Seven patients out of 8 (88%) that were found to have pathological evidence of CAA were classified as either having CAA pattern or no NHIM (and the remaining patient classified as having HTN-cSVD pattern). Six of these eight patients did not have LVH. Of the remaining two patients, the patient that was classified as having HTN-cSVD had LVH, and the other patient did not have any cardiac studies performed.
Discussion
In a cohort of patients with isolated-LICH, NHIM can aid in discriminating HTN-cSVD from CAA. Although probable CAA carries 100% specificity for the pathological diagnosis of CAA from the original validation study,3 the accuracy of possible CAA for a pathological diagnosis of CAA is much lower, at around 62%. In this study, we incorporate recently identified non-haemorrhagic cSVD markers to increase the accuracy of the cSVD etiologic diagnosis in patients with isolated-LICH. While lobar haemorrhage is classically thought to be due to CAA, recent evidence suggests that the underlying aetiology of patients with a lobar haemorrhage and any combination of deep and lobar CMBs is more likely due to HTN-cSVD.2 9 26 Furthermore, patients with mixed ICH/CMBs represent a more advanced form of HTN-cSVD given the higher burden of cSVD biomarkers and risk factors.2 These recent developments challenge the notion that all lobar ICH is due to CAA and raises the possibility that HTN-cSVD can give rise to lobar ICH, thereby offering an explanation for the reduced specificity of possible CAA.27 Since patients with possible CAA from one lobar ICH will not have any CMBs and cSS, additional markers of cSVD, including WMH patterns, lacunes and severe EPVS, may be useful in further delineating the underlying cSVD aetiology.
Although some studies have reported increased frequency of hypertension in patients with HTN-cSVD compared with those with CAA,2 several other studies have demonstrated similar burdens of vascular risk factors between these two cSVD groups.8 28 29 Our study also did not show any differences between the frequency of vascular risk factors between the four cSVD groups among patients with isolated-LICH. A single lobar ICH probably represents a relatively early phase of cerebral injury even among HTN-cSVD and this might explain the lack of significant differences in risk factor load. As reported in other studies, the admission blood pressure was not significantly different between patients with HTN-cSVD pattern and CAA pattern suggesting that the binary variable of hypertension (ie, the presence or absence) is a relatively non-specific risk factor that is unreliable at predicting underlying cSVD aetiology.8 To increase our ability to discriminate cSVD aetiologies, we employed LVH as sensitive non-invasive cardiac biomarker that has been associated with the presence of HTN-cSVD.8 28 30 As expected, we demonstrated that patients with CAA pattern had a low frequency of LVH, compared with patients with HTN-cSVD pattern, which had a high frequency of LVH even among isolated-LICH. Given that mixed-location ICH/CMBs represents a more advanced form of HTN-cSVD, it follows that patients with mixed NHIM would also have HTN-cSVD and exhibit LVH. Patients with no NHIM had a similar frequency of LVH as those with the CAA pattern suggesting that isolated-LICH without any additional NHIM might be more likely to represent CAA in its early stages. This notion was reinforced by the eight pathologically proven patients with CAA in which seven of them were classified as either CAA pattern or no NHIM.
In addition to LVH, we explored serum creatinine as a possible laboratory marker that may aid in discriminating subgroups of cSVD. However, no major differences were found in serum creatinine levels among the various subgroups. A previous study demonstrated that serum creatinine levels were higher in patients with mixed-location ICH/CMBs compared with those with CAA, although this finding was not replicated in a more recent cross-sectional study of East Asian patients.2 9 Given that mixed-location ICH/CMBs is a severe form of HTN-cSVD, we suspect that serum creatinine levels only rise in patients with advanced or long-standing cSVD pathology. In a cohort of patients with isolated-LICH and mixed NHIM, it is likely that the average cSVD burden is much lower than would be expected in a cohort of patients with mixed-location ICH/CMBs. Indeed, only a small percentage of patients in our study had four types of NHIM. We suspect that serum creatinine may not be as sensitive as LVH as a marker for HTN-cSVD. This notion is consistent with a prior study showing that LVH is associated with cardiovascular morbidity and stroke, whereas chronic kidney disease is only associated with cardiovascular morbidity (but not stroke).31
Our study has several limitations. Despite using a large cohort of patients with ICH, only 72% of them received an MRI of the brain, resulting in a small sample size of patients with isolated-LICH. Nevertheless, this group of patients with primary ICH is the largest cohort with clinical grade MRIs and detailed clinical, laboratory and cardiac assessments. Second, although 10% of patients in our study received a biopsy, the majority of these were non-diagnostic, thereby limiting pathological verification of our classification system. However, at least two of the NHIM biomarkers used in our study (EPVS and WMH patterns) were validated in the recently updated Boston criteria for probable CAA V.2.0 which used pathology as the gold standard.32 Our results confirm that additional NHIM can be used in cases of possible CAA, which carries the most diagnostic uncertainty for the underlying cSVD subtype. Because we used an ICH database, it is unclear whether our results apply to patients with isolated focal or disseminated cSS (without CMBs or macrobleeds), or patients with a single lobar CMB, both of whom meet criteria for possible CAA.25 Given that the present study analyses a late-stage sequelae of cSVD (namely, ICH), it may limit the relevance of our results to populations at earlier stages of cSVD.
While our results show a low frequency of LVH in patients with no NHIM (suggesting underlying CAA), future efforts should focus on additional verification of the predominant cSVD aetiology in this group using either histopathology or amyloid-β positron emission tomography studies.9 33 Furthermore, future efforts should be directed toward understanding the ICH recurrence risk of patients among our four categories of patients with isolated-LICH.
Conclusion
In a cohort of patients with isolated-LICH, the presence of additional NHIM may aid in discriminating patients with HTN-cSVD and CAA, using LVH as a surrogate marker for HTN-cSVD. Using NHIM led to enhanced diagnostic precision of cSVD aetiology in about two-third of patients with isolated-LICH. Such results may require additional verification with histopathology or positron emission tomography studies before they are incorporated into ICH classification systems.
Footnotes
Twitter: @alvindasMD, @rwregen, @braindoc_mgh
Contributors: ASD, EG and RWR: study concept and design, acquisition of data and manuscript drafting. MJH, KS, ND and AV: acquisition of data and critical revision of the manuscript. WTK, JNG, AB, NR, JR, LHS, SMG and MEG: study supervision, critical revision of the manuscript, and guarantor of the study.
Funding: This study was funded by support from the Andrew David Heitman Young Investigator Fund (grant numbers: R25NS065743 and R01NS11452).
Competing interests: ASD reports grants from the Andrew David Heitman Young Investigator Fund. RWR reports grants from the National Institute of Neurological Disorders and Stroke (R25 NS065743). JNG reports research support from National Institutes of Health (NIH), Pfizer, Takeda and Octapharma and consulting support from Alexion, CSL Behring, NControl and Cayuga. MEG receives funding from the NIH (R01NS11452).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Study approval was granted by our hospital’s institutional review board (protocol number: 2019P003026). Informed consent was waived for this study given the retrospective nature of analyses.
References
- 1. Greenberg SM, Rebeck GW, Vonsattel JP, et al. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol 1995;38:254–9. 10.1002/ana.410380219 [DOI] [PubMed] [Google Scholar]
- 2. Pasi M, Charidimou A, Boulouis G, et al. Mixed-location cerebral hemorrhage/microbleeds. Neurology 2018;90:e119–26. 10.1212/WNL.0000000000004797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knudsen KA, Rosand J, Karluk D, et al. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–9. 10.1212/WNL.56.4.537 [DOI] [PubMed] [Google Scholar]
- 4. Das AS, Regenhardt RW, Vernooij MW, et al. Asymptomatic cerebral small vessel disease: insights from population-based studies. J Stroke 2019;21:121–38. 10.5853/jos.2018.03608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charidimou A, Boulouis G, Haley K, et al. White matter hyperintensity patterns in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2016;86:505–11. 10.1212/WNL.0000000000002362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charidimou A, Boulouis G, Pasi M, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2017;88:1157–64. 10.1212/WNL.0000000000003746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pasi M, Boulouis G, Fotiadis P, et al. Distribution of lacunes in cerebral amyloid angiopathy and hypertensive small vessel disease. Neurology 2017;88:2162–8. 10.1212/WNL.0000000000004007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pallesen L-P, Wagner J, Lambrou D, et al. Association of hypertensive intracerebral hemorrhage with left ventricular hypertrophy on transthoracic echocardiography. J Clin Med 2020;9:2148. 10.3390/jcm9072148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsai H-H, Pasi M, Tsai L-K, et al. Microangiopathy underlying mixed-location intracerebral hemorrhages/microbleeds: a PIB-PET study. Neurology 2019;92:e774–81. 10.1212/WNL.0000000000006953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–7. 10.1161/01.STR.19.5.604 [DOI] [PubMed] [Google Scholar]
- 11. Sagie A, Benjamin EJ, Galderisi M, et al. Echocardiographic assessment of left ventricular structure and diastolic filling in elderly subjects with borderline isolated systolic hypertension (the Framingham heart study). Am J Cardiol 1993;72:662–5. 10.1016/0002-9149(93)90881-C [DOI] [PubMed] [Google Scholar]
- 12. Papadopoulos A, Palaiopanos K, Protogerou AP, et al. Left ventricular hypertrophy and cerebral small vessel disease: a systematic review and meta-analysis. J Stroke 2020;22:206–24. 10.5853/jos.2019.03335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Das AS, Regenhardt RW, Gokcal E, et al. Idiopathic primary intraventricular hemorrhage and cerebral small vessel disease. Int J Stroke : 2022;17:645-653. 10.1177/17474930211043957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 2015;28:1–39. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 15. Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949;37:161–86. 10.1016/0002-8703(49)90562-1 [DOI] [PubMed] [Google Scholar]
- 16. Sklyar E, Ginelli P, Barton A, et al. Validity of electrocardiographic criteria for increased left ventricular mass in young patients in the general population. World J Cardiol 2017;9:248. 10.4330/wjc.v9.i3.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verdecchia P, Porcellati C, Reboldi G, et al. Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation 2001;104:2039–44. 10.1161/hc4201.097944 [DOI] [PubMed] [Google Scholar]
- 18. Selvetella G, Notte A, Maffei A, et al. Left ventricular hypertrophy is associated with asymptomatic cerebral damage in hypertensive patients. Stroke 2003;34:1766–70. 10.1161/01.STR.0000078310.98444.1D [DOI] [PubMed] [Google Scholar]
- 19. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–38. 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993;43:1683–9. 10.1212/WNL.43.9.1683 [DOI] [PubMed] [Google Scholar]
- 21. Das AS, Regenhardt RW, Feske SK, et al. Treatment approaches to lacunar stroke. J Stroke Cerebrovasc Dis 2019;28:2055–78. 10.1016/j.jstrokecerebrovasdis.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Regenhardt RW, Das AS, Ohtomo R, et al. Pathophysiology of lacunar stroke: history's mysteries and modern interpretations. J Stroke Cerebrovasc Dis 2019;28:2079–97. 10.1016/j.jstrokecerebrovasdis.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009;8:165–74. 10.1016/S1474-4422(09)70013-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Charidimou A, Boulouis G, Roongpiboonsopit D, et al. Cortical superficial siderosis multifocality in cerebral amyloid angiopathy: a prospective study. Neurology 2017;89:2128–35. 10.1212/WNL.0000000000004665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010;74:1346–50. 10.1212/WNL.0b013e3181dad605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jung YH, Jang H, Park SB, et al. Strictly lobar microbleeds reflect amyloid angiopathy regardless of cerebral and cerebellar compartments. Stroke 2020;51:3600–7. 10.1161/STROKEAHA.119.028487 [DOI] [PubMed] [Google Scholar]
- 27. Das AS, Gurol ME. Not all lobar hemorrhages are created equal. Stroke 2020;51:3485–6. 10.1161/STROKEAHA.120.032404 [DOI] [PubMed] [Google Scholar]
- 28. Broderick J, Brott T, Tomsick T, et al. Lobar hemorrhage in the elderly. The undiminishing importance of hypertension. Stroke 1993;24:49–51. 10.1161/01.STR.24.1.49 [DOI] [PubMed] [Google Scholar]
- 29. Guidoux C, Hauw J-J, Klein IF, et al. Amyloid angiopathy in brain hemorrhage: a postmortem Neuropathological-Magnetic resonance imaging study. Cerebrovasc Dis 2018;45:124–31. 10.1159/000486554 [DOI] [PubMed] [Google Scholar]
- 30. Tsai H-H, Pasi M, Tsai L-K, et al. Distribution of lacunar infarcts in Asians with intracerebral hemorrhage: a magnetic resonance imaging and amyloid positron emission tomography study. Stroke 2018;49:1515–7. 10.1161/STROKEAHA.118.021539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsioufis C, Vezali E, Tsiachris D, et al. Left ventricular hypertrophy versus chronic kidney disease as predictors of cardiovascular events in hypertension: a Greek 6-year-follow-up study. J Hypertens 2009;27:744–52. 10.1097/HJH.0b013e32832401ff [DOI] [PubMed] [Google Scholar]
- 32. Charidimou A, Boulouis G, Frosch M, et al. Abstract 36: the Boston criteria v2.0 for cerebral amyloid angiopathy: updated criteria and multicenter MRI-Neuropathology validation. Stroke 2021;52. 10.1161/str.52.suppl_1.36 [DOI] [Google Scholar]
- 33. Gurol ME, Becker JA, Fotiadis P, et al. Florbetapir-PET to diagnose cerebral amyloid angiopathy: a prospective study. Neurology 2016;87:2043–9. 10.1212/WNL.0000000000003197 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.